A few years ago, autophagy would require a detailed background introduction, but more and more, this fundamental cell biology mechanism of lysosome-driven degradation and recycling of cellular organelles, long-lived proteins, and lipids is becoming a common knowledge. Its main pathway, macroautophagy (herein referred to as autophagy), sequesters material destined for degradation into autophagosomes, which then fuse with lysosomes, forming the autolysosomes where cargo is degraded. The degradation products are recycled for the cell’s energy and biogenesis needs. Autophagosome formation is a complex process governed by sequential formation of protein complexes controlled by evolutionarily conserved ATG (autophagy-related) genes; ATG5, ATG7, and ATG8/LC3 are among the key ATG proteins. Autophagy is a major quality control mechanism and is critical for cell survival in starvation and other stress conditions.
Numerous reviews (eg, references1–4) discuss diverse roles of autophagy in health and disease, which have been studied more extensively in some disease states (neurodegenerative diseases, cancer) and organs (heart, and more recently, liver5) than in others. The essential function of autophagy as a homeostatic mechanism is demonstrated by the embryonic lethality of mice with general deletion of major Atg proteins (eg, Atg5 or Atg7) and pathologic changes in specific organs targeted by conditional autophagy-gene knockouts.2,6 Further, impaired autophagy is increasingly implicated in the pathogenesis of various diseases.1–3,5–8 Autophagy impairment can be caused by defects in autophagosome formation, their fusion with lysosomes, and lysosomal degradative function. Impaired autophagy often manifests itself in accumulation of large cytoplasmic vacuoles containing undegraded or partially degraded material. Accumulation of such vacuoles in acinar cells is a long-noted feature of both human and experimental pancreatitis; however, the investigation of the status and roles of autophagy in normal and diseased exocrine pancreas has started only recently (reviewed in references7,8). In particular, it was shown9 that autophagic flux is impaired in various experimental models of acute pancreatitis, mediating not only vacuolization but also increased intra-acinar trypsin activity, a signature response of pancreatitis. However, genetic approaches to examine the role of autophagy in pancreatitis have not been actively pursued10,11; in fact, the study by Diakopolous et al12 in this issue of Gastroenterology represents the first detailed analysis of the effects of genetic ablation of a key autophagy mediator in pancreas.
The authors crossed “floxed” Atg5F/F mice and Ptf1a-Cre mice expressing Cre recombinase under control of the transcription factor Ptf1a that directs pancreas organogenesis, 13 to generate mice (termed A5) in which Atg5 is specifically deleted in acinar and islet cells at a distinct developmental age. Loss of Atg5 triggered the development of chronic pancreatitis (CP), with fibrosis, macrophage-type inflammation, acinar-to-ductal metaplasia, apoptosis, and pancreatic atrophy that progressed from 4 to 36 weeks of age. Such progression is also characteristic of human CP. Serum lipase was increased at the early (4 weeks) but not late (18 weeks) time point, reminiscent of human CP; intrapancreatic trypsin and cathepsin B activities were also increased at 4 weeks. A block in normal autophagy was manifest by a decrease in the autophagosomal marker LC3-II, a dramatic increase in p62/SQSTM1 (sequestosome 1), a protein degraded through autophagy, and the appearance of large (“improperly formed”12) autophagic vacuoles containing undegraded material. Dilated endoplasmic reticulum (ER) was also prominent in acinar cells early on.
Surprisingly, the development of CP, with all pathologic responses, was much less pronounced in female than in male A5 mice. Because the early pancreas damage (at 4 weeks) was morphologically similar between males and females, the authors postulate that pancreas regeneration in A5 mice is sex dependent, but another possibility is that female pancreas is more resistant to the effects of impaired autophagy. Whatever the mechanism, these findings present a mechanistic correlate to the known greater prevalence of CP in male human population.14
The Ptf1a-Cre–driven recombination occurs during pancreas development, and the defective autophagy impacted both acinar and islet cells in A5 mice. Similarly, both exocrine and endocrine pancreas destruction was found11 in Pdx-Cre;Atg7 and Pdx-Cre;Atg5 conditional knockouts (in which Cre expression is driven by Pdx1, another pancreatic progenitor transcription factor13), although the tissue damage was not studied in detail. These findings prompted a question about the primary role of the exocrine vs. endocrine compartment in pancreas injury. Diakopoulos et al12 present several lines of evidence indicating that β-cell dysfunction (strictly speaking, diabetes) is not the primary driver of CP in A5 mice. Thus, although insulin treatment of male A5 mice reduced serum glucose levels, it did not rescue acinar cells from damage. Furthermore, inducing loss of insulin by streptozotocin treatment did not cause pathologic alterations in acinar tissue of Ela-CreER;Atg5 mice (expressing inducible CreER under ElastaseI promoter) in which Atg5 is specifically deleted in adult acinar cells.
The focus of the Diakopoulos et al study12 is on cellular and molecular pathways perturbed by impaired autophagy in A5 pancreas. Transcriptomic analysis revealed marked enrichment of gene sets associated with pathways related to inflammation (ie, innate and adaptive immunity, cytokines), fibrosis, cell stress, and apoptosis. Autophagy is emerging as a major regulator of cellular metabolism,15 and the metabolomic analysis showed disturbances of multiple pathways in A5 pancreas. Mitochondrial dysfunction was manifest by abnormal ultrastructure, lower activities of respiratory complexes, and dramatic decreases in CREB, a key transcription factor driving mitochondrial biogenesis. Based on these analyses, the authors stress the importance of reactive oxygen species (ROS) accumulation and reduced availability of anabolic substrates caused by glutamate deficiency (although limited information is presented on the latter). They further focus on the p62/Nrf2/Nqo1/p53 pathway. Nrf2, a key transcription factor regulating antioxidant gene expression, is activated by ROS and p62; Nqo1, a target of Nrf2, stabilizes p53 protein, a major driver of apoptosis. The expression of Nrf2- and p53-regulated genes was enhanced in A5 pancreas. To elucidate the role of these pathways, Diakopoulos et al12 generated A5;p62 and A5;p53 pancreas-specific double knockouts by crossing corresponding “floxed” mouse strains. Pancreas damage was reduced in each of these double knockouts (compared with A5 alone), with less inflammation, fibrosis, acinar cell apoptosis, and compensatory proliferation. The results show conclusively that both p62 and p53 mediate the effects of impaired autophagy in A5 pancreas.
To validate the detrimental effects of ROS accumulation and metabolic disturbances, male A5 mice were given a diet containing palm oil, a rich source of antioxidants and fatty acids; a separate cohort received the antioxidant N-acetylcysteine. These treatments not only reduced ROS levels and cardiolipin oxidationin A5 pancreas (as might be expected), they improved pancreatic morphology, preserved acinar tissue, and alleviated apoptosis. However, there was no improvement of endocrine dysfunction. On a molecular level, palm oil diet counteracted the p62 increase and CREB decrease in A5 pancreas. (Of note, palm oil was shown16 to improve CP in an experimental model.) Finally, some features of the A5 pancreatic phenotype were displayed in human tissue from CP patients, including dilated ER, damaged mitochondria, and accumulation of p62 (previously reported17), Nqo1, and p53. However, exon sequencing of ATG5 in 267 patients with hereditary or idiopathic CP (159 female and 108 male) did not find any genetic alterations associated with CP.
The work by Diakopoulos et al12 is bursting with data that should stimulate broadly future basic research. These include issues related to the mechanisms of observed effects, for example, deficiencies in glutamate-dependent metabolism (does glutamate level decrease in A5 pancreas?); or the decreased mRNA expression of p53, which is usually regulated at the protein level. Another aspect is the effects of Nrf2 activation known to be “context dependent” (promoting apoptosis, inflammation, and fibrosis in Atg5-deficient liver,18 but suppressing oxidative stress and apoptosis in cardiomyocytes19).
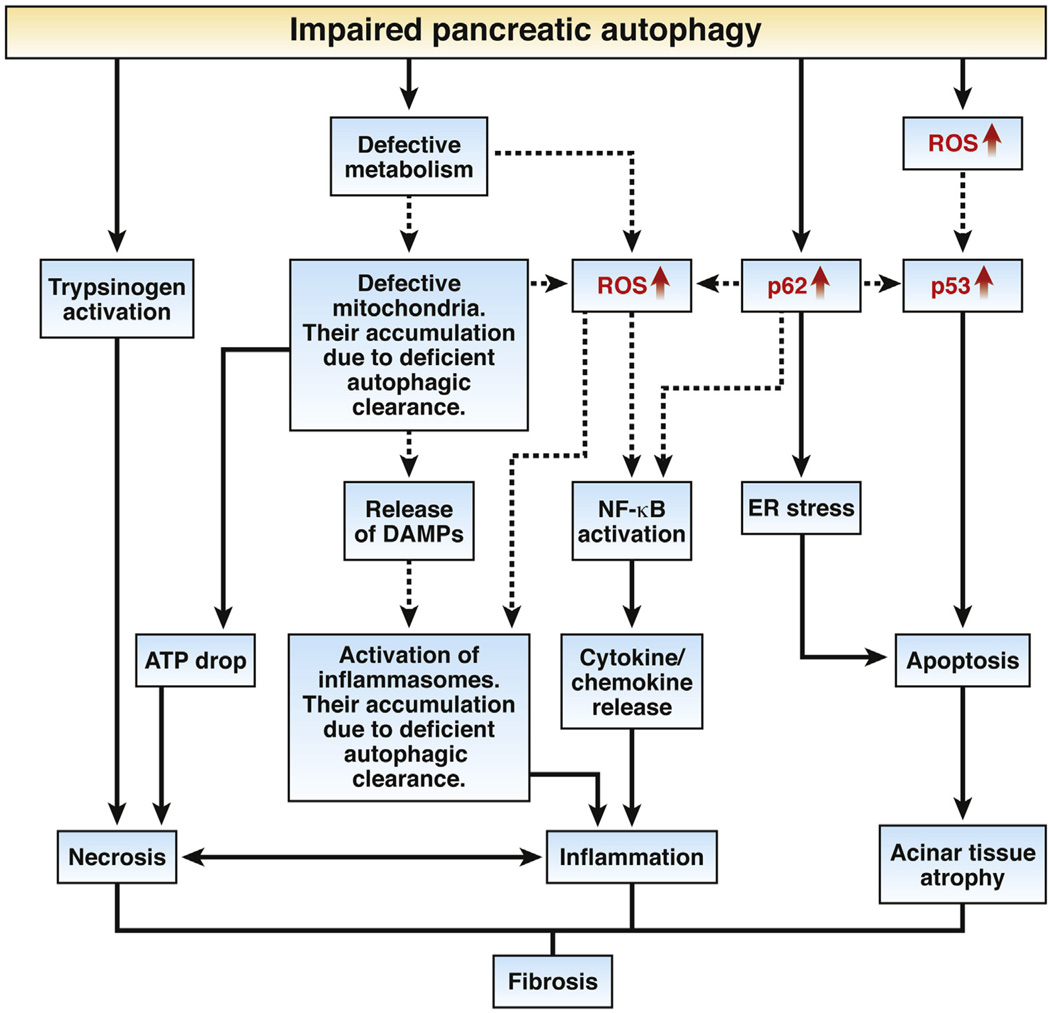
More important, there is much to be learned about the pathways that can link defective autophagy with pancreatitis pathologies, namely, inflammation, necrosis, and fibrosis. Some of these pathways, both established and putative, are illustrated in Figure 1 based on the findings from12 as well as other studies.7–9,17,20–24 In particular, 1 mechanism whereby impaired pancreatic autophagy can promote inflammation is through activation of the p62–TRAF6–nuclear factor-κB pathway, leading to cytokine/chemokine release.8,25 Another likely mechanism8,21 involves inflammasome activation caused by ROS increase and the release of DAMPs (danger/damage-associated pattern molecules) from damaged mitochondria and necrotic cells. Of note, deficient autophagic clearance hinders the elimination of both inflammasomes and damaged mitochondria.1–3,5 p62 accumulation also can promote pancreatic damage by activating ER stress, as shown in the CP model induced by IKKα deficiency.17 Recent evidence22 indicates an important role for maladaptive (“terminal”12) ER stress in the pathogenesis of pancreatitis, but the underlying mechanisms remain largely unknown. Both ER stress and p53 up-regulation promote apoptosis and thus pancreatic atrophy. The A5;p62 and A5;p53 double knockouts generated in Diakopoulos et al’s study12 should help to clarify these issues. Pathways linking impaired epithelial autophagy and fibrosis, in general, are only starting to be unraveled,5,26 but the vicious cycle of inflammation and necrosis is a major driver of pancreatic fibrosis, providing, in particular, cytokines and other factors that mediate the activation and proliferation of stellate cells.24 The mechanisms whereby impaired autophagy can induce pancreatic necrosis (Figure 1) include mitochondrial dysfunction that leads to decreased cellular ATP,23 and intra-acinar trypsinogen activation.8,9,20
Figure 1.
Pathways linking impaired autophagy to pancreatitis. Solid lines indicate pathways operating in pancreas, based on the results of Diakopoulos et al12 and other studies.7–9,17,20–24,28 Dashed lines indicate pathways that are likely to be involved but not yet proven in the pancreas. Key molecular signals identified in Diakopoulos et al12 are shown in red.
As stated, pancreas-specific Atg5 knockouts driven by the developmental regulators Ptf1a12 or Pdx111 both caused pancreas damage. In contrast, acinar tissue morphology remained normal in Ela-CreER;Atg5 mice12 in which Atg5 deletion was triggered by tamoxifen treatment specifically in adult acinar cells (expressing inducible CreER). This is in agreement with the results of an earlier study10 that used noninducible Ela-Cre to generate Ela-Cre;Atg5 mice in which Atg5 is deleted in acinar cells starting from late embryo. One possible reason for the limited pancreatic damage in Ela-Cre;Atg5 mice is the known suboptimal efficiency of the Ela-Cre driver.27 However, the previous study10 reported an improvement in acute cerulein pancreatitis in Ela-Cre;Atg5 mice; this issue needs to be revisited.
The study by Diakopoulos et al12 provides strong evidence that autophagy is critical to pancreas homeostasis and its impairment leads to pancreatitis. This conclusion is in accord with recent findings in other genetic models11,17 and experimental pancreatitis models.7–9,28 Further, the study uncovers cellular and molecular mechanisms mediating the effects of impaired autophagy in pancreas. One may speculate that autophagy is of particular importance to the pancreatic acinar cell, which relies on coordinated function of various organelles (ER, zymogen granules, endolysosomes, mitochondria) to maintain the high level of protein synthesis and secretion. Not only is there a profound autophagy impairment in various experimental models of pancreatitis (induced by administration of cerulein, choline-deficient/ethionine supplemented diet or l-arginine,9 or by a combination of ethanol diet and lipopolysaccharide28), but, remarkably, alterations in many disparate pathways, which in one way or another cause impaired autophagy, all result in pancreas damage. Besides Atg511,12 and Atg711 knockouts, the examples (discussed in references7,8,17) include genetic ablation of Spink3, IKKα, LAMP-2, and inactivation of the mannose-6-phospate pathway of hydrolase delivery to the lysosome. The fact that screening of CP patients12 has not (so far) found changes in ATG5 does not rule out a causative role for genetic alterations in autophagy regulating proteins in human disease. Although the search has only begun, it has revealed that p62 accumulation, a marker of dysfunctional autophagy, is prominent in human CP.12,17 Because multiple routes lead to defective autophagy, mutations in other proteins affecting ATG protein levels or ability to regulate autophagy, could promote pancreatitis.
The novel genetic model of CP,12 using deletion of a key autophagy mediator, opens new venues for both elucidating the pathogenic mechanism of pancreatitis and developing/testing therapeutic approaches. One question, which would need to be clinically tested, is whether palm oil12,16 may be effective in patients with CP (although, some say, it tastes terrible).
Acknowledgments
The authors thank Fred Gorelick for critical reading of this editorial.
Funding
Our research on autophagy in pancreatitis is supported, fully or in part, by NIH grants DK059936, AA019730, and DK098108.
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20:460–473. doi: 10.1089/ars.2013.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 4.Klionsky DJ, Abdalla FC, Abeliovich H, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czaja MJ, Ding WX, Donohue TM, Jr, et al. Functions of autophagy in normal and diseased liver. Autophagy. 2013;8:1131–1158. doi: 10.4161/auto.25063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ichimura Y, Komatsu M. Pathophysiological role of autophagy: lesson from autophagy-deficient mouse models. Exp Anim. 2011;60:329–345. doi: 10.1538/expanim.60.329. [DOI] [PubMed] [Google Scholar]
- 7.Gukovskaya AS, Gukovsky I. Autophagy and pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2012;303:G993–G1003. doi: 10.1152/ajpgi.00122.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gukovsky I, Li N, Todoric J, et al. Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1199–1209. doi: 10.1053/j.gastro.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mareninova OA, Hermann K, French SW, et al. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J Clin Invest. 2009;119:3340–3355. doi: 10.1172/JCI38674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto D, Ohmuraya M, Hirota M, et al. Involvement of autophagy in trypsinogen activation within the pancreatic acinar cells. J Cell Biol. 2008;18:1065–1072. doi: 10.1083/jcb.200712156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenfeldt MT, O’Prey J, Morton JP, et al. p53 status determines the role of autophagy in pancreatic tumour development. Nature. 2013;504:296–300. doi: 10.1038/nature12865. [DOI] [PubMed] [Google Scholar]
- 12.Diakopoulos KN, Lesina M, Wörmann S, et al. Impaired autophagy induces chronic atrophic pancreatitis in mice via sex- and nutrition-dependent processes. Gastroenterology. 2015;148:626–638. doi: 10.1053/j.gastro.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Stanger BZ, Hebrok M. Control of cell identity in pancreas development and regeneration. Gastroenterology. 2013;144:1170–1179. doi: 10.1053/j.gastro.2013.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim KH, Lee MS. Autophagy–a key player in cellular and body metabolism. Nat Rev Endocrinol. 2014;10:322–337. doi: 10.1038/nrendo.2014.35. [DOI] [PubMed] [Google Scholar]
- 16.González AM, Garcia T, Samper E, et al. Assessment of the protective effects of oral tocotrienols in arginine chronic-like pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2011;30:G846–G855. doi: 10.1152/ajpgi.00485.2010. [DOI] [PubMed] [Google Scholar]
- 17.Li N, Wu X, Holzer RG, et al. Loss of acinar cell IKKα triggers spontaneous pancreatitis in mice. J Clin Invest. 2013;123:2231–2243. doi: 10.1172/JCI64498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ni HM, Woolbright BL, Williams J, et al. Nrf2 promotes the development of fibrosis and tumorigenesis in mice with defective hepatic autophagy. J Hepatol. 2014;6:617–625. doi: 10.1016/j.jhep.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Li S, Wang H, et al. Nrf2 enhances myocardial clearance of toxic ubiquitinated proteins. J Mol Cell Cardiol. 2014;72:305–315. doi: 10.1016/j.yjmcc.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sah RP, Dudeja V, Dawra RK, et al. Cerulein-induced chronic pancreatitis does not require intra-acinar activation of trypsinogen in mice. Gastroenterology. 2013;144:1076–1085. doi: 10.1053/j.gastro.2013.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoque R, Sohail M, Malik A, et al. TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology. 2011;141:358–369. doi: 10.1053/j.gastro.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Logsdon CD, Ji B. The role of protein synthesis and digestive enzymes in acinar cell injury. Nat Rev Gastroenterol Hepatol. 2013;10:362–370. doi: 10.1038/nrgastro.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voronina SG, Barrow SL, Simpson AW, et al. Dynamic changes in cytosolic and mitochondrial ATP levels in pancreatic acinar cells. Gastroenterology. 2010;138:1976–1987. doi: 10.1053/j.gastro.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Apte MV, Pirola RC, Wilson JS. Pancreatic stellate cells: a starring role in normal and diseased pancreas. Front Physiol. 2012;3:344. doi: 10.3389/fphys.2012.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moscat J. Diaz-Meco MT p62: a versatile multitasker takes on cancer. Trends Biochem Sci. 2012;37:230–236. doi: 10.1016/j.tibs.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallat A, Lodder J, Teixeira-Clerc F, et al. Autophagy: a multifaceted partner in liver fibrosis. Biomed Res Int. 2014;2014:869390. doi: 10.1155/2014/869390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magnuson MA, Osipovich AB. Pancreas-specific Cre driver lines and considerations for their prudent use. Cell Metab. 2013;18:9–20. doi: 10.1016/j.cmet.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fortunato F, Bürgers H, Bergmann F, et al. Impaired autolysosome formation correlates with Lamp-2 depletion: role of apoptosis, autophagy, and necrosis in pancreatitis. Gastroenterology. 2009;137:350–360. doi: 10.1053/j.gastro.2009.04.003. [DOI] [PubMed] [Google Scholar]