Summary
Background
Recent phase 3 trials have shown an overall survival benefit in metastatic melanoma. We aimed to assess whether progression-free survival (PFS) could be regarded as a reliable surrogate for overall survival through a meta-analysis of randomised trials.
Methods
We systematically reviewed randomised trials comparing treatment regimens in metastatic melanoma that included dacarbazine as the control arm, and which reported both PFS and overall survival with a standard hazard ratio (HR). We correlated HRs for overall survival and PFS, weighted by sample size or by precision of the HR estimate, assuming fixed and random effects. We did sensitivity analyses according to presence of crossover, trial size, and dacarbazine dose.
Findings
After screening 1649 reports and meeting abstracts published before Sept 8, 2013, we identified 12 eligible randomised trials that enrolled 4416 patients with metastatic melanoma. Irrespective of weighting strategy, we noted a strong correlation between the treatment effects for PFS and overall survival, which seemed independent of treatment type. Pearson correlation coefficients were 0.71 (95% CI 0.29–0.90) with a random-effects assumption, 0.85 (0.59–0.95) with a fixed-effects assumption, and 0.89 (0.68–0.97) with sample-size weighting. For nine trials without crossover, the correlation coefficient was 0.96 (0.81–0.99), which decreased to 0.93 (0.74–0.98) when two additional trials with less than 50% crossover were included. Inclusion of mature follow-up data after at least 50% crossover (in vemurafenib and dabrafenib phase 3 trials) weakened the PFS to overall survival correlation (0.55, 0.03–0.84). Inclusion of trials with no or little crossover with the random-effects assumption yielded a conservative statement of the PFS to overall survival correlation of 0.85 (0.51–0.96).
Interpretation
PFS can be regarded as a robust surrogate for overall survival in dacarbazine-controlled randomised trials of metastatic melanoma; we postulate that this association will hold as treatment standards evolve and are adopted as the control arm in future trials.
Funding
None.
Introduction
Substantial advances have been made in the treatment of metastatic melanoma on the basis of insights gained into the unique molecular biology of this disease and the mechanisms by which immune effector cells are silenced. The identification of activating BRAF mutations in about 50% of advanced melanomas in 2002 was a watershed event that pointed the specialty toward a molecularly targeted treatment approach.1 In addition, identification of CTLA4 and PD1 as negative regulators of effector T-cell function that could be countered with monoclonal antibodies has made the prospect of reversal of immune tolerance a tractable therapeutic approach.2,3
Two BRAF inhibitors, vemurafenib and dabrafenib, and a MEK inhibitor, trametinib, have all proven superior to dacarbazine—the only cytotoxic chemotherapy approved by the US Food and Drug Administration for melanoma— in randomised phase 3 trials.4–6 Ipilimumab was superior to an investigational vaccine in patients refractory to chemotherapy and to dacarbazine alone when combined with dacarbazine in the first-line metastatic setting,2,7 and nab-paclitaxel improved progression-free survival (PFS) compared with dacarbazine.8 Numerous randomised trials that have compared investigational therapies with dacarbazine in recent years have failed to show a significant improvement in overall survival.9–15
As melanoma researchers continue to develop new treatment approaches to extend the effects of molecularly targeted treatments and immunotherapies, the next generation of investigational melanoma therapies will undergo definitive, randomised trials in an environment in which patients will have access to therapies with known effects on overall survival. Furthermore, targeted therapy or immunotherapy control arms are increasingly incorporated into such trials. Provided that patients remain on protocol-assigned therapy until disease progression, PFS is not confounded by post-protocol therapy in the same way that overall survival can be. If a strong correlation between treatment effects for PFS and overall survival can be established from analysis of previous randomised trials in metastatic melanoma, a significant improvement in progression-free survival noted in a future trial could be regarded as definitive evidence of clinical benefit. Establishment of such surrogacy would permit the next generation of experimental therapies in melanoma to be judged on their individual merits and diminish the risk that a potentially effective therapy is deemed ineffective because overall survival endpoints are affected by post-protocol therapy with other effective drugs. Similar analyses have been done for metastatic breast cancer and colorectal cancer. In both cases, the observed correlations between PFS and overall survival were regarded as sufficiently robust to support PFS as a surrogate endpoint in definitive phase 3 trials.16,17
Whether improved PFS can be regarded as a predictor of improved overall survival in metastatic melanoma needs to be understood. In several cancer types, including melanoma, PFS has been adopted as the primary endpoint in definitive phase 3 trials without supporting evidence in those cancers that an effect on PFS is a reliable predictor of overall survival. Therefore, we aimed to assess correlation between PFS and overall survival in randomised, dacarbazine-controlled trials of treatments for metastatic melanoma.
Methods
Search strategy and selection criteria
In September, 2013, we systematically searched Medline, Embase, the Cochrane Database of Systematic Review, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects, the National Health Service Economic Evaluation Database, and International Network of Agencies for Health Technology Assessment Database. The appendix contains full details of the search terms. We hand-searched conference abstracts to retrieve the latest studies, which had not been published in journals as full-text articles, or those that provided substantial supplemental data for previously published studies. We also searched abstracts from the following three conference proceedings from January, 2012 onward: American Society of Clinical Oncology (ASCO) annual meeting, the Society for Melanoma Research (SMR) International Melanoma Congress, and the European Society for Medical Oncology (ESMO)/European Cancer Organisation (ECCO) Congress.
We included randomised controlled trials of patients with non-resectable or metastatic melanoma that had dacarbazine as the control group and any systemic therapy as the experimental arm. We required trials to include data for hazard ratios (HRs) for overall survival and PFS, and conform to the convention of reporting HR showing benefit of experimental drug versus control (HR <1 favouring the experimental group and >1 favouring the control group).
The systematic literature review included a hand-search by two independent reviewers based at GlaxoSmithKline, who screened preselected abstracts resulting from the automated literature search. In a second step the independent reviewers also reviewed full publications.
Statistical analysis
Our analysis used overall survival and PFS data as defined and reported in the selected trials. For each trial, we extracted the data for sample sizes and HR (95% CI) for PFS and overall survival. We assessed surrogacy of PFS with overall survival by use of a correlation analysis, in which the HRs for both endpoints were considered for each study. To account for differences between studies, in terms of study size and precision of HR estimates, we weighted the analysis in proportion to the study sample size or to the precision of the observed treatment effects. In this scenario, we further distinguished between weights with a fixed-effects model and with a random-effects model, according to DerSimonian and Laird.18 The fixed-effects meta-analysis relies on the assumption of a common treatment effect underlying every study and uses the inverse variances of the estimates as weights. The random-effects meta-analysis allows for differences in the treatment effect from study to study and incorporates the underlying among-study variation of effects into the weights. In other words, the fixed-effects model assumes that the relative effect of therapy on PFS and overall survival would not vary based on type of therapy (molecularly targeted vs anti-angiogenic vs immunological), whereas the random-effects model allows for differences across the range of therapeutic mechanisms. For both models we applied the weights for the PFS endpoint. Overall, we applied three weighting strategies (sample size, fixed effects, and random effects).
We calculated weighted Pearson correlation coefficients (including 95% CI) on the basis of the natural log (HR) for PFS and overall survival, to meet our underlying linearity assumption. Because the estimate of the treatment effect on overall survival is potentially affected in studies allowing for a crossover from control to experimental drug at progression, we did several sensitivity analyses. In these analyses, we included studies without crossover, studies with a crossover rate of less than 50%, and studies using a crossover adjustment strategy. For studies with adjustment strategies, we used the rank-preserving structural failure time model (RPSFTM), according to Robins and Tsiatis.19 The RPSFTM uses a counterfactual framework to estimate the causal effect of the treatment in question, based on the randomisation done within a trial. The model assumes that if two patients have the same observed event time and neither have received treatment, those two patients would also have the same event time if they both received treatment. This assumption is linked to the associated assumptions that the treatment effect is equal for all patients irrespective of when the treatment is received (the common treatment effect assumption), and that the randomisation of the trial means that there are no differences between the treatment groups, apart from treatment allocated. The RPSFTM method has been shown to produce very low levels of bias when its assumptions are satisfied.20 Other sensitivity analysis included analysis of only phase 3 studies, restriction of analyses to large trials (>100 patients), restriction to trials with a dacarbazine dose of 1000 mg/m2, and by inclusion of mature follow-up data (vemurafenib and dabrafenib phase 3 trials).21,22
Statistical analyses were done with Comprehensive Meta Analysis version 2.2 and SAS version 9.2.
Role of the funding source
This study was designed by KTF, SJL, and DS in collaboration with GlaxoSmithKline's Department of Biostatistics (MH), without funding. All authors had full access to all of the data. The corresponding author had final responsibility for the decision to submit for publication.
Results
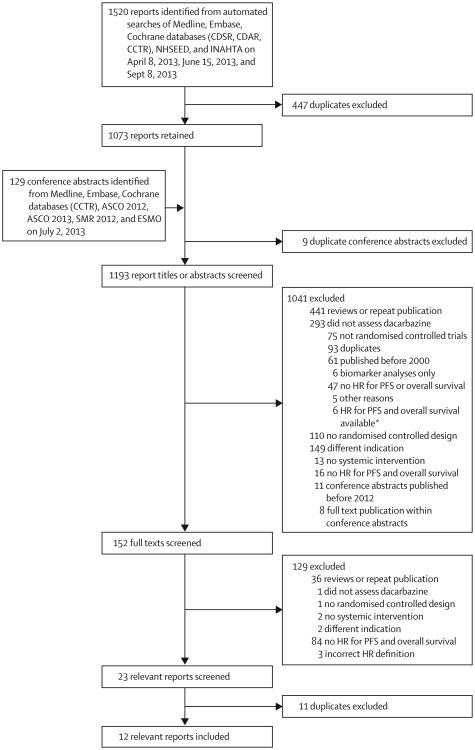
After screening of 1649 reports and meeting abstracts, we identified 12 trials4–15 that were eligible for inclusion (figure 1, table 1). One study included three comparisons between experimental therapy and control arms, so overall we included 14 comparisons (table 2). We did not require trials to be done in the first-line metastatic setting, but the requirement that dacarbazine be the control arm led to inclusion of only first-line trials.
Figure 1. Study flow chart.
*Details of these six trials are in the discussion section. CDSR=Cochrane Database of Systematic Reviews.
CDAR=Cochrane Database of Abstracts of Reviews of Effects. CCTR=Cochrane Central Register of Controlled Trials. NHSEED=National Health Service Economic Evaluation Database. INHATA=International Network of Agencies for Health Technology Assessment. ASCO=American Society of Clinical Oncology. SMR=Society for Melanoma Research. ESMO=European Society for Medical Oncology. HR=hazard ratio. PFS=progression-free survival.
Table 1. Key characteristics of the included trials.
| Population | Experimental drug | Control arm(s) | N | Crossover* | Follow-up | |
|---|---|---|---|---|---|---|
| Cui et al (2013)15 | Histologically, cytologically, or radiologically confirmed metastatic melanoma | Recombinant human endostatin plus dacarbazine | Dacarbazine plus placebo | 110 | 0% | Planned 1.5 years |
| Robert et al (2013)14 | Histologically or cytologically confirmed advanced, BRAF-mutant, cutaneous, or unknown primary melanoma | Selumetinib plus dacarbazine | Dacarbazine plus placebo | 91 | 0% | Median 12.3 months |
| Hersh et al (2012)8 | Previously untreated metastatic melanoma | Nab-paclitaxel | Dacarbazine | 529 | 0% | Not available |
| Hauschild et al (2012)5 | Stage IV or unresectable stage III, BRAFV600E, no previous systemic therapy apart from interleukin 2 | Dabrafenib | Dacarbazine | 250 | 44% | Median at cutoff date of Dec 19, 2011: 51 months for dabrafenib and 4.8 months for dacarbazine |
| Flaherty et al (2012)6 | Unresectable stage IIIC/IV, BRAFV600E/K, ≤1 previous systemic treatment | Trametinib | Dacarbazine or paclitaxel | 322 | 47% | Median at cutoff date of Oct 26, 2011: 4.9 months for trametinib and 4.8 months for chemotherapy |
| Chapman et al (2011)4† | Unresectable, previously untreated stage IIIC or stage IV | Vemurafenib | Dacarbazine | 672 | 0% | Median 3.8 months for vemurafenib and 2.3 months for dacarbazine |
| O'Day et al (2011)13 | Stage IV, no previous chemotherapy | Intetumumab 10 mg/kg, intetumumab 5 mg/kg, or dacarbazine plus intetumumab 10 mg/kg | Dacarbazine plus placebo | 129 | 63% | Patients were followed up for survival for up to 2 years |
| Robert et al (2011)7 | Previously untreated unresectable stage III or IV | Ipilimumab plus dacarbazine | Dacarbazine plus placebo | 502 | 0% | Median from start of study: 54.0 months; median from last patient randomly allocated to end of study 36.6 months |
| Patel et al (2011)12 | Stage IV, no previous chemotherapy or cytokine therapy for metastatic disease | Temozolomide | Dacarbazine | 859 | 0% | Median 19 months |
| Keff ord et al (2010)11 | Stage IV, no previous treatment with dacarbazine | Bosentan plus dacarbazine | Dacarbazine plus placebo | 80 | 0% | Not available |
| McDermott et al (2008)10 | Chemotherapy-naive patients with stage III or IV advanced melanoma (previous immunological, biological, or vaccine therapy allowed) | Sorafenib plus dacarbazine | Dacarbazine plus placebo | 101 | 0% | Not available |
| Bedikian et al (2006)9 | Unresectable stage III or IV, no previous chemotherapy | Oblimersen plus dacarbazine | Dacarbazine | 771 | 0% | Minimum 24 months |
| Overall | .. | .. | .. | 4416 | .. | .. |
Control to experimental drug.
Definitions of the intention-to-treat populations for the overall survival and for the PFS analysis are based on different criteria; for the overall survival analysis, patients who were randomly allocated at least 2 weeks before the cutoff date were included (336 patients in the experimental arm and 336 in the control arm); for the PFS analysis, patients who were randomised at least 9 weeks before the cutoff date were included (275 patients in the experimental arm and 274 in the control arm).
Table 2. Progression-free survival and overall survival estimates for 12 included randomised trials.
| Number of patients | Progression-free survival | Overall survival | ||||
|---|---|---|---|---|---|---|
| Experimental arm | Control arm (dacarbazine) | Hazard ratio | 95% CI | Hazard ratio | 95% CI | |
| Cui et al (2013)15 | 56 | 54 | 0.58 | 0.38–0.89 | 0.52 | 0.33–0.82 |
| Robert et al (2013)14 | 45 | 45 | 0.63 | 0.47–0.84* | 0.93 | 0.67–1.28* |
| Hersh et al (2012)8 | 264 | 265 | 079 | 0.63–0.99† | 0.83 | 0.58–1.20‡ |
| Hauschild et al (2012)5 | 187 | 63 | 0.30 | 0.18–0.51 | 0.61 | 0.25–1.48 |
| Flaherty et al (2012)6 | 214 | 108 | 045 | 0.33–0.63 | 0.54 | 0.32–0.92 |
| Chapman et al (2011)4§ | 336 | 336 | 0.26 | 0.20–0.33 | 0.37 | 0.26–0.55 |
| O'Day et al (2011)13 | ||||||
| Intetumumab 10 mg/kg | 33 | 32 | 1.25 | 073–214 | 0.61 | 0.35–1.07 |
| Intetumumab 5 mg/kg | 32 | 32 | 170 | 0.99–2.93 | 0.97 | 0.56–1.68 |
| Dacarbazine plus intetumumab 10 mg/kg | 32 | 32 | 079 | 0.46–1.37 | 0.78 | 0.45–1.33 |
| Robert et al (2011)7 | 250 | 252 | 076 | 0.63–0.93 | 0.72 | 0.59–0.87 |
| Patel et al (2011)12 | 429 | 430 | 0.92 | 0.80–1.06 | 1.00 | 0.86–1.17 |
| Keff ord et al (2010)11 | 40 | 40 | 1.06 | 0.66–1.70 | 1.04 | 0.58–1.87 |
| McDermott et al (2008)10 | 51 | 50 | 0.67 | 0.43–1.03 | 1.02 | 0.65–1.62 |
| Bedikian et al (2006)9 | 386 | 385 | 075 | 0.63–0.88 | 0.87 | 0.75–1.01 |
80% CI.
95.1% CI.
99.9% CI.
Definitions of the intention-to-treat populations for the overall survival and for the PFS analysis are based on different criteria; for the overall survival analysis, patients who were randomly allocated at least 2 weeks before the cutoff date were included (336 patients in the experimental arm and 336 in the control arm); for the PFS analysis, patients who were randomised at least 9 weeks before the cutoff date were included (275 patients in the experimental arm and 274 in the control arm).
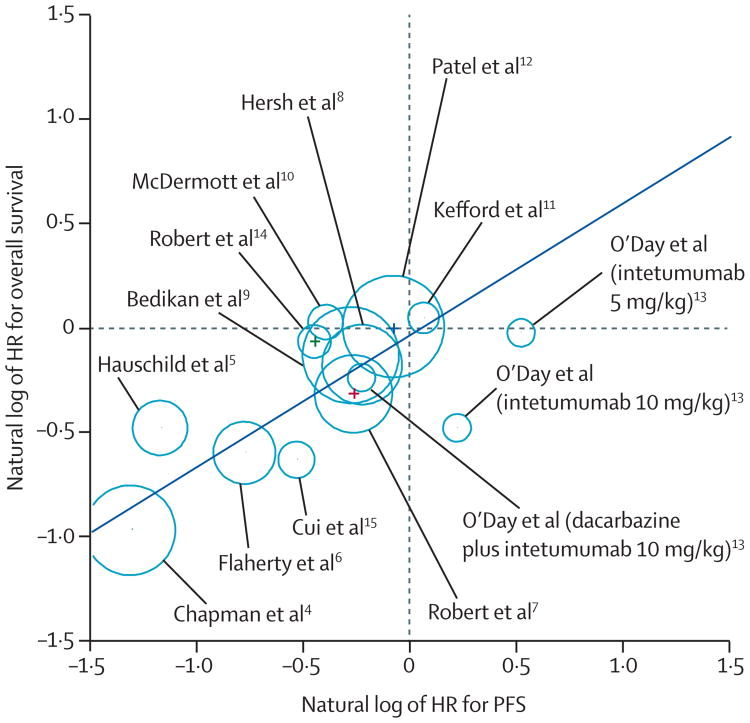
Eight comparisons reported an improvement in PFS (lower limit of the CI for HR <1.0) and four comparisons reported an improvement in overall survival. Plotting the natural log of the HRs for PFS and overall survival suggested a strong association (figure 2). This analysis assumes a linear association between the natural logarithms, but made no assumption that the absence of an improvement in PFS correlates with absence of improvement in overall survival. Thus, the observation that the PFS versus overall survival plot nearly crossed the origin was a true observation. The notable outliers were two comparisons made within a randomised phase 2 trial13 comparing two doses of intetumumab to dacarbazine for which there was no improvement in PFS, but rather in overall survival. The only other study that appeared to deviate was a dabrafenib versus dacarbazine phase 3 trial,5 in which crossover was allowed and 44% of patients in the control arm had done so at the time of reporting.
Figure 2. Correlation between treatment effects on overall survival and progression-free survival (PFS).
Size of circles is proportional to sample size. The regression equation can be used to predict the overall survival effect on the basis of an observed PFS effect; eg, an observed HR for PFS of 0.5 leads to an estimated HR for overall survival of 0.625.
We first derived a Pearson correlation coefficient by weighting for sample size and noted a strong correlation between PFS and overall survival (0.89, 95% CI 0.68–0.97). Assuming that no difference existed between type of therapy and effect on PFS and overall survival (fixed-effect model) only slightly weakened the association (0.85, 0.59–0.95), whereas allowing for different types of therapy to have a differential effect on PFS and overall survival (random-effects model) weakened the association (0.71, 0.29–0.90). Furthermore, this decreased effect on the correlation between PFS and overall survival was driven largely by the intetumumab trial,13 not the ipilimumab trial.7
In our sensitivity analyses, restriction of the analysis to phase 3 trials strengthened the correlation coefficient, which was not surprising because this method excluded the intetumumab trial for which the correlation between PFS and overall survival was especially weak (table 3).
Table 3. Sensitivity analyses.
| Weighted correlation-coefficient, R (95% CI) | |
|---|---|
| Phase 3 studies4–9,12 | |
|
| |
| Sample size | 095 (0.67 to 0.99) |
| Fixed effect | 095 (0.71 to 0.99) |
| Random effect | 0.91 (0.51 to 0.99) |
|
| |
| Studies without crossover4,7–12,14,15 | |
|
| |
| Sample size | 096 (0.81 to 0.99) |
| Fixed effect | 093 (0.70 to 0.99) |
| Random effect | 088 (0.52 to 0.97) |
|
| |
| Studies with <50% crossover4–12,14,15* | |
|
| |
| Sample size | 093 (0.74 to 0.98) |
| Fixed effect | 092 (0.71 to 0.98) |
| Random effect | 0.85 (0.51 to 0.96) |
|
| |
| Studies without crossover or crossover studies applying an RPSFTM crossover adjustment strategy4–12,14,15* | |
|
| |
| Sample size | 095 (0.83 to 0.99) |
| Fixed effect | 093 (076 to 0.98) |
| Random effect | 0.89 (0.63 to 0.97) |
|
| |
| All studies,4–15 with mature follow-up data for Chapman et al4† and Hauschild et al5‡ | |
|
| |
| Sample size | 0.55 (0.03 to 0.84) |
| Fixed effect | 0.54 (0.01 to 0.83) |
| Random effect | 041 (−0.16 to 077) |
|
| |
| Comparisons with >100 patients4–10,12,15 | |
|
| |
| Sample size | 0.93 (070 to 0.99) |
| Fixed effect | 0.93 (0.68 to 0.98) |
| Random effect | 0.84 (041 to 097) |
|
| |
| Studies with a dacarbazine doses of 1000 mg/m24–6,9,14 | |
|
| |
| Sample size | 0.91 (070 to 0.97) |
| Fixed effect | 0.88 (0.61 to 0.97) |
| Random effect | 072 (025 to 092) |
RPSFTM-adjustment data according to Latimer and colleagues18 and Abrams and colleagues.21 RPSFTM=rank-preserving structural failure time models.
For Hauschild and colleagues' study,5 the cutoff date shown in Latimer and colleagues18 was used; for Flaherty and colleagues' study,6 RPSFTM-adjustment data were used according to Abrams and colleagues.21
Crossover from control arm to highly active experimental therapy (eg, vemurafenib and dabrafenib) at the time of disease progression has the potential to weaken the correlation between PFS and overall survival if the experimental therapy has a significant effect on survival even when used late in the disease course. To eliminate the potential effect of crossover therapy, we analysed nine studies without crossover,4,7–12,14,15 including 3715 patients overall. The resulting weighted correlation coefficients (table 3) corroborated the very strong correlation between the treatment effects on PFS and overall survival. In a second step, we additionally included those studies with crossover rate of less than 50%, based on the work of Zhang and colleagues.23 The corresponding analysis was based on 11 of the 12 included publications, excluding the three comparisons from the O'Day study, and included data from 4287 patients. The resulting weighted correlation coefficients suggested a very strong correlation between the treatment effects on PFS and overall survival (table 3). However, inclusion of mature follow-up data (vemurafenib and dabrafenib phase 3 trials) weakened the correlation between PFS and overall survival (table 3).
Finally, we applied a crossover adjustment strategy for estimating the overall survival treatment effect in two studies with a crossover5,6 as previously described.21,24 The adjustment strategy was the RPSFTM in both cases. For the O'Day trial,13 no crossover adjustment data were available, therefore we decided to exclude this publication. The resulting weighted correlation coefficients remained strong (table 3).
Discussion
In our analysis of randomised, dacarbazine-controlled trials of metastatic melanoma, we noted a strong correlation between PFS and overall survival (correlation coefficients ranging from 0.55 to 0.96), irrespective of the weighting strategy applied. Randomised trials that permitted crossover to experimental therapy after disease progression on dacarbazine had the greatest effect on the correlation coefficients. Restriction of our analysis to those trials without crossover yielded a nearly perfect correlation between PFS and overall survival (correlation coefficient 0.96), whereas inclusion of trials with crossover and mature follow-up data produced a much weaker correlation coefficient (0.55).
A reliable surrogate marker for overall survival in metastatic melanoma is urgently needed, and PFS is the first available endpoint parameter for which data are available. Despite recent advances in systemic therapy, most patients with metastatic melanoma succumb to their disease and new approaches are sought. Novel targeted therapy and immunotherapy strategies, building on the benefits observed with BRAF, MEK, and CTLA4 inhibition, are already being assessed in phase 3 trials. Several ongoing randomised trials have been designed with PFS as the primary endpoint and some permit crossover at the time of disease progression on control therapy. Furthermore, patients who have not yet received the available targeted therapy or im muno therapies will have access to these treatments as back-up options. All of these issues create the immediate need for improved understanding of whether PFS can be regarded as a robust surrogate for overall survival in metastatic melanoma.
Dacarbazine has been regarded as the standard comparator arm in metastatic melanoma for a long time. Notable exceptions included trials investigating combination chemotherapy, chemoimmunotherapy, and high-dose interleukin 2: all regimens for which clinical trial eligibility and physician judgment introduce significant selection bias with regard to the fitness of enrolled patients. We preferred to focus our analysis on trials with a dacarbazine control because this therapy requires the least selection of patients. With this approach, we hoped to produce an analysis with the broadest clinical applicability and relevance. Compared with other common advanced cancers, for which significant associations between PFS and overall survival have been reported, we identified an even more robust association in dacarbazine-controlled melanoma trials. In breast cancer and colorectal cancer, Spearman rank correlation coefficients of 0.48 (95% CI 0.34–1.30) and 0.74 (0.47–0.88) have been reported and accepted as an established association between PFS and overall survival, supporting the use of PFS as a definitive endpoint of clinical benefit.16,17 We used the same methods as in previously published correlation analyses of PFS and overall survival, including both varied weighting strategies, additional exclusion criteria, and censoring or adjustment for crossover design effects. In view of all of these approaches and the range of correlation coefficient values reported, we selected a correlation coefficient of 0.85 (95% CI 0.51–0.96), which included trials with limited or no crossover and the random-effects assumption, as being representative of our findings.
Our study has limitations. By comparison with breast cancer or colon cancer, randomised trials of melanoma tend to be small and seek large treatment effects. Even after aggregation of all randomised trials that satisfied our inclusion criteria, we included only 4416 patients. This sample size accounts for the fairly wide CIs around our estimates of correlation coefficients. However, we regard even the lower boundaries of these CIs as showing strong associations between PFS and overall survival and they compare favourably with the estimates reported in other cancer types for which PFS is currently regarded as an acceptable surrogate endpoint. Notably, trials included in our analysis used RECIST as the basis for determination of PFS. For immunotherapy, new and more permissive criteria have been proposed that allow continuation of protocol therapy in the face of some cases of RECIST progression. Another limitation is that this meta-analysis was based on published data from 12 trials, and therefore a potential publication bias cannot be excluded. Further heterogeneity might have been introduced through the approaches used for calculation of the HRs, which differed slightly between the published trials.
Mature follow-up data were not available from all studies included in our analysis. Inherently, trials with relatively short follow-up will have their results affected by those patients with the most aggressive disease and the different effects of experimental versus control therapy can be difficult to discern for patients with indolent disease. Analyses such as ours thus gain most of their strength from those subpopulations with aggressive disease in which progression and survival events were reported. The most notable concern about our analysis is whether these observations will continue to hold in future years as further investigation of molecularly targeted treatments and immunotherapy occurs, and new control group therapies are adopted. Although our analysis included a large number of therapies targeting tumour cells directly or the tumour microenvironment, only one immunotherapy trial was included. We were reassured by the observation that inclusion or exclusion of the ipilimumab trial did not significantly affect the estimated correlation coefficient (figure 1). In this trial,7 the HR for PFS was 0.76 and overall survival was 0.72, reinforcing our hypothesis that PFS and overall survival effects are closely related in melanoma. Although we did not include the phase 3 trial of another CTLA4 blocking antibody (tremelimumab), the effect on PFS and overall survival seemed to be highly correlated as well.25 In this trial, patients on the dacarbazine arm underwent radiographic restaging every 6 weeks, whereas patients on the tremelimumab arm were restaged every 12 weeks. This method precluded derivation of Kaplan-Meier PFS. At 6 months, both populations were assessed synchronously and PFS was 20.5% for tremelimumab and 18.2% for dacarbazine (with an 11% reduction in relapse rate for tremelimumab). The reported HR for overall survival was 1.14 in favour of the experimental arm. Thus, both dacarbazine-controlled CTLA4 blocking antibody trials had a very close association between PFS and overall survival.
We believe that the robust association between PFS and overall survival in melanoma is most attributable to the fact that melanoma is an aggressive disease and that patients have not previously had the opportunity to pursue multiple lines of effective therapy. The opposite scenario in breast cancer has been suggested to explain the weaker correlation between PFS and overall survival noted in randomised trials done in the metastatic setting. Thus, although our results only provide direct evidence of the surrogacy of PFS for overall survival in dacarbazine-controlled trials, we postulate that this association would hold even as new treatment standards evolve and are included as the control arm. Beyond our statistical analysis of dacarbazine-controlled trials, we examined six randomised trials (making seven comparisons) that were excluded only because they lacked a dacarbazine control arm.2,26–30 Averaging of hazard ratios from these trials yields a mean PFS HR of 0.83 and an overall survival HR of 0.89, and supports the observed correlation between PFS and overall survival in dacarbazine-controlled trials. We did not intentionally censor second-line trials, however such studies had been far outnumbered by first-line trials and dacarbazine would generally be considered a standard comparator in previously untreated patients. It is beyond the scope of this analysis to suggest a magnitude of effect on PFS that would predict a clinically meaningful effect on overall survival. We regard establishment of a threshold as a matter for regulatory and health authorities accounting for costs such as a treatment-related toxicity or cost-effectiveness.
The most important outcomes of our analysis would be an evolution in the regulatory standards by which new melanoma therapies are considered and approved, and in the weight of evidence given to PFS by other bodies that set treatment guidelines. A move towards a clinical trial design standard in which PFS is used as the primary endpoint would allow small randomised trials to be done and allow for more efficient vetting of new treatment regimens. Furthermore, this approach would minimise the risk of abandoning potentially effective new treatments when overall survival endpoints are contaminated by post-protocol therapy with other effective drugs. Our results suggest that PFS can confidently be used as a reliable surrogate endpoint for overall survival in metastatic melanoma.
Supplementary Material
Acknowledgments
We thank Toni Ribas, the principal investigator of the tremelimumab versus dacarbazine phase 3 trial for providing clarifications about efficacy outcomes in that trial.
DS has received research funding from Merck, and has had consultancies or participated in advisory boards with GlaxoSmithKline, Roche, Bristol Myers-Squibb, Merck, Amgen, Delcath, and Novartis. KTF has had consultancies with Roche, GlaxoSmithKline, Celgene, and Novartis. GVL is consultant advisor to GlaxoSmithKline, Roche, Novartis, Bristol Myers-Squibb, and Amgen, and has received honoraria from GlaxoSmithKline and Roche. AM has participated in advisory boards with GlaxoSmithKline, Roche, and Bristol Myers-Squibb. PAA has received research funding from Bristol Myers-Squibb, and has had consultancies or participated in advisory boards for Bristol Myers-Squibb, Roche-Genentech, GlaxoSmithKline, and Novartis. PMP has had consultancies or participated in advisory boards with GlaxoSmithKline, Roche, Bristol Myers-Squibb, and Schering Plough Merck. CR is consultant for GlaxoSmithKline, Roche, Novartis, Bristol Myers-Squibb, Merck, Amgen, and Bayer. AH is consultant advisor and received honoraria from Roche, Genentech, Amgen, GlaxoSmithKline, Bristol Myers Squibb, Merck/MSD, Pfizer, and Novartis. RK has received institutional reimbursement for advisory board participation from GlaxoSmithKline, Novartis, Roche, BMS, and Sanofi-Aventis and institutional reimbursement-honoraria for educational symposia from Merck. SO'D has received research support from Merck, Roche, BMS, and Novartis and served on the speakers bureau of Merck and BMS. MH is an employee at GlaxoSmithKline in Germany. PL has had consultancies or participated in advisory boards of BMS, Roche, GlaxoSmithKline, Novartis, and Celgene. GM has had consultancies or participated in advisory boards with GlaxoSmithKline, Roche, Bristol Myers-Squibb, and Schering Plough/Merck. AMME received honorary fees for advisory board participation for Amgen, BMS, GlaxoSmithKline, MedImmune, and Merck. JMK has received honorary fees for advisory board participation for BMS, GlaxoSmithKline, Celgene, Vical, and Merck.
Footnotes
Contributors: All authors were involved in collection and interpretation of data. KTF, HM, SJL, and DS designed the analysis and had full access to the raw data. MH did the initial statistical analysis and the approaches and results were verified by SJL. KTF and DS created the tables and figures. All authors had the opportunity to review the analysis plan and outcome, participated in the preparation of this report, and provided final approval.
Conflicts of interest: All other authors declare that they have no conflicts of interest.
Contributor Information
Keith T Flaherty, Center for Melanoma, Massachusetts General Hospital Cancer Center, Boston, MA, USA.
Michael Hennig, Biostatistics and Epidemiology, GlaxoSmithKline, Munich, Germany.
Sandra J Lee, Department of Biostatistics and Computational Biology, Dana-Farber Cancer Institute and Harvard Medical School, Boston, MA, USA.
Paolo A Ascierto, Unit of Melanoma, Cancer Immunotherapy and Innovative Therapy—Istituto Nazionale Tumori Fondazione “G Pascale”, Napoli, Italy.
Reinhard Dummer, Prof, Department of Dermatology, University Hospital Zurich, Zurich, Switzerland.
Alexander M M Eggermont, Prof, Gustave Roussy Cancer Campus, Paris-Sud University Grand Paris, Villejuif, France.
Axel Hauschild, Department of Dermatology, University Hospital Schleswig-Holstein (UKSH), Campus Kiel, University Hospital Kiel, Kiel, Germany.
Richard Kefford, Prof, Westmead Hospital and Melanoma Institute Australia, University of Sydney, NSW, Australia.
John M Kirkwood, Prof, Skin Cancer Program University of Pittsburgh Cancer Institute, Pittsburgh, PA, USA.
Georgina V Long, Melanoma Institute Australia and the University of Sydney, Sydney, NSW, Australia.
Paul Lorigan, Institute of Cancer Sciences, Faculty of Medical & Human Sciences, University of Manchester, Manchester, UK.
Andreas Mackensen, Prof, Department of Internal Medicine 5—Hematology/Oncology, University of Erlangen, Erlangen, Germany.
Grant McArthur, Peter MacCallum Cancer Institute, St Andrews Place, East Melbourne, VIC, Australia.
Steven O'Day, Beverly Hills Cancer Center, Beverly Hills, CA, USA.
Poulam M Patel, Academic Unit of Oncology, University of Nottingham, Nottingham, UK.
Caroline Robert, Dermatology and INSERM Unit 981 Gustave Roussy Cancer Campus and Paris-Sud University Grand Paris, Villejuif, France.
Dirk Schadendorf, Prof, Department of Dermatology, University Hospital Essen, Essen, Germany.
References
- 1.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–65. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 6.Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–14. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 7.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 8.Hersh E, Del Vecchio M, Brown M, Kefford R, Loquai C, Testori A. Phase 3, randomized, open-label, multicenter trial of nab-paclitaxel (nab-P) vs dacarbazine (DTIC) in previously untreated patients with metastatic malignant melanoma (MMM) Pigment Cell Melanoma Res. 2012;25:863. [Google Scholar]
- 9.Bedikian AY, Millward M, Pehamberger H, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24:4738–45. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- 10.McDermott DF, Sosman JA, Gonzalez R, et al. Double-blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: a report from the 11715 study group. J Clin Oncol. 2008;26:2178–85. doi: 10.1200/JCO.2007.14.8288. [DOI] [PubMed] [Google Scholar]
- 11.Kefford RF, Clingan PR, Brady B, Ballmer A, Morganti A, Hersey P. A randomized, double-blind, placebo-controlled study of high-dose bosentan in patients with stage IV metastatic melanoma receiving first-line dacarbazine chemotherapy. Mol Cancer. 2010;9:69. doi: 10.1186/1476-4598-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel PM, Suciu S, Mortier L, et al. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032) Eur J Cancer. 2011;47:1476–83. doi: 10.1016/j.ejca.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 13.O'Day S, Pavlick A, Loquai C, et al. A randomised, phase II study of intetumumab, an anti-alphav-integrin mAb, alone and with dacarbazine in stage IV melanoma. Br J Cancer. 2011;105:346–52. doi: 10.1038/bjc.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robert C, Dummer R, Gutzmer R, et al. Selumetinib plus dacarbazine versus placebo plus dacarbazine as first-line treatment for BRAF-mutant metastatic melanoma: a phase 2 double-blind randomised study. Lancet Oncol. 2013;14:733–40. doi: 10.1016/S1470-2045(13)70237-7. [DOI] [PubMed] [Google Scholar]
- 15.Cui C, Mao L, Chi Z, et al. A phase II, randomized, double-blind, placebo-controlled multicenter trial of Endostar in patients with metastatic melanoma. Mol Ther. 2013;21:1456–63. doi: 10.1038/mt.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burzykowski T, Buyse M, Piccart-Gebhart MJ, et al. Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J Clin Oncol. 2008;26:1987–92. doi: 10.1200/JCO.2007.10.8407. [DOI] [PubMed] [Google Scholar]
- 17.Tang PA, Bentzen SM, Chen EX, Siu LL. Surrogate end points for median overall survival in metastatic colorectal cancer: literature-based analysis from 39 randomized controlled trials of first-line chemotherapy. J Clin Oncol. 2007;25:4562–68. doi: 10.1200/JCO.2006.08.1935. [DOI] [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Robins JM, Tsiatis AA. Correcting for noncompliance in randomized trials using rank preserving structural failure time models. Commun Stat Theory Methods. 1991;20:2609–31. [Google Scholar]
- 20.Morden JP, Lambert PC, Latimer N, Abrams KR, Wailoo AJ. Assessing methods for dealing with treatment switching in randomised controlled trials: a simulation study. BMC Med Res Methodol. 2011;11:4. doi: 10.1186/1471-2288-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latimer N, Abrams KR, Amonkar M, Stapelkamp C, Swann RS. Adjusting for treatment crossover in the BREAK-3 metastatic melanoma trial for dabrafenib: Preliminary analysis. Proc Am Soc Clin Oncol. 2013;31(suppl) abstr 9044. [Google Scholar]
- 22.National Institute for Health and Care Excellence. NICE technology appraisal guidance 269. [accessed Jan 9, 2014]; guidance.nice.org.uk/ta269. [PubMed]
- 23.Zhang L, Ko CW, Tang S, Sridhara R. Relationship between progression-free survival and overall survival benefit: a simulation study. Therapeutic Innovation & Regulatory Science. 2013;47:95–100. doi: 10.1177/0092861512459180. [DOI] [PubMed] [Google Scholar]
- 24.Abrams KR, Latimer N, Amonkar M, Stapelkamp C, Casey M. Adjusting for treatment crossover in the METRIC metastatic melanoma (MM) trial for trametinib: preliminary analysis. Proc Am Soc Clin Oncol. 2013;31(suppl) abstr 9040. [Google Scholar]
- 25.Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31:616–22. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keilholz U, Punt CJ, Gore M, et al. Dacarbazine, cisplatin, and interferon-alfa-2b with or without interleukin-2 in metastatic melanoma: a randomized phase III trial (18951) of the European Organisation for Research and Treatment of Cancer Melanoma Group. J Clin Oncol. 2005;23:6747–55. doi: 10.1200/JCO.2005.03.202. [DOI] [PubMed] [Google Scholar]
- 27.Atkins MB, Hsu J, Lee S, et al. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2008;26:5748–54. doi: 10.1200/JCO.2008.17.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiarion-Sileni V, Guida M, RidolfiL, et al. Central nervous system failure in melanoma patients: results of a randomised, multicentre phase 3 study of temozolomide- and dacarbazine-based regimens. Br J Cancer. 2011;104:1816–21. doi: 10.1038/bjc.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27:2823–30. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 30.Maio M, Mackiewicz A, Testori A, et al. Large randomized study of thymosin alpha 1, interferon alfa, or both in combination with dacarbazine in patients with metastatic melanoma. J Clin Oncol. 2010;28:1780–87. doi: 10.1200/JCO.2009.25.5208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.