Just a few years ago, molecular biologists hoping to alter the genome of their favorite organisms faced an arduous task and likely weeks of genetic tinkering. Today, those scientists can quickly destroy or edit a gene with a new technology called CRISPR (clustered regularly interspaced short palindromic repeat)/Cas9.
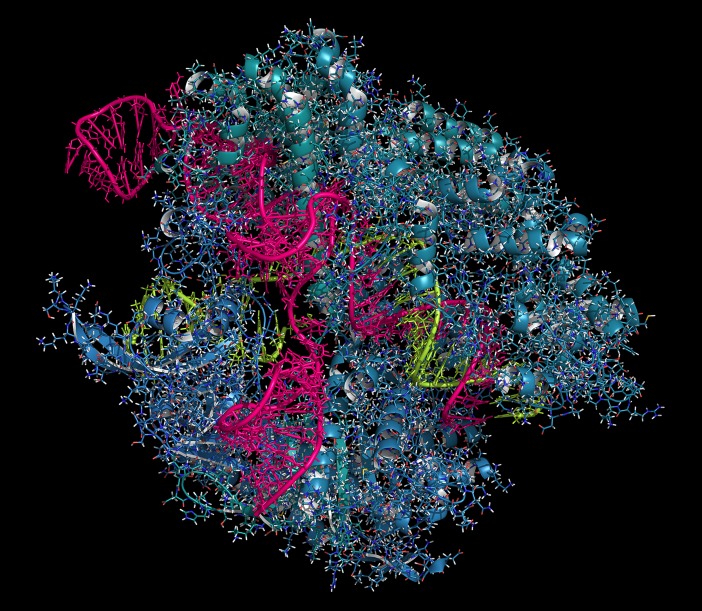
The use of the CRISPR-CAS9 gene-editing complex, illustrated here in Streptococcus pyogenes, has already had a major impact on multiple fields. Cas9 is shown in teal/blue, RNA in magenta and lime green. Image courtesy of Shutterstock/molekuul.be.
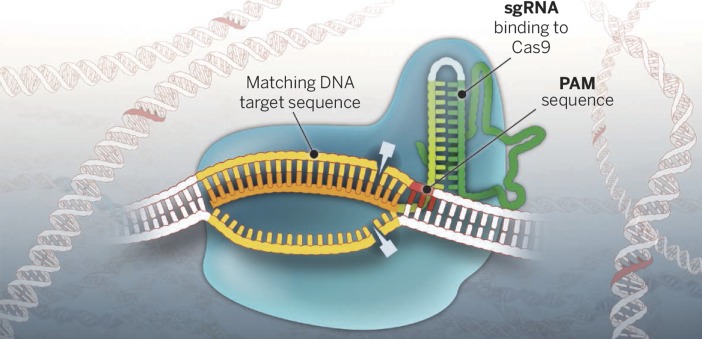
The Cas9 enzyme (blue), targeted by a guide RNA (orange), slices DNA to initiate genomic editing. Reprinted from ref. 20 with permission from AAAS.
“It really opens up the genome of virtually every organism that’s been sequenced to be edited and engineered,” says Jill Wildonger of the University of Wisconsin–Madison. CRISPR users have essentially co-opted the immune system of bacteria, using the Cas9 enzyme to chop up the genomes of invading viruses.
The trick is simple: the Cas9 enzyme cuts DNA at a specific sequence, determined by an accompanying bit of RNA called a guide RNA. Then, the cell’s own DNA repair machinery typically takes over in one of two different modes. In the first mode, it simply glues the two pieces back together, but imperfectly, so the leftover scar interrupts and disables the targeted gene. Or, in a second kind of repair, the cell can copy a nearby piece of DNA to fill in the missing sequence. By providing their own DNA template, scientists can induce the cell to fill in any desired sequence, from a small mutation to a whole new gene.
The CRISPR/Cas9 genetic engineering system has become nearly ubiquitous in biology laboratories over the past few years. However, the original CRISPR researchers had no such application in mind, according to Jennifer Doudna of the University of California, Berkeley, one of the developers of the technology. Some of the first researchers to investigate CRISPR were food scientists at Danisco USA Inc.; they were worried about viruses infecting the Streptococcus strains used to make yogurt and cheese (1). These researchers observed that bacterial chromosomes contain oddly repetitive sequences called “clustered regularly interspaced short palindromic repeats,” or CRISPR. Between those repeats are sequences from viruses that infect bacteria. The microbes use the viral sequences as a mnemonic to remember past invaders. If the same virus should try to get a foothold again, the microbes are ready with an immune response that includes a copy of the remembered sequences, called a crRNA, and a second RNA, dubbed tracrRNA, encoded near the CRISPR repeats. Together, these RNAs recruit the Cas9 protein to viral DNA, and the enzyme cuts it up.
CRISPRs were interesting mainly to microbiologists, Doudna points out, until 2012 when her group and that of collaborator Emmanuelle Charpentier, at Umeå University in Sweden, figured out they could combine crRNA and tracrRNA into a single, artificial guide RNA, which they could then use to aim the DNA-slicing enzyme at a sequence of their choosing (2). “We realized that we could use this for cutting DNA…in order to trigger repair,” Doudna recalls. The implications were thrilling. “I had chills going down my back,” she says. Doudna’s initial experiments were with molecules in test tubes, not living cells. But less than a year later, two other laboratories demonstrated the use of CRISPR/Cas9 to edit the genomes of cultured mouse and human cells in laboratory dishes (3, 4).
Genetic engineers had already designed similar systems to snip DNA at any desired location, but they required scientists to assemble a protein to home in on every new target sequence, a tedious process. “Then along came CRISPR and, boom! You can just order an oligo[nucleotide] and make any change in the genome you wish,” says Dan Voytas, director of the Center for Genome Engineering at the University of Minnesota in Minneapolis, who developed one of the protein-based systems.
Scientists can alter a gene—or several at once—and look for effects on their organism of choice. Moreover, researchers have altered the Cas9 enzyme to do other jobs. In one instance, instead of cleaving the DNA, it merely tunes the amount of a gene that is made up or down (5). In another application, a fluorescent, noncutting version of Cas9 sticks to guide RNA-targeted sequences and lights up where specific bits of a cell’s genome are hanging out (6).
Companies hoping to exploit CRISPR/Cas9 to repair the genes of people with diseases have already sprung up. The tool “should be regarded as being more precise” than other gene therapies, says George Church of Harvard University in Cambridge, Massachusetts. For example, researchers might attempt to cure HIV by cutting out viral genes that have taken up residence in the genome of infected people. Scientists have already done so with T cells in the laboratory (7, 8). Researchers have also repaired a mutant gene needed to break down the amino acid tyrosine in the liver cells of mice, protecting the animals from liver damage (9).
The field of agriculture also stands poised to reap the benefits of CRISPR/Cas9, says Voytas. He and others think that CRISPR/Cas9-based editing might raise fewer hackles with the public than genetically modified organisms made by transferring entire genes from one organism to another.
The simplicity of CRISPR/Cas9 has scientists thinking beyond the corner market or therapeutics. Church and Kevin Esvelt, of Harvard’s Wyss Institute for Biologically Inspired Engineering, have suggested that one could use CRISPR/Cas9 to modify an entire population of wild organisms (10). A prime target: mosquitoes that carry pathogens such as the malaria parasite. Researchers could, theoretically, spread a gene that makes mosquitos malaria-resistant, or get rid of mosquitos altogether with a gene that makes them infertile. “Malaria would have to either find another host or go extinct,” says Esvelt. The tactic requires a genetic element called a “gene drive,” which selfishly promotes its own inheritance, for example by copying itself onto other chromosomes (11). Although the gene drive idea has been around for decades, and scientists have shown the basic principle could work in insects, CRISPR will likely perform much better than previous gene-editing methods, Esvelt and Church predict (12–16). Keeping in mind unintended environmental effects, the scientists have proposed that researchers making gene drives should, at the same time, design “reversal” drives that could undo the effects if necessary.
Before human CRISPR/Cas9 use, scientists still need to make the templated DNA repair process, the only way to repair defective genes, more efficient and reliable, points out Blake Wiedenheft of Montana State University in Bozeman. If the repair enzymes don’t follow a template, they’ll reattach the DNA without the desired change. In some cell types, such as neurons, no one has yet succeeded with templated editing, notes Wildonger. Another concern, Wiedenheft adds, is the potential for the Cas9 enzyme to mistakenly cut the wrong bit of DNA. Any time DNA is broken, there is potential for chromosomes to rearrange and cause cancer, points out Esvelt.
Even so, CRISPR’s ease of use could very well intensify ongoing debates about human genetic modification. Esvelt thinks the tiny cancer risk, multiplied by the large number of cells required to edit, say, eye color, will probably prevent purely recreational use of CRISPR by adults. But would-be parents of designer babies could potentially select properly edited embryos. For example, Wiedenheft asks, should parents be allowed to delete any propensity for obesity in their offspring with the flick of a Cas9-based switch? CRISPR has brought such hypothetical scenarios much closer to reality, spurring Church, Doudna, and other scientists to recently recommend a moratorium on editing human sperm, eggs, or embryos until the consequences are better understood (17, 18). There are already reports of Chinese scientists using the technique on human embryos; however, in doing so, the researchers found many unexpected mutations (19).
Yet CRISPR also has the potential to improve lives, whether by altering foodstuffs to improve nutrition or yield, or by eliminating genetic or bug-borne disease. These ideas are not new, but the technology opens promising possibilities. Says Wiedenheft, “It’s no longer sci-fi.”
References
- 1.Barrangou R, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315(5819):1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- 2.Jinek M, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cong L, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mali P, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339(6121):823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert LA, et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154(2):442–451. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen B, et al. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell. 2013;155(7):1479–1491. doi: 10.1016/j.cell.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebina H, Misawa N, Kanemura Y, Koyanagi Y. Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus. Sci Rep. 2013;3:2510. doi: 10.1038/srep02510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu W, et al. RNA-directed gene editing specifically eradicates latent and prevents new HIV-1 infection. Proc Natl Acad Sci USA. 2014;111(31):11461–11466. doi: 10.1073/pnas.1405186111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin H, et al. Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat Biotechnol. 2014;32(6):551–553. doi: 10.1038/nbt.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esvelt KM, Smidler AL, Catteruccia F, Church GM. Concerning RNA-guided gene drives for the alteration of wild populations. eLife. 2014;3:e03401. doi: 10.7554/eLife.03401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venton D. Core concept: Synthetic biology-change, accelerated. Proc Natl Acad Sci USA. 2014;111(48):16978–16979. doi: 10.1073/pnas.1419688111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig GB, Jr, Hickey WA, Vandehey RC. An inherited male-producing factor in Aedes aegypti. Science. 1960;132(3443):1887–1889. doi: 10.1126/science.132.3443.1887. [DOI] [PubMed] [Google Scholar]
- 13.Wood RJ, Cook LM, Hamilton A, Whitelaw A. Transporting the marker gene re (red eye) into a laboratory cage population of Aedes aegypti (Diptera: Culicidae), using meiotic drive at the MD locus. J Med Entomol. 1977;14(4):461–464. doi: 10.1093/jmedent/14.4.461. [DOI] [PubMed] [Google Scholar]
- 14.Burt A. Site-specific selfish genes as tools for the control and genetic engineering of natural populations. Proc Biol Sci. 2003;270(1518):921–928. doi: 10.1098/rspb.2002.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan YS, Naujoks DA, Huen DS, Russell S. Insect population control by homing endonuclease-based gene drive: An evaluation in Drosophila melanogaster. Genetics. 2011;188(1):33–44. doi: 10.1534/genetics.111.127506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simoni A, et al. Development of synthetic selfish elements based on modular nucleases in Drosophila melanogaster. Nucleic Acids Res. 2014;42(11):7461–7472. doi: 10.1093/nar/gku387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baltimore BD, et al. A prudent path forward for genomic engineering and germline gene modification. Science. 2015;348(6230):36–38. doi: 10.1126/science.aab1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanphier E, Urnov F, Haecker SE, Werner M, Smolenski J. Don’t edit the human germ line. Nature. 2015;519(7544):410–411. doi: 10.1038/519410a. [DOI] [PubMed] [Google Scholar]
- 19. doi: 10.1007/s13238-015-0153-5. Liang P (2015) CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein & Cell. DOI: 10.1007/s13238-015-0153-5 ( http://link.springer.com/article/10.1007%2Fs13238-015-0153-5) [DOI] [PMC free article] [PubMed]
- 20.Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346(6213):1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]