Abstract
Despite classical expectations of a trade-off between immune activity and reproduction, an emergent view suggests that individuals experiencing activation of their immune system actually increase reproductive effort and allocation to offspring as a form of terminal investment in response to reduced survival probability. However, the components and mechanisms of increased parental investment following immunostimulation are currently unknown. We hypothesize that increased glucocorticoid production following immunostimulation modulates the increase in reproductive effort that constitutes terminal investment. We activated the immune system of breeding female house wrens (Troglodytes aedon) with an immunogen and cross-fostered the eggs they subsequently produced to separate pre- and post-natal components of maternal investment. Cross-fostering revealed an increase in both pre- and post-natal allocation from immunostimulated females, which was confirmed by quantification of egg constituents and maternal provisioning behavior. The increase in maternal provisioning was mediated, at least in part, by increased corticosterone in these females. Offspring immune responsiveness was also enhanced through transgenerational immune priming via the egg. Thus, our results indicate that maternal immunostimulation induces transgenerational effects on offspring through both pre- and post-natal parental effects, and support an important role for corticosterone in mediating parental investment.
Keywords: house wren, life history, parental care, predictive adaptive response, transgenerational immune priming, Troglodytes aedon
Investing resources into immunity is generally expected to reduce an animal’s ability to invest in reproduction, and vice versa, when resources are in short supply (Ilmonen et al. 2000; Casto et al. 2001; Ardia 2005; Martin et al. 2008; Knowles et al. 2009), and trade-offs such as this provide a robust framework for predicting how natural selection acts on parental investment and life histories (Sheldon and Verhulst 1996; Ricklefs and Wikelski 2002). However, despite the potential importance of a trade-off between immunity and reproduction, recent work has revealed that parents in a broad range of taxa actually respond to activation of their immune system by increasing reproductive effort and success (Williams et al. 1999; Bonneaud et al. 2004; Sadd et al. 2006; Schwanz 2008; Adamo et al. 2014). Such a result is consistent with the terminal-investment hypothesis, which predicts that individuals should increase reproductive effort as a response to declining residual reproductive value, i.e., a decline in their probability of survival and future reproduction (Williams 1966; Clutton-Brock 1984). Thus, increased reproductive effort and success following an immune challenge is expected if activation of the immune system signals to an individual that its residual reproductive value has been jeopardized (see also Hanssen 2006; Weil et al. 2006; Velando et al. 2006; 2014; Kivleniece et al. 2010).
We recently tested the terminal-investment hypothesis in a wild population of house wrens (Troglodytes aedon) by inducing an immune response in breeding females through injection of lipopolysaccharide (LPS), a potent but non-lethal immunogen that is commonly used to activate the immune system (McEwen et al. 1997; Klasing 1998). Rather than reducing reproductive effort and success, females treated with LPS subsequently produced higher-quality offspring than saline-injected controls, and in a sex-specific manner (Bowers et al. 2012). These findings contradict the long-held notion that immune-challenged parents should sacrifice investment in offspring to improve their own survival. However, house wrens are short-lived in temperate North America, and many that survive to adulthood breed in only one year (Johnson 2015); thus, the results are expected if mothers perceive that their current offspring may be their last. The physiological processes underlying this behavior, however, remain largely unexplored.
We hypothesize that the parental response to immunostimulation and induction of terminal investment involves the glucocorticoid corticosterone. Secreted by the adrenal gland, corticosterone serves as a signal of environmental conditions, promotes the mobilization of energetic reserves, and is intimately involved with the immune system through the action of cytokines in the brain (Besedovsky et al. 1986; Sapolsky et al. 1987; Turnbull and Rivier 1999; McEwen et al. 1997; Klasing 1998; Demas et al. 2011; see also Zimmerman et al. 2014). Immunostimulation has long been known to cause sustained increases in circulating corticosterone, as expected if mounting an immune response is energetically expensive. Moreover, this increase in corticosterone is likely to remain elevated for at least part of the time that immunostimulated parents are breeding (McEwen et al. 1997; Adelman et al. 2010), and although elevated corticosterone is often associated with a reduction in reproductive investment, there is evidence that low-level variation in corticosterone promotes foraging, parental care, and reproductive success because the energetic demands of parental care require the mobilization of energetic reserves (Landys et al. 2006; Doody et al. 2008; Hau et al. 2010; Ouyang et al. 2011, 2013; Crossin et al. 2012; Love et al. 2014). Although immunostimulation is often expected to reduce reproductive effort, neuroendocrine effects of immune activation that lead to the suppression of reproduction (e.g., reduced production of gonadotropin-releasing hormone by the hypothalamus) often occur over shorter time periods than the effect that immunostimulation has on glucocorticoid production (Adelman et al. 2010; Lopes et al. 2012a). For breeding animals, this should result in their producing eggs or rearing offspring following immunostimulation while circulating corticosterone remains elevated.
Circulating corticosteroids accumulate in the yolk during egg formation (Sinervo and DeNardo 1996; McCormick 1998; Hayward et al. 2005; Love et al. 2005, 2008; Okuliarová et al. 2010; Almasi et al. 2012); thus, egg-yolk corticosterone may serve as a non-invasive, integrated measure of maternal physiology reflecting what an individual produces over time and which is not subject to researcher- or restraint-induced stress. We hypothesize that increased corticosterone following immunostimulation is passed from mother to offspring during egg formation, and that the increase in corticosterone (i) reflects maternal physiology, with variation in corticosterone positively affecting maternal care (e.g., Sapolsky et al. 2000; Crossin et al. 2012; Love et al. 2014), and (ii) enhances offspring development via the egg by promoting begging and growth after hatching (Quillfeldt et al. 2006; Loiseau et al. 2008; Love and Williams 2008; Chin et al. 2009; Smiseth et al. 2011). In blue-footed boobies (Sula nebouxii), immune-challenged males reared more-productive broods than controls, indicating that terminal investment and its effects on offspring can be derived from a source other than the egg (Velando et al. 2006, 2014). Given that immunostimulation leads to the activation of the hypothalamic-pituitary-adrenal (HPA) axis, the subsequent increase in corticosterone may promote foraging and provisioning by parents (Kitaysky et al. 2001; Angelier et al. 2008; Crossin et al. 2012; Love et al. 2014) and increased allocation to eggs (Kouwenberg et al. 2013), thus providing potential mechanisms of terminal investment.
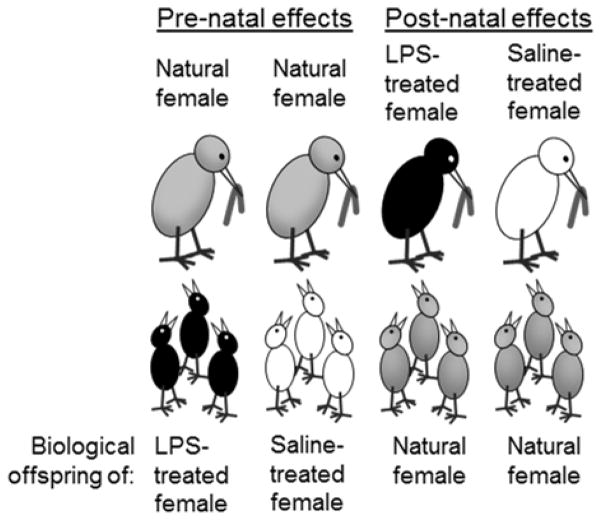
In this study, we tested the hypotheses that females shape offspring phenotype following immunostimulation through (i) increasing pre-natal allocation to eggs, (ii) enhancing post-natal allocation to nestlings, or (iii) by altering both pre- and post-natal allocation. To separate pre- and post-natal effects, we conducted a cross-fostering experiment with three treatment groups for use in the field: experimental females recently injected with LPS, control females recently injected with the saline vehicle, and natural females that were not manipulated. We transferred clutches of eggs among nests such that experimental and control females reared offspring from eggs that were produced by natural females, and natural females reared offspring from eggs produced either by experimental or by control females (fig. 1). Therefore, if immune-stimulated mothers increase allocation in the egg, offspring of LPS-injected females should grow larger than those of saline-injected females when each are reared by natural, unmanipulated mothers. We also predicted that LPS-injected females enhance offspring growth after hatching by provisioning neonates with extra food, in which case the condition and health of natural offspring reared by experimental females should be higher than that of nestlings reared by controls (fig. 1). In addition to the cross-fostering experiment, we conducted a concurrent experiment to study effects of immune stimulation on allocation to eggs. In this experiment, females were treated identically to those in the cross-fostering experiment, but we collected pre- and post-treatment eggs to quantify maternal allocation of yolk and yolk-steroids within the egg. We predicted that egg-yolk corticosterone would increase in post-treatment eggs for experimental, but not for control females, as the immunostimulation should increase HPA activity. For a subset of these females, we were then able to provide them with natural offspring to rear (as in the cross-fostering experiment); in these nests, we used the concentration of yolk-corticosterone in the eggs that females produced as a reflection of their physiology and tested whether corticosterone deposited in the egg predicted their subsequent provisioning effort to nestlings.
Figure 1.
Schematic representation of treatment groups in the cross-fostering experiment. For investigation of pre-natal effects, natural, previously unmanipulated females reared the biological offspring of females that had previously been treated either with LPS (experimental) or with saline (control). For investigation of post-natal effects, experimental and control females reared the biological offspring of natural females.
Methods
Study Area and Species
House wrens are small songbirds with a widespread distribution in North America (biology summarized in Johnson 2015). We studied a migratory population from 2012–2014 in Illinois, USA (40.665°N, 88.89°W). Nestboxes (N = 820; see Lambrechts et al. 2010 for details) were distributed at a density of 5.4 boxes/ha and protected, to an extent, from ground-dwelling predators by aluminum predator baffles placed beneath the nestboxes. Each year we attempted to capture and mark all individuals on the site. Adults were captured inside nestboxes or by using mist nets near the box; we measured their body mass and tarsus length and banded them with a unique U. S. Geological Survey leg band; males received three additional colored bands in a unique combination so that they could be identified visually and distinguished from females while provisioning. Clutch sizes typically range from four to eight eggs. Only females incubate and brood nestlings until they begin thermoregulating at 8–10 days of age, but both parents provision nestlings, and young fledge 14–16 d post-hatching (Bowers et al. 2013, 2014a). Both parents bring only one prey item to the nest on each visit (Barnett et al. 2012).
Field Procedures
We conducted this study during the second half of the 2012 and 2013 breeding seasons (as in Bowers et al. 2012) to ensure that females in the experiment would be breeding for the last time of the season. Because house wrens are short-lived (Johnson 2015), we predicted that intimations of impending mortality would elicit a terminal-investment response by females. Clutches initiated by single- and double-brooded females were equally represented in the experiment and yielded a sample that is closely representative of the larger population. We visited nestboxes at least twice weekly to check for evidence of female settlement, and visited nests daily during egg laying to mark eggs as laid using a non-toxic marker. Once females completed their clutches, we captured them shortly into incubation (3.2 ± 1.1 d; mean ± SD) and randomly assigned them to the control or experimental group (table 1). Experimental females were injected with 50 μL of phosphate-buffered saline (PBS) containing 0.1 mg × kg body mass−1 LPS (from Salmonella enterica serotype typhimurium; Sigma product number L7261) and control females with 50 μL of PBS.
Table 1.
Sample sizes.
| Females injected | N | Females re-nesting | N |
|---|---|---|---|
| Control | Control | ||
| 2012 | 32 | 2012 | 21 |
| 2013 | 34 | 2013 | 27 |
| Experimental | Experimental | ||
| 2012 | 33 | 2012 | 19 |
| 2013 | 33 | 2013 | 23 |
LPS is a component of the cell membrane of gram-negative bacteria that the vertebrate immune system has been selected to recognize, and the response to LPS is highly conserved across taxa. Binding of LPS by a toll-like receptor generates an acute-phase immune response, involving the production of inflammatory cytokynes and the downstream production of corticosterone (see Introduction). LPS also elicits humoral responses that take longer to develop (i.e., days to weeks). Each of these responses are often energetically costly to produce; thus, the production of glucocorticoids following an immune challenge may facilitate such responses, and even play a role in mediating or avoiding traditionally expected trade-offs associated with immune activation. Therefore, LPS can be used to elicit an immune response without causing an infection or direct harm, allowing for analysis of the behavioral effects of immunostimulation that are not generated by direct effects of a replicating pathogen (e.g., Bonneaud et al. 2003; Grindstaff et al. 2006).
After injection, we collected the females’ current clutch of eggs, forcing them to produce a replacement clutch (i.e., re-nest) and allowing time for development of an immune response while producing eggs (Grindstaff et al. 2006). Whether or not females re-nested occurred randomly with respect to the variables we analyze below (all P > 0.05). There were also no differences between control and experimental females in their pre-injection clutch size (treatment: F1, 128 = 0.30, P = .587; year: F1, 128 = 4.46, P = .037; treatment × year: F1, 128 = 1.26, P = .264), egg mass (treatment: F1, 47.9 = 0.21, P = .648; year: F1, 47.9 = 0.13, P = .720; treatment × year: F1, 33.7 = 1.92, P = .175), or body mass (treatment: F1, 128 = 0.73, P = .394; year: F1, 128 = 2.56, P = .115; treatment × year: F1, 128 = 0.13, P = 0.718) prior to the experiment, and females were treated identically in the two experiments (see below).
Cross-fostering Experiment
Once females re-nested, we randomly matched them for cross-fostering of eggs with unmanipulated, natural females breeding at the same time (fig. 1). Beginning approximately one day before eggs were expected to hatch, we cross-fostered whole clutches, such that natural females reared the biological offspring of either control or experimental females, and control and experimental females reared the offspring of natural females (fig. 1). We standardized the task of nestling rearing by providing all females with five eggs (the modal post-treatment clutch size in this study and in Bowers et al. 2012), allowing us to use the condition of foster offspring that females produced as a measure of post-hatching parental investment. Including the difference between the size of the clutch a female produced and the number of eggs she received after cross-fostering as a covariate had no appreciable effect on the outcome of our statistical tests (data not shown). A small number of surplus eggs were given to females not involved in the study.
We monitored the progress and status of nests over the following days and, 11 days after hatching began, weighed nestlings (± 0.1 g) on an electronic balance and measured their tarsus (± 0.1 mm) with dial calipers. We also drew a blood sample from the brachial vein at this time and used it to determine hematocrit, which is the percentage of whole blood comprised of erythrocytes and is a commonly used measure of health (Richner et al. 1993; Ots et al. 1998; Williams 2012). Although the significance of variation in hematocrit has been debated, our data suggest that it is a meaningful measure of condition and health state that has long-term consequences for fitness (Bowers et al. 2014b; see also Williams 2012). At this time, we also administered a phytohaemagglutinin (PHA) test in nestlings to obtain a measure of cutaneous immune activity (Martin et al. 2006). For this test, we used a digital thickness gauge (Mitutoyo no. 547–500) to measure pre-injection thickness of the wing web (prepatagium) as the mean of three measures, and we then injected the web with 50 μL of PBS containing PHA (Sigma product number L8754; concentration = 5 mg PHA/mL PBS). PHA is a plant-derived mitogen that stimulates inflammation and swelling upon injection, including immune cells derived from the innate and adaptive axes of the immune system, and large swellings indicate heightened immune activity (Martin et al. 2006; Forsman et al. 2010; Vinkler et al. 2014). We measured the swelling 24 h post-injection, and used the difference between the mean of three pre- and post-injection measures as the PHA response. The magnitude of the swelling at this age is positively associated with long-term reproductive success in our study population (Bowers et al. 2014b).
We monitored provisioning to the nest by each parent early in the nestling period (day 4 or 5 post-hatching), when nestling growth is most rapid and when parental provisioning positively affects offspring growth and survival (Bowers et al. 2014a, 2015b). We used digital video cameras (Kodak Sport Zx5, Eastman Kodak Company, Rochester, NY, USA) placed 1–2 m from the nestbox to record provisioning trips to the nest by parents, and all recordings were made between 0630 and 1100 hr Central Daylight Time. We set up a dummy camera outside the nestbox the day before filming to allow parents time to habituate to the presence of the camera, although its presence does not appear to affect parental behavior, and provisioning data obtained from videos are similar to those obtained when parents are undisturbed and observed using a spotting scope from a distance of approximately 30 m (DeMory et al. 2010; Bowers et al. 2014a). Once filming began, most parents returned to the nestbox within 1–2 min and provisioned at regular intervals thereafter. We filmed for approximately 100–120 min to ensure that we had one full hour of undisturbed provisioning for analyses; provisioning data were obtained from the 1-h period after parents resumed provisioning (DeMory et al. 2010; Barnett et al. 2012; Bowers et al. 2014a). We also used the time that females spent inside nestboxes as an estimate of brooding effort (i.e., the time [min] spent in the nestboxes providing warmth for ectothermic young during our observations of provisioning).
Allocation to Eggs
In this experiment, females were treated the same as those in the concurrent cross-fostering experiment. However, we collected eggs from the field on the day each was laid to quantify the amount of yolk deposited in eggs and concentrations of yolk steroids before and following treatment (N = 349 eggs from 38 pre- and 21 post-treatment clutches). We collected eggs on the morning each was laid, replacing the fresh eggs with artificial eggs that females readily accepted and incubated. We then measured the size (length and breadth) and mass of the eggs and stored them at −20°C prior to measuring concentrations of yolk steroids (corticosterone and testosterone) through competitive-binding radioimmunoassay (RIA) using a standardized protocol (Paitz et al. 2011). We also measured yolk testosterone, which also elicits post-hatching effects on offspring begging and growth (Barnett et al. 2011; Smiseth et al. 2011). For the RIAs, we diluted yolk samples in 500 μL water and added radiolabelled (tritiated) steroid tracer to quantify recoveries of the extracted steroids following column chromatography. Yolk steroids were extracted twice with 3 mL of an ether extraction solvent (30% petroleum ether: 70% diethyl ether). The organic fraction containing the extracted steroid was dried, reconstituted in 90% ethanol, and stored at −20°C overnight to precipitate neutral lipids. We then fractionated different steroids from yolk extracts using column chromatography; samples were applied directly to the column and eluted using hormone-specific ethyl acetate: isooctane ratios (testosterone = 20%, corticosterone = 50%; fractions containing progesterone and dihydrotestosterone were fractionated separately and discarded). We dried the corticosterone and testosterone elutes and measured concentrations using separate competitive-binding RIAs with tritiated steroid. Average recovery was 67% for corticosterone and 73% for testosterone. We conducted a total of six RIAs for each steroid, and obtained corticosterone and testosterone concentrations for 325 and 326 egg yolks, respectively (for corticosterone, we omitted one egg that had a concentration 9.8 SD above the population mean; inclusion of this datum has no effect on the results). Clutches from 2012 and 2013 were randomly assayed across the six RIAs, and all eggs from a given female were run within the same assay to reduce within-female variation that might be attributable to inter-assay variability. For corticosterone, the intra-assay coefficients of variation were 7.1%, 5.7%, 9.9%, 7.6%, 7.7%, and 8.2%; the inter-assay coefficient of variation was 8.5%. For testosterone, the intra-assay coefficients of variation were 9.4%, 2.1%, 6.9%, 3.8%, 10.5%, and 1.6%; the inter-assay coefficient of variation was 9.0%.
Data Analysis
We used SAS (version 9.3) for all analyses, all tests are two-tailed, and we converted data to z-scores prior to analysis to obtain standardized parameter estimates, which provide greater interpretability as measures of effect size (Schielzeth 2010). Much of the USA, including central Illinois, experienced a drought during the 2012 breeding season (fig. A1). Therefore, we included year as a random effect in all analyses, but year did not interact with our treatment in any analysis (all P > 0.05). We used mixed-model analyses of variance (ANOVA), with nest as a random effect in addition to year, to analyze treatment effects on nestling phenotype. We included nestling tarsus length as a covariate in our analysis of body mass, and nestling body mass as a covariate in our analyses of PHA responsiveness and hematocrit. We then analyzed maternal food-provisioning rates with treatment (control, experimental, and natural), male provisioning rates, female brooding time, and the time of day of our observations as fixed effects. We analyzed the effects of maternal provisioning on nestling asymptotic body mass 11 days after hatching, which positively predicts recruitment and reproductive success in the study population (Bowers et al. 2014b, 2015b), and we included paternal provisioning rate, maternal brooding time, and nestling tarsus length as fixed effects. We then analyzed allocation to eggs (egg mass, yolk mass, yolk corticosterone, and yolk testosterone) using mixed-model, repeated-measures ANOVA; we included treatment (control vs. experimental) and time (pre- vs. post-injection) as crossed main effects, and we also included female body mass and relative egg-laying order (egg number divided by the clutch size) as fixed effects. We included clutch as a random effect to account for the non-independence of eggs within clutches, and time (pre- vs. post-injection) as a within-subject effect. When analyzing yolk mass, we analyzed residuals of a linear regression of yolk mass on egg mass as the dependent variable, thus reflecting yolk mass adjusted for egg mass; eggs with positive and negative values have increased and decreased yolk, respectively, relative to what would be expected from the overall mass/size of the egg. For a random subset of these females (N = 13), we quantified concentrations of yolk-steroids from their eggs and also provided them with natural foster offspring to rear, as in the cross-fostering experiment; for these females, we tested whether the concentrations of yolk steroids predicted their provisioning rates to nestlings using a linear mixed model. Finally, we analyzed female clutch sizes using repeated-measures ANOVA (as above) and return rates to the breeding population the following year using a generalized linear mixed model with a binary response distribution and logit link function, similar to a logistic regression, with treatment (control, experimental, and natural) and the number of young fledged as fixed effects.
Appendix: Figure A1.
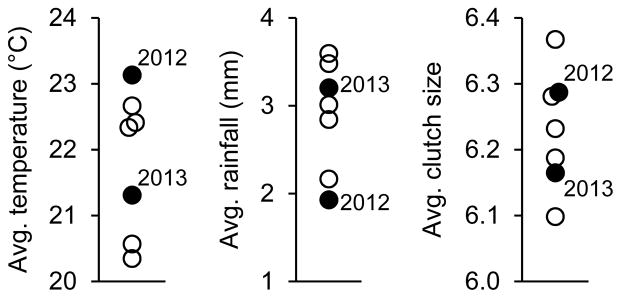
Variation in average temperature, rainfall, and clutch size during the 2012 and 2013 breeding seasons (filled symbols) and the five seasons preceding this study (open symbols). Data from the National Oceanographic and Atmospheric Association.
Results
Cross-fostering Experiment
Cross-fostering revealed effects on both pre- and post-natal allocation to offspring induced by maternal immunostimulation. Pre-natal effects manifested through the egg enhanced the body mass and immune responsiveness of the offspring of experimental females relative to those of controls, but hematocrit was not affected (table 2; fig. 2). We also detected post-natal effects, as experimental females reared natural offspring of greater body mass than those reared by controls (table 2; fig. 2A). However, neither offspring immune responsiveness nor hematocrit was affected by post-natal effects after correcting for nestling mass (table 2; fig. 2B,C). Removal of the term for mass in the analysis of post-natal effects on hematocrit (table 2) revealed a modestly significant treatment effect (P = .039); thus, post-natal effects influenced variation in nestling hematocrit, but through an effect on body mass.
Table 2.
Effects of pre- and post-natal allocation on offspring.
| Pre-natal effects | Post-natal effects | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate ± SE | F | df | P | Estimate ± SE | F | df | P | ||
|
|
|
||||||||
| Body mass | Body mass | ||||||||
| Treatment | 12.65 | 1, 29.4 | .001 | Treatment | 15.50 | 1, 40.6 | < .001 | ||
| Experimentala | .710 ± .199 | Experimentala | .663 ± .169 | ||||||
| Tarsus length | .420 ± .066 | 40.87 | 1, 127 | < .001 | Tarsus length | .508 ± .049 | 107.71 | 1, 185 | < .001 |
| Intercept | .063 ± .623 | Intercept | .415 ± .199 | ||||||
| PHA responsiveness | PHA responsiveness | ||||||||
| Treatment | 9.78 | 1, 29.7 | .004 | Treatment | 1.21 | 1, 48.8 | .276 | ||
| Experimentala | .823 ± .263 | Experimentala | .217 ± .197 | ||||||
| Body mass | .139 ± .098 | 2.02 | 1, 114 | .158 | Body mass | .106 ± .089 | 1.42 | 1, 177 | .234 |
| Intercept | .421 ± .193 | Intercept | .155 ± .140 | ||||||
| Hematocrit | Hematocrit | ||||||||
| Treatment | .00 | 1, 32.1 | .965 | Treatment | 2.39 | 1, 45.5 | .129 | ||
| Experimentala | −.012 ± .270 | Experimentala | .361 ± .233 | ||||||
| Body mass | .299 ± .088 | 11.45 | 1, 125 | .001 | Body mass | .197 ± .080 | 6.12 | 1, 164 | .014 |
| Intercept | −2.993 ± .907 | Intercept | .174 ± .199 | ||||||
relative to control offspring
Figure 2.
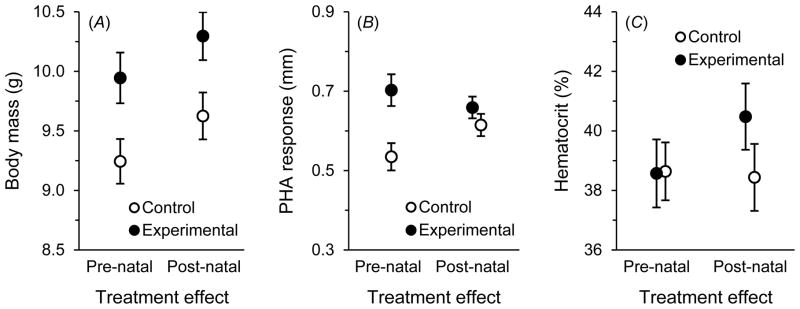
Treatment effects on nestling phenotype. Pre-natal treatment effects are manifested through the eggs produced by control (saline) and experimental (LPS) females, whereas post-natal effects are manifested by differences in post-natal care (least-squares means ± SE).
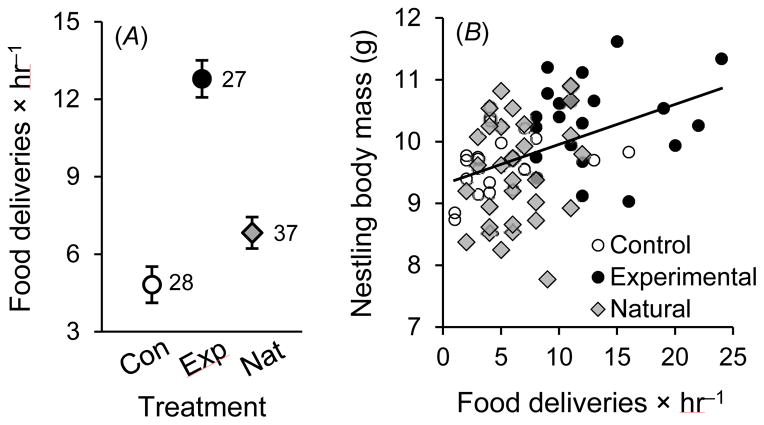
Consistent with the post-natal effects on offspring body mass, maternal food provisioning to nestlings was substantially elevated in response to immune stimulation (table 3A; fig. 3A). Although experimental females subsequently increased food provisioning to offspring, they did not reduce brooding effort, because brooding effort was not affected by the treatment (F2, 87.1 = 0.86, P = .428), even though provisioning and brooding were slightly negatively correlated among females (table 3A). Both maternal provisioning rate and, to a lesser extent, brooding effort positively affected the asymptotic body mass of nestlings after controlling for their structural size (table 3B, fig. 3B).
Table 3.
Maternal food provisioning and its effects on offspring.
| Estimate ± SE | F | df | P | |
|---|---|---|---|---|
| (A) Effects on maternal provisioning. | ||||
| Treatment | 34.24 | 2, 86 | < .001 | |
| Experimentala | 1.226 ± .193 | |||
| Controla | −.413 ± .190 | |||
| Male provisioning rate | −.175 ± .080 | 4.81 | 1, 86 | .031 |
| Time spent brooding | −.159 ± .081 | 3.89 | 1, 86 | .052 |
| Time of observation | .026 ± .080 | .10 | 1, 86 | .749 |
| Intercept | −.234 ± .125 | |||
| (B) Effects of parental care on offspring body mass. | ||||
| Female provisioning rate | .246 ± .070 | 12.50 | 1, 70.2 | < .001 |
| Female brooding time | .138 ± .067 | 4.22 | 1, 69.9 | .044 |
| Male provisioning rate | −.110 ± .068 | 2.60 | 1, 74.8 | .111 |
| Nestling tarsus length | .461 ± .041 | 125.70 | 1, 305 | < .001 |
| Intercept | −.080 ± .376 | |||
relative to natural (unmanipulated) females
Figure 3.
(A) Maternal food provisioning effort (food deliveries × hr−1) in relation to maternal treatment (least-squares means ± SE). (B) Nestling body mass on day 11 post-hatching in relation to maternal provisioning on day 4 or 5. In both panels, open circles represent control (Con) females, black circles represent experimental (Exp) females, and gray diamonds represent unmanipulated, natural (Nat) females (as in fig. 1).
Allocation to Eggs
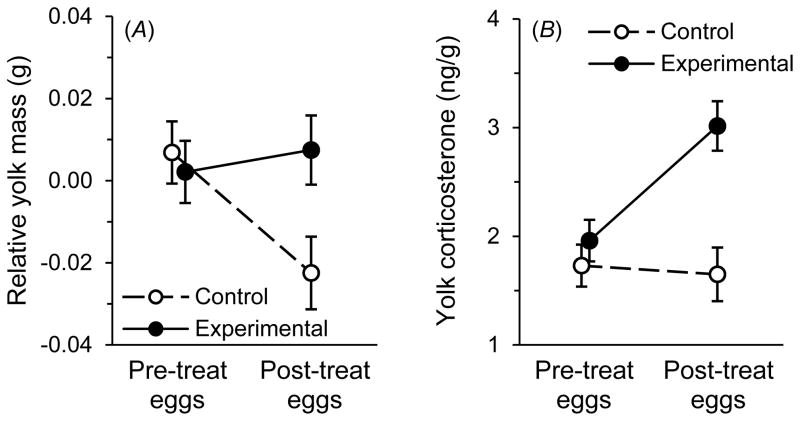
Egg mass was not affected by treatment with LPS (table 4A), but pre-natal allocation of egg yolk was affected by immune stimulation with LPS (table 4B, fig. 4A). Allocation of yolk did not differ between control and experimental females prior to the treatment, but their post-treatment eggs contained differing amounts of yolk, as revealed by an interaction between treatment and time in their effect on yolk mass (table 4B, fig. 4A). Follow-up tests revealed that immunostimulated females produced significantly more yolk per unit egg mass than control females post-treatment (F1, 55.1 = 10.38, P = .002; fig. 4A); this difference was manifested primarily by a reduction in yolk mass for control females from pre- to post-treatment clutches (F1, 27.4 = 16.13, P < .001), as the amount of yolk allocated by experimental females did not change between pre- and post-treatment clutches (F1, 25 = 0.61, P = .443; fig. 4A).
Table 4.
Components of pre-natal allocation.
| Estimate ± SE | F | df | P | |
|---|---|---|---|---|
| (A) Effects on egg mass. | ||||
| Treatment | .51 | 1, 36.5 | .480 | |
| Experimentala | −.127 ± .323 | |||
| Time | .76 | 1, 21.6 | .393 | |
| Pre-injectionb | −.162 ± .114 | |||
| Treatment × time | .176 ± .170 | 1.07 | 1, 21.8 | .312 |
| Relative egg-laying order | .860 ± .070 | 154.81 | 1, 287 | < .001 |
| Maternal body mass | .085 ± .103 | .68 | 1, 52.4 | .412 |
| Intercept | −.532 ± .227 | |||
| (B) Effects on egg-yolk massc. | ||||
| Treatment | 3.47 | 1, 31.9 | .072 | |
| Experimentala | 1.037 ± .322 | |||
| Time | 5.74 | 1, 26.3 | .024 | |
| Pre-injectionb | −.185 ± .238 | |||
| Treatment × time | 1.201 ± .347 | 11.97 | 1, 26.3 | .002 |
| Relative egg-laying order | .209 ± .119 | 3.09 | 1, 285 | .080 |
| Maternal body mass | .068 ± .111 | .37 | 1, 44.4 | .547 |
| Intercept | .131 ± .299 | |||
| (C) Effects on yolk corticosterone. | ||||
| Treatment | 7.95 | 1, 34.8 | .008 | |
| Experimentala | 1.174 ± .324 | |||
| Time | 7.06 | 1, 27.6 | .013 | |
| Pre-injectionb | −.974 ± .239 | |||
| Treatment × time | 1.018 ± .350 | 8.44 | 1, 27.7 | .007 |
| Relative egg-laying order | .171 ± .123 | 1.94 | 1, 259 | .165 |
| Maternal body mass | .065 ± .108 | .36 | 1, 44.6 | .551 |
| Intercept | .823 ± .229 | |||
| (D) Effects on yolk testosterone. | ||||
| Treatment | 2.03 | 1, 36 | .163 | |
| Experimentala | .410 ± .309 | |||
| Time | 2.69 | 1, 22 | .115 | |
| Pre-injectionb | −.123 ± .104 | |||
| Treatment × time | −.005 ± .154 | .00 | 1, 22 | .973 |
| Relative egg-laying order | .593 ± .121 | 24.16 | 1, 284 | < .001 |
| Maternal body mass | −.041 ± .097 | .18 | 1, 45 | .673 |
| Intercept | −.060 ± .225 | |||
relative to control females,
relative to post-injection eggs,
while controlling for egg mass (i.e., relative egg-yolk mass)
Figure 4.
(A) Change in relative egg-yolk mass (i.e., yolk mass adjusted for egg mass) in eggs produced by control and experimental females. (B) Change in the corticosterone concentration of the egg yolks produced by control and experimental females. Plotted are least-squares means ± SE.
Immunostimulation also caused an increase in the concentration of egg-yolk corticosterone (fig. 4B). Females did not differ in the corticosterone concentration of their yolks prior to receiving the treatment, but there was an interaction between treatment and time in their effect on yolk corticosterone (table 4C, fig. 4B). Follow-up tests revealed that, for post-treatment clutches, experimental females produced egg yolks with higher corticosterone concentrations than those produced by saline-injected controls (F1, 53.7 = 13.18, P < .001; fig. 4B); this difference came about primarily by an increase in egg-yolk corticosterone for experimental females (F1, 26.6 = 16.55, P < .001), as there was no change in control females from pre- to post-treatment clutches (F1, 28.6 = 0.03, P = .864; fig. 4B). In contrast to effects on corticosterone, immune stimulation had no effect on yolk-testosterone concentrations, although testosterone concentrations increased from earlier- to later-laid eggs within clutches (table 4D).
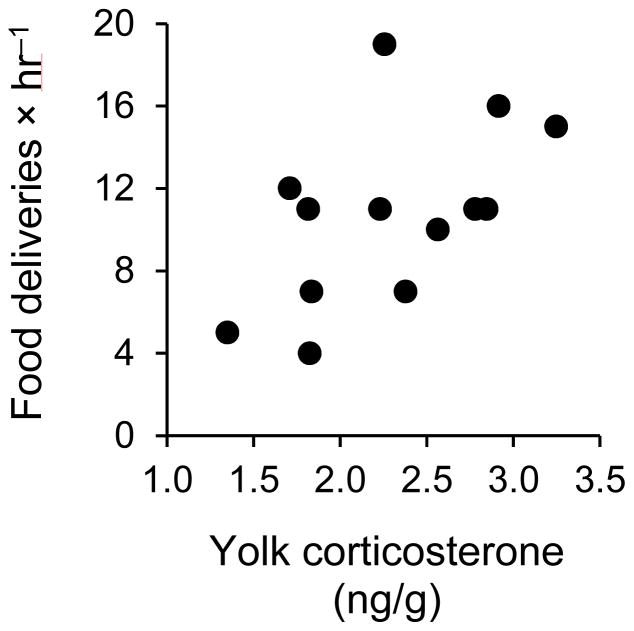
The increase in glucocorticoid production by females in response to LPS treatment was also associated with a change in maternal food provisioning to unmanipulated nestlings (fig. 5). Among the females for which we obtained both yolk-steroid data and food-provisioning rates, the rate at which they provisioned food to nestlings was positively predicted by the concentration of yolk corticosterone in the eggs they produced (estimate ± S.E. = 1.06 ± 0.35, F1, 10 = 8.90, P = .014; fig. 5), but food provisioning was not associated with variation in yolk testosterone (estimate ± S.E. = −0.79 ± 0.44, F1, 10 = 3.15, P = .106).
Figure 5.
Food-provisioning rate by females to unmanipulated offspring (as in fig. 1) in relation to the concentration of yolk corticosterone in the eggs they produced.
Effects on Females
Control and experimental females were equally likely to re-nest following the implementation of our treatment (table 1; F1, 129 = 0.17, P = .680), and the change in clutch size between pre- and post-treatment nests did not differ for control and experimental females (table 5A). However, experimental females were less likely to return to the study area than control and natural females the following year (table 5B). Among the control and experimental females that re-nested, 15 of 48 control females returned (31.3%) and 3 of 42 experimental females returned (7.1%), and 17 of 56 natural females that reared offspring returned (30.4%) the following year.
Table 5.
Effects on clutch size and maternal return rates.
| Estimate ± SE | F | df | P | |
|---|---|---|---|---|
| (A) Effects on clutch size. | ||||
| Treatment | 1.19 | 1, 107 | .278 | |
| Experimentala | −.336 ± .188 | |||
| Time | 38.90 | 1, 103 | < .001 | |
| Pre-injectionb | .820 ± .148 | |||
| Treatment × time | −.364 ± .204 | 3.18 | 1, 102 | .077 |
| Maternal body mass | .185 ± .066 | 7.76 | 1, 178 | .006 |
| Intercept | −.543 ± .252 | |||
| (B) Effects on female return rate. | ||||
| Treatment | 3.59 | 2, 130 | .030 | |
| Controlc | .107 ± .459 | |||
| Experimentalc | −1.687 ± .683 | |||
| Contrasts: | ||||
| Exp vs. Con | 6.63 | 1, 130 | .011 | |
| Exp vs. Nat | 6.10 | 1, 104.4 | .015 | |
| Con vs. Nat | .05 | 1, 130 | .816 | |
| Number of young fledged | .241 ± .220 | 1.20 | 1, 130 | .276 |
| Intercept | −.859 ± .349 | |||
relative to control females,
relative to post-injection clutches,
relative to natural (unmanipulated) females
Discussion
Our experiments revealed effects of immunostimulation on both pre- and post-natal investment as predicted by the terminal-investment hypothesis, and that these effects are associated with an increase in corticosterone production as evidenced by variation in egg-yolk corticosterone concentrations. Pre-natal allocation in the egg increased the asymptotic body mass and immune responsiveness of offspring (fig. 2A,B); the increase in post-natal provisioning also enhanced offspring body mass (figs. 2A, 3), but did not directly affect immune responsiveness or hematocrit, each of which is often influenced indirectly by effects of the rearing environment on offspring mass and condition (Sakaluk et al. 2014). The terminal-investment hypothesis predicts that individuals with reduced survival prospects should increase reproductive effort, regardless of any costs that the increased effort might impose on their future reproductive output, because they are unlikely to survive to breed again. Treatment with LPS elicits a strong immune response, but LPS is a non-replicating, non-pathogenic immunogen that does not directly harm the animals, thus allowing for analysis of costs that are not created by negative effects of a pathogen on host survival. In the current study, the increased reproductive effort by experimental females appears to have imposed a long-term cost, as experimental females were much less likely to return to breed on the study site the following year (but see Bowers et al. 2012). We cannot rule out, however, the possibility that differences in return rates among treatments reflect changes in site fidelity and dispersal generated by the immune activation and glucocorticoid production.
Variation in corticosterone levels has long been known to be influenced by environmental conditions, and rapid and dramatic increases in corticosterone can sometimes promote the reallocation of energy toward self-maintenance and survival rather than reproduction (Wingfield 2003; Romero et al. 2009; Angelier et al. 2009; Lothery et al. 2014). However, for short-lived animals with few reproductive opportunities during their lifetime, small increases in glucocorticoid production have been predicted to facilitate, not inhibit, reproductive effort (Sapolsky et al. 2000). Indeed, there is an increasing appreciation that variation in corticosterone levels below those that induce radical life-history transitions promote foraging, parental care, and reproductive success in breeding birds as this metabolic steroid mobilizes energetic reserves, thus helping parents meet the energetic demands associated with rearing dependent young (Romero 2002; Landys et al. 2006; Angelier et al. 2007; Doody et al. 2008; Hau et al. 2010; Ouyang et al. 2011, 2013; Crossin et al. 2012; Love et al. 2014; Crino et al. 2014). Moreover, variation in egg mass has recently been found to be positively associated with measures of circulating corticosterone prior to the breeding season (Kouwenberg et al. 2013). Both baseline and stress-induced glucocorticoid production appear to have a heritable genetic basis (Hayward et al. 2005; Jenkins et al. 2014), indicating that this trait may evolve through natural selection and provide a potentially widespread means through which immunostimulation can modulate reproductive effort. A critical observation is that the effect of immunostimulation on behavior can be context-dependent. Zebra finches (Taeniopygia guttata) treated with LPS displayed classic “sickness behavior” (lethargy, reduced appetite) when in isolation, without direct interaction with the opposite sex, but did not exhibit such behavior when in groups containing the opposite sex, despite the fact that the physiology underlying the immune response (cytokine and corticosterone production) was the same for individuals in the different social settings (Lopes et al. 2012b, 2013). Such a result is consistent with terminal investment, and indicates that ecological and behavioral inferences about sickness and the trade-off between immune activity and reproduction should be made with caution when studies are conducted on captive animals without the opportunity to breed.
We have treated yolk corticosterone primarily as a reflection of maternal physiology, yet there is also evidence, albeit mixed, that yolk corticosterone can promote post-hatching begging, growth, and size (Love and Williams 2008; Chin et al. 2009; Crino et al. 2011; Strange 2015), and even induce effects on immune responsiveness in offspring (Love and Williams 2008). Such effects are consistent with the pre-natal effects we detected in the cross-fostering experiment (fig. 2A,B). Effects of yolk corticosterone on offspring phenotype appear to be decidedly mixed (Chin et al. 2009; Love et al 2009; Henriksen et al. 2011); however, we do know that developing avian embryos metabolize maternally derived steroids in eggs (von Engelhardt et al. 2009; Paitz et al. 2011; Paitz and Casto 2012; Vassallo et al. 2014), and that, in our free-living study population, experimental elevation of corticosterone in the egg at laying enhances the post-hatching growth and asymptotic body mass of nestlings prior to fledging (Strange 2015), a trait positively associated with their long-term reproductive prospects (Bowers et al. 2014b). It should be noted, however, that the specific mechanism through which in ovo corticosterone acts on offspring is unresolved and is likely to be more complex than traditionally thought (von Engelhardt et al. 2009; Vassallo et al. 2014). It should also be noted that experimental manipulations of yolk steroids can be problematic if they cause embryos to develop under concentrations of steroids other than what the maternal phenotype has been selected to produce. Parent-offspring coadaptation should generally select for an optimization of maternally derived compounds within eggs to “match” the post-natal development of offspring with prevailing conditions (Wagner and Williams 2007; Hinde et al. 2009, 2010; Henriksen et al. 2011; Sheriff and Love 2013). Thus, if the concentration of any given compound (e.g., corticosterone) within an egg is optimized according to the availability of other resources both within the egg and outside the nest with which parents will eventually provision young after hatching, exogenous alterations to the hormonal milieu under which offspring develop may cause the offspring genotype to be expressed in a manner that does not maximize offspring or parental fitness, potentially contributing to such widely mixed results among studies (Wagner and Williams 2007; Williams 2012). In the current study, we did not manipulate yolk corticosterone directly, but control and experimental females differed in the deposition of both nutritional resources and corticosterone in eggs (fig. 4).
Our data suggest that females engage in terminal investment by altering their allocation to both eggs and nestlings. In conjunction with previous work on this population showing a positive effect of in ovo corticosterone elevation on nestling growth (Strange 2015), terminally investing females in our experiments appear to have matched their allocation to eggs with their provisioning effort to offspring post-hatching (fig. 5). The modification of both pre- and post-hatching modes of maternal investment is not surprising, though rarely reported, given that parents are expected to match the post-natal needs of offspring with the environmental conditions in which they are expected to develop (e.g., Wagner and Williams 2007; Tschirren et al. 2009; Jensen et al. 2013; Davis and Guinan 2014; Giordano et al. 2014). For example, Hinde et al. (2009) manipulated the diet of female canaries (Serinus canaria) during egg formation and cross-fostered offspring among nests prior to hatching; nestling begging intensity was affected by the maternal diet treatment, an effect manifested via the egg, and females on enhanced diets displayed increased food-provisioning to foster young after hatching. Thus, mothers fine-tuned the constituents of their eggs to match offspring begging with prevailing conditions.
Enhanced immune responsiveness of offspring following parental immune challenge has now been documented in a number of studies and is consistent with the idea that selection favors modulation of offspring immune function according to the environment they are likely to encounter in the future (Buechler et al. 2002; Sadd et al. 2005; Grindstaff et al. 2006; Sadd and Schmid-Hempel 2007; Love and Williams 2008; Hasselquist and Nilsson 2009; Martyka et al. 2011; Midamegbe et al. 2013; Sternberg et al. 2015). However, whether this constitutes terminal investment or an “anticipatory parental effect” remains unresolved, and its resolution requires a cross-fostering approach to separate pre- from post-natal conditions experienced by offspring. Anticipatory parental effects, or “predictive adaptive responses,” occur when the environment experienced by parents (e.g., risk of parasitism) has epigenetic effects on offspring, potentially matching their phenotype or behavior to the environment offspring are likely to encounter (Saastamoinen et al. 2010; Coslovsky and Richner 2011; Crews et al. 2012; Uller et al. 2013; Burgess and Marshall 2014; Burton and Metcalfe 2014; Merrill and Grindstaff 2014a,b). Unlike terminal investment, this hypothesis does not predict an increase in reproductive effort as a response to reduced residual reproductive value. Thus, our data on maternal provisioning behavior may help resolve this issue. The hypothesis of an anticipatory parental effect does not predict changes in parental food provisioning to nestlings; if anything, this hypothesis predicts that parents should invest less effort into progeny produced in an environment that turns out not to be conducive to maximizing parental fitness (e.g., an environment rich with parasites). Thus, although enhanced immune responsiveness of offspring is consistent with an anticipatory parental effect, elevated food provisioning to nestlings by parents with reduced prospects for survival through autumn and spring migration and to the next breeding season supports the terminal-investment hypothesis.
In conclusion, maternal immune stimulation can induce pronounced transgenerational effects on offspring that are manifested through both pre- and post-natal parental effects. Moreover, the increased investment by immunostimulated mothers is mediated, at least in part, by an increase in glucocorticoid production, providing a mechanism of terminal investment and challenging the widely held view that corticosterone production negatively affects parental care and offspring development.
Acknowledgments
We thank the 2012–2014 Wren Crews for field assistance and the ParkLands Foundation (Merwin Preserve), the Illinois Great Rivers Conference of the United Methodist Church, and the Sears and Butler families for the use of their properties. We also thank Ryan Paitz for thoughtful discussion, and two anonymous reviewers for helpful comments on the manuscript. All research activities complied with current laws of the United States of America and were in accordance with the Illinois State University Institutional Animal Care and Use Committee (Protocol Nos. 05-2010, 04-2013), United States Geological Survey banding permit 09211 and U.S. Fish and Wildlife Service collecting permit MB692148-0. Financial support was provided by NSF grants IBN-0316580, IOS-0718140, and IOS-1118160; NIH grant R15HD076308-01; the School of Biological Sciences, Illinois State University; and student-research grants from the Animal Behavior Society, the American Museum of Natural History’s Frank M. Chapman Memorial Fund, and the Beta Lambda Chapter of the Phi Sigma Biological Honor Society at Illinois State University. Data underlying the paper are deposited in the Dryad Digital Repository (Bowers et al. 2015a).
Contributor Information
Rachel M. Bowden, Email: rmbowde@ilstu.edu.
Scott K. Sakaluk, Email: sksakal@ilstu.edu.
Charles F. Thompson, Email: wrens@ilstu.edu.
Literature Cited
- Adamo SA, Kovalko I, Easy RH, Stolz D. A viral aphrodisiac in the cricket Gryllus texensis. Journal of Experimental Biology. 2014;217:1970–1976. doi: 10.1242/jeb.103408. [DOI] [PubMed] [Google Scholar]
- Adelman JS, Bentley GE, Wingfield JC, Martin LB, Hau M. Population differences in fever and sickness behaviors in a wild passerine: a role for cytokines. Journal of Experimental Biology. 2010;213:4099–4109. doi: 10.1242/jeb.049528. [DOI] [PubMed] [Google Scholar]
- Almasi B, Rettenbacher S, Müller C, Brill S, Wagner H, Jenni L. Maternal corticosterone is transferred into the egg yolk. General and Comparative Endocrinology. 2012;178:139–144. doi: 10.1016/j.ygcen.2012.04.032. [DOI] [PubMed] [Google Scholar]
- Angelier F, Bost C-A, Giraudeau M, Bouteloup G, Dano S, Chastel O. Corticosterone and foraging behavior in a diving seabird: the Adélie penguin, Pygoscelis adeliae. General and Comparative Endocrinology. 2008;156:134–144. doi: 10.1016/j.ygcen.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Angelier F, Clément-Chastel C, Welcker J, Gabrielsen GW, Chastel O. How does corticosterone affect parental behavior and reproductive success? A study of prolactin in black-legged kittiwakes. Functional Ecology. 2009;23:784–793. [Google Scholar]
- Angelier F, Shaffer SA, Weimerskirch H, Trouvé C, Chastel O. Corticosterone and foraging behavior in a pelagic seabird. Physiological and Biochemical Zoology. 2007;80:283–292. doi: 10.1086/512585. [DOI] [PubMed] [Google Scholar]
- Ardia DR. Tree swallows trade off immune function and reproductive effort differently across their range. Ecology. 2005;86:2040–2046. [Google Scholar]
- Barnett CA, Clairardin SG, Thompson CF, Sakaluk SK. Turning a deaf ear: a test of the manipulating androgens hypothesis in house wrens. Animal Behaviour. 2011;81:113–120. [Google Scholar]
- Barnett CA, Thompson CF, Sakaluk SK. Aggressiveness, boldness and parental food provisioning in male house wrens (Troglodytes aedon) Ethology. 2012;118:1–10. [Google Scholar]
- Besedovsky H, del Rey A, Sorki E, Dinarello CA. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986;233:652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- Bonneaud C, Mazuc J, Chastel O, Westerdahl H, Sorci G. Terminal investment induced by immune challenge and fitness traits associated with major histocompatibility complex in the house sparrow. Evolution. 2004;58:2823–2830. doi: 10.1111/j.0014-3820.2004.tb01633.x. [DOI] [PubMed] [Google Scholar]
- Bonneaud C, Mazuc J, Gonzalez G, Haussy C, Chastel O, Faivre B, Sorci G. Assessing the cost of mounting an immune response. American Naturalist. 2003;161:367–379. doi: 10.1086/346134. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Bowden RM, Sakaluk SK, Thompson CF. Data from: Immune activation generates corticosterone-mediated terminal reproductive investment in a wild bird. American Naturalist, Dryad Digital Repository. 2015a doi: 10.1086/681017. http://dx.doi.org/10.5061/dryad.6km3j. [DOI] [PMC free article] [PubMed]
- Bowers EK, Hodges CJ, Forsman AM, Vogel LA, Masters BS, Johnson BGP, Johnson LS, Thompson CF, Sakaluk SK. Neonatal body condition, immune responsiveness, and hematocrit predict longevity in a wild bird population. Ecology. 2014b;95:3027–3034. doi: 10.1890/14-0418.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Nietz D, Thompson CF, Sakaluk SK. Parental provisioning in house wrens: effects of varying brood size and consequences for offspring. Behavioral Ecology. 2014a;25:1485–1493. [Google Scholar]
- Bowers EK, Sakaluk SK, Thompson CF. Sibling cooperation influences the age of nest-leaving in an altricial bird. American Naturalist. 2013;181:775–786. doi: 10.1086/670244. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Smith RA, Hodges CJ, Zimmerman LM, Thompson CF, Sakaluk SK. Sex-biased terminal investment in offspring induced by maternal immune challenge in the house wren (Troglodytes aedon) Proceedings of the Royal Society B: Biological Sciences. 2012;279:2891–2898. doi: 10.1098/rspb.2012.0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Thompson CF, Sakaluk SK. Persistent sex-by-environment effects on offspring fitness and sex-ratio adjustment in a wild bird population. Journal of Animal Ecology. 2015b doi: 10.1111/1365-2656.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechler K, Fitze PS, Gottstein B, Jacot A, Richner H. Parasite-induced maternal response in a natural bird population. Journal of Animal Ecology. 2002;71:247–252. [Google Scholar]
- Burgess SC, Marshall DJ. Adaptive parental effects: the importance of estimating environmental predictability and offspring fitness appropriately. Oikos. 2014;123:769–776. [Google Scholar]
- Burton T, Metcalfe NB. Can environmental conditions experienced in early life influence future generations? Proceedings of the Royal Society B: Biological Sciences. 2014;281:20140311. doi: 10.1098/rspb.2014.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casto JM, Nolan V, Jr, Ketterson ED. Steroid hormones and immune function: experimental studies in wild and captive dark-eyed juncos (Junco hyemalis) American Naturalist. 2001;157:408–420. doi: 10.1086/319318. [DOI] [PubMed] [Google Scholar]
- Chin EH, Love OP, Verspoor JJ, Williams TD, Rowley K, Burness G. Juveniles exposed to embryonic corticosterone have enhanced flight performance. Proceedings of the Royal Society B: Biological Sciences. 2009;276:499–505. doi: 10.1098/rspb.2008.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock TH. Reproductive effort and terminal investment in iteroparous animals. American Naturalist. 1984;123:212–229. [Google Scholar]
- Coslovsky M, Richner H. Predation risk affects offspring growth via maternal effects. Functional Ecology. 2012;25:878–888. [Google Scholar]
- Crews D, Gillette R, Scarpino SV, Manikkam M, Savenkova MI, Skinner MK. Epigenetic transgenerational inheritance of altered stress responses. Proceedings of the National Academy of Sciences of the USA. 2012;109:9143–9148. doi: 10.1073/pnas.1118514109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crino OL, Prather CT, Driscoll SC, Good JM, Breuner CW. Developmental stress increases reproductive success in male zebra finches. Proceedings of the Royal Society B: Biological Sciences. 2014;281:20141266. doi: 10.1098/rspb.2014.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crino OL, Van Oorschot BK, Johnson EE, Malisch JL, Breuner CW. Proximity to a high traffic road: glucocorticoid and life history consequences for nestling white-crowned sparrows. General and Comparative Endocrinology. 2011;173:323–332. doi: 10.1016/j.ygcen.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Crossin GT, Trathan PN, Phillips RA, Gorman KB, Dawson A, Sakamoto KQ, Williams TD. Corticosterone predicts foraging behavior and parental care in macaroni penguins. American Naturalist. 2012;180:E31–E41. doi: 10.1086/666001. [DOI] [PubMed] [Google Scholar]
- Davis JE, Guinan JA. Parental behavior correlates to baseline corticosterone of mates and offspring in nesting eastern bluebirds (Sialia sialis) General and Comparative Endocrinology. 2014;201:1–7. doi: 10.1016/j.ygcen.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Demas GE, Adamo SA, French SS. Neuroendocrine-immune crosstalk in vertebrates and invertebrates: implications for host defence. Functional Ecology. 2011;25:29–39. [Google Scholar]
- DeMory ML, Thompson CF, Sakaluk SK. Male quality influences male provisioning in house wrens independent of attractiveness. Behavioral Ecology. 2010;21:1156–1164. [Google Scholar]
- Doody LM, Wilhelm SI, McKay DW, Walsh CJ, Storey AE. The effects of variable foraging conditions on common murre (Uria aalge) corticosterone concentrations and parental provisioning. Hormones and Behavior. 2008;53:140–148. doi: 10.1016/j.yhbeh.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Forsman AM, Sakaluk SK, Thompson CF, Vogel LA. Cutaneous immune activity, but not innate immune responsiveness, covaries with mass and environment in nestling house wrens (Troglodytes aedon) Physiological and Biochemical Zoology. 2010;83:512–518. doi: 10.1086/649894. [DOI] [PubMed] [Google Scholar]
- Giordano M, Groothuis TGG, Tschirren B. Interactions between prenatal maternal effects and posthatching conditions in a wild bird population. Behavioral Ecology. 2014;25:1459–1466. [Google Scholar]
- Grindstaff JL, Hasselquist D, Nilsson J-Å, Sandell M, Smith HG, Stjernman M. Transgenerational priming of immunity: maternal exposure to a bacterial antigen enhances offspring humoral immunity. Proceedings of the Royal Society B: Biological Sciences. 2006;273:2551–2557. doi: 10.1098/rspb.2006.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen SA. Cost of an immune challenge and terminal investment in a long-lived bird. Ecology. 2006;87:2440–2446. doi: 10.1890/0012-9658(2006)87[2440:coaica]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Hasselquist D, Nilsson J-Å. Maternal transfer of antibodies in vertebrates: trans-generational effects on offspring immunity. Philosophical Transactions of the Royal Society B. 2009;364:51–60. doi: 10.1098/rstb.2008.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hau M, Ricklefs RE, Wikelski M, Lee KA, Brawn JD. Corticosterone, testosterone and life-history strategies of birds. Proceedings of the Royal Society B: Biological Sciences. 2010;277:3203–3212. doi: 10.1098/rspb.2010.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward LS, Satterlee DG, Wingfield JC. Japanese quail selected for high plasma corticosterone response deposit high levels of corticosterone in their eggs. Physiological and Biochemical Zoology. 2005;78:1026–1031. doi: 10.1086/432854. [DOI] [PubMed] [Google Scholar]
- Henriksen R, Rettenbacher S, Groothuis TGG. Prenatal stress in birds: pathways, effects, function and perspectives. Neuroscience and Biobehavioral Reviews. 2011;35:1484–1501. doi: 10.1016/j.neubiorev.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Hinde CA, Buchanan KL, Kilner RM. Prenatal environmental effects match offspring begging to parental provisioning. Proceedings of the Royal Society B: Biological Sciences. 2009;276:2787–2794. doi: 10.1098/rspb.2009.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinde CA, Johnstone RA, Kilner RM. Parent-offspring conflict and coadaptation. Science. 2010;327:1373–1376. doi: 10.1126/science.1186056. [DOI] [PubMed] [Google Scholar]
- Ilmonen P, Taarna T, Hasselquist D. Experimentally activated immune defence in female pied flycatchers results in reduced breeding success. Proceedings of the Royal Society of London B. 2000;267:665–670. doi: 10.1098/rspb.2000.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins BR, Vitousek MN, Hubbard JK, Safran RJ. An experimental analysis of the heritability of variation in glucocorticoid concentrations in a wild avian population. Proceedings of the Royal Society B: Biological Sciences. 2014;281:20141302. doi: 10.1098/rspb.2014.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen N, Allen RM, Marshall DJ. Adaptive maternal and paternal effects: gamete plasticity in response to parental stress. Functional Ecology. 2013;28:724–733. [Google Scholar]
- Johnson LS. House wren (Troglodytes aedon) In: Poole A, editor. The birds of North America. 2. 380. Cornell Lab of Ornithology and American Ornithologists’ Union; Ithaca, NY: 2015. [Google Scholar]
- Kitaysky AS, Wingfield JC, Piatt JF. Corticosterone facilitates begging and affects resource allocation in the black-legged kittiwake. Behavioral Ecology. 2001;12:619–625. [Google Scholar]
- Kivleniece I, Krams I, Daukšte J, Krama T, Rantala MJ. Sexual attractiveness of immune-challenged male mealworm beetles suggests terminal investment in reproduction. Animal Behaviour. 2010;80:1015–1021. [Google Scholar]
- Klasing KC. Avian macrophages: regulators of local and systemic immune responses. Poultry Science. 1998;77:983–989. doi: 10.1093/ps/77.7.983. [DOI] [PubMed] [Google Scholar]
- Knowles SCL, Nakagawa S, Sheldon BC. Elevated reproductive effort increases blood parasitaemia and decreases immune function in birds: a meta-regression approach. Functional Ecology. 2009;23:405–415. [Google Scholar]
- Kouwenberg A-L, Hipfner JM, McKay DW, Storey AE. Corticosterone and stable isotopes in feathers predict egg size in Atlantic puffins Fratercula arctica. Ibis. 2013;155:413–418. [Google Scholar]
- Lambrechts MM, Adriaensen F, Ardia DR, Artemyev AV, Atiénzar F, Bańbura J, et al. The design of artificial nestboxes for the study of secondary hole-nesting birds: a review of methodological inconsistencies and potential biases. Acta Ornithologica. 2010;45:1–26. [Google Scholar]
- Landys MM, Ramenofsky M, Wingfield JC. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. General and Comparative Endocrinology. 2006;148:132–149. doi: 10.1016/j.ygcen.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Loiseau C, Sorci G, Dano S, Chastel O. Effects of experimental increase of corticosterone levels on begging behaviour, immunity and parental provisioning rate in house sparrows. General and Comparative Endocrinology. 2008;155:101–108. doi: 10.1016/j.ygcen.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Lopes PC, Adelman J, Wingfield JC, Bentley GE. Social context modulates sickness behavior. Behavioral Ecology and Sociobiology. 2012b;66:1421–1428. [Google Scholar]
- Lopes PC, Chan H, Demathieu S, González-Gómez PL, Wingfield JC, Bentley GE. The impact of exposure to a novel female on symptoms of infection and on the reproductive axis. Neuroimmunomodulation. 2013;20:348–360. doi: 10.1159/000353779. [DOI] [PubMed] [Google Scholar]
- Lopes PC, Wingfield JC, Bentley GE. Lipopolysaccharide injection induces rapid decrease in hypothalamic GnRH mRNA and peptide, but does not affect GnIH in zebra finches. Hormones and Behavior. 2012a;62:173–179. doi: 10.1016/j.yhbeh.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Lothery CJ, Thompson CF, Lawler ML, Sakaluk SK. Food supplementation fails to reveal a trade-off between incubation and self-maintenance in female house wrens. PLoS One. 2014;9:e106260. doi: 10.1371/journal.pone.0106260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love OP, Chin EH, Wynne-Edwards KE, Williams TD. Stress hormones: a link between maternal condition and sex-biased reproductive investment. American Naturalist. 2005;166:751–766. doi: 10.1086/497440. [DOI] [PubMed] [Google Scholar]
- Love OP, Gilchrist HG, Bêty J, Wynne-Edwards KE, Berzins L, Williams TD. Using life-histories to predict and interpret variability in yolk hormones. General and Comparative Endocrinology. 2009;163:169–174. doi: 10.1016/j.ygcen.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Love OP, Madliger CL, Bourgeon S, Semeniuk CAD, Williams TD. Evidence for baseline glucocorticoids as mediators of reproductive investment in a wild bird. General and Comparative Endocrinology. 2014;199:65–69. doi: 10.1016/j.ygcen.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Love OP, Williams TD. The adaptive value of stress-induced phenotypes: effects of maternally derived corticosterone on sex-biased investment, cost of reproduction, and maternal fitness. American Naturalist. 2008;172:E135–E149. doi: 10.1086/590959. [DOI] [PubMed] [Google Scholar]
- Love OP, Wynne-Edwards KE, Bond L, Williams TD. Determinants of within- and among-clutch variation in yolk corticosterone in the European starling. Hormones and Behavior. 2008;53:104–111. doi: 10.1016/j.yhbeh.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Martin LB, Han P, Lewittes J, Kuhlman JR, Klasing KC, Wikelski M. Phytohemagglutinin-induced skin swelling in birds: histological support for a classic immunoecological technique. Functional Ecology. 2006;20:290–299. [Google Scholar]
- Martin LB, Weil ZM, Nelson RJ. Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:321–339. doi: 10.1098/rstb.2007.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyka R, Rutkowska J, Cichoń M. Sex-specific effects of maternal immunization on yolk antibody transfer and offspring performance in zebra finches. Biology Letters. 2011;7:50–53. doi: 10.1098/rsbl.2010.0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick MI. Behaviorally induced maternal stress in fish influences progeny quality by a hormonal mechanism. Ecology. 1998;79:1873–1883. [Google Scholar]
- McEwen BS, Biron CA, Brunson KW, Bulloch K, Chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH, Spencer RL, Weiss JM. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Research Reviews. 1997;23:79–133. doi: 10.1016/s0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- Merrill L, Grindstaff JL. Maternal antibody transfer can lead to suppression of humoral immunity in developing zebra finches (Taeniopygia guttata) Physioloical and Biochemical Zoology. 2014a doi: 10.1086/677218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill L, Grindstaff JL. Pre and post-natal antigen exposure can program the stress axis of adult zebra finches: evidence for environment matching. Brain, Behavior, and Immunity. 2014b doi: 10.1016/j.bbi.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midamegbe A, Grégoire A, Staszewski V, Perret P, Lambrechts MM, Boulinier T, Doutrelant C. Female blue tits with brighter yellow chests transfer more carotenoids to their eggs after an immune challenge. Oecologia. 2013;173:387–397. doi: 10.1007/s00442-013-2617-8. [DOI] [PubMed] [Google Scholar]
- Okuliarová M, Šárniková B, Rettenbacher S, Škrobánek P, Zeman M. Yolk testosterone and corticosterone in hierarchical follicles and laid eggs of Japanese quail exposed to long-term restraint stress. General and Comparative Endocrinology. 2010;165:91–96. doi: 10.1016/j.ygcen.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Ots I, Murumägi A, Hõrak P. Haematological health state indices of reproducing great tits: methodology and sources of natural variation. Functional Ecology. 1998;12:700–707. [Google Scholar]
- Ouyang JQ, Muturi M, Quetting M, Hau M. Small increases in corticosterone before the breeding season increase parental investment but not fitness in a wild passerine bird. Hormones and Behavior. 2013;63:776–781. doi: 10.1016/j.yhbeh.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Ouyang JQ, Sharp PJ, Dawson A, Quetting M, Hau M. Hormone levels predict individual differences in reproductive success in a passerine bird. Proceedings of the Royal Society B: Biological Sciences. 2011;278:2537–2545. doi: 10.1098/rspb.2010.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paitz RT, Bowden RM, Casto JM. Embryonic modulation of maternal steroids in European starlings. Proceedings of the Royal Society B: Biological Sciences. 2011;278:99–106. doi: 10.1098/rspb.2010.0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paitz RT, Casto JM. The decline in yolk progesterone concentrations during incubation is dependent on embryonic development in the European starling. General and Comparative Endocrinology. 2012;176:415–419. doi: 10.1016/j.ygcen.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Quillfeldt P, Masello JF, Strange IF, Buchanan KL. Begging and provisioning of thin-billed prions, Pachyptila belcheri, are related to testosterone and corticosterone. Animal Behaviour. 2006;71:1359–1369. [Google Scholar]
- Richner H, Oppliger A, Christe P. Effect of an ectoparasite on reproduction in great tits. Journal of Animal Ecology. 1993;62:703–710. [Google Scholar]
- Ricklefs RE, Wikelski M. The physiology/life-history nexus. Trends in Ecology and Evolution. 2002;17:462–468. [Google Scholar]
- Romero LM. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. General and Comparative Endocrinology. 2002;128:1–24. doi: 10.1016/s0016-6480(02)00064-3. [DOI] [PubMed] [Google Scholar]
- Romero LM, Dickens MJ, Cyr NE. The reactive scope model – a new model integrating homeostasis, allostasis, and stress. Hormones and Behavior. 2009;55:375–389. doi: 10.1016/j.yhbeh.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Saastamoinen M, van der Sterren D, Vastenhout N, Zwaan BJ. Predictive adaptive responses: condition-dependent impact of adult nutrition and flight in the tropical butterfly Bicyclus anynana. American Naturalist. 2010;176:686–698. doi: 10.1086/657038. [DOI] [PubMed] [Google Scholar]
- Sadd B, Holman L, Armitage H, Lock F, Marland R, Siva-Jothy MT. Modulation of sexual signaling by immune challenged male mealworm beetles (Tenebrio molitor, L.): evidence for terminal investment and dishonesty. Journal of Evolutionary Biology. 2006;19:321–325. doi: 10.1111/j.1420-9101.2005.01062.x. [DOI] [PubMed] [Google Scholar]
- Sadd BM, Kleinlogel Y, Schmid-Hempel R, Schmid-Hempel P. Trans-generational immune priming in a social insect. Biology Letters. 2005;1:386–388. doi: 10.1098/rsbl.2005.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadd BM, Schmid-Hempel P. Facultative but persistent trans-generational immunity via the mother’s eggs in bumblebees. Current Biology. 2007;17:R1046–R1047. doi: 10.1016/j.cub.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Sakaluk SK, Wilson AJ, Bowers EK, Johnson LS, Masters BS, Johnson BGP, Vogel LA, Forsman AM, Thompson CF. Genetic and environmental variation in condition, cutaneous immunity, and haematocrit in house wrens. BMC Evolutionary Biology. 2014;14:242. doi: 10.1186/s12862-014-0242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238:522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schielzeth H. Simple means to improve the interpretability of regression coefficients. Methods in Ecology and Evolution. 2010;1:103–113. [Google Scholar]
- Schwanz LE. Persistent effects of maternal parasitic infection on offspring fitness: implications for adaptive reproductive strategies when parasitized. Functional Ecology. 2008;22:691–698. [Google Scholar]
- Sheldon BC, Verhulst S. Ecological immunology: costly parasite defenses and trade-offs in evolutionary ecology. Trends in Ecology and Evolution. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Love OP. Determining the adaptive potential of maternal stress. Ecology Letters. 2013;16:271–280. doi: 10.1111/ele.12042. [DOI] [PubMed] [Google Scholar]
- Sinervo B, DeNardo DF. Costs of reproduction in the wild: path analysis of natural selection and experimental tests of causation. Evolution. 1996;50:1299–1313. doi: 10.1111/j.1558-5646.1996.tb02370.x. [DOI] [PubMed] [Google Scholar]
- Smiseth PT, Scott MP, Andrews C. Hormonal regulation of offspring begging and mediation of parent-offspring conflict. Animal Behaviour. 2011;81:507–517. [Google Scholar]
- Sternberg ED, de Roode JC, Hunter MD. Trans-generational parasite protection associated with paternal diet. Journal of Animal Ecology. 2015;84:310–321. doi: 10.1111/1365-2656.12289. [DOI] [PubMed] [Google Scholar]
- Strange MS. Master’s thesis. Illinois State University; Normal, IL: 2015. Corticosterone in nestling house wrens: effects on fitness-related traits and the development of the stress response. [Google Scholar]
- Tschirren B, Sendecka J, Groothuis TGG, Gustafsson L, Doligez B. Heritable variation in maternal yolk hormone transfer in a wild bird population. American Naturalist. 2009;174:557–564. doi: 10.1086/605379. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiological Reviews. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- Uller T, Nakagawa S, English S. Weak evidence for anticipatory parental effects in plants and animals. Journal of Evolutionary Biology. 2013;26:2161–2170. doi: 10.1111/jeb.12212. [DOI] [PubMed] [Google Scholar]
- Vassallo BG, Paitz RT, Fasanello VJ, Haussmann MF. Glucocorticoid metabolism in the in ovo environment modulates exposure to maternal corticosterone in Japanese quail embryos (Coturnix japonica) Biology Letters. 2014;10:20140502. doi: 10.1098/rsbl.2014.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velando A, Beamonte-Barrientos R, Torres T. Enhanced male coloration after immune challenge increases reproductive potential. Journal of Evolutionary Biology. 2014;27:1582–1589. doi: 10.1111/jeb.12416. [DOI] [PubMed] [Google Scholar]
- Velando A, Drummond H, Torres R. Senescent birds redouble reproductive effort when ill: confirmation of the terminal investment hypothesis. Proceedings of the Royal Society B: Biological Sciences. 2006;273:1443–1448. doi: 10.1098/rspb.2006.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkler M, Svobodová J, Gabrielová B, Bainová H, Bryjová A. Cytokine expression in phytohaemagglutinin-induced skin inflammation in a galliform bird. Journal of Avian Biology. 2014;45:43–50. [Google Scholar]
- von Engelhardt N, Henriksen R, Groothuis TGG. Steroids in chicken egg yolk: metabolism and uptake during early embryonic development. General and Comparative Endocrinology. 2009;163:175–183. doi: 10.1016/j.ygcen.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Wagner EC, Williams TD. Experimental (antiestrogen-mediated) reduction in egg size negatively affects offspring growth and survival. Physiological and Biochemical Zoology. 2007;80:293–305. doi: 10.1086/512586. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Martin LB, Workman JL, Nelson RJ. Immune challenge retards seasonal reproductive regression in rodents: evidence for terminal investment. Biology Letters. 2006;2:393–396. doi: 10.1098/rsbl.2006.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC. Adaptation and natural selection: a critique of some current evolutionary thought. Princeton University Press; Princeton: 1966. [Google Scholar]
- Williams TD. Physiological adaptations for breeding in birds. Princeton University Press; Princeton: 2012. [Google Scholar]
- Williams TD, Christians JK, Aiken JJ, Evanson M. Enhanced immune function does not suppress reproductive output. Proceedings of the Royal Society of London B. 1999;266:753–757. [Google Scholar]
- Wingfield JC. Control of behavioural strategies for capricious environments. Animal Behaviour. 2003;66:807–816. [Google Scholar]
- Zimmerman LM, Bowden RM, Vogel LA. A vertebrate cytokine primer for eco-immunologists. Functional Ecology. 2014;28:1061–1073. [Google Scholar]