Abstract
Background:
Still there is no consensus on the best treatment for abdominal pain-related functional Gastrointestinal Disorders (FGIDs).
Objectives:
The purpose of this study was to compare the effects of a synbiotic Lactol (Bacillus coagulans + fructooligosaccharide (FOS)), peppermint oil (Colpermin) and placebo (folic acid) on abdominal pain-related FGIDs except for abdominal migraine.
Patients and Methods:
This placebo-controlled study was conducted on 120 children aged 4 - 13 years to compare the efficacy of pH-dependent peppermint oil (Colpermin) versus synbiotic Lactol (Bacillus coagulans + fructooligosaccharids (FOS)) in decreasing duration, severity and frequency of functional abdominal pain. The patients were randomly allocated into three equal groups (n = 40 in each group) and each group received Colpermin or Lactol or placebo.
Results:
Eighty-eight out of 120 enrolled patients completed a one-month protocol and analyses were performed on 88 patients’ data. Analyses showed that improvement in pain duration, frequency and severity in the Colpermin group was better than the placebo group (P = 0.0001, P = 0.0001 and P = 0.001, respectively). Moreover, pain duration and frequency were decreased in the Lactol group more than the placebo (P = 0.012 and P = 0.0001, respectively), but changes in pain severity were not significant (P = 0.373). Colpermin was superior to Lactol in decreasing pain duration and severity (P = 0.040 and P = 0.013, respectively). No known side effects or intolerance were seen with Colpermin or Lactol.
Conclusions:
The pH-dependent peppermint oil capsule and Lactol tablet (Bacillus coagulans+ FOS) as synbiotics seem to be superior to placebo in decreasing the severity, duration and frequency of pain in abdominal pain-related functional GI disorders.
Keywords: Mentha Piperita, Synbiotics, Abdominal Pain
1. Background
Functional Gastrointestinal Disorders (FGIDs) are a group of chronic or recurrent gastrointestinal symptoms not explained by structural, inflammatory, metabolic or neoplastic pathophysiologies (1). The Rome III committee aimed to update and revise the pediatric criteria in FGIDs. The Rome III process had two pediatric subcommittees based on the age range: neonate/toddler (0 - 4 years) and child/adolescent (4 - 18 years) (2). The child/adolescent committee classified FGIDs based on the main complaints and described three classes of FGIDs: vomiting and aerophagia, abdominal pain-related FGIDs and constipation and incontinence (1). Abdominal pain-related FGIDs consist of functional dyspepsia, irritable bowel syndrome, abdominal migraine, functional abdominal pain and functional abdominal pain syndrome. In functional GI disorders is no evidence of inflammatory, anatomic, metabolic or neoplastic process (1, 3, 4). Some changes in brain-gut axis seem to be an important mechanism for FGIDs (5). Except for abdominal migraine with episodic pattern, abdominal pain or discomfort must be reported for at least once per week in a two-steady month period and accompanying symptoms for more than 25% of the time (2, 6). Various interventions including special diets, conventional and alternative medical therapies and psychological therapy have been tried in patients with abdominal pain-related FGIDs. Trials with H2 receptor antagonists, proton pump inhibitors, sucralfate, prokinetic drugs and metoclopramide, tricyclic antidepressants, antispasmodic and anticholinergic drugs such as mebeverine and dicyclomine, probiotics such as Lactobacillus reuteri and Lactobacillus rhamnosus GG, botanicals including peppermint oil, diets with added fiber or restricted lactose, cognitive-behavioral therapy have been showed different results (7-13). However, still there is no consensus on the best treatment for abdominal pain-related FGIDs. Colpermin (pH-dependent peppermint oil) capsule comprises menthol that causes inhibition of smooth muscle contractions by blocking calcium channels. Moreover, it has a mild topical effect on ileal and colonic mucosa (14-16). As commensal bacteria of the GI tract are believed to play an important role in homeostasis, alterations to these populations have been implicated in dysmotility, visceral hypersensitivity, abnormal colonic fermentation and immunologic activation. Probiotics and synbiotics (Lactol in this study) may improve functional GI symptoms by restoring the microbial balance in barrier or by altering the intestinal inflammatory response (17-21).
2. Objectives
This study aimed to compare the effects of a synbiotic Lactol (Bacillus coagulans + fructooligosaccharide (FOS)), peppermint oil (Colpermin) and placebo (folic acid) on abdominal pain-related FGIDs except for abdominal migraine.
3. Patients and Methods
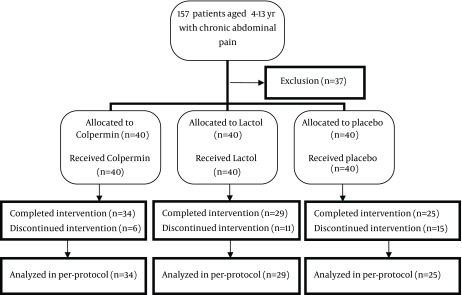
This randomized placebo-controlled trial was conducted on 120 patients referred to clinic of pediatric gastroenterology in Valiasr Hospital of Imam Khomeini Hospital Complex, an educational-governmental center, Tehran, Iran, from September 2012 to August 2014. The patients were randomly allocated into three equal groups for one-month treatment (n =40) (14, 18); group A: Colpermin (pH-dependent peppermint oil) (Tillotts company) capsule 187 mg Three times a day thirty minutes before meals (for patients over 45 kg weight, two caps per dose), group B: Lactol tablet (150 million spores of Bacillus coagulans + Fructooligosaccharide) (BIoPlus company) TDS after meals and group C: folic acid Table 1 mg (Jalinous company) daily thirty minutes before breakfast or lunch (as a placebo). From 157 patients with chronic abdominal pain (pain for more than 2 months with recurrence at least once a week) 120 patients were enrolled in this study based on the inclusion criteria including lack of red flags such as Right Lower Quadrant or Right Upper Quadrant pain, weight loss or growth impairment, dysphagia, vomiting, anemia, diarrhea (specially nocturnal), fever, arthritis, familial history of Inflammatory Bowel Disease (IBD) or any abnormal finding in physical examination or primary lab tests. Patients with mentioned red flags and probable diagnosis of abdominal migraine were excluded from the Figure 1. This trial has been evaluated in ethics committee of Tehran University of Medical Sciences (91-03-30-18354-74162) also registered on Iranian registry of Clinical Trials (IRCT 2012 1107 11392 N1). We recruited 120 patients aged between 4 - 13 years with abdominal pain-related FGIDs based on Rome III criteria in this study. Patients complained of abdominal pain at least weekly for past two months (Functional Abdominal Pain; FAP) and some of them reported dyspeptic symptoms such as early satiety, belching, fullness after meal, etc. (functional dyspepsia) or Irritable Bowel Syndrome (IBS) symptoms including alteration in bowel habit, constipation, pain relief after defecation, etc. (IBS) or dispersed symptoms such as headache, pain in extremities, difficulty in sleep, etc. Abdominal Pain Syndrome (APS). Abdominal migraine has been excluded based on its possible different pathophysiology and treatment. All of the cases were investigated with complete blood count and differentiation- Erythrocytes Sedimentation Rate (ESR)-urine analysis and stool exam (for WBC, RBC, occult blood, parasites) before enrollment. For patients with abdominal pain and growth deficiency, the celiac panel was checked too. These tests were checked in Valiasr Hospital laboratory to exclude common organic causes of abdominal pain. Patients with negative results were enrolled in the study (120 patients) (Figure 1). Eighty-eight patients completed the one-month trial and periodic visits (34 in Colpermin, 29 in Lactol and 25 in the placebo group). Thirty-two patients were excluded during trial because they didn’t complete one-month drug consumption (due to journey or lack of 2 weeks visit). We analyzed 88 patients’ data. All parents gave the written informed consent. For each patient a questionnaire was completed before and after intervention by a professional nurse who was unaware of the protocol. A two-week drug quota was prescribed and the remaining on next visit after two weeks. The outcome measure was changes in severity, duration and frequency of pain after the one-month intervention in each group and between groups. Pain severity assessment was done based on patients’ or their parents’ reports with numbers from zero to ten (numerical rating scale). Duration of pain as minutes per day and frequency as pain episodes in week was assessed. For each patient, before and after treatment changes in severity, duration and frequency of pain were recorded. Rectal burning, esophageal pain or heart burn and allergic reactions are side effects of Colpermin that we monitored them during the trial process. The ability of probiotic strains to survive in gastric conditions and strongly adhere to the intestinal epithelium may entail a risk of bacterial translocation, bacteremia and sepsis that we explained them for parents and monitored them. Data were analyzed using SPSS version 18 United States of America. Descriptive statistics were evaluated as frequencies, percentiles and mean ± SD (standard deviation). Consequently to normal distribution of variables, analytical statistics and comparison between groups were evaluated using ANOVA, a repeated-measures analysis, chi-square test and multivariate general linear regression. In each group, before and after intervention, changes were compared using a paired t-test. P < 0.05 was considered as statistically significant.
Table 1. Variables Between the Groupsa,b.
| Variable | A (Colpermin) | B (Lactol) | C (Folic acid) | P Value |
|---|---|---|---|---|
| Mean age c | 7.06 ± 2.38 | 7.44 ± 2.44 | 7.42 ± 2.49 | 0.783 |
| Gender d | 0.190 | |||
| Male | 19 | 13 | 8 | |
| Female | 15 | 16 | 17 | |
| Duration of pain, minutes per day c | 67.05 ± 36.97 | 56.37 ± 25.56 | 53.40 ± 16.81 | 0.154 |
| Frequency of pain, per week c | 4.24 ± 1.57 | 4.83 ± 1.64 | 4.44 ± 1.87 | 0.381 |
| Severity of pain, numerical rating scale c | 5.23 ± 1.34 | 5.75 ± 1.21 | 5.20 ± 1.15 | 0.172 |
| Pain localization d | 0.674 | |||
| Periumblical | 20 | 16 | 12 | |
| LLQ-Hypogastric | 2 | 1 | 2 | |
| Epigastric | 1 | 1 | 1 | |
| Diverse pain | 9 | 11 | 10 | |
| Clinical subgroups d | 0.608 | |||
| FAP | 19 | 15 | 11 | |
| Dyspepsia | 1 | 2 | 2 | |
| IBS | 9 | 10 | 11 | |
| APS | 5 | 2 | 1 |
aAbbreviations: APS, Abdominal Pain Syndrome; FAP, Functional Abdominal Pain; IBS, Irritable Bowel Syndrome.
bData are presented as Mean ± SD.
cANOVA analyse.
dChi-square test.
Figure 1. Consort Flow Chart.
4. Results
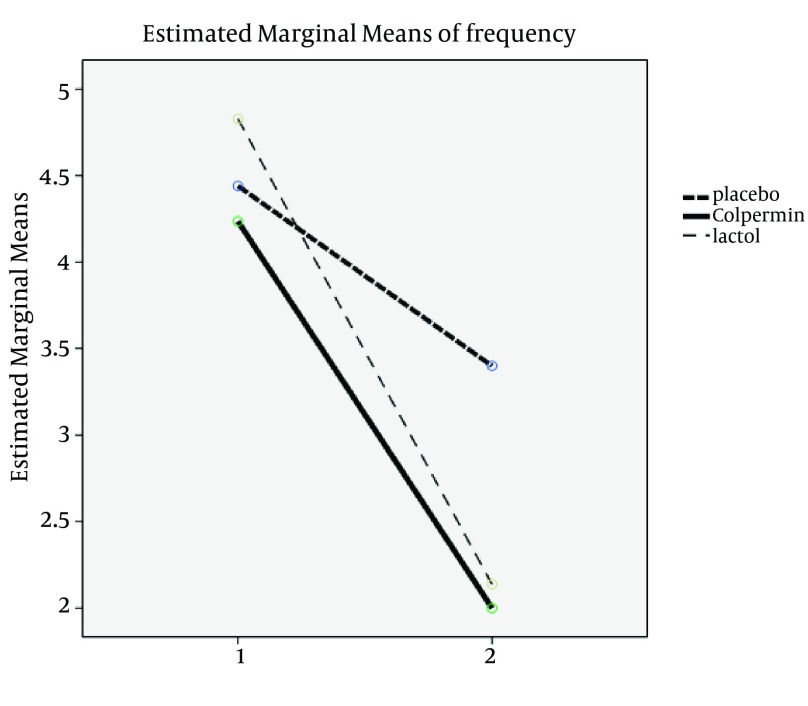
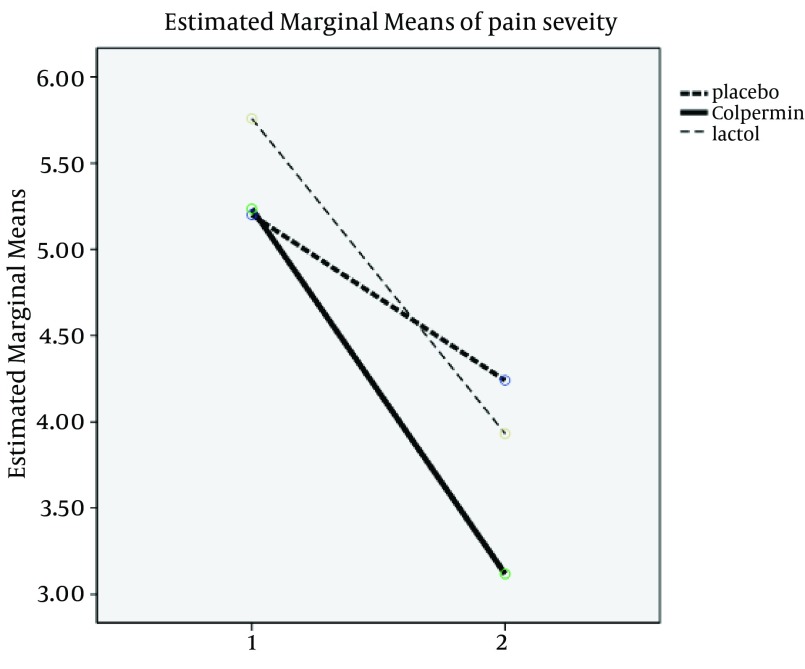
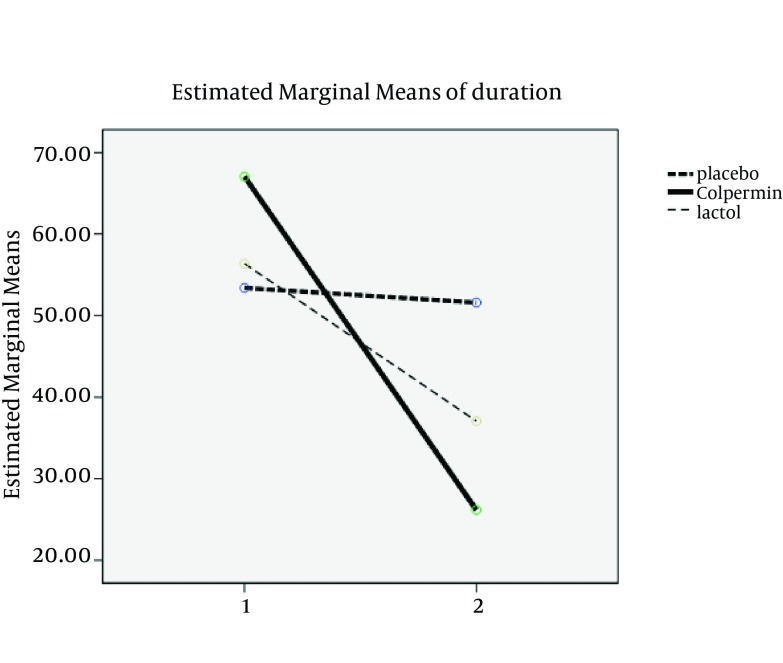
There were no statistically significant differences between the three groups in terms of age, sex, duration, frequency and severity of pain, pain localization and clinical subgroups (FAP-Dyspepsia-IBS-APS) before treatment (P > 0.05). There were no statistically significant differences in demographic variables between the groups (Table 1). Two main used drugs were Colpermin (peppermint oil) and Lactol (synbiotic). In group A (Colpermin), significant changes were seen in duration, frequency and severity of abdominal pain after intervention (P value = 0.0001). For Lactol group, changes in duration, frequency and severity of pain were significant too (P value = 0.008, 0.001 and 0.001, respectively). In placebo group, change in duration was not significant (P value = 0.730); however, the frequency and severity changes were significant (P = 0.032 and P = 0.002, respectively) (Table 2). Decrease in duration, frequency and severity of pain after intervention in the Colpermin group was significant compared to the placebo (P = 0.0001, P = 0.0001, and P = 0.001, respectively). Pain duration and frequency changes were also significant in the Lactol group compared to the placebo (P = 0.012, P = 0.0001, respectively), but pain severity changes were not significant (P = 0.373). Pain duration and severity in the Colpermin group were decreased more than the Lactol group (P = 0.040 and P = 0.013 respectively). Lactol reduced pain frequency more than the Colpermin, but this difference was not statistically significant (P = 0.619) (Table 3, Figures 2-4). Overall, pain duration and frequency were decreased in the Colpermin and Lactol groups more than the placebo Moreover, pain duration and severity were decreased in the Colpermin group more than the Lactol group.
Table 2. Means and Standard Deviations of Scores Before and After the Intervention a.
| Variable | A (Colpermin, n = 34) | B (Lactol, n = 29) | C (Placebo, n = 25) | |||
|---|---|---|---|---|---|---|
| Baseline | Final | Baseline | Final | Baseline | Final | |
| Duration of pain, minutes per day a | 67.05 ± 36.97 | 26.17 ± 11.61 | 56.37 ± 25.56 | 37.06 ± 25.51 | 53.40 ± 16.81 | 51.60 ± 23.74 |
| P Value | 0.0001 | 0.008 | 0.730 | |||
| Frequency of pain, per week a | 4.24 ± 1.57 | 2.00 ± 0.98 | 4.83 ± 1.64 | 2.14 ± 0.87 | 4.44 ± 1.87) | 3.40 ± 1.41) |
| P Value | 0.0001 | 0.0001 | 0.032 | |||
| Severity of pain, numerical rating scale a | 5.23 ± 1.34 | 3.11 ± 1.36 | 5.75 ± 1.21 | 3.93 ± 1.06 | 5.20 ± 1.15 | 4.24 ± 1.33 |
| P Value | 0.0001 | 0.0001 | 0.002 | |||
aPaired t-test.
Table 3. Comparison Between Drugs and Placebo and Between Two Drugsa.
| Mean Changes in Pain Duration | Mean Changes in Pain Frequency | Mean Changes in Pain Severity | |
|---|---|---|---|
| Colpermin | 40.882 | 2.235 | 2.117 |
| Placebo | 1.800 | 1.040 | 0.960 |
| P value a | 0.0001 | 0.0001 | 0.001 |
| Lactol | 19.310 | 2.690 | 1.827 |
| Placebo | 1.800 | 1.040 | 0.960 |
| P value a | 0.012 | 0.0001 | 0.373 |
| Colpermin | 40.882 | 2.235 | 2.117 |
| Lactol | 19.310 | 2.690 | 1.827 |
| P value a | 0.040 | 0.619 | 0.013 |
aGLM analysis.
Figure 2. Comparison of Frequency Changes Between Groups.
Figure 4. Comparison of Pain Severity Changes Between Groups.
Figure 3. Comparison of Duration Changes Between Groups.
5. Discussion
Abdominal pain of unknown origin, or nonspecific abdominal pain, is the most frequent diagnosis in children with abdominal pain (22). There are still questions on the definitive pathophysiological alterations found in children with abdominal pain-related FGIDs. Current theories include autonomic nervous system instability, visceral hyperalgesia, gut dysmotility, stressful life events or poor coping skills based on the complex interplay of genetic, physiological and psychological factors (5, 6, 23-26). It is known that both the gut and the nervous system are derived from the same tissue embryologically. Neuropeptides and neurotransmitters produced in the gastrointestinal tract regulate gastrointestinal motility, blood flow, secretion and absorption (5). The enteric and central nervous systems have direct effects on each other. This brain-gut communication seems to be a mechanism that links the psychoemotional state with gastrointestinal dysfunction (5). In this study we compared the efficacy and tolerability of pH-dependent peppermint oil (Colpermin) and synbiotic Lactol (Bacillus coagulans + FOS) with each other and with placebo in childhood functional abdominal pain. We evaluated the patients’ response as decrease in severity, duration and frequency of pain and found superiority of Colpermin and Lactol to placebo. In the one-month trial period, we didn’t see any adverse reaction or intolerance in our patients and it seems that Colpermin and Lactol can provide better outcome in controlling pain in children with functional abdominal pain compared to placebo (folic acid). Also, it seems that Colpermin compared with Lactol is better in decreasing duration and severity of pain. Both of them were tolerated without side effects. Kline et al. (14) used pH-dependent, enteric-coated peppermint oil capsule against placebo in 50 children with IBS for 2 weeks in a randomized, double-blind controlled trial and assessed changes in the severity of symptoms including abdominal pain, stool pattern, etc. They showed decreases in the severity of symptoms in 76% of patients received peppermint oil compared to only 19% who received placebo. However, they tried peppermint oil only for IBS patients, but we used it for four subclasses of abdominal pain-related FGIDs. Several meta-analyses showed the effectiveness of different antispasmodics in the treatment of IBS (16, 27). The significant improvement in severity, duration and frequency of pain in our patients may have several explanations. Colpermin (pH-dependent peppermint oil) capsule comprises menthol that causes the inhibition of smooth muscle contractions by blocking calcium channelsalso It has a mild topical effect on ileal and colonic mucosa (14-16). Romano et al. (17) showed the efficacy of Lactobacillus reuteri as a probiotic for children abdominal pain intensity in a randomized, double-blind placebo-controlled trial. Gawronska et al. (18) used Lactobacillus GG for treating functional abdominal pain disorders in children in a randomized, double-blind placebo-controlled trial and showed 25% response for probiotic compared to 9.6% for placebo and concluded that the Lactobacillus rhamnosus GG appears to moderately increase treatment success particularly among children with IBS. Bauserman et al. (19) also tried Lactobacillus rhamnosus GG versus placebo for six weeks in patients with IBS and showed no significant difference in abdominal pain relief. On the other hand, Francavilla et al. (20) showed that Lactobacillus GG significantly reduces the frequency and severity of abdominal pain in a randomized controlled trial. Bu et al. (21) randomized patients with chronic constipation and abdominal pain to receive either Lactobacillus casei rhamnosus, magnesium oxide or placebo for 4 weeks and showed that frequency of abdominal pain was significantly decreased in the probiotic group compared to both magnesium oxide and placebo. We tried the synbiotic Lactol (Bacillus coagulans + FOS) for 4 weeks and saw its superiority to placebo in decreasing the pain severity, frequency and duration. As commensal bacteria of the GI tract are believed to play an important role in homeostasis, alterations to these populations have been implicated in dysmotility, visceral hypersensitivity, abnormal colonic fermentation and immunologic activation, probiotics and synbiotics (Lactol in this study) may improve functional GI symptoms by restoring the microbial balance in barrier or by altering the intestinal inflammatory response (17-21). Because of efficacy and no side effect or intolerance, these drugs can be worthy and considerable. Peppermint oil is a botanical with rectal burning, esophageal pain or heart burn and allergic reactions, side effects that none of them has been reported or seen in our patients (14). The ability of probiotic strains to survive in gastric conditions and to strongly adhere to the intestinal epithelium may entail a risk of bacterial translocation, bacteremia and sepsis. However, in past studies, current preparations were safe for normal hosts (17-21). We applied a standard preparation and were sure of our patients’ immunity based on history and physical exam and complete blood count with differentiation. We didn’t seen any side effects or intolerance strong point of this study was the placebo-controlled, single-blind design of this study (our professional nurse who has completed questionnaires was unaware of the protocol), which aimed to compare the efficacy of a botanical and safe drug with a synbiotic product. Weak points of our study were the dropout of some patients during trial and using unsealed drugs. The pH-dependent peppermint oil capsule and Lactol tablet (Bacillus coagulans + FOS) as synbiotic seem to be superior to placebo in decreasing severity, duration and frequency of pain in abdominal pain-related FGIDs (except for abdominal migraine).None of these drugs showed side effect or intolerance and both are worthy and safe. Future studies based on biochemical and inflammatory changes in GI tract are suggested. This can be evaluated by stool examination for inflammatory markers, such as calprotectin.
Footnotes
Authors’ Contributions:Masoumeh Asgarshirazi: Study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and administrative, technical, and material support. Mamak Shariat: Drafting of the manuscript, critical revision of the manuscript for important intellectual content and statistical analysis. Hosein Dalili: Study supervision.
References
- 1.Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130(5):1527–37. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baber KF, Anderson J, Puzanovova M, Walker LS. Rome II versus Rome III classification of functional gastrointestinal disorders in pediatric chronic abdominal pain. J Pediatr Gastroenterol Nutr. 2008;47(3):299–302. doi: 10.1097/MPG.0b013e31816c4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korterink J, Devanarayana NM, Rajindrajith S, Vlieger A, Benninga MA. Childhood functional abdominal pain: mechanisms and management. Nat Rev Gastroenterol Hepatol. 2015;12(3):159–71. doi: 10.1038/nrgastro.2015.21. [DOI] [PubMed] [Google Scholar]
- 4.Chiou FK, How CH, Ong C. Recurrent abdominal pain in childhood. Singapore Med J. 2013;54(4):195–9. doi: 10.11622/smedj.2013072. [DOI] [PubMed] [Google Scholar]
- 5.Weydert JA, Ball TM, Davis MF. Systematic review of treatments for recurrent abdominal pain. Pediatrics. 2003;111(1):e1–11. doi: 10.1542/peds.111.1.e1. [DOI] [PubMed] [Google Scholar]
- 6.Rasquin-Weber A, Hyman PE, Cucchiara S, Fleisher DR, Hyams JS, Milla PJ, et al. Childhood functional gastrointestinal disorders. Gut. 1999;45(suppl 2):II60–8. doi: 10.1136/gut.45.2008.ii60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.See MC, Birnbaum AH, Schechter CB, Goldenberg MM, Benkov KJ. Double-blind, placebo-controlled trial of famotidine in children with abdominal pain and dyspepsia: global and quantitative assessment. Dig Dis Sci. 2001;46(5):985–92. doi: 10.1023/a:1010793408132. [DOI] [PubMed] [Google Scholar]
- 8.Kusunoki H, Haruma K, Hata J, Ishii M, Kamada T, Yamashita N, et al. Efficacy of Rikkunshito, a traditional Japanese medicine (Kampo), in treating functional dyspepsia. Intern Med. 2010;49(20):2195–202. doi: 10.2169/internalmedicine.49.3803. [DOI] [PubMed] [Google Scholar]
- 9.Monkemuller K, Malfertheiner P. Drug treatment of functional dyspepsia. World J Gastroenterol. 2006;12(17):2694–700. doi: 10.3748/wjg.v12.i17.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bijkerk CJ, Muris JW, Knottnerus JA, Hoes AW, de Wit NJ. Systematic review: the role of different types of fibre in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;19(3):245–51. doi: 10.1111/j.0269-2813.2004.01862.x. [DOI] [PubMed] [Google Scholar]
- 11.Pourmoghaddas Z, Saneian H, Roohafza H, Gholamrezaei A. Mebeverine for Pediatric Functional Abdominal Pain: A Randomized, Placebo-Controlled Trial. Bio Med Res Int. 2014;2014(2014) doi: 10.1155/2014/191026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huertas-Ceballos A, Macarthur C, Logan S. Dietary interventions for recurrent abdominal pain in childhood. Cochrane Library. 2002;(1) doi: 10.1002/14651858.CD010972. [DOI] [PubMed] [Google Scholar]
- 13.Camilleri M, Andresen V. Current and novel therapeutic options for irritable bowel syndrome management. Dig Liver Dis. 2009;41(12):854–62. doi: 10.1016/j.dld.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kline RM, Kline JJ, Di Palma J, Barbero GJ. Enteric-coated, pH-dependent peppermint oil capsules for the treatment of irritable bowel syndrome in children. J Pediatr. 2001;138(1):125–8. doi: 10.1067/mpd.2001.109606. [DOI] [PubMed] [Google Scholar]
- 15.Lacy BE, Wang F, Bhowal S, Schaefer E, study G. On-demand hyoscine butylbromide for the treatment of self-reported functional cramping abdominal pain. Scand J Gastroenterol. 2013;48(8):926–35. doi: 10.3109/00365521.2013.804117. [DOI] [PubMed] [Google Scholar]
- 16.Ford AC, Talley NJ, Spiegel BMR, Foxx-Orenstein AE, Schiller L, Quigley EMM, et al. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. Bri Med J. 2008;337(a2313) doi: 10.1136/bmj.a2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romano C, Ferrau V, Cavataio F, Iacono G, Spina M, Lionetti E, et al. Lactobacillus reuteri in children with functional abdominal pain (FAP). J Paediatr Child Health. 2014;50(10):E68–71. doi: 10.1111/j.1440-1754.2010.01797.x. [DOI] [PubMed] [Google Scholar]
- 18.Gawronska A, Dziechciarz P, Horvath A, Szajewska H. A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Aliment Pharmacol Ther. 2007;25(2):177–84. doi: 10.1111/j.1365-2036.2006.03175.x. [DOI] [PubMed] [Google Scholar]
- 19.Bauserman M, Michail S. The use of Lactobacillus GG in irritable bowel syndrome in children: a double-blind randomized control trial. J Pediatr. 2005;147(2):197–201. doi: 10.1016/j.jpeds.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Francavilla R, Miniello V, Magistà AM, De Canio A, Bucci N, Gagliardi F, et al. A randomized controlled trial of Lactobacillus GG in children with functional abdominal pain. Pediatrics. 2010;126(6):e1445–52. doi: 10.1542/peds.2010-0467. [DOI] [PubMed] [Google Scholar]
- 21.Bu LN, Chang MH, Ni YH, Chen HL, Cheng CC. Lactobacillus casei rhamnosus Lcr35 in children with chronic constipation. Pediatr Int. 2007;49(4):485–90. doi: 10.1111/j.1442-200X.2007.02397.x. [DOI] [PubMed] [Google Scholar]
- 22.Lin WC, Lin CH. Re-appraising the role of sonography in pediatric acute abdominal pain. Iran J Pediatr. 2013;23(2):177–82. [PMC free article] [PubMed] [Google Scholar]
- 23.Andresen V, Camilleri M. Irritable bowel syndrome: recent and novel therapeutic approaches. Drugs. 2006;66(8):1073–88. doi: 10.2165/00003495-200666080-00004. [DOI] [PubMed] [Google Scholar]
- 24.Lacy BE. Irritable bowel syndrome: a primer on management. Rev Gastroenterol Disord. 2003;3 Suppl 3:S32–42. [PubMed] [Google Scholar]
- 25.Sanders MR, Shepherd RW, Cleghorn G, Woolford H. The treatment of recurrent abdominal pain in children: a controlled comparison of cognitive-behavioral family intervention and standard pediatric care. J Consult Clin Psychol. 1994;62(2):306–14. doi: 10.1037//0022-006x.62.2.306. [DOI] [PubMed] [Google Scholar]
- 26.Chiou E, Nurko S. Management of functional abdominal pain and irritable bowel syndrome in children and adolescents. Expert Rev Gastroenterol Hepatol. 2010;4(3):293–304. doi: 10.1586/egh.10.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez-Vazquez MA, Vazquez-Elizondo G, Gonzalez-Gonzalez JA, Gutierrez-Udave R, Maldonado-Garza HJ, Bosques-Padilla FJ. Effect of antispasmodic agents, alone or in combination, in the treatment of Irritable Bowel Syndrome: systematic review and meta-analysis. Rev Gastroenterol Mex. 2012;77(2):82–90. doi: 10.1016/j.rgmx.2012.04.002. [DOI] [PubMed] [Google Scholar]