Abstract
It was once believed that tumor growth, progression, and metastasis were intrinsically driven by the tumor. Instead, recent research has demonstrated that a solid tumor is surrounded by a complex matrix of cells, particularly fibroblasts, which support and even promote tumor progression. This matrix of stromal cells, also known as the tumor microenvironment (TME), plays a critical role in cancer and may represent a novel therapeutic target. As such, understanding the complex nature of how the tumor initiates and maintains communication, or a “conversation”, with the TME is the focus of current investigations. We have previously shown that the most prevalent mutation found in melanoma, BRAFV600E, results in increased expression and secretion of several growth factors, cytokines, and matrix metalloproteinases, including factors that are able to activate fibroblasts. Targeted inhibition of the BRAFV600E mutation resulted in a decrease of secreted proteins into the TME and suggests that targeting the tumor also modifies the TME. Overall, this work, in combination with several additional studies discussed herein, provides strong evidence for the potential therapeutic benefits of targeting the TME, particularly signaling pathways within the fibroblasts, in conjunction with the tumor. This approach may result in extended drug resistance free survival, reduction in metastasis, and improved cytotoxic drug delivery.
Keywords: activated fibroblasts, tumor microenvironment, BRAFV600E, vemurafenib, IL-1β
The tumor microenvironment - more than structural support
It is well established that tumors are incredibly complex tissues comprised of a heterogeneous mixture of cells, and that aberrant gene expression and mutations in critical genes are major contributors to tumor development and progression. However, the picture of what influences tumor growth, and especially tumor metastasis, is a dynamic field, one that has expanded to include the cells that surround and interact with the tumor, i.e., the tumor microenvironment (TME). This microenvironment is a rich and robust mixture comprised of fibroblasts, leukocytes, pericytes, endothelial cells, and extra-cellular matrix[1]. No longer considered a supportive bystander, the TME acts as an active participant in a constant conversation with the tumor (Figure 1). A growing body of evidence has revealed that tumor cells do not act alone to promote tumorigenesis, progression, angiogenesis, and metastasis but instead collaborate with an activated TME[2–4].
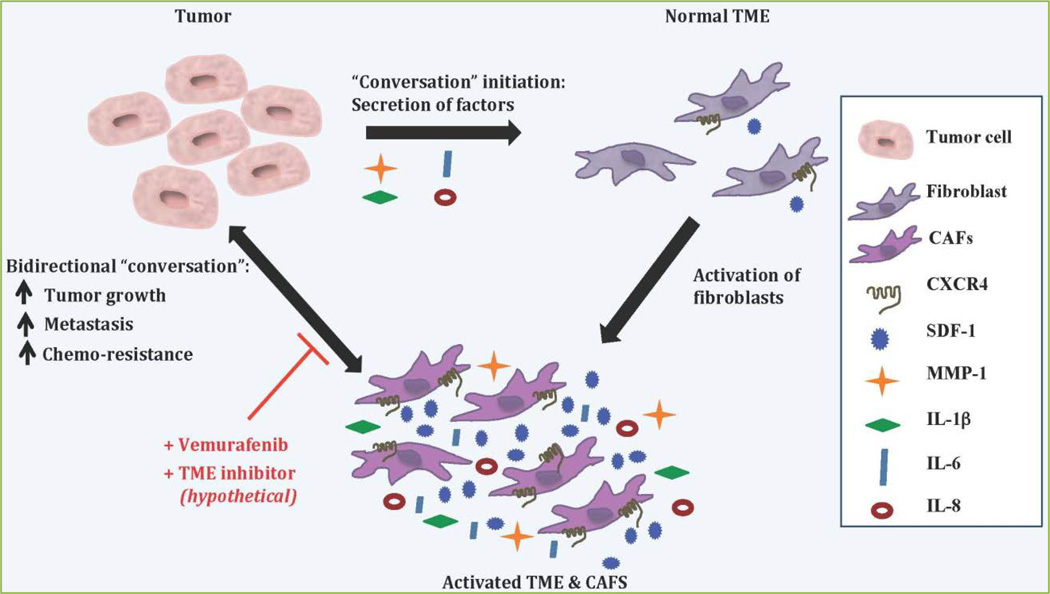
Figure 1. The conversation between the tumor and the stroma.
The tumor secretes factors, such as MMP-1, IL-1β, IL-6, and IL-8 into the surrounding tumor microenvironment (TME). These factors activate the fibroblasts that comprise the TME resulting in an increased expression of CXCR4, SDF-1, MMP-1, IL-1β, IL-6, and IL-8. The activated TME, and specifically the cancer-associated fibroblasts (CAFs), engages in a bidirectional “conversation” where it encourages and enables tumor growth, metastasis, and chemo-resistance. In turn, the tumor responds by continuing to secrete activation factors into the TME. Stopping this “conversation” by targeting the tumor cells with an anti-cancer drug such as Vemurafenib while simultaneously utilizing a (hypothetical) TME inhibitor, potentially one that specifically targets the CAFs, could result in an amplified therapeutic response.
Under normal conditions fibroblasts, which comprise the largest cellular component of the TME[4], exist in an inactive quiescent state and form a structural network by synthesizing several components of the extracellular matrix (ECM), including collagens, laminin, and fibronectin[4–6]. During wound healing and fibrosis, the fibroblasts become activated, which elicits tissue remodeling and expression of surface markers such as α–smooth muscle actin (α-SMA), Platelet-derived growth factor receptor (PDGFR), fibroblast specific protein (FSP)-1, fibroblast activation protein (FAP), as well as stromal-derived factor-1 (SDF-1: CXCL12) and its receptor CXCR4[1, 6–9]. Upon the completion of wound healing, the majority of the fibroblasts are removed by apoptosis[6, 10]. However, since tumors are often referred to as “wounds that do not heal”, they co-opt fibroblasts such that they become continuously activated and actually promote tumorigenesis[11, 12].
Once activated, these fibroblasts are referred to as cancer-associated fibroblasts (CAFs) and play an integral role in tumor-stromal interaction in several ways[13, 14]. First, fibroblasts contribute to tumor cell growth and invasion by increasing production of ECM proteins and proteases, thereby promoting tumor growth by degrading and remodeling components of the ECM[4, 5]. Next, fibroblasts suppress the immune response by recruiting inflammatory cells (such as monocytes and macrophages) to the tumor as well as by modifying immune cell function[4, 15]. Lastly, fibroblasts release growth factors and cytokines, including vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), hepatocyte growth factor (HGF), platelet derived growth factor (PDGF), SDF-1, cyclooxygenase 2 (COX-2), and several interleukins (IL-1β, IL-6, IL-8), which in turn can promote tumor angiogenesis and metastasis[16–18].
An active conversation between the tumor cells and the tumor microenvironment
Accumulating evidence emphasizes the importance of the TME in enhancing the aggressive behavior of several types of cancer, especially melanoma, pancreatic, and breast cancer[17, 19, 20]. The exact players and mechanisms that enable a tumor to activate the TME are not completely understood. In our recent study, we asked what effect the most common mutation found in melanoma, BRAFV600E, has on cytokine production by the tumor and how this impacted human dermal fibroblasts in vitro[17]. We found that melanoma cells engineered to express BRAFV600E expressed higher levels of the secreted proteins, IL-1β, IL-6, IL-8, and matrix metalloproteinase-1 (MMP-1), than wild-type cellular counterparts. Notably, conditioned media collected from the BRAFV600E melanoma cells promoted stromal fibroblast activation as indicated by an increased expression of SDF-1 (CXCL12) and its receptor CXCR4. Of particular interest, when the BRAFV600E specific inhibitor, vemurafenib, was added to the tumor cells, it reduced the expression of MMP-1 and cytokines IL-1β, IL-6, IL-8, thereby mitigating the activation of the fibroblasts (Figure1). Moreover, we found that exogenous addition of IL-1β was able to rescue expression of these cytokines, suggesting that IL-1β could act as a “master regulator” of gene expression in both tumor and stromal cells[17].
It has been demonstrated that IL-1β can induce the expression of several growth factors and chemokines, including SDF-1 and CXCR4[21–24]. Overexpression of SDF-1 and CXCR4 are not only important markers of activated fibroblasts, but also prognostic markers in various types of cancer[25, 26]. The CXCR4/SDF-1 signaling axis is involved in several critical aspects of inflammation and tumorigenesis, including: promoting tumor cell migration, invasion and site-specific metastasis[27, 28]. Notably, CXCR4/SDF-1 signaling has been shown to be pivotal in the trafficking of both normal and cancer stem cells to organs that express high levels of SDF-1, such as the lymph nodes, lungs, liver, and bone[25, 28, 29]. Additionally, several cancers with abundant CXCR4 expression (e.g. breast, ovarian, and prostate cancers, as well as neuroblastoma) have been shown to exhibit increased metastasis in a SDF-1 dependent manner[30]. Tumor progression is also enhanced via CXCR4/SDF-1 signaling. Specifically, CXCR4/SDF-1 activates several critical signal transduction pathways, including: adhesion (e.g. Fak, Paxillin, p130 CAS, PI3K, pMAPK p42/44), transcription factors, and phosphatases[29, 31]. CXCR4/SDF-1 signaling promotes matrix remodeling and invasion via the production of matrix metalloproteinases, especially MMP-1[26, 29, 32]. Considering the large number of studies that demonstrate the critical importance of CXCR4 and SDF-1 in cancer metastasis[28, 31], this interaction has emerged as a highly relevant target for hindering metastatic progression[28]. In fact, several small molecule CXCR4 antagonists, under various stages of development, have shown promising results in reducing CXCR4/SDF-1 signaling, inflammation, and metastasis[28, 33].
It is possible that IL-1β plays an integral role in tumor cells and CAFs as well as in additional stromal components such as macrophages and monocytes. It has been shown that tumor associated macrophages (TAMs), found in renal cell carcinoma (RCC), produced elevated levels of IL-1β and that the resulting high serum levels correlated with advanced disease[34]. Additionally, IL-1β contributed to disease progression by enhancing tumor cell invasion through an induction of expression in MMP-1, MMP-3, MMP-10 and MMP-14[34]. In a separate study, IL-1β, along with several additional proinflammatory cytokine, chemokines, and “protumor” genes, was upregulated in RCC and this elevated expression, which was detected in the plasma of RCC patients and in tumors, was significantly correlated with advanced tumor stages[35]. This study went on to show that in xenograft models, an antagonist of IL-1β and its receptor IL-1R1, not only impaired the expression of protumor genes and functions in TAMs but also reduced tumor growth, angiogenesis, and invasion[35]. Combined, these studies suggest that the tumorigenic effect of the IL-1β/IL-1R pathway is either intrinsic to transformed stromal cells or is mediated by the cross-communication between the tumor cells and stromal cells (e.g., immune cells, endothelial cells, and activated fibroblasts). Moreover, despite limited knowledge of the role that macrophages and monocytes play in promotion of human cancer, these studies suggest that the proinflammatory milieu expressed by TAMs, monocytes, and fibroblasts in the TME directly impacts the gene expression profile of the tumor cells[35]. Additional studies investigating the impact of targeting this subset of the TME through inhibition of IL-1β and its receptor may provide a novel avenue of combined treatment.
Targeting the stroma – potential approaches to reducing metastasis and extending response to treatment
Identifying the primary molecular signals, or key points of “conversation”, between the TME and the tumor could reveal viable novel approaches to inhibiting tumor growth and metastasis. It is known that tumor cells secrete growth factors and cytokines which recruit, or activate, the stroma and that this produces an autocrine effect where the stroma, in turn, modifies and even enables the proliferative and invasive behavior of the tumor cells[36, 37]. However, despite the advent of multiple therapeutic strategies aimed at eliminating tumor progression, many questions remain as to how targeting the tumor cells impacts the TME. For example, as our study suggests, does treatment with vemurafenib in metastatic melanoma ameliorate the TME such that the TME no longer promotes tumor progression? If so, is it possible that a reduced expression (and secretion) of inflammatory cytokines and growth factors could result in an enhanced or extended normalization or treatment window? This extended treatment window might provide an optimal time for introducing a novel inhibitor. This inhibitor could either A) specifically target activated stromal cells or B) enable a two-pronged approach where the inhibitor is aimed at two different cell populations: tumor cells and fibroblasts.
It is likely that targeting stromal cells, in conjunction with inhibition of the tumor, will be more successful at reducing tumor growth and metastasis than targeting the tumor alone, especially considering that these cells exhibit high genomic stability and thus are less likely to develop drug resistance[38]. Smalley et al. proposed three areas on which to focus the stromal therapeutic approach: inhibiting the ECM remodeling capability, blocking adhesive interactions between the stroma and the tumor, and impeding the downstream signaling pathways (especially receptor tyrosine kinase/growth factors)[38]. Although solid tumors are infiltrated by fibroblasts as well as inflammatory and endothelial cells, most of the dynamic interaction between the tumor and the stroma occurs exactly where the majority of the tumors active growth occurs - at the tumor periphery[39, 40]. This is of particular relevance as recent work has shown that CXCR4 expression is significantly increased at the tumor front versus the tumor center[41]. Combined, these data suggest that targeting the CXCR4/SDF-1 signaling axis, at the interface between the tumor and the stroma, could have a considerable impact on destabilizing the tumor-stroma “conversation”, resulting in inhibition of tumor progression and metastasis.
Several therapeutic agents targeting CXCR4, PDGF receptors, IL-1β, IL-6, IL-8, MMP inhibitors, CAF derived growth factors, and CAF derived glycoproteins (such as Tenascin-C) are already at various stages of investigation[1, 33, 42–50]. Optimally, at least one of these agents could prove highly efficacious, especially when used in combination with current treatments. It is also plausible that a novel TME inhibitor could generate a synergistic effect such that it amplifies the therapeutic effect of current drugs, potentially with few side effects. Previous studies have shown that the stroma plays a critical role in determining anticancer drug activity as well as enabling and supporting chemo-resistance[51–54]. In fact, stromal mediated alterations in malignant tumor cells not only correlate with clinical drug resistance but a high-through-put screen of over 3,000 anticancer compounds revealed that more than half of these compounds were less active in the presence of the stroma[52]. Thus, it is highly possible that targeting the stroma in conjunction with the tumor could result in a synergistic response of the anti-cancer drug as well as a reduction in clinical drug resistance.
Considering the large heterogeneity of the tumor-stroma interaction, it is unlikely that one single target will be able to stop tumorigenesis and metastasis. However, attaining a better understanding of the “conversation” between CAFs and the tumor, especially elucidating changes in gene expression in CAFs upon activation, may provide critical insights to make anti-cancer therapy more effective as well as to identify novel anti-cancer therapies. There are several key areas of the “conversation” to target: 1.) inhibiting tumor signals being sent to the fibroblasts 2.) inhibiting the return signal from the CAFs 3.) eliminating the CAFs themselves[55].
Arguably, the most beneficial target may prove to be the CAFs themselves. Gonda et al. summarized several lines of evidence for targeting CAFs that extends beyond the fact that they can support tumor proliferation, angiogenesis, and invasion[45]. First, CAFs are less likely (than tumor cells) to acquire new genetic mutations, thus they may be less prone to escape or to develop drug resistance due to genomic stability[45]. Secondly, current cancer treatments often lead to residual fibrosis, which suggests adjuvant therapy may be needed to target this fibrosis[45, 56]. Third, CAF derived factors can interfere with anti-cancer therapies, contribute to recruitment of bone-marrow derived cells to tumors, and may prevent effective immune surveillance of anti-tumor response[20, 45, 57, 58]. Lastly, a negative correlation may exist between the level of involvement and activation of the stroma and survival in certain cancers[45, 59].
Although targeting the CAFs directly may prove to be the most efficacious approach, it will likely involve several technical challenges, similar to those encountered when developing tumor cell specific antibodies. However, preliminary studies in pancreatic cancer, a cancer known for its large stromal reaction, have revealed that reduction of stromal cell proliferation can increase distribution of therapeutic agents to tumor cells[45, 60]. Specifically, in a xenograft model of pancreatic cancer, Olive et al. showed that when they inhibited stromal proliferation by targeting the hedgehog receptor, they normalized the tumor vasculature enabling enhanced delivery of the therapeutic drug to the tumor[60]. Importantly, these findings correlated with an increase in survival[60]. It may also be possible to inhibit CAF function and proliferation by targeting epigenetic alterations such as DNA methylation[45]. Experiments in mouse models of stroma rich human cancers with demethylating drugs are currently under investigation[61, 62].
In conclusion, understanding the TME and its interaction with the tumor is a complex and dynamic field. In order to significantly reduce the tumor promoting effects of the TME, it may be necessary to reduce the number of CAFs by targeting the tumor signal sent to the stroma, target the CAF signaling back to the tumor, or eliminate the CAFs themselves in order to abolish the “conversation” and help to normalize the TME. One promising area currently under investigation is aimed at understanding and comparing stromal differences across cancer types in order to discern the impact of these differences on tumor progression and cancer prognosis. It is possible that specific cancer types, especially cancers with a higher level of stromal interaction, will require an individualized approach to simultaneously target the tumor and the TME. Moreover, although fibroblasts are the predominant cell type surrounding the tumor[4]; the TME is a rich and diverse environment, consisting of a multitude of cells including: endothelial cells, pericytes, leukocytes, extra-cellular matrix. Thus, targeting other stromal components, either separately or in combination with activated fibroblasts is a promising avenue for future investigations, which may lead to significant progress in improving response to treatment for a range of cancer types.
Acknowledgements
Supported by NIH R01 AR-26599, NIH R01 CA-77267, and a Norris Cotton Cancer Center Pilot Grant awarded to Constance E. Brinckerhoff as well as NRSA- F32FCA144479A awarded to Chery A. Whipple.
References
- 1.Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Experimental Cell Research. 2010;316:1324–1331. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 2.Hu M, Peluffo G, Chen H, Gelman R, Schnitt S, Polyak K. Role of COX-2 in epithelial-stromal cell interactions and progression of ductal carcinoma in situ of the breast. Proc Natl Acad Sci U S A. 2009;106:3372–3377. doi: 10.1073/pnas.0813306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyan SW, Kuo WH, Huang CK, Pan CC, Shew JY, Chang KJ, et al. Breast cancer cells induce cancer-associated fibroblasts to secrete hepatocyte growth factor to enhance breast tumorigenesis. PLoS One. 2011;6:e15313. doi: 10.1371/journal.pone.0015313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Liu J. Tumor stroma as targets for cancer therapy. Pharmacol Ther. 2013;137:200–215. doi: 10.1016/j.pharmthera.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bremnes RM, Donnem T, Al-Saad S, Al-Shibli K, Andersen S, Sirera R, et al. The role of tumor stroma in cancer progression and prognosis: emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. Journal of Thoracic Oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6:209–217. doi: 10.1097/JTO.0b013e3181f8a1bd. [DOI] [PubMed] [Google Scholar]
- 6.Rasanen K, Vaheri A. Activation of fibroblasts in cancer stroma. Exp Cell Res. 2010;316:2713–2722. doi: 10.1016/j.yexcr.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 7.Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biology & Therapy. 2006;5:1640–1646. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- 8.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. The Journal of Cell Biology. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domanska UM, Kruizinga RC, Nagengast WB, Timmer-Bosscha H, Huls G, de Vries EG, Walenkamp AM. A review on CXCR4/CXCL12 axis in oncology: no place to hide. European Journal of Cancer. 2013;49:219–230. doi: 10.1016/j.ejca.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- 11.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haviv I, Polyak K, Qiu W, Hu M, Campbell I. Origin of carcinoma associated fibroblasts. Cell Cycle. 2009;8:589–595. doi: 10.4161/cc.8.4.7669. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 14.Desmouliere A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. The International Journal of Developmental Biology. 2004;48:509–517. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- 15.Silzle T, Kreutz M, Dobler MA, Brockhoff G, Knuechel R, Kunz-Schughart LA. Tumor-associated fibroblasts recruit blood monocytes into tumor tissue. European Journal of Immunology. 2003;33(5):1311–1320. doi: 10.1002/eji.200323057. [DOI] [PubMed] [Google Scholar]
- 16.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 17.Whipple CA, Brinckerhoff CE. BRAF(V600E) melanoma cells secrete factors that activate stromal fibroblasts and enhance tumourigenicity. Br J Cancer. 2014;111:1625–1633. doi: 10.1038/bjc.2014.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 19.Kleeff J, Beckhove P, Esposito I, Herzig S, Huber PE, Lohr JM, Friess H. Pancreatic cancer microenvironment. Int J Cancer. 2007;121:699–705. doi: 10.1002/ijc.22871. [DOI] [PubMed] [Google Scholar]
- 20.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 21.Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, et al. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Reviews. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 22.Apte RN, Voronov E. Is interleukin-1 a good or bad 'guy' in tumor immunobiology and immunotherapy? Immunol Rev. 2008;222:222–241. doi: 10.1111/j.1600-065X.2008.00615.x. [DOI] [PubMed] [Google Scholar]
- 23.Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal. 2010;3 doi: 10.1126/scisignal.3105cm1. cm1. [DOI] [PubMed] [Google Scholar]
- 24.Weber A, Wasiliew P, Kracht M. Interleukin-1beta (IL-1beta) processing pathway. Sci Signal. 2010;3 doi: 10.1126/scisignal.3105cm2. cm2. [DOI] [PubMed] [Google Scholar]
- 25.Furusato B, Mohamed A, Uhlen M, Rhim JS. CXCR4 and cancer. Pathol Int. 2010;60:497–505. doi: 10.1111/j.1440-1827.2010.02548.x. [DOI] [PubMed] [Google Scholar]
- 26.Eck SM, Blackburn JS, Schmucker AC, Burrage PS, Brinckerhoff CE. Matrix metalloproteinase and G protein coupled receptors: co-conspirators in the pathogenesis of autoimmune disease and cancer. J Autoimmun. 2009;33:214–221. doi: 10.1016/j.jaut.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weitzenfeld P, Ben-Baruch A. The chemokine system, and its CCR5 and CXCR4 receptors, as potential targets for personalized therapy in cancer. Cancer Lett. 2014;352:36–53. doi: 10.1016/j.canlet.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Liang Z, Zhan W, Zhu A, Yoon Y, Lin S, Sasaki M, et al. Development of a unique small molecule modulator of CXCR4. PLoS One. 2012;7:e34038. doi: 10.1371/journal.pone.0034038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem cells. 2005;23:879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 30.Takeyama K, Jung B, Shim JJ, Burgel PR, Dao-Pick T, Ueki IF, Protin U, et al. Activation of epidermal growth factor receptors is responsible for mucin synthesis induced by cigarette smoke. Am J Physiol Lung Cell Mol Physiol. 2001;280:L165–L172. doi: 10.1152/ajplung.2001.280.1.L165. [DOI] [PubMed] [Google Scholar]
- 31.Shim H, Oishi S, Fujii N. Chemokine receptor CXCR4 as a therapeutic target for neuroectodermal tumors. Semin Cancer Biol. 2009;19:123–134. doi: 10.1016/j.semcancer.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eck SM, Cote AL, Winkelman WD, Brinckerhoff CE. CXCR4 and matrix metalloproteinase-1 are elevated in breast carcinoma-associated fibroblasts and in normal mammary fibroblasts exposed to factors secreted by breast cancer cells. Molecular cancer research : MCR. 2009;7:1033–1044. doi: 10.1158/1541-7786.MCR-09-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Debnath B, Xu S, Grande F, Garofalo A, Neamati N. Small molecule inhibitors of CXCR4. Theranostics. 2013;3:47–75. doi: 10.7150/thno.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrella BL, Vincenti MP. Interleukin-1beta mediates metalloproteinase-dependent renal cell carcinoma tumor cell invasion through the activation of CCAAT enhancer binding protein beta. Cancer Medicine. 2012;1:17–27. doi: 10.1002/cam4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chittezhath M, Dhillon MK, Lim JY, Laoui D, Shalova IN, Teo YL, et al. Molecular profiling reveals a tumor-promoting phenotype of monocytes and macrophages in human cancer progression. Immunity. 2014;41:815–829. doi: 10.1016/j.immuni.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 37.Brown LF, Guidi AJ, Schnitt SJ, Van De Water L, Iruela-Arispe ML, Yeo TK, et al. Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin Cancer Res. 1999;5:1041–1056. [PubMed] [Google Scholar]
- 38.Smalley KS, Lioni M, Herlyn M. Targeting the stromal fibroblasts: a novel approach to melanoma therapy. Expert Rev Anticancer Ther. 2005;5:1069–1078. doi: 10.1586/14737140.5.6.1069. [DOI] [PubMed] [Google Scholar]
- 39.Labrousse AL, Ntayi C, Hornebeck W, Bernard P. Stromal reaction in cutaneous melanoma. Critical Reviews in Oncology/Hematology. 2004;49:269–275. doi: 10.1016/j.critrevonc.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 40.Ruiter D, Bogenrieder T, Elder D, Herlyn M. Melanoma-stroma interactions: structural and functional aspects. The Lancet Oncology. 2002;3:35–43. doi: 10.1016/s1470-2045(01)00620-9. [DOI] [PubMed] [Google Scholar]
- 41.Delongchamps NB, Beuvon F, Mathieu JR, Delmas S, Metzger I, Prats H, Cabon F. CXCR4 is highly expressed at the tumor front but not in the center of prostate cancers. World Journal of Urology. 2015;33:281–287. doi: 10.1007/s00345-014-1299-0. [DOI] [PubMed] [Google Scholar]
- 42.Gacche RN, Meshram RJ. Targeting tumor micro-environment for design and development of novel anti-angiogenic agents arresting tumor growth. Progress in Biophysics and Molecular Biology. 2013;113:333–354. doi: 10.1016/j.pbiomolbio.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nature Reviews Molecular Cell Biology. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 44.Pietras K, Pahler J, Bergers G, Hanahan D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Medicine. 2008;5:e19. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonda TA, Varro A, Wang TC, Tycko B. Molecular biology of cancer-associated fibroblasts: can these cells be targeted in anti-cancer therapy? Seminars in Cell & Developmental Biology. 2010;21:2–10. doi: 10.1016/j.semcdb.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim KJ, Wang L, Su YC, Gillespie GY, Salhotra A, Lal B, Laterra J. Systemic anti-hepatocyte growth factor monoclonal antibody therapy induces the regression of intracranial glioma xenografts. Clin Cancer Res. 2006;12:1292–1298. doi: 10.1158/1078-0432.CCR-05-1793. [DOI] [PubMed] [Google Scholar]
- 47.van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 48.Hofmeister V, Schrama D, Becker JC. Anti-cancer therapies targeting the tumor stroma. Cancer Immunol Immunother. 2008;57:1–17. doi: 10.1007/s00262-007-0365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Devine SM, Flomenberg N, Vesole DH, Liesveld J, Weisdorf D, Badel K, et al. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin's lymphoma. J Clin Oncol. 2004;22:1095–1102. doi: 10.1200/JCO.2004.07.131. [DOI] [PubMed] [Google Scholar]
- 50.Rubin JB, Kung AL, Klein RS, Chan JA, Sun Y, Schmidt K, et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci U S A. 2003;100:13513–13518. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McMillin DW, Delmore J, Negri JM, Vanneman M, Koyama S, Schlossman RL, et al. Compartment-Specific Bioluminescence Imaging platform for the high-throughput evaluation of antitumor immune function. Blood. 2012;119(15):e131–e138. doi: 10.1182/blood-2011-04-348490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McMillin DW, Delmore J, Weisberg E, Negri JM, Geer DC, Klippel S, et al. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nat Med. 2010;16:483–489. doi: 10.1038/nm.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitsiades CS, Mitsiades N, Munshi NC, Anderson KC. Focus on multiple myeloma. Cancer Cell. 2004;6:439–444. doi: 10.1016/j.ccr.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 54.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 55.Micke P, Ostman A. Tumour-stroma interaction: cancer-associated fibroblasts as novel targets in anti-cancer therapy? Lung Cancer. 2004;45(Suppl 2):S163–S175. doi: 10.1016/j.lungcan.2004.07.977. [DOI] [PubMed] [Google Scholar]
- 56.Harless WW. Revisiting perioperative chemotherapy: the critical importance of targeting residual cancer prior to wound healing. BMC cancer. 2009;9:118. doi: 10.1186/1471-2407-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crawford Y, Kasman I, Yu L, Zhong C, Wu X, Modrusan Z, et al. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15:21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Zhang B. Targeting the stroma by T cells to limit tumor growth. Cancer Res. 2008;68:9570–9573. doi: 10.1158/0008-5472.CAN-08-2414. [DOI] [PubMed] [Google Scholar]
- 59.Maeshima AM, Niki T, Maeshima A, Yamada T, Kondo H, Matsuno Y. Modified scar grade: a prognostic indicator in small peripheral lung adenocarcinoma. Cancer. 2002;95:2546–2554. doi: 10.1002/cncr.11006. [DOI] [PubMed] [Google Scholar]
- 60.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bloushtain-Qimron N, Yao J, Snyder EL, Shipitsin M, Campbell LL, Mani SA, et al. Cell type-specific DNA methylation patterns in the human breast. Proc Natl Acad Sci U S A. 2008;105:14076–14081. doi: 10.1073/pnas.0805206105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stresemann C, Brueckner B, Musch T, Stopper H, Lyko F. Functional diversity of DNA methyltransferase inhibitors in human cancer cell lines. Cancer Res. 2006;66:2794–2800. doi: 10.1158/0008-5472.CAN-05-2821. [DOI] [PubMed] [Google Scholar]