Significance
Modern life is enabled by the use of materials in its technologies. Over time, these technologies have used a larger and more diverse array of materials. Elemental life cycle analyses yield an understanding of these materials, and a definite concern that arises is that of possible scarcity of some of the elements as their use increases. We studied substitution potential for 62 different metals in their major uses. For a dozen different metals, the potential substitutes for their major uses are either inadequate or appear not to exist at all. Further, for not 1 of the 62 metals are exemplary substitutes available for all major uses.
Keywords: criticality, material substitution, product complexity, metal life cycle, sustainability
Abstract
It is indisputable that modern life is enabled by the use of materials in its technologies. Those technologies do many things very well, largely because each material is used for purposes to which it is exquisitely fitted. The result over time has been a steady increase in product performance. We show that this materials complexity has markedly increased in the past half-century and that elemental life cycle analyses characterize rates of recycling and loss. A further concern is that of possible scarcity of some of the elements as their use increases. Should materials availability constraints occur, the use of substitute materials comes to mind. We studied substitution potential by generating a comprehensive summary of potential substitutes for 62 different metals in all their major uses and of the performance of the substitutes in those applications. As we show herein, for a dozen different metals, the potential substitutes for their major uses are either inadequate or appear not to exist at all. Further, for not 1 of the 62 metals are exemplary substitutes available for all major uses. This situation largely decouples materials substitution from price, thereby forcing material design changes to be primarily transformative rather than incremental. As wealth and population increase worldwide in the next few decades, scientists will be increasingly challenged to maintain and improve product utility by designing new and better materials, but doing so under potential constraints in resource availability.
The degree to which the materials of modern technology enable and improve our state of life is not adequately appreciated. A century ago, or even half a century ago, less than 12 materials were in wide use: wood, brick, iron, copper, gold, silver, and a few plastics. Today, however, substantial materials diversity in products of every kind is the rule rather than the exception. [A modern computer chip, for example, employs more than 60 different elements (1).] This use of materials is not a whim of the designer, but a carefully calculated effort to achieve increasingly high performance in products simple to complex.
Rich Materials Palette of Modern Products
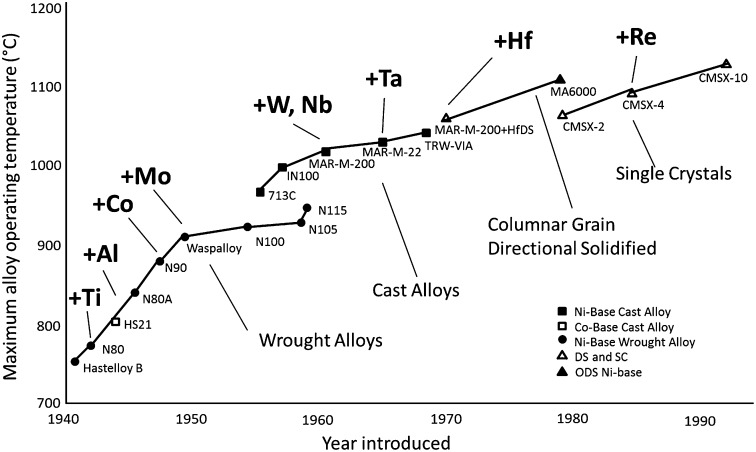
There are many examples of this evolution in material complexity (2), but a particularly vivid one is the increase in diversity of the “superalloy” metals used in aircraft turbine blades, as shown in Fig. 1. These nickel-rich alloys are corrosion resistant and stable at higher temperatures than most metals and alloys. As the figure shows, additional alloying elements have been added one by one over the years, and the alloys have assumed more complex structural forms as well. The result has been a relatively steady increase in the engine operating temperature over time, carrying with it increased engine efficiency and reduced greenhouse gas emissions. A similar story could be told for most of today’s more advanced technologies and products.
Fig. 1.
The progressive development of the metal palette in high-temperature superalloys (reprinted from ref. 2, which, in turn, was adapted from ref. 34). Reprinted with permission of ASM International. All rights reserved (www.asminternational.org). The maximum alloy operating temperature is the highest temperature at which the alloy maintains satisfactory physical properties for at least 100 h at 140-MPa pressure (2), without the use of aids like thermal coatings or cooling mechanisms.
This utilization of materials provides great benefits; faster computers, more dependable vehicles, and higher-resolution medical images, to cite a few. However, this design approach comes with a challenge: can robust supplies of all these materials be ensured? Doubts have been raised with respect to available metal supplies and escalating demands (3–5). If shortages of some materials emerge, it has been suggested that we substitute other materials for them, but is this a realistic idea? We explore these issues as we assess the degree to which modern society is materials-dependent, and to what degree that dependency carries with it benefits and challenges.
Life Cycles of Materials
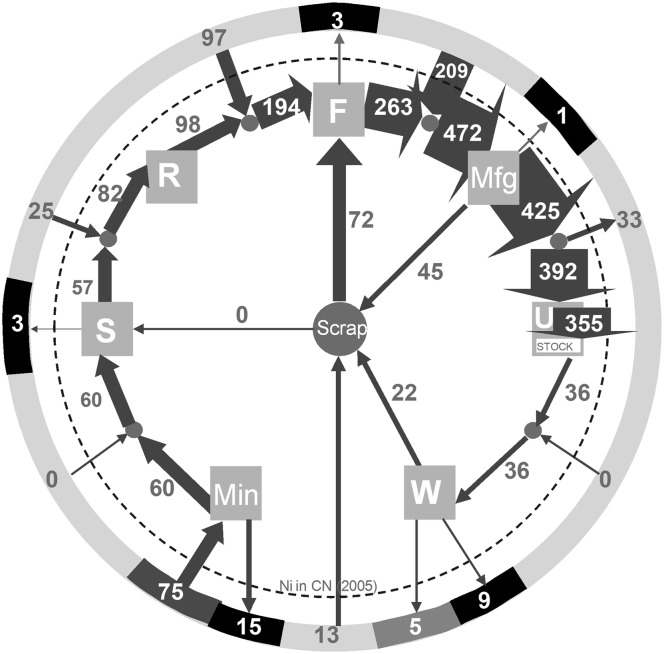
Metal supply and demand, nationally and globally, are illuminated by defining the processes that comprise metal life cycles and then by quantifying the flows from one life stage to another (6, 7). To illustrate, consider the circular display of Fig. 2, which shows the nickel cycle for China in 2005 (8). The cycle is characterized by processes that are linked through markets (9), each indicating trade with other regions at the respective life stages. The scrap market plays a central role in that it connects waste management with production and fabrication. The cycle is surrounded by entities other than trade partners lying outside of the system boundary: the lithosphere from which ore extraction takes place, repositories for nickel losses occurring in production, fabrication, and manufacturing (e.g., production wastes such as tailings and slags), and landfilling.
Fig. 2.
Material flow analysis for nickel in China, 2005 (8). The small dots between the life stages are the markets through which imports and exports flow. Min, mining; S, smelting; R, refining; F, fabrication; Mfg, manufacturing; U, use; W, waste management and recycling. The units are Gg Ni (thousand metric tons). The line width approximates the relative nickel flow from one node to another.
Production includes mining/milling, smelting, and refining: The blended or concentrated ore is smelted to a nickel matte and then refined into a variety of nickel products. In fabrication, virgin and recycled nickel is used for the production of intermediate nickel products (e.g., wire), which are then used in manufacturing for final goods (e.g., automobiles). At the use life stage, the inflow equals the outflow from manufacturing as adjusted by trade flows. The difference between in- and outflow into use is the net addition of nickel goods to in-use stock. The final stage of the cycle is waste management, which includes the collection, separation, treatment, recycling (mostly as scrap), and deposition (mostly as landfilling) of waste. The quantification of such a cycle requires assembling and harmonizing data from national and global statistics, industry associations, and technology experts, followed by harmonization of the results.
The specific example of the 2005 nickel cycle for China (Fig. 2) reveals several features. One is that import occurs at every life stage except for a modest outflow following manufacture. A second is that flow into use is more than 10 times the flow out of use, an indication of the rapid buildup of the Chinese economy. A third is that the recycling structure is reasonably well developed, although the bulk of the inputs to recycling are from manufacturing scrap and not obsolete products. The implications are that the Chinese nickel cycle was far from equilibrium and highly dependent on international trade, neither feature of which was properly understood before quantification of the cycle.
Quantified elemental cycles have particular resonance because of their policy implications, both for corporations [as in improving material efficiency (10)] and governments [as in minimizing loss during recycling (11)]. Analyses of rates of recycling show that many metals see little or no recycling (12). Where discards are not lost, however, they often become objects of international trade. There is obvious financial relevance here, but there has also been a realization that international trade permits countries to import materials for which they do not have domestic resources (13), or to lose material in export instead of using domestic recycling to provide secondary resources (14). Analysis and comparison of the trade of material among countries or regions (15) help to identify the different roles regions play in the global trade network, and provide insights into policy options to promote supply security and sustainable development of resources.
Criticality of Metals
A metal’s life cycle tells a great deal about the current situation but says nothing about possible changes in supply or demand at any point in the cycle. Those aspects can be addressed to at least some degree by studies of a metal’s criticality. This concept originated in 2006 when the US National Research Council (NRC) undertook a study to address the lack of understanding and of data on nonfuel minerals important to the US economy. The report (16) defined the criticality of minerals as a function of two variables: importance of uses and availability. The NRC committee carried out preliminary criticality analyses for several metals. Of those surveyed, a number were identified as critical: rhodium, platinum, manganese, niobium, indium, and the rare earths. Copper was not considered critical, not because of lack of importance but because supply risk was judged to be low. A number of other elements were located between these extremes. The evaluations were regarded as very preliminary but served to point out the potentially great differences in criticality among a number of the metals.
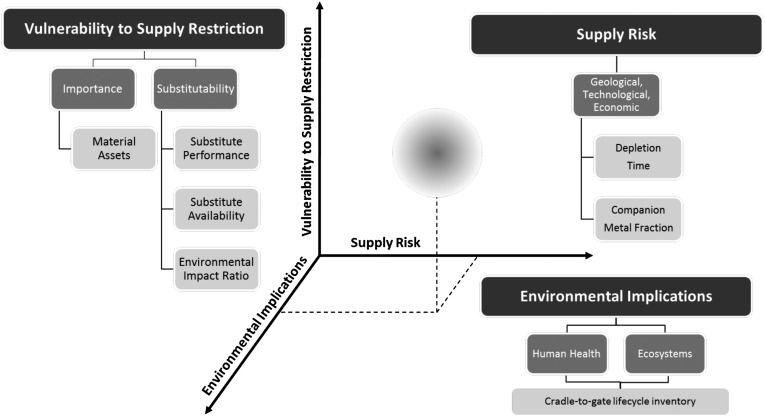
There has since been further interest in criticality work (17), enough to warrant the development of a more rigorous and quantitative methodology for assessing criticality. In perhaps the most comprehensive of these, a group at Yale University has extended the NRC approach to address three key dimensions, each of which comprises one axis of criticality space: supply risk (SR), environmental implications (EI), and vulnerability to supply restriction (VSR) (18). Fig. 3 illustrates the concept and shows that a number of indicators must be evaluated for each of the three axes to locate an element in this 3D criticality space. Additionally, many of the indicators are weighted by country-level production data for mining and/or refining processes for the corresponding metal. Using the methodology is an exercise in both data acquisition and expert judgment. For many of the geologically scarcer specialty metals, data are in short supply, and informed estimates are needed to paint a complete picture.
Fig. 3.
The Yale analytical framework for determining metal criticality at the global level, with the metrics described in detail in ref. 18.
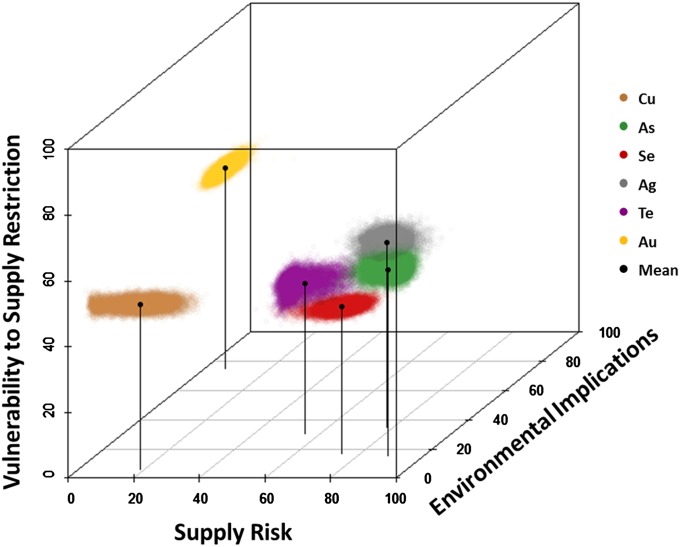
The results of the criticality study for a group of six elements commonly found in copper ores (copper, silver, gold, arsenic, selenium, and tellurium) is shown in Fig. 4, plotted in 3D criticality space. The best estimate for each metal is given by the central dot, with the “uncertainty cloud” generated by a Monte Carlo calculation in which the uncertainties of the various metrics are varied across their uncertainty ranges. An important realization is that the uncertainties are not significant enough to diminish the general level of conclusions that can be drawn from the diagram, on which several features stand out. First, the supply risk for some metals (arsenic, silver, tellurium) is much greater than for some others (copper, gold). Environmental implications also vary widely, from minimal for copper, selenium, and tellurium to significant for arsenic and high for gold. The high vulnerability to supply restriction for copper and gold reflects their very wide use in modern technology and, in the case of gold, the lower availability of its most viable substitute (i.e., silver). The degree to which metals may be substituted and how available these substitutes are play an important role in determining each metal’s criticality. Similar studies now nearing completion for other metals demonstrate that distinctions such as these are common among the metals, with scarce metals recently used in advanced products being more likely than the more commonly used metals to be at higher degrees of criticality.
Fig. 4.
Locations of the geological copper family of elements in criticality space, global level, 2008. The highest level of criticality is at 100, 100, 100 (back right top) (updated version of a display from ref. 35).
Substitution in Light of Possible Resource Constraints
The evaluations of criticality discussed above are contemporary snapshot assessments. A number of studies have attempted to look into the materials future, anticipating increasing demands on the supplies of one or more of the elements. Some of these investigations (4, 19) invoke the possibility of shortages. Others (20, 21) express more confidence in sustainable long-term supply. Because such factors as energy costs, technology transformations, and geopolitics are unpredictable, it is not possible to be certain as to the degree one should be concerned about possible resource constraints. However, a related question can be asked: what is the potential for substitution by something else if the supply of a given element becomes constrained?
It has long been a tenet of resource economics that if a material’s price should increase due to scarcity or some other factor, then the high price relative to historic norms will stimulate increased exploration and thereby increased supply, as well as encouraging the development by engineers and technologists of a suitable substitute (22, 23). It is certainly possible to cite instances in which the latter has occurred. A classic example is that of cobalt use in batteries in the 1970s. When a civil war in Zaire caused a sharp decrease in the cobalt supply, scientists at the General Motors Research Laboratories and elsewhere developed excellent magnets that used no cobalt (24). More recently, a shortage of rhenium, used in superalloys in gas turbines (1), stimulated the General Electric Research Laboratories to develop alternative alloys containing little or no rhenium (25). In each case, these were not direct metal for metal substitutions, but the development of alternative materials. However, do material transformations like these occur routinely or are these special cases? Were metal for metal substitution possibilities overlooked? Are there shortages that for one reason or another have not inspired substitute identification and use or have found substitution difficult or impossible?
To investigate substitution in more detail, we determined the major uses for all of the metals and metalloids in the periodic table (62 in number) for the year 2008. For each of these uses, we then determined the best-performing (or primary) substitute and how well that substitute performs. We then analyzed this package of information with the goal of gaining new perspectives on the likelihood of substitution when viewed comprehensively rather than for a few successful examples.
It is not straightforward, in general, to determine the major uses of metals except in the case of those used in traditional technologies and over long periods of time: iron, copper, zinc, and the like. For others, the various uses to which the metal is put may be known to technologists, but not more generally. Even for those specialists, the fractions of produced metal that enter the various uses are often not generally available. To estimate those use fractions, we surveyed those most likely to know: industry associations, metal producers, metal marketers, consultancies, and product designers. The results of our estimations should be viewed from that perspective. We do not attempt to determine substitutes for all possible uses of the metals, but for those that make up at least 80% by mass of total use (typically three to five uses). We regard the order of use as likely correct in most cases and the fractional distribution into those uses as approximate (and variable over time).
The best substitute for a metal in a particular use is not always readily apparent, as is known by every materials scientist. There are general frameworks for determining likely substitutes, such as the multiparameter plots of Ashby (26); but in general, each situation must be investigated on its own. Our approach was to review the literature of substitution to the degree possible and then to interview materials scientists and product designers. The goal we set is to determine the best potential substitute in each case, regardless of whether it is in fact a good substitute. For broad end use categories such as industrial machinery or catalysts, we select the substitute that could replace the largest mass fraction of the broad category. We then estimate the likely performance of the substitute in a particular use, again through literature searches and by consulting with product designers and materials scientists.
Through consideration of all of these factors, we define the substitutability for each element, that is, the evaluation of the degree to which material substitution in a metal’s major uses is likely to be relatively successful. Table 1 demonstrates features of this process, using tungsten as the example. Tungsten has five major use categories (or set of applications), each of which relates to specific physical and chemical properties of the element. For each use, and drawing extensively on the materials science literature and expert opinion, we identify the substitute element that will most closely approach the performance of tungsten in that application and then define on an ordinal basis how well that substitute performs. The ratings are exemplary, good, adequate, or poor, with the four designations in the analysis as 12.5, 37.5, 62.5, and 87.5 (i.e., the respective medians of the ranges 0–25, 25–50, 50–75, and 75–100). For three of tungsten’s five uses, the potential substitute is determined to provide good performance. For the use in of cemented carbides in Table 1 (which is, by far, tungsten’s major application), an adequate substitute exists. In the fifth case, substitution varies over the diverse uses, and there is no single substitute for this use.
Table 1.
Tungsten uses and potential substitutes
| Application | Fraction into application | Application details | Contributory tungsten properties | Primary substitutes | Substitute performance |
| Cemented carbides | 50%* (36) | Metal cutting and forming tools, and mining and construction equipment | High hardness and high compressive strength | Boron nitride (37) | Adequate |
| Mill products | 15%* (36) | Light filaments, electrodes, and welding applications | High melting point | Molybdenum | Good |
| Specialty steels | 8.5%† | Tool steels and dies | Resistance to mechanical and thermal shock | Molybdenum (37, 38) | Good |
| Superalloys | 8.5%† | Turbine engine components | Corrosion resistance and high temperature strength | Nickel and molybdenum alloys (37) | Good |
| Other | 18%* (36) | Includes pigments and counterweights | Low vapor pressure and high density | Diverse | Not applicable |
Value for 2005.
Value for 2005, and superalloys and steels were aggregated in global statistics (36) so we assumed an equal division of use.
Details of the overall analysis and a complete table of potential substitutes for the major uses of 62 metals are given in SI Text. We have compiled or determined the major uses and addressed materials substitution across these major uses of the metals of the periodic table. The result can be no more than an approximation of reality because of data limitations and because of the constant fluctuation in metal use in response to technological innovation, market forces, and the like. Nonetheless, it provides a foundation useful for analyses of the life cycles of metals, the dependence of technology development on competing uses for the same material, the market potential of substitute materials, and related issues.
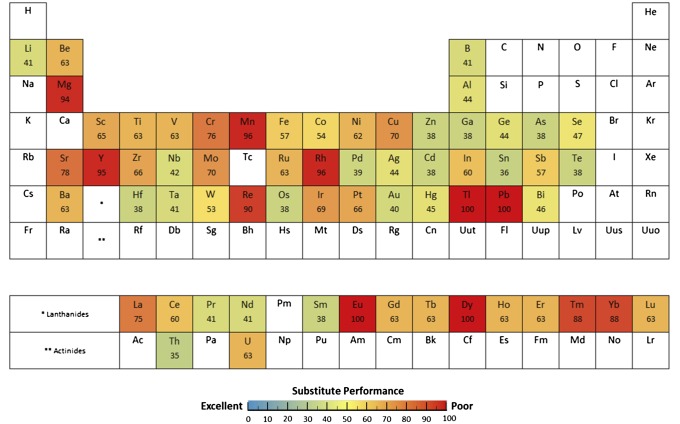
The aggregated summary of this research is shown in the substitute performance periodic table of Fig. 5. In a number of cases, the ratings seem unsurprising, but others are nonintuitive. For several widely used metals, aluminum and zinc, for example, substitute performance throughout their major uses is moderate to good. For others of the widely used metals, however (copper, chromium, manganese, and lead), this is not the case; for those elements, no good substitutes are presently available for their major uses. Other metals with low to very low substitute performance include the important superalloy metal rhenium, the platinum group metal rhodium, several of the rare earths (lanthanum, europium, dysprosium, thulium, and ytterbium), yttrium, strontium, and thallium.
Fig. 5.
The periodic table of substitute performance. The results are scaled from 0 to 100, with 0 indicating that exemplary substitutes exist for all major uses and 100 indicating that no substitute with even adequate performance exists for any of the major uses.
A further very interesting result is that absolutely none of the 62 metals have substitutes that provide exemplary performance across all its major applications. Therefore, one might say that facile substitution as a generic solution to supply risk fails in every case. There are considerable nuances and interpretations in our analysis, and one should be cautious of universal statements, but it seems very clear that substitution in the face of metal scarcity is not a general panacea. In fact, if the information in Supporting Information is combined with the relative willingness of users to pay, one could explore which application or applications of a metal “drop out” first if supply is disrupted.
It is important to recall the methodology used for this evaluation when interpreting the results. In each case, the result is heavily influenced by the use fractions of the major uses and the existence of suitable substitute performance. For the rare earths and platinum group metals, for example, the best substitute is generally a metal from the same group, thus posing the same supply risk as the target metal.
Future of Materials Use
It is paradoxical that the materials complexity of modern products brings with it a heightened level of risk. Because each constituent is carefully chosen to enable exquisite performance, precise physical and chemical properties essentially become requirements. Substitution by a different material, in those cases, is very likely to decrease product performance, raise the price, or both. The product thus becomes increasingly vulnerable to supply risks resulting from natural disasters, political unrest in crucial mining regions, energy restrictions, trade barriers, or other causes.
Complexity carries with it another attribute, which is that the ore deposits that yield the metals used in complex products are not uniformly distributed from a geographical perspective. No country or region, in fact, has substantial deposits of everything; platinum comes largely from South Africa and Russia, copper from Chile and the United States, strontium from China and Spain, and so on. The consequence is that modern technology is dependent on resources from every continent other than Antarctica, a situation that increases the potential for geopolitical machinations as far as resources are concerned.
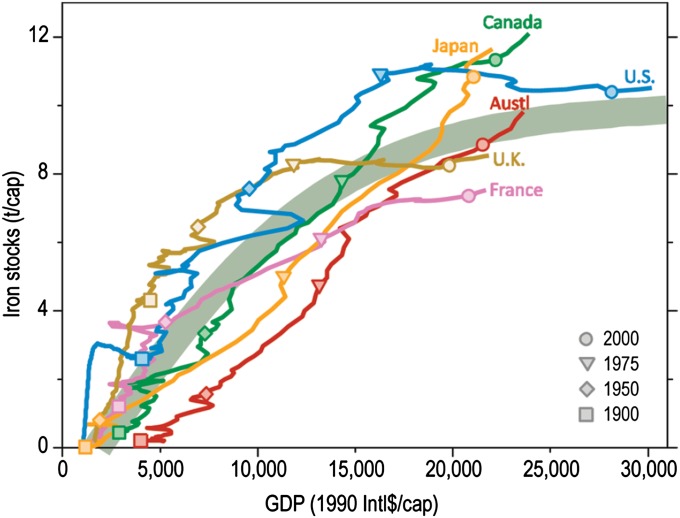
Over the longer term, some comfort might be drawn from evidence that mature societies may reach saturation points for the amount of metals in use on a per capita basis. A convincing case has been made for iron saturation for several countries (Fig. 6) (27), and a tentative confirmation is seen for aluminum in the United States (28). These data seem to be saying that, at a certain point, an individual’s share of iron and aluminum in buildings, bridges, automobiles, and other products is as much as is desired, and further injections of the metals need only replace metal that is discarded in obsolete products. As more metals data are acquired, we may expect to see this situation extended to other major metals, thus opening the door over the next few decades to a gradual shift from primary to secondary resources as the principal source of supply. This behavior seems less likely for the scarcer metals, which have been used only within the last two or three decades, whose use is increasing, and whose end-of-life recycling is generally low (29). For those metals, saturation of per capita demand may be a long time coming. Additionally, saturation at the per capita level should not be overemphasized, because absolute resource demands will continue to grow: emerging economies with their large and growing populations will more than compensate for any saturation seen in mature and industrialized countries during the coming years.
Fig. 6.
Per capita iron stocks in use vs. per capita gross domestic product (GDP) purchasing power parity (1990 international dollars). Iron stock data are based on medium lifetime assumptions, except for Japan, where lower lifetime estimates were applied. The thick gray-green line is a fitted logistic growth curve used to estimate the contemporary iron stocks in other countries. Reprinted with permission from ref. 27. Copyright 2011 American Chemical Society.
What does this enhanced understanding of metal demand and metal use say to the “if we run short, the market will produce a substitute” argument? The first thing to say is that for some materials and for some final products, we know of no suitable substitute. In other cases, product performance would suffer markedly under substitution. These arguments can, of course, be trumped by new and transformative technologies, many of which are under active investigation: advanced composite materials (30), bulk metallic glasses (31), and structural biological materials (32), to name a few. Transformations are unlikely to happen rapidly, however: a recent study by the European Parliament (33) states “The majority of substitutions are currently in the research and development stage, and market-ready solutions are rarely available.” Thus, the outstanding efforts of materials scientists over the last few decades appear to have effectively decoupled substitution from price in many cases, because often no suitable substitute can be found no matter what price is offered without performance and function being seriously compromised.
It thus appears that society will need to pay more attention to the acquisition and maintenance of nonrenewable resources than has been the case in the past. Growing populations, growing affluence, and the materials diversity of modern technologies are straining the resource capacities on which we draw. The situation need not inspire panic, but should instead stimulate more diligent and more comprehensive approaches to the balance between supply and demand across the entire periodic table.
Supplementary Material
Acknowledgments
This research is based on extensive metal statistics generated over the last 12 y. Wei-Qiang Chen, Zhouwei Diao, Xiaoyue Du, Goksin Kavlak, Philip Nuss, and Stefania Panousi provided research assistance. We thank the US Geological Survey, Roskill Information Services, SMR Steel & Metals Market Research, and many others for providing data. Sponsors included the US National Science Foundation, the Nickel Institute, the International Stainless Steel Forum, the International Chromium Development Association, the International Molybdenum Association, General Electric, Grundfos, Renault Group, Shell Global Development, and the Volkswagen Group.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312752110/-/DCSupplemental.
References
- 1.National Research Council . Minerals, Critical Minerals, and the U.S. Economy. Chap 2 National Academies Press; Washington: 2008. [Google Scholar]
- 2.Greenfield A, Graedel TE. The omnivorous diet of modern technology. Resour Conserv Recycling. 2013;74:1–7. [Google Scholar]
- 3.Harmsen J, Roes AL, Patel MK. The impact of copper scarcity on the efficiency of 2050 global renewable energy scenarios. Energy. 2013;50:62–73. [Google Scholar]
- 4.Kleijn R, van der Voet E. Resource constraints in a hydrogen economy based on renewable energy sources: An exploration. Renew Sustain Energy Rev. 2010;14(9):2784–2795. [Google Scholar]
- 5.Prior T, Giurco D, Mudd G, Mason L, Behrisch J. Resource depletion, peak minerals and the implications for sustainable resource management. Glob Environ Change. 2012;22(3):577–587. [Google Scholar]
- 6.Ayres RU. In: Industrial Metabolism: Theory and Policy. Allenby BR, Richards DJ, editors. National Academy Press; Washington: 1994. pp. 23–37. [Google Scholar]
- 7.Socolow R, Thomas V. The industrial ecology of lead and electric vehicles. J Ind Ecol. 1997;1(1):13–36. [Google Scholar]
- 8.Reck BK, Rotter VS. Comparing growth rates of nickel and stainless steel use in the early 2000s. J Ind Ecol. 2012;16(4):518–528. [Google Scholar]
- 9.Müller DB, Wang T, Duval B, Graedel TE. Exploring the engine of anthropogenic iron cycles. Proc Natl Acad Sci USA. 2006;103(44):16111–16116. doi: 10.1073/pnas.0603375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cullen JM, Allwood JM, Bambach MD. Mapping the global flow of steel: From steelmaking to end-use goods. Environ Sci Technol. 2012;46(24):13048–13055. doi: 10.1021/es302433p. [DOI] [PubMed] [Google Scholar]
- 11.Cullen JM, Allwood JM. Mapping the global flow of aluminum: From liquid aluminum to end-use goods. Environ Sci Technol. 2013;47(7):3057–3064. doi: 10.1021/es304256s. [DOI] [PubMed] [Google Scholar]
- 12. United Nations Environment Programme (2011) The Recycling Rates of Metals: A Status Report. A Report of the Working Group on the Global Metal Flows to the International Resource Panel. Graedel TE, Allwood J, Birat J-P, Reck BK, Sibley SF, Sonnemann G, Buchert M, Hagelüken C. (UNEP, Paris), pp 18–21.
- 13.Johnson J, Graedel TE. The “hidden” trade of metals in the United States. J Ind Ecol. 2008;12(5-6):739–753. [Google Scholar]
- 14.Fuse M, Yamasue E, Reck BK, Graedel TE. Regional development or resource preservation? A perspective from Japanese appliance exports. Ecol Econ. 2011;70(4):788–797. [Google Scholar]
- 15.Dittrich M, Bringezu S. The physical dimension of international trade Part I: Direct global flows between 1962 and 2005. Ecol Econ. 2010;69(9):1838–1847. [Google Scholar]
- 16.National Research Council . Minerals, Critical Minerals, and the U.S. Economy. Chap 1 National Academies Press; Washington: 2008. [Google Scholar]
- 17.Erdmann L, Graedel TE. Criticality of non-fuel minerals: a review of major approaches and analyses. Environ Sci Technol. 2011;45(18):7620–7630. doi: 10.1021/es200563g. [DOI] [PubMed] [Google Scholar]
- 18.Graedel TE, et al. Methodology of metal criticality determination. Environ Sci Technol. 2012;46(2):1063–1070. doi: 10.1021/es203534z. [DOI] [PubMed] [Google Scholar]
- 19.Mudd G. The environmental sustainability of mining in Australia: Key megatrends and looming constraints. Resour Policy. 2010;35(2):98–115. [Google Scholar]
- 20.Bloodworth A, Gunn G. The future of the global minerals and metals sector: Issues and challenges out to 2050. BRGM's J Sustainable Earth. 2012;15:90–97. [Google Scholar]
- 21.Sinding-Larsen R, Wellmer F-W. Non-renewable resource issues: An introduction. In: Sinding-Larsen R, Wellmer FW, editors. Non-Renewable Resource Issues: Geoscientific and Societal Challenges. Springer Science+Business Media; Berlin: 2012. pp. 35–43. [Google Scholar]
- 22.Solow R. The economics of resources or the resources of economics. Am Econ Rev. 1974;64(2):1–14. [Google Scholar]
- 23.Stiglitz J. Reply: Georgescu-Roegen versus Solow/Stiglitz. Ecol Econ. 1997;22(3):269–270. [Google Scholar]
- 24.US Congressional Budget Office . Cobalt: Policy Options for a Strategic Mineral. US Congressional Budget Office; Washington: 1982. p. 35. [Google Scholar]
- 25.Duclos S, Otto J, Konitzer D. Design in an era of constrained resources. Mech Eng. 2010;132(9):36–40. [Google Scholar]
- 26.Ashby M. Materials Selection in Mechanical Design. Elsevier; Amsterdam: 2004. [Google Scholar]
- 27.Müller DB, Wang T, Duval B. Patterns of iron use in societal evolution. Environ Sci Technol. 2011;45(1):182–188. doi: 10.1021/es102273t. [DOI] [PubMed] [Google Scholar]
- 28.Chen W-Q, Graedel TE. Dynamic analysis of aluminum stocks and flows in the United States: 1900-2009. Ecol Econ. 2012;81:92–102. [Google Scholar]
- 29.Graedel TE, et al. What do we know about metal recycling rates? J Ind Ecol. 2011;15(3):355–366. [Google Scholar]
- 30.Gupta N. Modeling and simulation in composite materials: Integration from nanostructure to component-level design. J Miner Metals Mater Soc. 2013;65(2):136–139. [Google Scholar]
- 31.Schroers J. Bulk metallic glasses. Phys Today. 2013;66(2):32–37. [Google Scholar]
- 32.Meyers MA, McKittrick J, Chen P-Y. Structural biological materials: Critical mechanics-materials connections. Science. 2013;339(6121):773–779. doi: 10.1126/science.1220854. [DOI] [PubMed] [Google Scholar]
- 33.European Parliament . Substitutionability of critical raw materials. European Commission, Department of Enterprise and Industry; Brussels: 2012. [Google Scholar]
- 34.Donachie M, Donachie S. Superalloys: A Technical Guide. 2nd Ed ASM International; Materials Park, OH: 2002. [Google Scholar]
- 35.Nassar NT, et al. Criticality of the geological copper family. Environ Sci Technol. 2012;46(2):1071–1078. doi: 10.1021/es203535w. [DOI] [PubMed] [Google Scholar]
- 36.Roskill Information Services . The Economics of Tungsten. Roskill Information Services; London: 2007. [Google Scholar]
- 37.US Department of Defense . Strategic and Critical Materials 2013 Report on Stockpile Requirements. Office of the Under Secretary of Defense for Acquisition, Technology, and Logistics, US Department of Defense; Washington: 2013. [Google Scholar]
- 38.International Molybdenum Association . International Molybdenum Association; London: 2013. Tool & High Speed Steel. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.