Significance
Descriptions of impaired coherent motion perception in autism spectrum disorder (ASD) underlie theories that individuals with ASD have difficulty integrating local “parts” into a global percept. This notion maintains widespread influence and motivates recent theories of defective multisensory integration in ASD. However, heightened sensitivity to sensory noise, used to manipulate task difficulty in predominant visual motion stimuli, may provide an alternative explanation for impaired performance. Here we indeed found increased sensitivity in ASD to sensory noise. Noiseless motion perception and multisensory integration (even with noise) were unimpaired. These findings question prevalent theories of global and multisensory integration deficits in ASD. Rather, they suggest increased reliance on—and sensitivity to—incoming sensory information and less use of prior knowledge in ASD.
Keywords: autism, multisensory integration, noise, coherence, Bayesian
Abstract
Perceptual processing in autism spectrum disorder (ASD) is marked by superior low-level task performance and inferior complex-task performance. This observation has led to theories of defective integration in ASD of local parts into a global percept. Despite mixed experimental results, this notion maintains widespread influence and has also motivated recent theories of defective multisensory integration in ASD. Impaired ASD performance in tasks involving classic random dot visual motion stimuli, corrupted by noise as a means to manipulate task difficulty, is frequently interpreted to support this notion of global integration deficits. By manipulating task difficulty independently of visual stimulus noise, here we test the hypothesis that heightened sensitivity to noise, rather than integration deficits, may characterize ASD. We found that although perception of visual motion through a cloud of dots was unimpaired without noise, the addition of stimulus noise significantly affected adolescents with ASD, more than controls. Strikingly, individuals with ASD demonstrated intact multisensory (visual–vestibular) integration, even in the presence of noise. Additionally, when vestibular motion was paired with pure visual noise, individuals with ASD demonstrated a different strategy than controls, marked by reduced flexibility. This result could be simulated by using attenuated (less reliable) and inflexible (not experience-dependent) Bayesian priors in ASD. These findings question widespread theories of impaired global and multisensory integration in ASD. Rather, they implicate increased sensitivity to sensory noise and less use of prior knowledge in ASD, suggesting increased reliance on incoming sensory information.
One in every 68 children in the United States is diagnosed with autism spectrum disorder (ASD) (1). ASD is characterized by a range of social, communication, cognitive, and behavioral impairments, as well as differences in perceptual processing (2). Intriguingly, individuals with ASD often demonstrate superior performance at low-level visual tasks, such as those that emphasize the perception of local elements (3–6). However, perceptual deficits are often described in more complex visual tasks (7), like those that require aggregating local elements into a global whole (8–10). However, many aspects of the latter remain unclear. Even the basic question of whether this difference represents an actual deficit of perceptual integration or whether it simply results from a bias for local processing is a matter of debate (5, 11, 12).
Visual-motion stimuli have been widely used to investigate global perception in ASD. These studies have predominantly used random dot kinematograms (RDKs), for which participants are required to discriminate the overall direction of motion of a field of dots, where some dots move coherently while the others are randomly displaced. Increased ASD thresholds are indeed reported in many RDK studies (refs. 13–17; but see refs. 18–20), as well as similar studies using Glass patterns (detecting a target area of coherent dot rotation within a random dot display) (21, 22).
Although RDK studies are recognized to reflect “coherent motion perception,” interpretation of this term may significantly affect the conclusions of these studies. Traditional visual research specifically associates the term coherent motion perception with RDK stimuli, and thus noise. However, interpretation in the ASD literature focuses primarily on its meaning as “global,” irrespective of noise [e.g., see noiseless coherent motion perception (23)]. This understanding of coherent is reflected already in the terminology of “weak central coherence” theories (11, 24) and extends to other uses, such as “coherent form perception” (13, 18, 25). Although such use may actually reflect a good literal understanding of coherent, it may be the root of a serious confound in the interpretation of RDK results in ASD. Specifically, reduced RDK performance in ASD may result from the inherent noise rather than global integration deficits.
Interestingly, a noiseless motion paradigm using plaid stimuli that can be perceived as a coherently moving pattern or as two transparent gratings sliding over each other found intact global motion perception in ASD (23). This finding, together with the RDK confound explained above and predominant descriptions of intact global form processing (refs. 9, 12, 13, 18, and 25; but see refs. 21, 22), all contribute to the already wide debate regarding the very existence of global integration deficits in ASD (5, 11, 12) and highlight the need to better understand the mechanisms of impaired RDK performance.
In the classic RDK paradigm, thresholds are measured as a percentage of coherently vs. randomly moving dots (thus, noise is always present). To dissociate sensory noise and global integration, we use here a different paradigm comprising simulated self-motion through a random dot cloud (star-field), in which discrimination thresholds are measured in spatial angles (26, 27). Thus, thresholds can be measured also for noiseless motion stimuli (100% coherence), as well as for varying levels of visual noise. Interestingly, we found that adolescents with ASD demonstrate unimpaired perception of self-motion through a noiseless (100% coherence) star-field. Rather, they demonstrate greater perceptual impairment (compared with controls) specifically in the presence of sensory noise.
In light of the pressing need to operationalize and study multisensory integration in ASD (28), global processing theories have also led to hypotheses that individuals with ASD may have deficits integrating across multiple senses. Reduced audiovisual facilitation has recently been described in ASD (29, 30), in conjunction with less effective neural integration (30). Altered audiovisual temporal processing has also been found (31, 32). However, the concurrent presence of low-level auditory deficits (33, 34), together with robust multisensory integration that was nonetheless still observed (30), makes it difficult to implicate multisensory integration per se vs. compounded effects of early sensory deficits.
Further complicating the matter, ASD is often studied in children, whereas multisensory integration is not fully developed until adolescence (35–37). There are few, and contradictory, results regarding the existence of multisensory deficits in adolescents with ASD. Smith and Bennetto (38) describe audiovisual speech integration deficits. However, other studies have found that adolescents with ASD catch up to their typically developing peers and that these deficits are ameliorated upon entering adolescence (39, 40). Also the terminology of “multisensory integration” is inconsistent (28, 41), making the interpretation of results complex. Thus, a second goal of the present study was to test for a specific, well-studied property of cue combination. We found no multisensory (visual–vestibular) integration impairment, even in the presence of visual noise.
Our results suggest that global and multisensory integration remain intact in ASD. Rather, adolescents with ASD may suffer from a heightened sensitivity to sensory noise, possibly due to higher reliance on incoming sensory signals and less use of prior knowledge about the world (which would have otherwise dampened the sensory noise) (42, 43).
Result
ASD Example.
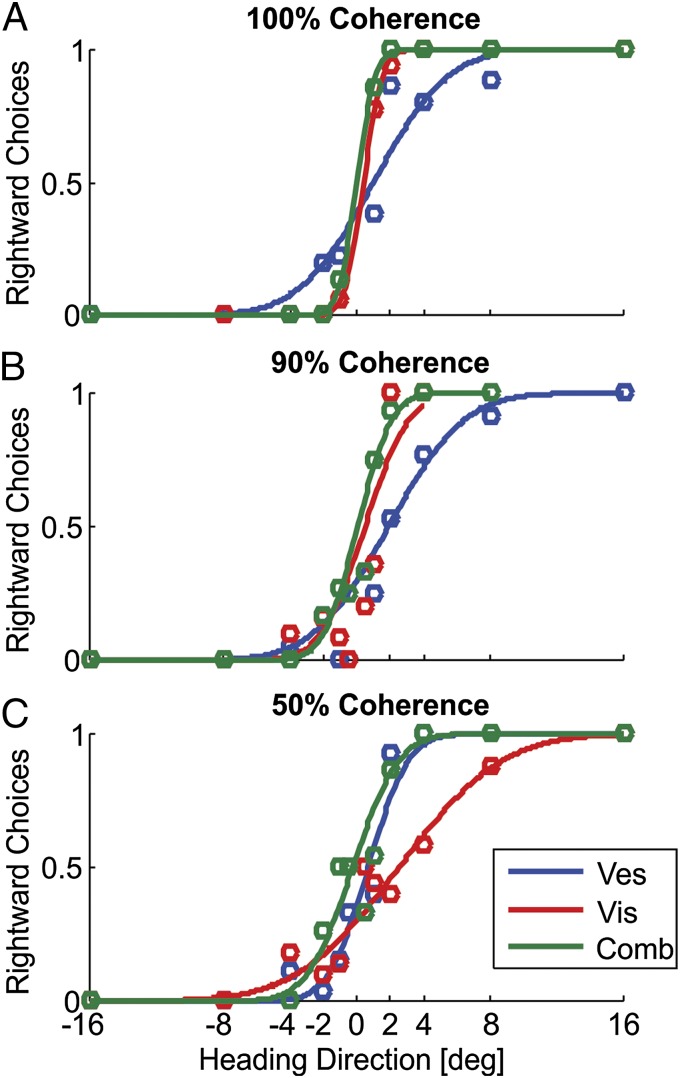
Adolescents with high-functioning ASD perform very well on our task of self-motion perception. Three blocks for an example participant with ASD are presented in Fig. 1. For 100% visual motion coherence (no visual noise), visual cue reliability is high, as demonstrated by the steep slope of the psychometric curve (Fig. 1A, red). High reliability means that the participant can easily discern the direction of simulated self-motion (right or left of straight ahead), even for small heading angles. In comparison, his vestibular cue here is less reliable, as demonstrated by the relatively less steep psychometric curve (Fig. 1A, blue). When experiencing simultaneous visual and vestibular stimuli (combined condition), the participant relies primarily on the more reliable visual cue and thus achieves reliable performance also in this multisensory case (Fig. 1A, green curve). These results are similar to those expected from typical adolescents or adults.
Fig. 1.
ASD example. Behavioral responses of an example participant with ASD are presented for three separate blocks, tested with 100% (A), 90% (B), and 50% (C) visual motion coherence. For each block, psychometric curves represent the ratio of rightward choices as a function of heading direction, based on visual-only (red), vestibular-only (blue), or combined visual–vestibular (green) cues. Data (circles) were fitted with cumulative Gaussian functions (solid lines).
The introduction of visual noise affects visual reliability. Reduced reliability is demonstrated by the deterioration (flattening) of the psychometric curve—seen already with slight noise (90% coherence; Fig. 1B, red curve) and more strongly for higher noise (50% coherence; Fig. 1C, red curve). Despite this steep deterioration in visual reliability, multisensory integration is still evident in the combined condition: In Fig. 1C, the participant relies primarily on the now-more-reliable vestibular cue (the green curve is now more similar to the blue curve). Also, when the cues are of more similar reliability (Fig. 1B), the combined cue is more reliable than each of the individual cues alone. Thus, important characteristics of optimal multisensory integration are fulfilled even in ASD.
We now look at the group data and investigate whether the effects of visual noise in ASD are more extreme than normal. To do so, we compare cue thresholds, defined as the SD of the psychometric fit (Data Analysis). Note that thresholds are inversely related to reliability, such that small thresholds (steep psychometric curves) indicate a reliable cue and large thresholds mark low reliability.
ASD Sensitivity to Visual Noise.
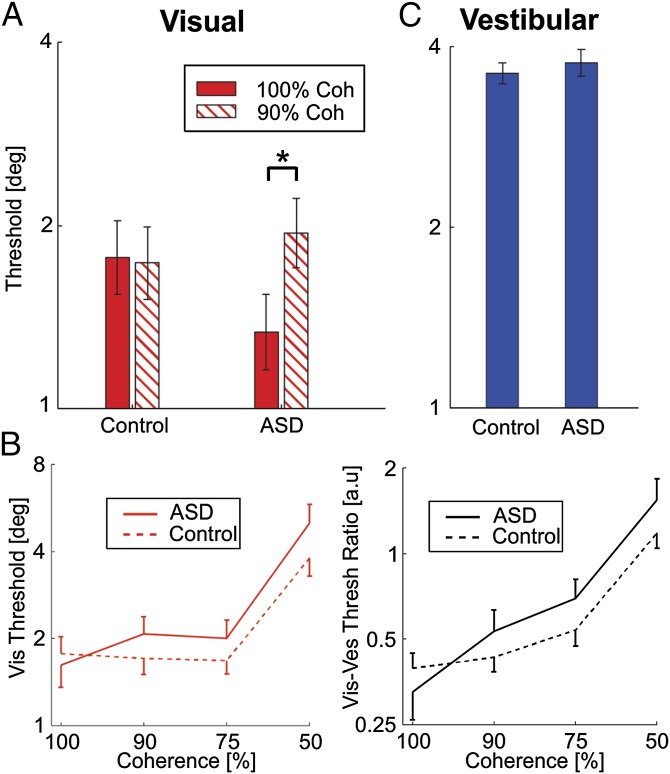
To compare the initial effect of visual noise, we looked at the data from the first visit, in which two blocks were tested: a 100% coherence block, followed by a 90% coherence block (only data in which both blocks were completed in the same visit were used for this comparison; 20 control and 12 ASD participants). Here, an interesting difference was seen between ASD and controls. For controls, visual thresholds at 100% and 90% coherence were comparable (Fig. 2A, left)—demonstrating that the little noise added during 90% coherence is largely undisruptive in normal participants. In contrast, this slight addition of noise largely affects participants with ASD, who demonstrated a significant increase in threshold (Fig. 2A, right).
Fig. 2.
Response to visual noise. (A) Filled and textured red bars represent the log-scale mean ± SEM visual psychometric thresholds for 100% and 90% motion coherence, respectively. These were similar for controls (left) but significantly different for participants with ASD (right). *P < 0.05. (B, Left) Red solid and dashed lines represent the log-scale mean visual thresholds, as a function of visual motion coherence for participants with ASD and controls, respectively. Error bars mark 1 SEM. (B, Right) Similarly, black solid and dashed lines represent the mean visual-to-vestibular threshold ratios for participants with ASD and controls, respectively. (C) Filled blue bars represent the log-scale mean ± SEM vestibular psychometric thresholds, which were similar for control and ASD participants. See also Figs. S1 and S2.
We now look at all of the data, gathered across different levels of coherence (Fig. 2B). Although, on average, ASD participants began with slightly better (lower) visual thresholds at 100%, this trend switched when noise was added to the stimulus—average ASD thresholds were worse for all three noisy conditions (90%, 75%, and 50% coherences; Fig. 2B, Left). An ANOVA comparison of the noisy-condition visual thresholds, in relation to the noiseless (100% coherence) condition, found a significant difference between the ASD and control groups (P = 0.004). This finding indicates that participants with ASD are more strongly affected by visual sensory noise.
Vestibular trials were interleaved in all of the blocks (together with visual and multisensory trials). No difference was seen between the vestibular thresholds for ASD vs. controls (P = 0.6; Fig. 2C). For this comparison, all vestibular data were pooled across the different coherence blocks (because no noise was added to the vestibular stimulus). However, also when grouped by visual coherence, no significant differences were seen in vestibular thresholds (ANOVA, P = 0.6; Fig. S1).
An alternative way to extract the effects of visual noise is to look at the relative reliability between the visual and vestibular cues, on a block-by-block basis. A main expected effect is that, as more visual noise is added (increasing visual thresholds), the visual-to-vestibular threshold ratio should increase—the question is by how much? An exaggerated response to visual noise would cause a larger increase in this ratio. Indeed, we found here that, although ASD participants started with a lower visual-to-vestibular threshold ratio than controls at 100% coherence, when noise was added (90%, 75%, and 50% coherences), their ratios overtook and became larger than controls’ (Fig. 2B, Right). An ANOVA comparing the noisy conditions found a significant difference between the ASD and control groups (P = 0.04). This finding shows an exaggerated response specifically in the visual cue. Also, reduced performance is not an effect of fatigue or attention, because concurrent vestibular performance was indistinguishable from controls’ (Fig. S1).
To confirm that the range of ages used in this study (13–19 y old) was not a confounding factor, we performed the same analysis presented above on a subset of participants with a narrower age range (15–17 y old). This subset demonstrated the same results as the whole cohort (Fig. S2). We showed here that individuals with ASD are more affected by visual noise than controls. Next, we tested their multisensory integration—i.e., whether or not individuals with ASD integrate visual and vestibular cues near-optimally, also in the presence of noise—as shown in normal individuals (26, 44).
Intact Multisensory Integration.
A strong characteristic of optimal multisensory integration is reduced variability of combined-cue performance (45–47). Thus, to assess multisensory integration in ASD, we compare the measured combined-cue thresholds to those predicted theoretically, as described here below. When two cues with Gaussian noise distributions—e.g., visual and vestibular—with respective SDs (thresholds) σvis and σves, are integrated optimally, the variance of the combined-cue measurement is given by:
| . | [1] |
The largest reduction in threshold (decrease by a factor of ) occurs when the two cues have equal thresholds (σvis = σves). When one cue is much more reliable than the other (e.g., visual more reliable: σvis << σves), behavior is captured mainly by the reliable cue such that σc ∼ σvis.
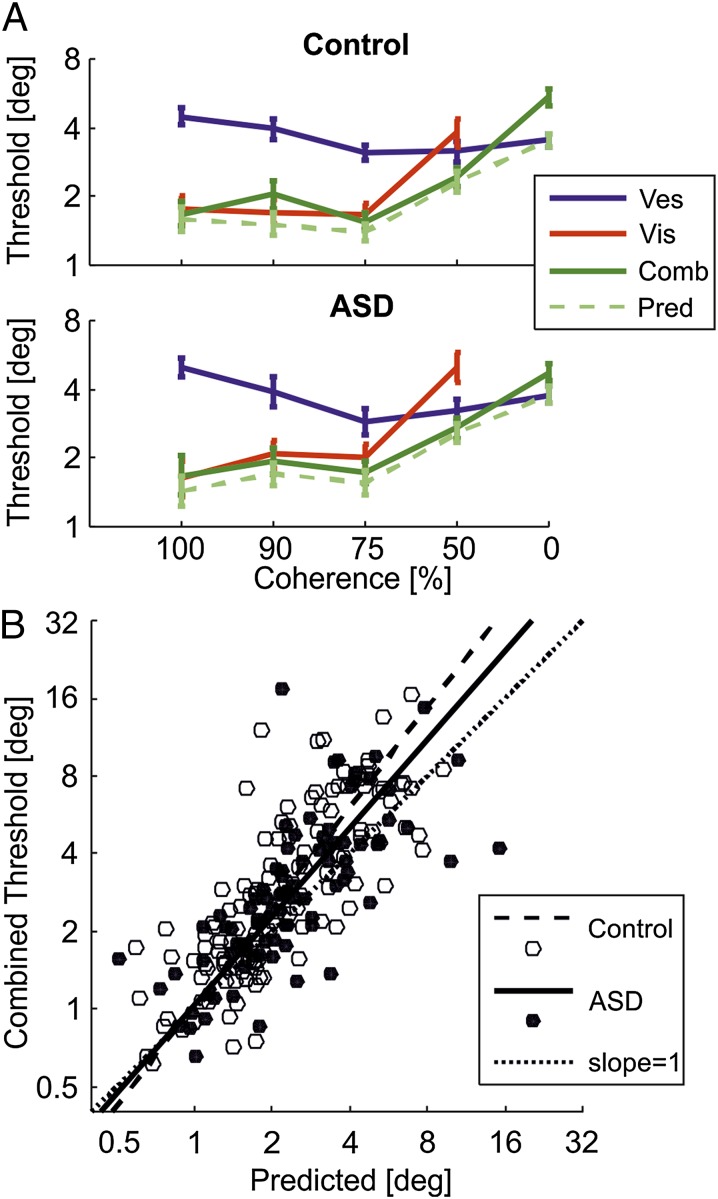
The average visual, vestibular, and combined cue thresholds, measured from the corresponding visual-only, vestibular-only, and multisensory (combined cue) trials, are presented in Fig. 3A (red, blue, and dark green, respectively). A comparison of the measured multisensory thresholds (dark green) to those predicted from the single cues using Eq. 1 (light green, dashed) demonstrates intact multisensory integration for both control and ASD groups (0% coherence is an exception for both groups and is dealt with separately in Reduced Multisensory Flexibility in the Presence of Pure Visual Noise).
Fig. 3.
Multisensory integration. (A) Mean ± SEM (log-scale) psychometric thresholds are presented for visual (red), vestibular (blue), and combined visual–vestibular (green) cues as a function of visual coherence for controls (Upper) and participants with ASD (Lower). The light green dashed line represents predicted combined cue thresholds, based on the single (visual and vestibular) cues. Data for 0% coherence was pooled across all four blocks (the other data comprise one block per coherence). (B) A scatter plot of actual combined cue thresholds vs. predicted thresholds for controls (open circles) and participants with ASD (filled circles) is presented. Dashed and solid lines represent type-II regressions for controls and participants with ASD, respectively. Ideal performance (y = x) is marked by the diagonal dotted line.
Firstly, for 100% and 90% coherence conditions, where the visual cue is considerably more reliable than vestibular, the combined cue is dominated by the visual cue (σc ∼ σvis). Thus, even though participants with ASD demonstrate an exaggerated response to slight visual noise at 90% coherence (described above), they nonetheless appropriately integrate the visual information, which still dominates the combined condition due to lower visual vs. vestibular thresholds. At 75% coherence, visual thresholds approach vestibular thresholds for ASD (not control), and a reduction in combined cue thresholds begins to emerge. This reduction is also seen at 50% coherence where visual thresholds are now worse than vestibular for both groups. Thus, when the visual cue carries any signal (100%, 90%, 75%, and 50% coherence), it is appropriately integrated with the vestibular cue, also in ASD.
Scatter plots and regressions of the measured vs. predicted combined cue thresholds across all conditions (Fig. 3B) indicate near-optimal cue integration for ASD and control participants. Optimal integration is marked by a slope of 1 (dotted line). The bootstrap (n = 1,000) calculated 95% confidence interval for the slope of the ASD linear regression (solid line) included 1, both when excluding and including 0% coherence sessions ([0.8 1.6] and [0.9 1.5], respectively). Thus, ASD performance was not significantly different from optimal. For controls, the confidence interval only included 1 when excluding, but not when including, the 0% coherence sessions ([0.9 1.5] and [1.1 1.5], respectively). Therefore, no deficit in multisensory integration was seen in ASD (if anything, they seemed more optimal here than controls).
Reduced Multisensory Flexibility in the Presence of Pure Visual Noise.
We now address the interesting condition of 0% coherence. Here, optimal observers should perform the task based solely on nonvisual cues and ignore dot motion. Given that individuals with ASD are more affected by visual noise than controls (Fig. 2), one may have expected that the presence of pure visual noise (0% coherence) would affect their multisensory performance more than controls. However, there is an important difference: Whereas Fig. 2 describes the effects of visual noise on the same (visual) cue, here we are testing how the noisy visual cue is integrated with another (vestibular) cue. Thus, it does not test sensitivity to noise per se, but rather multisensory integration of a completely noisy (visual) cue with another (vestibular) cue. In fact, we also showed (Fig. 3) that individuals with ASD integrate even noisy visual cues appropriately. The 0% coherence sessions simply test whether appropriate multisensory integration occurs, even in the extreme case of complete visual noise.
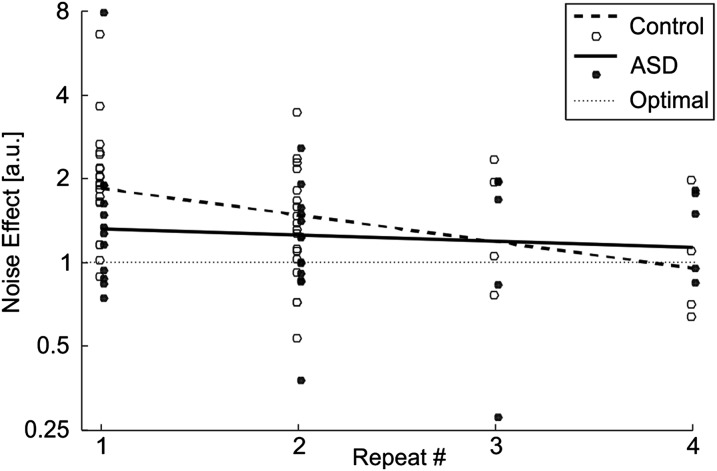
Specifically, for 0% coherence, four blocks were tested (Materials and Methods), thus progression of behavior (learning) could be studied as a function of block repeats (Fig. 4). Across these blocks, a very different picture emerges for the ASD and control groups. On their first exposure to 0% coherence noise, control participants tried to integrate the visual cue even though it carries no information. Overall (grouping all four repeats), significant integration of noise was found (Fig. 3A, Upper; paired t test, P < 10−6). However, a significant learning effect (Fig. 4, dashed line; Pearson correlation, P = 0.005) demonstrates that they quickly learned to down-weight the visual noise and achieved more optimal behavior by the fourth repeat. In contrast, although the ASD group also integrated visual noise (Fig. 3A, Lower; paired t test, P = 0.02), they did this to a lesser degree initially, and they showed no change over repeats (Fig. 4, solid line; Pearson correlation P = 0.6). Therefore, although individuals with ASD seemed to perform better initially, unlike controls, their strategy was static and did not change over time. This interesting result is addressed further in Discussion, SI Text, and Fig. S3.
Fig. 4.
Influence of complete visual noise. The noise effect (ratio of psychometric thresholds with/without the addition of 0% coherence visual noise) as a function of session repeats is presented for controls (open circles) and participants with ASD (filled circles). Linear regressions of the noise effect indicate habituation for controls (dashed line) but not for participants with ASD (solid line). The horizontal dotted line represents no noise effect (optimal noise filtering). See also Fig. S3.
Discussion
In our multisensory task of self-motion perception, we showed that adolescents with high-functioning ASD have a specific sensitivity to visual sensory noise. In contrast, their performance in noiseless visual (100% coherence), vestibular, and multisensory integration conditions was no worse (and often even better) than controls. Strikingly, even noisy visual signals were appropriately integrated with vestibular cues. These findings question prevalent theories that ASD is marked by specific deficits in the integration of local elements, or the integration of multiple senses, into a global percept (11, 48).
Rather, our results suggest that deficits of coherent motion perception, often described in ASD (13–17), result not from a spatial integration impairment, but from the sensory noise inherent in RDK tests. This idea can settle some apparent contradictions in the ASD literature. Specifically, it can concurrently explain impaired ASD performance found in most RDK and glass pattern studies (13–17, 21, 22), in conjunction with unimpaired global perception of plaid motion stimuli (23) and unimpaired form processing (9, 12, 13, 18, 25). Although all these studies test global spatial perception (integrating the parts to form a coherent percept), only the former contain dynamic visual noise. It does not explain why some RDK studies did not find a significant increase in ASD thresholds (18–20). However, these may result from methodological differences—e.g., very long (effectively unlimited until response) stimulus presentation times (19, 20), or unbalanced data collection (three vs. one sessions for ASD and controls, respectively, in ref. 18). Furthermore, this notion is also in line with Robertson et al. (49) who suggest that low-level (reflected as early as primary visual cortex), and not high-level, integrative, phenomena account for RDK differences in ASD.
Already the first description of autism (50) notes that autistic children who are distressed by loud external sounds are not bothered if they themselves generate the same sounds. Accordingly, Pellicano and Burr (42) suggest that events that are unexpected and unpredictable are particularly unsettling for autistic individuals and that understanding the effects of sensory uncertainty is key to explaining perception in ASD. This notion is supported by our primary findings of heightened sensitivity to visual sensory noise, which is an unexpected sensory experience for our novice participants. Pellicano and Burr go on to suggest that attenuated Bayesian priors (namely, a stronger reliance on incoming sensory information and less use of prior knowledge about the world) may be responsible for the different perceptual experience in ASD. Bayesian priors can be advantageous because they smooth noise in sensory input and reduce variability. Therefore, attenuated Bayesian priors predict an increase in cortical response variability, as indeed found in ASD (51, 52), and increased sensitivity to sensory noise, as described here.
Interestingly, the attenuated Bayesian prior hypothesis may also explain our finding of better initial performance in ASD when vestibular motion was paired with complete visual noise. Good performance here required correct estimation that the visual cue was completely noisy. However, all sessions preceding the first 0% coherence session contained visual stimuli which were not completely noisy and thus required integration also of the visual cue. Hence a cue-weighting prior might lead to integration even of complete visual noise, when first presented. Indeed, controls initially integrated complete visual noise, but quickly learned to become more optimal (adaptation of this prior). In contrast, individuals with ASD better sensed the new situation immediately and correctly down-weighted the visual cue from the outset. This result seems to be in line with the hypothesis that they have attenuated Bayesian priors and thus are more attuned to the external signal. Also, no change over time was observed for individuals with ASD (no adaptation of a prior). We present a simulation of the Bayesian prior hypothesis in SI Text and Fig. S3.
It has recently been suggested that underlying the diversity of ASD symptoms is impairment in predictive abilities (53). Accordingly, individuals with ASD live in a world in which events and sensory input seem to occur unexpectedly. This account is tightly related to the attenuated-priors hypothesis, because priors themselves represent an individual’s prediction of the world. Our results of no habituation in ASD performance during 0% coherence sessions suggest diminished Bayesian prior adaptation and inflexible predictive abilities—normally learned and updated dynamically through experience. Consequently, impoverished predictive abilities may lead individuals with ASD to rely more highly on (and be more sensitive to) sensory stimuli.
In conclusion, we found that high-functioning adolescents with ASD have a specific sensitivity to dynamic visual sensory noise, but demonstrate intact global and multisensory integration. These results question traditional theories of reduced global perception and more recent suggestions of impaired multisensory integration. Rather, they are consistent with the hypothesis that individuals with ASD have attenuated Bayesian priors—namely, a stronger reliance on incoming sensory information and less use of prior knowledge about the world.
Materials and Methods
Participants.
Fourteen adolescents with ASD and 22 age-matched controls (all male; ages 13–19) participated in this study. The study was approved by the Institutional Review Board for Baylor College of Medicine, and all participants (or their caregiver) signed informed consent. Participants with ASD were recruited through several sources, including the cohort of Simons Simplex Collection (SSC) families that had participated at the Baylor College of Medicine site (n = 6), autism clinics at Texas Children’s Hospital (n = 2) and University of Texas (n = 2), the Interactive Autism Network (n = 2), and online (n = 2). All participants with ASD had previous diagnoses that were confirmed either through prior research testing (e.g., SSC probands) or review of clinical information in school and medical records.
All probands with ASD were considered high-functioning and cooperative and fully understood the task, as demonstrated by their performance. For those who had previously been assessed with the Autism Diagnostic Observation Schedule (54), all received module 3, which is intended for use with individuals who have fluent speech. IQ was not tested at the time of the study, but previous scores were available for nine of the ASD participants (Table S1). The average IQ from these previous scores (101.8 ± 15.9) showed no apparent difference vs. the general population (which, by definition, has a mean of 100). Thus, based on the available scores, school and medical reports, and our observation that all participants fully cooperated and understood the task (and no difference was observed between controls and ASD participants for noiseless 100% coherence or vestibular conditions), there is no reason to believe that IQ affected the results.
All participants were screened at enrollment with the Social Communication Questionnaire (SCQ), current version (55) to afford both (i) a measure of current ASD symptomatology in affected probands and (ii) screen to rule out concerns for ASD in controls (i.e., scores ≥ 15 demonstrate increased concern for ASD). The SCQ scores were cleanly separable (nonoverlapping) for the ASD and control groups, creating a de facto boundary (≤10 for controls and ≥11 for participants with ASD). Participant details and SCQ scores are presented in Tables S1 and S2, along with other clinical scores, where available.
Experiment.
Details of the apparatus, stimuli, and basic task design, described in previous publications (26, 27, 56–58), are briefly summarized here, together with those specific for this study (see SI Text for details regarding the motion systems). The stimulus presented was either vestibular-only (inertial motion on a moving platform in darkness), visual-only (optic-flow motion simulation), or simultaneously combined vestibular and visual cues (inertial motion in conjunction with synchronized optic flow). Although additional proprioceptive or somatosensory cues could also be present during inertial motion, we refer to this condition as vestibular because performance depends strongly on intact vestibular labyrinths (59). The optic flow simulated self-motion of the participant through a random-dot cloud. Visual cue reliability was varied by manipulating the motion coherence of the optic-flow pattern—i.e., percentage of dots moving coherently. Vestibular reliability was fixed throughout the trials. For each block, thresholds of the visual, vestibular, and combined cues were extracted and used for analysis.
The stimulus velocity followed a 3-sigma Gaussian profile with duration of 1 s and total displacement of 13 cm. Peak velocity was 0.26 m/s, and peak acceleration was 0.81 m/s2. It primarily comprised a forward motion, with heading direction varied slightly about straight ahead. The participants’ task was to discriminate heading direction (two-alternative forced-choice, right or left of straight ahead), after presentation of a single-interval stimulus. Stimuli followed a staircase procedure (see SI Text for details). Participants were instructed to focus on a central fixation point throughout the duration of the trial. Trials were initiated and choice selection was reported via button press on a handheld unit. Participants received trial-timing-related feedback through headphones or speakers. However, no feedback about correct or incorrect choices was provided.
Five levels of coherence were tested: 100%, 90%, 75%, 50%, and 0%. Only one level of coherence was tested per block, with visual-only, vestibular-only, and combined cues interleaved. For 0% coherence, the visual-only stimulus was not tested because it represents complete visual noise. On the first visit, participants were given several practice rounds (100% coherence) before data collection to familiarize themselves with the experiment. Typically, two blocks were run per visit, often with a short (∼5 min) break in between: 100% and 90% coherence on the first visit, 75% and 0% coherence on the second visit, and 50% and 0% coherence on the third visit. Some participants returned for a fourth visit in which they were tested with two more 0% coherence blocks. Thus, 100%, 90%, 75%, and 50% coherence comprised a single block each, whereas 0% coherence comprised two to four blocks.
Data Analysis.
Data analysis was performed with custom software using Matlab (MathWorks) and the psignifit toolbox for Matlab (Version 2.5.6) (60). Psychometric plots were defined as the proportion of rightward choices as a function of heading angle and calculated by fitting the data with a cumulative Gaussian distribution function. For each block, separate psychometric functions were constructed for visual, vestibular, and combined cues. The psychophysical threshold and point of subjective equality were the SD (σ) and mean (μ), respectively, deduced from the fitted distribution function. Thresholds are nonnegative values that scale geometrically. Thus, geometric (logarithmic) means were used for all threshold calculations and plotting.
Supplementary Material
Acknowledgments
We thank Eric Raap and Alan Lin for help with data collection. This work was supported by Simons Foundation Grant SFARI 247992 (to D.E.A.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1506582112/-/DCSupplemental.
References
- 1.Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC) Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63(2):1–21. [PubMed] [Google Scholar]
- 2.American Psychiatric Association . The Diagnostic and Statistical Manual of Mental Disorders: DSM-5. American Psychiatric Publishing; Washington, DC: 2013. [Google Scholar]
- 3.Shah A, Frith U. An islet of ability in autistic children: A research note. J Child Psychol Psychiatry. 1983;24(4):613–620. doi: 10.1111/j.1469-7610.1983.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 4.O’Riordan M, Plaisted K. Enhanced discrimination in autism. Q J Exp Psychol A. 2001;54(4):961–979. doi: 10.1080/713756000. [DOI] [PubMed] [Google Scholar]
- 5.Dakin S, Frith U. Vagaries of visual perception in autism. Neuron. 2005;48(3):497–507. doi: 10.1016/j.neuron.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Foss-Feig JH, Tadin D, Schauder KB, Cascio CJ. A substantial and unexpected enhancement of motion perception in autism. J Neurosci. 2013;33(19):8243–8249. doi: 10.1523/JNEUROSCI.1608-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertone A, Mottron L, Jelenic P, Faubert J. Motion perception in autism: A “complex” issue. J Cogn Neurosci. 2003;15(2):218–225. doi: 10.1162/089892903321208150. [DOI] [PubMed] [Google Scholar]
- 8.Rinehart NJ, Bradshaw JL, Moss SA, Brereton AV, Tonge BJ. Atypical interference of local detail on global processing in high-functioning autism and Asperger’s disorder. J Child Psychol Psychiatry. 2000;41(6):769–778. [PubMed] [Google Scholar]
- 9.Blake R, Turner LM, Smoski MJ, Pozdol SL, Stone WL. Visual recognition of biological motion is impaired in children with autism. Psychol Sci. 2003;14(2):151–157. doi: 10.1111/1467-9280.01434. [DOI] [PubMed] [Google Scholar]
- 10.Behrmann M, et al. Configural processing in autism and its relationship to face processing. Neuropsychologia. 2006;44(1):110–129. doi: 10.1016/j.neuropsychologia.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Happé F, Frith U. The weak coherence account: Detail-focused cognitive style in autism spectrum disorders. J Autism Dev Disord. 2006;36(1):5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- 12.Koldewyn K, Jiang YV, Weigelt S, Kanwisher N. Global/local processing in autism: Not a disability, but a disinclination. J Autism Dev Disord. 2013;43(10):2329–2340. doi: 10.1007/s10803-013-1777-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spencer J, et al. Motion processing in autism: Evidence for a dorsal stream deficiency. Neuroreport. 2000;11(12):2765–2767. doi: 10.1097/00001756-200008210-00031. [DOI] [PubMed] [Google Scholar]
- 14.Milne E, et al. High motion coherence thresholds in children with autism. J Child Psychol Psychiatry. 2002;43(2):255–263. doi: 10.1111/1469-7610.00018. [DOI] [PubMed] [Google Scholar]
- 15.Pellicano E, Gibson L, Maybery M, Durkin K, Badcock DR. Abnormal global processing along the dorsal visual pathway in autism: A possible mechanism for weak visuospatial coherence? Neuropsychologia. 2005;43(7):1044–1053. doi: 10.1016/j.neuropsychologia.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Atkinson AP. Impaired recognition of emotions from body movements is associated with elevated motion coherence thresholds in autism spectrum disorders. Neuropsychologia. 2009;47(13):3023–3029. doi: 10.1016/j.neuropsychologia.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Robertson CE, Martin A, Baker CI, Baron-Cohen S. Atypical integration of motion signals in autism spectrum conditions. PLoS ONE. 2012;7(11):e48173. doi: 10.1371/journal.pone.0048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Viva MM, Igliozzi R, Tancredi R, Brizzolara D. Spatial and motion integration in children with autism. Vision Res. 2006;46(8-9):1242–1252. doi: 10.1016/j.visres.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 19.de Jonge MV, et al. Visual information processing in high-functioning individuals with autism spectrum disorders and their parents. Neuropsychology. 2007;21(1):65–73. doi: 10.1037/0894-4105.21.1.65. [DOI] [PubMed] [Google Scholar]
- 20.Jones CR, et al. No evidence for a fundamental visual motion processing deficit in adolescents with autism spectrum disorders. Autism Res. 2011;4(5):347–357. doi: 10.1002/aur.209. [DOI] [PubMed] [Google Scholar]
- 21.Spencer JV, O’Brien JM. Visual form-processing deficits in autism. Perception. 2006;35(8):1047–1055. doi: 10.1068/p5328. [DOI] [PubMed] [Google Scholar]
- 22.Tsermentseli S, O’Brien JM, Spencer JV. Comparison of form and motion coherence processing in autistic spectrum disorders and dyslexia. J Autism Dev Disord. 2008;38(7):1201–1210. doi: 10.1007/s10803-007-0500-3. [DOI] [PubMed] [Google Scholar]
- 23.Vandenbroucke MW, Scholte HS, van Engeland H, Lamme VA, Kemner C. Coherent versus component motion perception in autism spectrum disorder. J Autism Dev Disord. 2008;38(5):941–949. doi: 10.1007/s10803-007-0467-0. [DOI] [PubMed] [Google Scholar]
- 24.Frith U, Happé F. Autism: Beyond “theory of mind”. Cognition. 1994;50(1-3):115–132. doi: 10.1016/0010-0277(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 25.Koldewyn K, Whitney D, Rivera SM. The psychophysics of visual motion and global form processing in autism. Brain. 2010;133(Pt 2):599–610. doi: 10.1093/brain/awp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fetsch CR, Turner AH, DeAngelis GC, Angelaki DE. Dynamic reweighting of visual and vestibular cues during self-motion perception. J Neurosci. 2009;29(49):15601–15612. doi: 10.1523/JNEUROSCI.2574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaidel A, Ma WJ, Angelaki DE. Supervised calibration relies on the multisensory percept. Neuron. 2013;80(6):1544–1557. doi: 10.1016/j.neuron.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iarocci G, McDonald J. Sensory integration and the perceptual experience of persons with autism. J Autism Dev Disord. 2006;36(1):77–90. doi: 10.1007/s10803-005-0044-3. [DOI] [PubMed] [Google Scholar]
- 29.Collignon O, et al. Reduced multisensory facilitation in persons with autism. Cortex. 2013;49(6):1704–1710. doi: 10.1016/j.cortex.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Brandwein AB, et al. The development of multisensory integration in high-functioning autism: High-density electrical mapping and psychophysical measures reveal impairments in the processing of audiovisual inputs. Cereb Cortex. 2013;23(6):1329–1341. doi: 10.1093/cercor/bhs109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevenson RA, et al. Multisensory temporal integration in autism spectrum disorders. J Neurosci. 2014;34(3):691–697. doi: 10.1523/JNEUROSCI.3615-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevenson RA, et al. Evidence for diminished multisensory integration in autism spectrum disorders. J Autism Dev Disord. 2014;44(12):3161–3167. doi: 10.1007/s10803-014-2179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwakye LD, Foss-Feig JH, Cascio CJ, Stone WL, Wallace MT. Altered auditory and multisensory temporal processing in autism spectrum disorders. Front Integr Neurosci. 2011;4:129. doi: 10.3389/fnint.2010.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brandwein AB, et al. Neurophysiological indices of atypical auditory processing and multisensory integration are associated with symptom severity in autism. J Autism Dev Disord. 2015;45(1):230–244. doi: 10.1007/s10803-014-2212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gori M, Del Viva M, Sandini G, Burr DC. Young children do not integrate visual and haptic form information. Curr Biol. 2008;18(9):694–698. doi: 10.1016/j.cub.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 36.Nardini M, Jones P, Bedford R, Braddick O. Development of cue integration in human navigation. Curr Biol. 2008;18(9):689–693. doi: 10.1016/j.cub.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 37.Brandwein AB, et al. The development of audiovisual multisensory integration across childhood and early adolescence: A high-density electrical mapping study. Cereb Cortex. 2011;21(5):1042–1055. doi: 10.1093/cercor/bhq170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith EG, Bennetto L. Audiovisual speech integration and lipreading in autism. J Child Psychol Psychiatry. 2007;48(8):813–821. doi: 10.1111/j.1469-7610.2007.01766.x. [DOI] [PubMed] [Google Scholar]
- 39.Taylor N, Isaac C, Milne E. A comparison of the development of audiovisual integration in children with autism spectrum disorders and typically developing children. J Autism Dev Disord. 2010;40(11):1403–1411. doi: 10.1007/s10803-010-1000-4. [DOI] [PubMed] [Google Scholar]
- 40.Foxe JJ, et al. Severe multisensory speech integration deficits in high-functioning school-aged children with autism spectrum disorder (ASD) and their resolution during early adolescence. Cereb Cortex. 2015;25(2):298–312. doi: 10.1093/cercor/bht213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stein BE, et al. Semantic confusion regarding the development of multisensory integration: A practical solution. Eur J Neurosci. 2010;31(10):1713–1720. doi: 10.1111/j.1460-9568.2010.07206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pellicano E, Burr D. When the world becomes ‘too real’: A Bayesian explanation of autistic perception. Trends Cogn Sci. 2012;16(10):504–510. doi: 10.1016/j.tics.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 43.Ropar D, Mitchell P. Shape constancy in autism: The role of prior knowledge and perspective cues. J Child Psychol Psychiatry. 2002;43(5):647–653. doi: 10.1111/1469-7610.00053. [DOI] [PubMed] [Google Scholar]
- 44.Butler JS, Smith ST, Campos JL, Bülthoff HH. Bayesian integration of visual and vestibular signals for heading. J Vis. 2010;10(11):23. doi: 10.1167/10.11.23. [DOI] [PubMed] [Google Scholar]
- 45.Yuille AL. Bülthoff, H. H. In: Knill DC, Richards W, editors. Perception as Bayesian Inference. Cambridge Univ Press; New York: 1996. [Google Scholar]
- 46.Ernst MO, Banks MS. Humans integrate visual and haptic information in a statistically optimal fashion. Nature. 2002;415(6870):429–433. doi: 10.1038/415429a. [DOI] [PubMed] [Google Scholar]
- 47.Knill DC, Pouget A. The Bayesian brain: The role of uncertainty in neural coding and computation. Trends Neurosci. 2004;27(12):712–719. doi: 10.1016/j.tins.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Oberman LM, Ramachandran VS. Preliminary evidence for deficits in multisensory integration in autism spectrum disorders: The mirror neuron hypothesis. Soc Neurosci. 2008;3(3-4):348–355. doi: 10.1080/17470910701563681. [DOI] [PubMed] [Google Scholar]
- 49.Robertson CE, et al. Global motion perception deficits in autism are reflected as early as primary visual cortex. Brain. 2014;137(Pt 9):2588–2599. doi: 10.1093/brain/awu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- 51.Dinstein I, et al. Unreliable evoked responses in autism. Neuron. 2012;75(6):981–991. doi: 10.1016/j.neuron.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haigh SM, Heeger DJ, Dinstein I, Minshew N, Behrmann M. Cortical variability in the sensory-evoked response in autism. J Autism Dev Disord. October 19, 2014 doi: 10.1007/s10803-014-2276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinha P, et al. Autism as a disorder of prediction. Proc Natl Acad Sci USA. 20d14;111(42):15220–15225. doi: 10.1073/pnas.1416797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lord C, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 55.Rutter M, Bailey A, Lord C. The Social Communication Questionnaire: Manual. Western Psychological Services; Los Angeles: 2003. [Google Scholar]
- 56.MacNeilage PR, Banks MS, DeAngelis GC, Angelaki DE. Vestibular heading discrimination and sensitivity to linear acceleration in head and world coordinates. J Neurosci. 2010;30(27):9084–9094. doi: 10.1523/JNEUROSCI.1304-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gu Y, Fetsch CR, Adeyemo B, Deangelis GC, Angelaki DE. Decoding of MSTd population activity accounts for variations in the precision of heading perception. Neuron. 2010;66(4):596–609. doi: 10.1016/j.neuron.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zaidel A, Turner AH, Angelaki DE. Multisensory calibration is independent of cue reliability. J Neurosci. 2011;31(39):13949–13962. doi: 10.1523/JNEUROSCI.2732-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gu Y, DeAngelis GC, Angelaki DE. A functional link between area MSTd and heading perception based on vestibular signals. Nat Neurosci. 2007;10(8):1038–1047. doi: 10.1038/nn1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit. Percept Psychophys. 2001;63(8):1293–1313. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.