Significance
We reveal a novel and highly significant change in how items are held in mind in healthy aging. Using smartphones, data were collected from 29,631 participants, between the ages of 18–69 y. We compare the ability to exclude distractors when items are entered into working memory (WM) (encoding distraction, ED) and when items are held in mind (delay distraction, DD). In older adults, WM in the absence of distraction was more similar to ED exclusion than DD exclusion. A greater reliance on focused attention during encoding may reflect compensation for the more pronounced deterioration we observed in DD exclusion in older age. This can inform other areas of cognition and strategies to ameliorate or manage debilitating age-related cognitive decline.
Keywords: working memory, aging, distraction, attention
Abstract
A weakened ability to effectively resist distraction is a potential basis for reduced working memory capacity (WMC) associated with healthy aging. Exploiting data from 29,631 users of a smartphone game, we show that, as age increases, working memory (WM) performance is compromised more by distractors presented during WM maintenance than distractors presented during encoding. However, with increasing age, the ability to exclude distraction at encoding is a better predictor of WMC in the absence of distraction. A significantly greater contribution of distractor filtering at encoding represents a potential compensation for reduced WMC in older age.
The number of items that can be held in working memory (WM) declines with increasing age (1). Our ability to effectively exclude distractors is one basis for this limited working memory capacity (WMC) (2, 3), with impaired inhibitory processing of distraction contributing to an age-related reduction in WM performance (4). A specific impairment in suppressing distractor representations in older adults has been linked to reduced WMC (5). Typically distractors are presented either with the items to be remembered (encoding distraction, ED, e.g., 6, 7) or while these items are held in mind (delay distraction, DD, e.g., 5, 8). We recently highlighted a distinction between the effects of these two types of distraction in younger adults (9). Although greater WMC is associated with an enhanced ability to exclude distractors in both cases, each makes a unique contribution to WMC (9). Here we examine the well-known age-related reduction in WMC. Previous work has identified an age-related delay in ED filtering (7) and an early age-related deficit in DD suppression (8). We directly compare the age-related decline in ED and DD to assess whether an ability to ignore a distraction at encoding or at delay provides the best predictor of general WMC.
We obtained data from 29,631 users of a smartphone game (part of The Great Brain Experiment, www.thegreatbrainexperiment.com), a platform that has enabled us to replicate a range of laboratory studies (9, 10). Using this medium we implemented a WM task to enable us to directly compare the effects of age on WM in the absence of distractors (no distraction, ND; Fig. 1A), when distractors are presented at encoding (ED; Fig. 1B) and when distractors are presented during maintenance (DD; Fig. 1C). This large subject pool enabled us to consider data from six age groups (18–24 y: n = 7,658; 25–29 y: n = 5,702; 30–39 y: n = 8,225; 40–49 y: n = 4,667; 50–59 y: n = 2,359; and 60–69 y: n = 1,020). For each condition the number of items to be remembered (WM load) increased as a function of performance until either eight trials had been completed or a participant failed two successive trials of a given WM load. Data were excluded from participants who failed a “load 2” trial in any condition. For each condition, the participant’s score represents the maximum number of items for which they could report all items successfully, representing their WMC.
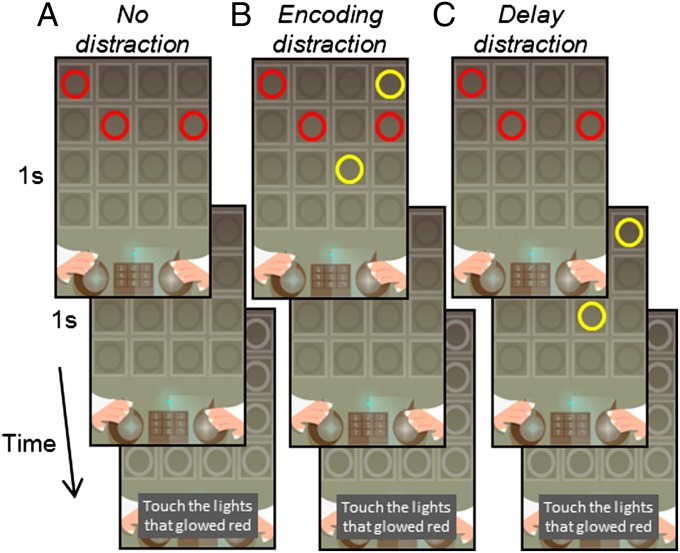
Fig. 1.
The smartphone game. Red circles are presented simultaneously, followed by a delay of 1 s. Participants should then indicate the positions of the red circles. (A) No distraction (ND) condition; only red circles are shown. (B) Encoding distraction (ED) condition; two yellow circles (distractors) are presented with the red circles. (C) Delay distraction (DD) condition; two yellow circles (distractors) are presented during the delay.
Results
Across all three conditions, performance declined with increasing age (Fig. 2A). Considering scores from the ED condition alone, an ANCOVA that controlled for ND score revealed a main effect of age (F5, 29,624 = 230.18, P < 0.0001). Similarly, when considering scores from the DD condition alone, controlling for ND score, we again found a significant main effect of age (F5, 29,624 = 329.01, P < 0.0001). A third ANCOVA, that controlled for ND score and that included both ED and DD scores, enabled us to compare the effects of age on each type of distraction. We observed a significant interaction between distractor type (ED or DD) and age (F5, 29,624 = 30.28, P < 0.0001), indicative of a greater decline in DD score than ED score with increasing age. To ensure ceiling effects do not account for these results, we repeated the analysis, excluding data from any individual with the maximum score of 10 for any of the three conditions (leaving n = 9,209). We again observed a main effect of age for ED (F5, 9,202 = 32.24, P < 0.0001) and DD (F5, 9,202 = 49.16, P < 0.0001), with a significant interaction between age and distractor type (F5, 9,202 = 4.37, P = 0.001). To ensure sample size differences between the age groups do not account for the results, we repeated the analysis using only the first 1,020 participants for each age group. We again observed a main effect of age for ED (F5, 6,113 = 67.29, P < 0.0001) and DD (F5, 6,113 = 93.88, P < 0.0001), with a significant interaction between age and distractor type (F5, 6,113 = 8.14, P < 0.0001).
Fig. 2.
Task performance. (A) The mean score for each condition, for each age group. (B) The performance cost associated with the inclusion of ED (red) or DD (blue) for each age group. Error bars represent means ± SEM.
Fig. 2B shows the extent to which performance was affected by distraction [distraction cost (%) = ((ND score − (ED or DD score))/ND score) × 100]. We observed a significant correlation between DD cost and age, with increasing age associated with increasing cost (r = 0.095, P < 0.0001). No such correlation was observed for the ED scores (r = 0.003, P = 0.602). Consistently, when using the first 1,020 participants from each age group, there was a significant correlation between DD cost and age, with increasing age associated with increasing cost (r = 0.102, P < 0.0001), but no such correlation for the ED scores (r = 0.002, P = 0.864).
Next we addressed the extent to which each type of distractor exclusion (DD or ED) predicted WM performance in the absence of overt distraction (ND score, used as a measure of WMC). We used the regression model: WMC = α + β1 ED + β2 DD + β3 age + β4 (ED × age) + β5 (DD × age) + β6 (ED × DD × age), where α is the intercept and β1–6 are the regression coefficients. As both ED and DD scores were included in the model, we could examine the unique contribution of each to WMC, as other sources of variance would be shared between the two distraction variables. The model accounted for a significant amount of variance (adjusted r2 = 0.32, P < 0.0001) and as predicted ED and DD score, as well as age, made a significant contribution in the model (β1 = 0.24, β2 = 0.27, β3 = −1.03, where β refers to standardized beta; P < 0.0001 for each). Importantly the three interaction terms also made significant contributions to the model (β4 = 0.89, β5 = 0.76, β6 = −0.77, P < 0.0001 for each). As illustrated by Fig. 3A, as age increased, the extent to which distractor exclusion predicted WMC increased for both types of distraction, but ED performance had a significantly greater contribution than DD with increasing age. Adding the two two-way interaction terms to the model (WMC = α + β1 ED + β2 DD + β3 age) explained significantly more variance (r2 change = 0.003, P < 0.0001), as did adding the three-way interaction term to that model (r2 change = 0.002, P < 0.0001). All of these results remained significant when we considered only the first 1,020 participants in each age group (adjusted r2 = 0.37, P < 0.0001; β4 = 0.62, β5 = 0.54, β6 = −0.52, P < 0.0001 for each; two-way interaction term: r2 change = 0.002, P < 0.0001; three-way interaction term: r2 change = 0.002, P < 0.0001).
Fig. 3.
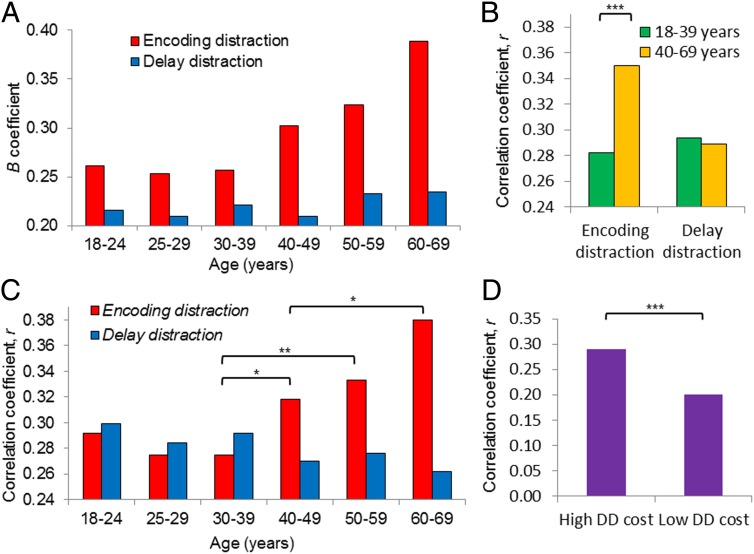
The association between ED and DD scores and WMC. (A) The B coefficients for ED (red) and DD (blue) for each age group from the regression model (ND score = α + B1 ED score + B2 DD score). (B and C) The correlation coefficients for partial correlations between ND score and ED score, controlling for DD score, and also between ND score and DD score, controlling for ED score. Results are shown for (B) participants of 18–39 y (green) and 40–69 y (orange) and (C) the six age groups. *P < 0.05; **P < 0.01; ***P < 0.001 (Fisher’s z test). (D) The correlation coefficients for partial correlations between ND score and ED score, controlling for DD score for older adults (40–69 y) with a high or low DD cost (a median split; the median was 10%).
Our results show that in the older age groups, who express an exaggerated reduction in DD performance (Fig. 2), WMC is better predicted by ED than in the younger age groups (Fig. 3A). To test the specificity of the association between WMC and ED exclusion, we divided the sample into two age bins of 18–39 y and 40–69 y and applied partial correlation analysis, controlling for DD score. As shown by Fig. 3B, we observed a greater correlation for the older compared with the younger age group (older group: r = 0.35, P < 0.0001; younger group: r = 0.28, P < 0.0001; Fisher’s z = −5.79, P < 0.0001). The association between WMC and DD (controlling for ED) was also significant for each age group (older group: r = 0.29, P < 0.0001; younger group: r = 0.29, P < 0.0001), but no difference was observed between the two age groups (Fisher’s z = 0.42, P = 0.674). When we repeated these analyses, but now for each of the six original age groups, we could see that the correlation between WMC and ED increased between the ages of 30 and 60 (as shown in Fig. 3C), with significant differences between the 30–39- and 40–49-y groups (Fisher’s z = −2.57, P = 0.010), between the 30–39- and 50–59-y groups (Fisher’s z = −2.74, P = 0.006), and between the 40–49- and 60–69-y groups (Fisher’s z = −2.04, P = 0.041). The results remained significant when we considered only the first 1,020 participants in each age group, (partial correlation between ED and WMC, controlling for DD: older group: r = 0.37, P < 0.0001; younger group: r = 0.303, P < 0.0001; Fisher’s z = −2.87, P < 0.005; partial correlation between DD and WMC, controlling for ED: older group: r = 0.28, P < 0.0001; younger group: r = 0.30, P < 0.0001; Fisher’s z = 0.60, P = 0.549). Repeating these analyses for each of the six original age groups revealed a significant difference in the correlation between WMC and ED between the 30–39- and 40–49-y groups (Fisher’s z = −2.19, P = 0.029). The difference between the 30–39- and 50–59-y groups (Fisher’s z = −1.7, P = 0.089) and the difference between the 40–49- and 60–69-y groups (Fisher’s z = −0.86, P = 0.390) no longer reached significance.
As an ability to hold information in mind in the presence of DD is impaired with advancing age, it becomes more important to ensure efficient encoding of items. This necessity leads WM task performance in the absence of distraction to become more similar to that seen under ED. Consistent with this relationship, we show that older adults (40–69 y) with a high DD cost (>10%, the median) have a significantly greater partial correlation between ND score and ED score, even when controlling for both DD score and age (r = 0.29, P < 0.0001), than is the case in those with a low DD cost (<10%; r = 0.20, P = 1.404; Fisher’s z = 4.02, P < 0.0001). Although age may still contribute to this result, given that age was measured in 10-y age ranges, the finding nevertheless supports the idea that an increased similarity between WM without distraction and WM with ED can compensate for worse DD exclusion.
Discussion
The greater similarity between WM performance (in the absence of distraction) on the one hand and encoding distractor exclusion on the other, with increasing age, argues for a greater involvement of focused attention during encoding. In older adults, there is presumably a greater reliance on focused attention during the encoding period of a WM task without distraction, as is required when ED is present. This reliance may be unnecessary in younger adults, who more successfully retain weakly encoded information during the delay period. Our results would also seem to complement findings showing a transfer of benefit from perceptual discrimination training to WM performance in older adults, where training-induced changes in early visual processing during encoding predict WM improvement (11). The idea that naturally occurring changes with increasing age represent an adaptive encoding change, promoted by impaired delay distractor exclusion, provides the most parsimonious explanation of our data, although we acknowledge other nonpsychological factors are likely to contribute, including reduced frontal neural responsivity reported in older adults during encoding (12) as well as age-related perceptual impairments (13).
To the best of our knowledge, the neural underpinnings of encoding and delay distractor exclusion have yet to be defined. One suggestion is that the basal ganglia plays a role in selectively initiating storage of new memories (3, 14,), whereas frontal cortex plays a role in buffering remembered items from delay distraction (15–18). The latter has been identified as a potential locus for greater interference effects in older adults (19).
Although we observed a significant age-related decline in both ED and DD exclusion, this decline was greater for DD. It may be possible to equalize performance on the ED and DD task conditions in older adults by changing task parameters, for example the encoding period duration. What we show is that when the presentation duration of targets and distractors are held constant at 1 s, DD shows greater age-related decline than ED. It is unclear why increasing age should affect delay distraction to a greater extent than encoding distraction; one possibility is that if delay distractors are not completely excluded, then judgments of temporal order are required to dissociate the relevant from irrelevant information. There is no such temporal order associated with encoding distractors as they appear together with the relevant, to be encoded, information. Such judgments are disrupted in older age, possibly due to emerging frontal dysfunction (20). It is also possible that generalized slowing in older age leads to longer latencies in the presence of delay distraction (the more difficult condition) compared with encoding distraction, resulting in a greater decline in WM performance (21). A tendency for older adults to perform WM tasks without distraction, as if encoding distractors were present, as we observe here, may help to preserve encoding relative to delay distractor exclusion during aging.
We show the effects of age on WM extend beyond a simple overall decline, and instead point to an age-related change in how information is remembered. WM in the absence of distraction becomes more similar to an individual’s ability to ignore distraction at encoding, perhaps reflecting an increasing reliance on focused attention at encoding with increasing age. This finding is relevant to a goal of ameliorating cognitive decline as well as highlighting the importance of a distinction between encoding and delay distraction exclusion. This distinction is also one that has relevance for neuropsychiatric disorders where WM and distractor filtering are likely to be impaired, such as attention deficit hyperactivity disorder (22).
Methods
Participants.
All participants gave informed consent and the study was approved by the Research Ethics Committee of University College London. Data from participants aged 18–69 y were considered. Data were excluded from 1,805 participants who failed at the easiest level of any of the six conditions (i.e., failed two consecutive trials of WM load 2). Following these exclusions, data from 29,631 participants remained for analysis.
Experimental Design and Task.
The smartphone game we used in our experiment forms part of the “The Great Brain Experiment,” which is funded by the Wellcome Trust (thegreatbrainexperiment.com). The game involves six conditions, three of which are considered here. Participants were asked to remember the positions of red circles that appeared on a 4 × 4 grid for 1 s, and ignore yellow circles. At the end of each trial, they were presented with an empty grid and asked to press on the grid positions in which red circles had appeared. In all three conditions there was a delay period of 1 s during which an empty grid was shown, after the red circles had disappeared and before participants could make their response. In the ND condition, only red circles were displayed. In the ED condition, two yellow distractor circles were shown together with the red circles. In the DD condition, two yellow distractor circles were displayed during the delay period. For each condition, there were three red circles to remember in the first trial. If the participant failed to respond correctly, there were two red circles to remember on the second trial. If that trial was not performed correctly, that condition ended. If a trial was performed correctly, the number of red circles (WM load) increased by one in the next trial. If a participant failed on any load from load 4 onwards, they were given one more trial of that load. If they failed two successive trials of a condition, that condition ended. A maximum of eight trials were given in each condition.
Data Analysis.
Here we consider data only from the first time each participant played the game. Performance in each condition was measured as the maximum WM load at which a trial was answered correctly. The maximum score for each condition was 10. The score from the ND condition was used as a measure of WMC. All analysis was performed using IBM SPSS Statistics version 21 and the accompanying P values were determined by two-tailed analysis.
To determine whether the ability to effectively ignore ED and DD declined with increasing age, we performed separate ANCOVAs for ED and DD scores to determine the effect of age, while controlling for WM performance in the ND condition. To compare the effects of age on ED and DD exclusion, a third ANCOVA was performed to determine the effects of distractor type (ED or DD), age, and their interaction, while again controlling for ND performance. To enable us to correlate the extent to which WM performance was affected by each type of distraction with age, we calculated distraction cost for both ED and DD using the formula: distraction cost (%) = ((ND score – ED or DD score)/ND score) × 100.
To determine whether there was an age-related change in the extent to which ED and DD exclusion uniquely predicts WMC, we performed a hierarchical regression analysis. Performance in the ND condition was our measure of WMC, and ED and DD scores were predictor variables, together with age. The interaction between ED score and age and the interaction between DD score and age were then added to the model. R2 change between the two models was used to assess the variability in WMC that could be explained by age-related change in the contribution of distractor exclusion. Finally the interaction between ED score, DD score, and age was added to the model. R2 change between this and the previous model was used to assess the variability in WMC that could be explained by the increasing contribution of ED relative to DD with increasing age. Standardized beta values are reported in the text, but Fig. 3 shows unstandardized B coefficients, as here all variables represent the maximum number of items successfully reported.
Having established a larger contribution of ED exclusion than DD exclusion to WMC with increasing age, we performed correlation analyses to determine whether ED exclusion becomes more similar to WM performance in the absence of distraction. Partial correlations were performed between ND score and ED score, controlling for DD score, for both younger (18–39 y) and older adults (40–69 y), and a Fisher z test was used to compare the results. For illustration, the results of partial correlation analyses between ND score and DD score, controlling for ED score, are also shown. Also for illustration, correlation analyses were performed for each of the six original age groups, with Fisher’s z statistics shown to highlight significant differences between directly neighboring age groups and between age groups with one intermediate age group.
Finally, to assess whether older adults with worse DD exclusion show a greater similarity between ND score and ED exclusion, the older adult group (40–69 y) was divided by a median split according to DD cost (greater than or less than 10%). Partial correlation analysis was performed between ND score and ED score, controlling for both DD score and age group (40–49 y, 50–59 y, or 60–69 y). Despite controlling for age, it should be noted that age may still contribute to this correlation, given our imprecise measure of age.
Acknowledgments
We thank Ulman Lindenberger and Howard Bowman for helpful discussion; Neil Millstone of White Bat Games for developing the application; and Ric Davis, Chris Freemantle, and Rachael Maddock for information technology support. This work was funded by a Wellcome Trust Engaging Science: Brain Awareness Week Award (101252/Z/13/Z). F.M. holds a Wellcome Trust Research Career Development Fellowship (091826/Z/10/Z). P.Z. was funded by the Brain Research Trust. R.J.D. holds a Wellcome Trust Senior Investigator Award (098362/Z/12/Z). P.S. is supported by a PhD Studentship from The Wellcome Trust (092859/Z/10/Z). The Wellcome Trust Centre for Neuroimaging is supported by core funding from the Wellcome Trust (091593/Z/10/Z).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper are available on request from The Wellcome Trust Centre for Neuroimaging.
References
- 1.Craik FI, Salthouse TA, editors. The Handbook of Aging and Cognition. Erlbaum; Mahwah, NJ: 2000. [Google Scholar]
- 2.Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428(6984):748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- 3.McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11(1):103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- 4.Hasher L, Zacks RT. 1988. Working memory, comprehension, and aging: A review and a new view. The Psychology of Learning and Motivation, ed Bower GH (Academic, San Diego), Vol 22, pp 193–225.
- 5.Gazzaley A, Cooney JW, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8(10):1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- 6.Chadick JZ, Zanto TP, Gazzaley A. Structural and functional differences in medial prefrontal cortex underlie distractibility and suppression deficits in ageing. Nat Commun. 2014;5:4223. doi: 10.1038/ncomms5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jost K, Bryck RL, Vogel EK, Mayr U. Are old adults just like low working memory young adults? Filtering efficiency and age differences in visual working memory. Cereb Cortex. 2011;21(5):1147–1154. doi: 10.1093/cercor/bhq185. [DOI] [PubMed] [Google Scholar]
- 8.Gazzaley A, et al. Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proc Natl Acad Sci USA. 2008;105(35):13122–13126. doi: 10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNab F, Dolan RJ. Dissociating distractor-filtering at encoding and during maintenance. J Exp Psychol Hum Percept Perform. 2014;40(3):960–967. doi: 10.1037/a0036013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown HR, et al. Crowdsourcing for cognitive science—the utility of smartphones. PLoS ONE. 2014;9(7):e100662. doi: 10.1371/journal.pone.0100662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berry AS, et al. The influence of perceptual training on working memory in older adults. PLoS ONE. 2010;5(7):e11537. doi: 10.1371/journal.pone.0011537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cabeza R, et al. Age-related differences in neural activity during memory encoding and retrieval: A positron emission tomography study. J Neurosci. 1997;17(1):391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider B, Pichora-Fuller M. Implications of perceptual deterioration for cognitive aging research. In: Craik FI, Salthouse TA, editors. The Handbook of Aging and Cogntion. Erlbaum; Mahwah, NJ: 2000. [Google Scholar]
- 14.Frank MJ, Loughry B, O’Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: A computational model. Cogn Affect Behav Neurosci. 2001;1(2):137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- 15.Artchakov D, et al. Distracters impair and create working memory-related neuronal activity in the prefrontal cortex. Cereb Cortex. 2009;19(11):2680–2689. doi: 10.1093/cercor/bhp037. [DOI] [PubMed] [Google Scholar]
- 16.Dolcos F, Miller B, Kragel P, Jha A, McCarthy G. Regional brain differences in the effect of distraction during the delay interval of a working memory task. Brain Res. 2007;1152:171–181. doi: 10.1016/j.brainres.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakai K, Rowe JB, Passingham RE. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nat Neurosci. 2002;5(5):479–484. doi: 10.1038/nn846. [DOI] [PubMed] [Google Scholar]
- 18.Bonnefond M, Jensen O. Alpha oscillations serve to protect working memory maintenance against anticipated distracters. Curr Biol. 2012;22(20):1969–1974. doi: 10.1016/j.cub.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 19.Jonides J, et al. Age differences in behavior and PET activation reveal differences in interference resolution in verbal working memory. J Cogn Neurosci. 2000;12(1):188–196. doi: 10.1162/089892900561823. [DOI] [PubMed] [Google Scholar]
- 20.Moscovitch M, Winocur G. Frontal lobes, memory, and aging. Ann N Y Acad Sci. 1995;769:119–150. doi: 10.1111/j.1749-6632.1995.tb38135.x. [DOI] [PubMed] [Google Scholar]
- 21.Cerella J. Handbook of The Psychology of Aging. Academic; San Diego: 1990. [Google Scholar]
- 22.Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: The search for endophenotypes. Nat Rev Neurosci. 2002;3(8):617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]