Abstract
In most primate societies, strong and enduring social bonds form preferentially among kin, who benefit from cooperation through direct and indirect fitness gains. Chimpanzees, Pan troglodytes, differ from most species by showing consistent female-biased dispersal and strict male philopatry. In most East African populations, females tend to forage alone in small core areas and were long thought to have weak social bonds of little biological significance. Recent work in some populations is challenging this view. However, challenges remain in quantifying the influence of shared space use on association patterns, and in identifying the drivers of partner preferences and social bonds. Here, we use the largest data set on wild chimpanzee behaviour currently available to assess potential determinants of female association patterns. We quantify pairwise similarities in ranging, dyadic association and grooming for 624 unique dyads over 38 years, including 17 adult female kin dyads. To search for social preferences that could not be explained by spatial overlap alone, we controlled for expected association based on pairwise kernel volume intersections of core areas. We found that association frequencies among females with above-average overlap correlated positively with grooming rates, suggesting that associations reflected social preferences in these dyads. Furthermore, when available, females preferred kin over nonkin partners for association and grooming, and variability was high among nonkin dyads. While variability in association above and below expected values was high, on average, nonkin associated more frequently if they had immature male offspring, while having female offspring had the opposite effect. Dominance rank, an important determinant of reproductive success at Gombe, influenced associations primarily for low-ranking females, who associated preferentially with each other. Our findings support the hypothesis that female chimpanzees form well-differentiated social relationships that are of potential adaptive value to females and their offspring.
Keywords: chimpanzee social structure, core area, dyadic association, kin bias, range estimation, social bonding, social preference
Kinship has long been recognized as an important factor mediating the distribution of social interactions among conspecifics, given the inclusive fitness benefits gained by cooperating with relatives (Hamilton, 1964). Empirical evidence from a range of social mammals supports the role of kinship for structuring social relationships within groups (spotted hyaenas, Crocuta crocuta: Holekamp et al., 1997; African elephants, Loxodonta africana: Archie et al., 2006; sperm whales, Physeter macrocephalus: Gero et al., 2008; killer whales, Orcinus orca: Pilot et al., 2010; yellow-bellied marmots, Marmota flaviventris: Wey & Blumstein, 2010; Thornicroft’s giraffes, Giraffa camelopardalis thornicrofti: Bercovitch & Berry, 2013), and this is particularly true for nonhuman primates (reviewed in Langergraber, 2012) and humans (Madsen et al., 2007). Because of sex differences in fitness-optimizing strategies that result in greater resource constraints on reproduction in females than in males, female mammals often benefit most from kin support in resource defence. As a result, mammalian societies with sex-biased dispersal often form around females and their related offspring (Dobson, 1982; Mabry et al., 2013; Pusey, 1987). Thus, it is not surprising that female mammals, more often than males, form the strongest and longest-lasting social bonds, and have evolved hormonal adaptations that facilitate such bonding (Taylor et al., 2000).
Chimpanzees, Pan troglodytes, deviate from the typical mammalian pattern of female philopatry by showing consistent female-biased dispersal and male philopatry (Nishida & Kawanaka, 1972; Pusey, 1979), a feature of social organization shared with their sister taxon, bonobos, Pan paniscus (Eriksson et al., 2006; Furuichi, 1989; Gerloff et al., 1999). Males defend a community range in which females settle, and female eastern chimpanzees, P. t. schweinfurthii, in at least two populations establish areas of preferential use within this range, often referred to as “core areas” (Gombe, Tanzania: Murray et al., 2007; Williams et al., 2002b; Kanyawara, Kibale National Park, Uganda: Emery Thompson et al., 2007; Kahlenberg et al., 2008b). Within the community range, both males and females freely join or leave subgroups known as “parties” (Goodall, 1986), a characteristic shared with other fission–fusion societies (Grove, 2009; Mann, 2000). As expected based on kin selection theory, male chimpanzees form strong social bonds with other males (Mitani, 2009; Watts, 2002), with measurable effects on rank acquisition and the likelihood of siring offspring (Gilby et al., 2013a).
In most East African populations of chimpanzees, females spend much of their time foraging alone with their dependent offspring (Murray et al., 2007; Wrangham & Smuts, 1980), and it is often assumed that they do so to minimize contest competition over limited resources (Wrangham, 1979). Empirical evidence supports the role of competition in determining female ranging patterns and social interactions. Female core areas vary in resource quality (Emery Thompson et al., 2007; Kahlenberg et al., 2008b; Murray et al., 2006), and higher-ranking females often occupy better habitats (Pusey & Schroepfer-Walker, 2013). Females aggressively defend core areas (Miller et al., 2014), and differences in resource-holding potential may lead to better foraging efficiency among high-ranking females (Emery Thompson et al., 2007; Murray et al., 2006; Williams et al., 2002b), which can influence reproductive success (Emery Thompson et al., 2007; Jones et al., 2010; Pusey et al., 1997).
Given competition over resources and the general absence of kin, female social relationships among chimpanzees were originally considered to be weak and of little significance (Goodall, 1986; Wrangham, 1979). However, the role of social bonding among nonkin as an important mediator of social structure in mammalian societies is increasingly being revealed through empirical studies in a variety of species (bottlenose dolphins, Tursiops aduncus: Connor et al., 2001; wild dogs, Lycaon pictus: de Villiers et al., 2003; black-and-white colobus monkeys, Colobus vellerosus: Wikberg et al., 2012), and evidence is accumulating that these bonds can have direct fitness benefits in both sexes (male lions, Panthera leo: Packer & Pusey, 1982; female feral horses, Equus caballus: Cameron et al., 2009; male Assamese macaques, Macaca assamensis: Schuelke et al., 2010; female chacma baboons, Papio hamadryas ursinus: Silk et al., 2010). In line with findings from other taxa, there is evidence from multiple study populations of chimpanzees suggesting that females can be more social than traditionally assumed (Lehmann & Boesch, 2008; Wakefield, 2008). For example, Gilby and Wrangham (2008) reported that some female dyads at Kanyawara have as high or higher association rates than the most strongly bonded males, and the highest party association rates among adult chimpanzees at Ngogo (Kibale National Park, Uganda) were recorded for female dyads (Langergraber et al., 2009). Some suggest that females may indeed form differentiated social relationships that reflect social preferences, like the social cliques identified by Wakefield (2013), and can be stable over time (Langergraber et al., 2009). However, questions remain about whether these relationships are similar to the social bonds among male chimpanzees and philopatric females of other primate societies, and about how females benefit from them.
Here, our main aims were to identify the correlates of female chimpanzee association patterns at Gombe National Park, Tanzania, with particular emphasis on factors that mediate variation in these associations, and to test for the existence of social preferences that could support the existence of social bonds among unrelated females. For this purpose, we analysed the most comprehensive set of behavioural data from any population of wild chimpanzees to date, spanning a period of 38 years.
Detecting and characterizing differentiated social relationships is challenging among female chimpanzees at Gombe, because affiliative interactions are rarely observed (Goodall, 1986). Instead, estimates of social preferences rely heavily on dyadic association indices derived from party composition data (Cairns & Schwager, 1987), that is, information on who was seen with whom. While the fission–fusion nature of chimpanzee social structure offers unique opportunities for individuals to express partner choices within their communities, it poses unique challenges for researchers attempting to distinguish actual social preferences from random associations due to shared space use, a problem shared with studies of other social mammals (tent-making bats, Artibeus watsoni: Chaverri et al., 2007; bottlenose dolphins: Frère et al., 2010; grey kangaroos, Macropus giganteus: Best et al., 2014).
Previous studies on female chimpanzee dyadic association have varied in their approaches to assess the influence of shared space use on association patterns. Some classified similarities in space use at the scale of “neighbourhoods”, and assumed little further influence of variation in core area overlap on association rates within these neighbourhoods (Gombe: Murray et al., 2007; Williams et al., 2002b; Kanyawara: Emery Thompson et al., 2007). In contrast, at Ngogo, Langergraber et al. (2009) used a more spatially explicit technique by testing for a relationship between (1) correlation coefficients representing similarity in grid cell usage frequencies between any two individuals and (2) the party association index for the given dyad. A matrix correlation of the two measures provided evidence that, across all female dyads, shared space was positively related to association rates. Similarly, Wakefield (2013) established that dyadic associations were positively correlated with space use overlap, assessed as the percentage overlap of any two minimum convex polygons drawn around 100% of sightings for a given female.
As association depends on being in the same location at the same time, a positive relationship between degree of shared space use and dyadic association rate is likely, though not guaranteed (e.g. De Villiers & Kok, 1997). Therefore, revealing active partner preferences generally relies on assessing rates of association that deviate from those expected based on random interactions among all individuals sharing the same area. Previous studies on female chimpanzee association patterns established random expectations for dyadic association rates based on permuting the rows and columns of an association matrix (Langergraber et al., 2009; Lehmann & Boesch, 2009; Wakefield, 2013). However, such matrix permutation tests do not account for spatial constraints on association, and are therefore most suitable in social groups where all individuals do indeed share the same space (e.g. Lehmann & Boesch, 2009). If individuals occupy distinct and only partially overlapping home ranges, as is the case for female chimpanzees at Gombe and other East African populations, spatially explicit methods for deriving expected association rates (e.g. Best et al., 2014) may provide a more powerful technique to assess social preferences.
In this paper, we search for evidence of female social bonds by assessing nonspatial (i.e. social) drivers of dyadic association while controlling for mean expected association at a given level of shared space use. If spatiotemporal association reflects active social partner preferences, we expected a positive relationship between dyadic association and grooming rates, similar to previous findings at Ngogo (Langergraber et al., 2009). Although social grooming is rare among female chimpanzees (Goodall, 1986), it is indicative of social bonding in males (Mitani, 2009), among females of other primate taxa (Cords, 2002; Dunbar, 2010; Silk et al., 2006), and in other social vertebrates (reviewed in Massen et al., 2010).
Preferential associations may also arise for reasons other than direct affiliation. For example, higher-quality feeding areas could simply facilitate association among neighbouring females regardless of their core area overlap, because abundant food supply may allow for larger parties (Mitani et al., 2002; Murray et al., 2006). Alternatively, preferential association might come with greater tolerance around feeding areas in general, or reduce vigilance and increase feeding efficiency (Carter et al., 2009; Kutsukake, 2006). While feeding competition is assumed to be an important factor in female eastern chimpanzee social structure and life history (Pusey & Schroepfer-Walker, 2013), whether access to food resources is mediated by variation in dyadic associations is still uncertain and requires further investigation.
Given the potential inclusive fitness benefits to be gained, kin may be more likely to cooperate to access food resources or for offspring rearing than unrelated individuals. Therefore, we expected that kin, if available, would associate more frequently than unrelated females, after controlling for spatial overlap. Despite the general pattern of male philopatry and female dispersal that limits opportunities for kin interactions, the Kasekela community of chimpanzees at Gombe has an unusually high proportion of females who remain in their natal community (Pusey et al., 1997). This unique context allows us to distinguish the role of kinship in female association patterns more clearly than any study thus far (Gilby & Wrangham, 2008; Langergraber et al., 2009), and to compare the relative importance of kin and nonkin in social partner choices.
Besides kinship, other potential nonspatial determinants of female association include cycling state, presence and sex of offspring, and dominance rank. We expected that females would become more social when sexually receptive (Matsumoto-Oda, 1999; Pepper et al., 1999) and therefore would be more likely to associate with other sexually receptive females (Pepper et al., 1999; Williams et al., 2002a), reflecting a shared tendency to associate with potential mates. Offspring presence can mediate changes in association among females for reasons such as avoidance of competition or harassment (decrease in association) or the socialization of offspring (increase in association) (Murray et al., 2014; Otali & Gilchrist, 2006; Williams et al., 2002a; Wrangham, 2000). Based on recent evidence from Gombe that mothers of male infants are more gregarious than mothers of female infants (Murray et al., 2014), as well as sex differences in behaviour that show more frequent social interactions between male offspring and adult males (Lonsdorf et al., 2014), we expected mothers of male offspring to be more social in general, and to associate more with each other than with mothers of female offspring or nonmothers. Such sex-biased socialization could have adaptive benefits, because males will become lifelong partners in cooperation and community defence (Gilby et al., 2013b; Goodall, 1986; Mitani, 2009).
Lastly, we examined rank-related variation in dyadic associations, which can reveal adaptive social strategies. Given the importance of dominance rank for mediating success in resource competition in at least two East African chimpanzee populations (Kanyawara: Emery Thompson et al., 2007; Gombe: Kahlenberg et al., 2008b; Murray et al., 2007), low-ranking females may attempt to gain access to better feeding sites by establishing predictable relationships with higher-ranking females, or they may increase their competitiveness by supporting each other against those females. Similarly, high-ranking females with preferred feeding sites within their core areas may attempt to exclude females with equivalent resource-holding power (i.e. females close in rank). Previous findings at Gombe indicated that females associate at higher rates with females of similar rank within larger neighbourhoods (Murray et al., 2006; Williams et al., 2002a), but it is still unclear whether these associations are mediated by spatial constraints on core area location (and shared space use as indicated by core area overlap) or reflect active social preferences.
METHODS
Study Site and Data Collection
Gombe National Park covers 35 km2 of forest and forest/grassland mosaic on the eastern shore of Lake Tanganyika in Tanzania. The park contains three communities of eastern chimpanzees (P. t. schweinfurthii). The Kasekela community has been studied since the early 1960s and was fully habituated by 1966 through banana provisioning at a feeding station, which continued until 2000. Since 1973, Tanzanian field assistants have conducted almost daily full-day focal observations on members of the Kasekela community (Goodall, 1986). During these follows, the location of the focal animal was recorded at 15 min intervals on a map, with an approximate accuracy of 133 m (Gilby, 2004), while party composition was recorded continuously to the nearest minute. Additionally, long-hand notes of focal and party behaviour and the reproductive state of any females encountered by the focal were recorded.
Female Ranging and Overlap of Core Areas
Previous studies of female association in our study population clustered core areas into two to three neighbourhoods, and assessed the influence of predictor variables on association rates within each neighbourhood (Murray et al., 2006; Williams et al., 2002b). As the distinction between large-scale neighbourhoods was not always clearly delineated in our long-term data set and therefore not always an effective way to control for shared space use among dyad partners, we developed a new approach that contrasts observed dyadic association rates with those expected based on the pairwise overlap of individual females’ core areas.
We estimated core area location and size for each female using kernel estimation methods. Specifically, we calculated utilization distributions based on all certain first encounters of a female with a focal party, and identified the smallest area that contained 50% of the estimated probability density function. As sample size influences the accuracy of home range estimates, we included only those females for which we had at least 20 such locations per 2-year period (mean ± SD number of locations per period, for all included females, was 135 ± 80), a sample size associated with unbiased estimates in other studies (Börger et al., 2006; Saïd et al., 2005). While previous work on ranging patterns in this population has focused on defining areas in which females are most likely to be encountered alone (‘alone core areas’), we modified our approach in this study for several reasons: (1) to make our methods more comparable with other studies on chimpanzee ranging, which generally use location information regardless of party size to assess ranging patterns (Amsler, 2010; Emery Thompson et al., 2007; Lehmann & Boesch, 2003) and (2) because our focus on socially mediated association requires the presence of others at recorded locations.
In exploratory analyses, we tested a variety of kernel estimation methods in their performance and representation of actual ranging patterns, paying particular attention to the choice of smoothing parameter h, the single most important factor in home range estimation using kernel algorithms (Kie et al., 2010). While optimal smoothing parameters are frequently identified using a least squares cross-validation technique, this method often failed to converge within a reasonable range of values for our data set (see also Seaman & Powell, 1996; Silverman, 1986). We therefore used an alternative method, which scales the reference smoothing parameter (empirically calculated for each set of points) by a fixed factor, with suggested values being 0.8 or 0.7 (Kie et al., 2010). After comparison of results obtained with different scaling factors, we settled on 0.8 as giving the best compromise between underestimating actual usage and excessively smoothed boundaries that extended into unused habitat.
We calculated kernels in R version 3.0.2 (R Core Team, 2013) using the adehabitatHR package version 1.8.14 (Calenge, 2006), which we modified to achieve the above adjustment of smoothing factors for each utilization distribution of a given female and time period. We constrained kernels to a fixed grid of 10 × 10 m to avoid inaccuracies in calculation of overlaps that would otherwise be introduced by differences in resolution of utilization distributions. We calculated pairwise overlap between core areas in each period using the kerneloverlapHR function of the adehabitatHR package. To obtain one symmetrical measure of overlap per dyad, we used the volume intersection of two utilization distributions (Seidel, 1992), which calculates the probability of finding two females in the area of overlap. This index is widely used in studies of home range overlap (Kernohan et al., 2001) and has been applied successfully in a variety of study systems (reviewed in Fieberg & Kochanny, 2005). Finally, to account for temporal changes in community range size, demography and ecology, we z-transformed overlap measures using the mean across all dyads in each period.
Dyadic Association
We quantified dyadic association and ranging data from 1974 to 2011, and grooming data from 1978 to 2011, aggregated into 2-year periods. Across all periods, the study includes 53 adult females and 624 unique dyads. We included females in a given period only if they were alive and present in the Kasekela community at the beginning and end of that period, and were either 12 years or older, or had experienced a full sexual swelling and mated with an adult male, by the start of the period. Across studies, mean age at first birth is about 13 years (Emery Thompson, 2013), and four known-aged Gombe females have given birth at 11–12 years (Pusey, n.d.). Therefore, most females would be adult within the first 2-year period that they were included in our analyses. We considered males to be adult if they were at least 12 years of age, which is the earliest recorded age that a male at Gombe has fathered offspring (Wroblewski et al., 2009).
In fission–fusion social systems, dyadic association indices are widely used to estimate the proportion of time two individuals spend together, given incomplete observation of parties that contain either one or both individuals. The choice of index determines the degree of potential bias in these estimates (Cairns & Schwager, 1987), based on the difference in probability between encountering two individuals either apart or together. As female chimpanzees at Gombe spend nearly half of their time or more alone with dependent offspring, on average (Murray et al., 2007; Wrangham & Smuts, 1980), and because the chance of finding any two neighbouring females together within the area of overlap of their respective core areas was low (mean probability as calculated by the volume intersection of two kernel distributions: 0.24 ± 0.09, N = 624 dyads), the chance of encountering two individuals together was probably lower than the chance of encountering either one without the other. In this context, the half-weight index provides a relatively unbiased estimate of actual time spent together (Cairns & Schwager, 1987), and we therefore follow Murray et al. (2006) in using it for quantifying dyadic associations among female chimpanzees.
In line with previous work (Murray et al., 2006; Williams et al., 2002b), we based calculation of the half-weight index on “first arrivals” (i.e. the first observed encounter of an individual or group of individuals by the focal). We decided against more traditional measures based on time spent in association or the frequency of group scans for the following reasons: (1) focal observations were not evenly distributed among all females, which would lead to biases in observed time and hence in the estimation of time spent together with other females; (2) repeated measures taken in the same party over the course of all-day focal observations would cause considerable autocorrelation in frequency data, which would complicate analyses and potentially be difficult to control for; and (3) dyadic association rates based on time or frequency are more likely to be influenced by individual variation in gregariousness (Pepper et al., 1999), which can mask true partner preferences.
Females were considered arriving together if they were first encountered by the focal within 5 min of each other, and we therefore assumed to have been together before they were encountered. We calculated the index as: YAB/(YAB + ½ (YA + YB)), where YAB is the number of times A and B arrived together, YA is the number of times A arrived into a group without B, and YB is the number of times B arrived into a group without A. Note that when observers first encountered a focal party, all individuals in that party were counted as “arriving” together. Subsequently, arriving individuals were not counted as “in association” with members of the focal party at the time of the first encounter, but only in association with those whom they arrived with. We did this to minimize observer bias, and because we could not distinguish between active arrivals into parties versus passive encounters by the focal party as it moved through the community range. To further minimize the effects of sampling bias on estimation of half-weight indices (Whitehead, 2008), we excluded females from analyses if they had 20 or fewer sightings for a given period.
Our index of association based on first arrivals was highly correlated with a dyadic association index based on time spent together (Pearson’s r = 0.934, N = 2222 dyad scores across all periods), yet preferred for its conceptual advantages listed above. We also repeated our analyses using the simple ratio index (again, calculated from first arrivals) as the dependent variable to assess the sensitivity of our results to inherent inaccuracies in the estimation of actual time spent together. The direction and relative magnitude of findings did not change, indicating that both indices provide comparable information in our study population. Both measures were correlated highly (Pearson’s r = 0.982, N = 2222 dyad scores across all periods).
Exploratory analyses indicated a positive temporal trend in the community-wide tendency to associate that was not of primary interest and could confound our assessment of differences in association between dyads. To remove this temporal variation we z-transformed association scores in each period using the mean across all dyads. Thus, all reported measures of dyadic association reflect differences in terms of standard deviations from the mean for each period.
Grooming Index
To quantify affiliative interactions among females, we calculated a grooming index that quantified the proportion of time two individuals spent grooming each other, controlling for time spent together (e.g. Machanda et al., 2013). We calculated this measure from two data sets. From 1978 to 1997, grooming involving the focal female was recorded at 5 min intervals during all-day follows, and we defined the grooming index as: SAB/(SA + SB), where SAB is the number of grooming scans where A and B were grooming, SA is the number of scans where A was focal and B was present, and SB is the number of scans where B was focal and A was present. Scans were included only when either A or B were the focal subject. From 1998 to 2011, grooming durations were extracted from long-hand behaviour notes, and calculated equivalently using duration of grooming in minutes instead of number of scans. As the grooming index was only calculated in a given period for dyads that were observed together in the same party when one was the focal subject (457 dyad scores), some dyads lacked this measure and the data set was reduced by about 20% when this measure was included in analyses. Although there was no consistent temporal trend in the period mean grooming index, we z-transformed this measure to control for period-to-period variation in community-wide grooming rates.
Assessment of Kinship
Maternal relations were determined based on genealogical records. Females who were born before data collection began or who immigrated into the community without records on their parental relationships (N = 30) were assumed to be unrelated to all other females. Thus, it is possible that some of the dyads identified as nonkin were in fact related, although their proportion is likely to be very small and decreasing over the course of the study. Some purely paternal relationships were known from DNA testing (N = 4 dyads of adult females), but due to incompleteness of genetic data we considered only mother–daughter and maternal sister relationships as kin in our analyses. In total, there were 17 such kin pairs across all study periods, 12 mother–daughter dyads and 5 maternal sister dyads (~3% of all dyads).
Cycling State
Female swelling state was recorded during daily focal follows for each female that joined the focal party, and assessed as flat, quarter swollen, half swollen, three-quarters swollen, or fully swollen. To evaluate the influence of cycling state on dyadic association, for each pair of females we calculated the proportion of joint first arrivals in which both partners were recorded as fully swollen, in each 2-year period.
Presence and Sex of Offspring
We classified offspring of each sex into three age groups: infants (<3.5 years) and juveniles (≥3.5 years and <7 years), following Williams et al. (2002a), and adolescents (≥7 years and <12 years). For each 2-year period, we classified a female as having an offspring of a given age/sex class if the offspring was alive for at least 365 days (i.e. 50% of the period length), and assessed offspring age at the midpoint of each period.
Dominance Rank
We derived ranks from single-recipient female–female pant-grunts (a vocalization directed at dominant individuals by subordinates; Bygott, 1979), which were extracted from all behavioural observations made during the study period (including focal follows of adults and mothers and offspring, observations at the feeding station, and student projects). For each period, we calculated modified David’s scores (de Vries et al., 2006), and then determined categorical ranks for each female as follows: high rank if her score was ≥ 0.5 SD above the mean score for a given period (27 ± 8% of females across all periods), and low rank if her score was ≤ 0.5 SD below the mean (24 ± 8% of females). We assigned all other females medium rank. If a female had insufficient data to calculate her score for a given period, we assigned her last known rank.
Statistical Analysis
We modelled variance in dyadic association for each dyad (N = 624) and 2-year period (N = 19) using general linear mixed models (GLMM), with dyad ID as a random effect to allow random variation among dyads in the predicted mean dyadic association (intercept) based on unknown characteristics of individuals in each dyad. While this model structure did not control for dependencies introduced by the same individual participating in multiple dyads, variance component analyses suggested that these dependencies have relatively little influence on our findings. If an individual strongly influences association rates across all its dyads, variance across these dyads would be relatively smaller than if the identity of partners was the driving force behind association rates for that same individual across its partners. We found that only about 25% of total variance in the data was accounted for by individual differences in mean dyadic association (calculated across all partners for a given female), while 75% of the variance was associated with within-female variation in dyadic association across partners. Thus, the influence of any given female on the dyadic association scores of all the dyads she was part of was much smaller than the influence of other factors that determined variation in dyadic association across partners.
To guide the parameterization of our models, we used a small sample size correction of the Akaike Information Criterion (AICC) to verify the relative fit of random intercept models with no fixed predictor variables (null model) but different covariance structures for repeated measures taken on the same dyads (Burnham & Anderson, 2002). We achieved the best model fit with a first-order heterogeneous autoregressive covariance structure, which assumes decreased correlation of residuals within dyads as the separation of data points in time increases, and allows for heterogeneous variances across dyads in each period. For one analysis (sex combination of offspring), using this covariance structure resulted in convergence failure, and we reduced model complexity by assuming a simple first-order autoregressive covariance structure instead.
Our first model focused on assessing the effects of spatial overlap on dyadic association, aimed at determining mean levels of association at any given level of core area overlap. We then continued to examine each potential predictor of variation in socially driven association independently, rather than conducting multivariate model selection. We did this because we were specifically interested in the individual effects of each predictor on social preferences and we wanted to maximize available data for each independent factor of interest, and because multiple categorical predictor variables resulted in very low sample sizes for each combination of predictor levels that made model estimation unreliable.
We set the significance level for assessing the influence of predictor variables in our mixed models at α = 0.05, and report tendencies if P < 0.1. As our analyses are not strictly confirmatory, and because the hypotheses we test are conceptually independent, we did not adjust P values of individual models for multiple testing (Bender & Lange, 2001; Quinn & Keough, 2002). For all categorical predictors that included more than two levels, we conducted pairwise comparisons of estimated marginal means, which we set to predict means for each level of a categorical predictor at the period mean level of spatial overlap of core areas across all dyads (z score = 0). As the mean of overlap across dyads could vary across levels of a predictor, we tested whether the effects of a predictor varied by the magnitude of spatial overlap by including a first-order interaction term in the model. We removed nonsignificant interactions to obtain final estimates, which we use for all group comparisons. We report estimated means with their standard errors and 95% confidence intervals, and P values adjusted for multiple comparisons using a sequential Bonferroni correction. All analyses were conducted in IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY, U.S.A.).
RESULTS
Differentiation of Dyadic Association among Dyads
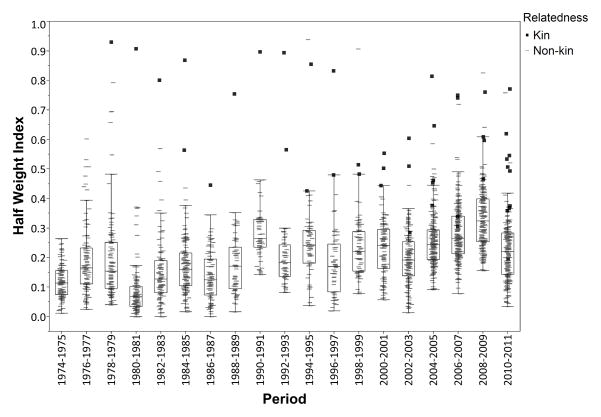
Dyadic association frequencies were well differentiated among dyads within a given 2-year period (Fig. 1), with period-specific means and standard deviations ranging from 0.07–0.33 and 0.04–0.1, respectively (N = 19 2-year periods). Across all periods, the mean ± SD of means in dyadic association across dyads was 0.19 ± 0.07. Minimum dyadic associations for a given period ranged from 0 to 0.16, and maximums from 0.2 to 0.94.
Figure 1.
Distribution of half-weight index scores among adult female chimpanzee kin (■) and nonkin (−) dyads within and across 2-year periods between 1974 and 2011. Box plots give median with interquartile range (IQR), and whiskers extend to furthest points within 1.5 × IQR.
Spatially Driven Variation in Dyadic Association
Spatial overlap of core areas had the predicted positive effects on dyadic association; for each standard deviation increase in overlap, dyadic association increased by about 0.46 SD, on average (t = 26.5, P < 0.001). Thus, a significant portion of the observed variance in association index can be explained by passive association due to chance encounters, given independent movement of females within their respective core areas. However, the finding that association rates did not increase in direct proportion to overlap, but instead increased considerably more slowly, suggests that shared space use is a necessary, but not sufficient, determinant of dyadic association.
In the following analyses, we account for expected mean association at any level of core area overlap by including core area overlap as a covariate, and focus on social factors that explain residual variation in dyadic association that is not explained by shared space use alone.
Social Correlates of Variation in Dyadic Association
Social affiliation
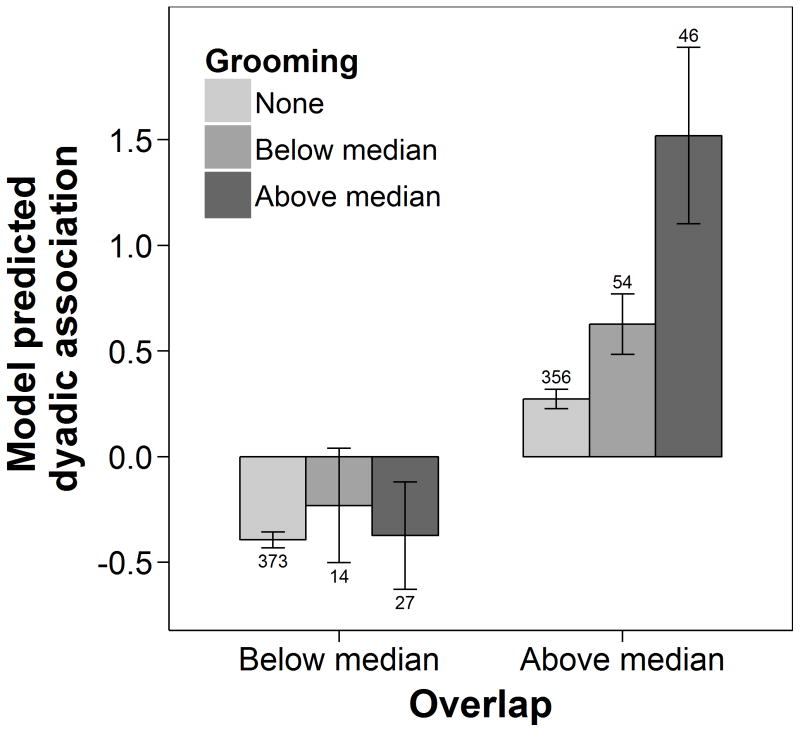
If dyadic association is an indicator of active partner preferences, we predicted that those dyads associating more than expected based on their core area overlap would also affiliate (i.e. groom) more frequently, while those who associated less than expected would affiliate less frequently. This prediction was only partially supported by the data. A significant interaction between overlap and grooming (Table 1) indicated that among dyads whose core areas overlapped more than average for a given period, dyadic association and grooming were positively correlated (Fig. 2). There was no such relationship for dyads whose core areas overlapped less than average for a given period. Thus, dyadic association was a good indicator of actual affiliation tendencies for spatially close females, while variation in dyadic association among more spatially separated females appears to have been driven by other motivating factors.
Table 1.
Parameter estimates of general linear mixed models assessing the influence of spatial overlap of core areas and grooming on dyadic association in female chimpanzees
| Parameter | Estimate | SE | df | P | 95% CI |
|
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Overlap | 0.50 | 0.02 | 1539.8 | <0.000 | 0.46 | 0.54 |
| Grooming | 0.02 | 0.02 | 1041.2 | 0.291 | −0.02 | 0.07 |
| Overlap*grooming | 0.08 | 0.02 | 886.8 | <0.001 | 0.05 | 0.12 |
Figure 2.
Model predicted mean (± 95% CI) z-transformed half-weight index of dyadic association in female chimpanzees in relation to z-transformed core area overlap and a dyadic grooming index that measures frequency of grooming in relation to observation time and opportunity (see text for definition of measures). For illustration purposes, we binned overlap z scores into below and above the median across all dyads and periods. As the grooming index was highly skewed towards zero values, we created three groups: no grooming recorded, and below and above median of all nonzero grooming indices in a given period. Numbers above or below error bars indicate the number of unique dyads in each group, across all periods.
Subsequently, we assess potential correlates of dyadic association that are nonaffiliative in nature (i.e. factors other than grooming that influenced variation in dyadic association).
Kin effects
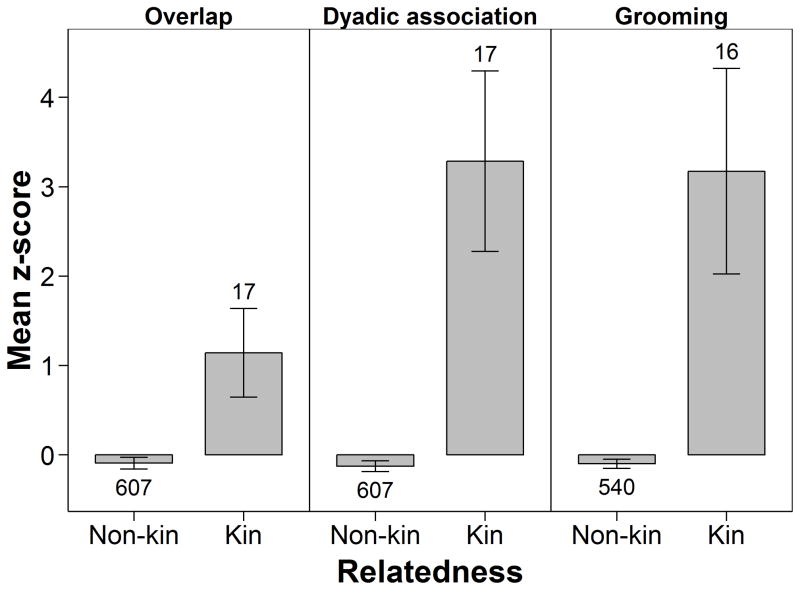
Because of sample size limitations, we limit our assessment of kin effects to descriptive statistics. Kin differed from nonkin in showing much higher levels of dyadic association, core area overlap and grooming (Fig. 3). Particularly, levels of association were much greater than expected based on core area overlap: while kin overlap was on average about 1 SD above the overall mean (i.e. for kin and nonkin), their mean dyadic association index was more than 3 SD above the overall mean. Although this pattern indicates generally stronger social relationships among kin than nonkin dyads, value ranges indicated that some nonkin dyads had similarly high levels of overlap and dyadic association (overlap range −1.24–2.36 SD versus −2.32–2.31 SD from the mean across all dyads, for kin and nonkin dyads, respectively; dyadic association index range −0.27– 6.4 SD versus −2.01–5.29 SD from the mean across all dyads, for kin and nonkin dyads, respectively). Furthermore, some nonkin dyads exceeded kin dyads in their grooming index (range 0–3.4% versus 0–8.8% for kin and nonkin dyads, respectively), suggesting the existence of strong partner preferences among certain nonkin dyads. Among the two different types of female kin that we examined, mother–daughter pairs had higher mean association and grooming indices than maternal sister dyads (association index: 0.63 ± 0.18, N = 11 mother–daughter dyad means across time periods, versus 0.43 ± 0.15, N = 5 sister dyads; grooming: 2.04 ± 0.75% versus 0.67 ± 0.71% for mother–daughter and sister dyads, respectively).
Figure 3.
Mean (± 95% CI) overlap of core areas (z score), dyadic association index (z score) and untransformed grooming index (%) for female chimpanzee nonkin and kin dyads, based on means calculated for each dyad across periods. Numbers above or below error bars indicate the number of unique dyads in each group, across all periods.
Because of the small sample of kin dyads, which precluded analyses of additional determinants of dyadic association while controlling for spatial overlap, we excluded kin dyads in the analyses of social determinants of dyadic association in all subsequent analyses.
Cycling state
Given equal levels of spatial overlap, estimated time spent together varied with the proportion of joint arrivals in which both females were fully swollen (Table 2). However, effect size was small; two females who were jointly swollen for 10% more time than another pair of females were estimated to spend 0.06 SD more time together in association. In other words, the difference in dyadic association between females who were never seen swollen together and those who were always swollen together was a maximum of about 6%, given a mean SD of 0.10 (i.e. 10% of time spent together) for the dyadic association index, calculated across all 19 periods and dyads.
Table 2.
Results of mixed model analyses estimating the influence of hypothesized individual traits on dyadic association among unrelated adult female chimpanzees
| Predictor | Group | N | F | P |
|---|---|---|---|---|
| Cycling state | 2177 | 19.2 | <0.001 | |
| Offspring presence a | Any offspring | 2177 | 5.9 | 0.003 |
| Female offspring | 2177 | 16.1 | <0.001 | |
| Male offspring | 2177 | 0.7 | 0.505 | |
| Male/female offspring combination | 2177 | 4.9 | <0.001 | |
| Infant female | 2177 | 1.7 | 0.181 | |
| Infant male | 2177 | 0.9 | 0.421 | |
| Juvenile female | 2177 | 15.6 | <0.001 | |
| Juvenile male | 2177 | 0.4 | 0.671 | |
| Adolescent female | 2177 | 0.4 | 0.669 | |
| Adolescent male | 2177 | 0.1 | 0.895 | |
| Offspring sex in dyads composed of two mothers b | Infants | 408 | 2.5 | 0.087 |
| Juveniles | 182 | 9.1 | <0.001 | |
| Adolescents | 203 | 0.004 | 0.996 | |
| Rank pairing | 1885 | 3.7 | 0.003 |
All models include overlap of core areas as a fixed effect covariate.
None, one of the partners, both partners.
Male–male, female–female, male–female.
Presence of offspring
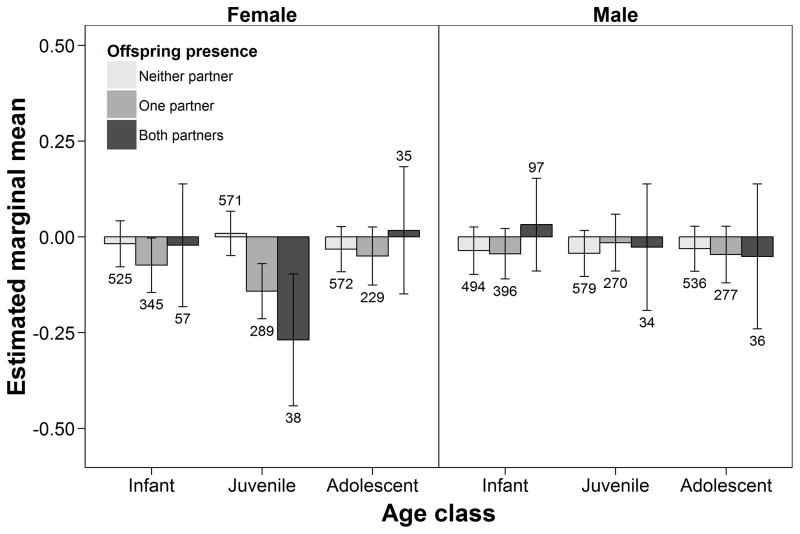
Given equal overlap of core areas, dyads in which both females lacked offspring <12 years old (i.e. immature) associated more frequently (z-score estimated marginal mean, EMM: 0.133 ± 0.055, N = 147 unique dyads) compared to dyads involving one (−0.029 ± 0.033, df = 1522.7, N = 440, P = 0.010) or two females with immature offspring (−0.072 ± 0.036, df = 1593.3, N = 322, P = 0.002). As females without immature offspring were more likely to be cycling, we repeated this analysis while controlling for the proportion of days in which both partners were swollen together, entered into the model as a covariate. Results changed very little (EMM: 0.133 ± 0.064 for dyads lacking immature offspring, compared to −0.027 ± 0.033 for dyads involving one female with immature offspring, and −0.048 ± 0.036 for dyads involving two females with immature offspring, P = 0.039 and P = 0.026, respectively).
We investigated the role of offspring sex on dyadic association by testing whether females were more or less likely to associate if both, only one, or neither of the partners had at least one immature male offspring, and did the same for the presence of female offspring (Table 2). Dyads in which both females had at least one female offspring associated less frequently and below what was expected based on their spatial overlap (EMM: −0.225 ± 0.047, N = 148), compared to dyads in which either one (−0.065 ± 0.032, df = 1190.3, N = 415, P = 0.001) or neither (0.109 ± 0.038, df = 1366, N = 312, P < 0.001) of the two females had a female offspring. The presence of male offspring did not influence female association above or below chance expectations.
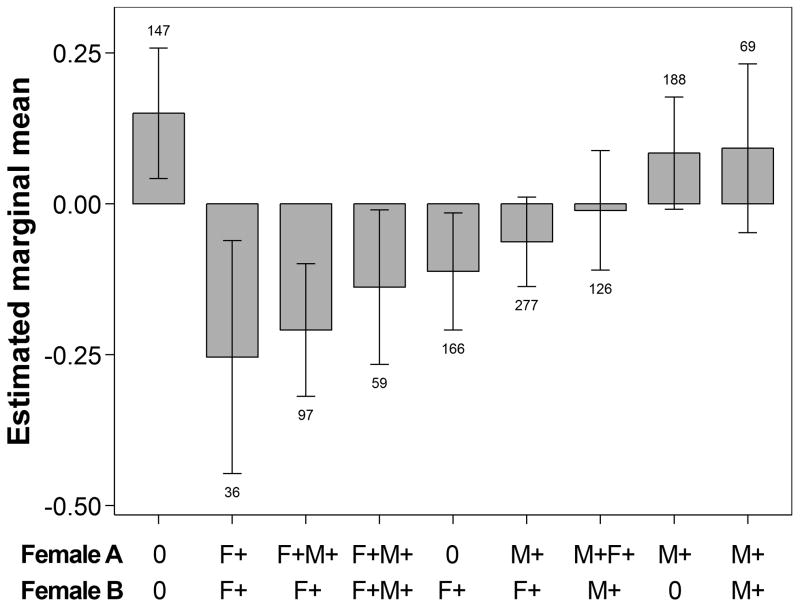
To account for simultaneous presence of offspring of the opposite sex and its possible influence on mediating female associations, we estimated the mean dyadic association for each level of combined male and female offspring presence in the dyad (Fig. 4). We found that predicted dyad association decreased as the presence of female offspring increased, indicating that the presence of female offspring was associated with lower levels of association regardless of the simultaneous presence of male offspring. Dyads with two females with immature male offspring and no simultaneous female offspring of any age were estimated to associate at significantly higher rates than dyads of two females with immature female offspring and no simultaneous presence of male offspring (mean z-score difference ± SE: 0.44 ± 0.12, df = 1265.8, P = 0.01; Fig. 4). Females with no immature offspring also associated at higher rates with females who had at least one male offspring and no female offspring than with those who had at least one female offspring and no male offspring (mean z-score difference: 0.22 ± 0.06, df = 1694.1, P = 0.016).
Figure 4.
Estimated marginal means (± 95% CI) of z-transformed half-weight index of dyadic association of female chimpanzees in relation to the presence of immature male versus female offspring of both members of the dyad, estimated at the population mean level of core area overlap for each group (z score = 0). Labels on X axis indicate, for females A and B in a given dyad, whether either one had at least one male (M+) or female (F+) offspring in a given period, or neither (0). The first group represents dyads of two females without immature offspring, and all subsequent groups are sorted by their mean estimated association index. Numbers above or below error bars indicate the number of unique dyads in each group, across all periods.
We further evaluated whether the effect of offspring sex varied by age by assessing whether females were more or less likely to associate if both, only one, or neither had offspring of a given age class, for each offspring sex. We found that the presence of juvenile females had significant effects on dyadic association (Table 2); females associated at significantly lower rates when they both had juvenile female offspring than when neither one of them had a female juvenile offspring (mean z-score difference: −0.29 ± 0.09, df = 568.6, P = 0.001; Fig. 5). The lack of effect of other female offspring age classes on dyadic association indicates that the general effect of female offspring presence (see above) is driven by the presence of juvenile females alone (Fig. 5). In contrast, the presence of male offspring of different age classes had no significant influence on dyadic association, given equal overlap of core areas (Table 2, Fig. 5).
Figure 5.
Estimated marginal means (± 95% CI) of z-transformed half-weight index of dyadic association of female chimpanzees in relation to the presence of offspring of different age and sex in a given dyad, with core area overlap held constant at the population mean for each group (z score = 0). For each age/sex class (N = 6), the model assessed whether females associated more or less than predicted based on the overlap of their core areas when neither one, only one, or both of the females had an offspring of that age/sex class, regardless of other offspring they may have had. Numbers above or below error bars indicate the number of unique dyads in each group, across all periods.
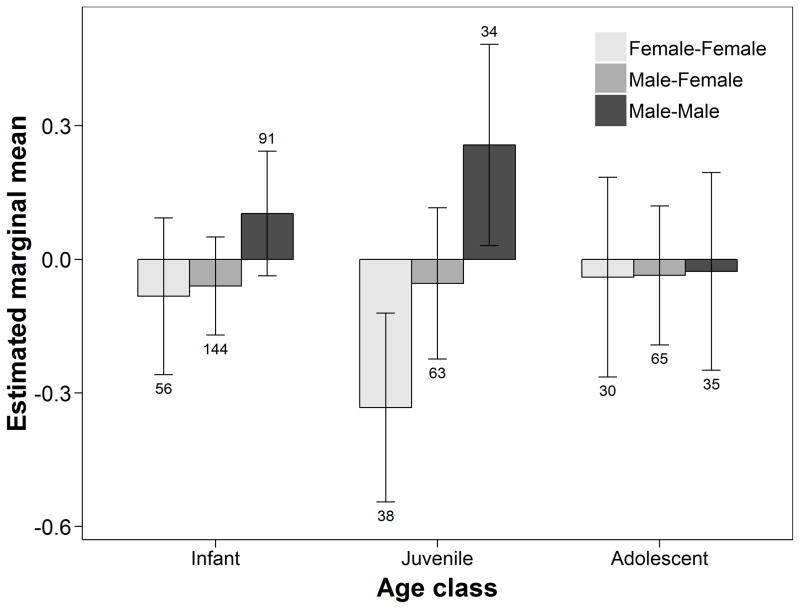
Lastly, we tested whether the simultaneous presence of offspring of different sex influenced association among dyads of two females with at least one immature offspring each, in relation to offspring age. We found that for pairs of mothers of infants and juveniles, offspring sex combination had significant effects on dyadic association (Table 2). Two females with immature offspring associated at higher rates if they both had at least one male infant, compared with dyads where both had offspring of different sex (mean z-score difference: 0.21 ± 0.08, df = 303, P = 0.041; Fig. 6), and tended to associate more than dyads of females with two female offspring (mean z-score difference: 0.25 ± 0.11, df = 274.5, P = 0.059). Females with immature offspring also associated at higher rates when they both had at least one male juvenile offspring than when they both had at least one female juvenile offspring (mean z-sore difference: 0.59 ± 0.15, df = 114, P < 0.001), and tended to associate more than dyads with offspring of different sex (mean z-score difference: 0.31 ± 0.13, df = 111.3, P = 0.058). The presence of adolescent offspring of different sex did not have any visible influence on dyadic association.
Figure 6.
Estimated marginal means (± 95% CI) of z-transformed half-weight index of dyadic association among pairs of chimpanzee mothers with different combinations of offspring sex within the same age class, regardless of the number of offspring in each class. Means are estimated at the population mean core area overlap (z score = 0). Dyads consisting of two mothers with at least one male infant or juvenile offspring each, and no female offspring of the same age class, associated at higher rates than dyads consisting of at least one mother with at least one female infant or juvenile offspring. Numbers above or below error bars indicate the number of unique dyads in each group, across all periods.
Dominance rank
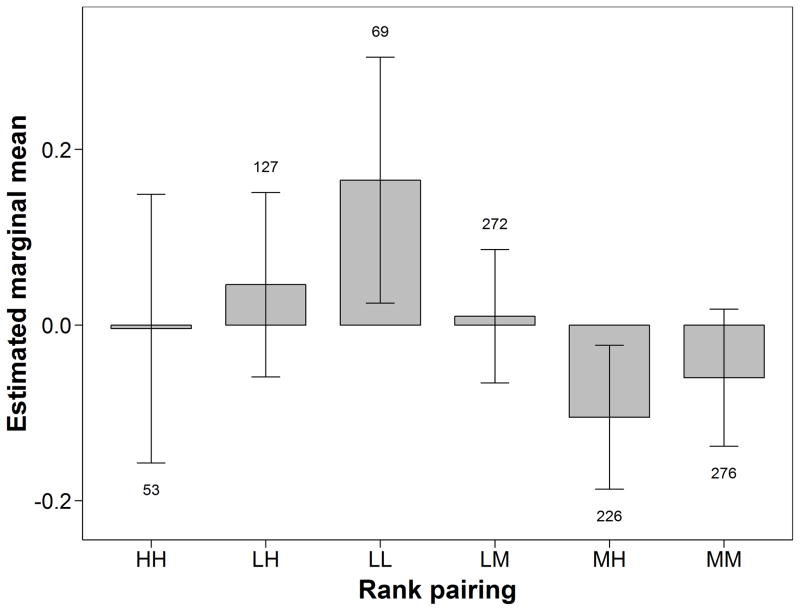
Rank of both dyad partners had significant effects on dyadic association (Table 2). Assuming equal overlap, dyads of two low-ranking females associated the most and above average for a given period (Fig. 7). Low-ranking females associated at higher rates with each other than dyads of two medium-ranking females (mean z-score difference: 0.23 ± 0.07, df = 1160.7, P = 0.036), suggesting that association preferences among low-ranking females were stronger than among medium-ranking females. High-ranking females tended to associate more with low-ranking than with medium-ranking females (mean z-score difference: 0.15 ± 0.06, df = 1337.0, P = 0.086). Overall, association rates did not deviate markedly from expected means based on spatial overlap of core areas except for pairs of low-ranking females (Fig. 7).
Figure 7.
Estimated marginal means (± 95% CI) of z-transformed half-weight index of dyadic association of female chimpanzees at the population mean level of core area overlap (z score = 0), for dyads of different rank class pairings (L: low; M: medium; H: high rank). Numbers above or below error bars indicate the number of unique dyads in each group, across all periods.
DISCUSSION
Here, our main objectives were to quantify variability in association patterns among adult female chimpanzees over a 38-year period, test for the existence of social preferences, and identify the drivers of such preferences. We controlled for the influence of shared space use on dyadic encounter rates, a nontrivial issue in the analysis and interpretation of spatiotemporal association patterns, by comparing observed association rates with those expected based on existing spatial constraints on female ranging patterns.
Our main results are that (1) despite relatively low mean levels of gregariousness compared with other populations (see Wakefield, 2013 for a cross-site comparison), female chimpanzees at Gombe form highly differentiated relationships with other females, which are not explained by variation in ranging overlap alone, (2) differentiated associations among spatially close females reflect grooming partner preferences, (3) related females associate and groom much more frequently, on average, than unrelated females, (4) cycling females associate more than expected with other cycling females, but only marginally so, which suggests only weak association preferences for each other, (5) mothers of immature female offspring associate with other females less than expected, while mothers of immature male offspring are attracted to each other and (6) low-ranking females preferentially associate with each other, but associations between females of other rank positions appear to be more a side-effect of spatial constraints on core area location than reflecting actual partner choices.
Social Affiliation
Contact affiliation (e.g. grooming) is a primary means by which most primates and many other social vertebrates form differentiated relationships with others (reviewed in Dunbar, 2010; Massen et al., 2010). We found that female chimpanzees whose core areas overlapped more than the average across all dyads groomed in direct relation to their rates of association, suggesting that affiliation was one of the motivating factors underlying spatial association among these pairs of females. In addition, the fact that only dyads with considerable spatial overlap showed such active affiliative tendencies may indicate either that social relationships were of particularly high quality among close neighbours only, or that social and spatial tolerance among close neighbours requires active maintenance.
In contrast to our findings, Lehmann and Boesch (2009) reported that grooming preferences did not explain variation in dyadic association and that dyadic association rates were not correlated with grooming rates, suggesting that association rates did not reflect true social affinities among female chimpanzees at Taï (as also suggested by Gilby & Wrangham, 2008 for females at Kanyawara). Female chimpanzees in the Taï Forest extensively overlap in their ranging patterns (Lehmann & Boesch, 2005) and associate with other females at relatively high rates (Wittiger & Boesch, 2013). As a result, there is relatively little potential for differentiation in association rates, compared to the spatially heterogeneous ranging pattern of females at Gombe, making it less likely that dyadic associations represent social affinities in the same way as grooming interactions.
As reported from other study sites, social affiliation was generally very infrequent. The vast majority of female dyads were observed to groom very little, if at all, in any given 2-year period, and only a few dyads showed high rates of observed affiliation (Gilby & Wrangham, 2008; Langergraber et al., 2009; Lehmann & Boesch, 2009; Wakefield, 2013). While we have yet to characterize the temporal stability of dyadic association partner preferences among females in our study population, findings from other sites suggest that at least some females maintain relatively stable association partners over time; about three-quarters of female chimpanzee dyads at Ngogo maintained association frequencies at consistent levels across a 4-year period (Langergraber et al., 2009), and association patterns were stable across years for female chimpanzees in the Taï Forest (Côte d’Ivoire) (Lehmann & Boesch, 2009). Further analyses are needed to investigate the potential drivers and adaptive benefits of long-term dyadic association patterns among unrelated female chimpanzees at Gombe and other study sites.
The Role of Kinship
Our data set with a total of 17 known adult kin dyads allowed us, for the first time, to test the influence of relatedness on female chimpanzee association, despite a social structure in which such associations are rarely possible. We predicted that the indirect fitness benefits of cooperating with close kin for competition and offspring rearing would cause kin dyads to have particularly high rates of association and affiliation. Our results are generally consistent with this prediction. Female kin tended to range near each other, but regardless of the extent of spatial overlap, kin dyads associated and groomed more frequently than nonkin dyads. Anecdotal evidence from other study sites supports the preference for kin among adult female association partners. For example, among female chimpanzees at Kanyawara, the only mother–daughter dyad associated as strongly as the strongest male dyad (Gilby & Wrangham, 2008), and at the nearby study site, Ngogo, a single mother–daughter dyad associated more frequently than 98.5% of all female dyads (Langergraber et al., 2009).
Despite this strong evidence for kin preference in our data, some kin dyads did not associate frequently, and we found that the range of association and grooming frequencies was similar for kin and nonkin dyads. Indeed, the highest grooming rates were recorded for three dyads classified as nonkin, involving females with unknown mothers, many years (6 or more) after each had immigrated into the Kasekela community. While it is possible that these dyads were in fact misclassified as nonkin, and actually represented unknown sister relationships, grooming rates among all other kin dyads were highest for mother–daughter dyads, which groomed on average three times more frequently than sister dyads. Thus, unless they represented exceptional social bonds among sisters, it is more likely that frequent affiliation in these dyads reflected strong social bonds formed for reasons other than kinship.
Questions remain about the benefits that female chimpanzees derive from staying in their natal communities, and how exactly such benefits may be obtained. Are female kin more tolerant around feeding sites, and thereby enhance each other’s feeding efficiency and energetic state? Do kin support each other in feeding competition against unrelated females? Research by Emery Thompson and colleagues at Kanyawara has shown how habitat quality is an important (and expected) driver of female reproduction. This effect is likely mediated by energy availability (Emery Thompson, 2013; Emery Thompson et al., 2007), a factor well known to influence reproductive function in primates, including humans (Ellison, 2003). At Gombe, there is evidence that higher-ranking females range in habitats with greater food availability (Murray et al., 2006), and that high rank is related to greater fertility (Jones et al., 2010; Pusey et al., 1997), likely through its effect on energetic status, reflected in greater and more constant body mass among high-ranking females (Pusey et al., 2005).
Given the ecological and social context at Gombe, any form of cooperation among females to maintain high-quality core areas is likely to come with significant benefits. These benefits could theoretically accrue regardless of whether or not females are related. However, the strong mother–offspring bond and predictability of behavioural interactions among familiar individuals (both mother–offspring and sister dyads) would facilitate continued cooperation, while stable cooperation among unrelated, unfamiliar females would be more difficult to achieve. On a proximate level, therefore, kin are more likely to engage in cooperative behaviour than nonkin, regardless of the theoretical (additional) benefits of kin selection. Still, if there are tangible fitness benefits to kin support in competition, then why don’t more females remain in their natal communities to enhance their fitness, especially in light of the severe resistance to immigration into another community by resident females and the risk of injury or even death from female aggression (Kahlenberg et al., 2008a; Pusey et al., 2008)? The answer may lie in the need to balance the benefits of kin selection against the costs of inbreeding with philopatric males (Pusey, 1980). Evidence shows that even those females who remain in their natal community can usually avoid inbreeding (Constable et al., 2001), but the success of this strategy would likely decrease with an increasing number of philopatric females. Further analyses of the determinants of female philopatry at Gombe are currently underway and will help in obtaining a more complete picture of the adaptive benefit of female dispersal strategies.
Cycling State
We expected that females would be more likely to associate with unrelated females if they both were swollen at the time of association, because swollen females generally travel farther (Wrangham & Smuts, 1980) and are more gregarious (Goodall, 1986; Pepper et al., 1999). While we confirmed this prediction, the effect size was extremely small and possibly not of biological significance. Through the association in larger mixed-sex groups, swollen females may change their ranging patterns to become more similar, which would lead to higher rates of association without reflecting active social preferences. The benefits of this behavioural change may lie not only in acquiring mates, but in obtaining protection from males against female aggression, sperm competition, or paternity confusion.
Offspring Presence
Both offspring sex and age influenced female association patterns. Dyads associated at the highest rates if neither female had immature offspring, or if either one or both females had an infant or juvenile male offspring but no immature female offspring at the same time (Fig. 4). Regardless of the simultaneous presence of male offspring, females associated below expectation when either one or both partners had a juvenile female offspring (Figs 4–6).
Homophily (i.e. the tendency to bond with similar others) in association patterns based on reproductive state is known from other social animals (Grevy’s zebra: Sundaresan et al., 2007; bottlenose dolphins: Möller & Harcourt, 2008; ring-tailed coatis, Nasua nasua: Hirsch et al., 2012; beef cows: Finger et al., 2014). Among female chimpanzees at Gombe, homophilic tendencies (i.e. individuals who are alike associate more with each other than with those who are different) appear limited to a subset of females with immature offspring, namely those with male offspring. Such preferred association may be adaptive, because interaction with peers should be particularly relevant for male offspring (the philopatric sex), where it can lay the foundation for long-lasting social bonds (Mitani, 2009). Conversely, in female-bonded primate societies, the opposite sex differences in offspring socialization would be considered adaptive, and are generally supported by empirical data on infant socialization (Fairbanks, 1993; Förster & Cords, 2005). The hypothesis that socialization of male offspring is an adaptive strategy is supported by recent evidence, which shows mothers are more gregarious when they have male offspring than when they have female offspring (Murray et al., 2014). In addition, a second recent analysis from a different data set found that young males interacted with more social partners than young females (Lonsdorf et al., 2014), suggesting that the sons themselves may be a driving factor behind dyadic associations of their mothers. Lastly, there is anecdotal evidence that associations among mothers of male offspring dissolve as the offspring matures or dies. In the mid-1970s, Gombe females Fifi and Winkle often travelled together, probably because of a ‘play-bond’ between their male infants, Freud and Wilkie (Goodall, 1986). The bond between Fifi and Winkle dissolved in 1979 as the young males became older and more aggressive with one another. Other offspring benefits may include preferential treatment by a mother’s bond partner, and in extreme cases the adoption of a juvenile by the bond partner, if its mother dies (Goodall, 1986).
Our finding that mothers of juvenile female offspring associated significantly below expected values based on their core area overlap awaits further explanation. While previous findings suggested that females with juvenile offspring associated preferentially (Williams et al., 2002a), analyses did not consider offspring sex and it is possible that the reported effect was driven by the presence of male offspring, which we report to have the greatest positive effect on female dyadic association during the juvenile period. Given that about half of all females at Gombe disperse (Pusey et al., 1997), and that coalitions among adult females are rare (Kahlenberg et al., 2008b), there may be few benefits for female offspring to socialize with peers. In addition, aggressive competition among females (Miller et al., 2014) may constrain association tendencies in the absence of factors that promote association, such as presence of male offspring or associating with males for the purpose of mating. Females may also reduce association with other females to avoid risk of injury to their offspring resulting from attacks, which in some cases can be fatal (Pusey et al., 2008; Townsend et al., 2007). If so, one might expect that mothers of infant female offspring would show the lowest rates of association, as infants are the most vulnerable age class. In contrast, we found that association was lowest, and well below expected mean levels, among mothers of juvenile female offspring. Perhaps daughters benefit nutritionally by avoiding feeding competition, allowing them to grow more quickly and experience an earlier age of first reproduction, which is an important component of fitness (Altmann & Alberts, 2005; Charnov & Berrigan, 1993). Indeed, a recent study found that juvenile female chimpanzees in our study population spend significantly more time feeding than their male counterparts (Wellens et al., n.d.).
Dominance Rank
We found that low-ranking females associated at higher rates with other low-ranking females, regardless of spatial overlap, and association frequencies among low-ranking females exceeded those of any other rank pairing (Fig. 7). These results resemble previous findings from a subset of periods, which showed that within a given neighbourhood, low-ranking females preferentially associated with each other, more than with medium- or high-ranking females (Murray et al., 2006; Williams et al., 2002a). However, both previous studies were unable to control for small-scale variation in spatial location of female core areas within a larger neighbourhood, which left it uncertain whether these rank differences in association were largely passive in nature or brought about by low-ranking females seeking each other out as preferred association partners. Passive association appeared as a plausible cause for greater association among low-ranking females, because low-ranking females have larger core areas and show lower site fidelity (Murray et al., 2007), and may therefore show greater levels of overlap with other females that result in higher levels of passive associations. Our analyses provide the first conclusive evidence that associations among low-ranking females are a result of active partner choices, while association among other rank pairings appear to be largely a result of passive association determined by the extent of overlap of their core areas. Further work is necessary to determine what benefits low-ranking females may obtain by preferential association.
In contrast to Williams et al. (2002a) and Murray et al. (2006) we found no evidence that high-ranking females associated preferentially with females of their own rank class, nor did we find evidence that medium-ranking females associated preferentially with high-ranking females. Instead, it appeared as if high-ranking females were more likely to associate above expectations with low-ranking females than with medium-ranking females. There are a number of possible reasons for these differences between studies on the same community of chimpanzees, in particular (1) our analyses included many more years of data, (2) we controlled for temporal changes in absolute levels of association by standardizing association scores by period, (3) we controlled for the influence of core area overlap on dyad-level association rather than neighbourhood, and (4) we used different statistical techniques that may be considered more powerful in adjusting for random individual variation and autocorrelation of data over time.
One remaining caveat to the interpretation of our data on association patterns remains the difficult distinction between factors that determine the choice of core area locations, and hence female ranging patterns, and those that determine social preferences independent of location preferences. While we interpret associations above or below what is expected based on random, independent movements within two overlapping core areas as driven by nonspatial factors, it may be that the choice of core area locations is in itself driven by social preferences, and that even seemingly passive associations are inherently influenced by social factors. Arguing against this possibility is the current evidence for competitive exclusion based on female rank, which limits settlement options for lower-ranking females. Nevertheless, opportunities remain for social influences on the location of female core areas, particularly for medium- and low-ranking females whose core areas are less stable over time and cover a larger area (Murray et al., 2007). Thus, our assessment of social determinants of association patterns remains conservative, and further analyses are underway to identify the social factors that influence spatial preferences.
Highlights.
We assess social partner preferences among adult female chimpanzees.
We control for expected dyadic association based on shared space use.
Among spatially close partners, association rates indicated affiliative preferences.
When available, females preferred kin over nonkin for association and grooming.
Nonkin partner preferences varied by age and sex of offspring and maternal rank.
Acknowledgments
We thank Tanzania National Parks, the Tanzania Wildlife Research Institute, and the Tanzanian Commission for Science and Technology for granting us permission to work on this project in Gombe National Park. We also thank the Jane Goodall Institute for funding data collection at Gombe, the Gombe Stream Research Center staff for data collection, and Dr Jane Goodall for granting us permission to work with the long-term data set. Additional funding was provided by National Science Foundation grants DBS-9021946, SBR-9319909, BCS-0452315, IOS-LTREB-1052693 and DGE-1106401, and by National Institutes of Health grants R01-AI058715 and R00-HD057992. We thank Allison Rogers for help with data extraction, Joseph Feldblum for his assistance with rank calculations and Roger Mundry for helpful discussions on multivariate modeling.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altmann J, Alberts SC. Growth rates in a wild primate population: ecological influences and maternal effects. Behavioral Ecology and Sociobiology. 2005;57:490–501. [Google Scholar]
- Amsler SJ. Energetic costs of territorial boundary patrols by wild chimpanzees. American Journal of Primatology. 2010;72:93–103. doi: 10.1002/ajp.20757. [DOI] [PubMed] [Google Scholar]
- Archie EA, Moss CJ, Alberts SC. The ties that bind: genetic relatedness predicts the fission and fusion of social groups in wild African elephants. Proceedings of the Royal Society B: Biological Sciences. 2006;273:513–522. doi: 10.1098/rspb.2005.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R, Lange S. Adjusting for multiple testing: when and how? Journal of Clinical Epidemiology. 2001;54:343–349. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- Bercovitch FB, Berry PSM. Herd composition, kinship and fission–fusion social dynamics among wild giraffe. African Journal of Ecology. 2013;51:206–216. [Google Scholar]
- Best EC, Dwyer RG, Seddon JM, Goldizen AW. Associations are more strongly correlated with space use than kinship in female eastern grey kangaroos. Animal Behaviour. 2014;89:1–10. [Google Scholar]
- Börger L, Franconi N, De Michele G, Gantz A, Meschi F, Manica A, et al. Effects of sampling regime on the mean and variance of home range size estimates. Journal of Animal Ecology. 2006;75:1393–1405. doi: 10.1111/j.1365-2656.2006.01164.x. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodel inference: A practical information-theoretic approach. 2. New York, NY: Springer; 2002. [Google Scholar]
- Bygott D. Agonistic behaviour and dominance in wild chimpanzees. In: Hamburg DA, McCown ER, editors. The great apes. Menlo Park, CA: Benjamin/Cummings; 1979. pp. 405–427. [Google Scholar]
- Cairns SJ, Schwager SJ. A comparison of association indexes. Animal Behaviour. 1987;35:1454–1469. [Google Scholar]
- Calenge C. The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecological Modelling. 2006;197:516–519. [Google Scholar]
- Cameron EZ, Setsaas TH, Linklater WL. Social bonds between unrelated females increase reproductive success in feral horses. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13850–13853. doi: 10.1073/pnas.0900639106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AJ, Macdonald SL, Thomson VA, Goldizen AW. Structured association patterns and their energetic benefits in female eastern grey kangaroos, Macropus giganteus. Animal Behaviour. 2009;77:839–846. [Google Scholar]
- Charnov EL, Berrigan D. Why do female primates have such long lifespans and so few babies? Or, life in the slow lane. Evolutionary Anthropology: Issues, News, and Reviews. 1993;1:191–194. [Google Scholar]
- Chaverri G, Gamba-Rios M, Kunz TH. Range overlap and association patterns in the tent-making bat Artibeus watsoni. Animal Behaviour. 2007;73:157–164. [Google Scholar]
- Connor RC, Heithaus MR, Barre LM. Complex social structure, alliance stability and mating access in a bottlenose dolphin ‘super-alliance’. Proceedings of the Royal Society B: Biological Sciences. 2001;268:263–267. doi: 10.1098/rspb.2000.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constable JL, Ashley MV, Goodall J, Pusey AE. Noninvasive paternity assignment in Gombe chimpanzees. Molecular Ecology. 2001;10:1279–1300. doi: 10.1046/j.1365-294x.2001.01262.x. [DOI] [PubMed] [Google Scholar]
- Cords M. Friendship among adult female blue monkeys (Cercopithecus mitis) Behaviour. 2002;139:291–314. [Google Scholar]
- de Villiers MS, Richardson PRK, van Jaarsveld AS. Patterns of coalition formation and spatial association in a social carnivore, the African wild dog (Lycaon pictus) Journal of Zoology. 2003;260:377–389. [Google Scholar]
- De Villiers PA, Kok OB. Home range, association and related aspects of elephants in the eastern Transvaal Lowveld. African Journal of Ecology. 1997;35:224–236. [Google Scholar]
- de Vries H, Stevens JMG, Vervaecke H. Measuring and testing the steepness of dominance hierarchies. Animal Behaviour. 2006;71:585–592. [Google Scholar]
- Dobson FS. Competition for mates and predominant juvenile male dispersal in mammals. Animal Behaviour. 1982;30:1183–1192. [Google Scholar]
- Dunbar RIM. The social role of touch in humans and primates: behavioural function and neurobiological mechanisms. Neuroscience and Biobehavioral Reviews. 2010;34:260–268. doi: 10.1016/j.neubiorev.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Ellison PT. Energetics and reproductive effort. American Journal of Human Biology. 2003;15:342–351. doi: 10.1002/ajhb.10152. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M. Reproductive ecology of female chimpanzees. American Journal of Primatology. 2013;75:222–237. doi: 10.1002/ajp.22084. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Kahlenberg SM, Gilby IC, Wrangham RW. Core area quality is associated with variance in reproductive success among female chimpanzees at Kibale National Park. Animal Behaviour. 2007;73:501–512. [Google Scholar]
- Eriksson J, Siedel H, Lukas D, Kayser M, Erler A, Hashimoto C, et al. Y-chromosome analysis confirms highly sex-biased dispersal and suggests a low male effective population size in bonobos (Pan paniscus) Molecular Ecology. 2006;15:939–949. doi: 10.1111/j.1365-294X.2006.02845.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA. Juvenile vervet monkeys: establishing relationships and practicing skills for the future. In: Pereira ME, Fairbanks LA, editors. Juvenile Primates. New York, NY: Oxford University Press; 1993. pp. 211–227. [Google Scholar]
- Fieberg J, Kochanny CO. Quantifying home-range overlap: the importance of the utilization distribution. Journal of Wildlife Management. 2005;69:1346–1359. [Google Scholar]
- Finger A, Patison KP, Heath BM, Swain DL. Changes in the group associations of free-ranging beef cows at calving. Animal Production Science. 2014;54:270–276. [Google Scholar]
- Förster S, Cords M. Socialization of infant blue monkeys (Cercopithecus mitis stuhlmanni): Allomaternal interactions and sex differences. Behaviour. 2005;142:869–896. [Google Scholar]
- Frère CH, Krützen M, Mann J, Watson-Capps JJ, Tsai YJ, Patterson EM, et al. Home range overlap, matrilineal and biparental kinship drive female associations in bottlenose dolphins. Animal Behaviour. 2010;80:481–486. [Google Scholar]
- Furuichi T. Social interactions and the life history of female Pan paniscus in Wamba, Zaire. International Journal of Primatology. 1989;10:173–197. [Google Scholar]
- Gerloff U, Hartung B, Fruth B, Hohmann G, Tautz D. Intracommunity relationships, dispersal pattern and paternity success in a wild living community of bonobos (Pan paniscus) determined from DNA analysis of faecal samples. Proceedings of the Royal Society B: Biological Sciences. 1999;266:1189–1195. doi: 10.1098/rspb.1999.0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gero S, Engelhaupt D, Whitehead H. Heterogeneous social associations within a sperm whale, Physeter macrocephalus, unit reflect pairwise relatedness. Behavioral Ecology and Sociobiology. 2008;63:143–151. [Google Scholar]
- Gilby IC. PhD thesis. St Paul, MN: University of Minnesota; 2004. Hunting and meat sharing among the chimpanzees of Gombe National Park, Tanzania. [Google Scholar]
- Gilby IC, Brent LJN, Wroblewski EE, Rudicell RS, Hahn BH, Goodall J, et al. Fitness benefits of coalitionary aggression in male chimpanzees. Behavioral Ecology and Sociobiology. 2013a;67:373–381. doi: 10.1007/s00265-012-1457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilby IC, Wilson ML, Pusey AE. Ecology rather than psychology explains co-occurrence of predation and border patrols in male chimpanzees. Animal Behaviour. 2013b;86:61–74. doi: 10.1016/j.anbehav.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilby IC, Wrangham RW. Association patterns among wild chimpanzees (Pan troglodytes schweinfurthii) reflect sex differences in cooperation. Behavioral Ecology and Sociobiology. 2008;62:1831–1842. [Google Scholar]
- Goodall J. The chimpanzees of Gombe: Patterns of behavior. Cambridge, MA: Belknap Press of Harvard University Press; 1986. [Google Scholar]
- Grove M. Hunter-gatherer movement patterns: causes and constraints. Journal of Anthropological Archaeology. 2009;28:222–233. [Google Scholar]
- Hamilton WD. The genetical evolution of social behaviour. Journal of Theoretical Biology. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Hirsch BT, Stanton MA, Maldonado JE. Kinship shapes affiliative social networks but not aggression in ring-tailed coatis. PLoS One. 2012;7:e37301. doi: 10.1371/journal.pone.0037301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holekamp KE, Cooper SM, Katona CI, Berry NA, Frank LG, Smale L. Patterns of association among female spotted hyenas (Crocuta crocuta) Journal of Mammalogy. 1997;78:55–64. [Google Scholar]
- Jones JH, Wilson ML, Murray C, Pusey A. Phenotypic quality influences fertility in Gombe chimpanzees. Journal of Animal Ecology. 2010;79:1262–1269. doi: 10.1111/j.1365-2656.2010.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlenberg SM, Emery Thompson M, Muller MN, Wrangham RW. Immigration costs for female chimpanzees and male protection as an immigrant counterstrategy to intrasexual aggression. Animal Behaviour. 2008a;76:1497–1509. [Google Scholar]
- Kahlenberg SM, Emery Thompson M, Wrangham RW. Female competition over core areas in Pan troglodytes schweinfurthii, Kibale National Park, Uganda. International Journal of Primatology. 2008b;29:931–947. [Google Scholar]
- Kernohan BJ, Gitzen RA, Millspaugh JJ. Analysis of animal space use and movements. In: Millspaugh JJ, Marzluff JM, editors. Radio Tracking and Animal Populations. San Diego, CA: Academic Press; 2001. pp. 125–166. [Google Scholar]
- Kie JG, Matthiopoulos J, Fieberg J, Powell RA, Cagnacci F, Mitchell MS, et al. The home-range concept: are traditional estimators still relevant with modern telemetry technology? Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365:2221–2231. doi: 10.1098/rstb.2010.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsukake N. The context and quality of social relationships affect vigilance behaviour in wild chimpanzees. Ethology. 2006;112:581–591. [Google Scholar]
- Langergraber K. Cooperation among kin. In: Mitani J, Call J, Kappeler P, Palombit R, Silk J, editors. The evolution of primate societies. Chicago, IL: University of Chicago Press; 2012. pp. 491–513. [Google Scholar]
- Langergraber K, Mitani J, Vigilant L. Kinship and social bonds in female chimpanzees (Pan troglodytes) American Journal of Primatology. 2009;71:840–851. doi: 10.1002/ajp.20711. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Boesch C. Social influences on ranging patterns among chimpanzees (Pan troglodytes verus) in the Taï National Park, Côte d’Ivoire. Behavioral Ecology. 2003;14:642–649. [Google Scholar]
- Lehmann J, Boesch C. Bisexually bonded ranging in chimpanzees (Pan troglodytes verus) Behavioral Ecology and Sociobiology. 2005;57:525–535. [Google Scholar]
- Lehmann J, Boesch C. Sexual differences in chimpanzee sociality. International Journal of Primatology. 2008;29:65–81. doi: 10.1007/s10764-007-9230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Boesch C. Sociality of the dispersing sex: the nature of social bonds in West African female chimpanzees, Pan troglodytes. Animal Behaviour. 2009;77:377–387. [Google Scholar]
- Lonsdorf EV, Anderson KE, Stanton MA, Shender M, Heintz MR, Goodall J, et al. Boys will be boys: sex differences in wild infant chimpanzee social interactions. Animal Behaviour. 2014;88:79–83. doi: 10.1016/j.anbehav.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabry KE, Shelley EL, Davis KE, Blumstein DT, Van Vuren DH. Social mating system and sex-biased dispersal in mammals and birds: a phylogenetic analysis. PLoS One. 2013;8:e57980. doi: 10.1371/journal.pone.0057980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machanda ZP, Gilby IC, Wrangham RW. Male–female association patterns among free-ranging chimpanzees (Pan troglodytes schweinfurthii) International Journal of Primatology. 2013;34:917–938. [Google Scholar]
- Madsen EA, Tunney RJ, Fieldman G, Plotkin HC, Dunbar RIM, Richardson JM, et al. Kinship and altruism: a cross-cultural experimental study. British Journal of Psychology. 2007;98:339–359. doi: 10.1348/000712606X129213. [DOI] [PubMed] [Google Scholar]
- Mann J. Cetacean societies: Field studies of dolphins and whales. Chicago, IL: University of Chicago Press; 2000. [Google Scholar]
- Massen JJM, Sterck EHM, de Vos H. Close social associations in animals and humans: functions and mechanisms of friendship. Behaviour. 2010;147:1379–1412. [Google Scholar]
- Matsumoto-Oda A. Mahale chimpanzees: grouping patterns and cycling females. American Journal of Primatology. 1999;47:197–207. doi: 10.1002/(SICI)1098-2345(1999)47:3<197::AID-AJP2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Miller JA, Pusey AE, Gilby IC, Schroepfer-Walker K, Markham AC, Murray CM. Competing for space: female chimpanzees are more aggressive inside than outside their core areas. Animal Behaviour. 2014;87:147–152. doi: 10.1016/j.anbehav.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani JC. Male chimpanzees form enduring and equitable social bonds. Animal Behaviour. 2009;77:633–640. [Google Scholar]
- Mitani JC, Watts D, Lwanga J. Ecological and social correlates of chimpanzee party size and composition. In: Boesch C, Hohmann G, Marchant LF, editors. Behavioral diversity in chimpanzees and bonobos. Cambridge, U.K: Cambridge University Press; 2002. [Google Scholar]
- Möller LM, Harcourt RG. Shared reproductive state enhances female associations in dolphins. Research Letters in Ecology. 2008;2008:5. [Google Scholar]
- Murray CM, Eberly LE, Pusey AE. Foraging strategies as a function of season and rank among wild female chimpanzees (Pan troglodytes) Behavioral Ecology. 2006;17:1020–1028. [Google Scholar]
- Murray CM, Lonsdorf EV, Stanton MA, Wellens KR, Miller JA, Goodall J, et al. Early social exposure in wild chimpanzees: mothers with sons are more gregarious than mothers with daughters. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:18189–18194. doi: 10.1073/pnas.1409507111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CM, Mane SV, Pusey AE. Dominance rank influences female space use in wild chimpanzees, Pan troglodytes: towards an ideal despotic distribution. Animal Behaviour. 2007;74:1795–1804. [Google Scholar]
- Nishida T, Kawanaka K. Inter-unit group relationships among wild chimpanzees of the Mahali Mountains. Kyoto University African Studies. 1972;7:131–169. [Google Scholar]
- Otali E, Gilchrist JS. Why chimpanzee (Pan troglodytes schweinfurthii) mothers are less gregarious than nonmothers and males: the infant safety hypothesis. Behavioral Ecology and Sociobiology. 2006;59:561–570. [Google Scholar]
- Packer C, Pusey AE. Cooperation and competition within coalitions of male lions: kin selection or game theory? Nature. 1982;296:740–742. [Google Scholar]
- Pepper JW, Mitani JC, Watts DP. General gregariousness and specific social preferences among wild chimpanzees. International Journal of Primatology. 1999;20:613–632. [Google Scholar]
- Pilot M, Dahlheim ME, Hoelzel AR. Social cohesion among kin, gene flow without dispersal and the evolution of population genetic structure in the killer whale (Orcinus orca) Journal of Evolutionary Biology. 2010;23:20–31. doi: 10.1111/j.1420-9101.2009.01887.x. [DOI] [PubMed] [Google Scholar]
- Pusey A. [Demographic records of Gombe chimpanzees] n.d Unpublished raw data. [Google Scholar]
- Pusey A, Murray C, Wallauer W, Wilson M, Wroblewski E, Goodall J. Severe aggression among female Pan troglodytes schweinfurthii at Gombe National Park, Tanzania. International Journal of Primatology. 2008;29:949–973. [Google Scholar]
- Pusey A, Williams J, Goodall J. The influence of dominance rank on the reproductive success of female chimpanzees. Science. 1997;277:828–831. doi: 10.1126/science.277.5327.828. [DOI] [PubMed] [Google Scholar]
- Pusey AE. Intercommunity transfer of chimpanzees in Gombe National Park. In: Hamburg DA, McCown ER, editors. The great apes. Menlo Park, CA: Benjamin/Cummings; 1979. pp. 464–479. [Google Scholar]
- Pusey AE. Inbreeding avoidance in chimpanzees. Animal Behaviour. 1980;28:543–552. [Google Scholar]
- Pusey AE. Sex-biased dispersal and inbreeding avoidance in birds and mammals. Trends in Ecology & Evolution. 1987;2:295–299. doi: 10.1016/0169-5347(87)90081-4. [DOI] [PubMed] [Google Scholar]
- Pusey AE, Oehlert GW, Williams JM, Goodall J. Influence of ecological and social factors on body mass of wild chimpanzees. International Journal of Primatology. 2005;26:3–31. [Google Scholar]
- Pusey AE, Schroepfer-Walker K. Female competition in chimpanzees. Philosophical Transactions of the Royal Society B: Biological Sciences. 2013;368 doi: 10.1098/rstb.2013.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn GP, Keough MJ. Experimental designs and data analysis for biologists. Cambridge, U.K: Cambridge University Press; 2002. [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. Retrieved from http://www.R-project.org/ [Google Scholar]
- Saïd S, Gaillard JM, Duncan P, Guillon N, Guillon N, Servanty S, et al. Ecological correlates of home-range size in spring–summer for female roe deer (Capreolus capreolus) in a deciduous woodland. Journal of Zoology. 2005;267:301–308. [Google Scholar]
- Schuelke O, Bhagavatula J, Vigilant L, Ostner J. Social bonds enhance reproductive success in male macaques. Current Biology. 2010;20:2207–2210. doi: 10.1016/j.cub.2010.10.058. [DOI] [PubMed] [Google Scholar]
- Seaman DE, Powell RA. An evaluation of the accuracy of kernel density estimators for home range analysis. Ecology. 1996;77:2075–2085. [Google Scholar]
- Seidel KD. PhD thesis. Seattle, WA: University of Washington; 1992. Statistical properties and applications of a new measure of joint space use for wildlife. [Google Scholar]
- Silk JB, Altmann J, Alberts SC. Social relationships among adult female baboons (Papio cynocephalus) I. Variation in the strength of social bonds. Behavioral Ecology and Sociobiology. 2006;61:183–195. [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, et al. Strong and consistent social bonds enhance the longevity of female baboons. Current Biology. 2010;20:1359–1361. doi: 10.1016/j.cub.2010.05.067. [DOI] [PubMed] [Google Scholar]
- Silverman BW. Density estimation for statistics and data analysis. London, U.K: Chapman & Hall; 1986. [Google Scholar]
- Sundaresan SR, Fischhoff IR, Dushoff J, Rubenstein DI. Network metrics reveal differences in social organization between two fission–fusion species, Grevy’s zebra and onager. Oecologia. 2007;151:140–149. doi: 10.1007/s00442-006-0553-6. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RAR, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychological Review. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Townsend SW, Slocombe KE, Emery Thompson M, Zuberbühler K. Female-led infanticide in wild chimpanzees. Current Biology. 2007;17:R355–R356. doi: 10.1016/j.cub.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Wakefield ML. Grouping patterns and competition among female Pan troglodytes schweinfurthii at Ngogo, Kibale National Park, Uganda. International Journal of Primatology. 2008;29:907–929. [Google Scholar]
- Wakefield ML. Social dynamics among females and their influence on social structure in an East African chimpanzee community. Animal Behaviour. 2013;85:1303–1313. [Google Scholar]
- Watts D. Reciprocity and interchange in the social relationships of wild male chimpanzees. Behaviour. 2002;139:343–370. [Google Scholar]
- Wellens KR, Lonsdorf EV, Stanton MA, O’Malley RC, Murray CM. Sex differences in wild juvenile chimpanzee (Pan troglodytes schweinfurthii) foraging and socializing behaviors. n.d Manuscript in preparation. [Google Scholar]
- Wey TW, Blumstein DT. Social cohesion in yellow-bellied marmots is established through age and kin structuring. Animal Behaviour. 2010;79:1343–1352. [Google Scholar]
- Whitehead H. Precision and power in the analysis of social structure using associations. Animal Behaviour. 2008;75:1093–1099. [Google Scholar]
- Wikberg EC, Sicotte P, Campos FA, Ting N. Between-group variation in female dispersal, kin composition of groups, and proximity patterns in a black-and-white colobus monkey (Colobus vellerosus) PLos One. 2012;7 doi: 10.1371/journal.pone.0048740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Liu H-Y, Pusey AE. Costs and benefits of grouping for female chimpanzees at Gombe. In: Boesch C, Hohmann G, Marchant LF, editors. Behavioral diversity in chimpanzees and bonobos. Cambridge, U.K: Cambridge University Press; 2002a. pp. 192–203. [Google Scholar]
- Williams JM, Pusey AE, Carlis JV, Farm BP, Goodall J. Female competition and male territorial behaviour influence female chimpanzees’ ranging patterns. Animal Behaviour. 2002b;63:347–360. [Google Scholar]
- Wittiger L, Boesch C. Female gregariousness in Western chimpanzees (Pan troglodytes verus) is influenced by resource aggregation and the number of females in estrus. Behavioral Ecology and Sociobiology. 2013;67:1097–1111. [Google Scholar]
- Wrangham R. On the evolution of ape social systems. Social Science Information. 1979;18:336–368. [Google Scholar]
- Wrangham RW. Why are male chimpanzees more gregarious than mothers? A scramble competition hypothesis. In: Kappeler P, editor. Primate males. Causes and consequences in group composition. Cambridge, U.K: Cambridge University Press; 2000. pp. 248–258. [Google Scholar]
- Wrangham RW, Smuts BB. Sex differences in the behavioural ecology of chimpanzees in the Gombe National Park, Tanzania. Journal of Reproduction and Fertility. 1980;28(Suppl):13–31. [PubMed] [Google Scholar]
- Wroblewski EE, Murray CM, Keele BF, Schumacher-Stankey JC, Hahn BH, Pusey AE. Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Animal Behaviour. 2009;77:873–885. doi: 10.1016/j.anbehav.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]