Abstract
Background
Long-term effects of behavioral weight loss interventions on diabetes complications are unknown. We assessed whether an intensive lifestyle intervention (ILI) affects the development of nephropathy in Look AHEAD, a multicenter randomized clinical trial in type 2 diabetes.
Methods
5145 overweight or obese persons aged 45–76 years with type 2 diabetes were randomized to ILI designed to achieve and maintain weight loss through reduced caloric consumption and increased physical activity or to a diabetes support and education (DSE) group. Randomization to ILI or DSE, in a 1:1 ratio, was implemented in a central web-based data management system, stratified by clinical center, and blocked with random block sizes. Outcomes assessors and laboratory staff were masked to treatment. The interventions ended early because of lack of effect on the primary outcome of cardiovascular disease events. Albuminuria and estimated glomerular filtration rate were prespecified “other” outcomes and were assessed from baseline through 9.6 years (median) of follow-up until the interventions ended. They were combined post-hoc to define the main outcome for this report: very-high-risk chronic kidney disease (CKD) based on the 2013 Kidney Disease Improving Global Outcomes classification. Data were analyzed by intention to treat. The trial is registered as Clinicaltrials.gov NCT00017953.
Findings
The incidence rate of very-high-risk CKD was 31% lower in ILI than DSE with hazard rates of 0.90 cases/100 person-years in DSE and 0.63 in ILI (difference=0.27 cases/100 person-years, hazard ratio and 95% confidence interval: HR=0.69, 0.55 to 0.87). This effect was partly attributable to reductions in weight, HbA1c, and blood pressure.
Interpretation
Weight loss should be considered as an adjunct to medical therapies to prevent or delay progression of CKD in overweight or obese persons with type 2 diabetes.
Primary Funding
National Institute of Diabetes and Digestive and Kidney Diseases.
INTRODUCTION
Diabetic nephropathy, a microvascular complication of diabetes, causes substantial individual and societal burdens as well as predicting mortality from cardiovascular disease (CVD) and other causes.1,2 It is associated with hyperglycemia, hypertension, and obesity. Improving glucose control decreases the development and retards the progression of microvascular complications in both type 13 and type 2 diabetes,4–6 and is projected to reduce its economic burden.7 This beneficial effect has been shown in type 2 diabetes with different classes of glucose-lowering medicines, typically in combination.4–6
While lifestyle modification is recommended as a primary treatment approach for type 2 diabetes,8 its impact on diabetic nephropathy has not been well studied. Look AHEAD (Action for Health in Diabetes) was a multicenter, randomized clinical trial of an Intensive Lifestyle Intervention (ILI) to achieve weight loss through caloric restriction and physical activity compared with a Diabetes Support and Education (DSE) group in overweight and obese adults with type 2 diabetes. Although the ILI did not reduce the incidence of the primary outcome of CVD morbidity and mortality,9 we also examined the impact of the ILI on other important health outcomes, including nephropathy, as described in this report.
METHODS
Participants and Interventions
Look AHEAD randomly assigned 5145 overweight/obese individuals with type 2 diabetes to ILI or DSE, the comparison condition, at 16 clinical sites in the United States (Figure 1). The design, power calculations, methods, and baseline characteristics9–12 have been published, and the protocol is available at https://www.lookaheadtrial.org/public/LookAHEADProtocol.pdf. Participating centers received local IRB approval, and written informed consent was obtained from all participants.
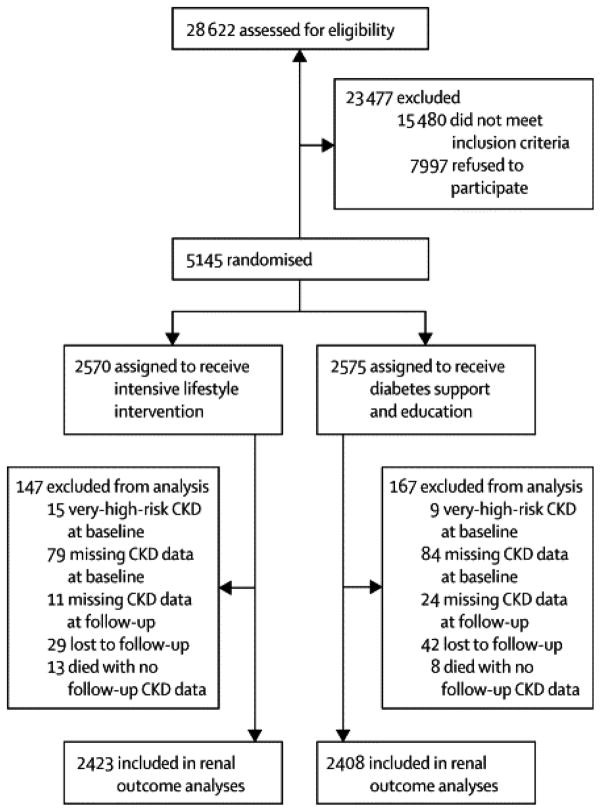
Figure 1.
Flowchart showing recruitment, randomization, and follow-up.
We ascertained self-reported age, sex, race, and ethnicity because of their potential risk modification and interaction with intervention effects. Ethnicity (Hispanic or not) and race were asked separately. Those reporting Hispanic ethnicity were classified as “Hispanic” regardless of self-reported race. Others were classified by their self-reported race according to categories provided by the investigators: white, African American, American Indian, Asian American or Pacific Islander, or other/mixed race. At randomization, participants were 45 to 76 years old, overweight or obese (body mass index, BMI ≥25 kg/m2 in those not taking insulin, and ≥27 kg/m2 in those taking insulin), able to complete a maximal exercise test suggesting it was safe to exercise, and had a primary health care provider. Exclusion criteria included inability to walk two blocks, amputation of a lower limb for non-traumatic causes, urine dipstick protein of 4+ (equivalent to approximately >1 g protein/day), serum creatinine exceeding 1.4 mg/dl in women or 1.5 mg/dl in men, or current treatment with dialysis. There were no exclusions based on other complications of diabetes. Participants could be using any type of glucose-lowering medicine, but the percentage of participants using insulin was limited to <30%. The trial included participants with and without a history of CVD, defined as prior myocardial infarction, stroke, congestive heart failure or interventional procedures for CVD (coronary artery bypass grafting, percutaneous transluminal coronary angioplasty, carotid endarterectomy, angioplasty of a lower extremity artery, or aortic aneurysm repair). Additional criteria are described elsewhere.10 The ILI aimed to achieve and maintain weight loss of at least 7% through reduced caloric intake and increased physical activity.9 Strategies included a calorie goal of 1200 to 1800 kcal per day (with <30% of calories from fat and >15% from protein), meal-replacement products, and at least 175 minutes of moderate-intensity physical activity per week. DSE group sessions focused on diet, exercise, and social support. The study did not set goals for control of glycemia or CVD risk factors in either group; these were managed by the participants’ health care providers outside of Look AHEAD.
There were three sets of stopping rules: 1) for feasibility of treatment group difference on weight loss and improved physical fitness, 2) for efficacy or futility of the treatment effect on the cardiovascular outcomes, and 3) for safety. The stopping rules did not address nephropathy. Data contributing to the current analyses were collected through September 14, 2012 when the ILI was stopped on the recommendation of the Data and Safety Monitoring Board because of futility of finding an effect on the incidence of major CVD events.9 This report describes the intervention effects on renal function, assessed by estimated glomerular filtration rate (eGFR), urine albumin-to-creatinine ratio (ACR), and renal replacement therapy. The study protocol specified obtaining these data to examine the effect of the intervention on renal function as another outcome, but did not define the analytic outcomes.
Randomization and Masking
Randomization to ILI or DSE, in a 1:1 ratio, occurred from August 2001 through April 2004. It was implemented in a web-based data management system at the Coordinating Center, stratified by clinical center, and blocked with random block sizes. Allocation was concealed to clinic staff; the assignment was revealed only after the participant was enrolled in the clinical trial. Outcomes assessors and laboratory staff were masked to treatment, but participants and interventionists were not because the intervention was behavioral. Those performing the statistical analyses for this report were not masked.
Outcomes
Data were obtained by trained, certified staff who were masked to the intervention. Nephropathy was assessed by urine albumin and creatinine concentrations, from which the ACR was calculated, and the serum creatinine concentration. These measures were made annually through year four and every other year thereafter. Laboratory tests were performed at the Northwest Lipid, Metabolism and Diabetes Research Laboratory, University of Washington, Seattle. Serum and urine creatinine concentrations were measured with the Roche Creatinine Plus enzymatic reagent on a Roche Modular Pautoanalyzer. The value of the assay calibrator is traceable to the Isotope Dilution Mass Spectrometry reference method. Data on initiation of treatment for renal failure (renal replacement therapy, defined as self-reported dialysis, kidney transplantation, or renal failure) were collected every six months from interviewer-administered questionnaires. Indications for renal replacement therapy were not ascertained, and such therapy was counted whether given acutely or chronically.
The effect of ILI on diabetic nephropathy was pre-specified as one of the “key objectives of additional data collection” with analyses by subgroups defined by history of cardiovascular disease, sex, ethnicity, and other factors being pre-specified.10 The protocol, however, did not specify how the renal variables would be analyzed. Thus, we chose post-hoc to use the 2013 Kidney Disease Improving Global Outcomes (KDIGO) classification,13 which are the most recent internationally recognized criteria. This classification is based on combinations of eGFR, calculated with the CKD-Epi equation,14 and ACR with abnormalities lasting at least 3 months. Our examinations were conducted every one or two years, so we classified each participant at each examination using eGFR and urine ACR at a single time without verifying chronicity. The KDIGO classification provides four categories based on risk of all-cause and cardiovascular mortality, renal failure, or CKD progression: 1) normal or low risk, 2) moderate risk, 3) high risk, and 4) very high risk. Very high risk is defined if a) eGFR <30 ml/min/1.73m2 regardless of ACR; b) eGFR <45 ml/min/1.73m2 and ACR ≥30 mg albumin/g creatinine; or c) eGFR <60 ml/min/1.73m2 and ACR >300 mg/g (Appendix Table 1). We also classified participants as very-high-risk if they had received renal replacement therapy regardless of measured eGFR or ACR. The other classifications and their prevalence at baseline are shown in Table 1 and Appendix Table 1.
Table 1.
Baseline characteristics among the 5145 randomized participants. None of these variables except systolic blood pressure differed significantly (p<0.05) between treatment groups.
| Variable | Statistic | DSEa N=2575 |
ILIb N=2570 |
|---|---|---|---|
| Sex | Number (%) female | 1537 (59.7) | 1526 (59.4) |
| Age | Mean (SD) | 58.9 (6.9) | 58.6 (6.8) |
| Race/ethnicityc | White, N (%) | 1631 (63.3) | 1621 (63.1) |
| African American, N (%) | 404 (15.7) | 400 (15.6) | |
| Hispanic, N (%) | 340 (13.2) | 340 (13.2) | |
| American Indian, N (%) | 128 (5.0) | 130 (5.1) | |
| Other/Mixed, N(%) | 72 (2.8) | 78 (3.0) | |
| Duration of diabetes (years) | Median (25, 75 %-ile) | 5 (2, 10) | 5 (2, 10) |
| Duration of diabetes ≥5 years | N (%) | 1411 (55.1) | 1357 (53.3) |
| History of CVDd | N (%) | 348 (13.5) | 366 (14.2) |
| Weight (kg) | Mean (SD) | 101 (19) | 101 (20) |
| BMI (kg/m2) | Mean (SD) | 36.0 (5.8) | 35.9 (6.0) |
| Waist circumference (cm) | Mean (SD) | 114 (14) | 114 (14) |
| HbA1c (% or mmol/L, IFCC units) | Mean (SD) | 7.3 (1.2) 56 (13.1) |
7.2 (1.1) 55 (12.0) |
| Systolic blood pressure (mm Hg) | Mean (SD) | 129 (17) | 128 (17) |
| ACR (mg/g) | Median (25, 75 %-ile) Geometric mean (CI)e |
8.9 (5.4, 19.4) 12.1 (11.5, 12.7) |
8.6 (5.2, 18.3) 11.7 (11.2, 12.3) |
| Serum creatinine (mg/dl or μmol/L) | Mean (SD) | 0.8 (0.2) 71 (18) |
0.8 (0.2) 71 (18) |
| eGFR (ml/min/1.73m2) | Mean (SD) | 90.0 (16.0) | 90.4 (16.2) |
| Insulin treatment | N (%) | 410 (16.5) | 382 (15.4) |
| ACR ≥300 mg/gf | N (%) | 69 (2.8) | 80 (3.2) |
| Serum creatinine ≥2 mg/dl (177 μmol/L)f | N (%) | 2 (0.1) | 4 (0.2) |
| eGFR <45 ml/min/1.73m2f | N (%) | 13 (0.5) | 16 (0.6) |
| Kidney disease categoryg | Normal/low risk, N (%) | 1968 (79.0) | 2000 (80.3) |
| Moderate risk, N (%) | 425 (17.1) | 377 (15.1) | |
| High-risk, N (%) | 89 (3.6) | 99 (4.0) | |
| Very-high-risk, N (%) | 9 (0.4) | 15 (0.6) |
DSE = Diabetes Support and Education.
ILI = Intensive Lifestyle Intervention.
Ethnicity (Hispanic or not) and race were asked separately. Those reporting Hispanic ethnicity were classified as “Hispanic” regardless of self-reported race. Others were classified by their self-reported race. “Other” includes Asian Americans and Pacific Islanders. “Mixed” includes those reporting more than one race.
Cardiovascular disease, defined as prior myocardial infarction, stroke, congestive heart failure or interventional procedures for CVD (coronary artery bypass grafting, percutaneous transluminal coronary angioplasty, carotid endarterectomy, angioplasty of a lower extremity artery, or aortic aneurysm repair).
CI = 95% confidence interval.
Also includes persons with history of renal replacement therapy regardless of laboratory test results
Kidney disease classification adapted from KDIGO (see Methods and Appendix Table 1). Persons with history of renal replacement therapy regardless of laboratory test results were classified as “very-high-risk”.
The main renal outcome was the development of very-high-risk CKD among participants not in this category at baseline. To examine the sensitivity of the results to this definition, we performed secondary analyses of treatment effects on other renal outcomes shown in Table 2.
Table 2.
Incidence rates and hazard ratios for renal outcomes.
| Outcome CKD categorya or other | Number at risk at baseline | DSEb | ILIc | Hazard Ratio (95% CI) | p-value | ||
|---|---|---|---|---|---|---|---|
| Eventsd | Ratee | Eventsd | Ratee | ||||
| 4 (very high risk) | 4831 | 173 | 0.91 | 123 | 0.63 | 0.69 (0.55 to 0.87) | 0.0016 |
| 4 twicef | 4831 | 109 | 0.75 | 69 | 0.46 | 0.63 (0.47 to 0.86) | 0.0031 |
| 4 twice or lastg | 4831 | 147 | 0.76 | 104 | 0.53 | 0.68 (0.53 to 0.88) | 0.0028 |
| ACRh ≥300 | 4713 | 187 | 1.00 | 155 | 0.82 | 0.81 (0.66 to 1.01) | 0.0566 |
| eGFRi <45 | 4976 | 246 | 1.26 | 201 | 1.01 | 0.79 (0.66 to 0.96) | 0.0151 |
| RRTj | 5111 | 36 | 0.16 | 29 | 0.13 | 0.80 (0.49 to 1.30) | 0.3672 |
| Double s. creatk | 5000 | 109 | 0.54 | 91 | 0.45 | 0.81 (0.61 to 1.07) | 0.1298 |
Numbers 1–4 represent the KDIGO categories of CKD as defined in Appendix Table 1: 1=normal or low risk, 2=moderate risk, 3=high risk, 4=very high risk.
DSE is the diabetes support and education group.
ILI is the intensive lifestyle intervention group.
Events are the first occurrence of this condition after randomization, in those without the condition at baseline.
First events/100 person-years at risk.
The outcome is first occurrence of KDIGO category 4 (very high risk CKD) confirmed at the next examination. Time to event is based on the date of the first occurrence.
Same as (f) except that first event occurring at the participant’s last examination was counted without confirmation, since such confirmation was not possible.
ACR = urine albumin-to-creatinine ratio (mg/g). The category ACR ≥300 also includes persons with history of renal replacement therapy regardless of laboratory test results.
eGFR = estimated glomerular filtration rate (ml/min/1.73m2). The eGFR < 45 also includes persons with history of renal replacement therapy regardless of laboratory test results.
RRT = renal replacement therapy (dialysis or transplantation). Of the 65 initial reports of renal replacement therapy, 32 for were for chronic renal failure and 33 were for replacement therapy of acute or unknown duration.
Double s. creat=serum creatinine concentration was at least two times the baseline value. This outcome also includes persons with history of renal replacement therapy regardless of laboratory test results.
Statistical Analyses
No power calculations were performed for nephropathy because it was not the primary outcome. Baseline characteristics were compared between intervention groups using chi-square tests for categorical variables and two-sample t-test or Wilcoxon rank sum tests for continuous variables. For each renal outcome of interest, among subgroups defined at baseline, the time until the first occurrence after randomization was modeled using Cox proportional hazards regression according to the intention-to-treat principle, with follow-up censored at the last available visit for participants who remained event free for that condition. Intervention effects were reported as hazard ratios (HR) with 95% confidence intervals (CI). Kaplan-Meier estimates were used to calculate the cumulative incidence of an event over time for both study groups and were compared using log-rank tests. The consistency of intervention effects among seven pre-specified subgroups defined by age, sex, race/ethnicity, BMI, history of CVD, insulin use, and diabetes duration was assessed with tests for interaction with intervention assignment. Additionally, we performed multivariable analyses for the incidence of very-high-risk CKD. We first fit a model with seven baseline variables listed above plus intervention assignment. Then we expanded the model to examine the effects of body weight, HbA1c, and systolic blood pressure as time-varying predictors.
The use of blockers of the renin-angiotensin system over time (through year 10) was examined as a binary outcome using generalized estimating equations with an unstructured variance-covariance matrix. Analyses were conducted with SAS 9.3 (Cary, NC) and a two-sided p-value of less than 0.05 was considered statistically significant. Because these outcomes were highly correlated with each other, and one was specified as primary, we did not adjust p-values for multiple comparisons. Sensitivity of the results to alternate definitions of the outcome was assessed by analyzing different definitions or manifestations of renal disease.
The trial is registered as Clinicaltrials.gov number NCT00017953.
Role of the Funding Source
The primary sponsor, the NIDDK, was represented on the Steering Committee and played a part in study design, management, and publication. The statistician (H.C.) had access to the raw data. The first author (W.C.K.) had full access to all results and the final responsibility to submit for publication.
RESULTS
Of the 5145 participants randomized (2570 in ILI and 2575 in DSE), the main renal outcome analyses (i.e., for very-high-risk CKD) were performed in 2422 (94%) in ILI and 2408 (94%) in DSE (Figure 1). Participant characteristics at baseline have been described.12 Most (63%) participants were non-Hispanic white, and 59% were women (Table 1). Mean age was 59 years, 14% had a history of CVD, and 16% were treated with insulin. At baseline, about 4% of participants were in the high-risk or very-high-risk CKD categories. Further details are in Appendix Table 1.
As previously reported, ILI produced sustained reductions in body weight, HbA1c, and systolic blood pressure compared with DSE.9,15 Mean one-year weight loss was 0.7% in DSE and 8.6% in ILI. Averaged over all post-baseline follow-up visits (median 9.6 years), weight in ILI participants was 4 kg lower than in DSE (p<0.05).9 During the first year of intervention, HbA1c (as a percent of total hemoglobin) declined by 0.14% in DSE and 0.64% in ILI.15 Averaged over time, HbA1c was 0.22% lower in ILI than in DSE (p<0.05).9 During the first year, systolic blood pressure declined by 2.8 mm Hg in DSE and 6.8 mmHg in ILI.15 Averaged over time, systolic blood pressure was 1.9 mm Hg lower in ILI than DSE (p<0.05).9 Averaged over time, use of antihypertensive medicines was less common in ILI than in DSE (odds ratio=0.88, 95% CI=0.78 to 0.89, p=0.026); insulin (odds ratio=0.74, 95% CI=0.66 to 0.82, p<0.001) and statins (odds ratio=0.86, 95% CI=0.78 to 0.94, p=0.001) were also less likely to be used by ILI participants.9 Furthermore, blockers of the renin-angiotensin system, a subset of antihypertensive medicines, were used less frequently in ILI than in DSE (odds ratio=0.76, 95% CI=0.68 to 0.84, p<0.0001).
Table 2 shows the number of events, incidence rates and hazard ratios for several different renal outcomes. The first row shows the primary renal outcome, very-high-risk CKD, and alternate outcomes are shown in the other rows. The hazard ratio for this outcome was 0.69 (95% CI= 0.55 to 0.87, p=0.002). The treatment effect was at least as great when the outcome of very-high-risk CKD required confirmation on the next examination (HR=0.63 (95% CI=0.47 to 0.86). Hazard ratios for intervention effects on the other renal variables ranged from 0.68 to 0.81 with 95% CIs differing among variables, but most excluding 1.0. There were also apparent reductions in risk of needing renal replacement therapy or doubling of serum creatinine (HR=0.80 or 0.81), but with the small numbers of such events, these estimates were imprecise with wide CIs.
We also examined geometric mean ACR and mean eGFR over time by treatment group, as an alternate to computing the incidence of reaching the arbitrary thresholds shown in Table 2. Mean ACR and eGFR did not differ significantly at baseline (Table 1) but diverged during follow-up, with ACR being significantly lower (p<0.0001) and eGFR higher (p=0.02) in the ILI group (appendix Figure 1), consistent with the treatment effects on crossing CKD thresholds.
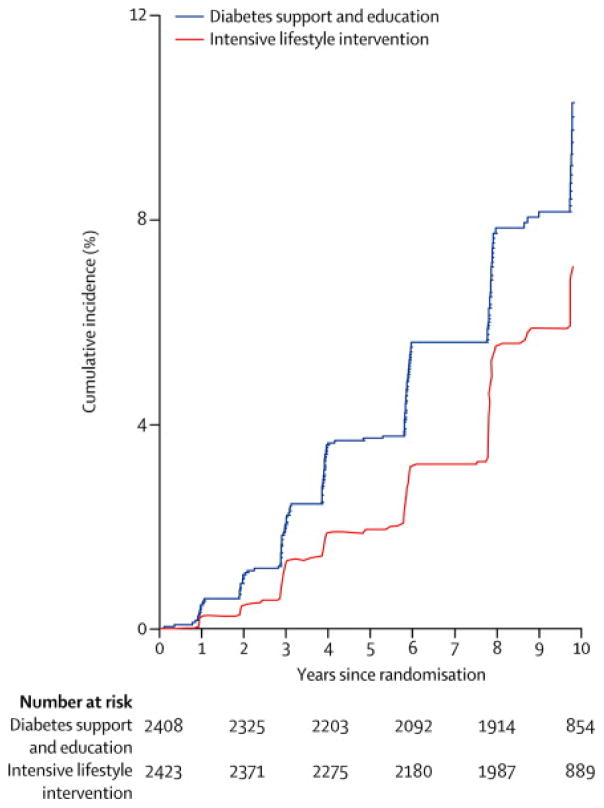
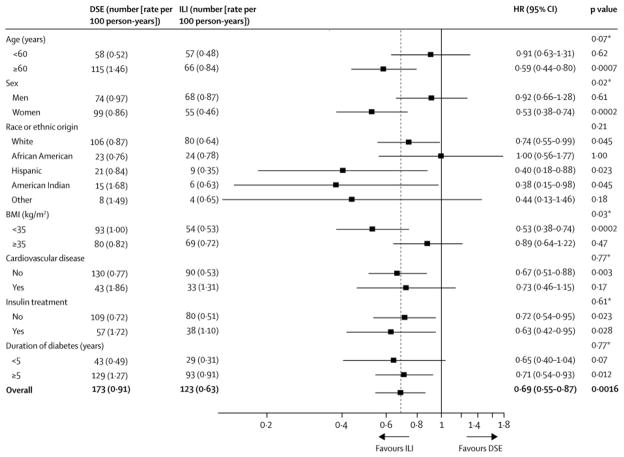
The cumulative incidence of very-high-risk CKD was lower in ILI than in DSE at all time points (Figure 2). Incidence rates of very-high-risk CKD were also examined within subgroups defined by age, sex, race/ethnicity, BMI, history of CVD, insulin treatment, and duration of diabetes (Appendix Figures 2–5). There were no significant interactions between intervention assignment and these variables except sex and BMI in their effects on development of very-high-risk CKD, i.e., the results are compatible with a uniform intervention effect over the other five subgroups. The ILI effect was greater in women and in those with BMI <35 kg/m2. These subgroup analyses are summarized in Figure 3.
Figure 2.
Cumulative incidence of very-high-risk CKD by treatment group through year 10. Too few observations were available beyond year 10 for reliable estimates. DSE is the Diabetes Support and Education group, and ILI is the Intensive Lifestyle Intervention group. The numbers of persons at risk at the beginning of the even-numbered years since randomization are shown. The hazard ratio (ILI vs. DSE) is 0.69, 95% confidence interval = 0.55 to 0.87, p=0.002.
Figure 3.
Numbers of first occurrence of very-high-risk CKD and incidence rates (events per 100 person-years) by treatment group, hazard ratios, and treatment by subgroup interaction tests by subgroups defined by age, sex, race/ethnicity, body mass index (BMI, kg/m2), history of CVD, insulin use, and diabetes duration. The p-values denoted “*” are for the tests of interaction between the subgroups and the intervention group. The dotted reference line refers to the overall hazard ratio. DSE is the Diabetes Support and Education group, and ILI is the Intensive Lifestyle Intervention group. The race/ethnicity variables are abbreviated as: AA=African American, AI=American Indian. Further details are in a footnote to Table 1.
We performed a multivariable proportional hazards analysis of baseline variables predicting very-high-risk CKD, including age, sex, race/ethnicity, BMI, diabetes duration, history of CVD, insulin treatment, and intervention assignment as covariates. All significantly predicted very-high-risk CKD when controlled for each other (Table 3, baseline predictors). There was an overall significant race/ethnic effect; in comparisons of each of the smaller groups with the larger white group, American Indians had a higher rate of very-high-risk CKD (HR=2.31, 95% CI=1.44 to 3.70). Because these variables were evenly distributed between the randomized intervention groups (Table 1), the overall intervention effect in this multivariable model (HR=0.70, Table 3) was virtually the same as in the unadjusted analysis (HR=0.69, Table 2).
Table 3.
Multivariable analysis of predictors of very-high-risk CKD testing for mediation of intervention effect by body weight, HbA1c, and systolic blood pressure. Predictor variables were defined at baseline except for the last three that are time-dependent.
| Predictor | Units or values | Baseline predictors | Baseline predictors plus time-varying weight, HbA1c, and blood pressure | ||||
|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | p-value | Hazard Ratio | 95% Confidence Interval | p-value | ||
| Age at baseline | Per 10 years | 2.05 | 1.70 to 2.47 | <.0001 | 2.06 | 1.68 to 2.52 | <.0001 |
| Sex | Female vs. male | 0.77 | 0.60 to 0.98 | 0.0363 | 0.88 | 0.64 to 1.21 | 0.4177 |
| Race/ethnicity | African American vs. White | 1.16 | 0.82 to 1.62 | 0.0124 | 1.02 | 0.72 to 1.44 | 0.0142 |
| Hispanic vs. White | 1.06 | 0.71 to 1.57 | 1.05 | 0.69 to 1.60 | |||
| American Indian vs. White | 2.31 | 1.44 to 3.70 | 2.36 | 1.45 to 3.85 | |||
| Other vs. White | 1.42 | 0.77 to 2.62 | 1.26 | 0.64 to 2.46 | |||
| BMI | Per 5 kg/m2 | 1.19 | 1.08 to 1.31 | 0.0007 | 1.00 | 0.85 to 1.17 | 0.9988 |
| Diabetes duration | Per 5 years | 1.21 | 1.12 to 1.30 | <.0001 | 1.18 | 1.10 to 1.28 | <.0001 |
| History of CVDa | Yes vs. no | 1.58 | 1.18 to 2.11 | 0.0019 | 1.56 | 1.16 to 2.11 | 0.0034 |
| Insulin treatment | Yes vs. no | 1.57 | 1.18 to 2.09 | 0.0020 | 1.39 | 1.03 to 1.88 | 0.0311 |
| Randomized intervention assignment | ILI vs. DSE | 0.70 | 0.56 to 0.89 | 0.0038 | 0.77 | 0.60 to 0.99 | 0.0381 |
| Body weightb | Per 5 kg | n/a | n/a | n/a | 1.06 | 1.01 to 1.12 | 0.0194 |
| HbA1cb | Per 1% (10.9 mmol/mol) | n/a | n/a | n/a | 1.11 | 1.02 to 1.20 | 0.0147 |
| Systolic blood pressureb | Per 5 mm Hg | n/a | n/a | n/a | 1.10 | 1.07 to 1.14 | <.0001 |
CVD is cardiovascular disease (defined in the Methods)
Analyzed as time-dependent variables, with the values updated at each assessment used to predict occurrence of the event at the next assessment. The hazard ratio for each of these variables corresponds with a higher value by the units given. That is, the hazard is higher for a higher value of each of these variables, or, conversely, lower for a lower value.
When time-dependent values of body weight, HbA1c, and systolic blood pressure were added to this multivariable model predicting very-high-risk CKD, the effect of ILI was modestly attenuated, with the adjusted HR for ILI effect changing from 0.70 to 0.77 (Table 3, baseline predictors plus time-varying covariates). That is, the changes in these three variables in response to ILI partly explained the intervention effect on very-high-risk CKD. Sex*treatment or BMI*treatment interaction terms added to this model were not statistically significant, indicating that the variables in Table 3 account for the treatment interactions shown in Figure 3, where there was no adjustment for these covariates.
Adverse events were described previously.9
DISCUSSION
In the Look AHEAD multicenter randomized clinical trial, the ILI, designed to produce weight loss through caloric restriction and physical activity, reduced the incidence of the very-high-risk category of CKD by 31%. Estimated treatment effects were similar for the other outcomes shown in Table 3. These outcomes are not independent of each other; many are components of each other. Therefore the analyses of alternate outcomes should be interpreted as an analysis of the sensitivity of the results to classification of the renal variables. The treatment benefit was most pronounced for incidence of very-high-risk CKD, especially when confirmed at the next examination.
Our summary definition of CKD, a composite of ACR and eGFR, was adapted from the recent KDIGO classification. Persons with very-high-risk CKD are at high risk of progressing to kidney failure, CVD, and death.13 Although ILI had no significant effect on CVD or mortality, there was a non-significant reduction in total mortality (HR=0.85, 95% CI=0.69 to 1.04, for ILI vs. DSE).9 The beneficial effects of the ILI on diabetic nephropathy might eventually lead to reduced mortality. Most of the excess mortality in persons with type 1 or type 2 diabetes occurs in those with diabetic nephropathy.16,17 In the UKPDS clinical trial in type 2 diabetes, the effects of improved glycemic control on CVD took much longer to appear than the effects on microvascular disease.18
The 31% risk reduction for very-high-risk CKD should be compared with other clinical trials of nephropathy in type 2 diabetes. (See panel: Research in Context.) Although outcome definitions differed among studies, risk of advanced nephropathy was reduced by 20 to 61% by angiotensin-receptor antagonists19–21 and by 61% with the Steno Diabetes Center’s multifactorial behavioral and pharmacological intervention focused on glucose, lipids, and blood pressure.22 Other studies have shown reductions in urine albumin excretion and stabilization of declining eGFR with voluntary weight loss.23 Maximal nephropathy risk reduction may, therefore, come from combining intensive weight loss and pharmacologic interventions, although, to our knowledge, this has not been tested.
Because the reductions in body weight, HbA1c, and systolic blood pressure with ILI could contribute to the reduced risk of nephropathy, these variables were entered into the multivariable proportional hazards model as time-dependent covariates to reflect their changes with intervention. Their inclusion attenuated the effect of ILI on very-high-risk CKD (Table 3), suggesting that these changes in response to intervention mediated some of the effect of ILI on the incidence of advanced diabetic nephropathy. Even with these risk factors in the model, there was a significant effect of time-dependent weight (p=0.019), suggesting effects of weight change beyond the effects of blood pressure, glycemic control, and other results of the randomly assigned ILI. By contrast, the ILI effect was not mediated by protective effects of antihypertensive medicines or the subset of renin-angiotensin system blockers, because they were used less frequently in the ILI group.
While our large, randomized clinical trial had many strengths, including excellent retention, objective measures, accepted CKD definitions, and long duration of follow-up, there are limitations. First, CKD was not the primary trial outcome, and the specific analyses of CKD were not pre-specified at the beginning of the trial; however, the protocol did specify that renal complications would be examined, and these outcomes were laboratory based and, therefore, not subject to observer bias. They were analyzed according to the latest international classification of CKD. Furthermore, the estimated treatment effects were minimally sensitive to alternate categorizations of renal disease. ILI appeared to lower the incidence of renal replacement therapy and doubling of serum creatinine, common endpoints in renal clinical trials, but these events were too infrequent for precise estimates. As indicated by the wide CIs, there was inadequate power to detect modest effects on these endpoints. In post-hoc power calculations, 247 events would give 80% power to detect a HR=0.70. Our total of 296 very-high-risk CKD events thus yielded a significant treatment effect. Detecting a HR=0.80, however, would require 631 events. We observed HR=0.80 for renal replacement therapy based on 65 cases - clearly too few for such an effect to be statistically significant. Second, we lacked data on causes of renal impairment. Third, participants were motivated to lose weight and successfully completed a maximal fitness test at baseline. Thus, the results cannot be generalized to all individuals with type 2 diabetes.
The ILI had many previously reported benefits, including improvements in HbA1c,15,24 CVD risk factors,15,24 depression,25 knee pain,26 urinary incontinence in women,27 self-reported mobility,28 and heart rate recovery after exercise.29 These benefits were attained with less use and cost for drugs during the intensive intervention phase.30 Here we report that ILI also protected against CKD, consistent with reports that improving glucose control prevents or delays microvascular complications, including nephropathy, in both type 13 and type 2 diabetes.4–6 This protection, however, was not accompanied by reduction in CVD event rates, consistent with three other large clinical trials in which intensive glucose control did not reduce cardiovascular events.5,31,32 Thus, one might not expect improved glucose control in the ILI to reduce CVD event rates. Moreover, statin use was less and LDL cholesterol was higher in ILI than in DSE. This might attenuate a beneficial effect of ILI on CVD without affecting renal outcomes. Despite not reducing cardiovascular outcomes,9 the current and previous reports from Look AHEAD suggest that in obese and overweight persons with type 2 diabetes, ILI improves many health outcomes including the microvascular complication of nephropathy.
Supplementary Material
Panel: Research in context.
Systematic Review
Nephropathy is an important microvascular complication of diabetes, and advanced diabetic nephropathy is related to increased risk of cardiovascular disease and death.13 Previous clinical trials of nephropathy in type 2 diabetes have tested pharmacologic agents, including angiotensin-receptor antagonists,19–21 glucose lowering drugs targeting intensive compared with less intensive control of glycemia,4–6 and an intensive medication and behavior modification intervention focused on glucose, lipids, and blood pressure.22 A recent review of surgical and non-surgical weight loss in obese patients noted improvements in proteinuria, albuminuria, and glomerular filtration rate.23 Generally, the studies reviewed were small and of short duration, and few were randomized trials. The reviewed studies were not large or long enough to establish clinical benefits of weight loss. To date, there have been no large, long-term randomized trials of lifestyle intervention focused on weight loss in type 2 diabetes testing whether such intervention could prevent diabetic nephropathy.
Interpretation
An intensive lifestyle intervention designed to produce and retain weight loss reduced the incidence of very-high-risk chronic kidney disease, as measured with estimated glomerular filtration rate and albumin-creatinine ratio, over a median follow-up of 9.6 years in middle aged and older US adults with type 2 diabetes. The effect was greater in women and those with baseline BMI < 35 kg/m2, and was partially attributable to reductions in weight, HbA1c, and blood pressure. These results are consistent with those of other trials of treatments that improve glucose control or blood pressure in type 1 and type 2 diabetes, and that prevent or delay the onset of nephropathy.
Acknowledgments
The full membership of the Look AHEAD Research Group is shown in the Appendix. The Look AHEAD Research Group acknowledges the contributions of Drs. Frederick Brancati and Richard Rubin, who passed away during the completion of this manuscript. Both colleagues were fundamental to the successful development and execution of our trial.
Funding and Support
Funded by the National Institutes of Health and other Department of Health and Human Services agencies through cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. Additional funding was provided by the National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; NIH Office of Research on Women’s Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (I.H.S.) provided personnel, medical oversight, and use of facilities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the I.H.S. or other funding sources.
Additional support was received from The Johns Hopkins Medical Institutions Bay view General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); the VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs; and the Frederic C. Bartter General Clinical Research Center (M01RR01346).
Footnotes
Author Contributions: Drs. Knowler and Chen had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Knowler, Bahnson, Bantle, Bertoni, Bray, Chen, Cheskin, Clark, Egan, Evans, Foreyt, Glasser, Greenway, Gregg, Hazuda, Hill, Horton, Hubbard, Jakicic, Jeffery, Johnson, Kahn, Kitabchi, Korytkowski, Krakoff, Kure, Lewis, Maschak-Carey, Michaels, Montez, Nathan, Nyenwe, Patricio, Peters, Pi-Sunyer, Pownall, Wadden, Wagenknecht, Williamson, Wing, Wyatt, Yanovski.
Acquisition of data: Knowler, Bahnson, Bantle, Bertoni, Bray, Chen, Cheskin, Clark, Egan, Evans, Foreyt, Glasser, Greenway, Hazuda, Hill, Horton, Hubbard, Jakicic, Jeffery, Johnson, Kahn, Kitabchi, Korytkowski, Krakoff, Kure, Lewis, Maschak-Carey, Michaels, Montez, Nathan, Nyenwe, Patricio, Peters, Pi-Sunyer, Pownall, Wadden, Wagenknecht, Williamson, Wing, Wyatt.
Analysis and interpretation of data: Knowler, Bahnson, Bantle, Chen, Greenway, Johnson, Kahn, Kitabchi, Lewis, Nathan.
Drafting of the manuscript: Knowler, Bahnson, Bantle, Chen, Greenway, Johnson, Kahn, Kitabchi, Lewis, Nathan.
Critical revision of the manuscript for important intellectual content: Knowler, Bahnson, Bantle, Bertoni, Bray, Chen, Cheskin, Clark, Egan, Evans, Foreyt, Glasser, Greenway, Gregg, Hazuda, Hill, Horton, Hubbard, Jakicic, Jeffery, Johnson, Kahn, Kitabchi, Korytkowski, Krakoff, Kure, Lewis, Maschak-Carey, Michaels, Montez, Nathan, Nyenwe, Patricio, Peters, Pi-Sunyer, Pownall, Wadden, Wagenknecht, Williamson, Wing, Wyatt, Yanovski.
Statistical analysis: Chen and Knowler.
The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; Life Scan, Inc., a Johnson & Johnson Company; OPTIFAST® of Nestle Health Care Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America.
Some of the information contained herein was derived from data provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662–73. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302–8. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 5.The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 6.Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–30. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The DCCT Research Group. Lifetime benefits and costs of intensive therapy as practiced in the Diabetes Control and Complications Trial. JAMA. 1996;276:1409–15. [PubMed] [Google Scholar]
- 8.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diab Care. 2012;35:1364–79. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Look AHEAD Research Group. Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. New Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Look AHEAD Research Group. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–28. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 11.The Look AHEAD Research Group. The Look AHEAD Study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737–52. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Look AHEAD Research Group. Baseline characteristics of the randomised cohort from the Look AHEAD (Action for Health in Diabetes) research study. Diab Vasc Dis Res. 2006;3:202–15. doi: 10.3132/dvdr.2006.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidney Disease Improving Global Outcomes (KDIGO) Chronic Kidney Disease Work Group. KDIGO Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013;(Suppl 3):1–150. [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. Chronic Kidney Disease Epidemiology Collaboration: A new equation to estimate glomerular filtration rate. Ann Int Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diab Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borch-Johnsen K, Andersen PK, Deckert T. The effect of proteinuria on relative mortality in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1985;28:590–596. doi: 10.1007/BF00281993. [DOI] [PubMed] [Google Scholar]
- 17.Nelson RG, Pettitt DJ, Carraher MJ, Baird HR, Knowler WC. The effect of proteinuria on mortality in non-insulin-dependent diabetes mellitus. Diabetes. 1988;37:1499–1504. doi: 10.2337/diab.37.11.1499. [DOI] [PubMed] [Google Scholar]
- 18.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 19.Lewis EL, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the Angiotensin-receptor antagonist Irbesartan in patients with nephropathy due to type 2 diabetes. New Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 20.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. New Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 21.Parving HH, Lehnert H, Bröchner-Mortensen J, et al. The effect of Irbesartan on the development diabetic nephropathy in patients with type 2 diabetes. New Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 22.Gæde P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Mutifactorial intervention and cardiovascular disease in patients with type 2 diabetes. New Enl J Med. 2003;348:383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 23.Ibrahim HN, Weber ML. Weight loss: a neglected intervention in the management of chronic kidney disease. Current Opinion in Nephrology and Hypertension. 2010;19:534–538. doi: 10.1097/MNH.0b013e32833f13de. [DOI] [PubMed] [Google Scholar]
- 24.Look AHEAD Research Group. Long term effects of lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes: four year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faulconbridge LF, Wadden TA, Rubin RR, et al. for the Look AHEAD Research Group. One-year changes in symptoms of depression and weight in overweight/obese individuals with type 2 diabetes in the Look AHEAD study. Obesity. 2012;20:783–793. doi: 10.1038/oby.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foy CG, Lewis CE, Hairston KG, et al. the Look AHEAD Research Group. Intensive lifestyle intervention improves physical function among obese adults with knee pain: findings from the Look AHEAD trial. Obesity. 2011;19:83–93. doi: 10.1038/oby.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phelan S, Kanaya AM, Subak LL, et al. the Look AHEAD Research Group. Weight loss prevents urinary incontinence in women with type 2 diabetes: results from the Look AHEAD trial. J Urology. 2012;187:947–952. doi: 10.1016/j.juro.2011.10.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rejeski WJ, Ip EH, Bertoni AG, et al. for the Look AHEAD Research Group. Lifestyle change and mobility in obese adults with type 2 diabetes. New Engl J Med. 2012;366:1209–1217. doi: 10.1056/NEJMoa1110294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribisl PM, Gaussoin SA, Lang W, et al. the Look AHEAD Research Group. Lifestyle intervention improves heart rate recovery from exercise in adults with type 2 diabetes: results from the Look AHEAD study. J Obesity. 2012;2012:309196. doi: 10.1155/2012/309196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redmon JB, Bertoni AG, Connelly S, et al. the Look AHEAD Research Group. Effect of the Look AHEAD study intervention on medication use and related cost to treat cardiovascular disease risk factors in individuals with type 2 diabetes. Diab Care. 2010;33:1153–1158. doi: 10.2337/dc09-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.