Highlights
-
•
Expression of AQP2 restores drug susceptibility in a resistant Trypanosoma brucei gambiense isolate.
-
•
The AQP2/3 chimera from the resistant isolate does not complement AQP2 deletion.
-
•
Hence AQP2/3 chimerization accompanied by loss of AQP2 is the cause of drug resistance.
Keywords: Human African trypanosomiasis, Sleeping sickness, Trypanosoma brucei gambiense, Drug resistance, Melarsoprol, Pentamidine, Aquaporin, Reverse genetics
Graphical Abstract
Abstract
Aquaglyceroporin-2 is a known determinant of melarsoprol–pentamidine cross-resistance in Trypanosoma brucei brucei laboratory strains. Recently, chimerization at the AQP2–AQP3 tandem locus was described from melarsoprol–pentamidine cross-resistant Trypanosoma brucei gambiense isolates from sleeping sickness patients in the Democratic Republic of the Congo. Here, we demonstrate that reintroduction of wild-type AQP2 into one of these isolates fully restores drug susceptibility while expression of the chimeric AQP2/3 gene in aqp2–aqp3 null T. b. brucei does not. This proves that AQP2–AQP3 chimerization is the cause of melarsoprol–pentamidine cross-resistance in the T. b. gambiense isolates.
1. Introduction
Trypanosoma brucei gambiense is the causative agent of West-African sleeping sickness and responsible for 98% of today's cases of human African trypanosomiasis (HAT) (Brun et al., 2010). HAT is a fatal disease whose treatment exclusively relies on chemotherapy. Only five drugs are available: suramin and pentamidine for the first, haemolymphatic stage of the disease, melarsoprol and nifurtimox/eflornithine combination therapy for the second stage, when the parasites have infested the central nervous system. These drugs cause severe side effects and are difficult to administer (Brun et al., 2010). New drug candidates are in clinical development (Mäser et al., 2012), but until they are available for treatment, the current drugs must be used sustainably. Therefore understanding the molecular mechanism of drug resistance is a prerequisite. Drug resistance studies with Trypanosoma brucei brucei lab strains have identified loss of drug uptake as the major mechanism of drug resistance in trypanosomes. This is due to mutations in the transporters responsible for drug uptake. The clinical drugs melarsoprol and pentamidine share two common transporter systems, the adenosine transporter 1 (TbAT1, also called P2; Carter and Fairlamb, 1993; Carter et al., 1995; Mäser et al., 1999) and aquaglyceroporin 2 (Baker et al., 2012). Genetic knock-out of either transporter gene, but particularly of AQP2, led to melarsoprol–pentamidine cross-resistance (MPXR) (Matovu et al., 2003; Baker et al., 2012, 2013).
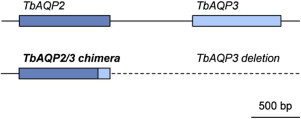
Drug resistance of T. b. gambiense in the field has been controversial. The occurrence of mutant TbAT1 alleles correlated to some extent with melarsoprol treatment failures (Matovu et al., 2001; Maina et al., 2007; Kazibwe et al., 2009), but no unambiguous genetic marker for resistance has been established so far. Recently, mutations at the AQP2–AQP3 (Tb927.10.14170/Tb927.10.14160) tandem locus were found in T. b. gambiense isolates from the Democratic Republic of the Congo (Graf et al., 2013). In particular, a set of 41 isolates from Mbuji-Mayi, a HAT focus of exceptionally high melarsoprol treatment failure rates (Pyana et al., 2014), all carried a chimeric aquaglyceroporin, presumably formed by homologous recombination between AQP2 and AQP3; a putative single-strand annealing mechanism accompanied by deletion of segments of AQP2 and AQP3 (Graf et al., 2013). The AQP2/3(814) chimera, the first 813 b derived from AQP2 and the last 126 b from AQP3, was in-frame, transcribed and homozygous. A second AQP2/3 chimeric gene, AQP2/3(880) with just the last 60 bp derived from AQP3, had been described in the T. b. gambiense isolates from Mbuji-Mayi (Pyana et al., 2014). In the present study we did not detect the AQP2/3(880) gene either with direct sequencing of PCR products or after cloning of the PCR products into expression vectors. The isolates carrying the chimeric gene exhibited a markedly decreased melarsoprol sensitivity in vivo (Pyana et al., 2014). Those that were tested in vitro were cross-resistant to melarsoprol and pentamidine; to our knowledge, the first example of MPXR from clinical T. b. gambiense isolates (Graf et al., 2013).
Thus, the important question remained: Is the MPXR phenotype of the T. b. gambiense isolates from Mbuji-Mayi caused by the observed chimerization at the AQP2–AQP3 locus? And if so, is it the presence of the AQP2/3(814) chimera or the absence of wild-type AQP2 that causes drug resistance? Here, we answer these questions by (i) re-introducing wild-type AQP2 into one of the mutant T. b. gambiense isolates and (ii) expressing the chimeric AQP2/3(814) gene from T. b. gambiense in T. b. brucei.
2. Materials and methods
2.1. Cell lines, cell culture and in vitro drug sensitivity assay
T. b. brucei 2T1 cells (Alsford et al., 2005) and 2T1 aqp2–aqp3 double knock-out cells (Alsford et al., 2012) were maintained in HMI-11 medium. Puromycin (0.2 µg/ml) and phleomycin (0.5 µg/ml) were added for 2T1 cells. For 2T1 aqp2–aqp3 double knock-out cells blasticidin (10 µg/ml) and G418 (2 µg/ml) were added in addition. Hygromycin (2.5 µg/ml), instead of puromycin, was added after transfection with chimeric AQP2/3(814). T. b. gambiense 40AT (MHOM/CD/INRB/2006/07; Pyana et al., 2011) were cultured in HMI-9 medium with 15% FCS and 5% human serum, plus blasticidin (5 µg/ml) after transfection. In vitro drug sensitivities were determined as described (Graf et al., 2013). For the inducible cells, 1 µg/ml tetracycline (tet) was added 24 h prior to the assay.
2.2. Plasmids and transfection
The chimeric AQP2/3(814) gene (GenBank accession KF564935) was amplified by PCR with primers AQP_HindIII_F (ccgcaagcttatgcagagccaaccagac) and AQP_BamH1_R (ccgcggatccttagtgtggcacaaaatatt), or AQP_Xba1_F (ccgctctagaatgcagagccaaccagac) and AQP_BamH1_R, and cloned into the pRPa-series of tetracycline-inducible expression vectors (http://www.lifesci.dundee.ac.uk/groups/david-horn/resources). Vector inserts were checked for fidelity by Sanger sequencing (Microsynth). Bloodstream-form T. b. brucei were transfected as previously described (Baker et al., 2012). Clones were obtained by limiting dilution in standard HMI-11 medium plus antibiotics (see above). The AQP2 gene was amplified from wild-type T. brucei 427 parasites and the AQP2/3(569–841) gene from the derived, pentamidine-resistant, strain B48, using proof-reading polymerase and oligonucleotides which added an ApaI site to the 5′ end and a BamHI site to the 3′ end of the genes. The genes were ligated into pGEM-T Easy vector, and digested out using the added restriction sites. They were then ligated into similarly digested pHD1336 vector, to give plasmids pHDK21 (AQP2) and pHDK34 (AQP2/3(569–841)). Both plasmids were checked by Sanger Sequencing (Eurofins MWG Operon). Bloodstream-form T. b. gambiense were transfected with pHDK21 and pHDK34 as follows: 4 × 107 cells were resuspended in 100 µl Tb-BSF nucleofection buffer (Schumann Burkard et al., 2011) (90 mM NaHPO3, 5 mM KCl, 0.15 mM CaCl2, 50 mM HEPES, pH 7.3) including 10 µg linearized plasmid DNA and placed in the nucleofection cuvette in the Amaxa Nucleofector (Lonza). Cells were electroporated using the program Z-001 and immediately transferred into 25 ml of pre-warmed HMI-9 medium containing 15% FCS, 5% human serum, and 20% sterile-filtered conditioned medium. Stable clones were obtained by limiting dilution and blasticidin selection (5 µg/ml). Correct integration was assessed by PCR on genomic DNA with primers AQP2_int_F (gtattggtgtggctgtcacg), AQP3_int_R (cccgttgagtaaccgatgtt), pAQP_F (aacacaccggtaccgtcatt) and pAQP_R (cttctcttgtgcgctgtacg).
Western blots of GFP-AQP2/3(814) in 2T1 aqp2–aqp3 null cells were performed as described (Baker et al., 2012). Western blots with GFP-AQP2/3(814) in 2T1 wild-type cells were performed as follows: cells were lysed in NUPAGE® LDS sample buffer (Life Technologies), samples separated on precast 4–12% Bis-Tris Gradient Gels (NuPAGE Novex®, Life Technologies) and transferred to nitrocellulose membranes using the iBlot dry-blotting system (Novex®, Life Technologies) according to the manufacturer's recommendations. Western blots were developed with the ECL Western Blotting Substrate (Pierce) using a ChemiDoc™ MP Gel Imaging System (Biorad). Primary Antibody: rabbit anti-GFP (Abcam, Ab290); secondary antibody: goat anti-rabbit (SouthernBiotech, 4050-05).
3. Results and discussion
3.1. Expression of wild-type AQP2 re-sensitizes drug-resistant T. b. gambiense
To test whether the lack of bona fide AQP2 activity contributes to drug resistance in the isolates from Mbuji-Mayi, we introduced a ‘wild-type’ copy of AQP2 into T. b. gambiense 40AT, isolated from a melarsoprol-relapse patient after treatment (Pyana et al., 2011). The gene was integrated into the highly transcribed rRNA-spacer locus. This shifted the IC50 of pentamidine from 108 nM to 2 nM and the IC50 of melarsoprol from 47 nM to 10 nM (Fig. 1), a level similar to the fully susceptible T. b. gambiense reference isolate STIB930 (which had an IC50 of 2 nM for pentamidine and 10 nM for melarsoprol; Graf et al., 2013). No shifts were observed with diminazene aceturate, a diamidine that is not an AQP2 substrate (Munday et al., 2014), or with phenylarsine oxide (data not shown), an arsenical that diffuses through the plasma membrane. The same results were obtained with three additional clones. As a negative control, we transfected the 40AT cells with a non-functional AQP2 mutant from the MPXR T. b. brucei clone B48 (Munday et al., 2014). As expected, this did not affect susceptibility to melarsoprol or pentamidine (Fig. 1). These results demonstrate that AQP2 is key to drug susceptibility in the MPXR T. b. gambiense isolate.
Fig. 1.
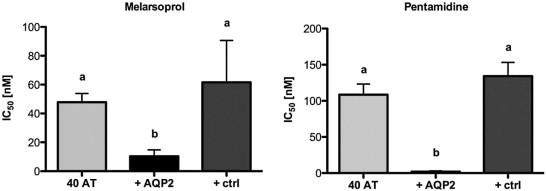
Introduction of AQP2 into mutant T. b. gambiense. In vitro drug sensitivity of bloodstream-form T. b. gambiense 40AT (grey) transfected with AQP2 (black) or dysfunctional AQP2 (ctrl, dark grey). Error bars are standard errors of the mean. n = 6 independent experiments, each in duplicate. Small letters indicate significance groups as determined by one-way ANOVA and Tukey's post test using GraphPad Prism 5.0.
3.2. Expression of the chimeric AQP2/3(814) in an aqp2–aqp3 null background
To test whether the chimeric AQP2/3(814) can complement AQP2 function with regard to drug uptake, we stably integrated the chimeric AQP2/3(814) gene from T. b. gambiense 40AT, either untagged or GFP-tagged, under the control of the tetracycline operator in a T. b. brucei host strain that expressed the tet repressor, and that carried a complete deletion of the AQP2–AQP3 locus (Alsford et al., 2011). Tetracycline-inducible (1 µg/ml) expression of the chimeric AQP2/3(814) protein was confirmed by immuno-fluorescence microscopy (data not shown) and by Western blotting with an anti-GFP antibody (Fig. 2C). Drug sensitivities were determined in vitro for melarsoprol and pentamidine. None of the transfected cell lines showed a significant difference in IC50 to pentamidine or melarsoprol when expression of AQP2/3(814) had been induced with tetracycline as compared to non-induced cells (Fig. 2A). This held true irrespective of the presence of the GFP tag. Thus no potential function in drug susceptibility could be attributed to the AQP2/3 chimera. Expression of ‘wild-type’ AQP2 using the same over-expression system (untagged and GFP-tagged) did not just reverse MPXR but actually hypersensitized the aqp2–aqp3 double null T. b. brucei to pentamidine and melarsoprol (Baker et al., 2012).
Fig. 2.
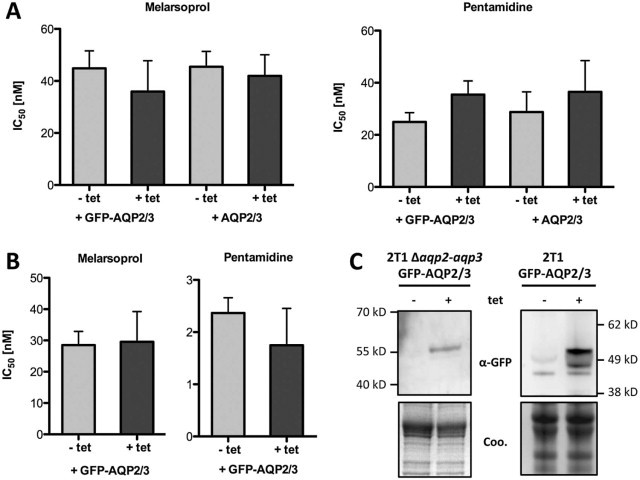
Expression of the AQP2/3(814) chimera in T. b. brucei. In vitro drug sensitivity of bloodstream-form T. b. brucei 2T1 aqp2–aqp3 double null mutants (A) and parental 2T1 cells (B) transfected with a tetracycline (tet) inducible AQP2/3(814) chimera. Dark bars, tet (1 µg/ml) was added 24 h prior to the drug assay. Error bars are standard error of the mean. n = 4–5 independent experiments, each in duplicate. (C) Western blot with anti-GFP antibody demonstrating inducible expression of GFP-tagged AQP2/3(814) (Coo, Coomassie stain). The GFP-AQP2/3(814) fusion proteins ran below their predicted molecular mass (approximately 60 kDa), which often applies for proteins with many transmembrane domains. The lower of the inducible bands in the blot on the right may represent unprocessed (e.g. unglyosylated) GFP-AQP2/3.
3.3. Expression of chimeric AQP2/3(814) in wild-type cells does not affect drug sensitivity
Aquaporins form homotetramers where each monomer constitutes a single pore. Work on human aquaporins involved in diabetes insipidus has revealed that the expression of a mutant aquaporin can give rise to dominant negative effects (Mulders et al., 1998). To test for negative interactions of AQP2/3(814) with ‘wild-type’ AQP2, the chimera was expressed in parental T. b. brucei 2T1 cells. The same tetracycline-inducible over-expression system was used. Again, no significant difference was observed regarding sensitivity to pentamidine and melarsoprol in tetracycline-induced versus uninduced cells (Fig. 2B). Hence, the AQP2/3(814) chimera does not interfere with endogenous AQP2 function in T. b. brucei bloodstream-form cells.
4. Conclusion
Previous work on the correlation of occurrence of the chimeric AQP2/3(814) gene in T. b. gambiense isolates from the DRC with in vitro drug sensitivity (Graf et al., 2013) suggested a functional link between the chimera and MPXR. However, proof of a causal relationship was lacking. The AQP2/3(814) chimeric protein consists mostly of AQP2 sequence, including the atypical second filter sequence (Baker et al., 2012). Overall, AQP2/3(814) of the T. b. gambiense from Mbuji-Mayi has only 9 amino acid differences with AQP2. Moreover, the different T. b. gambiense isolates that harboured the chimeric gene were probably of clonal origin (Pyana et al., 2015) and may therefore not count as independent samples for the correlation of AQP2/3(814) genotype to MPXR phenotype. Thus reverse genetic engineering of bloodstream-form trypanosomes was required to establish a direct link between chimerization at the AQP2–AQP3 locus in T. b. gambiense isolates and MPXR. This was only feasible because some of the isolates from Mbuji-Mayi had been adapted to axenic growth in vitro (Pyana et al., 2011).
The MPXR T. b. gambiense isolate 40AT was completely re-sensitized to melarsoprol and pentamidine when transfected with a wild-type copy of AQP2. This proves that the observed chimerization at the AQP2–AQP3 locus is indeed the genetic basis of MPXR. The AQP2/3(814) chimeric protein did not exhibit any role in conferring drug sensitivity when over-expressed in T. b. brucei, neither in a aqp2–aqp3 null background nor in the AQP2–AQP3 wild-type background. This further demonstrates that it is the absence of ‘wild-type’ AQP2, and not the presence of the AQP2/3(814) chimera, that causes the MPXR phenotype. Deletion-based gene-fusion at the AQP2–AQP3 locus by homologous recombination is likely facilitated by the high degree of sequence identity between AQP2 and AQP3. Taken together, our findings strongly indicate that chimerization at the AQP2–AQP3 locus causes melarsoprol–pentamidine cross-resistance in T. b. gambiense. This consequently increases the risk of treatment failures in problematic HAT foci such as Mbuji-Mayi of the Democratic Republic of the Congo. This is the first example where a genetic basis for drug-resistant sleeping sickness has been confirmed.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgements
We wish to thank Remo Schmidt and Christina Kunz-Renggli for their help in the lab. This work was supported by the Swiss National Science Foundation (grant 31003A_135746 to PM), from the UK Medical Research Council (MRC; grant 84733 to HdK), from the MRC and Department for International Development, UK under the MRC/DFID Concordat agreement (grant MR/K000500/1 to DH), and from the Wellcome Trust (grant 100320/Z/12/Z; Senior Investigator Award to DH).
References
- Alsford S., Kawahara T., Glover L., Horn D. Tagging a T. brucei RRNA locus improves stable transfection efficiency and circumvents inducible expression position effects. Mol. Biochem. Parasitol. 2005;144:142–148. doi: 10.1016/j.molbiopara.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsford S., Turner D.J., Obado S.O., Sanchez-Flores A., Glover L., Berriman M. High-throughput phenotyping using parallel sequencing of RNA interference targets in the African trypanosome. Genome Res. 2011;21:915–924. doi: 10.1101/gr.115089.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsford S., Eckert S., Baker N., Glover L., Sanchez-Flores A., Leung K.F. High-throughput decoding of antitrypanosomal drug efficacy and resistance. Nature. 2012;482:232–236. doi: 10.1038/nature10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N., Glover L., Munday J.C., Aguinaga Andres D., Barrett M.P., de Koning H.P. Aquaglyceroporin 2 controls susceptibility to melarsoprol and pentamidine in African trypanosomes. Proc. Natl. Acad. Sci. U.S.A. 2012;109:10996–11001. doi: 10.1073/pnas.1202885109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N., de Koning H.P., Mäser P., Horn D. Drug resistance in African trypanosomiasis: the melarsoprol and pentamidine story. Trends Parasitol. 2013;29:110–118. doi: 10.1016/j.pt.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun R., Blum J., Chappuis F., Burri C. Human African trypanosomiasis. Lancet. 2010;375:148–159. doi: 10.1016/S0140-6736(09)60829-1. [DOI] [PubMed] [Google Scholar]
- Carter N.S., Fairlamb A.H. Arsenical-resistant trypanosomes lack an unusual adenosine transporter. Nature. 1993;361:173–175. doi: 10.1038/361173a0. [DOI] [PubMed] [Google Scholar]
- Carter N.S., Berger B.J., Fairlamb A.H. Uptake of diamidine drugs by the P2 nucleoside transporter in melarsen-sensitive and -resistant Trypanosoma brucei brucei. J. Biol. Chem. 1995;270:28153–28157. doi: 10.1074/jbc.270.47.28153. [DOI] [PubMed] [Google Scholar]
- Graf F.E., Ludin P., Wenzler T., Kaiser M., Brun R., Pyana P.P. Aquaporin 2 mutations in Trypanosoma brucei gambiense field isolates correlate with decreased susceptibility to pentamidine and melarsoprol. PLoS Negl. Trop. Dis. 2013;7:e2475. doi: 10.1371/journal.pntd.0002475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazibwe A.J., Nerima B., de Koning H.P., Mäser P., Barrett M.P., Matovu E. Genotypic status of the TbAT1/P2 adenosine transporter of Trypanosoma brucei gambiense isolates from Northwestern Uganda following melarsoprol withdrawal. PLoS Negl. Trop. Dis. 2009;3:e523. doi: 10.1371/journal.pntd.0000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina N., Maina K.J., Mäser P., Brun R. Genotypic and phenotypic characterization of Trypanosoma brucei gambiense isolates from Ibba, South Sudan, an area of high melarsoprol treatment failure rate. Acta Trop. 2007;104:84–90. doi: 10.1016/j.actatropica.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Matovu E., Geiser F., Schneider V., Mäser P., Enyaru J.C.K., Kaminsky R. Genetic variants of the TbAT1 adenosine transporter from African trypanosomes in relapse infections following melarsoprol therapy. Mol. Biochem. Parasitol. 2001;117:73–81. doi: 10.1016/s0166-6851(01)00332-2. [DOI] [PubMed] [Google Scholar]
- Matovu E., Stewart M.L., Geiser F., Brun R., Mäser P., Wallace L.J. Mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot. Cell. 2003;2:1003–1008. doi: 10.1128/EC.2.5.1003-1008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäser P., Sütterlin C., Kralli A., Kaminsky R. A nucleoside transporter from Trypanosoma brucei involved in drug resistance. Science. 1999;285:242–244. doi: 10.1126/science.285.5425.242. [DOI] [PubMed] [Google Scholar]
- Mäser P., Wittlin S., Rottmann M., Wenzler T., Kaiser M., Brun R. Antiparasitic agents: new drugs on the horizon. Curr. Opin. Pharmacol. 2012;12:562–566. doi: 10.1016/j.coph.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Mulders S.M., Bichet D.G., Rijss J.P., Kamsteeg E.J., Arthus M.F., Lonergan M. An aquaporin-2 water channel mutant which causes autosomal dominant nephrogenic diabetes insipidus is retained in the Golgi complex. J. Clin. Invest. 1998;102:57–66. doi: 10.1172/JCI2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday J.C., Eze A.A., Baker N., Glover L., Clucas C., Aguinaga Andres D. Trypanosoma brucei aquaglyceroporin 2 is a high-affinity transporter for pentamidine and melaminophenyl arsenic drugs and the main genetic determinant of resistance to these drugs. J. Antimicrob. Chemother. 2014;69:651–663. doi: 10.1093/jac/dkt442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyana P., Van Reet N., Mumba Ngoyi D., Ngay Lukusa I., Karhemere Bin Shamamba S., Buscher P. Melarsoprol sensitivity profile of Trypanosoma brucei gambiense isolates from cured and relapsed sleeping sickness patients from the Democratic Republic of the Congo. PLoS Negl. Trop. Dis. 2014;8:e3212. doi: 10.1371/journal.pntd.0003212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyana P.P., Ngay Lukusa I., Mumba Ngoyi D., Van Reet N., Kaiser M., Karhemere Bin Shamamba S. Isolation of Trypanosoma brucei gambiense from cured and relapsed sleeping sickness patients and adaptation to laboratory mice. PLoS Negl. Trop. Dis. 2011;5:e1025. doi: 10.1371/journal.pntd.0001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyana P.P., Sere M., Kabore J., De Meeus T., MacLeod A., Bucheton B. Population genetics of Trypanosoma brucei gambiense in sleeping sickness patients with treatment failures in the focus of Mbuji-Mayi, Democratic Republic of the Congo. Infect. Genet. Evol. 2015;30:128–133. doi: 10.1016/j.meegid.2014.12.017. [DOI] [PubMed] [Google Scholar]
- Schumann Burkard G., Jutzi P., Roditi I. Genome-wide RNAi screens in bloodstream form trypanosomes identify drug transporters. Mol. Biochem. Parasitol. 2011;175:91–94. doi: 10.1016/j.molbiopara.2010.09.002. [DOI] [PubMed] [Google Scholar]


