Abstract
Background and purpose
It has been suggested that the risk of prosthetic joint infection (PJI) in patients with total hip arthroplasty (THA) may be underestimated if based only on arthroplasty registry data. We therefore wanted to estimate the “true” incidence of PJI in THA using several data sources.
Patients and methods
We searched the Danish Hip Arthroplasty Register (DHR) for primary THAs performed between 2005 and 2011. Using the DHR and the Danish National Register of Patients (NRP), we identified first revisions for any reason and those that were due to PJI. PJIs were also identified using an algorithm incorporating data from microbiological, prescription, and clinical biochemistry databases and clinical findings from the medical records. We calculated cumulative incidence with 95% confidence interval.
Results
32,896 primary THAs were identified. Of these, 1,546 had first-time revisions reported to the DHR and/or the NRP. For the DHR only, the 1- and 5-year cumulative incidences of PJI were 0.51% (0.44–0.59) and 0.64% (0.51–0.79). For the NRP only, the 1- and 5-year cumulative incidences of PJI were 0.48% (0.41–0.56) and 0.57% (0.45–0.71). The corresponding 1- and 5-year cumulative incidences estimated with the algorithm were 0.86% (0.77–0.97) and 1.03% (0.87–1.22). The incidences of PJI based on the DHR and the NRP were consistently 40% lower than those estimated using the algorithm covering several data sources.
Interpretation
Using several available data sources, the “true” incidence of PJI following primary THA was estimated to be approximately 40% higher than previously reported by national registries alone.
PJI is the third most common indication for revision of total hip arthroplasty (THA), accounting for approximately 15% of all revisions (Bozic and Ries 2005, Dale et al. 2012). Studies have shown that the incidence of PJI is currently on the rise (Kurtz et al. 2008, Dale et al. 2012). Despite this increase, the reported 5-year cumulative incidence of infection following primary THA in the Scandinavian countries—based on the validated arthroplasty registries alone (Soderman et al. 2000, Pedersen et al. 2004, Arthursson et al. 2005)—is less than 1%.
However, reliance on a single arthroplasty registry for identification of revision due to infection may lead to an underestimation of the true incidence (Swedish Hip Arthroplasty Register, Arthursson et al. 2005, Espehaug et al. 2006, Jamsen et al. 2009, Dale et al. 2011). The definitive diagnosis of revision due to joint infection is based on intraoperative cultures, the results of which are often unknown at the time of registration, introducing the problem of misclassification of diagnosis when the indication is based entirely on the judgment of the surgeon. In addition, lower registration of revisions than of primary procedures is a general problem in arthroplasty registries. A more realistic estimate of the incidence of PJI may be found by combining different data sources (Huotari et al. 2010).
The aim of this study was to determine the “true” incidence of first-time revision due to PJI following primary THA using several data sources, and to compare it with that derived from single registries.
Material and methods
Setting
We conducted this population-based cohort study using prospectively collected data available from nationwide medical registries in Denmark, a country with 5.6 million inhabitants. The Danish National Health Service provides tax-supported healthcare for all Danish citizens. Free medical care is guaranteed for emergency and general hospital admissions, and for outpatient clinic visits. All Danish citizens are assigned a unique civil registration number, either at birth or upon immigration; this encodes date of birth and sex. The number is recorded for all healthcare contacts, and this allows unambiguous linkage between all the Danish population-based administrative and health registers (Pedersen 2011).
The Danish Arthroplasty Register (DHR) was used to identify a cohort of patients undergoing primary THA. The patients in this cohort were then followed until death, emigration, or first-time revision. First-time revisions were identified using the DHR or the National Register of Patients (NRP). In addition, the DHR, the NRP, and additional information from databases on microbiology, prescriptions, and clinical biochemistry together with medical records were used to classify the revisions as being due to PJI or to other causes.
The primary THA study population
The Danish Hip Arthroplasty Register (DHR) was used to identify a cohort of primary THA patients, from here on referred to as the study population. The DHR is a nationwide database covering primary THAs, revisions, and postoperative complications. Registration of primary THAs and revisions is compulsory for orthopedic departments in public hospitals and private clinics in Denmark. The data are filled out by the operating surgeon on a standard form immediately after surgery. A detailed description of the DHR is available elsewhere (www.dhr.dk). The DHR was validated in 2004 (Pedersen et al. 2004) and this is an ongoing activity as part of annual reports. The registry has information on the date of surgery and the operative side.
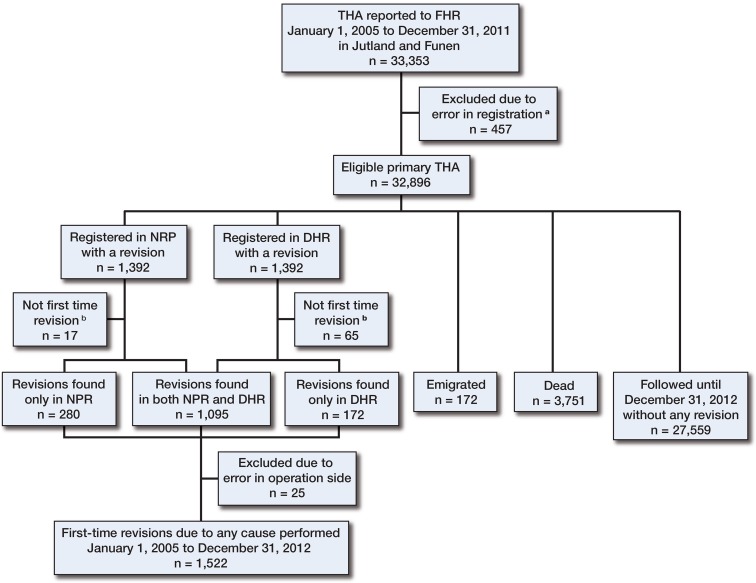
The study population included all the patients who were registered in the DHR as having had a primary THA operation performed between January 1, 2005 and December 31, 2011 in a hospital or clinic in the Jutland or Funen province. The 2 provinces have a total population of 3 million, representing 54% of the entire Danish population, and the age and sex distribution is the same as in the rest of the Danish population (Statistics Denmark); the completeness of registration is also similar. The patients were checked for missing or incorrect information regarding civil registration number, operative side, date of operation, and indication (Figure 1). All revised patients from the study population were linked to their subsequent revision.
Figure 1.
Revisions identified in either the DHR or the NRP.
a Missing or incorrect information regarding the civil registration number, operative side, date of operation, or indication.
b Excluded, as a previous revision was reported to the other registry.
Identification of first-time revision for any reason
The following sources of data were used for identification of first-time revision for any reason.
The Danish Hip Arthroplasty Register (DHR): In the DHR, the indication for revision THA is recorded as aseptic loosening, wear of polyethylene without loosening, deep infection, fracture of the femur, dislocation of the hip, pain, osteolysis, and other less frequent causes. Procedures that involved exchange of only part of the prosthesis (e.g. exchange of cup or liner alone) and debridement without exchange or removal of any part of the prosthesis were also included in the study and defined as revision.
The Danish National Register of Patients (NRP): The NRP was also used for identification of any revision. The NRP has been a national administrative health registry for all admissions and discharges from somatic public hospitals since 1977, and for all hospital outpatient and emergency visits since 1995. Since 1995, compulsory reporting to the NRP has included the civil registration number, date of admission, and physician-derived discharge diagnoses according to the Danish version of the International Classification of Diseases Tenth Edition (ICD-10), and surgical procedure codes according to the Nordic Medico-Statistical Committee (Nordic Centre for Classifications in Health Care 2008). The following surgical procedure codes are defined as revision surgery in the NRP: KNFC20-99, “revision with secondary insertion of hip prosthesis”; KNFU1, “removal of hip prosthesis”; KNFG09-29, “other kind of hip arthroplasty”; KNFW69, “revision because of deep infection”; and KNFS19 or KNFS49, “incision and revision because of prosthetic infection”.
Using the unique civil registration number, operative side, and date of primary THA operation for patients in the study population, we searched the DHR and the NRP for any subsequent revisions performed in Denmark from January 1, 2005 to December 31, 2012, which allowed a follow-up period of at least 1 year (Figure 1). First-time revisions were defined as the earliest registered revision after primary THA. If the date of operation reported to the DHR was different from that reported to the NRP, the earlier of the 2 was included and the second one was considered a second revision (and was therefore excluded) (Figure 1).
Identification of first-time revision due to prosthetic joint infection
Information on revision due to PJI was collected from the DHR (using deep infection as indication for revision) and the NRP (using the diagnosis DT845*, “infection or inflammation in the area of a prosthesis” in combination with one of the surgical procedure codes for revision described above).
In addition, the following data sources were used:
The microbiological laboratory information system (LIS): Each Department of Clinical Microbiology (DCM) serving Danish hospitals maintains an electronic LIS. All the DCMs that served a hospital in which a revision was performed provided culture reports for this study, i.e. the DCM at Aalborg University Hospital, Aarhus University Hospital, West and Central Jutland Regional Hospital, Vejle Hospital, Southern Jutland Hospital, Southwest Jutland Regional Hospital, Odense University Hospital, Slagelse Hospital, Rigshospitalet, and Naestved Hospital. Information was collected on cultures taken intraoperatively by tissue biopsy. As a rule, a number of samples (preferably 5) were obtained in the vicinity of the prosthesis and handled separately in the laboratory according to the principles of Kamme and Lindberg (Kamme and Lindberg 1981, Lorentzen and Sørensen 1986). All intraoperative samples were in culture for at least 4 days.
Prescription databases: The Universities of Aarhus and Southern Denmark maintain prescription databases, with data submitted by community pharmacies on all reimbursable prescription drugs for outpatient use (Johannesdottir et al. 2012). Patients were regarded as having received antibiotic treatment before revision if a prescription for an antibiotic had been redeemed and the treatment had lasted to within 2 weeks before the revision, which was determined by the dispensing date, defined daily dose (DDD), pack size, strength, and the number of packets or units and the information could be corroborated by review of the medical records. Lists of the ATC (Anatomic Therapeutic Chemical Classification System) codes used to identify antibiotics and the antibiotic prescriptions redeemed by patients in the study population are given in Appendix A (see Supplementary data).
Clinical biochemistry databases: The databases are maintained by the departments of clinical biochemistry at the hospitals undertaking the THA revisions (Grann et al. 2011). Information was retrieved for plasma C-reactive protein (CRP). If the database reported the CRP in nmol/L, a conversion factor of 9.524 mg/nmol was used. The normal range was set at < 10 mg/L.
Medical records: The medical records were either accessed at the hospital that performed the revision surgery or through the e-journal database maintained by the Ministry of Public Health, jointly with the Danish Regions. We extracted information about previous use of antibiotics, presence of a sinus tract, date of operation, operative side, and description of purulence in the operation notes.
Algorithm
To identify a PJI, we developed an algorithm based on multiple data sources from microbiology, clinical findings described in the medical record, antibiotic treatment prior to the revision, and preoperative CRP levels < 10 mg/L (Figures 2 and 3). A more detailed explanation of the algorithm is presented in Appendix B (see Supplementary data). We defined a PJI following a primary THA as a first-time revision reported to the DHR and/or the NRP that could be classified as having been performed due to deep infection, based on our algorithm:
Figure 2.
Algorithm for classification of the1,522 first-time revisions performed because of deep infection or other causes.
Figure 3.
Algorithm (continued) for classification of the revisions performed due to periprosthetic joint infection or other causes.
A:A PJI was defined by microbiology if the same microorganism was isolated from 3 or more biopsies taken during surgery (Kamme and Lindberg 1981, Atkins et al. 1998, Mikkelsen et al. 2006) and the microorganism isolated was not a spore-forming bacterium of questionable significance.
B:Revision THA was classified as being due to causes other than infection if: ≥ 5 intraoperative cultures were taken and all of them were negative (Atkins et al. 1998, Trampuz et al. 2007), the indication for revision in either the DHR or the NRP was reported as a cause other than infection, the patient was not treated with antibiotics prior to revision (Trampuz et al. 2007, Malekzadeh et al. 2010), and there was no positive culture from the aspiration of joint fluid (Lachiewicz et al. 1996, Malhotra and Morgan 2004, Williams et al. 2004).
C:If any of the statements in B were positive, the medical record was reviewed to identify whether a sinus tract communicating with the prosthesis had been observed (Zimmerli et al. 2004, Parvizi and Gehrke 2013).
D:If more than 5 intraoperative cultures had been taken, an audit was performed by the authors in order to classify the revision as having been performed due to PJI or no infection. The audit was done to ensure that 2 or more positive cultures did not automatically lead to a revision being classified as deep infection if the 2 positive cultures were the result of a large number of intraoperative cultures (e.g. 10–15).
E:If the CRP was >10 mg/L and had been measured within 30 days before the date of revision, the indication for performing the revision was classified according to the algorithm (continued in Figure 3) using the result of aspiration, intraoperative cultures (with prior use of antibiotics taken into account), and the presence of purulence. If CRP was not measured or it was <10 mg/L, the revision was classified as being due to other causes (Bernard et al. 2004, Savarino et al. 2004, Schinsky et al. 2008).
Note: For all stages in Figure 3, it is an underlying premise that the CRP was measured and found to be >10 mg/L.
F: 2 positive intraoperative cultures with growth of the same microorganism, as judged by phenotypic characters, were diagnostic for a PJI (Atkins et al. 1998, Mikkelsen et al. 2006, Trampuz et al. 2007, Schafer et al. 2008). A single positive culture or 2 positive cultures revealing different microorganisms (or different phenotypes) with the presence of purulence or a positive culture from joint fluid aspiration were diagnostic for a PJI (Atkins et al. 1998, Zimmerli et al. 2004, Trampuz et al. 2007, Schafer et al. 2008). If none of the cultures were positive, the likelihood of infection was considered to be low (Kamme and Lindberg 1981, Atkins et al. 1998, Mikkelsen et al. 2006, Trampuz et al. 2007, Schafer et al. 2008) unless the patient had been given antimicrobial therapy prior to the revision (Trampuz et al. 2007, Malekzadeh et al. 2010). Thus, if all intraoperative cultures were negative, the revisions were defined as having been done for causes other than infection, unless the patient had received antibiotics prior to the revision. Aspirations of joint fluid are considered to have low to moderate sensitivity and high specificity (Lachiewicz et al. 1996, Malhotra and Morgan 2004, Williams et al. 2004). Thus, a positive aspiration was given considerable weight in determining that a revision had been performed due to infection, whereas a negative aspiration had less of an influence in ruling out infection.
G:The presence of purulence has been regarded as a definite sign of infection (Zimmerli et al. 2004, Parvizi et al. 2011b) but it remains a subjective measurement (Biant et al. 2010, Blumenfeld et al. 2010, Molvik et al. 2010). The presence of purulence was therefore only regarded as a definite sign of infection if supported by other observations (e.g. elevated CRP or positive intraoperative cultures) or if negative intraoperative cultures could be explained by previous use of antibiotics.
Statistics
The cumulative incidence with 95% confidence interval (CI) was calculated as the proportion of the cohort in which a revision was performed. In accordance with the guidelines for statistical analysis of arthroplasty data, the cumulative incidence function was used to adjust for competing risks (death, emigration, and— in the estimation of PJI—revision due to causes other than infection) (Ranstam et al. 2011). The cumulative incidence of revisions for any reason was estimated for the DHR and NRP, separately and in combination. The cumulative incidence of PJI was calculated for the DHR, for the NRP, and according to our algorithm. For the 2- and 5-year cumulative incidences (with 95% CI), only primary THAs that were operated on between January 1, 2005 and December 31, 2010, and between January 1, 2005 and December 31, 2007 were included. All cases were observed until the date of the first-reported revision in the DHR and/or NRP, death, emigration, or 1, 2, or 5 years after the primary THA. Bilateral hip prostheses are not independent observations (Ranstam et al. 2011), but as none of the patients had a PJI in both hips, the effect of bilaterality was considered negligible. Data management and statistical analyses were performed with STATA12 software.
Ethics
The study was approved by the National Board of Health (journal no. 3-3013-303/1/) and the Danish Data Protection Agency (journal no. 2008-58-0035).
Results
We identified 32,896 primary THAs in 29,077 patients in our study population (Figure 1). The median age of patients with a primary THA was 69 (11–98) years. 55% of the patients were female.
First-time revisions for any reason
For the entire study period (January 1, 2005 through December 31, 2011), 1,332 and 1,392 first-time revisions were identified in the DHR and NRP, respectively. A combined total of 1,546 first-time revisions were identified; of these, 1,095 (71%) were found in both registries. The main reasons for lack of agreement between the DHR and the NRP were erroneous registration of the operative side and registration of a procedure code in the NRP that we had not defined as a revision.
The cumulative incidence of first-time revisions for any reason in the first year of follow-up was 2.83 (2.65–3.01) when the combined registries were used. Separately, the incidence for any reason was 2.22 (2.06–2.38) in the DHR, and it was 2.55 (2.39–2.73) in the NRP (Table 1). For all time periods (1, 2, and 5 years), the NRP consistently underestimated the incidence of first-time revisions by 10%, and the DHR by 20%, compared to the combined estimates using both the DHR and the NRP. There was a statistically significant increase in the cumulative incidence from 1 to 5 years of follow-up.
Table 1.
Cumulative incidence of revision for any reason following total hip arthroplasty (THA)
| Register | No. of primary THAs | No. of revisions | Cumulative incidence (%) (95% CI) |
|---|---|---|---|
| 1 year | |||
| DHR | 32,896 | 730 | 2.22 (2.06–2.38) |
| NRP | 32,896 | 839 | 2.55 (2.39–2.73) |
| Combined | 32,896 | 930 | 2.83 (2.65–3.01) |
| 2 years | |||
| DHR | 27,906 | 802 | 2.87 (2.68–3.08) |
| NRP | 27,906 | 893 | 3.20 (3.00–3.41) |
| Combined | 27,906 | 990 | 3.55 (3.34–3.77) |
| 5 years | |||
| DHR | 13,175 | 560 | 4.25 (3.92–4.60) |
| NRP | 13,175 | 589 | 4.54 (4.19–4.90) |
| Combined | 13,175 | 662 | 5.02 (4.66–5.41) |
DHR: the Danish Hip Arthroplasty Register;
NRP: the National Register of Patients.
First-time revision due to prosthetic joint infection
Of all the 1,332 revisions identified in the DHR, 227 (17%) were reported to be due to infection. For the NRP, 207 out of 1,392 revisions (16%) were due to infection. The 1-year cumulative incidences of PJI were 0.51% (0.44–0.59) for the DHR and 0.48% (0.41–0.56) for the NRP (Table 2). There was no statistically significant difference in the cumulative incidences of infection estimated from the DHR alone and from the NRP alone.
Table 2.
Cumulative incidence of prosthetic joint infection following primary total hip arthroplasty (THA)
| Register | No. of primary THAs | No. of revisions | Cumulative incidence (%) (95% CI) |
|---|---|---|---|
| 1 year | |||
| DHR | 32,896 | 167 | 0.51 (0.44–0.59) |
| NRP | 32,896 | 158 | 0.48 (0.41–0.56) |
| Algorithm | 32,893 | 285 | 0.86 (0.77–0.97) |
| 2 years | |||
| DHR | 27,906 | 185 | 0.61 (0.52–0.70) |
| NRP | 27,906 | 150 | 0.54 (0.46–0.63) |
| Algorithm | 27,903 | 266 | 0.96 (0.85–1.08) |
| 5 years | |||
| DHR | 13,175 | 84 | 0.64 (0.51–0.79) |
| NRP | 13,175 | 75 | 0.57 (0.45–0.71) |
| Algorithm | 13,172 | 136 | 1.03 (0.87–1.22) |
DHR: the Danish Hip Arthroplasty Register;
NRP: the National Register of Patients.
After review of the medical records for information on sinus tract, presence of purulence, and previous use of antibiotics, 3 primary THAs and 25 first-time revisions were excluded from the study due to misclassification of operative side, procedure code, or date of operation. 1,522 first-time revisions remained, 343 (23%) of which could be classified as PJI according to our algorithm (Figure 2).
Intraoperative samples had been obtained for culture in 1,210 revisions (80%). Most infections, 285 (83%) in the present study, had occurred within the first year of the primary THA. The cumulative incidence of infection estimated by our algorithm was approximately 40% higher than reported by the DHR and NRP for the entire study period (Table 2 and Figure 4).
Figure 4.
Prosthetic joint infections over 1 year, with 95% CI.
Discussion
To our knowledge, this is the first study to use several data sources, including microbiology, for estimation of the incidence of surgically treated PJI in primary THA. We found that the “true” cumulative incidence using our algorithm was approximately 40% higher for the 1-, 2-, and 5-year follow-up periods than the incidences reported by the validated Danish registries (the DHR and the NRP).
Methodological considerations
A key strength of this study was the number of data sources used for identification of prosthetic infections, combined with the ability to perform accurate linkage and follow-up by means of the unique civil registration number assigned to all residents of Denmark. Thus, only 40 patients (0.1%) were lost to follow-up, all of them due to emigration. The microbiology databases, which provided information on the intraoperative cultures of a minimum of 3 samples for 1,210 revisions (80%), contributed especially to the diagnosis of a large number of previously unidentified infections.
More than 90% of the intraoperative cultures included 5 samples (4–6 samples in more than 95% of the sets). This appears to reflect the work of Kamme and Lindberg in 1981, and the efforts of the scientific societies in Denmark to promote a consensus on the diagnosis, treatment, and prevention of PJI (Lorentzen and Sørensen 1986). Thus, even though data were collected from 29 orthopedic departments and intraoperative cultures were examined in 9 different microbiology departments, there was great similarity in the way that cultures were obtained.
However, building of a study on several data sources is also a weakness. Most diagnoses in the DHR and NRP have been validated (Andersen et al. 1999, Pedersen et al. 2004), but some of the codes for revision procedures have not yet been validated. So erroneous misclassifications of revisions cannot be fully accounted for. Both the DHR and the NRP have previously been shown to have a high degree of completeness (Pedersen et al. 2004, Arthursson et al. 2005), but our results indicate that for our study population, the registries have a completeness of between 80–90%. There is therefore a risk that some revisions might have been missed by both registers, which may have affected our estimate of first-time revisions.
Estimation of the true incidence of PJI is problematic, due to the inherent difficulties in making an unquestionable diagnosis of a PJI due to the lack of a universally accepted definition, as described in several papers and reviews (Bauer et al. 2006, Della Valle et al. 2010, Parvizi et al. 2011a). The International Consensus Meeting on Periprosthetic Joint Infection (Parvizi and Gehrke 2013) formulated a definition that was accepted by 85% of the physicians present, but that definition requires analyses that are not in routine use in Denmark, such as erythrocyte sedimentation rate, aspiration of joint fluid, white blood cell count in joint fluid, and histological examination of intraoperative tissue biopsies. We have tried to cover this by basing the algorithm on the most widely accepted definitions of a PJI, bestowing great importance on intraoperative cultures (Zimmerli et al. 2004, Parvizi and Gehrke 2013).
In our algorithm, a normal CRP (< 10 mg/L) or missing CRP value was used to determine whether a revision should be classified as being due to causes other than infection. A normal CRP has a high negative predictive value, but some cases of late low-grade infection may be classified as false negative—as the CRP is not always elevated in these kinds of infections. By not using the CRP in the algorithm, we might avoid classification of these cases as false negatives, but this would undoubtedly lead to a larger percentage of false positives.
For some of the patients, we were not able to identify a preoperative CRP. Naturally, it is not possible to estimate the negative predictive value of a missing CRP. However, as most Danish surgeons would request a CRP before revision if there was any doubt concerning aseptic loosening or infection, we believe that classification of revisions as not having been infected based on missing CRP values does not affect our results greatly; however, any effect would result in an even higher incidence. This was confirmed in the 146 revisions where CRP was not measured prior to the revision, as only 15 cultures showed growth and only 3 of them had more than one positive culture. Purulence was not found in any of these revisions, and none of them were reported in the DHR or the NRP as being due to infection.
Purulence has previously been regarded as a definitive sign of infection (Zimmerli et al. 2004, Parvizi et al. 2011a), but in recent years it has been downgraded due to subjective assessment. In a number of case reports, purulence was found during revision but infection remained unconfirmed (Biant et al. 2010, Blumenfeld et al. 2010, Molvik et al. 2010). In the present study, only 7 cases were diagnosed as infected based solely on elevated CRP and the presence of purulence. These patients had been treated with antibiotics before the revision, resulting in all intraoperative cultures being negative or cultures not being taken. This is a weakness, but it also reflects problems in the daily routine of revision surgery. In future studies, this conundrum may be solved by the use of molecular diagnostic methods without the limitations of culture techniques.
Comparisons with previous studies
The 5-year cumulative infection rates reported by the DHR (0.64%) and the NRP (0.57%) are similar to the 0.62% (0.60–0.65) reported by Dale et al. (2012) in a study based on the NARA register that included 2,778 revisions due to prosthetic infection from a total of 432,168 primary THAs. This indicates that our study population and the registration of infection represent the general population of primary THAs reported in Scandinavia. Ong et al. (2009) reported an incidence of infection for elective THA in the United States Medicare population of 0.78 over 2 years. This is higher than the 2-year incidence proportion reported by the DHR (0.61%) and the NRP (0.54%), but less than the 0.94% that we found using the algorithm. However, a comparison between the studies is difficult, as the Medicare population is older than 65 years of age and was different from our population regarding socioeconomic structure.
The Swedish Hip Arthroplasty Register Annual Report of 2011 described a study in which a linkage between the registry and the Swedish prescription database was used to identify patients treated with at least 1 month of antibiotics within 2 years after a primary THA. For each patient treated with antibiotics, both a questionnaire and a list of the antibiotics prescribed for the patients were sent to the clinic that performed the primary THA, asking the clinic to extract data from medical records on whether PJI was the cause of antibiotic treatment and whether the patient had been operated due to infection. The study showed that only 67% of the revisions due to infection were reported to the register. Of all the infections diagnosed, 94% underwent revision to treat the infection (Swedish Hip Arthroplasty Register). This supports the findings of our study that PJI is underestimated by national registries, but it also indicates that we did not estimate the true incidence of infection—but rather estimated the true incidence of infection that necessitated revision surgery. We chose not to include patients treated only with antibiotics, as it was considered impossible to differentiate wound drainage and a superficial surgical site infection from a true PJI when a revision was not performed.
2 or more prosthetic cultures with phenotypically identical organisms or a sinus tract communicating with the joint are generally used to diagnose infection (Zimmerli et al. 2004, Parvizi and Gehrke 2013). We chose not to use this definition, as we did not find enough studies that could validate a limit of 2 or more positive intraoperative cultures. If, however, we applied this definition (of 2 or more prosthetic cultures with phenotypically identical organisms or a sinus tract) to our data, 325 revisions could be classified as being due to PJI; of these, 9 were not classified as infected according to our algorithm. The estimated cumulative incidence based on this generally accepted definition was lower, but it was not statistically significantly different from our estimate.
The major reason for underestimating prosthetic infection in the DHR and the NRP may be that reporting to the registries is often based on subjective assessments of purulence or tissue inflammation, while other information such as definitive culture reports may not always be available at the time of reporting. In addition, surgeons most likely do not change their reporting to the registry if later culture reports show that a presumed aseptic loosening has in fact been a deep infection.
The national registries in Scandinavia have proven to be a useful tool in research, monitoring improvements in THA surgery. How the underestimation of PJI will affect the results of previous and future studies from the registries will depend entirely on the research question raised in individual studies. While absolute estimates of the incidence of PJI reported so far in studies from national hip registries is lower and only a conservative estimate of the true incidence, the relative risk estimates reported in numerous studies may not have been affected.
Conclusion
In the Danish national registries, the 1- and 5-year cumulative incidences of surgically treated prosthetic joint infection were approximately 0.50% and 0.60%. The corresponding 1- and 5-year cumulative incidences estimated by the algorithm were 0.86% (0.77–0.97) and 1.03% (0.87–1.22). Thus, the use of multiple data sources for estimation of the “true” incidence of surgically treated PJIs led to a 40% higher estimate than reported by national arthroplasty and patient registries alone.
Supplementary data
Appendices A and B are available at Acta’s website (www.actaorthop.org), identification number 7544.
Acknowledgments
PG: design of research question and study, collection and analysis of data, and writing of the article. ABP, PKA, SO: design of research question and the study, analysis of data, and revision of the manuscript. JKM, HCS: design of the study, the collection and analysis of data and revision of the manuscript.
We thank Flemming Christensen for help with extraction of data from the Microbiology Databases of Region Midtjylland and Britta Vandet Gundtoft for english language proofreading.
The study was supported by the Southern Denmark Region and Lillebaelt Hospitals.
No competing interests declared.
References
- Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences . Dan Med Bull. 1999;46(3):263–8. [PubMed] [Google Scholar]
- Arthursson AJ, Furnes O, Espehaug B, Havelin LI, Soreide JA. Validation of data in the Norwegian Arthroplasty Register the Norwegian Patient Register: 5,134 primary total hip arthroplasties and revisions operated at a single hospital between 1987 and 2003 . Acta Orthop. 2005;76(6):823–8. doi: 10.1080/17453670510045435. [DOI] [PubMed] [Google Scholar]
- Atkins BL, Athanasou N, Deeks JJ, Crook DW, Simpson H, Peto TE, McLardy-Smith P, Berendt AR. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. The OSIRIS Collaborative Study Group . J Clin Microbiol. 1998;36(10):2932–9. doi: 10.1128/jcm.36.10.2932-2939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer TW, Parvizi J, Kobayashi N, Krebs V. Diagnosis of periprosthetic infection . J Bone Joint Surg Am. 2006;88(4):869–82. doi: 10.2106/JBJS.E.01149. [DOI] [PubMed] [Google Scholar]
- Bernard L, Lubbeke A, Stern R, Bru JP, Feron JM, Peyramond D, Denormandie P, Arvieux C, Chirouze C, Perronne C, Hoffmeyer P. Value of preoperative investigations in diagnosing prosthetic joint infection: retrospective cohort study and literature review . Scand J Infect Dis. 2004;36(6)(7):410–6. doi: 10.1080/00365540410015240. [DOI] [PubMed] [Google Scholar]
- Biant LC, Bruce WJ, van der Wall H, Walsh WR. Infection or allergy in the painful metal-on-metal total hip arthroplasty? . J Arthroplasty. 2010;25(2):334. doi: 10.1016/j.arth.2008.08.015. e11-6. [DOI] [PubMed] [Google Scholar]
- Blumenfeld TJ, Bargar WL, Campbell PA. A painful metal-on-metal total hip arthroplasty: a diagnostic dilemma . J Arthroplasty. 2010;25(7):1168. doi: 10.1016/j.arth.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization . J Bone Joint Surg Am. 2005;87(8):1746–51. doi: 10.2106/JBJS.D.02937. [DOI] [PubMed] [Google Scholar]
- Dale H, Skramm I, Lower HL, Eriksen HM, Espehaug B, Furnes O, Skjeldestad FE, Havelin LI, Engesaeter LB. Infection after primary hip arthroplasty: a comparison of 3 Norwegian health registers . Acta Orthop. 2011;82(6):646–54. doi: 10.3109/17453674.2011.636671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale H, Fenstad AM, Hallan G, Havelin LI, Furnes O, Overgaard S, Pedersen AB, Karrholm J, Garellick G, Pulkkinen P, Eskelinen A, Makela K, Engesaeter LB. Increasing risk of prosthetic joint infection after total hip arthroplasty . Acta Orthop. 2012;83(5):449–58. doi: 10.3109/17453674.2012.733918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danish Hip Arthroplasty Register Annual report 2012. Annual report 2012. 20122014 [Google Scholar]
- Della Valle C, Parvizi J, Bauer TW, Dicesare PE, Evans RP, Segreti J, Spangehl M, Watters WC, 3rd, Keith M, Turkelson CM, Wies JL, Sluka P, Hitchcock K. Diagnosis of periprosthetic joint infections of the hip and knee . J Am Acad Orthop Surg. 2010;18(12):760–70. doi: 10.5435/00124635-201012000-00006. [DOI] [PubMed] [Google Scholar]
- Espehaug B, Furnes O, Havelin LI, Engesaeter LB, Vollset SE, Kindseth O. Registration completeness in the Norwegian Arthroplasty Register . Acta Orthop. 2006;77(1):49–56. doi: 10.1080/17453670610045696. [DOI] [PubMed] [Google Scholar]
- Grann AF, Erichsen R, Nielsen AG, Froslev T, Thomsen RW. Existing data sources for clinical epidemiology: The clinical laboratory information system (LABKA) research database at Aarhus University. Denmark. Clin Epidemiol. 2011;3:133–8. doi: 10.2147/CLEP.S17901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huotari K, Lyytikainen O, Ollgren J, Virtanen MJ, Seitsalo S, Palonen R, Rantanen P. Disease burden of prosthetic joint infections after hip and knee joint replacement in Finland during. 1999-: capture-recapture estimation . J Hosp Infect. 2010;75(3):205–8. doi: 10.1016/j.jhin.2009.10.029. [DOI] [PubMed] [Google Scholar]
- Jamsen E, Huotari K, Huhtala H, Nevalainen J, Konttinen YT. Low rate of infected knee replacements in a nationwide series--is it an underestimate? . Acta Orthop. 2009;80(2):205–12. doi: 10.3109/17453670902947432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesdottir SA, Horvath-Puho E, Ehrenstein V, Schmidt M, Pedersen L, Sorensen HT. Existing data sources for clinical epidemiology: The Danish National Database of Reimbursed Prescriptions . Clin Epidemiol. 2012;4:303–13. doi: 10.2147/CLEP.S37587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamme C, Lindberg L. Aerobic and Anaerobic Bacteria in Deep Infections after Total Hip Arthroplasty . Clinical Orthopaedics and Related Research. 1981;154:201–7. [PubMed] [Google Scholar]
- Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States . J Arthroplasty. 2008;23(7):984–91. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Lachiewicz PF, Rogers GD, Thomason HC. Aspiration of the hip joint before revision total hip arthroplasty. Clinical and laboratory factors influencing attainment of a positive culture . J Bone Joint Surg Am. 1996;78(5):749–54. doi: 10.2106/00004623-199605000-00015. [DOI] [PubMed] [Google Scholar]
- Lorentzen JE, Sørensen TS. Proteseinfektioner I Kar- Og Ortopædkirurgi. Mohns Bogtrykkeri. Copenhagen. 1986.
- Malekzadeh D, Osmon DR, Lahr BD, Hanssen AD, Berbari EF. Prior use of antimicrobial therapy is a risk factor for culture-negative prosthetic joint infection . Clin Orthop Relat Res. 2010;468(8):2039–45. doi: 10.1007/s11999-010-1338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra R, Morgan DA. Role of core biopsy in diagnosing infection before revision hip arthroplasty . J Arthroplasty. 2004;19(1):78–87. doi: 10.1016/s0883-5403(03)00453-4. [DOI] [PubMed] [Google Scholar]
- Mikkelsen DB, Pedersen C, Hojbjerg T, Schonheyder HC. Culture of multiple peroperative biopsies and diagnosis of infected knee arthroplasties . Apmis. 2006;114(6):449–52. doi: 10.1111/j.1600-0463.2006.apm_428.x. [DOI] [PubMed] [Google Scholar]
- Molvik H, Hanna SA, de Roeck NJ. Failed metal-on-metal total hip arthroplasty presenting as painful groin mass with associated weight loss and night sweats . Am J Orthop (Belle Mead NJ) 2010;39(5):E46–9. [PubMed] [Google Scholar]
- Nordic Centre for Classifications in Health Care NOMESCO Classification of Surgical Procedures (NCSP), version 1.14. AN:sats - Tryk & Design a-s,, Copenhagen. 2008.
- Ong KL, Kurtz SM, Lau E, Bozic KJ, Berry DJ, Parvizi J. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population . J Arthroplasty. 2009;24(6 Suppl):105–9. doi: 10.1016/j.arth.2009.04.027. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Gehrke T. Proceedings of the International Consensus Meeting on Periprosthetic Joint Infection; Proceedings of the International Consensus Meeting on Periprosthetic Joint Infection; 2013. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Jacovides C, Zmistowski B, Jung KA. Definition of periprosthetic joint infection: is there a consensus? . Clin Orthop Relat Res. 2011a;469(11):3022–30. doi: 10.1007/s11999-011-1971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society . Clin Orthop Relat Res. 2011b;469(11):2992–4. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen A, Johnsen S, Overgaard S, Soballe K, Sorensen HT, Lucht U. Registration in the danish hip arthroplasty registry: completeness of total hip arthroplasties and positive predictive value of registered diagnosis and postoperative complications . Acta Orthop Scand. 2004;75(4):434–41. doi: 10.1080/00016470410001213-1. [DOI] [PubMed] [Google Scholar]
- Pedersen CB. The Danish Civil Registration System . Scand J Public Health. 2011;39(7 Suppl):22–5. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- Ranstam J, Karrholm J, Pulkkinen P, Makela K, Espehaug B, Pedersen AB, Mehnert F, Furnes O. Statistical analysis of arthroplasty data. II. Guidelines. Acta Orthop. 2011;82(3):258–67. doi: 10.3109/17453674.2011.588863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino L, Baldini N, Tarabusi C, Pellacani A, Giunti A. Diagnosis of infection after total hip replacement . J Biomed Mater Res B Appl Biomater. 2004;70(1):139–45. doi: 10.1002/jbm.b.30030. [DOI] [PubMed] [Google Scholar]
- Schafer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy . Clin Infect Dis. 2008;47(11):1403–9. doi: 10.1086/592973. [DOI] [PubMed] [Google Scholar]
- Schinsky MF, Della Valle CJ, Sporer SM, Paprosky WG. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty . J Bone Joint Surg Am. 2008;90(9):1869–75. doi: 10.2106/JBJS.G.01255. [DOI] [PubMed] [Google Scholar]
- Soderman P, Malchau H, Herberts P, Johnell O. Are the findings in the Swedish National Total Hip Arthroplasty Register valid? A comparison between the Swedish National Total Hip Arthroplasty Register, the National Discharge Register, and the National Death Register . J Arthroplasty. 2000;15(7):884–9. doi: 10.1054/arth.2000.8591. [DOI] [PubMed] [Google Scholar]
- Statistic Denmark
- Swedish Hip Arthroplasty Register Annual report 2011 page 56-58. Annual report 2011 page 56-58. 2012.
- Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. Sonication of removed hip and knee prostheses for diagnosis of infection . N Engl J Med. 2007;357(7):654–63. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- Williams JL, Norman P, Stockley I. The value of hip aspiration versus tissue biopsy in diagnosing infection before exchange hip arthroplasty surgery . J Arthroplasty. 2004;19(5):582–6. doi: 10.1016/j.arth.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Yi PH, Cross MB, Moric M, Sporer SM, Berger RA, Della Valle CJ. The 2013 Stinchfield Award: Diagnosis of infection in the early postoperative period after total hip arthroplasty . Clin Orthop Relat Res. 2014;472(2):424–9. doi: 10.1007/s11999-013-3089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections . N Engl J Med. 2004;351(16):1645–54. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.