Abstract
The TARP syndrome (Talipes equinovarus, Atrial septal defect, Robin sequence, and Persistent left superior vena cava) is an X-linked disorder that was determined to be caused by mutations in RBM10 in two families, and confirmed in a subsequent case report. The first two original families were quite similar in phenotype, with uniform early lethality although a confirmatory case report showed survival into childhood. Here we report on five affecteds from three newly recognized families, including patients with atypical manifestations. None of the five patients had talipes and others also lacked cardinal TARP features of Robin sequence and atrial septal defect. All three families demonstrated de novo mutations, and one of the families had two recurrences, with demonstrable maternal mosaicism.
Keywords: TARP, RBM10
INTRODUCTION
Gorlin et al [1970] described a large kindred with a distinctive syndrome with early lethality that affected only boys comprising Talipes equinovarus, Atrial septal defect (ASD), Robin sequence and Persistence of the left superior vena cava (SVC). This was originally termed ‘Robin's syndrome’ [Gorlin et al., 1970] but the acronym TARP was later used [Kurpinski et al., 2003]. A second family with TARP syndrome was identified at Mt. Sinai in Toronto and massively parallel sequencing was used to identify loss of function alleles in RBM10 in both families [Johnston et al., 2010b]. Subsequently, an additional child with an RBM10 mutation was identified who survived beyond 3 ½ years of age [Gripp et al., 2011].
The affected probands in these three previously reported families displayed a phenotype that encompassed most of the cardinal features of the disorder, which is how the TARP acronym was derived. As well, all were familial cases with the mothers of the affected boys confirmed to be heterozygotes. We describe here three families in which the affected males were more phenotypically diverse, expanding our understanding of the genetics and the phenotypic spectrum of this disorder.
MATERIALS AND METHODS
The evaluation of Family 1 was performed in a clinical care setting at Stanford University Medical Center and Genetic Medicine Central California/UCSF. The molecular work was done under the 10-HG-0065 research protocol approved by the National Human Genome Research Institute Institutional Review Board. Families 2 and 3 were evaluated in a clinical setting at the Children's Hospital of Philadelphia and the University of Utah, respectively. The former family underwent clinical exome analysis, whereas the latter family underwent a directed single gene test at NHGRI, under an approved research protocol 97-HG-0193.
Molecular analysis for Family 1
DNA was isolated from whole blood on the first affected boy and his mother using the salting out method (Qiagen, Germantown, MD) following the manufacturer's instructions. X-chromosome inactivation analysis was performed in the mother as described [Ng et al., 2004]. Exome sequencing was performed using exome enrichment (TruSeq v2, Illumina Corp, La Jolla, CA), indexed and pooled. Each captured exome pool was sequenced in two HiSeq2000 lanes using version 3 chemistry. At least 40 million paired-end 100 base reads were obtained for each sample. Data were processed using RTA version 1.13.48 and CASAVA 1.8.2. Reads were aligned to UCSC assembly hg18, NCBI build 36 using ELAND, as described [Johnston et al., 2012]. The filters were implemented using the VarSifter software program for exome and whole genome data management [Teer et al., 2012]. Variants were initially filtered for predicted loss of function including frameshift, nonsense and splice site alterations. The resulting set of variants was further filtered for absence from 870 controls. Sanger sequence analysis of RBM10 c.448C>T was performed as described [Gripp et al., 2011]. Sequence data were compared with the published RBM10 sequence (GenBank reference number NM_005676.4) using Sequencher 5.0.1 (Gene Codes Corp., Ann Arbor, MI). Additionally, confirmation of this alteration was performed using restriction enzyme digestion with PstI (New England Biolabs, Ipswich, MA) using standard procedures.
Molecular analysis for Family 2
A peripheral blood sample was submitted to the Baylor College of Medicine clinical exome sequencing service. This analysis was performed based on standard procedures within that laboratory, based on published methods [Bainbridge et al., 2011] and information at the Baylor Human Genome Sequencing Center: https://hgsc.bcm.edu/sites/default/files/documents/Illumina_Barcoded_Paired-End_Capture_Library_Preparation.pdf
And analyzed by their clinical testing pipeline: https://github.com/dsexton2/Mercury-Pipeline
Molecular analysis for Family 3
Following clinical evaluation, a tentative diagnosis of TARP syndrome was made in this child and samples on the patient and his unaffected mother were sent to NHGRI for Sanger sequencingof RBM10, which was performed as described [Gripp et al., 2011].
For all mutations, nucleotide numbering reflects cDNA numbering with +1 corresponding to the A of the ATG translation initiation codon in the reference sequence, according to the conventions specified by the Human Genome Variation Society. The initiation codon is codon 1.
CLINICAL REPORTS
Family 1 – Clinical data
Individual 1
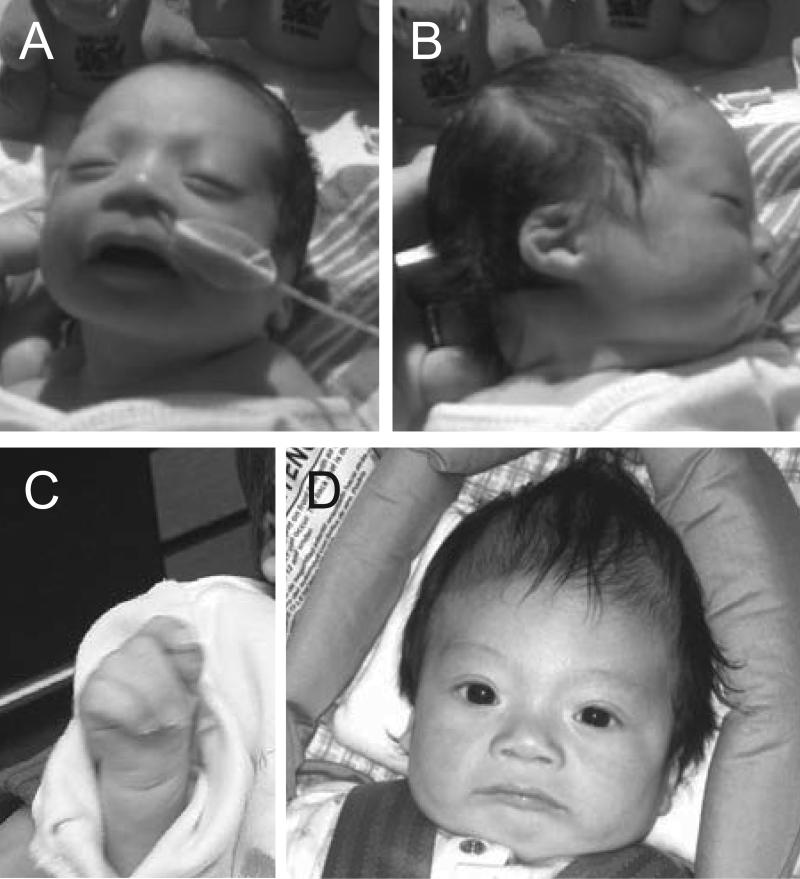
This child was the product of a 38 week gestation to a 33-year-old primigravida Asian mother and Caucasian father. The parents had a negative family history and the mother had eight healthy brothers. A first trimester choroid plexus cyst was detected that resolved in the third trimester. Prenatal echocardiography showed atrial enlargement with pericardial effusion and there was a possible tricuspid valve abnormality. By the mother's report, prenatal ultrasound at 27 weeks at an outside facility showed a possible Dandy-Walker malformation. Amniocentesis showed a 46,XY karyotype. His birth weight (BW) was 2162 gm (just <10th centile), birth length (BL) 48 cm (~50th centile), and birth occipitofrontal circumference (BOFC) 31 cm (~10th centile). In the nursery he was noted to have a broad, wide nasal bridge with an inner canthal distance of 2.3 cm (50th – 97th centile), short palpebral fissures (1.5 cm) and small ears with simple helices and prominent antihelices (ear length 2.7 cm (<3rd centile). He had moderate to severe micrognathia and a U-shaped cleft palate (Robin sequence). A mild pectus excavatum was noted. His limbs were notable for a unilateral single transverse palmar crease, mild 2-3 cutaneous toe syndactyly and unilateral postaxial toe polydactyly. A sacral dimple was present. He did not have talipes equinovarus.
Cranial MRI at 9 days of age showed small frontal hemispheres and frontal horns with a cortical pattern that was mildly immature for age. The corpus callosum was thin, with an absent rostrum, small genu and body, and a very small splenium. The septum pellucidum was absent. There was an unusually large cystic space, probably perivascular cystic spaces in the right caudal basal ganglia. He had a moderately small cerebellar vermis and left hemisphere, mildly small right cerebellar hemisphere, and mildly enlarged posterior fossa consistent with mega-cisterna magna (Fig. 1, A-C). An echocardiogram showed bilateral superior venae cavae, patent ductus arteriosus, and a small secundum ASD. Renal ultrasound showed a horseshoe kidney with mild hydronephrosis.
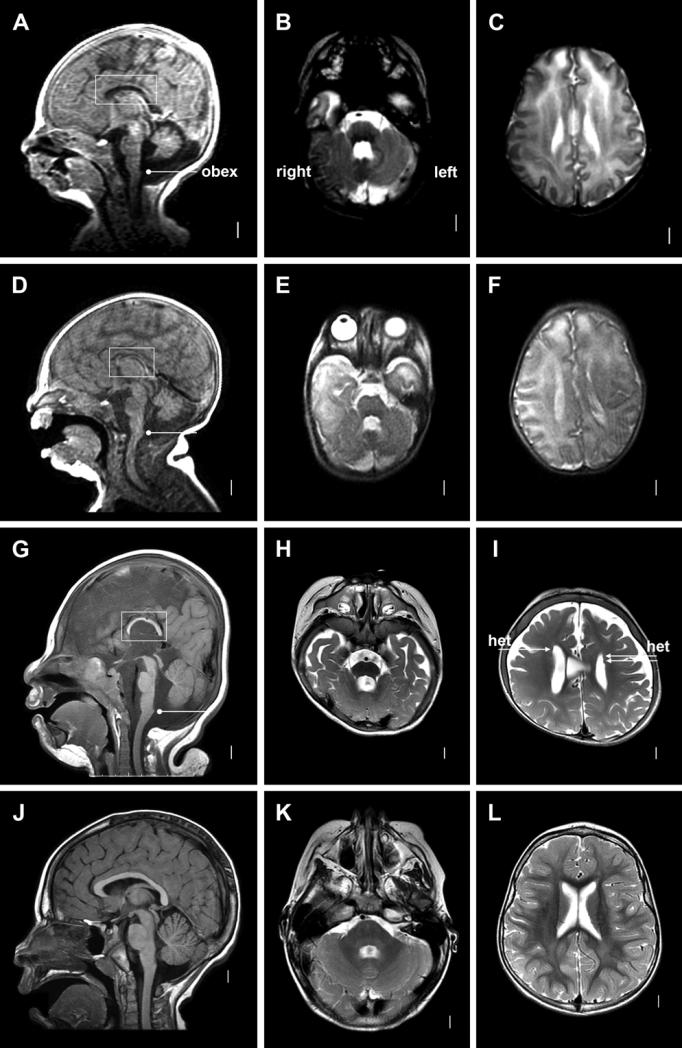
Figure 1.
Brain imaging in Individual 1 from Family 1 (A-C), Individual 2 from Family 1 (D-F), Patient 1 in Gripp et al 2011 (G-I) and a normal 3-year-old girl (J-L) including mid-sagital T1-weighted (left column), T2-weighed axial images through the cerebellum (middle column) and T2-weighted axial images through the lateral ventricles (right column). The mid-sagital images show thin and short corpus callosum (boxes in A, D, G), and moderately (A, D) or borderline (G) small cerebellar vermis. The horizontal lines with bullets mark the obex, the lower border of the vermis in most individuals. The low axial images show mildly small cerebellar hemisphere and mildly enlarged 4th ventricle (B, E, H), while the higher axial images show poorly-developed or simplified gyral pattern over the anterior and mid-frontal lobes (C, F, I). The arrows in I point to a few small periventricular nodular heterotopia.
The patient's clinical course was notable for obstructive and central sleep apnea with hypoxemia. He died at 2 ½ months of age secondary to respiratory failure.
Individual 2
This boy was born at 37 weeks gestation following an uncomplicated second pregnancy. Multiple ultrasounds by several sonographers in separate centers showed no convincing abnormalities. His BW was 2.3 kg (3rd – 10th centile), BL was 46.5 cm (10th – 50th centile), and his BOFC was 33 cm (25th – 50th centile). Apgar scores were 4/6/8 and intubation was required for respiratory distress. He was noted to have anomalies similar to those seen in his brother including Robin sequence with U-shaped cleft of soft and hard palate, small ears with prominent antihelices and overfolded superior helices (2.5 and 2.7 cm, both <3rd centile), short palpebral fissures, a broad flat nasal bridge, and 2-3 cutaneous toe syndactyly bilaterally and unilateral 4-5 cutaneous toe syndactyly (Fig. 2). The cutaneous toe syndactyly on one foot was Y-shaped. He did not have talipes equinovarus. He did have 3-4-5 cutaneous syndactyly of one hand and 4-5 cutaneous syndactyly of the other hand. He also had a horseshoe kidney with mild pelviectasis and a small ASD. Additional findings at 4 weeks included a wide mouth, downturned mouth corners, anteverted nares, and sublingual tongue masses. He had diffuse hypotonia and an abnormal, squeaky cry.
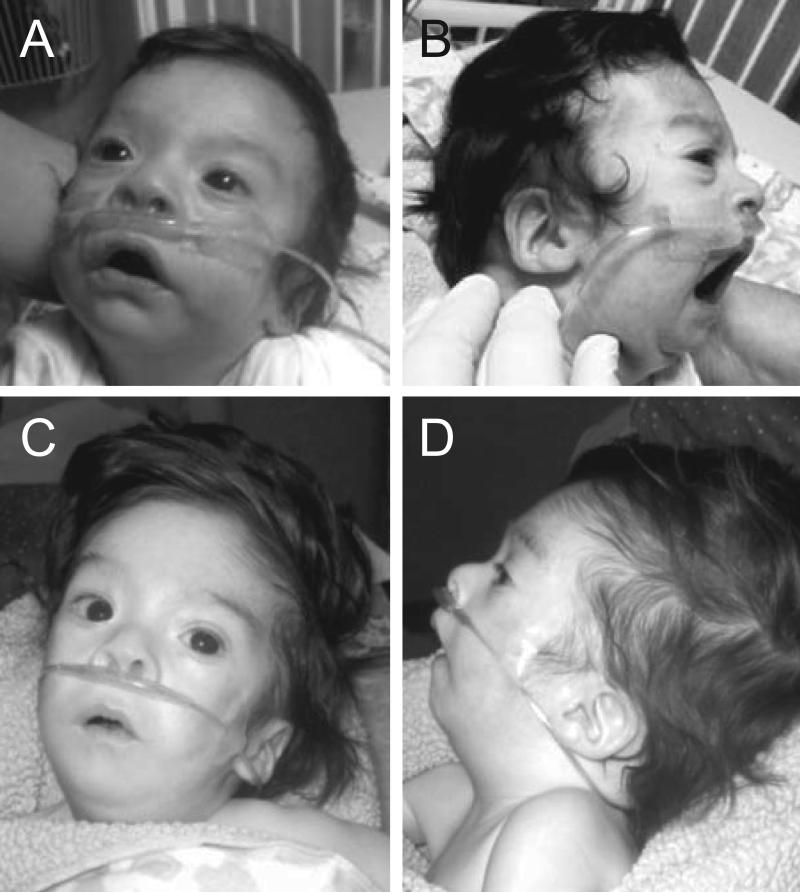
Figure 2.
Clinical features of Individual 2 from Family 1 in the neonatal period (A, B) at four weeks (C), and at 3 months of age (D). The images show small ears with prominent antihelices and overfolded superior helices, short palpebral fissures, a broad flat nasal bridge, micrognathia, and 3-4-5 cutaneous syndactyly of the right hand.
A cranial MRI at 12 days of age showed an immature gyral pattern for age. The corpus callosum was short and thin, more severe than seen in his brother. The cerebellar findings were similar to his brother with a moderately small vermis, mildly small but symmetric hemispheres, and mildly enlarged posterior fossa consistent with mega-cisterna magna (Fig. 1,D-F).
His clinical course was remarkable for poor feeding that led to placement of a gastrostomy tube. He died at 4 months of age due to respiratory failure.
Individual 3
The third pregnancy for this family was followed closely by prenatal ultrasound. At 12 weeks gestation, the nuchal translucency was markedly increased at 5 mm. A CVS was performed and chromosome analysis was 46,XY. Ultrasound at 15 weeks 4 days showed a unilateral cleft lip, a possible ventricular septal defect and a receding chin. A fetal echocardiogram was normal. Ultrasound at 16 weeks 1 day confirmed the cleft lip and showed some suggestion of an abnormal posterior fossa. Based on these findings and likely recurrence, the pregnancy was terminated at 18 weeks gestation. Postmortem examination by genetics showed a left cleft lip, cleft palate, mild widely spaced eyes, redundant nuchal skin, 2-3 partial cutaneous syndactyly of the toes bilaterally and a unilateral short third toe. Additional anatomical diagnoses included bilateral frontal bone hypoplasia, and bowel malrotation. The brain was not examined.
Individuals 4 and 5
The fourth and fifth pregnancies resulted in a healthy boy and girl with none of the findings in common with their siblings.
Family 1 – Molecular Data
The proband was initially enrolled in a research protocol targeted at understanding the contribution of GLI3 mutations to complex phenotypes including polydactyly [Johnston et al., 2010a]. No variants were identified in GLI3 and the subsequent (after the GLI3 analysis) occurrence of a third affected male fetus suggested X-linked inheritance in this family. X-chromosome inactivation studies, however, showed absence of skewing in maternal DNA. Based on the phenotypic overlap with OFD/GLI3 disorders, and the potential to filter variants for interaction with known pathways, the proband was submitted for whole exome sequencing. A target-selected library yielded 178,336 variant genotypes. Initial filtering for loss of function mutations and absence in a control set of 870 individuals reduced the set of possible variants to 15 including RBM10 c.448C>T, which predicts p.Gln150X. Reviewing the phenotypic features of the affected infants it was determined that this was likely the causative mutation. Confirmation sequencing and restriction enzyme digestion confirmed the presence of this alteration in the proband and found low-level mosaicism in the mother. Samples from other family members were not available.
Family 2 - Clinical Data
This was the first pregnancy for a 22-year-old healthy mother and her nonconsanguineous partner. Maternal past medical and family history were non-contributory. Enlarged lateral cerebral ventricles were detected on ultrasound at 23 weeks gestation. A fetal echocardiogram was read as a possible ventricular septal defect with overriding aorta. Amniocentesis was performed and a 46,XY karyotype and normal FISH for 22q11.2 were reported. Follow-up ultrasound at 30 weeks gestation again demonstrated ventriculomegaly with incomplete formation of the inferior vermis and cerebellum, rocker bottom feet, and dilated bowel. A fetal body MRI at 32 weeks gestation showed asymmetric cerebral ventricles, left greater than right; mild inferior vermian hypoplasia; possible low set ears and mild micrognathia; minimal distal sacral curvature; and foreshortened long bones. Rocker bottom feet and the dilated bowel were not confirmed by MRI. Repeat fetal echocardiogram demonstrated findings consistent with tetralogy of Fallot (TOF) with a large malalignment type ventricular septal defect (VSD) and moderate degree of pulmonary valve stenosis. On room air the ductus arteriosus was visualized but there was no flow seen across the ductus and very little blood flow seen in the branch pulmonary arteries. Maternal hyperoxygenation studies were performed that resulted in dramatic increase in the pulmonary blood flow in association with increased pulmonary venous return. Flow and hyperoxygenation studies suggested fetal pulmonary hypertension.
A spontaneous vaginal delivery occurred at 35 weeks gestation. His BW was 2025 g (~25th centile). The BL and BOFC were not available. The infant spent 59 days in the neonatal intensive care unit for respiratory distress, cardiac disease, poor weight gain, and a grade 3 intraventricular hemorrhage.
The infant presented to the Children's Hospital of Philadelphia at 3 months of age for a second opinion on his cardiac status. A genetics consultation was requested due to his multiple congenital anomalies and dysmorphic features. His weight was 3250 g (<<2nd centile), length was 50 cm (<<2nd centile), and head circumference was 37 cm (~2nd centile). On physical exam he had a wide anterior fontanelle, sloping forehead, relatively widely spaced eyes, low set and posteriorly rotated ears, a high arched palate with no cleft, micrognathia, clinodactyly of the fifth fingers, and prominent heels (Fig. 3). He did not have talipes equinovarus. His neurologic examination was notable for hypotonia and lethargy.
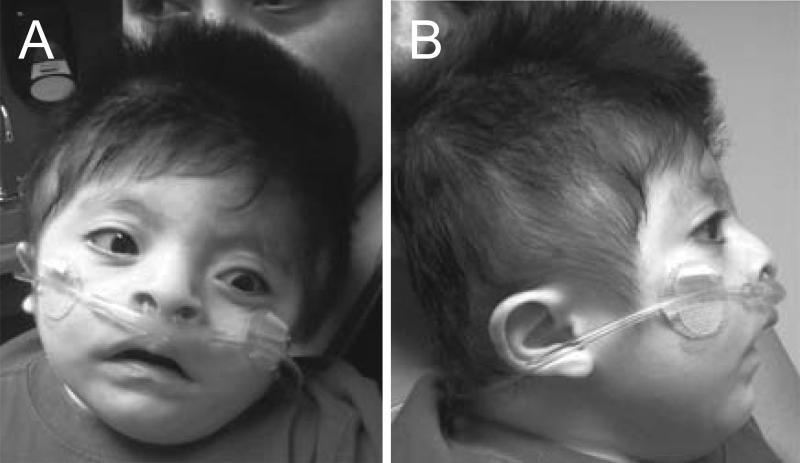
Figure 3.
Clinical features of the affected patient from Family 2 at 3 months of age (A,B) and 18 months of age (C,D). He had a sloping forehead, relatively widely spaced eyes, low set and posteriorly rotated ears, and micrognathia.
An echocardiogram showed TOF along with a new finding of hypertrophic cardiomyopathy, characterized by disproportionate thickening of the ventricular septum and classic systolic anterior motion of the mitral valve. Later review of the echocardiogram showed persistent left superior vena cava draining into the coronary sinus, which was initially considered as a variant of TOF and not commented on separately.
Normal results included karyotype, SNP microarray, hypertrophic cardiomyopathy panel, Noonan syndrome panel, Prader-Willi methylation testing, mitochondrial respiratory chain enzyme analysis, transferrin isoelectric focusing, long chain fatty acids, creatine phophokinase, acylcarnitine profile, and muscle biopsy. Ophthalmology evaluation showed pale optic discs with optic nerve hypoplasia and central visual impairment.
He had signs consistent with progressive pulmonary hypoplasia with chronic respiratory insufficiency. He required tracheostomy and became ventilator-dependent. He developed pancreatic insufficiency and pancreatic ultrasound showed a small and echogenic pancreas.
At 18 months of age his length was 75.0 cm (<2nd centile), weight was 9.9 kg (5-10th centile). He had global developmental delay with hypotonia but was able to smile and transfer objects. In addition to the physical findings described above, he had a small phallus with retractile left testicle, severe hyperkeratosis pilaris, and a slightly enlarged liver. Ultrasound of a palpable mass of the right thigh showed a 2 cm heterogeneously echogenic solid mass in the subcutaneous tissue of the right thigh. Differential diagnosis included involuting vascular malformation or lipoma. It is being followed clinically. Chronic respiratory issues have led to ongoing ventilator dependence. He had persisting gastroesophageal reflux following gastrostomy tube placement and fundoplication. His hypertrophic cardiomyopathy has gradually resolved. He underwent TOF repair via non-transannular patch with subsequent VSD repair. A subsequent pregnancy showed a normal male by fetal MRI and echocardiogram. Amniotic fluid karyotype was 46,XY.
Family 2 - Molecular Data
Exome sequencing identified a hemizygous c.724+2T>C mutation of RBM10. This mutation has not been previously reported, but was predicted to be deleterious. The mutation was confirmed by Sanger sequencing of the targeted region of RBM10. Sanger sequence of the maternal sample did not detect the mutation, suggesting that this change arose apparently de novo or was mosaic in peripheral blood DNA from the mother. Molecular testing of the clinically unaffected brother did not show the mutation.
Exome sequencing also elucidated deleterious mutations in two genes for recessive conditions not related to the clinical phenotype and variants of unknown clinical significance in 14 genes for autosomal dominant conditions not related to the clinical phenotype. Review of the additional variants reported did not suggest another possible diagnosis.
Family 3 - Clinical Data
This was a second, 37 week gestation pregnancy to healthy 23 and 27-year-old nonconsanguineous Hispanic parents. The family history was remarkable for a maternal uncle with hip dysplasia and cardiac murmur whose co-twin died shortly after birth from complications of oligohydramnios. A paternal uncle died of sudden infant death syndrome. The infant's BW was 2145 g (~5th centile), BL was 44 cm (~3rd centile), and BOFC was 29 cm (<3rd centile). He had poor respiratory effort and bradycardia. His Apgar scores were 3, 5, and 7. He required three days of continuous positive airway pressure. Physical examination at five days of life was remarkable for symmetrical growth restriction, abnormal hair patterning, large anterior and posterior fontanels with widely split sagittal sutures, and overriding lambdoid sutures. There was a wide nasal bridge (inner canthal distance 2.8 cm, >95%), underdeveloped supraorbital ridges, sparse eyelashes, and anteverted nares. He had a wide mouth with downturned corners, high arched palate, midface hypoplasia, and severe micrognathia without a cleft palate (Fig. 4). Right fifth finger clindodactyly and slightly decreased supination on the elbows were appreciated. He did not have talipes equinovarus. A prominent chest with a short sternum (<10th centile) was noticed along with small nipples and a decreased internipple distance (<10th centile). Epispadias and superficial midline sacral dimple were present. Hypotonia with a weak suck, jerky movements of the extremities and an intermittent upward eye deviation were noted.
Figure 4.
Clinical features of the affected patient from Family 3 at 6 months of age. He had an abnormal hair pattern, wide nasal bridge, underdeveloped supraorbital ridges, sparse eyelashes, and anteverted nares, wide mouth with downturned corners, midface hypoplasia, and micrognathia.
Studies included a cranial MRI that showed a left cerebellar parenchymal hemorrhage and T2 hyperintensities in the right frontal region. Auditory brainstem response testing showed mild to moderate hearing loss from 500 to 4000 Hz bilaterally. An upper GI contrast study showed a tortuous duodenum. A CGH microarray was normal. Ophthalmology evaluation was normal. A skeletal survey showed small facial bones and dysplastic proximal radii.
He underwent mandibular distraction at 2 weeks of age and a gastrostomy tube was placed at six weeks of age. After three months he was discharged from the neonatal intensive care unit. At 5 months he was admitted to the pediatric intensive care unit with the diagnosis of parainfluenza pneumonia requiring intubation. An echocardiogram showed a small secundum ASD, mild right heart enlargement, and dilated coronary suggestive of a left SVC. Pulmonic and aortic valve thickening with trivial pericardial effusion were also observed.
Studies included an EEG, which showed polymorphic posterior slowing along with infrequent right front to central sharp waves. A bronchoscopy showed laryngomalacia with severe and persistent recurrent upper airway obstruction with infolding and very large arytenoids causing obstruction of the glottis. A swallowing function study showed disorganized swallowing but no aspiration. He had absent otoacoustic emission responses bilaterally. A retroperitoneal ultrasound was normal. Additional studies included spinal radiographs, which showed flattening of the occipital condyles with C2-C3 fusion.
He was discharged home after three months of serial hospitalizations due to respiratory distress. At six months his weight was 6.2 kg (~2nd centile), his length 60 cm (<3rd centile) and OFC was 40 cm (<3rd centile). Physical examination showed pronounced plagiocephaly with a large anterior fontanel but no overriding sutures. He had abundant, unruly, long black hair. Palpebral fissures were long and he had sparse eyebrows with synophrys. The ears were small (3 cm; <3rd centile) low set and posteriorly rotated with squared off superior helices. He also had a low anterior hairline, round face, and short neck. Palmar creases were deep and his feet were small (<10th centile). Visual tracking was poor. At 10 months his head control was poor and he was unable to sit up or roll over. Bilateral myringotomy and tube placement were performed due to chronic effusions and small ear canals.
At 9 months of age a laryngoscopy performed for persistent respiratory distress and upper airway obstruction showed a floppy and posteriorly placed epiglottis with enlarged cuneiform cartilage. His parents declined a tracheostomy tube placement and he died at 14 months of age due to respiratory failure.
Family 3 – Molecular Data
Sanger sequencing of the coding exons of RBM10 showed a c.2176C>T variant, which predicts p.Arg726X. This variant was not present in peripheral blood DNA in his unaffected mother.
DISCUSSION
The TARP syndrome was initially described in a single family by Gorlin et al [1970] with a pattern of anomalies including Talipes equinovarus, Atrial septal defect, the Robin sequence (micrognathia, and tongue occlusion of palatal closure leading to cleft palate), and Persistence of the left superior vena cava. They designated this disorder as “Robin's syndrome”, which was problematic and confusing because of the possessive form of the proper noun, and the coupling of the words “Robin” and “syndrome”. For these reasons, the disorder was re-designated as TARP syndrome to capture the anomalies (Talipes, ASD, Robin Sequence, and Persistent superior vena cava) but as demonstrated by the families described here, this designation also fails to capture the phenotypic diversity and variability seen in this condition.
In 2003 the disorder was mapped to Xp11.23-q13.3 [Kurpinski et al., 2003]. A strikingly similar family was identified by Karen Chong and colleagues and linkage analysis mapped the gene in this family to the same locus. Subsequently, massively parallel sequencing was used to identify two null mutations in RBM10 [Johnston et al., 2010].
One of the major strengths of genomic approaches to genetic disorders caused by single gene mutations is that the techniques facilitate an unbiased examination of cases, substantially alleviating the problems inherent in biased ascertainment of individuals with recognizable patterns of malformations. Prior to the advent of genomic tools, limitations of candidate gene testing mandated that a gene would be sequenced only if the patient had phenotypic features that closely matched a known disorder, because it was typically a large amount of work (or expensive) to sequence many genes. This is the legacy of a phenotype-driven approach to multiple malformations, which is why one of the primary textbooks for our discipline is entitled “Recognizable Patterns of Human Malformation” [Jones and Smith, 2006]. For this reason, our literature is biased by the inclusion of typically or characteristically affected individuals in genotype-phenotype studies. Over time, the phenotypic parameters of recognizable disorders has slowly expanded as clinicians recognize progressively less typically affected patients, what we refer to as enlarging the clinical spectrum of the disease. Whole genome approaches massively accelerate this process because essentially all genes are tested at once. This allows us to more rapidly recognize atypical patients and better identify patients with highly atypical presentations (which could only be done previously if the family was large enough for linkage analysis). In this way, we can begin to achieve an increasing appreciation of the spectrum of human genomic variation.
The patients presented here are emblematic of this issue. Family 1 presented with two boys initially without a key finding in TARP syndrome, talipes equinovarus, and with additional findings including polydactyly, cutaneous syndactyly, and sublingual tongue masses, which led to a tentative diagnosis of atypical oral-facial-digital syndrome. It should be noted, with some humility, that several authors of the present study (LGB, JJJ, JCS) were also authors on the 2010 publication describing TARP syndrome, yet even we did not recognize the diagnosis in this case. Several other authors on the present paper are senior dysmorphologists, yet again the clinical diagnosis was not made. The patient in Family 2 had only one of the cardinal features, which explains why he was not clinically diagnosed. The proband in Family 3 had two of the four recognized manifestations of TARP, which led an astute clinician to refer him to our study for candidate gene analysis. Our experience with this syndrome emphasizes that a genomic approach to pleiotropic syndromes can identify conditions that escape clinical recognition even by senior clinicians.
The genetics of TARP syndrome is X-linked with high penetrance in males and no known effects in heterozygote females recognized to date. The first three families [Gripp et al., 2011; Johnston et al., 2010b] all included heterozygous carrier mothers of affected boys. Here we have identified three families where the mothers were not heterozygous. Family 1 had three affected children while X inactivation studies in the mother did not demonstrate skewing, supporting a negative mutation status. However, Sanger sequence analysis suggested possible low-level mosaicism for the RBM10 mutation in blood and mosaicism was confirmed with restriction enzyme digestion. Families 2 and 3 were simplex cases and both mothers showed no evidence of the RBM10 mutation identified in their children, most likely explained by de novo RBM10 mutations. Given that this disorder appears to be a genetic lethal (high penetrance and complete pre-reproductive lethality) population genetics principles would predict that 1/3 of the simplex cases would be de novo mutations.
The mutational spectrum of this disorder now includes six alleles (c.1235G>A p.Trp412X; c.1893dupA p.Pro632ThrfsX41; c.159delC p.Lys54SerfsX80; c.448C>T, p.Gln150X; c.724+2T>C; c.2176C>T p.Arg726X). We find it interesting that all of these alleles are null or highly likely to be null. The ClinSeq® (~900 individuals) and NHLBI exome sequencing datasets (~10,000 alleles) for this gene shows that there are no nonsense, splice, or frameshift variants in this gene and only 20 missense variants, most of which are quite rare, being identified in only one or two alleles each. Comparing the affecteds to the control exomes suggests that loss of function mutations in this gene are poorly tolerated and very rare. We speculate that hypomorphic alleles in this gene cause an as yet unrecognized syndrome or phenotype. In summary, we have identified three additional families with mutations in RBM10 causing variable manifestations of TARP syndrome as well as previously undescribed syndromic features. Probably the most specific of the craniofacial findings include the Robin sequence but this too is variable. A wide mouth with downturned corners was seen in all the children and most had ear abnormalities that did not seem specific. This said, it may be difficult for clinicians to recognize this syndrome. The relative frequency of the Robin sequence in clinical practice and the non-specificity of the cardiac findings compound these difficulties. The persistence of the superior vena cava may be the most specific of the cardiac anomalies but again we feel that this finding could be easily overlooked in the context of more serious cardiac anomalies.
The brain findings also deserve mention as they could help refine the differential diagnosis and narrow the diagnostic odyssey. These include cerebellar vermis hypoplasia, mega cisterna magna, and cerebellar hypoplasia with or without abnormalities of the corpus callosum. These are all abnormalities within the spectrum of the Dandy Walker malformation sequence and indeed this was suggested in the first child in Family 1. Further highlighting the variability of the disorder, the affected child in Family 3 had, by report, none of these posterior fossa abnormalities.
Current knowledge of this disorder makes clinical diagnosis challenging. The affected children from the six reported families (this report, Johnston et al [2010b] and Gripp et al [2011]) with the disorder show that the clinical presentations are substantially variable. The cardinal finding of talipes was seen in none of the patients reported here. The inclusion of talipes in the acronym TARP may lead to clinical underascertainment of this condition. The findings in the present report of additional phenotypic features including postaxial foot polydactyly, cutaneous syndactyly, and tongue nodules also complicate the differential, as these findings are prominent in disorders of the cholesterol pathway or the oral-facial-digital syndromes. Other previously undescribed findings include horseshoe kidney and short proximal radii. As well, we provide data on CNS findings, the most distinctive of which include cerebellar and more specifically, vermis hypoplasia. Finally, there are a number of other dysmorphic features such as underdeveloped supraorbital ridges, upturned noses, abnormal palm creases, etc. Reports such as this should assist physicians in making a clinical diagnosis. Given that there are still a relatively small number of described patients and the clinical manifestations are inconsistently characterized, we do not feel that we can provide clear conclusions on the relative utility of these manifestations for use by geneticists for clinical pattern recognition. While it is true that exome sequencing is not widely available, we predict that clinical and exome sequencing will identify yet further patients prior to a correct clinical diagnosis. In such cases, clinical acumen is still essential for validating the findings by correlating these variable clinical features with the identified genomic alteration [Hennekam and Biesecker, 2012].
Our observations expand our understanding of the genetic and the phenotypic spectrum of this disorder. The mutational spectrum of the disorder is limited to loss of function alleles and we hypothesize that the clinical spectrum of the disorder and mutations is yet wider than that represented by the six families identified to date. We look forward to future ascertainment of additional patients with this disorder to provide a full understanding of the clinical spectrum, facilitate its clinical recognition, appreciate the remarkable developmental pleiotropy of RBM10 dysfunction, and understand the pathophysiology of RBM10 mutations.
TABLE I.
Clinical findings of individuals with TARP syndrome.
| Family 1 II-1 | Family 1 II-2 | Family 1 II-3 | Family 2 | Family 3 | Prior reports* | |
|---|---|---|---|---|---|---|
| Talipes | - | - | - | - | - | +++ |
| ASD | + | + | -1 | -2 | + | ++ |
| Robin sequence | + | + | CP | - | M | +++ |
| Persistent left superior vena cava | + | ? | - | + | ++ | |
| SGA | + | + | + | - | ||
| FTT | + | + | + | |||
| Abnormal skull shape | + | + | ||||
| Round face & malar flattening | + | + | ||||
| Short palpebral fissures | + | + | ||||
| Small / abnl shaped ears | + | + | + | + | ||
| Cutaneous syndactyly | + | + | + | + | ||
| Hypotonia | + | + | + | + | + | |
| Developmental delay | + | + | + | + | + | |
| CNS | Small frontal horns, immature cortex, abnl CC, vermis & cerebellar hemispheres | Vermis & cerebellar emisphere small, abnl CC | Dilated ventricles, vermis hypoplasia, IVH | Only IVH, frontal T2 hyperintensity | ||
| Eyes | Optic atrophy/cortical visual impairment | Optic atrophy/cortical visual impairment | ||||
| Hearing loss | + | + | ||||
| Airway /pulmonary abnormalities | + | + | + | + | + | |
| Age at death | 1 month | 4 mos. | Terminated 18 weeks | Alive at 20 mos. | 14 mos. | Median few mos. |
Ventricular septal defect
Tetralogy of Fallot; ASD atrial septal defect; SGA small for gestational age; FTT failure to thrive; CNS central nervous system; CC corpus callosum; CP cleft palate only; IVH intaventricular hemorrhage; M micrognathia only
Much of the specific clinical data beyond the cardinal features is from Gripp et al., [2011].
ACKNOWLEDGMENTS
We thank Julia Fekecs for graphic design services. Whole exome sequence analysis for Family 1 Individual 1 was performed at the NIH Intramural Sequencing Center. This research was supported in part by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health.
Footnotes
Conflict of interest
LGB is an uncompensated advisor to the Illumina Corp. and his group receives in-kind support for collaborative work distinct from that described here. All other authors declare no conflict of interest.
REFERENCES
- Bainbridge MN, Wang M, Wu Y, Newsham I, Muzny DM, Jefferies JL, Albert TJ, Burgess DL, Gibbs RA. Targeted enrichment beyond the consensus coding DNA sequence exome reveals exons with higher variant densities. Genome Biol. 2011;12:R68. doi: 10.1186/gb-2011-12-7-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlin RJ, Cervenka J, Anderson RC, Sauk JJ, Bevis WD. Robin's syndrome. A probably X-linked recessive subvariety exhibiting persistence of left superior vena cava and atrial septal defect. Am J Dis Child. 1970;119:176–178. [PubMed] [Google Scholar]
- Gripp KW, Hopkins E, Johnston JJ, Krause C, Dobyns WB, Biesecker LG. Long-term survival in TARP syndrome and confirmation of RBM10 as the disease-causing gene. American Journal of Medical Genetics. Part A. 2011;155A:2516–2520. doi: 10.1002/ajmg.a.34190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennekam RC, Biesecker LG. Next-generation sequencing demands next-generation phenotyping. Hum Mutat. 2012;33:884–886. doi: 10.1002/humu.22048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JJ, Rubinstein WS, Facio FM, Ng D, Singh LN, Teer JK, Mullikin JC, Biesecker LG. Secondary variants in individuals undergoing exome sequencing: screening of 572 individuals identifies high-penetrance mutations in cancer-susceptibility genes. Am J Hum Genet. 2012;91:97–108. doi: 10.1016/j.ajhg.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JJ, Sapp JC, Turner JT, Amor D, Aftimos S, Aleck KA, Bocian M, Bodurtha JN, Cox GF, Curry CJ, Day R, Donnai D, Field M, Fujiwara I, Gabbett M, Gal M, Graham JM, Hedera P, Hennekam RC, Hersh JH, Hopkin RJ, Kayserili H, Kidd AM, Kimonis V, Lin AE, Lynch SA, Maisenbacher M, Mansour S, McGaughran J, Mehta L, Murphy H, Raygada M, Robin NH, Rope AF, Rosenbaum KN, Schaefer GB, Shealy A, Smith W, Soller M, Sommer A, Stalker HJ, Steiner B, Stephan MJ, Tilstra D, Tomkins S, Trapane P, Tsai AC, Van Allen MI, Vasudevan PC, Zabel B, Zunich J, Black GC, Biesecker LG. Molecular analysis expands the spectrum of phenotypes associated with GLI3 mutations. Hum Mutat. 2010a;31:1142–1154. doi: 10.1002/humu.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JJ, Teer JK, Cherukuri PF, Hansen NF, Loftus SK, Chong K, Mullikin JC, Biesecker LG. Massively parallel sequencing of exons on the X chromosome identifies RBM10 as the gene that causes a syndromic form of cleft palate. Am J Hum Genet. 2010b;86:743–748. doi: 10.1016/j.ajhg.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Smith's Recognizable Patterns of Human Malformation. Elsevier Saunders; Philadelphia: 2006. p. 954. [Google Scholar]
- Kurpinski KT, Magyari PA, Gorlin RJ, Ng D, Biesecker LG. Designation of the TARP syndrome and linkage to Xp11.23-q13.3 without samples from affected patients. Am J Med Genet A. 2003;120A:1–4. doi: 10.1002/ajmg.a.10201. [DOI] [PubMed] [Google Scholar]
- Ng D, Thakker N, Corcoran CM, Donnai D, Perveen R, Schneider A, Hadley DW, Tifft C, Zhang L, Wilkie AO, van der Smagt JJ, Gorlin RJ, Burgess SM, Bardwell VJ, Black GC, Biesecker LG. Oculofaciocardiodental and Lenz microphthalmia syndromes result from distinct classes of mutations in BCOR. Nature Genet. 2004;36:411–416. doi: 10.1038/ng1321. [DOI] [PubMed] [Google Scholar]
- Teer JK, Green ED, Mullikin JC, Biesecker LG. VarSifter: visualizing and analyzing exome-scale sequence variation data on a desktop computer. Bioinformatics. 2012;28:599–600. doi: 10.1093/bioinformatics/btr711. [DOI] [PMC free article] [PubMed] [Google Scholar]