Abstract
The Pennsylvanian lowlands of western Pangea are best known for their diverse wetland floras of arborescent and herbaceous ferns, and arborescent horsetails and clubmosses. In apparent juxtaposition, a very different kind of flora, dominated by a xerophilous assemblage of conifers, taeniopterids and peltasperms, is occasionally glimpsed. Once believed to represent upland or extrabasinal floras from well-drained portions of the landscape, these dryland floras more recently have been interpreted as lowland assemblages growing during drier phases of glacial/interglacial cycles. Whether Pennsylvanian dryland and wetland floras were separated spatially or temporally remains an unsettled question, due in large part to taphonomic bias toward preservation of wetland plants. Previous paleobotanical and sedimentological analysis of the Markley Formation of latest Pennsylvanian (Gzhelian) age, from north central Texas, U.S.A, indicates close correlation between lithofacies and distinct dryland and wetland megaflora assemblages. Here we present a detailed analysis one of those localities, a section unusual in containing abundant palynomorphs, from the lower Markley Formation. Paleobotanical, palynological and lithological data from a section thought to represent a single interglacial/glacial phase are integrated and analyzed to create a complex picture of an evolving landscape. Megafloral data from throughout the Markley Formation show that conifer-dominated dryland floras occur exclusively in highly leached kaolinite beds, likely eroded from underlying soils, whereas a mosaic of wetland floras occupy histosols, ultisols, and fluvial overbank deposits. Palynological data largely conform to this pattern but reveal a more complex picture. An assemblage of mixed wetland and dryland palynofloral taxa is interpolated between a dryland assemblage and an overlying histosol containing wetland taxa. In this section, as well as elsewhere in the Markley Formation, kaolinite and overlying organic beds appear to have formed as a single genetic unit, with the kaolinite forming an impermeable aquiclude upon which a poorly drained wetland subsequently formed. Within a single inferred glacial/interglacial cycle, lithological data indicate significant fluctuations in water availability tracked by changes in palynofloral and megafloral taxa. Palynology reveals that elements of the dryland floras appear at low abundance even within wetland deposits. The combined data indicate a complex pattern of succession and suggest a mosaic of dryland and wetland plant communities in the Late Pennsylvanian. Our data alone cannot show whether dryland and wetland assemblages succeed one another temporally, or coexisted on the landscape. However, the combined evidence suggests relatively close spatial proximity within a fragmenting and increasingly arid environment.
Keywords: Palaeoecology, Palynology, Environmental change, Pennsylvanian, Texas, Markley Formation
1 Introduction
The Pennsylvanian lowlands in western equatorial Pangaea are characterized by the iconic wetland Coal Age floras, an assemblage of arborescent and herbaceous lycopods, calamites, ferns and seed ferns associated with vast peat deposits (DiMichele and Phillips, 1994, 1996). In contrast, a quite different, apparently contemporaneous flora dominated by walchian conifers, cordaitaleans, and an array of poorly understood seed plants of uncertain affinity is occasionally glimpsed (Cridland and Morris, 1963; Winston, 1983; Mapes and Rothwell, 1988; Lyons & Darrah, 1989; DiMichele et al., 2010; Falcon-Lang et al., 2011a, 2011b; Bashforth et al., 2014). Based on ecomorphological characters, especially of conifers (Kerp, 1996, 2000) and their sedimentological context (Rueger, 1996, Falcon-Lang et al., 2011b), these gymnosperm-dominated assemblages are believed to represent plants adapted to drier or better-drained conditions. Over the course of the Late Pennsylvanian, these dryland floras become increasingly common until they dominate lowland basins in western Pangaea in the early Permian. Concurrently, the wetland floras contract to spatially and temporally constricted ‘wet spots’ on the landscape (Rees et al., 2002; DiMichele et al., 2006; Fielding et al., 2008; Montañez et al., 2007; Tabor et al., 2008; DiMichele, 2014). This long-term trend toward aridity is attributed to continental movement accompanied by shifts in atmospheric circulation, orogenic processes and probably most importantly, increased atmospheric pCO2 (Tabor and Poulsen, 2008; Montañez and Poulsen, 2013).
Increasing aridity is by no means monotonic throughout the Late Pennsylvanian. Superimposed on this long-term trend are short-term climate oscillations driven by orbital forcing (Milankovich cycles) at scales of 106 and 105 years (Heckel, 2008; Tabor and Poulsen, 2008; Pointon et al., 2012; Eros et al., 2012). At high southern latitudes, waxing and waning of glacial ice sheets can be inferred directly from the sedimentological record (e.g., Fielding et al., 2008; Montañez and Poulsen, 2013). At equatorial latitudes in shelf settings, these glacial/interglacial cycles find expression in cyclothems, repeating packages of coal and terrestrial clastic sediments overlain by marine limestones, as eustatic sea level rose and fell in response to waning and waxing ice sheets (Wanless and Shepard, 1936; Archer, 2009). Although there is general consensus that eustatic sea level change was driven by advance and retreat of high latitude southern ice sheets in response to shifts in atmospheric pCO2, the precise rainfall and temperature patterns that prevailed during glacial and interglacial cycles remain uncertain. Many models hypothesize that Pennsylvanian interglacials were warm and wet, followed by cooler and drier glacial phases, analogous to climate cycles during the Pleistocene (Feldman et al., 2005; Falcon-Lang, 2004). In contrast, other models posit that interglacials were warm and seasonally arid, whereas glacial phases were cooler and wetter, with major commercial coal formed during maximum glacial advance (Peyser and Poulsen, 2008; Horton et al., 2010; Horton et al., 2012; Cecil et al., 2014). Resolution of this question is beyond the scope of this paper; for our purposes, recognition of cyclicity at short time scales is sufficient.
Similarly, the spatial and temporal relationship between the Pennsylvanian wetland and dryland floral assemblages remains unclear. Peat and clastic swamp floras are usually preserved as autochthonous or parautochthonous assemblages, so their distribution on the landscape is known with a remarkable degree of precision (Gastaldo et al., 2004; Bashforth et al., 2010; DiMichele and Falcon-Lang, 2011). Short and long term taphonomic factors favor their preservation: a high water table and rapid burial in a dysaerobic, acidic environment that discourages decay, within a rapidly aggrading landscape (Gastaldo and Demko, 2011). In contrast, dryland assemblages are at a taphonomic disadvantage because they are buried in sediments exposed to a fluctuating water table, where aerobic conditions foster oxidative attack and microbial decay (Gastaldo and Demko, 2011; Falcon-Lang et al., 2011b). Additionally, during the Pennsylvanian, taphonomic bias against preservation of dryland floras may have been accentuated if they were widespread during times of rapid sea level fall and an erosional regime (DiMichele, 2014).
Two contrasting interpretations of the relationship between wetland and dryland assemblages have been advanced. The first (and older) view posits that wetland and dryland floras were coeval, but that the latter occupied better-drained, upland or extrabasinal sites distant from depositional basins (Chaloner, 1958a; Cridland and Morris, 1963; Pfefferkorn, 1980; Plotnick et al., 2009; Dimitrova et al., 2011). The observed oscillation between wetland and dryland assemblages can be attributed to normal landscape evolution, such as channel migration, stream avulsion and delta switching, i.e., to autocyclic processes (Beerbower, 1964). Climate is often held constant or not considered in this model, although there is no requirement for this assumption. Under an autocyclic model, one would predict some overlap of wet and dry (wetland and better-drained) elements as normal landscape evolution captures wetter or drier parts of the depositional system. A contrasting interpretation views dryland and wetland assemblages as temporally separate, responding to allocyclic shifts imposed by climate change attributable to glacial-interglacial cycles or broader secular trends (DiMichele,et al., 2010, Dimichele, 2014; Tabor et al., 2013). In this interpretation, little overlap between wetland and dryland assemblages should be observed, and one would never expect to see lateral gradation from one type to the other.
Distinguishing between these two hypotheses is not straightforward, especially given the taphonomic bias against preservation in seasonally dry and erosional regimes. Allocyclic and autocyclic processes are by no means mutually exclusive; both likely operate at different times and at different scales. To further complicate matters, sedimentary processes are themselves inextricably tied with climate and vegetation. For example, lower vegetation cover in seasonally dry climates leads to higher erosion and intermittently high (flashy) sedimentation rates (Cecil and Dulong, 2003; Birgenheier et al., 2009; Allen et al., 2011). Humid to perhumid climate with little seasonal variation is characterized by dense vegetation cover, significantly reducing erosion and clastic input (Cecil and Dulong, 2003; Cecil et al., 2003). Significant peat accumulation occurs only when precipitation is greater than evapotranspiration rates for 8–10 months of the year, resulting in a permanently high water table (Cecil and Dulong, 2003).
Outcrops exposed in north central Texas, U.S.A., capture the reorganization of plant assemblages from the Late Pennsylvanian to the Middle Permian in the Midland Basin. Much has been published on the megafloras and sedimentology of this region (e.g., Mamay et al., 1988; DiMichele et al., 2000, 2001, 2004; Tabor and Montañez, 2004; Looy, 2007, 2013, Looy and Duijnstee, 2013; Looy and Stevenson, 2014; Tabor et al., 2008, 2013), but very little on the palynology, in large part due to the low preservation potential in the extensive redbeds of the Early Permian part of the sequence (for an exception, see Dickey and Gupta, 1980). In this study we focus on palynology of the latest Pennsylvanian-earliest Permian (Gzehlian-Asselian) Markley Formation, and compare our data to recent studies focused on megafloras and facies relationships (DiMichele et al., 2005a; Tabor et al., 2013). Palynology offers a source of data complementary to the megafloral record. The small size and resistance to physical abrasion of pollen and spores allows greater dispersal and thus broader sampling of habitats normally not preserved in the sedimentary record, as well as recognition of rarer and more fragile floral elements unlikely to be preserved as megafossils. The presence of palynomorphs in sediments unconducive to megafossil preservation (e.g., certain paleosols) offers an assessment of the vegetation characterizing that lithofacies. Here we present one of the few studies that compares detailed palynological data from both wetland and dryland assemblages in the Late Pennsylvanian. We integrate these data with the sedimentary and plant megafossil rccords in an attempt to assess the relationships between dryland and wetland flora assemblages and to test two competing hypotheses of plant distribution on the landscape. The preservation of palynologically productive beds in this formation offers a rare window into the broader composition and distribution of plants in a tropical coastal plain setting during the latest Pennsylvanian.
2 Geology and megaflora of the Markley Formation
The Markley Formation was located in the western Pangaean tropics between 0° and 5° N (Scotese, 1999). Lithostratigraphic correlation from conodont changes within the marine equivalent Harpersville Formation places the Pennsylvanian-Permian boundary near the top of the Markley Formation on a roadcut exposed along US Highway 281, 29.9 km at heading 136.2 from Jacksboro, Texas (Wardlaw, 2005). The formation was deposited in a fluvial-dominated coastal plain setting on the eastern shelf of the Midland Basin, with sediments derived from the Ouachita fold belt to the east and the Wichita-Amarillo and Arbuckle highlands to the north (Hentz, 1988; Tabor and Montañez, 2004; Tabor, 2007). Its areal extent in outcrop is about 1700 km2. The Markley Formation is characterized by a distinctive, repeating package of sedimentation. Each sedimentary package typically consists of a basal paleosol overlain by a light-gray or white, kaolinite-rich siltstone unit between 0.1 and 1 m thick underlying thin coals or organic-rich shales of limited lateral extent. This organic unit is in turn overlain by coarsening-upward mudstones, siltstones and fine sandstones (“heterolithic”beds) and capped by medium to coarse sandstones (DiMichele et al., 2005a; also see Fig. 4 of Tabor et al., 2013). Approximately seven to nine of these repeating sedimentary packages can be recognized within the Formation (W.A. DiMichele, pers. comm., 2011). A distinct suite of plant megafossils characterizes each of these lithological facies. Paleosols (lithofacies 1) contain no body fossils but do display vertically penetrating root casts 3–5 cm in diameter and extending up to a meter below the soil surface, indicating the presence of relatively large trees (DiMichele et al., 2005a). Based on our knowledge of Pennsylvanian plants, the most likely candidates producing these rhizoliths would have been either Cordaites or conifers (DiMichele et al., 2005a). Kaolinitic beds (lithofacies 2) contain a distinctive, low diversity megaflora of walchian conifers, the seed ferns Sphenopteridium manzanitanum and Neuropteris sp., and sphenopsids Asterophyllites and Calamites. The organic shale facies (lithofacies 3) is dominated by Macroneuropteris scheuzeri and other medullosans, species of Marattiales, Calamites and Sigillaria brardii, the last surviving arborescent lycopsid in paleotropical Euramerica. The heterolithic facies (lithofacies 4) preserves a somewhat similar wetland assemblage that is dominated by Pseudomariopteris cordato-ovata and Pecopteris spp., but also includes abundant medullosan seed ferns, Calamites, and sphenophylls, as well as rare Cordaites, walchian conifers and Taeniopteris. Fossils are rare in the sandstone facies (lithofacies 5), but, when present, include conifers, cordaitaleans, and rare Calamites, Neuropteris and Pecopteris. For more details of the megaflora, see Tabor et al. (2013). Paleosol analysis indicates that there is a long-term drying trend upsection in the Markley Formation. Histosols and ultisols, formed under humid to perhumid conditions, are common in the lower half of the formation but disappear in the upper half (above sandstone 11 of Hentz, 1988). These are replaced by vertisols, which are formed in conditions of fluctuating moisture with four to eight dry months per year (Tabor and Montañez, 2004).
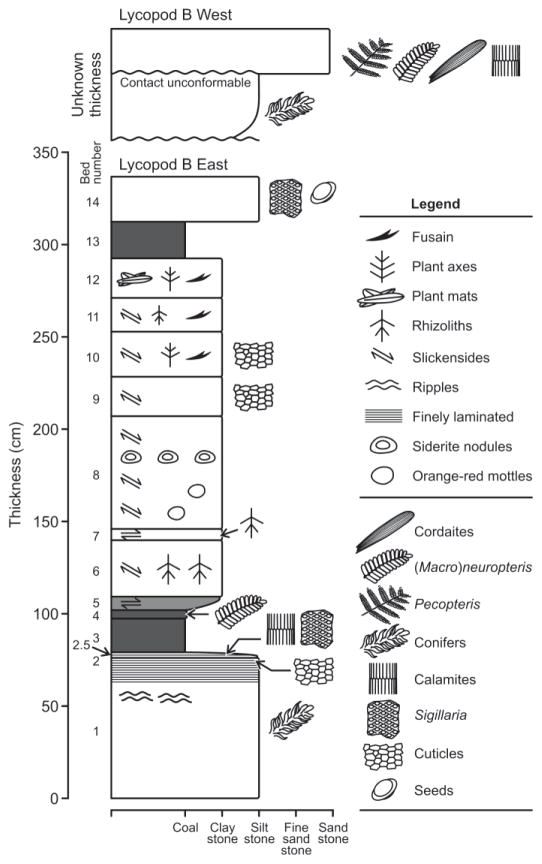
The locality termed ‘Lycopod B’, the focus of this study, occurs in Young County immediately underlying sandstone 10 of Hentz, 1988; also see Fig. 2 of Tabor and Montañez, 2004). It consists of two separate sites, Lycopod B West and East, about 150 m apart, from which a composite section was drafted (Fig. 1). The two exposures together comprise a single typical sedimentary package with a paleosol marking the base and a cap sandstone at the top. The palynologically productive exposure is located at the Lycopod B East site, which has subsequently eroded away. The following description is based on detailed field notes from W.A. DiMichele supplemented by additional field notes from one of the authors (CLH).
Fig. 1.
Composite section of localities Lycopod B West and East, Markley Formation, north-central Texas.
The beds at Lycopod B are lenticular, tilting upward at the margin toward the north (Fig. 2). The exposure at Lycopod B East is approximately 3.4 m thick and rests on a paleosol. At the base lies a gray, relatively indurated, kaolinitic siltstone bearing compressions of walchian conifer branch fragments, up to 10 cm long (bed 1). The bed is obscurely cross-laminated, but becomes increasingly finer grained, planar-laminated and organic-rich in the upper 2–3 cm (bed 2). Fine rhizoliths, several centimeters in diameter, are present in the top of this bed but are rare; otherwise there is no evidence of pedogenesis. A thin layer containing numerous flexible, strap-shaped cuticle fragments of unknown affinities occurs within bed 2. The top of the kaolinitic unit merges gradually into a black, microlaminated mudstone about 3 cm thick containing compressions of Calamites and Sigillaria (bed 2.5). This unit underlies a thin friable, 18 cm thick coal with vitric streaks (bed 3), and is overlain by a thin highly carbonaceous clay or ‘bone coal’ containing pinnules of Macroneuropteris scheuchzeri (bed 4). Above the coal lie a series of medium gray to almost black claystones and mudstones that, together with the coals, comprise the organic facies of this sedimentary package. These clastic units display contorted or obscure laminations, as well as vertical rhizoliths up to 5 mm in diameter, slickenplanes, vertical cracking, manganese coatings, orange mottles and fragments of plant axes (beds 5–11). Fusain fragments occur in beds 10–11. Near the top of the exposure lies a thin, organic-rich paper shale, consisting of highly compressed, unidentifiable plant fragments (bed 12), and overlain by a thin, highly friable coal with vitric streaks at the top (bed 13). The coal is overlain by an organic-rich indurated siltstone (bed 14) containing large compressions of Sigillaria brardii and two types of seeds of unknown affinities (Figs. 1 and 2).
Fig. 2.
Lycopod B East locality outcrop (informal collection name 1990-31; USNM localities 40081, 40682, and 43546). Indicated are the bottom and top of the sampled and measured section. Numbers on the image indicate the lithologically distinct units, each sampled for palynological analysis, except for beds 5 and 11. The people are Ken Craddock (seated left), Christopher Durden (standing center) and Dan Chaney (right; DiMichele pers. comm. 2011).
The exposure at Lycopod B West correlates precisely with Lycopod B East, and comprises a complete ‘typical’ Markley Formation sedimentary package from paleosol to cap sandstone (Fig. 1). Lycopod B West was not sampled for palynomorphs, but it contains significant suites of plant megafossils. The organic interval is about one half the thickness of the interval at Lycopod B East, and the coals at Lycopod B East grade into organic-rich clastics at Lycopod B West. A unit corresponding to bed 12 of Lycopod B East contains Macroneuropteris scheuchzeri, Calamites and Sigillaria. Sigillaria also occurs in a siltstone correlative with bed 14 of Lycopod B East. This fossiliferous siltstone is overlain by a dark gray claystone, which underlies a light, kaolinitic siltstone containing compressions of walchian conifers. This kaolinitic bed, in contrast to the one at Lycopod B East underlies coarsening upward siltstones, mudstones and inceptisols (heterlithic beds) unconformably overlain by a resistant, coarse-grained cap sandstone. The base of the sandstone contains a megafloral assemblage unusual in its abundant cordaitalean leaves, along with rare Pecopteris and Neuropteris ovata pinnules, and a calamite (Fig. 1).
3 Materials and methods
3.1 Sample collection, preparation and imaging
A trench was dug through the exposure at Lycopod B East to expose fresh sediment (Fig. 2). Palynological samples (sample code LycB90) were collected from each lithologically distinct unit except beds 5 and 11 (Fig. 1 and 2). Approximately 10 g of material for each sample was processed by Global Geolab Ltd. (hydrofluoric and hydrochloric acid maceration, heavy liquid separation and sieving through a 15 micrometer mesh). Palynological residues were strew-mounted in glycerine jelly. Twelve out of thirteen samples were productive. Slides were observed with Nikon Eclipse 80i and Leica DM2500 microscopes. Three hundred grain counts were made, and an additional 200–400 grains were scanned for rare taxa. High-resolution images were taken with the Leica microscope using Differential Interference Contrast, a Plan Apo 63× Oil objective, and a Nikon DS-Fi1 Digital Camera. Extended depth of field (EDF) images were generated using CombineZP (Alan Hadley, http://www.hadleyweb.pwp.blueyonder.co.uk/CZP/News.htm). Illustrated specimens are stored in the Paleobotanical Type and Illustrated Collections under the USNM catalog numbers USNM 546150-546189 (see also figure legends).
3.2 Data analysis
A list of pollen and spore taxa and their per-sample abundance was compiled. The initial count data were then reduced by combining certain dispersed pollen taxa that were judged to be likely derived from the same whole plant taxon, in order to create a condensed tally list. Exploratory data analysis was carried out by first examining the composition of each palynological assemblage with respect to abundance, species richness and botanical affinities. Abundance was binned according to the following categories: dominant, 25–100%; abundant, 10–25%; frequent, 5–10%; common, 1–5%; rare, < 1%. Detrended Correspondence Analyses (DCA) was chosen as a data ordination technique to explore the relationship between palynofloral composition and depositional environments. In order to make an informed choice between available ordination techniques, an initial DCA (detrended by segments) was carried out, in which sample scores are expressed in standard deviation. The total lengths of gradient on the first axis were almost 3 standard deviations, justifying the use of an ordination method that assumes a unimodal response of species abundance along environmental gradients (e.g. Correspondence Analysis), rather than methods assuming a linear response model (e.g. Principal Component Analysis). The detrending method in the analyses was based on 26 segments. The data set utilized 30 taxa or groups of taxa with an abundance of 2% or higher in at least one of the samples. Rarer taxa were combined with other taxa from the same plant group, except for Nuskoisporites and Potonieisporites, which are of particular ecological interest. The DCA was carried out using PAST 3.02 software (Hammer et al. 2001). Palynofloral diversity of all samples was summarized in two metrics that reflect two important aspects of diversity: simple diversity (S) to express taxon richness (or the number of taxa encountered in a subset of 300 palynomorphs), and equitability (EH) as a measure of evenness. The latter is the Shannon index for entropy divided by the natural logarithm of the simple diversity. It is calculated as follows:
where pi is the proportion of individuals of the ith species in the total number of specimens, and S is the number of taxa in the sample.
4 Interpreting plant distribution from dispersed pollen and spores
4.1 Botanical affinities of pollen and spores
Reconstructing landscapes from dispersed pollen and spores requires knowledge of the affinities of the parent plants. For Pennsylvanian wetland species, affinities of many pollen/spore taxa are well known at the family or ordinal level, e.g., Sigillariales, Chaloneriaceae, Medullosales, Sphenophyllales, Calamitales, Marrattiales, Tedeleaceae, Gleicheniaceae (Table 1). Parent plants for dryland taxa, because of their relative rarity, are much more poorly understood. However, some generalizations can be made. Monosaccate prepollen (Potonieisporites and Nuskoisporites) can be connected with confidence to walchian conifers (Table 1; Poort et al., 1997). Non-taeniate bisaccate pollen (Falcisporites, Pityosporites) has been attributed to Permian peltasperms (Zavialova and van Konijnenburg-van Cittert, 2011). Vesicaspora is well established as pollen of Carboniferous Callistophytaceae (Millay and Taylor, 1979; Rothwell, 1981), but has also been associated with Angaran Permian and Triassic peltasperms (see review in Zavialova and van Konijnenburg-van Cittert, J.H. 2011). Either the Alis porites/Falcisporites complex or Vesicaspora, or both, may represent spores of local peltasperms (DiMichele et al., 2005b; Table 1 and references therein). This association is plausible, as peltaspermales may be related to or possibly even descended from callistophytes (Hilton and Bateman, 2006; DiMichele et al., 2013). Several taeniate saccate pollen (Protohaploxypinus, Striatoabietites, Hamiapollenites) and taeniate non-saccate pollen (Vittatina) have not been associated with parent plants in the Pangaean tropics. Taeniate bisaccates are characteristic of the Southern Hemisphere order Glossopteridales (e.g., Protohaploxypinus, Striatopodocarpidites; Table 1; Zavada, M.S., 1991; Lindström et al., 1997). However, no glossopterid megafossil has ever been reported from the equatorial lowlands, so Glossopteridales are considered highly unlikely sources for taeniate pollen in the Markley Formation. Taeniate pollen (Protohaploxypinus, Vittatina) has been found in association with the Upper Permian Angaran peltasperm Tatarina (e.g., Gomankov and Meyen, 1986). Thus, peltasperms may have been one group that produced taeniate pollen in the Late Pennsylvanian and Early Permian of tropical Pangea, although there is no direct evidence of this. Colatisporites decorus is another problematic taxon. It bears a strong resemblance to Angulisporites intonsus Wilson 1962, originally described as a spore by Williams in Neves et al. (1973) and treated as such by Dolby et al. (2011). However, it has a pseudoalveolar outer layer and bears a strong resemblance to in situ spores in ovules of Lyginopteris (Retallack and Dilcher, 1988). We treat it here as a likely seed fern of unknown affinities.
Table 1.
Lycopod B identified pollen and spores and botanical affinity. First column: Taxonomic resolution: ● = known from a single order or family; ◉ = known from a single class, but in multiple or unknown orders; ○ = known from a single division, but in multiple or unknown classes; ✕ = known from multiple divisions or division unknown; Second column: Taxa; Third column: (1) Literature references to in situ records and previous authors who have assigned particular spore/pollen taxa to macrofloral elements; (2) information where these micro-macro correlations were established (locality, area and age); (3) and comments on the reliability of correlations, indicating how trustworthy specific assignments to taxa are.
| Spore or (pre)pollen category | Botanical affinity | |
|---|---|---|
| ✕ | Alisporites cf. plicatus Jizba 1962 |
Peltaspermales (seed ferns) and Voltziales
(conifers) Alisporites s.l. has been described in situ from the peltamales pollen organ Pterispermostrobus (Permian, Germany; Balme, 1995), pollen organ Lelestrobus (? Peltaspermales, Permian/Triassic, India; Srivastava, 1984), and Triassic Corystospermales (Osborn and Taylor, 1993). It has also been isolated from conifer cones, including Ullmannia (Voltziales, Permian, Germany; Florin, 1944; Potonié, 1962) and Willsiostrobus (Triassic, France; Grauvogel-Stamm, 1978). |
| ✕ | Anapiculatisporites vegrandis (Upshaw et Creath) Ravn 1986 |
Botryopteridaceae (herbaceous ferns) and unknown
Lycopsida (lycopods) SEM studies have shown that spores found in Botryopteris sp. (Carboniferous, USA) belong to Anapiculatisporites (Balme, 1995), but it has also been also described from a lycopsid of unknown affinity (Carinostrobus, Carboniferous, USA; Baxter, 1971). |
| ● |
Apiculatasporites aculeatus (Ibrahim)
Ravn 1986 A. latigranifer (Loose) Ravn 1986 A. spinulistratus (Loose) Ibrahim 1933 A. variocorneus (Sullivan) Ravn 1986 A. variusetosus (Peppers) Ravn 1986 |
Zygopteridales (herbaceous
ferns) Apiculatasporites spores (including A. spinulistratus) were found in situ in Corynepteris (Carboniferous, USA, England, Germany and France; Brousmiche, 1983; Balme, 1995). |
| ● | Apiculatisporites saetiger (Peppers) Ravn 1986 |
Zygopteridales (herbaceous
ferns) Apiculatisporites saetiger spores have been found in situ in Corynepteris (Carboniferous, Scotland, Hemsley et al., 1994). |
| ● | Cadiospora magna Kosanke 1950 |
Lepidodendrales: Sigillariaceae;
Sigillaria (lycopods) Cadiospora is known from the heterosporous strobilus Thomasostrobus, which resembles those of Sigillaria brardii (Pennsylvanian; Czech Republic; Opluštil et al., 2009). |
| ○ |
Calamospora breviradiata Kosanke
1950 C. flexilis Kosanke 1950 C. parva Guennel 1958 C. pedata Kosanke 1950 C. straminea Wilson et Kosanke 1944 |
Equisetales, Sphenophyllales (horsetails), and
Noeggerathiales (progymnosperms) Carboniferous Calamospora is known in situ from strobili of Equisetales (e.g., Calamostachys, Palaeostachya) and Sphenophyllales (Bowmanites), and cones belonging to Noeggerathiales, of unknown affinity (e.g., USA, Germany, France, Czech Republic, China; for an overview see Balme, 1995; Taylor et al., 2009). Calamospora in Lycopod B probably do not represent Noeggerathiales; megafossils attributable to that group are rare or absent in the Texas flora. |
| × | Colatisporites decorus (Bharadwaj and Venkatachala) Williams, in Neves et al. 1973 |
? Lyginopteridales (seed ferns) The prepollen Colatisporites has been found in ovules of Lyrasperma scotica, but the pollen organs are unknown (Retallack and Dilcher 1988). |
| ● | Columinisporites ovalis Peppers 1964 |
Sphenophyllales
(horsetails) Columinisporites is known in situ from Euramerican Carboniferous sphenopsid cone taxa Bowmanites (England, Potonié, 1962), Peltastrobus (USA, Taylor, 1986), and Sentisporites (USA; Riggs and Rothwell, 1985). |
| ✕ |
Convolutispora florida Hoffmeister et al.
1955 C. tessellata Hoffmeister et al. 1955 |
Polypodiidae, Marattiales (both ferns) and
Lyginopteridales (seed ferns) Convolutispora spores have been described in situ from Carboniferous Euramerican Zygopteridaceae, Botryopteridaceae, and Marattiales, and Lyginopterdales (Balme, 1995). In Lycopod B Convolutispora likely represents ferns. |
| ● |
Crassispora kosankei (Potonié et
Kremp) Bhardwaj 1957 C. annulata Ravn 1979 |
Lepidodendrales: Sigillariaceae;
Sigillaria (lycopods) Spores attributable to Crassispora (including C. kosankei) have been found in situ in Carboniferous Sigillaria strobili (Mazocarpon, Sigillariostrobus, Megalocarpon; USA, England, Germany; for an overview see Opluštil and Bek (2009)). |
| ✕ |
Cyclogranisporites aureus (Loose) Potonie
et Kremp 1955 C. flavus (Konsanke) Potonie et Kremp 1955 C. microgranus Bhardwaj 1957 C. minutus Bhardwaj 1957 C. lasius (Waltz) Playford 1962 (syn: C. multigranus Smith et Butterworth 1967) C. obliquus (Kosanke) Upshaw et Hedlund 1967 (syn: Punctatisporites obliquus Kosanke 1950) C. orbicularis (Kosanke) Potonié et Kremp 1955 |
Marattiales (tree ferns) and ? Medullosales (seed
ferns) Many species of Cyclogranisporites spores (including cf. C. aureus, minutus and orbicularis) have been found in situ in Asterotheca, Scolecopteris and other fertile fronds of Marattiales (USA, France, Germany; Pfefferkorn et al., 1971; Zodrow et al., 2006) and tentative Medullosan pollen organ Potoniea (Carboniferous, England; for an overview see Balme, 1995). In Lycopod B Cyclogranisporites likely represents ferns. |
| ◉ |
Deltoidospora gracilis (Imgrund) Ravn 1986 D. sphaerotriangularis (Loose) Ravn 1986 |
Polypodiidae
(ferns) Deltoidospora is found a wide range of Mesozoic ferns, but has not been described from Paleozoic representatives. However, Leiotriletes, a junior synonym of Deltoidospora (Balme, 1995) has been reported in Zygopteridales (including spores similar to L. sphaerotriangularis, Carboniferous, England; Chaphekar and Alvin, 1972), and numerous Carboniferous Euramerican Botryopteriaceae (including spores comparable to L. sphaerotriangularis, Carboniferous, USA; Eggert and Delevoryas, 1967). Several of these spores are likely immature; for an overview see Balme (1995). |
| ✕ | Diaphanospora parvigracila (Peppers) Ravn 1979 |
Parent plants unknown These trilete spores have not been found in situ. They could represent one of a number of ferns or seeds ferns. |
| ✕ | Dictyomonolites swadei Ravn 1986 |
Parent plants unknown These spores have not been found in situ. They could represent one of a number of ferns or seeds ferns, but their morphology (small, irregular amb, ornamented, monolete) suggests an association with Marattiales. |
| ● | Endosporites globiformis (Ibrahim) Schopf et al. 1944 |
Isoetales;
Chaloneria/Polysporia
(lycopods) Known in situ from Late Pennsylvanian strobili of Polysporia (USA, United Kingdom, Czech Republic; Chaloner 1958b; Bek et al., 2008). |
| ● | Fabasporites pallidus Clendening 1979 |
Marattiales (tree ferns) Spores assignable to Fabasporites have been found in situ in fertile Acaulangium fronds (Carboniferous, USA, Millay, 1977). |
| ◉ | Falcisporites zapfei (Leschik) Klaus 1963 |
Peltaspermales and Corystospermales (seed
ferns) Falcisporites has been found in a large number of Peltaspermales and some Corystospermales, albeit mainly from the Permian and younger. Permian taxa, including Nidistrobus and Pteruchus (both Permo-Triassic, India; see Balme (1995) and references therein). It is also known from the pollen organ Permotheca (Permian, Russia; Meyen, 1984; Naugolnykh, 2013). |
| ● |
Florinites florinii Imgrund
1960 F. junior Potonié et Kremp 1956 F. mediapudens (Loose) Potonié et Kremp 1956 F. millottii Butterworth et Williams 1954 F. occultus Habib 1966 F. pumicosus (Ibrahim) Schopf et al. 1944 F. similis Kosanke 1950 F. visendus (Ibrahim) Schopf et al. 1944 F. volans (Loose) Potonié et Kremp 1956 |
Cordaitales:
Cordaites Florinites-type pollen has been described from several Cordaitanthus cones (Carboniferous; USA, France; for an overview see Balme, 1995). |
| ◉ | Gillespieisporites venustus Clendening 1969 (?= Triquitrites spp., esp. T. sculptilis) |
Gleicheniaceae (herbaceous
ferns) These trilete acavate spores have not been found in situ. However, they closely resemble Triquitrites spores, which have been found in situ in the fern Szea sinensis (Gleicheniales, Permian, South China; Yao and Taylor, 1988). |
| ✕ |
Granulatisporites adnatus Kosanke 1950
(syn: G. adnatoides (Potonié et Kremp 1955) G. parvus Kosanke 1950 |
Lycopsida, Filicopsida, Equisetopsida,
Lyginopteridales Granulatisporites is an undiagnostic morphotaxon, known from very different plant groups (see Balme, 1995). However, the taxa found in Lycopod B likely represent herbaceous ferns. G. adnatus is found in Botryopteris (England; Holden, 1962) and spores similar to G. parvus in Oligocarpia (Sermayaceae, France; Grauvogel-Stamm and Doubinger, 1975; Brousmiche, 1983, Pšenička et al., 2005). |
| ◉ | Guthoerlisporites sp. |
? Peltaspermales (seed
ferns) According to Balme (1995), some of the spores found in situ in Schuetzia are assignable to Guthoerlisporites (see information under Wilsonites). |
| ✕ | Hymenospora sp. cf. H. caperata Felix et Burbridge 1967 |
Parent plants unknown These trilete cavate spores have not been found in situ. Their parent plant is unknown. |
| ◉ | Horriditriletes sp. |
Polypodiidae: Osmundales and unknown
ferns Spores similar to Horriditriletes have been found in situ in Skaaripteris (Osmundales, Permian, Antactica; Galtier and Taylor, 1994), and in ferns of unknown affinities (Permian, India, and Australia; Balme, 1995). |
| ✕ | Illinites unicus Kosanke 1950 |
Voltziales
(conifers) Illinites pollen has been described from the conifer pollen cone Willsiostrobus (Voltziales, Triassic, France; Grauvogel-Stamm and Grauvogel, 1973; Grauvogel-Stamm, 1978). They have not been found in Carboniferous or Permian pollen organs. |
| ● |
Laevigatosporites globosus Schemel 1951
(intergrades w/ C. obliquus) L. minimus (Wilson et Coe) Schopf et al. 1944 L. minor Loose 1934 |
Marattiales (tree ferns) Both L. globosus and minimus have been isolated from Scolecopteris fronds (Marattiales, Carboniferous; USA, Canada; Pfefferkorn et al., 1971; Millay and Taylor 1984; Pšenicka et al., 2003). Small Laevigatosporites (≤30–35μm) are generally assigned to Marattiales and larger ones to Sphenophyllales (Ravn, 1986; Libertín et al., 2014). |
| ● |
Laevigatosporites desmoinesensis (Wilson
et Coe) Schopf et al. 1944 L. maximus Loose 1934 em. Potonié et Kremp 1956 L. medius Kosanke 1950 L. vulgaris (Ibrahim) Ibrahim 1933 |
Sphenophyllales (horsetails) Several Sphenophyllales produce large monolete spores (≥35μm) with ridged perispores (Columinisporites). When the perispore is not preserved the spores are morphologically compatible with the dispersed spore genus Laevigatosporites (e.g., Playford and Dino, 2000; Balme, 1995; Libertín et al., 2014). |
| ✕ | Leioaletes circularis Ravn et Fitzgerald 1982 |
? Marattiales (tree ferns) Ravn (1986) compares L. circularis to spores described in situ from Radstockia (Carboniferous, USA; Taylor, 1967). However, Libertin et al. (2014) consider Leioaletes to represent immature spores of Laevigatosporites or Latisporites. |
| ● |
Lophotriletes granoornatus Artüz
1957 L. rarispinosus Peppers 1970 |
Botryopteridaceae (herbaceous
ferns) Lophotriletes spores have been described from several Carboniferous taxa, e.g. Botryopteris, Psalixochlaena, Sphyropteris, and Renaultia (USA, England, Germany, France; Remy and Remy, 1957; Good, 1981; Millay and Taylor, 1982; Potonié, 1962; Brousmiche, 1986; Balme, 1995). |
| ● | Microreticulatisporites nobilis (Wicher) Knox 1955 |
Botryopteridaceae and Gleicheniaceae (herbaceous
ferns) Microreticulatisporites spores have been found in Botryopteris (Botryopteridaceae, Carboniferous, USA and France; for an overview see Balme, 1995) and Radiitheca (Gleicheniaceae, Carboniferous, France and Czech Republic; Brousmiche et al., 1997). |
| ● | Nuskoisporites crenulatus Wilson 1962 |
Voltziales (conifers) The prepollen produced by members of the Late Permian conifer genus Ortiseia fall within the dispersed pollen genus Nuskoisporites (Clement-Westerhof, 1984; Poort et al., 1997). N. crenulatus was likely also produced by a conifer. |
| × | Paravesicaspora cf. P. splendens (Leschik) Klaus 1963 |
Parent plants unknown (but seed plant based on
morphology) This pollen type has not been found in situ, but the morphology closely resembles that of Vesicaspora and may represent Callistophytaceae or Peltaspermales, which have similar, alveolate bisaccate or pseudobisaccate, sulcoid pollen. In any case, it almost certainly represents a seed fern. |
| ✕ | Pityosporites sp. cf. P. westphalensis Williams 1955 |
Parent plants unknown (but seed plant based on
morphology) Pityosporites pollen has been found in situ in the gymnosperm microsporophyll Pramelreuthia (Triassic, USA; Ash and Litwin, 1996). The alveolar bisaccate morphology indicates that this form is produced by a seed plant, most likely a seed fern. |
| ✕ | Platysaccus saarensis (Bharadwaj) Jizba 1962 |
Parent plants unknown (but seed plant based on
morphology) Platysaccus has been found in cones associated with the Triassic seed fern Dicroidium (Corystospermaceae) (South Africa, Anderson and Anderson, 1983). The parent plants of the Carboniferous forms are unknown, but the alveolar bisaccate morphology indicates that it represents a seed plant, most likely a seed fern. |
| ● |
Potonieisporites novicus Bharadwaj
1954 P. bharadwaji Remy et Remy 1961 P. elegans (Wilson and Kosanke) Wilson et Venkatachala 1964 P. simplex Wilson 1962 |
Voltziales (conifers) The prepollen Potonieisporites was produced by members of several Pennsylvanian and Early Permian walchian conifer families, including Emporiaceae, Bartheliaceae, Thucydiaceae, Utrechtiaceae (e.g., Hernandez-Castillo et al. 2001; Rothwell et al., 2005). |
| ○ | Protohaploxypinus sp. |
Glossopteridales and Peltaspermales (seed
ferns) Protohaploxypinus pollen has been found in Permian pollen organs associated with Gondwanan Glossopteridales (e.g., Gould and Delevoryas, 1977; Zavada, 1991; Lindström et al., 1997). Protohaploxypinus was found in situ in pollen organ Permotheca, and in association with Peltaspermalean cuticles in a coprolite (Russia, Gomankov and Meyen, 1986; Meyen, 1997). Their parent plants in the Euramerican realm are, however, unknown. |
| ✕ |
Punctatisporites edgarensis Peppers
1970 P. flavus (Kosanke) Potonié et Kremp 1955 P. glaber (Naumova) Playford 1962 [syn: P. curviradiatus Staplin 1960] P. obesus (Loose) Potonié et Kremp 1955 Punctatisporites minutus (Kosanke) Peppers 1964 Punctatisporites aerarius Butterworth et Williams 1958 Punctatisporites nitidus Hoffmeister et al.1955 |
Filicopsida, Equisetopsida, Cycadopsida,
Noeggerathians, and Calamopityales Mature Carboniferous forms resembling Punctatisporites have been described from many plant groups (for an overview see Balme, 1995). Punctatisporites aerarius has been reported from Scolecopteris majopsis (Lesnikowska, 1989; see also Eble et al., 2003). P. obesus is known in situ from Bowmanites (sphenophylls, Czech Republic; Libertín et al. 2008). However, most of the species of Punctatisporites in the Lycopod B flora likely represent Marattiales or herbaceous ferns (Ravn, 1986. |
| ● |
Punctatosporites punctatus Ibrahim
1933 P. rotundus Bhardwaj 1957 em. Alpern et Doubinger 1973 |
Marattiales (tree
ferns) Punctatosporites has been reported in situ from several Marratialean sporangia (USA, Pfefferkorn et al., 1971; France, Germany, Spain; for an overview see Balme, 1995). |
| ✕ | Pustulatisporites sp. cf. P. papillosus (Knox) Potonié et Kremp |
Parent plants unknown These spores have not been found in situ. They could represent one of a variety of ferns or seeds ferns. |
| ✕ | Reinschospora speciosa (Loose) Schopf et al. 1944 |
Parent plants unknown These trilete spores have not been found in situ. However, most likely they represent some kind of fern or lycophyte. |
| ✕ | Reticulatisporites reticulatus (Ibrahim) Ibrahim 1933 |
Sphenophyllales (horsetails) and unknown
ferns Reticulatisporites reticulatus is known from Bowmanites cones (Sphenophyllales, Carboniferous, Czech Republic, Bek and Libertín, 2010), and in sporangial aggregates of unknown filicopsid affinity (Eopteridangium, Carboniferous, USA; Andrews and Agashe, 1962; Potonié, 1967). |
| ✕ |
Rugospora gracilirugosa
Ravn 1986 R. radiata Ravn et Fitzgerald 1982 |
Parent plants unknown These acavate trilete spores have not been found in situ. They could represent one of a variety of ferns or seeds ferns. |
| ○ | Sahnisporites sp. cf. S. saarensis Bharadwaj 1954 |
Voltziales (conifers) These monolete bisaccate pollen have not been found in situ. However, they bear a close resemblance to Potonieisporites (Walchian Voltziales). |
| ● | Schopfipollenites ellipsoides (Ibrahim) Potonié et Kremp 1954 |
Medullosales (seed
ferns) Schopfipollenites (Monoletes) type pollen has been found in situ in Aulacotheca, Bernaulthia, Dolerotheca (Carboniferous; USA, United Kingdom, France; e.g., Millay and Taylor, 1979; Taylor and Rothwell, 1982; Balme, 1995). |
| ● | Spinosporites exiguus Upshaw and Hedlund 1967 |
Marattiales (tree ferns) Spores resembling Spinosporites exiguus have been described from Scolecopteris monothrix (Millay, 1979, Millay and Taylor, 1984). |
| ✕ | Tantillus triquetrus Felix et Burbridge 1967 |
Parent plants unknown These acavate trilete spores have not been found in situ. They could represent one of a variety of ferns or seeds ferns, but in the Lycopod B flora most likely represent an herbaceous fern. |
| ● |
Thymospora obscura (Kosanke) Wilson et
Venkatachala 1963 T. thiessenii (Kosanke) Wilson et Venkatachala 1963 |
Marattiales (tree
ferns) Thymospora (including T. obscura and thiessenii) are known from synangia of fertile marattialean fronds (USA, France, Germany; Doubinger and Grauvogel-Stamm, 1971; Millay 1979; Lesnikowska and Willard, 1997). |
| ● |
Triquitrites sculptilis Balme 1952 em.
Smith et Butterworth 1967 T. spinosus Kosanke 1943 |
Gleicheniaceae (herbaceous
ferns) Triquitrites spores have been found in situ in Szea sinensis (Gleicheniaceae, Permian, South China; Yao and Taylor, 1988). |
| ● | Tuberculatosporites robustus (Kosanke) Peppers 1970 |
Marattiales (tree
ferns) Tuberculatosporites spores are known in situ from the following Marattiales: Pecopteris (Carboniferous, France), Qasimia (Permian, Saudi Arabia), and Scolecopteris (Carboniferous USA). For an overview see Balme (1995). |
| ✕ |
Verrucosisporites donarii Potonié
et Kremp 1955 V. sp. cf. V. morulatus (Knox) Potonié et Kremp 1955 em. Smith et Butterworth 1967 Verrucosisporites verrucosus (Ibrahim) Ibrahim 1933 V. microtuberosus (Ibrahim) Smith et Butterworth 1967 |
Zygopteridales, Marattiales, Lyginopteridales,
Medullosales, Isoetales The genus Verrucosisporites is an undiagnostic morphotaxon that has been attributed to, Marattiales, Lyginopteridales, Medullosales and even Isoetales (for overview see Balme, 1995). Most or all of the Verrucosisporites spores in Lycopod B likely represent Zygopteridales and Marattiales. V. verrucosus has been reported in situ from Corynepteris (Zygopteridales, Carboniferous, U.S.A, Pfefferkorn et al., 1971). Spores resembling V. microtuberosus were produced by Acitheca (Marattiales, Carboniferous, Canada, Czech Republic; Zodrow et al., 2006). |
| ○ |
Vesicaspora wilsonii Schemel 1951 em.
Wilson et Venkatachala 1963 V. ovata (Balme and Hennelly) Hart 1960 |
Callistophytales and Peltaspermales (seed
ferns) Vesicaspora has been found in situ in pollen organs (Idanothekion) borne on Callistophyton pinnules (Carboniferous, USA, Millay and Taylor, 1979; Rothwell 1981), and in the pollen organs Pterispermostrobus (Peltaspermales, Permian, Germany, Kerp, 1988) and Permotheca (Permian, Russia; Krassilov et al., 1999; Zavialova and Van Konijnenburg-van Cittert, 2011). |
| ✕ |
Waltzispora sagittata Playford
1962 Waltzispora prisca (Kosanke) Sullivan 1964 |
Parent plants unknown These trilete spores have not been found in situ. They could represent one of a variety of ferns or seeds ferns. |
| ◉ | Wilsonites circularis (Guennel) Peppers et Ravn, in Ravn 1979 |
? Peltaspermales (seed
ferns) Wilsonites is known from pollen organs (Schuetzia) associated with Sphenopteris germanica; a potential peltasperm (Permian; Germany; Remy and Rettschlag, 1954; Balme, 1995). |
| ✕ | Zosterosporites triangularis Kosanke 1973 |
Parent plants unknown These trilete spores have not been found in situ. They could represent one of a variety of ferns or seeds ferns. |
Two factors complicate the interpretation of parent plant from dispersed pollen and spores. The first is rampant homoplasy in palynomorph morphology, especially in taxa with simple morphology. For example, the morphotaxon Granulatisporites was produced at various times by most of the major vascular plant clades (lycophytes, monilophytes, seed plants) (Table 1). The megaflora in the Markley helps constrain possible candidates; in this case, the source plant for Granulatisporites was probably some kind of fern. Another, more problematic example comes from the smooth monolete spore Laevigatosporites, produced by both Marattiales and Sphenophyllales. Generally, smaller forms less than about 30 micrometers are attributed to Marattiales and larger forms are attributed to Sphenophyllales; however there is some overlap in size distribution between these two orders (Ravn, 1986). A second confounding factor is the evident inflation of dispersed spore and pollen species and genera, given the diversity of dispersed morphotaxa that can be recognized in a single sporangium or pollen (e.g., Zavada, 1991; Lindström et al., 1997; Bek and Pšenička, 2001). For these reasons, we have adopted a conservative approach to palynomorph species circumscriptions, but it should be borne in mind that some degree of error in taxonomic assignments will remain in any paleoecological analysis using dispersed pollen and spores.
4.2 Pollen and spore taphonomy
Taphonomy of dispersed pollen and spores must be considered before they can be used as proxies for plant assemblages in ecological space. Pollen and spores are generally deposited as silt-sized particles, so they are usually not present in coarse grained sediments such as sandstones or in very pure claystones (Traverse, 2007). The distribution and abundance of pollen and spores in sediments is affected by numerous factors, including abundance and height of parent plant, pollen productivity and wall thickness, phenology and mode of pollen/spore dispersal, canopy structure, prevailing wind patterns and air currents, rainfall patterns, position on the landscape relative to depocenters, and environment of deposition. Trees that are heavy pollen or spore producers, such as Marattiales (tree ferns), will be particularly overrepresented in a given sample. Common taxa will overwhelm rare taxa, species growing close to a catchment area will be more abundant than those distant from the basin, and taller plants will disperse their spores or pollen farther than plants of low stature. Herbaceous ferns, for example, tend not to disperse their spores far from where they are growing. How pollen or spores are distributed also plays a role in pollen productivity and distribution. It is often assumed that all Pennsylvanian plants were anemophilous, but the large size of pre-pollen of Medullosaceae (200–600 micrometers) suggests zoophily (Taylor and Millay, 1979; Labandeira, 2000; Schwendemann et al., 2007), or perhaps hydrophily (DiMichele, 2014). Zoophily could explain the apparent underrepresentation of Medullosaceae in the pollen record relative to their abundance in megafossil assemblages, because zoophilous plants tend to produce less pollen. The structure of the canopy also affects pollen and spore distribution, since open canopy allows dispersal by air currents over a greater distance. Coal swamps dominated by Marattiales are thought to have possessed closed canopies (DiMichele et al., 2009), which may be one of the factors accounting for the hyperabundance of tree fern spores in many samples. Data on spacing of trees in dryland habitats are limited; however, what little evidence exists suggests a relatively open canopy (Falcon-Lang et al., 2011a; Bashforth et al., 2014). In any case, plant cover in general tends to be significantly lower in drier environments, because water is the single most important physiological constraint for land plants, so it is reasonable to suppose that canopy in dryland environments was not as dense compared to wetland habitats.
Knowledge of facies type is also critical to making accurate ecological extrapolations from dispersed palynofloras. As a general rule, depositional environments with little or no clastic input, such as peat or carbonaceous mudstones, will represent primarily spores or pollen carried by wind from vegetation growing in or near the periphery of the depositional site (Bunting, 2008). These kinds of depositional environments will more closely resemble megafloral assemblages (Nichols, 2005). In contrast, sediments deposited at the mouth of a large river can potentially include palynomorphs drawn from great distances and highly varied environments (as a modern example, pollen from North Dakota can be carried downstream for hundreds of kilometers and deposited at the mouth of the Mississippi River (Traverse, 2007)). In general, pollen/spore assemblages, especially those derived primarily from water lain deposits that include a mixture of sediments eroded and re-deposited from stream banks, are time-averaged, in contrast to most megafloral assemblages, which represent ‘snapshots’ or near instantaneous events (Burnham, 1993; Wing and DiMichele, 1995).
5 Results
5.1 Palynology
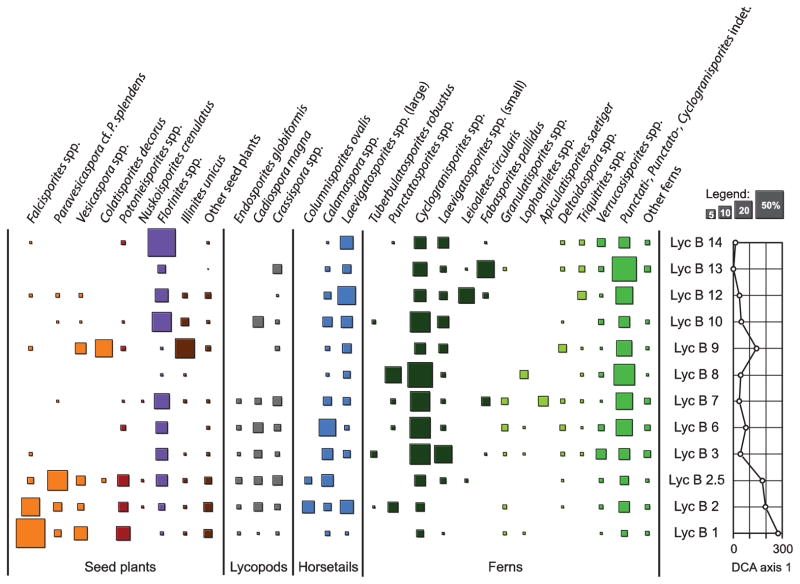
Two endpoint assemblages may be recognized among the samples. Assemblage I occurs in the organic facies (LycB90-3-14), and is dominated by characteristic Late Pennsylvanian wetland taxa (Fig. 3, Plates 1 and 2, Table 1). Spores of Marattiales (e.g., Cyclogranisporites, Punctatisporites spp., Punctatosporites spp., Laevigatosporites globosus, Thymospora spp. and Verrucosisporites microtuberosus (Plate 1, Figs. 16–20) are consistently present and collectively are abundant to dominant. Arborescent lycopsids are represented by low levels of Crassispora kosankei (Sigillaria brardii), Endosporites globiformis (Chaloneria spp.), as well as Cadiospora magna (Thomasostrobus, possibly Sigillariales) (Plate 2, Figs. 1–3). Spores of Calamites (Calamospora spp.) as well as Sphenophyllales (e.g., Laevigatosporites medius and L. desmoinesensis) are abundant in most of the organic facies (Plate 1, Figs 3–6). Herbaceous ferns display considerable diversity, although usually in low abundance (Plate 1, Figs. 7–15). Cordaitalean prepollen (Florinites spp.) varies from common to abundant (Plate 2, Figs. 4–7). Pollen or prepollen of other seed plants, such as the medullosan seed fern Schopfipollenites, are rare (Plate 2, Fig. 14). Conifer prepollen such as Potonieisporites, Nuskoisporites and other non-taeniate bisaccates and pseudosaccates occur sporadically at extremely low abundance (one or two grains on a slide) throughout the section.
Fig. 3.
Taxonomic composition and percent relative abundance of selected pollen and spore types per sample. Palynomorph taxa or groups of taxa are clustered according to their botanical affinity. The surface area of each square is a measure for a taxon’s realtive abundance in a sample. As a summarizing measure for the dominant compositional changes along the section, the sample scores on the first axis of a DCA (see Fig. 5) are plotted on the right. Colors (from left to right): orange = seed ferns; red = conifers; purple = cordaitaleans; brown = other seed plants; grey = lycopods; blue = horsetails; dark green = tree ferns; light green = herbaceous ferns; intermediate green = other ferns.
Plate I.
Selected simple spores from the Lycopod B East locality, Markley Formation, north-central Texas. Specimen names are followed by USNM numbers, slide code and England Finder graticule coordinates. Scale bar is 20 μm.
1. Leioaletes circularis (USNM 546150, LycB90-12-1, B38-3)
2. Diaphonosporites parvigracilis (USNM 546151, LycB90-10-1, H45-4)
3. Calamospora breviradiata (USNM 546152, 40081-1, G44)
4. Columinisporites ovalis (USNM 546153, LycB90-2-6, L38-2)
5. Laevigatosporites medius (USNM 546154, LycB90-7-1, D62-4)
6. Tuberculatosporites robustus (USNM 546155, LycB90-10-3, T41-2)
7. Anapiculatisporites vegrandis (USNM 546156, TX2007-5-2, Q48-2)
8. Converrucosporites sp. (USNM 546157, LycB90-6 GG2, E18-2)
9. Convolutispora sp. (USNM 546158, LycB90-9-2, C43-2)
10. Gillespiesporites venusta (USNM 546159, LycB90-10-1, O51-4)
11. Granulatisporites sp. (USNM 546160, LycB90-6 GG2, P18-2)
12. Gillespiesporites venustus (USNM 546161, LycB90-10-1, W63-3)
13. Triquitrites sculptilis (USNM 546162, LycB90-7-1, Y62-1)
14. Triquitrites spinosus (USNM 546163, LycB90-10-3, O53)
15. Verrucosiporites donarii (USNM 546164, LycB90-14-1, S47)
16. Cyclogranisporites aureus (USNM 546165, LycB90-10-3, V37-4)
17. Cyclogranisporites flavus (USNM 546166, LycB90-8-2, D43)
18. Cyclogranisporites minutus (USNM 546167, LycB90-9-2, K55)
19. Dictyomonolites swadei (USNM 546168, LycB90-1-1, N41-1)
20. Punctatisporites minutus (USNM 546169, LycB90-10-1, V37-2)
Plate II.
Selected camerate spores and pollen from the Lycopod B East locality, Markley Formation, north-central Texas. Specimen names are followed by USNM numbers, slide code and England Finder graticule coordinates. Scale bar is 20 μm.
1. Cadiospora magna (USNM 546170, LycB90-10-1, M35-2)
2. Crassispora kosankei (USNM 546171, LycB90-7-1, M56-4)
3. Endosporites globiformis (USNM 546172, LycB90-10-3, H47)
4. Florinites mediapudens (USNM 546173, LycB90-10-1, U37-2)
5. Florinites millottii (USNM 546174, LycB90-7-1, P60-2)
6. Florinites pumicosus (USNM 546175, LycB90-10-1, X43-1)
7. Florinites volans (USNM 546176, LycB90-101, D36-3)
8. Nuskoisporites sp. (USNM 546177, LycB90-10-3, S37-1)
9. Nuskoisporites sp. (USNM 546178, LycB90-7-1, X62-2)
10. Potonieisporites elegans (USNM 546179, LycB90-2.5, U46-1)
11. Potonieisporites simplex (USNM 546180, LycB90-1-1, U46-3)
12. Potonieisporites sp. (USNM 546181, LycB90-6-GG2, P17-1)
13. Vesicaspora wilsonii (USNM 546182, TX2007-5-2, Q54-1)
14. Schopfipollenites ellipsoides (USNM 546183, LycB90-7-1, X63)
15. Alisporites sp. (USNM 546184, LycB90-10-3, L34-2)
16. Alisporites cf. plicatus (USNM 546185, LycB90-1-1, K44-1)
17. Falcisporites zapfei sensu Jizba (USNM 546186, LycB90-1-1, S43-1)
18. Illinites cf. unicus (USNM 546187, LycB90-10-1, K58-3)
19. Platysaccus sp. (USNM 546188, TX2007-5-2, T39)
20. Protohaploxypinus sp. (USNM 546189, 2007-5-2, H45-1)
Within the organic mudstones, two samples, LycB90-8 and 9 (from beds 8 and 9) are somewhat anomalous in displaying low diversity and possessing unusual taxa or unusually high abundance of certain taxa. Sample 8 is strongly dominated by the marattialean tree fern spore Cyclogranisporites minutus and a complex of spores resembling Punctatispories punctatus, P. obesus and P. flavus, many with anomalous germination apertures (Fig. 3). Presumed sphenophyte spores (large Laevigatosporites spp.) are common, whereas other fern and seed fern spores are subordinate. Sample 9 is characterized by unusual taxa, especially the putative seed fern taxon Colatisporites decorus and seed plant Illinites unicus. I. unicus (Plate 2, Fig. 18) is a taeniate bisaccate suggesting a phylogenetic relationship to Permian forms, but it is locally common in Late Pennsylvanian wetland floras (Peppers, 1996).
Assemblage II occurs only in the kaolinitic siltstone at the base of the exposure (LycB90-1) and comprises a markedly different flora compared to the rest of the section (Fig. 3). Dominants in this assemblage include pollen of seed ferns such as the non-taeniate bisaccates Falcisporites and Alisporites, and species of the pseudobisaccate genus Vesicaspora (Plate 2, Fig. 13, 15–17). Species of Potonieisporites (Plate 2, Fig. 8–12), representing walchian conifers, are abundant. Diversity and abundance of spores in this assemblage is low, and only species of Cyclogranisporites (Marattiales) are common. Sphenophyte spores (Calamospora and Laevigatosporites), lycopsid spores (Cadiospora, Crassispora, and Endosporites), and cordaitalean prepollen (Florinites) are all rare. Most of the less common taxa found in Assemblage I are absent.
A mixed assemblage that includes taxa from both Assemblage I and II is found in samples from the top of the kaolinitic siltstone immediately underlying the first coal bed (samples LycB90-2 and 2.5 from beds 2 and 2.5; Fig. 3). Just as in Assemblage II, the non-taeniate bisaccates Falcisporites and Alisporites are dominant. Prepollen of walchian conifers is abundant and includes Potonieisporites and Nuskoisporites. A pseudobisaccate or monosccate form that we term here cf. Sahnisporites may also represent a conifer, based on its morphological similarity to Potonieisporites. Most unexpected, several grains of Protohaploxypinus occur in sample LycB90-2—this becomes much more common in the Permian of north Texas and elsewhere but is extremely rare in the Pennsylvanian (Peppers, 1996). The proportion of lycophyte, sphenophyte, and marratialean tree fern spores, and cordaitalean prepollen, however, is much higher compared to Assemblage II; in this respect, the mixed assemblage more closely resembles Assemblage I (Fig. 3). The sphenophyll Columinisporites ovalis is common in these two samples, in marked contrast to its rarity elsewhere in the section (Fig. 3).
Per sample taxon richness ranges from 30 to 55 taxa (Fig 4). Most of the samples in the organic facies display moderate evenness and diversity. Exceptions are samples 8 and 9, which display low species diversity and low evenness. Sample 2 combines the highest diversity (55 taxa) with high evenness and sample 2.5 displays moderate diversity and evenness. The assemblages with the highest number of taxa also display high equitability, whereas the less diverse samples lack evenness. Compared to the other samples, Assemblage II has low mean diversity, coupled with the lowest evenness.
Fig. 4.
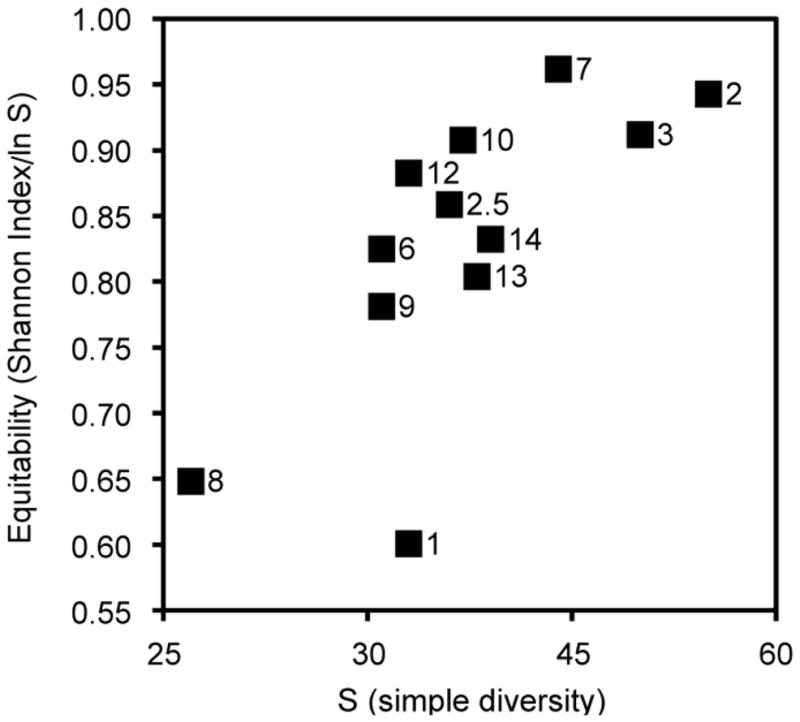
Diversity aspects of palynological samples. Equitability values (based on the Shannon index of entropy) are plotted against taxon richness (as simple diversity). LycB90 sample numbers are shown next to the data points.
5.2 Ordination analysis
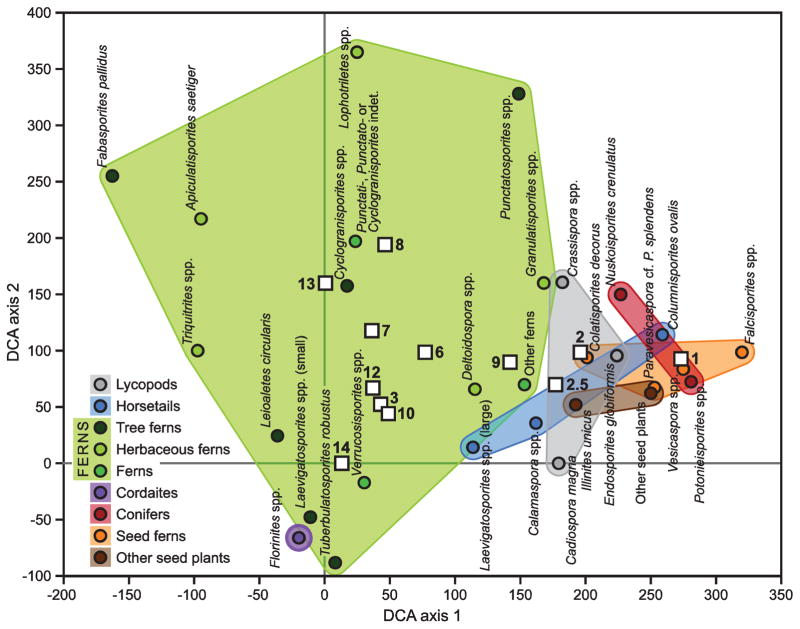
Ordination of palynological abundance data against sample (i.e., bed) shows clearly that samples are arrayed along Axis 1, which accounts for 26.4% of the variance in the data (Fig. 5). Axis 2 accounts for another 10.5% of the variance in the data. The taxa that drive these loadings are outlined in convex hulls of color for visual clarity. Fern spores, primarily those of Marattiales but also including herbaceous ferns such as Triquitrites (Gleicheniaceae), as well as Florinites prepollen (Cordaitales) occupy an area in ordination space on the central to left side of Axis 1 where they peak in abundance. Seed plant peak abundances, except for Cordaitales, are strongly clustered on the positive side of Axis 1, with none having scores lower than about 200. Sphenophytes display greater range along Axis 1. Notably, Columinisporites ovalis (Sphenophyllales) lies well within the seed plant cluster; this species is one of the few spore types to persist into the Permian. Perhaps surprisingly, because lycophytes tend to occupy the wettest habitats in many Pennsylvanian wetland assemblages, lycophytes here lie outside the cluster formed by Florinites plus fern spores. Like Columinisporites ovalis, these lycophyte taxa represent forms that persist into the Permian.
Fig. 5.
Ordination of palynomorphs from the Lycopod B sequence, based on a Detrended Correspondence Analysis (DCA) of the data. Selected palynomorph taxa (or groups of taxa) are plotted as colored dots on the first two DCA axes, which respectively account for 26.4 and 10.5% of the variance in the species data. The palynomorphs are grouped in colored convex envelopes, based on their botanical affinity. In this biplot, samples are shown as white squares; their numbers refer to the ‘LycB90’ sample numbers.
6. Discussion
6.1 Lithofacies interpretation of Lycopod B
Ecological inference rests on a detailed understanding of the environments of deposition in which the palynofloras and megafloral assemblages are preserved. The contact with the underlying paleosol at Lycopod B is buried, so its composition and geometry of overlying beds is uncertain. However, typical paleosols in the lower Markley Formation underlying coal/carbonaceous shales correspond to Pedotype B of Tabor and Montañez (2004), representing paleoultisols developed on sites of low relief during periods of sedimentary stasis in a humid to subhumid climate experiencing some periodic drying. Comparable kaolinite/organic sedimentary packages elsewhere in the Markley Formation display an erosional contact with the underlying paleosol and are shallowly trough-shaped (Tabor et al., 2013). The upward tilt of the kaolinite unit and overlying beds at one end of the exposure at Lycopod B East suggests that this outcrop exhibits a similar geometry.
Throughout the Markley Formation, kaolinite units closely resemble underlying paleosols in mineralogical composition, indicating that they were eroded from the paleosols (Tabor et al., 2013). Kaolinite beds indicate a high degree of leaching and formation in hot, humid climatic conditions. Obscure cross laminations and absence of pedogenesis indicates both kaolinite beds at Lycopod B were deposited relatively rapidly, probably as sheet wash or channel overflow. Fining upward, fine laminations and increased organic content at the top of the kaolinite at Lycopod B East indicate settling out in a brief ponding interval. The bulk of sedimentation of the kaolinite at Lycopod B East carries allochthonous elements such as small branches of walchian conifers, but the top includes parachthonous elements, including plants such as Calamites. In the Markley Formation, almost all kaolinites underlie organic rich facies (Tabor et al., 2013). Exceptions to this rule include the kaolinite within heterolithic beds at Lycopod B West, and in similar deposits at two older localities. This close correlation between kaolinitic units and overlying organic beds implies a genetic association between the two units: once deposited, the clay rich kaolinite created a poorly drained aquiclade upon which a wetland could form (Gardner et al., 1988; DiMichele et al., 2006; Gastaldo, 2010).
Thinning of beds and grading of coals into clastics from Lycopod East to Lycopod West indicate that the organic facies was, like other lower Markley Formation organic-rich beds, a spatially limited, poorly drained wetland fill within a relatively shallow, broad channel, probably a fluvial channel cutoff. Thin peats formed at the base and top of the organic facies at Lycopod B East, indicating a high water table, but there is evidence of fluctuating water table within this unit. Both coals contain vitrain, representing degraded woody material, likely derived from degraded sheets of lycopsid bark (here, almost certainly Sigillaria) (Boyce et al., 2010). Extremely sluggish streams or occasional sheet flow into the swamp were probably responsible for the deposition of beds 6 through 12. The high organic content, good palynomorph preservation, gley color and limited pedogenesis in these beds indicate deposition in water-saturated, dysaerobic and acidic conditions. Beds 3 through 7 correspond to Pedotype A of Tabor and Montañez (2004), interpreted as a histosol formed under conditions of poor drainage and low sedimentation. Bed 8 displays evidence of better drainage and increased exposure to oxidation, from the presence of orange mottles, manganese/iron coatings, and iron oxide nodules, indicating a drop in water table and partial drying. Beds 9 through 14 become increasingly organic-rich, culminating in another thin coal in bed 13, and display other signs of immature pedogenesis and a high water table. The presence of fusain in beds 10–12, however, indicates periods of drought on the landscape with consequent wildfires, indicating increasing seasonality.
The heterolithic beds above Bed 14, marked by a coarsening upward set of shales, mudstones, fine sandstone channels and immature paleosols, indicate a shift toward more active sedimentation on a low energy, regressive coastal flood plain. Collectively the heterolithic beds are considered floodplain deposits. Coarsening up sedimentation indicates movement from an initially more distal position on the floodplain to a proximal position relative to the channel. The cap sandstone represents a channel of unknown size at Lyopod B West. At other localities, channels range from a few meters to a few tens of meters thick and contain fine to coarse and occasional conglomerates, indicating low to relatively high stream energies. Channel cutting and sand deposition is probably contemporaneous with formation of paleosols, so cap sandstones and paleosols likely represent part of the same, more active sedimentary phase in the sedimentological cycle (Tabor and Montañez, 2004, Tabor et al., 2013).
6.2. Palynological interpretation of Lycopod B
Since seed plants as a group possess reproductive traits such as enclosed gametophytes and conductive tissue that are advantageous in water limited conditions compared to pteridophytes as a group, water availability is reasonably interpreted as the primary factor affecting Axis 1 (Fig. 5). Samples in the organic facies lie toward the left (wet) side of the graph, with considerable spread, while the seed-plant basal kaolinite (Assemblage II) lies to the very right of the graph, on the ‘dry’ end of Axis 1. The two mixed assemblages lie between the kaolinite and the organic facies, and clearly represent some kind of transition between dryland and wetland assemblages, or a mosaic of wetland and dryland communities (Fig. 5). Within the organic cluster, which represents Assemblage 1, sample 9 compositionally most closely resembles the dry and mixed floras, and lies close to them along Axis 1.
The ordination analysis clearly shows the existence of strong phylogenetic niche partitioning in the Lycopod B flora (Webb et al., 2002). Ferns and cordaitaleans form the core of the wetland community (Assemhlage I). Lycophytes lie between the wetland and dryland floras and represent Late Pennsylvanian, more xerophytic survivors within this clade. Hygrophilic lycophytes such as Lepidodendrales mostly disappeared by this time (DiMichele et al., 2009). Sphenophytes display a fairly broad range along the moisture gradient, with Calamites and certain Sphenophyllales displaying a greater affinity for wetland habitats. Seed ferns and walchian conifers cluster along the dry side of the moisture gradient, with the putative seed fern Colatisporites decorus and seed plant Illinites unicus and lying somewhat closer to the wet side of the moisture gradient. These taxa both represent Pennsylvanian holdovers, occupying drier parts of the wetland assemblage. An important element that is missing from this discussion, because of its rarity in the pollen record, is Schopfipollenites (Medullosales), which should lie well within Assemblage I based on megafloral data (see next section).
The factor that accounts for Axis 2 is likely disturbance. Inspection of the data suggests that abundance of Florinites is driving the spread along the axis (Fig. 3). Florinites occupies the most negative space in the graph, and is widely separated from a number of Marattiales taxa, both suggesting that tree ferns and Cordaitales occupied different ecological niches within the wetland community. Evidence from other sources indicates that some species of Marattiales replaced Lepidodendrales in the wettest parts of the wetland habitat in the Late Pennsylvanian, but that as a group they occupied a wide range of wetland habitats (DiMichele et al., 2009). Cordaitales, on the other hand, occupy a wide range of wetland to well-drained, but not dryland habitats (Falcon-Lang and Bashforth, 2005; Bashforth et al., 2014). However, they tended to occupy more stable habitats within fluvial systems, in contrast to Marattiales, which tended to occupy more disturbed habitats, although not as pioneering species (Bashforth et al., 2010, 2011).
Low species evenness and richness may be caused by constraints on biomass production (stress), disturbance (biomass destruction) or as evidence of a climax community (e.g., Begon et al., 2006). Samples LycB-90 1 and 8 are both relatively poor in taxa, and display by far the lowest equitability. Interestingly, these two lie at opposites ends in the palynofloral compositional spectrum, as shown in the DCA ordination (Fig. 5). Based on the composition of Assemblage II, sample 1 likely represents a homogeneous gymnosperm dryland community with relatively few spore producing taxa present. The low species richness in sample 8 may reflect disturbance, as this bed displays the highest degree of both pedogenesis and oxidation within the organic facies.
6.3 Comparison of palynofloral and megafloral assemblages
Higher taxonomic diversity in the palynofloras indicates that they capture a larger fraction of the actual floristic diversity, compared to megafossils. For example, palynology reveals a much larger variety of herbaceous ferns, which are absent in the megafossil record, as well as the consistent presence of cordaitaleans throughout the section. Cordaitaleans tend to be underestimated in the megafossil record due to the difficulty of distinguishing cordaitalean leaf fragments from rachis fragments (Tabor et al., 2013).
A certain degree of taxonomic congruence between megafloral and palynofloral elements exists in both organic and kaolinite facies, but it is greater in the organic facies. Megafossils recognized in the organic facies at both Lycopod B West and Lycopod B East include Sigillaria brardii (Crassispora kosankei), Calamites spp. (Calamospora spp.), Pecopteris spp. (numerous spore taxa), and Macroneuropteris scheuchzeri and Neuropteris ovata (Schopfipollenites). These are typical elements of the wetland flora and are largely reflected in the palynological record. The most important exception to this is Schopfipollenites (Medullosales), which is quite rare in the pollen record. In contrast, Macroneuropteris scheuchzeri is the dominant species in the organic facies (Tabor et al., 2013). Numerically, spores of Marattiales are dominant throughout the organic facies, with sphenophylls, Calamites and Sigillaria very much subdominant. Marattiales are known to be prolific spore producers, so palynological abundance is unlikely an accurate representation of the composition of this wetland. Nevertheless, the palynoflora indicates that other groups were at least as important components as medullosan seed ferns, in contrast to by the megafloral record, which indicates that medullosans (Macroneuropteris scheuzeri) was dominant (Tabor et al., 2013)..
In contrast, megafloras and palynofloras are relatively incongruent in the kaolinitic unit at Lycopod B. In part, this is due to more incomplete knowledge of spore/pollen affinities of the dryland assemblage. Only walchian conifers and Calamites occur in the kaolinite bed at Lycopod B East (as well as another kaolinite bed in Lycopod B West) (Fig. 1), although this is no doubt due to limited sampling. Comparable kaolinitic units elsewhere in the Markley Formation typically contain low diversity floras dominated by Sphenopteridium manzanitum (?Lyginopteridaceae). They also include walchian conifers, Medullosales and Calamitales (Tabor et al., 2013). Only Potonieisporites and Calamospora represent common elements in both the palynoflora and megaflora. Palynologically, the dominant elements in the kaolinite bed are non-taeniate bisaccate and pseudobisaccates which may represent peltasperms, callistophytes or perhaps even callipterids; these taxa are rare or absent in the kaolinite megafloras. The affinities of Sphenopteridium manzanitum are uncertain, and its pollen is unknown. If it is indeed lyginopteridalean, a connection with Colatisporites would be possible, but Colatisporites does not occur in the Lycopod B East kaolinite bed (although it does occur in drier assemblages in the Lower Permian of Texas (Looy and Hotton, unpublished data). Marattiales are diverse but at low abundance in the palynoflora, and are represented in the megaflora by rare Pecopteris pinnules. In this instance, megaflora and palynoflora agree. Walchian conifer prepollen (Potonieisporites, Nuskoisporites, and possibly cf. Sahnisporites) and seed fern pollen (Alisporites/Falcisporites), occurs throughout the wetland facies at very low abundance. These taxa are not reflected in the megafossil record at all. Similarly, Florinites (Cordaitales) is common to dominant throughout the organic facies and rare in the kaolinite unit, but occurs in only one megaflora in Lycopod B West. This agrees with other evidence that cordaitaleans were important elements of the vegetation in both wetland and better-drained riparian habitats (Bashforth et al., 2014). Florinites reaches its highest abundance in bed 14, which contains no megafloral remains of Cordaitales. Sigillaria occurs in this same bed, but no Crassispora was observed in the palynoflora at this level. These incongruities caution against reading too much into absences in either the palynoflora or megaflora at a given level, because incomplete sampling and imperfect preservation can and do distort floral content in a given bed. The megafloral record does record a broader sampling of landscape diversity: only two of the five Markley facies-associated megafloral assemblage types per Tabor et al. (2013) are represented in the palynofloral record at Lycopod B (Lithofacies 2 and 3). In addition to dryland and wetland megafloral assemblages at Lycopod B East, Lycopod B West records another kaolinite unit containing walchian conifers above a wetland muck soil, much like the basal kaolinite unit at Lycopod B East. Another megafloral assemblage occurs at the base of the cap sandstone at Lycopod B West, unusual for its abundance of cordaitalean leaves, as well as rare medullosans and tree ferns. It presumably represents Lithofacies 5 (channel sandstone facies), and probably reflects typical elements of riparian vegetation, given its position at the base of a channel.
6.4 Sedimentary cycles and time in the Markley Formation
The Markley Formation lacks the marine fossils or volcanic ashes that would allow biostratigraphic or chronometric correlation with marine cyclothems exposed along the coastal margins of Pangaea. Nevertheless, sedimentation in this formation progresses in a repeating and predictable cycle, suggesting a correlation with glacioeustatic sea level and climate change linked to glacial/interglacial cycles. Critical to this inference is determining how much time is represented by a single sedimentary package (paleosol to cap sandstone, Fig. 1). Without firm chronometric or stratigraphic controls, time estimates based on sedimentary rates are imprecise, at best. The basal conifer bearing kaolinitic siltstone was probably deposited as a single, rapid sedimentation event from a flood event. There does not appear to have been a significant lag between deposition of the kaolinite and initiation of peat accumulation. By analogy with measurements of accumulation in recent peats on Papua New Guinea, one to several thousand years may separate these two beds, at most (Staub and Gastaldo, 2003). Rates of peat accumulation are quite variable, but under tropical conditions peats can accumulate up to 20 mm/year (Page et al., 2006). Given a compression factor of 5:1 (based on the significant fractions of mineral matter in these peats), a 20 cm thick coal, such as the ones at Lycopod B, could represent as little as 100 years. Realistically, most Markley coals represent a complex interplay of intermittent decay, accumulation and auto-compression prior to burial. Thus, the minimum rate is almost certainly a significant underestimate of the actual time represented by these thin peats; a more plausible estimate for their formation is several thousands of years. The approximately two meters of organic mudstones exposed at Lycopod B East show evidence of pedogenesis but retain some evidence of bedding planes and recognizable plant fragments, so are clearly immature. These units probably accumulated over hundreds or thousands of years. Although there are no obvious unconformities, numerous short hiatuses undoubtedly exist within this interval. Overall, the roughly 3.4 m of organic-rich sediments exposed at Lycopod B East could represent accumulation of as little as a few thousand years, to as much as several tens of thousands of years. Estimating the accumulation rate of the floodplain deposits above the organic beds exposed at Lycopod B West poses even more challenges, since they include a variety of paleosols and small channels encompassing diastems of unknown length. A reasonable assumption of an average deposition rate of 100 mm/ky yields an estimate of approximately 20,000 years for the two meters of heterolithic beds exposed Lycopod B West. Thus, Lycopod B, from kaolinite to cap sandstone, could represent as little as 25,000 to as much as 40,000 to 100,000 years. The longer estimate seems more likely, given the significant gaps represented by paleosols, and agrees with estimates of 800,000 to 1,000,000 years for the entire Markley Formation (DiMichele et al. 2005a).
These estimates represent time scales (105 years) congruent with glacial/interglacial periodicity as a driver of repetitive sedimentary patterns in the Markley Formation. Other possible explanations, such as tectonic movement or base level changes, operate at longer time scales and do not exhibit the same level of periodicity. Furthermore, the Midland Basin appears to have been tectonically inactive during deposition of the Markley Formation (Hentz, 1988). Basin subsidence and accommodation appears to have been nearly constant during this time as well, again indicating that base level changes were not responsible for shifts in sedimentation patterns (Tabor et al., 2013). Where the boundary between glacial and interglacial lies in the Markley Formation depends on the preferred atmospheric circulation model. If higher rainfall and/or low seasonality is supposed to occur during glacial maxima, the initiation of organic deposition marks the beginning of the glacial cycle, and glacial minimum is marked by the onset of evidence of greater seasonality, either at the beginning of the heterolithic beds or with the formation of paleosols and channel sandstones. Alternatively, if glacial minima were correlated with higher rainfall patterns and/or lower seasonality, they would be marked by initiation of organic facies. At present, there is insufficient evidence to determine exactly where glacial cycles end or interglacials begin in the Markley Formation.
6.5 Do autocyclic or allocyclic processes account for floral shifts at Lycopod B?
The question remains: were wetland and dryland floras separated temporally or spatially? Invoking strictly autocyclic processes, the sedimentary sequence at Lycopod B can be interpreted as normal fluvial landscape evolution from backswamp to floodplain to active channel. Under this model, dryland plants (conifers, peltasperms, and others, many unknown) occupied driest parts of the landscape, such as elevated interfluves, while cordaitaleans, sphenopsids and some seed ferns occupied stream banks and wetter sites within the flood plain, and wetland plants such as ferns, lycopsids and seed ferns occupied sites with the highest water table and poor drainage. Interpretation of kaolinite units as aquicludes suggests that organic facies formed independently of climate. Palynological patterns can be similarly interpreted. For example, the mixed dryland/wetland assemblage at the top of the kaolinite could be interpreted as an ecotone between the two communities. Furthermore, the presence of low levels (<1%) of dryland taxa in wetlands (organic facies) indicates that their source may have been nearby. Long-distance transport of dryland taxa, although possible, is considered less likely because of low clastic input into the organic facies, indicating that most of the pollen and spores were locally derived from vegetation growing in or very close to the wetland. Especially if the wetlands at Lycopod B were dominated largely by species of Marattiales, which are believed to have formed closed canopy forests (DiMichele, 2014), little pollen from plants growing more distally would be likely to penetrate by means of pollen rain.
The complex sedimentary patterns observed at Lycopod B, and the apparent cyclicity of sedimentation throughout the Markley, both seem difficult to explain through autocyclic processes alone. Allocyclic processes, especially changes in precipitation and seasonality, are considered more likely responsible for these observed changes. Under this model, climate controls both sedimentary patterns and distribution of vegetation. Wetland and dryland taxa are predicted to succeed one another temporally but rarely coexist; they would normally be spatially separated, perhaps by hundreds of kilometers. Kaolinites might indeed form aquicludes, but that is alone is insufficient for peat formation: in addition, rainfall must exceed evapotranspiration for most of the year. Absence of peat or carbonaceous shales above the kaolinite unit at Lycopod B West, and several other localities, indicates that climate may have been too dry to allow peat formation at those times. The mixed palynological assemblage at the top of the kaolinite could represent a brief interval of rapid climate change from drier to wetter conditions where both communities coexisted on the landscape in close proximity. Perhaps more likely, though, kaolinite beds represent sheet wash transporting allochthonous elements from some unknown distance, but as the slurries ponded and settled out, local wetland vegetation colonized the site and contributed plant debris and pollen and spores to the deposit. In other words, the mixed assemblage is likely a taphonomic mixture of two spacially separate floras (although what degree of spatial separation remains unclear). There is abundant evidence of seasonality throughout the Lycopod B locality. Evidence of a fluctuating water table and fusain near the top of the organic facies suggests some degree of seasonality even within the wettest phase of Lycopod B. The predominance of carbonaceous shales and limited peat development in the organic facies indicates that perhumid climate existed for a very short time; carbonaceous shales generally develop in subhumid or seasonal conditions. Although paleosols are not well characterized at Lycopod B, elsewhere at comparable levels in the Markley, they are characterized as ultisols or vertisols, both indicating periods of dryness (Tabor and Montañez, 2004).
Climate appears to be the primary factor in controlling variability in precipitation throughout the Markley Formation. The coals at Lycopod B appear to have formed during a somewhat wetter phase, as they correlate with significant coals formed elsewhere at approximately the same stratigraphic level within the Markley Formation (Tabor and Montañez, 2004). Nevertheless, the long-term trend within the Markley Formation was toward increasing aridity up section, and even within the Lycopod B site, some degree of water limitation appears to have existed throughout all but the wettest phases. From a global perspective, the Lycopod B organic facies represents one of the last gasps of the once mighty coal forests. It seems entirely plausible that the strict delineation of dryland and wetland floras was beginning to break down during this time of climate change. Perhaps parts of the landscape had already begun to fragment into “wet spots” that supported typical Late Pennsylvanian wetland plants and much drier areas of the landscape that supported conifers and other markers of the dryland assemblage. The apparent shift within the kaolinite bed at Lycopod B East could, in fact, represent actual physical proximity. It has been emphasized that, because little topographic relief existed within hundreds of kilometers of the Midland Basin, there was no place for “upland” plants to exist contemporaneously with wetland communities (DiMichele, 2014). However, plant communities in modern fluvial systems are controlled by hydrologic, edaphic and topographic gradients (Hupp and Osterkamp, 1985; Walling and He, 1998; Osterkamp and Hupp, 2010). Even in areas of low topography, fluvial systems create their own relief, in the form of levees, channel bars, backswamps, crevasse-splays, oxbows, interfluve ponds and other microhabitats, each occupied by distinct suites of plant species. Similarly, vegetation heterogeneity occurred among microhabitats within Pennsylvanian wetlands (Bashforth et al., 2010, 2011, 2014).
Because of its stratigraphic and spatial limitations, this study alone cannot resolve the vexed question of spatial vs. temporal relations between dryland and wetland floral assemblages. Nevertheless, it shows that palynology expands and in some ways complicates our understanding of how dryland and wetland assemblages were distributed on the landscape. Resolution of how these two types of plant communities were related to one another requires study of exceptional floral assemblages, where dryland and wetland assemblages are laterally juxtaposed, and precisely correlated to marine sequences, as the key to understanding how vegetation was distributed across the landscape in the Late Pennsylvanian.
Highlights.
A palynological analysis from a latest Pennsylvanian locality from Texas is described
The LycopodB section represents one single interglacial/glacial phase
Plant communities are reconstructed using the palynomorphs’ botanical affinities
Dryland and wetland floras succeed one another with little species turnover
Dryland and wetland assemblages are in close spatial proximity on the landscape
Acknowledgments
We acknowledge William A. DiMichele for his generous sharing of samples and field notes, helpful discussions and support for this work. Ivo Duijnstee and Robert Stevenson assisted with imaging, figure and plate preparation. Critiques by Henk Visscher, Tom van Hoof and especially Hans Kerp greatly improved the manuscript. Cindy Looy was supported by the Hellman Family Foundation. The research of Carol Hotton was supported in part by the Intramural Research Program of the National Institutes of Health, National Library of Medicine. This is ETE publication 337, and UCMP Contribution 2055.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Cindy V. Looy, Email: looy@berkeley.edu.
Carol L. Hotton, Email: hotton@ncbi.nlm.nih.gov, hottonc@si.edu.
References
- Allen JP, Fielding CR, Gibling MR, Rygel MC. Fluvial response to paleoequatorial climate fluctuations during the late Paleozoic ice age. Geological Society of America Bulletin. 2011;123:1524–1538. [Google Scholar]
- Anderson JM, Anderson HM. Palaeoflora of southern Africa, Molteno Formation (Triassic) 1. (Part 1. Introduction; Part 2. Dicroidium) Balkema; Rotterdam: 1983. [Google Scholar]
- Andrews HN, Agashe SN. A new sporangium from the American Carboniferous. Palaeobotanist. 1962;11:46–48. [Google Scholar]
- Archer AW. Cyclic sedimentation (cyclothem) In: Gornitz V, editor. Encyclopedia of paleoclimatology and ancient environments. Springer; Dordrecht: 2009. pp. 226–228. [Google Scholar]
- Ash SR, Litwin RJ. New species of the pinnate microsporophyll Pramelreuthia from the Upper Triassic of the southwestern United States. American Journal of Botany. 1996;83:1091–1099. [Google Scholar]
- Balme BE. Fossil in situ spores and pollen grains: an annotated catalogue. Review of Palaeobotany and Palynology. 1995;87:81–323. [Google Scholar]
- Bashforth AR, Falcon-Lang HJ, Gibling MR. Vegetation heterogeneity on a Late Pennsylvanian braided-river plain draining the Variscan Mountains, La Magdalena Coalfield, northwestern Spain. Palaeogeography, Palaeoclimatology, Palaeoecology. 2010;292:367–390. [Google Scholar]
- Bashforth A, Drábková J, Opluštil S, Gibling MR, Falcon-Lang HJ. Landscape gradients and patchiness in riparian vegetation on a Middle Pennsylvanian braided-river plain prone to flood disturbance (N any Member, Central and Western Bohemian Basin, Czech Republic) Review of Palaeobotany and Palynology. 2011;163:153–189. [Google Scholar]
- Bashforth AR, Cleal CJ, Gibling MR, Falcon-Lang HJ, Miller RF. Paleoecology of Early Pennsylvanian vegetation on a seasonally dry tropical landscape (Tynemouth Creek Formation, New Brunswick, Canada) Review of Palaeobotany and Palynology. 2014;200:229–263. [Google Scholar]
- Baxter RW. Carinostrobus foresmani, a new lycopod genus from the Middle Pennsylvanian of Kansas. Palaeontographica Abteilung B. 1971;134:124–130. [Google Scholar]
- Beerbower JR. Cyclothem and cyclic depositional mechanisms in alluvial plain sedimentation. Kansas Geological Survey Bulletin. 1964;169:31–42. [Google Scholar]
- Begon M, Townsend CR, Harper JL. Ecology from individuals to ecosystems. 4. Blackwell Publishing; Oxford: 2006. [Google Scholar]
- Bek J, Pšenička J. Senftenbergia plumosa (Artis) emend., and its spores from the Carboniferous of the Kladno and Pilsen basins, Bohemian Massif, and some related and synonymous taxa. Review of Palaeobotany and Palynology. 2001;116:213–232. [Google Scholar]
- Bek J, Drábková D, Daškov J, Libertín M. The sub-arborescent lycopsid genus Polysporia Newberry and its spores from the Pennsylvanian (Bolsovian–Stephanian B) continental basins of the Czech Republic. Review of Palaeobotany and Palynology. 2008;152:176–199. [Google Scholar]
- Bek J, Libertín M. In situ reticulate sphenophyllalean spores, Pennsylvanian (Bolsovian) of the Czech Republic. Review of Palaeobotany and Palynology. 2010;159:56–61. [Google Scholar]
- Birgenheier LP, Fielding CR, Rygel MC, Frank TD, Roberts J. Evidence for dynamic climate change on sub-106-year scales from the late Paleozoic glacial record, Tamworth Belt, New SouthWales, Australia. Journal of Sedimentary Research. 2009;79:56–82. [Google Scholar]
- Boyce CK, Abrecht M, Zhou D, Gilbert PUPA. X-ray photoelectron emission spectromicroscopic analysis of arborescent lycopsid cell wall composition and Carboniferous coal ball preservation. International Journal of Coal Geology. 2010;83:146–153. [Google Scholar]
- Brousmiche C. Les fougères sphenopteridiennes du bassin houiller sarro-lorrain (Systematique-Stratigraphie) Annales de la Société géologique du Nord. 1983;10:1–480. [Google Scholar]
- Brousmiche C. Precisions sur les spores produites par quelques sphenopteridiennes appartenant aux genres Boweria Kidston, Crossotheca Zeiller, Discopteris Stur, Myriotheca Zeiller et Urnatopteris Kidston. Revue de Paléobiologie. 1986;5:231–248. [Google Scholar]
- Brousmiche-Delcambre C, Lugardon B, Coquel R, Goubet P. Découverte dans le bassin houiller sarro-lorrain du genre Radiitheca (organes reproducteurs de Filicophytes) et ultrastructure du genre Microreticulatisporites. Geobios. 1997;30:3–14. [Google Scholar]
- Bunting MJ. Pollen in wetlands: using simulations of pollen dispersal and deposition to better interpret the pollen signal. Biodiversity and Conservation. 2008;17:2079–2096. [Google Scholar]
- Burnham RJ. Reconstructing richness in the plant fossil record. Palaios. 1993;8:376–384. [Google Scholar]
- Cecil CB, Dulong FT. Precipitation models for sediment supply in warm climates. In: Cecil CB, Edgar TN, editors. Climate controls on stratigraphy. 2003. pp. 21–27. SEPM Special Publication 77. [Google Scholar]
- Cecil CB, Dulong FT, West RR, Stamm R, Wardlaw B, Edgar NT. Climate controls on the stratigraphy of a Middle Pennsylvanian cyclothem in North America. In: Cecil CB, Edgar TN, editors. Climate controls on stratigraphy. 2003. pp. 151–182. SEPM Special Publication 77. [Google Scholar]
- Cecil CB, DiMichele WA, Elrick SD. Middle and Late Pennsylvanian cyclothems, American Midcontinent: Ice-age environmental changes and terrestrial biotic dynamics. Comptes Rendus Geoscience. 2014;346:159–168. [Google Scholar]
- Chaloner WG. The Carboniferous upland flora. Geological Magazine. 1958a;95:261–262. [Google Scholar]
- Chaloner WG. Polysporia mirabilis Newberry, a fossil lycopod cone. Journal of Palaeontology. 1958b;32:199–209. [Google Scholar]
- Chaphekar M, Alvin KL. On the fertile parts of the coenopterid fern Metaclepsydropsis duplex (Williamson) Review of Palaeobotany and Palynology. 1972;14:63–76. [Google Scholar]
- Clement-Westerhof JA. Aspects of Permian palaeobotany and palynology. IV. The conifer Ortiseia Florin from the Val Gardena Formation of the Dolomites and Vicentinian Alps (Italy) with special reference to a revised concept of the Walchiaceae (Göppert) Schimper. Review of Palaeobotany and Palynology. 1984;41:51–166. [Google Scholar]
- Cridland AA, Morris JE. Taeniopteris, Walchia, and Dichophyllum in the Pennsylvanian system in Kansas. University of Kansas Science Bulletin. 1963;44:71–85. [Google Scholar]
- Dickey BC, Gupta S. Palynostratigraphy of the Newcastle Coal of north central Texas, U.S.A. Pollen et Spores. 1980;22:545–553. [Google Scholar]
- DiMichele WA, Phillips TL. Paleobotanical and paleoecological constraints on models of peat formation in the Late Carboniferous of Euramerica, Palaeogeography, Palaeoclimatology, Palaeoecology. 1994;106:39–90. [Google Scholar]
- DiMichele WA, Phillips TL. Climate change, plant extinctions, and vegetational recovery during the Middle-Late Pennsylvanian transition: the case of tropical peat-forming environments in North America. Geological Society Special Publication. 1996;102:201–221. [Google Scholar]
- DiMichele WA, Chaney DS, Dixon WH, Nelson WJ, Hook RW. An Early Permian coastal flora from the Central Basin Platform of Gaines Country, West Texas. Palaios. 2000;15:524–534. [Google Scholar]
- DiMichele WA, Mamay SH, Chaney DS, Hook RW. An Early Permian flora with Late Permian and Mesozoic affinities from North-Central Texas. Journal of Paleontology. 2001;75:449–460. [Google Scholar]
- DiMichele WA, Hook RW, Nelson WJ, Chaney DS. An unusual middle Permian flora from the Blaine Formation (Pease River Group, Leonardian-Guadalupian Series) of King County, West Texas. Journal of Paleontology. 2004;78:765–782. [Google Scholar]
- DiMichele WA, Kerp H, Krings M, Chaney DS. The Permian peltasperm radiation: evidence from the southwestern United States. In: Lucas SG, Zeigler KE, editors. The nonmarine Permian. Vol. 30. 2005b. pp. 67–79. New Mexico Museum of Natural History and Science Bulletin. [Google Scholar]
- DiMichele WA, Tabor NJ, Chaney DS. Outcrop-scale environmental heterogeneity and vegetational complexity in the Permo-Carboniferous Markley Formation of north-central Texas. In: Lucas SG, Zeigler KE, editors. The Nonmarine Permian. Vol. 30. 2005a. pp. 60–66. New Mexico Museum of Natural History and Science Bulletin. [Google Scholar]
- DiMichele WA, Tabor NJ, Chaney DS, Nelson WJ. From wetlands to wet spots: environmental tracking and the fate of Carboniferous elements in Early Permian tropical floras. In: Greb SF, DiMichele WA, editors. Wetlands through time. Vol. 399. 2006. pp. 223–248. Geological Society of America Special Paper. [Google Scholar]
- DiMichele WA, Montañez IP, Poulsen CJ, Tabor NJ. Climate and vegetational regime shifts in the late Paleozoic ice age earth. Geobiology. 2009;7:200–226. doi: 10.1111/j.1472-4669.2009.00192.x. [DOI] [PubMed] [Google Scholar]
- DiMichele WA, Cecil CB, Montañez IP, Falcon-Lang HJ. Cyclic changes in Pennsylvanian paleoclimate and effects on floristic dynamics in tropical Pangaea. International Journal of Coal Geology. 2010;83:329–344. [Google Scholar]
- DiMichele WA, Falcon-Lang HJ. Pennsylvanian ‘fossil forests’ in growth position (T0 assemblages): origin, taphonomic bias and palaeoecological insights. Journal of the Geological Society, London. 2011;168:585–605. [Google Scholar]
- DiMichele WA, Kerp H, Sirmons R, Fedorko N, Skema V, Blake BM, Jr, Cecil CB. Callipterid peltasperms of the Dunkard Group, Central Appalachian Basin. International Journal of Coal Geology. 2013;119:56–78. [Google Scholar]
- DiMichele WA. Wetland-dryland vegetational dynamics in the Pennsylvanian ice age tropics. International Journal of Plant Science. 2014;175:123–164. [Google Scholar]
- Dimitrova T, Cleal CJ, Thomas BA. Palynological evidence for Pennsylvanian extra-basinal vegetation in Atlantic Canada. Journal of the Geological Society, London. 2011;168:559–569. [Google Scholar]
- Dolby G, Falcon-Lang HJ, Gibling M. A conifer-dominated palynological assemblage from Pennsylvanian (late Moscovian) alluvial drylands in Atlantic Canada: implications for the vegetation of tropical lowlands during glacial phases. Journal of the Geological Society, London. 2011;168:571–584. [Google Scholar]
- Doubinger J, Grauvogel-Stamm L. Présence de spores du genre Thymospora chez Pecopteris hemiteliodes du Mont-Pélé (Stephanian moyen du Bassin d’Autun) Pollen et Spores. 1971;12:598–607. [Google Scholar]
- Eble CF, Blake BM, Gillespie WH, Pfefferkorn HW. Appalachian basin fossil floras. In: Greb SF, Chesnut D, editors. Carboniferous of the Appalachian and Black Warrior Basins. Vol. 10. 2009. pp. 46–58. Kentucky Geological Survey Special Publication. [Google Scholar]
- Eggert DA, Delevoryas T. Studies of Paleozoic ferns: Sermeya, gen. nov. and its bearing on filicalean evolution in the Paleozoic. Palaeontographica Abteilung B. 1967;118:169–180. [Google Scholar]
- Eros JM, Montañez IP, Osleger DA, Davydov VI, Nemyrovska TI, Poletaev VI, Zhykalyak MV. Sequence stratigraphy and onlap history of the Donets Basin, Ukraine: insight into Carboniferous icehouse dynamics. Palaeogeography, Palaeoclimatology, Palaeoecology. 2012;313–314:1–25. [Google Scholar]
- Falcon-Lang HJ. Pennsylvanian tropical rain forests responded to glacial-interglacial rhythms. Geology. 2004;32:689–692. [Google Scholar]
- Falcon-Lang HJ, Bashforth AR. Morphology, anatomy, and upland ecology of large cordaitalean trees from the Middle Pennsylvanian of Newfoundland. Review of Palaeobotany and Palynology. 2005;135:223–243. [Google Scholar]
- Falcon-Lang HJ, Jud NA, Nelson WJ, DiMichele WA, Chaney DS, Lucas SG. Pennsylvanian coniferopsid forests in sabkha facies reveal the nature of seasonal tropical biomes. Geology. 2011a;39:371–374. [Google Scholar]
- Falcon-Lang HJ, Pendleton JL. Dryland plant communities in the Pennsylvanian (mid- to late Bolsovian) Winterbourne Formation of Bristol, southern Britain: Further evidence for taphonomic megabias. Review of Palaeobotany and Palynology. 2011b;166:268–285. [Google Scholar]
- Feldman HR, Franseen EK, Joeckel RM, Heckel PH. Impact of longer-term modest climate shifts on architecture of high-frequency sequences (cyclothems), Pennsylvanian of Midcontinent USA. Journal of Sedimentary Research. 2005;75:350–368. [Google Scholar]
- Fielding CR, Frank TD, Isbell JL. The late Paleozoic ice age—A review of current understanding and synthesis of global climate patterns. Geological Society of America Special Papers. 2008;441:343–354. [Google Scholar]
- Florin R. Die Koniferen des Oberkarbons und des Unteren Perms. Palaeontographica Abteilung B. 1944;85:365–456. [Google Scholar]
- Galtier J, Taylor TN. The first record of ferns from the Permian of Antarctica. Review of Palaeobotany and Palynology. 1994;83:227–239. [Google Scholar]
- Gastaldo RA, Stevanovic-Walls IM, Ware WN, Greb SF. Community heterogeneity of Early Pennsylvanian peat mires. Geology. 2004;32:693–696. [Google Scholar]
- Gastaldo RA. Peat or no peat: Why do the Rajang and Mahakam Deltas differ? International Journal of Coal Geology. 2010;83:162–172. [Google Scholar]
- Gastaldo RA, Demko TM. Long term hydrology controls the plant fossil record. Taphonomy: processes and bias through time. In: Allison PA, Bottjer DJ, editors. Topics in Geobiology. 2. Vol. 32. 2011. pp. 249–286. [Google Scholar]
- Gardner TW, Williams EG, Holbrook PW. Pedogenesis of some Pennsylvanian underclays: ground-water, topographic and tectonic controlls. In: Reinhardt J, Sigleo WR, editors. Paleosols and weathering through geological time: principles and applications. Vol. 216. 1988. pp. 81–102. Geological Society of America Special Paper. [Google Scholar]
- Gomankov AV, Meyen SV. Tatarian flora (composition and distribution in the Late Permian of Eurasia) Transactions of Geological Institute of Academy of Sciences USSR. 1986;401:1–173. (in Russian) [Google Scholar]
- Good CW. A petrified fern sporangium from the British Carboniferous. Palaeontology. 1981;24:483–492. [Google Scholar]
- Gould RE, Delevoryas T. The biology of Glossopteris: evidence from petrified seed-bearing and pollen-bearing organs. Alcheringia. 1977;1:387–399. [Google Scholar]
- Grauvogel-Stamm L, Doubinger J. Deux fougères fertiles du Stéphanien du Massif Central (France) Geobios. 1975;6:409–421. [Google Scholar]
- Grauvogel-Stamm L. La flore du Grès à Voltzia (Buntsandstein súperieur) des Vosges du Nord (France). Morphologie, anatomie, interprétations phylogénique et paléogéographique. Université Louis-Pasteur de Strasbourg Institut de Géologie. 1978;50:1–225. [Google Scholar]
- Grauvogel-Stamm L, GrauvogeL L. Masculostrobus acuminatus nom. nov., un nouvel organe reproducteur mâle de Gymnosperme de Grès à Voltzia (Trias inférieur) des Vosges (France) Geobios. 1973;6:101–114. [Google Scholar]
- Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica. 2001;4:1–9. [Google Scholar]
- Heckel PH. Pennsylvanian cyclothems in Midcontinent North America as far-field effects of waxing and waning of Gondwana ice sheets. In: Fielding CR, Frank TD, Isbell JL, editors. Resolving the Late Paleozoic Ice Age in time and space. Vol. 441. 2008. pp. 275–289. Geological Society of America Special Paper. [Google Scholar]
- Hemsley AR. Exine ultrastructure in the spores of enigmatic Devonian plants: its bearing on the interpretation of relationships and on the origin of the sporophyte. In: Kurmann MH, Doyle JA, editors. Ultrastructure of fossil spores and pollen. The Royal Botanic Gardens; Kew, London: 1994. pp. 1–21. [Google Scholar]
- Hentz TF. Lithostratigraphy and paleoenvironments of Upper Paleozoic continental red beds, north-central-central Texas: Bowie (new) and Wichita (revised) Groups. Bureau of Economic Geology Report of Investigations. 1988;170:1–55. [Google Scholar]
- Hernandez-Castillo GR, Rothwell GW, Mapes G. Thucydiaceae fam. nov., with a review and reevaluation of Paleozoic walchian conifers. International Journal of Plant Science. 2001;162:1155–1185. [Google Scholar]
- Hilton J, Bateman RM. Pteridosperms are the backbone of seed-plant phylogeny. Journal of the Torrey Botanical Society. 2006;133:119–168. [Google Scholar]
- Holden HS. The morphology of Botryopteris antiqua Kidston. Br Mus Nat Hist Bull. 1962;5:361–380. [Google Scholar]
- Horton DE, Poulsen CJ, Pollard D. Influence of high-latitude vegetation feedbacks on late Palaeozoic glacial cycles. Nature Geoscience. 2010;3:572–577. [Google Scholar]
- Horton DE, Poulsen CJ, Montañez IP, DiMichele WA. Eccentricity-paced late Paleozoic climate change. Palaeogeography, Palaeoclimatology, Palaeoecology. 2012;331–332:150–161. [Google Scholar]
- Hupp CR, Osterkamp WR. Bottomland vegetation distribution along Passage Creek, Virginia, in relation to fluvial landforms. Ecology. 1985;66:670–681. [Google Scholar]
- Kerp JHF. Aspects of Permian palaeobotany and palynology. X. The Western and Central European species of the genus Autunia Krasser emend. Kerp (Peltaspermaceae) and the form genus Rhachiphyllum Kerp (callipterid foliage) Review of Palaeobotany and Palynology. 1988;54:249–360. [Google Scholar]
- Kerp H. Post-Variscan Late Palaeozoic Northern Hemisphere gymnosperms: the onset to the Mesozoic. Review of Palaeobotany and Palynology. 1996;90:263–285. [Google Scholar]
- Kerp H. The modernization of landscapes during the Late Paleozoic-Early Mesozoic. In: Gastaldo RA, DiMichele WA, editors. Phanerozoic terrestrial ecosystems. Vol. 6. 2000. pp. 79–113. Paleontological Society Papers. [Google Scholar]
- Krassilov VA, Afonin SA, Naugolnykh SV. Permotheca with in situ pollen grains from the Lower Permian of the Urals. Palaeobotanist. 1999;48:19–25. [Google Scholar]
- Labandeira CC. The paleobiology of pollination and its precursors. In: Gastaldo RA, DiMichele WA, editors. Phanerozoic terrestrial ecosystems, Paleontological Society Papers. Vol. 6. 2000. pp. 233–269. [Google Scholar]
- Lesnikowska AD. Dissertation. University of Illinois; Urbana-Champaign: 1989. Anatomically preserved Marattiales from coal swamps of the Desmoinesian and Missourian of the Midcontinent United States: systematics, ecology and evolution; pp. 1–227. [Google Scholar]
- Lesnikowska AD, Willard DA. Two new species of Scolecopteris (Marattiales), sources of Torispora securis Balme and Thymospora thiessenii (Kosanke) Wilson et Venkatachala. Review of Palaeobotany and Palynology. 1997;95:211–225. [Google Scholar]
- Libertín M, Bek J, Drábková J. Two new Carboniferous fertile sphenophylls and their spores from the Czech Republic. Acta Palaeontologica Polonica. 2008;53:723–732. [Google Scholar]
- Libertín M, Bek J, Drábková J. New sphenophyllaleans from the Pennsylvanian of the Czech Republic. Review of Palaeobotany and Palynology. 2014;200:196–210. [Google Scholar]
- Lindström S, McLoughlin S, Drinnan AN. Intraspecific variation of taeniate bisaccate pollen within Permian glossopterid sporangia, from the Prince Charles Mountains, Antarctica. International Journal of Plant Sciences. 1997;158:673–684. [Google Scholar]
- Looy CV. Extending the range of derived Late Paleozoic conifers: Lebowskia gen. nov. (Majonicaceae) International Journal of Plant Sciences. 2007;168:957–972. [Google Scholar]
- Looy CV. Natural history of a plant trait: branch system abscission in Paleozoic conifers and its environmental, autecological and ecosystem implications. Paleobiology. 2013;39:235–252. [Google Scholar]
- Looy CV, Duijnstee IAP. Characterizing morphologic variability in foliated Paleozoic conifer branches – a first step in testing its potential as proxy for taxonomic position. In: Lucas SG, DiMichele WA, Barrick JE, Schneider JW, Spielmann JA, editors. The Carboniferous-Permian transition. Vol. 60. 2013. pp. 215–223. New Mexico Museum of Natural History and Science Bulletin Series. [Google Scholar]
- Looy CV, Stevenson RA. Earliest occurrence of autorotating seeds in conifers: the Permian (Kungurian-Roadian) Manifera talaris gen. et sp. nov. International Journal of Plant Sciences. 2014;175:841–854. [Google Scholar]
- Lyons PC, Darrah WC. Earliest conifers of North America: Upland and/or paleoclimatic indicators? Palaios. 1989;4:480–486. [Google Scholar]
- Mamay SH, Miller JM, Rohr DR, Stein WE., Jr Foliar morphology and anatomy of the gigantopterid plant Delnortea abbottiae, from the Lower Permian of West Texas. American Journal of Botany. 1988;75:1409–1433. [Google Scholar]
- Mapes G, Rothwell GW. Diversity among Hamilton conifers. In: Mapes G, Mapes RH, editors. Regional geology and paleontology of Upper Paleozoic Hamilton quarry area in southeastern Kansas. Vol. 6. 1988. pp. 225–244. Kansas Geological Survey Guidebook Series. [Google Scholar]
- Meyen SV. Basic features of gymnosperm systematics and phylogeny as shown in the fossil record. Botanical Reviews. 1984;50:1–111. [Google Scholar]
- Meyen SV. Permian conifers of western Anagaraland. Review of Palaeobotany and Palynology. 1997;96:351–447. [Google Scholar]
- Millay MA. Acaulangium gen. n., a fertile marattialean from the Upper Pennsylvanian of Illinois. Amer J Bot. 1977;64:223–229. [Google Scholar]
- Millay MA. Studies of Paleozoic Marattialeans: A monograph of the American species of Scolecopteris. Palaeontographica Abteilung B. 1979;169:1–69. [Google Scholar]
- Millay MA, Taylor TN. Paleozoic seed fern pollen organs. Bot Rev. 1979;45:301–375. [Google Scholar]
- Millay MA, Taylor TN. The ultrastructure of Paleozoic fern spores: I. Botryopteris. American Journal of Botany. 1982;69:1148–1155. [Google Scholar]
- Millay MA, Taylor TN. The ultrastructure of Paleozoic fern spores. II. Scolecopteris (Marattiales) Palaeontographica Abteilung B. 1984;194:1–13. [Google Scholar]
- Montañez IP, Tabor NJ, Niemeier D, DiMichele WA, Frank TD, Fielding CR, Isbell JL, Birgenheier LT, Rygel M. CO2-forced climate and vegetation instability during Late Paleozoic glaciation. Science. 2007;315:87–91. doi: 10.1126/science.1134207. [DOI] [PubMed] [Google Scholar]
- Montañez IP, Poulsen CJ. The Late Paleozoic Ice Age: an evolving paradigm. Annual Reviews in Earth and Planetary Science. 2013;41:629–656. [Google Scholar]
- Naugolnykh SV. New male reproductive organs of gymnosperms Permotheca colovratica sp. nov. from the Lower Permian of the Ural Mountains. Paleontological Journal. 2013;47:114–126. [Google Scholar]
- Neves R, Gueinn KJ, Clayton G, Ioannides NS, Kruszewska K. Palynological correlations within the Lower Carboniferous of Scotland and Northern England. Transactions of the Royal Society of Edinburgh. 1973;69:23–76. [Google Scholar]
- Nichols DJ. Palynology in the coal systems analysis - the key to floras, climate, and stratigraphy of coal forming environments. Geological Society of America Special Papers. 2005;387:51–58. [Google Scholar]
- Opluštil S, Bek J. Some Pennsylvanian arborescent lycopsid cones and their microspores from the British coalfields. Bulletin of Geosciences. 2009;84:203–226. [Google Scholar]
- Opluštil S, Bek J, Drábková J. A new bisporangiate lycopsid cone genus Thomasostrobus gen. nov. from the Late Pennsylvanian of the Intra-Sudetic Basin (Czech Republic) Bulletin of Geosciences. 2009;84:283–300. [Google Scholar]
- Osborn JM, Taylor TN. Pollen morphology and ultrastructure of the Corystospermales: permineralized in situ grains from the Triassic of Antarctica. Review of Palaeobotany and Palynology. 1993;79:205–219. [Google Scholar]
- Osterkamp WR, Hupp CR. Fluvial processes and vegetation - glimpses of the past, the present, and perhaps the future. Geomorphology. 2010;116:274–285. [Google Scholar]
- Page SE, Rieley JO, Wuest R. Lowland tropical peatlands of Southeast Asia. In: Martini P, Martínez Cortizas A, Chesworth W, editors. Peatlands: evolution and records of environmental and climate changes. Elsevier; Amsterdam: 2006. pp. 145–172. [Google Scholar]
- Peppers RA. Palynological correlation of major Pennsylvanian (Middle and Upper Carboniferous) chronostratigraphic boundaries in the Illinois and other coal basins. Geological Society of America Memoirs. 1996;188:1–112. [Google Scholar]
- Peyser CE, Poulsen CJ. Controls on Permo-Carboniferous precipitation over tropical Pangaea: a GCM sensitivity study. Palaeogeography, Palaeoclimatology, Palaeoecology. 2008;268:181–192. [Google Scholar]
- Pfefferkorn HW, Peppers RA, Phillips TL. Some fern-like fructifications and their spores from the Mazon Creek compression flora of Illinois (Pennsylvanian) Illinois State Geological Survey, Circular. 1971;463:1–54. [Google Scholar]
- Pfefferkorn HW. A note on the term “upland”flora. Review of Palaeobotany and Palynology. 1980;30:157–158. [Google Scholar]
- Playford G, Dino R. Palynostratigraphy of upper Palaeozoic strata (Tapajós Group), Amazonas Basin, Brazil: part one. Palaeontographica Abteilung B. 2000;255:1–46. [Google Scholar]
- Plotnick RE, Kenig F, Scott AC, Glasspool IJ, Eble CF, Lang WJ. Pennsylvanian paleokarst and cave fills from northern Illinois, USA: a window into late Carboniferous environments and landscapes. Palaios. 2009;24:627–637. [Google Scholar]
- Pointon MA, Chew DM, Ovtcharova M, Sevastopulo GD, Crowley QG. New high-precision U-Pb dates from western European Carboniferous tuffs; implications for timescale calibration, the periodicity of late Carboniferous cycles and stratigraphical correlation. Journal of the Geological Society, London. 2012;169:713–721. [Google Scholar]
- Poort R, Clement-Westerhof JA, Looy CV, Visscher H. Conifer extinction in Europe at the Permian-Triassic junction: morphology, ultrastructure and geographic-stratigraphic distribution of Nuskoisporites dulhuntyi (prepollen of Ortiseia, Walchiaceae) Review of Palaeobotany and Palynology. 1997;97:9–39. [Google Scholar]
- Potonié R. Synopsis der Sporae in situ. Beihefte zum Geologischen Jahrbuch. 1962;52:1–204. [Google Scholar]
- Potonié R. Versuch der Einordnung der fossilen Sporae dispersae in das phylogenetische System der Pfianzenfamilien. I. Thallophyta bis Gnetales. II. Angiospermae. Forschungsberichte des Landes Nordrhein-Westfalen. 1967;1761:1–310. [Google Scholar]
- Pšenička J, Bek J, Zodrow EL, Cleal CJ, Hemsley A. A new late Westphalian fossil marattialean fern from Nova Scotia. Botanical Journal of the Linnean Society. 2003;142:199–212. [Google Scholar]
- Pšenička J, Zodrow EL, Bek J. A reassessment of the taxonomy of Oligocarpia bellii (Late Pennsylvanian, Sydney Coalfield, Nova Scotia, Canada) Palaeontographica, Abteilung B. 2005;272:51–65. [Google Scholar]
- Ravn RL. Palynostratigraphy of the Lower and Middle Pennsylvanian coals of Iowa. Iowa Geological Survey Technical Paper. 1986;7:1–245. [Google Scholar]
- Rees PM, Ziegler AM, Gibbs MT, Kutzbach JE, Behrling PJ, Rowley DB. Permian phytogeographic patterns and climate data/model comparisons. Journal of Geology. 2002;110:1–31. [Google Scholar]
- Remy W, Remy R. Durch Mazeration fertiler Farne des Paläozoikums gewonnene Sporen. Paläontologische Zeitschrift. 1957;31:55–65. [Google Scholar]
- Remy W, Rettschlag R. Neue Untersuchungen über die Pollen von Schuetzia anomala H. B. Geinitz. Geologie. 1954;3:582–589. [Google Scholar]
- Retallack GJ, Dilcher DL. Reconstructions of selected seed ferns. Annals of the Missouri Botanical Garden. 1988;75:1010–1057. [Google Scholar]
- Riggs SD, Rothwell GW. Sentistrobus goodii n. gen. and sp., a permineralized sphenophyllalean cone from the Upper Pennsylvanian of the Appalachian Basin. Journal of Paleontology. 1985;59:1194–1202. [Google Scholar]
- Rothwell GW. The Callistophytales (Pteridospermopsida): Reproductively sophisticated Palaozoic gymnosperms. Review of Palaeobotany and Palynology. 1981;32:103–121. [Google Scholar]
- Rothwell GW, Mapes G, Hernandez-Castillo GR. Hanskerpia gen. nov. and phylogenetic relationships among the most ancient conifers (Voltziales) Taxon. 2005;54:733–750. [Google Scholar]
- Rueger BF. Palynology and its relationship to climatically induced depositional cycles in the Middle Pennsylvanian (Desmoinesian) Paradox Formation of southeastern Utah. US Geological Survey Bulletin. 1996;2000-K:K1–K22. [Google Scholar]
- Schwendemann AB, Wang G, Mertz ML, McWilliams RT, Thatcher SL, Osborn JM. Aerodynamics of saccate pollen and its implications for wind pollenation. American Journal of Botany. 2007;94:1371–1381. doi: 10.3732/ajb.94.8.1371. [DOI] [PubMed] [Google Scholar]
- Scotese C. PALEOMAP Project. Department of Geology, University of Texas; Arlington, TX: 1999. PALEOMAP Animations ‘Paleogeography’. [Google Scholar]
- Srivastava SC. Lelestrobus: a new microsporangiate organ from the Triassic of Nidpur, India. Palaeobotanist. 1984;32:86–90. [Google Scholar]
- Staub JR, Gastaldo RA. Late Quaternary incised-valley fill and deltaic sediments in the Rajang River Delta. In: Sidi HF, Nummedal D, Imbert P, Darman H, Posamentier HW, editors. Tropical deltas of southeast Asia – sedimentology, stratigraphy, and petroleum geology. Vol. 76. 2003. pp. 71–87. SEPM Special Publication. [Google Scholar]
- Tabor NJ. Permo-Pennsylvanian palaeotemperatures from Fe-Oxide and phyllosilicate δ18O values. Earth Planet Sci Lett. 2007;253:159–171. [Google Scholar]
- Tabor NJ, Montañez IP. Permo-Pennsylvanian alluvial paleosols (north-central Texas): High-resolution proxy records of the evolution of early Pangean paleoclimate. Sedimentology. 2004;51:851–884. [Google Scholar]
- Tabor NJ, Montañez IP, Scotese CR, Poulsen CJ, Mack GH. Paleosol archives of environmental and climatic history in paleotropical western Pangea during the latest Pennsylvanian through Early Permian. In: Fielding CR, Frank TD, Isbell JL, editors. Resolving the Late Paleozoic Ice Age in Time and Space. Vol. 441. 2008. pp. 291–303. Geological Society of America Special Paper. [Google Scholar]
- Tabor NJ, Poulsen CJ. Palaeoclimate across the Late Pennsylvanian–Early Permian tropical palaeolatitudes: A review of climate indicators, their distribution, and relation to palaeophysiographic climate factors. Palaeogeography, Palaeoclimatology, Palaeoecology. 2008;268:293–310. [Google Scholar]
- Tabor NJ, Romanchock CM, Looy CV, Hotton C, DiMichele WA, Chaney DS. Conservatism of Late Pennsylvanian vegetational patterns during short-term cyclic and long-term directional environmental change, western equatorial Pangaea. Geological Society, London, Special Publications. 2013;376:201–234. doi: 10.1144/sp376.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TN. On the structure and phylogenetic relationships of the fern Radstockia Kidston. Palaeontology. 1967;10:43–46. [Google Scholar]
- Taylor WA. Ultrastructural studies of sphenophyllalean spores. Review of Paleobotany and Palynology. 1986;47:105–128. [Google Scholar]
- Taylor TN, Millay MA. Pollination biology and reproduction in early seed plants. Review of Paleobotany and Palynology. 1979;27:329–355. [Google Scholar]
- Taylor TN, Rothwell GW. Studies of seed fern pollen: development of the exine in Monoletes (Medullosales) American Journal of Botany. 1982;69:570–578. [Google Scholar]
- Taylor TN, Taylor EL, Krings M. Paleobotany: the biology and evolution of fossil plants. Academic Press; Burlington, MA: 2009. [Google Scholar]
- Traverse A. Topics in Geobiology. 2. Vol. 28. Springer; Dordrecht: 2007. Paleopalynology. [Google Scholar]
- Walling DE, He Q. The spatial variability of overbank sedimentation on river floodplains. Geomorphology. 1998;24:209–223. [Google Scholar]
- Wanless HR, Shepard FP. Sea level and climatic changes related to late Paleozoic cycles. Geological Society of America Bulletin. 1936;47:1177–1206. [Google Scholar]
- Wardlaw BR. Age assignment of the Pennsylvanian-Early Permian succession of North Central Texas. Permophiles. 2005;46:21–22. [Google Scholar]
- Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. Phylogenies and community ecology. Annual Review of Ecology, Evolution, and Systematics. 2002;33:475–505. [Google Scholar]
- Wing SL, DiMichele WA. Conflict between local and global changes in plant diversity through geological time. Palaios. 1995;10:551–564. [Google Scholar]
- Winston RB. A Late Pennsylvanian upland flora in Kansas: systematics and environmental implications. Review of Palaeobotany and Palynology. 1983;40:5–31. [Google Scholar]
- Yao Z, Taylor TN. On a new gleicheniaceous fern from the Permian of South China. Review of Palaeobotany and Palynology. 1988;54:121–134. [Google Scholar]
- Zavada MS. The ultrastructure of pollen found in dispersed sporangia of Arberiella (Glossopteridaceae) Botanical Gazette. 1991;152:248–255. [Google Scholar]
- Zavialova N, Van Konijnenburg-van Cittert JHA. Exine ultrastructure of in situ peltasperm pollen from the Rhaetian of Germany and its implications. Review of Palaeobotany and Palynology. 2011;168:7–20. [Google Scholar]
- Zodrow EL, Šimünek Z, Cleal CJ, Bek J, Pšenička J. Taxonomic revision of the Palaeozoic marattialean fern Acitheca Schimper. Review of Palaeobotany and Palynology. 2006;138:239–280. [Google Scholar]