Abstract
Pericytes are embedded within basal lamina and play multiple roles in the perivascular niche in brain. Recently, oligodendrocyte precursor cells (OPCs) have also been reported to associate with cerebral endothelium. Is it possible that within this gliovascular locus, there may also exist potential spatial and functional interactions between pericytes and OPCs? Here, we demonstrated that in the perivascular region of cerebral white matter, pericytes and OPCs may attach and support each other. Immunostaining showed that pericytes and OPCs are localized in close contact with each other in mouse white matter at postnatal days 0, 60 and 240. Electron microscopic analysis confirmed that pericytes attached to OPCs via basal lamina in the perivascular region. The close proximity between these two cell types was also observed in postmortem human brains. Functional interaction between pericytes and OPCs was assessed by in vitro media transfer experiments. When OPC cultures were treated with pericyte-conditioned media, OPC number increased. Similarly, pericyte number increased when pericytes were maintained in OPC-conditioned media. Taken together, our data suggest a potential anatomical and functional interaction between pericytes and OPCs in cerebral white matter.
Keywords: pericyte, oligodendrocyte precursor cell, perivascular niche, white matter
Introduction
Pericytes are embedded within basal lamina and localized at the abluminal side of perivascular space in microvessels. Brain is one of the most vascularized organs, and pericytes in brain have a higher density compared to other organs [4, 5, 9]. Consistent with their higher density, pericytes play multiple roles on neurovascular function in brain [33]. For example, pericytes regulate blood-brain barrier (BBB) integrity and microcirculation, promote vasculo-angiogenesis, enhance clearance and phagocytosis of cellular debris or byproducts, and modulate inflammation and immune system. In addition, disruption of crosstalk between pericytes and other cell types by pericyte dysfunction may lead to various brain disorders including stroke, Alzheimer's disease, and other neurodegenerative diseases.
OPCs comprise the primary source for myelin-expressing mature oligodendrocytes [22, 24], and in adult brain, OPCs are uniformly distributed in both grey and white matter areas, comprising around 5% of all brain cells [10, 22, 28]. Notably, some oligodendrocyte precursor cells (OPCs) may also interact with cerebral endothelium [30, 31]. As pericytes and OPCs co-exist in a perivascular location, is it possible that there are also unsuspected potential interactions between these two cell types? In this study, we use a combination of anatomic mapping and cell culture studies to address these questions.
Materials and Methods
Animals
C57Bl/6 mice and SD rats were obtained from Jackson Laboratories. PDGFR-α-creERT2 mice (Jackson Laboratories) were crossed with the Cre-reporter line ROSA26-green fluorescent protein (GFP) (Jackson Laboratories) and utilized in this study at adult stages. Please see Supplementary Information for preparation of GFP-labeled OPC transgenic mice. Animal procedures were performed according to the guidelines of the Animal Use and Care Committee in the authors’ research institutes.
Immunohistochemistry for mouse and rat brains
After perfusion with PBS, brains were quickly frozen using powdered dry ice. Coronal sections of 16-μm thicknesses were cut on cryostat at −20°C and collected on glass slides. According to our previous report [30], sections were stained with anti-PDGFR-α (1:100, R&D systems), anti-PDGFR-β (1:100, eBioscience or R&D systems), lectin (1:200, Vector Laboratories), or anti-CD31 (1:100, BD Biosciences).
Immuno-Electron Microscopy
SD rats were perfused with 0.1% glutaraldehyde-4% PFA fixative, and brains were immersed in the 4% PFA at 4°C for 5 hr and sectioned at a thickness of 50 μm with a microslicer (Dosaka EM). According to our previous report [30], OPCs were stained with anti-PDGFR-α antibody (1: 100, Santa Cruz). Electron micrographs were taken at 80 kV on a EM-1400 Plus electron microscope.
Histological examination of postmortem human brains
Autopsied human brains were obtained from Kyoto University Hospital through a process approved by an institutional research committee. Deparaffinized 20-μm-thick sections were used for immunostaining with anti-PDGFR-α antibody (1:150, Cell Signaling) and anti-PDGFR-β antibody (1:500, R&D systems). Four postmortem human brains were examined in this study; 76-year old female (diagnosed with subcortical ischemic vascular dementia, SIVD, died of small bowel volvulus), 52-year old male (diagnosed with SIVD and cerebral amyloid angiopathy, died of pneumonia), 77-year old male (diagnosed with Alzheimer's disease, died of pneumonia), and 55-year old female (diagnosed with Mashado-Joseph disease, died of pneumonia).
Cell Culture
OPCs were prepared from cerebral cortices of 1–2 day old SD rats according to our previous report [30]. OPCs were maintained in Neurobasal medium containing 2% B27 supplement, 10 ng/mL PDGF, and 10 ng/mL FGF-2. Human brain vascular pericytes were cultured in pericyte basal medium (Sciencell research laboratories) containing 2% fetal bovine serum and pericyte growth supplement (Sciencell research laboratories) onto poly-l-lysine-coated plates.
Preparation pericyte and OPC conditioned medium
Pericyte cultures were maintained in the pericyte basal media for 24 hr. OPC cultures were maintained in Neurobasal media containing 2% B27 supplement for 24 hr. These conditioned media were then collected and centrifuged at 10,000 g for 5 min at 4°C to remove cells and debris. For the media transfer experiments, pericyte-conditioned media was diluted with Neurobasal/2%-B27 media at a ratio of 50:50. Similarly, OPC-conditioned media was diluted with the pericyte basal media at a ratio of 50:50 before experiments. Control media (mixture of 50% the pericyte basal media and 50% Neurobasal/2%-B27) were prepared in the same way from empty wells.
Cell proliferation measurement
Cell proliferation/survival was assessed by WST reduction assay (Cell Counting Kit-8, Dojindo). Direct cell count was conducted in a blind manner. Percentage of cell number was calculated based on the number in control wells. Please see Supplementary Information for detailed WST methods.
Immunocytochemistry
After cells were confluent, they were washed with ice-cold PBS (pH 7.4), followed by 4% PFA for 15 min. After being further washed in PBS, they were incubated with 3% BSA in PBS for 1 hr. Anti-PDGFR-α (1:200, R&D systems) and anti-PDGFR-β (1:100, eBioscience or R&D systems) antibodies were used to stain OPCs and pericytes, respectively.
Statistical Analysis
Statistical significance was evaluated using one-way ANOVA followed by Tukey’s honestly significant difference test. Data are expressed as mean ± S.D. A p-value of <0.05 was considered statistically significant.
Results
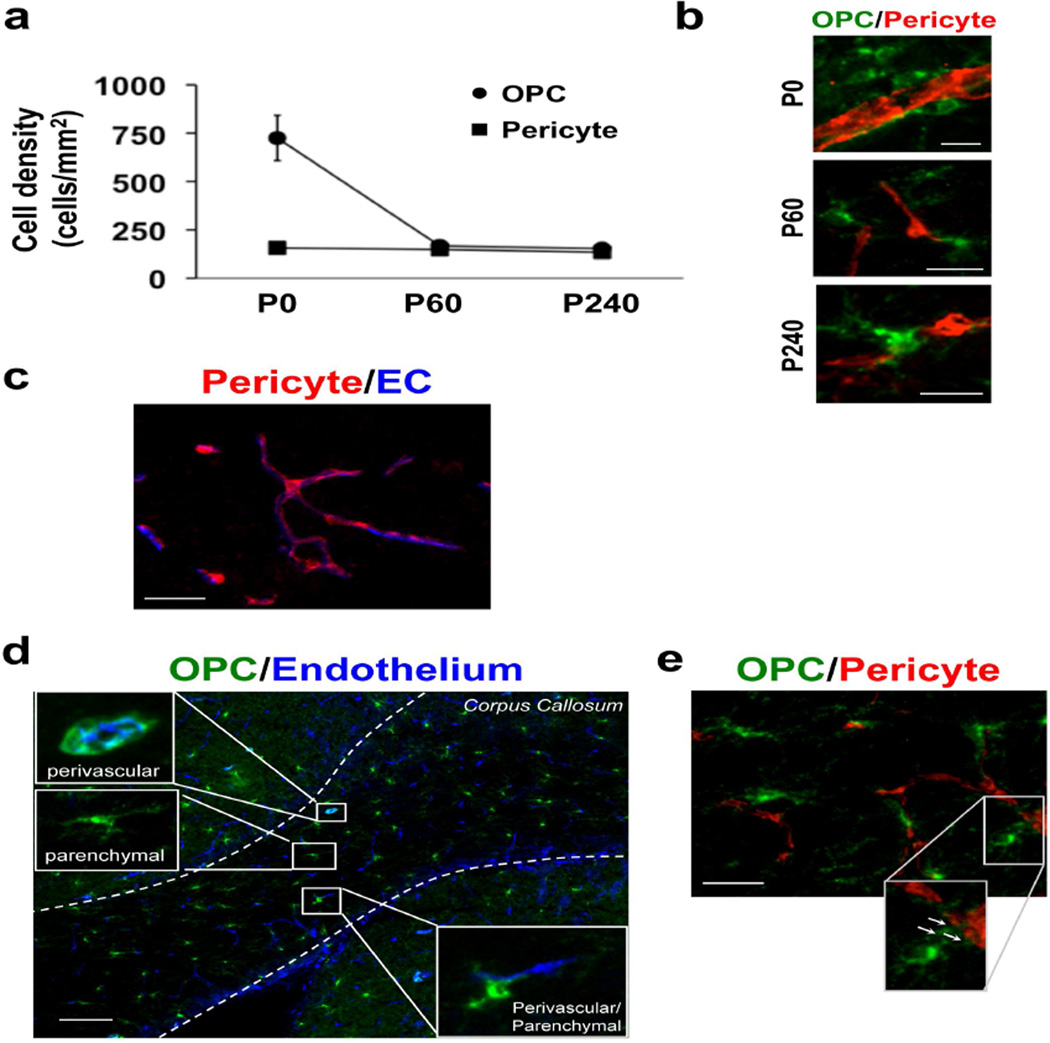
First, we examined the difference of distribution of OPCs and pericytes in mouse white matter (i.e. corpus callosum) at different ages. In this study, OPCs were stained with anti-PDGFR-α antibody, and pericytes were stained with anti-PDGFR-β antibody. There was no overlap between PDGFR-α-positive and PDGFR-β-positive cells in the corpus callosum region (Suppl Figure S1a). Those PDGFR-α- or PDGFR-β-positive cells expressed NG2 that was well known to be detected in both OPCs and pericytes (Suppl Figure S1b-c), confirming that in our system, PDGFR-α- and PDGFR-β-positive cells in the corpus callosum can be considered as OPCs and pericytes, respectively. The number of OPCs in corpus callosum dramatically decreases from postnatal days 0 to 60, and thereafter, slightly decreases from postnatal days 60 to 240 (Figure 1a). In contrast, pericyte number does not show remarkable changes between postnatal days 0 and 240 (Figure 1a). Spatial assessments suggest that OPCs are located in close proximity to pericytes within the perivascular area at all three-time points (Figure 1b). Notably, although all the pericytes reside near the cerebral endothelial cells in mouse corpus callosum (Figure 1c), the OPC phenotype per se (i.e. morphology, positional relation to cerebral endothelium) seem more variable (Figure 1d). Some OPCs wrap microvessels entirely with their fine processes (i.e. perivascular OPCs). Other OPCs are localized away from microvessels (i.e. parenchymal OPCs). In addition, there are OPCs with intermediate phenotype (i.e. perivascular/parenchymal OPCs), whose processes are partly localized in close vicinity to cerebral endothelial cells. Double-immunostaining of PDGFR-α (green color, OPC marker) and PDGFR-β (red color, pericyte marker) show that “perivascular/parenchymal OPCs” extend their processes to pericytes (Figure 1e).
Figure 1. Interaction between pericytes and OPCs in mouse corpus callosum.
(a) Histograms showing the density of PDGFR-α-positive OPCs and PDGFR-β-positive pericytes in corpus callosum in mice at postnatal days 0, 60, and 240. (b) Representative images of PDGFR-α (green: OPC) and PDGFR-β (red: Pericyte) immunostaining in corpus callosum in mice at postnatal days 0, 60, and 240. Scale bar = 20 μm. (c) Representative images of PDGFR-β (red: Pericyte) and lectin (blue: Endothelium) immunostaining in mouse corpus callosum at postnatal day 60. Scale bar = 50 μm. (d) Representative images of PDGFR-α (green: OPC) and lectin (blue: Endothelium) immunostaining in mouse corpus callosum at postnatal day 60. There are "perivascular" OPCs, parenchymal OPCs, and "intermediate (perivascular/parenchymal) " OPCs in the mouse white matter. Scale bar = 100 μm. (e) Double-immunostaining of PDGFR-α (green, OPCs) and PDGFR-β (red, pericytes) show that “perivascular/parenchymal OPCs” extend their processes to pericytes. Arrows indicate OPC processes that attach to pericytes. Scale bar = 50 μm.
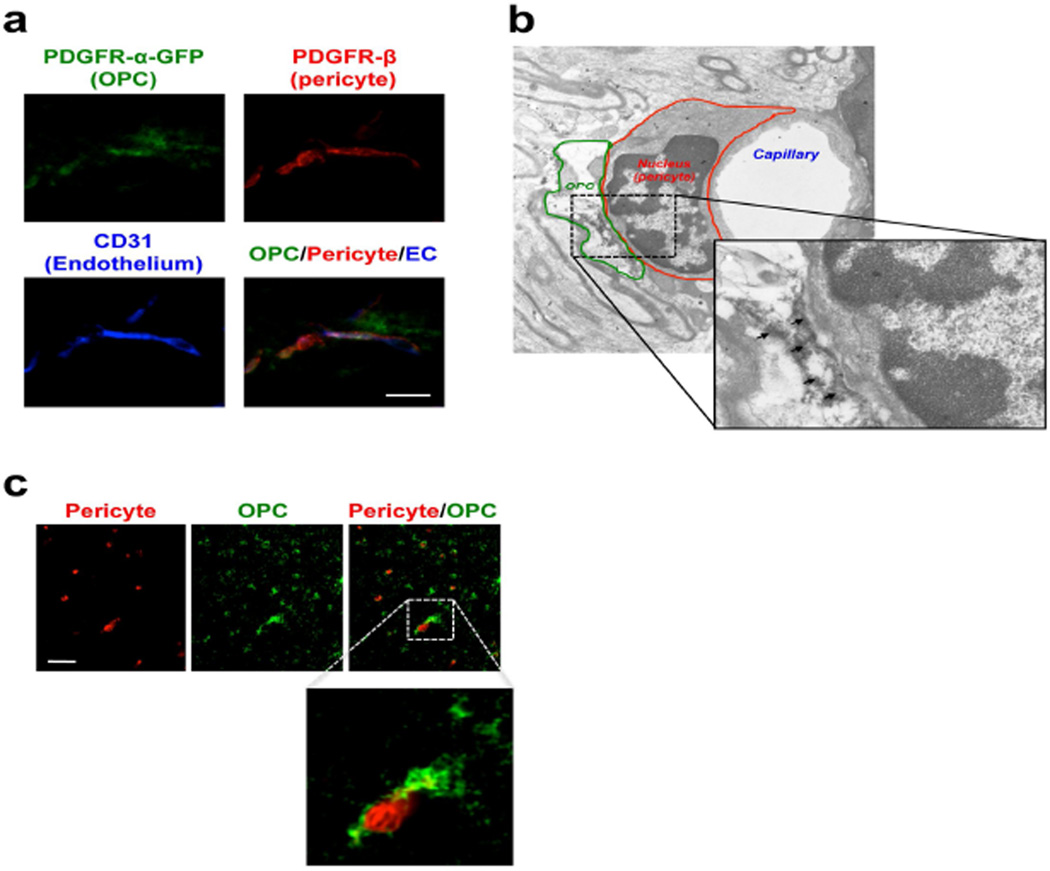
To further confirm pericyte-OPC interactions, we used genetically fluorescent-labeled mice. PDGFR-α-creERT2 mice were crossed with Cre-reporter line ROSA26-green fluorescent protein (GFP). After recombination was induced by three daily intraperitoneal injections of 6 mg tamoxifen, we performed co-staining with PDGFR-β (red) and CD31 (blue color, endothelial cell marker). In these genetically-modified mice, some GFP-labeled OPCs are located in close apposition to pericytes immediately adjacent to cerebral endothelium in corpus callosum (Figure 2a), consistent with the findings in normal mouse brains. We then conducted immuno-electron microscopic analyses to further evaluate structural relationship between OPCs, pericytes, and cerebral endothelium. Similar to our findings in standard immunostaining above, PDGFR-α-positive OPCs are attached to pericytes via basal lamina in the perivascular region (Figure 2b). In some organ systems, there may be differences between rodent vs human systems. So we also asked whether the potential interaction between pericytes and OPCs exist in human brains. Four postmortem human brains were examined (Suppl Table S1), and we confirmed that pericytes and OPCs also appear to exist in close proximity to each other within perivascular areas in postmortem human brains (Figure 2c).
Figure 2. The close vicinity between OPCs and pericytes at perivascular region.
(a) Representative images of PDGFR-β (red: Pericyte) and CD31 (blue: EC) immunostaining with GFP-labeled PDGFR-α-cells. Scale bar = 50 μm. (b) Representative images of immuno-electron microscopic analysis using anti-PDGFR-α antibody in corpus callosum in 2 month-old rat brain. Arrows indicate PDGFR-α positive signals. (c) Representative images of PDGFR-α (green: OPC) and PDGFR-β (red: Pericyte) immunostaining in corpus callosum in postmortem human brain (76 year-old). The region marked by a dashed square is enlarged in the inset. Scale bar = 50 μm.
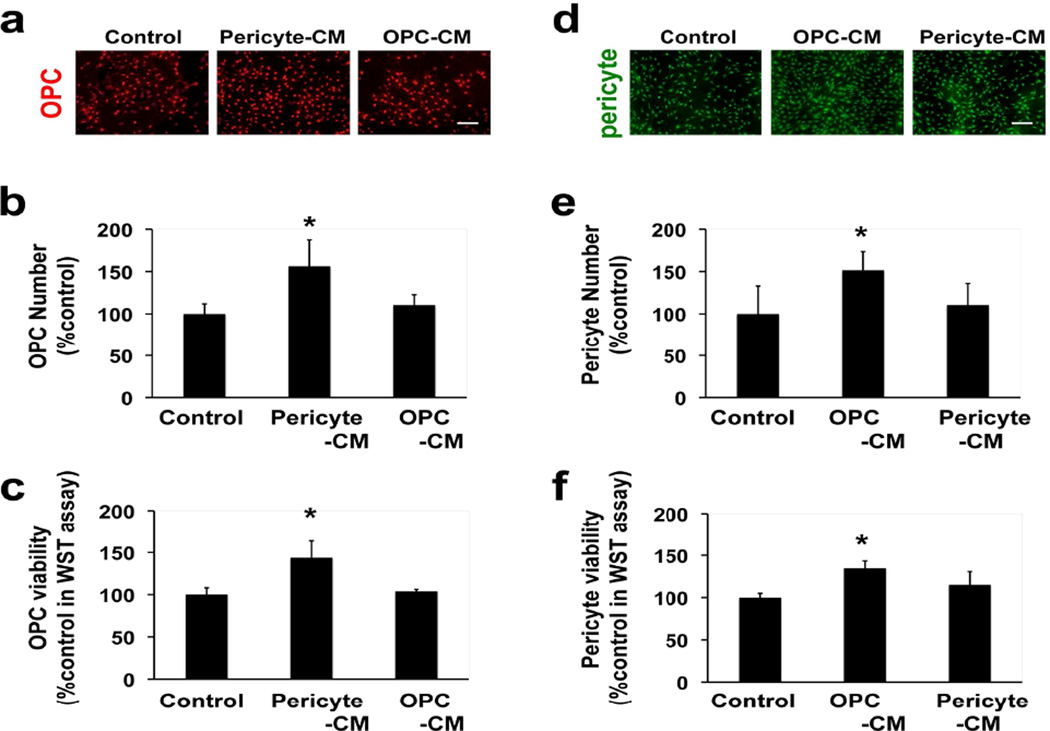
Since our immunostaining and electron microscopy data so far are consistent with pericyte-OPC interactions, we finally asked whether cell-cell signaling could also be observed between these two cell types. We conducted in vitro media transfer experiments to investigate whether OPCs receive functional support from pericytes, and vice versa. Our cultured OPCs and pericytes were pure and functional [1, 32], and in this study, we maintained OPCs and pericytes in separate culture plates. Conditioned media from OPCs or pericytes were then obtained, and control media were prepared in the same way from empty wells without OPCs or pericytes. When OPCs were treated with pericyte-conditioned media (Pericyte-CM), direct-cell-counting and the WST assay showed that OPC numbers significantly increased at day 2, but OPC-CM did not increase OPC number (Figure 3a-c). Similarly, pericyte numbers increased when pericytes were maintained in OPC-CM, but not in Pericyte-CM, for 2 days (Figure 3d-f). These in vitro data suggest a potential functional interaction in cell survival/proliferation between pericytes and OPCs, which are mediated by secreting soluble factors.
Figure 3. OPC-pericyte interaction in vitro.
(a) Representative images of PDGFR-α staining in OPC cultures that were treated with control media, Pericyte-CM, or OPC-CM for 2 days. Scale bar = 100 μm. (b) Results of direct cell count of OPCs after 2-day Pericyte-CM treatment. Values are mean ± SD from 3 independent experiments. *p<0.05 vs control. (c) Results of WST assay for assessing OPC proliferation. Values are mean ± SD from 4 independent experiments. *p<0.05 vs control. (d) Representative images of PDGFR-β staining in pericyte cultures that were treated with control media, OPC-CM, or Pericyte-CM for 2 days. Scale bar = 100 μm. (e) Results of direct cell count of pericytes after 2-day OPC-CM treatment. Values are mean ± SD from 3 independent experiments. *p<0.05 vs control. (f) Results of WST assay for assessing pericyte proliferation. Values are mean ± SD from 4 independent experiments. *p<0.05 vs control.
Discussion
Our data with rodent and human brain sections suggest that pericytes and OPCs are in close apposition to each other in the perivascular region of cerebral white matter. In addition, in vitro experiments indicate that OPCs and pericytes may exchange soluble factors to support their function each other. These findings provide initial proof-of-concept that OPCs and pericytes may comprise a novel mode of cell-cell interactions in the perivascular region.
Many tissues including brain contain multiple stem cell lineages. Coordination of self-renewal and differentiation among different stem cell populations should be essential for maintaining tissue homeostasis [21, 34]. For example, in the bone marrow, endothelial cells and perivascular components create critical niche for haematopoietic stem cells maintenance through secretion of various factors [11, 20]. Also in the brain, neural stem/progenitor cells reside in the so-called neurovascular niche for sustainable neurogenesis both in developing and maturation periods [15]. In addition, mesenchymal stem cells may reside in the perivascular area and exhibit certain characteristics identical to a subclass of pericytes, presumably behaving as pericyte progenitor cells [8, 27]. In cerebral white matter, OPCs receive support from cerebral endothelial cells for maintaining their function and for self-renewal [3]. Hence, perivascular area should provide a specific microenvironment (i.e. niche) that allows stem cells to retain their multi-lineage potential and self-renewal capacity [13, 26]. Our current findings may reveal novel roles of perivascular niche that pericytes interact with OPCs to regulate their function each other in cerebral white matter.
Residual OPCs in adult brain retain proliferative ability throughout life, and in fact, may play critical roles in white matter maintenance and remodeling [23, 35]. Fate mapping studies using PDGFR-α-creERT2/ROSA26-YFP transgenic mice confirmed that the majority of adult OPCs generate myelin-forming oligodendrocytes in white matter and, to a much lesser extent, in gray matter [17, 29]. Non-cell autonomous mechanisms may be important for OPC maturation. For example, astrocytes are known to support OPCs. OPCs are adjacent to astrocytes [6, 25], and OPCs seeded on astrocytes exhibit higher motility than OPCs on laminin-coated plates in vitro [14]. Besides the direct interaction, astrocytic-derived factors are also reported to help OPCs against several kinds of insults in vitro [2, 19]. Similarly, cerebral endothelial cells may also regulate OPC function. In vitro cell culture studies showed that cerebral-endothelium-derived BDNF/FGF-2 promoted the proliferation and survival of OPCs [3] and cerebral-endothelium-derived VEGF-A increased OPC motility [16]. Our initial findings here may offer yet another non-cell autonomous pathway, i.e. pericytes may also interact with and support OPC function in cerebral white matter.
Although our whole brain and cell culture data support the idea of a potential interaction between OPCs and pericytes, there are still some caveats in this study. First, we only focused on pericytes and OPCs. But there are several types of cells in the perivascular region, such as NSPCs, microglia, and astrocytes. How are pericyte-OPC interactions regulated in the context of these other cellular signals? This question should be carefully assessed in future studies for elucidating mechanisms that orchestrate dynamic interactions in the perivascular niche. Second, we used rat OPCs and human pericytes for our in vitro experiments. Some growth factors such as FGF-2 show high cross-species homology between human and rat, and both OPCs and pericytes are known to secrete a battery of growth factors. In addition, we previously showed that human endothelial cells secreted FGF-2 and BDNF to promote the proliferation of rat OPCs in vitro [1]. So, our rat-OPC/human-pericyte models can be utilized to dissect mechanisms of interactions between OPCs and pericytes in vitro to some extent. Nevertheless, OPCs and pericytes from the same species would be ideal for media transfer experiments in future studies. Third, although our in vitro data suggest that pericytes and OPCs may support their survival/proliferation each other, we have not examined whether those two cell types support their differentiation. In adult brain, OPCs differentiate into mature oligodendrocytes as needed to oligodenrocyte renewal and repair. Also, under some conditions, pericytes exhibit stem-cell-like properties, and in fact, pericytes have been shown to be reprogrammed into neurons [18]. Therefore, mechanisms of their interactions in cell differentiation/reprogramming need to be carefully examined in future studies. Fourth, in addition to the primary observation of potential pericyte-OPC interactions, our studies may also confirm the presence of heterogeneity in OPC-endothelial relationships. Perivascular OPCs surround the entire microvessel, whereas parenchymal OPCs are located away from microvessels. Classical textbooks state that oligodendrocytes occupy three distinctive positions; next to neurons (perineuronal satellite oligodendrocytes), between myelin sheaths (interfascicular oligodendrocytes), and along blood vessels (perivascular oligodendrocytes) [7, 12]. However, how different types of OPCs relate to different types of mature oligodendrocytes remain to be elucidated. Finally, we should investigate the pericyte-OPC interaction under pathological conditions as well. Would it be possible that disruption of pericyte-OPC trophic coupling lead to white matter dysfunction? If so, understanding the difference of pericyte-OPC interaction between physiological and pathological conditions would provide us a novel therapeutic target for white matter-related diseases.
In conclusion, this proof-of-concept study provided initial anatomic and cell culture evidence to suggest a potential interaction between pericytes and OPCs in the perivascular niche of cerebral white matter. Further studies are warranted to explore how these gliovascular interactions may contribute to maintaining white matter homeostasis and integrity in mammalian brain.
Supplementary Material
Highlights.
Pericytes and oligodendrocyte precursor cells (OPCs) are localized in close contact with each other in cerebral white matter
Pericytes attached to OPCs via basal lamina in the perivascular region
OPC-derived factors support pericyte survival in vitro
Pericyte-derived factors support OPC survival in vitro
Acknowledgements
Supported in part by the National Institutes of Health, the Uehara Memorial Foundation, and the Japan Society for the Promotion of Science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest statement
The authors have declared that no conflict-of-interest exists.
References
- 1.Arai K, Lo E. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:4351–4356. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai K, Lo EH. Astrocytes protect oligodendrocyte precursor cells via MEK/ERK and PI3K/Akt signaling. Journal of neuroscience research. 2010;88:758–763. doi: 10.1002/jnr.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:4351–4355. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Developmental cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butt AM, Ibrahim M, Ruge FM, Berry M. Biochemical subtypes of oligodendrocyte in the anterior medullary velum of the rat as revealed by the monoclonal antibody Rip. Glia. 1995;14:185–197. doi: 10.1002/glia.440140304. [DOI] [PubMed] [Google Scholar]
- 7.Cammermeyer J. Reappraisal of the perivascular distribution of oligodendrocytes. The American journal of anatomy. 1960;106:197–231. doi: 10.1002/aja.1001060303. [DOI] [PubMed] [Google Scholar]
- 8.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell stem cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Dalkara T, Gursoy-Ozdemir Y, Yemisci M. Brain microvascular pericytes in health and disease. Acta neuropathologica. 2011;122:1–9. doi: 10.1007/s00401-011-0847-6. [DOI] [PubMed] [Google Scholar]
- 10.Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Molecular and cellular neurosciences. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 11.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodson RF, Cheung LW. Perivascular oligodendrocytes in the striatum of the squirrel monkey. Journal of the neurological sciences. 1972;17:237–244. doi: 10.1016/0022-510x(72)90029-9. [DOI] [PubMed] [Google Scholar]
- 13.Ergun S, Tilki D, Klein D. Vascular wall as a reservoir for different types of stem and progenitor cells. Antioxidants & redox signaling. 2011;15:981–995. doi: 10.1089/ars.2010.3507. [DOI] [PubMed] [Google Scholar]
- 14.Fok-Seang J, DiProspero NA, Meiners S, Muir E, Fawcett JW. Cytokine-induced changes in the ability of astrocytes to support migration of oligodendrocyte precursors and axon growth. The European journal of neuroscience. 1998;10:2400–2415. doi: 10.1046/j.1460-9568.1998.00251.x. [DOI] [PubMed] [Google Scholar]
- 15.Goldman SA, Chen Z. Perivascular instruction of cell genesis and fate in the adult brain. Nature neuroscience. 2011;14:1382–1389. doi: 10.1038/nn.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayakawa K, Seo JH, Pham LD, Miyamoto N, Som AT, Guo S, Kim KW, Lo EH, Arai K. Cerebral endothelial derived vascular endothelial growth factor promotes the migration but not the proliferation of oligodendrocyte precursor cells in vitro. Neuroscience letters. 2012;513:42–46. doi: 10.1016/j.neulet.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karow M, Sanchez R, Schichor C, Masserdotti G, Ortega F, Heinrich C, Gascon S, Khan MA, Lie DC, Dellavalle A, Cossu G, Goldbrunner R, Gotz M, Berninger B. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell stem cell. 2012;11:471–476. doi: 10.1016/j.stem.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Kato S, Aoyama M, Kakita H, Hida H, Kato I, Ito T, Goto T, Hussein MH, Sawamoto K, Togari H, Asai K. Endogenous erythropoietin from astrocyte protects the oligodendrocyte precursor cell against hypoxic and reoxygenation injury. J Neurosci Res. 2011;89:1566–1574. doi: 10.1002/jnr.22702. [DOI] [PubMed] [Google Scholar]
- 20.Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, Mar JC, Bergman A, Frenette PS. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leatherman J. Stem cells supporting other stem cells. Frontiers in genetics. 2013;4:257. doi: 10.3389/fgene.2013.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- 23.Maki T, Liang AC, Miyamoto N, Lo EH, Arai K. Mechanisms of oligodendrocyte regeneration from ventricular-subventricular zone-derived progenitor cells in white matter diseases. Front Cell Neurosci. 2013;7:275. doi: 10.3389/fncel.2013.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orthmann-Murphy JL, Abrams CK, Scherer SS. Gap junctions couple astrocytes and oligodendrocytes. Journal of molecular neuroscience : MN. 2008;35:101–116. doi: 10.1007/s12031-007-9027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozen I, Boix J, Paul G. Perivascular mesenchymal stem cells in the adult human brain: a future target for neuroregeneration? Clinical and translational medicine. 2012;1:30. doi: 10.1186/2001-1326-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paul G, Ozen I, Christophersen NS, Reinbothe T, Bengzon J, Visse E, Jansson K, Dannaeus K, Henriques-Oliveira C, Roybon L, Anisimov SV, Renstrom E, Svensson M, Haegerstrand A, Brundin P. The adult human brain harbors multipotent perivascular mesenchymal stem cells. PloS one. 2012;7:e35577. doi: 10.1371/journal.pone.0035577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pringle NP, Mudhar HS, Collarini EJ, Richardson WD. PDGF receptors in the rat CNS: during late neurogenesis. PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development. 1992;115:535–551. doi: 10.1242/dev.115.2.535. [DOI] [PubMed] [Google Scholar]
- 29.Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nature neuroscience. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seo JH, Maki T, Maeda M, Miyamoto N, Liang AC, Hayakawa K, Pham LD, Suwa F, Taguchi A, Matsuyama T, Ihara M, Kim KW, Lo EH, Arai K. Oligodendrocyte precursor cells support blood-brain barrier integrity via TGF-beta signaling. PLoS One. 2014;9:e103174. doi: 10.1371/journal.pone.0103174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo JH, Miyamoto N, Hayakawa K, Pham LD, Maki T, Ayata C, Kim KW, Lo EH, Arai K. Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. J Clin Invest. 2013;123:782–786. doi: 10.1172/JCI65863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi Y, Maki T, Liang AC, Itoh K, Lok J, Osumi N, Arai K. p38 MAP kinase mediates transforming-growth factor-beta1-induced upregulation of matrix metalloproteinase-9 but not-2 in human brain pericytes. Brain Res. 2014;1593:1–8. doi: 10.1016/j.brainres.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Woodhead GJ, Swaminathan SK, Noles SR, McQuinn ER, Pisarek AJ, Stocker AM, Mutch CA, Funatsu N, Chenn A. Cortical neural precursors inhibit their own differentiation via N-cadherin maintenance of beta-catenin signaling. Developmental cell. 2010;18:472–479. doi: 10.1016/j.devcel.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang R, Chopp M, Zhang ZG. Oligodendrogenesis after cerebral ischemia. Front Cell Neurosci. 2013;7:201. doi: 10.3389/fncel.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.