Summary
Stem cell-based therapies hold considerable promise for many currently devastating neurological disorders. Substantial progress has been made in the derivation of disease-relevant human donor cell populations. Behavioral data in relevant animal models of disease have demonstrated therapeutic efficacy for several cell-based approaches. Consequently, GMP grade cell products are currently being developed for first in human clinical trials in select disorders. Despite the therapeutic promise, the presumed mechanism of action of donor cell populations often remains insufficiently validated. It depends greatly on the properties of the transplanted cell type and the underlying host pathology. Several new technologies have become available to probe mechanisms of action in real time and to manipulate in vivo cell function and integration to enhance therapeutic efficacy. Results from such studies generate crucial insight into the nature of brain repair that can be achieved today and push the boundaries of what may be possible in the future.
Introduction
Most degenerative, vascular, inflammatory or traumatic neurological diseases lead to an irreversible demise of brain tissue at some point during the disease course which commonly goes along with deteriorating physical or intellectual function. Apart from the limited potential for endogenous regeneration in the human brain, which can be enhanced by rehabilitative training, treatment of such disorders is largely symptomatic. Symptomatic treatment usually involves the modulation of neurotransmitter systems and, for a growing number of pathologies, deep brain stimulation. However, symptomatic therapies often achieve only transient and partial efficacy and remain ineffective for several disorders. The identification of disease modifying drugs is highly desirable and is being pursued by the pharmaceutical industry (AlDakheel et al., 2014; Caraci et al., 2013). However, for most neurological disorders such drugs have not yet reached the clinic with a few notable exceptions such as in the case of relapsing-remitting multiple sclerosis (Smith et al., 2010). Given this medical dilemma, which represents a major socio-economic burden for many ageing societies, experimental stem cell therapies hold considerable promise for brain repair. Research activities in neural transplantation have steadily increased since the initial reports of fetal tissue grafting in experimental models of Parkinson’s disease (PD) (Brundin et al., 1986; Dunnett et al., 1981) followed by early clinical trials in PD (Lindvall et al., 1990; Lindvall et al., 1989) and HD patients (Bachoud-Levi et al., 2000; Reuter et al., 2008). Here we review the progress and remaining challenges towards the generation of unlimited numbers of defined human donor cell populations with therapeutic relevance to CNS disorders. We continue to describe the benefits and caveats that go along with the use of these cell populations in preclinical studies and impending clinical trials. We highlight the use of emerging technologies, which are geared towards increasing therapeutic efficacy, mapping connectivity or interrogating mechanisms and therapeutic rationale. The potential for endogenous regeneration has been reviewed elsewhere recently (Dimyan and Cohen, 2011; Saha et al., 2012) and is not discussed here except for selective examples that highlight specific mechanisms or experimental approaches. We acknowledge, that many therapeutic principles have been first described using rodent primary or mouse embryonic stem cell derived donor cells. However, since this review focuses on the prospect for human therapy, studies employing non-human cells are only mentioned if they demonstrate a unique principle not yet recapitulated with human cells.
I. Generation of neural cell types from various sources
Primary cells
While a number of non-neural tissue sources such as adrenal medulla autografts in Parkinson’s disease (PD) have been used in the past (Backlund et al., 1985; Madrazo et al., 1987), the main era of neurotransplantation started with the use of fetal brain tissue as human donor tissue source. Early preclinical studies employed rodent (Dunnett et al., 1981), and later human (Brundin et al., 1986) cells derived from the fetal ventral midbrain in experimental models of PD. These studies provided strong evidence for the survival and therapeutic efficacy of mesencephalic dopaminergic grafts. As a consequence, the first clinical transplantation trials utilizing these cells in PD patients ensued swiftly. Despite promising data indicating motor recovery in the initial open label studies (Lindvall et al., 1990; Lindvall et al., 1989; Wenning et al., 1997) the two double-blind, placebo-controlled trials in PD patients (Freed et al., 2001; Olanow et al., 2003) failed to reach their primary endpoints. These studies also revealed graft induced dyskinesias as a troubling side effect, which may be caused by contaminating serotonergic neurons in the donor cell population (Politis et al., 2010) though other factors may contribute as well. In some cases however, grafts have been shown to survive for more than 15 years, to grow axonal projections and to secrete dopamine as shown by [18F]Fluorodopa PET scans and postmortem analysis. Also, subgroup analysis revealed significant effects in patients receiving a transplant under the age of 60 and patients followed for longer periods of time (Ma et al., 2010). Therefore, multiple factors such as patient selection (age, disease severity, L-Dopa responsiveness), trial design (target site, immunosuppression, end points) as well as issues related to the donor cell populations are likely critical factors for success (Barker et al., 2013a). The donor cell populations in those studies varied with respect to gestational age, number of donors, pre-transplantation derivation and storage as well as dopamine neuron content. However, beyond confounding biological and technical factors, obtaining up to seven donors simultaneously for transplantation of a single PD patient represents a serious logistical challenge and raises ethical concerns for a disease affecting millions of patients. In a related approach, Huntington’s disease (HD) patients have been grafted with fetal striatal tissue in small open label studies as well as in ongoing multicenter efforts involving several hundred patients. Long-term follow up in at least a subset of those studies suggests benefits on motor and cognitive function for a period of several years following transplantation (Bachoud-Levi et al., 2006; Bachoud-Levi et al., 2000; Reuter et al., 2008). Functional benefits may correlate with the extent of graft survival as determined by PET imaging (Bachoud-Levi et al., 2006; Barker et al., 2013b; Reuter et al., 2008), though many other factors likely contribute.
Primary neural stem cells
Given the concerns related to the use of primary fetal cells, a major focus in the field has been the generation of scalable cell populations that can be developed into standardized and quality controlled products for future therapeutic use. The ability to isolate neural stem cells in vitro and evidence of lifelong neurogenesis in some regions of the mammalian brain, reviewed in (Gage and Temple, 2013), argue for neural stem cells as one potential cell source. In vitro expanded rat fetal midbrain precursors have been shown to recover motor deficits in Parkinsonian animals (Studer et al., 1998), but the extent of cell expansion is limited using this approach and has never been developed into robust technology for use with human cells (Cave et al., 2014; Sanchez-Pernaute et al., 2001). Human neural stem cells obtained from the fetal telencephalon were shown to be expandable in vitro after immortalization (Flax et al., 1998), as neurospheres (Caldwell et al., 2001; Uchida et al., 2000) or in adherent monolayer cultures (Sun et al., 2008) in the presence of epidermal and/or fibroblast growth factors (EGF, FGF). Similarly, multipotent neural progenitor cells were expanded from several regions of the adult human brain (Nunes et al., 2003; Walton et al., 2006). These populations were shown to survive transplantation into immuno-compromised rodents and differentiate mostly into neurons and astrocytes in vivo. However, fate specification and therapeutic potential of the resulting neurons appears restricted and differentiation into defined, authentic neuronal subtypes such as striatal projection neurons or midbrain dopamine neurons has never been shown. In fact, continuous exposure to mitogens such as EGF and FGF in the absence of additional patterning factors seems to interfere with the fate potential of the region of origin (Jain et al., 2003). In addition, the progressive switch from neurogenesis to gliogenesis occurring in many of the primary expanded populations (Naka-Kaneda et al., 2014; Patterson et al., 2014) can further complicate the use of neural stem cells in regenerative therapies. Nevertheless, despite their limited differentiation potential, neural stem cells are currently being tested in a variety of preclinical and clinical applications (see section 2).
Neural cells derived from pluripotent stem cells
The isolation of human embryonic stem cells (hESCs) (Thomson et al., 1998) and subsequently induced pluripotent stem cells (iPSCs) (Takahashi et al., 2007) offered a new strategy to potentially generate any cell type in unlimited numbers. The generation of differentiated neural cell types from human embryonic stem cells (Reubinoff et al., 2001; Zhang et al., 2001) was followed by the derivation of several developmentally distinct neural stem cell (NSC) populations (Elkabetz et al., 2008; Koch et al., 2009) reviewed in (Conti and Cattaneo, 2010). These pluripotent stem cell (PSC)-derived NSC populations displayed properties comparable to primary NSC sources with respect to expandability and differentiation potential with improved but still limited ability to control neuron subtype specific fates. In parallel, several strategies were developed to generate region specific neurons from human PSCs without relying on a NSC intermediate. The strategy of directed differentiation has gradually evolved towards defined culture systems. Initial protocols commonly made use of stromal feeder cells or embryoid body cultures to enhance neural induction and further neuronal differentiation (Roy et al., 2006; Vazin et al., 2008). The increasing understanding of processes that regulate early mammalian CNS development and the availability of recombinant morphogens and growth factors led to a transition towards more defined differentiation protocols (Pera et al., 2004). An additional level of sophistication and efficacy was achieved with the use of small molecules, which activate or inhibit key developmental pathways such as WNT, SHH, Activin/NODAL, BMP, TGF signaling (Smith et al., 2008). Harnessing these developments, a rapid, highly efficient and surprisingly facile protocol has been devised, which generates PAX6+ primitive neuroectoderm within 10 days by inhibition of TGFβ and BMP signaling, also known as dual SMAD inhibition (dSMADi) (Chambers et al., 2009). The most attractive feature of dSMADi however is its malleability and modularity. Using the timed addition of one or several other patterning factors, a multitude of disease-relevant human neural cell populations can been derived in a systematic manner and with unprecedented efficiency and purity.
Projection neurons
Excitatory glutamatergic projection neurons represent the main building blocks of the human telencephalon (Lui et al., 2011) and are affected in a large number of neurological diseases with different etiology. Interestingly, most neural differentiation protocols, whether feeder or embryoid body (EB) based (Elkabetz et al., 2008; Koch et al., 2009; Li et al., 2005) or based on any form of SMAD inhibition (Chambers et al., 2009; Espuny-Camacho et al., 2013) pass through a dorsal telencephalic PAX6/OTX2 double positive intermediate. The acquisition of telencephalic fates is believed to represent a ground state of neuroepithelial cells during human PSC differentiation in the absence of additional patterning factors. Small molecule inhibition of canonical WNT signaling was shown to further enhance telencephalic (FOXG1) induction (Maroof et al., 2013). Further in vitro differentiation of such ground state telencephalic cultures mimics the sequential generation of cortical layers based on the expression of layer-specific markers. However, extended in vitro differentiation periods of 100 days or longer are required to efficiently generate upper layer neurons under those conditions (Eiraku et al., 2008; Espuny-Camacho et al., 2013; Shi et al., 2012; van de Leemput et al., 2014). Morphologically, the resulting neurons have a pyramidal shape and express markers of glutamatergic cortical neurons. Ongoing studies are focused on further directing cell fates towards specific cortical areas such as frontal or occipital cortex or the cortical hem. For example, a recent study has shown the derivation of cortical precursors enriched in hippocampal granule neurons following exposure of cortical neuroepithelial precursors to Wnt3a (Yu et al., 2014).
Interneurons
Cortical and striatal interneurons arise from the ganglionic eminence, a ventral forebrain structure in the developing mammalian brain and undergo tangential migration to reach their appropriate targets (Anderson et al., 2001). Human PSC-derived primitive ground state neuroepithelium can be ventralized using SHH agonists to induce robust NKX2.1 expression (Liu et al., 2013; Maroof et al., 2013; Nicholas et al., 2013). Several classes of interneurons and other ventral forebrain cell types (i.e basal forebrain cholinergic neurons) may be generated by varying the timing of SHH activation (Liu et al., 2013; Maroof et al., 2013). Cortical interneurons initially display an immature GABAergic phenotype and mature functionally over extended periods of time in vitro and in vivo (Maroof et al., 2013; Nicholas et al., 2013). It is currently unclear what percentage of hPSC-derived interneurons correspond to striatal versus cortical interneurons and the current protocols appear to generate primarily somatostatin+ rather than parvalbumin+ interneurons. This is likely due to the fact that parvalbumin expression occurs late at postnatal stages of development and may not be detectable at the time of analysis in most interneuron protocols. A recent protocol has reported the derivation of interneurons enriched for a Calretinin+ fate suggesting a caudal ganglionic eminence origin of those neurons (Nestor et al., 2014).
Dopamine neurons
The generation of midbrain dopamine neurons for cell replacement therapy in PD has been a longstanding goal. Accordingly, several groups have reported the derivation of midbrain dopamine neuron-like cells that were generated through a neuroepithelial intermediate and the addition of SHH agonists and FGF8 (Perrier et al., 2004; Sonntag et al., 2007; Yan et al., 2005). However these cells did not display all the cardinal features of authentic midbrain DA neurons and did not survive well after transplantation. In contrast, the stepwise recapitulation of developmental processes, namely the generation of a midbrain floorplate intermediate by activation of SHH signaling (Fasano et al., 2010) followed by their neurogenic conversion through WNT activation ultimately produced cells with transcriptional, biochemical and physiological features of bona fide midbrain dopamine neurons (Kirkeby et al., 2012; Kriks et al., 2011). These protocols have been validated by various independent groups (Doi et al., 2014; Sundberg et al., 2013; Xi et al., 2012). A remaining challenge in the directed differentiation of midbrain dopamine neurons from human PSCs is the selective derivation of substantia nigra (SN, A9) as opposed to ventral tegmental area (VTA, A10) dopamine neurons. Despite claims in some papers to selectively generate cells of A9 identity, formal proof that this has been achieved remains pending. The main challenge is the lack of specific early markers of A9 versus A10 subtypes. Current markers such as Calbindin for A10 or GIRK2 for A9 subtype identity appear not sufficiently specific to resolve cell identity at early stages of dopamine neuron development. Therefore functional studies such as migration assays, axonal pathfinding assays (Cord et al., 2010; Grealish et al., 2014) or careful physiological experiments (Chan et al., 2010) will be required to validate A9 versus A10 properties of grafted cells in vitro and in vivo. In addition, expression profiling including single cell analysis as has been done for rodent dopamine neurons (Panman et al., 2014; Poulin et al., 2014) will be necessary to compare bona fide human fetal ventral midbrain explants to their PSC derived counterparts.
Motor neurons
Spinal motoneurons (MNs) are affected in several degenerative neurological diseases (e.g. amyotrophic lateral sclerosis or spinal muscular atrophy) as well as at the site of local damage in spinal cord injury. Therefore, the generation of this cholinergic neuronal cell type has been at the center of intense research efforts since human pluripotent cells became available, reviewed in (Davis-Dusenbery et al., 2014). Similar to dopamine neurons, spinal motoneurons can be generated in vitro from human PSCs through the recapitulation of developmental signals. Neural induction initially relied on embryoid body formation (Boulting et al., 2011; Karumbayaram et al., 2009; Li et al., 2005; Singh Roy et al., 2005) or co-culture with neural inducing cells such as MS5 or PA6 (Lee et al., 2007). The use of SMAD inhibitors for neuralization (Amoroso et al., 2013; Patani et al., 2011) has simplified, accelerated and boosted efficacy of spinal MN production (Boulting et al., 2011). Most studies have relied on the use of high dose retinoic acid (RA) for caudalization and SHH or one of its small molecule agonists for ventralization of primitive neuroepithelium, resulting in MNs with a mostly cervical/brachial HOX code. A combinatorial small molecule screening approach revealed the derivation of cranial in addition to spinal motoneurons (Maury et al., 2014) and further optimized yield. The recapitulation of MN diversity and subtype specification within the spinal cord is however less well understood. Reducing or eliminating RA treatment during MN induction can yield fates posterior to the brachial/cervical level though at the expense of MN yield (Peljto et al., 2010). In addition, FGF and WNT signaling pathways have been reported to induce more caudal MN fates (Mazzoni et al., 2013). However, those findings are awaiting implementation in human cells. Also, recreating the intricate columnar organization of MNs in the spinal cord, which defines axonal projections to specific muscle groups, remains a challenge to the directed differentiation field (Amoroso et al., 2013; Patani et al., 2011).
Striatal medium spiny neurons
Another neuronal cell type of great interest in regenerative medicine are neostriatal medium spiny projection neurons, which degenerate in Huntington’s disease (HD). A major motivating factor for the directed differentiation of hPSCs into medium spiny neurons are the ongoing clinical trials in HD using human fetal tissue to replace functional medium spiny neurons lost in the disease. Considerable progress has been made over the last few years starting with feeder-based hPSC neuronal induction protocols and low dose treatment with both SHH agonists and WNT inhibitors (Perrier et al., 2008). More recent refinements of this protocol have improved efficiency and demonstrated the authentic nature and in vivo potential of PSC-derived medium spiny neurons (Delli Carri et al., 2013; Ma et al., 2012).
Oligodendrocytes
Significant progress has also been achieved regarding the generation of myelinating oligodendrocytes (Hu et al., 2009; Wang et al., 2013). Oligodendrocytes have a broad potential in regenerative medicine ranging from applications in spinal cord injury to the use in various genetic, chemical or radiation-induced demyelinating disorders (Goldman et al., 2012). The guiding principles behind the generation of medium spiny neurons and oligodendrocytes and their significance in regenerative medicine have been discussed in more detail recently (Tabar and Studer, 2014).
Pluripotent cell sources
The prospect of generating patient specific human pluripotent cells (Takahashi et al., 2007) for regenerative therapies has been hailed as a breakthrough on the road to personalized medicine. However, several scientific and economic considerations make this a challenging approach to enter routine clinical use. First of all, the timeframe and resources required to generate GMP quality autologous iPSCs and differentiated transplantable progeny for every single patient seem to preclude mass application with current technology. An alternative could be the development of iPSC banks homozygous for major HLA haplotypes, which would serve as universal donors. This strategy could result in the generation of about 150 iPSC or hESC lines with sufficient homology to >90% of a population such as the UK (Turner et al., 2013). While an attractive approach, there remains some controversy regarding the immunogenicity of even syngenic iPSC-derived transplants in mice, reviewed in (Fu, 2014). Furthermore, the immunogenicity of human cells, whether autologous, HLA matched or unmatched can only be inadequately assessed in preclinical models, such as in mice with humanized immune systems. In one such study reduced immunogenicity of unmatched hESC-derived tissue has been reported (Drukker et al., 2006). Also the need for autologous or matched donor cells, especially for transplantation into the immunoprivileged CNS (Muldoon et al., 2013) is not entirely clear. Early fetal grafting trials showed that unmatched donor neurons survive for more than a decade in patient brains after cessation of immunosuppression (Cooper et al., 2009; Li et al., 2010). Nonetheless clinical outcomes of these trials were suboptimal, which could in part relate to chronic inflammation or actual immune rejection of the graft (Barker et al., 2013a). The acquisition of genetic and epigenetic alterations occurs in any cultured cell type (Narva et al., 2010). However, reprogramming to pluripotency may confer an additional risk (Pera, 2011). Lately, reprogramming to pluripotency has also been achieved in human cells by somatic cell nuclear transfer (SCNT) into oocytes (Tachibana et al., 2013; Yamada et al., 2014). Genetic comparisons of iPSCs to isogenic SCNT-ESCs have produced contradictory results (Ma et al., 2014). In a recent study, high quality iPSCs generated by RNA reprogramming showed similar frequencies of coding mutations and loss of imprinting as SCNT ESCs (Johannesson et al., 2014). Given all these variables, the optimal pluripotent cell source for clinical translation remains to be determined.
Alternative cell sources and direct reprogramming
In addition to primary and pluripotent-derived cell types there is increasing interest in deriving specific neural cell types using direct reprogramming approaches. The use of transcription factors to enforce ectopic cell fates in somatic cell types builds on a long history of basic cell fate specification studies such as the derivation of skeletal muscle cells from fibroblasts by MyoD, or the expression of C/EBPa to convert pre-B cells or even fibroblasts into macrophages, reviewed in (Graf and Enver, 2009). However this approach has only received serious consideration for regenerative medicine applications since the Wernig lab demonstrated the efficient conversion of fibroblasts into functional neurons both in mouse and human cells (Pang et al., 2011; Vierbuchen et al., 2010). Subsequently it was shown that not only transcription factors but also miRNAs (Ambasudhan et al., 2011; Yoo et al., 2011) can induce new cell fates. Several of the cell types discussed previously such as midbrain dopamine neurons, motoneurons, oligodendrocytes or cortical-like neurons have been reported (Ang and Wernig, 2014). However, their therapeutic potential remains insufficiently validated. Particular points of concern relate to the genetic modification of the cells and the fact that these strategies often yield postmitotic neurons with unclear scalability and engraftment capabilities. In addition, it is also unclear how faithfully transdifferentiated cells adopt the intended fate and how completely the transcriptional program of the cell of origin is silenced. To address this issue, a novel computational tool called CellNet has been introduced (Morris et al., 2014). It allows to quantitatively compare whether engineered cells are equivalent to their primary target population with respect to gene expression. Other cutting edge approaches in the field of cell fate conversion include the cell fate switch of various cortical neuron subtypes based on the increasing understanding of the transcriptional circuits underlying cell fate identity within the telencephalon (Amamoto and Arlotta, 2014), and the conversion of endogenous brain tissue into disease-relevant cells in vivo (De la Rossa et al., 2013; Niu et al., 2013; Rouaux and Arlotta, 2013). While a fascinating perspective, the therapeutic potential of this approach remains to be determined. The need for the intracerebral injection of viral vectors and the possibility of generating partially reprogrammed cells represent additional risks. Also, the strategy utilizes resident cells, whose original function would likely be lost after transdifferentiation with unclear consequences for host brain function.
II. Efficacy in preclinical models
Therapeutic efficacy of human donor cells of various sources (Fig. 1A) is usually evaluated in animal models of neurological disease. Given the protracted timeframe for functional maturation of human cells, which resembles the timing of normal human brain development, behavioral recovery of neurological function may only be observed over the course of several months. The putative mechanisms through which the graft induces behavioral recovery are diverse and challenging to pinpoint. Proposed mechanisms include actual neuronal integration and network repair (Fig. 1B), the provision of trophic support (Fig. 1C), enzyme replacement (Fig. 1D), secretion of neuromodulators and immunomodulation (Fig. 1E), prevention of scar formation and re-myelination (Fig. 1F). Moreover it is conceivable that several mechanisms work in combination to promote recovery while others may also be responsible for graft induced adverse effects. We therefore believe that a mechanistic understanding of the principles that underlie behavioral recovery is necessary to evolve stem cell therapies into safe and effective clinical therapies. In the following section we review the therapeutic benefits that have been reported with a special emphasis on the mechanisms that have been proposed to drive recovery.
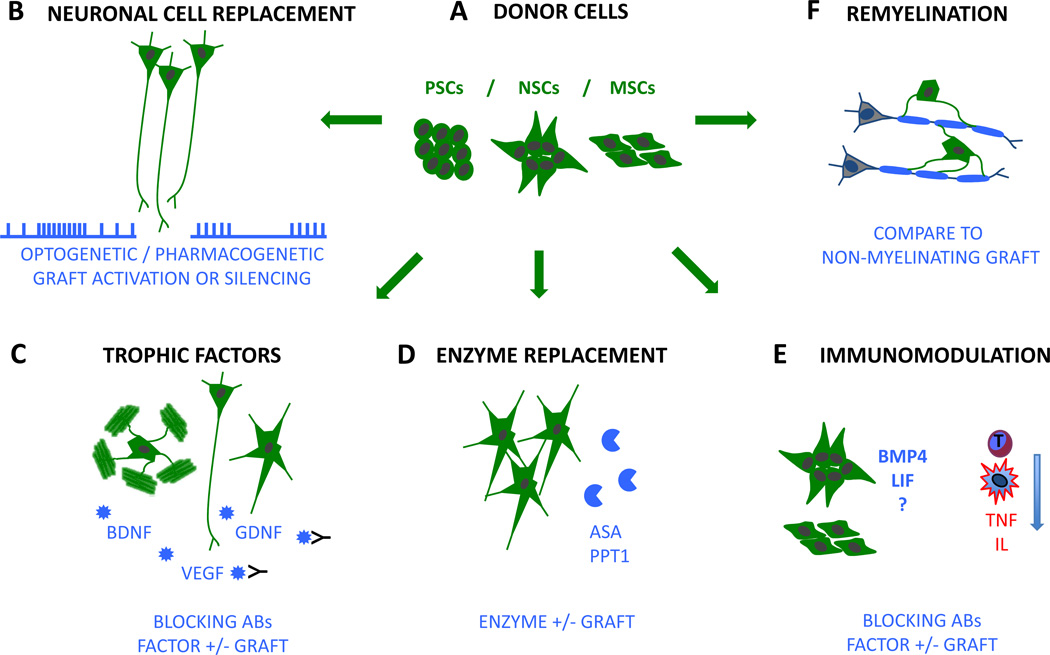
Figure 1. Mechanisms of stem cell-based brain repair.
A, neural cells derived from pluripotent stem cells (PSCs) as well as adult neural stem cells (NSCs) and mesenchymal stem cells (MSCs) are used for therapeutic purposes. B, donor neurons may drive repair by neuronal cell replacement and integration into host networks. Network integration can be tested by optogenetic or pharmacogenetic manipulation of the graft. C, various neural and non-neural donor populations may release therapeutic factors (such as BDNF, GDNF, VEGF) to promote recovery in the host brain. Trophic effects could be assessed using blocking antibodies (black) or by engineering grafts, which overexpress a candidate factor or are deficient in it. D, astroglial cells have been used for the delivery of missing enzymes in metabolic disorders. The effect of enzyme replacement can be assessed by engineering grafts that overexpress an enzyme or are deficient in it (e.g. ASA or PPT1). E, the immune response of the host (depicted in red, e.g. T-cell response, microglia, TNF or interleukins) can be modulated by NSC as well as MSC transplantation. Factors mediating these effects are largely unknown (?) but secretion of BMP4 and leukemia inhibitory factor (LIF) has been implicated. F, transplanted oligodendrocytes have been shown to re-myelinate endogenous axons. The effect of re-myelination could be assessed by comparing the effect to a non-myelinating graft. Donor cells are depicted in green. Proposed therapeutic mechanisms and experimental strategies to test for such mechanisms are depicted in blue.
Primary neural stem cells
Human CNS stem cells (hCNS-SC) can be expanded in vitro while maintaining the ability to generate neurons, astrocytes and oligodendrocytes (Uchida et al., 2000). Such cells have shown beneficial effects in several animal models of neurological diseases. For example, locomotor recovery was demonstrated after spinal cord lesions in mice (Cummings et al., 2005; Salazar et al., 2010). In these studies the multipotent cell population has been shown to both remyelinate axons and form synapses onto host neurons. The experimental design is noteworthy, since the therapeutic effect was reversed by selective ablation of the graft. This finding was among the first to provide clear evidence for the functional integration of grafted cells into host circuits. However, since the entire graft was ablated, this approach does not prove whether the neuronal component (Fig 1B), re-myelination (Fig 1F), both in conjunction or something else was necessary to induce recovery. In another application, the same cell population (hCNS-SC) was found to slow neurodegeneration and motor decline in an animal model of an infantile lysosomal storage disorder (Tamaki et al., 2009). After transplantation into newborn mice harboring the disease, hCNS-SCs migrated significantly and expressed the missing enzyme. The neuroprotective effect is believed to be dependent on enzyme substitution stemming from human cells (Fig. 1D). Which cell type of the human neural lineage may be most effective regarding enzyme substitution remains unclear. However, forced overexpression of arylsufatase A (ASA) in glial precursors transplanted into an animal model of a different lysosomal disease conferred an additional benefit (Klein et al., 2006). Regarding enzyme substitution, astrocytes may appear as a preferred donor cell type since a therapeutic factor could be distributed via gap junctions to the host. hCNS-SCs were also shown to promote recovery in stroke-lesioned rats. The therapeutic benefit, which was found to occur in two temporally and mechanistically distinct waves, was attributed to stem cell-mediated repair of the blood brain barrier, immunomodulation (Fig. 1E) as well as VEGF dependent neovascularization (Fig. 1C) (Horie et al., 2011). Importantly, the authors showed that the effect on neovascularization was blocked by a human specific VEGF antibody, which is a rare demonstration of therapeutic specificity. In another application, hCNS-SCs were further shown to substantially remyelinate the CNS of newborn and juvenile mice that lack endogenous myelin (Fig 1F) (Uchida et al., 2012). Moreover, these cells have been shown to protect photoreceptors and preserve retinal integrity in a rat model of macular degeneration (McGill et al., 2012). Interestingly, progeny of hCNS-SCs of unclear fate targeted to the subretinal space formed a cellular layer replacing defective retinal pigment epithelium (RPE) and phagocytizing outer segments of photoreceptors (Cuenca et al., 2013). In summary, hCNS-SCs have been reported to generate therapeutic effects in a variety of animal models with surprisingly diverse etiologies. The proposed mechanisms comprise neuronal replacement, re-myelination, enzyme replacement, immunomodulation, neovascularization, trophic support and phagocytosis for this particular donor cell population. This opens up the possibility that the multipotent donor cell population undergoes differentiation according to the needs of the diseased tissue. However, the identity of the cell type conferring the benefit remains unclear in most studies. This raises the concern that despite evidence of therapeutic efficacy, the subset of hCNS-SC progeny not contributing to the therapeutic effect in a particular paradigm may cause side effects, which could go unnoticed in preclinical models. Furthermore, the lack of a clear mechanism raises concerns about how to assess the potency of the cells and to define an optimal cell dose in addition to defining risk related to potential side effects.
An additional human neural stem cell line that has been shown to induce substantial benefit is the fetal spinal cord derived line NSI566RSC (Guo et al., 2011; Yan et al., 2007). A comprehensive regenerative therapy for amyotrophic lateral sclerosis (ALS, Lou Gehrig’s disease) may be difficult to conceive because cortical and spinal motoneurons degenerate along the entire neuraxis. An ideal therapy would therefore replace or protect cortical as well as spinal neurons. In a first promising step however, intraspinal transplantation of NSI566RSCs in a genetic animal model of ALS has been shown to preserve endogenous alpha-motoneurons near the graft (Hefferan et al., 2012) and extend lifespan by approximately two weeks (Xu et al., 2011). The benefit is largely attributed to NSC-dependent trophic effects (Fig 1C) but formal proof regarding the mechanism is pending. NSI566RSCs have also been tested in a severe model of spinal cord injury alongside human ES and iPS cells (discussed below).
Immunomodulation
Over the past decade the bi-directional interplay between endogenous or transplanted neural stem cells and the immune system has been recognized, a subject discussed in detail in a recent review (Kokaia et al., 2012). A direct immunomodulatory role of primary multipotent neural precursors has been demonstrated in murine models of neuroinflammation (Pluchino et al., 2003; Pluchino et al., 2005) and subsequently reproduced using human NSCs in non-human primate models (Pluchino et al., 2009a). The neural stem cell-dependent mechanisms providing immunomodulation are potentially complex and remain largely unexplored. In some studies the secretion of BMP4 (Pluchino et al., 2009b) and leukemia inhibitory factor (Cao et al., 2011) from grafted NSCs have been proposed as mechanisms to mediate immunomodulation via inhibition of dendritic and T-cell function. Beyond the action of NSCs in primary inflammatory disease, immunomodulatory or neuroprotective effects may also play a role in the action of human stem/progenitor cells in neurodegenerative disease such as in animal models of Parkinson’s (Redmond et al., 2007) or Huntington’s disease (McBride et al., 2004).
For most primary NSC populations there is a limitation in scalability due to telomere erosion and changes in fate potential following long-tern in vitro culture often resulting in cells with increasing glial differentiation propensities. Those are challenges for clinical translation that may limit clinical usefulness. Immortalization has been proposed as a strategy to preserve the initial differentiation potential and the use of immortalized cells has been proposed for several clinical applications (www.reneuron.com). However, given the safety concerns associated with immortalization and the availability of alternative strategies and cell sources, such a strategy should only be pursued with the utmost caution.
Mesenchymal stem cells
Despite the fact that mesenchymal stem cells (MSCs) have no known physiological function in the brain, a large body of preclinical studies as well as initial clinical trials have reported beneficial effects after MSC transplantation. MSC transplantation is thought to act via trophic (Fig. 1C) and immunomodulatory mechanisms (Fig. 1E) whereas their potential for cell replacement remains controversial. Findings and perspectives regarding the use of MSCs in neurological diseases are the topics of several recent review articles (Uccelli et al., 2011; Wan et al., 2014).
Neural cells derived from pluripotent stem cells
As outlined in section I, various specialized cell types have been generated from PSCs. In most cases, donor cells for therapeutic applications are pre-differentiated in vitro but injected into adequate animals models before the donor cell type reaches a fully mature status. Also, most donor cell populations are not pure even if enriched by means of cell sorting. They usually contain minority fractions of other neuronal populations and often astroglial precursors.
Retinal pigment epithelium (RPE) for the treatment of macular degeneration
RPE has been successfully generated from human PSCs, reviewed in (Ramsden Coffey 2013). To assess efficacy, ES-derived RPE has been injected into the subretinal space of royal college of surgeon (RCS) rats, which develop a genetic form of macular degeneration. Notably, the preservation of photoreceptors, physiologic retinal activity and the improvement of visual performance were shown in a number of studies (Lu et al., 2009; Vugler et al., 2008; Zhu et al., 2013). It is particularly encouraging that PSC-derived RPE recapitulate bona-fide RPE morphology, phagocytose photoreceptor outer segments and cycle retinol. PSC-derived RPE transplanted into immunodeficient mice also shows a favorable long-term safety profile (i.e. no teratoma formation or other type of overgrowth).
Dopamine neurons for use in PD cell therapy
The therapeutic efficacy of donor cell populations for Parkinson’s disease is usually assessed in a chemical lesion model in rodents (Grealish et al., 2010) where 6-hydroxydopamine is used to ablate the endogenous dopaminergic innervation unilaterally in the dorsal striatum. Transplantation of human PSC-derived dopamine neurons into the denervated striatum (Kirkeby et al., 2012; Kriks et al., 2011) (i.e. ectopic transplantation) or potentially the midbrain (Grealish et al., 2014) reverses the resulting motor asymmetry in various behavioral tests. Prevailing thought holds that such grafted dopamine neurons extend fibers into the denervated striatum, form synaptic connections with resident medium spiny striatal projection neurons, mature physiologically over the course of several months and ultimately start to release dopamine resulting in circuit repair and behavioral recovery. This view is supported by one study showing that re-lesioning ablates recovery (Dunnett et al., 1988). However, chemical re-lesioning of the dopaminergic system ablates all dopamine neurons, including any recovering host dopamine fibers. Additional support for the idea to prefer bona-fide midbrain dopamine neurons as donor source comes from studies showing that non-DA neural transplants cannot induce behavioral recovery (Cenci et al., 1992; Dunnett et al., 1988). This claim has been challenged however, since other cell populations like GABAergic interneurons (Martinez-Cerdeno et al., 2010), growth factor releasing astrocytes (Proschel et al., 2014) and even mesenchymal stem cells (Glavaski-Joksimovic et al., 2010) have been proposed to induce at least partial recovery. Also, current protocols do not yield pure midbrain dopamine neurons and it is unclear whether pure dopamine grafts would confer and additional benefit. Similar to early studies utilizing fetal tissue, PSC-derived dopaminergic cultures contain at least serotonergic and GABAergic contaminants as well as glial precursors. The role of these additional populations regarding the induction of a therapeutic benefit remains poorly understood. The presence of serotonergic neurons is particularly troubling since graft induced dyskinesias that were observed in clinical trials have been linked to serotonergic contaminants (Politis et al., 2010). Nonetheless, the robustness of recovery in preclinical models is encouraging. Therefore, a thorough understanding of graft function and connectivity and the elimination of contaminants through cell sorting strategies should contribute to the generation of a standardized PSC-derived cell product on the road towards clinical application.
Cortical neurons for the treatment of experimental stroke
Cortical neurons have also been specified from an iPSC-derived highly neurogenic neural stem cell population and tested in a rat stroke model (Tornero et al., 2013). In contrast to the study employing hCNS-SCs (Horie et al., 2011) this study focused on the potential of neuronal cell replacement. Cortical neurons were targeted to the ischemic rat cortex and found to differentiate and function physiologically according to their pre-specified fate. Axonal outgrowth was widespread and mainly along white matter tracts. Behaviorally, animals showed a modest recovery, which was correlated with the presence of the graft. Which aspect of graft function causes behavioral recovery e.g. neuronal function and integration or the previously proposed mechanisms such repair of the blood brain barrier, immunomodulation, neovascularization or a complex combination thereof remains unclear.
Interneurons for modulating excitability and plasticity
The rationale for the application of interneurons in neurological disease has recently been reviewed (Southwell et al., 2014). Interneurons have shown beneficial effects in a number of animal models of disorders associated with an imbalance between excitation and inhibition of neuronal networks (e.g. epilepsy, schizophrenia or autism). Of particular interest is also their migratory capacity, which could be utilized for the widespread delivery of therapeutic agents (Liu et al., 2013; Maroof et al., 2013; Nicholas et al., 2013). Protracted functional maturation of these neurons, which reflects ontogeny, may impose significant experimental challenges. For example hPSC-derived interneurons grafted into neonatal hosts retained highly immature properties even 7 months after transplantation (Nicholas et al., 2013). Nonetheless one recent study showed potent seizure suppression and amelioration of associated behavioral abnormalities even before full electrophysiological maturation in a temporal lobe epilepsy model (Cunningham et al., 2014). A different group described a therapeutic benefit, of a population containing interneurons and basal forebrain cholinergic neurons. It was shown to correct a memory deficit induced by septo-hippocampal lesioning. The benefit was specific to the ventral forebrain population as opposed to a spinal cord GABAergic population but the study was not designed to clarify the therapeutic mechanism in more detail (Liu et al., 2013).
Spinal cord injury
In spinal cord injury the lesion pattern is complex involving destruction of spinal motoneurons and local interneurons at the site of injury as well as discontinuation of myelinated axons transmitting information from the brain to the spinal cord and vice versa. Therefore several cell types may contribute to functional recovery through a variety of mechanisms (Lu et al., 2014a). Human ESC-derived oligodendrocytes were demonstrated to remyelinate spared axons and restore locomotion in a model of incomplete (compression) spinal cord injury (Keirstead et al., 2005). Recently established, myelin-defective iPSC-derived oligodendrocytes (Numasawa-Kuroiwa et al., 2014) could now be used to assess the extent to which myelination versus different donor cell dependent mechanisms contribute to recovery. Alternative approaches have focused on the introduction of neural stem cells to bridge the site of injury and reconnect the distal and the proximal ends at the lesion site. Dramatic findings were reported when (Lu et al., 2012) embedded human NSI566RSCs and ESC derived NSCs into a fibrin and growth factor matrix. The matrix served to bridge the lesion cavity caused by complete T3 transsection and supported the survival and differentiation of donor cells. Axonal outgrowth extended several centimeters in rostral and caudal directions into the injured spinal cord and significantly restored locomotion. In a follow up study human iPSC-derived neural precursor cells were grafted into C5 lateral spinal cord lesions (Lu et al., 2014b). Axonal outgrowth extended over the entire length of the rat CNS. However, locomotion did not recover, which may be due to the majority of grafts showing central collagen-rich rifts or insufficient behavioral follow up, which was terminated at 3 months. Nonetheless these findings are altogether very encouraging since they indicate that human neural stem cells may be able to functionally re-connect the severely lesioned spinal cord.
Restorative approaches for the pyramidal motor system
The functional reconstruction of the pyramidal motor system spanning from the motor cortex via the cortico-spinal tract (CST), the lower motoneurons in the ventral horn to the skeletal muscle has been a challenging and longstanding goal. If accomplished, such an approach could contribute to the restoration of motor function in a number of disease paradigms (e.g. stroke, ALS, spinal cord injury). Encouraging results were reported when homotopic mouse E14 cortical tissue blocks were grafted into the acutely lesioned adult mouse sensori-motor cortex (Gaillard et al., 2007). Transplants integrated well into cortical lesions and axonal outgrowth reached appropriate cortical, subcortical and spinal cord targets. Unfortunately, the behavioral consequences of lesioning and grafting were not assessed; therefore also the functional connectivity of these transplants remains unclear. The embryonic, homotopic nature and organotypic preservation of the graft were believed to be the determinants of successful axonal pathfinding in this study. However, significant axonal outgrowth from cortical transplants to cortical, subcortical and cervical spinal cord targets has also been reported after transplantation of human ESC-derived neural stem cells (Steinbeck et al., 2012). CST terminals synapse onto lower spinal cord motoneurons, which convey motor control to the skeletal muscle. Owing to the success in generating human PSC-derived spinal motoneurons, several studies have evaluated their intraspinal engraftment. Human motoneurons can engraft in the spinal cord and axons project into the ventral root of embryonic chick (Amoroso et al., 2013; Lee et al., 2007) and adult rodent hosts (Lee et al., 2007). Efficient engraftment and axonal projections into the ventral root were also observed after transplantation of NSI566RSCs (Yan et al., 2007) into nude rats. Apart from survival and choosing the appropriate trajectory, grafted MNs would have to innervate skeletal muscle and receive cortical inputs to restore connectivity. In consideration of the distance between the spinal cord and limb and lacking guidance cues in the adult host this is an enormous challenge, which despite extensive efforts (Deshpande et al., 2006) has not yet been resolved in a relevant animal model. However, mouse ESC-derived spinal motoneurons, directly injected into the lesioned sciatic nerve were shown to functionally connect to limb muscles in vivo (Bryson et al., 2014).
General safety considerations
Major determinants of the safety profile are related to the sterility of the cell source, the delivery of the cells to the CNS and to the long-term proliferative capacity of the donor cell population. Injection of donor cells into various regions of the brain and spinal cord has been optimized and can be performed safely (see clinical trials section). Intra-operative MRI also allows real time monitoring of stereotactic injections, which should further increase accuracy and safety (Larson et al., 2012). With regard to the proliferative potential of the donor population primary cells have the principle advantage that they do not contain pluripotent contaminants, which harbor the potential for teratoma formation. Nevertheless, tumors arising from clinical transplantation of primary NSCs have been described (Amariglio et al., 2009) though the exact nature of the cells engrafted in this study remains unclear. Potentially oncogenic genetic alterations can occur in any cultured cell type. Therefore close monitoring of genetic integrity is mandatory for any donor cell population awaiting clinical application. Integration of suicide genes (Di Stasi et al., 2011) into genomic safe harbors (Papapetrou et al., 2011) has been proposed as a strategy to ablate a graft in case it produces intolerable effects as reviewed in detail elsewhere (Kiuru et al., 2009; Lee et al., 2013). The long-term proliferative potential of a donor cell population is also dependent on its maturation status at the time of transplantation. Efficient pre-differentiation of pluripotent donor cells towards the desired fate greatly reduces the risk of teratoma formation or unwanted over-proliferation of cells resisting final differentiation. Such differentiation strategies can be based on the exposure to extrinsic cues (Chambers et al., 2009) or to intrinsic factors such as neurogenic transcription factors that can drive neuronal fates to near purity (Zhang et al., 2013). However, the capabilities for successful engraftment and integration of donor cells may decline with increasing maturity at the time of engraftment. In many neuron replacement paradigms neurons at the stage of cell cycle exit (e.g. neuroblasts) are regarded as a reasonable compromise to ensure cell type specificity and integration while reducing the risk of tumor formation (Kriks et al., 2011; Liu et al., 2013). However, the specific safety concerns have to be assessed individually for each candidate cell population. Beyond the risk of overproliferation and tumor formation, grafts may exhibit the risk of aberrant host innervation, ectopic distribution of cells outside the target area, immune-rejection and CNS inflammation among others. Several of these caveats may remain unnoticed in preclinical studies. Therefore the clinical translation of candidate populations must be conducted under closely monitored clinical trial conditions.
III. Derivation of donor cells under GMP condition
Once efficacy is proven in a preclinical model and before a clinical trial may be initiated, the donor cell population has to be produced under clinical-grade standards. In the US, current Good Manufacturing Practice (cGMP) regulations are enforced by the Food and Drug Administration (FDA) and comprise standard operating procedures for manufacturing and quality control in a dedicated facility. This is not a trivial matter since most cell lines used in preclinical studies were not initially derived under cGMP standards. New human pluripotent cell lines have therefore been derived and banked under cGMP conditions (Crook et al., 2007; Tannenbaum et al., 2012). In the US, this process includes the derivation of the cells from tested, traceable donors and replacement of all animal products i.e. feeder cells, serum, growth factors, enzymes for passaging, and extracellular matrices. It is interesting to note that some regulatory bodies accept animal components and therefore the process in such countries will differ from the US process and even within the US, not all cell products that move into early stage clinical trials are xeno-free (see below). Several non-cGMP cell lines have been transferred into cGMP facilities, which requires propagation and expansion of the line for several passages along with extensive testing for adventitious pathogens (Durruthy-Durruthy et al., 2014), (www.gmpbio.org). Cell lines should be generated and maintained in a clean room and have to be monitored for maintenance of the correct karyotype, identity and sterility. The transition to cGMP qualified cell lines, animal-free, cGMP pluripotent culture systems and the substitution of non-cGMP compliant components of the differentiation protocol represent frequent challenges in the transition from pre-clinical to clinical studies. In some cases, cGMP reagents do not exist and in others the cost to manufacture custom batches is prohibitive. Likewise, many processes can be qualified in the early stages of clinical trials. The process of qualification means presenting the regulatory authorities with as much information as possible about purity, processing, raw ingredients, sterility, etc. For each reagent, risk mitigation is a top priority for scientists and regulators. A “clean” reagent that creates a less potent product might create greater risk for a patient. As such, some of the early groups that treated patients with hESC-derived cells have decided to maintain their hESCs on mouse feeder cells (Schwartz et al., 2012). The mouse feeders used had to be derived under cGMP conditions and were subject to the normal extensive battery of tests used for any cGMP cell bank. Such concessions are often made during the early phases of a clinical trial but are expected to be less likely as a treatment approaches to become a commercial product.
IV. Clinical trials
The success in inducing behavioral recovery in several preclinical paradigms led to the initiation of clinical studies using these GMP qualified cells. The company Neuralstem has completed a phase I clinical study which pioneered the transplantation of their NSI566RSC spinal cord derived stem cells into the lumbar spinal cord of ALS patients, reporting no significant adverse effects (Glass et al., 2012). A dose escalation phase II trial has been initiated (www.clinicaltrials.gov, NCT01730716). NSI566RSC phase I clinical trials are currently ongoing for chronic complete spinal cord injury (www.clinicaltrials.gov, NCT01772810) and acute spinal cord injury in Korea. For the treatment of chronic motor residues after ischemic stroke a combined phase I/II clinical trial was initiated in China in 2013. Efforts regarding the clinical translation of HuCNS-SCs by StemCells Inc. have been recently reviewed (Tsukamoto et al., 2013). Phase I clinical trials have been completed for an infantile lysosomal storage disorder (Selden et al., 2013) and a myelination disorder (Gupta et al., 2012) reporting a favorable safety profile. Phase I trials are ongoing for cervical and thoracic spinal cord injury (www.clinicaltrials.gov, NCT02163876, NCT01321333) and age related macular degeneration (www.clinicaltrials.gov, NCT01632527). The first human ESC-derived product developed for clinical application were oligodendrocyte precursors intended to remyelinate spinal cord lesions. After overcoming substantial regulatory hurdles and significant delays, five patients were treated before Geron abandoned the trial for strategic and financial reasons (Frantz, 2012). The patients are still being followed with an initial report of “no serious adverse events related to AST-OPC1 administration” presented at the ASGCT meeting in 2014 (http://asteriasbiotherapeutics.com/wp-content/uploads/2014/05/Lebkowski-ASGCT-5-22-14.pdf). Asterias Biopharmaceuticals took over the portfolio from Geron and recently received FDA clearance and $14.3 million from CIRM (California Institute of Regenerative Medicine) to initiate a Phase 1/2a clinical trial including dose escalation in 2015. Human ESC-derived retinal pigment epithelium is currently in phase I/II clinical trials in patients with macular degeneration. The first report of two treated patients showed no significant adverse effects and possible improvement in visual parameters (Schwartz et al., 2012) with a recent follow-up report indicating therapeutic efficacy (Schwartz et al., 2014). There is also renewed interest in the grafting of fetal tissue for Parkinson’s disease under optimized conditions in Europe (www.transeuro.org.uk) (Barker et al., 2013a) in preparation for pluripotent-derived dopamine neuron grafts being planned in the US, Japan and Sweden (GForce-PD.com). Significant further challenges for the implementation of restorative therapies relate to the internationally diverse regulatory requirements (Andrews et al., 2014). Despite the considerable progress in translating neural and pluripotent-derived products for early stage trails in patients with neurological disorders, there is remarkable paucity of information regarding the specific mechanisms by which those cells are thought to provide therapeutic benefit.
V. Current strategies for the enhancement of structural integration
The structural integration of donor neurons into diseased brain tissue is a key challenge for any therapy aimed at actual cell replacement. Several aspects related to the structural integration of donor neurons (i.e. maturity, migration, axonal outgrowth) have been the focus of recent studies (Figure 2).
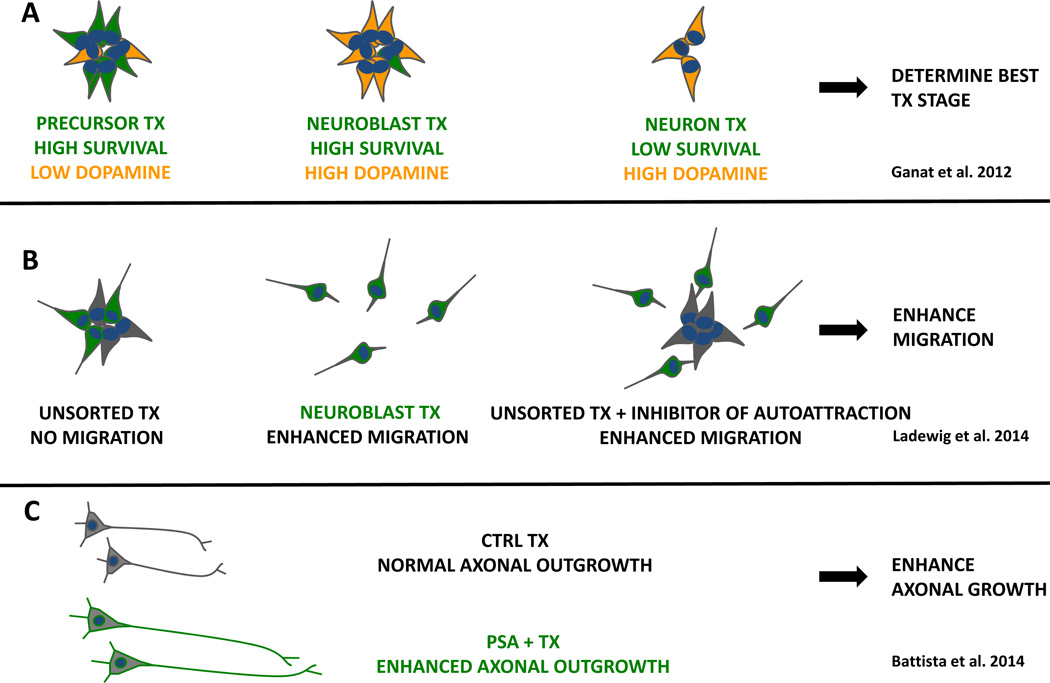
Figure 2. Strategies to enhance structural integration.
A, Grafts of developmentally distinct dopaminergic populations were compared. Transplantations (TX) of neural precursors, neuroblasts and mature neurons varied with respect to survival and dopamine neuron content. B, mixed grafts of NSCs (grey) and neuroblasts (green) form clusters, whereas pure neuroblasts migrated into the host brain. In mixed grafts, chemoattraction of neuroblasts to NSCs can be overcome by blocking FGF and VEGF pathways. C, axonal outgrowth into the host brain and therapeutic efficacy can be enhanced by expression of polysialyltransferase (PST) in grafted neurons.
Donor cell maturity
One important question is how donor cell maturity affects survival, migration, axonal outgrowth and ultimately therapeutic efficacy. This question was systematically addressed by purifying developmentally early (Hes5+ midbrain precursors), midstage (Nurr1+ neuroblasts) and late (Pitx3+ differentiated neurons) derived from murine ESCs (Figure 2A). Each population was subsequently transplanted into Parkinsonian mice to compare the in vivo therapeutic potential (Ganat et al., 2012). The results show that dopamine neuron survival and therapeutic efficacy was particularly high in the donor cell population selected at the Nurrl+ neuroblast stage. Those cells are at an intermediate stage of differentiation with high specificity for midbrain dopamine neuron phenotype but retain sufficient developmental plasticity for functional integration. Future studies are required to further test the contribution of non-dopamine neuron subtypes in PD grafting studies and to apply similar stage-specific transplantation paradigms to other models of neurological disease.
Migration
The migratory capacity of donor cells is another aspect that can greatly affect the regenerative potential. The ability of transplanted cells to migrate varies substantially, depending on the developmental age (Wang et al., 2013) and region (Tabar et al., 2005) of the host CNS. Most neural donor cell populations however show little, if any migration if transplanted into the adult or diseased CNS (Kriks et al., 2011; Steinbeck et al., 2012) unless they represent a cell type with a particular migratory capacity (Maroof et al., 2013; Nicholas et al., 2013). The restricted migratory potential of most populations may limit therapeutic efficacy where a widespread distribution of donor neurons or delivery of a therapeutic factor is desirable (Li et al., 2007). Results from a recent study demonstrate that the restricted migratory potential of a neural stem cell population may be overcome (Ladewig et al., 2014). The work showed evidence for chemo-attraction between NSCs and their differentiating progeny that prevents neuroblasts from migrating into host tissue (Figure 2B). Purification of postmitotic neuroblasts before transplantation or pharmacological inhibition of the chemoattraction between neurons and NSCs resulted in enhanced migration. It remains to be shown however if such enhanced migration may indeed be beneficial in the context of a therapeutic paradigm.
Axonal outgrowth
Another possibility to increase tissue integration of a transplant is to enhance its axonal outgrowth. This strategy was pursued recently by increasing polysialic acid (PSA) in mouse ESC-derived neural precursors via transgenic expression of the polysialyltransferase (PST) enzyme. PSA, if added to the neural cell adhesion molecule (PSA-NCAM) reduces inhibitory cell-cell interactions by changing biophysical membrane properties and thereby facilitates migration and axonal growth (Rutishauser, 2008). Overexpression of PSA in dopamine precursors significantly increased axonal growth of tyrosine hydroxylase positive fibers into the dopamine-depleted striatum, which was correlated with more efficient amelioration of the Parkinsonian phenotype in these animals (Battista et al., 2014) (Figure 2C). In a related approach, spinal motorneuron precursors overexpressing PSA transplanted into the lesioned sciatic nerve extended axons in higher numbers towards the denervated muscle (El Maarouf et al., 2014). A recent strategy for PSA addition was developed that does not require the genetic manipulation of the target cell and relies on direct exposure of purified enzyme and substrate, a technology suitable for increasing PSA in various cell types and tissues (El Maarouf et al., 2012).
As an alternative to manipulating the cells to be grafted, approaches have been developed for rendering the host brain more permissive for donor cell migration or axonal outgrowth. PSA overexpression in astrocytes has been shown to enhance endogenous axonal regeneration in a model of spinal cord injury and facilitate recruitment of endogenous progenitors to a cortical lesion site (El Maarouf et al., 2006). The external, non-genetic addition of PSA not only to donor cells, but also in vivo to host tissue provides an interesting perspective. Co-injection of a donor cell population with the PSA producing enzyme and its substrate (El Maarouf et al., 2012) may result in optimized polysialylation of donor and host and therefore superior tissue integration of grafted cells. Other inhibitory constituents of the extracellular matrix have been targeted as well. For example, growth-inhibitory chondroitin sulfate proteoglycans (CSPGs) are upregulated in spinal cord lesions and can be degraded by intraspinal delivery of chondroitinase ABC (C-ABC), which induces endogenous axonal regeneration and behavioral recovery (Busch and Silver, 2007). When C-ABC treatment of a spinal cord lesion was combined with a neural stem cell graft, migration of donor cells, as well as axonal integration into the lesioned spinal cord was enhanced (Ikegami et al., 2005). Interestingly the growth promoting effect of C-ABC is not restricted to the spinal cord. In rats with nigro-striatal tract axotomies C-ABC infusion resulted in the re-growth of the nigro-striatal tract into its correct striatal target region (Moon et al., 2001). Furthermore, mechanistically distinct growth promoting strategies can be combined to achieve optimized outcomes (Fouad et al., 2005; Zhao et al., 2013). However, most of these growth-promoting strategies have not yet been explored in the context of stem cell based grafts.
Biomaterials
The use of biomaterials has been implemented for conditions where the disease process produces large lesion cavities. A degradable biomaterial scaffold could be combined with extracellular matrix proteins, growth factors and potentially drugs in addition to the seeded donor cells (Orive et al., 2009). The goal of such technologies is to create a microenvironment that facilitates the regenerative process. Such scaffolds have also proven useful in supporting regenerative responses of the surrounding brain tissue as it provides a substrate for host cell infiltration and axonal outgrowth. Several studies employing donor cells in scaffolds are particularly noteworthy. Large ischemia-induced telencephalic lesion cavities were successfully re-populated by mouse NSCs seeded onto polyglycolic acid biodegradable polymers (Park et al., 2002). Axonal outgrowth from the graft as well as host-derived connections into the graft were surprisingly robust and apparently specific over long distances. The second study employed rat and human NSCs in a fibrin matrix, as has been discussed for spinal cord lesions (Lu et al., 2012). An example where biomaterials are in use to assure proper structural organization is the in vitro generation of transplantable human retinal pigment epithelium. RPEs, if transplanted in a pre-specified monolayer (as opposed to a cell suspension) may display enhanced functionality and efficacy in animal models of macular degeneration (Stanzel et al., 2014; Subrizi et al., 2012).
VI. Emerging approaches for the assessment of graft function and connectivity
The therapeutic potential of human cells is commonly assessed using long-term behavioral assays following transplantation into a relevant animal model of neurological disease (Kriks et al., 2011; Liu et al., 2013; Steinbeck et al., 2015). However, such experiments do not necessarily define the biological mechanisms responsible for behavioral recovery.
Optogenetics and Pharmacogenetics
The goal of neuronal cell replacement or graft-mediated neuronal network repair (Fig. 1B) is often implied as a therapeutic rationale, but this concept remains insufficiently validated. It is clear that human neurons functionally mature, generate action potentials in vivo and receive synaptic input from the host (Koch et al., 2009; Tornero et al., 2013). Whether donor neurons are however also able to relay information to the host and whether such functional graft-to-host connectivity may be responsible for graft-induced therapeutic benefits has been difficult to assess. Initial strategies to address this issue sought to selectively ablate the graft via the expression of diphtheria toxin in transplanted cells (Cummings et al., 2005) or via chemical re-lesioning (Dunnett et al., 1988). However, those approaches lead to a complete elimination of the transplanted cells without addressing a specific mechanism. In contrast, two new techniques, optogenetics (Zhang et al., 2011) and pharmacogenetics (Rogan and Roth, 2011) allow the reversible functional manipulation of genetically and spatially defined neurons with unprecedented precision. In the case of optogenetics, light sensitive ion channels or pumps (opsins) are expressed in the cell population to be studied. Depending on the properties of the opsin, neurons can be activated or silenced by exposure to light within milliseconds in vitro and in vivo. Pharmacogenetic strategies depend on the expression of a designer receptor exclusively activated by a designer drug (DREADD). Such DREADDs are coupled to inhibitory or excitatory G-proteins, which activate or silence the neuronal population expressing the receptor upon binding of the designer drug. Optogenetics offers considerable advantages because of its exquisite temporal resolution, which is required for many physiological experiments. Pharmacogenetic strategies are in contrast less invasive and enable the manipulation of larger tissue volumes. Regardless of the technology, functional control of specific neuronal populations can link network activity to behavior in freely moving animals in real time, including animals with neurological disease (Chaudhury et al., 2013; Kravitz et al., 2010). Despite the transformative role of optogenetics and pharmacogenetics in neuroscience, these technologies are only starting to impact our understanding of graft function and connectivity. Some of the reasons for the slow adoption may relate to the fact that human PSC-derived neurons require several months for functional maturation (Johnson et al., 2007). The first report showed that matured human ESC-derived neurons expressing Channelrhodopsin 2 (ChR2) reliably fire action potentials upon every light pulse and that such controlled activity induces postsynaptic responses in nearby ChR2-negative neurons in vitro and after transplantation (Weick et al., 2010). In follow up studies, ChR2-expressing human neurons were shown to modulate the activity of mouse cortical networks in vitro (Weick et al., 2011) and of organotypic rat hippocampal slices upon light activation (Pina-Crespo et al., 2012). For the interpretation of many xeno-grafting studies this is essential information, since it proves that human neurons can efficiently modulate the activity of a rodent network. Exciting results, which unequivocally demonstrated the usefulness of optogenetics to analyze graft-to-host connectivity in a behavioral regenerative paradigm were reported recently. ChR2 expressing spinal motoneurons derived from murine ES cells were implanted into the crushed sciatic nerve (Bryson et al., 2014). After re-growth of donor axons into lower limb muscles, light activation of the graft induced muscle twitches (Figure 3A). Two studies addressed the function of transplanted dopamine neurons in an animal model of PD (Figure 3B). In a pharmacogenetic approach, murine induced dopamine neurons (iDA) activated via a DREADD receptor during behavioral testing, generated an additional behavioral improvement (Dell’Anno et al., 2014). We recently reported that optogenetic silencing of tonically active human ESC-derived dopaminergic grafts re-introduced the pre-transplantation behavioral deficit, mimicking the effect of chemical re-lesioning (Dunnett et al., 1988). We also provide evidence that optogenetic graft silencing modulates the endogenous glutamatergic transmission onto host medium spiny neurons in a manner reminiscent of the function of endogenous substantia nigra DA neurons, which are lost in PD (Steinbeck et al., 2015). However the implications of these tools for regenerative medicine are even broader. The function of newly formed endogenous connections, which emerged in the spinal cord after degeneration of the corticospinal tract has also been tested (Figure 3C). DREADD-mediated inactivation of the new connections resulted in a dramatic loss of previously recovered forelimb use (Wahl et al., 2014). Together, these studies demonstrate that the application of optogenetic or pharmacogenetic strategies in regenerative paradigms can establish the causative link between grafting, behavioral recovery and histological analysis. These technologies therefore enable the clarification of mechanisms that induce recovery in animal models of neurological disease. Accordingly, we predict that the selective manipulation of graft function will be instrumental in understanding and refining therapeutic approaches in many more disease paradigms and in much greater detail. New generations of spectrally optimized optogenetic tools with enhanced light sensitivity (Berndt et al., 2014; Chuong et al., 2014; Wietek et al., 2014) should facilitate in vivo applications in a broader set of regenerative medicine paradigms.
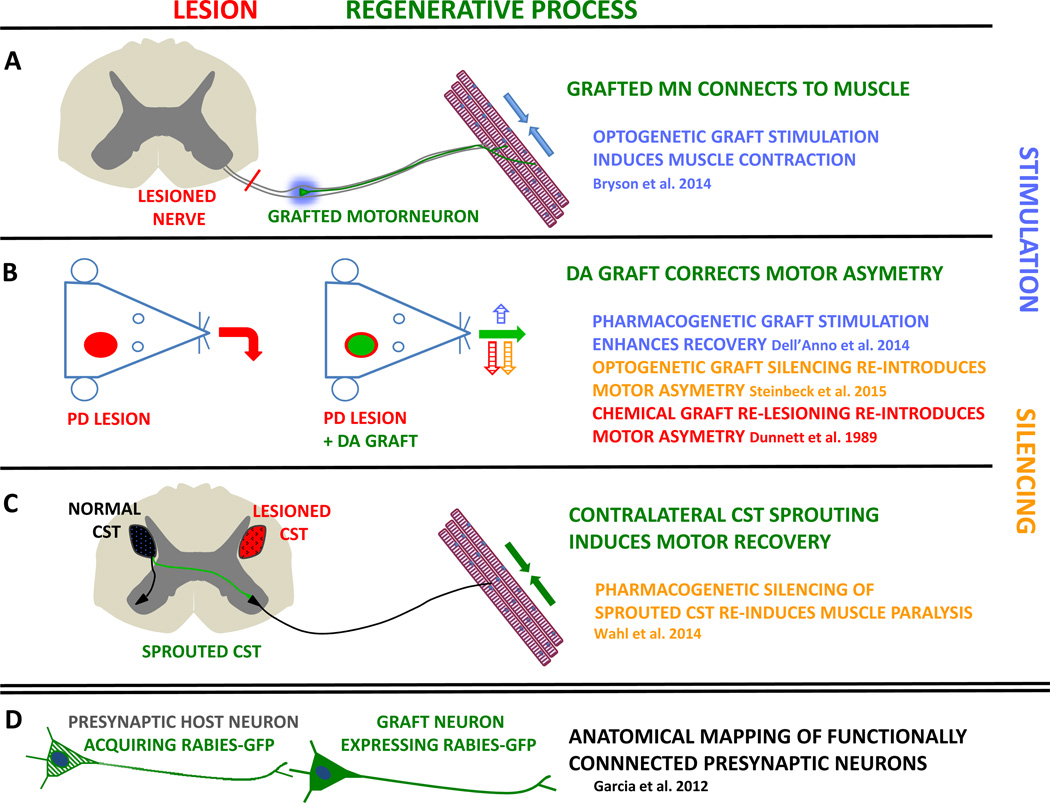
Figure 3. Strategies to assess neuronal graft-to-host connectivity.
For functional experiments optogenetic or pharmacogenetic strategies have been used to stimulate (blue) or silence (orange) donor cells after recovery in behavioral experiments. The initial lesion is depicted in red and the regenerative mechanism in green. A, Spinal motoneurons were injected into the crushed sciatic nerve of mice and donor axons reconnected to limb muscles. Blue light optogenetic stimulation of the graft induced muscle contractions in the leg. B, in a Parkinson’s disease (PD) model with unilateral lesions, dopamine (DA) grafts correct the movement asymmetry. Pharmacogenetic graft stimulation enhances recovery whereas optogenetic graft silencing re-introduces the previous deficit, mimicking the effect chemical re-lesioning. C, rats with unilateral cortico-spinal tract (CST) lesions were subjected to a combination therapy to enhance axonal sprouting (green) from the contralateral CST. This therapy induced motor recovery in the impaired paw. Pharmacogenetic silencing of the new connection resulted in muscle paralysis, demonstrating that axonal sprouting from the healthy CST drives recovery. D, grafted neurons infected with a GFP-tagged rabies virus transfer rabies-GFP (green) to presynaptic host neurons in vivo, thereby revealing the upstream functional connectome of the graft.
Optogenetics and pharmacogenetics are not limited to studying graft function in behavioral experiments. Optogenetics in particular has proven extremely useful in analyzing the connectivity of a brain region in physiological experiments. In such experiments the brain region under study (potentially a graft) is stimulated optogenetically while activity is assessed in the expected target region, such as shown recently for human GABAergic interneurons transplanted into the epileptic hippocampus (Cunningham et al., 2014). To date, the readout in connected brain regions still depends largely on patch clamp physiological measurements, which are time consuming. Also, unexpected connections may go unnoticed, since researchers may concentrate on one or two target regions in which they expect to find connectivity. In contrast, functional imaging strategies, which are becoming more and more powerful, should allow a more integrative assessment of the functional connectome of stem cell grafts and the brain in general, which could feedback into transplantation paradigms. Calcium Imaging has been used for the assessment of neural network dynamics in vitro and in vivo for several decades (Grienberger and Konnerth, 2012). Recent developments include the ability to image assemblies of neurons in vivo in freely moving animals using head-fixed miniature microscopes (Flusberg et al., 2008; Ghosh et al., 2011; Sawinski et al., 2009). Also, genetically encoded calcium indicators have been engineered to reliably achieve single spike resolution (Chen et al., 2013; Thestrup et al., 2014). Therefore, calcium imaging should be a suitable tool to image the activity of both grafts and functionally connected neurons in vivo and in slice preparations. Similarly, new types of engineered optical voltage sensors (Cao et al., 2013; Hochbaum et al., 2014) have recently been shown to faithfully report action potentials. When combined with optogenetic stimulation, separation of the light stimulus from the optical readout is crucial. This separation can be spatial, temporal or spectral. The pairing of a bright and fast voltage sensor with a spectrally non-overlapping activating opsin has been reported, representing an all-optical system for simultaneous stimulation and readout. The activity of dozens of neurons was recorded simultaneously and linked to the activity of the stimulated population (Hochbaum et al., 2014) in vitro. Using this technology, one could optogenetically stimulate a graft and at the same time record neuronal activity in the host brain in a slice preparation. Such experiments should not only define the functional connectome of stem cell grafts in the healthy or diseased brain, but also make graft-to-host connectivity amenable to pharmacological interrogation. While experiments using genetically encoded voltage or calcium indicators offer single neuron resolution they will likely remain restricted to slice preparations or imaging of neuronal assemblies near the brain surface owing to light scattering. In the intact brain functional magnetic resonance imaging (fMRI) is routinely applied to monitor changes in blood oxygenation level-dependent (BOLD) signals. BOLD signals can be modulated by optogenetic stimulation of endogenous neurons at the site of stimulation and in connected brain regions distant from the stimulus (Lee et al., 2010). If applied to a stem cell graft, optogenetic fMRI could reveal the functional impact of the graft in the entire host nervous system.
In vivo imaging
Established techniques to monitor the functional consequences of stem cell grafts mostly rely on positron emission tomography (PET) imaging. For example, various aspects of dopaminergic re-innervation have been imaged in preclinical models (Grealish et al., 2014) and in PD patients with fetal grafts (Ma et al., 2010). Structurally, grafts can be followed after labeling with superparamagnetic iron oxide nanoparticles (SPIONS) (Thu et al., 2012) or reporter genes (i.e luciferase, transferrin) that have been successfully used in clinical studies. These in vivo imaging techniques designed to trace stem cell grafts are the topic of a recent detailed review (Duffy et al., 2014).
Transsynaptic tracing
In addition to the tools that define functional connectivity, several anterograde and retrograde transsynaptic tracers exist (Callaway, 2008), which, if successfully applied to a stem cell graft could anatomically map the grafts functional connections. Wheat germ agglutinin (WGA) is transported anterogradely and retrogradely and can be coupled to CRE recombinase to initiate expression of a floxed marker protein in a trans-synaptically connected population (Gradinaru et al., 2010). Similarly, the non-toxic fragment of tetanus toxin coupled to a fluorescent marker (GFP-TTC) can be used to label retrograde connections (Maskos et al., 2002). The only trans-synaptic labeling strategy so far successfully applied to a stem cell graft employed a pseudo-typed rabies virus (Garcia et al., 2012). Retrograde transfer of an EGFP tagged rabies virus labeled functionally connected upstream neurons efficiently in vitro and in vivo (Figure 3D).
Gene editing
Another recent advance that will greatly facilitate mechanistic studies in neural repair is the use of novel genome engineering techniques such as CRISPR/Cas9 based gene editing (Hsu et al., 2014), a technique already in routine use for human PSC studies (Hou et al., 2013; Mali et al., 2013). Beyond the applications in basic stem cell biology, these technologies should also be useful in generating hPSC-derived donor cells deficient in one or several candidate therapeutic factors in analogy to classic loss of function experiments in the mouse. Thereby, adequate control populations could be generated for paradigms where the therapeutic mechanism is likely to involve factors such as trophic support, enzyme replacement, immunomodulation or myelination.
Conclusions
Over the last two decades the field of stem cell-based repair has moved from basic science to promising preclinical results and early stage clinical trials. The push towards translation will only increase over the next few years driven in part by the successes in generating disease-relevant human cell types at scale and under cGMP conditions. There is also considerable pressure from the patient community, the public at large as well as the various funding agencies that have supported stem cell research to make human stem cell-based therapies a reality. The next few years will likely be an exciting period with many potential cell sources, differentiation strategies and disease targets to be pursued. However, we argue that for the long-term implementation of stem cell based therapies, it will be essential to critically assess their mechanism of action. Only then can we safely transplant cells into patients where they will assume lifelong function – a setting nearly impossible to mimic in preclinical models. The use of novel technologies to interrogate graft function, such as optogenetics, transsynaptic tracers or genetic gain or loss of function experiments should become routine in guiding therapeutic development and in providing scientific rationale for cell choice or dose. The time has come to translate basic findings towards the clinic. In this process, mechanism-based approaches will allow the field to progress effectively, to move back and forth from basic research to clinical application to generate a cell product with optimal efficacy. Only if stem cell therapies achieve disease-modifying activity or long-term functional improvements that are competitive or superior to existing therapies will the field be able to progress beyond “first in human” studies towards meaningful therapies that may enter routine medical practice over the next two decades to come.
Acknowledgements
JAS was supported by a DFG fellowship. The own work of the authors described in this review was supported in part by NIH/NINDS (NS052671), NYSTEM (C028503), and the Starr Foundation. We thank Mark Tomishima, Stuart Chambers and Stefan Irion for valuable comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Julius A. Steinbeck, Email: steinbej@mskcc.org.
Lorenz Studer, Email: studerl@mskcc.org.
References
- AlDakheel A, Kalia LV, Lang AE. Pathogenesis-targeted, disease-modifying therapies in Parkinson disease. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2014;11:6–23. doi: 10.1007/s13311-013-0218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amamoto R, Arlotta P. Development-inspired reprogramming of the mammalian central nervous system. Science. 2014;343:1239882. doi: 10.1126/science.1239882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, Leider-Trejo L, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS medicine. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambasudhan R, Talantova M, Coleman R, Yuan X, Zhu S, Lipton SA, Ding S. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell stem cell. 2011;9:113–118. doi: 10.1016/j.stem.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoroso MW, Croft GF, Williams DJ, O’Keeffe S, Carrasco MA, Davis AR, Roybon L, Oakley DH, Maniatis T, Henderson CE, Wichterle H. Accelerated high-yield generation of limb-innervating motor neurons from human stem cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:574–586. doi: 10.1523/JNEUROSCI.0906-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- Andrews PW, Cavanagro J, Deans R, Feigel E, Horowitz E, Keating A, Rao M, Turner M, Wilmut I, Yamanaka S. Harmonizing standards for producing clinical-grade therapies from pluripotent stem cells. Nature biotechnology. 2014;32:724–726. doi: 10.1038/nbt.2973. [DOI] [PubMed] [Google Scholar]
- Ang CE, Wernig M. Induced neuronal reprogramming. The Journal of comparative neurology. 2014;522:2877–2886. doi: 10.1002/cne.23620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachoud-Levi AC, Gaura V, Brugieres P, Lefaucheur JP, Boisse MF, Maison P, Baudic S, Ribeiro MJ, Bourdet C, Remy P, et al. Effect of fetal neural transplants in patients with Huntington’s disease 6 years after surgery: a long-term follow-up study. The Lancet Neurology. 2006;5:303–309. doi: 10.1016/S1474-4422(06)70381-7. [DOI] [PubMed] [Google Scholar]
- Bachoud-Levi AC, Remy P, Nguyen JP, Brugieres P, Lefaucheur JP, Bourdet C, Baudic S, Gaura V, Maison P, Haddad B, et al. Motor and cognitive improvements in patients with Huntington’s disease after neural transplantation. Lancet. 2000;356:1975–1979. doi: 10.1016/s0140-6736(00)03310-9. [DOI] [PubMed] [Google Scholar]
- Backlund EO, Granberg PO, Hamberger B, Knutsson E, Martensson A, Sedvall G, Seiger A, Olson L. Transplantation of adrenal medullary tissue to striatum in parkinsonism. First clinical trials. Journal of neurosurgery. 1985;62:169–173. doi: 10.3171/jns.1985.62.2.0169. [DOI] [PubMed] [Google Scholar]
- Barker RA, Barrett J, Mason SL, Bjorklund A. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson’s disease. The Lancet Neurology. 2013a;12:84–91. doi: 10.1016/S1474-4422(12)70295-8. [DOI] [PubMed] [Google Scholar]
- Barker RA, Mason SL, Harrower TP, Swain RA, Ho AK, Sahakian BJ, Mathur R, Elneil S, Thornton S, Hurrelbrink C, et al. The long-term safety and efficacy of bilateral transplantation of human fetal striatal tissue in patients with mild to moderate Huntington’s disease. Journal of neurology, neurosurgery, and psychiatry. 2013b;84:657–665. doi: 10.1136/jnnp-2012-302441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista D, Ganat Y, El Maarouf A, Studer L, Rutishauser U. Enhancement of polysialic acid expression improves function of embryonic stem-derived dopamine neuron grafts in Parkinsonian mice. Stem cells translational medicine. 2014;3:108–113. doi: 10.5966/sctm.2013-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt A, Lee SY, Ramakrishnan C, Deisseroth K. Structure-guided transformation of channelrhodopsin into a light-activated chloride channel. Science. 2014;344:420–424. doi: 10.1126/science.1252367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulting GL, Kiskinis E, Croft GF, Amoroso MW, Oakley DH, Wainger BJ, Williams DJ, Kahler DJ, Yamaki M, Davidow L, et al. A functionally characterized test set of human induced pluripotent stem cells. Nature biotechnology. 2011;29:279–286. doi: 10.1038/nbt.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin P, Nilsson OG, Strecker RE, Lindvall O, Astedt B, Bjorklund A. Behavioural effects of human fetal dopamine neurons grafted in a rat model of Parkinson’s disease. Experimental brain research. 1986;65:235–240. doi: 10.1007/BF00243848. [DOI] [PubMed] [Google Scholar]
- Bryson JB, Machado CB, Crossley M, Stevenson D, Bros-Facer V, Burrone J, Greensmith L, Lieberam I. Optical control of muscle function by transplantation of stem cell-derived motor neurons in mice. Science. 2014;344:94–97. doi: 10.1126/science.1248523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Current opinion in neurobiology. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Caldwell MA, He X, Wilkie N, Pollack S, Marshall G, Wafford KA, Svendsen CN. Growth factors regulate the survival and fate of cells derived from human neurospheres. Nature biotechnology. 2001;19:475–479. doi: 10.1038/88158. [DOI] [PubMed] [Google Scholar]
- Callaway EM. Transneuronal circuit tracing with neurotropic viruses. Current opinion in neurobiology. 2008;18:617–623. doi: 10.1016/j.conb.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, Platisa J, Pieribone VA, Raccuglia D, Kunst M, Nitabach MN. Genetically targeted optical electrophysiology in intact neural circuits. Cell. 2013;154:904–913. doi: 10.1016/j.cell.2013.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Yang Y, Wang Z, Liu A, Fang L, Wu F, Hong J, Shi Y, Leung S, Dong C, Zhang JZ. Leukemia inhibitory factor inhibits T helper 17 cell differentiation and confers treatment effects of neural progenitor cell therapy in autoimmune disease. Immunity. 2011;35:273–284. doi: 10.1016/j.immuni.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Caraci F, Bosco P, Leggio GM, Malaguarnera M, Drago F, Bucolo C, Salomone S. Clinical pharmacology of novel anti-Alzheimer disease modifying medications. Current topics in medicinal chemistry. 2013;13:1853–1863. doi: 10.2174/15680266113139990141. [DOI] [PubMed] [Google Scholar]
- Cave JW, Wang M, Baker H. Adult subventricular zone neural stem cells as a potential source of dopaminergic replacement neurons. Frontiers in neuroscience. 2014;8:16. doi: 10.3389/fnins.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci MA, Kalen P, Mandel RJ, Wictorin K, Bjorklund A. Dopaminergic transplants normalize amphetamine- and apomorphine-induced Fos expression in the 6-hydroxydopamine-lesioned striatum. Neuroscience. 1992;46:943–957. doi: 10.1016/0306-4522(92)90196-9. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nature biotechnology. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Gertler TS, Surmeier DJ. A molecular basis for the increased vulnerability of substantia nigra dopamine neurons in aging and Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2010;25(Suppl 1):S63–S70. doi: 10.1002/mds.22801. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong AS, Miri ML, Busskamp V, Matthews GA, Acker LC, Sorensen AT, Young A, Klapoetke NC, Henninger MA, Kodandaramaiah SB, et al. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nature neuroscience. 2014;17:1123–1129. doi: 10.1038/nn.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti L, Cattaneo E. Neural stem cell systems: physiological players or in vitro entities? Nature reviews Neuroscience. 2010;11:176–187. doi: 10.1038/nrn2761. [DOI] [PubMed] [Google Scholar]
- Cooper O, Astradsson A, Hallett P, Robertson H, Mendez I, Isacson O. Lack of functional relevance of isolated cell damage in transplants of Parkinson’s disease patients. Journal of neurology. 2009;256(Suppl 3):310–316. doi: 10.1007/s00415-009-5242-z. [DOI] [PubMed] [Google Scholar]
- Cord BJ, Li J, Works M, McConnell SK, Palmer T, Hynes MA. Characterization of axon guidance cue sensitivity of human embryonic stem cell-derived dopaminergic neurons. Molecular and cellular neurosciences. 2010;45:324–334. doi: 10.1016/j.mcn.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Crook JM, Peura TT, Kravets L, Bosman AG, Buzzard JJ, Horne R, Hentze H, Dunn NR, Zweigerdt R, Chua F, et al. The generation of six clinical-grade human embryonic stem cell lines. Cell stem cell. 2007;1:490–494. doi: 10.1016/j.stem.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Cuenca N, Fernandez-Sanchez L, McGill TJ, Lu B, Wang S, Lund R, Huhn S, Capela A. Phagocytosis of photoreceptor outer segments by transplanted human neural stem cells as a neuroprotective mechanism in retinal degeneration. Investigative ophthalmology & visual science. 2013;54:6745–6756. doi: 10.1167/iovs.13-12860. [DOI] [PubMed] [Google Scholar]
- Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M, Cho JH, Leung A, Savvidis G, Ahn S, Moon M, Lee PK, Han JJ, Azimi N, Kim KS, et al. hPSC-derived maturing GABAergic interneurons ameliorate seizures and abnormal behavior in epileptic mice. Cell stem cell. 2014;15:559–573. doi: 10.1016/j.stem.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Dusenbery BN, Williams LA, Klim JR, Eggan K. How to make spinal motor neurons. Development. 2014;141:491–501. doi: 10.1242/dev.097410. [DOI] [PubMed] [Google Scholar]
- De la Rossa A, Bellone C, Golding B, Vitali I, Moss J, Toni N, Luscher C, Jabaudon D. In vivo reprogramming of circuit connectivity in postmitotic neocortical neurons. Nature neuroscience. 2013;16:193–200. doi: 10.1038/nn.3299. [DOI] [PubMed] [Google Scholar]
- Dell’Anno MT, Caiazzo M, Leo D, Dvoretskova E, Medrihan L, Colasante G, Giannelli S, Theka I, Russo G, Mus L, et al. Remote control of induced dopaminergic neurons in parkinsonian rats. The Journal of clinical investigation. 2014;124:3215–3229. doi: 10.1172/JCI74664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delli Carri A, Onorati M, Lelos MJ, Castiglioni V, Faedo A, Menon R, Camnasio S, Vuono R, Spaiardi P, Talpo F, et al. Developmentally coordinated extrinsic signals drive human pluripotent stem cell differentiation toward authentic DARPP-32+ medium-sized spiny neurons. Development. 2013;140:301–312. doi: 10.1242/dev.084608. [DOI] [PubMed] [Google Scholar]
- Deshpande DM, Kim YS, Martinez T, Carmen J, Dike S, Shats I, Rubin LL, Drummond J, Krishnan C, Hoke A, et al. Recovery from paralysis in adult rats using embryonic stem cells. Annals of neurology. 2006;60:32–44. doi: 10.1002/ana.20901. [DOI] [PubMed] [Google Scholar]
- Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. The New England journal of medicine. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nature reviews Neurology. 2011;7:76–85. doi: 10.1038/nrneurol.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi D, Samata B, Katsukawa M, Kikuchi T, Morizane A, Ono Y, Sekiguchi K, Nakagawa M, Parmar M, Takahashi J. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem cell reports. 2014;2:337–350. doi: 10.1016/j.stemcr.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drukker M, Katchman H, Katz G, Even-Tov Friedman S, Shezen E, Hornstein E, Mandelboim O, Reisner Y, Benvenisty N. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem cells. 2006;24:221–229. doi: 10.1634/stemcells.2005-0188. [DOI] [PubMed] [Google Scholar]
- Duffy BA, Weitz AJ, Lee JH. In vivo imaging of transplanted stem cells in the central nervous system. Current opinion in genetics & development. 2014;28C:83–88. doi: 10.1016/j.gde.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnett SB, Bjorklund A, Stenevi U, Iversen SD. Behavioural recovery following transplantation of substantia nigra in rats subjected to 6-OHDA lesions of the nigrostriatal pathway. I. Unilateral lesions. Brain research. 1981;215:147–161. doi: 10.1016/0006-8993(81)90498-4. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Hernandez TD, Summerfield A, Jones GH, Arbuthnott G. Graft-derived recovery from 6-OHDA lesions: specificity of ventral mesencephalic graft tissues. Experimental brain research. 1988;71:411–424. doi: 10.1007/BF00247501. [DOI] [PubMed] [Google Scholar]
- Durruthy-Durruthy J, Briggs SF, Awe J, Ramathal CY, Karumbayaram S, Lee PC, Heidmann JD, Clark A, Karakikes I, Loh KM, et al. Rapid and efficient conversion of integration-free human induced pluripotent stem cells to GMP-grade culture conditions. PloS one. 2014;9:e94231. doi: 10.1371/journal.pone.0094231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell stem cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- El Maarouf A, Moyo-Lee Yaw D, Lindhout T, Pearse DD, Wakarchuk W, Rutishauser U. Enzymatic engineering of polysialic acid on cells in vitro and in vivo using a purified bacterial polysialyltransferase. The Journal of biological chemistry. 2012;287:32770–32779. doi: 10.1074/jbc.M112.377614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Maarouf A, Moyo-Lee Yaw D, Rutishauser U. Improved Stem Cell-Derived Motoneuron Survival, Migration, Sprouting and Innervation with Enhanced Expression of Polysialic Acid. Cell transplantation. 2014 doi: 10.3727/096368914X679228. [DOI] [PubMed] [Google Scholar]
- El Maarouf A, Petridis AK, Rutishauser U. Use of polysialic acid in repair of the central nervous system. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16989–16994. doi: 10.1073/pnas.0608036103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkabetz Y, Panagiotakos G, Al Shamy G, Socci ND, Tabar V, Studer L. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes & development. 2008;22:152–165. doi: 10.1101/gad.1616208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espuny-Camacho I, Michelsen KA, Gall D, Linaro D, Hasche A, Bonnefont J, Bali C, Orduz D, Bilheu A, Herpoel A, et al. Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron. 2013;77:440–456. doi: 10.1016/j.neuron.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Fasano CA, Chambers SM, Lee G, Tomishima MJ, Studer L. Efficient derivation of functional floor plate tissue from human embryonic stem cells. Cell stem cell. 2010;6:336–347. doi: 10.1016/j.stem.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Flax JD, Aurora S, Yang C, Simonin C, Wills AM, Billinghurst LL, Jendoubi M, Sidman RL, Wolfe JH, Kim SU, Snyder EY. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nature biotechnology. 1998;16:1033–1039. doi: 10.1038/3473. [DOI] [PubMed] [Google Scholar]
- Flusberg BA, Nimmerjahn A, Cocker ED, Mukamel EA, Barretto RP, Ko TH, Burns LD, Jung JC, Schnitzer MJ. High-speed, miniaturized fluorescence microscopy in freely moving mice. Nature methods. 2008;5:935–938. doi: 10.1038/nmeth.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz S. Embryonic stem cell pioneer Geron exits field, cuts losses. Nature biotechnology. 2012;30:12–13. doi: 10.1038/nbt0112-12. [DOI] [PubMed] [Google Scholar]
- Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. The New England journal of medicine. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- Fu X. The immunogenicity of cells derived from induced pluripotent stem cells. Cellular & molecular immunology. 2014;11:14–16. doi: 10.1038/cmi.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH, Temple S. Neural stem cells: generating and regenerating the brain. Neuron. 2013;80:588–601. doi: 10.1016/j.neuron.2013.10.037. [DOI] [PubMed] [Google Scholar]
- Gaillard A, Prestoz L, Dumartin B, Cantereau A, Morel F, Roger M, Jaber M. Reestablishment of damaged adult motor pathways by grafted embryonic cortical neurons. Nature neuroscience. 2007;10:1294–1299. doi: 10.1038/nn1970. [DOI] [PubMed] [Google Scholar]
- Ganat YM, Calder EL, Kriks S, Nelander J, Tu EY, Jia F, Battista D, Harrison N, Parmar M, Tomishima MJ, et al. Identification of embryonic stem cell-derived midbrain dopaminergic neurons for engraftment. The Journal of clinical investigation. 2012;122:2928–2939. doi: 10.1172/JCI58767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia I, Huang L, Ung K, Arenkiel BR. Tracing synaptic connectivity onto embryonic stem cell-derived neurons. Stem cells. 2012;30:2140–2151. doi: 10.1002/stem.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh KK, Burns LD, Cocker ED, Nimmerjahn A, Ziv Y, Gamal AE, Schnitzer MJ. Miniaturized integration of a fluorescence microscope. Nature methods. 2011;8:871–878. doi: 10.1038/nmeth.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Boulis NM, Johe K, Rutkove SB, Federici T, Polak M, Kelly C, Feldman EL. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients. Stem cells. 2012;30:1144–1151. doi: 10.1002/stem.1079. [DOI] [PubMed] [Google Scholar]
- Glavaski-Joksimovic A, Virag T, Mangatu TA, McGrogan M, Wang XS, Bohn MC. Glial cell line-derived neurotrophic factor-secreting genetically modified human bone marrow-derived mesenchymal stem cells promote recovery in a rat model of Parkinson’s disease. Journal of neuroscience research. 2010;88:2669–2681. doi: 10.1002/jnr.22435. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Nedergaard M, Windrem MS. Glial progenitor cell-based treatment and modeling of neurological disease. Science. 2012;338:491–495. doi: 10.1126/science.1218071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T, Enver T. Forcing cells to change lineages. Nature. 2009;462:587–594. doi: 10.1038/nature08533. [DOI] [PubMed] [Google Scholar]
- Grealish S, Diguet E, Kirkeby A, Mattsson B, Heuer A, Bramoulle Y, Van Camp N, Perrier AL, Hantraye P, Bjorklund A, Parmar M. Human ESC-Derived Dopamine Neurons Show Similar Preclinical Efficacy and Potency to Fetal Neurons when Grafted in a Rat Model of Parkinson’s Disease. Cell stem cell. 2014;15:653–665. doi: 10.1016/j.stem.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grealish S, Mattsson B, Draxler P, Bjorklund A. Characterisation of behavioural and neurodegenerative changes induced by intranigral 6-hydroxydopamine lesions in a mouse model of Parkinson’s disease. The European journal of neuroscience. 2010;31:2266–2278. doi: 10.1111/j.1460-9568.2010.07265.x. [DOI] [PubMed] [Google Scholar]
- Grienberger C, Konnerth A. Imaging calcium in neurons. Neuron. 2012;73:862–885. doi: 10.1016/j.neuron.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Guo X, Gonzalez M, Stancescu M, Vandenburgh HH, Hickman JJ. Neuromuscular junction formation between human stem cell-derived motoneurons and human skeletal muscle in a defined system. Biomaterials. 2011;32:9602–9611. doi: 10.1016/j.biomaterials.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Henry RG, Strober J, Kang SM, Lim DA, Bucci M, Caverzasi E, Gaetano L, Mandelli ML, Ryan T, et al. Neural stem cell engraftment and myelination in the human brain. Science translational medicine. 2012;4 doi: 10.1126/scitranslmed.3004373. 155ra137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefferan MP, Galik J, Kakinohana O, Sekerkova G, Santucci C, Marsala S, Navarro R, Hruska-Plochan M, Johe K, Feldman E, et al. Human neural stem cell replacement therapy for amyotrophic lateral sclerosis by spinal transplantation. PloS one. 2012;7:e42614. doi: 10.1371/journal.pone.0042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochbaum DR, Zhao Y, Farhi SL, Klapoetke N, Werley CA, Kapoor V, Zou P, Kralj JM, Maclaurin D, Smedemark-Margulies N, et al. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nature methods. 2014;11:825–833. doi: 10.1038/nmeth.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie N, Pereira MP, Niizuma K, Sun G, Keren-Gill H, Encarnacion A, Shamloo M, Hamilton SA, Jiang K, Huhn S, et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem cells. 2011;29:274–285. doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z, Zhang Y, Propson NE, Howden SE, Chu LF, Sontheimer EJ, Thomson JA. Efficient genome engineering in human pluripotent stem cells using Cas9 from Neisseria meningitidis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15644–15649. doi: 10.1073/pnas.1313587110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BY, Du ZW, Li XJ, Ayala M, Zhang SC. Human oligodendrocytes from embryonic stem cells: conserved SHH signaling networks and divergent FGF effects. Development. 2009;136:1443–1452. doi: 10.1242/dev.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami T, Nakamura M, Yamane J, Katoh H, Okada S, Iwanami A, Watanabe K, Ishii K, Kato F, Fujita H, et al. Chondroitinase ABC combined with neural stem/progenitor cell transplantation enhances graft cell migration and outgrowth of growth-associated protein-43-positive fibers after rat spinal cord injury. The European journal of neuroscience. 2005;22:3036–3046. doi: 10.1111/j.1460-9568.2005.04492.x. [DOI] [PubMed] [Google Scholar]
- Jain M, Armstrong RJ, Tyers P, Barker RA, Rosser AE. GABAergic immunoreactivity is predominant in neurons derived from expanded human neural precursor cells in vitro. Experimental neurology. 2003;182:113–123. doi: 10.1016/s0014-4886(03)00055-4. [DOI] [PubMed] [Google Scholar]
- Johannesson B, Sagi I, Gore A, Paull D, Yamada M, Golan-Lev T, Li Z, LeDuc C, Shen Y, Stern S, et al. Comparable frequencies of coding mutations and loss of imprinting in human pluripotent cells derived by nuclear transfer and defined factors. Cell stem cell. 2014;15:634–642. doi: 10.1016/j.stem.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Weick JP, Pearce RA, Zhang SC. Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:3069–3077. doi: 10.1523/JNEUROSCI.4562-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karumbayaram S, Novitch BG, Patterson M, Umbach JA, Richter L, Lindgren A, Conway AE, Clark AT, Goldman SA, Plath K, et al. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem cells. 2009;27:806–811. doi: 10.1002/stem.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkeby A, Grealish S, Wolf DA, Nelander J, Wood J, Lundblad M, Lindvall O, Parmar M. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell reports. 2012;1:703–714. doi: 10.1016/j.celrep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Kiuru M, Boyer JL, O’Connor TP, Crystal RG. Genetic control of wayward pluripotent stem cells and their progeny after transplantation. Cell stem cell. 2009;4:289–300. doi: 10.1016/j.stem.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D, Schmandt T, Muth-Kohne E, Perez-Bouza A, Segschneider M, Gieselmann V, Brustle O. Embryonic stem cell-based reduction of central nervous system sulfatide storage in an animal model of metachromatic leukodystrophy. Gene therapy. 2006;13:1686–1695. doi: 10.1038/sj.gt.3302834. [DOI] [PubMed] [Google Scholar]
- Koch P, Opitz T, Steinbeck JA, Ladewig J, Brustle O. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3225–3230. doi: 10.1073/pnas.0808387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokaia Z, Martino G, Schwartz M, Lindvall O. Cross-talk between neural stem cells and immune cells: the key to better brain repair? Nature neuroscience. 2012;15:1078–1087. doi: 10.1038/nn.3163. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladewig J, Koch P, Brustle O. Auto-attraction of neural precursors and their neuronal progeny impairs neuronal migration. Nature neuroscience. 2014;17:24–26. doi: 10.1038/nn.3583. [DOI] [PubMed] [Google Scholar]
- Larson PS, Starr PA, Bates G, Tansey L, Richardson RM, Martin AJ. An optimized system for interventional magnetic resonance imaging-guided stereotactic surgery: preliminary evaluation of targeting accuracy. Neurosurgery. 2012;70:95–103. doi: 10.1227/NEU.0b013e31822f4a91. discussion 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS, Tang C, Rao MS, Weissman IL, Wu JC. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nature medicine. 2013;19:998–1004. doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Shamy GA, Elkabetz Y, Schofield CM, Harrsion NL, Panagiotakos G, Socci ND, Tabar V, Studer L. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem cells. 2007;25:1931–1939. doi: 10.1634/stemcells.2007-0097. [DOI] [PubMed] [Google Scholar]
- Lee JH, Durand R, Gradinaru V, Zhang F, Goshen I, Kim DS, Fenno LE, Ramakrishnan C, Deisseroth K. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature. 2010;465:788–792. doi: 10.1038/nature09108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Englund E, Widner H, Rehncrona S, Bjorklund A, Lindvall O, Brundin P. Characterization of Lewy body pathology in 12- and 16-year-old intrastriatal mesencephalic grafts surviving in a patient with Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2010;25:1091–1096. doi: 10.1002/mds.23012. [DOI] [PubMed] [Google Scholar]
- Li T, Steinbeck JA, Lusardi T, Koch P, Lan JQ, Wilz A, Segschneider M, Simon RP, Brustle O, Boison D. Suppression of kindling epileptogenesis by adenosine releasing stem cell-derived brain implants. Brain : a journal of neurology. 2007;130:1276–1288. doi: 10.1093/brain/awm057. [DOI] [PubMed] [Google Scholar]
- Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nature biotechnology. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Brundin P, Widner H, Rehncrona S, Gustavii B, Frackowiak R, Leenders KL, Sawle G, Rothwell JC, Marsden CD, et al. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson’s disease. Science. 1990;247:574–577. doi: 10.1126/science.2105529. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Rehncrona S, Brundin P, Gustavii B, Astedt B, Widner H, Lindholm T, Bjorklund A, Leenders KL, Rothwell JC, et al. Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson’s disease. A detailed account of methodology and a 6-month follow-up. Archives of neurology. 1989;46:615–631. doi: 10.1001/archneur.1989.00520420033021. [DOI] [PubMed] [Google Scholar]
- Liu Y, Weick JP, Liu H, Krencik R, Zhang X, Ma L, Zhou GM, Ayala M, Zhang SC. Medial ganglionic eminence-like cells derived from human embryonic stem cells correct learning and memory deficits. Nature biotechnology. 2013;31:440–447. doi: 10.1038/nbt.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Malcuit C, Wang S, Girman S, Francis P, Lemieux L, Lanza R, Lund R. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem cells. 2009;27:2126–2135. doi: 10.1002/stem.149. [DOI] [PubMed] [Google Scholar]
- Lu P, Kadoya K, Tuszynski MH. Axonal growth and connectivity from neural stem cell grafts in models of spinal cord injury. Current opinion in neurobiology. 2014a;27:103–109. doi: 10.1016/j.conb.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Woodruff G, Wang Y, Graham L, Hunt M, Wu D, Boehle E, Ahmad R, Poplawski G, Brock J, et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. 2014b;83:789–796. doi: 10.1016/j.neuron.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell. 2011;146:18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Morey R, O’Neil RC, He Y, Daughtry B, Schultz MD, Hariharan M, Nery JR, Castanon R, Sabatini K, et al. Abnormalities in human pluripotent cells due to reprogramming mechanisms. Nature. 2014;511:177–183. doi: 10.1038/nature13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Hu B, Liu Y, Vermilyea SC, Liu H, Gao L, Sun Y, Zhang X, Zhang SC. Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell stem cell. 2012;10:455–464. doi: 10.1016/j.stem.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Tang C, Chaly T, Greene P, Breeze R, Fahn S, Freed C, Dhawan V, Eidelberg D. Dopamine cell implantation in Parkinson’s disease: long-term clinical and (18)F-FDOPA PET outcomes. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2010;51:7–15. doi: 10.2967/jnumed.109.066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrazo I, Drucker-Colin R, Diaz V, Martinez-Mata J, Torres C, Becerril JJ. Open microsurgical autograft of adrenal medulla to the right caudate nucleus in two patients with intractable Parkinson’s disease. The New England journal of medicine. 1987;316:831–834. doi: 10.1056/NEJM198704023161402. [DOI] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroof AM, Keros S, Tyson JA, Ying SW, Ganat YM, Merkle FT, Liu B, Goulburn A, Stanley EG, Elefanty AG, et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell stem cell. 2013;12:559–572. doi: 10.1016/j.stem.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Cerdeno V, Noctor SC, Espinosa A, Ariza J, Parker P, Orasji S, Daadi MM, Bankiewicz K, Alvarez-Buylla A, Kriegstein AR. Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6-OHDA-lesioned rats. Cell stem cell. 2010;6:238–250. doi: 10.1016/j.stem.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskos U, Kissa K, St Cloment C, Brulet P. Retrograde trans-synaptic transfer of green fluorescent protein allows the genetic mapping of neuronal circuits in transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10120–10125. doi: 10.1073/pnas.152266799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury Y, Come J, Piskorowski RA, Salah-Mohellibi N, Chevaleyre V, Peschanski M, Martinat C, Nedelec S. Combinatorial analysis of developmental cues efficiently converts human pluripotent stem cells into multiple neuronal subtypes. Nature biotechnology. 2014 doi: 10.1038/nbt.3049. [DOI] [PubMed] [Google Scholar]
- Mazzoni EO, Mahony S, Peljto M, Patel T, Thornton SR, McCuine S, Reeder C, Boyer LA, Young RA, Gifford DK, Wichterle H. Saltatory remodeling of Hox chromatin in response to rostrocaudal patterning signals. Nature neuroscience. 2013;16:1191–1198. doi: 10.1038/nn.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride JL, Behrstock SP, Chen EY, Jakel RJ, Siegel I, Svendsen CN, Kordower JH. Human neural stem cell transplants improve motor function in a rat model of Huntington’s disease. The Journal of comparative neurology. 2004;475:211–219. doi: 10.1002/cne.20176. [DOI] [PubMed] [Google Scholar]
- McGill TJ, Cottam B, Lu B, Wang S, Girman S, Tian C, Huhn SL, Lund RD, Capela A. Transplantation of human central nervous system stem cells - neuroprotection in retinal degeneration. The European journal of neuroscience. 2012;35:468–477. doi: 10.1111/j.1460-9568.2011.07970.x. [DOI] [PubMed] [Google Scholar]
- Moon LD, Asher RA, Rhodes KE, Fawcett JW. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nature neuroscience. 2001;4:465–466. doi: 10.1038/87415. [DOI] [PubMed] [Google Scholar]
- Morris SA, Cahan P, Li H, Zhao AM, San Roman AK, Shivdasani RA, Collins JJ, Daley GQ. Dissecting engineered cell types and enhancing cell fate conversion via CellNet. Cell. 2014;158:889–902. doi: 10.1016/j.cell.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon LL, Alvarez JI, Begley DJ, Boado RJ, Del Zoppo GJ, Doolittle ND, Engelhardt B, Hallenbeck JM, Lonser RR, Ohlfest JR, et al. Immunologic privilege in the central nervous system and the blood-brain barrier. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:13–21. doi: 10.1038/jcbfm.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka-Kaneda H, Nakamura S, Igarashi M, Aoi H, Kanki H, Tsuyama J, Tsutsumi S, Aburatani H, Shimazaki T, Okano H. The miR-17/106-p38 axis is a key regulator of the neurogenic-to-gliogenic transition in developing neural stem/progenitor cells. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:1604–1609. doi: 10.1073/pnas.1315567111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narva E, Autio R, Rahkonen N, Kong L, Harrison N, Kitsberg D, Borghese L, Itskovitz-Eldor J, Rasool O, Dvorak P, et al. High-resolution DNA analysis of human embryonic stem cell lines reveals culture-induced copy number changes and loss of heterozygosity. Nature biotechnology. 2010;28:371–377. doi: 10.1038/nbt.1615. [DOI] [PubMed] [Google Scholar]
- Nestor MW, Jacob S, Sun B, Pre D, Sproul AA, Hong SI, Woodard C, Zimmer M, Chinchalongporn V, Arancio O, Noggle SA. Characterization of a subpopulation of developing cortical interneurons from human iPSCs within serum free embryoid bodies. American journal of physiology Cell physiology, ajpcell. 2014;00263:02014. doi: 10.1152/ajpcell.00263.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas CR, Chen J, Tang Y, Southwell DG, Chalmers N, Vogt D, Arnold CM, Chen YJ, Stanley EG, Elefanty AG, et al. Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell stem cell. 2013;12:573–586. doi: 10.1016/j.stem.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, Zhang CL. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nature cell biology. 2013;15:1164–1175. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numasawa-Kuroiwa Y, Okada Y, Shibata S, Kishi N, Akamatsu W, Shoji M, Nakanishi A, Oyama M, Osaka H, Inoue K, et al. Involvement of ER stress in dysmyelination of Pelizaeus-Merzbacher Disease with PLP1 missense mutations shown by iPSC-derived oligodendrocytes. Stem cell reports. 2014;2:648–661. doi: 10.1016/j.stemcr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, Jiang L, 2nd, Kang J, Nedergaard M, Goldman SA. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nature medicine. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Annals of neurology. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- Orive G, Anitua E, Pedraz JL, Emerich DF. Biomaterials for promoting brain protection, repair and regeneration. Nature reviews Neuroscience. 2009;10:682–692. doi: 10.1038/nrn2685. [DOI] [PubMed] [Google Scholar]
- Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Sudhof TC, Wernig M. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panman L, Papathanou M, Laguna A, Oosterveen T, Volakakis N, Acampora D, Kurtsdotter I, Yoshitake T, Kehr J, Joodmardi E, et al. Sox6 and Otx2 control the specification of substantia nigra and ventral tegmental area dopamine neurons. Cell reports. 2014;8:1018–1025. doi: 10.1016/j.celrep.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Papapetrou EP, Lee G, Malani N, Setty M, Riviere I, Tirunagari LM, Kadota K, Roth SL, Giardina P, Viale A, et al. Genomic safe harbors permit high beta-globin transgene expression in thalassemia induced pluripotent stem cells. Nature biotechnology. 2011;29:73–78. doi: 10.1038/nbt.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KI, Teng YD, Snyder EY. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nature biotechnology. 2002;20:1111–1117. doi: 10.1038/nbt751. [DOI] [PubMed] [Google Scholar]
- Patani R, Hollins AJ, Wishart TM, Puddifoot CA, Alvarez S, de Lera AR, Wyllie DJ, Compston DA, Pedersen RA, Gillingwater TH, et al. Retinoid-independent motor neurogenesis from human embryonic stem cells reveals a medial columnar ground state. Nature communications. 2011;2:214. doi: 10.1038/ncomms1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M, Gaeta X, Loo K, Edwards M, Smale S, Cinkornpumin J, Xie Y, Listgarten J, Azghadi S, Douglass SM, et al. let-7 miRNAs Can Act through Notch to Regulate Human Gliogenesis. Stem cell reports. 2014 doi: 10.1016/j.stemcr.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peljto M, Dasen JS, Mazzoni EO, Jessell TM, Wichterle H. Functional diversity of ESC-derived motor neuron subtypes revealed through intraspinal transplantation. Cell stem cell. 2010;7:355–366. doi: 10.1016/j.stem.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera MF. Stem cells: The dark side of induced pluripotency. Nature. 2011;471:46–47. doi: 10.1038/471046a. [DOI] [PubMed] [Google Scholar]
- Pera MF, Andrade J, Houssami S, Reubinoff B, Trounson A, Stanley EG, Ward-van Oostwaard D, Mummery C. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. Journal of cell science. 2004;117:1269–1280. doi: 10.1242/jcs.00970. [DOI] [PubMed] [Google Scholar]
- Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, Harrison NL, Studer L. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pina-Crespo JC, Talantova M, Cho EG, Soussou W, Dolatabadi N, Ryan SD, Ambasudhan R, McKercher S, Deisseroth K, Lipton SA. High-frequency hippocampal oscillations activated by optogenetic stimulation of transplanted human ESC-derived neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:15837–15842. doi: 10.1523/JNEUROSCI.3735-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Gritti A, Blezer E, Amadio S, Brambilla E, Borsellino G, Cossetti C, Del Carro U, Comi G, t Hart B, et al. Human neural stem cells ameliorate autoimmune encephalomyelitis in non-human primates. Annals of neurology. 2009a;66:343–354. doi: 10.1002/ana.21745. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Brambilla E, Rovere-Querini P, Capobianco A, Alfaro-Cervello C, Salani G, Cossetti C, Borsellino G, Battistini L, et al. Immune regulatory neural stem/precursor cells protect from central nervous system autoimmunity by restraining dendritic cell function. PloS one. 2009b;4:e5959. doi: 10.1371/journal.pone.0005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- Politis M, Wu K, Loane C, Quinn NP, Brooks DJ, Rehncrona S, Bjorklund A, Lindvall O, Piccini P. Serotonergic neurons mediate dyskinesia side effects in Parkinson’s patients with neural transplants. Science translational medicine. 2010;2 doi: 10.1126/scitranslmed.3000976. 38ra46. [DOI] [PubMed] [Google Scholar]
- Poulin JF, Zou J, Drouin-Ouellet J, Kim KY, Cicchetti F, Awatramani RB. Defining midbrain dopaminergic neuron diversity by single-cell gene expression profiling. Cell reports. 2014;9:930–943. doi: 10.1016/j.celrep.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proschel C, Stripay JL, Shih CH, Munger JC, Noble MD. Delayed transplantation of precursor cell-derived astrocytes provides multiple benefits in a rat model of Parkinsons. EMBO molecular medicine. 2014;6:504–518. doi: 10.1002/emmm.201302878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond DE, Jr, Bjugstad KB, Teng YD, Ourednik V, Ourednik J, Wakeman DR, Parsons XH, Gonzalez R, Blanchard BC, Kim SU, et al. Behavioral improvement in a primate Parkinson’s model is associated with multiple homeostatic effects of human neural stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12175–12180. doi: 10.1073/pnas.0704091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, Ben-Hur T. Neural progenitors from human embryonic stem cells. Nature biotechnology. 2001;19:1134–1140. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- Reuter I, Tai YF, Pavese N, Chaudhuri KR, Mason S, Polkey CE, Clough C, Brooks DJ, Barker RA, Piccini P. Long-term clinical and positron emission tomography outcome of fetal striatal transplantation in Huntington’s disease. Journal of neurology, neurosurgery, and psychiatry. 2008;79:948–951. doi: 10.1136/jnnp.2007.142380. [DOI] [PubMed] [Google Scholar]
- Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacological reviews. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouaux C, Arlotta P. Direct lineage reprogramming of post-mitotic callosal neurons into corticofugal neurons in vivo. Nature cell biology. 2013;15:214–221. doi: 10.1038/ncb2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nature medicine. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- Rutishauser U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nature reviews Neuroscience. 2008;9:26–35. doi: 10.1038/nrn2285. [DOI] [PubMed] [Google Scholar]
- Saha B, Jaber M, Gaillard A. Potentials of endogenous neural stem cells in cortical repair. Frontiers in cellular neuroscience. 2012;6:14. doi: 10.3389/fncel.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar DL, Uchida N, Hamers FP, Cummings BJ, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in an early chronic spinal cord injury NOD-scid mouse model. PloS one. 2010;5:e12272. doi: 10.1371/journal.pone.0012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pernaute R, Studer L, Bankiewicz KS, Major EO, McKay RD. In vitro generation and transplantation of precursor-derived human dopamine neurons. Journal of neuroscience research. 2001;65:284–288. doi: 10.1002/jnr.1152. [DOI] [PubMed] [Google Scholar]
- Sawinski J, Wallace DJ, Greenberg DS, Grossmann S, Denk W, Kerr JN. Visually evoked activity in cortical cells imaged in freely moving animals. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19557–19562. doi: 10.1073/pnas.0903680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2014 doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- Selden NR, Al-Uzri A, Huhn SL, Koch TK, Sikora DM, Nguyen-Driver MD, Guillaume DJ, Koh JL, Gultekin SH, Anderson JC, et al. Central nervous system stem cell transplantation for children with neuronal ceroid lipofuscinosis. Journal of neurosurgery Pediatrics. 2013;11:643–652. doi: 10.3171/2013.3.PEDS12397. [DOI] [PubMed] [Google Scholar]
- Shi Y, Kirwan P, Smith J, Robinson HP, Livesey FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nature neuroscience. 2012;15:477–486. S471. doi: 10.1038/nn.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Roy N, Nakano T, Xuing L, Kang J, Nedergaard M, Goldman SA. Enhancer-specified GFP-based FACS purification of human spinal motor neurons from embryonic stem cells. Experimental neurology. 2005;196:224–234. doi: 10.1016/j.expneurol.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Smith B, Carson S, Fu R, McDonagh M, Dana T, Chan BKS, Thakurta S, Gibler A. In Drug Class Review: Disease-modifying Drugs for Multiple Sclerosis: Final Update 1 Report (Portland (OR)) 2010 [PubMed] [Google Scholar]
- Smith JR, Vallier L, Lupo G, Alexander M, Harris WA, Pedersen RA. Inhibition of Activin/Nodal signaling promotes specification of human embryonic stem cells into neuroectoderm. Developmental biology. 2008;313:107–117. doi: 10.1016/j.ydbio.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Sonntag KC, Pruszak J, Yoshizaki T, van Arensbergen J, Sanchez-Pernaute R, Isacson O. Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. Stem cells. 2007;25:411–418. doi: 10.1634/stemcells.2006-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwell DG, Nicholas CR, Basbaum AI, Stryker MP, Kriegstein AR, Rubenstein JL, Alvarez-Buylla A. Interneurons from embryonic development to cell-based therapy. Science. 2014;344:1240622. doi: 10.1126/science.1240622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanzel BV, Liu Z, Somboonthanakij S, Wongsawad W, Brinken R, Eter N, Corneo B, Holz FG, Temple S, Stern JH, Blenkinsop TA. Human RPE stem cells grown into polarized RPE monolayers on a polyester matrix are maintained after grafting into rabbit subretinal space. Stem cell reports. 2014;2:64–77. doi: 10.1016/j.stemcr.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbeck JA, Choi SJ, Mrejeru A, Ganat Y, Deisseroth K, Sulzer D, Mosharov EV, Studer L. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson’s disease model. Nature biotechnology. 2015 doi: 10.1038/nbt.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbeck JA, Koch P, Derouiche A, Brustle O. Human embryonic stem cell-derived neurons establish region-specific, long-range projections in the adult brain. Cellular and molecular life sciences : CMLS. 2012;69:461–470. doi: 10.1007/s00018-011-0759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer L, Tabar V, McKay RD. Transplantation of expanded mesencephalic precursors leads to recovery in parkinsonian rats. Nature neuroscience. 1998;1:290–295. doi: 10.1038/1105. [DOI] [PubMed] [Google Scholar]
- Subrizi A, Hiidenmaa H, Ilmarinen T, Nymark S, Dubruel P, Uusitalo H, Yliperttula M, Urtti A, Skottman H. Generation of hESC-derived retinal pigment epithelium on biopolymer coated polyimide membranes. Biomaterials. 2012;33:8047–8054. doi: 10.1016/j.biomaterials.2012.07.033. [DOI] [PubMed] [Google Scholar]
- Sun Y, Pollard S, Conti L, Toselli M, Biella G, Parkin G, Willatt L, Falk A, Cattaneo E, Smith A. Long-term tripotent differentiation capacity of human neural stem (NS) cells in adherent culture. Molecular and cellular neurosciences. 2008;38:245–258. doi: 10.1016/j.mcn.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Sundberg M, Bogetofte H, Lawson T, Jansson J, Smith G, Astradsson A, Moore M, Osborn T, Cooper O, Spealman R, et al. Improved cell therapy protocols for Parkinson’s disease based on differentiation efficiency and safety of hESC-, hiPSC-, and non-human primate iPSC-derived dopaminergic neurons. Stem cells. 2013;31:1548–1562. doi: 10.1002/stem.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabar V, Panagiotakos G, Greenberg ED, Chan BK, Sadelain M, Gutin PH, Studer L. Migration and differentiation of neural precursors derived from human embryonic stem cells in the rat brain. Nature biotechnology. 2005;23:601–606. doi: 10.1038/nbt1088. [DOI] [PubMed] [Google Scholar]
- Tabar V, Studer L. Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nature reviews Genetics. 2014;15:82–92. doi: 10.1038/nrg3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Amato P, Sparman M, Gutierrez NM, Tippner-Hedges R, Ma H, Kang E, Fulati A, Lee HS, Sritanaudomchai H, et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153:1228–1238. doi: 10.1016/j.cell.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tamaki SJ, Jacobs Y, Dohse M, Capela A, Cooper JD, Reitsma M, He D, Tushinski R, Belichenko PV, Salehi A, et al. Neuroprotection of host cells by human central nervous system stem cells in a mouse model of infantile neuronal ceroid lipofuscinosis. Cell stem cell. 2009;5:310–319. doi: 10.1016/j.stem.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Tannenbaum SE, Turetsky TT, Singer O, Aizenman E, Kirshberg S, Ilouz N, Gil Y, Berman-Zaken Y, Perlman TS, Geva N, et al. Derivation of xeno-free and GMP-grade human embryonic stem cells--platforms for future clinical applications. PloS one. 2012;7:e35325. doi: 10.1371/journal.pone.0035325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thestrup T, Litzlbauer J, Bartholomaus I, Mues M, Russo L, Dana H, Kovalchuk Y, Liang Y, Kalamakis G, Laukat Y, et al. Optimized ratiometric calcium sensors for functional in vivo imaging of neurons and T lymphocytes. Nature methods. 2014;11:175–182. doi: 10.1038/nmeth.2773. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Thu MS, Bryant LH, Coppola T, Jordan EK, Budde MD, Lewis BK, Chaudhry A, Ren J, Varma NR, Arbab AS, Frank JA. Self-assembling nanocomplexes by combining ferumoxytol, heparin and protamine for cell tracking by magnetic resonance imaging. Nature medicine. 2012;18:463–467. doi: 10.1038/nm.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornero D, Wattananit S, Gronning Madsen M, Koch P, Wood J, Tatarishvili J, Mine Y, Ge R, Monni E, Devaraju K, et al. Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain : a journal of neurology. 2013;136:3561–3577. doi: 10.1093/brain/awt278. [DOI] [PubMed] [Google Scholar]
- Tsukamoto A, Uchida N, Capela A, Gorba T, Huhn S. Clinical translation of human neural stem cells. Stem cell research & therapy. 2013;4:102. doi: 10.1186/scrt313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M, Leslie S, Martin NG, Peschanski M, Rao M, Taylor CJ, Trounson A, Turner D, Yamanaka S, Wilmut I. Toward the development of a global induced pluripotent stem cell library. Cell stem cell. 2013;13:382–384. doi: 10.1016/j.stem.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. The Lancet Neurology. 2011;10:649–656. doi: 10.1016/S1474-4422(11)70121-1. [DOI] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Chen K, Dohse M, Hansen KD, Dean J, Buser JR, Riddle A, Beardsley DJ, Wan Y, Gong X, et al. Human neural stem cells induce functional myelination in mice with severe dysmyelination. Science translational medicine. 2012;4 doi: 10.1126/scitranslmed.3004371. 155ra136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Leemput J, Boles NC, Kiehl TR, Corneo B, Lederman P, Menon V, Lee C, Martinez RA, Levi BP, Thompson CL, et al. CORTECON: a temporal transcriptome analysis of in vitro human cerebral cortex development from human embryonic stem cells. Neuron. 2014;83:51–68. doi: 10.1016/j.neuron.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Vazin T, Chen J, Lee CT, Amable R, Freed WJ. Assessment of stromal-derived inducing activity in the generation of dopaminergic neurons from human embryonic stem cells. Stem cells. 2008;26:1517–1525. doi: 10.1634/stemcells.2008-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vugler A, Carr AJ, Lawrence J, Chen LL, Burrell K, Wright A, Lundh P, Semo M, Ahmado A, Gias C, et al. Elucidating the phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantation. Experimental neurology. 2008;214:347–361. doi: 10.1016/j.expneurol.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Wahl AS, Omlor W, Rubio JC, Chen JL, Zheng H, Schroter A, Gullo M, Weinmann O, Kobayashi K, Helmchen F, et al. Neuronal repair. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science. 2014;344:1250–1255. doi: 10.1126/science.1253050. [DOI] [PubMed] [Google Scholar]
- Walton NM, Sutter BM, Chen HX, Chang LJ, Roper SN, Scheffler B, Steindler DA. Derivation and large-scale expansion of multipotent astroglial neural progenitors from adult human brain. Development. 2006;133:3671–3681. doi: 10.1242/dev.02541. [DOI] [PubMed] [Google Scholar]
- Wan H, Li F, Zhu L, Wang J, Yang Z, Pan Y. Update on therapeutic mechanism for bone marrow stromal cells in ischemic stroke. Journal of molecular neuroscience : MN. 2014;52:177–185. doi: 10.1007/s12031-013-0119-0. [DOI] [PubMed] [Google Scholar]
- Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, Maherali N, Studer L, Hochedlinger K, Windrem M, Goldman SA. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell stem cell. 2013;12:252–264. doi: 10.1016/j.stem.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weick JP, Johnson MA, Skroch SP, Williams JC, Deisseroth K, Zhang SC. Functional control of transplantable human ESC-derived neurons via optogenetic targeting. Stem cells. 2010;28:2008–2016. doi: 10.1002/stem.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weick JP, Liu Y, Zhang SC. Human embryonic stem cell-derived neurons adopt and regulate the activity of an established neural network. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:20189–20194. doi: 10.1073/pnas.1108487108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning GK, Odin P, Morrish P, Rehncrona S, Widner H, Brundin P, Rothwell JC, Brown R, Gustavii B, Hagell P, et al. Short- and long-term survival and function of unilateral intrastriatal dopaminergic grafts in Parkinson’s disease. Annals of neurology. 1997;42:95–107. doi: 10.1002/ana.410420115. [DOI] [PubMed] [Google Scholar]
- Wietek J, Wiegert JS, Adeishvili N, Schneider F, Watanabe H, Tsunoda SP, Vogt A, Elstner M, Oertner TG, Hegemann P. Conversion of channelrhodopsin into a light-gated chloride channel. Science. 2014;344:409–412. doi: 10.1126/science.1249375. [DOI] [PubMed] [Google Scholar]
- Xi J, Liu Y, Liu H, Chen H, Emborg ME, Zhang SC. Specification of midbrain dopamine neurons from primate pluripotent stem cells. Stem cells. 2012;30:1655–1663. doi: 10.1002/stem.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Shen P, Hazel T, Johe K, Koliatsos VE. Dual transplantation of human neural stem cells into cervical and lumbar cord ameliorates motor neuron disease in SOD1 transgenic rats. Neuroscience letters. 2011;494:222–226. doi: 10.1016/j.neulet.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Johannesson B, Sagi I, Burnett LC, Kort DH, Prosser RW, Paull D, Nestor MW, Freeby M, Greenberg E, et al. Human oocytes reprogram adult somatic nuclei of a type 1 diabetic to diploid pluripotent stem cells. Nature. 2014;510:533–536. doi: 10.1038/nature13287. [DOI] [PubMed] [Google Scholar]
- Yan J, Xu L, Welsh AM, Hatfield G, Hazel T, Johe K, Koliatsos VE. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS medicine. 2007;4:e39. doi: 10.1371/journal.pmed.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, Pearce RA, Thomson JA, Zhang SC. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem cells. 2005;23:781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DX, Di Giorgio FP, Yao J, Marchetto MC, Brennand K, Wright R, Mei A, McHenry L, Lisuk D, Grasmick JM, et al. Modeling hippocampal neurogenesis using human pluripotent stem cells. Stem cell reports. 2014;2:295–310. doi: 10.1016/j.stemcr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Vierock J, Yizhar O, Fenno LE, Tsunoda S, Kianianmomeni A, Prigge M, Berndt A, Cushman J, Polle J, et al. The microbial opsin family of optogenetic tools. Cell. 2011;147:1446–1457. doi: 10.1016/j.cell.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nature biotechnology. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, Marro S, Patzke C, Acuna C, Covy J, et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78:785–798. doi: 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao RR, Andrews MR, Wang D, Warren P, Gullo M, Schnell L, Schwab ME, Fawcett JW. Combination treatment with anti-Nogo-A and chondroitinase ABC is more effective than single treatments at enhancing functional recovery after spinal cord injury. The European journal of neuroscience. 2013;38:2946–2961. doi: 10.1111/ejn.12276. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Carido M, Meinhardt A, Kurth T, Karl MO, Ader M, Tanaka EM. Three-dimensional neuroepithelial culture from human embryonic stem cells and its use for quantitative conversion to retinal pigment epithelium. PloS one. 2013;8:e54552. doi: 10.1371/journal.pone.0054552. [DOI] [PMC free article] [PubMed] [Google Scholar]