Significance
Transgenes can be permanently silenced in a single generation via a previously described small RNA-induced epigenetic silencing (RNAe) mechanism, which is promoted by the presence of a perfect Piwi-interacting RNA (piRNA) target site. In this study, we identify a previously unidentified mechanism capable of silencing single-copy transgenes that lack perfect piRNA target sites and that is triggered by a lack of chromosomal pairing during meiosis for multiple generations. Multigenerational RNAe can lead to reversible or permanent transgene silencing and may provide insight into variability in the expression of single-copy transgenes or single-copy genomic insertions, which are commonly used in experimental biology. Our analysis of “multigenerational RNAe” also offers new insights into potentially common epigenetic silencing events relevant to genome expression in the germline and embryo.
Keywords: epigenetics, epigenome, RNAe, transgenerational silencing, siRNAs
Abstract
Single-copy transgenes in Caenorhabditis elegans can be subjected to a potent, irreversible silencing process termed small RNA-induced epigenetic silencing (RNAe). RNAe is promoted by the Piwi Argonaute protein PRG-1 and associated Piwi-interacting RNAs (piRNAs), as well as by proteins that promote and respond to secondary small interfering RNA (siRNA) production. Here we define a related siRNA-mediated silencing process, termed “multigenerational RNAe,” which can occur for transgenes that are maintained in a hemizygous state for several generations. We found that transgenes that contain either GFP or mCherry epitope tags can be silenced via multigenerational RNAe, whereas a transgene that possesses GFP and a perfect piRNA target site can be rapidly and permanently silenced via RNAe. Although previous studies have shown that PRG-1 is typically dispensable for maintenance of RNAe, we found that both initiation and maintenance of multigenerational RNAe requires PRG-1 and the secondary siRNA biogenesis protein RDE-2. Although silencing via RNAe is irreversible, we found that transgene expression can be restored when hemizygous transgenes that were silenced via multigenerational RNAe become homozygous. Furthermore, multigenerational RNAe was accelerated when meiotic pairing of the chromosome possessing the transgene was abolished. We propose that persistent lack of pairing during meiosis elicits a reversible multigenerational silencing response, which can lead to permanent transgene silencing. Multigenerational RNAe may be broadly relevant to single-copy transgenes used in experimental biology and to shaping the epigenomic landscape of diverse species, where genomic polymorphisms between homologous chromosomes commonly result in unpaired DNA during meiosis.
Small RNAs can repress expression of endogenous genes as well as parasites such as transposons or viruses. Small RNA-mediated repression has the potential to result in epigenetic silencing of genomic loci, which can yield a permanent, heritable state of expression in germ cells in metazoans.
RNA interference (RNAi) is a conserved biological process in which small noncoding RNA molecules promote gene silencing (1). RNAi was originally identified in Caenorhabditis elegans, but has been observed in a large number of eukaryotes ranging from fungi to plants to humans (1–6). Small interfering RNAs (siRNAs) can be produced from a variety of sources (7, 8) and include endogenous siRNAs, which are produced by genes, transposons, or aberrant transcripts, and exogenous siRNAs, which target foreign nucleic acids. siRNAs interact with Argonaute proteins that potentiate their functions (9). When exogenous double-stranded RNA is introduced into C. elegans, it is processed by the Dicer nuclease into primary 5′ monophosphorylated 22G siRNA duplexes that interact with the Argonaute RDE-1 whose slicer activity promotes degradation of one strand of the duplex (10). RDE-1 and associated primary siRNAs then interact with target mRNAs to recruit RNA-dependent RNA polymerases that synthesize secondary 5′ triphosphorylated siRNAs (11–15). Secondary Argonaute proteins then bind with the secondary siRNAs, and it is this effector complex that directly targets mRNA transcripts in the cytoplasm for degradation (16).
A second class of primary siRNAs in C. elegans is the Piwi-interacting RNAs (piRNAs) that are highly abundant in the germline and interact with the C. elegans Piwi Argonaute protein PRG-1 (17, 18). C. elegans piRNAs are termed 21U-RNAs as they are 21 nucleotides long and possess a 5′ uracil. PRG-1 and associated piRNAs target transposons and some genes, typically based on imperfect homology to their targets, which recruits RNA-dependent RNA polymerases to promote biogenesis of 22G secondary siRNAs that bear perfect homology to their targets (17, 18). These secondary 22G-RNAs interact with WAGO-class Argonaute proteins to promote transcriptional silencing of germline loci. The vast repertoire of C. elegans piRNAs and their ability to target nucleic acids with mismatches may allow them to target both endogenous loci as well as foreign nucleic acids such as transposons or viruses (19, 20). Although C. elegans piRNAs can target many endogenous transposons, prg-1 mutants displayed transposition for only one of three DNA transposons tested, even though all three transposons become active if secondary siRNA populations are disrupted with Mutator gene mutations (21). These results indicate that small RNA-mediated epigenetic silencing of many transposons initially depends on PRG-1 and associated piRNAs, but then a downstream secondary siRNA system is capable of maintaining silencing of many transposon classes in the absence of piRNAs.
A silencing process that relies on the secondary siRNA system of C. elegans is “cosuppression,” where microinjection of plasmids into the germline leads to creation of repetitive extrachromosomal transgenic arrays that can be expressed in somatic cells but typically become rapidly and permanently silenced in the germline (22). In cosuppression, germline silencing can act in trans on the endogenous locus, leading to its silencing as well (23, 24). The diffusible transposon silencing factor depends on Mutator class proteins that promote secondary siRNA production, but not on proteins that initiate the response to exogenous double-stranded RNA such as RDE-1 or RDE-4 (23). Cosuppression was originally observed in plants in response to high copy number transgenes and was reported to be associated with a population of siRNAs targeting the transgenes, which foreshadowed the discovery of RNA interference (1, 25–27). Methylation-mediated silencing of multicopy transgenes has also been observed in zebrafish, where transgenes inserted as high copy concatemeric arrays become completely silenced in two to three generations (28, 29). Similar results are seen in mice, where copy number is inversely correlated to expression (30, 31). Therefore, epigenetic silencing of high copy number transgenes in the C. elegans germline is consistent with a natural defense response to foreign nucleic acids such as transposons that is seen in diverse organisms.
To stably express transgenes in C. elegans, an elegant method to create single-copy transgene insertions was developed with the aid of a unique copy of the Drosophila melanogaster transposon MosI to induce a chromosomal double-strand break that promotes site-specific recombination with a plasmid-derived template (32–35). Although single-copy transgenes can be stably expressed in the germline using this method, a piRNA sensor transgene that encodes GFP and also has a perfect piRNA target site in its 3′ UTR is expressed if created in a background that is mutant for prg-1, but the piRNA sensor is silenced if created in a background that is wild type for prg-1 (17). If males containing a silenced piRNA sensor are crossed directly with prg-1 mutant hermaphrodites, F2 lines that are homozygous for both the piRNA sensor and the prg-1 mutation then display expression of GFP from the piRNA sensor (17). Thus, both initiation and maintenance of epigenetic silencing of the piRNA sensor transgene can depend on prg-1.
Three independent studies subsequently showed that the piRNA sensor transgene, as well as other transgenes possessing GFP, can be subjected to a permanent epigenetic silencing process in the germline, termed small RNA-induced epigenetic silencing (RNAe) where prg-1 is required for initiation but not maintenance of transgene silencing (32, 35, 36). In the first study, the group that created the piRNA sensor transgene crossed it in trans to a dpy-10 unc-4 balancer chromosome, and this transformed the piRNA sensor transgene into a permanently silent state whose maintenance was independent of PRG-1 (32). A second group showed that propagation of the piRNA sensor in a henn-1 mutant background, which affects the stability of a subset of endogenous siRNAs (37), resulted in stochastic levels of RNAe, where initiation but not maintenance of transgene silencing depended on PRG-1 (35). A third group showed that certain single-copy GFP transgenes were prone to permanent silencing if created in a wild-type background, that this did not happen if they were created in a prg-1 mutant background, and that once silenced via RNAe, these GFP transgenes failed to reactivate if combined with a prg-1 mutation (36).
Together, the latter three studies suggest that transgenes can be subjected to a permanent silencing process, termed RNAe, where initiation of silencing depends on PRG-1/piRNAs, but subsequent maintenance of transgene silencing depends on Mutator class secondary siRNA proteins as well as nuclear RNAi and chromatin proteins (32, 35, 36). This implies that initial targeting of transgenes by PRG-1 and associated piRNAs elicits production of a secondary siRNA population whose maintenance, in conjunction with siRNA-directed histone marks, is sufficient to enforce a stable, heritable silent state.
The permanent silencing process termed RNAe occurs very rapidly, typically within a single generation (32, 35, 36). Herein we describe a distinct siRNA-mediated silencing process that we term “multigenerational RNAe.” We identified single-copy transgenes that become silent if propagated in the hemizygous state for multiple generations. We found that multigenerational transgene silencing can either become permanent or, crucially, that it can be reversed, an unexpected result given the permanence of transgene silencing previously described for RNAe. Further, we identify molecular triggers that distinguish RNAe from multigenerational RNAe. Our study provides insight into the genesis and maintenance of epialleles, relevant not only to transgenes, transposons, and some viruses, but also to dynamic regulation of germline gene expression across generations for the vast majority of metazoans.
Results
Discovery of Multigenerational Transgene Silencing.
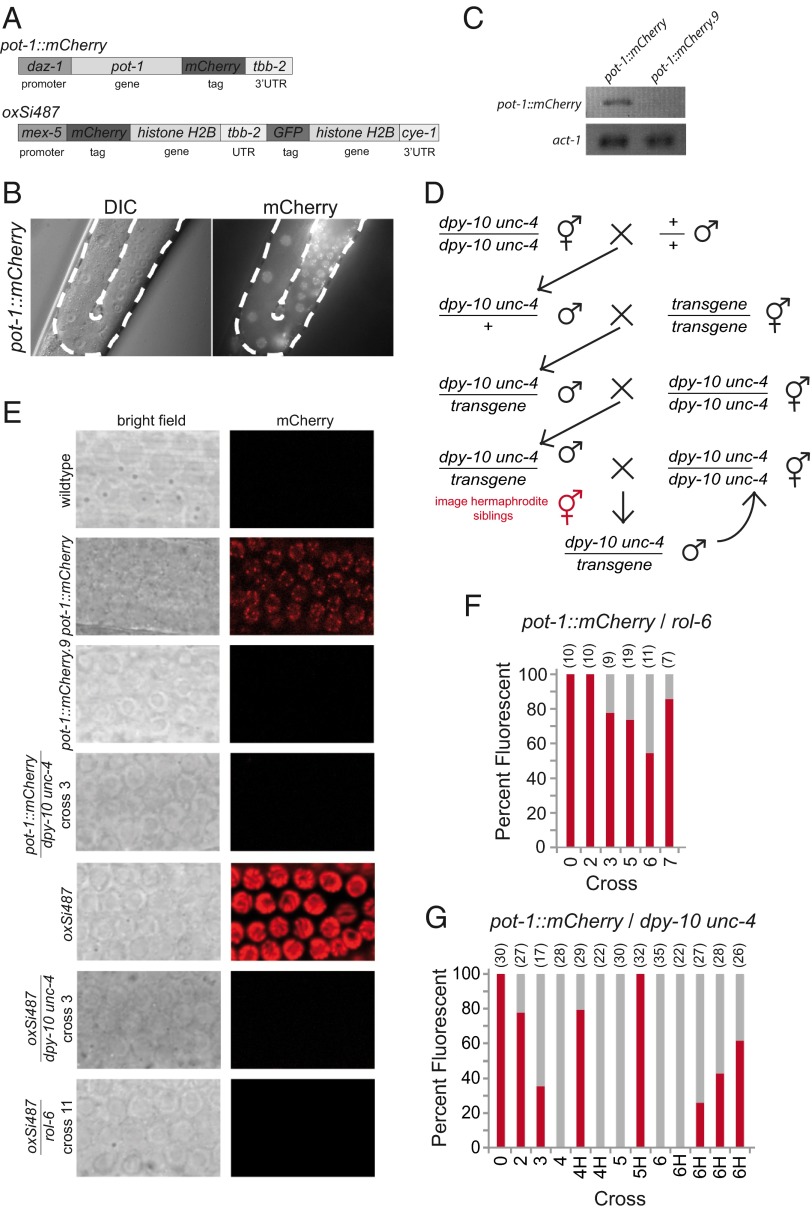
The Protection of Telomeres-1 (POT-1) single-stranded telomere binding protein inhibits telomerase and forms discrete foci at C. elegans telomeres in vivo (38). We previously created three independent single-copy transgene insertions that express POT-1::mCherry, ypSi1, ypSi2, and ypSi3 (38), each inserted via a transposon-induced double-strand break at the MosI locus ttTi5605, located near the center of chromosome II (Fig. 1A) (33, 34). POT-1::mCherry fluorescence can be detected throughout the germline and is particularly evident in meiotic pachytene germ cells as fluorescent punctae (Fig. 1 B and E) (38). Although several single-copy transgenes inserted in the ttTi5605 MosI locus are silenced in the F1 cross-progeny of a single outcross (36), we previously found that pot-1::mCherry transgenes remained robustly expressed following two successive crosses: one to combine pot-1::mCherry with a marker mutation and a second to cross in a mutation with relevance to telomere biology (38). However, when we crossed pot-1::mCherry in trans to the recessive marker mutation rol-6(e189) for nine successive crosses—by crossing pot-1::mCherry/rol-6 heterozygous males with rol-6−/− homozygous hermaphrodites and then selecting for pot-1::mCherry homozygotes in the F3—we found that POT-1::mCherry fluorescence was either weak or abolished (n = 2 crosses each for three independent pot-1::mCherry insertions). Further propagation of these strains for a number of generations revealed that POT-1::mCherry expression was robustly restored for ypSi1.9 and ypSi3.9, but that the ypSi2.9 transgene had become permanently silenced (Fig. S1). Neither nuclear nor telomeric POT-1::mCherry fluorescence was observed for the ypSi2.9 strain during 3 years of culture in our laboratory (Fig. 1E), whereas the original noncrossed ypSi2 strain always displayed robust expression during this time. The presence of the pot-1::mCherry transgene in ypSi2.9 was confirmed by PCR from genomic DNA, and rtPCR indicated that pot-1::mCherry mRNA is present in the original ypSi2 transgene, hereafter referred to as pot-1::mCherry, but not for the crossed ypSi2.9 transgene, hereafter referred to as pot-1::mCherry.9 (Fig. 1C). Together, these results led us to hypothesize that a partially penetrant multigenerational silencing process can occur when single-copy transgenes are repeatedly crossed in C. elegans. This process can ultimately result in a state of permanent transgene silencing that is stable for many generations.
Fig. 1.
mCherry-expressing transgenes can be silenced via multigenerational RNAe. (A) Structures of pot-1::mCherry and oxSi487 transgenes. (B) DIC (Left) and mCherry fluorescence image (Right) of pot-1::mCherry. Dotted white line outlines the mitotic and meiotic germline. (C) RT-PCR of strains containing the pot-1::mCherry transgene. (D) Crossing schema used to cross chromosome II transgenes. (E) Confocal images of brightfield (Left column) and mCherry fluorescence (Right column) in the mitotic germline nuclei in various strains (60x). (F and G) Percent of population fluorescent at each round of crossing for pot-1::mCherry using either rol-6 or dpy-10 unc-4 marker strain. Numbers at Top in parentheses indicate number of worms scored. Bar 0 indicates original transgenic strain before crossing. H denotes homozygous F3 lines derived from parental heterozygous transgene/marker.
We repeated the above cross by placing the pot-1::mCherry transgene in trans to the rol-6 marker mutation and found that a fraction of pot-1::mCherry/rol-6 animals were mCherry negative starting at cross 3, and transgene silencing was sustained for hemizygous pot-1::mCherry/rol-6 animals until cross 7 (Fig. 1F). Notably, transgene silencing was never observed for the original pot-1::mCherry transgene homozygotes (n > 200), nor in the pot-1::mCherry/rol-6 progeny from cross 1 or 2. These results confirmed that a partially penetrant multigenerational silencing process occurs when the pot-1::mCherry transgene is crossed in trans to the rol-6 marker mutation.
We next placed pot-1::mCherry in trans to the marker mutations dpy-10 unc-4, which flank the pot-1::mCherry transgene, and repeatedly crossed pot-1::mCherry/dpy-10 unc-4 heterozygous males with dpy-10 unc-4 hermaphrodites (Fig. 1D). A proportion of silent pot-1::mCherry heterozygotes were observed for crosses 2 and 3, followed by uniform silencing for crosses 4–6 (Fig. 1 E and G and Fig. S2J). F2 animals were singled and pot-1::mCherry homozygotes were identified by selecting against the dpy-10 unc-4 balancer mutations, and a number of pot-1::mCherry homozygotes gave rise to F3 progeny that showed POT-1::mCherry expression (Fig. 1G). One pot-1::mCherry homozygous line remained completely silent (Fig. 1G column 6H). We therefore conclude that a single-copy transgene that is transmitted in the hemizygous state for multiple generations can be subjected to a powerful silencing process. Although transgene expression is often restored when the hemizygous transgene becomes homozygous, a fraction of pot-1::mCherry homozygotes remain permanently epigenetically silenced, as observed during our original outcrosses of pot-1::mCherry in trans to rol-6.
Independent Transgenes Succumb to Multigenerational Silencing.
We next tested multigenerational silencing of an independent single-copy transgene, oxSi487, which expresses both mCherry- and GFP-tagged proteins from an operon integrated into the ttTi5605 MosI locus, Pmex-5::mCherry::H2B::tbb-2 3′UTR::gpd-2 operon::GFP::H2B::cye-1 3′UTR (Fig. 1 A and E). The mCherry expression in this transgene was highly robust, whereas the GFP expression was substantially weaker; therefore, we used the mCherry fluorescence to score for transgene expression. Silencing was never observed in the original oxSi487 strain (n > 100). However, crosses of oxSi487 in trans to a balancer chromosome containing dpy-10 and unc-4 mutations resulted in silencing for some progeny in cross 2 and complete silencing by cross 3 (Fig. 1E and Fig. S2I). We also crossed oxSi487 in trans to rol-6 and found that a fraction of animals for four independent crosses were mCherry negative from crosses 4–11 (Fig. 1E and Fig. S2H). Together, the above pot-1::mCherry and oxSi487 experiments indicate that multigenerational transgene silencing occurs for independent mCherry transgene strains with balancer chromosomes containing distinct marker mutations, that permanent transgene silencing can occur in either circumstance, and that weaker multigenerational silencing occurs when transgenes are crossed in trans to a balancer chromosome containing rol-6.
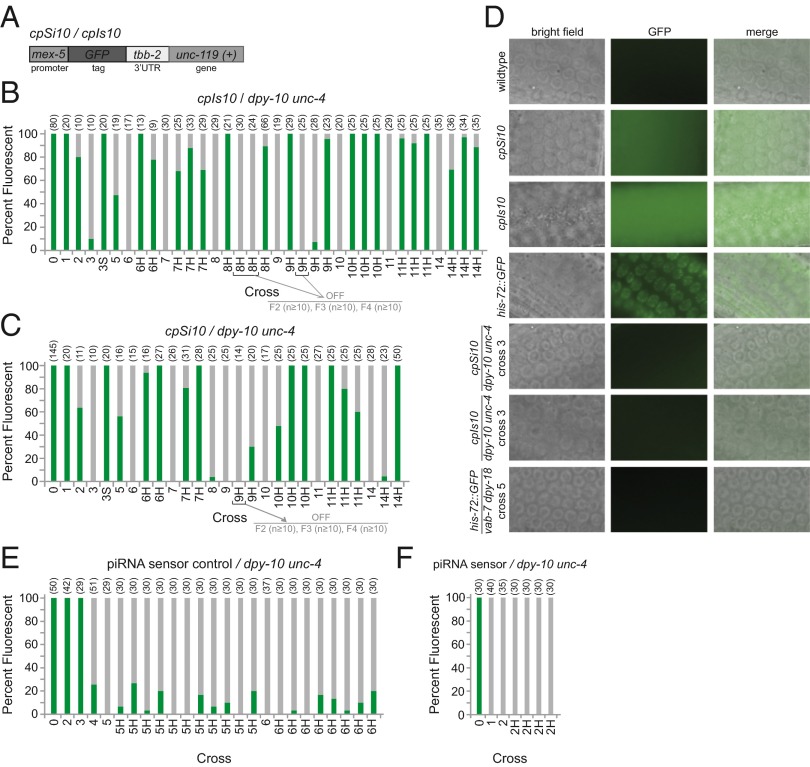
We next asked whether multigenerational transgene silencing also occurs for transgenes that only contain GFP-epitope tags, and whether transgene insertion via a double-strand break created by the Drosophila MosI transposon ttTi5605 has any impact on transgenerational silencing. We therefore tested identical transgenes that express Pmex-5::GFP::tbb-2 3′UTR that were inserted either via MosI-mediated gene conversion at ttTi5605, cpSi10[Pmex-5::GFP::tbb-2 3′UTR + unc-119(+)] or via CRISPR/Cas9-mediated gene conversion at the identical position on chromosome II, cpIs10[Pmex-5::GFP::tbb-2 3′UTR + unc-119(+)] (Fig. 2 A and D) (39). We found that GFP was expressed uniformly in worm populations from cpSi10 and cpIs10 stocks (n > 100), but that hemizygous cpSi10 and cpIs10 transgenes underwent multigenerational silencing with very similar kinetics (Fig. 2 B and C). Thus, the presence of the Drosophila MosI transposon at the ttTi5605 locus before transgene insertion does not affect transgene silencing. When cpSi10 and cpIs10 were crossed in trans to our dpy-10 unc-4 marker strain, cpSi10 was silenced by cross 3 and cpIs10 at cross 6 (Fig. 2 B–D). Propagating worms from starved cross plates yielded F3 progeny homozygous for the transgene, where transgene expression was fully restored (Fig. 2 B, column 3S and C, column 3S). To determine if starvation was necessary for restoration of transgene expression in transgene homozygotes, we singled unstarved F1 progeny from silent cpSi10/ dpy-10 unc-4 and cpIs10/ dpy-10 unc-4 heterozygotes and found that the majority of F3 cpSi10 or cpIs10 homozygotes expressed GFP in the absence of starvation (Fig. 2 B and C, columns marked H). Several of the homozygous F3 progeny that did not express GFP were isolated (Fig. 2 B, columns 8H and 9H and C, column 9H). These lines remained GFP negative, and fully penetrant silencing was transmitted for at least five generations.
Fig. 2.
Silencing of GFP-expressing transgenes via multigenerational RNAe. (A) Structures of cpSi10 and cpIs10 transgenes. cpSi10 was inserted in an inverse orientation to that of cpIs10. (B and C) Percent of population fluorescent at each round of crossing for cpIs10 and cpSi10 using dpy-10 unc-4 as a marker strain. Numbers at Top in parentheses indicate number of worms scored. Bar 0 indicates original transgenic strain before crossing. S denotes homozygous F3 worms derived from starved heterozygous transgene/marker F1. H denotes homozygous F3 worms derived from heterozygous transgene/marker F1. Call out indicates the number of generations for which silencing was scored. (D) Widefield microscopy images of the mitotic germline nuclei in brightfield (Left column), GFP fluorescence (Center column), and merged (Right column) (60x). (E and F) Percent of population fluorescent at each round of crossing for piRNA sensor control or piRNA sensor using a dpy-10 unc-4 balancer chromosome. Note that the initial piRNA sensor strain was mutant for prg-1 and that the status of the prg-1 mutation was not followed during crosses in trans to dpy-10 unc-4. Numbers at Top in parentheses indicate number of worms scored. Bar 0 indicates original transgenic strain before crossing. H denotes homozygous F3 worms derived from heterozygous transgene/marker F.
Because we saw variability in the kinetics of multigenerational silencing for mCherry transgenes using our dpy-10 unc-4 and rol-6 balancer chromosomes, we further examined the effect of balancer chromosome identity on multigenerational silencing of hemizygous cpSi10 and cpIs10 transgenes. We found that multigenerational transgene silencing could be promoted in trans to three distinct balancer chromosomes, one containing dpy-2 and unc-4 mutations (Figs. S2 A and D and S3), one carrying an inversion between dpy-10 and unc-4 mutations (Figs. S2 B and E and S3), and a new dpy-10 unc-4 chromosome ordered directly from the C. elegans stock center (Figs. S2 C and F and S3). Furthermore, when transgene homozygotes were isolated from completely silent hemizygous populations, we once again saw desilencing of the transgene in some cases (Fig. S2 A and D). We conclude that a strong yet reversible multigenerational silencing process occurs for transgene hemizygotes in trans to a range of distinct balancer chromosomes, and that hemizygous transgene silencing is potent enough to become permanent when transgene homozygotes are isolated.
We next asked whether a CRISPR/Cas9-mediated GFP insertion at a distinct genomic location, the his-72 locus on chromosome III (39), could also be subjected to multigenerational silencing. his-72::GFP was placed in trans to the marker mutations vab-7 dpy-18 and crossed versus these mutations (Fig. S4). Similar to our other transgenes tested, a significant fraction of his-72::GFP/vab-7 dpy-18 heterozygotes displayed transgene silencing for crosses 3–22 (Fig. 2D and Fig. S2G). Thus, multigenerational transgene silencing is not specific to single-copy transgenes at the ttTi5605 locus on chromosome II and can regulate the expression of GFP cassettes inserted at endogenous genomic loci.
A piRNA Target Site Dictates RNAe Pathway Choice.
It was previously observed that a “piRNA sensor” transgene could be subjected to fully penetrant, irreversible RNAe if crossed using dpy-10 unc-4 as a balancer chromosome. To determine how this example of RNAe is related to the multigenerational RNAe process that we report here, we used the previously characterized piRNA sensor transgene that was prone to RNAe, as well as the “piRNA sensor control” transgene that was resistant to RNAe (32, 35, 36). The piRNA sensor contains the reverse complement of an endogenous piRNA sequence in its 3′ UTR, whereas the piRNA sensor control instead contains the piRNA sequence in opposite orientation. In agreement with published results, we saw expression of the piRNA sensor transgene in a prg-1; piRNA sensor strain, but a single cross in trans to a dpy-10 unc-4 balancer chromosome resulted in silencing of the piRNA sensor (Fig. 2F) (32, 35, 36). We confirmed that isolation of piRNA sensor transgene homozygotes from piRNA sensor/dpy-10 unc-4 hemizygotes resulted in fully penetrant silencing that reflects the rapid, irreversible RNAe process that was previously reported (Fig. 2F) (32). In contrast, when the piRNA sensor control transgene was outcrossed in trans to a dpy-10 unc-4 balancer chromosome (Fig. 1D), we observed that it underwent multigenerational RNAe (Fig. 2E). Complete transgene silencing was observed for piRNA sensor control transgene hemizygotes at cross 3, and some transgene homozygotes derived from progeny of silent hemizygotes expressed the fluorescent transgene (Fig. 2E, columns H). Thus, one factor that distinguishes the previously described permanent RNAe process from the multigenerational RNAe process that we report is the presence of a piRNA target site.
PRG-1/piRNAs and Secondary siRNAs Promote Initiation and Maintenance of Multigenerational RNAe.
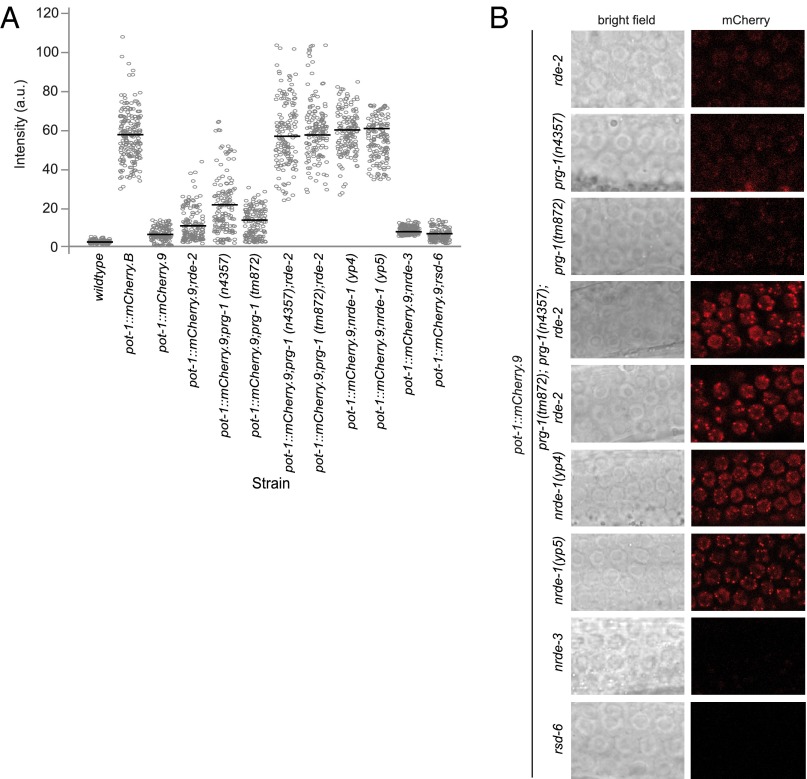
RDE-2 is a component of the Mutator-class of RNA interference proteins that acts to promote biogenesis of secondary siRNA populations, which were previously shown to be required for maintenance of silencing of single-copy transgenes via RNAe (32, 35, 36) as well as for cosuppression-mediated silencing of repetitive transgenes (18, 23, 40–42). Consistently, we found that POT-1::mCherry fluorescence was partially restored when the silenced pot-1::mCherry.9 transgene was placed in an rde-2–deficient background (Fig. 3 A and B).
Fig. 3.
prg-1 and rde-2 coordinately promote maintenance of the silencing of the pot-1::mCherry transgene. (A) Intensity measurements for individual nuclei (n = 150 for each genotype, five nuclei counted per animal). Solid horizontal bars indicate the mean intensity for each population. (B) Confocal images of both brightfield (Left column) and mCherry fluorescence (Right column) in the mitotic germline nuclei in various strains in the pot-1::mCherry.9 transgenic background (60x).
The piRNA-interacting Argonaute protein PRG-1 is typically required for initiation but not maintenance of RNAe (17, 32, 35, 36). We found that independent alleles of prg-1, tm872 and n4357, elicited partial desilencing of pot-1::mCherry.9 (Fig. 3 A and B). As prg-1 and rde-2 mutations both resulted in partial desilencing of pot-1::mCherry.9, we constructed rde-2 prg-1(tm872); pot-1::mCherry.9 and rde-2 prg-1(n4357); pot-1::mCherry.9 strains and found that POT-1::mCherry fluorescence was fully restored in both cases (Fig. 3 A and B). Thus, proteins that play distinct roles in initiation or maintenance of single-generation single-copy transgene silencing via RNAe coordinately promote maintenance of multigenerational transgene silencing.
NRDE-1 is required for nuclear RNA interference, where it functions downstream of siRNAs to promote transcriptional gene silencing (32, 43). We found that when the silent pot-1::mCherry.9 transgene was placed in a nrde-1–deficient background, silencing was completely abolished (Fig. 3 A and B). In contrast, the NRDE-3 Argonaute protein, which promotes transcriptional silencing in the soma in response to exogenous dsRNAs (44), was dispensable for pot-1::mCherry silencing (Fig. 3 A and B). These results are consistent with the possibility that a nuclear silencing process in the germline promotes maintenance of multigenerational RNAe.
As a control, we tested the RSD-6 Tudor domain protein, which has been previously described to promote spreading of RNAi from the soma to the germline and generation of robust secondary siRNA populations in response to exogenous dsRNA triggers, but is dispensable for single-generation silencing of the piRNA sensor transgene (32, 45, 46). pot-1::mCherry silencing was maintained when rsd-6 was mutant (Fig. 3 A and B).
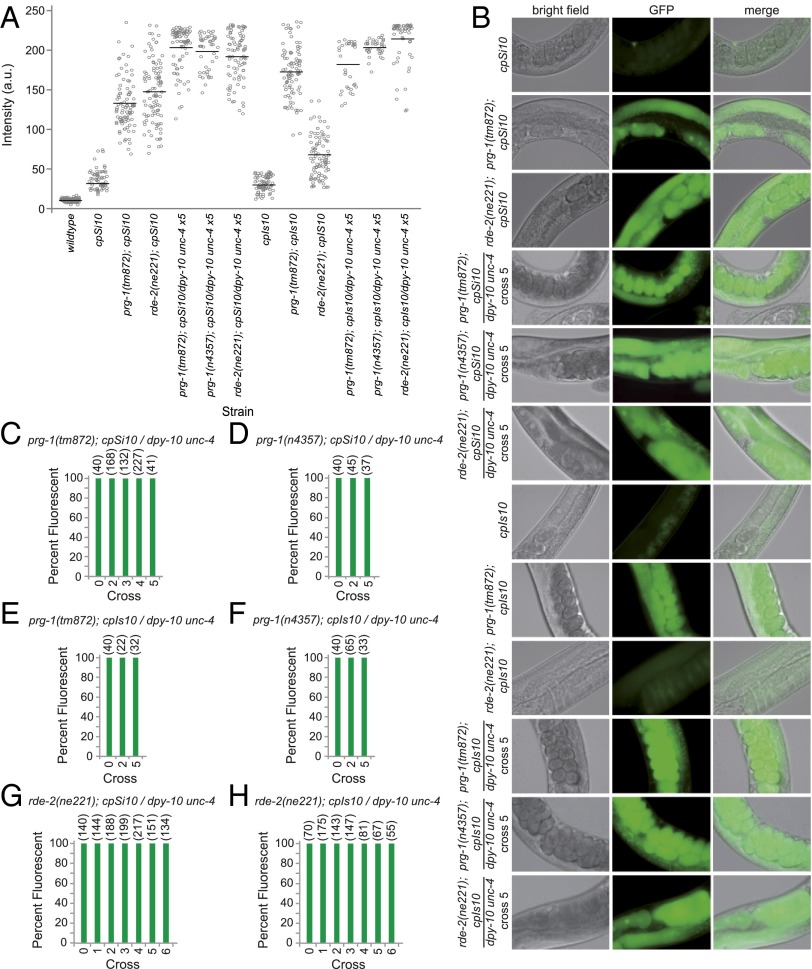
Having established roles for prg-1 and rde-2 in maintenance of multigenerational transgene silencing, we next asked whether these genes also played roles in initiation of multigenerational RNAe. We created strains with active GFP transgenes that have mutations in prg-1 or rde-2 and crossed these with a marker strain containing a dpy-10 unc-4 balancer chromosome and either prg-1 or rde-2 mutations, respectively. Silencing of hemizygous GFP transgenes was not observed in either prg-1 or rde-2 mutant backgrounds (Fig. 4 B–H), indicating that both PRG-1/piRNAs and secondary siRNA biogenesis are required for initiation of multigenerational RNAe. Surprisingly, when we initiated these active transgene experiments by crossing either prg-1 or rde-2 mutations into cpSi10 or cpIs10 transgene backgrounds, we observed robust increases in GFP expression in comparison with the parental transgene strains (Fig. 4 A and B), which did not diminish during multigenerational RNAe, indicating that PRG-1 and RDE-2 promote a state of incomplete silencing for both of these active transgenes.
Fig. 4.
prg-1 and rde-2 are not required for initiation of multigenerational RNAe. (A) Intensity measurements for germlines of various strains. (n > 25 for each genotype, three independent measurements of each germline). Solid horizontal bars indicate the mean intensity for each population. (B) Representative widefield microscopy images of the mitotic germline nuclei in brightfield (Left column), GFP fluorescence (Center column), and merged (Right column) (from A) (40x). Due to robust expression in rde-2 and prg-1 mutant backgrounds and uniform exposure time to accurately measure intensities, active transgene intensities appear extremely dim although they are significantly expressed. (P < 0.0001 wild-type vs. transgene unpaired t test). (C–H) Percent of population fluorescent at each round of crossing in prg-1 or rde-2 mutant backgrounds in either cpSi10 or cpIs10 using dpy-10 unc-4 balancer chromosome. Numbers at Top in parentheses indicate number of worms scored. Bar 0 indicates original transgenic strain before crossing (combined data from three or more independent experiments).
Lack of Chromosomal Pairing Induced Rapid Silencing.
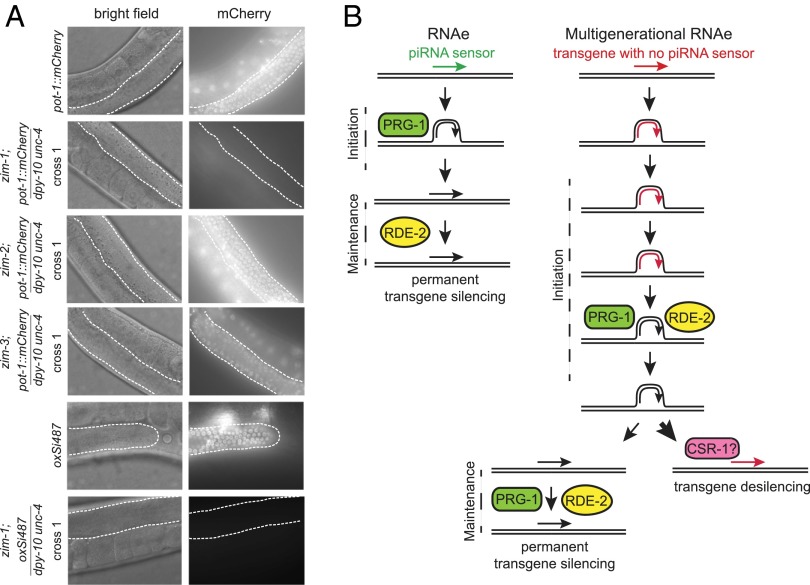
The ZIM proteins are a family of four related C2H2 zinc-finger domains proteins that promote pairing of specific chromosome homologs during meiosis (47). ZIM-1 promotes pairing of chromosomes II and III, ZIM-2 promotes pairing of chromosome V, and ZIM-3 promotes pairing of chromosomes I and IV (47). When mutated, these genes result in a loss of meiotic pairing of their respective chromosomes (48, 49). When pot-1::mCherry; zim-1 males were crossed with dpy-10 unc-4; zim-1 hermaphrodites, uniform mCherry silencing occurred in the progeny of cross 1 (Fig. 5A). The same result was observed for progeny of oxSi487; zim-1 males crossed with dpy-10 unc-4; zim-1 hermaphrodites (Fig. 5A). We confirmed that rapid transgene silencing in a zim-1 mutant background was due to complete lack of pairing the chromosome containing the pot-1::mCherry transgene, chromosome II, by testing zim-2 or zim-3 mutations that promote pairing of distinct autosomes. Neither zim-2 nor zim-3 promoted immediate transgene silencing (Fig. 5A), indicating that lack of pairing during meiosis promotes multigenerational transgene silencing.
Fig. 5.
Complete disruption of meiotic chromosome pairing leads to rapid transgene silencing. (A) Representative widefield microscopy images of brightfield (Left column) and mCherry fluorescence (Right column) of pot-1::mCherry (n > 75 expressed mCherry), pot-1::mCherry/ dpy-10 unc-4; zim-1 (n = 28 were silent), pot-1::mCherry/ dpy-10 unc-4; zim-2 (n = 30), pot-1::mCherry/ dpy-10 unc-4; zim-3 (n = 30 were silent), oxSi487 (n > 75 expressed mCherry), oxSi487/ dpy-10 unc-4; zim-1 (n = 21; 19/21 were silent, 2/21 displayed faint mCherry fluorescence). Dotted white line outlines mitotic germline; all images are 40×. (B) Model depicting RNAe, as previously described, and multigenerational RNAe.
Discussion
Lack of a pairing partner in meiosis is known to activate an RNA directed-silencing mechanism termed meiotic silencing of unpaired DNA (MSUD), which was first identified in Neurospora crassa (50). MSUD suppresses the expression of genes that fail to pair with their homologs during the first prophase of meiosis (51–53). MSUD requires small RNA factors, including an Argonaute protein and an RNA-dependent RNA polymerase, which likely respond to unpaired meiotic DNA to produce double-stranded RNAs that feed into an siRNA-mediated silencing system (50, 54–56). We found that transgene silencing occurred when hemizygous single-copy transgene/marker mutation heterozygotes were crossed with marker mutation homozygotes for multiple generations. Although potent, this multigenerational silencing process was reversible, as transgene activation was commonly observed when transgene homozygotes were isolated from silent single-copy transgene/marker mutation heterozygotes. These results imply that the trigger of multigenerational transgene silencing corresponds to unpaired transgene DNA during meiosis, which occurs if one homolog possesses a transgene and the other does not. In support of this model, we found that complete disruption of meiotic pairing of the chromosome containing a transgene normally targeted for multigenerational RNAe resulted in rapid transgene silencing in a single generation (Fig. 5A). These data imply that the molecular trigger of multigenerational transgene silencing is lack of pairing during meiosis (Fig. 5B), which is a physical hallmark of the siRNA-mediated genomic silencing process MSUD. Our study integrates the process of piRNA-mediated silencing, where targets are commonly identified based on imperfect homology to piRNAs (17), with small but nevertheless potent structural aberrations that can occur when meiotic chromosomes pair.
We observed silencing via multigenerational RNAe for single-copy transgene/marker mutation heterozygotes using various transgenes and marker mutations on different chromosomes (Figs. 1 and 2 and Fig. S2). However, for a single locus on chromosome II, we found that the kinetics and penetrance of multigenerational RNAe for independent transgenes was variable and dependent on the balancer chromosome containing the marker mutation used for the hemizygous transgene crosses (Figs. 1 and 2 and Fig. S2). Thus, although the hemizygous state represents the molecular trigger of multigenerational RNAe, factors on a balancer chromosome can influence the severity and rate of silencing. An interesting future line of investigation will be to identify the trans-acting factor on the dpy-10 unc-4 balancer chromosome that promotes robust and completely penetrant multigenerational RNAe by crosses 3–5 (Figs. 1 F and G and 2 and Fig. S2).
The reversibility of multigenerational RNAe contrasts sharply with permanent transgene silencing of RNAe (32, 35, 36), indicating that multigenerational RNAe is a distinct, although related, form of siRNA-mediated genome silencing. RNAe was previously reported for a piRNA sensor transgene containing a perfect piRNA target site and for GFP transgenes that lack a perfect piRNA target site but are prone to silencing and are targeted at least in part by secondary siRNAs that may be generated by piRNAs with imperfect homology to GFP (32, 35, 36). We found that the piRNA sensor control transgene, which lacks a piRNA target site but is otherwise identical to the piRNA sensor transgene (32, 35, 36), succumbs to multigenerational RNAe instead of RNAe (Fig. 2F). Because the piRNA sensor and the piRNA sensor control both contain GFP and likely possess imperfect piRNA target sites, we conclude that the presence of a perfect piRNA target site can be a decisive trigger of RNAe. The piRNA sensor control transgene contains a control piRNA target site that is in reverse orientation (on the opposite strand of transgene DNA in comparison with the piRNA sensor) and so is not expressed (17). Therefore, the perfect piRNA target site in RNA transcripts from the piRNA sensor transgene is crucial for interactions with PRG-1/piRNAs that promote RNAe-mediated transcriptional silencing of this transgene. Note that the hypothesis that a perfect piRNA target site can promote RNAe has not been rigorously tested. For example, it remains uncertain whether a perfect piRNA target site is sufficient to promote RNAe and how piRNA identity or piRNA target site homology might affect interactions with RNAe.
How might PRG-1/piRNAs promote multigenerational RNAe of the piRNA sensor control transgene that does not possess a perfect piRNA target site? We suggest that piRNAs scan GFP and possibly other segments of RNA expressed from a transgene to promote the synthesis and/or stability of secondary siRNA populations, specifically in response to lack of pairing of the transgene during meiosis (57). How PRG-1 and associated piRNAs specifically target RNA derived from unpaired meiotic DNA for silencing is presently unclear.
As the presence of a perfect piRNA target site can elicit rapid and permanent RNAe, we suggest that the degree of homology of a piRNA with its mRNA target could reflect the history of a piRNA. For example, if C. elegans piRNA genes are occasionally created in response to foreign nucleic acids and initially possess perfect homology to their targets, then when PRG-1 encounters a transcript that matches a piRNA perfectly, this could suggest the expression of a recently integrated foreign genetic element that has not accumulated mutations in its piRNA target site. We speculate that such elements might be perceived as especially threatening and would then be targeted for immediate silencing via RNAe.
As we identified multiple GFP transgenes that succumb to multigenerational RNAe, we suggest that GFP transgenes that are prone to RNAe but lack a perfect piRNA target site (35) likely contain an additional factor that promotes RNAe-mediated transgene silencing. This factor could be an unusual piRNA target site or the expression of a transgenic protein that promotes RNAe-mediated silencing. For example, GFP transgenes that are prone to RNAe could repress an siRNA-mediated antisilencing system that was recently identified in C. elegans, which is mediated by the Argonaute protein CSR-1 and protects endogenous germline genes from silencing (58–61). Transgenes that are active in the germline and licensed by CSR-1 have been shown to elicit a multigenerational antisilencing effect, and active transgenes can act in trans via 22G-RNAs to awaken distinct silent transgenes (58, 60). The direct competition between CSR-1 and PRG-1 to mark a gene for either licensing and protection or for silencing and repression, respectively, is likely to potentiate both RNAe and multigenerational RNAe (35).
The interaction of pro- and antisilencing siRNA pathways is likely reflected by independent GFP transgenes that suffer from reduced levels of GFP expression due to PRG-1– and RDE-2–dependent silencing (Fig. 4), yet can be completely silenced via multigenerational RNAe (Fig. 2 A–D). We suggest that a significant number of transgenes that are routinely created in research laboratories may be incompletely silenced as a consequence of being hemizygous for several generations when they are created (32–35). Another general concern raised by our study is that once a transgenic strain has been created, the transgene may be subjected to transient or permanent silencing when manipulated in crossing schemes where it is hemizygous for several generations.
Previous reports have suggested that initiation and maintenance of transgene silencing are typically separable for single-copy GFP transgenes that are permanently silenced via RNAe, which can be initiated by the germline Argonaute protein PRG-1 but then maintained by secondary siRNA biogenesis proteins and small RNA-mediated nuclear silencing factors (36, 60). We show that maintenance of silencing for transgenes silenced by multigenerational RNAe can require both PRG-1 as well as the secondary siRNA protein RDE-2 (Fig. 3) and found that robust transgene desilencing occurred only when both prg-1 and rde-2 were disrupted. We conclude that multigenerational RNAe can lead to a state of epigenetic silencing where the maintenance phase of transgene silencing remains mechanistically coupled to PRG-1/piRNAs (Fig. 5B). Consistently, maintenance of silencing of the piRNA sensor transgene created in a wild-type background requires PRG-1/piRNAs (17), although outcrossing the piRNA sensor with a balancer chromosome will transform it into a permanently silent state that is no longer maintained by PRG-1/piRNAs (32, 35, 36). Overall, our results are consistent with genome silencing activities that can remain at least partially dependent on PRG-1 function, in contrast to the discrete role of PRG-1 in initiation, but not maintenance of silencing that has been typically reported for RNAe (32, 35, 36).
Our results are in consonance with a model derived from studies of epigenetic silencing in plants, where initiation and maintenance loops can promote transcriptional silencing mediated by small RNA-directed DNA methylation, for example in response to viral DNA (26, 62). Once silencing is established, the initiation loop can become dispensable for silencing, as is the case for PRG-1–initiated rapid silencing of a single-copy GFP transgene. However, the initiation loop can potentially contribute to maintenance of a silent state (26), as we show for PRG-1 in multigenerational silencing of the pot-1::mCherry.9 transgene. These results are consistent with a known role for PRG-1 in maintenance of silencing for a subset of transposons—the Tc3 DNA transposon becomes desilenced and active when prg-1 is mutant, but not Tc1 or Tc4 transposons, all of which become active when secondary siRNA biogenesis is disrupted (20). We further show that RDE-2 is required for initiation of silencing of active transgenes in multigenerational RNAe (Fig. 4), which is also consistent with examples in plants where initiation and maintenance phases of epigenetic silencing can be coupled (26). We note that the possibility that secondary siRNA biogenesis is required for initiation of RNAe has not been formally tested.
The formation of epialleles in reaction to foreign DNA has been well documented in Arabidopsis thaliana. Although plants possess a system of DNA methylation, and C. elegans lacks this mechanism, other epigenetic alterations that affect chromatin states do occur in the nematode (63–65). Epigenetic modification in plants is accomplished via the RNA-directed DNA methylation pathway, where RNA-dependent RNA polymerases bind single-stranded RNAs and create small dsRNAs that are then cleaved by Dicer and loaded into an Argonaute complex (66–70), which target both CG and non-CG sites for methylation (71). Once established, the cytosine methyl marks are associated with an epigenetic state that is transmitted to future generations. Maintenance is mediated by specific DNA methyltransferases, which copy epigenetic information to the daughter strand of DNA during meiosis, resulting in the establishment of an epiallele that can be passed on from one generation to the next (66, 72).
Genome-wide association studies have revealed that DNA sequence polymorphisms explain only a minor fraction of variability for a number of common human traits such as type II diabetes. Some of this missing heritability may be due to epigenetic modification of the genome (73–75), as reflected by creation of numerous de novo epialleles in F2 lines derived from crosses between different A. thaliana ecotypes (66, 76). Although there are a number of explanations for creation of such epialleles, genomes of metazoans typically display numerous copy number changes between homologous chromosomes. For example, gain or loss of copies of a specific segment of the genome has been estimated to account for up to 5% of the variability between human individuals (77, 78), implying that significant levels of unpaired DNA occur during most meioses. We propose that the multigenerational epigenetic silencing process described in our study, likely triggered by lack of pairing during meiosis, can elicit both permanent and reversible forms of silencing that may be relevant to heritable epigenomic variation in many species, with pertinent yet complex implications for understanding and predicting heritability in the context of human disease.
Materials and Methods
Strains.
All strains were cultured and maintained at 20 °C on nematode growth medium plates seeded with Escherichia coli OP50. Strains used include: Wild type (N2 Bristol ancestral strain), WM161 prg-1(tm872) I, SX922 prg-1(n4357) I, WM29 rde-2(ne221) I, rsd-6(yp11) I, GE1708 dpy-2(e8) unc-4(e120) II, DR103 dpy-10(e128) unc-4(e120) II, mIn1[unc-4(e120) dpy-10(e128)] II derived from DR2054, YA1198 ypIn3 [Pdaz-1:pot-1::mCherry::tbb-2utr], EG6787 oxSi487 [Pmex-5::mCherry::H2B::tbb-2 3′UTR::gpd-2 operon::GFP::H2B::cye-1 3′UTR + unc-119(+)] II, CB187 rol-6 (e187) II, SX1888 prg-1(n4357) I; mjIs144 II, nrde-1(yp4) III, nrde-1(yp5) III, LP135 cpSi10 [Pmex-5::GFP::tbb-2 3′UTR + unc-119(+)] II; unc-119(ed3) III, LP136 cpIs10 [Pmex-5::GFP::tbb-2 3′UTR + unc-119(+)] II; unc-119(ed3) III, vab-7(e1562) dpy-18(e364) III, LP148 unc-119(ed3) his-72(cp10[his-72::gfp + LoxP unc-119 (+) LoxP]) III, CA258 zim-2(tm574) IV, CA324 zim-1(tm1813) IV, zim-3(yp8) IV, YY158 nrde-3 (gg66) X, and SX1287 mjIs145 II; unc-119 (ed3) III. For zim experiments, males homozygous for a transgene and a zim mutation were created, crossed with zim; dpy-10 unc-4 triple mutant hermaphrodites, and F1 cross-progeny were scored for mCherry fluorescence.
Microscopy.
Live 1-d-old young adult worms were mounted on 2% (wt/vol) agarose pads in 7 µL of 2 mg/mL levamisole. Strains were examined in widefield using a Nikon Eclipse E800 microscope both under differential interference contrast (DIC) and a 595-nm excitation wavelength at 20×, 60×, or 100× magnifications. Confocal microscopy was performed using a Zeiss LSM710 laser-scanning microscope with a C-APO 40×/1.2 N.A. objective at 2.0× optical zoom. Images were obtained using a 561-nm diode laser for excitation, and emission was collected at 563–701 nm. Brightfield images were collected simultaneously.
Fluorescence Quantification.
Animals were mounted as described above, and Z stacks were taken using confocal microscopy as described above, within 2 h of mounting. Zeiss ZEN 2009 software was used to collect and compile the stacks. Analysis of fluorescence in the nuclear volume (arbitrary units) was performed using ImageJ v1.45S and the “measure stack” volume measurement plugin (developed by R. F. Dougherty and available at www.optinav.com/imagej.html). For each genotype, 30 animals were selected and five nuclei were quantified from each animal. GFP transgenic lines were mounted and examined as described above. Pictures were taken at 40× with an exposure time of 300 ms. To determine intensities, background was subtracted out using NIS Elements software. In ImageJ, a circle (100 pixels high ×100 pixels wide) was used to measure the intensity of three independent sections of each germline. Each intensity measurement was plotted using an x-axis jitter.
rtPCR.
Control and experimental animals were grown alongside each other under identical conditions. RNA extraction with TRIzol was performed using standard protocols. cDNA was synthesized using SuperScript III reverse transcriptase (Invitrogen) with random hexamers. Primer pair sequences used are as follows: actin control (F-GATATGGAGAAGATCTGGCATCA and R-GGGCAAGAGCGGTGATT) and mCherry (F-TGGTCCAATTTCGTGGTTTATATCCTC and R-CTTTGCTCTTCGCCATTGTTTCC).
Supplementary Material
Acknowledgments
We thank Dan Dickinson and Bob Goldstein for strains, Tony Perdue for technical assistance, and Alexandra Sapetschnig and Eric Miska for information regarding crosses of the piRNA sensor with prg-1. Some strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources. This research was supported by NIH Grant T32-CA009156 (to B.N.H.) and NIH Grant GM083048 (to S.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1501979112/-/DCSupplemental.
References
- 1.Fire A, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 3.Blevins T, et al. Four plant Dicers mediate viral small RNA biogenesis and DNA virus induced silencing. Nucleic Acids Res. 2006;34(21):6233–6246. doi: 10.1093/nar/gkl886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brodersen P, Voinnet O. The diversity of RNA silencing pathways in plants. Trends Genet. 2006;22(5):268–280. doi: 10.1016/j.tig.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Cogoni C, Macino G. Isolation of quelling-defective (qde) mutants impaired in posttranscriptional transgene-induced gene silencing in Neurospora crassa. Proc Natl Acad Sci USA. 1997;94(19):10233–10238. doi: 10.1073/pnas.94.19.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton AJ, Baulcombe DC. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science. 1999;286(5441):950–952. doi: 10.1126/science.286.5441.950. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein E, Denli AM, Hannon GJ. The rest is silence. RNA. 2001;7(11):1509–1521. [PMC free article] [PubMed] [Google Scholar]
- 8.Hutvágner G, et al. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293(5531):834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 9.Hutvagner G, Simard MJ. Argonaute proteins: Key players in RNA silencing. Nat Rev Mol Cell Biol. 2008;9(1):22–32. doi: 10.1038/nrm2321. [DOI] [PubMed] [Google Scholar]
- 10.Steiner FA, Okihara KL, Hoogstrate SW, Sijen T, Ketting RF. RDE-1 slicer activity is required only for passenger-strand cleavage during RNAi in Caenorhabditis elegans. Nat Struct Mol Biol. 2009;16(2):207–211. doi: 10.1038/nsmb.1541. [DOI] [PubMed] [Google Scholar]
- 11.Gent JI, et al. Distinct phases of siRNA synthesis in an endogenous RNAi pathway in C. elegans soma. Mol Cell. 2010;37(5):679–689. doi: 10.1016/j.molcel.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu C, et al. Structure-function analysis of mutant RNA-dependent RNA polymerase complexes with VPg. Biochemistry (Mosc) 2009;74(10):1132–1141. doi: 10.1134/s0006297909100095. [DOI] [PubMed] [Google Scholar]
- 13.Pak J, Fire A. Distinct populations of primary and secondary effectors during RNAi in C. elegans. Science. 2007;315(5809):241–244. doi: 10.1126/science.1132839. [DOI] [PubMed] [Google Scholar]
- 14.Sijen T, et al. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell. 2001;107(4):465–476. doi: 10.1016/s0092-8674(01)00576-1. [DOI] [PubMed] [Google Scholar]
- 15.Sijen T, Steiner FA, Thijssen KL, Plasterk RH. Secondary siRNAs result from unprimed RNA synthesis and form a distinct class. Science. 2007;315(5809):244–247. doi: 10.1126/science.1136699. [DOI] [PubMed] [Google Scholar]
- 16.Yigit E, et al. Analysis of the C. elegans Argonaute family reveals that distinct Argonautes act sequentially during RNAi. Cell. 2006;127(4):747–757. doi: 10.1016/j.cell.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 17.Bagijn MP, et al. Function, targets, and evolution of Caenorhabditis elegans piRNAs. Science. 2012;337(6094):574–578. doi: 10.1126/science.1220952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee RC, Hammell CM, Ambros V. Interacting endogenous and exogenous RNAi pathways in Caenorhabditis elegans. RNA. 2006;12(4):589–597. doi: 10.1261/rna.2231506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batista PJ, et al. PRG-1 and 21U-RNAs interact to form the piRNA complex required for fertility in C. elegans. Mol Cell. 2008;31(1):67–78. doi: 10.1016/j.molcel.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das PP, et al. Piwi and piRNAs act upstream of an endogenous siRNA pathway to suppress Tc3 transposon mobility in the Caenorhabditis elegans germline. Mol Cell. 2008;31(1):79–90. doi: 10.1016/j.molcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon M, et al. 2014. Reduced insulin/IGF-1 signaling restores germ cell immortality to Caenorhabditis elegans Piwi mutants Cell Rep 7(3):762–773.
- 22.Adamo A, et al. Transgene-mediated cosuppression and RNA interference enhance germ-line apoptosis in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2012;109(9):3440–3445. doi: 10.1073/pnas.1107390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dernburg AF, Zalevsky J, Colaiácovo MP, Villeneuve AM. Transgene-mediated cosuppression in the C. elegans germ line. Genes Dev. 2000;14(13):1578–1583. [PMC free article] [PubMed] [Google Scholar]
- 24.Ketting RF, Plasterk RH. A genetic link between co-suppression and RNA interference in C. elegans. Nature. 2000;404(6775):296–298. doi: 10.1038/35005113. [DOI] [PubMed] [Google Scholar]
- 25.Dalmay T, Hamilton A, Rudd S, Angell S, Baulcombe DC. An RNA-dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell. 2000;101(5):543–553. doi: 10.1016/s0092-8674(00)80864-8. [DOI] [PubMed] [Google Scholar]
- 26.Baulcombe D. RNA silencing in plants. Nature. 2004;431(7006):356–363. doi: 10.1038/nature02874. [DOI] [PubMed] [Google Scholar]
- 27.Jorgensen RA. Cosuppression, flower color patterns, and metastable gene expression states. Science. 1995;268(5211):686–691. doi: 10.1126/science.268.5211.686. [DOI] [PubMed] [Google Scholar]
- 28.Akitake CM, Macurak M, Halpern ME, Goll MG. Transgenerational analysis of transcriptional silencing in zebrafish. Dev Biol. 2011;352(2):191–201. doi: 10.1016/j.ydbio.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goll MG, Anderson R, Stainier DY, Spradling AC, Halpern ME. Transcriptional silencing and reactivation in transgenic zebrafish. Genetics. 2009;182(3):747–755. doi: 10.1534/genetics.109.102079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grosveld F, van Assendelft GB, Greaves DR, Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- 31.Sharpe JA, et al. Analysis of the human alpha-globin gene cluster in transgenic mice. Proc Natl Acad Sci USA. 1993;90(23):11262–11266. doi: 10.1073/pnas.90.23.11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashe A, et al. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell. 2012;150(1):88–99. doi: 10.1016/j.cell.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frøkjær-Jensen C, Davis MW, Ailion M, Jorgensen EM. Improved Mos1-mediated transgenesis in C. elegans. Nat Methods. 2012;9(2):117–118. doi: 10.1038/nmeth.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frøkjaer-Jensen C, et al. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat Genet. 2008;40(11):1375–1383. doi: 10.1038/ng.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shirayama M, et al. piRNAs initiate an epigenetic memory of nonself RNA in the C. elegans germline. Cell. 2012;150(1):65–77. doi: 10.1016/j.cell.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luteijn MJ, et al. Extremely stable Piwi-induced gene silencing in Caenorhabditis elegans. EMBO J. 2012;31(16):3422–3430. doi: 10.1038/emboj.2012.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamminga LM, et al. Differential impact of the HEN1 homolog HENN-1 on 21U and 26G RNAs in the germline of Caenorhabditis elegans. PLoS Genet. 2012;8(7):e1002702. doi: 10.1371/journal.pgen.1002702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shtessel L, et al. Caenorhabditis elegans POT-1 and POT-2 repress telomere maintenance pathways. G3 (Bethesda) 2013;3(2):305–313. doi: 10.1534/g3.112.004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dickinson DJ, Ward JD, Reiner DJ, Goldstein B. Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nat Methods. 2013;10(10):1028–1034. doi: 10.1038/nmeth.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grishok A, Tabara H, Mello CC. Genetic requirements for inheritance of RNAi in C. elegans. Science. 2000;287(5462):2494–2497. doi: 10.1126/science.287.5462.2494. [DOI] [PubMed] [Google Scholar]
- 41.Sijen T, Plasterk RH. Transposon silencing in the Caenorhabditis elegans germ line by natural RNAi. Nature. 2003;426(6964):310–314. doi: 10.1038/nature02107. [DOI] [PubMed] [Google Scholar]
- 42.Tops BB, et al. RDE-2 interacts with MUT-7 to mediate RNA interference in Caenorhabditis elegans. Nucleic Acids Res. 2005;33(1):347–355. doi: 10.1093/nar/gki183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burkhart KB, et al. A pre-mRNA-associating factor links endogenous siRNAs to chromatin regulation. PLoS Genet. 2011;7(8):e1002249. doi: 10.1371/journal.pgen.1002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhuang JJ, Banse SA, Hunter CP. The nuclear argonaute NRDE-3 contributes to transitive RNAi in Caenorhabditis elegans. Genetics. 2013;194(1):117–131. doi: 10.1534/genetics.113.149765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han W, Sundaram P, Kenjale H, Grantham J, Timmons L. The Caenorhabditis elegans rsd-2 and rsd-6 genes are required for chromosome functions during exposure to unfavorable environments. Genetics. 2008;178(4):1875–1893. doi: 10.1534/genetics.107.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang C, et al. The Caenorhabditis elegans RDE-10/RDE-11 complex regulates RNAi by promoting secondary siRNA amplification. Curr Biol. 2012;22(10):881–890. doi: 10.1016/j.cub.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phillips CM, Dernburg AF. A family of zinc-finger proteins is required for chromosome-specific pairing and synapsis during meiosis in C. elegans. Dev Cell. 2006;11(6):817–829. doi: 10.1016/j.devcel.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 48.Hawley RS, Gilliland WD. Homologue pairing: Getting it right. Nat Cell Biol. 2009;11(8):917–918. doi: 10.1038/ncb0809-917. [DOI] [PubMed] [Google Scholar]
- 49.Phillips CM, et al. Identification of chromosome sequence motifs that mediate meiotic pairing and synapsis in C. elegans. Nat Cell Biol. 2009;11(8):934–942. doi: 10.1038/ncb1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maine EM, et al. EGO-1, a putative RNA-dependent RNA polymerase, is required for heterochromatin assembly on unpaired dna during C. elegans meiosis. Curr Biol. 2005;15(21):1972–1978. doi: 10.1016/j.cub.2005.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aramayo R, Metzenberg RL. Meiotic transvection in fungi. Cell. 1996;86(1):103–113. doi: 10.1016/s0092-8674(00)80081-1. [DOI] [PubMed] [Google Scholar]
- 52.Shiu PK, Metzenberg RL. Meiotic silencing by unpaired DNA: Properties, regulation and suppression. Genetics. 2002;161(4):1483–1495. doi: 10.1093/genetics/161.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shiu PK, Raju NB, Zickler D, Metzenberg RL. Meiotic silencing by unpaired DNA. Cell. 2001;107(7):905–916. doi: 10.1016/s0092-8674(01)00609-2. [DOI] [PubMed] [Google Scholar]
- 54.Hammond TM, et al. Identification of small RNAs associated with meiotic silencing by unpaired DNA. Genetics. 2013;194(1):279–284. doi: 10.1534/genetics.112.149138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hammond TM, et al. SAD-3, a putative helicase required for meiotic silencing by unpaired DNA, interacts with other components of the silencing machinery. G3 (Bethesda) 2011;1(5):369–376. doi: 10.1534/g3.111.000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hynes MJ, Todd RB. Detection of unpaired DNA at meiosis results in RNA-mediated silencing. BioEssays. 2003;25(2):99–103. doi: 10.1002/bies.10241. [DOI] [PubMed] [Google Scholar]
- 57.de Albuquerque BF, Ketting RF. Is this mine? Small RNAs help to decide. Dev Cell. 2013;27(6):599–601. doi: 10.1016/j.devcel.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 58.Avgousti DC, Palani S, Sherman Y, Grishok A. CSR-1 RNAi pathway positively regulates histone expression in C. elegans. EMBO J. 2012;31(19):3821–3832. doi: 10.1038/emboj.2012.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conine CC, et al. Argonautes promote male fertility and provide a paternal memory of germline gene expression in C. elegans. Cell. 2013;155(7):1532–1544. doi: 10.1016/j.cell.2013.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seth M, et al. The C. elegans CSR-1 argonaute pathway counteracts epigenetic silencing to promote germline gene expression. Dev Cell. 2013;27(6):656–663. doi: 10.1016/j.devcel.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wedeles CJ, Wu MZ, Claycomb JM. Protection of germline gene expression by the C. elegans Argonaute CSR-1. Dev Cell. 2013;27(6):664–671. doi: 10.1016/j.devcel.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 62.Waterhouse PM, Graham MW, Wang MB. Virus resistance and gene silencing in plants can be induced by simultaneous expression of sense and antisense RNA. Proc Natl Acad Sci USA. 1998;95(23):13959–13964. doi: 10.1073/pnas.95.23.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui M, Han M. Roles of chromatin factors in C. elegans development. WormBook. 2007;3:1–16. doi: 10.1895/wormbook.1.139.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gonzalez-Aguilera C, Palladino F, Askjaer P. C. elegans epigenetic regulation in development and aging. Brief Funct Genomics. 2013;13(3):223–234. doi: 10.1093/bfgp/elt048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wenzel D, Palladino F, Jedrusik-Bode M. Epigenetics in C. elegans: Facts and challenges. Genesis. 2011;49(8):647–661. doi: 10.1002/dvg.20762. [DOI] [PubMed] [Google Scholar]
- 66.Bond DM, Baulcombe DC. Small RNAs and heritable epigenetic variation in plants. Trends Cell Biol. 2014;24(2):100–107. doi: 10.1016/j.tcb.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 67.Haag JR, et al. In vitro transcription activities of Pol IV, Pol V, and RDR2 reveal coupling of Pol IV and RDR2 for dsRNA synthesis in plant RNA silencing. Mol Cell. 2012;48(5):811–818. doi: 10.1016/j.molcel.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Havecker ER, et al. The Arabidopsis RNA-directed DNA methylation argonautes functionally diverge based on their expression and interaction with target loci. Plant Cell. 2010;22(2):321–334. doi: 10.1105/tpc.109.072199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Law JA, Vashisht AA, Wohlschlegel JA, Jacobsen SE. SHH1, a homeodomain protein required for DNA methylation, as well as RDR2, RDM4, and chromatin remodeling factors, associate with RNA polymerase IV. PLoS Genet. 2011;7(7):e1002195. doi: 10.1371/journal.pgen.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xie Z, et al. Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2004;2(5):E104. doi: 10.1371/journal.pbio.0020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chan SW, et al. RNA silencing genes control de novo DNA methylation. Science. 2004;303(5662):1336. doi: 10.1126/science.1095989. [DOI] [PubMed] [Google Scholar]
- 72.Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet. 2010;11(3):204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jablonka E, Lamb MJ. The evolution of information in the major transitions. J Theor Biol. 2006;239(2):236–246. doi: 10.1016/j.jtbi.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 74.Park S, Lehner B. Epigenetic epistatic interactions constrain the evolution of gene expression. Mol Syst Biol. 2013;9:645. doi: 10.1038/msb.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weigel D, Colot V. Epialleles in plant evolution. Genome Biol. 2012;13(10):249. doi: 10.1186/gb-2012-13-10-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmitz RJ, et al. Transgenerational epigenetic instability is a source of novel methylation variants. Science. 2011;334(6054):369–373. doi: 10.1126/science.1212959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bailey JA, et al. Recent segmental duplications in the human genome. Science. 2002;297(5583):1003–1007. doi: 10.1126/science.1072047. [DOI] [PubMed] [Google Scholar]
- 78.Zhang F, Gu W, Hurles ME, Lupski JR. Copy number variation in human health, disease, and evolution. Annu Rev Genomics Hum Genet. 2009;10:451–481. doi: 10.1146/annurev.genom.9.081307.164217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.