Abstract
Background and purpose
Concern has emerged about local soft-tissue reactions after hip resurfacing arthroplasty (HRA). The Birmingham Hip Resurfacing (BHR) was the most commonly used HRA device at our institution. We assessed the prevalence and risk factors for adverse reaction to metal debris (ARMD) with this device.
Patients and methods
From 2003 to 2011, BHR was the most commonly used HRA device at our institution, with 249 implantations. We included 32 patients (24 of them men) who were operated with a BHR HRA during the period April 2004 to March 2007 (42 hips; 31 in men). The mean age of the patients was 59 (26–77) years. These patients underwent magnetic resonance imaging (MRI), serum metal ion measurements, the Oxford hip score questionnaire, and physical examination. The prevalence of ARMD was recorded, and risk factors for ARMD were assessed using logistic regression models. The mean follow-up time was 6.7 (2.4–8.8) years.
Results
6 patients had a definite ARMD (involving 9 of the 42 hips). 8 other patients (8 hips) had a probable ARMD. Thus, there was definite or probable ARMD in 17 of the 42 hips. 4 of 42 hips were revised for ARMD. Gender, bilateral metal-on-metal hip replacement and head size were not factors associated with ARMD.
Interpretation
We found that HRA with the Birmingham Hip Resurfacing may be more dangerous than previously believed. We advise systematic follow-up of these patients using metal ion levels, MRI/ultrasound, and patient-reported outcome measures.
The medium-term revision risk of many hip resurfacing arthroplasty (HRA) devices is high (AOA 2012, NJR 2012). Concern has emerged about soft-tissue reactions after HRA (Pandit et al. 2008, Glyn-Jones et al. 2009). Patients whose devices are failing often experience pain and swelling in the groin (Macpherson and Breusch 2011). The finding of large sterile effusions of the hip and/or macroscopic necrosis/metallosis associated with joint failure and pain may be referred to as adverse reactions to metal debris (ARMD) (Langton et al. 2010). Furthermore, asymptomatic pseudotumors are common after HRA (Kwon et al. 2011, Matthies et al. 2012). The reaction to excess metal wear debris is often associated with increased serum metal ion levels (Langton et al. 2010, Kwon et al. 2010). Magnetic resonance imaging (MRI) optimized to reduce image artifacts and distorsions caused by metallic implants is an important tool in diagnosing local soft-tissue abnormalities and mass lesions (Haddad et al. 2011). MRI analysis is useful in delineating soft-tissue abnormalities and mass lesions even when radiographs are normal (Hart et al. 2012).
HRA has been popular in Finland during the last 10 years (Seppänen et al. 2012). From 2003 to 2011, the BHR HRA (Smith and Nephew, Warwick, UK) was the most commonly used HRA device at our institution, with 249 implantations. We analyzed the prevalence of ARMD in an early BHR cohort consisting of 42 BHR HRA implantations performed from April 2004 to March 2007. BHR HRA is considered to be the best-performing HRA, with 10-year registry follow-up (AOA 2012). For the assessment, in addition to a physical examination, we used radiographs and MRI of the hip, serum metal ion concentrations, and the Oxford hip score (OHS) questionnaire. On the basis of these results, we tried to identify risk factors for ARMD.
Patients and methods
32 patients (42 hips) had undergone a BHR HRA between April 2004 and the end of March 2007 (Table 1). There were 24 male patients (31 study hips). The mean age of the patients was 59 (26–77) years. The patients were examined between March 2012 and June 2012 with MRI, assessment of serum metal ion measurements, the Oxford hip score (OHS) questionnaire, and physical examination. The mean follow-up time was 6.7 (2.4–8.8) years. None of the patients had undergone BHR HRA of both hips in 1 session; 10 patients had had both hips operated during the study period with BHR HRA, but in separate sessions (20 hips). 1 patient with a study implant also had a BHR HRA in the contralateral hip, but it was inserted outside the study period (2010). 1 patient had a Synergy-BHR (Smith and Nephew) large-head metal-on-metal (MoM) replacement (THR) in the contralateral hip; 1 patient had a cemented Muller THR (Zimmer, Warsaw, IN) in the contralateral hip. Posterior approach was used in all cases. 1 hip had recurrent dislocations. There were no femoral neck fractures, infections, nerve damage, or other complications.
Table 1.
Characteristics of 32 patients and results for 42 corresponding hips. Data on swelling, clicking, and subluxation sensation are given hipwise for 41 hips (the data on 1 hip are missing). Data on mean OHS (range) and the OHS classification are given hipwise for 40 hips (the data on 2 hips are missing). Data on mean (range) age, follow-up, and inclination angle of the cup are given hipwise for 42 hips
| Total | ARMD | Probable ARMD | ARMD not found | ||
|---|---|---|---|---|---|
| Patients, n | 32 | 6 | 8 | 18 | |
| Males, n | 24 | 6 | 7 | 11 | |
| Serum cobalt, µg/La | 2.5 (0.8–14.9) | 6.9 (1.2–14.9) | 1.5 (0.8–2.6) | 1.5 (0.8–2.6) | |
| Serum chromium, µg/La | 2.1 (0.6–7.6) | 4.4 (1.1–7.6) | 1.5 (1.0–2.4) | 1.6 (0.6–2.5) | |
| Hips, n | 42 | 8 | 8 | 24 | |
| Age, years a | 59 (26–77) | 63 (49–70) | 58 (26–76) | 58 (38–77) | |
| Follow-up, years a | 6.7 (2.4–8.8) | 6.0 (2.4–7.0) | 6.8 (6.3–7.3) | 7.0 (6.2–8.8) | |
| Swelling, n | 2 | 2 | 0 | 0 | |
| Clicking, n | 2 | 2 | 0 | 0 | |
| Subluxation sensation, n | 6 | 2 | 1 | 3 | |
| Inclination angle of the cup, degrees a | 47 (37–64) | 47 (42–61) | 50 (39–64) | 46 (37–60) | |
| OHS a | 44 (21–48) | 40 (33–48) | 45 (32–48) | 44 (21–48) | |
| OHS excellent, n | 30 | 3 | 7 | 20 | |
| OHS good, n | 6 | 4 | 0 | 2 | |
| OHS fair, n | 2 | 1 | 1 | 0 | |
| OHS poor, n | 2 | 0 | 0 | 2 |
Mean (range)
ARMD: adverse reaction to metal debris;
OHS: Oxford hip score (42–48 = excellent, 34–41 = good, 27–33 = fair, and 0–26 = poor).
The BHR cup has a hemispherical design with the cast-in POROCAST ingrowth surface. This HA-coated ingrowth surface does not require heat treatment to attach beads, and therefore preserves the carbide structure. This surface is integral to the cup and is not a spray-on coating. The BHR femoral component is cemented to femoral bone. The BHR HRA uses an as-cast cobalt chrome metal-on metal-bearing surface with a highly polished finish. In theory, cobalt chrome in its as-cast form has superior wear resistance to other forms of the alloy (BHR Product Manual).
MRI was used to identify fluid collections and soft-tissue masses (Toms et al. 2008, Hart et al. 2012). MRI was performed on 40 hips regardless of the patient’s symptoms. 1 patient refused MRI examination due to claustrophobia. For 1 patient, a revision operation had been performed earlier for ARMD without MRI imaging. We used 3 1.5T MR imagers (Philips Ingenia (2012); Philips Medical Systems, Best, the Netherlands; Siemens Avanto (2008) and Siemens Aera (2012); Siemens, Erlangen, Germany). The pulse sequences used were optimized to reduce metal-induced artifacts (Hargreaves et al. 2011). MARS (metal artifact reduction sequence) MRI is a recently developed technique that provides good metal artifact suppression while minimizing image blurring and scanning time (Eustace et al. 1998, Hart et al. 2012). One imager (Siemens Aera) was equipped with an advanced metal artifact reduction technique—Slice Encoding for Metal Artifact Correction—with view angle tilting (SEMAC-VAT) (Sutter et al. 2012). At least 2 sequences covering the whole pelvic area were obtained in the coronal and axial planes (STIR and T2 or T1) followed by smaller field-of-view images in 3 planes centralized in the joint with implant (STIR, T1, and T2).
Images were examined by radiologists experienced in ARMD-related MRI diagnostics. Special attention was paid to detection of periarticular fluid collections and soft-tissue masses. Pathology was measured in 3 planes and stored for analysis. For this, MRI images were examined in 3 planes for measurement of the maximal anterior-posterior, superior-inferior, and medial-lateral diameters.
All patients underwent conventional radiography of the pelvis and hip; the radiographs were used to measure the inclination angle of the cup. Radiographs were taken in upright position. Cup inclination angles were analyzed from digital pelvic radiographs using digital angle measurement. There was no osteolysis or heterotopic ossification in any of the hips. In 1 patient, there was a partial radiolucent line under the cup in Gruen zone I, but the cup position was not changed and it was considered stable.
Serum metal ion measurements (cobalt and chromium) were performed at follow-up. For ion measurements, 5–7 mL of whole blood was taken in a test tube containing heparin (for example, Venosafe or Vacuette trace elements). The Finnish Institute of Occupational Health performs all the cobalt and chromium ion measurements in Finland using inductively coupled plasma mass spectrometry. The analyses have been accredited (FINAS T013).
The OHS questionnaire was completed by 31 patients at the time of follow-up (40 hips). Clicking, a sensation of subluxation, and swelling of the hip were considered separately. The OHS questionnaire was not filled out preoperatively or at routine outpatient visits. All patients were clinically evaluated by 1 of the 5 orthopedic surgeons who performed revision surgery at the Turku University Hospital.
The prevalence of ARMD after the BHR HRA was assessed and risk factors for ARMD were evaluated: age, sex, head size (≥ 54 mm vs. ≤ 50 mm), diagnosis (secondary vs. primary OA), inclination of the cup, and bilaterality. The association of patient symptoms with ARMD was analyzed separately. The symptoms assessed were clicking, subluxation sensation, swelling, OHS total score, and relation of poor/fair versus good/excellent OHS score. OHS group 1 was considered excellent, group 2 good, group 3 fair, and group 4 poor.
ARMD was considered definite if the patient was revised for ARMD and the operative finding was compatible with ARMD. ARMD was also considered definite in those cases where a revision operation had not been performed but the serum chromium or cobalt level was ≥ 10 µg/L, and/or where there was a solid mass or a fluid collection of ≥ 50 mm in MRI (in any plane). In patients who had not undergone surgery, ARMD was considered to be probable either if the serum chromium or cobalt concentration was ≥ 5 µg/L and/or if there was a fluid collection of any size by MRI.
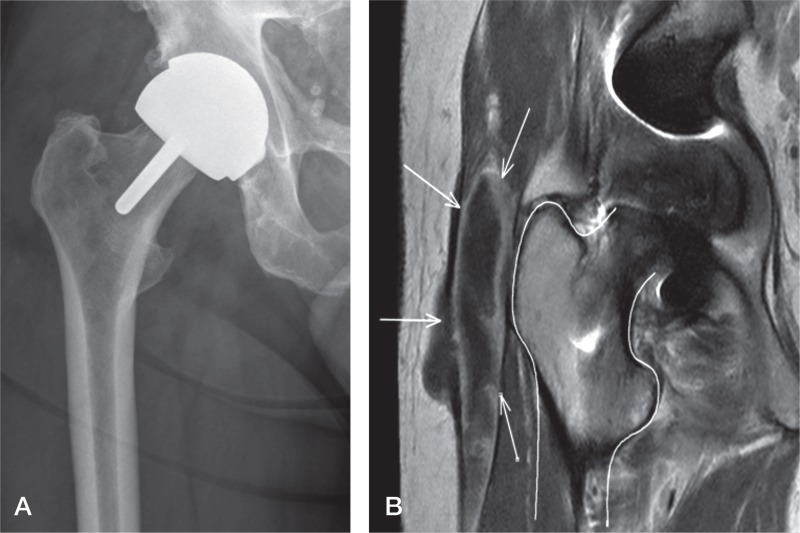
A radiograph and an MRI image of a BHR hip with a pseudotumor are presented in Figure 1.
Figure 1.
A radiograph (panel A) and an MRI image (panel B) of a BHR hip with a pseudotumor.
Statistics
Potential risk factors for ARMD were analyzed by binary logistic regression with random intercept for patient. The dependent variable ARMD consisted of 2 groups (definite or probable cases and no ARMD), with no ARMD being used as the reference group. Results are expressed as crude odds ratios (ORs) with 95% confidence intervals (CIs). Multiple binary logistic regression including risk factors with p < 0.40 in a bivariable model, forward selection, and backward elimination methods (inclusion criteria, p < 0.20) were used to investigate the potential confounding effect of other risk variables. Exact chi-square test was used to analyze clicking and swelling due to 0 cell counts. were considered statistically significant. Statistical analysis was carried out using SAS for Windows version 9.3.
Ethics
Ethical approval was not required due to adherence to national guidelines on the follow-up of metal-on-metal hip arthroplasty patients. The study was performed according to the ethical standards of Turku University Hospital and the Helsinki Declaration.
Results
6 patients (9 of 42 hips) were considered to have a definite ARMD. 4 of these hips were revised for ARMD (Tables 1 and 2). 8 patients (8 hips) were considered to have a probable ARMD. Altogether, there were 17 hips with a definite or probable ARMD. 18 patients were considered not to have ARMD.
Table 2.
Data on the 6 patients (9 hips) with a definite adverse reaction to metal debris (ARMD). None of the patients had major muscle destruction. The 64 M, 69 M, and 62 M patients had both hips with ARMD. The ARMD diagnosis of the right hip of 64 M was based on operative findings in a revision operation in 2009
| Age | Sex | Side | OHS | Pain | Clicking | Sublux. | Swelling | s-Cr, µg/L | s-Co, µg/L | Cup incl. (°) | MRI | Revision or follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 64 | M | Right | NA | Moderate | No | Yes | No | NA | NA | 48 | NA | Revised |
| 64 | M | Left | 35 | Moderate | Yes | Yes | Yes | 3.9 | 4.5 | 43 | Solid and fluid | Revised 55 × 35 × 110 mm |
| 69 | M | Right | 44 | Mild | No | No | No | 7.6 | 13.5 | 61 | Fluid 30 × 40 × 65 mm | Revised and 85 × 80 × 30 and solid 20 × 20 × 50 |
| 69 | M | Left | 44 | No | No | No | No | 7.6 | 13.5 | 47 | Fluid 57 × 46 × 10 mm | Follow-up |
| 49 | M | Right | 33 | Hard | Yes | Yes | Yes | 4.3 | 4.5 | 42 | Fluid 70 × 26 × 23 mm | Follow-up |
| 62 | M | Right | 39 | No | No | No | No | 7.6 | 14.9 | 48 | No findings | Follow-up |
| 62 | M | Left | 39 | No | No | No | No | 7.6 | 14.9 | 43 | Some fluid | Revised |
| 59 | M | Right | 41 | Moderate | No | No | No | 1.6 | 2.9 | 47 | Fluid 50 × 5 × 5 mm | Follow-up |
| 67 | M | Right | 48 | No | No | No | No | 1.1 | 1.2 | 47 | Fluid 13 × 19 × 50 mm | Follow-up |
OHS: See Table 1. Sublux.: subluxation sensation; s-Cr: serum chromium level; s-Co: serum cobalt level; Cup incl.: cup inclination angle;
MRI: magnetic resonance imaging; NA: not available.
Male sex was associated with definite ARMD, although not statistically significantly so (OR = 11, CI: 0.7–165; p = 0.08). However, sex (p = 0.2), bilateral MoM (p = 0.3), and head size (p = 0.7) were not statistically significant in the multiple logistic regression model (Tables 3 and 4). Sex was the only risk factor included in the final model using forward selection and backward elimination methods.
Table 3.
Results of testing of associations between risk factors and ARMD using logistic regression with random intercept for patient, with crude odds ratios (ORs) and 95% confidence intervals (CIs)
| ARMD definite or probable (n = 17) vs. ARMD not found (n = 25) |
|||
|---|---|---|---|
| Risk factor | OR (95% CI) | p-value | |
| Age at follow-up | 1.03a | (0.93–1.13) | 0.5 |
| Sex (male vs. female) | 10.8 | (0.7–165) | 0.08 |
| Inclination angle of the cup | 1.05a | (0.93–1.2) | 0.4 |
| Bilateral MoM | 0.33 | (0.05–2.1) | 0.2 |
| Bilateral THA | 0.55 | (0.09–3.4) | 0.5 |
| Diagnosis secondary vs. primary OA | 2.0 | (0.27–14) | 0.5 |
| Head size (≥ 54 vs. ≤ 50 mm) | 4.1 | (0.66–25) | 0.1 |
ARMD: adverse reaction to metal debris;
MoM: metal-on-metal implant; THR: total hip arthroplasty
OA: osteoarthritis.
For 1 unit increase (continuous variable).
Table 4.
Results of testing of associations between risk factors and ARMD using a multiple logistic regression model with random intercept for patient, with adjusted odds ratios (ORs) (including risk factors with p < 0.40 in bivariable model) and 95% confidence intervals (CIs)
| ARMD definite or probable (n = 17) vs. ARMD not found (n = 25) |
|||
|---|---|---|---|
| Risk factor | OR (95% CI) | p-value | |
| Sex (male vs. female) | 7.6 | (0.29–204) | 0.2 |
| Bilateral MoM | 0.40 | (0.05–3.2) | 0.3 |
| Head size (≥ 54 vs. ≤ 50 mm) | 1.6 | (0.16–16) | 0.7 |
For abbreviations: See Table 3.
OHS score (crude OR = 0.97, CI: 0.85–1.1; p = 0.7, for 1 unit increase in this continuous variable) or OHS poor/fair vs. good/excellent relation (crude OR = 1.6, CI: 0.09–27; p = 0.7) were not associated with ARMD. Furthermore, subluxation sensation (crude OR = 1.7, CI: 0.16–18; p = 0.6) was not associated with ARMD. Clicking and swelling were not associated with ARMD either (p = 0.07 for both; Fisher’s exact test).
Discussion
We found that BHR HRA may be more dangerous than previously thought. 4 of 42 hips were revised for ARMD. There was a trend of male sex being associated with definite ARMD.
One limitation of the present study was that the definition of a non-revised ARMD was not clear. Persistent pain after metal-on-metal hip implants has been shown to be associated with higher serum metal ion levels with a probable cutoff of 8 µg/L (Lardanchet et al. 2012). A cutoff level of 10 µg/L has been used previously in assessing ARMD in association with metal-on-metal hip implants (Mokka et al. 2013). There was 1 hip in our study that we considered to have ARMD due to high serum ion levels, without MRI findings. Another limitation was that we included patients with bilateral metal-on-metal implants, which may have biased metal ion analyses. However, we increased the cutoff level from 8 µg/L suggested by Lardanchet et al. (2012) to 10 µg/L due to the inclusion of bilateral HRAs. We used a metal ion level of ≥ 5 µg/L as a criterion for probable ARMD. Due to the possible bias caused by inclusion of bilateral HRAs, we performed further analysis to assess bilaterality and found that it was not associated with ARMD. 4 of our 6 definite ARMD patients had normal serum metal ion levels (< 5 µg/L). 1 of these patients was revised, and ARMD was verified at the operation. Normal serum metal ion levels may be misleading in detecting ARMD, and metal ion measurements alone should not be used for ARMD screening (Macnair et al. 2013).
Another limitation of the present study was that the approximate size of the fluid collections by MRI was used to define definite ARMD and to differentiate it from probable ARMD. All fluid collections with a solid component were considered to be definite ARMDs. The dichotomy between MRI findings ≥ 50 mm in any dimension and < 50 mm is artificial. We thus hypothesize that a fluid collection ≥ 50 mm in any dimension is a clinically significant amount of fluid with regard to a diagnosis of AMRD. Furthermore, 1 of the limitations of the present study was the lack of CT-based evaluation of implant position. However, no association has been found between MRI-detected pseudotumor formation and CT-detected HRA cup position (Hart et al. 2012), which is in accordance with our findings.
Another limitation of our study was that not all patients who were operated in our unit during the period April 2004 to March 2007 were included. At the start, we wanted to follow up patients who had been operated 2004–2005. However, the contralateral hips of many of these patients were operated with a BHR implant later, up to 2007. We decided to include these patients with bilateral hips (although one was operated later). However, there were many BHR operations in 2006 and 2007 that were not included in this screening study due to lack of resources. The total number of BHR hips inserted at our unit during the period April 2004 through March 2007 was 116 (42 of which were included in the study). We understand that there may have been selection bias, although it was not intentional. However, we believe that this did not undermine our results. ARMD was common, and several revisions for ARMD were performed.
Possible association of the risk factors with ARMD was determined using binary logistic regression (definite or probable cases vs. no ARMD). Results were expressed using ORs. When interpreting these results, the reader should be aware that OR is not equivalent to relative risk (RR) (Schmidt and Kohlmann 2008). The risk factors assessed were not statistically significantly associated with ARMD, probably due to the relatively small number of hips in the study. The same was true of possible associations between symptoms of the patients and ARMD (OHS score, relation of OHS poor/fair versus good/excellent, subluxation sensation, clicking, swelling).
Concern has been raised recently about the high failure rate of HRA due to ARMD. In May 2012, the Finnish Arthroplasty Association recommended that performance of HRAs should not be continued (FAA 2012). However, the first reports of the clinical success of BHR were promising (Treacy et al. 2005, Steffen et al. 2008, Heilpern et al. 2008). The short-term survival of the BHR was found to be comparable to that of conventional cemented THR, based on data from the Finnish Arthroplasty Register (Seppänen et al. 2012). The cumulative revision percentage of BHR at 5 years (3.6%, 95% CI: 3.2–4.0) and at 10 years (6.7%, 95% CI: 6.0–7.5) is relatively low, based on Australian registry data (AOA 2012). However, registry studies are poor at detecting early implant failure, since radiological data on osteolysis and ARMD emerge late. Early clinical trials may focus solely on radiographic findings. Bisschop et al. (2013) reported a 28% prevalence of CT-verified pseudotumors in 149 BHR HRAs after an average follow-up of 3 years. These results are in accordance with our findings. However, we based the radiological diagnosis of fluid collections and soft-tissue masses solely on MRI, except in 2 cases. The prevalence of fluid collections verified by MRI in our study was higher than that of CT-verified pseudotumor in the study by Bisschop et al. (2013). The follow-up time in the present study was longer, which is probably related to the high prevalence of ARMD. However, our aim was to detect the prevalence of ARMD based on MRI findings, serum metal ion levels, and surgical findingsand not only the prevalence of radiologically detected pseudotumors. The clinical relevance of asymptomatic fluid collections detected by MRI in patients with normal metal ion levels is unclear. The prevalence of MRI-verified pseudotumors in HRA patients with a painful hip is similar to that in asymptomatic HRA patients (Hart et al. 2012). However, the high rate of fluid collections seen by MRI and the soft-tissue destruction at the time of revision found in our patients is a cause for great concern. A systematic follow-up of these patients using metal ion levels, MRI/ultrasound, and symptom-based questionnaires is advisable.
Acknowledgments
MJ, JM, MS, PV, and KTM designed the study protocol. MJ, JM, MS, PV, JR, VÄ, AI, and KTM performed the surgery, recorded the intraoperative data, and wrote the manuscript. TP, TV, and KTM analyzed the data. KM and ET designed the MRI protocol and participated in image interpretation and revision of the manuscript for intellectual content.
This study was funded by a research grant from Turku University Hospital and a grant from the Orion-Farmos Research Foundation.
No competing interests declared.
References
- AOA 2012. Australian Orthopaedic Association National Joint Replacement Registry. http://www.dmac.adelaide.edu.au/aoanjrr/documents/aoanjrrreport_2012.pdf Annual Report. 2012
- BHR Product Manual http://www.smith-nephew.com/global/assets/pdf/products/surgical/bhr
- Bisschop R, Boomsma MF, Van Raay J J A M, Tiebosch A T M G, Maas M, Gerritsma C L E. High prevalence of pseudotumors in patients with a Birmingham hip resurfacing prosthesis. A prospective cohort study of one hundred and twenty-nine patients . J Bone Joint Surg (Am) 2013;95:1554–60. doi: 10.2106/JBJS.L.00716. [DOI] [PubMed] [Google Scholar]
- Eustace S, Goldberg R, Williamson D, Melhem ER, Oladipo O, Yucel EK, Jara H. MR imaging of soft tissues adjacent to orthopaedic hardware: techniques to minimize susceptibility artefact. Clin Radiol. 1997;52:589–94. doi: 10.1016/s0009-9260(97)80250-4. [DOI] [PubMed] [Google Scholar]
- FAA 2012. The Finnish Arthroplasty Association. http://www.suomenartroplastiayhdistys.fi
- FINAS T013 (Finnish Accreditation Service) http://www.finas.fi/
- Glyn-Jones S, Pandit H, Kwon YM, Doll H, Gill HS, Murray DW. Risk factors for inflammatory pseudotumour formation following hip resurfacing. J Bone Joint Surg (Br) 2009;91(12):1566–74. doi: 10.1302/0301-620X.91B12.22287. [DOI] [PubMed] [Google Scholar]
- Haddad FS, Thakrar RR, Hart AJ, Skinner JA, Nargol A V F, Nolan JF, Gill HS, Murray DW, Blom AW, Case CP. Metal-on-metal bearings. The evidence so far. J Bone Joint Surg (Br) 2011;93(5):572–9. doi: 10.1302/0301-620X.93B4.26429. [DOI] [PubMed] [Google Scholar]
- Hargreaves BA, Worters PW, Pauly KB, Pauly JM, Koch KM, Gold GE. Metal-induced artifacts in MRI. Am J Roentgenol. 2011;197(3):547–55. doi: 10.2214/AJR.11.7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AJ, Satchithananda K, Liddle AD, Sabah SA, McRobbie D, Henckel J, Cobb JP, Skinner JA, Mitchell AW. Pseudotumors in association with well-functioning metal-on-metal hip prostheses. A case-control study using three-dimensional computed tomography and magnetic resonance imaging . J Bone Joint Surg (Am) 2012;94(4):317–25. doi: 10.2106/JBJS.J.01508. [DOI] [PubMed] [Google Scholar]
- Heilpern GN, Shah NN, Fordyce MJ. Birmingham hip resurfacing arthroplasty: a series of 110 consecutive hips with a minimum five-year clinical and radiological follow-up . J Bone Joint Surg (Br) 2008;90:1137–42. doi: 10.1302/0301-620X.90B9.20524. [DOI] [PubMed] [Google Scholar]
- Kwon YM, Glyn-Jones S, Simpson DJ, Kamali A, McLardy-Smith P, Gill HS, Murray DW. Analysis of wear of retrieved metal-on-metal hip resurfacing implants revised to pseudotumours . J Bone Joint Surg (Br) 2010;92(3):356–61. doi: 10.1302/0301-620X.92B3.23281. [DOI] [PubMed] [Google Scholar]
- Kwon YM, Ostlere DJ, McLardy-Smith P, Athanasou NA, Gill HS, Murray DW. “Asymptomatic” pseudotumors after metal-on-metal hip resurfacing arthroplasty . J Arthroplasty. 2011;26(4):511–8. doi: 10.1016/j.arth.2010.05.030. [DOI] [PubMed] [Google Scholar]
- Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear . J Bone Joint Surg (Br) 2010;92(1):38–46. doi: 10.1302/0301-620X.92B1.22770. [DOI] [PubMed] [Google Scholar]
- Lardanchet JF, Taviaux J, Arnalsteen D, Gabrion A, Mertl P. One-year prospective comparative study of three large-diameter metal-on-metal total hip prostheses: serum metal ion levels and clinical outcomes . Orthop Traumatol Surg Res. 2012;98(3):265–74. doi: 10.1016/j.otsr.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Macnair RD, Wynn-Jones H, Wimhurst JA, Toms A, Cahir J. Metal ion levels not sufficient as a screening measure for adverse reactions in metal-on-metal hip arthroplasties . J Arthroplasty. 2013;28(1):78–83. doi: 10.1016/j.arth.2012.05.029. [DOI] [PubMed] [Google Scholar]
- Macpherson GJ, Breusch SJ. Metal-on-metal hip resurfacing: a critical review . Arch Orthop Trauma Surg. 2011;131(1):101–10. doi: 10.1007/s00402-010-1153-9. [DOI] [PubMed] [Google Scholar]
- Matthies AK, Skinner JA, Osmani H, Henckel J, Hart AJ. Pseudotumors are common in well-positioned low-wearing metal-on-metal hips . Clin Orthop Relat Res. 2012;470:1895–1906. doi: 10.1007/s11999-011-2201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokka J, Junnila M, Seppänen M, Virolainen P, Pölönen T, Vahlberg T, Mattila K, Tuominen EK, Rantakokko J, Aärimaa V, Kukkonen J, Mäkelä KT. Adverse reaction to metal debris after ReCap-M2A-Magnum large-diameter-head metal-on-metal total hip arthroplasty . Acta Orthop. 2013;84(6):549–54. doi: 10.3109/17453674.2013.859419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NJR 2012. National Joint Registry for England and Wales (NJR England-Wales) 9th Annual Report 2012 www.njrcentre.org.uk
- Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings . J Bone Joint Surg (Br) 2008;90(7):847–51. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- Schmidt CO, Kohlmann T. When to use the odds ratio or the relative risk? . Int J Public Health. 2008;53(3):165–7. doi: 10.1007/s00038-008-7068-3. [DOI] [PubMed] [Google Scholar]
- Seppänen M, Mäkelä K, Virolainen P, Remes V, Pulkkinen P, Eskelinen A. Hip resurfacing arthroplasty: short-term survivorship of 4,401 hips from the Finnish Arthroplasty Register . Acta Orthop. 2012;83(3):207–13. doi: 10.3109/17453674.2012.693016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen RT, Pandit HP, Palan J, Beard DJ, Gundle R, McLardy-Smith P, Murray DW, Gill HS. The five-year results of the Birmingham Hip Resurfacing Arthroplasty: an independent series . J Bone Joint Surg (Br) 2008;90(4):436–41. doi: 10.1302/0301-620X.90B4.19648. [DOI] [PubMed] [Google Scholar]
- Sutter R, Ulbrich EJ, Jellus V, Nittka M, Pfirrmann CW. Reduction of metal artifacts in patients with total hip arthroplasty with slice-encoding metal artifact correction and view-angle tilting MR imaging . Radiology. 2012;265(1):204–14. doi: 10.1148/radiol.12112408. [DOI] [PubMed] [Google Scholar]
- Toms AP, Marshall TJ, Cahir J, Darrah C, Nolan J, Donell ST, Barker T, Tucker JK. MRI of early symptomatic metal-on-metal total hip arthroplasty: a retrospective review of radiological findings in 20 hips . Clin Radiol. 2008;63(1):49–58. doi: 10.1016/j.crad.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Treacy RB, McBryde CW, Pynsent PB. Birmingham hip resurfacing Arthroplasty: a minimum follow-up of five years . J Bone Joint Surg (Br) 2005;87:167–70. doi: 10.1302/0301-620x.87b2.15030. [DOI] [PubMed] [Google Scholar]