Abstract
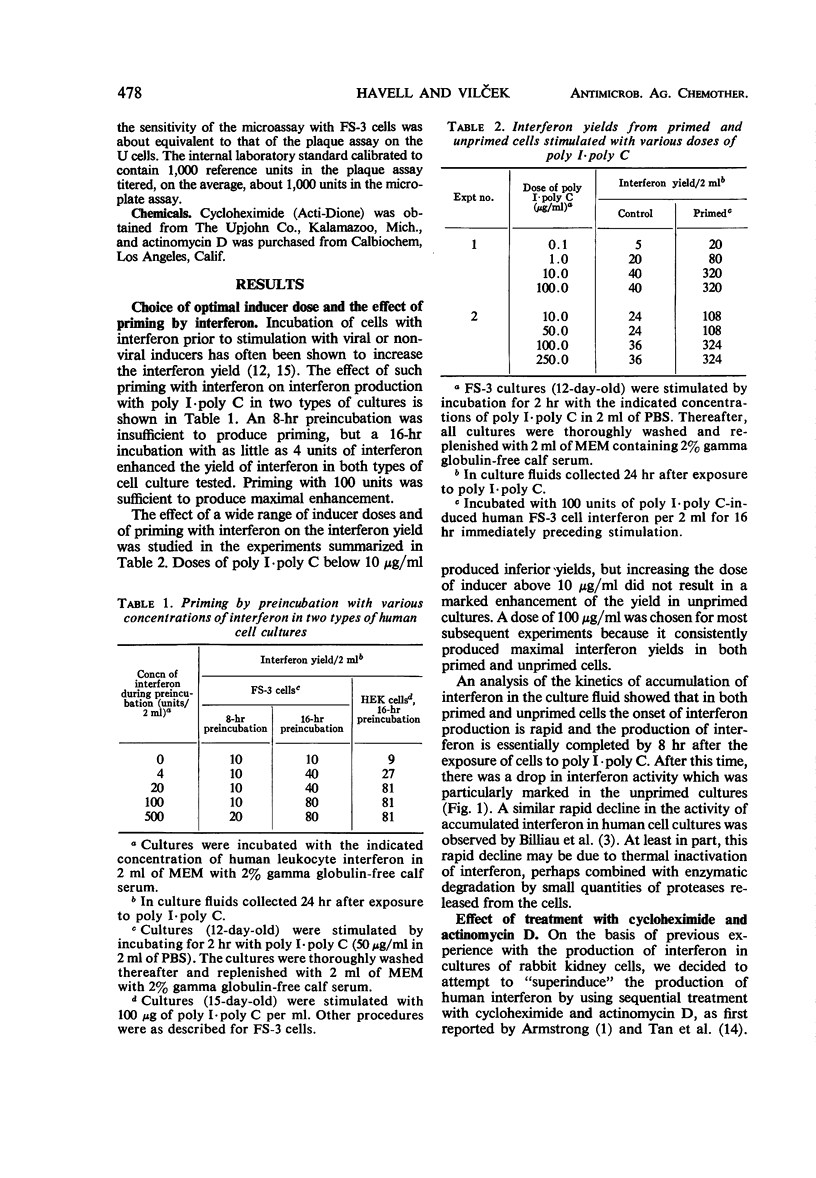
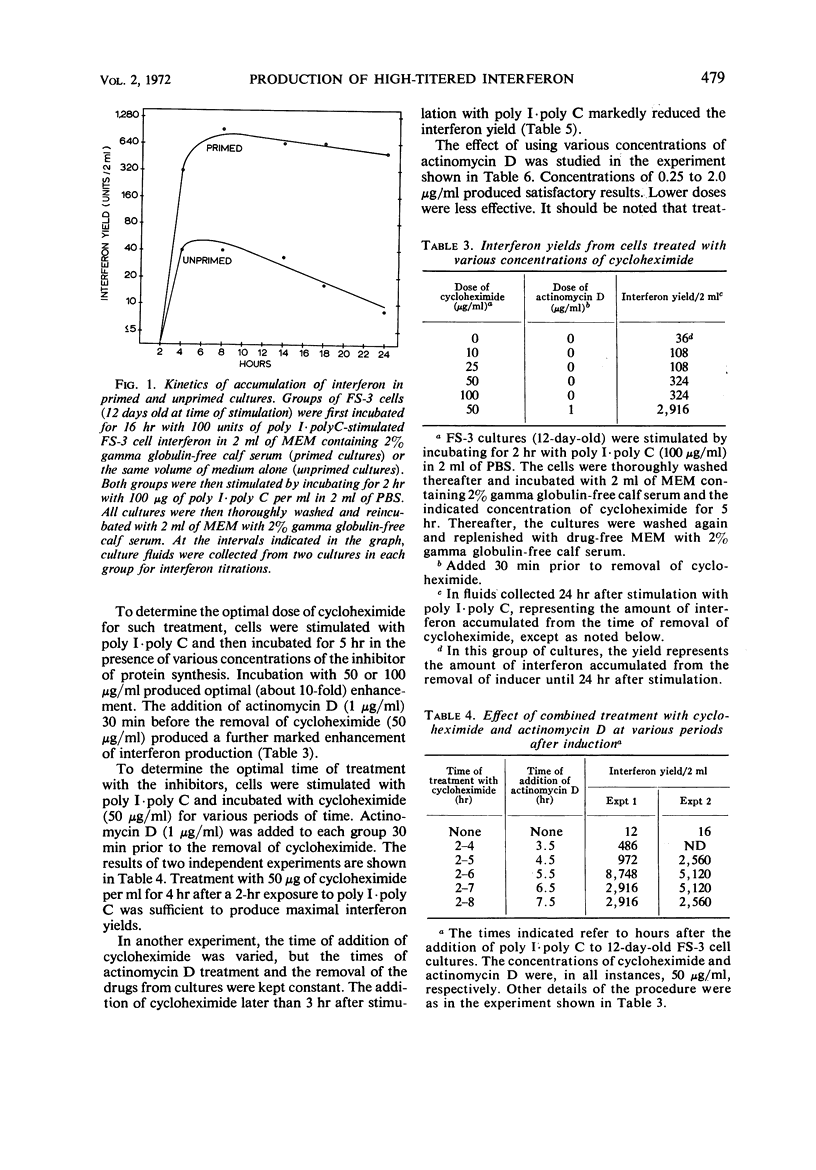
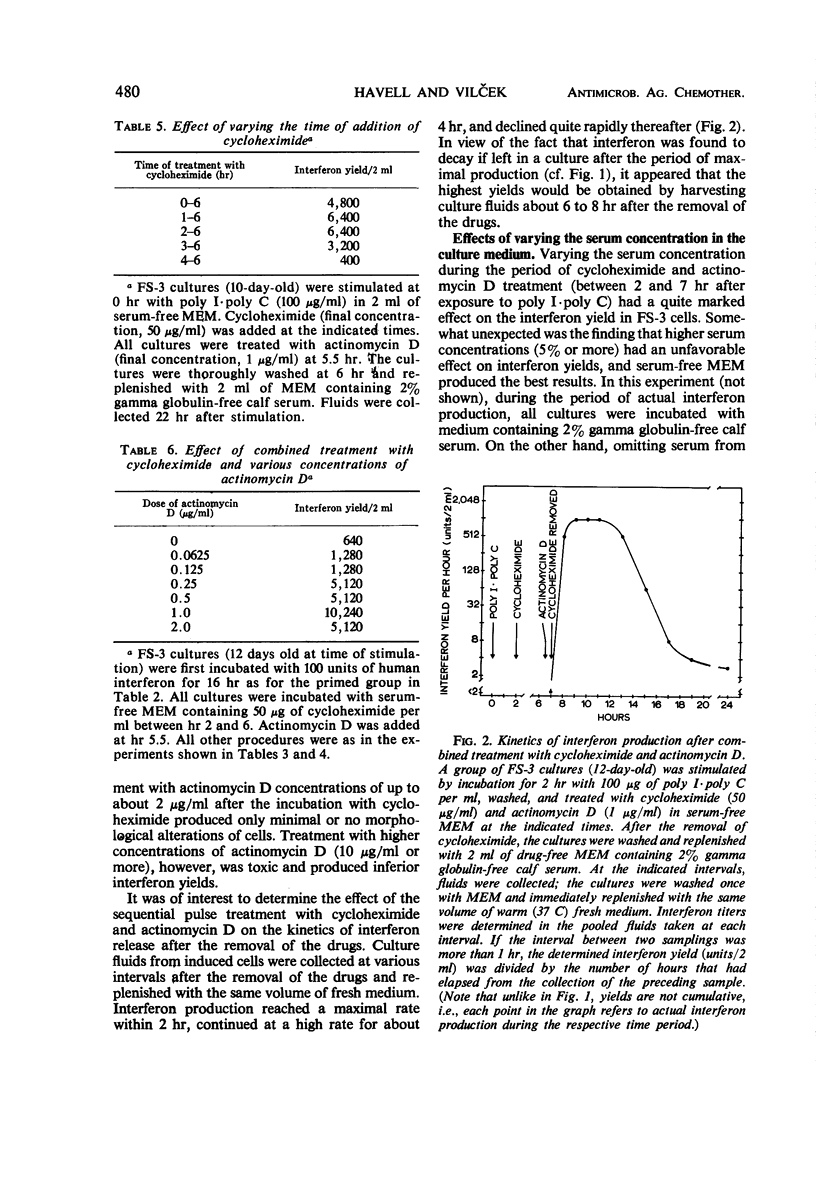
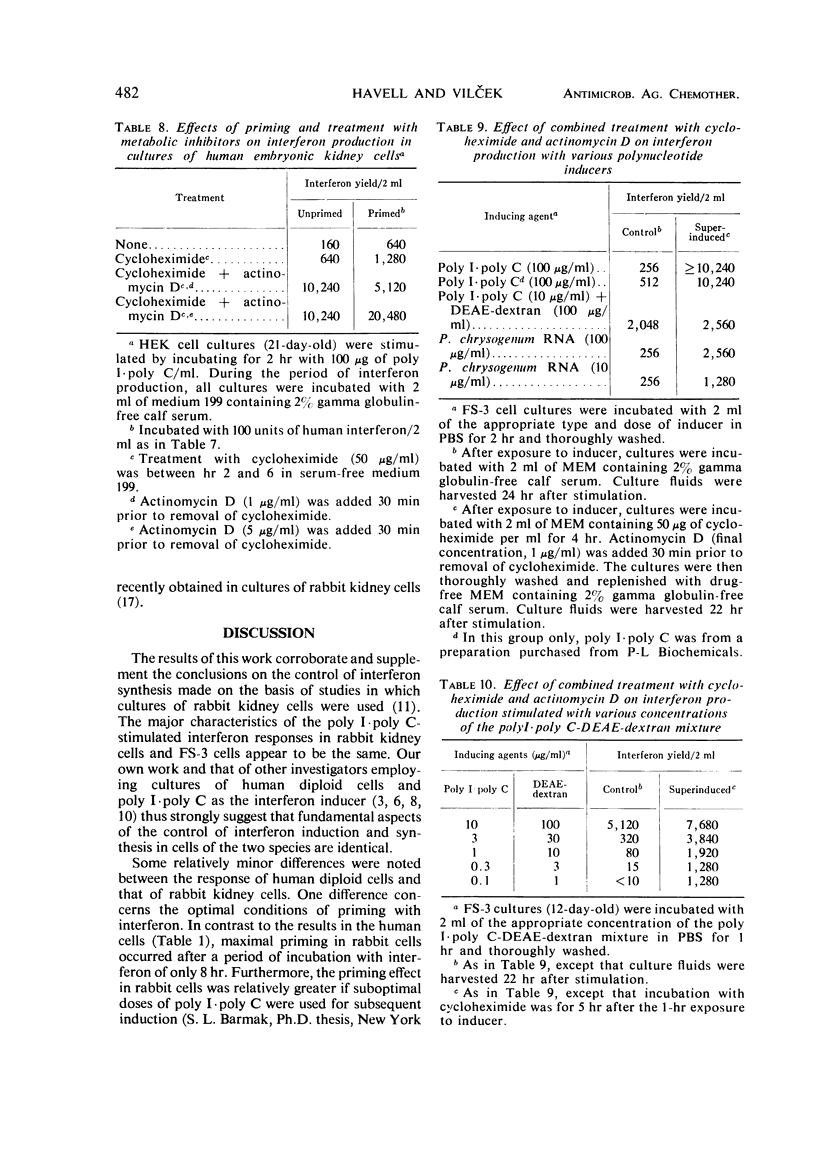
The effect of incubation with interferon prior to the stimulation of interferon production (priming) and of sequential treatment with cycloheximide and actinomycin D (superinduction) on the interferon yield from polyinosinic-polycytidylic acid (poly I·poly C)-induced diploid human foreskin cell cultures (FS-3 strain) was studied. Suitable priming with interferon produced, on the average, about an eightfold increase over the control yield, with a greater increase noted on some occasions when the control interferon yield was very low. Under the optimal conditions carefully defined in our experiments, superinduction produced about a 100-fold increase over the average control yield, resulting in interferon yields of about 10,000 reference units from cultures containing about 106 cells. Combined superinduction and priming did not produce yields markedly higher than obtainable by superinduction alone. Essentially similar results were obtained in cultures of human embryonic kidney cells and in FS-3 cells stimulated with other double-stranded polynucleotide inducers. However, stimulation of cells with certain concentrations of a mixture of diethylaminoethyl-dextran and poly I·poly C altered the interferon response; the yield was considerably higher than in cells stimulated with poly I·poly C alone, but it could not be markedly increased further by superinduction.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A. Semi-micro, dye-binding assay for rabbit interferon. Appl Microbiol. 1971 Apr;21(4):723–725. doi: 10.1128/am.21.4.723-725.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiau A., Joniau M., De Somer P. Production of crude human interferon with high specific activity. Proc Soc Exp Biol Med. 1972 Jun;140(2):485–491. doi: 10.3181/00379727-140-36486. [DOI] [PubMed] [Google Scholar]
- Falcoff E., Lévy H., Colin J., Chany C. Estimation du poids moléculaire de l'interféron produit par les globules blancs humains. C R Acad Sci Hebd Seances Acad Sci D. 1965 May 17;260(20):5405–5407. [PubMed] [Google Scholar]
- Finkelstein M. S., Bausek G. H., Merigan T. C. Interferon inducers in vitro: difference in sensitivity to inhbitiros of RNA and protein synthesis. Science. 1968 Aug 2;161(3840):465–468. doi: 10.1126/science.161.3840.465. [DOI] [PubMed] [Google Scholar]
- Ho M., Tan Y. H., Armstrong J. A. Accentuation of production of human interferon by metabolic inhibitors. Proc Soc Exp Biol Med. 1972 Jan;139(1):259–262. doi: 10.3181/00379727-139-36122. [DOI] [PubMed] [Google Scholar]
- Merigan T. C., Gregory D. F., Petralli J. K. Physical properties of human interferon prepared in vitro and in vivo. Virology. 1966 Aug;29(4):515–522. doi: 10.1016/0042-6822(66)90276-5. [DOI] [PubMed] [Google Scholar]
- Myers M. W., Friedman R. M. Potentiation of human interferon production by superinduction. J Natl Cancer Inst. 1971 Oct;47(4):757–764. [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Gosser L. B., Lockart R. Z., Jr Priming: a nonantiviral function of interferon. J Virol. 1971 Jun;7(6):792–801. doi: 10.1128/jvi.7.6.792-801.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strander H., Cantell K. Further studies on the production of interferon by human leukocyte in vitro. Ann Med Exp Biol Fenn. 1967;45(1):20–29. [PubMed] [Google Scholar]
- Tan Y. H., Armstrong J. A., Ke Y. H., Ho M. Regulation of cellular interferon production: enhancement by antimetabolites. Proc Natl Acad Sci U S A. 1970 Sep;67(1):464–471. doi: 10.1073/pnas.67.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovell D., Cantell K. Kinetics of interferon production in human leukocyte suspensions. J Gen Virol. 1971 Dec;13(3):485–489. doi: 10.1099/0022-1317-13-3-485. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Barmak S. L., Havell E. A. Control of interferon synthesis: effect of diethylaminoethyl-dextran on induction by polyinosinic-polycytidylic acid. J Virol. 1972 Oct;10(4):614–621. doi: 10.1128/jvi.10.4.614-621.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilcek J., Ng M. H. Post-transcriptional control of interferon synthesis. J Virol. 1971 May;7(5):588–594. doi: 10.1128/jvi.7.5.588-594.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilcek J., Rossman T. G., Varacalli F. Differential effects of actinomycin D and puromycin on the release of interferon induced by double stranded RNA. Nature. 1969 May 17;222(5194):682–683. doi: 10.1038/222682a0. [DOI] [PubMed] [Google Scholar]
- Wood H. A., Bozarth R. F. Properties of viruslike particles of Penicillium chrysogenum: one double-stranded RNA molecule per particle. Virology. 1972 Mar;47(3):604–609. doi: 10.1016/0042-6822(72)90549-1. [DOI] [PubMed] [Google Scholar]



