Highlights
-
•
Monepantel resistance was selected in three isolates of Teladorsagia circumcincta.
-
•
Selection for resistance was generated through rounds of sub-optimal dosing.
-
•
Selection for monepantel resistance occurred within 9–13 generations.
-
•
Selection for resistance impacted on the life history traits of the isolates.
-
•
Increased egg output and shortened time to patency was seen in a resistant isolate.
Keywords: Efficacy, In vitro, Monepantel, Resistance, Selection, Teladorsagia circumcincta
Graphical Abstract

Abstract
Monepantel (MPTL) is one of two new anthelmintic compounds introduced onto the sheep market to control gastro-intestinal nematodes. Resistance to this compound is rare but has been reported. In order to preserve the efficacy of this and other anthelmintics, it is essential to understand both (a) the mechanisms involved in the selection of resistance and (b) how the parasites evolve to deal with these compounds.
To address these questions three MPTL-resistant Teladorsagia circumcincta isolates (MTci2-11, MTci5-13 and MTci7-12) have been artificially selected in vivo from phenotypically characterised parent isolates (MTci2, MTci5, MTci7 respectively). The selection process involved collecting and culturing eggs from surviving worms from sheep administered sub-optimal dosages of MPTL (Zolvix®) to provide infective larvae to infect further sheep until resistant isolates were generated (between 9 and 13 rounds of selection). A controlled efficacy test was conducted using the original parental isolates and the newly generated MPTL resistant isolates (n = 5 per group). Selected isolates were assessed both under anthelmintic stress (Zolvix®, 2.5 mg/kg bodyweight; MTci-MPTL) and at rest (untreated, MTci-CON). A number of life-history traits were assessed, namely, worm establishment rates, time to patency, faecal egg output, body length of adults and eggs in utero. The estimated resistance status of the selected isolates was confirmed with 48%, 28% and 9% reductions in worm burden at 7-days post Zolvix® administration for MTci2-11-MPTL, MTci5-13-MPTL and MTci7-12-MPTL, respectively, compared with untreated controls. One of the selected isolates MTci7-12-CON showed significantly greater total worm burden (p = 0.025), greater establishment rate (p = 0.033), decreased time to patency (p = 0.048), higher cumulative egg outputs (p = 0.002) compared with its parental derivative MTci7. The trial results suggest that anthelmintic selection in T. circumcincta, albeit under experimental conditions, can select for more prolific/fecund and quicker maturing populations. These data provide an insight into how parasites evolve in response to anthelmintic pressure.
1. Introduction
Monepantel (trade name Zolvix®) was launched, firstly in New Zealand in 2009, and then in Australia and the UK in 2010 by Novartis Animal Health. It was the first new drug family of anthelmintic to appear on the livestock healthcare market for over 25 years (Kaminsky et al., 2008). In an effort to prolong the useful life of this new compound and protect the efficacy of the older anthelmintic families, monepantel has been sold as prescription only medicine (POM-V) in the UK. Although efforts have been made to “protect” the compound, the first cases of resistance have been reported only two years after its launch. The first case of resistance was reported in sheep and goats in New Zealand (Leathwick et al., 2013; Scott et al., 2013) and another case was reported on a goat farm in New South Wales, Australia (https://wormmailinthecloud.wordpress.com/?s=monepantel+resistance; last accessed 30APR15). All reports have found that Teladorsagia circumcincta was one of the parasites surviving treatment.
An understanding of the development of anthelmintic resistance and evolvement of parasites in the face of anthelmintic pressure is key to developing strategies to preserve their efficacy. Previously different selection processes for the assessment of anthelmintic resistance have been used: (a) comparison of anthelmintic resistant and sensitive field derived isolates (Le Jambre et al., 1977; Hall et al., 1978; Kelly et al., 1978; Hunt et al., 2008, 2010); (b) experimental selection via increasing sub-therapeutic dosages in vivo (Kates et al., 1973; Colglazier et al., 1974a; Egerton et al., 1988; Giordano et al., 1988; Shoop et al., 1990; Rohrer et al., 1994; Ranjan et al., 2002; Coles et al., 2005); (c) in vitro pressurisation and selection of individuals within a population with an inherent insensitivity to a compound and use them for in vivo trials (Maingi et al., 1990; Kaminsky et al., 2008); (d) comparison of populations pre- and post-in vitro anthelmintic exposure (Laing, 2010; De Graef et al., 2013); (e) introgression of resistant genes into sensitive populations through the process of backcrossing individuals (Redman et al., 2012); (f) comparison of clade V free living model nematode systems to extrapolate information on parasitic nematodes (Driscoll et al., 1989; Chehresa, 1996).
Phenotypic variation in life history traits following selection for benzimidazole (BZ; Hall et al., 1978, 1981; Kelly et al., 1978; Maingi et al., 1990; Leignel and Cabaret, 2001) and ivermectin (IV; Echevarria et al., 1993a, 1993b) resistance has been examined previously in a number of parasite species. Historically, it was believed that chemotherapy that impacts on adult parasite life expectancy would result in one of the two outcomes: either earlier development to sexual maturity of parasites in order to propagate prior to treatment, or delayed development to avoid the effects of treatment (Arneberg et al., 1998; Skorping and Read, 1998). The finding from the aforementioned selection studies would suggest that the impact of the development of resistance on phenotypes, as one might expect, is not so clear cut, and it is influenced by a number of host related/physiological (e.g. age, size, diet, immune status, presence of conspecifics and heterospecifics) and environmental factors (Poulin, 1996).
The aims of this study were: firstly, to generate T. circumcincta isolates selected for monepantel resistance from previously phenotypically characterised parental isolates to improve our understanding of the development, dissemination and detection of MPTL resistance and secondly, to assess the extent of phenotypic changes that occur in selected isolates compared to parental isolates following selection for resistance to MPTL.
2. Materials and methods
2.1. Parasite isolates
Three T. circumcincta (designated MTci2, MTci5 and MTci7) isolates were used to generate the MPTL resistant isolates, as outlined in section 2.2 (Table 1). MTci2 is phenotypically sensitive to all broad spectrum anthelmintics (unpublished data) whilst MTci5 and MTci7 are phenotypically BZ, levamisole (LV) and IV resistant (Sargison et al., 2001; Bartley et al., 2004, 2005). MTci7 also exhibits moxidectin (MX) resistance (Sargison et al., 2005; Wilson and Sargison, 2007). The isolates were passaged through parasite naive lambs that were housed under conditions that precluded contamination with other nematode species prior to use. During the period of selection, the parental isolates were independently passaged four times.
Table 1.
Teladorsagia circumcincta isolate designations pre- (parent) and post-selection for monepantel resistance.
| Parental designation | Anthelmintic sensitivity | Designation post monepantel selection | Designation during phenotypic characterisation | Original parental characterisation references | |||
|---|---|---|---|---|---|---|---|
| BZ | LV | IV | MX | ||||
| MTci2 | S | S | S | S | MTci2-11 | MTci2-11-CON | NA |
| MTci2-11-MPTL | |||||||
| MTci5 | R | R | R | S | MTci5-13 | MTci5-13-CON | (Sargison et al., 2001; Bartley et al., 2005) |
| MTci5-13-MPTL | |||||||
| MTci7 | R | R | R | R | MTci7-12 | MTci7-12-CON | (Sargison et al., 2005; Wilson and Sargison, 2007) |
| MTci7-12-MPTL | |||||||
BZ, fenbendazole; LV, levamisole; IV, ivermectin; MX, moxidectin; S, sensitive to anthelmintic class; R, resistant to anthelmintic class; NA, not available.
2.2. Monepantel resistance selection process
Three MPTL resistant T. circumcincta isolates were artificially selected in vivo from phenotypically characterised isolates using an adaptation of a previously outlined methodology (Coles et al., 2005). In brief, lambs were initially mono-specifically infected with approximately 20,000 infective larvae of MTci2, MTci5 or MTci7. Due to availability of stocks, the infective larvae used to initiate the infections were 1, 4 and 11 months old for MTci7, MTci2 and MTci5, respectively. All subsequent infections were less than 1 month old when administered. All larvae were assessed visually to ensure they appeared active. Prior to infection all larvae were stored in 100 ml tap water in 75 cm2 suspension culture flasks (Startsedt Ltd, Germany) between 4 and 8 °C. At patency, lambs were administered monepantel (Zolvix®, Novartis Animal Heath) at 0.125 mg/kg bodyweight (BW), 1/20th of the manufacturer's recommended dose rate. The lowest dose used was selected because previous work had demonstrated that even a dose of 1.25 mg/kg BW had been effective at removing >99% of T. circumcincta populations (Kaminsky et al., 2009). Eggs from surviving worms were collected and cultured to generate infective larvae. Lambs with positive faecal egg counts (FEC) were re-treated at increasing dose rates of MPTL (e.g. 0.25, 0.5, 0.63, 0.83, 1.25, 1.88 and 2.5 mg/kg BW) until egg counts dropped. At this point, the larvae obtained from the highest dosage were either used to re-infect the donor that had been previously treated or used to infect a new donor. At patency, the process was repeated using increasing dose rates until a fully resistant isolate, i.e. able to survive a full therapeutic dose rate (2.5 mg/kg BW), was generated. Further information can be found in the supplementary material file attached.
2.3. Monepantel efficacy trial design
The monepantel efficacy trial overall included 10 groups including parent (without anthelmintic treatment) and selected (with and without monepantel administration on day 28, i.e. -CON and -MPTL) isolates. Five lambs were randomly allocated to each of the 10 groups: MTci2, MTci5, MTci7, MTci7-MPTL, MTci2-11-CON, MTci5-13-CON, MTci7-12-CON, MTci2-11-MTPL, MTci5-13-MTPL, MTci7-12-MTPL (Table 1). The group MTci7-MPTL is a treatment validation group, included for those infected with the parental isolate MTci7, to confirm that MPTL was effective at removing adult populations in the original parental isolate. On day 0, Suffolk greyface worm-free crossbred lambs of 5 to 9 months were challenged per os with 7000 infective larvae from one of the three parental isolates (MTci2, MTci5 or MTci7) or three monepantel selected isolates (MTci2-11, MTci5-13 or MTci7-12) according to the constituent of group. Faecal egg counts were conducted on the lambs prior to the start of the trial to confirm their worm-free status, and on the day of treatment (day 28) to allow the formation of groups within which all lambs have similar egg counts. Further FECs were conducted throughout the trial to assess time to patency and magnitude of egg output (details in section 2.3.1). On day 28 post-infection, the lambs were weighed and dosed orally by syringe at the manufacturer's recommended dose rate with either Zolvix® (2.5 mg/kg BW; -MPTL) or left untreated to act as controls (-CON). All anthelmintic administrations were rounded up to the nearest 0.2 ml (dosage range 2.5–2.7 mg/kg BW). All of the animals were necropsied seven days post-treatment; the methods used to euthanase the animals, remove and process their organs for worm burden recovery were as described by Patterson et al. (1996). The total worm burdens were estimated from 2% sub-samples of the abomasal washings and saline digests. Recovered worms were sexed and staged (MAFF, 1986).
Due to animal, material and time constraints the above trials were conducted at two separate time points. The controlled efficacy test (CET) using MTci7 and its selected isolate (MTci7-12) was investigated in one trial whilst MTci2, MTci5, MTci2-11 and MTci5-13 were investigated in a second trial. All experimental procedures described here were approved by the Moredun Research Institute Experiments and Ethics Committee and were conducted under the legislation of a UK Home Office License (reference PPL 60/03899) in accordance with the Animals (Scientific Procedures) Act of 1986.
2.3.1. Faecal egg counts
Faecal samples were taken per rectum from each lamb prior to infection to confirm that they were negative for nematode eggs: from day 8 post infection (PI) egg counts, eggs per gram (EPG), were performed three times a week to assess pre-patency of the isolates, on day 27 PI prior to the anthelmintic treatment (day 28), and then daily until necropsy seven days later. Faecal egg counts were conducted throughout the trials using a modification of a salt flotation method with a sensitivity of up to one egg per gram (Christie and Jackson, 1982), allowing the evaluation of the effectiveness of the anthelmintic on faecal egg count reduction.
2.3.2. Eggs in utero and worm body lengths
Twenty individual adult worms (20 male and 20 female worms where available) were randomly selected from each lamb within a group, mounted in a drop of lactophenol (GCC Diagnostics) on a microscope slide and assessed for fecundity. The numbers of eggs in utero in female worms were enumerated at magnification (×40) on a compound microscope. Images were captured (Nikon D70) of each individual for length determination; where multiple images were captured, the images were compiled to form a single composite image using an image editing program (Adobe Photoshop Elements 4.0). Worm lengths were estimated using image processing software (ImageJ 1.46r).
2.4. Statistical analysis
The main effects to be investigated were: comparisons between groups corresponding to the parental isolates (MTci2, MTci5, MTci7), comparisons between groups corresponding to the parent and selected isolates (versus MTci2-11-CON, MTci5-13-CON, MTci7-12-CON), and finally, comparisons between groups corresponding to the selected isolates without treatment (-CON) and with treatment (-MPTL) (versus MTci2-11-MTPL, MTci5-13-MTPL, MTci7-12-MTPL).
2.4.1. Parasite establishment and worm burden
The data on each stage of worm burden (juvenile, male, female and total) in individual lamb were investigated by fitting a generalised linear mixed model (GLMM) to the counts using a logarithmic link function and assuming the Poisson distribution. Estimates of establishment rate (the proportion of juvenile and adult worms recovered from the initial challenge dose i.e. 7000) and development rate (the proportion of adult worms out of total worms established) were obtained for each group by fitting a GLMMs using a logit link function and assuming the binomial distribution. All models included group as a fixed effect, and lamb (the level of each observation) as a random effect to allow for over-dispersion.
2.4.2. Treatment efficacy
The percentage efficacy of the treatment for each selected isolate was calculated using the equation: (1-(T/C)) × 100, where C and T are the arithmetic mean total worm burdens or faecal egg counts at 35 days PI of the control (-CON) and treated (-MPTL) groups, respectively (Coles et al., 1992). According to Coles et al. (1992), anthelmintic resistance was deemed to be present when the mean percentage efficacy at reducing faecal egg output or worm burden was less than 95%.
2.4.3. Adult worm body length
The data on length of male and female worms were each analysed using a linear mixed model (LMM). The model included group as a fixed effect and lamb as a random effect.
2.4.4. Per capita fecundity
The worm egg counts in utero were analysed using a GLMM with a logarithmic link function and assuming the Poisson distribution. The model incorporated the group as a fixed effect and lamb as a random effect, and worm as a random effect to allow for over-dispersion. Two separate GLMMs were investigated, with and without an offset variable of the logarithm of the female worm length. An additional GLMM was also fitted to estimate the effect of logarithm of the female worm length on the egg counts.
2.4.5. Time to patency
The data on time to patency for each animal were calculated as the time when the faecal egg count is greater than or equal to 25 eggs per gram. This was analysed using a linear model (LM). The model included the isolate, combining the two groups for each selected isolate (-CON and -MPTL), as a fixed effect, because days to patency are before the treatment day (i.e. day 28).
2.4.6. Daily and cumulative faecal egg count
To investigate the effect of group on the faecal egg counts of lambs, two different approaches were used. First, the logarithm of faecal egg counts (count+1) data for each lamb on each time point (day) until day 35 of the experimental period (including both pre and post-anthelmintic treatment phases) were analysed by an additive LMM. The model included group as a fixed effect and separate smoothing curves for each group to consider the non-linear relationship of faecal egg count with day. The model incorporated lamb as a random effect and heterogeneity in variance for each group. Model selection was based on the Akaike's information criterion (AIC). Secondly, to compare the overall faecal egg counts, the cumulative egg count for each lamb during the entire experimental period was estimated by calculating the area under the curve (AUC) of EPG using the trapezoid method. A linear model (LM) was fitted to the square root transformed AUC data for control lambs only, incorporating the group as a fixed effect.
2.4.7. General modelling strategy
If all lambs within a group recorded zero values for a response variable (commonly occurred in the MTci7-MPTL group), there was no information about lambs within the group; hence the corresponding group was removed before fitting the model to the data. The overall statistical significance of the group (or isolate) was assessed using the F-statistic for the LM, LMM and additive LMM or chi-square statistic for the GLMM. If the overall test for the group was statistically significant (p < 0.05), a set of biologically interesting contrasts to compare among groups was formulated, and two-sided probabilities for each contrast were obtained. Finally, these probabilities were adjusted using a False Discovery Rate (FDR) approach (Benjamini and Hochberg, 1995) to take into account multiple comparisons of means so that the expected proportion of false positives among all positives (i.e. rejecting the null hypothesis) was less than 5%. The FDR value, denoted in the results here as ‘pf’, therefore summarises the strength of evidence for there being a real difference in a way analogous to a standard p-value. For GLMMs, and LMs applied to transformed data, estimated means and confidence intervals are back transformed onto the original scale (i.e. count, proportion, AUC) and tabulated.
All statistical analyses were carried out using R software version 3.1.0 with appropriate R packages (stats, lme4, mgcv, multcomp, ggplot2) (R Core Team, 2014).
3. Results
3.1. Parasite isolates
Resistance was generated in all three previously susceptible isolates. Parasite survival at a dose rate of 2.5 mg/kg BW was achieved after 9, 12 and 13 rounds of selection for MTci5-13, MTci7-12 and MTci2-11 respectively (see Supplementary material).
3.2. Parasite establishment and worm burden
Group had a statistically significant effect on the mean juvenile (p = 0.004), male (p < 0.001) and total (p = 0.010), and weak evidence for female (p = 0.060) worm burdens in lambs. Table 2 presents the estimated means and 95% confidence limits of juvenile worms, males, females and total worm burdens for different isolates. Lambs from the MTci7 group showed significantly lower worm burdens on average: less males compared with MTci2 (pf = 0.048), MTci5 (pf < 0.001) and MTci7-12-CON (pf = 0.002); less females compared with MTci5 (pf = 0.009) and less total worm burdens compared with MTci5 (pf = 0.003) and MTci7-12-CON (pf = 0.025).
Table 2.
Mean (lower, upper 95% confidence limits) worm burdens of Teladorsagia circumcincta differentiated into male, female, juvenile and expressed as total worm counts as well as the establishment and development rates of worms estimated from GLMMs. The table also presents the percentage efficacy of anthelmintic treated groups of lambs relative to untreated control lambs seven days post-treatment. Percentage efficacy is estimated for selected lines only based on the worm burdens of treated lambs (i.e. MTci2-11-MPTL, MTci5-13-MPTL and MTci7-12-MPTL) relative to untreated control lambs (i.e. MTci2-11-CON, MTci5-13-CON and MTci7-12-CON).
| Isolate | Worm burden | Establishment rate† | Development rate$ | Percentage efficacy | |||
|---|---|---|---|---|---|---|---|
| Male | Female | Juvenile | Total | ||||
| MTci2 | 1275 (862, 1885) | 1555 (1026, 2356) | 85 (40, 179) | 2937 (1990, 4335) | 0.45 (0.28, 0.63) | 0.97 (0.92, 0.99) | NA |
| MTci5 | 1783 (1220, 2607) | 2562 (1713, 3830) | 19 (4, 81) | 4395 (2996, 6447) | 0.66 (0.48, 0.80) | 1.00 (0.96, 1.00) | NA |
| MTci7 | 560 (361, 868) | 948 (612, 1468) | 139 (75, 258) | 1581 (1051, 2376) | 0.22 (0.12, 0.38) | 0.92 (0.83, 0.97) | NA |
| MTci2-11-CON | 2417 (1666, 3507) | 2248 (1499, 3371) | 0 | 4675 (3189, 6854) | 0.71 (0.54, 0.84) | 1.00 | NA |
| MTci2-11-MPTL | 1100 (740, 1635) | 1306 (858, 1987) | 9 (1, 73) | 2444 (1651, 3617) | 0.35 (0.21, 0.53) | 1.00 (0.90, 1.00) | 48 |
| MTci5-13-CON | 974 (651, 1459) | 1363 (896, 2074) | 37 (13, 106) | 2409 (1626, 3570) | 0.36 (0.21, 0.54) | 0.98 (0.92, 1.00) | NA |
| MTci5-13-MPTL | 910 (607, 1366) | 1232 (807, 1880) | 28 (8, 94) | 2182 (1470, 3240) | 0.32 (0.19, 0.50) | 0.99 (0.92, 1.00) | 9 |
| MTci7-12-CON | 1654 (1129, 2424) | 1900 (1262, 2861) | 140 (76, 260) | 3699 (2515, 5440) | 0.57 (0.39, 0.74) | 0.96 (0.91, 0.98) | NA |
| MTci7-12-MPTL | 1168 (785, 1737) | 1467 (965, 2229) | 56 (23, 136) | 2654 (1792, 3929) | 0.44 (0.28, 0.63) | 0.98 (0.93, 1.00) | 28 |
NA, Not applicable since the percentage efficacy is estimated for the selected lines only.
The establishment rate is defined as the proportion of juvenile and adult worms out of total infected worms (i.e. 7000).
The development rate is defined as the proportion of adult worms out of total worms established.
Group had a statistically significant (p = 0.006) effect on the mean parasite establishment rate in lambs (Table 2). Lambs in the MTci7 group showed the lowest mean establishment rate (0.22; 95% CI: 0.12, 0.38) whilst lambs in the MTci2-11-CON group showed the highest mean establishment rate (0.71; 95% CI: 0.54, 0.84). The MTci7 group showed a significantly lower mean establishment rate compared with MTci5 (pf = 0.003) and MTci7-12-CON (pf = 0.033). However, no evidence of differences in development rates of worms among groups was observed (p = 0.101).
3.3. Treatment efficacy
Treatment efficacies based on the percentage reduction in average estimated total worm burden between treated (-MPTL) and untreated (-CON) groups were 48%, 28% and 9% for MTci2-11, MTci7-12 and MTci5-13, respectively (Table 2).
3.4. Adult worm body length
Average body length of adult female worms obtained from each of the groups measured between 8.6 mm and 9.7 mm in length (Table 3). Average length of adult male worms ranged between 6.2 mm and 6.9 mm in length. However, there was no evidence that average worm body length differed between the groups (p = 0.242 and 0.193 for male and female, respectively).
Table 3.
Summary of life history traits of Teladorsagia circumcincta isolates before and after selection for monepantel resistance. Values are means (lower, upper 95% confidence limits) estimated from GLMMs, LMMs and LMs.
| Isolate | Adult worm length (mm) | Eggs in utero | Eggs in utero per mm body length | Time to patency† (days PI) | Total cumulative faecal egg count$ | |
|---|---|---|---|---|---|---|
| Female | Male | |||||
| MTci2 | 8.9 (8.3, 9.5) | 6.5 (6.1, 6.9) | 21 (16, 28) | 2.4 (1.9, 3.1) | 19 (16, 22) | 2826 (1037, 5495) |
| MTci5 | 9.5 (8.9, 10.2) | 6.7 (6.3, 7.2) | 28 (21, 37) | 3.0 (2.3, 3.8) | 18 (15, 22) | 6898 (3855, 10820) |
| MTci7 | 8.6 (7.9, 9.2) | 6.2 (5.8, 6.6) | 19 (14, 25) | 2.2 (1.7, 2.9) | 20 (18, 22) | 1546 (337, 3635) |
| MTci2-11-CON | 9.7 (9.1, 10.3) | 6.7 (6.3, 7.1) | 25 (19, 33) | 2.6 (2.1, 3.3) | 16 (14, 18) | 8067 (4741, 12273) |
| MTci2-11-MPTL | 9.6 (8.9, 10.2) | 6.9 (6.5, 7.2) | 13 (10, 17) | 1.4 (1.1, 1.8) | NA | NA |
| MTci5-13-CON | 9.0 (8.4, 9.7) | 6.7 (6.3, 7.1) | 18 (13, 24) | 2.0 (1.6, 2.6) | 19 (17, 21) | 2949 (1111, 5665) |
| MTci5-13-MPTL | 9.0 (8.4, 9.6) | 6.4 (6.1, 6.8) | 23 (18, 31) | 2.6 (2.0, 3.3) | NA | NA |
| MTci7-12-CON | 9.7 (9, 10.3) | 6.8 (6.4, 7.2) | 28 (21, 37) | 2.9 (2.3, 3.7) | 15 (13, 18) | 10215 (6417, 14893) |
| MTci7-12-MPTL | 9.4 (8.7, 10.0) | 6.7 (6.2, 7.1) | 30 (23, 40) | 3.3 (2.5, 4.2) | NA | NA |
PI, post infection.
Time to patency was calculated as faecal egg count >25 eggs per gram; data for selected lines combined, therefore no data quoted for monepantel (MPTL) treated groups (marked as NA, not applicable).
Estimated total faecal egg counts over the course of the trial; the outcomes for selected lines of monepantel (MPTL) treated groups were not estimated (marked as NA, not applicable).
3.5. Per capita fecundity
Average number of eggs in utero (EIU) for each of the groups ranged between 13 and 30 (Table 3). The female worm length had a statistically significant effect (p < 0.001) on mean EIU, and each mm increase in the female worm length resulted in a 30% increase of mean EIU. Group also had a statistically significant (p = 0.005) effect on the mean EIU. Results suggested that the mean EIU did not differ significantly (pf > 0.310) among the parental isolates, but the group MTci2-11-CON had significantly (pf = 0.010) higher mean EIU (25, 95% confidence limits: 19, 33) than the MTci2-11-MPTL (13, 95% confidence limits: 10, 17). Results were similar when the estimates of EIU for different groups were adjusted for the female worm length.
3.6. Time to patency
The time to patency, for this study, is defined as the time when a lamb produced a faecal egg count ≥ 25 eggs per gram. The estimated mean time to patency (lower, upper 95% confidence limits) were 19 (16, 22), 18 (15, 22) and 20 (18, 22) days post-infection for the parental isolates MTci2, MTci5 and MTci7, respectively; and 16 (14, 18), 19 (17, 21) and 15 (13, 18) days for the MPTL selected isolates MTci2-11, MTci5-13 and MTci7-12, respectively (Table 3). The effect of group on the mean time to patency showed a weak evidence (p = 0.052). The mean time to patency among the parental isolates and among the selected isolates were not statistically significant (pf > 0.925). Although the mean time to patency shortened for two of the three selected isolates following selection, only MTci7-12 had significant (pf = 0.048) shortening compared with its parental isolates MTci7.
3.7. Daily faecal egg count
Figure 1 presents the observed FEC data as well as the mean FECs and corresponding 95% confidence limits for each of the groups over the time course of the study. The interaction effect of group and smooth term for day was statistically significant (p < 0.001). Lambs of all groups started excreting eggs between days 14 and17 PI, and showed an increasing trend until the anthelmintic treatment on day 28 PI (Figure 1). Estimated mean faecal egg counts (95% lower, upper confidence limits) at day 28 PI for different isolates of parent and selected control lambs were: MTci2 (125; 95% confidence limits 57, 273); MTci2-11-CON (317; 136, 738); MTci5 (294; 142, 606); MTci5-13-CON (94; 42, 208); MTci7 (69; 31, 149) and MTci7-12-CON (499; 217, 1146) EPG. The mean egg count in the parental isolate MTci5 was higher than the parental isolate MTci7 on day 28 post infection, but the strength of evidence was weak (pf = 0.057). At the same time point, the mean egg count in the selected isolate MTci7-12-CON was significantly (pf = 0.006) higher compared with its corresponding parental isolate MTci7.
Fig. 1.
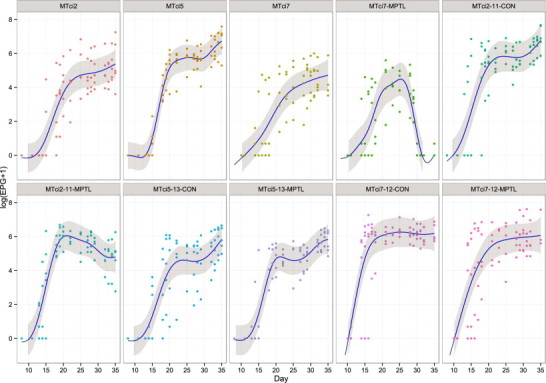
The mean (line) faecal eggs counts (egg per gram, EPG) of lambs infected with either parental (MTci2, MTci5, MTci7) or monepantel selected (MTci2-11, MTci5-13, MTci7-12) isolates over the course of the study and corresponding 95% confidence limits (shaded region) estimated from the linear mixed model (LMM). Observed individual faecal egg counts are shown as closed circles. Groups in each panel represent parent isolates and selected isolates with (-MPTL) or without (-CON) monepantel treatment (on day 28 post-infection) i.e. MTci2, MTci5, MTci7, MTci7-MPTL, MTci2-11-CON, MTci2-11-MTPL, MTci5-13-CON, MTci5-13- MTPL, MTci7-12-CON, MTci7-12-MTPL.
At post mortem examination (day 35 PI), the anthelmintic treatment efficacy based on the percentage reduction in FEC of treated versus untreated animals were 86%, 11% and 1% for MTci2-11, MTci7-12 and MTci5-13, respectively.
3.8. Cumulative faecal egg count
Figure 1 shows scatter plots of individual animals of each of the groups over the time course of the study. Table 3 presents the estimated mean cumulative faecal egg count for isolates of control parent and selected lambs and corresponding 95% lower and upper confidence limits for the different groups. Group had a statistically significant (p = 0.002) effect on the mean cumulative faecal egg counts in lambs. Among the parental isolates, the MTci7 had the lowest (1546; 95% CI: 337, 3635) and MTci5 had the highest (6898; 95% CI: 3855, 10820) estimated mean cumulative faecal egg counts, and these two parental isolates were significantly different (pf = 0.040). Among the selected isolates, MTci5-13-CON had the lowest estimate (2949; 95% CI: 1111, 5665) and MTci7-12-CON had the highest estimate (10215; 95% CI: 6417, 14893) of cumulative faecal egg counts. The mean cumulative egg count in the selected isolate MTci7-12-CON was significantly higher compared with the parental isolate MTci7 (pf = 0.002). Interestingly, MTci5-13-CON showed lower mean cumulative egg counts compared with its parental isolate (MTci5), although the difference was not statistically significant (pf = 0.278).
4. Discussion
Selection for monepantel resistance was “successful” in three discrete T. circumcincta isolates with different phenotypic sensitivities to “traditional” broad-spectrum anthelmintics. Selection was achieved after 9, 12 and 13 rounds of selection for MTci5-13, MTci7-12 and MTci2-11 respectively.
All selected parasite isolates were confirmed as MPTL resistant; the estimated resistance status of the selected isolates was confirmed with 48%, 28% and 9% reductions in worm burden at 7-days post Zolvix® administration for MTci2-11-MPTL, MTci5-13-MPTL and MTci7-12-MPTL, respectively, compared with untreated controls.
A selection process of administering increasing sub-therapeutic dosages to phenotyically characterised parental isolates was chosen for this work because it allowed the generation of a resistant population arising from a common ancestry which would allow future genotypic assessments. This process has been successfully used earlier to generate material to examine a number of candidate genes associated with benzimidazole (Beech et al., 1994; Lubega et al., 1994) and macrocyclic lactone (Blackhall et al., 1998a, 1998b, 2003; Paiement et al., 1999) resistance as well as to investigate non-specific mechanism of resistance (Molento and Prichard, 2001). Very little work has been conducted on T. circumcincta, but previous reports of artificial selection for anthelmintic resistance appear to be longer in this species than in other species. Workers using similar procedures to select BZ (Kates et al., 1973; Colglazier et al., 1974b) or ML (Egerton et al., 1988; Echevarria et al., 1993a; Rohrer et al., 1994; Ranjan et al., 2002; Coles et al., 2005) resistance in Haemonchus contortus generated it within 3 to 22 generations. Giordano et al. (1988) reported that selection pressures applied to a mixed population of nematode species selected for ivermectin resistance within four generations in Trichostrongylus colubriformis whilst T. circumcincta and H. contortus remained sensitive to the treatment in that time period. In T. circumcincta, morantel, levamisole and thiabendazole resistance was selected after around three years of selection pressure (no actual generations times stated; Le Jambre et al., 1977). Potential reasons for these inter- and intra-species differences may reflect the isolates' previous exposure to compounds of interest (Kates et al., 1973), inherent sensitivities to the compounds, e.g., dose limiting species (Thomas and Reid, 1980; Armour et al., 1982); initial frequency of “potential” gene(s) for resistance within the population; dominance/recessive nature of inheritance; whether resistance is monogenic or polygenic or the impact of non-specific mechanisms of resistance on the ability of an isolate to survive the effects of an anthelmintic. The complexity of the situation means that changes in the phenotypes and genotypes of nematode populations as a result of anthelmintic usage is unlikely to be the same across the board.
The length of time to generate resistance is difficult to accurately calculate within this type of selection process, although a conservative estimate would suggest that resistance was selected within 9–13 generations, albeit the genes for resistance may have been selected sooner. In the field the first reported cases of MPTL resistance occurred two years after the launch of the Zolvix® in New Zealand, where treatment failure against T. circumcincta and T. colubriformis occurred after intense (17 separate occasions) dosing strategies (Leathwick et al., 2013; Scott et al., 2013).
Although the intensity of the selection process is significantly more rapid than that seen in the field, it is conceivable that re-infection of hosts predominantly with the progeny of worms that have survived anthelmintic treatment could occur. An example would be when the number of infective larvae in refugia, i.e. the parasite population that is unexposed to anthelmintic treatment that is available to be ingested by a host, is extremely low. This phenomenon has been shown to rapidly select for anthelmintic resistance in the field. Benzimidazole (Papadopoulos et al., 2001) and IVM (Besier and Love, 2003; Suter et al., 2004) resistance is believed to have been selected when animals were treated around dry season and subsequently moved to “safe/clean” pasture (Cawthorne and Whitehead, 1983; Martin et al., 1985). Rapid selection occurs because, even with only a small number of survivors, they form the only individuals available for re-infection of stock.
Because health programmes alter nematode mortality, they force the parasites to evolve or face extinction. One way that organisms do this is through changing their strategies/life history traits (Paterson and Barber, 2007; Lynch et al., 2008). In addition to differences in the time scale of the selection for resistance, it would be expected that phenotypes such as life history traits would be different within different isolates/populations and species. Differences in life history traits have been observed both following artificial and field selection of resistance to a number of different anthelmintic drug classes (Kelly et al., 1978; Hall et al., 1981; Echevarria et al., 1993a; Elard et al., 1998; Skorping and Read, 1998; Leignel and Cabaret, 2001; Sutherland et al., 2002). Historically, it has been stated that interventions such as vaccination or chemotherapy, which potentially impact on the survival of the organism, would result in smaller, faster developing, less fecund worms (Medley, 1994; Poulin and Morand, 1997; Lynch et al., 2008). In situations where chances of survival are perceived to be high, the slower growing, larger and more fecund individuals would proliferate (Lynch et al., 2008) even though larger size has been correlated with increased pathogenicity (Stear et al., 1999). It is believed that the environmental conditions, be that within or out with the host, under which a parasite develops can influence the phenotype of that organism. Changes in growth, fecundity or mortality, for example, can traditionally occur in two ways; phenotypic plasticity whereby an organism produces a number of progeny of different phenotypes under different conditions, or genotypic adaptation where there is a shift in genotype frequency to one that is better suited to new conditions (Stearns, 1992). Phenotypic plasticity was believed to be the reserve of prokaryotic organisms such as bacteria, but work on trematodes has shown that even within parasite populations there is the capacity to accelerate development and reach precocious maturity sooner if conditions require (Poulin, 2003).
Assessment of the life history traits of the MPTL selected isolates showed that in one of the cases (MTci7-12) statistically significant differences were observed in a number of the phenotypic traits assessed in relationship to survivability and propagation i.e. establishment rate, time to patency, per capita fecundity and overall egg output. Establishment has been suggested as the most important of the life history traits and also the one most difficult to compare experimentally (Chehresa, 1996). The findings from this current study suggested that the selected isolates had higher establishment rates compared with their derivatives with the exception MTci5-13. The findings are in general agreement with previous ones which showed that the establishment of resistant isolates were the same or higher than unselected isolates in resistant H. contortus (Kelly et al., 1978; Hall et al., 1981; Echevarria et al., 1993a) and T. circumcincta (Hall et al., 1981; Elard et al., 1998).
Overall net cumulative egg production and per capita fecundity increased in magnitude for MTci2-11 and MTci7-12 whilst a reduction was observed with MTci5-13 compared with corresponding parental isolates, although the increase was statistically significant only for the cumulative egg production in the MTci7-12 isolate. Taking into account the greater female burdens carried by these individual hosts, cumulative egg outputs were 3 to 7 times greater than the parental isolates. Previous studies reported similar findings in populations selected for IVM resistance (Kelly et al., 1978; Sutherland et al., 2002) whilst others found only transient increases in egg excretion (Maingi et al., 1990). As previously observed, the increase in egg numbers in utero was related to increase in the worm body length (Skorping and Read, 1998; Leignel and Cabaret, 2001). In the current study, one mm increase in female body length resulted in 30% increase in eggs in utero.
In the early stages of resistance, particularly with the ML's, suppression in the generation and/or oviposition of eggs in adult female worms that survive treatment has been observed (McKellar et al., 1988; Sargison et al., 2005; Bartley et al., 2012). The impact is believed to be due to temporary paralysing effects of the anthelmintics on uterine musculature (Scott et al., 1991; McKenna, 1997). The result is the suppression in egg output in surviving worms whilst the drug is at a particular level which resumes when the drug concentration falls. McKellar et al. (1988) also suggested that dosing events may damage the worms so that although they survive, they will not contribute greatly to future populations. Analysis of historical faecal egg count data in conjunction with data from passage donors would suggest that the long term survivability of the selected isolates is no shorter than their parental counterparts, i.e. patency in donor animals used for propagation of isolates is similar irrespective of resistance status.
Although the work highlighted here would suggest that changes in life history traits may occur in response to anthelmintic selection, it must also be acknowledged that other factors may play roles. Phenotypic changes between the parental and/or selected isolates could (a) have occurred due to chance linkage events (Gilleard, 2006; Gilleard and Beech, 2007), with the life history trait being passed down to the progeny through the selection process, (b) be confounded by trial to trial effects and/or (c) through the passaging/culturing of the isolates for generations through lambs under laboratory conditions.
In conclusion, the study has established that MPTL resistance can be artificially selected in T. circumcincta in as short a time frame as nine generations. Selection for resistance impacted on the life history traits of the isolates, but the findings re-iterate the points stated earlier that it is difficult to predict what may occur to a parasite population following anthelmintic treatment.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgements
We gratefully acknowledge funding from The Scottish Government's Rural and Environment Science and Analytical Services Division (RESAS). We are grateful to the Bioservices Division, Moredun Research Institute, for expert care and assistance with animals. We also thank the staff of Parasitology Department at Moredun Research Institute for their technical assistance with the controlled efficacy test, and Steve Paterson for his assistance in analysing initial life history data.
Footnotes
Note: Supplementary material associated with this article.
Appendix. Supplementary material
The following is the supplementary data to this article:
Tables S1–S3.
References
- Armour J., Bairden K., Preston J.M. Anthelmintic efficiency of ivermectin against naturally occurring gastrointestinal nematodes of sheep. Vet. Rec. 1982;111:80–81. doi: 10.1136/vr.111.4.80. [DOI] [PubMed] [Google Scholar]
- Arneberg P., Skorping A., Read A.F. Parasite abundance, body size, life histories, and the energetic equivalence rule. Am. Nat. 1998;151:497–513. doi: 10.1086/286136. [DOI] [PubMed] [Google Scholar]
- Bartley D.J., Jackson F., Jackson E., Sargison N. Characterisation of two triple resistant field isolates of Teladorsagia from Scottish lowland sheep farms. Vet. Parasitol. 2004;123:189–199. doi: 10.1016/j.vetpar.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Bartley D.J., Jackson E., Sargison N., Jackson F. Further characterisation of a triple resistant field isolate of Teladorsagia from a Scottish lowland sheep farm. Vet. Parasitol. 2005;134:261–266. doi: 10.1016/j.vetpar.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Bartley D.J., McArthur C.L., Devin L.M., Sutra J.F., Morrison A.A., Lespine A. Characterisation of macrocyclic lactone resistance in two field-derived isolates of Cooperia oncophora. Vet. Parasitol. 2012;190:454–460. doi: 10.1016/j.vetpar.2012.07.022. [DOI] [PubMed] [Google Scholar]
- Beech R.N., Prichard R.K., Scott M.E. Genetic variability of the beta-tubulin genes in benzimidazole-susceptible and -resistant strains of Haemonchus contortus. Genetics. 1994;138:103–110. doi: 10.1093/genetics/138.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the False Discovery Rate – a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B (Methodol.) 1995;57:289–300. [Google Scholar]
- Besier R.B., Love S.C.J. Anthelmintic resistance in sheep nematodes in Australia: the need for new approaches. Aust. J. Exp. Agric. 2003;43:1383–1391. [Google Scholar]
- Blackhall W.J., Liu H.Y., Xu M., Prichard R.K., Beech R.N. Selection at a P-glycoprotein gene in ivermectin- and moxidectin-selected strains of Haemonchus contortus. Mol. Biochem. Parasitol. 1998;95:193–201. doi: 10.1016/s0166-6851(98)00087-5. [DOI] [PubMed] [Google Scholar]
- Blackhall W.J., Pouliot J.F., Prichard R.K., Beech R.N. Haemonchus contortus: selection at a glutamate-gated chloride channel gene in ivermectin- and moxidectin-selected strains. Exp. Parasitol. 1998;90:42–48. doi: 10.1006/expr.1998.4316. [DOI] [PubMed] [Google Scholar]
- Blackhall W.J., Prichard R.K., Beech R.N. Selection at a gamma-aminobutyric acid receptor gene in Haemonchus contortus resistant to avermectins/milbemycins. Mol. Biochem. Parasitol. 2003;131:137–145. doi: 10.1016/s0166-6851(03)00201-9. [DOI] [PubMed] [Google Scholar]
- Cawthorne R.J., Whitehead J.D. Isolation of benzimidazole resistant strains of Ostertagia circumcincta from British sheep. Vet. Rec. 1983;112:274–277. doi: 10.1136/vr.112.12.274. [DOI] [PubMed] [Google Scholar]
- Chehresa A. 1996. Benzimidazole-resistance and associated changes in life history traits of Heligosmoides polygyrus (Nematoda) in mice. PhD Thesis, McGill University, Montreal. [Google Scholar]
- Christie M., Jackson F. Specific identification of strongyle eggs in small samples of sheep faeces. Res. Vet. Sci. 1982;32:113–117. [PubMed] [Google Scholar]
- Coles G.C., Bauer C., Borgsteede F.H.M., Geerts S., Klei T.R., Taylor M.A. World-Association-for-the-Advancement-of-Veterinary- Parasitology (WAAVP) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 1992;44:35–44. doi: 10.1016/0304-4017(92)90141-u. [DOI] [PubMed] [Google Scholar]
- Coles G.C., Rhodes A.C., Wolstenholme A.J. Rapid selection for ivermectin resistance in Haemonchus contortus. Vet. Parasitol. 2005;129:345–347. doi: 10.1016/j.vetpar.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Colglazier M.L., Kates K.C., Enzie F.D. Cambendazole-resistant Haemonchus contortus strain in sheep: further experimental development. J. Parasitol. 1974;60:289–292. [PubMed] [Google Scholar]
- Colglazier M.L., Kates K.C., Enzie F.D. Cambendazole-resistant Haemonchus contortus strain in sheep: further experimental development. J. Parasitol. 1974;60:289–292. [PubMed] [Google Scholar]
- De Graef J., Demeler J., Skuce P., Mitreva M., von Samson-Himmelstjerna G., Vercruysse J. Gene expression analysis of ABC transporters in a resistant Cooperia oncophora isolate following in vivo and in vitro exposure to macrocyclic lactones. Parasitology. 2013;140:499–508. doi: 10.1017/S0031182012001849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll M., Dean E., Reilly E., Bergholz E., Chalfie M. Genetic and molecular analysis of a Caenorhabditis elegans beta-tubulin that conveys benzimidazole sensitivity. J. Cell Biol. 1989;109:2993–3003. doi: 10.1083/jcb.109.6.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echevarria F.A., Armour J., Bairden K., Duncan J.L. Laboratory selection for ivermectin resistance in Haemonchus contortus. Vet. Parasitol. 1993;49:265–270. doi: 10.1016/0304-4017(93)90125-7. [DOI] [PubMed] [Google Scholar]
- Echevarria F.A., Armour J., Borba M.F., Duncan J.L. Survival and development of ivermectin-resistant or susceptible strains of Haemonchus contortus under field and laboratory conditions. Res. Vet. Sci. 1993;54:133–139. doi: 10.1016/0034-5288(93)90048-k. [DOI] [PubMed] [Google Scholar]
- Egerton J.R., Suhayda D., Eary C.H. Laboratory selection of Haemonchus contortus for resistance to ivermectin. J. Parasitol. 1988;74:614–617. [PubMed] [Google Scholar]
- Elard L., Sauve C., Humbert J.F. Fitness of benzimidazole-resistant and -susceptible worms of Teladorsagia circumcincta, a nematode parasite of small ruminants. Parasitology. 1998;117:571–578. doi: 10.1017/s0031182098003436. [DOI] [PubMed] [Google Scholar]
- Gilleard J.S. Understanding anthelmintic resistance: the need for genomics and genetics. Int. J. Parasitol. 2006;36(12):1227–1239. doi: 10.1016/j.ijpara.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Gilleard J.S., Beech R.N. Population genetics of anthelmintic resistance in parasitic nematodes. Parasitology. 2007;134:1133–1147. doi: 10.1017/S0031182007000066. [DOI] [PubMed] [Google Scholar]
- Giordano D.J., Tritschler J.P., Coles G.C. Selection of ivermectin-resistant Trichostrongylus colubriformis in lambs. Vet. Parasitol. 1988;30:139–148. doi: 10.1016/0304-4017(88)90161-6. [DOI] [PubMed] [Google Scholar]
- Hall C.A., Kelly J.D., Campbell N.J., Whitlock H.V., Martin I.C. The dose response of several benzimidazole anthelmintics against resistant strains of Haemonchus contortus and Trichostrongylus colubriformis selected with thiabendazole. Res. Vet. Sci. 1978;25:364–367. [PubMed] [Google Scholar]
- Hall C.A., Kelly J.D., Whitlock H.V., Martin I.C., McDonell P.A., Gunawan M. Five generations of selection with benzimidazole and non-benzimidazole anthelmintics against benzimidazole resistant strains of Haemonchus and Ostertagia spp in sheep. Res. Vet. Sci. 1981;30:138–142. [PubMed] [Google Scholar]
- Hunt P.W., Knox M.R., Le Jambre L.F., McNally J., Anderson L.J. Genetic and phenotypic differences between isolates of Haemonchus contortus in Australia. Int. J. Parasitol. 2008;38:885–900. doi: 10.1016/j.ijpara.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Hunt P.W., Kotze A.C., Knox M.R., Anderson L.J., McNally J., Le Jambre L.F. The use of DNA markers to map anthelmintic resistance loci in an intraspecific cross of Haemonchus contortus. Parasitology. 2010;137:705–717. doi: 10.1017/S0031182009991521. [DOI] [PubMed] [Google Scholar]
- Kaminsky R., Ducray P., Jung M., Clover R., Rufener L., Bouvier J. A new class of anthelmintics effective against drug-resistant nematodes. Nature. 2008;452:176–180. doi: 10.1038/nature06722. [DOI] [PubMed] [Google Scholar]
- Kaminsky R., Mosimann D., Sager H., Stein P., Hosking B. Determination of the effective dose rate for monepantel (AAD 1566) against adult gastro-intestinal nematodes in sheep. Int. J. Parasitol. 2009;39:443–446. doi: 10.1016/j.ijpara.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Kates K.C., Colglazier M.L., Enzie F.D. Experimental development of a cambendazole-resistant strain of Haemonchus contortus in sheep. J. Parasitol. 1973;59:169–174. [PubMed] [Google Scholar]
- Kelly J.D., Whitlock H.V., Thompson H.G., Hall C.A., Martin I.C., Le Jambre L.F. Physiological characteristics of free-living and parasitic stages of strains of Haemonchus contortus, susceptible or resistant to benzimidazole anthelmintics. Res. Vet. Sci. 1978;25:376–385. [PubMed] [Google Scholar]
- Laing R. 2010. The cytochrome P450 family in the parasitic nematode Haemonchus contortus. PhD Thesis, University of Glasgow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Jambre L.F., Southcott W.H., Dash K.M. Resistance of selected lines of Ostertagia circumcincta to thiabendazole, morantel tartrate and levamisole. Int. J. Parasitol. 1977;7:473–479. doi: 10.1016/0020-7519(77)90009-1. [DOI] [PubMed] [Google Scholar]
- Leathwick D., Miller C., McMurty L. 2013. Resistance to monepantel in two nematode species in goats. World Association for the Advancement of Veterinary Parasitology: The 24th International Conference of the World Association for the Advancement of Veterinary Parasitology. [Google Scholar]
- Leignel V., Cabaret J. Massive use of chemotherapy influences life traits of parasitic nematodes in domestic ruminants. Funct. Ecol. 2001;15:569–574. [Google Scholar]
- Lubega G.W., Klein R.D., Geary T.G., Prichard R.K. Haemonchus contortus: the role of two beta-tubulin gene subfamilies in the resistance to benzimidazole anthelmintics. Biochem. Pharmacol. 1994;47:1705–1715. doi: 10.1016/0006-2952(94)90551-7. [DOI] [PubMed] [Google Scholar]
- Lynch P.A., Grimm U., Read A.F. How will public and animal health interventions drive life-history evolution in parasitic nematodes? Parasitology. 2008;135:1599–1611. doi: 10.1017/S0031182008000309. [DOI] [PubMed] [Google Scholar]
- Maingi N., Scott M.E., Prichard R.K. Effect of selection pressure for thiabendazole resistance on fitness of Haemonchus contortus in sheep. Parasitology. 1990;100(Pt 2):327–335. doi: 10.1017/s0031182000061345. [DOI] [PubMed] [Google Scholar]
- Martin P.J., Anderson N., Jarrett R.G. Resistance to benzimidazole anthelmintics in field strains of Ostertagia and Nematodirus in sheep. Aust. Vet. J. 1985;62:38–43. doi: 10.1111/j.1751-0813.1985.tb14230.x. [DOI] [PubMed] [Google Scholar]
- McKellar Q.A., Bogan J.A., Horspool L., Reid K. Effect of Ivermectin on the reproductive potential of Cooperia curticei. Vet. Rec. 1988;122:444. doi: 10.1136/vr.122.18.444. [DOI] [PubMed] [Google Scholar]
- McKenna P.B. Anthelmintic treatment and the suppression of egg production in gastro-intestinal nematodes of sheep and cattle: fact or fallacy? N. Z. Vet. J. 1997;45:173–177. doi: 10.1080/00480169.1997.36021. [DOI] [PubMed] [Google Scholar]
- Medley G.F. Chemotherapy. In: Scott M.E., Smith G., editors. Parasitic and Infectious Diseases: Epidemiology and Ecology. Academic Press; San Diego: 1994. pp. 141–157. [Google Scholar]
- Ministry of Agriculture, Fisheries and Food . Her Majesty's Stationery Office; London: 1986. Manual of Veterinary Parasitological Laboratory Techniques. [Google Scholar]
- Molento M.B., Prichard R.K. Effect of multidrug resistance modulators on the activity of ivermectin and moxidectin against selected strains of Haemonchus contortus infective larvae. Pesqui. Vet. Brasil. 2001;21:117–121. [Google Scholar]
- Paiement J.P., Leger C., Ribeiro P., Prichard R.K. Haemonchus contortus: effects of glutamate, ivermectin, and moxidectin on inulin uptake activity in unselected and ivermectin-selected adults. Exp. Parasitol. 1999;92:193–198. doi: 10.1006/expr.1999.4413. [DOI] [PubMed] [Google Scholar]
- Papadopoulos E., Himonas C., Coles G.C. Drought and flock isolation may enhance the development of anthelmintic resistance in nematodes. Vet. Parasitol. 2001;97:253–259. doi: 10.1016/s0304-4017(01)00435-6. [DOI] [PubMed] [Google Scholar]
- Paterson S., Barber R. Experimental evolution of parasite life-history traits in Strongyloides ratti (Nematoda) Proc. Biol. Sci. 2007;274:1467–1474. doi: 10.1098/rspb.2006.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson D.M., Jackson F., Huntley J.F., Stevenson L.M., Jones D.G., Jackson E. Studies on caprine responsiveness to nematodiasis: segregation of male goats into responders and non-responders. Int. J. Parasitol. 1996;26:187–194. doi: 10.1016/0020-7519(95)00121-2. [DOI] [PubMed] [Google Scholar]
- Poulin R. The evolution of life history strategies in parasitic animals. Adv. Parasitol. 1996;37:107–134. doi: 10.1016/s0065-308x(08)60220-1. [DOI] [PubMed] [Google Scholar]
- Poulin R. Information about transmission opportunities triggers a life-history switch in a parasite. Evolution. 2003;57:2899–2903. doi: 10.1111/j.0014-3820.2003.tb01530.x. [DOI] [PubMed] [Google Scholar]
- Poulin R., Morand S. Parasite body size distributions: interpreting patterns of skewness. Int. J. Parasitol. 1997;27:959–964. doi: 10.1016/s0020-7519(97)00055-6. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2014. R: A Language and Environment for Statistical Computing.http://www.R-project.org/ accessed 18.05.15. [Google Scholar]
- Ranjan S., Wang G.T., Hirschlein C., Simkins K.L. Selection for resistance to macrocyclic lactones by Haemonchus contortus in sheep. Vet. Parasitol. 2002;103(1):109–117. doi: 10.1016/s0304-4017(01)00551-9. [DOI] [PubMed] [Google Scholar]
- Redman E., Sargison N., Whitelaw F., Jackson F., Morrison A., Bartley D.J. Introgression of ivermectin resistance genes into a susceptible Haemonchus contortus strain by multiple backcrossing. PLoS Pathog. 2012;8:e1002534. doi: 10.1371/journal.ppat.1002534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer S.P., Birzin E.T., Eary C.H., Schaeffer J.M., Shoop W.L. Ivermectin binding sites in sensitive and resistant Haemonchus contortus. J. Parasitol. 1994;80:493–497. [PubMed] [Google Scholar]
- Sargison N., Scott P., Jackson F. Multiple anthelmintic resistance in sheep. Vet. Rec. 2001;149:778–779. [PubMed] [Google Scholar]
- Sargison N.D., Jackson F., Bartley D.J., Moir A.C.P. Failure of moxidectin to control benzimidazole-, levamisole- and ivermectin-resistant Teladorsagia circumcincta in a sheep flock. Vet. Rec. 2005;156:105–109. doi: 10.1136/vr.156.4.105. [DOI] [PubMed] [Google Scholar]
- Scott E.W., Baxter P., Armour J. Fecundity of anthelmintic resistant adult Haemonchus contortus after exposure to ivermectin or benzimidazoles in vivo. Res. Vet. Sci. 1991;50:247–249. doi: 10.1016/0034-5288(91)90117-7. [DOI] [PubMed] [Google Scholar]
- Scott I., Pomroy W.E., Kenyon P.R., Smith G., Adlington B., Moss A. Lack of efficacy of monepantel against Teladorsagia circumcincta and Trichostrongylus colubriformis. Vet. Parasitol. 2013;198:166–171. doi: 10.1016/j.vetpar.2013.07.037. [DOI] [PubMed] [Google Scholar]
- Shoop W.L., Egerton J.R., Eary C.H., Suhayda D. Laboratory selection of a benzimidazole-resistant isolate of Trichostrongylus colubriformis for ivermectin resistance. J. Parasitol. 1990;76:186–189. [PubMed] [Google Scholar]
- Skorping A., Read A.F. Drugs and parasites: global experiments in life history evolution? Ecol. Lett. 1998;1:10–12. [Google Scholar]
- Stear M.J., Bairden K., McKeller Q.A., Scott I., Strain S., Bishop S.C. The relationship between the number and size of nematodes in the abomasum and the concentration of pepsinogen in ovine plasma. Res. Vet. Sci. 1999;67:89–92. doi: 10.1053/rvsc.1998.0301. [DOI] [PubMed] [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford: 1992. Evolution of Life Histories. [Google Scholar]
- Suter R.J., Besier R.B., Perkins N.R., Robertson I.D., Chapman H.M. Sheep-farm risk factors for ivermectin resistance in Ostertagia circumcincta in Western Australia. Prev. Vet. Med. 2004;63:257–269. doi: 10.1016/j.prevetmed.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Sutherland I.A., Moen I.C., Leathwick D.M. Increased burdens of drug-resistant nematodes due to anthelmintic treatment. Parasitology. 2002;125:375–381. doi: 10.1017/s0031182002002184. [DOI] [PubMed] [Google Scholar]
- Thomas R.J., Reid J.F. Efficacy of oxfendazole against Nematodirus battus and inhibited stages of sheep nematodes. Res. Vet. Sci. 1980;28:134–136. [PubMed] [Google Scholar]
- Wilson D., Sargison N. Anthelmintic resistance in Teladorsagia circumcincta in sheep in the UK. Vet. Rec. 2007;161:535–536. doi: 10.1136/vr.161.15.535-a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3.


