Abstract
In this paper, different concentrations of multi-walled carbon nanotube (MWCNT) were homogeneously dispersed throughout the chitosan (CS) matrix. A simple solvent-cast method was used to fabricate chitosan films with 0.1, 0.5, and 1% of MWCNT with the average diameter around 30 nm. The CS/MWCNT films were characterized for structural, viscous and mechanical properties with optical microscopy, wide-angle X-ray diffraction, Raman spectroscopy, tensile test machine, and microindentation testing machine. Murine osteoblasts were used to examine the cell viability and attachment of the nanocomposite films at two time points. In comparison to the pure chitosan film, the mechanical properties, including the tensile modulus and strength of the films were greatly improved by increasing the percentage of MWCNT. Furthermore, adding MWCNT up to 1% increased the viscosity of the chitosan solution by 15%. However, adding MWCNT decreased the samples ductility and transparency. In biological point of view, no toxic effect on osteoblasts was observed in the presence of different percentages of MWCNT at day 3 and day 7. This investigation suggested MWCNT could be a promising candidate for improving chitosan mechanical properties without inducing remarkable cytotoxicity on bone cells.
Keywords: multi-walled carbon nanotube, mechanical properties, chitosan, nanocomposite, cytotoxicity
1. Introduction
The need for optimized designs of tissue engineering scaffolds has resulted in the advancement of biomaterial composites with unexceptional properties [1, 2]. Following the discovery of carbon nanotube (CNT) in 1991 [3], these nanosized materials have quickly become a technological platform for researchers in diverse fields of science including materials and biomedical engineering. CNT is one of the most useful nanoscale agents for diverse material properties reinforcement due to its extraordinary mechanical, electrical and thermal properties [4–6]. CNT’s have shown controversial and complex properties. On one hand, pristine CNT has displayed cytotoxicity on certain cell lines , which can be attributed to the usage of metal catalysts such as nickel during CNT fabrication [7]. On the other hand, CNT incorporated with biopolymers has shown great biocompatibility [8–10]. Despite a growing debate of the cytotoxicity nature of CNT, the published articles showing interest in this field have doubled in recent years [11]. Researchers are continually improving their knowledge on CNT effects on the biological surroundings by designing novel methods to use CNT in biomedical engineering. CNT has been used to enhance the mechanical properties of different forms of biomaterials such as hydrogels [12, 13] and conventional polymer solutions [14, 15].
Chitosan (CS) is one of the natural polysaccharides derived from chitin with wide current and potential range of applications in biology, medicine and biotechnology [16]. It has been widely used as the base material for bone tissue engineering, specifically for bone tissue scaffolds. In addition, diverse forms of CS such as microparticles, film and gel have been developed [17]. The CS/CNT combination was also previously used for biosensor applications [18] and recently proposed for CNT-based scaffolds fabrication [19]. Despite the great properties of CS such as biocompatibility and biodegradability, CS has demonstrated poor mechanical properties when CS does not cross-linked. Moreover, CS solution can be used as aqueous injectable gel for inducing new bone formation, therefore the surgical implantation is not necessary [20–22]. Injectable chitosan gel supplemented with bone marrow proteins has been reported to drastically enhance new bone tissue formation due to more efficient cell delivery than a solid scaffold [21]. In this sense, investigating and improving the injectability and viscosity properties of CS solution is vital for potential usage of CS injectable gels. Among diverse nanomaterials, CNT is one of the promising candidates to improve CS scaffold and gel mechanical and injectability properties as well as the electrical and thermal properties [23–26].
To the best of author’s knowledge, most of the related papers investigated either the material properties or cell compatibility of CS/CNT composites. Considering the high capability of CNTs, the targets of investigation for CS/CNT nanocomposite films were used material characterization, mechanical properties, and cell biocompatibility. We selected multi-walled carbon nanotube (MWCNT) to reinforce CS matrix since MWCNT is proven to be more beneficial in biomedical engineering applications than single-walled carbon nanotube (SWCNT) is [27, 28].
The aim of this paper is to present the comprehensive investigation of CS/MWCNT nanocomposite films fabricated with a simple procedure. We used low CS concentration as used to make microparticles and films in our laboratory [29, 30]. Moreover, lower CS concentration has been shown to be beneficial for CNT dispersion [31]. Four concentrations of CS/MWCNT films were fabricated and used in this study. We reported structural, optical, injectability and mechanical properties as well as cell cytotoxicity of the films.
2. Experimental section
2.1. Materials
CS of medium molecular weight (75–85% deacetylated) was purchased from Sigma-Aldrich. MWCNT was obtained from Alfa Aesar. MWCNT’s mixture typically has nanotubes with 10–30 μm length and other carbon particulates as mentioned in the manufacturer catalog. As prepared MWCNT was gently washed with deionized water and filtered to remove any impurities.
2.2. CS/MWNCT nanocomposite film fabrication
To make MWCNT suspension, 25 mg of MWCNT was first suspended in 100 mL of 1% acetic acid (v/v) diluted with deionized water. MWCNT suspension was homogenized in ultrasonic bath (BRANSON 2510) for 90 min at 35°C. CS solution (2%) was separately made by dissolving 400 mg of CS (w/v) into 20 mL of 1% acetic acid. Similar CS solutions have been widely used for bone tissue scaffold fabrication in different forms including injectable gel and exhibited great biocompatibility and degradability [32]. Using a magnetic stirrer, 2% CS solution was completely mixed for 60 min. By adding appropriate volume of MWCNT suspension into CS solution, CS/MWCNT mixtures with 0.1, 0.5 and 1% of MWCNT were fabricated. The final mixtures were also stirred for 60 min followed by sonication for 20 min to remove air bubbles. The injection tests were performed on these solutions. A part of each prepared solutions was placed in the glass vial to examine the homogeneity and stability after 3 days in a stationary position. To fabricate CS/MWCNT films, the solutions with different MWCNT percentages were casted into a clean microscope glass slides (7.5 cm x 2.5 cm x 1 mm) and dried at room temperature. Dried uniform thin films with an average thickness of 0.06 mm (measured by caliper) were easily removed from the glass slides by initially peeling off the edges using a sharp tip. The entire film came off without any particular treatment.
2.3. Injection and tensile test
To calculate the effect of MWCNT on the viscosity of CS, CS/MWCNT solutions were injected from a 10 mL syringe with the needle gauge of 19 G. Injection tests were performed at room temperature with the injection speed of 100 mm/min using an ADMET universal testing machine (expert 2611) with customized grips. Assuming Newtonian fluid passing through a narrow tube, the Hagen-Poiseuille equation can describe the relation of viscosity and applied force during the injection test [33]:
In this equation, Qi is fluid flow rate running through the syringe, Dn is the needle length, μp is the fluid viscosity, ΔPi is the pressure drop and Ln is the length of needle. The geometric parameters were easily obtained from measuring instruments or from the manufacturer’s manual and available standards.
Tensile tests were carried out using the same machine at room temperature with a gauge length of 50 mm and crosshead speed of 25 mm/min. Each test was done at least 3 times and the reported values here are the average of the obtained data.
2.4. Raman spectra and X-ray diffraction (XRD)
Raman spectra of the films were obtained at room temperature with a Jobin Yvon Horiba confocal Raman spectrometer in backscattering geometry with the laser excitation of 632.8 nm at a power level of 1.7 mV. XRD patterns of the films were obtained by PANanalytical X’Pert Pro MPD using Cu kα radiation under a voltage of 45 kV and current of 40 mA.
2.5. Scanning Electron microscopy (SEM), optical microscopy, transmittance spectroscopy and microhardness test
SEM images were obtained using Hitachi S4800 operating with an accelerating voltage of 10 kV under high vacuum. The obtained images were quantitatively analyzed using imageJ software. The optical and fluorescence microscopy images of CS/MWCNT films were taken by a FSX-100 Olympus microscope. The transmittance tests of CS/MWCNT films on glass substrate were performed by an ultraviolet-visible (UV-VIS-NIR) spectrometer. Microhardness tests were conducted using a Clark CM-400AT hardness test machine at 10 gf load.
2.6. pH measurement and Film-Cell interaction study
To track the effect of MWCNT on the films pH level, two phosphate buffered saline (PBS) solutions with the molarity of 10 mM and adjusted pH of 7.4 and 5 were prepared. CS/MWCNT films were cut into small pieces (2 cm x 2 cm) and placed in the glass vial filled with 12 mL of the PBS. The vials were kept in incubator at 37 °C. pH values were measured every week for 22 weeks by using a Mettler Toledo S20-K pH meter kit.
Murine osteoblast cell line (OB6) was used for propagation and further studies. Cells were plated on a petri dish with 100 mm diameter. Two time points (day 3 and day 7) were selected to examine the cytotoxicity and cell attachment of CS/MWCNT films. Films were cut into small pieces (1 cm x 1 cm), sterilized under UV light for 10 min before adding original cell suspension. Four types of sterilized films were placed in 24-well plates and seeded with OBs with the concentration of 25000 cells per well. Well plates were incubated at 37°C in a humidified 5% CO2/95% air atmosphere in an osteogenic medium. Cell culture media was changed every 2–3 days.
To prepare cell culture medium, alpha minimum essential medium (α-MEM) containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (Pen Strep) were purchased from Gibco. An Invitrogen Live/Dead cell assay kit with Dulbecco’s phosphate buffered saline (DPBS) (Thermal Scientific) was used to identify live and dead cells.
3. Result and discussion
3.1. Structure and morphology
As presented in supporting information section, characterization of the fibers using SEM showed the MWCNT structure and morphology (Figure S1(A)). MWCNT adhered together and formed bulk shape containing various numbers of individual MWCNT [34]. Images were used to analyze MWCNT size distribution (Figure S1(B)). In terms of the diameters, bare MWCNT were measured to be ~ 31.48±16 nm.
MWCNT has poor wetting properties in water such that it aggregated and precipitated in a stationary position. As shown in figure S2, MWCNT powder that was suspended into 1% acetic acid and sonicated for 1 h, mostly settled down after 20 min in a stationary position.
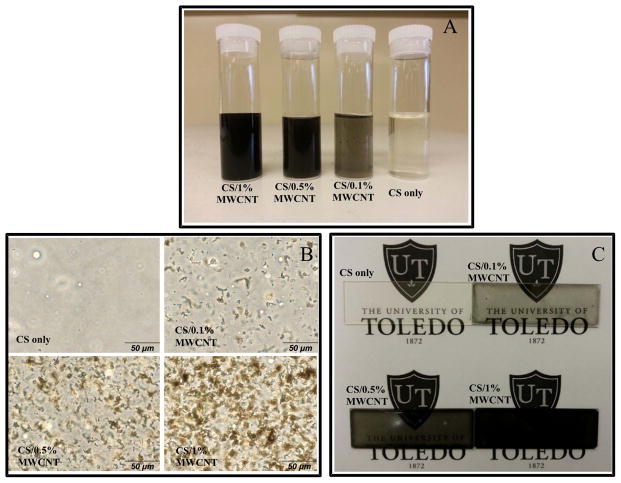
CS/MWCNT mixtures displayed different appearance. As shown in Figure 1(A), after adding, mixing and sonicating of MWCNT into CS solution, the dispersion and solubility of MWCNT were significantly improved. This image was taken after 3 days of placing solutions in a stationary position. It also showed that the MWCNT and CS interaction improved the dispersion of MWCNT. Generally, MWCNT’s floated on water, thus it was difficult to obtain a uniform solution of MWCNT spreading over on the water or 1% acetic acid. This is due to the large aspect ratio of MWCNT as well as the water surface tension. The higher percentage of MWCNT dispersed in CS solution, the darker black color of final mixture. The ultimate composition was completely stable and no degradation, separation or floccule was observed after one month of visual tracking.
Figure 1.
(A) CS/MWCNT nanocomposite films with different percentages of MWCNT after 3 days in a stationary position. (B) Optical microscopy images and (C) normal camera images of CS/MWCNT films with different concentrations of MWCNT.
The deposition of MWCNT in the CS matrix was examined using an optical microscopy as shown in figure 1(B). Deposited MWCNT absorbed the light and seen as the black particles on the image. By increasing the percentage of MWCNT in CS films, the number of black spots showing aggregated MWCNT increased. However, this figure demonstrates that MWCNT’s were uniformly distributed in CS matrix. The level of distribution was acceptable compared with other previous similar works [35, 36]. The small isotropic aggregations were unavoidable because the sonication process did not induce enough energy to overcome depletion and nanotube van der Waals attraction between MWCNT or their bundles [37]. In addition, the high-resolution camera photographs of CS/MWCNT films with various MWCNT loadings provided direct evidence for evaluating the homogeneity of films (Figure 1(C)). This image gives an insight of how the fabricated films look like. CS film with 1% MWCNT was almost completely opaque, which was verified by later transmittance measurement.
3.2. XRD and optical properties
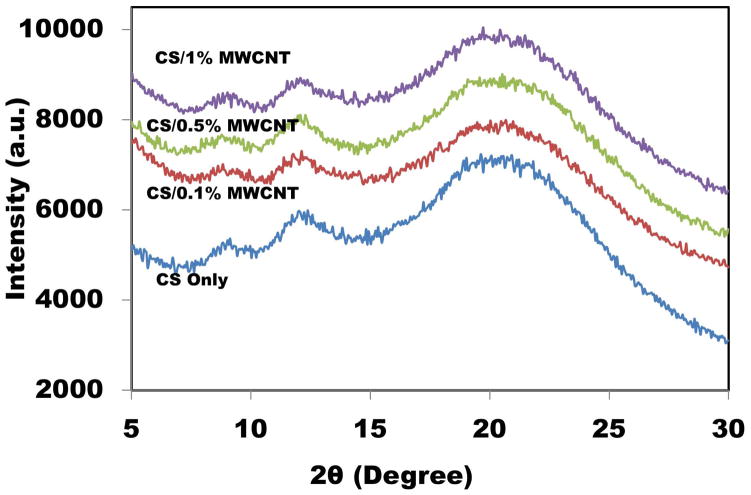
Generally, seven polymorph models have been suggested for CS [35, 38]. CS has three peaks correspond to two crystal structures [39]. XRD pattern of the samples is demonstrated in Figure 2. Two former characteristic peaks around 2θ=8.5° and 11.5° indicated a hydrated (tendon) crystalline, whereas the latter peak at 19° showed an amorphous structure of CS. The broadening of the peaks was due to the amorphous nature of polymers [40]. For all CS/MWCNT films, nearly the same XRD graphs were obtained showing the same crystalline structure. MWCNT including a strong peak at 2θ=25° corresponds to (002) crystal plane [41, 42]. This figure displays the incorporation of small percentages of MWCNT into CS films did not affect the crystalline structure of CS films because all four samples had approximately same peaks.
Figure 2.
XRD patterns of CS/MWCNT films with different concentrations of MWCNT.
The optical properties and dispersion quality of CS/MWCNT films were also examined by UV/VIS transmittance measurements (Figure 3(A)). At wavelengths higher than 450 nm, adding up to 1% MWCNT can decrease the transmittance value to 40%. Based on Lamber-Beer’s law, absorbance can be easily calculated from the graph. As shown in Figure 3(B), a linear relation was observed between the percentage of MWCNT and absorbance which is in agreement with other works [43, 44]. Furthermore, the linear fashion is usually attributed to the rich dispersion of CNT [45]. Small error in linear behavior is negligible and can be explained as the effect of glass substrate used as a film support.
Figure 3.
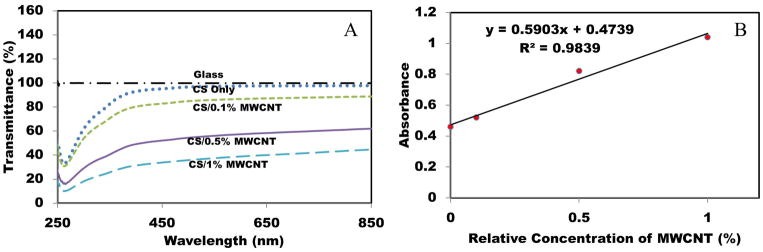
(A) Transmittance curves for CS films with different concentrations of MWCNT. (B) Absorbance of CS/MWCNT films as a function of elative concentration of MWCNTs.
3.3. Raman spectra analysis
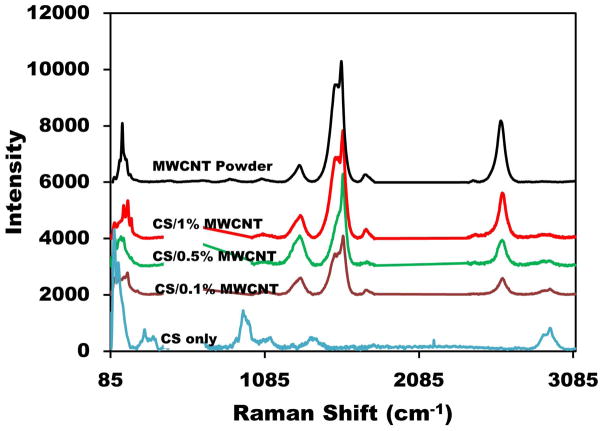
Raman spectra demonstrated the differences between CS and CS/MWCNT composite films. A major application of Raman spectroscopy is to recognize specific groups where relatively large molecules are involved. Raman spectrometry of the films is shown in Figure 4. For pure CS and CS/MWCNT films a portion of graph ranging from 480–700 cm−1 was eliminated due to the large peak of silicon substrate. Silicon was used as an opaque substrate for the best laser reflection and Raman spectra results. Normal CS Raman bands were observed at 100, 280 and 935 cm−1 [46]. A later peak corresponded to a C–O–C band. The first and second peaks indicated lattice vibration and δ(C–C) band stretching, respectively. It also displayed a broadening peak at 1100 cm−1 which corresponded to ν(C–C) band. Another typical intense band was due to ν(C=O) stretching at 1355 cm−1. Finally, a peak at 2850 cm−1 corresponded to a C–CH3 band [47, 48]. Furthermore, MWCNT powder Raman spectra revealed a number of main peaks. A low energy mode observed at Raman shift between 100–200 cm−1 is considered a “radial breathing modes” (RBM) where all atoms vibrate radially in phase [45, 49]. D and G band peaks at Raman shift were between 1200–1340 cm−1, respectively. The last peak corresponded to G’ (D*) band at Raman shift between 2450–2650 cm−1 [50, 51]. CS/MWCNT films with different percentage of MWCNT had approximately the same peaks and peak ratios as MWCNT powder. However, all peaks of MWCNT shifted to higher wavelengths upon blending with CS, showing strong interactions between CNT and CS. This was due to an increase of the energy of CS coated MWCNTs [52]. The hydrophobic and π–π interactions between the CS (–C=OCH3) and (C=C) bands increased the required vibrational energy which resulted in upshift of Raman peaks [53].
Figure 4.
Raman spectra of CS films with different MWCNT concentrations and MWCNT powder.
3.4. pH measurement of films
Since pH is a critical and important factor in physiological applications, we measured the pH level of samples in two PBS solutions with the initial pH values of 7.4 (Figure 5(A)) and (Figure 5(B)). CS is a pH-responsive polymer because of the existing amino groups. As shown in Figure 5, the pH changed rapidly during the early weeks and finally leveled off to about 7 and 6.8 for the initial pH of 7.4 and 5, respectively. Samples in both PBS solutions swelled after a few days and they completely disappeared after 5–6 weeks showing the degradability of the films in normal pHs. MWCNTs settled down at the bottom of the plastic vials. With the initial pH value of 7.4, the pH changing fluctuation was less than one. This fluctuation was even smaller for CS/1% MWCNT at early weeks. For all samples with the initial pH value of 5, the pHs almost reached their final value after six weeks. An increase in pH demonstrated a decrease in hydrogen ion concentration. Adding MWCNT did not show significant effect on pH changing of CS films. Moreover, films with different percentages of MWCNT balanced the final pH value which is useful for biomedical engineering applications. Different parameters such as temperature have shown to affect the pH of CS [54]. For this paper, the experiments were done at 37°C since we are mostly interested in biomedical applications of the films.
Figure 5.
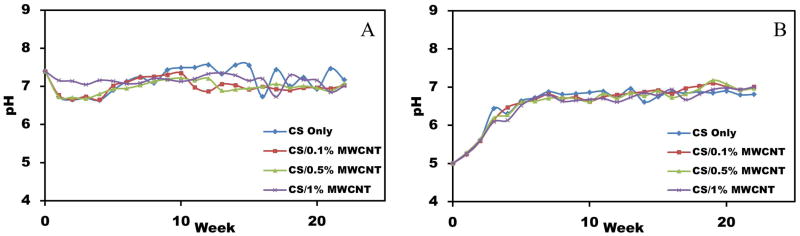
pH value changing of CS/MWCNT films with (A) initial pH value of 7.4, (B) initial pH value of 5.
3.5. Injectability and viscosity
For potential clinical application as injectable biomaterial, an acceptable injectability and viscosity is necessary. In this part of the experiments, relatively low injection speed was applied in order to allow the fluid to exhibit Newtonian flow. Based on Hagen-poiseuille equation [33, 55], viscosity of the fluid can be obtained by injection test. Figure 6(A) shows the injection test results for different concentrations of MWCNT dispersed into CS matrix. The force exerted to the syringe plunger during the injection process is dissipated in the following ways: (1) prevailing the resistance force of the syringe plunger. (2) transferring energy to the liquid, and (3) extruding the liquid through the needle [56]. After the applied force reached the required value to overcome the sum of the forces, the graph demonstrated a constant value, which was used to calculate the fluid viscosity.
Figure 6.
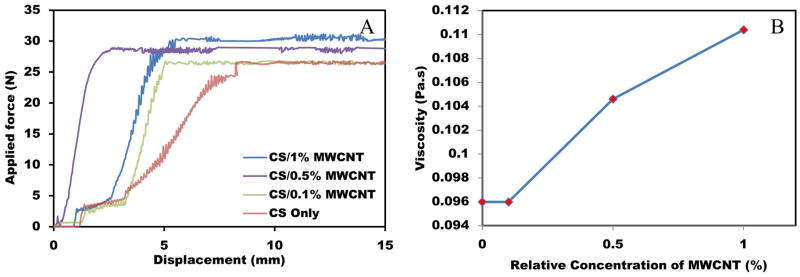
(A) Typical injection curves obtained for CS/MWCNT pastes with different percentages of MWCNT. (B) Relationship between viscosity and MWCNT concentration.
Figure 6(B) presents the effect of MWCNT percentage on the solution viscosity. Adding 1% MWCNT into CS solution increased the viscosity by 15%. Based on Figure 8, adding up to 0.1% MWCNT did not have a remarkable effect on the ultimate magnitude of applied force and subsequently the viscosity. However, by increasing the percentage of MWCNT, the required injection force was also increased showing higher viscosity of the samples. This can be explained as the effect of interaction between CS/MWCNT solution and the syringe wall. Carbon nanosized particles and tubes are prone to attach to the syringe and needle walls due to the Van der Waals interaction forces.
Figure 8.
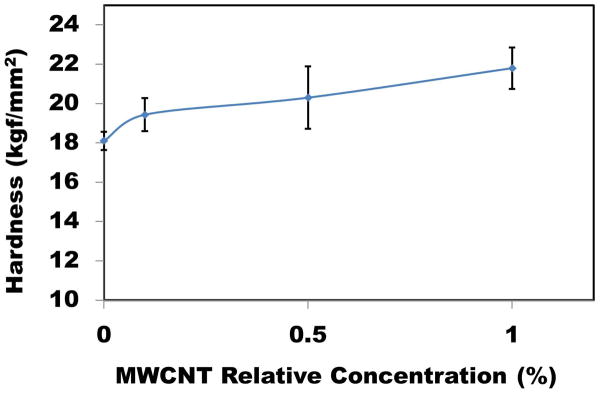
Microhardness of CS/MWCNT films at 10 gf load as a function of MWCNT concentration.
3.6. Tensile and microhardness test
To investigate the effects of incorporating MWCNT on the mechanical properties of CS films, a tensile test was done at room temperature. The typical stress-strain curves for CS/MWCNT films are shown in Figure 7(A) As shown in this figure, the presence of MWCNT in CS matrix significantly enhanced the mechanical properties. All four samples showed mostly brittle behavior since yield strength and ultimate strength were not clearly distinguished. As shown in Figure 7(B) the elastic modulus and tensile strength of the CS film increased with the increase of MWCNT loading. For instance, by adding 1% MWCNT into the CS matrix, the elastic modulus and tensile strength increased by about 47% and 33%, respectively. However, as shown in Figure 9(B), by increasing the percentage of MWCNT in CS films, the elasticity and elongation at break decreased. As an example, CS films with 1% MWCNT had 33% more rigidity (i.e. less elongation) in comparison with neat CS films. Another interesting point perceived from this figure is adding 0.1% MWCNT induced drastic improvement in mechanical properties of CS films. However, due to the MWCNT aggregation at higher percentage, with further increase of MWCNT concentration, elastic modulus and tensile strength only increased slightly. This observation is in agreement with other similar studies [35, 57]. Noted in the referred article, higher CS concentration resulted in films with more flexibility (i.e. higher percentage of elongation at break) and lower elastic modulus.
Figure 7.
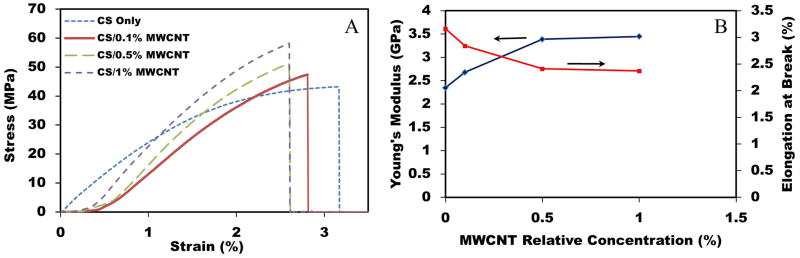
(A) Tensile test of CS/MWCNT films. (B) The effect of MWCNT concentration on Young’s modulus and ductility of samples.
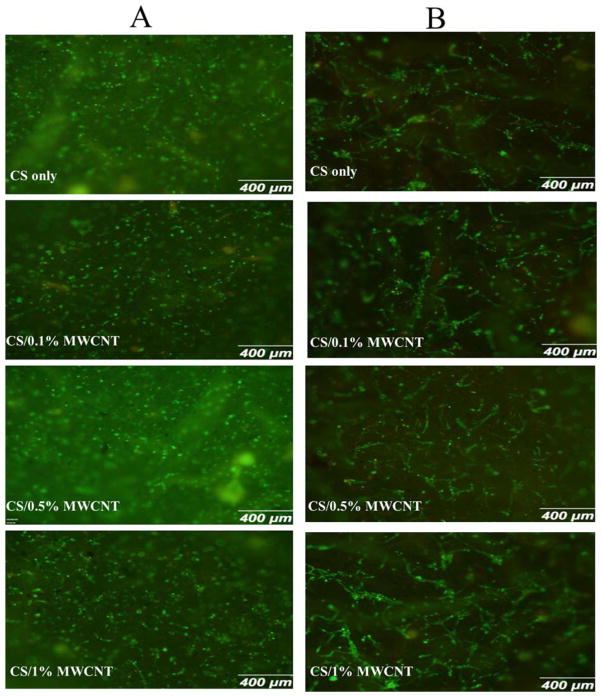
Figure 9.
Fluorescent microscopy images of osteoblasts (A) after 3 days and (B) after 7 days treated with Live/Dead assay.
Improving mechanical properties of CS films in the presence of MWCNT can be attributed to the interaction of MWCNT as the reinforced fibers and CS as the matrix. Due to the strong interactions between CS and MWCNT, higher energy was needed to overcome the molecular bonding energy and subsequently, elastic modulus and tensile strength increased. Nevertheless, after reaching certain energy, nanocracks started initiating at the MWCNT and CS boundary, finally resulting in the failure of films in tensile test at lower elongation. Moreover, with increasing the percentage of MWCNT, the statistical chance of having nanocracks at the boundary of MWCNT and CS increased. In this way, further increase of MWCNT did not demonstrate significant improvement in tensile strength and elastic modulus.
Microhardness tests were performed at the lowest possible load and the maximum indentation depth was 10% of the total film thickness to minimize the substrate effect [58]. Ten different locations were examined and the average value of hardness at 10 gf is shown in Figure 8. Dispersing MWCNT into CS matrix hardened the CS film surface. Carbon and its derivative such as CNTs are one of the most rigid and hard materials [59], and adding small percentage of MWCNT can induce hardness to the CS films. In another point of view, having harder material means lower deformability and elasticity, which is in agreement with tensile test. For example, adding just 1% MWCNT, can increase the surface hardness up to 20%.
3.7. CS/MWCNT film cytotoxicity
To evaluate cell proliferation and film cytotoxicity, Live/Dead cell assay was employed to investigate the cell viability of the CS/MWCNT films. This assay has the benefit of simultaneous detection of live and dead cells. Green fluorescence from calcein dye binded to the active esterase in healthy cells, and red fluorescence from EthD-1 interconnected to nucleic acids of damaged membranes of dead cells. Two time points (i.e. day 3 and day 7) were selected to study cell behavior as stated earlier.
Osteoblasts were used to examine the film-induced cytotoxicity. Overall, osteoblasts adhered strongly to CS/MWCNT films and showed normal morphology over 3 and 7 days, indicating nontoxic effects of MWCNT to the osteoblasts. After 3 days of cell seeding, as observed from Figure 9(A), cells proliferated and attached to the all kinds of film surfaces and the viability percentage was high, showing cell-friendly property of the films. At this time point, majority of cells were not completely elongated and did not demonstrate well-distributed morphology. Some parts of the figures are not clear and vivid due to the rugged and scaly morphology of the films in micrometer scale. Moreover, the approximate circular shape of cells altered to elongated structure after 7 days and they started proliferating (Figure 9(B)). Maximum diameter of a single elongated cell was 75 μm, and they aggregated and created big cell colonies as shown in this figure. Randomly aligned cells were observed in most of the samples. This can be attributed to the surface swelling on the samples. Swelling of the surface was not perfectly uniform and resulted trapping cells in the surface micro grooves and elongating them in the specific direction. The fraction of dead cells to the total number of attached cells was negligible. This confirmed that MWCNT is not toxic for osteoblasts as reported in literature [60].
4. Conclusions
This paper investigated the structural, optical, mechanical, and biological properties of CS/MWCNT nanocomposite films. By applying a simple fabricating method, we obtained homogeneous dispersion of MWCNT in the CS matrix. Adding low percentages of as-prepared MWCNT had significant effects on optical, mechanical properties and bonding energy of molecules. For instance, adding just 1 wt% of MWCNT to CS matrix can enhance the tensile elastic modulus and tensile strength by about 47% and 33%, respectively. In addition, the added amount of MWCNT, using two different initial pH values, was demonstrated to not highly affect the polymer crystal structure and pH level of samples. We have also studied the ability of osteoblasts to attach and proliferate on the CS/MWCNT at two selected time points. No cell toxicity effects were observed at days 3 and 7. In summary, we showed that the CS/MWCNT nanocomposite films with improved mechanical and bioactive properties are well suited as a novel biomaterial. MWCNT incorporated into CS film increased the viscosity of CS solution, which may be useful for injecting low viscous CS pastes.
Supplementary Material
Acknowledgments
This work was supported by NSF grants 0652024, 0925783, and NIH grant DE019508.
Footnotes
Conflict of interest statement
We declare that none of authors involved in this research work have conflict of interest.
References
- 1.Mohanty A, Misra M, Hinrichsen G. Biofibres, biodegradable polymers and biocomposites: An overview. Macromol Mater Eng. 2000;276:1–24. [Google Scholar]
- 2.MacDonald RA, Laurenzi BF, Viswanathan G, Ajayan PM, Stegemann JP. Collagen–carbon nanotube composite materials as scaffolds in tissue engineering. J Biomed Mater Res A. 2005;74:489–496. doi: 10.1002/jbm.a.30386. [DOI] [PubMed] [Google Scholar]
- 3.Iijima S. Helical microtubules of graphitic carbon. Lett Nat. 1991;354:56–58. [Google Scholar]
- 4.Shokrieh M, Rafiee R. A review of the mechanical properties of isolated carbon nanotubes and carbon nanotube composites. Mech Compos Mater. 2010;46:155–172. [Google Scholar]
- 5.Balandin AA. Thermal properties of graphene and nanostructured carbon materials. Nat Mater. 2011;10:569–581. doi: 10.1038/nmat3064. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald RA, Voge CM, Kariolis M, Stegemann JP. Carbon nanotubes increase the electrical conductivity of fibroblast-seeded collagen hydrogels. Acta Biomater. 2008;4:1583–1592. doi: 10.1016/j.actbio.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal S, Zhou X, Ye F, He Q, Chen GC, Soo J, Boey F, Zhang H, Chen P. Interfacing live cells with nanocarbon substrates. Langmuir. 2010;26:2244–2247. doi: 10.1021/la9048743. [DOI] [PubMed] [Google Scholar]
- 8.Misra SK, Ohashi F, Valappil SP, Knowles JC, Roy I, Silva SRP, Salih V, Boccaccini AR. Characterization of carbon nanotube (MWCNT) containing P (3HB)/bioactive glass composites for tissue engineering applications. Acta Biomater. 2010;6:735–742. doi: 10.1016/j.actbio.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Tercero JE, Namin S, Lahiri D, Balani K, Tsoukias N, Agarwal A. Effect of carbon nanotube and aluminum oxide addition on plasma-sprayed hydroxyapatite coating's mechanical properties and biocompatibility. Mater Sci Eng C. 2009;29:2195–2202. [Google Scholar]
- 10.Lobo A, Antunes E, Machado A, Pacheco-Soares C, Trava-Airoldi V, Corat E. Cell viability and adhesion on as grown multi-wall carbon nanotube films. Mater Sci Eng C. 2008;28:264–269. [Google Scholar]
- 11.Harrison BS, Atala A. Carbon nanotube applications for tissue engineering. Biomaterials. 2007;28:344–353. doi: 10.1016/j.biomaterials.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 12.Shin SR, Bae H, Cha JM, Mun JY, Chen Y-C, Tekin H, Shin H, Farshchi S, Dokmeci MR, Tang S. Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS nano. 2011;6:362–372. doi: 10.1021/nn203711s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abarrategi A, Gutiérrez MC, Moreno-Vicente C, Hortigüela MJ, Ramos V, López-Lacomba JL, Ferrer ML, del Monte F. Multiwall carbon nanotube scaffolds for tissue engineering purposes. Biomaterials. 2008;29:94–102. doi: 10.1016/j.biomaterials.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 14.Price RL, Waid MC, Haberstroh KM, Webster TJ. Selective bone cell adhesion on formulations containing carbon nanofibers. Biomaterials. 2003;24:1877–1887. doi: 10.1016/s0142-9612(02)00609-9. [DOI] [PubMed] [Google Scholar]
- 15.Koh LB, Rodriguez I, Venkatraman SS. A novel nanostructured poly (lactic-co-glycolic-acid)–multi-walled carbon nanotube composite for blood-contacting applications: Thrombogenicity studies. Acta Biomater. 2009;5:3411–3422. doi: 10.1016/j.actbio.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Jayakumar R, Menon D, Manzoor K, Nair S, Tamura H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr Polym. 2010;82:227–232. [Google Scholar]
- 17.Di Martino A, Sittinger M, Risbud MV. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26:5983–5990. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, Smith A, Gorski W. Carbon nanotube-chitosan system for electrochemical sensing based on dehydrogenase enzymes. Anal Chem. 2004;76:5045–5050. doi: 10.1021/ac049519u. [DOI] [PubMed] [Google Scholar]
- 19.Nardecchia S, Serrano MC, Gutierrez MC, Ferrer ML, del Monte F. Modulating the cytocompatibility of tridimensional carbon nanotube-based scadffolds. J Mater Chem B. 2013:3064–3072. doi: 10.1039/c3tb20253d. [DOI] [PubMed] [Google Scholar]
- 20.Chenite A, Chaput C, Wang D, Combes C, Buschmann M, Hoemann C, Leroux J, Atkinson B, Binette F, Selmani A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials. 2000;21:2155–2161. doi: 10.1016/s0142-9612(00)00116-2. [DOI] [PubMed] [Google Scholar]
- 21.Park DJ, Choi BH, Zhu SJ, Huh JY, Kim BY, Lee SH. Injectable bone using chitosan-alginate gel/mesenchymal stem cells/BMP-2 composites. J Craniomaxillofac Surg. 2005;33:50–54. doi: 10.1016/j.jcms.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Zhou G, Lu Y, Zhang H, Chen Y, Yu Y, Gao J, Sun D, Zhang G, Zou H, Zhong Y. A novel pulsed drug-delivery system: polyelectrolyte layer-by-layer coating of chitosan–alginate microgels. Int J Nanomedicine. 2013;8:877–887. doi: 10.2147/IJN.S38144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau C, Cooney MJ, Atanassov P. Conductive Macroporous Composite Chitosan Carbon Nanotube Scaffolds. Langmuir. 2008;24:7004–7010. doi: 10.1021/la8005597. [DOI] [PubMed] [Google Scholar]
- 24.Thompson BC, Moulton SE, Gilmore KJ, Higgins MJ, Whitten PG, Wallace GG. Carbon nanotube biogels. Carbon. 2009;47:1282–1291. [Google Scholar]
- 25.Liu Y-L, Chen W-H, Chang Y-H. Preparation and properties of chitosan/carbon nanotube nanocomposites using poly (styrene sulfonic acid)-modified CNTs. Carbohydr Polym. 2009;76:232–238. [Google Scholar]
- 26.Carson L, Kelly-Brown C, Stewart M, Oki A, Regisford G, Luo Z, Bakhmutov VI. Synthesis and characterization of chitosan–carbon nanotube composites. Mater Lett. 2009;63:617–620. doi: 10.1016/j.matlet.2008.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraczek A, Menaszek E, Paluszkiewicz C, Blazewicz M. Comparative in vivo biocompatibility study of single-and multi-wall carbon nanotubes. Acta Biomater. 2008;4:1593–1602. doi: 10.1016/j.actbio.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 28.Matsuoka M, Akasaka T, Totsuka Y, Watari F. Strong adhesion of Saos-2 cells to multi-walled carbon nanotubes. Mater Sci Eng B. 2010;173:182–186. [Google Scholar]
- 29.Aryaei A, Jayatissa AH, Jayasuriya AC. Nano and micro mechanical properties of uncross-linked and cross-linked chitosan films. J Mech Behav Biomed Mater. 2012;5:82–89. doi: 10.1016/j.jmbbm.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jayasuriya AC, Bhat A. Optimization of scaled-up chitosan microparticles for bone regeneration. Biomed Mater. 2009;4:055006. doi: 10.1088/1748-6041/4/5/055006. [DOI] [PubMed] [Google Scholar]
- 31.Lynam C, Grosse W, Wallace GG. Carbon-nanotube biofiber microelectrodes. J Electrochem Soc. 2009;156:117–121. [Google Scholar]
- 32.Dreifke MB, Ebraheim NA, Jayasuriya AC. Investigation of potential injectable polymeric biomaterials for bone regeneration. J Biomed Mater Res A. 2013:2436–2447. doi: 10.1002/jbm.a.34521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bohner M, Baroud G. Injectability of calcium phosphate pastes. Biomaterials. 2005;26:1553–1563. doi: 10.1016/j.biomaterials.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Tseng S-H, Palathinkal TJ, Tai N-H. Nondestructive purification of single-walled carbon nanotube rope through a battery-induced ignition and chemical solution approach. Carbon. 2010;48:2159–2168. [Google Scholar]
- 35.Wang S-F, Shen L, Zhang Wei-De, Tong Y-J. Preparation and mechanical properties of chitosan/carbon nanotubes composites. Biomacromolecules. 2005;6:3067–3072. doi: 10.1021/bm050378v. [DOI] [PubMed] [Google Scholar]
- 36.Spinks GM, Shin SR, Wallace GG, Whitten PG, Kim SI, Kim SJ. Mechanical properties of chitosan/CNT microfibers obtained with improved dispersion. Sensors Actuators B: Chem. 2006;115:678–684. [Google Scholar]
- 37.Badaire S, Zakri C, Maugey M, Derré A, Barisci JN, Wallace G, Poulin P. Liquid crystals of DNA-stabilized carbon nanotubes. Adv Mater. 2005;17:1673–1676. [Google Scholar]
- 38.Saito H, Tabeta R, Ogawa K. High-resolution solid-state carbon-13 NMR study of chitosan and its salts with acids: conformational characterization of polymorphs and helical structures as viewed from the conformation-dependent carbon-13 chemical shifts. Macromolecules. 1987;20:2424–2430. [Google Scholar]
- 39.Rhim JW, Hong SI, Park HM, Ng PKW. Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity. J Agric Food Chem. 2006;54:5814–5822. doi: 10.1021/jf060658h. [DOI] [PubMed] [Google Scholar]
- 40.Govindan S, Nivethaa EAK, Saravanan R, Narayanan V, Stephen A. Synthesis and characterization of chitosan–silver nanocomposite. Appl Nanosci. 2012;2:1–5. [Google Scholar]
- 41.Stylianakis MM, Mikroyannidis JA, Kymakis E. A facile, covalent modification of single-wall carbon nanotubes by thiophene for use in organic photovoltaic cells. Sol Energy Mater Sol Cell. 2010;94:267–274. [Google Scholar]
- 42.Ng S, Wang J, Guo Z, Chen J, Wang G, Liu H. Single wall carbon nanotube paper as anode for lithium-ion battery. Electrochim Acta. 2005;51:23–28. [Google Scholar]
- 43.Wu Z, Feng W, Feng Y, Liu Q, Xu X, Sekino T, Fujii A, Ozaki M. Preparation and characterization of chitosan-grafted multiwalled carbon nanotubes and their electrochemical properties. Carbon. 2007;45:1212–1218. [Google Scholar]
- 44.Bahr JL, Mickelson ET, Bronikowski MJ, Smalley RE, Tour JM. Dissolution of small diameter single-wall carbon nanotubes in organic solvents? Chem Commun. 2001:193–194. [Google Scholar]
- 45.Tang C, Zhou T, Yang J, Zhang Q, Chen F, Fu Q, Yang L. Wet-grinding assisted ultrasonic dispersion of pristine multi-walled carbon nanotubes (MWCNTs) in chitosan solution. Colloids Surf B Biointerfaces. 2011;86:189–197. doi: 10.1016/j.colsurfb.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 46.Vasconcelos HL, Camargo TP, Gonçalves NS, Neves A, Laranjeira M, Fávere VT. Chitosan crosslinked with a metal complexing agent: Synthesis, characterization and copper (II) ions adsorption. React Funct Polym. 2008;68:572–579. [Google Scholar]
- 47.Smith E, Dent G, Wiley J. Modern Raman spectroscopy: a practical approach. England: J. Wiley Hoboken, NJ; 2005. [Google Scholar]
- 48.Mai TTT, Ha PT, Pham HN, Le TTH, Pham HL, Phan TBH, Nguyen XP. Chitosan and O-carboxymethyl chitosan modified Fe3O4 for hyperthermic treatment. Adv Nat Sci: Nanosci Nanotechnol. 2012;3:015006. [Google Scholar]
- 49.Graupner R. Raman spectroscopy of covalently functionalized single-wall carbon nanotubes. J Raman Spectrosc. 2007;38:673–683. [Google Scholar]
- 50.Dresselhaus M, Dresselhaus G, Jorio A, Souza Filho A, Saito R. Raman spectroscopy on isolated single wall carbon nanotubes. Carbon. 2002;40:2043–2061. [Google Scholar]
- 51.Dresselhaus MS, Dresselhaus G, Saito R, Jorio A. Raman spectroscopy of carbon nanotubes. Phys Rep. 2005;409:47–99. [Google Scholar]
- 52.Liu Y, Liang P, Zhang HY, Guo DS. Cation-Controlled Aqueous Dispersions of Alginic-Acid-Wrapped Multi-Walled Carbon Nanotubes. Small. 2006;2:874–878. doi: 10.1002/smll.200600099. [DOI] [PubMed] [Google Scholar]
- 53.Sinani VA, Gheith MK, Yaroslavov AA, Rakhnyanskaya AA, Sun K, Mamedov AA, Wicksted JP, Kotov NA. Aqueous dispersions of single-wall and multiwall carbon nanotubes with designed amphiphilic polycations. J Am Chem Soc. 2005;127:3463–3472. doi: 10.1021/ja045670+. [DOI] [PubMed] [Google Scholar]
- 54.Jang JH, Choi YM, Choi YY, Joo MK, Park MH, Choi BG, Kang EY, Jeong B. pH/temperature sensitive chitosan-g-(PA-PEG) aqueous solutions as new thermogelling systems. J Mater Chem. 2011;21:5484–5491. [Google Scholar]
- 55.Baroud G, Schleyer A. In: Biomechanics of cement injection in vertebroplasty. Becker S, Ogon M, editors. Austria: Springer Wien NewYork; 2008. p. 23. [Google Scholar]
- 56.Rungseevijitprapa W, Bodmeier R. Injectability of biodegradable in situ forming microparticle systems (ISM) Eur J Pharm Sci. 2009;36:524–531. doi: 10.1016/j.ejps.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 57.Chen L, Hu J, Shen X, Tong H. Synthesis and characterization of chitosan–multiwalled carbon nanotubes/hydroxyapatite nanocomposites for bone tissue engineering. J Mater Sci Mater Med. 2013:1–9. doi: 10.1007/s10856-013-4954-x. [DOI] [PubMed] [Google Scholar]
- 58.Saha R, Nix WD. Effects of the substrate on the determination of thin film mechanical properties by nanoindentation. Acta Mater. 2002;50:23–38. [Google Scholar]
- 59.Treacy M, Ebbesen T, Gibson J. Exceptionally high Young's modulus observed for individual carbon nanotubes. Lett Nat. 1996;381:678–680. [Google Scholar]
- 60.Czarnecki JS, Lafdi K, Tsonis PA. A novel approach to control growth, orientation, and shape of human osteoblasts. Tissue Eng A. 2008;14:255–265. doi: 10.1089/tea.2007.0169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.