Abstract
Background
Eye disease due to herpes simplex virus (HSV) commonly presents as epithelial keratitis which, though usually self‐limiting, may persist or progress without treatment.
Objectives
To compare the relative effectiveness of antiviral agents, interferon, and corneal debridement in the treatment of HSV epithelial keratitis.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2014, Issue 12), PubMed (January 1946 to 31 December 2014), EMBASE (January 1980 to 31 December 2014), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to 31 December 2014), System for Information on Grey Literature in Europe (OpenGrey) (January 1995 to 31 December 2014), BIOSIS (January 1926 to 5 May 2014), Scopus (January 1966 to 31 December 2014), Japan Science and Technology Institute (J‐Global) (January 1975 to 31 December 2014), China National Knowledge Infrastructure (CNKI) (January 1979 to 31 December 2014), British Library's Electronic Table of Contents (Zetoc) (January 1993 to 7 May 2014). We looked for trials listed on the the metaRegister of Controlled Trials (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov), the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en), Chinese Clinical Trial Registry, the U.S. Food and Drug Administration (FDA) (www.fda.gov/), National Institute for Health and Clinical Excellence (NICE) (www.evidence.nhs.uk) and the European Medicines Agency (EMA) (www.ema.europa.eu/ema/) as of 31 December 2014. There were no language or date restrictions in the search for trials. We also culled literature digests and conference proceedings as of 15 April 2014. There were no language or date restrictions in the search for trials.
Selection criteria
Randomised and quasi‐randomised trials of HSV dendritic or geographic epithelial keratitis were included that reported the proportion of eyes healed at one week, two weeks, or both after enrolment.
Data collection and analysis
We tabulated data on study characteristics, risk of bias, and outcomes and used direct comparisons to estimate a risk ratio (RR) and, when feasible, a hazard ratio (HR) with a 95% confidence interval (CI). Heterogeneity was assessed by an inconsistency index. A multiple treatment comparison meta‐analysis consolidated direct and indirect comparisons of relative healing at 14 days.
Main results
One hundred thirty‐seven studies involving 8333 eyes met the inclusion criteria. Placebo‐controlled studies were heterogeneous in comparison with idoxuridine (RR 1.74; 95% CI 1.03 to 2.91) and few in number for vidarabine (RR 1.81; 95% CI 1.09 to 3.01), interferon (RR 1.32; 95% CI 1.06 to 1.64), and debridement. Vidarabine (RR 1.13; 95% CI 1.02 to 1.25), trifluridine (RR 1.30; 95% CI 1.18 to 1.43), acyclovir (RR 1.23; 95% CI 1.14 to 1.34), and brivudine (RR 1.34; 95% CI 1.18 to 1.51) were more effective than idoxuridine. Trifluridine (RR 1.17; 95% CI 1.03 to 1.32) and acyclovir (RR 1.11; 95% CI 1.03 to 1.19) were more effective than vidarabine. No significant differences in healing emerged among trifluridine, acyclovir, brivudine, and foscarnet although few studies compared brivudine or foscarnet with other antivirals. Any potential advantage of ganciclovir compared to acyclovir was mitigated by study heterogeneity and possible publication bias. Only one study evaluated the joint use of two topical antivirals. In a limited number of studies, oral acyclovir (RR 0.92; 95% CI 0.79 to 1.07) or the combination of oral acyclovir with a topical antiviral (RR 1.36; 95% CI 0.68 to 2.74) appeared as effective as a single topical antiviral agent. Compared to topical antiviral monotherapy, the combination of an antiviral with either interferon or debridement had inconsistent effects on expediting healing and improving outcome.
Authors' conclusions
Placebo‐controlled studies of HSV epithelial keratitis are limited to superseded interventions. Trifluridine and acyclovir are more effective than idoxuridine or vidarabine and similar in therapeutic effectiveness. Brivudine and foscarnet do not substantially differ in effectiveness from trifluridine or acyclovir. Ganciclovir is at least as effective as acyclovir. The addition of interferon to a nucleoside antiviral agent and the combination of debridement with antiviral treatment need to be further assessed to substantiate any possible advantage in healing.
Plain language summary
Antiviral medicines, interferon, and corneal surface removal in the treatment of herpes simplex virus infection of the eye
Review question We compared different treatments of people's eyes infected with herpes simplex virus (HSV).
Background HSV infection of the eye causes pain and hazy vision. Antiviral eye medicines, interferon drops, and superficial wiping have been used to cure HSV infection of the corneal surface.
Study characteristics This update, current to December 2014, uses a network of 137 studies of 8333 eyes to compare antiviral medicines and to find out if interferon or debridement would help. Between one and 28 studies were available to compare seven ophthalmic antiviral drugs, an antiviral taken by mouth, interferon, office procedures to remove the eye's infected surface, and other medicines.
Key results The first antivirals, idoxuridine and vidarabine, seem better than no treatment in healing HSV dendritic keratitis within two weeks. Topically applied trifluridine, acyclovir, or brivudine are better and safer than idoxuridine, cure about 90% of treated eyes within two weeks, and have no significant differences in effectiveness. The evidence is conflicting whether ganciclovir is as good as or better than acyclovir. Determining the role of antiviral pills is limited by few studies and inconsistent findings. Interferon, a natural part of the immune system that can be given as an eye drop, is active against HSV infection of the cornea. The integrated use of interferon and an antiviral drug might be slightly better than an antiviral drug by itself. Another treatment is to rub off the infected surface of the eye, but using a wiping method followed by an antiviral drug is not consistently better than just an antiviral medication.
Quality of the evidence Comparisons of one ophthalmic antiviral drug to another have a moderate quality of evidence, except for the appraisal comparing ganciclovir and acyclovir where studies are inconsistent. The quality of the evidence is moderate to low when an ophthalmic antiviral drug was compared to combined antiviral and interferon treatments or to combined antiviral treatment and debridement. Evidence is scarce or poor for placebo‐controlled comparisons, comparisons of antiviral treatment to interferon or to debridement, and evaluations of antiviral pills. Proper randomisation could not be assured in nearly a quarter of the studies. Patients or examiners could have known which treatment was assigned in at least half of the studies.
Summary of findings
Summary of findings for the main comparison. Network analysis of antiviral agents and combination interventions.
| Network analysis of antiviral agents and combination interventions | |||||
|
Study population: trial participants with dendritic or geographic epithelial keratitis Outcomes: relative corneal epithelial healing at two weeks following trial enrolment | |||||
| Treatment | Comparison | Pooling method 1 | Risk ratio (95% CI)2 | No. trials (no. participants) of direct comparisons | No. indirect network intermediates studied |
| Idoxuridine | Inactive control | Combined | 1.74 (1.03‐2.91)8 | 2 (63) | 1 |
| Vidarabine | Inactive control | Combined | 1.81 (1.09‐3.01) | 1 (43) | 1 |
| Idoxuridine | Combined | 1.13 (1.02‐1.25) | 3 (243) | 3 | |
| Trifluridine | Idoxuridine | Combined | 1.30 (1.18‐1.43) | 5 (256) | 3 |
| Vidarabine | Combined | 1.17 (1.03‐1.32)8 | 3 (188) | 2 | |
| Acyclovir | Idoxuridine | Combined | 1.23 (1.14‐1.34)8 | 11 (606) | 3 |
| Vidarabine | Combined | 1.11 (1.03‐1.19) | 7 (342) | 2 | |
| Trifluridine | Combined | 0.96 (0.90‐1.04) | 4 (178) | 3 | |
| Brivudine | Idoxuridine | Combined | 1.34 (1.18‐1.51) | 2 (99) | 2 |
| Trifluridine | Combined | 1.01 (0.92‐1.12) | 3 (147) | 2 | |
| Acyclovir | Combined | 1.04 (0.95‐1.15) | 1 (40) | 2 | |
| Ganciclovir | Acyclovir | Combined | 1.34 (1.20‐1.51)8 | 28 (2062) | 1 |
| Foscarnet | Trifluridine | Combined | 1.09 (0.92‐1.29) | 1 (20) | 1 |
| Acyclovir | Combined | 1.15 (1.01‐1.32) | 1 (104) | 2 | |
| Ganciclovir | Combined | 0.92 (0.75‐1.13) | 1 (60) | 1 | |
| Interferon | Inactive control | Direct | 1.32 (1.06‐1.64) | 2 (110) | 0 |
| Antiviral3 | Direct | 1.22 (0.91‐1.62) | 4 (222) | 0 | |
| Interferon + antiviral4 | Antiviral4 | Direct | 1.06 (0.99‐1.13)8 | 12 (718) | 0 |
| Antiviral5 | Debridement | Direct | 0.98 (0.72‐1.32)8 | 7 (317) | 0 |
| Debridement + Antiviral6 | Debridement | Direct | 1.25 (0.78‐2.00)8 | 3 (99) | 0 |
| Debridement + Antiviral7 | Antiviral7 | Direct | 1.05 (0.94‐1.17)8 | 7 (334) | 0 |
1 Combined method uses direct and indirect risk ratios in a multiple treatment meta‐analysis; direct method is the risk ratio of the direct meta‐analysis. 2 Adjusted RRs of antiviral comparisons are taken from Table 2 for combined direct and indirect estimates in network meta‐analysis. Corresponding direct RRs for antiviral comparisons are tabulated in Table 4 and are supplemented with HRs in Table 5. Direct RRs of interferon comparisons are taken from Analysis 3.1; Analysis 3.5; and Analysis 3.7. Direct RRs of debridement comparisons are taken from Analysis 4.2; Analysis 4.4; and Analysis 4.6. Direct comparisons that are restricted to studies of randomized, double‐masked trials are tabulated in Table 3. 3 Idoxuridine or acyclovir 4 Trifluridine, acyclovir, brivudine, or ganciclovir 5 Idoxuridine, trifluridine, acyclovir, or brivudine 6 Trifluridine or interferon 7 Idoxuridine, trifluridine, brivudine, or ganciclovir
8 I2 > 50% of direct comparison suggests diversity among studies
1. Combined direct and indirect comparisons: relative healing of HSV epithelial keratitis at 14 days between topical antiviral agents.
| Treatment comparisons | Type of comparison (intermediate comparator) | No. trials1 | Risk ratio (95% CI) | Combined risk ratio (95% CI)2 |
| Idoxuridine versus placebo | Direct | 2 | 1.31 (0.45‐3.84)* | 1.74 (1.03‐2.91) |
| Indirect (vidarabine) | 3, 1 | 1.89 (1.04‐3.40) | ||
| Vidarabine versus placebo | Direct | 1 | 1.96 (1.10‐3.49) | 1.81 (1.09‐3.01) |
| Indirect (idoxuridine) | 3, 2 | 1.36 (0.46‐4.01)* | ||
|
Vidarabine versus idoxuridine |
Direct | 3 | 1.04 (0.92‐1.18) | 1.13 (1.02‐1.25) |
| Indirect (inactive control) | 1, 2 | 1.67 (0.55‐5.12) | ||
| Indirect (trifluridine) | 3, 5 | 1.23 (0.89‐1.70)* | ||
| Indirect (acyclovir) | 7, 11 | 1.12 (0.96‐1.30) | ||
|
Trifluridine versus idoxuridine |
Direct | 5 | 1.38 (1.19‐1.60) | 1.30 (1.18‐1.43) |
| Indirect (vidarabine) | 3, 3 | 1.17 (0.85‐1.59)* | ||
| Indirect (acyclovir) | 4, 11 | 1.23 (1.06‐1.44)* | ||
| Indirect (brivudine) | 3, 2 | 1.38 (1.02‐1.87) | ||
|
Acyclovir versus idoxuridine |
Direct | 11 | 1.22 (1.08‐1.38)* | 1.23 (1.14‐1.34) |
| Indirect (vidarabine) | 7, 3 | 1.13 (0.98‐1.32) | ||
| Indirect (trifluridine) | 4, 5 | 1.37 (1.15‐1.63) | ||
| Indirect (brivudine) | 1, 2 | 1.31 (0.97‐1.78) | ||
|
Brivudine versus idoxuridine |
Direct | 2 | 1.38 (1.05‐1.81) | 1.34 (1.18‐1.51) |
| Indirect (trifluridine) | 3, 5 | 1.38 (1.13‐1.68) | ||
| Indirect (acyclovir) | 1, 11 | 1.28 (1.07‐1.54)* | ||
|
Trifluridine versus vidarabine |
Direct | 3 | 1.12 (0.84‐1.49)* | 1.17 (1.03‐1.32) |
| Indirect (idoxuridine) | 5, 3 | 1.33 (1.09‐1.61) | ||
| Indirect (acyclovir) | 4, 7 | 1.10 (0.97‐1.25) | ||
|
Acyclovir versus vidarabine |
Direct | 7 | 1.09 (1.00‐1.18) | 1.11 (1.03‐1.19) |
| Indirect (idoxuridine) | 11, 3 | 1.17 (0.99‐1.40)* | ||
| Indirect (trifluridine) | 4, 3 | 1.11 (0.82‐1.50)* | ||
|
Acyclovir versus trifluridine |
Direct | 4 | 0.99 (0.90‐1.09) | 0.96 (0.90‐1.04) |
| Indirect (idoxuridine) | 11, 5 | 0.90 (0.76‐1.06)* | ||
| Indirect (vidarabine) | 7, 3 | 0.97 (0.72‐1.31)* | ||
| Indirect (brivudine) | 1, 3 | 0.95 (0.79‐1.15) | ||
| Brivudine versus trifluridine | Direct | 3 | 1.00 (0.88‐1.14) | 1.01 (0.92‐1.12) |
| Indirect (idoxuridine) | 2, 5 | 1.00 (0.73‐1.36) | ||
| Indirect (acyclovir) | 1, 4 | 1.04 (0.88‐1.22) | ||
|
Brivudine versus acyclovir |
Direct | 1 | 1.05 (0.92‐1.20) | 1.04 (0.95‐1.15) |
| Indirect (idoxuridine) | 2, 11 | 1.13 (0.84‐1.53) | ||
| Indirect (trifluridine) | 4, 3 | 1.01 (0.86‐1.19) | ||
| Ganciclovir versus acyclovir | Direct | 28 | 1.38 (1.22‐1.57)* | 1.34 (1.20‐1.51) |
| Indirect (foscarnet) | 1, 1 | 1.20 (0.92‐1.57) | ||
| Foscarnet versus trifluridine | Direct | 1 | 1.00 (0.75‐1.34) | 1.09 (0.92‐1.29) |
| Indirect (acyclovir) | 1, 4 | 1.14 (0.92‐1.41) | ||
| Foscarnet versus acyclovir | Direct | 1 | 1.15 (0.95‐1.40) | 1.15 (1.01‐1.32) |
| Indirect (trifluridine) | 1, 4 | 1.01 (0.74‐1.37) | ||
| Indirect (ganciclovir) | 1, 17 | 1.27 (0.99‐1.63)* | ||
| Foscarnet versus ganciclovir | Direct | 1 | 0.96 (0.80‐1.16) | 0.92 (0.75‐1.13) |
| Indirect (acyclovir) | 1, 12 | 0.86 (0.62‐1.20)* |
1 Data derive from a network of antiviral treatment trials (Figure 3). Comparisons are based on reported outcomes at 14 days in which two antivirals were directly compared in at least one clinical trial. Indirect comparisons are limited to a linked network of trials having a single shared intermediate intervention since multiple intermediates were not considered for indirect adjustment. The number of trials indicate the number of direct comparisons made in either head‐to‐head trials or the number of trials between the first antiviral and the intermediate antiviral followed by the number of trials between the intermediate antiviral and the second antiviral.
2 While I2 < 25% for all combined direct and indirect comparisons estimated by the DerSimonian‐Laird random‐effects model method, networks that had at least one heterogeneous head‐to‐head comparison are identified (*).
CI: confidence interval; NA: not applicable (only direct or indirect comparison available)
2. Sensitivity analysis including only higher‐quality studies: relative healing at 14 days using only randomised, double‐masked trials.
| Treatment comparisons | Randomised, double‐masked studies1 | Risk ratio (95% CI) all studies | I2 (with all studies) | Risk ratio (95% CI) ‐ only randomised, double‐masked studies | I2 (with only randomised, double‐masked studies) |
| Idoxuridine versus inactive control | Markham 1977 | 1.31 (0.45‐3.84) | 92% | 1.79 (0.98‐3.26) | NA |
| Vidarabine versus idoxuridine | Markham 1977; Pavan‐Langston 1976 | 1.04 (0.92‐1.18) | 0% | 1.03 (0.90‐1.17) | 0% |
| Trifluridine versus idoxuridine | Panda 1995; Sugar 1980; Wellings 1972 | 1.38 (1.19‐1.60) | 0% | 1.47 (1.23‐1.75) | 0% |
| Acyclovir versus idoxuridine | Colin 1981; Collum 1980; Coster 1980; Kitano 1985; Kumar 1987; McCulley 1982; Panda 1995 | 1.22 (1.08‐1.38) | 63% | 1.17 (1.02‐1.34) | 69% |
| Brivudine versus idoxuridine | Panda 1995 | 1.38 (1.05‐1.81) | 30% | 1.64 (1.15‐2.35) | NA |
| Trifluridine versus vidarabine | Coster 1976 | 1.12 (0.84‐1.49) | 74% | 1.07 (0.98‐1.17) | NA |
| Acyclovir versus vidarabine | Collum 1985; Denis 1983; Genée 1987; Pavan‐Langston 1981; Yeakley 1981; Young 1982 | 1.09 (1.00‐1.18) | 0% | 1.07 (0.98‐1.18) | 0% |
| Acyclovir versus trifluridine | Høvding 1989; La Lau 1982; Panda 1995 | 0.99 (0.90‐1.09) | 0% | 1.01 (0.91‐1.12) | 0% |
| Brivudine versus trifluridine | Panda 1995; Power 1991 | 1.00 (0.88‐1.14) | 0% | 0.98 (0.89‐1.08) | 0% |
| Brivudine versus acyclovir | Panda 1995 | 1.05 (0.92‐1.20) | NA | NA | NA |
| Ganciclovir versus acyclovir | Colin 2007a | 1.38 (1.22‐1.57) | 79% | 1.21 (0.95‐1.54) | NA |
| Foscarnet versus trifluridine | Behrens‐Baumann 1992 | 1.00 (0.75‐1.34) | NA | NA | NA |
| Acyclovir, vidarabine versus acyclovir | Colin 1987 | 1.00 (0.89‐1.12) | NA | NA | NA |
| Oral versus topical antiviral | Collum 1986 | 0.92 (0.79‐1.07) | NA | NA | NA |
| Oral + topical antivirals versus topical antiviral | HEDS Group 1997 | 1.36 (0.68‐2.74) | 90% | 1.01 (0.93‐1.09) | NA |
| Interferon + antiviral versus antiviral | Colin 1983; de Koning 1982; de Koning 1983; Meurs 1985; Sundmacher 1987; van Bijsterveld 1989 | 1.06 (0.99‐1.13) | 67% | 1.02 (0.98‐1.06) | 0% |
1 Thirteen of 46 randomised, double‐masked trials are not tabulated because two‐week outcome data were not provided (Burns 1963; Wang 2004) or because comparative interventions not listed in this table were studied (Cellini 1994; Colin 1984; Coster 1977a; Guerra 1979; Parlato 1985; Sundmacher 1976b; Sundmacher 1978a; Sundmacher 1984a; Sundmacher 1985; Uchida 1981; Uchida 1982).
NA: not applicable (single study available for evaluation)
Summary of findings 2. Relative healing outcomes with topical antiviral therapy.
| Relative healing outcomes with topical antiviral therapy | ||||||
|
Study population: trial participants with dendritic or geographic epithelial keratitis Outcomes: corneal epithelial healing at 7 and 14 days | ||||||
| Treatment comparisons | Illustrative comparative healing percentages* (95% CI) |
Relative risk** (95% Cl) |
No. of participants (no. studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed healing | Corresponding healing | |||||
| Treatment A | Treatment B | |||||
| Idoxuridine (B) versus inactive control (A) | 7 days | ⊕⊕⊝⊝ low1,2 |
Different substances used as inactive control; random‐effects model used | |||
| 25% | 52% (31%‐88%) | 2.09 (1.24‐3.51) | 392 (10) | |||
| 14 days | ||||||
| 50% | 65% (22%‐100%) | 1.31 (0.45‐3.84) | 63 (2) | |||
| Vidarabine (B) versus inactive control (A) | 7 days | ⊕⊕⊝⊝ low4 |
Direct analysis limited to one study | |||
| 25% | 54% (20%‐100%) | 2.17 (0.81‐5.87) | 23 (1) | |||
| 14 days | ||||||
| 50% | 98% (55%‐100%) | 1.96 (1.10‐3.49) | 23 (1) | |||
| Vidarabine (B) versus idoxuridine (A) | 7 days | ⊕⊕⊕⊝ moderate4 |
Combined direct and indirect comparisons indicate vidarabine more effective than idoxuridine; neither antiviral commercially marketed | |||
| 50% | 55% (43%‐71%) | 1.10 (0.85‐1.42) | 243 (3) | |||
| 14 days | ||||||
| 75% | 78% (69%‐89%) | 1.04 (0.92‐1.18) | 243 (3) | |||
| Trifluridine (B) versus idoxuridine (A) | 7 days | ⊕⊕⊕⊝ moderate1,2 |
Indirect comparison shows similar results | |||
| 50% | 100% (87%‐100%) | 2.52 (1.74‐3.63) | 223 (4) | |||
| 14 days | ||||||
| 75% | 100% (89%‐100%) | 1.38 (1.19‐1.60) | 256 (5) | |||
| Acyclovir (B) versus idoxuridine (A) | 7 days | ⊕⊕⊕⊝ moderate1,2 |
Indirect comparison shows similar results; random‐effects model used | |||
| 50% | 99% (68%‐100%) | 1.98 (1.35‐2.90) | 468 (9) | |||
| 14 days | ||||||
| 75% | 92% (81%‐100%) | 1.22 (1.08‐1.38) | 606 (11) | |||
| Brivudine (B) versus idoxuridine (A) | 7 days | ⊕⊕⊕⊝ moderate4 |
Few studies, with slow healing of idoxuridine‐treated eyes, but indirect analysis yields similar relative effect at 14 days | |||
| 50% | 100% (100%‐100%) | 7.94 (2.80‐22.53) | 99 (2) | |||
| 14 days | ||||||
| 75% | 100% (79%‐100%) | 1.38 (1.05‐1.81) | 99 (2) | |||
| Trifluridine (B) versus vidarabine (A) | 7 days | ⊕⊕⊕⊝ moderate1,2 |
Results partly influenced by one study restricted to geographic epithelial keratitis; random‐effects model used | |||
| 65% | 70% (61%‐80%) | 1.08 (0.94‐1.23) | 288 (4) | |||
| 14 days | ||||||
| 82% | 92% (69%‐100%) | 1.12 (0.84‐1.49) | 188 (3) | |||
| Acyclovir (B) versus vidarabine (A) | 7 days | ⊕⊕⊕⊝ moderate2 |
Indirect analysis also favours acyclovir but one study restricted to geographic epithelial keratitis does not | |||
| 58% | 71% (61%‐84%) | 1.23 (1.05‐1.44) | 314 (6) | |||
| 14 days | ||||||
| 84% | 92% (84%‐99%) | 1.09 (1.00‐1.18) | 342 (7) | |||
| Acyclovir (B) versus trifluridine (A) | 7 days | ⊕⊕⊕⊝ moderate |
Studies differ in trifluridine formulation, in solution and as ointment | |||
| 71% | 70% (58%‐85%) | 0.99 (0.82‐1.20) | 178 (4) | |||
| 14 days | ||||||
| 90% | 89% (81%‐98%) | 0.99 (0.90‐1.09) | 178 (4) | |||
| Brivudine (B) versus trifluridine (A) | 7 days | ⊕⊕⊕⊝ moderate1,2 |
Heterogeneity among studies at 7‐day outcome | |||
| 61% | 57% (43%‐74%) | 0.93 (0.71‐1.21) | 147 (3) | |||
| 14 days | ||||||
| 88% | 88% (77%‐100%) | 1.00 (0.88‐1.14) | 147 (3) | |||
| Brivudine (B) versus acyclovir (A) | 7 days | ⊕⊕⊕⊝ moderate4 |
Direct analysis limited to one study, but indirect analysis yields similar relative effect | |||
| 80% | 95% (74%‐100%) | 1.19 (0.93‐1.51) | 40 (1) | |||
| 14 days | ||||||
| 95% | 100% (87%‐100%) | 1.05 (0.92‐1.20) | 40 (1) | |||
| Ganciclovir (B) versus acyclovir (A) | 7 days | ⊕⊕⊝⊝ low1,2,5 |
Slow healing in some studies results in unusually low assumed healing rate with acyclovir; substantial heterogeneity among studies; random‐effects model used to estimate relative risk | |||
| 43% | 49% (41%‐58%) | 1.14 (0.96‐1.35) | 551 (7) | |||
| 14 days | ||||||
| 55% | 76% (67%‐86%) | 1.38 (1.22‐1.57) | 2062 (28) | |||
| Foscarnet (B) versus trifluridine (A) | 7 days | ⊕⊕⊕⊝ moderate4 |
Direct analysis limited to one study | |||
| ‐ | ‐ | ‐ | ‐ | |||
| 14 days | ||||||
| 90% | 90% (68%‐100%) | 1.00 (0.75‐1.34) | 20 (1) | |||
| Foscarnet (B) versus acyclovir (A) | 7 days | ⊕⊕⊕⊝ moderate4 |
Direct analysis limited to one study | |||
| ‐ | ‐ | ‐ | ‐ | |||
| 14 days | ||||||
| 75% | 86% (71%‐100%) | 1.15 (0.95‐1.40) | 104 (1) | |||
| Foscarnet (B) versus ganciclovir (A) | 7 days | ⊕⊕⊕⊝ moderate4 |
Direct analysis limited to one study | |||
| 83% | 80% (63%‐100%) | 0.96 (0.76‐1.22) | 60 (1) | |||
| 14 days | ||||||
| 90% | 86% (72%‐100%) | 0.96 (0.80‐1.16) | 60 (1) | |||
| *The assumed risk for inactive control is chosen to be 25% at 7 days and 50% at 14 days. The assumed risk for idoxuridine, based on observational studies and trials using idoxuridine as a control, is chosen to be 50% at 7 days and 75% at 14 days. For other comparisons, the basis for each assumed risk is the mean baseline risk estimated as the overall healing percentage, at 7 and 14 days respectively, among all included studies for participants who received treatment A. Each corresponding risk (and its 95% confidence interval, with an upper bound of 100%) is based on the assumed risk and the risk ratio directly comparing treatment B to treatment A. **The relative risk is the pooled risk ratio. Pooling was based on fixed‐effects models except for heterogenous comparisons in which a random‐effects model was used. CI: confidence interval. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Some studies had a potential risk of bias 2 Pooling was limited by inconsistent results for either the 7‐day or 14‐day outcome 3 Analysis based only on indirect comparison 4 Few studies 5 Possible publication bias (Figure 1)
Summary of findings 3. Relative healing rates with antiviral agents and combination interventions.
| Relative healing rates with antiviral agents and combination interventions | |||
|
Study population: trial participants with dendritic or geographic epithelial keratitis Outcomes: rate of corneal epithelial healing following trial enrolment | |||
| Treatment | Comparison | Hazard ratio (95% CI) | No. of participants (no. studies) |
| Idoxuridine | Inactive control | 1.62 (1.00‐2.65)1 | 95 (3) |
| Vidarabine | Inactive control | 2.47 (1.14‐5.33) | 43 (1) |
| Idoxuridine | 1.36 (0.81‐2.28) | 74 (2) | |
| Trifluridine | Idoxuridine | 2.29 (1.37‐3.83) | 78 (1) |
| Vidarabine | 1.31 (0.96‐1.79)1 | 188 (3) | |
| Acyclovir | Idoxuridine | 2.15 (1.70‐2.72)1 | 355 (8) |
| Vidarabine | 1.13 (0.86‐1.47) | 259 (5) | |
| Trifluridine | 0.92 (0.65‐1.32) | 140 (3) | |
| Brivudine | Trifluridine | 0.60 (0.35‐1.02) | 60 (1) |
| Interferon + antiviral2 | Antiviral2 | 2.84 (2.13‐3.79)1 | 229 (5) |
| Debridement + antiviral3 | Antiviral3 | 1.76 (1.32‐2.35)1 | 248 (6) |
1 I2 > 50% 2 Trifluridine or acyclovir 3 Idoxuridine, trifluridine, or acyclovir
Summary of findings 4. Relative healing outcomes with combined topical or topical and oral antiviral therapy.
| Relative healing outcomes with combined topical and/or oral antiviral therapy | ||||||
|
Study population: trial participants with dendritic or geographic epithelial keratitis Outcomes: corneal epithelial healing at 7 and 14 days | ||||||
| Treatment comparisons | Illustrative comparative healing percentages* (95% CI) | Relative risk** (95% CI) | No. of participants (no. studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed healing | Corresponding healing | |||||
| Treatment A | Treatment B | |||||
| Topical acyclovir/vidarabine (B) versus topical acyclovir (A) | 7 days | ⊕⊕⊕⊝ moderate |
Analysis limited to one study | |||
| 62% | 93% (63%‐100% | 1.50 (1.01‐2.24) | 32 (1) | |||
| 14 days | ||||||
| 100% | 100% (89%‐100%) | 1.00 (0.89‐1.12) | 32 (1) | |||
| Oral antiviral (B) versus topical antiviral (A) | 7 days | ⊕⊕⊝⊝ low1 |
Only oral and topical acyclovir studied in comparative treatment trials; substantial heterogeneity between 2 trials at 7‐day outcome | |||
| 52% | 79% (59%‐100%) | 1.51 (1.13‐2.02) | 116 (2) | |||
| 14 days | ||||||
| 97% | 89% (77%‐100%) | 0.92 (0.79‐1.07) | 56 (1) | |||
| Oral antiviral + topical antiviral (B) versus topical antiviral (A) | 7 days | ⊕⊕⊝⊝ low1 |
Oral acyclovir was studied with topical trifluridine and with topical idoxuridine; random‐effects model used | |||
| 63% | 71% (60%‐84%) | 1.13 (0.95‐1.33) | 287 (1) | |||
| 14 days | ||||||
| 84% | 100% (57%‐100%) | 1.36 (0.68‐2.74) | 327 (2) | |||
| *The basis for the assumed risk is the overall 7‐day or 14‐day healing percentage among included studies for participants who received treatment A of both treatment comparisons (oral versus topical and oral/topical versus topical). The corresponding risk (and its 95% confidence interval) is based on the assumed risk and the risk ratio comparing treatment B to treatment A (and its 95% CI), with upper limits bounded at 100%. **The relative risk is the pooled risk ratio. CI: confidence interval. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Inconsistent and insufficient studies
Summary of findings 5. Relative healing outcomes with topical interferon.
| Relative healing outcomes with topical interferon | ||||||
|
Study population: trial participants with dendritic or geographic epithelial keratitis Outcomes: corneal epithelial healing at 7 and 14 days | ||||||
| Treatment comparisons | Illustrative comparative healing percentages* (95% CI) | Relative risk** (95% CI) | No. of participants (no. studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed healing | Corresponding healing | |||||
| Treatment A | Treatment B | |||||
| Interferon (B) versus inactive control (A) | 7 days | ⊕⊝⊝⊝ very low1 |
One study used debridement | |||
| 25% | 37% (27%‐52%) | 1.48 (1.07‐2.06) | 178 (3) | |||
| 14 days | ||||||
| 50% | 66% (53%‐82%) | 1.32 (1.06‐1.64) | 110 (2) | |||
| Interferon (B) versus antiviral (A) | 7 days | ⊕⊕⊝⊝ low1,2 |
Different antiviral agents used as comparative treatment; random‐effects model used | |||
| 55% | 57% (45%‐70%) | 0.99 (0.81‐1.21) | 85 (3) | |||
| 14 days | ||||||
| 69% | 84% (63%‐100%) | 1.22 (0.91‐1.62) | 222 (4) | |||
| Interferon + antiviral (B) versus antiviral (A) | 7 days | ⊕⊕⊕⊝ moderate2 |
Heterogeneity among trials at 7 and 14 days; slow healing with antiviral monotherapy in some trials; smaller relative effect size in sensitivity analyses | |||
| 44% | 83% (72%‐96%) | 1.64 (1.35‐2.55) | 475 (9) | |||
| 14 days | ||||||
| 86% | 95% (90%‐100%) | 1.06 (0.99‐1.13) | 718 (12) | |||
| *The assumed risk for inactive control is chosen to be 25% at 7 days and 50% at 14 days. The basis for the assumed risk of antiviral therapy is the overall 7‐day or 14‐day healing percentage among included studies for participants who received treatment A. The corresponding risk (and its 95% confidence interval) is based on the assumed risk and the risk ratio comparing treatment B to treatment A (and its 95% CI), with upper limits bounded at 100%. **The relative risk is the pooled risk ratio. CI: confidence interval. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Inconsistent results among studies using different interferon dosages 2 Different antiviral agents and different types and routes of interferon administration among studies
Summary of findings 6. Relative healing outcomes with corneal debridement.
| Relative healing outcomes with corneal debridement | ||||||
|
Study population: trial participants with dendritic or geographic epithelial keratitis Outcomes: corneal epithelial healing at 7 and 14 days | ||||||
| Treatment comparisons | Illustrative comparative healing percentages* (95% CI) | Relative risk** (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed healing | Corresponding healing | |||||
| Treatment A | Treatment B | |||||
| Antiviral (B) versus debridement (A) | 7 days | ⊕⊕⊝⊝ low1,2 |
Debridement limited by recrudescent epithelial keratitis during healing stage; random‐effects model used | |||
| 61% | 57% (45%‐73%) | 0.94 (0.74‐1.20) | 372 (7) | |||
| 14 days | ||||||
| 72% | 71% (52%‐95%) | 0.98 (0.72‐1.32) | 317 (7) | |||
| Debridement + antiviral or interferon (B) versus debridement (A) | 7 days | ⊕⊕⊕⊝ moderate1 |
Debridement limited by recrudescent epithelial keratitis during healing stage; random‐effects model used | |||
| 71% | 80% (67%‐97%) | 1.13 (0.94‐1.36) | 347 (8) | |||
| 14 days | ||||||
| 81% | 100% (63%‐100%) | 1.25 (0.78‐2.00) | 99 (3) | |||
| Debridement + antiviral (B) versus antiviral (A) | 7 days | ⊕⊕⊕⊝ moderate1 |
Different antiviral agents and different interferon dosages; random‐effects model used | |||
| 53% | 68% (57%‐81%) | 1.28 (1.07‐1.53) | 305 (7) | |||
| 14 days | ||||||
| 75% | 79% (70%‐88%) | 1.05 (0.94‐1.17) | 334 (7) | |||
| *The basis for the assumed risk is the overall 7‐day or 14‐day healing percentage among included studies for participants who received treatment A. The corresponding risk (and its 95% confidence interval) is based on the assumed risk and the risk ratio comparing treatment B to treatment A (and its 95% CI), with upper limits bounded at 100%. **The relative risk is the pooled risk ratio. CI: confidence interval. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Inconsistent results among studies using different physicochemical methods of corneal epithelial debridement 2 Disparate relative effect found in sensitivity analysis
Background
Herpes simplex virus (HSV) affects most people (Smith 2002). Upon initial exposure, HSV type 1 often infects subclinically but sometimes produces a primary viral syndrome afflicting the eyelids and ocular surface. After establishing latency within neurons of the trigeminal ganglion, periodic shedding of HSV into the tear film is commonly asymptomatic. On occasion, usually years to decades after initial exposure, reactivation of latent HSV erupts into infection and inflammation of the eye. Epithelial keratitis is its most frequent and conspicuous manifestation.
Description of the condition
Epidemiology
Public health importance
HSV is an epidemiologically important cause of infectious and inflammatory eye disease among children, adolescents, adults, and the elderly (Liesegang 2001; Wilhelmus 2008a). The incidence of first‐episode ocular HSV in population studies from Europe, North America, and South America is 4 to 13 per 100,000 person‐years (Adhin 2012; Labetoulle 2005; Liesegang 1989b; Mortensen 1979; Ribaric 1976; Stanzel 2014; Young 2010). The incidence of subsequent HSV keratitis is 6 to 18 per 100,000 person‐years (Labetoulle 2005; Liesegang 1989b; Stanzel 2014). The joint incidence of new or recurrent HSV keratitis is 12.5 to 31.5 people per 100,000 person‐years (Labetoulle 2005; Liesegang 1989b; Stanzel 2014). Worldwide, an estimated 1.5 million people experience HSV keratitis each year (Farooq 2012).
The prevalence of a history of ocular HSV disease is about 2 to 15 per 10,000 in the U.S. population (Liesegang 1989a; Stanzel 2014). Approximately 3% have visual acuity of the previously diseased eye worse than 20/200 (Wilhelmus 1981b; Young 2010). Globally, between one and ten million persons have had herpetic eye disease, and many are left with severely impaired vision of the affected eye. Ocular herpes is an important infective cause of corneal blindness.
Epithelial keratitis
Epithelial keratitis is the most common form of HSV eye disease, accounting for 50% to 80% of ocular herpes (Labetoulle 2005; Liesegang 1989a; Uchio 1994; Young 2010). The incidence of new or recurrent HSV epithelial keratitis is estimated at 5 to 22 people per 100,000 person‐years (Labetoulle 2005; Liesegang 1989b; Mortensen 1979; Stanzel 2014). On a global scale, about one million people suffer a new or repeat episode of HSV epithelial keratitis each year.
Dendritic epithelial keratitis, a branching pattern of painful infection affecting the corneal surface, is the customary configuration (Tabery 2010). Geographic epithelial keratitis is a macroulcerative form of corneal HSV infection that complicates topical corticosteroid use or systemic immunosuppression.
HSV epithelial keratitis is typically unilateral. The contralateral eye is infrequently affected, either simultaneously or subsequently (Liesegang 2001), in healthy individuals. People with atopy or an immune deviation may be predisposed to HSV epithelial keratitis of both eyes (Souza 2003; Wilhelmus 1981c).
Etiology
Clinical virology
Branching corneal disease was first mentioned in a 10th‐century medical text (Wood 1936) and redescribed in the 19th century with febrile illness (Kipp 1880) or as a spontaneous episode (Hansen Grut 1886) to which the term "keratitis dendritica" was given (Emmert 1885). Onset of dendritic corneal inflammation was realised to be part of an acute syndrome (Horner 1871; Verhoeff 1909) and by 1920 was shown to be due to a transmissible virus (Grüter 1920).
HSV‐1 is the cause of nearly all infective dendritic epithelial keratitis. HSV‐2 is much less commonly isolated from the cornea than HSV‐1 (Kaneko 2008; Neumann‐Haefelin 1978; Vannini 1986). Varicella‐zoster virus epithelial keratitis can resemble HSV epithelial keratitis (Bierly 1994; Hu 2010; Pavan‐Langston 1973) and may occur with or without dermatoblepharitis during chickenpox or shingles. Rare causes of dendritic epithelial keratitis include cytomegalovirus (Wilhelmus 1996b), Epstein‐Barr virus (Pflugfelder 1990), human herpesvirus 6 (Boto‐de‐los‐Bueis 2013; Okuno 2011), and adenovirus (Chodosh 1995). Laboratory testing can help to establish the etiology of atypical cases (Chanzy 2002; Farhatullah 2004; Kowalski 1993).
Differential diagnosis
Dendritiform keratopathy is a condition of the corneal surface characterized by a curvilinear or arborescent pattern that may simulate HSV epithelial keratitis but is due to various noninfective causes. Ramous epithelial changes can be associated with various disorders of the ocular surface. For example, a pseudodendrite can be made up of regenerating or hypertrophic epithelium. Whorling epithelial granularity may be due to dysfunction of the limbal stem cells. Intraepithelial deposits and drug‐induced changes can also take on a vortex formation. Fungal and amœbic infections of the cornea have been mistaken as HSV keratitis.
Reactivation and recurrence
Primary ocular herpes may cause dendritic keratitis in susceptible children and adults (Darougar 1985). HSV epithelial keratitis can occur following primary transmission from mother to neonate (Liesegang 2001) and from donor to corneal transplant recipient (Borderie 2004; Hassan 2009; Remeijer 1997; Robert 2005). Reinfection with a different HSV strain is also possible (Remeijer 2002). Much more commonly, HSV epithelial keratitis follows viral reactivation in a latently infected person. After a recurrence, 5% to 10% of people develop a subsequent recurrence of epithelial keratitis each year (Stanzel 2014; Young 2010), regardless of gender or ethnicity (HEDS Group 2001). Events that induce viral reactivation and shedding or that enhance susceptibility of the eye to viral infection can precipitate an infective episode (Webre 2012). Identifying triggers is difficult since any suspected causal association that is made in hindsight is liable to be faulty. For example, a suspected link between psychological stress and herpetic keratitis remains indecisive (HEDS Group 2000a; Kip 2001). With the caveat of recall bias, the following are situations thought to instigate HSV epithelial keratitis:
Reactivation of latent virus
Altered homeostasis
Fever (Ashaye 2008; Yorston 1992)
Advancing age (Young 2010; Stanzel 2014)
Radiation exposure
Excess sunlight (Ludema 2014a; Stan 2000)
Photodynamic therapy of corneal neovascularisation (Dantas 1997; Yoon 2010)
Corneal cross‐linking with ultraviolet light (Kymionis 2007; Yuksel 2011)
Ocular irritants or injections
Contact lens wear (Hamroush 2014; Mucci 2009)
Corneal injury with a foreign body (Sundmacher 1986)
Periocular injection (Lingua 1985)
Intraocular injection (Khalili 2009)
Topical ophthalmic drugs
Prostaglandin analogues and other glaucoma drugs (Alm 2008; Villegas 2008)
Mitomycin‐C and other cytotoxic agents (Rao 2009; Siddique 2011)
Anterior segment surgery
Laser iridotomy (Hou 2004)
Cataract extraction (Du 2010; Gu 2014; Lu 2011; Patel 2009; Sun 2013b; Yang 2011)
Keratorefractive surgery (Santos 1983)
Laser‐assisted in situ keratomileusis (LASIK), photorefractive keratectomy (PRK), laser astigmatic keratotomy, and phototherapeutic keratectomy (PTK) (Di 2011; Gómez García 2004; Hamoudi 2013; Lu 2006; Nagy 2003; Nataneli 2013)
Lamellar keratoplasty (Jezegabel 1967)
Penetrating keratoplasty (Ambrósio 2001; Miyajima 2003; Rezende 2004)
Endothelial keratoplasty (Prasher 2009)
Susceptibility to reactivated virus
Ophthalmic corticosteroids
Topical corticosteroid (Patterson 1967a; Sundmacher 1978c; Williams 1977)
Subconjunctival or subtenon corticosteroid (Hashizume 2009; Inoue 2014)
Intravitreal corticosteroid (Gulkilik 2007; Shtein 2007)
Other immunosuppressive treatment
Topical ophthalmic calcineurin inhibitor (Field 1995; Joseph 2005)
Inhaled corticosteroid (Garcia‐Medina 2011; McDonald 2015)
Systemic immunosuppressants following organ or bone marrow transplantation (Hayashi 2008; Kremer 1991; Ng 1998)
Systemic immunosuppressants given for autoimmune disorders (Larrañaga Fragoso 2015)
Immune dysregulation
Atopy (Borkar 2014; Kaiserman 2006; Prabriputaloong 2006; Rezende 2006)
HIV infection (Hodge 1997)
Diabetes mellitus (Kaiserman 2005)
Malnutrition (Ukety 1991)
Natural history
Without treatment, dendritic epithelial keratitis tends to be self‐limited (Thygeson 1976). Some eyes heal in a few days, but natural healing often takes longer than two weeks (Liesegang 1989a). Inappropriate treatment can worsen corneal inflammation and contribute to visual loss. Any treatment that speeds healing would reduce the opportunity for unsuitable management (Kaufman 1972).
Description of the intervention
Antiviral drugs
Antiviral compounds emerged as potential treatments for herpetic keratitis during the last half of the 20th century (Bauer 1985; Graupner 1969). In 1962 idoxuridine (iododeoxyuridine, IDU), a pyrimidine analogue, was the first effective antiherpetic drug (Kaufman 1962). In the following decade a purine analogue, vidarabine (adenine arabinoside, ARA‐A), entered ophthalmic practice (Whitley 1980). Idoxuridine and vidarabine were then progressively superseded by other nucleoside analogues: trifluridine (trifluorothymidine, TFT) (Carmine 1982), acyclovir (acycloguanosine, ACV) (Richards 1983; Wagstaff 1994), brivudine (bromovinyldeoxyuridine, BVDU) (De Clerq 2005), and ganciclovir (dihydroxypropoxymethylguanine, DHPG) (Chou 2014; Croxtall 2011; Tabbara 2010). Cidofovir (hydroxyphosphonylmethoxypropylcytosine, HPMPC) is an acyclic nucleotide analogue. Foscarnet (phosphonoformic acid, PFA) is a non‐nucleoside antiviral compound.
Interferon
As synthetic antivirals were under development, a cytokine that could interfere with viral replication was discovered in 1957. Interferon, found to be active against HSV in 1960, was first evaluated for HSV epithelial keratitis in 1963 (Tommila 1963) and emerged as a novel management strategy for viral eye disease (Jones 1967). Comparative treatment trials of topical interferon that began in 1976 showed type I interferons-interferon‐α (leukocyte interferon) and interferon‐β (fibroblast interferon)-to be effective for treating HSV epithelial keratitis (Pollard 1982; Sundmacher 1982). Several formulations and dosages of exogenous interferon were tested: alone, with an antiviral nucleoside, and after debridement (Cantell 1995; Sundmacher 1983). Formerly available in limited amounts, purified interferon is now produced by recombinant DNA technology. Though not marketed for ophthalmic use, interferon eye drops can be prepared extemporaneously (Ruiz 2007).
Corneal surface debridement
Curettage and cauterisation of the ocular surface came into use in 1890 (Kipp 1890), followed by the application of cytotoxic reagents in 1900 (Friedenwald 1900). Corneal epithelial removal, usually by iodinisation or carbolisation, became a customary remedy for dendritic keratitis during the first half of the 20th century. Initial clinical trials of corneal epithelial removal involved the application of noxious solutions such as tincture of iodine (Austin 1974; Davidson 1964; Graupner 1969; Matthäus 1970; Struck 1989) or phenol (Fulhorst 1972; MacKenzie 1964; Patterson 1967a; Patterson 1967b), sometimes in combination with mechanical scraping. Subsequent methods of corneal debridement included cryoapplication (Fulhorst 1972; Struck 1989), photoinactivation (Bartholomew 1977; Daniel 1972; O'Day 1975), and thermocautery (Sundmacher 1976a; Sundmacher 1976b; Sundmacher 1978a; Sundmacher 1978b). Minimal wiping debridement (Whitcher 1976) offered a simpler, safer procedure and was used in several clinical studies (Altinisik 1987; Coster 1977a; Hung 1984; Jensen 1982; Kato 1979; Parlato 1985; Richter 1986; Serifoglu 1987; Uchida 1981; Wilhelmus 1981a; Yamazaki 1984a).
How the intervention might work
Treatment of HSV epithelial keratitis aims to halt active viral infection of the cornea quickly and safely, thereby controlling symptoms and allowing a normal ocular surface to become re‐established. Antiviral nucleosides include purine analogues such as vidarabine; pyrimidine analogues such as idoxuridine, trifluridine, and brivudine; and acyclic analogues such as acyclovir and ganciclovir. These prodrugs are phosphorylated by viral thymidine kinase and inhibit HSV replication by interfering with viral DNA synthesis during transcription of the viral genome. Foscarnet and cidofovir directly inhibit viral DNA polymerase. Mutations in viral genes encoding thymidine kinase or DNA polymerase can confer antiviral resistance.
Interferons are cytokines capable of activating an intracellular pathway that upregulates host genes affecting antiviral responses. Interferon and a nucleoside antiviral could have an additive or synergistic interaction.
Corneal epithelial debridement or chemical cauterisation removes or destroys virus‐infected cells of the corneal surface and is followed by corneal epithelial regeneration if residual or persistently shed virus does not cause recrudescent ocular infection. A combination of debridement with either a nucleoside antiviral drug or interferon has been suggested to avert early recrudescence. An occlusion patch applied immediately after debridement could affect the epithelial healing rate (Turner 2006) or even raise the corneal temperature by a few degrees to hinder HSV replication (Wheeler 1959).
Ancillary topical ophthalmic agents such as lubricants or growth factors might facilitate corneal epithelial regeneration.
Why it is important to do this review
An antiviral agent is a favoured treatment for HSV epithelial keratitis (Sundmacher 2009). However, controversies persist about the optimal antiviral agent, the role of interferon, and the effectiveness of debridement methods (Epstein 1989; Guess 2007; Kastner 1984). Surveys among ophthalmologists reveal some differences of opinion in treatment preferences for HSV epithelial keratitis (Guess 2010; Labetoulle 2005; McAllum 2003; Sundmacher 1977; Ziahosseini 2009). To provide an evidence‐based analysis, this systematic review evaluates the collective evidence on the treatment of HSV epithelial keratitis.
Objectives
To compare the relative effectiveness of antiviral agents, interferon, and corneal debridement in the treatment of HSV epithelial keratitis.
Methods
Criteria for considering studies for this review
Types of studies
This review included clinical trials that compared the relative effectiveness of therapeutic interventions on the corneal epithelial healing of participants who had clinically diagnosed or virologically confirmed HSV epithelial keratitis. Trials limited to the prevention of recurrent herpetic eye disease were not considered in this review.
Types of participants
Participants had active dendritic or geographic epithelial keratitis. Punctate, stellate, or linear epithelial keratitis was considered to be a form of dendritic epithelial keratitis. Trials of herpes simplex virus stromal keratitis, keratouveitis, or uveitis were not considered in this review.
Types of interventions
Antiviral agents included idoxuridine, vidarabine, trifluridine, acyclovir, ganciclovir, brivudine, foscarnet, and cidofovir. Interferon preparations included interferon‐α, interferon‐β, and interferon inducers. Debridement of the corneal epithelium was done by wiping, scraping, chemical corrosion, cryotherapy, thermal application, or photoinactivation. Supplemental agents included lubricants, growth factors, anti‐inflammatory medications, and immunomodulators. Trials of herbal extracts, traditional medicines, acupuncture, and other alternative interventions were not considered in this review. Studies of gene therapy were not part of this review (Elbadawy 2012).
Types of outcome measures
Primary outcomes
The primary outcome was the proportion of eyes healed at 14 days after study entry.
Secondary outcomes
Two secondary outcomes were used to evaluate the pace of healing: the proportion healed at seven days after study entry and the comparative rate of healing between interventions as estimated by logrank analysis of survival curves or life tables. Ancillary measures that were recorded when available were the effect on viral recovery, the prevalence of adverse events during treatment, the occurrence of episodes of recrudescent keratitis during or immediately after treatment (arbitrarily defining recrudescence as reappearing epithelial keratitis occurring less than two weeks after completing short‐term therapy), and the number of episodes of recurrent keratitis during follow up over the ensuing months (arbitrarily defining recurrence as a new episode of epithelial keratitis occurring two weeks or longer after completing short‐term therapy).
Search methods for identification of studies
We attempted to identify all relevant trials, regardless of language or publication status, using search strategies recommended by The Cochrane Collaboration. The search for studies was performed with the assistance of the Cochrane Eyes and Vision Group (CEVG) and the United States Cochrane Center (USCC). The author selected potentially relevant studies by reviewing the titles and abstracts of studies found by the searches. The full text was obtained for any report of a possibly relevant clinical trial. Articles were translated as needed. The sequence of searching and assessment of studies was in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA).
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 12), PubMed (January 1946 to 31 December 2014), EMBASE (January 1980 to 31 December 2014), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to 31 December 2014), System for Information on Grey Literature in Europe (OpenGrey) (January 1995 to 31 December 2014), BIOSIS (January 1926 to 5 May 2014), Scopus (January 1966 to 31 December 2014), Japan Science and Technology Institute (J‐Global) (January 1975 to 31 December 2014), China National Knowledge Infrastructure (CNKI) (January 1979 to 31 December 2014), and British Library's Electronic Table of Contents (Zetoc) (January 1993 to 7 May 2014). The following resources were last searched on 31 December 2014: the metaRegister of Controlled Trials (mRCT), ClinicalTrials.gov, the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), and the Chinese Clinical Trial Registry (ChiCTR). There were no language or date restrictions in the search for trials. Spanish, Portuguese, Japanese, and Chinese databases were searched with English language terms and with pertinent language terms.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), PubMed (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), OpenGrey (Appendix 5), BIOSIS (Appendix 6), Scopus (Appendix 7), J‐Global (Appendix 8), CNKI (Appendix 9), Zetoc (Appendix 10), mRCT (Appendix 11), ClinicalTrials.gov (Appendix 12), ICTRP (Appendix 13), and ChiCTR (Appendix 14).
Searching other resources
Handsearching
Manual searching of printed databases, using the terms ‘cornea’, ‘herpes’, and ‘keratitis’, was undertaken for Index Medicus (1960 to 1965), Excerpta Medica Ophthalmology (1960 to 1973), and Ophthalmic Literature (1947 to 1998).
Grey literature
We searched titles and abstracts of presentations and posters from 1960 to 2014 for clinical reports of herpetic keratitis submitted to the following meetings: Association for Research in Vision and Ophthalmology (ARVO), World Ophthalmology Congress, American Academy of Ophthalmology (AAO), Ocular Microbiology and Immunology Group (OMIG), and International Conference on Herpetic Eye Diseases. Websites of the U.S.Food and Drug Administration (FDA) (Appendix 15), National Institute for Health and Clinical Excellence (NICE) (Appendix 16), the European Medicines Agency (EMA) (Appendix 17) were searched for unpublished or unregistered clinical trials on herpetic keratitis.
Reference lists
The bibliographies of included study reports, ocular virology review articles, and corneal textbooks were surveyed for relevant publications that included the word ‘herpes’ or ‘herpetic’ and the word 'keratitis' in the article title.
Correspondence
Selected authors were contacted, when feasible, if insufficient data were provided in the published report.
Data collection and analysis
Selection of studies
Human studies were selected based on study design, type of corneal disease, interventions, and outcome measures. Included studies had an impartial allocation of two or more interventions to participants who were clinically diagnosed or virologically confirmed, or both, with HSV epithelial keratitis and who were evaluated for corneal healing at seven or 14 days, or both, after study entry. Trials on the relative effectiveness of botanical or herbal preparations were not included in this review. For multiple publications that described all or part of the same study population the most detailed report with the largest sample was used (Wilhelmus 2007a). This review did not examine the management of HSV stromal keratitis, endotheliitis, keratouveitis, or iritis (Knickelbein 2009).
Data extraction and management
The author recorded relevant information for each included study onto a data collection form. Extracted data comprised attributes of study participants, method of treatment allocation, any co‐interventions or adjunctive treatment, and the examination method used to evaluate healing. Data from trials restricted to geographic epithelial keratitis were incorporated into pooled comparisons but also tabulated separately.
The cumulative number of eyes that healed in each intervention group was recorded for each day of follow up, including days seven and 14. For reports using graphs of healing curves to describe outcome, plotted lines were projected onto the ordinate axis for each follow‐up day along the abscissa to estimate the daily proportion healed in each treatment group. Adjustment for censoring was not part of the estimation of risk ratios but was considered when estimating hazard ratios.
Assessment of risk of bias in included studies
Appraisal of study design, analysis, and presentation considered potential sources of selection bias, performance bias, detection bias, and attrition bias. Selection bias was evaluated by examining randomisation of treatment allocation and concealment of assigned treatment group. Studies that did not explain the method of sequence generation but did describe a controlled clinical trial design were considered to have an unclear risk of bias for sequence generation. The remaining studies that did not identify a randomisation process were considered to have a high risk of bias for random sequence generation. Performance bias and detection bias were evaluated by noting the use of masking of participants, trialists, and outcome assessors and by the method that was used to ascertain corneal epithelial healing. Attrition bias was evaluated by the extent of withdrawals and other losses to follow up. Because the primary endpoint of corneal epithelial healing was measured within two weeks of enrolment, withdrawals and losses to follow up were expected to be relatively low. Studies were judged to be liable to have incomplete outcome reporting if the status was not provided for participants who did not achieve healing and who were not assessed at two weeks. Each component of internal validity (sequence generation, allocation concealment, masking, completeness of outcome data, outcome reporting, and other sources of bias) was categorically judged as having a low, unclear, or high risk of bias.
Measures of treatment effect
Relative measures of corneal healing between treatment groups were estimated by risk ratios at seven days and at 14 days after trial enrolment. A hazard ratio was also estimated when additional time‐to‐event data could be extracted from tables or cumulative healing curves. Ratios were reported with 95% confidence intervals. Reports of treatment comparisons that provided only the mean time to resolution were not used in estimating survival rates because all interventions did not yield consistently similar healing distributions (Michiels 2005).
Unit of analysis issues
The unit of analysis was the individual eye with HSV keratitis. If a person with bilateral or recurrent keratitis was enrolled more than once into a trial, the unit of analysis was the eye designated to receive study treatment for each episode. Potential correlation of outcomes between fellow eyes was not considered in pooled analyses.
Dealing with missing data
Whenever possible, data on all participants were extracted from studies that reported sufficient information for an intention‐to‐treat analysis. Censoring was assumed to be non‐informative and to occur at a constant rate during the initial two weeks of treatment.
Assessment of heterogeneity
Heterogeneity among trials evaluating similar interventions was investigated using forest plots. The I2 statistic was used as an index of inconsistency among pooled comparisons of interventions (Higgins 2002). I2 greater than 50% indicated substantial heterogeneity. Any treatment comparison having high heterogeneity when estimating a pooled risk ratio at 14 days was re‐analysed in a random‐effects model.
Assessment of reporting biases
Publication bias was explored by funnel plots for treatment comparisons that had at least ten trials.
Data synthesis
Treatment comparisons
Interventions were compared to inactive controls, to each other, or to combinations of interventions. Data from trials with multiple treatment groups were evaluated by selecting paired comparisons of two interventions or by combining data from similar treatment groups.
Placebo controls
Some placebo‐controlled trials used the vehicle of the relevant study drug for the inactive control. Other topical agents that were classified as inactive controls in this review included solutions of tissue‐culture medium, neomycin, albumin, non‐specific gamma‐globulin, and low‐dose interferon preparations.
Combining treatments within studies
Interventions that were considered sufficiently similar and that were amalgamated within studies having more than two treatment groups were photoinactivation and carbolisation; cryotherapy and carbolisation; interferon‐α at either 1 million IU/ml or 10 million IU/ml; thermomechanical debridement and thermomechanical debridement with interferon‐α 62,500 IU/ml, a dosage probably too low to exert clinical effects; debridement with interferon‐α at either 11 million IU/ml or 33 million IU/ml; and trifluridine with interferon‐α at either one million IU/ml or 30 million IU/ml.
Combining treatments between studies
Different formulations of the same nucleoside antiviral agent (for example, idoxuridine solution and idoxuridine ointment; acyclovir ointment and acyclovir solution; and ganciclovir gel and ganciclovir solution) were combined in pooled analyses. Data for ganciclovir 0.15% gel rather than ganciclovir 0.05% gel were tabulated for comparison to topical acyclovir since ganciclovir 0.15% is the commercially available concentration. Multi‐arm studies comparing more than two interventions were recombined into appropriate pairs of interventions in the data tables. One study (Li 2013a) that compared ganciclovir to acyclovir plus interferon was tabulated in the ganciclovir‐acyclovir comparison.
Statistical estimation
Direct risk ratio
Tables were constructed of the cumulative number of eyes healed after trial enrolment for each treatment group. Direct comparisons between interventions estimated risk ratios (Deeks 2002) at seven days and at 14 days using a fixed‐effect model with the Mantel‐Haenszel method. Pooled comparisons with I2 > 50% for the 14‐day outcome were re‐estimated in a random‐effects model.
Indirect risk ratio
Treatment comparisons were organised into networks. Within the network of antiviral treatment trials, indirect risk ratios were estimated in which different antivirals could be compared by way of a mutual antiviral agent (Bucher 1997; Glenny 2005; Song 2009). Using formulæ (Wells 2009) implemented in software from the Canadian Agency for Drugs and Technologies in Health, indirect risk ratios and corresponding 95% confidence intervals were computed for topical antiviral treatment comparisons at 14 days. Consistency within a set of indirect risk ratios was examined with random‐effects meta‐regression (White 2012).
Combined direct and indirect risk ratio
An adjusted risk ratio was estimated by pooling direct and indirect relative treatment effects (Song 2003) in a software program using a random‐effects model (Harris 2008). The I2 statistic and graphs (Chaimani 2013) were used to interpret the network meta‐analysis.
Hazard ratio
Healing times were estimated for studies that reported outcomes at more than two time points within two weeks after enrolment by extracting healing and censoring data from reported survival curves and life tables. The observed (O) and the logrank expected (E) numbers of events were used to compute logrank statistics (O‐E) and to estimate the hypergeometric variance (V) with formulæ (Tierney 2007) implemented in a spreadsheet calculator. Hazard ratios were then pooled with a fixed‐effect model to describe the relative therapeutic effect of interventions over time (Parmar 1998).
Healing rate
Studies providing healing curves were integrated into a graph to illustrate the relative rates of corneal re‐epithelialisation over two weeks of treatment. The cumulative healing probability was estimated by collating participant‐level data from a large antiviral treatment trial.
Subgroup analysis and investigation of heterogeneity
Two trials that restricted enrolment to persons with geographic epithelial keratitis were tabulated with other trials but also analysed separately. Studies that enrolled eyes with either dendritic or geographic epithelial keratitis did not undergo stratified analysis by type of keratitis in this systematic review. Other characteristics evaluated as possible prognostic factors of corneal epithelial healing time in any post hoc analysis of individual trials were recorded.
Sensitivity analysis
Sensitivity analyses assessed the robustness of pooled relative effect measures and explored possible reasons for heterogeneity. In one sensitivity analysis, studies were selectively omitted from the meta‐analysis if there was a potentially high risk of bias because of possible non‐random sequence generation, unconcealed allocation, or lack of masking that could have influenced outcome assessment. Another sensitivity analysis used only studies with an explicit randomised, double‐masked trial design. Studies that were excluded because cumulative healing proportions of the treatment arms were not given were tabulated to explore how their findings compared with the pooled results of included studies.
Results
Description of studies
Results of the search
Electronic and manual searches identified 2430 discrete reports of HSV keratitis. After screening, 280 therapeutic studies were identified that compared two or more interventions in the treatment of HSV epithelial keratitis (Figure 2). One hundred thirty‐seven studies that met this review's inclusion criteria are tabulated in Characteristics of included studies. Characteristics of excluded studies outlines which of the five main reasons why 143 studies were excluded along with the treatment groups of each excluded study. Two unpublished antiviral treatment trials are listed in Characteristics of studies awaiting classification.
2.
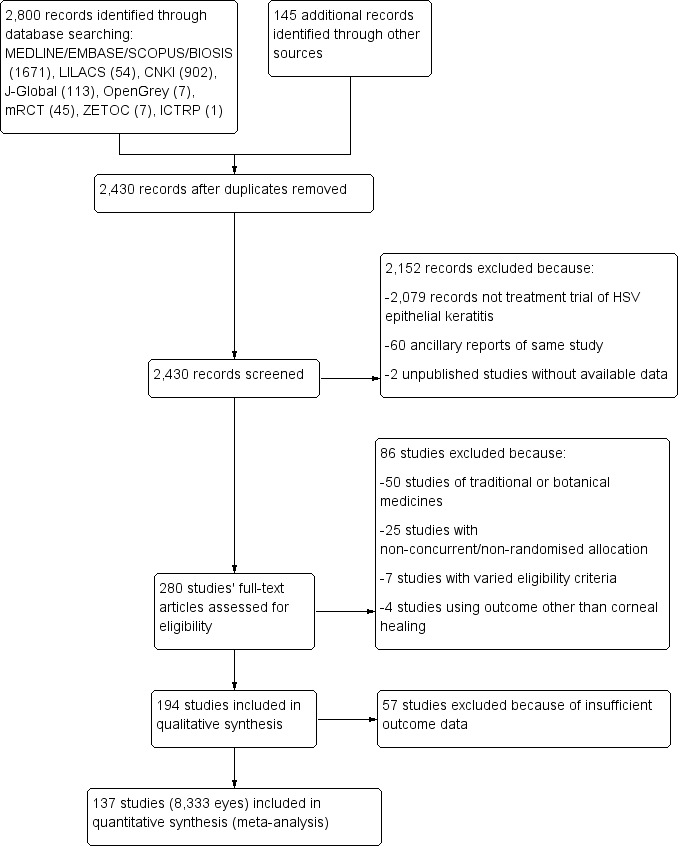
- Study flow diagram.
Included studies
Interventions
Trials compared various pharmacological and non‐pharmacological interventions. One hundred sixteen trials compared an intervention to a control or compared two interventions. The others had multiple treatment arms: 16 studies had three treatment groups (Altinisik 1987; Bartholomew 1977; Carmassi 1993; Colin 1997a; Colin 2007a; Coster 1977a; Davidson 1964; Huang 2008a; MacKenzie 1964; Maichuk 1988; Markham 1977; Meurs 1985; Parlato 1985; Sundmacher 1976a; Sundmacher 1981b; Uchida 1981), four studies had four groups (Matthäus 1970; Panda 1995; Struck 1989; Sundmacher 1987), and one study had five groups (Fulhorst 1972).
For this systematic review, treatment comparisons were organised into two‐way comparisons and allocated into five categories: comparisons of topical antivirals, trials involving oral antiviral treatments, studies examining the effect of interferon, studies of corneal epithelial debridement, and trials of miscellaneous drugs. Among multiple treatment comparisons (Figure 3), the number of direct, two‐way comparisons ranged from one to 28 studies.
3.
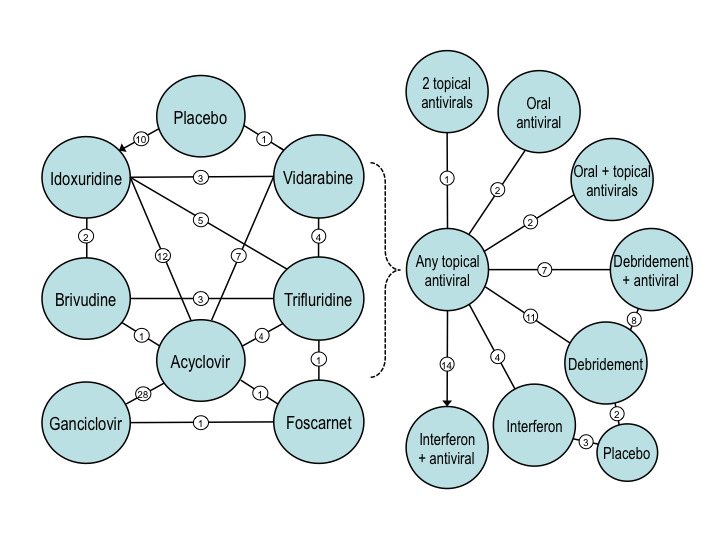
Networks of clinical trials included in this systematic review. Left diagram shows a network of two‐way comparisons among topical antiviral agents. Not shown in the left diagram are trials evaluating para‐fluorophenylalanine, different trifluridine vehicles, and different ganciclovir concentrations. Right diagram illustrates a network of comparisons between topical antiviral agents and various interventions that included dual antiviral therapy, oral acyclovir, interferon without or with an antiviral, and debridement without or with an antiviral. Not shown in the right diagram are trials evaluating different interferon dosages or types, an interferon inducer, different methods of debridement, debridement with two different antivirals, and miscellaneous agents (hyaluronate, epidermal growth factor, panthenol, methyluracil, oxyphenbutazone, or inosine pranobex). Lines show direct treatment comparisons reporting outcomes at one week or two weeks. Circled numerals indicate the number of direct treatment comparisons that were included in this systematic review. The placebo groups consist of a variety of inactive controls. The number of acyclovir‐idoxuridine (IDU) comparisons includes one trial using iododeoxycytidine (IDC), even though IDC was not pooled with IDU in the meta‐analysis. The number of direct comparisons exceeds the number of studies because 21 studies compared more than two interventions.
Supplemental medications
Use of a topical cycloplegic agent was reported in 51 included trials: atropine in 29 studies, scopolamine in six, homatropine in five, and a non‐specified cycloplegic or mydriatic agent in 11. A concomitant topical antibiotic was dispensed in 19 studies: ofloxacin in seven studies, chloramphenicol in four, gentamicin in one, micronomicin in one, and an unspecified antibiotic in six.
Sample sizes and study statistics
A total of 8333 eyes with HSV epithelial keratitis were enrolled and analysed in 137 studies included for analysis in this systematic review. Study sizes ranged from 15 to 287 participants, and the distribution of study sizes skewed toward small to moderately sized samples. The median number enrolled per study was 53 (interquartile range [IQR] 33 to 78). Multicentre studies enrolled more eyes: the median study size was 50 (IQR 32 to 76) among 118 single‐centre studies and 64 (IQR 51 to 109) among 19 multicentre studies.
None of the studies reported a pretrial sample size estimation based on a priori assumptions, although one trial did provide a statistical computation that was used to plan enrolment based on recurrence risk rather than epithelial healing (HEDS Group 1997). The statistical analysis of individual studies, when reported, was based on detecting a statistically significant difference between treatment groups in corneal epithelial healing. One study provided a post hoc non‐inferiority analysis (Colin 2007b). An independent committee monitored accrued data for some multicentre studies (HEDS Group 1997; Tanaka 1988a; Tanaka 1988b).
Study participants
Table 9 summarizes the characteristics of participants and interventions. Among 66 trials that described age statistics of enrolled participants (including one trial (Li 2013b) that gave median rather than mean age), the overall average age was 43 years (standard deviation (SD) 6 years). Of 78 trials reporting the gender of study participants (omitting one trial (Hart 1965) that only gave the gender of cured participants), 71 (91%) enrolled more men than women. The median male‐female ratio was 1.5 (IQR 1.2 to 2.1).
3. Overview of trial and participant characteristics among included studies.
| Study characteristics | Categories | No. (%) trials (n=137) |
| No. centres | One Two or more | 118 (86%) 19 (14%) |
| No. eyes per study | < 50 50 to 100 > 100 |
62 (45%) 58 (42%) 17 (12%) |
| Average age of participants | < 40 years 40 to 50 years > 50 years Not stated | 24 (18%) 36 (26%) 6 (4%) 71 (52%) |
| Gender of participants | Males exceed females Females exceed males Not stated | 71 (52%) 7 (5%) 59 (43%) |
| Type of epithelial keratitis | Dendritic Dendritic or geographic Geographic Not specified | 74 (54%) 59 (43%) 2 (1%) 2 (1%) |
| No. of intervention groups1 | Two Three Four Five | 116 (85%) 16 (12%) 4 (3%) 1 (1%) |
| Cycloplegic or mydriatic eye drops | Yes None, variable, or not stated | 51 (37%) 86 (63%) |
| Antibacterial eye drops | Yes None or not stated | 19 (14%) 118 (86%) |
1 Of 142 excluded studies, 121 had two intervention groups, ten had three groups, six had four groups, two had five groups, two had six groups, and one had eight groups.
Setting
Seventy‐one trials included in this review took place in Europe, 51 in Asia, 13 in North America (including one performed in the United States and the United Kingdom), one in Africa, and one in Australia. One hundred seventeen trials included in this review took place at a single centre. The primary report was published in the English language for 79 included trials, Chinese for 33, Japanese for seven, German for seven, French for six, Italian for two, Turkish for two, and Russian for one.
Type of epithelial keratitis
Seventy‐four trials specified dendritic epithelial keratitis as an eligibility criterion. Two other trials (Burns 1963; Davidson 1964) provided insufficient information to confirm that all participants had dendritic epithelial keratitis. Fifty‐nine trials enrolled participants with either dendritic or geographic epithelial keratitis. In these studies dendritic epithelial keratitis was, on average, five times more prevalent than geographic epithelial keratitis. Two trials were restricted to geographic epithelial keratitis (Collum 1985; Coster 1979).
Laboratory confirmation
Viral isolation was performed in 34 included trials. Five trials enrolled only HSV culture‐confirmed eyes (Colin 1983; Meurs 1985; Sundmacher 1981a; Sundmacher 1981b; Sundmacher 1984a). Four trials performed HSV isolation without providing the number of culture‐positive eyes (Bartholomew 1977; Hoang‐Xuan 1984; Luntz 1963; van Bijsterveld 1989). Of 25 trials that reported the prevalence of 759 positive viral culture results (including one trial that reported viral culture and immunofluorescent test results) among 1148 eyes tested at trial entry, the median prevalence of study participants who had laboratory‐confirmed HSV ocular infection, from corneal or conjunctival samples, was 68% (IQR 50% to 78%). HSV‐2 was rarely isolated (Vannini 1986). No study reported whether a topical anæsthetic (Goldschmidt 2006; Weinberg 1977) or stain (Roat 1987; Seitzman 2006) might have curtailed viral detection.
Outcome evaluation
Outcome assessment was based on clinical observation using slit‐lamp biomicroscopy, except for one study (Hart 1965) that used a magnifying loupe. Of 117 trials specifying the use of a stain, 96 (82%) used fluorescein (including one study stating only that a "stain" was used), nine used rose‐Bengal, and 12 used both fluorescein and rose‐Bengal.
The description of outcome assessment, when stated, assigned the day of healing as the time when re‐epithelialisation of the corneal surface was first verified. If a topical dye was used then the day of healing was the first day when no confluent staining was observed. The following extracts exemplify overlapping ways by which the primary endpoint of resolution of active epithelial keratitis were expressed:
"Disappearance of [fluorescein] dendritic staining, despite the occasional persistence of fine, superficial punctate keratitis" (Parlato 1985).
"Fluorescein‐negative healing of the corneal epithelium, which was defined as complete closure of all erosions except for some single dye‐positive micropunctations" (Sundmacher 1976a).
"Disappearance of specific Bengal‐rose staining of the precise site of the healing dendritic ulceration...[not considering] fine microscopic punctate staining diffusely arranged on the epithelial surface" (Wilhelmus 1981a).
"2 criteria for healing: (1) partial healing...defined as closure of the epithelial wound only, i.e., no staining with fluorescein; and (2) complete healing...defined as closure of the epithelial wound without any epithelial oedema or cystic changes in the area of the previous dendrite" (de Koning 1982).
"Healing was defined as the situation in which no staining with fluorescein was observed and no epithelial oedema and cystic changes were present in the epithelium covering the site of the original ulcer" (van Bijsterveld 1980).
Following fluorescein 1% and rose‐Bengal 1%, the initial dendritic pattern "was red, stained by rose bengal, and outlined by a green fluorescent double contour…. The red‐stained dendriform pattern represented virus‐affected cells, while the green double contour disclosed presence of epithelial defects as well. After on an average five days only uncharacteristic remains were left of the previous dendritic pattern in the form of accumulated vital‐stained dots localized within parts of the previous pattern. Some such remains were fluorescein‐stained epithelial defects and others rose‐bengal‐stained, degenerate epithelial cells…. This second phase was followed by a third one, during which punctate fluorescein and/or rose bengal staining might still be seen, but now only represented by a few dots, as a rule scattered over the whole cornea, also outside the original dendritic pattern" (Norn 1973).
The median time to healing of dendritic epithelial keratitis with trifluridine, acyclovir, brivudine, or ganciclovir was seven days. The median time to healing in both trials of antiviral‐treated geographic epithelial keratitis was nine days (Collum 1985; Coster 1979). Based on 49 trials that published survival graphs, the maximum separation of healing curves for different treatments occurred at a median of six days. The healing curve of one large antiviral treatment trial (HEDS Group 1997) was examined in parametric statistical models and found to fit a log‐logistic distribution (Wilhelmus 2000). In that study, the cumulative probability of corneal epithelial healing at each day (t) of trifluridine chemotherapy for dendritic epithelial keratitis could be estimated by [(7/t)4 + 1]‐1.
Excluded studies
One hundred forty‐three comparative treatment trials of HSV epithelial keratitis were excluded from analysis because study treatment, study design, or available data did not meet eligibility criteria (Characteristics of excluded studies). One excluded study did not report the sample size (Kuyama 1979). A total of 12,367 participants with HSV epithelial keratitis were enrolled in the other 142 excluded studies that had study populations ranging from nine to 416 participants.
Alternative study treatment
Fifty studies were ineligible because at least one of two treatment groups included an ethnobotanical preparation or traditional Chinese medication, used either alone or integrated with a synthetic antiviral agent. Several studies of complementary or alternative interventions were pre‐emptively identified before full articles were reviewed for eligibility, precluded based on their titles or abstracts, and do not appear in this subtotal.
Ineligible study design
Twenty‐five studies were excluded because of non‐concurrent or non‐randomised treatment allocation. Seven studies were excluded because their eligibility criteria did not ensure that enrolled patients had active epithelial keratitis. Four studies were excluded because outcome was based on an endpoint other than epithelial healing.
Insufficient outcome information
Fifty‐seven studies were excluded because insufficient outcome data were available at seven and 14 days. Trials lacking survival data generally assessed relative outcome by comparing mean healing times or clinical scores of symptoms and signs (Table 10). Thirty‐three studies reported mean healing times of treatment groups; 18 studies reported a P value for a different endpoint; and six studies provided neither outcome analysis.
4. Outcomes reported in studies excluded due to insufficient healing data.
|
Study (n=57) |
Treatment group (no. eyes)1 | Mean healing time ± SD, days; or P value2 |
| Antiviral agents | ||
| Babushkin 1993 | Idoxuridine (60) Acyclovir (24) | NS 5.9 ± 1.2 |
| Huang 2007 | Acyclovir (62) Ganciclovir (62) |
12 9.6 |
| Inocencio 1982 | Idoxuridine (9) Acyclovir (14) | 10.4 7.0 |
| Jing 2010 | Acyclovir (22) Ganciclovir (24) |
P = 0.005 |
| Leopold 1965 | Idoxuridine (5) Cytarabine (5) | NS NS |
| McGill 1981 | Vidarabine (29) Acyclovir (28) | 6.2 ± 1.8 4.5 ± 2.7 |
| Mohan 1987 | Vidarabine (19) Acyclovir (21) | 8.3 ± 0.9 6.5 ± 0.6 |
| Pavan‐Langston 1972 | Idoxuridine (14) Vidarabine (15) | 3.0 ‐ 4.5 3.5 ‐ 4.5 |
| Pavan‐Langston 1977 | Idoxuridine (17) Trifluridine (23) | 5.3 5.5 |
| Pietruschka 1968 | Fluorophenylalanine (30) Idoxuridine (28) Fluorophenylalanine, idoxuridine (40) | 15 18 22 |
| Wang 2009 | Acyclovir (39) Ganciclovir (39) | P < 0.05 |
| Debridement | ||
| Assetto 1981 | Debridement (8) Idoxuridine (16) Cytarabine (16) Trifluridine (8) | 3 ‐ 19 10 ‐ 18 13 ‐ 21 6 ‐ 13 |
| Fellinger 1980 | Idoxuridine (13) Trifluridine (16) Debridement, idoxuridine (13) Debridement, trifluridine (18) | 8.1 ± 2.5 5.5 ± 2.5 13.4 ± 9 6.8 ± 4.2 |
| Guo 2003 | Antiviral (34) Cryotherapy, antiviral (35) | NS NS |
| Hilsdorf 1969 | Iodinisation, idoxuridine (20) Cryotherapy, idoxuridine (20) | 4.3 14.0 |
| Koev 2007 | Acyclovir (25) Acyclovir, pandavir (24) Debridement, acyclovir, pandavir (26) | 9.3 ± 1.2 8.2 ± 1.1 6.2 ± 1.3 |
| Mathur 1984 | Iodinisation (50) Cryotherapy (20) Cryotherapy, autologous serum (24) Debridement (30) Debridement, idoxuridine (30) | 11 9 7 9 6 |
| Patterson 1967c | Debridement (9) Idoxuridine (15) | P < 0.01 |
| Shimomura 1987 | Debridement, idoxuridine (16) Idoxuridine (15) | P > 0.05 |
| Tarakji 1978 | Cryotherapy (21) Idoxuridine (14) | 2.4 6.2 |
| Whitcher 1976 | Debridement (20) Idoxuridine (31) | 5 13 |
| Interferon and interferon/antiviral combinations | ||
| Chen 2007 | Acyclovir (43) Acyclovir, interferon (69) | P < 0.01 |
| Gu 2005 | Acyclovir (20) Acyclovir, interferon (19) | P < 0.05 |
| Huang 2009 | Acyclovir (36) Acyclovir, interferon (29) | 39.6 30.4 |
| Jin 1992 | Acyclovir (41) Interferon (59) | 8.2 ‐ 9.6 9.9 ‐ 12.4 |
| Kuyama 1979 | Interferon‐α (NS) Interferon‐β (NS) | NS NS |
| Lin 2013b | Ganciclovir (33) Ganciclovir, interferon (33) |
P < 0.05 |
| Liu 2003 | Antiviral (21) Antiviral/interferon (21) | NS NS |
| Scialdone 1986 | Idoxuridine (8) Interferon (12) | 10 ‐ 14 7 ‐ 10 |
| Shiota 1988 | Interferon 100,000 IU/ml (14) Interferon 1 million IU/ml (46) | 4.4 ± 3.8 4.2 ± 4.1 |
| Su 2010 | Ganciclovir (40) Ganciclovir, interferon (38) |
P < 0.05 |
| Tamburi 1990 | Acyclovir (8) Interferon (8) Acyclovir, interferon (8) | 5.2 9.0 5.0 |
| Wan 2014 | Ganciclovir (24) Ganciclovir, interferon (26) |
6.5 ± 2.1 4.2 ± 1.1 |
| Weng 2014 | Acyclovir (35) Acyclovir, interferon (35) |
P < 0.05 |
| Zhang 2003 | Interferon‐α‐1b (29) Interferon‐α‐2b (17) | 12.9 ± 2.1 9.4 ± 3.0 |
| Zhao 2001 | Acyclovir (89) Acyclovir, interferon (97) | P < 0.01 |
| Zhou 2008 | Acyclovir (35) Acyclovir, interferon (66) | P < 0.05 |
| Interferon inducers | ||
| Galin 1976 | Idoxuridine (20) Poly I:C (19) | NS NS |
| Kasparov 1972 | Idoxuridine (16) Poly A:U (43) Immunomodulators (167) Debridement, immunomodulators (33) | P < 0.01 |
| Kasparov 1974 | Control (30) Idoxuridine (28) Poly A:U (27) Idoxuridine/poly A:U (15) | 14.1 ± 2.0 14.5 ± 2.0 10.2 ± 1.3 13.8 ± 3.2 |
| Kasparov 1991 | Acyclovir (45) Poly A:U (65) Acyclovir, poly A:U (40) | 13.8 ± 0.8 14.6 ± 1.2 9.5 ± 1.1 |
| Lin 2009 | Acyclovir (27) Acyclovir, poly I:C (22) | P < 0.05 |
| Liu 2014b | Acyclovir (NS) Poly I:C (NS) Acyclovir, poly I:C (NS) |
NS |
| Wei 2012 | Ganciclovir (24) Poly I:C (24) |
22.75 ± 5.09 13.54 ± 4.06 |
| Cytokines, growth factors, and immunomodulators | ||
| He 2014 | Antiviral (34) Autologous serum (34) |
P = 0.015 |
| Kolomiets 1986 | Control (10) HSV‐immunoglobulin (14) | 28.6 16.8 |
| Liu 2007 | Acyclovir (50) Acyclovir, fibroblast growth factor (46) | P < 0.05 |
| Li 2014c | Ganciclovir, acyclovir (21) Ganciclovir, fibroblast growth factor (21) |
P < 0.05 |
| Pivetti‐Pezzi 1985 | Placebo (13) Thymic extract (11) | 23 ± 13 23 ± 9 |
| Prost 1986 | Cryotherapy (20) Cryotherapy, oral inosine pranobex (19) | 6.8 ± 3.9 6.5 ± 3.8 |
| Salcedo Hernandez 2007 | Autologous serum (9) Acyclovir (8) | 10 7 |
| Sellitti 1982 | Topical/oral inosine pranobex (20) Oral inosine pranobex (20) | 13.3 25 |
| Topciu 1992 | Idoxuridine, foscarnet (120) Idoxuridine, foscarnet, immunostimulant (113) | 30 3 ‐ 4 |
| Xu 2009b | Acyclovir (30) Acyclovir, interleukin‐2 (32) | 11 ± 2.7 ‐ 41 ± 6.7 9 ± 2.5 ‐ 28 ± 4.5 |
| Yu 2012b | Ganciclovir (30) Ganciclovir, measles vaccine (30) |
P < 0.05 |
| Zhang 1992 | Acyclovir, interferon (20) Acyclovir, interferon, transfer factor (21) | P < 0.05 |
| Zhi 2001 | Acyclovir (30) Acyclovir, interleukin‐2 (38) | 9 ± 2.5 ‐ 30 ± 4.5 13 ± 2.7 ‐ 25 ± 3.1 |
1 Similar treatment groups were combined when possible; interventions are tabulated in Characteristics of excluded studies.
2 A range of average healing times indicates that mean estimates were provided for subcategories (e.g., dendritic vs. geographic epithelial keratitis) without summary estimation. For studies not reporting mean healing times, P values are given that either compare the proportion healed in study groups at a time other than 14 days or compare clinical scores of symptoms and signs between study groups.
NS, not stated; SD, standard deviation (when reported)
Risk of bias in included studies
Potential biases affected several studies (Figure 4).
4.
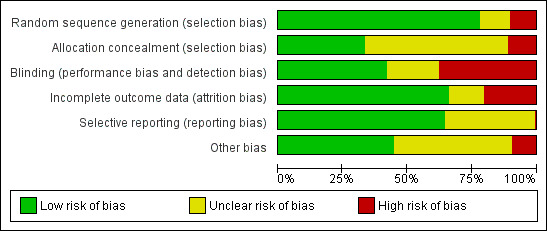
Methodological quality graph: review author's judgements about each methodological quality item presented as percentages across all included studies.
Allocation
Among 137 included trials, 107 (78%) stated that a randomisation process was used to allocate study interventions. Sixteen studies had an unclear method of generating the sequence of treatment assignment. Fourteen studies were considered to have a potentially high risk of bias of random sequence generation, including two studies that assigned study treatment to alternating participants (Daniel 1972; Luntz 1963) and two studies that apparently allocated treatment by availability (Abe 1987; Altinisik 1987). Allocation concealment was judged to have a low risk of bias for 46 (34%) of the studies. Seventy‐six studies had an unclear risk of bias of allocation concealment, and 15 were considered to have a high risk of bias.
Blinding
Fifty‐seven (42%) studies had a double‐masked study design, including two triple‐masked trials that used a different outcome assessor than the investigator who allocated treatment (Parlato 1985; Sundmacher 1978a). Twenty‐eight trials had a single‐masked or unclear scheme, and 52 trials used an unmasked design.
Incomplete outcome data
Ninety (66%) of the studies were considered to have a low risk of attrition bias. Nineteen studies had an unclear risk of attrition bias. Among 28 studies considered to have a high risk of having incomplete outcome data, the percentage of missing data varied from 5% to 40%. Eight of these studies provided outcome data only for culture‐positive eyes.
Selective reporting
Eighty‐eight (64%) of the studies had a low risk of reporting bias. Forty‐eight studies had an unclear risk of selective reporting, mainly because study protocols were rarely available and the published report was used to determine relevant events within two weeks of follow up. One study judged to have a high risk of reporting bias apparently enrolled patients with bilateral keratitis but used participants rather than eyes as the unit of analysis (Dai 2009a).
Other potential sources of bias
Thirteen studies were judged to have a high risk of other sources of bias. Two trials added supplemental treatment during follow up: additional debridement in one trial (Sundmacher 1978a) and corticosteroid eye drops in another trial (Luntz 1963). One trial allowed some participants to cross over to the other treatment arm (Patterson 1967a). Two trials were stopped early (Behrens‐Baumann 1992; O'Day 1975). In addition to one study that enrolled an unclear number of eyes with stromal keratitis (Zhen 2012), seven studies randomised between 13% and 34% of study eyes having disciform or stromal keratitis; these studies did not separately report healing outcomes by type of keratitis (Li 2009; Lin 2011; Liu 2012a; Liu 2012b; Liu 2014a; Wang 2014a; Zhao 2006).
Investigation of heterogeneity
Substantial heterogeneity (I2 > 50%) among study findings was found for ten treatment comparisons at the 14‐day outcome assessment, including one placebo‐controlled comparison (Analysis 1.1), three comparisons of topical nucleoside antivirals (Analysis 1.10; Analysis 1.13; Analysis 1.22), a comparison involving oral acyclovir (Analysis 2.2), the comparison between interferon (Analysis 3.5) or combined interferon and an antiviral (Analysis 3.7) with nucleoside antivirals, and three comparisons examining debridement (Analysis 4.2; Analysis 4.4; Analysis 4.6). Six of these comparisons also showed heterogeneous findings at seven days (Analysis 1.1; Analysis 1.10; Analysis 3.7; Analysis 4.2; Analysis 4.4; Analysis 4.6). Possible treatment‐related reasons for inconsistency among studies were differences in dosage or formulation of topical study medicines, non‐uniform methods of corneal debridement, dissimilar use of adjunctive drugs, and possible enrolment of some eyes that did not have HSV epithelial keratitis.
1.1. Analysis.
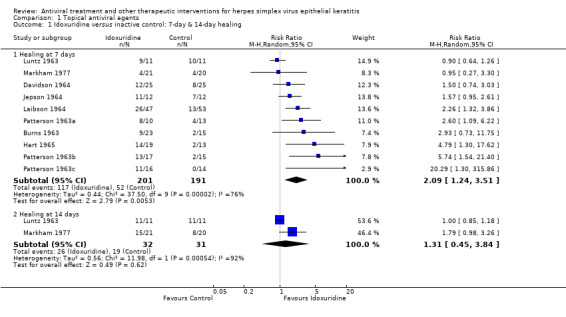
Comparison 1 Topical antiviral agents, Outcome 1 Idoxuridine versus inactive control: 7‐day & 14‐day healing.
1.10. Analysis.
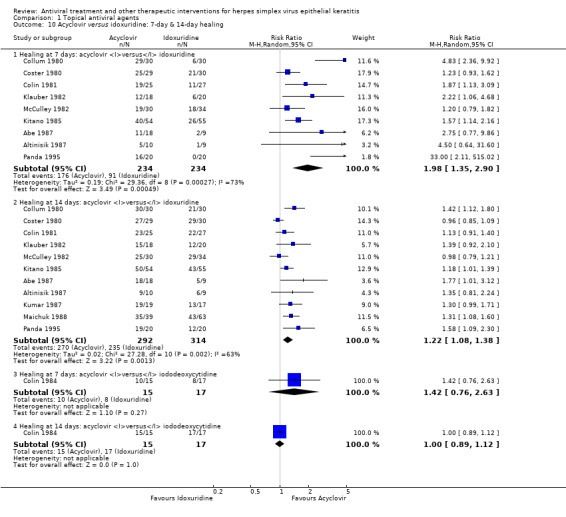
Comparison 1 Topical antiviral agents, Outcome 10 Acyclovir versus idoxuridine: 7‐day & 14‐day healing.
1.13. Analysis.
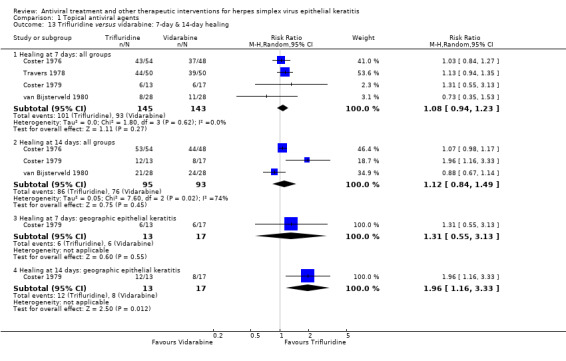
Comparison 1 Topical antiviral agents, Outcome 13 Trifluridine versus vidarabine: 7‐day & 14‐day healing.
1.22. Analysis.
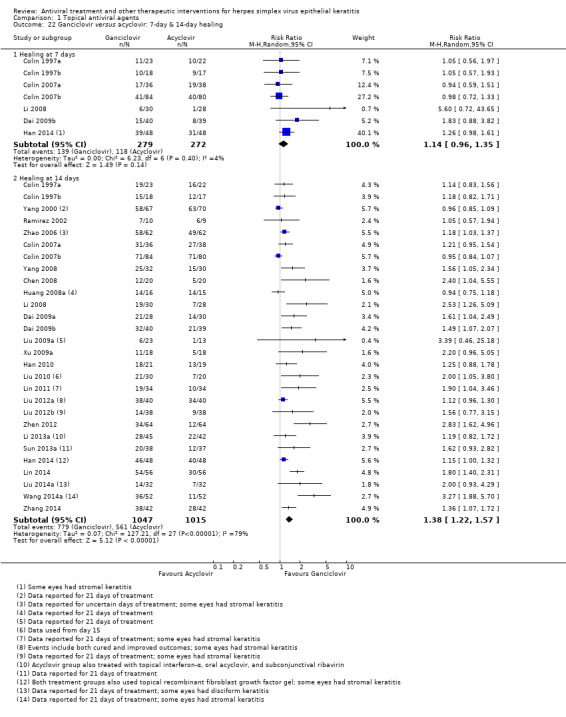
Comparison 1 Topical antiviral agents, Outcome 22 Ganciclovir versus acyclovir: 7‐day & 14‐day healing.
2.2. Analysis.
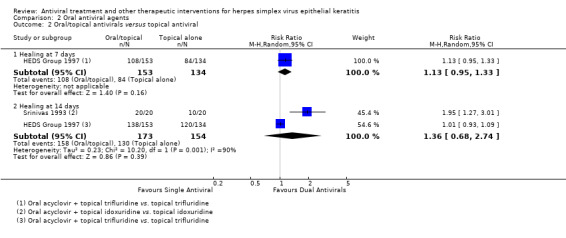
Comparison 2 Oral antiviral agents, Outcome 2 Oral/topical antivirals versus topical antiviral.
3.5. Analysis.
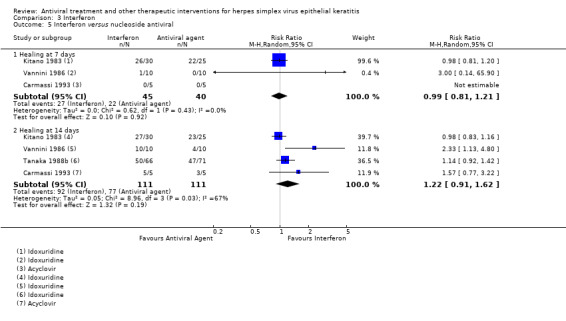
Comparison 3 Interferon, Outcome 5 Interferon versus nucleoside antiviral.
3.7. Analysis.
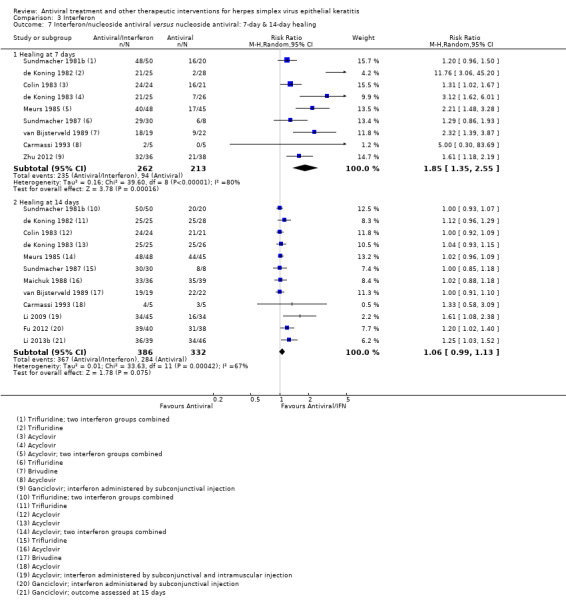
Comparison 3 Interferon, Outcome 7 Interferon/nucleoside antiviral versus nucleoside antiviral: 7‐day & 14‐day healing.
4.2. Analysis.
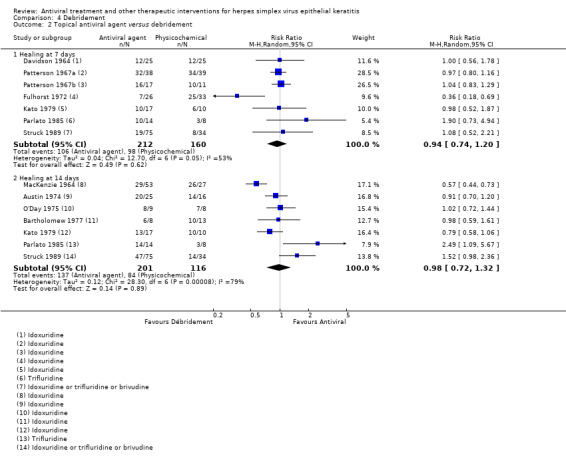
Comparison 4 Debridement, Outcome 2 Topical antiviral agent versus debridement.
4.4. Analysis.
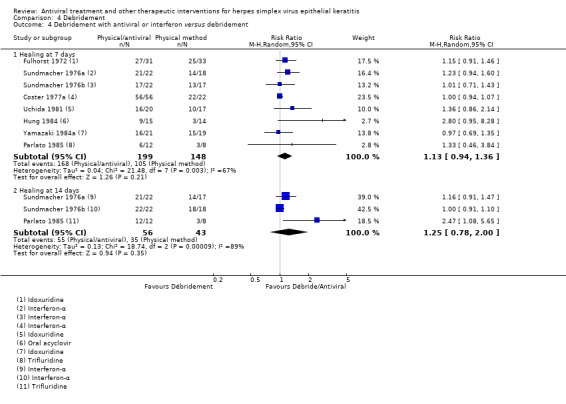
Comparison 4 Debridement, Outcome 4 Debridement with antiviral or interferon versus debridement.
4.6. Analysis.
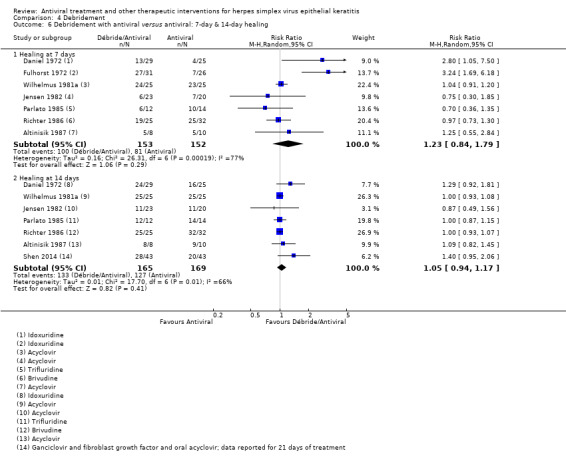
Comparison 4 Debridement, Outcome 6 Debridement with antiviral versus antiviral: 7‐day & 14‐day healing.
Sensitivity analyses
After selectively omitting studies that had a potentially inadequate or indeterminate process of random treatment assignment, studies that may have had unconcealed allocation, and studies that used incomplete masking, precision declined but relative effect measures did not shift substantially (Table 11). The findings of randomised, double‐masked trials were also generally comparable to the results of all studies (Table 3).
5. Sensitivity analysis omitting potentially biased studies: relative healing at 14 days after omitting trials with incomplete randomisation, concealment, or masking.
| Treatment comparisons1 | Studies under investigation2 | Risk ratio (95% CI) with studies included | I2 (with studies included) | Risk ratio (95% CI) with studies omitted | I2 (with studies omitted) |
| Idoxuridine versus inactive control | Luntz 1963 | 1.31 (0.45‐3.84) | 92% | 1.79 (0.98‐3.26) | NA |
| Trifluridine versus idoxuridine | Struck 1989 | 1.38 (1.19‐1.60) | 32% | 1.43 (1.23‐1.67) | 0% |
| Acyclovir versus idoxuridine | Abe 1987; Altinisik 1987; Maichuk 1988 | 1.22 (1.08‐1.38) | 63% | 1.18 (1.03‐1.35) | 66% |
| Brivudine versus idoxuridine | Struck 1989 | 1.38 (1.05‐1.81) | 30% | 1.64 (1.15‐2.35) | NA |
| Trifluridine versus vidarabine | Coster 1979 | 1.12 (0.84‐1.49) | 74% | 0.99 (0.79‐1.25) | 65% |
| Brivudine versus trifluridine | Struck 1989 | 1.00 (0.88‐1.14) | 0% | 0.98 (0.89‐1.08) | 36% |
| Ganciclovir versus acyclovir | Colin 1997a; Colin 1997b; Colin 2007b; Chen 2008; Dai 2009a; Dai 2009b; Han 2010; Han 2014; Huang 2008a; Li 2008; Li 2013a; Lin 2011; Lin 2014; Liu 2009a; Liu 2010; Liu 2012a; Liu 2012b; Liu 2014a; Ramirez 2002; Sun 2013a; Wang 2014a; Xu 2009a; Yang 2000; Yang 2008; Zhang 2014; Zhao 2006; Zhen 2012 | 1.38 (1.22‐1.57) | 79% | 1.21 (0.95‐1.54) | NA |
| Oral + topical antivirals versus topical antiviral | Srinivas 1993 | 1.36 (0.68‐2.74) | 90% | 1.01 (0.93‐1.09) | NA |
| Interferon versus nucleoside antiviral | Kitano 1983; Tanaka 1988b; Vannini 1986 | 1.22 (0.91‐1.62) | 67% | 1.57 (0.77‐3.22) | NA |
| Interferon + antiviral versus antiviral | Fu 2012; Li 2009; Li 2013b; Maichuk 1988 | 1.06 (0.99‐1.13) | 67% | 1.02 (0.98‐1.05) | 0% |
| Topical antiviral versus debridement | Bartholomew 1977; Kato 1979; MacKenzie 1964; O'Day 1975; Patterson 1967a; Struck 1989 | 0.98 (0.72‐1.32) | 79% | 1.43 (0.44‐4.67) | 87% |
| Debridement + antiviral or interferon versus debridement | Sundmacher 1976a | 1.25 (0.78‐2.00) | 89% | 1.54 (0.21‐11.5) | 96% |
| Debridement + antiviral versus antiviral | Daniel 1972; Richter 1986; Shen 2014; Wilhelmus 1981a | 1.05 (0.99‐1.17) | 66% | 1.01 (0.89‐1.15) | 0% |
1 Three comparisons that included a study judged to have a potentially high risk of bias were limited to a single study (Matthäus 1970; Serifoglu 1987; Sundmacher 1976a).
2 Two studies judged to have a potentially high risk of bias did not provide data for the 14‐day outcome (Patterson 1967a; Travers 1978).
NA: not applicable (single study available for evaluation)
Assessment of publication bias
Five treatment comparisons had enough studies to assess asymmetry in funnel plots. No obvious publication bias was found for the idoxuridine‐placebo comparison. Publication bias may have existed in favour of acyclovir for the acyclovir‐idoxuridine comparison at seven days, but no serious publication bias was apparent for the 14‐day outcome. Publication bias was possible for the ganciclovir‐acyclovir comparison (Figure 1), suggesting a bias toward publication of studies favouring ganciclovir. The comparisons between antiviral treatment and either interferon plus antiviral or debridement did not have apparent publication bias.
1.
Funnel plot of studies comparing ganciclovir to acyclovir.
Secular variation
To explore whether response to therapy may have changed over time, outcome were examined for 59 studies in which topical acyclovir ointment or solution was assigned to one treatment arm. The 14‐day cure rate with acyclovir averaged 91% (SD 10%) among 27 studies published between 1980 and 1989 (Abe 1987; Altinisik 1987; Colin 1981; Colin 1983; Colin 1987; Collum 1980; Collum 1985; Collum 1986; Coster 1980; de Koning 1983; Denis 1983; Genée 1987; Hoang‐Xuan 1984; Høvding 1989; Jackson 1984; Jensen 1982; Kitano 1985; Klauber 1982; Kumar 1987; La Lau 1982; Maichuk 1988; McCulley 1982; Meurs 1985; Pavan‐Langston 1981; Wilhelmus 1981a; Yeakley 1981; Young 1982), 75% (SD 15%) for four studies published between 1990 and 1999 (Carmassi 1993; Colin 1997a; Colin 1997b; Panda 1995), 56% (SD 27%) for 15 studies published between 2000 and 2009 (Cao 2001; Chen 2008; Colin 2007a; Colin 2007b; Dai 2009a; Dai 2009b; Huang 2008a; Li 2008; Li 2009; Liu 2009a; Ramirez 2002; Xu 2009a; Yang 2000; Yang 2008; Zhao 2006), and 46% (SD 24%) for 13 studies published between 2010 and 2014 (Han 2010; Han 2014; Li 2013a; Lin 2011; Lin 2014; Liu 2010; Liu 2012a; Liu 2012b; Sun 2013a; Wang 2014a; Zhang 2014; Zhen 2012). Among 32 studies evaluating ganciclovir gel, the 14‐day outcome with ganciclovir averaged 83% (SD 0.5%) for two studies published in 1997 (Colin 1997a; Colin 1997b), 73% (SD 18%) for 13 studies published between 2000 and 2009 (Chen 2008; Colin 2007a; Colin 2007b; Dai 2009a; Dai 2009b; Huang 2008a; Li 2008; Liu 2009a; Ramirez 2002; Xu 2009a; Yang 2000; Yang 2008; Zhao 2006), and 73% (SD 20%) for 17 studies published between 2010 and 2014 (Fu 2012; Han 2010; Han 2014; Li 2013a; Li 2013b; Lin 2011; Lin 2014; Liu 2010; Liu 2012a; Liu 2012b; Sun 2013a; Wang 2014a; Yu 2012a; Zhang 2014; Zhen 2012; Zheng 2010).
Effects of interventions
See: Table 1; Table 4; Table 5; Table 6; Table 7; Table 8
Interventions were divided into five categories: topical antiviral agents, oral antiviral therapy, interferon, corneal epithelial debridement, and adjunctive drugs. The primary treatment effect was assessed by a risk ratio at 14 days, adjusted by available indirect risk ratios for antiviral treatment comparisons (Table 2). Any difference in early resolution was examined by a risk ratio at seven days. The relative pace of healing was examined by a hazard ratio.
Topical antiviral therapy
Eight‐three studies made head‐to‐head comparisons among inactive control, idoxuridine, vidarabine, trifluridine, acyclovir, brivudine, ganciclovir, or foscarnet (Figure 3).
Idoxuridine and vidarabine
The analyses of seven‐day and 14‐day outcomes (Analysis 1.1) and of relative healing rates (Analysis 1.2) comparing idoxuridine to inactive control were limited by heterogeneity. In one trial, vidarabine was significantly better than inactive control at 14 days (Analysis 1.3) and increased the rate of healing (Analysis 1.4). While direct comparisons of vidarabine to idoxuridine did not show a significant difference in healing outcome (Analysis 1.5) or healing rate (Analysis 1.6), a combined direct and indirect comparison using studies in which vidarabine and idoxuridine were compared to a mutual antiviral suggested that vidarabine might have better therapeutic effectiveness than idoxuridine (Table 2). Additionally, para‐fluorophenylalanine, an amino acid analogue having antiviral activity, did not significantly differ from idoxuridine (Analysis 1.7).
1.2. Analysis.
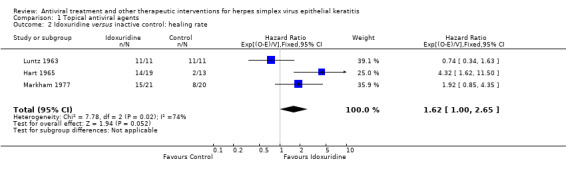
Comparison 1 Topical antiviral agents, Outcome 2 Idoxuridine versus inactive control: healing rate.
1.3. Analysis.
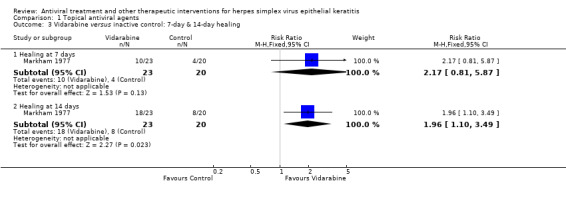
Comparison 1 Topical antiviral agents, Outcome 3 Vidarabine versus inactive control: 7‐day & 14‐day healing.
1.4. Analysis.
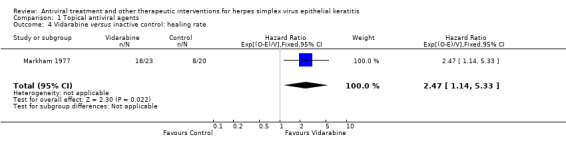
Comparison 1 Topical antiviral agents, Outcome 4 Vidarabine versus inactive control: healing rate.
1.5. Analysis.
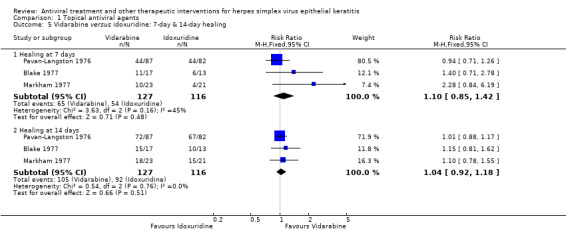
Comparison 1 Topical antiviral agents, Outcome 5 Vidarabine versus idoxuridine: 7‐day & 14‐day healing.
1.6. Analysis.
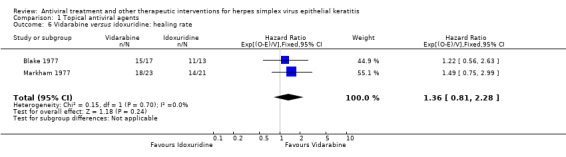
Comparison 1 Topical antiviral agents, Outcome 6 Vidarabine versus idoxuridine: healing rate.
1.7. Analysis.
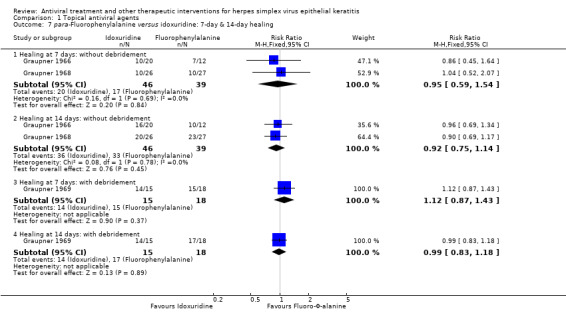
Comparison 1 Topical antiviral agents, Outcome 7 para‐Fluorophenylalanine versus idoxuridine: 7‐day & 14‐day healing.
Trifluridine, acyclovir, brivudine, and ganciclovir
Compared to idoxuridine, trifluridine (Analysis 1.8; Analysis 1.9), acyclovir (Analysis 1.10; Analysis 1.11), and brivudine (Analysis 1.12) were significantly better, although the acyclovir‐idoxuridine comparison had high heterogeneity and possible bias toward publication of trials that showed faster healing with acyclovir. One trial compared acyclovir to iododeoxycytidine, but a significant difference in healing outcome was not detected (Analysis 1.10.3). Network analysis indicated that healing outcomes of trifluridine, acyclovir, or brivudine were significantly better than for idoxuridine (Table 2). Assimilated healing curves also furnished evidence that trifluridine and acyclovir provided faster healing than idoxuridine (Figure 5). Trifluridine (Analysis 1.13; Analysis 1.14) and acyclovir (Analysis 1.15; Analysis 1.16), while not determined to be significantly different from vidarabine in direct comparisons, were more effective than vidarabine in network analyses (Table 2). Neither acyclovir (Analysis 1.17; Analysis 1.18) nor brivudine (Analysis 1.19; Analysis 1.20) was found to be significantly different from trifluridine. Brivudine was not different from acyclovir in one trial (Analysis 1.21) and in network analysis (Table 2). The direct comparison of ganciclovir with acyclovir was limited by heterogeneity among included studies (Analysis 1.22) and a possible publication bias toward studies favouring ganciclovir (Figure 1).
1.8. Analysis.
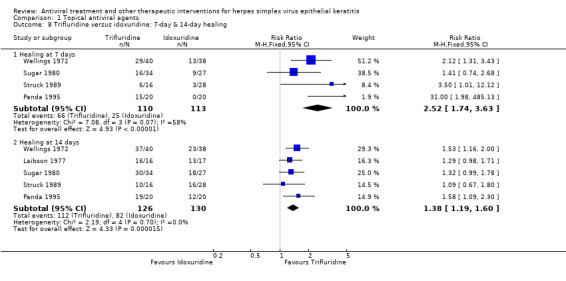
Comparison 1 Topical antiviral agents, Outcome 8 Trifluridine versus idoxuridine: 7‐day & 14‐day healing.
1.9. Analysis.
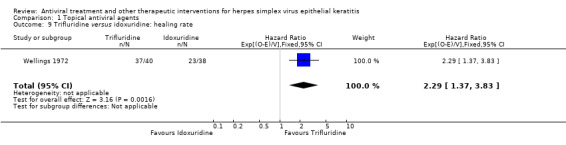
Comparison 1 Topical antiviral agents, Outcome 9 Trifluridine versus idoxuridine: healing rate.
1.11. Analysis.
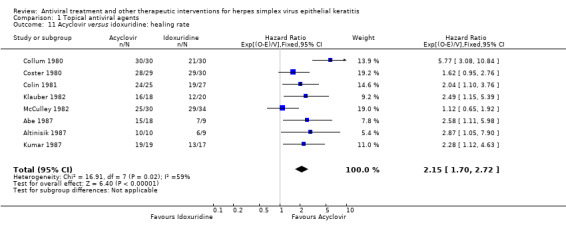
Comparison 1 Topical antiviral agents, Outcome 11 Acyclovir versus idoxuridine: healing rate.
1.12. Analysis.
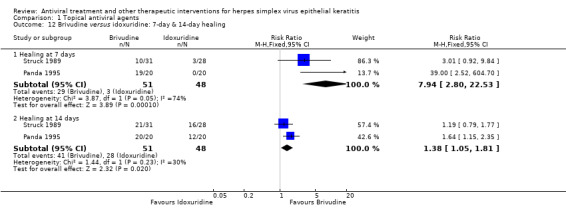
Comparison 1 Topical antiviral agents, Outcome 12 Brivudine versus idoxuridine: 7‐day & 14‐day healing.
5.
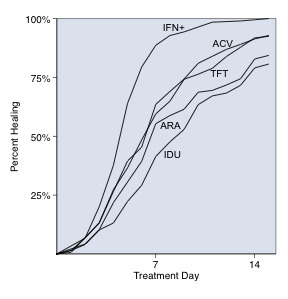
Cumulative healing data from trials evaluating idoxuridine, vidarabine, trifluridine, acyclovir, or interferon plus an antiviral agent. Antiviral healing curves were blended from 24 studies (Abe 1987; Altinisik 1987; Blake 1977; Colin 1981; Collum 1980; Collum 1985; Coster 1976; Coster 1979; Coster 1980; Denis 1983; Hart 1965; Hoang‐Xuan 1984; Høvding 1989; Jackson 1984; Klauber 1982; Kumar 1987; La Lau 1982; Luntz 1963; Markham 1977; McCulley 1982; van Bijsterveld 1980; Wellings 1972; Yeakley 1981; Young 1982) that reported healing data for idoxuridine (278 eyes), vidarabine (262 eyes), trifluridine (279 eyes), or acyclovir (480 eyes). Corneal healing with combined interferon‐antiviral was estimated from seven studies (Carmassi 1993; Colin 1983; de Koning 1982; de Koning 1983; Meurs 1985; Sundmacher 1981b; van Bijsterveld 1989) that evaluated 196 participants who received interferon with trifluridine (75), acyclovir (102), or brivudine (19). (ACV, acyclovir; ARA, vidarabine; IDU, idoxuridine; IFN+, interferon with an antiviral (trifluridine, acyclovir, or brivudine); TFT, trifluridine).
1.14. Analysis.
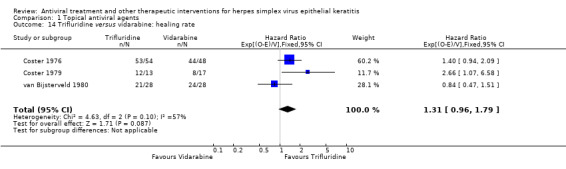
Comparison 1 Topical antiviral agents, Outcome 14 Trifluridine versus vidarabine: healing rate.
1.15. Analysis.
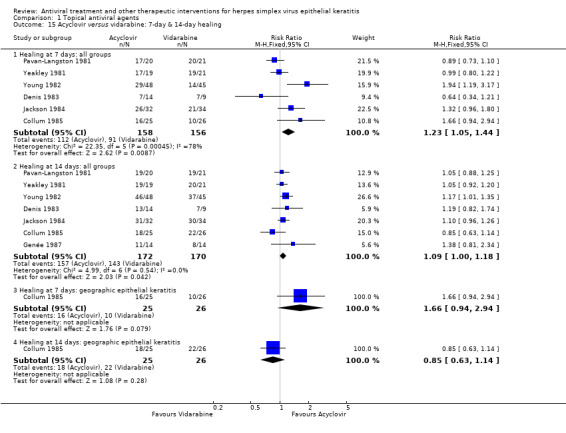
Comparison 1 Topical antiviral agents, Outcome 15 Acyclovir versus vidarabine: 7‐day & 14‐day healing.
1.16. Analysis.
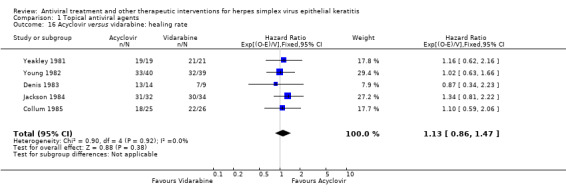
Comparison 1 Topical antiviral agents, Outcome 16 Acyclovir versus vidarabine: healing rate.
1.17. Analysis.
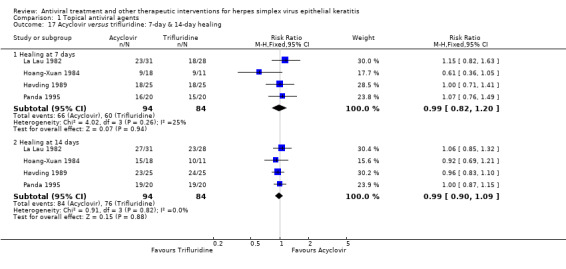
Comparison 1 Topical antiviral agents, Outcome 17 Acyclovir versus trifluridine: 7‐day & 14‐day healing.
1.18. Analysis.
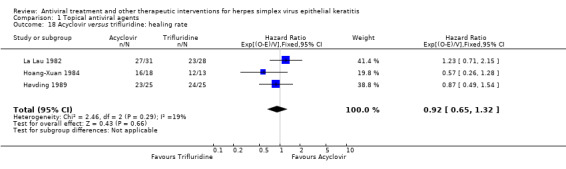
Comparison 1 Topical antiviral agents, Outcome 18 Acyclovir versus trifluridine: healing rate.
1.19. Analysis.
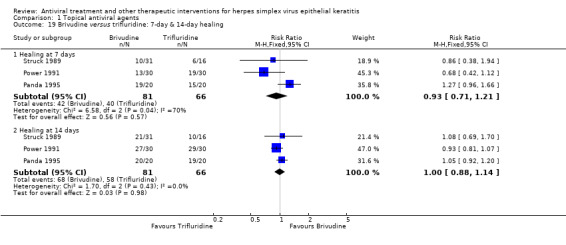
Comparison 1 Topical antiviral agents, Outcome 19 Brivudine versus trifluridine: 7‐day & 14‐day healing.
1.20. Analysis.
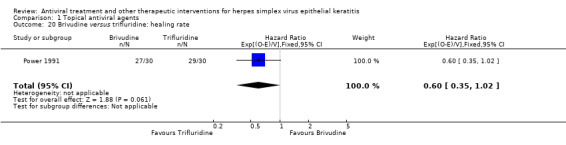
Comparison 1 Topical antiviral agents, Outcome 20 Brivudine versus trifluridine: healing rate.
1.21. Analysis.
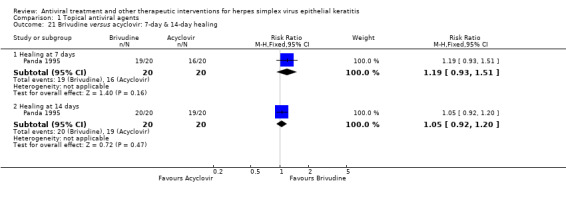
Comparison 1 Topical antiviral agents, Outcome 21 Brivudine versus acyclovir: 7‐day & 14‐day healing.
Foscarnet
Foscarnet was not significantly different from trifluridine in one trial (Analysis 1.23), from acyclovir in one trial (Analysis 1.24), or from ganciclovir in one trial (Analysis 1.25). Network analysis did not reveal statistically significant differences between foscarnet and trifluridine or ganciclovir (Table 2). The indirect comparison of foscarnet and acyclovir involved a heterogeneous group of studies comparing acyclovir and ganciclovir.
1.23. Analysis.
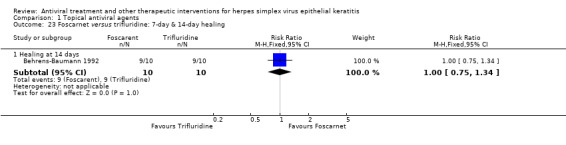
Comparison 1 Topical antiviral agents, Outcome 23 Foscarnet versus trifluridine: 7‐day & 14‐day healing.
1.24. Analysis.
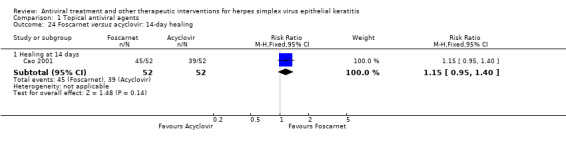
Comparison 1 Topical antiviral agents, Outcome 24 Foscarnet versus acyclovir: 14‐day healing.
1.25. Analysis.
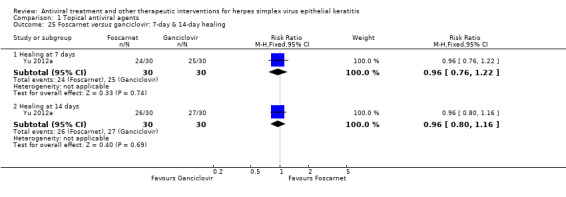
Comparison 1 Topical antiviral agents, Outcome 25 Foscarnet versus ganciclovir: 7‐day & 14‐day healing.
Antiviral combination
Although dual antiviral treatment was more effective at seven days, the combination of topical acyclovir and vidarabine was not significantly different at 14 days than topical acyclovir in one trial (Analysis 1.26).
1.26. Analysis.
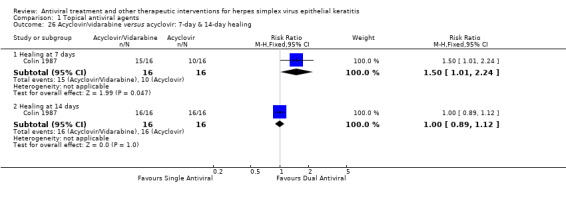
Comparison 1 Topical antiviral agents, Outcome 26 Acyclovir/vidarabine versus acyclovir: 7‐day & 14‐day healing.
Formulation and dosage of topical antivirals
No differences were apparent among few studies that evaluated different vehicles (Analysis 1.27) or concentrations (Analysis 1.28) of topical antiviral agents. No study compared different frequencies of administration of the same topical antiviral agent.
1.27. Analysis.
Comparison 1 Topical antiviral agents, Outcome 27 Trifluridine (aqueous) versus trifluridine (viscous).
1.28. Analysis.
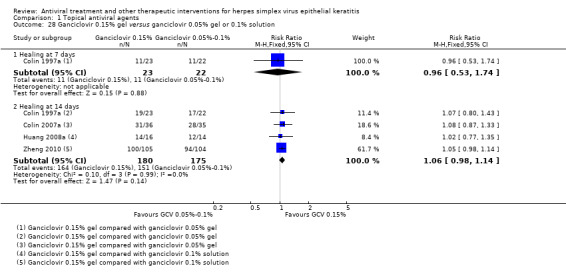
Comparison 1 Topical antiviral agents, Outcome 28 Ganciclovir 0.15% gel versus ganciclovir 0.05% gel or 0.1% solution.
Safety of topical antivirals
Forty‐four studies reported the prevalence of ocular adverse reactions other than stinging or discomfort that was attributable to topical antiviral medications. Treatment‐related effects on the ocular surface that were reported in trials included in this review were allergic blepharoconjunctivitis, toxo‐allergic follicular conjunctivitis, and superficial keratopathy. In these antiviral treatment trials the median percentage of eyes that developed superficial keratopathy or punctate epithelial erosions was 10% with idoxuridine, 11% with vidarabine, 4% with trifluridine, 10% with acyclovir, 0% with brivudine, and 4% with ganciclovir (Table 12).
6. Safety assessment among antiviral treatment studies.
| Topical antiviral agent | No. studies reporting corneal toxicity prevalence | No. antiviral‐treated participants assessed for corneal toxicity | Median prevalence of punctate epithelial erosions during antiviral use (interquartile range) |
| Idoxuridine | 141 | 430 | 10% (5%‐20%) |
| Vidarabine | 122 | 395 | 11% (4%‐17%) |
| Trifluridine | 93 | 220 | 4% (2%‐7%) |
| Acyclovir | 254 | 667 | 10% (0%‐17%) |
| Brivudine | 35 | 72 | 0% |
| Ganciclovir | 46 | 156 | 4% (0%‐11%) |
| All of above | 40 | 1940 | 8% (0%‐17%) |
1 Included idoxuridine studies: Altinisik 1987; Blake 1977; Colin 1981; Collum 1980; Kitano 1985; Klauber 1982; Laibson 1977; MacKenzie 1964; Markham 1977; McCulley 1982; Panda 1995; Pavan‐Langston 1976; Vannini 1986; Wellings 1972
2 Included vidarabine studies: Blake 1977; Collum 1985; Coster 1976; Coster 1979; Denis 1983; Jackson 1984; Markham 1977; Pavan‐Langston 1976; Pavan‐Langston 1981; van Bijsterveld 1980; Yeakley 1981; Young 1982
3 Included trifluridine studies: Behrens‐Baumann 1992; Coster 1976; Coster 1979; Hoang‐Xuan 1984; Panda 1995; Parlato 1985; Power 1991; van Bijsterveld 1980; Wellings 1972 (omitting La Lau 1982 because of imprecise assessment)
4 Included acyclovir studies: Altinisik 1987; Carmassi 1993; Colin 1981; Colin 1983; Colin 1984; Colin 1987; Colin 1997a; Colin 1997b; Colin 2007b; Collum 1980; Collum 1985; Collum 1986; Denis 1983; Hoang‐Xuan 1984; Huang 2008a; Jackson 1984; Jensen 1982; Kitano 1985; Klauber 1982; McCulley 1982; Panda 1995; Pavan‐Langston 1981; Wilhelmus 1981a; Yeakley 1981; Young 1982 (omitting La Lau 1982 because of imprecise assessment)
5 Included brivudine studies: Panda 1995; Power 1991; van Bijsterveld 1989
6 Included ganciclovir studies: Colin 1997a; Colin 1997b; Colin 2007b; Huang 2008a
Oral antiviral therapy
The comparison of oral antiviral to topical antiviral therapy (Analysis 2.1) and the comparison of combined oral and topical antiviral to topical antiviral therapy (Analysis 2.2) were limited by few trials that tended to be heterogeneous.
2.1. Analysis.
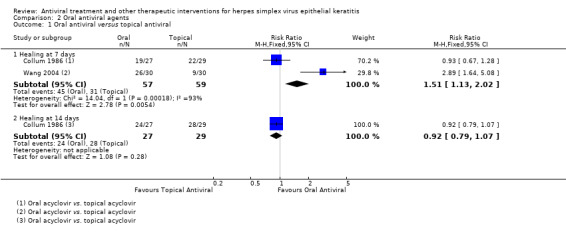
Comparison 2 Oral antiviral agents, Outcome 1 Oral antiviral versus topical antiviral.
Interferon therapy
Interferon monotherapy
Topical interferon therapy was significantly better than inactive control (Analysis 3.1), but few studies were available. A higher interferon concentration (≥ 1 million IU/ml) was more effective than a low concentration (1000 IU/ml) (Analysis 3.2). Interferon‐α and interferon‐β did not differ significantly when used with debridement (Analysis 3.3). Recombinant interferon was not significantly different from naturally‐derived interferon in combination with trifluridine (Analysis 3.4).
3.1. Analysis.
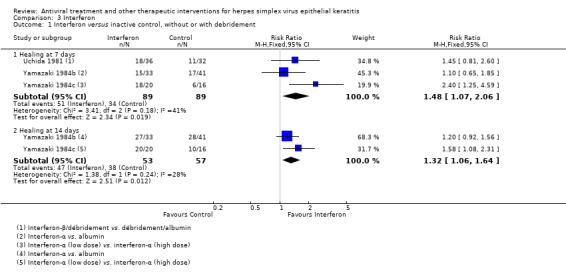
Comparison 3 Interferon, Outcome 1 Interferon versus inactive control, without or with debridement.
3.2. Analysis.
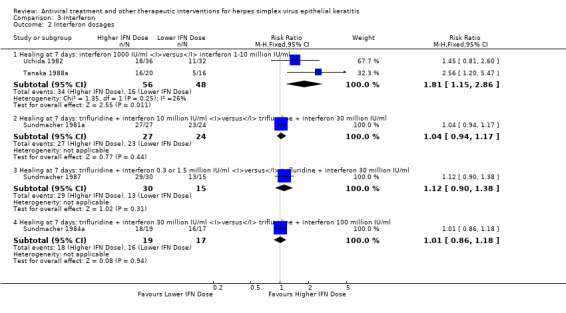
Comparison 3 Interferon, Outcome 2 Interferon dosages.
3.3. Analysis.
Comparison 3 Interferon, Outcome 3 Interferon‐α with debridement versus interferon‐β with debridement.
3.4. Analysis.
Comparison 3 Interferon, Outcome 4 Natural interferon‐α with trifluridine versus recombinant interferon‐α with trifluridine.
Interferon compared to nucleoside antivirals
The comparison of topical interferon to a topical nucleoside antiviral agent (Analysis 3.5) was limited by heterogeneity among included studies. A topical interferon inducer did not differ significantly from a topical antiviral agent in a single study (Analysis 3.6).
3.6. Analysis.
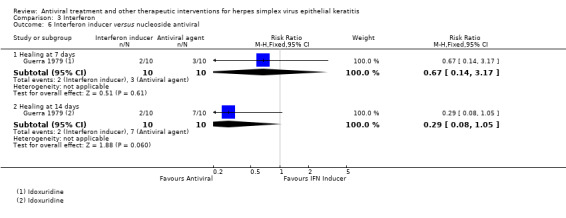
Comparison 3 Interferon, Outcome 6 Interferon inducer versus nucleoside antiviral.
Interferon‐antiviral combination therapy
The combination of interferon and an antiviral agent appeared to be significantly more effective than antiviral monotherapy (Analysis 3.7) and to offer earlier and significantly faster healing (Analysis 3.8), but these comparisons were limited by heterogeneity among studies.
3.8. Analysis.
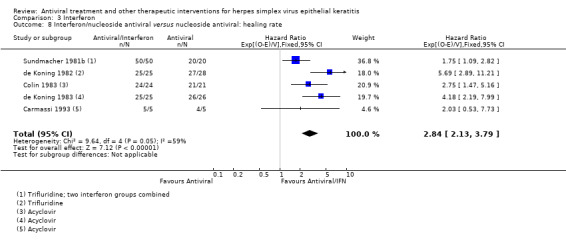
Comparison 3 Interferon, Outcome 8 Interferon/nucleoside antiviral versus nucleoside antiviral: healing rate.
Safety of topical interferons
Adverse effects attributable to topical interferon were rarely mentioned in studies, although between 5% and 25% of treated patients had a mild, transient reaction (Tanaka 1988a; Tanaka 1988b). Adverse events described with the combined use of interferon and an antiviral drug were limited to punctate keratopathy (Colin 1983; de Koning 1983).
Debridement
Methods of corneal debridement
Few studies of limited duration compared physicochemical debridement with control (Analysis 4.1). The comparison of debridement to topical antiviral therapy was limited by heterogeneity among studies (Analysis 4.2). No clinically significant differences were found in the few studies that compared different methods of taking off the corneal epithelium (Analysis 4.3).
4.1. Analysis.
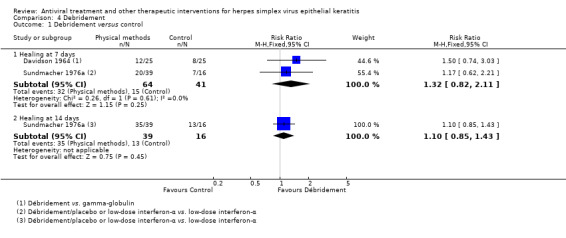
Comparison 4 Debridement, Outcome 1 Debridement versus control.
4.3. Analysis.
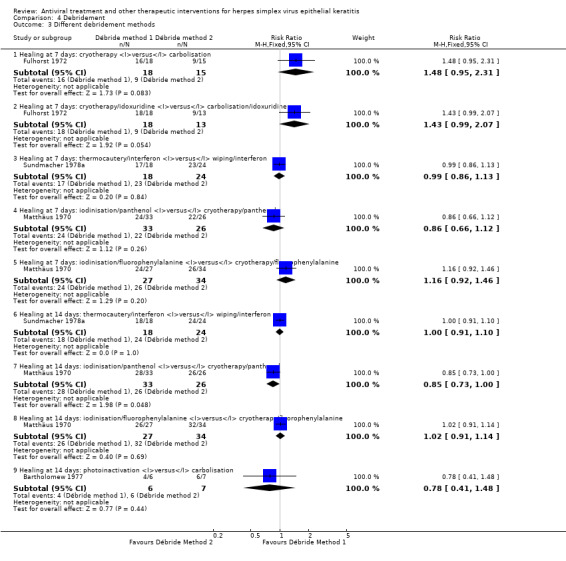
Comparison 4 Debridement, Outcome 3 Different debridement methods.
Debridement and antiviral chemotherapy
The comparison of physicochemical debridement followed by an antiviral agent compared to debridement alone was limited by heterogeneity among studies (Analysis 4.4). One trial comparing debridement and acyclovir to debridement and idoxuridine did not show a significant difference in the proportion of participants healed at seven or 14 days (Analysis 4.5). Heterogeneity was present among studies comparing combined debridement‐antiviral treatment to antiviral therapy (Analysis 4.6). Evidence that combination therapy might allow faster healing was also constrained by heterogeneity among studies (Analysis 4.7).
4.5. Analysis.
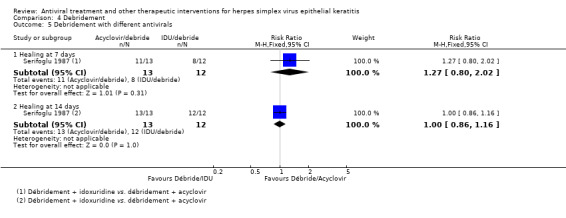
Comparison 4 Debridement, Outcome 5 Debridement with different antivirals.
4.7. Analysis.
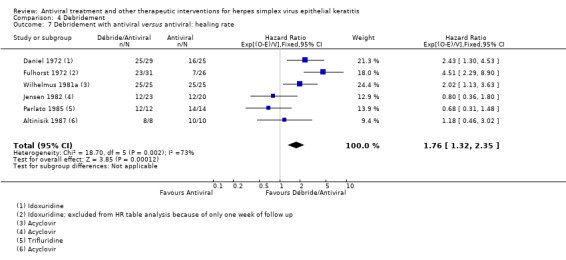
Comparison 4 Debridement, Outcome 7 Debridement with antiviral versus antiviral: healing rate.
Safety of debridement
Most studies that used a method of corneal debridement did not mention side effects. Four studies stated that no adverse reactions occurred (Bartholomew 1977; Jensen 1982; Kato 1979; Parlato 1985). Phototherapy was reported to be painful (Daniel 1972) and was associated with superficial punctate keratopathy and iritis in one study (O'Day 1975). Carbolisation produced superficial punctate keratopathy in 11% of patients in one study (MacKenzie 1964) and one episode of shallow ulceration in another study (Patterson 1967a).
Supplemental agents
Hyaluronate contributed to healing at one week but did not yield a better outcome at two weeks in one trial (Analysis 5.1). The combination of epidermal growth factor with an antiviral did not significantly enhance the antiviral agent's effect on corneal epithelial healing in one trial (Analysis 5.2). Topical panthenol, a precursor to pantothenic acid that might promote epithelial wound healing, was not significantly different from para‐fluorophenylalanine in one trial (Analysis 5.3). Methyluracil, an antioxidant thought to facilitate cell proliferation, did not have an additive effect with interferon in one trial (Analysis 5.4).
5.1. Analysis.
Comparison 5 Adjunctive and alternative agents: lubricant, growth factor, nonsteroidal anti‐inflammatory drug, immunomodulator, Outcome 1 Antiviral/lubricant versus antiviral.
5.2. Analysis.
Comparison 5 Adjunctive and alternative agents: lubricant, growth factor, nonsteroidal anti‐inflammatory drug, immunomodulator, Outcome 2 Antiviral/epidermal growth factor versus antiviral.
5.3. Analysis.
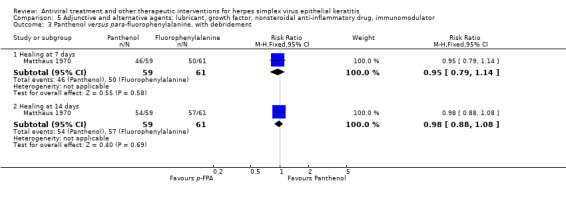
Comparison 5 Adjunctive and alternative agents: lubricant, growth factor, nonsteroidal anti‐inflammatory drug, immunomodulator, Outcome 3 Panthenol versus para‐fluorophenylalanine, with debridement.
5.4. Analysis.
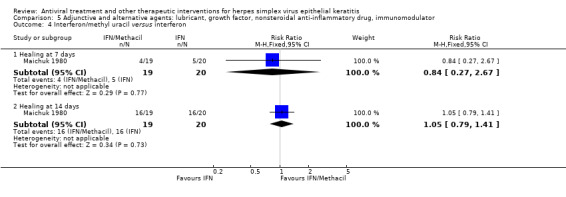
Comparison 5 Adjunctive and alternative agents: lubricant, growth factor, nonsteroidal anti‐inflammatory drug, immunomodulator, Outcome 4 Interferon/methyl uracil versus interferon.
The addition of the nonsteroidal anti‐inflammatory agent oxyphenbutazone to idoxuridine treatment did not differ from treatment with idoxuridine alone in one trial (Analysis 5.5). Inosine pranobex, an immune modulator (Campoli‐Richards 1986) that has been used both topically and orally (Sellitti 1982), had an uncertain effect on healing at one week and, without or with an antiviral agent, did not significantly affect the 14‐day outcome in two included studies (Analysis 5.6). No adverse reactions were reported in this cluster of studies.
5.5. Analysis.
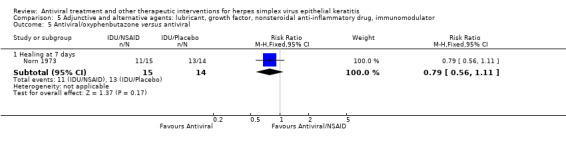
Comparison 5 Adjunctive and alternative agents: lubricant, growth factor, nonsteroidal anti‐inflammatory drug, immunomodulator, Outcome 5 Antiviral/oxyphenbutazone versus antiviral.
5.6. Analysis.
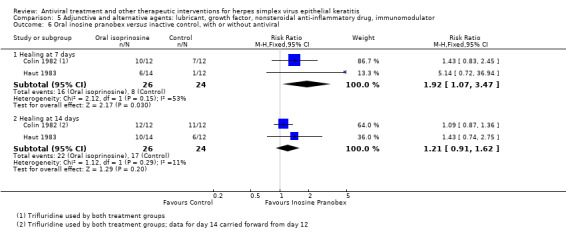
Comparison 5 Adjunctive and alternative agents: lubricant, growth factor, nonsteroidal anti‐inflammatory drug, immunomodulator, Outcome 6 Oral inosine pranobex versus inactive control, with or without antiviral.
Discussion
HSV infection is a prevalent and potentially damaging eye disease that inflicts personal distress and socioeconomic costs. Safe, effective treatment aims to alleviate symptoms, to enable corneal healing, and to expedite visual recovery. This systematic review synthesises an interconnected web of clinical trials comparing therapeutic options for HSV infection of the corneal surface (Figure 3) and presents the direct and indirect evidence on the relative effectiveness of antivirals, interferon, and debridement in the treatment of HSV epithelial keratitis (Table 1).
Summary of main results
Antiviral therapy
Placebo‐controlled studies were inconsistent for idoxuridine and sparse for vidarabine. No significant difference in effectiveness was found between idoxuridine and vidarabine (Pavan‐Langston 1972). Another out‐of‐use compound, para‐fluorophenylalanine, did not significantly differ from idoxuridine (Pietruschka 1968). Trifluridine and acyclovir were more effective than either idoxuridine or vidarabine. Excluded studies also suggested that trifluridine was better than idoxuridine (Assetto 1981; Pavan‐Langston 1977) and that acyclovir was better than idoxuridine (Babushkin 1993; Inocencio 1982) or vidarabine (McGill 1981; Mohan 1987).
Analysis of a network of antiviral treatment comparisons indicated that trifluridine, acyclovir, and brivudine had no significant differences in treatment (Table 1), a finding supported by one‐week results (Table 4) and by their relative rates of healing (Table 5). Based on two trials restricted to geographic epithelial keratitis (Collum 1985; Coster 1979), the two‐week healing outcome was better with trifluridine compared to vidarabine but was not significantly different between acyclovir and vidarabine.
Ganciclovir was possibly as or more effective as acyclovir in included studies and in excluded studies (Huang 2007; Jing 2010; Wang 2009), but interpretation was limited by heterogeneity among the studies. Foscarnet seemed as effective as trifluridine, acyclovir, and ganciclovir but needs further study. The effect of two topical antivirals was examined in only one study. Oral acyclovir may be an alternative to topical antiviral therapy, but few studies examined an oral antiviral or combined oral and topical antivirals in comparison to topical antiviral monotherapy (Table 6). The dosage, formulation, and frequency of administration of antiviral therapy were evaluated in few studies.
Interferon therapy
Interferon at a concentration of 1 million IU/ml or higher was more effective than control in included studies and an excluded study (Shiota 1988). Used either alone or in combination with debridement (Cantell 1995; Sundmacher 1982; Sundmacher 1984b) interferon was as effective as a nucleoside antiviral agent. Excluded studies (Jin 1992; Scialdone 1986; Tamburi 1990) had insufficient outcome data to adequately determine relative effectiveness between interferon and nucleoside antivirals. A topical interferon inducer did not compare favourably with a topical antiviral agent in a single included study (Analysis 3.6) but had encouraging clinical activity in excluded studies (Galin 1976; Kasparov 1972; Kasparov 1974; Kasparov 1991; Lin 2009). Interferon‐α and interferon‐β did not differ in included studies or among excluded studies (Kuyama 1979; Zhang 2003).
The combination of interferon and a nucleoside antiviral agent offers the prospect of faster healing than antiviral therapy alone (Table 7). The possible advantage of combining interferon and an antiviral agent was supported by observational experience (Figure 5) and by several excluded studies (Chen 2007; Gu 2005; Huang 2009; Lin 2013b; Liu 2003; Tamburi 1990; Wan 2014; Weng 2014; Zhao 2001; Zhou 2008), but evidence is limited by heterogeneity among included studies.
Debridement
Trials of physicochemical debridement were relatively few and inconsistent. The comparison of debridement to topical antiviral therapy was limited by heterogeneity among included studies and by different findings among excluded studies (Patterson 1967c; Tarakji 1978; Whitcher 1976). Various methods of curettage, cauterisation, and chemoablation were used to remove the corneal epithelium and likely produced variously sized corneal epithelial defects. Thermomechanical scraping, cryotherapy, and chemical abrasion could leave a large denuded area of the corneal surface while wiping and swabbing aim to selectively remove loosened, infected epithelial cells along the dendrite (Wilhelmus 1989). Potential shortcomings of debridement included damage to Bowman's layer (Coster 1977b) and exacerbation of corneal inflammation and opacification (Parlato 1985).
Epithelial keratitis occurred soon after debridement in some debridement‐treated eyes. Presumably, post‐debridement viral shedding (Sundmacher 1976a; Sundmacher 1976b) or infection of remaining cells of the ocular surface can lead to recrudescent epithelial keratitis (Coster 1977b). While a relapsing episode could be treated by repeating debridement, recrudescent keratitis is preventable by using an antiviral agent for several days after debridement (Coster 1977b). However, any healing advantage that might be gained by following debridement with an antiviral agent or interferon was uncertain because of study heterogeneity (Table 8). Excluded studies were also difficult to interpret: some inferred that the combination of debridement and an antiviral agent healed more quickly than antiviral treatment alone (Koev 2007; Mathur 1984) while others found little difference in average healing times (Fellinger 1980; Guo 2003; Shimomura 1987).
Ancillary agents
Trials that evaluated the addition of a lubricant, growth factors, or a nonsteroidal anti‐inflammatory drug to topical antiviral therapy were few in number and did not provide convincing evidence of effectiveness. The role of an immunomodulator such as inosine pranobex was uncertain in included studies and in an excluded study (Prost 1986). Studies did not examine justifications for using cycloplegic or antibacterial eye drops. Secondary microbial infection during herpetic epithelial keratitis (Boisjoly 1983; Wilhelmus 1982) was not reported in any included study.
Overall completeness and applicability of evidence
This systematic review aimed to synthesise the relative effectiveness among available interventions that can be used in the treatment of HSV epithelial keratitis. The literature was systematically searched for all relevant comparative clinical trials using electronic databases, handsearching, and personal contact.
Participant characteristics and potential effect modifiers
Age, gender and ethnicity
The incidence of HSV keratitis rises with advancing age (Labetoulle 2005; Young 2010). Participants in studies included in this systematic review averaged 43 years of age, but few studies described the shape of the age distribution. Males outnumbered females in 91% of studies, and studies enrolled, on average, 50% more men than women. While some cohort investigations have suggested that men might be more likely to be diagnosed and treated for HSV epithelial keratitis (Gold 1965; Gundersen 1936; HEDS Group 1997; Liesegang 1989a; Wilhelmus 1981b), population‐based studies show that men and women have similar rates of ocular herpes (Labetoulle 2005; Young 2010; Stanzel 2014). Selective enrolment according to age or gender could reflect under‐representation of children, the elderly, and women in clinical drug trials (Van Spall 2007). Women of childbearing potential have often been systematically deemed ineligible for topical ophthalmic antiviral trials because of an unproven concern about antiviral teratogenicity during pregnancy (Ahrens 2013; Chung 2004; Itoi 1975; Pasternak 2010). Among studies that examined the effect of patient characteristics on outcome, neither age nor gender influenced the rate of corneal healing during antiviral therapy for HSV epithelial keratitis (de Koning 1982; de Koning 1983; Jackson 1984; McCulley 1982; van Bijsterveld 1980; van Bijsterveld 1989). Observational studies have suggested that some ethnic groups may be susceptible to recurrent HSV epithelial keratitis (McDonald 2015; Wilhelmus 1996a), but ethnicity was not significantly associated with recurrence in a cohort followed for 18 months (HEDS Group 2001). Insufficient information was available to examine age, gender, or ethnicity as possible effect modifiers in this review.
Immune status
Medical conditions may modulate viral eradication and epithelial regeneration during HSV infection. Poorly controlled diabetes (Kaiserman 2005), atopy (Rezende 2006), and immunosuppression (Field 1995; Oshry 1998) might slow resolution of HSV epithelial keratitis. Health status was not examined as a possible effect modifier in studies included in this review.
Previous herpetic eye disease
Neither the number of previous episodes nor the duration of symptoms prior to initiating antiviral treatment affected healing rates in most studies that evaluated these factors (Blake 1977; Høvding 1989; Jensen 1982; La Lau 1982; McCulley 1982; Pavan‐Langston 1981; Sugar 1980; van Bijsterveld 1989; Yeakley 1981). Neither the prior use of antiviral agents (Jensen 1982; McCulley 1982) nor the recent use of corticosteroids (McCulley 1982; Pavan‐Langston 1981; Sugar 1980; Yeakley 1981) affected relative treatment response.
Duration between onset and treatment
One study found that the interval between the start of symptoms and the beginning of antiviral treatment correlated with healing time: patients who received earlier treatment healed faster (van Bijsterveld 1980).
Pattern of epithelial keratitis
Approximately 90% of eyes enrolled in studies included in this systematic review had dendritic epithelial keratitis. Geographic epithelial keratitis was much less prevalent. In epidemiological studies geographic epithelial keratitis accounts for 5% to 15% of HSV epithelial keratitis (Labetoulle 2005; Liesegang 1989a; Pramod 1999), although a slightly higher frequency was evident in some studies enrolling both dendritic and geographic epithelial keratitis.
Corneal epithelial wound healing follows a distribution function (Callaghan 2006) similar to the logistic healing curve of HSV epithelial keratitis (Wilhelmus 2000). The rate of healing depends on the area of the corneal epithelial defect (Chung 1998). Larger HSV epithelial keratitis re‐epithelialises at a greater rate than smaller lesions (Jackson 1984; Sugar 1980; Wellings 1972). Even so, geographic epithelial keratitis resolves more slowly than dendritic epithelial keratitis (Blake 1977; Coster 1976; Jackson 1984) and, on average, takes longer to heal (Altinisik 1987; Blake 1977; Jackson 1984; Wellings 1972; Wilhelmus 1981a; Young 1982). Investigators have stated that eyes with geographic epithelial keratitis "constitute the most difficult therapeutic challenge for antiviral drugs" (Coster 1979), "respond least well to conventional treatment" (Bartholomew 1977), and "frequently require more prolonged therapy than simple dendritic ulcers" (Collum 1985). On the other hand, one study found that the relative effect between antivirals was not necessarily affected by lesion size (Colin 2007b). It is unclear whether geographic epithelial keratitis offers a more stringent test of antiviral effectiveness.
Corneal stromal inflammation
Corneal characteristics of HSV epithelial keratitis that may be associated with slow healing include a peripheral corneal location (Wilhelmus 1981a) and the presence of stromal inflammation (Altinisik 1987; Daniel 1972; Klauber 1982; Wilhelmus 1981a). While inflammatory cells may slow corneal epithelial wound healing (Wagoner 1984), studies have not demonstrated that stromal keratitis impacts the rate of re‐epithelialisation (Høvding 1989; Jackson 1984). Insufficient data were available to undertake metaregression of these and other potential effect modifiers.
Virology and antiviral susceptibility
Viral infection
Laboratory methods can detect herpes simplex virus in specimens from the ocular surface (Satpathy 2011). One quarter of studies included in this review undertook viral isolation, including five studies that required viral confirmation before randomisation. The overall sensitivity of HSV recovery from the ocular surface was 68% at trial entry. One study reported that viral culture positivity did not affect healing (McCulley 1982). Antiviral therapy usually eliminates HSV from the ocular surface within the first few days of therapy (Sundmacher 1984a; Sundmacher 1985), although HSV could sometimes be recovered up to ten days after beginning antiviral therapy (Colin 1997a). HSV strains may differ in pathogenicity (Brandt 2005), but how virulence genes might impact the healing rate of herpetic epithelial keratitis remains to be determined.
Antiviral resistance
Genetic mutations of HSV affecting thymidine kinase or DNA polymerase can impart resistance to antiviral agents (Duan 2009). HSV strains that are not readily susceptible to acyclovir can emerge among immunocompromised persons and during antiviral treatment of otherwise healthy individuals (Burrel 2013; van Velzen 2013; Zhang 2007). Mutant HSV strains may recur more frequently and heal more slowly (Pan 2014) but usually respond to an alternative antiviral (Hlinomazová 2012; Turner 2013; Yao 1996), a combination of nucleoside antivirals, or co‐administration of an antiviral with interferon (Minkovitz 1995). Susceptibility testing could be considered for treatment‐unresponsive infection due to HSV (Choong 2010).
In this review, the 14‐day outcome with topical acyclovir declined during a 25‐year period while the response rate with topical ganciclovir remained stable over 15 years. Although the emergence of acyclovir resistance is a potential reason for this observed longitudinal trend, studies did not do susceptibility testing. Dissimilarities in defining cure in diverse studies might be a more likely explanation for this ebb in reported responses to topical acyclovir.
Quality of the evidence
This systematic review endeavoured to encapsulate a pragmatic and quantitative digest of clinical research. Many trials in this review were inconclusive because of inadequate sample size. In addition, trials concluding a similarity between treatments were not designed to demonstrate equivalence (Musch 2006). Systematic pooling of studies and network analysis endeavoured to improve precision through an objective appraisal of clinical evidence.
Data were extracted from journal articles and other sources to estimate relative effect measures of corneal healing for different interventions. Healing curves (Graupner 1968; Wellings 1972) and survival analysis (Coster 1976; Coster 1979) are recommended for reporting treatment trials of HSV epithelial keratitis, but these analytical methods were unevenly applied. Nearly a third of otherwise eligible studies were not included in this review because of incompletely reported data. The estimation of cumulative risk and the use of hazard functions that consider variable follow‐up durations need to be more widely implemented in clinical trials of ophthalmic interventions (Hosmer 2009; Jewell 2009).
Publication of studies included in this review peaked during the 1980s (Figure 6), an era of antiviral discovery and investigation (Gordon 2000). After a brief lull, the number of published studies began to rise again after 2005 with the advent of antiviral trials undertaken in China. In comparison, the number of excluded studies that had remained fairly stable throughout the late 20th century also increased during the early 21st century, largely driven by studies of traditional Chinese medicines. The inclusion of studies that lack adherence to proper study design, random allocation and analysis would dampen the quality of the cumulative evidence (Panagiotou 2013; Wu 2009).
6.
Histograms of publication year for included studies and excluded studies. Most of the studies during the late 20th century took place in Europe or North America. An upswing in studies reported since 2005 is due to trials conducted in China.
Potential biases in the review process
Internal and external validity
Attention to detail in study design and reporting
Methodological rigour varied among studies. Nearly a quarter of studies did not clarify the method of random sequence generation, and authenticating randomisation was problematic for brief reports (Wu 2009). Why the number of study eyes sometimes exceeded the number of participants was unclear; presumably, persons with bilateral keratitis or participants experiencing a recurrence were re‐enrolled into some studies. Half of the included studies lacked masking of both patients and trialists. Withdrawals were infrequent during the initial two weeks of observation and therapy, even among studies that had a risk of attrition bias. For studies did not specify censoring times, extrapolation of data from healing curves could have misinterpreted the number of healed eyes. Sensitivity analyses that omitted studies with a higher risk of bias left few trials for each treatment comparison.
Issues of consistency
Heterogeneity of trial results limited the interpretation of some treatment comparisons. Some trials of the ganciclovir trials enrolled patients with disciform or stromal keratitis and did not stratify outcome by type of corneal disease. Diverse treatment dosages and formulations of antivirals and interferons and varied physical and chemical methods of removing corneal epithelium tempered the comparability of studies. Pooling different antiviral agents in the interferon‐antiviral and debridement‐antiviral comparisons did not take into consideration potential differences in antiviral effectiveness. The use of a cycloplegic or antibiotic could have affected therapeutic effects since atropine and neomycin are capable of inhibiting HSV (Alarcón 1984; Langeland 1987).
The asymmetric geometry of the network of treatment comparisons was a web of open and closed loops. To contend with multiple treatment comparisons this systematic review integrated direct head‐to‐head comparisons of antivirals with indirect comparisons. As noted in the Cochrane Handbook, "indirect comparisons are not randomized comparisons, and cannot be interpreted as such" (Higgins 2011). Blending direct and indirect risk ratios offers increased precision but is liable to confounding and bias (Mills 2012). The validity of network analysis depends on homogeneity among studies and consistency between direct and indirect results.
Possible shortcomings in selecting and reviewing studies
Eighty‐six of 143 excluded studies were not appropriate for this review due to use of an alternative intervention or problematic design. The remaining 57 studies were excluded because data on cumulative healing proportions at one or two weeks of study treatment were not reported. The exclusion of adequately designed studies because relevant outcome information was not available in the published report could dampen the robustness of the review (Felton 1992).
Safety
Topically applied antivirals and other medications that delay epithelial regeneration (Lass 1984) could impact the assessment of relative treatment effects. Thus, while safety was an auxiliary concern, the evaluation of effectiveness could be affected by side effects. Less than half of the studies included in this review reported the incidence of drug‐related ocular allergy or toxicity. The relative safety of antivirals, interferon, and other interventions summarised in this review is likely to be inexact. Ophthalmic studies and case reports have described adverse reactions at the ocular surface due to antivirals (Chen 1989; Falcon 1981; Naito 1987) and interferon (Aldave 2007) that were more serious than those detected in treatment trials. Postmarketing surveillance and pharmacovigilance are needed to define the prevalence of common adverse reactions and to detect uncommon complications such as lacrimal punctal stenosis, conjunctival cicatrisation, corneal dysplasia, and anterior segment ischæmia.
Agreements and disagreements with other studies or reviews
Medical therapy
Literature reviews have deduced that currently available topical ophthalmic antiviral agents are effective for HSV epithelial keratitis (AAO 2013; Barker 2008; Behrens‐Baumann 2010; Hergeldzhieva 2012; Labetoulle 2012; Wei 2008). A systematic review of 28 randomised, double‐masked, controlled, English‐language clinical trials (including two trials that were subsets of larger trials) found that either trifluridine or acyclovir is effective for the treatment of HSV epithelial keratitis and that the combination of an antiviral with interferon might offer additional benefit (Guess 2007). While literature reviews have considered ganciclovir gel to be similar in efficacy to acyclovir ointment (Kaufman 2012; Sahin 2012), a systematic review of 14 randomized trials that compared topical ophthalmic acyclovir and ganciclovir in the treatment of HSV epithelial keratitis concluded that despite heterogeneity (I2=55%) ganciclovir improves the cure rate (RR, 1.22; 95% CI, 1.10, 1.36), has a lower risk of adverse reactions (RR, 0.12; 95% CI, 0.03 to 0.46), and reduces the risk of recurrence during the ensuing year (0.22; 92% CI, 0.11‐0.45) (Li 2014d). An evidence‐based guideline from the American Academy of Ophthalmology and Ocualr Microbiology and Immunology Group concluded that topical trifluridine, acyclovir, and ganciclovir are effective, that debridement is an alternative treatment, that combining debridement with a topical antiviral agent confers "limited or no benefit," and that oral antiviral agents such as acyclovir, valacyclovir, and famciclovir "appear to be as effective as topical antiviral agents" (White 2014). The therapeutic effect of oral acyclovir for treating herpetic epithelial keratitis is consistent with its efficacy for treating labial or genital herpes (Cernik 2008). and an oral antiviral might be preferred to topical antivirals when treating herpetic keratitis in children (Revere 2013).
Complementary and alternative medicine
This systematic review excluded clinical studies of ethnomedicinal products and alternative practices. A review of traditional herbal medicines in the treatment of herpetic keratitis found variable study quality and inconsistent results among 29 comparative trials of botanical and herbal preparations (Ma 2006b). Rather than reporting the proportion of eyes healed at each follow‐up visit, the outcome was often based on clinical scoring of symptoms and signs, measurements of the length of residual dendrites, or visual acuity. Systematic evaluation is needed to assess how herbal and botanical products and other alternative or complementary interventions such as acupuncture compare to or might integrate with antiviral chemotherapy.
Prevention
This review did not systematically evaluate whether interventions for treating HSV epithelial keratitis influenced subsequent inflammatory episodes or future recurrences. Limited evidence exists that short‐term antiviral therapy for dendritic epithelial keratitis can forestall consequent HSV stromal keratitis (Maudgal 1979; Wilhelmus 1981b). The evidence that a topical antiviral might also affect future episodes of epithelial keratitis is conflicting. Several studies included in this systematic review found no differential effect between interventions on later recurrences (HEDS Group 1997; McGill 1981; Patterson 1963a; Power 1991; Wilhelmus 1981a; Yamagami 1998). On the other hand, several included studies comparing ganciclovir and acyclovir observed that topical ganciclovir reduced the risk of ocular recurrence during the year after completing treatment (Han 2010; Lin 2011; Lin 2014; Liu 2012a; Liu 2012b; Liu 2014a; Wang 2014a; Yang 2008; Zhen 2012). Whether a short‐term intervention for HSV epithelial keratitis affects future recurrences deserves to be critically evaluated.
The prevention of recurrent ocular herpes is feasible through chronic suppressive antiviral prophylaxis (Jones 1977) and by drugs that inhibit viral reactivation (Kaufman 2002; Luzi 1983). Prolonged use of a topical antiviral (Wilhelmus 1983; Romano 1988) or oral antiviral (HEDS Group 2000b; Uchoa 2003; Wu 2002; Young 2010) can deter recurrent herpetic eye disease. This issue is examined in another systematic review (de la Parra 2013). Though costly (Lairson 2003), prolonged antiviral use may be helpful for previously infected people who have an increased risk of recurrence, such as with increased sunlight exposure (Ludema 2014b), topical corticosteroid use (Wilhelmus 1996a), or ocular surgery (Bhatt 2009). A safe and effective vaccine is awaited (Chentoufi 2012).
Authors' conclusions
Implications for practice.
Ophthalmic formulations of trifluridine, acyclovir, ganciclovir, brivudine, and foscarnet are effective in the treatment of HSV epithelial keratitis. The combination of an antiviral with interferon might be considered for clinically recalcitrant dendritic keratitis. Wiping debridement may be an option to avoid chemotherapeutic drugs, but the relative value of debridement accompanied by an antiviral agent is unclear.
Implications for research.
The design and reporting of future clinical trials of HSV epithelial keratitis should follow guidelines for Enhancing the Quality and Transparency of Health Research (EQUATOR), including the Consolidated Standards of Reporting Trials (CONSORT). Studies should specify the eligibility and healing criteria, methods of randomisation and allocation concealment, means of masking outcome evaluation, and rationale for the numbers of enrolled eyes and participants. Since dendritic epithelial keratitis heals relatively rapidly with currently available treatment, trialists may consider estimating sample size based on a non‐inferiority comparison. The time to corneal re‐epithelialisation is a key measure of therapeutic efficacy and days by which healing or censoring occur during the initial weeks of therapy should be given in graphs or tables. The effects of characteristics that might modify healing would be a useful supplementary analysis. Virological evaluation will have increasing relevance if optimal therapy is affected by the emergence of viral strains with enhanced resistance or virulence. Adverse events should be recorded, and a comprehensive evaluation of the safety of antivirals and other interventions should extend to data sources beyond clinical trials. Additional research is needed to clarify the roles of topical ganciclovir, topical foscarnet, oral antiviral treatment, concurrent use of an antiviral with interferon, and corneal epithelial debridement. However, future clinical trials should avoid unnecessarily randomising patients without contributing new information or addressing reasons for heterogeneity among previous studies. The potential value of integrative and alternative therapy with herbal medicines remains to be systematically reviewed.
What's new
History
Protocol first published: Issue 1, 2001 Review first published: Issue 1, 2001
| Date | Event | Description |
|---|---|---|
| 24 May 2011 | Amended | Issue 7, 2011: The review was edited. While new searches were not done for this interim update, two citations (Croxtall 2011; Guess 2010) were added to the 'Background' section. Satpathy 2010 citation updated to Satpathy 2011. |
| 9 November 2010 | New search has been performed | Issue 12, 2010: This review was substantively updated with the following changes:
|
| 6 November 2008 | Feedback has been incorporated | Converted to new review format. |
| 29 October 2007 | New citation required and conclusions have changed | Issue 1, 2008: two new studies (Colin 2007a and Colin 2007b) added. |
| 29 August 2006 | New citation required and minor changes | Issue 1, 2007: one new study (Kitano 1985) added. |
Notes
Issue 12, 2010: the original title of 'Therapeutic interventions for herpes simplex virus epithelial keratitis' was changed.
Acknowledgements
The United States Cochrane Center and the Cochrane Eyes and Vision Group editorial teams developed and executed electronic searches and provided translations. Peer reviewers of previous and current version of this review included Rainer Sundmacher, John Dart, Jayne Tierney, Christopher J Rapuano, David B Glasser, Prashant Garg, and Barbara Hawkins. An initial version of this work was published by the American Ophthalmological Society (Wilhelmus 2000).
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Keratitis, Herpetic #2 MeSH descriptor Keratitis, Dendritic #3 (herpe* or simplex) near (cornea* or kerati* or dendr* or ocular) #4 (#1 OR #2 OR #3)
Appendix 2. PubMed search strategy
((randomized controlled trial [pt]) OR (controlled clinical trial [pt]) OR (clinical trial [pt]) OR (random allocation [mh]) OR (randomized controlled trials [mh])) AND ((herpes OR herpetic OR simplex) AND (cornea OR corneal OR keratitis OR ocular))
Appendix 3. EMBASE.com search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'herpes simplex keratitis'/exp #34 ((herpe* OR simplex) NEAR/4 (cornea* OR kerati* OR dendr* OR ocular)):ab,ti #35 #33 OR #34 #36 #32 AND #35
Appendix 4. LILACS search strategy
English Language Search Terms: (herpe$ or simplex) and (cornea$ or kerati$ or dendr$ or ocular)
Spanish Language Search Terms: (herpes simplex) and (dendrítica or queratitis)
Portuguese Language Search Terms (herpes simples) and (dendr$ or ceratite)
Appendix 5. OpenGrey search strategy
(herpe* OR simplex) AND (cornea* OR kerati* OR dendr* OR ocular)
Appendix 6. BIOSIS search strategy
TS=(herpe* OR simplex) AND TS=(cornea* OR kerati* OR dendr* OR ocular) AND TS=(random* OR trial*)
Appendix 7. Scopus search strategy
Article Title, Abstract, Keywords=(herpe* OR simplex) AND (keratitis OR cornea*) AND (trial* OR RCT* OR random* OR blind*)
Appendix 8. J‐Global search strategy
English Language Search Terms: (herpes OR simplex OR dendritic) AND (keratitis OR cornea)
Japanese Language Individual Search Terms: (ヘルペス OR 単純ヘルペス OR 樹枝状の OR 樹木状の) AND (角膜炎 OR 角膜)
Japanese Language Combined Search Terms: ヘルペス性角膜炎 OR 角膜ヘルペス OR 純ヘルペス性角膜炎 OR 樹枝状角膜炎
Appendix 9. CNKI search strategy
English Language Search Term (subject, precise): herpes simplex keratitis
Chinese Language Search Term (subject, precise): 单纯疱疹性角膜炎
Appendix 10. ZETOC search strategy
herpe* simplex kerati* random*
herpe* simplex kerati* trial*
Appendix 11. metaRegister of Controlled Trials search strategy
herpe* simplex AND keratitis
Appendix 12. ClinicalTrials.gov search strategy
Condition: (herpes OR simplex) AND (corneal OR keratitis OR dendritic OR ocular)
Appendix 13. ICTRP search strategy
keratitis
Appendix 14. ChiCTR search strategy
Target disease=eye or cornea or keratitis
Appendix 15. U.S. Food and Drug Administration search strategy
"herpes simplex keratitis" and "dendritic keratitis"
Appendix 16. National Institute for Health and Clinical Excellence search strategy
"herpes simplex keratitis" and "dendritic keratitis"
Appendix 17. European Medicines Agency search strategy
"herpes simplex keratitis" and "dendritic keratitis"
Data and analyses
Comparison 1. Topical antiviral agents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Idoxuridine versus inactive control: 7‐day & 14‐day healing | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Healing at 7 days | 10 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.24, 3.51] |
| 1.2 Healing at 14 days | 2 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.45, 3.84] |
| 2 Idoxuridine versus inactive control: healing rate | 3 | 95 | Hazard Ratio (95% CI) | 1.62 [1.00, 2.65] |
| 3 Vidarabine versus inactive control: 7‐day & 14‐day healing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Healing at 7 days | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.81, 5.87] |
| 3.2 Healing at 14 days | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.10, 3.49] |
| 4 Vidarabine versus inactive control: healing rate | 1 | 43 | Hazard Ratio (95% CI) | 2.47 [1.14, 5.33] |
| 5 Vidarabine versus idoxuridine: 7‐day & 14‐day healing | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Healing at 7 days | 3 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.85, 1.42] |
| 5.2 Healing at 14 days | 3 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.92, 1.18] |
| 6 Vidarabine versus idoxuridine: healing rate | 2 | 74 | Hazard Ratio (95% CI) | 1.36 [0.81, 2.28] |
| 7 para‐Fluorophenylalanine versus idoxuridine: 7‐day & 14‐day healing | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Healing at 7 days: without debridement | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.59, 1.54] |
| 7.2 Healing at 14 days: without debridement | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.75, 1.14] |
| 7.3 Healing at 7 days: with debridement | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.87, 1.43] |
| 7.4 Healing at 14 days: with debridement | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.83, 1.18] |
| 8 Trifluridine versus idoxuridine: 7‐day & 14‐day healing | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Healing at 7 days | 4 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [1.74, 3.63] |
| 8.2 Healing at 14 days | 5 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.19, 1.60] |
| 9 Trifluridine versus idoxuridine: healing rate | 1 | 78 | Hazard Ratio (95% CI) | 2.29 [1.37, 3.83] |
| 10 Acyclovir versus idoxuridine: 7‐day & 14‐day healing | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Healing at 7 days: acyclovir versus idoxuridine | 9 | 468 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.35, 2.90] |
| 10.2 Healing at 14 days: acyclovir versus idoxuridine | 11 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.08, 1.38] |
| 10.3 Healing at 7 days: acyclovir versus iododeoxycytidine | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.76, 2.63] |
| 10.4 Healing at 14 days: acyclovir versus iododeoxycytidine | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.89, 1.12] |
| 11 Acyclovir versus idoxuridine: healing rate | 8 | 355 | Hazard Ratio (95% CI) | 2.15 [1.70, 2.72] |
| 12 Brivudine versus idoxuridine: 7‐day & 14‐day healing | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Healing at 7 days | 2 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.94 [2.80, 22.53] |
| 12.2 Healing at 14 days | 2 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.05, 1.81] |
| 13 Trifluridine versus vidarabine: 7‐day & 14‐day healing | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Healing at 7 days: all groups | 4 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.94, 1.23] |
| 13.2 Healing at 14 days: all groups | 3 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.84, 1.49] |
| 13.3 Healing at 7 days: geographic epithelial keratitis | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.55, 3.13] |
| 13.4 Healing at 14 days: geographic epithelial keratitis | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [1.16, 3.33] |
| 14 Trifluridine versus vidarabine: healing rate | 3 | 188 | Hazard Ratio (95% CI) | 1.31 [0.96, 1.79] |
| 15 Acyclovir versus vidarabine: 7‐day & 14‐day healing | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Healing at 7 days: all groups | 6 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.05, 1.44] |
| 15.2 Healing at 14 days: all groups | 7 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.00, 1.18] |
| 15.3 Healing at 7 days: geographic epithelial keratitis | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.94, 2.94] |
| 15.4 Healing at 14 days: geographic epithelial keratitis | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.63, 1.14] |
| 16 Acyclovir versus vidarabine: healing rate | 5 | 259 | Hazard Ratio (95% CI) | 1.13 [0.86, 1.47] |
| 17 Acyclovir versus trifluridine: 7‐day & 14‐day healing | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Healing at 7 days | 4 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.82, 1.20] |
| 17.2 Healing at 14 days | 4 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.90, 1.09] |
| 18 Acyclovir versus trifluridine: healing rate | 3 | 140 | Hazard Ratio (95% CI) | 0.92 [0.65, 1.32] |
| 19 Brivudine versus trifluridine: 7‐day & 14‐day healing | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Healing at 7 days | 3 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.71, 1.21] |
| 19.2 Healing at 14 days | 3 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.88, 1.14] |
| 20 Brivudine versus trifluridine: healing rate | 1 | 60 | Hazard Ratio (95% CI) | 0.60 [0.35, 1.02] |
| 21 Brivudine versus acyclovir: 7‐day & 14‐day healing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 Healing at 7 days | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.93, 1.51] |
| 21.2 Healing at 14 days | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.92, 1.20] |
| 22 Ganciclovir versus acyclovir: 7‐day & 14‐day healing | 28 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 22.1 Healing at 7 days | 7 | 551 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.96, 1.35] |
| 22.2 Healing at 14 days | 28 | 2062 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.22, 1.57] |
| 23 Foscarnet versus trifluridine: 7‐day & 14‐day healing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.1 Healing at 14 days | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.75, 1.34] |
| 24 Foscarnet versus acyclovir: 14‐day healing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 24.1 Healing at 14 days | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.95, 1.40] |
| 25 Foscarnet versus ganciclovir: 7‐day & 14‐day healing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 25.1 Healing at 7 days | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.76, 1.22] |
| 25.2 Healing at 14 days | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.80, 1.16] |
| 26 Acyclovir/vidarabine versus acyclovir: 7‐day & 14‐day healing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 26.1 Healing at 7 days | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [1.01, 2.24] |
| 26.2 Healing at 14 days | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.89, 1.12] |
| 27 Trifluridine (aqueous) versus trifluridine (viscous) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 27.1 Healing at 7 days | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.58, 4.05] |
| 27.2 Healing at 14 days | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.96, 2.41] |
| 28 Ganciclovir 0.15% gel versus ganciclovir 0.05% gel or 0.1% solution | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 28.1 Healing at 7 days | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.53, 1.74] |
| 28.2 Healing at 14 days | 4 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.98, 1.14] |
Comparison 2. Oral antiviral agents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral antiviral versus topical antiviral | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healing at 7 days | 2 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.13, 2.02] |
| 1.2 Healing at 14 days | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.79, 1.07] |
| 2 Oral/topical antivirals versus topical antiviral | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Healing at 7 days | 1 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.95, 1.33] |
| 2.2 Healing at 14 days | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.68, 2.74] |
Comparison 3. Interferon.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Interferon versus inactive control, without or with debridement | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healing at 7 days | 3 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.07, 2.06] |
| 1.2 Healing at 14 days | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.06, 1.64] |
| 2 Interferon dosages | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Healing at 7 days: interferon 1000 IU/ml versus interferon 1‐10 million IU/ml | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.15, 2.86] |
| 2.2 Healing at 7 days: trifluridine + interferon 10 million IU/ml versus trifluridine + interferon 30 million IU/ml | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.94, 1.17] |
| 2.3 Healing at 7 days: trifluridine + interferon 0.3 or 1.5 million IU/ml versus trifluridine + interferon 30 million IU/ml | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.90, 1.38] |
| 2.4 Healing at 7 days: trifluridine + interferon 30 million IU/ml versus trifluridine + interferon 100 million IU/ml | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.86, 1.18] |
| 3 Interferon‐α with debridement versus interferon‐β with debridement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Healing at 7 days | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.81, 1.09] |
| 4 Natural interferon‐α with trifluridine versus recombinant interferon‐α with trifluridine | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Healing at 7 days | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.89, 1.12] |
| 5 Interferon versus nucleoside antiviral | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Healing at 7 days | 3 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.81, 1.21] |
| 5.2 Healing at 14 days | 4 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.91, 1.62] |
| 6 Interferon inducer versus nucleoside antiviral | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Healing at 7 days | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.14, 3.17] |
| 6.2 Healing at 14 days | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.08, 1.05] |
| 7 Interferon/nucleoside antiviral versus nucleoside antiviral: 7‐day & 14‐day healing | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Healing at 7 days | 9 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [1.35, 2.55] |
| 7.2 Healing at 14 days | 12 | 718 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.99, 1.13] |
| 8 Interferon/nucleoside antiviral versus nucleoside antiviral: healing rate | 5 | 229 | Hazard Ratio (95% CI) | 2.84 [2.13, 3.79] |
Comparison 4. Debridement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Debridement versus control | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healing at 7 days | 2 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.82, 2.11] |
| 1.2 Healing at 14 days | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.85, 1.43] |
| 2 Topical antiviral agent versus debridement | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Healing at 7 days | 7 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.74, 1.20] |
| 2.2 Healing at 14 days | 7 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.72, 1.32] |
| 3 Different debridement methods | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Healing at 7 days: cryotherapy versus carbolisation | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.95, 2.31] |
| 3.2 Healing at 7 days: cryotherapy/idoxuridine versus carbolisation/idoxuridine | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.99, 2.07] |
| 3.3 Healing at 7 days: thermocautery/interferon versus wiping/interferon | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.86, 1.13] |
| 3.4 Healing at 7 days: iodinisation/panthenol versus cryotherapy/panthenol | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.66, 1.12] |
| 3.5 Healing at 7 days: iodinisation/fluorophenylalanine versus cryotherapy/fluorophenylalanine | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.92, 1.46] |
| 3.6 Healing at 14 days: thermocautery/interferon versus wiping/interferon | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.91, 1.10] |
| 3.7 Healing at 14 days: iodinisation/panthenol versus cryotherapy/panthenol | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.73, 1.00] |
| 3.8 Healing at 14 days: iodinisation/fluorophenylalanine versus cryotherapy/fluorophenylalanine | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.91, 1.14] |
| 3.9 Healing at 14 days: photoinactivation versus carbolisation | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.41, 1.48] |
| 4 Debridement with antiviral or interferon versus debridement | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Healing at 7 days | 8 | 347 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.94, 1.36] |
| 4.2 Healing at 14 days | 3 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.78, 2.00] |
| 5 Debridement with different antivirals | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Healing at 7 days | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.80, 2.02] |
| 5.2 Healing at 14 days | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.86, 1.16] |
| 6 Debridement with antiviral versus antiviral: 7‐day & 14‐day healing | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Healing at 7 days | 7 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.84, 1.79] |
| 6.2 Healing at 14 days | 7 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.94, 1.17] |
| 7 Debridement with antiviral versus antiviral: healing rate | 6 | 248 | Hazard Ratio (95% CI) | 1.76 [1.32, 2.35] |
Comparison 5. Adjunctive and alternative agents: lubricant, growth factor, nonsteroidal anti‐inflammatory drug, immunomodulator.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Antiviral/lubricant versus antiviral | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healing at 7 days | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.86 [1.03, 14.50] |
| 1.2 Healing at 14 days | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.73, 1.91] |
| 2 Antiviral/epidermal growth factor versus antiviral | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Healing at 7 days | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.54, 1.86] |
| 2.2 Healing at 14 days | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.91, 1.10] |
| 3 Panthenol versus para‐fluorophenylalanine, with debridement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Healing at 7 days | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.79, 1.14] |
| 3.2 Healing at 14 days | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.88, 1.08] |
| 4 Interferon/methyl uracil versus interferon | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Healing at 7 days | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.27, 2.67] |
| 4.2 Healing at 14 days | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.79, 1.41] |
| 5 Antiviral/oxyphenbutazone versus antiviral | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Healing at 7 days | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.56, 1.11] |
| 6 Oral inosine pranobex versus inactive control, with or without antiviral | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Healing at 7 days | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.07, 3.47] |
| 6.2 Healing at 14 days | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.91, 1.62] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abe 1987.
| Methods | Allocation method: not given Masking: none Number of centres: one | |
| Participants | Country: Japan Number enrolled: 27 Average age (range): 35 (2‐77) Sex: 17 males, 10 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=9): idoxuridine solution Treatment two (n=18): acyclovir 3% ointment 3 to 5 times per day (one patient received intravenous acyclovir 5 mg/kg every 8 hours) | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: antibiotic solution 3 to 5 times per day Report language: Japanese Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Unequal (1:2) treatment assignment, with allocation of treatment based on availability of the intervention and on judgement of the clinician |
| Allocation concealment (selection bias) | High risk | Unconcealed procedure |
| Blinding (performance bias and detection bias) All outcomes | High risk | Study personnel not masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | The study interventions may not have occurred concurrently |
Altinisik 1987.
| Methods | Allocation method: not given Masking: single Number of centres: one | |
| Participants | Country: Turkey Number enrolled: 27 Average age (range): 38 (4‐73) Sex: 16 males, 11 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=9): idoxuridine 0.5% ointment 5 times per day Treatment two (n=10): acyclovir 3% ointment 5 times per day Treatment three (n=8): acyclovir 3% ointment 5 times per day + wiping débridement | |
| Outcomes | Epithelial healing (resolution of 'punktat epitelial defektler') | |
| Notes | Nonstudy interventions: atropine Report language: Turkish Study date: 1985‐1986 Financial support: not given Adverse reactions: One case of punctate epithelial erosions observed in the idoxuridine group, and none in the acyclovir group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation apparently by judgment of the clinician |
| Allocation concealment (selection bias) | High risk | Unconcealed procedure |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No masking, although outcome may not have been influenced by lack of masking |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | Insufficient information to determine if patients were treated concurrently or sequentially during 9 months of study enrolment |
Austin 1974.
| Methods | Allocation method: not given Masking: none Number of centres: one | |
| Participants | Country: Great Britain Number enrolled: 41 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=16): iodinisation Treatment two (n=25): idoxuridine | |
| Outcomes | 'When no corneal staining remained' | |
| Notes | Nonstudy interventions: mydriatic, antibiotic (iodinisation group), pad Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Insufficient information provided about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Treatment failures (area of corneal staining increased from one visit to the next) included 5 in idoxuridine treatment group and 2 in iodinisation group |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | Insufficient information |
Bartholomew 1977.
| Methods | Allocation method: randomised Masking: none Number of centres: one HSV isolation (conjunctiva and cornea): performed but results not reported | |
| Participants | Country: Great Britain Number enrolled: 21 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=6): photodynamic inactivation (neutral red 1% solution x 2 then blue light for 15 minutes) Treatment two (n=7): carbolization Treatment three (n=8): idoxuridine ointment 6 times per day | |
| Outcomes | 'When the ulcerated area no longer stained with fluorescein (scattered superficial punctate erosions were ignored)' | |
| Notes | Nonstudy interventions: chloramphenicol ointment, corneal scraping
Report language: English
Study date: not given
Financial support: private foundation Adverse reactions (Quote): "none of our patients experienced any adverse reactions." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | No masking was likely because of disparate interventions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data, although times to treatment failure (2 in idoxuridine group, 1 in carbolization group, and 2 in 'dye light' group) were not provided |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | Insufficient information Quote: "none of our patients experienced any adverse reactions." |
Behrens‐Baumann 1992.
| Methods | Allocation method: randomised Masking: double Number of centres: one HSV isolation (conjunctiva): 19 positive of 20 tested | |
| Participants | Country: Germany Number enrolled: 20 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one: (n=10): trifluridine 1% solution 5 times per day Treatment two (n=10): foscarnet 3% solution 5 times per day | |
| Outcomes | Fluorescein staining with 'closure of the corneal epithelium' | |
| Notes | Nonstudy interventions: scopolamine Report language: English Study date: not given Financial support: not given Adverse reactions (Quote): "No punctate keratitis or allergic reactions were observed." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data, although times to treatment failure (1 in trifluridine group and 1 in foscarnet group) were not described |
| Selective reporting (reporting bias) | Unclear risk | The method of performing paired comparison that "was made prospectively and at random" is unclear |
| Other bias | High risk | The trial was apparently stopped early when "no significant difference between [treatment groups was found]...after 10 paired analyses." |
Blake 1977.
| Methods | Allocation method: randomised Masking: double Number of centres: one | |
| Participants | Country: Ireland Number enrolled: 30 Average age (range): 45 (7‐75) Sex: 16 males, 14 females Inclusion criteria: dendritic (23) or geographic (7) epithelial keratitis | |
| Interventions | Treatment one (n=13): idoxuridine ointment 0.5% 4 times daily Treatment two (n=17): vidarabine ointment 3% 4 times daily | |
| Outcomes | 'Complete re‐epithelialisation' | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given Adverse reactions (Quote): "Two patients in the Ara‐A group developed mild punctate keratitis...in both cases the condition cleared spontaneously on discontinuing medication." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | The drug allocation code was not "broken" unless "treatment was considered a failure." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data within 14 days of treatment onset |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | While "patients with disciform keratitis...were excluded" the table of patient characteristics lists one patient with "stromal" keratitis. In addition, a baseline imbalance of treatment groups occurred with respect to patient age |
Burns 1963.
| Methods | Allocation method: randomised Masking: double Number of centres: several (42 sites in entire study) Quote: "The signs and symptoms of this infection are clear cut and pathognomonic, thus no confirmatory laboratory tests will be necessary to establish the diagnosis." | |
| Participants | Country: United States Number enrolled: 38 Average age (range): not given Sex: not given Inclusion criteria: acute epithelial keratitis | |
| Interventions | Control (n=15): distilled water hourly day, 2‐hourly night Treatment one (n=23): idoxuridine solution hourly day, 2‐hourly night | |
| Outcomes | Fluorescein staining with 'healing of the ulcer' | |
| Notes | Nonstudy interventions: not given Report language: English Study date: 1962‐1963 Financial support: pharmaceutical industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The bottles were numbered consecutively, and active drug and placebo were randomly distributed." |
| Allocation concealment (selection bias) | Low risk | Study medications were distributed from a central location and "dispensed consecutively to ophthalmologists when requested." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study treatment medications "were supplied in identical brown bottles, labelled only with a code number." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition |
| Selective reporting (reporting bias) | Unclear risk | The time to "healing of the ulcer" was not clearly described |
| Other bias | Unclear risk | Quote: "The physician was instructed that, if after a week of therapy, he was dissatisfied with the patient's results, he could telephone this report, and the code would be broken." |
Cao 2001.
| Methods | Allocation method: randomised Masking: none Number of centres: 3 | |
| Participants | Country: China Number enrolled: 104 dendritic epithelial keratitis (123 total cases of epithelial or disciform keratitis + 41 others with open allocation of study treatments) Average age (range): 39 (18‐65) Sex (imputed from total, for dendritic sample): 75 males, 29 females Inclusion criteria: dendritic (104) epithelial keratitis, geographic epithelial keratitis (11), disciform keratitis (8) in trial; and in open allocation, dendritic (14), geographic (9), and disciform (18) cases, for total population of 164 | |
| Interventions | Treatment one (n=52): acyclovir 0.1% solution 6 times per day Treatment two (n=52): foscarnet 3% solution 6 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: atropine 1% Report language: Chinese Study date: 1999‐2000 Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The investigators describe a random component in the sequence generation process. However, additional study groups had open allocation of study treatments |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐masked trial design described but no details of masking |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Primary outcome data available for dendritic epithelial keratitis but not for other strata (e.g., geographic epithelial keratitis) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Carmassi 1993.
| Methods | Allocation method: not given Masking: none Number of centres: one HSV isolation (cornea): 10 positive of 15 tested | |
| Participants | Country: Italy Number enrolled: 15 Average age (range): 44 (25‐71) Sex: 7 males, 8 females Inclusion criteria: dendritic or geographic epithelial keratitis | |
| Interventions | Treatment one (n=5): acyclovir 3% ointment 5 times per day Treatment two (n=5): interferon solution 8 times per day (total of 200,000 units) Treatment three (n=5): acyclovir 3% ointment 5 times per day and interferon solution 8 times per day | |
| Outcomes | 'Corneal reepithelialization and negativity of the fluorescein test' ('riepitelizzazione corneale e negativizzazione del test alla fluoresceina') | |
| Notes | Nonstudy interventions: none Report language: Italian Study date: not given Financial support: not given Adverse reactions: Two cases of "cheratite puntata" occurred in the acyclovir group, and one case occurred in the acyclovir‐interferon group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Insufficient information ("i pazienti sono stati suddivisi in 3 gruppi di 5 pazienti ciascuno e sottoposti a 3 differenti trattamenti") |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Cellini 1994.
| Methods | Allocation method: randomised Masking: double Number of centres: one | |
| Participants | Country: Italy Number enrolled: 40 Average age (range): 43 (19‐70) Sex: 24 males, 16 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=20): acyclovir 3% ointment 4 times per day Treatment two (n=20): acyclovir 3% ointment 4 times per day and murine epidermal growth factor 10 mg/ml 4 times per day | |
| Outcomes | 'Negativity of the fluorescein staining test' | |
| Notes | Nonstudy interventions: none
Report language: English
Study date: not given
Financial support: pharmaceutical industry Adverse reactions (Quote): "The tolerability of EGF was always good, and there have not been signs of interaction or incompatibility between EGF and the antiviral agent." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | Insufficient information |
Chen 2008.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 40 Average age (range): 44 Sex: 23 males, 17 females Inclusion criteria: dendritic (9) epithelial keratitis | |
| Interventions | Treatment one (n=20): acyclovir 0.1% solution 4 times per day Treatment two (n=20): ganciclovir 0.15% gel 4 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: not given Report language: Chinese Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | No masking was undertaken, and outcome assessment could have been influenced by the lack of a masked assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Colin 1981.
| Methods | Allocation method: randomised Masking: double Number of centres: one | |
| Participants | Country: France Number enrolled: 52 Average age (range): 49 (8‐84) Sex: 31 males, 21 females Inclusion criteria: dendritic or geographic epithelial keratitis | |
| Interventions | Treatment one (n=27): idoxuridine 0.5% ointment 5 times per day Treatment two (n=25): acyclovir 3% ointment 5 times per day | |
| Outcomes | 'The absence of epithelial ulceration after instillation of fluorescein' ('l'absence d'ulcération epithéliale après instillation de fluorescéine') | |
| Notes | Nonstudy interventions: atropine
Report language: French
Study date: not given
Financial support: pharmaceutical industry
Adverse reactions: Two cases of "kératite ponctuée" occurred in the idoxuridine group, and one case in the acyclovir group [Presumed typographical error in publication's table of 11 males, instead of 17 males, randomised to idoxuridine] |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked ("en double insu") trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data, although results of treatment failure ("échecs") not described |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Colin 1982.
| Methods | Allocation method: not given ("traitement comparatif") Masking: none Number of centres: one | |
| Participants | Country: France Number enrolled: 24 Average age (range): 55 Sex: 13 males, 11 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=12): trifluridine solution 5 times per day Treatment two (n=12): trifluridine solution 5 times per day + oral isoprinosine 50 mg/kg/day (one tablet per 10 kg body weight per day) for 5 days | |
| Outcomes | Florescein staining: 'l'absence d'ulcération épithéliale après instillation de fluorescéine' | |
| Notes | Nonstudy interventions: atropine 1% 2 times per day Report language: French Study date: not given Financial support: not given Adverse reactions (Quotes): one patient treated with trifluridine had not healed by day 12 when he still had a 2‐mm epithelial defect and "kératite ponctuée diffuse et conjonctivite folliculaire." "Tolerance: en dehors de cette observation il n'a pas été noté de signes to toxicité dus à la TFT. Cependant 9 patients ont signalé une sensation de brûlure oculaire lors de l'installation des gouttes. Aucune manifestation générale n'a été signalée pendant cet essai chez les patients recevant de l'Isoprinosine." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Colin 1983.
| Methods | Allocation method: randomised Masking: double Number of centres: one HSV isolation (conjunctiva and cornea): inclusion criterion | |
| Participants | Country: France Number enrolled: 45 Average age (range): not given Sex: 36 males, 9 females Inclusion criteria: dendritic epithelial keratitis (culture‐confirmed) | |
| Interventions | Treatment one (n=21): acyclovir 3% ointment 5 times per day and albumin once per day Treatment two (n=24): acyclovir 3% ointment 5 times per day and human leukocyte interferon 30 million units/ml once per day | |
| Outcomes | 'The absence of fluorescein staining in the area of the previous corneal ulceration' | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given Adverse reactions: "Diffuse superficial punctate epitheliopathy was noted in five patients" including three in the acyclovir‐placebo group and two in the acyclovir‐interferon group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked ("en double insu") trial design described in the preliminary report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported. The trial was limited to "virologically identified dendritic keratitis." |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Colin 1984.
| Methods | Allocation method: randomised Masking: double Number of centres: one | |
| Participants | Country: France Number enrolled: 32 Average age (range): 48 (7‐79) Sex: 20 males, 12 females Inclusion criteria: dendritic or geographic epithelial keratitis | |
| Interventions | Treatment one (n=17): iododeoxycytidine 1% ointment 5 times per day Treatment two (n=15): acyclovir 3% ointment 5 times per day | |
| Outcomes | 'The absence of epithelial ulceration after the instillation of fluorescein, using the biomicroscope' ('l'absence d'ulcération après instillation de fluorescéïne') | |
| Notes | Nonstudy interventions: atropine
Report language: French
Study date: not given
Financial support: not given
Adverse reactions: One case of kératite ponctuée" was observed in the iododeoxycytidine group and one case in the acyclovir group. Another case of "allergie oculo‐palpébrale" occurred in the acyclovir group [Day 15 healing data of iododeoxycytidine group used for day 14 data table in this systematic review] |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked ("en double insu") trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Colin 1987.
| Methods | Allocation method: randomised Masking: double Number of centres: one HSV isolation (conjunctiva): 24 positive of 32 tested | |
| Participants | Country: France Number enrolled: 32 Average age (range): 49 (8‐77) Sex: 19 males, 13 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=16): acyclovir 3% ointment 4 times per day and placebo ointment 3 times per day Treatment two (n=16): acyclovir 3% ointment 4 times per day and vidarabine 3% ointment 3 time per day | |
| Outcomes | 'The absence of fluorescein staining in the area of previous corneal ulceration' | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: governmental agency Adverse reactions (Quote): "Diffuse superficial punctate epitheliopathy was noted in four patients (two in each group)...and resolved after the treatment was discontinued." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "The coded treatment were randomly allocated by the pharmacist." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "All patients were examined by one ophthalmologist (JC), who did not know whether the patient was receiving vidarabine or placebo." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Colin 1997a.
| Methods | Allocation method: randomised Masking: none Number of centres: three HSV isolation (conjunctiva): 35 positive of 59 tested | |
| Participants | Country: Mali and Tunisia Number enrolled: 67 Average age (range): 41 Sex: 37 males, 30 females Inclusion criteria: dendritic (51) or geographic (16) epithelial keratitis | |
| Interventions | Treatment one (n=22): acyclovir 3% ointment 5 times per day Treatment two (n=23): ganciclovir 0.15% gel 5 times per day Treatment three (n=22): ganciclovir 0.05% gel 5 times per day | |
| Outcomes | 'Absence of fluorescein staining at ulcer site' | |
| Notes | Nonstudy interventions: none Report language: English Study date: 1990‐1992 Financial support: pharmaceutical industry Adverse reactions (from Colin 2007a, Table 3): "Toxic superficial punctate keratitis related to treatment at Day 14" was observed in two cases in the acyclovir group, three cases in the ganciclovir 0.15% group, and no cases in the ganciclovir 0.05% group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomly allocated to the various treatment groups." |
| Allocation concealment (selection bias) | Low risk | There is no evidence that treatment assignments were not concealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "Double‐blind conditions were not possible because of the obviously different formulations of the two antiviral agents." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Eight case‐report forms from seven of the 67 patients included were found to be faulty during on‐site audits and were excluded from the efficacy analysis. Therefore, only 59 patients were assessed fro the efficacy (per protocol analysis)." |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | Insufficient information |
Colin 1997b.
| Methods | Allocation method: randomised Masking: none Number of centres: four HSV isolation (conjunctiva): 7 positive of 35 tested (Quote: "Because of problems of refrigeration during transportation, percentages of positive conjunctival swabs were very low.") | |
| Participants | Country: France, Switzerland, and Great Britain Number enrolled: 37 Average age (range): 48 Sex: 26 males, 11 females Inclusion criteria: dendritic (36) or geographic (1) epithelial keratitis | |
| Interventions | Treatment one (n=18): acyclovir 3% ointment 5 times per day Treatment two (n=19): ganciclovir 0.15% gel 5 times per day | |
| Outcomes | 'Absence of fluorescein staining at ulcer site' | |
| Notes | Nonstudy interventions: none Report language: English Study date: 1990‐1992 Financial support: pharmaceutical industry Adverse reactions (Quote from Colin, 2007, Study B): "There were no cases of superficial punctate keratitis that were found to be toxic." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | There is no evidence that treatment assignments were not concealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | No masking performed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Two patients were found to have been misdiagnosed (one in each treatment group): they were excluded from efficacy analysis." |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcomes was adequately reported |
| Other bias | Unclear risk | Quote: "There was a longer duration of disease before presentation" in the acyclovir group compared to the ganciclovir group that "occurred entirely by accident despite the randomization procedure." |
Colin 2007a.
| Methods | Allocation method: randomised Masking: none Number of centres: multiple | |
| Participants | Country: Pakistan Number enrolled: 109 Average age (range): not given Sex: not given Inclusion criteria: dendritic or geographic epithelial keratitis | |
| Interventions | Treatment one (n=38): acyclovir 3% ointment 5 times per day Treatment two (n=36): ganciclovir 0.15% gel 5 times per day Treatment three (n=35): ganciclovir 0.05% gel 5 times per day | |
| Outcomes | 'Lack of fluorescein staining' | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: pharmaceutical industry Adverse reactions (Quote): "The number of superficial punctate keratitis cases that appeared or were exacerbated while receiving treatment was similar across the treatment groups." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process ("étude clinique comparative randomisée") |
| Allocation concealment (selection bias) | Low risk | Centralized distribution process |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single‐blind ("en simple insu") trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | Outcome data are provided for both the intention‐to‐treat analysis and the per protocol analysis |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Colin 2007b.
| Methods | Allocation method: randomised Masking: none Number of centres: 28 | |
| Participants | Country: 28 European centres Number enrolled: 164 Average age (range): 45 Sex: not given Inclusion criteria: dendritic (138) or geographic (26) epithelial keratitis | |
| Interventions | Treatment one (n=80): acyclovir 3% ointment 5 times per day (n=67 dendrites) Treatment two (n=84): ganciclovir 0.15% gel 5 times per day (n=71 dendrites) | |
| Outcomes | 'When there was no fluorescein uptake' | |
| Notes | Nonstudy interventions: none Report language: English Study date: 1992‐1994 Financial support: not given Adverse reactions (Quote): "The frequency of toxic superficial punctate keratitis was reduced by half in the GCV0.15% group compared with the ACV group." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Centralized distribution scheme |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "The trial could not be made double masked owing to the obvious difference in the appearance between the gel and ointment formulations of the two drugs." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Collum 1980.
| Methods | Allocation method: randomised Masking: double Number of centres: one HSV isolation (conjunctiva): 19 positive of 54 tested | |
| Participants | Country: Ireland Number enrolled: 60, including 8 idoxuridine‐treated censored patients (7 who were not improved or worse at day 4 and one who defaulted) Average age (range): 41 (4‐79) Sex: 44 males, 16 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=30): idoxuridine 0.5% ointment 5 times per day Treatment two (n=30): acyclovir 3% ointment 5 times per day | |
| Outcomes | 'No fluorescein uptake' | |
| Notes | Nonstudy interventions: homatropine, pad Report language: English Study date: not given Financial support: pharmaceutical industry Adverse reactions: Six cases of "superficial punctate keratopathy" were observed in the idoxuridine group, and no cases in the acyclovir group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "The ointments were of similar appearance and packed in identical tubes." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Seven patients treated with idoxuridine were "withdrawn" and 1 "defaulted." |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Collum 1985.
| Methods | Allocation method: randomised Masking: double Number of centres: two | |
| Participants | Country: Ireland and UK Number enrolled: 51 Average age (range): 55 (range not given) Sex: 29 males, 22 females Inclusion criteria: geographic epithelial keratitis | |
| Interventions | Treatment one (n=26): vidarabine 3% ointment 5 times per day Treatment two (n=25): acyclovir 3% ointment 5 times per day | |
| Outcomes | 'No further staining of the ulcer area' | |
| Notes | Nonstudy interventions: pad "as appropriate" Report language: English Study date: not given Financial support: pharmaceutical industry Adverse reactions (Quote): "The only adverse reaction noted was superficial punctate epitheliopathy in two patients receiving acyclovir and five patients receiving Ara‐A." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "Both drugs were identically packaged and coded." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Three patients were withdrawn from the study owing to failure of treatment." Quote: "Three patients randomly allocated to acyclovir treatment failed to return after their initial assessment and were excluded from the analysis." |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Collum 1986.
| Methods | Allocation method: randomised Masking: double Number of centres: one | |
| Participants | Country: Ireland Number enrolled: 56 Average age (range): 47 (range not given) Sex: 45 males, 11 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=29): acyclovir 3% ointment 5 times per day and oral placebo 5 times per day Treatment two (n=27): placebo ointment 5 times per day and oral acyclovir 400 mg 5 times per day | |
| Outcomes | 'When there was no further staining with fluorescein, though slight irregularity or cystic change in the epithelium might still exist' | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given Adverse reactions (Quote): "In four patients receiving acyclovir ointment large punctate epithelial defects were noted...one other patient receiving acyclovir ointment had a minor degree of superficial punctate epitheliopathy." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "On a random basis patients were allocated either 3% acyclovir ophthalmic ointment and placebo tablets, or placebo ointment and acyclovir tablets." |
| Allocation concealment (selection bias) | Low risk | There is no evidence that participants or investigators could foresee treatment assignment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the 60 patients who entered the study three receiving the oral medication failed to come for follow‐up, and one was lost from the local therapy group." |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Coster 1976.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: Great Britain Number enrolled: 102 Average age (range): not given Sex: not given Inclusion criteria: dendritic (87) or geographic (15) epithelial keratitis | |
| Interventions | Treatment one (n=48): vidarabine 3.3% ointment 5 times per day Treatment two (n=54): trifluridine 1% solution 5 times per day | |
| Outcomes | 'Absence of staining with fluorescein' | |
| Notes | Nonstudy interventions: atropine Report language: English Study date: not given Financial support: not given Adverse reactions (Quote): "The only indication of toxicity observed was punctate epithelial staining with Bengal rose. This effect was considered to be due to drug toxicity if it affected areas of the cornea remote from the original lesion. Such a staining pattern was seen in nine patients, of whom eight had been treated with ara‐A and one with F3T | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "Each patient received coded treatment, randomly allocated by the pharmacist, who followed a stratification table." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 107 patients "who consented to the study...five patients were subsequently withdraw—four because of failure to attend the clinic for assessment and one because of an error dispensing." |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Coster 1977a.
| Methods | Allocation method: randomised Masking: double Number of centres: one | |
| Participants | Country: Great Britain Number enrolled: 78 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Control (n=22): minimal wiping débridement and placebo solution once per day Treatment one (n=26): minimal wiping débridement and human leukocyte interferon 11 million units/ml once per day Treatment two (n=30): minimal wiping débridement and human leukocyte interferon 33 million units/ml once per day | |
| Outcomes | Not given | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A preliminary communication (Jones et al, 1976) described treatment allocation in a "randomised manner." |
| Allocation concealment (selection bias) | Low risk | According to a preliminary report (Jones et al, 1976), "the coded, randomized allocation of treatment was stratified for size of the ulcer." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design implied (Note: Another systematic review judged this trial as not double‐blind (Guess 2007)) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All the ulcers healed within 48 hours." |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Coster 1979.
| Methods | Allocation method: randomised Masking: double Number of centres: one | |
| Participants | Country: Great Britain Number enrolled: 30 Average age (range): not given Sex: not given Inclusion criteria: geographic epithelial keratitis | |
| Interventions | Treatment one (n = 17): vidarabine 3.3% ointment 5 times per day Treatment two (n = 13): trifluridine 1% solution 5 times per day | |
| Outcomes | 'The absence of staining with fluorescein' | |
| Notes | Nonstudy interventions: atropine Report language: English Study date: not given Financial support: not given Adverse reactions (Quote): "The only manifestation of antiviral toxicity observed was punctate staining of the corneal epithelium with Bengal rose. This occurred in 3 patients treated with Ara‐A and 4 treated with F3T." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "The coded treatments were randomly allocated by the pharmacist within closely matched strata." |
| Blinding (performance bias and detection bias) All outcomes | High risk | The different treatment formulations (ointment versus drops) indicate probable lack of masking |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "In 6 patients the ulcers failed to heal." |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Coster 1980.
| Methods | Allocation method: randomised Masking: double Number of centres: one | |
| Participants | Country: Great Britain Number enrolled: 59 Average age (range): not given Sex: not given Inclusion criteria: dendritic (54) or geographic (5) epithelial keratitis | |
| Interventions | Treatment one (n=30): idoxuridine 1% ointment 5 times per day Treatment two (n=29): acyclovir 3% ointment 5 times per day | |
| Outcomes | 'No epithelial defect was demonstrable with rose‐Bengal and fluorescein staining' | |
| Notes | Nonstudy interventions: atropine Report language: English Study date: not given Financial support: not given Adverse reactions (Quote); "There were no untoward reactions that necessitated withdrawal of therapy." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were allocated to strata on the basis of clinical features likely to be significant in prognosis....Within these strata patients were randomly allocated to 1 of 2 treatment groups." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Neither the patient nor the ophthalmologist knew which treatment was being used." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One patient treated with acyclovir failed to present regularly for follow‐up [and]...is not included in the analysis." |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Dai 2009a.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 58 patients (71 eyes) Average age (range): 38 (15‐66) Sex: 40 males, 18 females Inclusion criteria: dendritic (59) or geographic (12) epithelial keratitis | |
| Interventions | Treatment one (n=30 patients, 36 eyes): acyclovir 0.1% solution 4 times per day Treatment two (n=28 patients, 35 eyes): ganciclovir 0.15% gel 4 times per day | |
| Outcomes | Fluorescein staining ('角膜荧光索染色阴性') | |
| Notes | Nonstudy interventions: atropine 1% for iridocyclitis Report language: Chinese Study date: 2008 Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Incomplete primary outcome data (outcome day unclear) |
| Selective reporting (reporting bias) | High risk | Outcomes reported for 58 patients rather than for 71 eyes |
| Other bias | Unclear risk | Insufficient information |
Dai 2009b.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 79 Average age (range): 32 (11‐57) Sex: 45 males, 34 females Inclusion criteria: dendritic (51) or geographic (28) epithelial keratitis | |
| Interventions | Treatment one (n=39): acyclovir 0.1% solution 6 times per day Treatment two (n=40): ganciclovir 0.15% gel 4 times per day | |
| Outcomes | Fluorescein staining ('荧光素染色阴性') | |
| Notes | Nonstudy interventions: ofloxacin 0.3% 4 times per day Report language: Chinese Study date: 2007‐2008 Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Incomplete primary outcome data (outcome day unclear) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Daniel 1972.
| Methods | Allocation method: alternate patients Masking: none Number of centres: one | |
| Participants | Country: Great Britain Number enrolled: 54 Average age (range): not given Sex: not given Inclusion criteria: dendritic or geographic epithelial keratitis | |
| Interventions | Treatment one (n=25): idoxuridine ointment 5 times per day Treatment two (n=29): idoxuridine ointment 5 times per day and ultraviolet (2536 Å) light for 2‐4 minutes once or twice | |
| Outcomes | Fluorescein and rose‐Bengal staining | |
| Notes | Nonstudy interventions: mydriatic, pad
Report language: English
Study date: 1970‐1971
Financial support: not given Adverse reactions (Quote): "Considerable pain resulted from the phototherapy, because of the resulting photochemical keratitis....In some patients pain prevented the further exposure that was necessary to achieve a confluent corneal stain." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The investigators describe a non‐random component in the sequence generation process |
| Allocation concealment (selection bias) | High risk | Quote: "The first four patients who presented were treated with the combined therapy...; thereafter alternate patients were treated" with combined idoxuridine and ultraviolet therapy or with idoxuridine alone |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "A double‐blind controlled trial was not possible as it was obvious to any observer which patients had received ultraviolet therapy." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three censored patients underwent débridement |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | This study appears to be free of other sources of bias |
Davidson 1964.
| Methods | Allocation method: randomised with table Masking: none Number of centres: one HSV isolation (cornea): 51 positive of 75 tested | |
| Participants | Country: Great Britain Number enrolled: 75 Average age (range): not given Sex: not given Inclusion criteria: not given | |
| Interventions | Control (n=25): gamma‐globulin 1% solution hourly during day, 2‐hourly during night Treatment one (n=25): débridement (with orange stick) and iodinisation (with alcohol solution of iodine and potassium iodide on soaked cotton wool) Treatment two (n=25): idoxuridine 0.1% solution hourly during day, 2‐hourly during night | |
| Outcomes | 'The absence of staining with two per cent fluorescein' | |
| Notes | Nonstudy interventions: pad, atropine (débridement group), chloramphenicol ointment (débridement group) Report language: English Study date: not given Financial support: not given Adverse reactions (Quote): "There were no allergic reactions to the drops, nor were there any complaints of burning or irritation. Some areas of punctate stain were noted after healing with IDU or gamma globulin drops, but there were no more frequent than after iodization." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "The patients admitted to the trial were allocated to one of the three groups using randomized sample tables, with which the pharmacist was provided." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "The usual precautions of a double‐blind clinical trial were followed, e.g. the observer was unaware of the type of drop being administered." While the nature of the interventions (chemical débridement and topical antiviral agent) would not permit a double‐masked design, it is unclear whether lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcomes were reported only for virologically confirmed cases |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
de Koning 1982.
| Methods | Allocation method: randomised Masking: double Number of centres: one HSV isolation (cornea): 58 positive of 61 tested | |
| Participants | Country: Netherlands Number enrolled: 53 Average age (range): not given Sex: 33 males, 20 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=28): trifluridine 1% solution 5 times per day and albumin solution Treatment two (n=25): trifluridine 1% solution 5 times per day and human leukocyte interferon 10 million units/ml | |
| Outcomes | 'No staining with fluorescein' and 'closure of the epithelial wound without any epithelial edema or cystic change in the area of the previous dendrite' | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe "a randomised arrangement" of the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Eight patients were excluded from the study"—two for failure to comply with the examination protocol, 3 for negative herpes simplex virus isolation, 2 for stromal keratitis, and 1 for metaherpes |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
de Koning 1983.
| Methods | Allocation method: randomised Masking: double Number of centres: one HSV isolation (cornea): 55 positive of 59 tested | |
| Participants | Country: Netherlands Number enrolled: 51 (actually 59, but 8 patients dropped from description and analysis) Average age (range): not given Sex: 30 males, 21 females Inclusion criteria: dendritic (42) or geographic (9) epithelial keratitis | |
| Interventions | Treatment one (n=26): acyclovir 3% ointment 5 times per day and albumin solution every morning Treatment two (n=25): acyclovir 3% ointment 5 times per day and human leukocyte interferon 30 million units/ml every morning | |
| Outcomes | 'No staining with fluorescein' and 'absence of epithelial edema and cystic changes' | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatment with either interferon or placebo was assigned randomly to patients." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: Of 59 patients who were enrolled and treated, "eight patients were excluded form the trial, two because they did not follow the instructions and four because all herpes simplex virus isolations were negative" and two treatment failures of geographic epithelial keratitis |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Denis 1983.
| Methods | Allocation method: randomised Masking: single Number of centres: one | |
| Participants | Country: France Number enrolled: 23 Average age (range): not given Sex: not given Inclusion criteria: dendritic or geographic epithelial keratitis | |
| Interventions | Treatment one (n=9): vidarabine 3% ointment 5 times per day Treatment two (n=14): acyclovir 3% ointment 5 times per day | |
| Outcomes | Ulcer healing ('cicatrisation de l'ulcère') | |
| Notes | Nonstudy interventions: mydriatic, antibiotic (rarely) Report language: French Study date: 1980‐1981 Financial support: not given Adverse reactions (Quote): "La tolérance locale à l'acyclovir et à l'Ara A est bonne, identique pour les deux substances. Chacune d'elle a occasionné dans cette étude: 5 kératites ponctuées superficielles qui n'ont pas empêché la poursuite du traitement et one disparu spontanément dès l'arrêt; et, chez 3 malades, une douleur modérée à chaque application de pommade." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked ("en double insu") trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Fu 2012.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 78 Average age (range): 34 (18‐75) Sex: 48 males, 30 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=38): ganciclovir 0.15% gel 4 times per day Treatment two (n=40): ganciclovir 0.15% gel 4 times per day + subconjunctival interferon (0.5 ml of 1 million units/ml every other day for 14 days) | |
| Outcomes | Fluorescein staining ('荧光染色阴性') | |
| Notes | Nonstudy interventions: none Report language: Chinese Study date: 2010‐2011 Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | No masking was likely because one treatment group received subconjunctival injections |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data, although survival data of healing were not tabulated |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Fulhorst 1972.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: Great Britain Number enrolled: 90 Average age (range): not given Sex: not given Inclusion criteria: dendritic (74) or geographic (16) epithelial keratitis | |
| Interventions | Treatment one (n=26): idoxuridine ointment 5 times per day Treatment two (n=18): cryotherapy (multiple applications at ‐70° to ‐80° C) and idoxuridine ointment 5 times per day Treatment three (n=18): cryotherapy (multiple applications at ‐70° to ‐80° C) and placebo ointment 5 times per day Treatment four (n=13): carbolization (débridement with orange stick followed by touching epithelial edge with phenol) and idoxuridine ointment 5 times per day Treatment five (n=15): carbolization (débridement with orange stick followed by touching epithelial edge with phenol) and placebo ointment 5 times per day | |
| Outcomes | 'When the lesion no longer stained with rose Bengal' | |
| Notes | Nonstudy interventions: scopolamine, chloramphenicol ointment, patching for 48 hours Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patient's treatment was determined by stratified randomization." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | While the trial is described as "double‐blind," the trial design is unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Genée 1987.
| Methods | Allocation method: randomised Masking: double Number of centres: one | |
| Participants | Country: Germany Number enrolled: 28 Average age (range): not given Sex: 21 males, 7 females Inclusion criteria: dendritic or geographic epithelial keratitis | |
| Interventions | Treatment one (n=14): acyclovir 3% ointment 5 times per day Treatment two (n=14): vidarabine 3% ointment 5 times per day | |
| Outcomes | Epithelial healing | |
| Notes | Nonstudy interventions: none Report language: German Study date: 1981‐1984 Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design ("Doppelblindstudie") described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 30 enrolled patients, 2 were excluded—1 for incorrect enrolment (recent vidarabine treatment) and 1 for non‐compliance. Note that there is an apparent typographical error in the table (13 instead of 14 patients in one treatment arm) |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Graupner 1966.
| Methods | Allocation method: not given Masking: double (partial) Number of centres: one | |
| Participants | Country: Germany Number enrolled: 32 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=12): idoxuridine 0.1% solution every 2 hours Treatment two (n=20): para‐fluorophenylalanine 0.1% solution every 2 hours | |
| Outcomes | Fluorescein ('Der Behandlungserfolg wurde an Hand der Anfärbbarkeit der Cornea beurteilt') | |
| Notes | Nonstudy interventions: atropine Report language: German Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Graupner 1968.
| Methods | Allocation method: randomised Masking: single Number of centres: one | |
| Participants | Country: Germany Number enrolled: 53 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=27): idoxuridine 0.1% ointment 5 times per day Treatment two (n=26): para‐fluorophenylalanine 0.1% ointment 5 times per day | |
| Outcomes | Fluorescein ('vollständige Epithelisation der Hornhaut') | |
| Notes | Nonstudy interventions: atropine Report language: German Study date: note given Financial support: pharmaceutical industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process: "Alle Patienten, bei denen in unserer Klinik die Diagnose Keratitis dendritica gestellt werden konnte, wurden zufällig einer der beiden Therapiegruppen zugeordnet." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome dat |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Graupner 1969.
| Methods | Allocation methods: randomised Masking: single Number of centres: one | |
| Participants | Country: Germany Number enrolled: 33 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=18): iodinization /débridement plus idoxuridine 0.1% ointment every 4 hours Treatment two (n=15): iodinization /débridement plus para‐fluorophenylalanine 0.1% ointment every 4 hours | |
| Outcomes | Fluorescein ('Anfärbbharkeit der Hornhaut mit Fluorescein beurteilt') | |
| Notes | Nonstudy interventions: atropine Report language: German Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process: "Alle Patienten, bei denen in unserer Klinik die Diagnose Keratitis dendritica gestellt werden konnte, wurden zufällig einer der beiden Therapiegruppen." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Guerra 1979.
| Methods | Allocation method: randomised by table Masking: single Number of centres: one HSV isolation (cornea): 11 positive of 20 tested | |
| Participants | Country: Italy Number enrolled: 20 Average age (range): 46 (9‐76) Sex: 13 males, 7 females Inclusion criteria: dendritic (18) or geographic (2) epithelial keratitis | |
| Interventions | Treatment one (n=10): polyinosinic‐polycytidylic 1000 μg/ml hourly Treatment two (n=10): idoxuridine 0.2% solution hourly | |
| Outcomes | Healing ('cicatrizzato') | |
| Notes | Nonstudy interventions: atropine Report language: Italian Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process Quote: "Il trattamento iniziale è stato assegnato su di una base prerandomizzata con la terz'ultima fila della tabella dei numeri casuali." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single‐masked study design Quote: "I due farmaci erano stati preparati della Cattedra di Virologia dell'Università di Siena in contenitori etichettati A e B, l'identità del contenuto dei quali era sconosciuta ai ricercatori clinici." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Han 2010.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 40 Average age (range): not give Sex: not given Inclusion criteria: epithelial keratitis | |
| Interventions | Treatment one (n=19): acyclovir solution Treatment two (n=21): ganciclovir gel | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: not given
Report language: Chinese
Study date: not given
Financial support: not given Recurrence rate: in one year, 2 episodes in acyclovir group and 1 episode in ganciclovir group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a randomised method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Han 2014.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 96 Average age (range): 40 (22‐58) Sex: 58 males, 38 females Inclusion criteria: dendritic or geographic epithelial keratitis (possibly also stromal keratitis) | |
| Interventions | Treatment one (n=48): acyclovir solution hourly and recombinant fibroblast growth factor gel every 2 hours Treatment two (n=48): ganciclovir gel hourly and recombinant fibroblast growth factor every 2 hours (apparent typographical error stating 46 patients enrolled in this treatment arm) | |
| Outcomes | Fluorescein staining ('痊愈: 眼部疼痛、畏光、流泪等刺激症状消失,角膜溃疡愈合,荧光素钠染色阴性') | |
| Notes | Nonstudy interventions: not given Report language: Chinese Study date: January 2012 ‐ December 2012 Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a randomised method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | Some patients had concomitant stromal keratitis |
Hart 1965.
| Methods | Allocation method: randomised by table Masking: double Number of centres: one HSV isolation (cornea): 25 positive of 32 tested | |
| Participants | Country: Australia Number enrolled: 32 Average age (range): 48 (14‐80) Sex: only given for healed patients (20 males, 6 females) Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Control (n=13): neomycin 0.3% solution hourly day, 2‐hourly night Treatment one (n=19): idoxuridine 0.1% solution hourly day, 2‐hourly night | |
| Outcomes | Quote: "the criterion of epithelial healing being the absence of discrete fluorescein staining of the cornea...in the assessment of epithelial healing, when such staining was no greater on the ulcer region than elsewhere, the ulcer would be deemed healed, but when punctate stains were confined to the ulcer region, it would be deemed not healed." | |
| Notes | Nonstudy interventions: mydriatic ("reserved for those patients in whom iritis was diagnosed"), pad Report language: English Study date: not given Financial support: pharmaceutical industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: Study medications "were supplied by [the manufacturer] in plastic dispensing bottles of identical appearance...[that were] then arranged...in random order." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The investigators were unaware of the nature of the drops being used to treat a particular patient until after assessment of the results had been made on the seventh day of treatment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two patients "were admitted to the trial but were subsequently discarded"—1 for loss to follow up and 1 for worsened corneal disease |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Haut 1983.
| Methods | Allocation method: not given Masking: double Number of centres: one | |
| Participants | Country: France Number enrolled: 26 Average age (range): not given Sex: not given Inclusion criteria: dendritic (15) or geographic (11) epithelial keratitis | |
| Interventions | Control (n=12): oral placebo Treatment one (n=14): oral isoprinosine 500 mg 6 times per day | |
| Outcomes | Healing ('amélioration') | |
| Notes | Nonstudy interventions: atropine, antibiotic
Report language: French
Study date: not given
Financial support: not given Adverse reactions (Quote): "Nous n'avons remarqué aucun signe secondaire important ni constant, et le produit a été bien toleré par tous les malades." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors describe this study as a "essai clinique en double insue" but do not describe the method of treatment allocation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked ("en double‐insu") trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information. The time to healing was not adequately provided |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | Insufficient information |
HEDS Group 1997.
| Methods | Allocation method: randomised by permuted blocks Masking: double Number of centres: 60 | |
| Participants | Country: USA Number enrolled: 287 Average age (range): 48 (range not given) Sex: 179 males, 108 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=134): trifluridine 1% solution 8 times per day and oral placebo 5 times per day Treatment two (n=153): trifluridine 1% solution 8 times per day and oral acyclovir 400 mg 5 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: none Report language: English Study date: 1992‐1995 Financial support: governmental agency | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe the use of "a computer‐generated list of random numbers" in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "Each patient was randomly assigned to receive either oral acyclovir...or oral placebo...identical in appearance and taste." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Patients and clinic personnel were masked to the oral medication treatment assignments; the data analysts and the data and safety monitoring committee were not." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Hoang‐Xuan 1984.
| Methods | Allocation method: not given Masking: none Number of centres: one HSV isolation (cornea and conjunctiva): results not reported | |
| Participants | Country: France Number enrolled: 29 Average age (range): not given Sex: only given for total group with herpetic keratitis (28 males, 9 females) Inclusion criteria: dendritic or geographic epithelial keratitis (n=29), or keratouveitis (n=8) | |
| Interventions | Treatment one (n=11): trifluridine 1% solution 6 times per day Treatment two (n=18): acyclovir 3% ointment 5 times per day | |
| Outcomes | No fluorescein staining ('lorsque la coloration épithéliale par la fluorescéine à l'examen biomicroscopique de la cornée a disparu') | |
| Notes | Nonstudy interventions: cycloplegic, antibiotic, timolol Report language: French Study date: 1980‐1982 Financial support: not given Adverse reactions (Quote): "Effets secondaires...sont limités à de simples kératites ponctuées superficielles: 8 dans le groupe ACV et 3 dans le groupe TFT, et à une conjonctivite folliculaire dans le groupe ACV." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors describe this study as "une étude comparative en clinique humaine" but do not describe the method of treatment allocation |
| Allocation concealment (selection bias) | High risk | Allocation method based on availability of the drug at the time of treatment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment of whether lack of masking influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Huang 2008a.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 45 Average age (range): 32 Sex: 31 males, 14 females Inclusion criteria: dendritic (29) or geographic (16) epithelial keratitis | |
| Interventions | Treatment one (n=15): acyclovir 0.1% solution 4 times per day Treatment two (n=16): ganciclovir 0.15% gel 4 times per day Treatment three (n=14): ganciclovir 0.1% solution 4 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: not given Report language: Chinese Study date: not given Financial support: not given Adverse reactions (Quote): "No obviously adverse drug reaction was found in all of three agents." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The investigators describe a double‐masked design, but different formulations (ointment, gel, and solution) of study medications were used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Hung 1984.
| Methods | Allocation method: randomised Masking: double Number of centres: one | |
| Participants | Country: Great Britain Number enrolled: 29 Average age (range): 56 (range not given) Sex: 23 males, 6 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=14): minimal wiping débridement and oral placebo 5 times per day Treatment two (n=15): minimal wiping débridement and oral acyclovir 400 mg 5 times per day | |
| Outcomes | 'Ulcer stained with rose‐Bengal to assess healing' | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: pharmaceutical industry Adverse reactions (Quote): "No adverse reaction was found in the acyclovir treated group." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Two patients were excluded from the analysis because no clinical assessment was made on day 6,7, or 8." |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | Insufficient information |
Høvding 1989.
| Methods | Allocation method: randomised Masking: double Number of centres: one | |
| Participants | Country: Norway Number enrolled: 50 Average age (range): 46 (range not given) Sex: 27 males, 23 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=25): trifluridine 2% ointment 5 times per day Treatment two (n=25): acyclovir 3% ointment 5 times per day | |
| Outcomes | 'Disappearance of epithelial ulceration(s) staining with fluorescein' | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given Adverse reactions (Quote): "Apart from a slight epithelial punctate keratopathy in a few patients in both treatment groups, no drug adverse effects were observed." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigator describes a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "Each patient received 2 identical, masked tubes containing either 3% acyclovir or 2% [trifluridine] ophthalmic ointment." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data during 14 days of follow up |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately assessed |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Jackson 1984.
| Methods | Allocation method: randomised Masking: double Number of centres: three HSV isolation (conjunctiva): 14 positive of 29 tested | |
| Participants | Country: Canada Number enrolled: 66 Average age (range): 45 (12‐80) Sex: 39 males, 27 females Inclusion criteria: dendritic (59) or geographic (7) epithelial keratitis | |
| Interventions | Treatment one (n=34): vidarabine 3% ointment 5 times per day Treatment two (n=32): acyclovir 3% ointment 5 times per day | |
| Outcomes | Using fluorescein and rose bengal staining 'treatment failure was defined as follows: ulceration worse or unchanged by day 7, healing incomplete by day 15 or recurrence between days 15 and 21' | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: pharmaceutical industry Adverse reactions (Quote): "Lid swelling with burning were each reported by one patient treated with vidarabine. Four patients treated with acyclovir and two treated with vidarabine were thought to exhibit drug‐related punctate epithelial keratitis." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component of the sequence generation process (personal communication) in this multicentre "double‐blind comparative study." |
| Allocation concealment (selection bias) | Low risk | Quote: "Each patient [received] a numbered tube of ointment containing either 3% acyclovir or 3% vidarabine." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Jensen 1982.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: Denmark Number enrolled: 43 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=20): acyclovir 3% ointment 6 times per day Treatment two (n=23): acyclovir 3% ointment 6 times per day and wiping débridement | |
| Outcomes | 'No epithelial defect was demonstrated after staining' with fluorescein and rose bengal | |
| Notes | Nonstudy interventions: cycloplegic, chloramphenicol ointment Report language: English Study date: not given Financial support: pharmaceutical industry Adverse reactions: "Superificial punctate keratitis" was observed in four cases in the acyclovir group and in three cases in the acyclovir‐débridement group. Six patients "complained of stinging after application of acyclovir ointment." "No other side effects were observed, and no patients were withdrawn due to side effects." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Whether the lack of masking influenced outcome assessment could not be determined |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Jepson 1964.
| Methods | Allocation method: not given Masking: double Number of centres: one Quote: "No viral identification studies were done. We considered the acute initial or recurrent dendritic figure to be definitely of herpetic etiology." | |
| Participants | Country: United States Number enrolled: 24 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Control (n=12): distilled water with thimerosal hourly day, 2‐hourly night Treatment one (n=12): idoxuridine 0.1% solution hourly day, 2‐hourly night | |
| Outcomes | 'Absence of fluorescein staining pattern' | |
| Notes | Nonstudy interventions: scopolamine Report language: English Study date: 1962‐1963 Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors describe this study as a "double‐blind study" but do not describe the method of treatment allocation |
| Allocation concealment (selection bias) | Low risk | Quote: "The hospital pharmacy coded the two groups" of study medications |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Neither the physician nor the patient knew whether the active substance or placebo was being used." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately assessed |
| Other bias | Unclear risk | Insufficient information |
Kato 1979.
| Methods | Allocation method: not given Masking: none Number of centres: one | |
| Participants | Country: Japan Number enrolled: 27 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=10): minimal wiping débridement Treatment two (n=17): idoxuridine | |
| Outcomes | 'Fluorescein staining' | |
| Notes | Nonstudy interventions: pad (débridement group)
Report language: Japanese
Study date: not given
Financial support: not given Adverse reactions (Quote): "No adverse reactions were observed in eyes treated with debridement." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Insufficient information |
| Allocation concealment (selection bias) | High risk | Unconcealed allocation procedure |
| Blinding (performance bias and detection bias) All outcomes | High risk | No masking was undertaken, and outcome assessment could have been influenced by the lack of a masked assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | Insufficient information |
Kitano 1983.
| Methods | Allocation method: randomised Masking: single (partial) Number of centres: 21 | |
| Participants | Country: Japan Number enrolled: 55 Average age (range): not given Sex: 34 males, 21 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=25): idoxuridine every 1 or 2 hours Treatment two (n=30): interferon‐β 20,000 IU/ml 4 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: none Report language: Japanese Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Incomplete masking |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kitano 1985.
| Methods | Allocation method: randomised Masking: double Number of centres: several | |
| Participants | Country: Japan Number enrolled: 109 (excluding 5 withdrawals) Average age (range): not given Sex: not given Inclusion criteria: dendritic or geographic epithelial keratitis | |
| Interventions | Treatment one (n=55, excluding 3 drop outs): idoxuridine 0.25% ointment 5 times per day Treatment two: (n=54, excluding 2 drop outs): acyclovir 3% ointment 5 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: none Report language: Japanese Study date: August 1981 ‐ May 1982 Financial support: not given Adverse reactions: "Multiple erosion" (Table 6) occurred in seven of 56 eyes treated with idoxuridine and in 12 of 54 eyes treated with acyclovir | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component of the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Centralised allocation scheme |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Klauber 1982.
| Methods | Allocation method: not given Masking: double Number of centres: one | |
| Participants | Country: Denmark Number enrolled: 38 Average age (range): 51 (range not given) Sex: 25 males, 13 females Inclusion criteria: "typical dendritic corneal ulcerations" | |
| Interventions | Treatment one (n=20): idoxuridine 0.5% ointment 5 times per day Treatment two (n=18): acyclovir 3% ointment 5 times per day | |
| Outcomes | 'Fluorescein and Rose‐Bengal' | |
| Notes | Nonstudy interventions: scopolamine Report language: English Study date: not given Financial support: not given Adverse reactions (Quote): "Punctate staining with Rose‐Bengal was the only adverse reaction in 6 patients. A transient staining was localised at the inferior cornea and adjacent conjunctiva in 1 patient treated with ACV, corresponding to the application of the ointment. In the other 5 patients (1 treated with ACV and 4 with IDU) the staining was temporarily localised around the previous ulcer and disappeared with the discontinuation of the ointment." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors describe this study as "a double‐blind clinical trial" but do not describe the method of treatment allocation |
| Allocation concealment (selection bias) | Low risk | Quote: "The Burroughs Wellcome Foundation, Copenhagen supplied the coded 3% acyclovir eye ointment and the coded 0.5% idoxuridine eye ointment." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kumar 1987.
| Methods | Allocation method: randomised Masking: double Number of centres: one | |
| Participants | Country: India Number enrolled: 36 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=17): idoxuridine 0.5% ointment 5 times per day Treatment two (n=19): acyclovir 3% ointment 5 times per day | |
| Outcomes | 'Fluorescein staining' | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors describe a random component in the sequence generation process. |
| Allocation concealment (selection bias) | Low risk | Quote: "[Idoxuridine] and acyclovir were in form of ointments, these were wrapped in similar paper and were labelled with different numbers randomly distributed. The master list was kept with the person who was dispensing the drug and was not connected with the study." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
La Lau 1982.
| Methods | Allocation method: randomised Masking: double Number of centres: four | |
| Participants | Country: Netherlands Number enrolled: 59 Average age (range): not given Sex: 34 males, 25 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=28): trifluridine 2% ointment 5 times per day Treatment two (n=31): acyclovir 3% ointment 5 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given Adverse reactions (Quote): "The most frequently observed side effect was superficial punctate keratopathy, showing fluorescein staining outside the area of the herpetic lesions. This occurred in about 70& of patients in both groups...In both groups a few patients complained of a stinging sensation after application of the ointment, but extensive conjunctival hyperaemia was seen in only 3 patients receiving TFT." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "We used 3% acyclovir and 2% trifluorothymidine ointment, both specially prepared for this trial and packed in identical tubes." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Laibson 1964.
| Methods | Allocation method: randomised Masking: double Number of centres: one HSV isolation (cornea): 18 positive of 21 tested | |
| Participants | Country: United States Number enrolled: 100 Average age (range): 45 (range not given) Sex: 72 males, 28 females Inclusion criteria: dendritic or geographic epithelial keratitis, without or with stromal keratitis | |
| Interventions | Control one (n=53): distilled water with thimerosal 0.002% hourly day, 2‐hourly night Treatment one (n=47): idoxuridine 0.1% solution hourly day, 2‐hourly night | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: none Report language: English Study date: 1962‐1963 Financial support: governmental agency | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component of the sequence generation process (personal communication) in this "double‐blind study." |
| Allocation concealment (selection bias) | Low risk | Quote: "All patients...were given a plain brown bottle sealed at the neck with a label on it bearing code numbers. The sealed code was held by the supplier (SKF) and was not known to the investigators. The bottles...were used in sequence." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Cautery was necessary in 5 cases because of failure to respond." |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Laibson 1977.
| Methods | Allocation method: randomised Masking: double Number of centres: one | |
| Participants | Country: United States Number enrolled: 33 Average age (range): not given Sex: 25 males, 8 females Inclusion criteria: dendritic (30) or geographic (3) epithelial keratitis | |
| Interventions | Treatment one (n=17): idoxuridine solution hourly day, 2‐hourly night Treatment two (n=16): trifluridine solution hourly day, 2‐hourly night | |
| Outcomes | Epithelial healing | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given Adverse reactions: One patient in the idoxuridine group "developed a drug toxicity." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component of the sequence generation process (personal communication) in this "double controlled study." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Unclear risk | Quote: "Patients who developed signs of toxicity to the drug were also construed as treatment failures." Thus, the investigators did not distinguish between effectiveness outcomes and withdrawals for other reasons |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Li 2008.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 58 Average age (range): 38 (10‐65) Sex: 22 males, 36 females Inclusion criteria: dendritic (48) or geographic (10) epithelial keratitis | |
| Interventions | Treatment one (n=28): acyclovir 0.1% solution 6 times per day Treatment two (n=30): ganciclovir 0.15% gel 4 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: not given Report language: Chinese Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | No masking was undertaken, and outcome assessment could have been influenced by the lack of a masked assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Li 2009.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 79 Average age (range): 44 (11‐66) Sex: 35 males, 44 females Inclusion criteria: dendritic (32) or geographic (29) epithelial keratitis or disciform keratitis (18) | |
| Interventions | Treatment one (n=34): acyclovir 0.1% solution 6 to 8 times per day Treatment two (n=45): acyclovir 0.1% solution 6 to 8 times per day and subconjunctival interferon 100,000 units every 1 to 2 days for 6 to 8 injections and intramuscular interferon (1 million units for adults and 1 million units/square meter for children) every 1 to 2 days for 6 to 8 injections | |
| Outcomes | Fluorescein staining ('治愈或基本治愈: 自觉症状消失, 充血与刺激症状消退, 角膜溃疡愈合, 荧光素染色转阴或残留极少上皮着色点, 角膜实质浸润吸收, 水肿和后弹力层皱褶消失, 角膜光切面厚度恢复正常或变薄, KP 消失或极少, Tyndall征转阴') | |
| Notes | Nonstudy interventions: atropine 1% (if iridocyclitis) Report language: Chinese Study date: November 2005 ‐ January 2008 Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a randomised method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Healing outcome presumed to be measured on day 14 |
| Selective reporting (reporting bias) | Unclear risk | The pre‐specified primary outcome was adequately reported, but the report did not stratify outcomes for participants treated with topical corticosteroid for stromal keratouveitis |
| Other bias | High risk | Among 79 eyes, the investigators enrolled 23% of eyes having disciform keratitis and did not separately report outcomes for epithelial keratitis (61) and stromal keratitis (18) |
Li 2013a.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 87 Average age (range): 35 (19‐56) Sex: 49 males, 38 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=42): acyclovir 0.1% solution 6 times per day + recombinant interferon α‐2b 6 times per day + oral acyclovir 200 mg 6 times per day + subconjunctival ribavirin 0.2 ml every other day Treatment two (n=45): ganciclovir 0.15% gel 4 times per day | |
| Outcomes | Fluorescein staining ('荧光素染色阴性') | |
| Notes | Nonstudy interventions: atropine 1% Report language: Chinese Study date: 2010‐2012 Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Incomplete primary outcome data (outcome day unclear) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Li 2013b.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 85 Average age (range): 38, median (16‐67) Sex: 52 males, 33 females Inclusion criteria: dendritic or geographic epithelial keratitis | |
| Interventions | Treatment one (n=46): ganciclovir 0.15% gel 4‐6 times per day Treatment two (n=39): ganciclovir 0.15% gel 4‐6 times per day + recombinant human interferon α‐1b 10 gm/mL 4‐6 times per day | |
| Outcomes | Fluorescein staining ('荧光素钠染色阴性') | |
| Notes | Nonstudy interventions: not given Report language: Chinese Study date: 2008‐2010 Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a randomised method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Healing outcome presumed to be measured on day 15 |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | The investigators enrolled more eyes (85) than patients (68), making it difficult to determine the primary unit of analysis |
Lin 2011.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 68 Average age (range): 36 (17‐72) Sex: 39 males, 29 females Inclusion criteria: epithelial keratitis (59, including 6 with stromal keratitis) or stromal keratitis (9) | |
| Interventions | Treatment one (n=34): acyclovir 0.1% solution 10 times per day Treatment two (n=34): ganciclovir 0.15% gel 5 times per day | |
| Outcomes | Fluorescein staining ('治愈或基本治愈: 自觉症状消失, 充血与刺激症状消退, 角膜溃疡愈合, 荧光素染色转阴或残留极少上皮着色点, 角膜实质浸润吸收, 水肿和后弹力层皱褶消失, 角膜光切面厚度恢复正常或变薄, KP 消失或极少, Tyndall征转阴') | |
| Notes | Nonstudy interventions: ofloxacin 0.3% 3 times per day
Report language: Chinese
Study date: January 2006 ‐ September 2009
Financial support: not given Recurrence rate: in one year, 8 episodes in acyclovir group and 2 episodes in ganciclovir group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a randomised method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Healing outcome reported at 3 weeks instead of 2 weeks |
| Selective reporting (reporting bias) | Unclear risk | The pre‐specified primary outcome was adequately reported, but the report did not stratify outcomes for participants treated with topical corticosteroid for stromal keratouveitis |
| Other bias | High risk | Among 68 eyes, the investigators enrolled 13% of eyes having stromal keratitis and did not separately report outcomes for epithelial keratitis (59) and stromal keratitis (9) |
Lin 2014.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 112 Average age (range): 44 (17‐72) Sex: 59 males, 53 females Inclusion criteria: dendritic (60) or geographic (52) epithelial keratitis | |
| Interventions | Treatment one (n=56): acyclovir 0.1% solution 8 times per day Treatment two (n=56): ganciclovir 0.15% gel 8 times per day | |
| Outcomes | Fluorescein staining ('治愈:荧光素染色阴性,流泪、畏光、疼痛、等刺激症状消失,角膜溃疡修复') | |
| Notes | Nonstudy interventions: mydriatic if uveitis, oral indomethacin, oral 'antiviral', oral anti‐glaucoma drug(s) if increased intraocular pressure
Report language: Chinese
Study date: February 2011 ‐ February 2013
Financial support: not given Adverse reactions: 7 occurrences in acyclovir group and 2 occurrences in ganciclovir group Recurrence rate: in one year, 12 episodes in acyclovir group and 3 episodes in ganciclovir group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a randomised method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Incomplete masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Liu 2009a.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 36 eyes (29 patients) Average age (range): 40 Sex: 17 males, 12 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=13 eyes, 11 patients): acyclovir 0.3% solution 6 times per day Treatment two (n=23 eyes, 18 patients): ganciclovir 0.15% gel 4 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: not given Report language: Chinese Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The investigators describe a random component in the sequence generation process ("随机选择"); however, no explanation for imbalance in the numbers of eyes assigned to each intervention |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | No masking was undertaken, and outcome assessment could have been influenced by the lack of a masked assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Liu 2010.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 67 eyes (50 patients) Average age (range): 30 (16‐68 Sex: 30 males, 20 females (based on estimation of GCV group's eye data) Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=28 eyes, 20 patients): acyclovir 0.1% solution 6 times per day Treatment two (n=39 eyes, 30 patients): ganciclovir 0.15% gel 4 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: ofloxacin 0.3% 4 times per day Report language: Chinese Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process but do not account for the unequal group sizes |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | Insufficient information |
Liu 2011.
| Methods | Allocation method: randomised Masking: single Number of centres: one | |
| Participants | Country: China Number enrolled: 26 Average age (range): not given (21‐53) Sex: 14 males, 12 females Inclusion criteria: dendritic or geographic epithelial keratitis | |
| Interventions | Treatment one (n=12): ganciclovir 0.15% gel 4 times per day + ofloxacin 0.3% 4 times per day Treatment two (n=14): ganciclovir 0.15% gel 4 times per day + sodium hyaluronate 0.1% 4 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: none Report language: Chinese Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | Insufficient information |
Liu 2012a.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 80 Average age (range): not given (17‐46) Sex: 48 males, 32 females Inclusion criteria: dendritic (38) or geographic (21) epithelial keratitis or disciform keratitis (21) | |
| Interventions | Treatment one (n=40): acyclovir 0.1% solution 6 times per day and intravenous ribavirin 500 mg/day Treatment two (n=40): ganciclovir 0.15% gel 4 times per day and intramuscular interferon every other day and oral vitamin C 200 mg 3 times per day | |
| Outcomes | Fluorescein staining, with disappearance of signs of corneal inflammation | |
| Notes | Nonstudy interventions: oral indomethacin
Report language: Chinese
Study date: January 2010 ‐ January 2012
Financial support: not given Recurrence rate: in one year, 7 episodes in acyclovir group and 1 episode in ganciclovir group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a randomised method of sequence generation |
| Allocation concealment (selection bias) | High risk | Possible open enrolment since treatment plans were complex, involving different topical and systemic medications for each study arm |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Healing outcome reported for combined cured and improved criteria |
| Selective reporting (reporting bias) | Unclear risk | The pre‐specified primary outcome was adequately reported, but the report did not stratify outcomes for participants treated with topical corticosteroid for stromal keratouveitis |
| Other bias | High risk | Among 80 eyes, the investigators enrolled 26% of eyes having disciform keratitis and did not separately report outcomes for epithelial keratitis (59) and stromal keratitis (21) |
Liu 2012b.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 76 Average age (range): 35 (21‐67) Sex: 35 males, 41 females Inclusion criteria: epithelial keratitis (51, including 15 with stromal keratitis) or stromal keratitis (25) | |
| Interventions | Treatment one (n=38): acyclovir 0.1% solution 8 times per day Treatment two (n=38): ganciclovir 0.15% gel 5 times per day (presumed typographic error of 0.5% gel) | |
| Outcomes | Fluorescein staining ('治愈: 患者不存在眼部刺激症状, 同时其眼部充血情况消退, 对其进行荧光素染色, 呈阴性, 患者角膜基质的水肿及浸润情况消失, 角膜后沉着物减退等') | |
| Notes | Nonstudy interventions: ofloxacin 0.3% 3 times per day
Report language: Chinese
Study date: October 2009 ‐ June 2011
Financial support: not given Recurrence rate: in six months, 10 episodes in acyclovir group and 2 episodes in ganciclovir group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a randomised method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Healing outcome reported at 3 weeks instead of 2 weeks |
| Selective reporting (reporting bias) | Unclear risk | The pre‐specified primary outcome was adequately reported, but the report did not stratify outcomes for participants treated with topical corticosteroid for stromal keratouveitis |
| Other bias | High risk | Among 76 eyes, the investigators enrolled 33% of eyes having stromal keratitis without epithelial keratitis and did not separately report outcomes for epithelial keratitis (36), combined epithelial and stromal keratitis (15), and stromal keratitis (25) |
Liu 2014a.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 64 Average age (range): 38 (not given) Sex: 38 males, 28 females Inclusion criteria: dendritic (34) or geographic (19) epithelial keratitis or disciform keratitis (11) | |
| Interventions | Treatment one (n=32): acyclovir 0.1% solution 4 times per day Treatment two (n=32): ganciclovir 0.15% gel 4 times per day | |
| Outcomes | Fluorescein staining ('痊愈: 治疗后眼部刺激, 充血症状完全消失, 角膜溃疡完全修复, 突光素染色转阴, 角膜后沉着物消失或呈色素性') | |
| Notes | Nonstudy interventions: ofloxacin 0.3% 4 times per day
Report language: Chinese
Study date: January 2007 ‐ July 2011
Financial support: not given Adverse reactions: 5 occurrences in acyclovir group and 3 occurrences in ganciclovir group Recurrence rate: in one year, 6 episodes in acyclovir group and 2 episodes in ganciclovir group; in two years, 10 episodes in acyclovir group and 4 episodes in ganciclovir group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a randomised method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Incomplete masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Healing outcome reported at 3 weeks instead of 2 weeks |
| Selective reporting (reporting bias) | Unclear risk | The pre‐specified primary outcome was adequately reported, but the report did not stratify outcomes for participants treated with topical corticosteroid for stromal keratouveitis |
| Other bias | High risk | Among 64 eyes, the investigators enrolled 17% of eyes having disciform keratitis and did not separately report outcomes for epithelial keratitis (53) and disciform keratitis (11) |
Luntz 1963.
| Methods | Allocation method: alternate patients Masking: none Number of centres: one HSV isolation (cornea): results not reported | |
| Participants | Country: Great Britain Number enrolled: 22 Average age (range): 49 (20‐83) Sex: 15 males, 7 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Control (n=11): neomycin 1% ointment 2 times daily Treatment one (n=11): idoxuridine 0.1% solution hourly | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: atropine, pad, small scraping Report language: English Study date: 1962 Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Alternate patients were put into 'treated' (with I.D.U.) and 'control' (without I.D.U.) groups." |
| Allocation concealment (selection bias) | High risk | Allocation based on non‐random alternation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | High risk | Quote: "Three 'treated' cases were given topical cortisone drops." |
MacKenzie 1964.
| Methods | Allocation method: not given Masking: none Number of centres: one | |
| Participants | Country: Great Britain Number enrolled: 80 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=27): carbolisation "followed by local chemotherapy" Treatment two (n=25): idoxuridine 0.1% solution hourly day, 2‐hourly night Treatment three (n=28): idoxuridine 0.5% ointment 4 times per day | |
| Outcomes | 'The disappearance of staining with 2 per cent fluorescein' | |
| Notes | Nonstudy interventions: mydriatic Report language: English Study date: not given Financial support: not given Adverse reactions: One case of "S.P.K." was observed in the idoxuridine 0.1% solution group, four cases in the idoxuridine 0.5% ointment group, and three in the carbolisation group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Insufficient information on how "the eighty eyes were divided into three groups." |
| Allocation concealment (selection bias) | High risk | Apparent open allocation scheme |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking may have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | Insufficient information |
Maichuk 1980.
| Methods | Allocation method: not given Masking: none Number of centres: one | |
| Participants | Country: Russia Number enrolled: 39 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=20): human leukocyte interferon 500‐700 units/mL 5‐6 times per day Treatment two (n=19): human leukocyte interferon 500‐700 units/mL + methacil (methyluracil) 3 times per day | |
| Outcomes | Quote: "corneal epithelialization" | |
| Notes | Nonstudy interventions: not given Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Insufficient information |
| Allocation concealment (selection bias) | High risk | Open allocation schedule |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Maichuk 1988.
| Methods | Allocation method: not given Masking: none Number of centres: one | |
| Participants | Country: Russia Number enrolled: 138 Average age (range): not given Sex: not given Inclusion criteria: dendritic (76) or geographic (62) epithelial keratitis | |
| Interventions | Treatment one (n=63): idoxuridine 0.1% solution Treatment two (n=39): acyclovir 3% ointment Treatment three (n=36): acyclovir 3% ointment and leukocyte interferon 10,000 units/ml | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: variable, including corticosteroid Report language: Russian Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Insufficient information |
| Allocation concealment (selection bias) | High risk | Open allocation schedule |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Markham 1977.
| Methods | Allocation method: randomised Masking: double Number of centres: one | |
| Participants | Country: Great Britain Number enrolled: 64 Average age (range): 51 (18‐87) Sex: 44 males, 20 females Inclusion criteria: dendritic (60) or geographic (4) epithelial keratitis | |
| Interventions | Control (n=20): placebo ointment 4 times per day Treatment one (n=21): idoxuridine 0.5% ointment 4 times per day Treatment two (n=23): vidarabine 3% ointment 4 times per day | |
| Outcomes | Rose‐Bengal staining | |
| Notes | Nonstudy interventions: homatropine Report language: English Study date: not given Financial support: governmental agency Adverse reactions (Quote): "No patient had to be removed from the trial because of drug toxicity. In the group receiving Ara‐A, two patients developed punctate epithelial keratitis over the lower cornea and away from the ulcer area; two others developed a mild follicular reaction chiefly on the lower tarsal plate and fornix; a further patient developed an allergic skin rash, but this might equally have been due to homatropine. In the group receiving IDU, one patient developed a punctate epithelial keratitis, and three others a mild follicular reaction....All reactions were short‐lived." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "The drugs were issued according to a fully randomized code, so that neither the patient nor the observer was at any time aware of which therapy had been allocated." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Of the original 69 patients four failed to attend for sufficient follow‐up examinations, and one received incorrect treatment, leaving 64 patients in the trial." |
| Selective reporting (reporting bias) | Low risk | Quote: "No patient had to be removed from the trial because of drug toxicity." |
| Other bias | Low risk | No other sources of bias were found |
Matthäus 1970.
| Methods | Allocation method: randomised ("wurden zufällig...zugeordnet") Masking: none Number of centres: one | |
| Participants | Country: Germany Number enrolled: 120 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=33): iodinisation plus panthenol ointment Treatment two (n=27): iodinisation plus para‐fluorophenylalanine 0.1% ointment Treatment three (n=26): cryotherapy plus panthenol ointment Treatment four (n=34): cryotherapy plus para‐fluorophenylalanine 0.1% ointment | |
| Outcomes | Fluorescein staining ('Anfärbbarkeit der Hornhaut mit Fluoreszein beurteilt') | |
| Notes | Nonstudy interventions: atropine Report language: German Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Alle Patienten, bei denen die Diagnose einer Keratitis dendritica...wurden zufällig einer der folgenden 4 Therapiegruppen." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | Insufficient information |
McCulley 1982.
| Methods | Allocation method: randomised by table Masking: double Number of centres: five | |
| Participants | Country: United States Number enrolled: 64 Average age (range): 46 (15‐80) Sex: 43 males, 21 females Inclusion criteria: dendritic (52) or geographic (12) epithelial keratitis | |
| Interventions | Treatment one (n=34): idoxuridine 0.5% ointment 5 times per day Treatment two (n=30): acyclovir 3% ointment 5 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given Adverse reactions (Quote): "Few adverse reactions were encountered in either treatment group....The most frequently encountered adverse reaction was superficial punctate keratopathy, which was found in 11% (sic) of patients receiving ACV and 42.4% (sic) of those receiving IDU...and was mild to moderate, not severe." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component to the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "Acyclovir ophthalmic ointment, 3%, and IDU ophthalmic ointment, 0.5%, were assigned to patients by a predesignated random code test." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
McGill 1974.
| Methods | Allocation method: randomised Masking: single Number of centres: one | |
| Participants | Country: Great Britain Number enrolled: 20 Average age (range): 48 (21‐79) Sex: not given Inclusion criteria: dendritic (15) or geographic (5) epithelial keratitis | |
| Interventions | Treatment one (n=9): trifluridine 1% aqueous solution 5 times per day Treatment two (n=11): trifluridine 1% viscous solution 5 times per day | |
| Outcomes | Not given | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Meurs 1985.
| Methods | Allocation method: randomised Masking: double Number of centres: one HSV isolation (cornea and conjunctiva): eligibility criterion | |
| Participants | Country: Netherlands Number enrolled: 93 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis (culture‐confirmed) | |
| Interventions | Treatment one (n=45): acyclovir ointment 5 times per day and albumin solution every morning Treatment two (n=24): acyclovir ointment 5 times per day and recombinant human interferon‐α‐2 30 million units/ml every morning Treatment three (n=24): acyclovir ointment 5 times per day and recombinant human interferon‐α‐2 rod every morning | |
| Outcomes | Partial healing ('no staining of the epithelium of the cornea with fluorescein') and complete healing ('absence of the epidelial oedema and microcystic changes') | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | Study limited to virologically confirmed participants |
Norn 1973.
| Methods | Allocation method: not given Masking: double Number of centres: one Quote: "Virus culture was not performed." | |
| Participants | Country: Denmark Number enrolled: 29 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=14): idoxuridine 1% solution hourly and idoxuridine 2% ointment at bedtime and placebo ointment 6 times per day Treatment two (n=15): idoxuridine 1% solution hourly and idoxuridine 2% ointment at bedtime and oxyphenbutazone 10% ointment 6 times per day | |
| Outcomes | Fluorescein and rose‐Bengal staining | |
| Notes | Nonstudy interventions: none
Report language: English
Study date: 1990‐1993
Financial support: not given Adverse reactions (Quote): "No instances were observed of allergy caused by Tanderil or ointment base." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The author describes this study as "a prospective study, based on double‐blind trials" but does not describe the method of treatment allocation |
| Allocation concealment (selection bias) | Low risk | Quote: "Each tube was provided with a code number (1‐30). The code was broken after the recording of the results." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
O'Day 1975.
| Methods | Allocation method: randomised Masking: none Number of centres: one Quote: "Viral isolation was not attempted routinely." | |
| Participants | Country: Great Britain Number enrolled: 17 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=9): idoxuridine 0.5 % ointment 5 times per day Treatment two (n=8): proflavine hemisulphate 0.1% solution with fluorescent light exposure daily for 3 or more days | |
| Outcomes | Rose‐Bengal staining | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe an allocation sequence process that aimed "to evaluate photoinactivation in comparison with IDU by random selection," although it is unclear how "these eight eyes were matched to nine randomly selected eyes that received IDU therapy for dendritic ulcers." |
| Allocation concealment (selection bias) | High risk | The investigators apparently used an open allocation schedule |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Idoxuridine‐treated eyes "healed in approximately seven days," but "one of the nine eyes was a failure." |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | High risk | The investigators "suspended the study because several of the patients [treated with proflavine photoinactivation]...had mild adverse reactions." These consisted "of a generalized epithelial keratitis and an anterior uveitis, possibly of phototoxic origin." |
Panda 1995.
| Methods | Allocation method: randomised Masking: double Number of centres: one | |
| Participants | Country: India Number enrolled: 80 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis (first episode) | |
| Interventions | Treatment one (n=20): idoxuridine 1% ointment 5 times per day Treatment two (n=20): trifluridine 2% ointment 5 times per day Treatment three (n=20): acyclovir 3% ointment 5 times per day Treatment four (n=20): brivudine 1% ointment 5 times per day | |
| Outcomes | "Fluorescein staining and reduced corneal sensitivity" | |
| Notes | Nonstudy interventions: none Report language: English Study date: 1988‐1993 Financial support: not given Adverse reactions: Follicular conjunctivitis occurred in one case in the idoxuridine group. Epithelial keratopathy was observed in four cases: three in the idoxuridine group and one in the trifluridine group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data. Thirteen patients were classified as "treatment failures" by the pre‐specified definition of being "not healed after 14 days of therapy |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Parlato 1985.
| Methods | Allocation method: randomised by table Masking: single (partial) Number of centres: one HSV isolation (cornea or conjunctiva): 11 positive of 22 tested, including 10 of 13 corneal specimens and 1 of 9 conjunctival specimens | |
| Participants | Country: United States Number enrolled: 34 (actually 39, but 5 dropped from description and analysis) Average age (range): 46 (15‐80) Sex: 25 males, 9 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=8): minimal wiping débridement Treatment two (n=14): trifluridine 1% solution 9 times per day Treatment three (n=12): minimal wiping débridement and trifluridine 1% solution 9 times per day (beginning on second day) | |
| Outcomes | 'The lesions were stained with fluorescein.' 'The disappearance of dendritic staining, despite the occasional persistence of fine superficial punctate keratitis' | |
| Notes | Nonstudy interventions: cycloplegic, patch (débridement group) Report language: English Study date: 1981‐1984 Financial support: pharmaceutical industry Adverse reactions (Quote): "Adverse reactions to treatment were minimal. One patient developed superficial punctate keratitis that resolved when treatment was stopped. Mechanical débridement was not associated with notable discomfort in any patients." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were placed by random number selections in three treatment categories." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Healing was determined in a masked fashion by one of two investigators (E.J.C. and P.R.L.) who did not participate in other aspects of patient care in the study...including débridement, patching, and dispensing of medication." (Note: Another systematic review judged this trial as not double‐blind (Guess 2007). While participants were aware of the choice of intervention, this review classifies the study's blinding status as adequate) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Thirty‐nine patients with a clinical diagnosis of herpes simplex dendritic keratitis were enrolled in the study...[and]...thirty‐four (87%) completed the study." Patients assigned to the minimal‐wiping débridement group were considered a treatment failure and received trifluridine "if new dendrites were noted at any follow‐up before healing occurred." |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Patterson 1963a.
| Methods | Allocation method: randomised (personal communication) "in a double‐blind controlled trial" Masking: double Number of centres: one | |
| Participants | Country: Great Britain Number enrolled: 23 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Control (n=13): 199 tissue‐culture medium hourly day, 2‐hourly night Treatment one (n=10): idoxuridine 0.1% solution hourly day, 2‐hourly night | |
| Outcomes | Fluorescein and rose‐Bengal staining | |
| Notes | Nonstudy interventions: atropine 1% twice daily, hot bathings three times daily, pad Report language: English Study date: 1962 Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators used a random component in the sequence generation process (personal communication) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described ("in accordance with the design of a double‐blind trial the observer was at no time conversant with the type of medication being administered" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Patterson 1963b.
| Methods | Allocation method: randomised Masking: double Number of centres: one | |
| Participants | Country: Great Britain Number enrolled: 32 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Control (n=15): phenyl mercuric nitrate 0.004% solution hourly day, 2‐hourly night Treatment one (n=17): idoxuridine 0.1% solution hourly day, 2‐hourly night | |
| Outcomes | Fluorescein and rose‐Bengal staining | |
| Notes | Nonstudy interventions: atropine, heat, pad Report language: English Study date: 1962 Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators used a random component in the sequence generation process (personal communication) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Patterson 1963c.
| Methods | Allocation method: randomised Masking: double Number of centres: one | |
| Participants | Country: Great Britain Number enrolled: 30 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Control (n=14): placebo hourly day, 2‐hourly night Treatment one (n=16): idoxuridine 0.1% solution hourly day, 2‐hourly night | |
| Outcomes | Fluorescein and rose‐Bengal staining | |
| Notes | Nonstudy interventions: atropine, heat, pad Report language: English Study date: 1962 Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators used a random component in the sequence generation process (personal communication) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Patterson 1967a.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: Great Britain Number enrolled: 77 Average age (range): 33 (1‐66) Sex: 54 males, 23 females Inclusion criteria: dendritic (74) or geographic (3) epithelial keratitis | |
| Interventions | Control (n=39): carbolization Treatment one (n=38): idoxuridine ointment 5 times per day | |
| Outcomes | Rose‐Bengal staining | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process Quote: "the 77 cases of simplex dendritic ulceration were divided randomly into groups." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | High risk | Quote: "Those cases treated with IDU which did not show progression to healing at 5 days were classed as failures and cauterized; ulcers treated initially with cauterization which showed dendritic figures in the next few days were classed as failures and either cauterized again or treated with IDU." |
Patterson 1967b.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: Great Britain Number enrolled: 28 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis with disciform keratitis | |
| Interventions | Control (n=11): carbolization Treatment one (n=17): idoxuridine ointment 5 times per day | |
| Outcomes | Rose‐Bengal staining | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Insufficient information. No explanation for the imbalance in numbers of participants assigned to each intervention |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Pavan‐Langston 1976.
| Methods | Allocation method: randomised Masking: double Number of centres: several | |
| Participants | Country: United States Number enrolled: 169 Average age (range): 47 (2‐85) Sex: 115 males, 54 females Inclusion criteria: dendritic (133) or geographic (36) epithelial keratitis | |
| Interventions | Treatment one (n=82): idoxuridine ointment 5 times per day Treatment two (n=87): vidarabine ointment 5 times per day | |
| Outcomes | Epithelial healing | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: pharmaceutical industry Adverse reactions: There were six cases of "drug‐related adverse experiences" but no case of superficial keratopathy in the idoxuridine group. There were ten cases of "drug‐related adverse experiences" including one case of "marked superficial punctate keratitis" in the vidarabine group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "Drugs were identified for patient and investigator by number only; the code was not broken until the end of the study, or until a patient was removed." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Pavan‐Langston 1981.
| Methods | Allocation method: randomised Masking: double Number of centres: two HSV isolation (conjunctiva): 3 positive of 81 tested before treatment | |
| Participants | Country: United States Number enrolled: 41 Average age (range): not given (16‐82) Sex: 23 males, 18 females Inclusion criteria: dendritic (36) or geographic (5) epithelial keratitis | |
| Interventions | Treatment one (n=21): vidarabine 3% ointment 5 times per day Treatment two (n=20): acyclovir 3% ointment 5 times per day | |
| Outcomes | Epithelial healing | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: pharmaceutical industry and governmental agency Adverse reactions (Quote): "Adverse reactions for vidarabine included two instances of superficial punctate keratitis and one instance of severe pain (possibly related but lasting only one day). In the acyclovir‐treated group, one patient reported itching and discontinued the drug within one week of entering the study...[but] there was no objective sign of allergic reaction. Approximately 25% of patients in both groups had superficial punctate keratitis...not believed to be drug‐related." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "Drugs were packaged in a sequential order by random code number designation and were also dispensed sequentially," according to an ancillary, subsequent report (Laibson et al 1982) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Power 1991.
| Methods | Allocation method: randomised Masking: double Number of centres: one | |
| Participants | Country: Ireland Number enrolled: 60 Average age (range): 45 (9‐72) Sex: 43 males, 17 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=30): trifluridine 1% solution 5 times per day Treatment two (n=30): brivudine 0.1% solution 5 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given Adverse reactions (Quote): "Side effects were seen infrequently. Stinging on initial application of the drops was noted by three patients in the BVDU group and in five who were treated with TFT....One patient in the TFT group developed a punctate epitheliopathy...There was no evidence of epithelial toxicity in any of the patients treated with BVDU." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment consisted of either BVDU 0.1% or TFT 1.0% ophthalmic drops, which were packaged in identical bottles and prescribed five times daily." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One patient in the BVDU group was lost to follow‐up and was not included in the analysis." |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Ramirez 2002.
| Methods | Allocation method: randomised Masking: not given Number of centres: one | |
| Participants | Country: Mexico Number enrolled: 19 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=9): acyclovir 3% ointment 5 times per day Treatment two (n=10): ganciclovir 0.15% gel 5 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: not given Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked study design could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Richter 1986.
| Methods | Allocation method: not given Masking: none Number of centres: one | |
| Participants | Country: Germany Number enrolled: 57 Average age (range): not given (11‐71) Sex: 34 males, 23 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=32): brivudine solution Treatment two (n=25): brivudine solution and epithelial abrasion | |
| Outcomes | Fluorescein staining ('den fluoreszeinnegativen Schluß der Dendriticafigur') | |
| Notes | Nonstudy interventions: cycloplegic, antibiotic Report language: German Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Insufficient information on how patients "2 Gruppen zugeordnet." |
| Allocation concealment (selection bias) | High risk | The investigators apparently used an open allocation schedule |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked study design could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | Insufficient information |
Serifoglu 1987.
| Methods | Allocation method: not given Masking: single Number of centres: one | |
| Participants | Country: Turkey Number enrolled: 25 Average age (range): 36 (4‐78) Sex: 17 males, 8 females Inclusion criteria: dendritic (21) or geographic (4) epithelial keratitis | |
| Interventions | Treatment one (n=12): minimal wiping débridement and idoxuridine 0.5% ointment 5 times per day Treatment two (n=13): minimal wiping débridement and acyclovir 3% ointment 5 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: none Report language: Turkish Study date: 1985‐1986 Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of apparent masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | Insufficient information |
Shen 2014.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 86 Average age (range): not given (8‐72) Sex: 40 males, 38 females (78 patients; 86 eyes) Inclusion criteria: punctate or dendritic epithelial keratitis | |
| Interventions | Treatment one (n=43): ganciclovir gel 4 times per day + bovine fibroblast growth factor solution 4 times per day + oral acyclovir Treatment two (n=43): debridement with sterile needle + ganciclovir gel 4 times per day + bovine fibroblast growth factor solution 4 times per day + oral acyclovir | |
| Outcomes | Fluorescein staining ('治愈 : 角膜刺激症状消失, 溃疡愈合, 荧光素染色(‐), 基质水肿消失, KP(‐), 部分遗留角膜云翳') | |
| Notes | Nonstudy interventions: ofloxacin eye drops 4 times per day; atropine 1% if uveitis; gatifloxacin ointment following debridement Report language: Chinese Study date: January 2009 ‐ June 2013 Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Incomplete primary outcome data (outcome day unclear) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Srinivas 1993.
| Methods | Allocation method: not given Masking: none Number of centres: one | |
| Participants | Country: India Number enrolled: 40 Average age (range): not given Sex: 18 males, 14 females, 8 children Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=20): idoxuridine solution 5 times per day Treatment two (n=20): idoxuridine solution 5 times per day and oral acyclovir 200 mg 3 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: cycloplegic Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The investigators state that this was a "clinical trial" but do not describe whether a random component was used in the sequence generation process |
| Allocation concealment (selection bias) | High risk | Quote: "The first twenty cases were treated with Acyclovir...along with IDU drops....The second 20 cases received the IDU eye drops." It is unclear whether this statement means an unconcealed (i.e., open) allocation process or whether the authors are merely summarizing the number of participants (20 per group) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition or delayed healing |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Struck 1989.
| Methods | Allocation method: not given Masking: none Number of centres: one | |
| Participants | Country: Germany Number enrolled: 109 Average age (range): not given (1‐89) Inclusion criteria: dendritic epithelial keratitis without or with stromal keratitis | |
| Interventions | Control (n=34): débridement, débridement with iodinisation, cryoapplication, or other Treatment one (n=28): idoxuridine 0.1% solution 5 times per day Treatment two (n=16): trifluridine 1% solution 5 times per day Treatment three (n=31): brivudine 0.1% solution | |
| Outcomes | Fluorescein and rose‐Bengal straining | |
| Notes | Nonstudy interventions: none Report language: German Study date: 1981‐1985 Financial support: not given Adverse reactions (superficial keratopathy): none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors describe this study "Im Rahmen einer multizentrischen Prüfung" but do not provide information about the method of treatment allocation |
| Allocation concealment (selection bias) | High risk | Investigators enrolling participants could possibly have foreseen treatment assignment |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of apparent masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data within first 14 days of treatment and follow up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Sugar 1980.
| Methods | Allocation method: randomised Masking: double Number of centres: six | |
| Participants | Country: United States Number enrolled: 61 Average age (range): 46 (range not given) Sex: 43 males, 18 females Inclusion criteria: dendritic (44) or geographic (17) epithelial keratitis | |
| Interventions | Treatment one (n=27): idoxuridine 0.1% solution 19 times per day Treatment two (n=34): trifluridine 1% solution 9 times per day | |
| Outcomes | Not given | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: pharmaceutical industry Adverse reactions: "One patient taking IDU developed follicular conjunctivitis." "No patients developed allergic reactions to F3T." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "An administrative assistant dispensed medication in the double‐masked protocol on a random basis." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "During the double‐masked study the ophthalmologist who examined the patient was not aware of the drug or dosage schedules being used." [Note: Another systematic review judged this trial as not double‐blind (Guess 2007)] |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Sun 2013a.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 75 Average age (range): 30 (17‐63) Sex: 51 males, 25 females (no explanation for total of 76 instead of 75) Inclusion criteria: dendritic (51) or geographic (25) epithelial keratitis (no explanation for total of 76 instead of 75) | |
| Interventions | Treatment one (n=37): acyclovir 0.1% solution 4 times per day Treatment two (n=38): ganciclovir 0.15% gel 4 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: ofloxacin 4 times per day Report language: Chinese Study date: September 2010 ‐ January 2013 Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a randomised method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Incomplete masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Healing outcome reported at 3 weeks instead of 2 weeks |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | 75 eyes were allocated to two treatment groups but demographic information (gender and type of epithelial keratitis) were given for 76 eyes |
Sundmacher 1976a.
| Methods | Allocation method: randomised Masking: single (partial) Number of centres: one HSV isolation (cornea and conjunctiva): 55 positive of 73 tested | |
| Participants | Country: Germany Number enrolled: 55 (actually 73, but only 55 were culture‐positive) Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=17): thermomechanical débridement and placebo solution 3 times per day Treatment two (n=22): thermomechanical débridement and human leukocyte interferon 62,500 units/ml 3 times per day Treatment three (n=16): human leukocyte interferon 62,500 units/ml 3 times per day | |
| Outcomes | 'Fluorescein‐negative healing of the corneal epithelium, which was defined as complete closure of all erosions except for some single dye‐positive micropunctations' | |
| Notes | Nonstudy interventions: scopolamine, lubricating ointment, pad Report language: English Study date: not given Financial support: governmental agency | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | "Group 1 received uncoded interferon, and groups 2 and 3 were treated with coded interferon or mock interferon, respectively," using sequentially numbered drug containers of similar appearance |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Group 1 was treated with human leukocyte interferon...[while] groups 2 and 2 were studied on a double‐blind basis." However, the same investigators who performed débridement performed the outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Fifty‐five (75%) of 73 patients in the study yielded isolated herpes simplex virus before treatment, and further discussion will be confined to the results in these patients." |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Sundmacher 1976b.
| Methods | Allocation method: randomised Masking: double Number of centres: one HSV isolation (cornea): 40 positive of 51 tested | |
| Participants | Country: Germany Number enrolled: 40 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=18): thermomechanical débridement and human albumin 2 times per day Treatment two (n=22): thermomechanical débridement and human leukocyte interferon 3 million units/ml 2 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: homatropine, lubricating ointment, pad Report language: English Study date: not given Financial support: governmental agency | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Centralised allocation of coded vials, although "one vial could be used for more than one patient." Quote: "After the treatment of 51 cases, the coded results were mailed from Freiburg to Helskinki and the code from Helsinki to Freiburg on the same day." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Only those 40 cases...in which the diagnosis was virologically confirmed and the virologic follow‐up was without technical failures were accepted for final evaluation." |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Sundmacher 1978a.
| Methods | Allocation method: randomised Masking: none Number of centres: one HSV isolation (cornea): 42 positive of 42 tested | |
| Participants | Country: Germany Number enrolled: 42 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=18): thermomechanical débridement plus human leukocyte interferon 6 million units/ml once per day Treatment two (n=24): minimal wiping débridement plus human leukocyte interferon 6 million units/ml once per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: homatropine Report language: English Study date: not given Financial support: governmental agency | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The outcome assessor differed from the treating investigator. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data. |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported. |
| Other bias | High risk | Fifteen of 42 patients underwent additional débridement. |
Sundmacher 1978b.
| Methods | Allocation method: randomised Masking: double Number of centres: one HSV isolation (cornea): 38 positive of 53 tested | |
| Participants | Country: Germany Number enrolled: 38 (actually 53, but only 38 were culture‐positive) Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=18): thermomechanical débridement plus human leukocyte interferon 1 million units/ml once per day Treatment two (n=20): thermomechanical débridement plus human fibroblast interferon 1 million units/ml once per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: homatropine Report language: English Study date: not given Financial support: governmental agency | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 53 enrolled patients, "these were reduced to 38 persons with virologically proven herpes simplex virus disease" for analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Sundmacher 1981a.
| Methods | Allocation method: randomised Masking: double Number of centres: one HSV isolation (conjunctiva): eligibility criterion | |
| Participants | Country: Germany Number enrolled: 51 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis (culture‐confirmed) | |
| Interventions | Treatment one (n=24): trifluridine 1% solution 5 times per day plus human leukocyte interferon 10 million units/ml once per day Treatment two (n=27): trifluridine 1% solution 5 times per day plus human leukocyte interferon 30 million units/ml once per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Use of coded eyedropper vials |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only "virologically proven dendritic keratitis" cases were reported and analysed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Study limited to virologically confirmed participants |
Sundmacher 1981b.
| Methods | Allocation method: randomised Masking: double Number of centres: one HSV isolation (conjunctiva): eligibility criterion | |
| Participants | Country: Germany Number enrolled: 70 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis (culture‐confirmed) | |
| Interventions | Treatment one (n=20): trifluridine 1% solution 5 times per day and albumin solution once per day Treatment two (n=24): trifluridine 1% solution 5 times per day and human leukocyte interferon 1 million units/ml once per day Treatment three (n=26): trifluridine 1% solution 5 times per day and human leukocyte interferon 30 million units/ml once per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Use of coded eyedropper vials |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "For final evaluation, only the results of those patients were used who had delivered positive virus cultures before initiation of therapy." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Study limited to virologically confirmed participants |
Sundmacher 1984a.
| Methods | Allocation method: randomised Masking: double Number of centres: one HSV isolation (conjunctiva): eligibility criterion | |
| Participants | Country: Germany Number enrolled: 36 Average age (range): not given Sex: not given Inclusion criteria: dendritic (29) or geographic (7) epithelial keratitis (culture‐confirmed) | |
| Interventions | Treatment one (n=17): trifluridine 1% solution 5 times per day plus human leukocyte interferon 30 million units/ml once per day Treatment two (n=19): trifluridine 1% solution 5 times per day plus human leukocyte interferon 100 million units/ml once daily | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "α‐interferon was prepared and bottled in identical calibrated dispensing systems and then coded" by a central facility |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "The only patients accepted for final evaluation were those in whom a virus culture from the cul‐de‐sac before the initiation of therapy had yielded herpes simplex virus." |
| Selective reporting (reporting bias) | Unclear risk | "Thirty‐eight patients...were selected for the study," but the report gives results for 36, 19 in one treatment group and 17 in the other treatment group |
| Other bias | Unclear risk | Study limited to virologically confirmed participants |
Sundmacher 1985.
| Methods | Allocation method: randomised Masking: double Number of centres: one HSV isolation (conjunctiva): 32 positive of 42 tested | |
| Participants | Country: Germany Number enrolled: 32 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=17): trifluridine 1% solution 5 times per day plus human interferon‐α 30 million units/ml once per day Treatment two (n=15): trifluridine 1% solution 5 times per day plus recombinant interferon‐α‐2 23 million units/ml once per day | |
| Outcomes | Fluorescein staining 'except for minor punctate stainings' | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Centralised allocation facility |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only virologically confirmed participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Sundmacher 1987.
| Methods | Allocation method: randomised Masking: double Number of centres: one HSV isolation (conjunctiva): 45 positive of 75 tested | |
| Participants | Country: Germany Number enrolled: 45 (actually 75, but only 45 were culture‐positive and compliant) Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=16): trifluridine 1% solution 5 times per day plus recombinant interferon‐α 30 million units/ml once per day Treatment two (n=14): trifluridine 1% solution 5 times per day plus recombinant interferon‐γ 30 million units/ml once per day Treatment three (n=8): trifluridine 1% solution 5 times per day plus recombinant interferon‐α 0.3 million units/ml once per day and interferon‐γ 0.3 million units/ml once per day Treatment four (n=7): trifluridine 1% solution 5 times per day plus recombinant interferon‐α 1.5 million units/ml once per day and interferon‐γ 1.5 million units/ml once per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Coded eyedropper vials dispensed from centralized facility |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of 75 patients who entered the study 30 had to be excluded because all virus cultures turned out negative or because of noncompliance." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Tanaka 1988a.
| Methods | Allocation method: randomised Masking: double Number of centres: 18 | |
| Participants | Country: Japan Number enrolled: 36 Average age (range): 47 (not given) Sex: 19 males, 17 females Inclusion criteria: dendritic (33) or geographic (3) epithelial keratitis | |
| Interventions | Treatment one (n=16): recombinant interferon‐α‐2a (Ro22‐8181) 1000 IU/ml 4 times per day Treatment two (n=20): recombinant interferon‐α‐2a (Ro22‐8181) 10 million IU/ml 4 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: atropine, micronomicin Report language: Japanese Study date: 1983 Financial support: not given (study medicines were provided by pharmaceutical firm) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Uncertain masking despite double‐blind method |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Three patients withdrew from the study, and after the study was completed a judgment committee that reviewed eligibility forms and photographs decided to exclude 11 patients |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | Insufficient information |
Tanaka 1988b.
| Methods | Allocation method: randomised Masking: single (partial "double blind method") Number of centres: 31 | |
| Participants | Country: Japan Number enrolled: 137 Average age (range): not given Sex: 75 males, 62 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=71): idoxuridine solution every 1 or 2 hours Treatment two (n=30): recombinant interferon‐α (Ro22‐8181) 10 million IU/ml 4 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: none Report language: Japanese Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Incomplete masking despite "double blind method." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Travers 1978.
| Methods | Allocation method: not given Masking: none Number of centres: one | |
| Participants | Country: Great Britain Number enrolled: 100 Average age (range): 50 (range not given) Sex: 52 males, 48 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=50): vidarabine ointment 5 times per day Treatment two (n=50): trifluridine solution 5 times per day | |
| Outcomes | Rose‐Bengal staining | |
| Notes | Nonstudy interventions: "mydriatics and antiglaucoma therapy was given when necessary" Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors describe this study as "a controlled trial" but do not describe the method of treatment allocation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Uchida 1981.
| Methods | Allocation method: randomised by code Masking: double Number of centres: eight | |
| Participants | Country: Japan Number enrolled: 54 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Control (n=17): minimal wiping débridement and human albumin 4 times per day Treatment one (n=17): minimal wiping débridement and idoxuridine solution hourly day, 2‐hourly night Treatment two (n=20): minimal wiping débridement and fibroblast interferon 1 million units/ml 4 times per day | |
| Outcomes | 'Disappearance of gross staining areas with fluorescein' | |
| Notes | Nonstudy interventions: gentamicin solution Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "the code was kept by National Institute of Health, Tokyo, and was broken after the results had been evaluated." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Uchida 1982.
| Methods | Allocation method: randomised Masking: double Number of centres: one HSV isolation (cornea): 41 positive (by culture or immunofluorescent antigen) of 44 tested | |
| Participants | Country: Japan Number enrolled: 68 (5 others disqualified) Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=32): human fibroblast interferon 1000 units/ml 4 times per day Treatment two (n=36): human fibroblast interferon 1 million units/ml 4 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: none Report language: Japanese Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigator describes a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "Samples to be tested were coded....The code was kept by National Institute of Health Tokyo, and broken after the result had been evaluated." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Out of 73 patients subjected to the double blind test, 5 were excluded from the final evaluation; three patients did not follow the medication schedule, and two were considered to have dendrites with coexisting metaherpetic alteration before treatment." |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
van Bijsterveld 1980.
| Methods | Allocation method: randomised Masking: double Number of centres: one | |
| Participants | Country: Netherlands Number enrolled: 56 Average age (range): 46 (range not given) Sex: 36 males, 20 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=28): vidarabine 3% ointment 5 times per day Treatment two (n=28): trifluridine 2% ointment 5 times per day | |
| Outcomes | Fluorescein and rose‐Bengal staining; 'no epithelial edema and cystic changes were present in the epithelium covering the site of the original ulcer' | |
| Notes | Nonstudy interventions: scopolamine Report language: English Study date: not given Financial support: not given Adverse reactions (Quote): "In 5 out of 17 patients that received the virostatic treatment longer than 14 days a very mild diffuse epithelial staining was observed with Bengal rose. Four of these patients received ara‐A and 1 received treatment with TFT." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. The investigators did not describe how the sequence generation process was developed |
| Allocation concealment (selection bias) | Low risk | Quote: "Each patient received a coded treatment sequentially allocated by the central dispensing unit....The ointment tubes were identical in design." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Seven out of 63 treated ulcers failed to heal within 23 days, which was our arbitrary limit. These cases were excluded from the analysis." |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
van Bijsterveld 1989.
| Methods | Allocation method: randomised Masking: double Number of centres: one HSV isolation (cornea): results not reported | |
| Participants | Country: Netherlands Number enrolled: 41 Average age (range): 39 (7‐81) Sex: 22 males, 19 females Inclusion criteria: dendritic (33) or geographic (8) epithelial keratitis | |
| Interventions | Treatment one (n=22): brivudine 1% ointment 5 times per day and albumin rod daily Treatment two (n=19): brivudine 1% ointment 5 times per day and recombinant interferon‐α‐2 rod (1.5 million units) daily | |
| Outcomes | 'Wound closure' | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given Adverse reactions (Quote): "In the present investigation no toxic effects, including drug allergy, were observed." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Vannini 1986.
| Methods | Allocation method: randomised Masking: single Number of centres: one HSV isolation (conjunctiva): 13 positive of 20 tested, including 11 HSV‐1 and 2 HSV‐2 | |
| Participants | Country: Italy Number enrolled: 20 Average age (range): 41 (18‐76) Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=10): idoxuridine solution 7 times per day Treatment two (n=10): interferon‐β 1 million units/ml 7 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: pharmaceutical industry Adverse reactions: Seven cases in the idoxuridine group developed "superficial punctate keratitis [that] was evident in the days subsequent to the disappearance of dendritis (sic), which must be interpreted as a toxic effect of IDU on the corneal epithelium." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | High risk | Investigators enrolling participants could possibly have foreseen open treatment assignments |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Wang 2004.
| Methods | Allocation method: randomised Masking: single Number of centres: one | |
| Participants | Country: China Number enrolled: 60 Average age (range): 36 (7‐65) Sex: 32 males, 28 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=30): topical acyclovir 0.1% solution 5 to 8 times per day Treatment two (n=30): oral acyclovir 200 mg 5 times per day | |
| Outcomes | Epithelial healing | |
| Notes | Nonstudy interventions: none Report language: Chinese Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Wang 2014a.
| Methods | Allocation method: randomised Masking: single Number of centres: one | |
| Participants | Country: China Number enrolled: 104 Average age (range): 38 (18‐71) Sex: 76 males, 28 females Inclusion criteria: epithelial keratitis (shallow, deep, combined) | |
| Interventions | Treatment one (n=52): topical acyclovir solution 8 times per day Treatment two (n=52): topical ganciclovir gel 5 times per day | |
| Outcomes | Fluorescein staining ('治愈:患者患眼的刺激症状消失,充血消退, 角膜基质无水肿和浸润, 后弹力层褶皱消退, 角膜后沉着物吸收, 溃疡愈合良好, 荧光色染色呈阴性, 房水闪辉呈阴性') | |
| Notes | Nonstudy interventions: none
Report language: Chinese
Study date: December 2011 to December 2013
Financial support: not given Adverse reactions: 11 occurrences of pain or discomfort in acyclovir group and 3 occurrences of pain or discomfort in ganciclovir group Recurrence rate: in one year, 11 episodes in acyclovir group and 3 episodes in ganciclovir group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a randomised method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Healing outcome reported at 3 weeks instead of 2 weeks |
| Selective reporting (reporting bias) | Unclear risk | The pre‐specified primary outcome was adequately reported, but the report did not stratify outcomes for participants treated with topical corticosteroid for stromal keratouveitis |
| Other bias | High risk | Among 104 eyes, the investigators enrolled 41 eyes with shallow keratitis, 35 eyes with deep keratitis, and 28 eyes with mixed type. |
Wellings 1972.
| Methods | Allocation method: randomised by table Masking: double Number of centres: two | |
| Participants | Country: Great Britain and United States Number enrolled: 78 Average age (range): not given Sex: not given Inclusion criteria: dendritic (69) or geographic (9) epithelial keratitis | |
| Interventions | Treatment one (n=38): idoxuridine 0.1% solution 5 times per day Treatment two (n=40): trifluridine 1% solution 5 times per day | |
| Outcomes | Fluorescein and rose‐Bengal staining | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: governmental agency Adverse reactions: "No symptoms or signs were observed in either center during the two‐week period of administration of either drug that could be ascribed to side effect of toxicity from, or allergy to either preparation." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "Each patient received randomly allocated coded treatment by the pharmacist, according to prearranged tables held in the pharmacy....The two preparations were indistinguishable other than by the code." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "In no case during the trial was any observer, or any patient, aware of the identity of the antiviral drug being used." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Patients whose ulcers failed to heal were taken out of the trial and treated by débridement or if slow healing had been taking place under the coded antiviral therapy, this was continued for a further short period." |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Wilhelmus 1981a.
| Methods | Allocation method: randomised by table Masking: none Number of centres: one | |
| Participants | Country: Great Britain Number enrolled: 50 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=25): minimal wiping débridement and acyclovir 3% ointment 5 times per day Treatment two (n=25): acyclovir 3% ointment 5 times per day | |
| Outcomes | 'Disappearance of specific Bengal‐rose staining of the precise site of the healing dendritic ulceration' | |
| Notes | Nonstudy interventions: atropine Report language: English Study date: not given Financial support: private foundation Adverse reactions (Quote): "Whether debridement was performed or not, the only adverse symptom related to topical acyclovir was a mild transient stinging sensation immediately after application of the antiviral ointment in nine (18%) patients. We found mild punctate epithelial staining with Bengal rose of the inferior bulbar conjunctiva and corneoscleral limbus remote from the original lesion in eight (16%) patients...[that] disappeared within three days after discontinuation of the antiviral medication." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Central allocation of treatment assignment |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Xu 2009a.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 36 Average age (range): 45 (10‐68) Sex: 19 males, 17 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=18): acyclovir 0.1% solution 4 times per day Treatment two (n=18): ganciclovir 0.15% gel 4 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: none Report language: Chinese Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | Insufficient information |
Yamazaki 1984a.
| Methods | Allocation method: not given Masking: single Number of centres: one | |
| Participants | Country: Japan Number enrolled: 40 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=21): minimal wiping débridement and idoxuridine 0.1% solution Treatment two (n=19): minimal wiping débridement and fibroblast interferon 20,000 units/ml | |
| Outcomes | Not given | |
| Notes | Nonstudy interventions: not given Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors describe these studies as "clinical trials" (and reference collaborators whose trial was randomised) but do not provide sufficient information on the method of treatment allocation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Yamazaki 1984b.
| Methods | Allocation method: not given Masking: single Number of centres: one | |
| Participants | Country: Japan Number enrolled: 74 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=41): albumin solution 4 times per day Treatment two (n=33): human leukocyte interferon 20 million units/ml 4 times per day | |
| Outcomes | Not given | |
| Notes | Nonstudy interventions: not given Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors describe these studies as "clinical trials" (and reference collaborators whose trial was randomised) but do not provide sufficient information on the method of treatment allocation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Yamazaki 1984c.
| Methods | Allocation method: not given Masking: single Number of centres: one | |
| Participants | Country: Japan Number enrolled: 36 Average age (range): not given Sex: not given Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=16): recombinant interferon‐α 1000 units/ml 4 times per day Treatment two (n=20): recombinant interferon‐α 10 million units/ml 4 times per day | |
| Outcomes | Not given | |
| Notes | Nonstudy interventions: not given Report language: English Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors describe these studies as "clinical trials" (and reference collaborators whose trial was randomised) but do not provide sufficient information on the method of treatment allocation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Yang 2000.
| Methods | Allocation method: not given Masking: double Number of centres: one | |
| Participants | Country: China Number enrolled: 137 Average age (range): 33 (1‐65) Sex: 93 males, 44 females Inclusion criteria: dendritic (55) epithelial keratitis | |
| Interventions | Treatment one (n=70): acyclovir 0.1% solution Treatment two (n=67): ganciclovir 0.1% solution | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: not given Report language: Chinese Study date: not given Financial support: governmental agency | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors describe "a double blind clinical investigation" but do not describe the method of treatment allocation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Yang 2008.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 62 Average age (range): 40 (20‐73) Sex: 27 males, 35 females Inclusion criteria: dendritic (49) or geographic (13) epithelial keratitis | |
| Interventions | Treatment one (n=30): acyclovir 0.1% solution 6 times per day Treatment two (n=32): ganciclovir 0.15% gel 4 times per day | |
| Outcomes | Fluorescein staining ('荧光素钠染色阴性') | |
| Notes | Nonstudy interventions: atropine 1% for iridocyclitis; oral indomethacin 25 mg 3 times per day Report language: Chinese Study date: 2010‐2012 Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Incomplete primary outcome data (outcome day unclear) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Yeakley 1981.
| Methods | Allocation method: randomised by code Masking: double Number of centres: one HSV isolation (conjunctiva): 13 positive of 40 tested | |
| Participants | Country: United States Number enrolled: 40 Average age (range): 49 (7‐81) Sex: 27 males, 13 females Inclusion criteria: dendritic (38) or geographic (2) epithelial keratitis | |
| Interventions | Treatment one (n=21): vidarabine 3% ointment 5 times per day Treatment two (n=19): acyclovir 3% ointment 5 times per day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: none Report language: English Study date: not given Financial support: not given Adverse reactions (Quote): "Diffuse superficial punctate epitheliopathy was noted in one patient on acyclovir...and resolved within two days after the drug was discontinued. No other adverse effects were noted in either drug treatment group during the course of therapy." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "The drugs were packaged in sequential order by random code number designation and were dispensed sequentially." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients used the medication as directed, and no patient was lost to follow‐up prior to complete corneal re‐epithelialization." |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Young 1982.
| Methods | Allocation method: randomised Masking: double Number of centres: one HSV isolation (conjunctiva): 35 positive of 93 tested | |
| Participants | Country: Great Britain Number enrolled: 93 Average age (range): 51 (range not given) Sex: 62 males, 31 females Inclusion criteria: dendritic (85) or geographic (8) epithelial keratitis | |
| Interventions | Treatment one (n=45): vidarabine 3% ointment 5 times per day Treatment two (n=48): acyclovir 3% ointment 5 times per day | |
| Outcomes | Rose Bengal | |
| Notes | Nonstudy interventions: atropine Report language: English Study date: not given Financial support: pharmaceutical industry Adverse reactions (Quote): "Two patients developed adverse symptoms during therapy possibly due to their antiviral therapy. One patient on acyclovir experienced an allergic response; this could also have been caused by the atropine drops being administered concurrently. One patient on ara‐A complained of grittiness after application of the ointment. No other adverse effect was seen in this group of patients." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "The ophthalmic ointments were provided in identical tubes bearing a trial number, and the entire study was carried out in a double‐blind manner." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐masked trial design described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Yu 2012a.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 60 Average age (range): 39 (17‐60) Sex: 32 males, 28 females Inclusion criteria: dendritic (36) or geographic (24) epithelial keratitis | |
| Interventions | Treatment one (n=30): ganciclovir 0.1% solution 8 times per day Treatment two (n=30): foscarnet 3% solution 6 times per day | |
| Outcomes | Epithelial healing | |
| Notes | Nonstudy interventions: none Report language: Chinese Study date: 2008‐2009 Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Unclear risk | Insufficient information |
Zhang 2014.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 84 Average age (range): 39 (22‐60) Sex: 46 males, 38 females Inclusion criteria: dendritic (55) or geographic (29) epithelial keratitis | |
| Interventions | Treatment one (n=42): acyclovir 0.1% solution every 2 hours Treatment two (n=42): ganciclovir 0.15% gel 5 times per day | |
| Outcomes | Fluorescein staining ('患者的眼部刺激症状消失, 角膜充血消退, 溃疡得到修复, 并且荧光素染色转为阴性, 浸润和水肿消失, 角膜后的沉着物呈现色素性或者消失') | |
| Notes | Nonstudy interventions: none Report language: Chinese Study date: January 2012 ‐ June 2013 Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a randomised method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified primary outcome was adequately reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Zhao 2006.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 124 Average age (range): 37 (12‐64) Sex: 81 males, 43 females Inclusion criteria: epithelial keratitis (106) or stromal keratitis (18) | |
| Interventions | Treatment one (n=62): acyclovir solution every 1‐2 hours Treatment two (n=62): ganciclovir gel 4 times per day | |
| Outcomes | Fluorescein staining ('刺激症状消失, 知觉恢复, 溃疡面愈合, 基质层水肿消失, 荧光素染色阴性') | |
| Notes | Nonstudy interventions: atropine 1% and "antibiotic eye drop" Report language: Chinese Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a randomised method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Incomplete masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Uncertain time when healing outcome reported |
| Selective reporting (reporting bias) | Unclear risk | The pre‐specified primary outcome was adequately reported, but the report did not stratify outcomes for participants treated with topical corticosteroid for stromal keratouveitis |
| Other bias | High risk | Among 124 eyes, the investigators enrolled 15% of eyes having stromal keratitis and did not separately report outcomes for epithelial keratitis (106) and disciform keratitis (18) |
Zhen 2012.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 128 Average age (range): not given (15‐65) Sex: 55 males, 73 females Inclusion criteria: dendritic or geographic epithelial keratitis (some eyes may have had disciform stromal keratitis) | |
| Interventions | Treatment one (n=64): acyclovir 0.1% solution 8 times per day Treatment two (n=64): ganciclovir 0.15% gel 4 times per day | |
| Outcomes | Fluorescein staining ('荧光素染色阴性') | |
| Notes | Nonstudy interventions: atropine 1% for iridocyclitis; dexamethasone 0.05% for keratouveitis Report language: Chinese Study date: 2009‐2012 Financial support: university | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Incomplete primary outcome data (outcome day unclear) |
| Selective reporting (reporting bias) | Unclear risk | The pre‐specified primary outcome was adequately reported, but the report did not stratify outcomes for participants treated with topical corticosteroid for stromal keratouveitis |
| Other bias | High risk | Insufficient information |
Zheng 2010.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 210 (not including 38 cases of stromal keratitis) Average age (range): not given (18‐65) Sex: 185 males, 62 females (gender distribution for epithelial keratitis patients not reported separately) Inclusion criteria: dendritic (178) or geographic (31) epithelial keratitis (38 cases, including 35 disciform keratitis and 3 necrotizing keratitis, are being excluded from this analysis) | |
| Interventions | Treatment one (n=104): ganciclovir 0.1% solution 6 times per day Treatment two (n=105): ganciclovir 0.15% gel 4 times per day | |
| Outcomes | Healing ('治愈: 刺激症状消失, 知觉恢复, 溃疡面愈合') | |
| Notes | Nonstudy interventions: mydriatic and subconjunctival dexamethasone 3 mg for stromal keratitis Report language: Chinese Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a randomised method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Incomplete masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data |
| Selective reporting (reporting bias) | Unclear risk | Vague description of assessing healing |
| Other bias | Unclear risk | Among 247 eyes, outcome data for 209 eyes having epithelial keratitis were extracted while ignoring 38 eyes with corticosteroid‐treated disciform or stromal keratitis |
Zhu 2012.
| Methods | Allocation method: randomised Masking: none Number of centres: one | |
| Participants | Country: China Number enrolled: 74 eyes (68 participants) Average age (range): not given (16‐67) Sex: 32 males, 36 females Inclusion criteria: dendritic epithelial keratitis | |
| Interventions | Treatment one (n=38): ganciclovir 0.15% gel 4 times per day Treatment two (n=36): ganciclovir 0.15% gel 4 times per day + interferon 1 million units/ml (0.5 ml) subconjunctival injection every other day | |
| Outcomes | Fluorescein staining | |
| Notes | Nonstudy interventions: none Report language: Chinese Study date: not given Financial support: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Lack of masking could have influenced outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Incomplete primary outcome data (outcome day unclear) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abboud 1967 | Non‐concurrent treatment allocation Treatment one (n=39): idoxuridine 0.1% solution Treatment two (n=24, subset of treatment one following failure to heal within one week using idoxuridine): iodine cauterisation |
| Akberova 2000 | Non‐concurrent treatment allocation Treatment one (n=118): para‐aminobenzoic acid 0.007% (Aktipal), an interferon inducer, 4 to 6 times per day Treatment two (n=34): idoxuridine solution 8 to 10 times per day Treatment three (n=41): acyclovir ointment 5 times per day |
| Assetto 1981 | Insufficient data provided Treatment one (n=8): wiping debridement Treatment two (n=8): idoxuridine 0.1% solution 5 times per day Treatment three (n=8): cytarabine solution 5 times per day Treatment four (n=8): idoxuridine 0.1% solution and oral isoprinosine 3 times per day Treatment five (n=8): cytarabine solution and oral isoprinosine 3 times per day Treatment six (n=8): trifluridine 1% solution 5 times per day |
| Babushkin 1993 | Insufficient data provided Treatment one (n=60): idoxuridine 0.1% solution Treatment two (n=24): acyclovir 3% ointment |
| Bianchetti 1964 | Non‐concurrent treatment allocation Treatment one (n=31): idoxuridine 0.1% solution (Stoxil or Dispersa) Treatment two (n=33): idoxuridine ointment or gel (Dispersa or Chauvin‐Blache) |
| Butikova 1990 | Non‐concurrent treatment allocation Control (n=10): historical controls Treatment one (n=18): antiherpetic immunoglobulin |
| Cao 2012 | Botanical or herbal preparation Treatment one (n=30): ganciclovir 0.15% gel Treatment two (n=30): ganciclovir 0.15% gel and botanical extract |
| Cao 2013 | Botanical or herbal preparation Treatment one (n=28): acyclovir 0.1% solution 4 times daily and oral antiviral (plus dexamethsone and tobramycin for stromal keratitis) Treatment two (n=30): acyclovir 0.1% solution 4 times daily and oral antiviral and oral botanical extract twice daily (plus dexamethonse and tobramycin for stromal keratitis) |
| Chen 2000 | Botanical or herbal preparation Treatment one (n=80): antiviral solution Treatment two (n=86): antiviral solution and botanical extract |
| Chen 2007 | Insufficient data provided Treatment one (n=43): acyclovir 0.1% solution Treatment two (n=69): acyclovir 0.1% solution and recombinant interferon‐α‐2b and intravenous acyclovir |
| Chen 2009 | Botanical or herbal preparation Treatment one (n=54): antiviral Treatment two (n=46): antiviral and botanical extract |
| Corina 1998 | Outcome not based on epithelial healing Treatment one (n=65): idoxuridine Treatment two (n=76): acyclovir ointment |
| Corina 1999 | Botanical or herbal preparation Treatment one (n=26): acyclovir ointment Treatment two (n=26): acyclovir ointment with botanical extract |
| Deng 2003 | Botanical or herbal preparation Treatment one (n=53): acyclovir 0.1% solution Treatment two (n=53): acyclovir 0.1% solution and subconjunctival ribavirin and botanical extract |
| Du 2008 | Botanical or herbal preparation Treatment one (n=71): antiviral Treatment two (n=78): antiviral and traditional Chinese medicine |
| Dundarov 1998 | Non‐concurrent treatment allocation Control (n=19): "symptomatic medicines" Treatment one (n=55): pandavir (nigericin 0.001%) 2 times on first day then 10 to 12 times per day Treatment two (n=52): pandavir (nigericin 0.001%) with interferon‐α 10,000 to 50,000 IU/ml 2 times on first day then 10 to 12 times per day |
| Elze 1979 | Non‐concurrent treatment allocation Treatment one (n=97): ethyldeoxyuridine 0.15% solution topically and 0.5% subconjunctivally daily Treatment two (n=71): ethyldeoxyuridine 2% solution topically and 0.5% subconjunctivally and 0.3% gel, with initial iodinisation debridement |
| Fellinger 1980 | Insufficient data provided Treatment one (n=13): idoxuridine 0.1% solution and 0.5% ointment Treatment two (n=16): trifluridine 1% solution and 2% ointment Treatment three (n=13): iodinisation debridement and idoxuridine Treatment four (n=18): iodinisation debridement and trifluridine |
| Feng 2004 | Botanical or herbal preparation Treatment one (n=52): acyclovir 0.1% solution Treatment two (n=52): acyclovir 0.1% solution and botanical extract |
| Galin 1976 | Insufficient data provided Treatment one (n=20): idoxuridine 0.5% solution Treatment two (n=19): polyinosinic‐polycytidylic acid 0.1% solution |
| Gao 2006 | Botanical or herbal preparation Treatment one (n=42): antiviral therapy Treatment two (n=44): antiviral therapy and botanical extract |
| Gilkes 1963 | Non‐concurrent treatment allocation Treatment one (n=77): historical controls treated by "carbolization or iodization" debridement Treatment two (n=82): idoxuridine 0.1% solution hourly or 0.5% ointment 2‐hourly |
| Gong 2013 | Botanical or herbal preparation Treatment one (n=55): antiviral Treatment two (n=55): antiviral and botanical extract |
| Gu 2005 | Insufficient data provided Treatment one (n=20): acyclovir 0.1% solution 4 to 6 times per day Treatment two (n=19): acyclovir 0.1% solution 4 to 6 times per day and recombinant interferon‐α‐2b 3 to 4 times per day |
| Gulinuer 2011 | Non‐concurrent/non‐randomised treatment allocation Treatment one (n=37): acyclovir 0.1% solution 6 times per day Treatment two (n=48): ganciclovir 0.15% gel 4 times per day |
| Gundersen 1936 | Non‐concurrent treatment allocation Control (n=53): historical controls using "various mydriatics and antiseptics" Treatment one (n=97): focal iodinisation debridement Treatment two (n=45): entire corneal iodinisation debridement |
| Guo 2003 | Insufficient data provided Treatment one: (n=34): "conventional antiviral therapy" Treatment two (n=35): cryotherapy and "conventional antiviral therapy" |
| Hao 2008 | Botanical or herbal preparation Treatment one (n=60): antiviral Treatment two (n=68): antiviral and botanical extract |
| He 2014 | Insufficient data provided Treatment one (n=34): antiviral Treatment two (n=34): autologous serum |
| Herbort 1987 | Non‐concurrent treatment allocation Treatment one (n=20): scraping debridement with trifluridine 1% solution 8 times daily for one day then 5 times daily Treatment two (n=11): trifluridine 1% solution 8 times daily for one day then 5 times daily |
| Hilsdorf 1969 | Insufficient data provided Treatment one (n=20): iodinisation debridement with idoxuridine ointment Treatment two (n=20): cryotherapy with idoxuridine ointment |
| Horodeňscy 1979 | Non‐concurrent treatment allocation Treatment one (n=17): idoxuridine 0.1% solution Treatment two (n=17): cytarabine 0.1% solution |
| Hu 2013 | Botanical or herbal preparation Treatment one (n=30): ganciclovir Treatment two (n=40): ganciclovir and botanical extract |
| Huang 2007 | Insufficient data provided Treatment one (n=62): acyclovir 0.1% solution Treatment two (n=62): ganciclovir 0.15% gel |
| Huang 2008b | Botanical or herbal preparation Treatment one (n=33): antiviral Treatment two (n=33): antiviral and botanical extract |
| Huang 2009 | Insufficient data provided Treatment one (n=36): acyclovir 0.1% solution Treatment two (n=29): acyclovir 0.1% solution and recombinant interferon‐α solution |
| Huang 2013 | Botanical or herbal preparation Treatment one (n=34): ganciclovir 0.15% gel 4 times daily Treatment two (n=34): ganciclovir 0.15% gel 4 times daily and oral botanical extract (qinggan jiedu) |
| Inocencio 1982 | Insufficient data provided Treatment one (n=9): idoxuridine 0.5% ointment 5 times daily Treatment two (n=14): acyclovir 3% ointment 5 times daily |
| Jiang 2011 | Varied eligibility criteria (both shallow and deep forms) Treatment one (n=22, shallow type): acyclovir 0.1% solution + basic fibroblast growth factor Treatment two (n=23, shallow type): acyclovir 0.1% solution + tobramycin‐dexamethasone |
| Jin 1992 | Insufficient data provided Treatment one (n=41): acyclovir 0.1% solution Treatment two (n=59): recombinant human interferon‐α 1 million IU/ml |
| Jing 2010 | Insufficient data provided Treatment one (n=22): acyclovir 0.1% solution 5 times daily Treatment two (n=24): ganciclovir 0.15% gel 5 times daily |
| Jones 1979 | Outcome not based on epithelial healing Treatment one (n=12): wiping debridement and placebo ointment 5 times per day Treatment two (n=13): wiping debridement and acyclovir 3% ointment 5 times per day |
| Kasparov 1972 | Insufficient data provided Treatment one (n=16): idoxuridine Treatment two (n=43): polyadenylic:polyuridylic acid solution Treatment three (n=167): various immunomodulators such as mebavin (IBC) Treatment four (n=33): immunomodulator(s) with debridement by either diathermy or cryotherapy |
| Kasparov 1974 | Insufficient data provided Control (n=30): "nonspecific drugs, such as antibiotics, sulphanyl amides, vitamins" Treatment one (n=28): idoxuridine 0.1% solution Treatment two (n=27): polyadenylic‐polyuridylic acid solution Treatment three (n=15): idoxuridine 0.1% solution and polyadenylic‐polyuridylic acid solution |
| Kasparov 1990 | Non‐concurrent treatment allocation Treatment one (n=46): idoxuridine Treatment two (n=80): thermal debridement and polyadenylic‐polyuridylic acid solution in soft contact lens |
| Kasparov 1991 | Insufficient data provided Treatment one (n=45): acyclovir 3% ointment Treatment two (n=65): polyadenylic‐polyuridylic acid solution Treatment three (n=40): acyclovir 3% ointment and polyadenylic‐polyuridylic acid solution |
| Koev 2007 | Insufficient data provided Treatment one (n=25): acyclovir ointment Treatment two (n=24): acyclovir ointment and pandavir Treatment three (n=26): acyclovir ointment and pandavir and laser debridement |
| Kolomiets 1986 | Insufficient data provided Treatment one (n=10): non‐specific immunoglobulin Treatment two (n=14): antiherpetic immunoglobulin |
| Kuyama 1979 | Insufficient data provided Treatment one (n=not stated): interferon‐α 1 million IU/ml Treatment two (n=not stated): interferon‐β 1 million IU/ml |
| Leopold 1965 | Insufficient data provided Treatment one (n=5): idoxuridine solution Treatment two (n=5): cytarabine solution |
| Li 1998 | Botanical or herbal preparation Treatment one (n=30): acyclovir 0.1% solution and botanical extract (Ban Lan Gen) by subconjunctival and intramuscular injection Treatment two (n=31): acyclovir 0.1% solution and ribavirin by subconjunctival injection |
| Li 2012 | Botanical or herbal preparation Treatment one (n=39): acyclovir 0.1% solution Treatment two (n=41): acyclovir 0.1% solution and botanical extract |
| Li 2013c | Botanical or herbal preparation Treatment one (n=85): acyclovir Treatment two (n=90): acyclovir and botanical extract |
| Li 2014a | Botanical or herbal preparation Treatment one (n=44): antiviral Treatment two (n=48): antiviral and botanical extract and oral transfer factor |
| Li 2014b | Botanical or herbal preparation Treatment one (n=40): antiviral Treatment two (n=40): botanical extract |
| Li 2014c | Insufficient data provided Treatment one (n=21): ganciclovir gel and acyclovir solution Treatment two (n=21): ganciclovir gel and basic fibroblast growth factor |
| Lin 2009 | Insufficient data provided Treatment one (n=27): acyclovir Treatment two (n=22): acyclovir and polyinosinic‐polycytidylic acid injection |
| Lin 2013a | Outcome not based on epithelial healing (total score of symptoms and signs) Treatment one (n=114): ganciclovir 0.15% gel Treatment two (n=116): ganciclovir 0.15% in situ gel |
| Lin 2013b | Insufficient data provided (unclear time until outcome assessment) Treatment one (n=33): ganciclovir 0.1% solution 4 times daily + levofloxacin solution 4 times daily Treatment two (n=33): ganciclovir 0.1% solution 4 times daily + levofloxacin solution 4 times daily + subconjunctival interferon one million units/ml (0.3 ml) every other day |
| Liu 2003 | Insufficient data provided Treatment one (n=21): topical antiviral Treatment two (n=21): topical antiviral and subconjunctival interferon |
| Liu 2007 | Insufficient data provided Treatment one (n=50): acyclovir 0.1% solution Treatment two (n=46): acyclovir 0.1% solution and fibroblast growth factor |
| Liu 2009b | Botanical or herbal preparation Treatment one (n=48): acyclovir Treatment two (n=48): acyclovir and botanical extract |
| Liu 2014b | Insufficient data provided Treatment one (n=not given): acyclovir solution Treatment two (n=not given): periocular injection of polyinosinic acid 0.5 mg every other day Treatment three (n=not given): acyclovir solution and periocular polyinosinic acid 0.5 mg every other day (Total enrolled=38 patients, 60 eyes) |
| Long 2011 | Varied eligibility criteria (25 eyes with HSV keratitis and 19 eyes with herpes zoster keratitis) Treatment one (n=13, HSV keratitis): acyclovir 0.1% solution 6‐8 times per day Treatment two (n=12, HSV keratitis): acyclovir 0.1% solution 6‐8 times per day + interferon eye drops 4 times per day + subconjunctival (1 mL) interferon one million units/mL every 1‐2 days (6‐8 injections) |
| Ma 1982 | Non‐concurrent treatment allocation Treatment one (n=29): idoxuridine 0.1% solution Treatment two (n=8): vidarabine 0.5% solution Treatment three (n=8): acyclovir 0.1% solution Treatment four (n=11): foscarnet 1% to 5% solution Treatment five (n=58): cyclocytidine 0.05% solution Treatment six (n=14): cyclocytidine 0.1% solution Treatment seven (n=6): cytarabine 0.025% solution Treatment eight (n=4): cyclocytidine monophosphate 0.5% solution |
| Ma 2006a | Botanical or herbal preparation Treatment one (n=60): acyclovir 0.1% solution 4 times per day Treatment two (n=60): acyclovir 0.1% solution 4 times per day and botanical extract 4 times per day |
| Ma 2010 | Botanical or herbal preparation Treatment one (n=42): acyclovir solution Treatment two (n=40): botanical extract |
| Ma 2011 | Botanical or herbal preparation Treatment one (n=34): acyclovir solution Treatment two (n=34): botanical extract |
| Ma 2013 | Botanical or herbal preparation Treatment one (n=29): antiviral Treatment two (n=29): botanical extract |
| Maichuk 1990 | Varied eligibility criteria Treatment one (n=28): oral pirprofen Treatment two (n=24): intramuscular pirprofen Treatment three (n=29): oral indomethacin |
| Mal'khanov 1991 | Varied eligibility criteria Treatment one (n=15): thymalin (thymic peptide) Treatment two (n=12): tactivin (thymic peptide) Treatment three (n=48): pirogenal (bacterial lipopolysaccharide) Treatment four (n=20): gamma‐globulin |
| Marquardt 1971 | Non‐concurrent treatment allocation Treatment one (n=87): thermocauterisation debridement Treatment two (n=75): scraping debridement Treatment three (n=64): idoxuridine Treatment four (n=64): unspecified treatment |
| Martenet 1979 | Non‐concurrent treatment allocation Treatment one (n=8): idoxuridine 0.1% solution hourly during day and 0.25% ointment at night Treatment two (n=1): iododeoxycytidine 0.15% solution hourly during day and 1% ointment at night |
| Matalia 1987 | Non‐concurrent treatment allocation Treatment one (n=102, eyes with epithelial keratitis): idoxuridine Treatment two (n=104, eyes with epithelial keratitis): vidarabine Treatment three (n=22, eyes with epithelial keratitis): trifluridine Treatment four (n=100, eyes with epithelial keratitis): acyclovir Treatment five (n=36, eyes with epithelial keratitis): vidarabine and idoxuridine Treatment six (n=52, eyes with epithelial keratitis): vidarabine and acyclovir |
| Mathur 1984 | Insufficient data provided Treatment one (n=50): iodinisation debridement Treatment two (n=20): cryotherapy debridement Treatment three (n=24): cryotherapy debridement and topical autologous serum Treatment four (n=30): wiping debridement Treatment five (n=30): wiping debridement and idoxuridine 0.1% solution 3 times per day |
| McGill 1981 | Insufficient data provided Treatment one (n=29): vidarabine 3% ointment 5 times per day Treatment two (n=28): acyclovir 3% ointment 5 times per day |
| Mohan 1987 | Insufficient data provided Treatment one (n=19): vidarabine 3% ointment 5 times per day Treatment two (n=21): acyclovir 3 ointment 5 times per day |
| Morimoto 1986 | Non‐concurrent treatment allocation Treatment one (n=22): idoxuridine ointment Treatment two (n=15): acyclovir 3% ointment |
| Patterson 1967c | Insufficient data provided Inclusion criteria: 24 eyes with corticosteroid‐enhanced epithelial keratitis: 22 eyes with geographic "amoeboid" epithelial keratitis and two eyes with "multiple linear ulcers"; "a severe stromal keratitis was often present." Treatment one (n=9): cauterisation, debridement Treatment two (n=15): idoxuridine ointment 5 times per day |
| Pavan‐Langston 1972 | Insufficient data provided Treatment one (n=14): idoxuridine 0.5% ointment 3 to 5 times per day Treatment two (n=15): vidarabine 3.3% ointment 3 to 5 times per day |
| Pavan‐Langston 1977 | Insufficient data provided Treatment one (n=17): idoxuridine 0.1% solution 19 times per day Treatment two (n=23): trifluridine 1% solution 9 times per day |
| Pietruschka 1968 | Insufficient data provided Treatment one (n=30): para‐fluorophenylalanine 0.1% solution hourly Treatment two (n=28): idoxuridine 0.1% solution hourly Treatment three (n=40): para‐fluorophenylalanine 0.1 % solution and idoxuridine 0.1% solution hourly, alternating |
| Pintér 1973 | Non‐concurrent treatment allocation Treatment one (n=16): photoinactivation with acridine orange Treatment two (n=6): idoxuridine with or without oral moroxydine Treatment three (n=2): idoxuridine and thermal debridement Treatment four (n=8): iodinisation debridement Treatment five (n=2): miscellaneous interventions including autologous blood injection (1) |
| Pivetti‐Pezzi 1985 | Insufficient data provided Treatment one (n=13): intramuscular placebo daily with "local conventional therapy" Treatment two (n=11): intramuscular thymic extract daily with "local conventional therapy" |
| Prost 1986 | Insufficient data provided Treatment one (n=20): cryotherapy and oral placebo Treatment two (n=19): cryotherapy and oral isoprinosine |
| Rykun 1988 | Non‐concurrent, non‐randomised treatment allocation Treatment one (n=38, dendritic type): antiviral (idoxuridine) Treatment two (n=22, dendritic type): antiviral + α‐tocopherol 10% solution |
| Salcedo Hernandez 2007 | Insufficient data provided Treatment one (n=9): diluted autologous serum 4 times per day Treatment two (n=8): acyclovir 3% ointment 4 times per day |
| Scialdone 1986 | Insufficient data provided Treatment one (n=8): idoxuridine solution 6 times per day and oral isoprinosine and intramuscular thymic extract Treatment two (n=12): interferon‐β solution 6 times per day and oral isoprinosine and intramuscular thymic extract |
| Sellitti 1982 | Insufficient data provided Treatment one (n=20): isoprinosine solution and oral isoprinosine and cytosine solution every 2 hours Treatment two (n=20): oral isoprinosine and cytosine solution every 2 hours |
| Shimomura 1987 | Insufficient data provided Treatment one (n=16): wiping debridement and idoxuridine solution every hour Treatment two (n=15): idoxuridine solution every hour |
| Shiota 1979 | Non‐concurrent treatment allocation Treatment one (n=16): vidarabine 3% ointment Treatment two (n=9): trifluridine 1% solution |
| Shiota 1988 | Insufficient data provided Treatment one (n=14): interferon‐β 100,000 IU/ml Treatment two (n=46): interferon‐β 1 million IU/ml |
| Sozen 2006 | Varied eligibility criteria Treatment one (n=15): acyclovir 3% ointment 5 times per day Treatment two (n=15, of 13 patients): oral valacyclovir 1 g 2 times per day |
| Stambuk 1995 | Outcome not based on epithelial healing Treatment one (n=8): idoxuridine ointment or acyclovir ointment Treatment two (n=12): bovine thymic extract and either idoxuridine ointment or acyclovir ointment |
| Su 2010 | Insufficient data provided Treatment one (n=40 eyes, 29 patients): ganciclovir 0.15% gel 4 times per day Treatment two (n=38 eyes, 30 patients): ganciclovir 0.15% gel 4 times per day + subconjunctival interferon one million units/mL (1 ml) every other day (6‐8 injections) |
| Sun 2012 | Botanical or herbal preparation Treatment one (n=35): acyclovir solution 4‐6 times per day Treatment two (n=33): acyclovir solution 4‐6 times per day + intravenous yan hu ning (dehydroandrographolide succinate) 400 mg in 250 mL saline, repeated every third day for 10 days |
| Sun 2014 | Botanical or herbal preparation Treatment one (n=34): acyclovir solution Treatment two (n=34): acyclovir solution + oral vitamin C |
| Tamburi 1990 | Insufficient data provided Treatment one (n=8): acyclovir 3% ointment 4 times per day Treatment two (n=8): interferon‐α 3 million IU/ml 8 times per day Treatment three (n=8): acyclovir 3% ointment 4 times per day and interferon‐α 3 million IU/ml 8 times per day Note: 3 randomised groups were also compared to a historical group treated with idoxuridine (n=8) |
| Tarakji 1978 | Insufficient data provided Treatment one (n=21): cryotherapy Treatment two (n=14): idoxuridine solution hourly and ointment at night |
| Tommila 1963 | Non‐concurrent treatment allocation Treatment one (n=17): iodinisation debridement Treatment two (n=17): interferon‐β solution every 1 to 3 hours |
| Topciu 1992 | Insufficient data provided Treatment one (n=120): idoxuridine and foscarnet Treatment two (n=113): idoxuridine and foscarnet and oral moroxydine and percutaneous vaccinia virus antigens and subcutaneous heat‐killed Corynebacterium parvum |
| Wan 2014 | Insufficient data provided (average healing time and outcome assessment at 4 weeks) Treatment one (n=24): ganciclovir 0.15% gel 6 times daily Treatment two (n=26): ganciclovir 0.15% gel 6 times daily + recombinant human interferon α‐2b solution 6 times daily |
| Wang 2009 | Insufficient data provided Treatment one (n=39): acyclovir 0.1% solution 10 times per day Treatment two (n=39): ganciclovir 0.15% gel 5 times per day |
| Wang 2010a | Botanical or herbal preparation Treatment one (n=102): ganciclovir gel + intravenous acyclovir Treatment two (n=111): ganciclovir gel + intravenous acyclovir + botanical extract |
| Wang 2010b | Botanical or herbal preparation Treatment one (n=42): acyclovir 0.1% solution + subconjunctival acyclovir Treatment two (n=45): acyclovir 0.1% solution + subconjunctival acyclovir + botanical extract |
| Wang 2011 | Botanical or herbal preparation Treatment one (n=48): acyclovir 0.1% solution Treatment two (n=108): acyclovir 0.1% solution + botanical extract |
| Wang 2014b | Botanical or herbal preparation Treatment one (n=106): ganciclovir 0.15% gel Treatment two (n=107): ganciclovir 0.15% gel and botanical extract (yiqi jiedu) |
| Wei 2012 | Insufficient data provided Treatment one (n=24): ganciclovir 0.15% gel 6 times per day Treatment two (n=24): ganciclovir 0.15% gel 6 times per day + polyinosinic acid (poly I:C) 0.5 mg subconjunctival injection every 3 days + poly I:C 1.5 mg intramuscular injection every other day |
| Wen 2011 | Botanical or herbal preparation Treatment one (n=41): acyclovir 0.1% solution Treatment two (n=40): acyclovir 0.1% solution + botanical extract |
| Weng 2014 | Insufficient data provided (outcome assessment at 6 weeks) Treatment one (n=35): acyclovir 0.1% solution 6‐8 times daily + tobramycin 0.3% 3 times daily Treatment two (n=35): acyclovir 0.1% solution 6‐8 times daily + tobramycin 0.3% 3 times daily + subconjunctival recombinant human interferon α‐2b one million U |
| Whitcher 1976 | Insufficient data provided Treatment one (n=20): wiping debridement Treatment two (n=31): idoxuridine solution hourly day and ointment at night |
| Xiao 1998 | Varied eligibility criteria Treatment one (n=10, epithelial type): acyclovir 0.1% solution Treatment two (n=20, epithelial type): acyclovir 0.1% solution and recombinant interleukin‐2 |
| Xie 2005 | Botanical or herbal preparation Treatment one (n=34): acyclovir solution Treatment two (n=26): botanical extract |
| Xu 2009b | Insufficient data provided Treatment one (n=30): acyclovir 0.1% solution Treatment two (n=32): acyclovir 0.1% solution and recombinant interleukin‐2 |
| Xu 2012 | Botanical or herbal preparation Treatment one (n=43): acyclovir 0.1% solution + intravenous acyclovir Treatment two (n=67): acyclovir 0.1% solution + intravenous acyclovir + botanical extract |
| Yamamoto 1984 | Non‐concurrent treatment allocation Treatment one (n=7): zinc sulfate 0.3% Treatment two (n=7): zinc sulfate 0.3% and idoxuridine Treatment three (n=2): zinc sulfate 0.3% and collagenase |
| Yang 2012 | Botanical or herbal preparation Treatment one (n=33): acyclovir 0.1% solution Treatment two (n=35): botanical extract |
| Yi 2011 | Botanical or herbal preparation Treatment one (n=60): acyclovir 0.1% solution + ganciclovir gel + intravenous acyclovir Treatment two (n=67): acyclovir 0.1% solution + botanical extract + acupuncture |
| Yu 1999 | Botanical or herbal preparation Treatment one (n=51): acyclovir 0.1% solution every 2 hours Treatment two (n=53): botanical extract (miedulin 0.8%) every 2 hours |
| Yu 2012b | Insufficient data provided (outcome based on clinical scores and corneal sensation) Treatment one (n=30): ganciclovir 0.15% gel 8 times per day Treatment two (n=30): ganciclovir 0.15% gel 8 times per day + subconjunctival live attenuated measles vaccine (0.5 ml) every other day |
| Yu 2014 | Botanical or herbal preparation Treatment one (n=46): acyclovir Treatment two (n=50): acyclovir and botanical extract |
| Yuan 2011 | Varied eligibility criteria (both epithelial and stromal keratitis) Treatment one (n=35, epithelial type): acyclovir 0.1% solution 4 times per day + interferon 1 million units/mL (0.5 ml) subconjunctival injection every other day Treatment two (n=34, epithelial type): ganciclovir 0.15% gel 4 times per day + interferon 1 million units/mL (0.5 ml) subconjunctival injection every other day |
| Zagaigora 1971 | Non‐concurrent treatment allocation Treatment one (n=41): gamma‐globulin Treatment two (n=37): desoxyribonuclease Treatment three (n=28): idoxuridine 0.1% solution |
| Zajácz 1968 | Non‐concurrent treatment allocation Control (n=84): "conservative treatment" with chloramphenicol Treatment one (n=20): corneal scraping débridement with chloramphenicol Treatment two (n=42): thermocoagulation with chloramphenicol ointment |
| Zhai 2007 | Botanical or herbal preparation Treatment one (n=58): acyclovir 0.1% solution and oral acyclovir Treatment two (n=57): acyclovir 0.1% solution and oral acyclovir and intravenous botanical extract |
| Zhan 2011 | Botanical or herbal preparation Treatment one (n=50): acyclovir 0.1% solution + oral acyclovir Treatment two (n=50): botanical extract |
| Zhang 1992 | Insufficient data provided Treatment one (n=20): acyclovir and interferon Treatment two (n=21): acyclovir and interferon and transfer factor |
| Zhang 1995 | Botanical or herbal preparation Treatment one (n=20): idoxuridine 0.1% solution every 3 to 4 hours Treatment two (n=20): herbal extract |
| Zhang 1997 | Botanical or herbal preparation Treatment one (n=52): acyclovir 0.1% solution every 2 hours Treatment two (n=60): botanical extract one time per day |
| Zhang 2003 | Insufficient data provided Treatment one (n=29): interferon‐α‐1b Treatment two (n=17): interferon‐α‐2b |
| Zhang 2008 | Botanical or herbal preparation Treatment one (n=50): acyclovir solution Treatment two (n=100): acyclovir solution and oral botanical extract |
| Zhang 2010 | Botanical or herbal preparation Treatment one (n=57): acyclovir 0.1% solution (or ganciclovir gel) Treatment two (n=61): acyclovir 0.1% solution (or ganciclovir gel) + botanical extract |
| Zhao 2001 | Insufficient data provided Treatment one (n=89): acyclovir 0.1% solution Treatment two (n=97): acyclovir 0.1% solution and recombinant interferon‐α 10 μg/ml and diclofenac 0.1% solution |
| Zhao 2007 | Botanical or herbal preparation Treatment one (n=111): acyclovir 0.1% solution Treatment two (n=112): topical botanical extract |
| Zhao 2010 | Botanical or herbal preparation Treatment one (n=54): botanical extract Treatment two (n=52): periocular injection of polyinosinic acid |
| Zhao 2013 | Botanical or herbal preparation Treatment one (n=48): antiviral Treatment two (n=48): antiviral and botanical extract (yu ping feng) |
| Zheng 2008 | Botanical or herbal preparation Treatment one (n=45): topical antiviral Treatment two (n=45): topical antiviral and systemic botanical extracts |
| Zheng 2014 | Botanical or herbal preparation Treatment one (n=39): acyclovir solution Treatment two (n=45): acyclovir solution and botanical extract |
| Zhi 2001 | Insufficient data provided Treatment one (n=30): topical acyclovir Treatment two (n=38): topical acyclovir and systemic interleukin‐2 |
| Zhong 2003 | Botanical or herbal preparation Treatment one (n=30): acyclovir 0.1% solution 6 times per day + intramuscular polyinosinic acid Treatment two (n=30): re du qing solution 6 times per day + intramuscular polyinosinic acid Treatment three (n=50): re du qing nebuliser for 15 minutes 2 times per day + intramuscular polyinosinic acid |
| Zhou 2007 | Botanical or herbal preparation Treatment one (n=34): acyclovir 0.1% solution Treatment two (n=38): acyclovir 0.1% solution and botanical extract |
| Zhou 2008 | Insufficient data provided Treatment one (n=35): acyclovir Treatment two (n=66): acyclovir and recombinant interferon‐α‐2b |
| Zirm 1981 | Non‐concurrent treatment allocation Treatment one (n=23): iodinisation debridement Treatment two (n=14): trifluridine or idoxuridine 3 to 5 times per day Treatment three (n=49): scraping debridement and trifluridine or idoxuridine Treatment four (n=33): trifluridine and antiherpetic immunoglobulin |
Characteristics of studies awaiting assessment [ordered by study ID]
Ajanta Pharma 2013.
| Methods | Allocation method: randomised |
| Participants | Country: India |
| Interventions | Treatment one: acyclovir 3% ointment five times per day Treatment two: ganciclovir 0.15% gel five times per day |
| Outcomes | Corneal epithelial healing |
| Notes | Financial support: pharmaceutical industry |
Bausch & Lomb 2003.
| Methods | Allocation method: randomised |
| Participants | Country: United States |
| Interventions | Treatment one: cidofovir 0.3% solution five times per day Treatment two: cidofovir 0.5% solution five times per day Treatment three: trifluridine 1.0% solution five times per day |
| Outcomes | Corneal epithelial healing and adverse reactions (e.g., punctal occlusion) |
| Notes | Financial support: pharmaceutical industry |
Differences between protocol and review
This updated review uses 14 days, rather than seven days, as the primary treatment outcome time and estimates risk ratios and hazard ratios instead of odds ratios.
Contributions of authors
KRW developed the protocol, selected studies, extracted data, and wrote the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Grant 1 U01 EY020522 National Eye Institute, National Institutes of Health, USA.
Methodological support provided by the Cochrane Eyes and Vision Group, US Project
Research to Prevent Blindness, Inc, USA.
Sid W Richardson Foundation, USA.
-
National Institute for Health Research, UK.
- Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision Group (CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEVG Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
None known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abe 1987 {published data only}
- Abe T, Hara S. Use of acyclovir in herpetic ocular infections [ヘルペス性眼感染症と桐沢型ぶどう膜炎に対する acyclovir の使用経験]. Japanese Journal of Clinical Ophthalmology [Rinsho Ganka] 1987;41(1):73‐7. [Google Scholar]
Altinisik 1987 {published data only}
- Altinisik C. Treatment of herpetic keratitis with IDU, acyclovir and debridement + acyclovir and comparing of their results [Herpetik keratitlerin IDU, acyclovir ve debritman + acyclovir ile tedavileri ve sonuçlarinin karsilastirilmasi]. Türk Oftalmoloji Gazetesi 1987;17:39‐48. [Google Scholar]
Austin 1974 {published data only}
- Austin DJ, Walker WM. Idoxuridine and iodization in the treatment of simple dendritic ulceration. Transactions of the Ophthalmological Societies of the United Kingdom 1974;94:553. [PubMed] [Google Scholar]
Bartholomew 1977 {published data only}
- Bartholomew RS, Clarke M, Phillips CI. "Dye/light". Dye‐induced photosensitization of herpes virus. A clinical trial on humans. Transactions of the Ophthalmological Societies of the United Kingdom 1977;97:508‐9. [PubMed] [Google Scholar]
Behrens‐Baumann 1992 {published data only}
- Behrens‐Baumann W. Phosphonoformate (foscarnet, PFA) versus trifluorthymidine in the treatment of keratitis dendritica in the human. A double‐blind, randomized, preliminary trial. Acta Ophthalmologica 1992;70:690‐2. [DOI] [PubMed] [Google Scholar]
Blake 1977 {published data only}
- Blake J, Browne M. Treatment of herpetic keratitis. Documenta Ophthalmologica 1977;44:23‐33. [DOI] [PubMed] [Google Scholar]
Burns 1963 {published data only}
- Burns RP. A double‐blind study of IDU in human herpes simplex keratitis. Archives of Ophthalmology 1963;70:381‐4. [DOI] [PubMed] [Google Scholar]
Cao 2001 {published data only}
- Cao K. Clinical research on treatment of herpes simplex keratitis with foscarnet sodium eye drops [膦甲酸钠滴眼液治疗单纯疱疹性角膜炎的临床研究]. Ophthalmology in China [Yanke] 2001;10(6):343‐6. [Google Scholar]
Carmassi 1993 {published data only}
- Carmassi L, Prantera M, Bergamini F. Use of acyclovir and interferon in the treatment of herpetic keratitis [L'uso del'acyclovir e dell'interferone nel trattamento della cheratite herpetica]. Annali di Ottalmologia e Clinica Oculistica 1993;119:737‐42. [Google Scholar]
Cellini 1994 {published data only}
- Cellini M, Baldi A, Caramazza N, Felice GP, Gazzaniga A. Epidermal growth factor in the topical treatment of herpetic corneal ulcers. Ophthalmologica 1994;208:37‐40. [DOI] [PubMed] [Google Scholar]
Chen 2008 {published data only}
- Chen J, Jin M, Gao ZY. Clinical observation of ganciclovir ophthalmic gel in the treatment of viral keratitis [更昔洛韦眼用凝胶治疗病毒性角膜炎临床观察]. Chinese Journal of Practical Ophthalmology [Zhongguo Shiyong Yanke Zazhi] 2008;26(3):248‐51. [Google Scholar]
Colin 1981 {published data only}
- Colin C, Chastel C. Human herpes infections and their treatment [L'infection herpétique humaine et ses traitements]. Revue Internationale du Trachome et de Pathologie Oculaire Tropicale et Subtropicale et de Santé Publique 1981;3‐4:111‐5. [PubMed] [Google Scholar]
- Colin J, Tournoux A, Chastel C, Renard G. Superficial herpes simplex keratitis. Double‐blind comparative trial of acyclovir and idoxuridine [Kératite herpétique superficielle. Traitement comparatif en double insu par acyclovir et idoxuridine]. La Nouvelle Presse Médicale 1981;10:2969‐70, 2975. [PubMed] [Google Scholar]
Colin 1982 {published data only}
- Colin J, Bonisset JF, Renard G. Superficial herpetic keratitis: comparative treatment with trifluorothymidine or the combination isoprinosine‐trifluorothymidine [Kératite herpétique superficielle: traitement comparatif par trifluorothymidine ou l'association isoprinosine‐trifluorothymidine]. Bulletin des Sociétes d'Ophtalmologie de France 1982;82(6‐7):779‐81. [PubMed] [Google Scholar]
Colin 1983 {published data only}
- Colin J, Chastel C, Renard G. Human leukocyte interferon and aciclovir versus albumin placebo and aciclovir in the treatment of dendritic keratitis. Acta XXIV International Congress of Ophthalmology. Philadelphia: JB Lippincott Company, 1982; Vol. 1:225‐6.
- Colin J, Chastel C, Renard G, Cantell K. Combination therapy for dendritic keratitis with human leukocyte interferon and acyclovir. American Journal of Ophthalmology 1983;95:346‐8. [DOI] [PubMed] [Google Scholar]
- Colin J, Chastel C, Renard G, Cantell K. Interferon: major therapeutic contributions in ocular herpes [L'interféron: apport thérapeutique majeur dans l'herpès oculaire]. Bulletin des Sociétés d'Ophtalmologie de France 1983;83:435‐6. [PubMed] [Google Scholar]
- Colin J, Chastel C, Renard G, Cantell K. Ocular herpes: synergistic antiviral activity of acyclovir and human leukocyte interferon [Herpès oculaire: activité antivirale synergique de l'acyclovir et de l'interféron leucocytaire humain]. La Nouvelle Presse Médicale 1982;11:2783. [Google Scholar]
Colin 1984 {published data only}
- Colin J. Superficial herpetic keratitis: comparative double‐blind treatment with iododeoxycytidine and acyclovir [Kératite herpétique superficielle: traitement comparatif en double‐insu par iododesoxycytidine et acyclovir]. Bulletin des Sociétés d'Ophtalmologie de France 1984;84:1283‐6. [PubMed] [Google Scholar]
Colin 1987 {published data only}
- Colin J, Chastel C, Kaufman HE, Kissling GE. Combination therapy for dendritic keratitis with acyclovir and vidarabine. Journal of Ocular Pharmacology 1987;3:39‐42. [DOI] [PubMed] [Google Scholar]
Colin 1997a {published data only}
- Colin J, Hoh HB, Easty DL, Herbort CP, Resnikoff S, Rigal D, et al. Ganciclovir ophthalmic gel (Virgan; 0.15%) in the treatment of herpes simplex keratitis. Cornea 1997;16:393‐9. [PubMed] [Google Scholar]
Colin 1997b {published data only}
- Colin J, Hoh HB, Easty DL, Herbort CP, Resnikoff S, Rigal D, et al. Ganciclovir ophthalmic gel (Virgan; 0.15%) in the treatment of herpes simplex keratitis. Cornea 1997;16:393‐9. [PubMed] [Google Scholar]
- Hoh HB, Hurley C, Claoué C, Viswalingham M, Easty DL, Goldschmidt P, et al. Randomised trial of ganciclovir and acyclovir in the treatment of herpes simplex dendritic keratitis: a multicentre study. British Journal of Ophthalmology 1996;80:140‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colin 2007a {published data only}
- Colin J. Ganciclovir ophthalmic gel 0.15%: a valuable tool for treating ocular herpes. Clinical Ophthalmology 2007;1:441‐53. [PMC free article] [PubMed] [Google Scholar]
Colin 2007b {published data only}
- Colin J. Ganciclovir ophthalmic gel 0.15%: a valuable tool for treating ocular herpes. Clinical Ophthalmology 2007;1:441‐53. [PMC free article] [PubMed] [Google Scholar]
- Colin J, Resnikoff S, Adrien JLM. Comparative efficacy of ganciclovir and acyclovir in the treatment of herpes simplex dendritic keratitis. Annual Meeting of the American Academy of Ophthalmology. 1992:125.
- Dart JKG. Randomised, parallel comparative multicentre study of LCM 1316 (ganciclovir) suspension versus acyclovir ointment 3% in herpes simplex keratitis (N0141164542). National Research Register, Department of Health, Great Britain. Unpublished clinical trial sponsored by Chauvin Pharmaceuticals, 2000‐2001.
- Hoh HB, Hurley C, Claoué C, Viswalingham M, Easty DL, Goldschmidt P, et al. Randomised trial of ganciclovir and acyclovir in the treatment of herpes simplex dendritic keratitis: a multicentre study. British Journal of Ophthalmology 1996;80:140‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley C, Hillery M, Hoh HB, Claoué E, Easty DL, Kenna P, et al. Randomised trial of ganciclovir 0.15% gel and acyclovir 3% ointment in the treatment of herpes simplex dendritic keratitis: a multicentre study [abstract]. Irish Journal of Medical Science 1995;164:332 (abstract 294). [Google Scholar]
- Hurley C, Hillery M, O'Doherty D, Collum LMT. Acyclovir vs ganciclovir in the treatment of herpes simplex keratitis. Irish Journal of Medical Science 1994;163:309. [Google Scholar]
Collum 1980 {published data only}
- Collum L, Logan P. Acyclovir in herpes simplex keratitis [abstract]. Irish Journal of Medical Science 1981;150:225. [Google Scholar]
- Collum LMT, Benedict Smith A, Hillary IB. Randomised double‐blind trial of acyclovir and idoxuridine in dendritic corneal ulceration. British Journal of Ophthalmology 1980;64:766‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collum LMT, Benedict‐Smith A, Hillary IB. Acyclovir in dendritic corneal ulceration. The Cornea in Health and Disease. VIth Congress of the European Society of Ophthalmology. London: Royal Society of Medicine, 1980:765‐71.
- Collum LMT, Logan P, Hillary IB, Ravenscroft T. Acyclovir in herpes keratitis. American Journal of Medicine 1982;73 Suppl 1A:290‐3. [DOI] [PubMed] [Google Scholar]
Collum 1985 {published data only}
- Collum LMT, Logan P. Acyclovir in complicated herpetic keratitis [abstract]. Irish Journal of Medical Science 1982;151:291‐2. [Google Scholar]
- Collum LMT, Logan P, McAuliffe‐Curtin D, Hung SO, Patterson A, Rees PJ. Randomised double‐blind trial of acyclovir (Zovirax) and adenine arabinoside in herpes simplex amoeboid corneal ulceration. British Journal of Ophthalmology 1985;69:847‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collum 1986 {published data only}
- Collum LMT, Akhtar J, McGettrick P. Oral acyclovir in herpetic keratitis. Transactions of the Ophthalmological Societies of the United Kingdom 1985;104:629‐32. [PubMed] [Google Scholar]
- Collum LMT, MacGettrick P, Akhtar J, Rees PJ. Oral acyclovir (Zovirax) in herpetic keratitis. In: Maudgal PC, Missotten L editor(s). Herpetic Eye Diseases. Dordrecht: Dr W Junk Publishers, 1985:233‐40. [Google Scholar]
- Collum LMT, McGettrick P, Akhtar J, Lavin J, Rees PJ. Oral acyclovir (Zovirax) in herpes simplex dendritic corneal ulceration. British Journal of Ophthalmology 1986;70:435‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coster 1976 {published data only}
- Coster DJ, McKinnon JR, McGill JI, Jones BR, Fraunfelder FT. Clinical evaluation of adenine arabinoside and trifluorothymidine in the treatment of corneal ulcers caused by herpes simplex virus. Journal of Infectious Diseases 1976;133 Suppl:A173‐7. [DOI] [PubMed] [Google Scholar]
- Jones BR, McGill JI, McKinnon JR, Holt‐Wilson AD, Williams HP. Preliminary experience with adenine arabinoside in comparison with idoxuridine and trifluorothymidine in the management of herpetic keratitis. In: Pavan‐Langston D, Buchanan RA, Alford CA Jr editor(s). Adenine‐Arabinoside: An Antiviral Agent. New York: Raven Press, 1975:411‐6. [Google Scholar]
- McGill JI, Coster D, Fraunfelder T, Holt‐Wilson AD, Williams H, Jones BR. Adenine arabinoside in the management of herpetic keratitis. Transactions of the Ophthalmological Societies of the United Kingdom 1975;95:246‐9. [PubMed] [Google Scholar]
- McKinnon JR, McGill JI, Jones BR. A coded clinical evaluation of adenine arabinoside and trifluorothymidine in the treatment of ulcerative herpetic keratitis. In: Pavan‐Langston D, Buchanan RA, Alford CA Jr editor(s). Adenine‐Arabinoside: An Antiviral Agent. New York: Raven Press, 1975:401‐10. [Google Scholar]
Coster 1977a {published data only}
- Coster DJ, Falcon MG, Cantell K, Jones BR. Clinical experience of human leucocyte interferon in the management of herpetic keratitis. Transactions of the Ophthalmological Societies of the United Kingdom 1977;97:327‐9. [PubMed] [Google Scholar]
- Jones BR, Coster DJ, Falcon MG, Cantell K. Clinical trials of topical interferon therapy of ulcerative viral keratitis. Journal of Infectious Diseases 1976;133 Suppl:A169‐72. [DOI] [PubMed] [Google Scholar]
- Jones BR, Coster DJ, Falcon MG, Cantell K. Topical therapy of ulcerative herpetic keratitis with human interferon. Lancet 1976;2:128. [DOI] [PubMed] [Google Scholar]
Coster 1979 {published data only}
- Coster DJ, Jones BR, McGill JI. Treatment of amoeboid herpetic ulcers with adenine arabinoside or trifluorothymidine. British Journal of Ophthalmology 1979;63:418‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coster DJ, McKinnon JR, McGill JI, Jones BR, Fraunfelder FT. Clinical evaluation of adenine arabinoside and trifluorothymidine in the treatment of corneal ulcers caused by herpes simplex virus. Journal of Infectious Diseases 1976;133 Suppl:A173‐7. [DOI] [PubMed] [Google Scholar]
Coster 1980 {published data only}
- Coster DJ, Wilhelmus KR, Michaud R, Jones BR. A comparison of acyclovir and idoxuridine as treatment for ulcerative herpetic keratitis. British Journal of Ophthalmology 1980;64:763‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BR, Coster DJ, Michaud R, Wilhelmus KR. Acyclovir (Zovirax) in the management of herpetic keratitis. In: Sundmacher R editor(s). Herpetische Augenerkrankungen. München: JF Bergmann, 1981:295‐301. [Google Scholar]
- Jones BR, Coster DJ, Wilhelmus KR, Michaud R. Acyclovir (Zovirax) an effective antiviral for herpetic keratitis. The Cornea in Health and Disease. VIth Congress of the European Society of Ophthalmology. London: Royal Society of Medicine, 1980:773‐9.
Dai 2009a {published data only}
- Dai X, Huang HH, Hu XJ, Liu Q, Liu ZR. Use of ganciclovir for herpes simplex virus keratitis [更昔洛韦滴眼液在单纯疱疹病毒性角膜炎中的应用]. Strait Pharmaceutical Journal [Haixia Yaoxue] 2009;21(10):145. [Google Scholar]
Dai 2009b {published data only}
- Dai ZX, Zhang H, Lin MY, Liang XY. Clinical observation of treatment of epithelial herpes simplex virus keratitis by ganciclovir ophthalmic gel [更昔洛韦眼用凝胶治疗上皮型单纯疱疹病毒性角膜炎临床疗效观察]. Asia‐Pacific Traditional Medicine [Yatai Chuantong Yiyao] 2009;5(7):100‐1. [Google Scholar]
Daniel 1972 {published data only}
- Daniel R, Karseras A. Assessment of possible potentiating action of ultraviolet light on 5 iodo‐2' deoxyuridine in superficial herpetic keratitis. British Journal of Ophthalmology 1972;56:604‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davidson 1964 {published data only}
- Davidson SI, Jameson Evans P. IDU and the treatment of herpes simplex keratitis. British Journal of Ophthalmology 1964;48:678‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Koning 1982 {published data only}
- Koning EWJ, Bijsterveld OP, Cantell K. Combination interferon‐trifluorothymidine in the treatment of herpetic keratitis [La combinaison interféron‐trifluorothymidine dans le traitement des kératites herpétiques]. Bulletins et Mémoires de la Société Française d'Ophtalmologie 1982;94:130‐2. [PubMed] [Google Scholar]
- Koning EWJ, Bijsterveld OP, Cantell K. Combination therapy for dendritic keratitis with human leucocyte interferon and trifluorothymidine. British Journal of Ophthalmology 1982;66:509‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning EWJ, Bijsterveld OP, Cantell K. Human leukocyte interferon and trifluorothymidine versus albumin placebo and trifluorothymidine in the treatment of dendritic keratitis. In: Maeyer E, Galasso G, Schellekens H editor(s). The Biology of the Interferon System. Amsterdam: Elsevier/North‐Holland Biomed Press, 1981:351‐4. [Google Scholar]
de Koning 1983 {published data only}
- Koning EWJ, Bijsterveld OP, Cantell K. Combination therapy for dendritic keratitis with acyclovir and α‐interferon. Archives of Ophthalmology 1983;101:1866‐8. [DOI] [PubMed] [Google Scholar]
Denis 1983 {published data only}
- Denis J, Thenault‐Giono S, Ray‐Cohen M‐L, Tournoux A, Pouliquen Y. Double‐blind treatment of ocular herpes simplex: Vira‐A and acyclovir [Traitement de l'herpès oculaire en double insu: Vira‐A et acyclovir]. Bulletin des Sociétés d'Ophtalmologie de France 1983;83:25‐9. [PubMed] [Google Scholar]
Fu 2012 {published data only}
- Fu JY. Clinical research of ganciclovir ophthalmic gel in combination with interferon treatment for herpes simplex virus keratitis [更昔洛韦眼用凝胶联合干扰素治疗单纯疱疹病毒性角膜炎临床研究]. Strait Pharmaceutical Journal [Haixia Yaoxue] 2012;19(6):174‐5. [Google Scholar]
Fulhorst 1972 {published data only}
- Fulhorst HW, Richards AB, Bowbyes J, Jones BR. Cryotherapy of epithelial herpes simplex keratitis. American Journal of Ophthalmology 1972;73:46‐51. [DOI] [PubMed] [Google Scholar]
Genée 1987 {published data only}
- Genée E, Maith J. Comparison of the effectiveness of acyclovir and vidarabine in superficial herpes keratitis [Wirksamkeitsvergleich von Aciclovir und Vidarabin bei oberflächlichen Herpeskeratitiden]. Klinische Monatsblätter für Augenheilkunde 1987;191:30‐2. [PubMed] [Google Scholar]
Graupner 1966 {published data only}
- Graupner K, Müller F. On the treatment of dendritic keratitis with para‐fluorophenylalanine (Preliminary report) [Zur Behandlung der Keratitis dendritica mit para‐Fluorphenylalanin (Vorlaufige Mitteilung)]. Albrecht von Graefes Archiv für Klinische und Experimentelle Ophthalmologie 1966;171:103‐4. [DOI] [PubMed] [Google Scholar]
Graupner 1968 {published data only}
- Graupner K, Klein S, Müller F. Treatment of dendritic keratitis [Die Behandlung der Keratitis dendritica]. Advances in Ophthalmology 1969;21:113‐31. [PubMed] [Google Scholar]
- Graupner K, Klein S, Müller F. Treatment of dendritic keratitis [Die Behandlung der Keratitis dendritica]. Bibliotheca Ophthalmologica 1969;80:113‐31. [PubMed] [Google Scholar]
- Graupner K, Müller F. On the treatment of dendritic keratitis with para‐fluorophenylalanine [Zur Behandlung der Keratitis dendritica mit para‐Fluorophenylalanin]. Albrecht von Graefes Archiv für Klinische und Experimentelle Ophthalmologie 1968;174:231‐5. [DOI] [PubMed] [Google Scholar]
Graupner 1969 {published and unpublished data}
- Graupner K, Klein S, Müller F. Studies on the effectiveness of non‐specific and virostatic treatment of dendritic keratitis with special reference to the combination of 5‐iodo‐2‐deoxyuridine and para‐fluorophenylalanine [Untersuchungen über die Wirksamkeit unspezifischer und virostatischer Behandlung der Keratitis dendritica unter besonderer Berucksichtigung der Kombination von 5‐Jod‐2‐deoxyuridin und para‐Flurophenylalanin]. Albrecht von Graefes Archiv für Klinische und Experimentelle Ophthalmologie 1969;177:89‐96. [DOI] [PubMed] [Google Scholar]
Guerra 1979 {published data only}
- Guerra R, Frezzotti R, Bonanni R, Dianzani F, Rita G. A preliminary study on treatment of human herpes simplex keratitis with an interferon inducer. Annals of the New York Academy of Sciences 1970;173:823‐30. [Google Scholar]
- Guerra R, Frezzotti R, Bonanni R, Rita G, Dianzani F. Possibilities of treatment of human herpetic keratitis with an inducer of the interferon system [Possibilita di trattamento della cheratite erpetica umana con un induttore del sistema interferone]. Atti dell Accademia Dei Fisiocritici in Siena 1968;17:1513‐7. [PubMed] [Google Scholar]
- Guerra R, Frezzotti R, Caporossi A, Speri A, Russi M, Bargigli V. Clinical appraisal of the therapeutic effectiveness of an interferon inducer in human herpetic keratitis [Una sperimentazione clinica sull'efficacia terapeutica di un induttore di interferone nella cheratite erpetica umana]. Minerva Oftalmologica 1979;21:97‐100. [Google Scholar]
- Guerra R, Galin MA, Frezzotti R. Interferon and viral infections of man. Texas Reports on Biology and Medicine 1981‐1982;41:555‐8. [PubMed] [Google Scholar]
- Guerra R, Galin MA, Frezzotti R. Use of interferon in eye infections of man. Texas Reports on Biology and Medicine 1977;35:497‐500. [PubMed] [Google Scholar]
Han 2010 {published data only}
- Han K. Clinical observation of ganciclovir ophthalmic gel in combination with interferon treatment of herpes simplex virus keratitis [更昔洛韦眼用凝胶联合干扰素治疗单纯疱疹病毒性角膜炎的临床观察]. World Health Digest [Zhongguo Qi Kan Wang] 2010;19(7):148‐9. [Google Scholar]
Han 2014 {published data only}
- Han P. Ganciclovir in combination with Beifushu (recombinant‐bFGF) in treatment of herpes simplex keratitis [更昔洛韦滴眼液联合贝复舒治疗单疱病毒性角膜炎疗效分析]. Journal of Otolaryngology and Ophthalmology of Shandong University [Shandong Daxue Er Bi Hou Yan Xuebao] 2014;28(3):73‐4. [Google Scholar]
Hart 1965 {published data only}
- Brightman VJF, Hart DRL, Porter GTJ, Readshaw GG, Tully MJ. A controlled study of the effectiveness of IDU in the treatment of human herpes simplex keratitis. Transactions of the Asia‐Pacific Academy of Ophthalmology 1965;2:152‐9. [Google Scholar]
- Hart DRL, Brightman VJF, Readshaw GG, Porter GTJ, Tully MJ. Treatment of human herpes simplex keratitis with idoxuridine. A sequential double‐blind controlled study. Archives of Ophthalmology 1965;73:623‐34. [DOI] [PubMed] [Google Scholar]
Haut 1983 {published data only}
- Haut J, Debrat G, Moulin F, Monin C, Sarnikovski C. Double‐blind clinical trial of isoprinosine and placebo in herpetic keratitis [Essai clinique en double insue de l'isoprinosine contre placebo dans la kératite herpétique]. Bulletin des Sociétés d'Ophtalmologie de France 1983;83:617‐20. [PubMed] [Google Scholar]
HEDS Group 1997 {published and unpublished data}
- Herpetic Eye Disease Study Group. A controlled trial of oral acyclovir for the prevention of stromal keratitis or iritis in patients with herpes simplex virus epithelial keratitis. Archives of Ophthalmology 1997;115:703‐12. [DOI] [PubMed] [Google Scholar]
Hoang‐Xuan 1984 {published data only}
- Hoang‐Xuan T, Denis J, Renard G, Pouliquen Y. Comparative study of the effect of trifluorothymidine and acyclovir in herpetic anterior segmentitis [Étude comparative de l'action de la TFT et de l'aciclovir sur la segmentite antérieure herpétique]. Bulletin des Sociétés d'Ophtalmologie de France 1983;83:31‐5. [PubMed] [Google Scholar]
- Hoang‐Xuan T, Frot P, Denis J, Pouliquen Y. Acyclovir and trifluorothymidine in herpetic kerato‐uveitis. A comparative clinical study. Indications for corticoid therapy [Aciclovir et trifluorothymidine dans la kérato‐uvéite herpétique. Une étude comparative en clinique humaine. Indications de la corticothérapie]. Journal Français d'Ophtalmologie 1984;7:125‐31. [PubMed] [Google Scholar]
Huang 2008a {published data only}
- Gao N, Huang T. Ganciclovir ophthalmic gel for herpes simplex keratitis and tear film stability [更昔洛韦眼用凝胶对单纯疱疹病毒性角膜炎角膜上皮及泪膜稳定性的影响]. Recent Advances in Ophthalmology [Yanke Xin Jinzhan] 2008;29(10):775‐7. [Google Scholar]
- Huang T, Gao N, Wang YJ. Observation of efficacy of ganciclovir gel in treatment of epithelial herpes simplex keratitis [更昔洛韦眼用凝胶治疗上皮型单纯疱疹病毒性角膜炎的疗效观察]. Recent Advances in Ophthalmology [Yanke Xin Jinzhan] 2008;29(4):297‐9. [Google Scholar]
Hung 1984 {published data only}
- Hung SO, Patterson A, Clark DI, Rees PJ. Oral acyclovir in the management of dendritic herpetic corneal ulceration. British Journal of Ophthalmology 1984;68:398‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Høvding 1989 {published data only}
- Høvding G. A comparison between acyclovir and trifluorothymidine ophthalmic ointment in the treatment of epithelial dendritic keratitis. A double blind, randomized parallel group trial. Acta Ophthalmologica 1989;67:51‐4. [DOI] [PubMed] [Google Scholar]
Jackson 1984 {published data only}
- Jackson WB, Breslin CW, Lorenzetti DWC, Michaud R, Dubé I. Treatment of herpes simplex keratitis: comparison of acyclovir and vidarabine. Canadian Journal of Ophthalmology 1984;19:107‐11. [PubMed] [Google Scholar]
Jensen 1982 {published data only}
- Jensen KB, Nissen SH, Jessen F. Acyclovir in the treatment of herpetic keratitis. Acta Ophthalmologica 1982;60:557‐63. [DOI] [PubMed] [Google Scholar]
Jepson 1964 {published data only}
- Jepson CN. Treatment of herpes simplex of the cornea with IDU. A double‐blind study. American Journal of Ophthalmology 1964;57:213‐7. [DOI] [PubMed] [Google Scholar]
Kato 1979 {published data only}
- Kato F, Ohno S, Matsuda H. Treatment of epithelial herpes simplex keratitis with simple debridement [角膜単純 療過迂による上皮性角ヘルペスヘルペスの治治療の試み]. Japanese Review of Clinical Ophthalmology [Ganka Rinsho Iho] 1979;33(9):1241‐4. [Google Scholar]
Kitano 1983 {published data only}
- Kitano S. Clinical trials of interferon in ophthalmology [眼科におけるインターフェロンの臨床]. Cancer and Chemotherapy [Gan To Kagaku Ryoho] 1984;11(2):212‐20. [PubMed] [Google Scholar]
- Kitano S. Clinical trials of interferon in ophthalmology [眼科におけるインターフェロンの臨床]. Japanese Journal of Cancer and Chemotherapy [Gan To Kagaku Ryoho] 1984;11(2):212‐20. [PubMed] [Google Scholar]
- Kitano S, Yamanishi M, Matsuda H, Hyoudo T, Aoki K. Clinical results of topical human fibroblast interferon for herpetic keratitis: comparison with IDU eyedrops [ヘルペス性角膜炎に対するヒト線維芽細胞インターフェロン点眼の臨床成績-IDU点眼剤との比較試験]. Japanese Review of Clinical Ophthalmology [Ganka Rinsho Iho] 1983;77(11):1777‐86. [Google Scholar]
Kitano 1985 {published data only}
- Kitano S. Efficacy of acyclovir in dendritic keratitis: a double‐blind comparative study with IDU [樹枝状角膜炎に対するAcyclovirの効果 二重盲検法によるIDUとの比較]. Therapeutic Research [Serapyuttiku Risachi] 1985;2(4):643‐7. [Google Scholar]
- Kitano S, Yamanishi M, Shudo M, Uchida Y, Kaneko M, Tanaka N, et al. A double‐blind comparative study of acyclovir (ACV) and IDU ophthalmic ointment in the treatment of herpes simplex keratitis [アシクロビル(ACV)とIDU眼軟こうとの単純ヘルペス性角膜炎に対する治療効果の二重盲検法による比較検討]. Japanese Review of Clinical Ophthalmology [Ganka Rinsho Iho] 1983;77(8):1273‐80. [Google Scholar]
Klauber 1982 {published data only}
- Klauber A, Ottovay E. Acyclovir and idoxuridine treatment of herpes simplex keratitis—a double blind clinical study. Acta Ophthalmologica 1982;60:838‐44. [DOI] [PubMed] [Google Scholar]
Kumar 1987 {published data only}
- Kumar S, Singh TP, Satsangi SK, Bist HK. Comparative study of acyclovir and 5 I.D.U. in the management of viral corneal ulcer. Indian Journal of Ophthalmology 1987;35:139‐42. [PubMed] [Google Scholar]
Laibson 1964 {published data only}
- Laibson PR, Leopold IH. An evaluation of double‐blind IDU therapy in 100 cases of herpetic keratitis. Transactions of the American Academy of Ophthalmology and Otolaryngology 1964;68:22‐34. [PubMed] [Google Scholar]
Laibson 1977 {published data only}
- Laibson PR, Arentsen JJ, Mazzanti WD, Eiferman RA. Double controlled comparison of IDU and trifluorothymidine in thirty‐three patients with superficial herpetic keratitis. Transactions of the American Ophthalmological Society 1977;75:316‐24. [PMC free article] [PubMed] [Google Scholar]
La Lau 1982 {published data only}
- Lau C, Oosterhuis JA, Rij G, Benardel de Lavalette JGC, Craandijk A, Lamers WR, et al. Double‐blind study of aciclovir and trifluorothymidine ointment in the treatment of dendritic or geographic herpes simplex [Dubbelblind onderzoek van aciclovir‐ en trifluorthymidinezalf bij de behandeling van herpes simplex dendritica of geografica]. Nederlands Tijdschrift voor Geneeskunde 1980;124:2204. [Google Scholar]
- Lau C, Oosterhuis JA, Versteeg J, Rij G, Renardel de Lavalette JGC, Craandijk A, et al. Aciclovir and trifluorothymidine in herpetic keratitis. Preliminary report of a multicentered trial. Documenta Ophthalmologica 1981;50:287‐90. [DOI] [PubMed] [Google Scholar]
- Lau C, Oosterhuis JA, Versteeg J, Rij G, Renardel de Lavalette JGC, Craandijk A, et al. Acyclovir and trifluorothymidine in herpetic keratitis: a multicentre trial. British Journal of Ophthalmology 1982;66(8):506‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Oosterhuis JA, Versteeg J, Rij G, Renardel de Lavalette JGC, Craandijk A, et al. Multicenter trial of acyclovir and trifluorothymidine in herpetic keratitis. American Journal of Medicine 1982;73(1):305‐6. [DOI] [PubMed] [Google Scholar]
- Lau C, Oosterhuis JA, Rij G, Renardel de Lavalette JGC, Craandijk A, Lamers WR, et al. Double‐blind study of aciclovir and trifluorothymidine ointment in the treatment of dendritic or geographic herpes simplex. Ophthalmologica 1980;181:293. [Google Scholar]
Li 2008 {published data only}
- Li Y, Yan JF, Zhang Q, Huang DL, Liu Y. Treatment of relapsing herpes simplex keratitis with ganciclovir ophthalmic gel [更昔洛韦眼用凝胶治疗复发性单纯疱疹性角膜炎的临床研究]. Medical Journal of the Chinese People's Armed Police Forces [Wujing Yixue] 2008;19(3):232‐4. [Google Scholar]
Li 2009 {published data only}
- Li GX, Sun AP. Interferon combined with antiviral drug for herpes simplex viral keratitis [干扰素联合抗病毒药物治疗单纯疱疹病毒性角膜炎]. Recent Advances in Ophthalmology [Yanke Xinjinzhan] 2009;29(9):703‐4. [Google Scholar]
Li 2013a {published data only}
- Li JP, Zhou PH, Guo XY. Observation on the effect of ganciclovir ophthalmic gel in the treatment of herpes simplex virus keratitis [更昔洛韦眼用凝胶在单纯疱疹病毒性角膜炎中应用的效果观察]. Clinical Medicine and Engineering [Linchuang Yixue Gongcheng] 2013;20(3):306‐7. [Google Scholar]
Li 2013b {published data only}
- Li L, Zhou H, Wang P. Clinical effects of the combination of recombinant human interferon and ganciclovir in the treatment of herpes simplex keratitis [重组人干扰素联合更昔洛韦治疗单纯疤疹病毒性角膜炎的效果观察]. China Medical Herald [Zhongguo Yiyao Daobao] 2013;10(10):77‐81. [Google Scholar]
Lin 2011 {published data only}
- Lin TF, Xue SY, Zhuo WY, Shi CC. Efficacy of ganciclovir ophthalmic gel and acyclovir eye drops in the treatment of herpes simplex viral keratitis [更昔洛韦眼用凝胶和阿昔洛韦滴眼液治疗单疱性病毒性角膜炎的疗效比较]. China Pharmaceuticals [Zhongguo Yaoye] 2011;17(13):53‐4. [Google Scholar]
Lin 2014 {published data only}
- Lin HB, Zhuo X, He AQ. Efficacy of treatment with ganciclovir and acyclovir for herpes simplex keratitis [更昔洛韦与阿昔洛韦治疗单纯疱疹性角膜炎的疗效观察]. Hainan Medical Journal [Hainan Yixue] 2014;25(1):96‐8. [Google Scholar]
Liu 2009a {published data only (unpublished sought but not used)}
- Liu L, Wang LQ. Clinical observation of ganciclovir ophthalmic gel in the treatment of herpes simplex keratitis [更昔洛韦眼用凝胶治疗单纯疱疹病毒性角膜炎的临床观察]. Chinese Journal of Drug Application and Monitoring [Zhongguo Yaowu Yingyong Yu Jiance] 2009;6(1):1‐3. [Google Scholar]
Liu 2010 {published data only}
- Liu YZ. Clinical efficacy of ganciclovir gel in the treatment of herpes simplex virus keratitis [更昔洛韦凝胶治疗单纯疱疹病毒性角膜炎的临床疗效观察]. Journal of Medical Forum [Yiyao Luntan Zazhi] 2010;18(15):161‐2. [Google Scholar]
Liu 2011 {published data only}
- Liu J. Effect of sodium hyaluronate eye drops on epithelial healing of herpes simplex virus keratitis [透明质酸钠滴眼液对单纯疱疹病毒角膜炎上皮愈合的影响]. Journal of Hauihai Medicine [Huaihai Yiyao] 2011;29(6):522‐3. [Google Scholar]
Liu 2012a {published data only}
- Liu LJ. Clinical efficacy of ganciclovir ophthalmic gel for herpes simplex keratitis [更昔洛韦眼凝胶对单纯疱疹性角膜炎的临床疗效观察]. China Health Care and Nutrition [Zhongguo Bao Jian Ying Yang] 2012;22(8):2819‐20. [Google Scholar]
Liu 2012b {published data only}
- Liu L, Zhong RY, Yu L. The efficacy and safety of ganciclovir ophthalmic gel and acyclovir eye drops in the treatment of herpes simplex viral keratitis [更昔洛韦眼用凝胶和阿昔洛韦滴眼液治疗单疱性病毒性角膜炎的疗效及安全性]. Chinese Community Doctors [Zhongguo Shequ Yishi] 2012;9(20):175‐6. [Google Scholar]
Liu 2014a {published data only}
- Liu J, Jin ZT, Sun L. Curative effect of ganciclovir ophthalmic gel on herpes simplex keratitis [更昔洛韦眼用凝胶治疗单纯疱疹病毒性角膜炎的疗效观察]. Chinese Journal of Pharmacoepidemiology [Zhongguo Difangbing Xue Zazhi] 2014;23(2):80‐2. [Google Scholar]
Luntz 1963 {published data only}
- Luntz MH, MacCallum FO. Treatment of herpes keratitis. Lancet 1963;1:830‐1. [DOI] [PubMed] [Google Scholar]
- Luntz MH, MacCallum FO. Treatment of herpes simplex keratitis with 5‐iodo‐2'‐deoxyuridine. British Journal of Ophthalmology 1963;47:449‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacKenzie 1964 {published data only}
- MacKenzie AD. A comparison of IDU solution, IDU ointment, and carbolization in the treatment of dendritic corneal ulcer. British Journal of Ophthalmology 1964;48:274‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maichuk 1980 {published data only}
- Maichuk YF. Interferon and interferon inducers in the treatment of herpesvirus and adenovirus eye diseases. Revue Internationale du Trachome et de Pathologie Oculaire Tropicale et Subtropicale 1980;57(2‐3):61‐9. [PubMed] [Google Scholar]
Maichuk 1988 {published data only}
- Maichuk YF, Kazachenko MA. Acyclovir and acyclovir in combination with interferon in the treatment of ophthalmic herpes [Ацикловир и ацикловир в cочeтании с интерфероном в лечении глазной офтальмогерпеса]. Oftalmologicheskii Zhurnal 1988;43(7):402‐5. [PubMed] [Google Scholar]
Markham 1977 {published data only}
- Markham RHC, Carter C, Scobie MA, Metcalf C, Easty DL. Double‐blind clinical trial of adenine arabinoside and idoxuridine in herpetic corneal ulcers. Transactions of the Ophthalmological Societies of the United Kingdom 1977;97:333‐40. [PubMed] [Google Scholar]
- Markham RHC, Easty DL, Carter C, Scobie MA, Metcalf C. The effect of adenine arabinoside in ulcerative herpetic keratitis. In: Silverstein AM, O'Connor AM editor(s). Immunology and Immunopathology of the Eye. New York: Masson, 1979:269‐75. [Google Scholar]
Matthäus 1970 {published data only}
- Matthäus W, Klein S, Graupner K, Dietze H. Comparative studies on the effect of cryotherapy and iodization in combination with unspecific and virostatic after‐treatment in dendritic keratitis [Vergleichende Untersuchungen über die Wirksamkeit der Kalteapplikation und der Jod‐Abrasio in Kombination mit unspezifischer und virostatischer Nachlehandlung bei der Keratitis dendritica]. Ophthalmologica 1970;161:484‐90. [DOI] [PubMed] [Google Scholar]
McCulley 1982 {published data only}
- McCulley JP, Binder PS, Kaufman HE, O'Day DM, Poirier RH. A double‐blind, multicenter clinical trial of acyclovir vs idoxuridine for treatment of epithelial herpes simplex keratitis. Ophthalmology 1982;89:1195‐200. [DOI] [PubMed] [Google Scholar]
McGill 1974 {published data only}
- McGill J, Holt‐Wilson AD, McKinnon JR, Williams HP, Jones BR. Some aspects of the clinical use of trifluorothymidine in the treatment of herpetic ulceration of the cornea. Transactions of the Ophthalmological Societies of the United Kingdom 1974;94:342‐52. [PubMed] [Google Scholar]
Meurs 1985 {published data only}
- Meurs PJ, Bijsterveld OP. Acyclovir and recombinant human alpha 2 arg interferon treatment for dendritic keratitis. In: Maudgal PC, Missotten L editor(s). Herpetic Eye Diseases. Dordrecht: Dr W Junk Publishers, 1985:367‐76. [Google Scholar]
- Meurs PJ, Bijsterveld OP. Berofor® alpha 2 (r‐HUIFN‐alpha 2 arg) and acyclovir in the treatment of dendritic keratitis [Berofor® alpha 2 (r‐Hu IFN‐alpha 2 arg) und Aciclovir in der Behandlung der Herpes‐Keratitis‐dendritica]. Klinische Monatsblätter für Augenheilkunde 1985;187:40‐2. [DOI] [PubMed] [Google Scholar]
- Meurs PJ, Bijsterveld OP. Combination therapy of recombinant human alpha 2 interferon and acyclovir in the treatment of herpes simplex keratitis. Antiviral Research 1985;5 Suppl 1:225‐8. [DOI] [PubMed] [Google Scholar]
- Meurs PJ, Bijsterveld OP. Combination therapy of recombinant human interferon and acyclovir in the treatment of herpetic keratitis [La combinaison interféron récombinante humain et acyclovir dans le traitement des kératites herpétiques]. Bulletins et Mémoires de la Société Française d'Ophtalmologie 1986;97:434‐6. [Google Scholar]
Norn 1973 {published data only}
- Norn MS. Oxyphenbutazonum (Tanderil®) as an adjuvant in treatment of dendritic keratitis. Double‐blind trial using fluorescein‐rose bengal vital staining. Acta Ophthalmologica 1973;51:591‐8. [DOI] [PubMed] [Google Scholar]
O'Day 1975 {published data only}
- O'Day DM, Jones BR, Poirier R, Pilley S, Chisholm I, Steele A, et al. Proflavine photodynamic viral inactivation in herpes simplex keratitis. American Journal of Ophthalmology 1975;79:941‐8. [DOI] [PubMed] [Google Scholar]
Panda 1995 {published and unpublished data}
- Panda A, Das GK, Khokhar S, Rao V. Efficacy of four antiviral agents in the treatment of uncomplicated herpetic keratitis. Canadian Journal of Ophthalmology 1995;30:256‐8. [PubMed] [Google Scholar]
Parlato 1985 {published data only}
- Parlato CJ, Cohen EJ, Sakauye CM, Dreizen NG, Galentine PG, Laibson PR. Role of debridement and trifluridine trifluorothymidine in herpes simplex dendritic keratitis. Archives of Ophthalmology 1985;103:673‐5. [DOI] [PubMed] [Google Scholar]
Patterson 1963a {published data only}
- Patterson A, Fox AD, Davies G, Maguire C, Holmes Sellers PJ, Wright P, et al. Controlled studies of IDU in the treatment of herpetic keratitis. Transactions of the Ophthalmological Societies of the United Kingdom 1963;83:583‐91. [PubMed] [Google Scholar]
Patterson 1963b {published data only}
- Patterson A, Fox AD, Davies G, Maguire C, Holmes Sellers PJ, Wright P, et al. Controlled studies of IDU in the treatment of herpetic keratitis. Transactions of the Ophthalmological Societies of the United Kingdom 1963;83:583‐91. [PubMed] [Google Scholar]
Patterson 1963c {published data only}
- Patterson A, Fox AD, Davies G, Maguire C, Holmes Sellers PJ, Wright P, et al. Controlled studies of IDU in the treatment of herpetic keratitis. Transactions of the Ophthalmological Societies of the United Kingdom 1963;83:583‐91. [PubMed] [Google Scholar]
Patterson 1967a {published data only}
- Patterson A, Jones BR. The management of ocular herpes. Transactions of the Ophthalmological Societies of the United Kingdom 1967;87:59‐84. [PubMed] [Google Scholar]
Patterson 1967b {published data only}
- Jones BR. Prospects in treating viral disease of the eye. Transactions of the Ophthalmological Societies of the United Kingdom 1967;87:537‐79. [PubMed] [Google Scholar]
- Patterson A, Jones BR. The management of ocular herpes. Transactions of the Ophthalmological Societies of the United Kingdom 1967;87:59‐84. [PubMed] [Google Scholar]
Pavan‐Langston 1976 {published data only}
- Buchanan RA. Advances in antiviral chemotherapy. Canadian Medical Association Journal 1979;120(1):7‐10. [PMC free article] [PubMed] [Google Scholar]
- Chin GN. Treatment of herpes simplex keratitis with a new antiviral agent‐‐vidarabine (Vira‐A). Journal of the Kentucky Medical Association 1977;75(7):322‐4. [PubMed] [Google Scholar]
- Chin GN. Treatment of herpes simplex keratitis with idoxuridine and vidarabine: a double‐blind study. Annals of Ophthalmology 1978;10:1171‐4. [PubMed] [Google Scholar]
- Dresner AJ, Seamans ML. Evidence of the safety and efficacy of adenine arabinoside in the treatment of herpes simplex epithelial keratitis. In: Pavan‐Langston R, Buchanan RA, Alford CA, Jr editor(s). Adenine‐Arabinoside: An Antiviral Agent. New York: Raven Press, 1975:381‐92. [Google Scholar]
- Hyndiuk RA, Schultz RO, Hull DS. Herpetic keratitis—clinical evaluation of adenine arabinoside and idoxuridine. In: Pavan‐Langston D, Buchanan RA, Alford CA Jr editor(s). Adenine‐Arabinoside: An Antiviral Agent. New York: Raven Press, 1975:331‐5. [Google Scholar]
- Keeney RE, Buchanan RA. Clinical application of adenine arabinoside. Annals of the New York Academy of Sciences 1975;255:185‐9. [DOI] [PubMed] [Google Scholar]
- Laibson PR, Hyndiuk R, Krachmer JH, Schultz RO. Ara‐A and IDU therapy of human superficial herpetic keratitis. Investigative Ophthalmology 1975;14:762‐3. [PubMed] [Google Scholar]
- Laibson PR, Krachmer JH. Controlled comparison of adenine arabinoside and idoxuridine therapy of human superficial dendritic keratitis. In: Pavan‐Langston D, Buchanan D, Alford CA Jr editor(s). Adenine‐Arabinoside: An Antiviral Agent. New York: Raven Press, 1975:323‐30. [Google Scholar]
- Pavan‐Langston D. Clinical evaluation of adenine arabinoside and idoxuridine in the treatment of ocular herpes simplex. American Journal of Ophthalmology 1975;80:495‐502. [DOI] [PubMed] [Google Scholar]
- Pavan‐Langston D. Use of vidarabine in ophthalmology: a review. Annals of Ophthalmology 1977;9:835‐9. [PubMed] [Google Scholar]
- Pavan‐Langston D, Buchanan R. Vidarabine therapy of simple and IDU‐complicated herpetic keratitis. Transactions of the American Academy of Ophthalmology and Otolaryngology 1976;81:813‐25. [PubMed] [Google Scholar]
Pavan‐Langston 1981 {published data only}
- Laibson PR, Pavan‐Langston D, Yeakley WR, Lass J. Acyclovir and vidarabine for the treatment of herpes simplex keratitis. American Journal of Medicine 1982;73 Suppl 1A:281‐5. [DOI] [PubMed] [Google Scholar]
- Pavan‐Langston D, Lass J, Hettinger M, Udell I. Acyclovir and vidarabine in the treatment of ulcerative herpes simplex keratitis. American Journal of Ophthalmology 1981;92:829‐35. [DOI] [PubMed] [Google Scholar]
Power 1991 {published data only}
- Power WJ, Benedict‐Smith A, Hillery M, Brady K, Collum LMT. Brivudine and trifluorothymidine (TFT) in dendritic corneal ulceration: a double blind controlled study. Current Eye Research 1991;10:183‐7. [DOI] [PubMed] [Google Scholar]
- Power WJ, Benedict‐Smith A, Hillery M, Brady K, Collum LMT. Randomised double‐blind trial of brivudine and trifluorothymidine (TFT) in dendritic corneal ulceration. British Journal of Ophthalmology 1991;75:649‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramirez 2002 {published data only}
- Ramirez JB, Baca O, Velasco R, Viggiano D, Erazo J. Topical treatment for epithelial herpetic keratitis: ganciclovir gel versus aciclovir ointment. Investigative Ophthalmology & Visual Science 2002;43:abstract 3072. [Google Scholar]
Richter 1986 {published data only}
- Richter S, Matthes H. Results of treating herpetic keratitis with bromovinyl‐deoxyuridine [Ergebnisse der Therapie herpetischer Hornhauterkrankungen mit (E)‐5‐(2‐Bromvinyl)‐2'‐Desoxyuridin]. Folia Ophthalmologica 1986;11:95‐9. [Google Scholar]
Serifoglu 1987 {published data only}
- Şerifoğlu A, Karakurt A. Clinical evaluation of acyclovir and idoxuridine with debridement in the treatment of superficial herpetic keratitis [Vüzeysel herpetik keratitlerin tedavisinde idoxuridine ve acyclovirin debridmanla beraber kullanilmasiyla alinan sonuçlar]. Türk Oftalmoloji Gazetesi 1987;17:380‐5. [Google Scholar]
Shen 2014 {published data only}
- Shen AJ, Chen XJ, Wang BZ. Efficacy of combination therapy with corneal debridement for herpes simplex epithelial keratitis [角膜清创联合药物治疗上皮型单纯疱疹病毒性角膜炎疗效观察]. China Practical Medicine [Zhongguo Shiyong Yiyao] 2014;9(6):157‐8. [Google Scholar]
- Xiao F, Zheng L, Lai Y. Efficacy of combination therapy with corneal debridement for herpes simplex epithelial keratitis [角膜清创联合药物治疗上皮型单纯疱疹病毒性角膜炎疗效观察]. China Medical Tribune [Zhongguo Yixue Luntan Bao] 2014; Vol. http://gp.cmt.com.cn/detail/555817.html.
Srinivas 1993 {published data only}
- Srinivas C. Combination therapy of acyclovir and idurine in herpetic keratitis. Afro‐Asian Journal of Ophthalmology 1993;12:336‐7. [Google Scholar]
Struck 1989 {published and unpublished data}
- Struck HG, Schäfer K. Investigations on the topical treatment of herpes simplex virus infection of the eye [Untersuchungen zur lokalen Herpestherapie im Augenbereich]. Folia Ophthalmologica 1989;14:335‐40. [Google Scholar]
Sugar 1980 {published data only}
- Sugar J, Stark W, Binder PS, Meyer R, Chandler JW, Waring GO III, et al. Trifluorothymidine treatment of herpes simplex epithelial keratitis and comparison with idoxuridine. Annals of Ophthalmology 1980;12:611‐5. [Google Scholar]
Sun 2013a {published data only}
- Sun CX. Efficacy of ganciclovir ophthalmic gel in treatment of herpes simplex viral keratitis [更昔洛韦眼用凝胶治疗单疱性病毒性角膜炎的疗效分析]. China Practical Medicine [Zhongguo Shiyong Yiyao] 2013;8(14):164‐5. [Google Scholar]
Sundmacher 1976a {published data only}
- Sundmacher R, Neumann‐Haefelin D, Manthey KF. Human‐interferon therapy in dendritic keratitis [Humaninterferon‐therapie der keratitis dendritica]. Deutsche Ophthalmologische Gesellschaft 1977;74:615‐8. [PubMed] [Google Scholar]
- Sundmacher R, Neumann‐Haefelin D, Manthey KF, Müller O. Interferon in treatment of dendritic keratitis in humans: a preliminary report. Journal of Infectious Diseases 1976;133 Suppl A:A160‐4. [DOI] [PubMed] [Google Scholar]
Sundmacher 1976b {published data only}
- Sundmacher R, Neumann‐Haefelin D, Cantell K. Interferon treatment of dendritic keratitis. Lancet 1976;1:1406‐7. [DOI] [PubMed] [Google Scholar]
- Sundmacher R, Neumann‐Haefelin D, Cantell K. Successful treatment of dendritic keratitis with human leukocyte interferon. A controlled clinical study. Albrecht von Graefes Archiv für Klinische und Experimentelle Ophthalmologie 1976;201:39‐45. [DOI] [PubMed] [Google Scholar]
Sundmacher 1978a {published data only}
- Sundmacher R, Cantell K, Haug P, Neumann Haefelin D. Role of debridement and interferon in the treatment of dendritic keratitis. Albrecht von Graefes Archiv für Klinische und Experimentelle Ophthalmologie 1978;207:77‐82. [DOI] [PubMed] [Google Scholar]
Sundmacher 1978b {published data only}
- Sundmacher R, Cantell K, Skoda R, Hallermann C, Neumann Haefelin D. Human leukocyte and fibroblast interferon in a combination therapy of dendritic keratitis. Albrecht von Graefes Archiv für Klinische und Experimentelle Ophthalmologie 1978;208:229‐33. [DOI] [PubMed] [Google Scholar]
Sundmacher 1981a {published data only}
- Sundmacher R, Cantell K, Neumann‐Haefelin D. Evaluation of interferon in ocular viral diseases. In: Maeyer E, Galasso G, Schellekens H editor(s). The Biology of the Interferon System. Amsterdam: Elsevier/North‐Holland Biomed Press, 1981:343‐50. [Google Scholar]
- Sundmacher R, Neumann‐Haefelin D, Cantell K. The clinical value of interferon in ocular viral disease. In: Munk K, Kirchner H editor(s). Interferon: Properties, Mode of Action, Production, Clinical Application. Contributions to Oncology. Vol. 11, Basel: Karger, 1982:191‐6. [Google Scholar]
Sundmacher 1981b {published data only}
- Sundmacher R, Cantell K, Neumann‐Haefelin D. Combination therapy of dendritic keratitis with trifluorothymidine and interferon. Lancet 1978;2:687. [DOI] [PubMed] [Google Scholar]
- Sundmacher R, Cantell K, Neumann‐Haefelin D. Evaluation of interferon in ocular viral diseases. In: Maeyer E, Galasso G, Schellekens H editor(s). The Biology of the Interferon System. Amsterdam: Elsevier/ North‐Holland Biomed Press, 1981:343‐50. [Google Scholar]
- Sundmacher R, Neumann‐Haefelin D, Cantell K. Therapy and prophylaxis of dendritic keratitis with topical human interferon. Herpetische Augenerkrankungen. München: JF Bergmann, 1981:401‐7.
Sundmacher 1984a {published data only}
- Sundmacher R, Cantell K, Mattes A. Combination therapy for dendritic keratitis. High‐titer alpha‐interferon and trifluridine. Archives of Ophthalmology 1984;102:554‐5. [DOI] [PubMed] [Google Scholar]
Sundmacher 1985 {published data only}
- Sundmacher R, Neumann‐Haefelin D, Mattes A, Merk W, Adolf G, Cantell K. Human leukocyte interferon plus trifluorothymidin versus recombinant alpha 2 arg interferon plus trifluorothymidin for therapy of dendritic keratitis. A controlled clinical study. In: Maudgal PC, Missotten L editor(s). Herpetic Eye Diseases. Dordrecht: Dr W Junk Publishers, 1985:359‐66. [Google Scholar]
Sundmacher 1987 {published data only}
- Sundmacher R, Mattes A, Neumann‐Haefelin D, Adolf G, Kruss B. The potency of interferon‐alpha 2 and interferon‐gamma in a combination therapy of dendritic keratitis. A controlled clinical study. Current Eye Research 1987;6:273‐6. [DOI] [PubMed] [Google Scholar]
Tanaka 1988a {published data only}
- Tanaka N, Kitano S, Uchida Y, Hosaka A, Matsuda H, Kamada K, et al. Clinical results of Ro22‐8181 ophthalmic solution for herpes simplex keratitis: a comparison test of 103 IU/ml and 107 IU/ml [単純ヘルペス性角膜炎に対するRo22‐8181点眼液の臨床成績:103IU/mlと107IU/mlの比較試験]. Japanese Review of Clinical Ophthalmology [Ganka Rinsho Iho] 1988;82(4):699‐705. [Google Scholar]
Tanaka 1988b {published data only}
- Tanaka N, Kitano S, Uchida Y, Akio H, Matsuda H, Matsuyama S, et al. Effect of eye drop solution of recombinant human leukocyte interferon‐α on herpes simplex keratitis: double‐blind comparative study with IDU eye drop [単純ヘルペス性角膜炎に対する組換え型ヒト白血球インターフェロンA点眼液の効果: IDU点眼剤との二重盲検比較]. Japanese Review of Clinical Ophthalmology [Ganka Rinsho Iho] 1988;82(5):908‐15. [Google Scholar]
Travers 1978 {published data only}
- Travers JP, Patterson A. A controlled trial of adenine arabinoside and trifluorothymidine in herpetic keratitis. Journal of International Medical Research 1978;6:102‐4. [DOI] [PubMed] [Google Scholar]
Uchida 1981 {published data only}
- Uchida Y, Kaneko M, Yamanishi R, Kobayashi S. Effect of human fibroblast interferon on dendritic keratitis. Herpetische Augenerkrankungen. München: JF Bergmann, 1981:409‐13.
- Yamazaki S. Further studies on clinical trials of interferon in Japan. Japanese Journal of Medical Science and Biology 1984;37:209‐23. [DOI] [PubMed] [Google Scholar]
Uchida 1982 {published data only}
- Uchida Y. Therapeutic effect of human fibroblast interferon on herpes simplex keratitis [インターフェロン点眼による単純ヘルペス角膜炎の治療]. Folia Ophthalmologica Japonica [Nippon Ganka Kiyo] 1982;33(8):1661‐3. [Google Scholar]
- Uchida Y. Therapeutic effect of human fibroblast interferon on herpes simplex keratitis. In: Shiota H, Cheng Y‐C, Prusoff WH editor(s). Herpesvirus: Clinical, Pharmacological and Basic Aspects. Amsterdam: Excerpta Medica, 1982:302‐7. [Google Scholar]
- Uchida Y, Kaneko M, Yamanishi R, Kobayashi S. Effect of human fibroblast interferon on dendritic keratitis. Herptische Augenerkrankungen. München: JF Bergmann, 1981:409‐13.
van Bijsterveld 1980 {published data only}
- Bijsterveld OP, Post H. Trifluorothymidine and adenine arabinoside in the treatment of dendritic keratitis. In: Sundmacher R editor(s). Herpetische Augenerkrankungen. München: JF Bergmann, 1981:380‐3. [Google Scholar]
- Bijsterveld OP, Post H. Trifluorothymidine versus adenine arabinoside in the treatment of herpes simplex keratitis. British Journal of Ophthalmology 1980;64(1):33‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Bijsterveld 1989 {published data only}
- Bijsterveld OP, Meurs PJ. Herpetic keratitis [Keratitis herpetica]. Tijdschrift voor Therapie Geneesmiddel en Onderzoek 1985;10:641‐9. [Google Scholar]
- Bijsterveld OP, Meurs PJ, Clerq E, Maudgal PC. Brivudine and interferon treatment in ulcerative herpetic keratitis: a double masked study. British Journal of Ophthalmology 1989;73(8):604‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vannini 1986 {published data only}
- Vannini A, Cembrano S, Assetto V, Giannitelli A. Interferon‐beta treatment of herpes simplex keratitis. Ophthalmologica 1986;192:6‐10. [DOI] [PubMed] [Google Scholar]
Wang 2004 {published data only}
- Wang J, Ni N, Yang Z, Han F. Two ways of using acyclovir in treatment of herpes simplex virus keratitis [阿昔洛韦治疗基本型单纯疱疹病毒性角膜炎两种用药途径的比较]. International Journal of Ophthalmology [Guoji Yanke Zazhi] 2004;4(2):349‐51. [Google Scholar]
Wang 2014a {published data only}
- Wang Y. Observation on curative effect of ganciclovir ophthalmic gel and aciclovir eye drops in the treatment of herpes simplex keratitis [更昔洛韦眼用凝胶和阿昔洛韦滴眼液治疗单疱病毒性角膜炎疗效观察]. Journal of Beihua University (Natural Science) [Beihua Daxue Xuebao (Zirankexueban)] 2014;15(5):633‐6. [Google Scholar]
Wellings 1972 {published data only}
- Wellings PC, Awdry PN, Bors FH, Jones BR, Brown DC, Kaufman HE. Clinical evaluation of trifluorothymidine in the treatment of herpes simplex corneal ulcers. American Journal of Ophthalmology 1972;73:932‐42. [DOI] [PubMed] [Google Scholar]
Wilhelmus 1981a {published data only}
- Wilhelmus KR, Coster DJ, Jones BR. Acyclovir and debridement in the treatment of ulcerative herpetic keratitis. American Journal of Ophthalmology 1981;91:323‐7. [DOI] [PubMed] [Google Scholar]
Xu 2009a {published data only}
- Xu JJ. Clinical observation of ganciclovir ophthalmic gel in treatment of viral keratitis [更昔洛韦眼用凝胶治疗单纯疱疹病毒性角膜炎的临床观察]. China Pharmaceuticals [Zhongguo Yaoye] 2009;18(7):42‐3. [Google Scholar]
Yamazaki 1984a {published data only}
- Yamazaki S. Further studies on clinical trials of interferon in Japan. Japanese Journal of Medical Science and Biology 1984;37:209‐23. [DOI] [PubMed] [Google Scholar]
Yamazaki 1984b {published data only}
- Yamazaki S. Further studies on clinical trials of interferon in Japan. Japanese Journal of Medical Science and Biology 1984;37:209‐23. [DOI] [PubMed] [Google Scholar]
Yamazaki 1984c {published data only}
- Yamazaki S. Further studies on clinical trials of interferon in Japan. Japanese Journal of Medical Science and Biology 1984;37:209‐23. [DOI] [PubMed] [Google Scholar]
Yang 2000 {published data only}
- Yang BL, Jin XY, Wu ZJ, Sun XQ. A clinical investigation of ganciclovir in the treatment of 137 cases of herpes simplex keratitis [更昔洛韦滴眼剂治疗单疱病毒角膜炎137例临床研究]. Ophthalmology in China [Yanke] 2000;9(5):297‐9. [Google Scholar]
Yang 2008 {published data only}
- Yang X, Wang BW, Ye QY, Liang XD. Ganciclovir ophthalmic gel in the treatment of 32 patients with herpes simplex virus keratitis [更昔洛韦眼用凝胶治疗单纯疱疹病毒性角膜炎32例疗效观察]. Journal of Guangdong Medical College [Guangdong Yixueyuan Xuebao] 2008;26(6):628‐9. [Google Scholar]
Yeakley 1981 {published data only}
- Laibson PR, Pavan‐Langston D, Yeakley WR, Lass J. Acyclovir and vidarabine for the treatment of herpes simplex keratitis. American Journal of Medicine 1982;73:281‐5. [DOI] [PubMed] [Google Scholar]
- Yeakley WR, Laibson PR, Michelson MA, Arentsen JJ. A double‐controlled evaluation of acyclovir and vidarabine for the treatment of herpes simplex epithelial keratitis. Transactions of the American Ophthalmological Society 1981;79:168‐79. [PMC free article] [PubMed] [Google Scholar]
Young 1982 {published data only}
- Young B, Patterson A, Ravenscroft T. Double‐blind clinical trial of acyclovir and adenine arabinoside in herpetic corneal ulceration. American Journal of Medicine 1982;73 Suppl 1A:311‐2. [DOI] [PubMed] [Google Scholar]
- Young BJ, Patterson A, Ravenscroft T. A randomised double‐blind clinical trial of acyclovir (Zovirax) and adenine arabinoside in herpes simplex corneal ulceration. British Journal of Ophthalmology 1982;66:361‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2012a {published data only}
- Yu J, Zhang MC. Clinical investigation of foscarnet sodium eye drops for the treatment of epithelial herpes simplex keratitis [膦甲酸钠滴眼液治疗上皮型单纯疱疹病毒性角膜炎]. International Eye Science [Guoji Yanke Zazhi] 2012;12(5):899‐901. [Google Scholar]
Zhang 2014 {published data only}
- Zhang DY. Comparison of ganciclovir ophthalmic gel and acyclovir eye drops in the treatment of herpes simplex virus keratitis [单纯疱疹病毒性角膜炎应用更昔洛韦眼用凝胶和阿昔洛韦滴眼液治疗的比较]. Chinese Journal of Modern Drug Application [Zhong Guo Xian Dai Yao Wu Ying Yong] 2014;8(12):126‐7. [Google Scholar]
Zhao 2006 {published data only}
- Zhao L, Liu X, Wang SG, Huang FY. Comparative efficacy of treatment of viral keratitis with ganciclovir ophthalmic gel and acyclovir eye drops [更昔洛韦眼用凝胶在单纯疱疹病毒性角膜炎中的应用]. Recent Advances in Ophthalmology [Yanke Xin Jinzhan] 2006;26(1):809. [Google Scholar]
Zhen 2012 {published data only}
- Zhen YJ. Effect comparison of two drugs on herpes simplex keratitis [两种药物治疗单纯疱疹性角膜炎疗效比较]. Journal of Hainan Medical University [Hainan Yixue] 2012;18(7):945‐6. [Google Scholar]
Zheng 2010 {published data only}
- Zheng W, Cui YX. Clinical efficacy of ganciclovir ophthalmic gel for herpes simplex keratitis [更昔洛韦眼凝胶对单纯疱疹性角膜炎的临床疗效观察]. Chinese Journal of Hospital Pharmacy [Zhongguo Yiyuan Yaoxue Zazhi] 2010;30(8):707‐8. [Google Scholar]
Zhu 2012 {published data only}
- Zhu Y. Efficacy analysis of ganciclovir ophthalmic gel in combination with interferon treatment of herpes simplex virus keratitis [更昔洛韦眼用凝胶联合干扰素治疗单纯疱疹病毒性角膜炎的疗效分析]. Guide of China Medicine [Zhongguo Yiyao Xuekan] 2012;10(17):214‐5. [Google Scholar]
References to studies excluded from this review
Abboud 1967 {published data only}
- Abboud I. Evaluation of I.D.U. in the treatment of dendritic ulcer. Bulletin of the Ophthalmological Society of Egypt 1967;60:245‐9. [PubMed] [Google Scholar]
Akberova 2000 {published data only}
- Akberova SI, Musaev‐Galbinur PI. The new interferon inducer Aktipol in the treatment of herpetic keratitis [Новый индуктор интерферона Актипол в лечении герпетического кератита]. Vestnik Oftalmologii 2000;116(2):16‐8. [PubMed] [Google Scholar]
Assetto 1981 {published data only}
- Assetto V, Lega M, Vinci V. Evaluation of the clinical effectiveness of methods of treating herpetic keratitis [Valutazione dell'efficacia clinica della terapia della cheratite erpetica]. Minerva Oftalmologica 1981;23:301‐6. [Google Scholar]
Babushkin 1993 {published data only}
- Babushkin AE, Mal'khanov VB. The results of using Virolex (acyclovir) in patients with herpetic keratitis [Результаты применения виролекса (ацикловира) у пациентов герпетическим кератитом]. Vestnik Oftalmologii 1993;109(5):26‐8. [PubMed] [Google Scholar]
Bianchetti 1964 {published data only}
- Bianchetti M, Witmer R. Herpes simplex keratitis [Ein jahr erfahrung mit Iododeoxyuridine]. Ophthalmologica 1964;147:251‐6. [DOI] [PubMed] [Google Scholar]
Butikova 1990 {published data only}
- Butikova VA, Soldatenkova NA, Ostrovskaya OV, Vlasova MA, Nefedova OA. The usage of an immunoglobulin in a complex treatment of ophthalmoherpes [Primenenie imunoglobulina v kompleksnom lechenii oftal'mogerpesa]. Oftalmologicheskii Zhurnal 1990;3:162‐3. [PubMed] [Google Scholar]
Cao 2012 {published data only}
- Cao SX, Zhang J. The clinical research of yin qiao jing fang decoction on epithelial herpes simplex keratitis of the wind‐heat in liver meridian system [银翘荆防汤治疗上皮型单纯疱疹病毒性角膜炎肝经风热证的临床研究]. China Foreign Medical Treatment [Zhongwai Yiliao] 2012;14(16):109‐10. [Google Scholar]
Cao 2013 {published data only}
- Cao BY, Zhang R. Clinical observation on combination of traditional Chinese and western medicine in treatment of herpes simplex virus keratitis [中西药治疗单纯疱疹病毒性角膜炎临床观察]. Liaoning Journal of Traditional Chinese Medicine [Liaoning Zhongyi Zazhi] 2013;40(5):966‐7. [Google Scholar]
Chen 2000 {published data only}
- Chen F. Treatment of herpes simplex viral conjunctivitis by traditional Chinese medicine combined with Western medicine: a clinical observation of 86 cases [中西医结合治疗单疱病毒性角膜炎86例疗效观察]. New Journal of Traditional Chinese Medicine [Xin Zhong Yi] 2000;32(7):23‐4. [Google Scholar]
Chen 2007 {published data only}
- Chen XD, Tang LS. Effect evaluation of aciclovir eye drops combined with recombinant human interferon‐α‐2b eye drops in treatment of herpes simplex keratitis [正大捷普联合重组人干扰素a‐2b治疗疱疹性角膜炎]. Food and Drug [Shipin Yu Yaopin] 2007;14(5):17‐9. [Google Scholar]
Chen 2009 {published data only}
- Chen JX, Li H, Zhang DX. Combination of Chinese and Western medicine treatment of herpes simplex virus keratitis in 100 cases [中西医结合治疗单疱病毒性角膜炎100例]. Gansu Journal of Traditional Chinese Medicine [Gansu Zhong Yi] 2009;22(9):38‐9. [Google Scholar]
Corina 1998 {published data only}
- Corina P. Acyclovir treatment—its effect on visual acuity and the rate of recurrences in herpetic keratitis [Tratamentul cu acyclovir—efectul asupra acuitatii vizuale si a ratei recurentelor in keratita herpetica]. Oftalmologia 1998;42:37‐41. [PubMed] [Google Scholar]
Corina 1999 {published data only}
- Corina P, Dimitris S, Emanuil T, Nora R. Treatment with acyclovir combined with a new Romanian product from plants [Tratamentul cu acyclovir asociat cu un nou produs romanesc din plante]. Oftalmologia 1999;46:55‐7. [PubMed] [Google Scholar]
Deng 2003 {published data only}
- Deng Z, Han J. Combination of Chinese and western medicine treatment for herpes simplex keratitis: clinical observation of 53 cases [中西医结合治疗单纯疱疹毒性角膜炎53例疗效观察]. New Journal of Traditional Chinese Medicine [Xin Zhong Yi] 2003;35(3):51‐2. [Google Scholar]
Du 2008 {published data only}
- Du C, Yu QZ. Combination of Chinese and western medicine treatment of herpes simplex virus keratitis [中西医结合治疗单纯疱疹病毒性角膜炎的疗效观察]. Journal of Zhejiang University of Traditional Chinese Medicine [Zhe Jiang Zhong Yi Yao Da Xue Xue Bao] 2008;32(4):475‐6. [Google Scholar]
Dundarov 1998 {published data only}
- Dundarov S, Petrunova S, Panova N, Petrova A. Treatment of viral eye diseases with pandavir and alphaferon. Problems in Infectious and Parasitic Diseases 1998;25:30‐1. [Google Scholar]
Elze 1979 {published data only}
- Elze KL. Ten years of clinical experience with ethyldeoxyuridine. Advances in Ophthalmology 1979;38:134‐9. [PubMed] [Google Scholar]
Fellinger 1980 {published data only}
- Fellinger C, Bartl G. Clinical experience with different methods of treating herpes corneæ [Klinische Erfahrungen über die Therapie des herpes corneæ mit verschiedenen Behandlungsmethoden]. Klinische Monatsblätter für Augenheilkunde 1980;176:239‐41. [DOI] [PubMed] [Google Scholar]
Feng 2004 {published data only}
- Feng Z, Jin H. Combination of Chinese and Western medicine treatment of herpes simplex virus keratitis: clinical observation of 52 cases [中西医结合治疗单疱病毒性角膜炎52例疗效观察]. Journal of Ningxia Medical College [Ningxia Yike Daxue Xuebao] 2004;26(6):467‐8. [Google Scholar]
Galin 1976 {published data only}
- Galin MA, Chowchuvech E, Kronenberg B. Therapeutic use of inducers of interferon on herpes simplex keratitis in humans. Annals of Ophthalmology 1976;8:72‐6. [PubMed] [Google Scholar]
Gao 2006 {published data only}
- Gao Y. Combination of Chinese and Western medicine treatment of herpes simplex virus keratitis: clinical analysis of 86 cases [中西医结合治疗单纯疱疹病毒性角膜炎86例临床分析]. Journal of Shanxi Medical College for Continuing Education [Shanxi Zhigong Yixueyuan Xuebao] 2006;16(1):32‐3. [Google Scholar]
Gilkes 1963 {published data only}
- Gilkes MJ. Further experiences in the therapy of herpetiform and other keratitis with 5‐iodo, deoxyuridine (IDU) in hospital practice. Transactions of the Ophthalmological Societies of the United Kingdom 1963;83:593‐601. [PubMed] [Google Scholar]
Gong 2013 {published data only}
- Gong J. Clinical efficacy of integrative medicine in 55 cases of herpes simplex keratitis [中西医结合治疗单纯疱疹性角膜炎55例临床疗效观察]. Modern Diagnosis and Treatment [Xiandai Zhenduan Yu Zhiliao] 2013;24(18):4124‐5. [Google Scholar]
Gu 2005 {published data only}
- Gu J, Lin Y, Qiu P, Huang G, Wu C. The treatment of herpes simplex keratitis with interferon‐α‐2b and acyclovir [α‐干扰素联合无环鸟苷滴眼液治疗单纯疱疹病毒性角膜炎]. Chinese Journal of Primary Medicine and Pharmacy [Zhongguo Ji Ceng Yi Yao] 2005;12(10):1395‐6. [Google Scholar]
Gulinuer 2011 {published data only}
- Gulinuer T, Liu XQ. Retrospective analysis of the effects of ganciclovir and acyclovir in the treatment of patients with herpes simplex keratitis in our hospital [回顾性分析本院用更昔洛韦与阿昔洛韦治疗单纯疱疹病毒性角膜炎的疗效]. Chinese Journal of Clinical Pharmacology [Zhongguo Linchuang Yaolixue Zazhi] 2011;27(6):425‐6. [Google Scholar]
Gundersen 1936 {published data only}
- Gundersen T. Herpes corneæ. With special reference to its treatment with strong solution of iodine. Archives of Ophthalmology 1936;15:225‐49. [Google Scholar]
Guo 2003 {published data only}
- Guo XW. The treatment of herpes simplex keratitis with antivirus drugs combined with deep freezing method [抗病毒药物联合低温冷冻治疗单疱病毒性角膜炎的临床研究]. West China Medical Journal [Huaxi Yixue] 2003;13(4):462‐3. [Google Scholar]
Hao 2008 {published data only}
- Hao Z. Combination of Chinese and Western medicine treatment of herpes simplex virus keratitis: clinical observation [中西医结合治疗单纯疱疹病毒性角膜炎临床观察]. Hebei Journal of Traditional Chinese Medicine [He Bei Zhong Yi] 2008;30(11):1197‐8. [Google Scholar]
He 2014 {published data only}
- He GJ. Efficacy of autologous serum treatment of herpes simplex virus keratitis [自体血清治疗单纯疱疹病毒性角膜炎的疗效观察]. Anhui Medical and Pharmaceutical Journal [Anhui Yiyao] 2014;18(4):755‐6. [Google Scholar]
Herbort 1987 {published data only}
- Herbort CP, Buechi ER, Matter M. Blunt spatula debridement and trifluorothymidine in epithelial herpetic keratitis. Current Eye Research 1987;6:225‐9. [DOI] [PubMed] [Google Scholar]
Hilsdorf 1969 {published data only}
- Hilsdorf C, Forudastan E. Therapeutic results of keratitis herpetica simplex with cryotherapy [Behandlungsergebnisse der Keratitis herpetica simplex mit Kälte]. Klinische Monatsblätter für Augenheilkunde 1969;154:627‐33. [PubMed] [Google Scholar]
Horodeňscy 1979 {published data only}
- Horodeňscy A, Horodeňscy J. Treatment of corneal herpes with cytarabine [Leczenie cytarbinq zapaleń herpetycznych rogówki]. Klinika Oczna 1979;81:217‐9. [PubMed] [Google Scholar]
Hu 2013 {published data only}
- Hu B, Dai J, Lei MS, Huang HY. Clinical study of Tujia Minority medicine on herpes simplex keratitis [土家药治疗单纯疱疹性角膜炎的临床研究]. Guiding Journal of Traditional Chinese Medicine and Pharmacy [Zhong Yi Yao Dao Bao] 2013;19(4):13‐5. [Google Scholar]
Huang 2007 {published data only}
- Huang Z. Clinical observation of ganciclovir ophthalmic gel in the treatment of herpes simplex keratitis [更昔洛韦眼用凝胶治疗单纯疱疹性角膜炎临床观察]. Sichuan Medical Journal [Sichuan Yixue] 2007;28(2):221‐2. [Google Scholar]
Huang 2008b {published data only}
- Huang CL, Chen ZG, Yu JF. Observation on herpes simplex keratitis treated by traditional Chinese medicine and Western medicine combined in clinic [中西医结合治疗单疱病毒性角膜炎临床观察]. Liaoning Journal of Traditional Chinese Medicine [Liao Ning Zhong Yi Zazhi] 2008;36(8):1212‐3. [Google Scholar]
Huang 2009 {published data only}
- Huang ZW, Wu ZF, Huang ZC. The clinical effect of acyclovir combined with interferon on herpes simplex keratitis [无环鸟苷‐干扰素治疗单疱病毒性角膜炎疗效观察]. China Tropical Medicine [Zhongguo Hedai Yixue] 2009;15(4):686, 695. [Google Scholar]
Huang 2013 {published data only}
- Huang XY. Curative effect and preventive reoccurrence function of liver detoxification soup [qing gan jie yu tang] combined with ganciclovir ophthalmic gel on herpes simplex keratitis [清肝解毒汤联合更昔洛韦眼用凝胶治疗单纯疱疹病毒性角膜炎的疗效及预防复发作用]. China Modern Doctor [Zhongguo Xiandai Yisheng] 2013;51(25):101‐3. [Google Scholar]
Inocencio 1982 {published data only}
- Inocencio FP, Valenton MJ, Dy‐Liacco JU, Batungbakal RT, Chua NG. Acycloguanosine and idoxuridine in herpes simplex keratitis. Philippine Journal of Ophthalmology 1982;14:39‐41. [Google Scholar]
Jiang 2011 {published data only}
- Jiang M, Jiang J, Zhang Y. Efficacy of Tobradex adjuvant therapy for herpes simplex keratitis [典必殊辅助治疗病毒性角膜炎的疗效观察]. China Medical Herald [Zhongguo Yiyao Daobao] 2011;8(1):80‐1. [Google Scholar]
Jin 1992 {published data only}
- Jin XY. A clinical investigation of rHuIFN alpha‐1 in the treatment of herpes simplex virus keratitis [基因工程干扰素α—1治疗单疱角膜炎的临床研究]. Chinese Journal of Ophthalmology [Zhonghua Yanke Zazhi] 1992;28(3):134‐7. [PubMed] [Google Scholar]
Jing 2010 {published data only}
- Jing GL, Jing SY, Zhou YZ. Clinical observation of ganciclovir ophthalmic gel in treatment of herpes simplex keratitis [更昔洛韦眼用凝胶治疗单纯疱疹病毒性角膜炎的临床观察]. Henan Medical Research [Henan Yixue Yanjiu] 2010;19(1):74‐5, 80. [Google Scholar]
Jones 1979 {published data only}
- Jones BR, Coster DJ, Fison PN, Thompson GM, Cobo LM, Falcon MG. Efficacy of acycloguanosine (Wellcome 248U) against herpes‐simplex corneal ulcers. Lancet 1979;1:243‐4. [DOI] [PubMed] [Google Scholar]
Kasparov 1972 {published data only}
- Kasparov AA. Significance of interferon inductors in the contemporary antiviral therapy of herpetic eye diseases [Значение индукторов интерферона в современной противовирусной терапии герпетическиой болезни глаз]. Vestnik Oftalmologii 1972;2:63‐7. [PubMed] [Google Scholar]
Kasparov 1974 {published data only}
- Kasparov AA, Fadeeva LL, Avetisov SE. Experience with combined use of polyribonucleotide complex and iododesoxyuridine in treatment of herpetic keratitis [Опыт комбинированого использования полирибонуклеотидного комплекса и 5‐йод‐2‐дезоксиуридина в лечении герпетического кератита]. Antibiotiki 1974;19:458‐62. [PubMed] [Google Scholar]
Kasparov 1990 {published data only}
- Kasparov AA, Oganesiants VA, Riabokon' BV, Gorbovitskaia GE. Microdiathermocoagulation in the treatment of herpetic keratitis [Mikrodiatermokoaguliatsiia v lechenii gerpeticheskogo keratita]. Vestnik Oftalmologii 1990;106:37‐41. [PubMed] [Google Scholar]
Kasparov 1991 {published data only}
- Kasparov AA, Pereverzina OK, Gorbovitskaya GE. A new combination in medicamentous treatment (poludan + aciclovir) of different forms of ophthalmoherpes [Новое в комбинированной медикаментозной терапии (Полудан + ацикловир) различных протявлений oфтальмогерпеса]. Oftalmologicheskii Zhurnal 1991;46:196‐200. [Google Scholar]
Koev 2007 {published data only}
- Koev K, Tanev V, Avramov L, Borisova E. Clinical investigation of combined therapy influence over keratitis herpetica dendritica with He‐Ne laser, pandavir and acyclovir. In: Atanasov PA, Dreischuh TN, Gateva SV, Kovachev LM editor(s). Proceedings of SPIE, the International Society for Optical Engineering. 14th International School on Quantum Electronics: Laser Physics and Applications. Vol. 6604, Bellingham, Washington: Society of Photo‐Optical Instrumentation Engineers, 2007:66042E‐1‐5. [Google Scholar]
Kolomiets 1986 {published data only}
- Kolomiets AG, Chekina AY, Birach TV, Kolomiets ND, Votiakov VI. Clinical studies of the therapeutic efficacy of antiherpes immunoglobulin in ophthalmoherpes [Klinicheskoe izuchenie teraperticheskoi effektivnosti protirogerpeticheskoga imunoglobulina pri oftal'mogerpene]. Oftalmologicheskii Zhurnal 1986;2:109‐12. [PubMed] [Google Scholar]
Kuyama 1979 {published data only}
- Kuyama H, Imanishi J, Itoi M. Comparative evaluation of human leukocyte and fibroblast interferon in the treatment of herpetic keratitis. Investigative Ophthalmology & Visual Science 1979;18 Suppl:57‐8. [Google Scholar]
Leopold 1965 {published data only}
- Leopold IH. Clinical experience with nucleosides in herpes simplex eye infections in man and animals. Annals of the New York Academy of Sciences 1965;130:181‐91. [DOI] [PubMed] [Google Scholar]
Li 1998 {published data only}
- Li SF, Wu X, Zhuge P, Zhang ZQ. Clinical observations on treating herpes simplex virus keratitis with Ban Lan Gen injection: report of 31 cases [板蓝根注射液治疗单疱病毒性角膜炎临床观察(附31例报告)]. Journal of Hebei Medical University [He Bei Yike Daxue Xuebao] 1998;19(4):215‐7. [Google Scholar]
Li 2012 {published data only}
- Li LJ, Yuan RJ. Clinical analysis on increased and decreased dosage of yin qiao san in treating herpes simplex viral keratitis [银翘散加减治疗单纯疱疹病毒性角膜炎临床分析]. Journal of Hebei North University (Natural Science Edition) [Hebei Beifang Xueyuan Xuebao (Ziran Kexueban)] 2012;10(3):83‐5. [Google Scholar]
Li 2013c {published data only}
- Li HY. Traditional Chinese medicine combined with acyclovir treatment of herpes simplex keratitis [中药联合无环鸟苷治疗单纯疱疹性角膜炎]. Guide of China Medicine [Zhongguo Yiyao Xuekan] 2013;11(13):448‐9. [Google Scholar]
Li 2014a {published data only}
- Li SM, He RX. Observation of integrative effect of combined traditional Chinese and Western medicine in the treatment of 92 patients with recurrent herpes simplex keratitis [中西医结合治疗复发性单纯疱疹性角膜炎92例疗效观察]. Nei Mongol Journal of Traditional Chinese Medicine [Nei Mung‐gu Zhong Yi Yao] 2014;33(3):67‐8. [Google Scholar]
Li 2014b {published data only}
- Li CJ, Zhang CH, Ding LL, Yu JL. Clinical experience with traditional Chinese medicine for herpes simplex keratitis [单纯疱疹性角膜炎进行中医辨证治疗临床体会]. Medicine and Health Care [Yi Yao Yu Bao Jian] 2014;21(1):134. [Google Scholar]
Li 2014c {published data only}
- Li XH, Jubo L, Lu X, Hong Y. Eye drops of basic fibroblast growth factor for herpes simplex keratitis [碱性成纤维细胞生长因子滴眼液在单纯疱疹性角膜炎中的应用]. International Medicine and Health Guidance News [Guoji Yiyao Weisheng Daobao] 2014;20(10):1392‐4. [Google Scholar]
Lin 2009 {published data only}
- Lin YY. Effect of acyclovir combined with polyinosinic‐polycytidylic acid injection on herpes simplex keratitis [无环鸟苷联合聚肌胞治疗单纯疱疹病毒性角膜炎临床观察]. China Tropical Medicine [Zhongguo Hedai Yixue] 2009;9(4):681, 769. [Google Scholar]
Lin 2013a {published data only}
- Lin T, Gong L, Sun XH, Zhao NQ, Chen W, Yuan HP, et al. Effectiveness and safety of 0.15% ganciclovir in situ ophthalmic gel for herpes simplex keratitis-a multicenter, randomized, investigator‐masked, parallel group study in Chinese patients. Drug Design, Development and Therapy 2013;7:361‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2013b {published data only}
- Lin X. Efficacy of the treatment of herpes simplex virus keratitis with ganciclovir ophthalmic solution in combination with interferon [更昔洛韦滴眼液联合干扰素治疗单纯疱疹病毒性角膜炎的疗效分析]. Modern Diagnosis and Treatment [Xiandai Zhenduan Yu Zhiliao] 2013;23(10):2324. [Google Scholar]
Liu 2003 {published data only}
- Liu T, Shi HW, Xie CZ. Subconjunctival interferon injection on 36 patients with herpes simplex keratitis [球结膜下注射干扰素治疗单疱病毒性角膜炎36例]. International Journal of Ophthalmology [Guoji Yanke Zazhi] 2003;3(2):118‐9. [Google Scholar]
Liu 2007 {published data only}
- Liu ZW, Li XC, Wen SZ. Evaluation of the therapeutic effects of basic fibroblast growth factor (bFGF) on herpes simplex keratitis [无环鸟苷滴眼液联合碱性成纤维细胞生长因子滴眼液治疗单纯疱疹病毒性角膜炎疗效观察]. Journal of Clinical Ophthalmology [Linchuang Yanke Zazhi] 2007;15(2):155‐7. [Google Scholar]
Liu 2009b {published data only}
- Liu Y. Combination of Chinese and Western medicine treatment of herpes simplex virus keratitis: clinical observation [中西医结合治疗单疱病毒性角膜炎临床观察]. Hebei Journal of Traditional Chinese Medicine [He Bei Zhong Yi] 2009;31(6):896‐7. [Google Scholar]
- Liu YH. Clinical study of qiang huo sheng feng decoction combined with acyclovir treatment on herpes simplex keratitis [羌活胜风汤加减联合无环鸟苷治疗单纯疱疹病毒性角膜炎的临床研究]. Fujian Medical University 2008; Vol. Master's Thesis.
Liu 2014b {published data only}
- Liu YH, Dong XH. Curative effect observation of combination therapy of aciclovir joint muscle cell [polyinosinic acid] on herpes simplex keratitis [阿昔洛韦联合聚肌胞治疗单疱病毒性角膜炎疗效观察]. Heilongjiang Medical Journal [Heilonghang Yixue] 2014;38(4):430‐1. [Google Scholar]
Long 2011 {published data only}
- Long KL. Effect of different treatments on tear EGF, Ig, and complement C3 of viral keratitis patients [不同治疗方案对病毒性角膜炎泪液EGF、Ig和补体C3 的影响研究]. Modern Preventive Medicine [Xiandai Yufang Yixue] 2011;20(15):3120‐1. [Google Scholar]
Ma 1982 {published data only}
- Ma ZX. Clinical investigation of anti‐herpes simplex virus agents [抗单纯疱疹病毒药疹的临床研究]. Chinese Journal of Ophthalmology [Zhonghua Yanke Zazhi] 1982;18(2):92‐5. [PubMed] [Google Scholar]
Ma 2006a {published data only}
- Ma YD. Combination of Chinese and Western medicine treatment of herpes simplex virus keratitis [中西医结合治疗单纯疱疹病毒性角膜炎疗效观察]. Liaoning Journal of Traditional Chinese Medicine [Liaoning Zhongyi Zazhi] 2006;33(9):1158. [Google Scholar]
Ma 2010 {published data only}
- Ma XL, Zhi Z, Liu J, Qi BY, Qu J. Efficacy of traditional Chinese medicine for herpes simplex virus keratitis and its anti‐retroviral role [中药内服外薰对单纯疱疹病毒性角膜炎疗效及抗复发作用的研究]. Chinese Journal of Difficult and Complicated Cases [Yinanbing Zazhi] 2010;9(8):613‐4. [Google Scholar]
Ma 2011 {published data only}
- Ma HX, Bao ZS, Xu CX, Sun L. Efficacy of curcuma oil eye gel in the treatment of herpes simplex virus keratitis [莪术油眼用凝胶治疗单纯疱疹病毒性角膜炎的疗效观察]. China Practical Medicine [Zhongguo Shiyong Yiyao] 2011;6(15):188‐9. [Google Scholar]
Ma 2013 {published data only}
- Ma JP. Differential treatment of 29 cases of herpes simplex keratitis [辨证治疗单纯疱疹性角膜炎29例]. Chinese Medicine Modern Distance Education of China [Zhongguo Zhongyiyao Xiandai Yuancheng Jiaoyu] 2013;11(22):33. [Google Scholar]
Maichuk 1990 {published data only}
- Maichuk YF, Kazachenko MA, Dyadina UV. Rengazyl (pyroprophene) in treatment of inflammatory eye diseases [Rengazil (piprofen) v lechenii vospalitel'nykh zabolevanii glaz]. Oftalmologicheskii Zhurnal 1990;45:290‐2. [PubMed] [Google Scholar]
Mal'khanov 1991 {published data only}
- Mal'khanov VB, Gripas' IA, Nasibullina GKh, Belousova MA. Use of T‐activin and thymalin in the treatment of ophthalmic herpes [Применение Тактивина и тималина при лечении офтальмогерпеса]. Vestnik Oftalmologii 1991;107(5):51‐4. [PubMed] [Google Scholar]
Marquardt 1971 {published data only}
- Marquardt R, Roth HW. Susceptibility of corneal herpes to treatment [Über die therapeutische Beeinflußbarkeit der Herpes corneæ]. Klinische Monatsblätter für Augenheilkunde 1971;158:517‐26. [PubMed] [Google Scholar]
Martenet 1979 {published data only}
- Martenet AC. Experiences with iododeoxycytidine in the treatment of ocular herpes simplex infections. Advances in Ophthalmology 1979;38:105‐9. [PubMed] [Google Scholar]
Matalia 1987 {published data only}
- Matalia PD. Herpetic keratitis newer modality of better approach: study of 440 cases/460 eyes. Indian Journal of Ophthalmology 1987;35:94‐102. [PubMed] [Google Scholar]
Mathur 1984 {published data only}
- Mathur AG, Elhence A. Cryotherapy, immunotherapy and wiping in herpes keratitis. Indian Journal of Ophthalmology 1984;32:382‐4. [PubMed] [Google Scholar]
McGill 1981 {published data only (unpublished sought but not used)}
- McGill J, Tormey P. Use of acyclovir in herpetic ocular infection. American Journal of Medicine 1982;73 Suppl 1A:286‐9. [DOI] [PubMed] [Google Scholar]
- McGill J, Tormey P, Walker CB. Comparative trial of acyclovir and adenine arabinoside in the treatment of herpes simplex corneal ulcers. British Journal of Ophthalmology 1981;65:610‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormey P, McGill J, Walker C. Use of acyclovir in herpes simplex corneal ulcers. Transactions of the Ophthalmological Societies of the United Kingdom 1981;101:6‐8. [PubMed] [Google Scholar]
Mohan 1987 {published data only (unpublished sought but not used)}
- Mohan M, Gupta SK, Vajpayee RB, Kalra VK. Acyclovir and adenine arabinoside in the treatment of herpes simplex keratitis: a prospective controlled clinical trial. Medical Science Research 1987;15:1173. [Google Scholar]
Morimoto 1986 {published data only}
- Morimoto K, Yagi J, Miyamoto Y, Yasumoto K, Nishida T, Otori T. Zovirax for epithelial herpetic keratitis [上皮型角膜ヘルペスに対するゾビラックスの治療効果について]. Folia Ophthalmologica Japonica [Nippon Ganka Kiyo] 1986;37(4):602‐6. [Google Scholar]
Patterson 1967c {published data only}
- Patterson A, Jones BR. The management of ocular herpes. Transactions of the Ophthalmological Societies of the United Kingdom 1967;87:59‐84. [PubMed] [Google Scholar]
Pavan‐Langston 1972 {published data only (unpublished sought but not used)}
- Pavan‐Langston D, Dohlman CH. A double blind clinical study of adenine arabinoside therapy of viral keratoconjunctivitis. American Journal of Ophthalmology 1972;74:81‐8. [DOI] [PubMed] [Google Scholar]
Pavan‐Langston 1977 {published data only (unpublished sought but not used)}
- Pavan‐Langston D. Current trends in therapy of ocular herpes simplex: experimental and clinical studies. Advances in Ophthalmology 1979;38:82‐8. [PubMed] [Google Scholar]
- Pavan‐Langston D, Foster CS. Trifluorothymidine and idoxuridine therapy of ocular herpes. American Journal of Ophthalmology 1977;84:818‐25. [DOI] [PubMed] [Google Scholar]
Pietruschka 1968 {published data only}
- Pietruschka G. On the epidemiology and therapy of herpes corneæ [Zur Epidemiologie und Therapie des Herpes corneæ]. Das Deutsche Gesundheitswesen 1968;23:1249‐54. [PubMed] [Google Scholar]
Pintér 1973 {published data only}
- Pintér L, Biró A, Berencsi G. Treatment of corneal herpes with a photosensitising dye [Die Behandlung des Herpes corneæ mit einem photosensibilisierenden Farbstoff]. Klinische Monatsblätter für Augenheilkunde 1973;163:600‐4. [PubMed] [Google Scholar]
Pivetti‐Pezzi 1985 {published data only (unpublished sought but not used)}
- Pivetti‐Pezzi P, Liso P, Tamburi S, Luzi G, Sirianni MC, Palmisano L, et al. Thymic factor therapy for herpetic keratitis. Annals of Ophthalmology 1985;17:327‐31. [PubMed] [Google Scholar]
Prost 1986 {published data only}
- Prost M, Gerkowicz K, Kozak U. Clinical investigations on the therapeutic effectiveness of isoprinosine in herpetic keratitis [Badania kliniczne uad skutecznoscia terapeutyczna preparatu isoprinosine w opryszczkocoym zapaleniu rogowki]. Klinika Oczna 1986;88:276‐7. [PubMed] [Google Scholar]
Rykun 1988 {published data only}
- Rykun VS, Maichuk YF. Clinico‐experimental substantiation of the use of tocopherol in the treatment of ocular herpes simplex [Клинико‐экспериментальное обоснование применения токоферола в лечении офтальмогерпеса]. Vestnik Oftalmologii 1988;104(4):65‐8. [PubMed] [Google Scholar]
Salcedo Hernandez 2007 {published data only}
- Salcedo Hernandez CI, Velasco R, Baca O, Babayan A, Salas F. Autologous serum vs topical acyclovir in the treatment of epithelial herpetic keratitis. ARVO Annual Meeting. 2007:withdrawn abstract 381.
Scialdone 1986 {published data only}
- Scialdone D, Chisci C, Volpe C. Valuation of efficacy of beta interferon in the treatment of herpes simplex virus (HSV) keratoconjunctivitis [Valutazione dell'efficacia del beta interferone nella terapia delle cheratocongiuntiviti da virus herpes simplex (HSV)]. Annali di Ottalmologia e Clinica Oculistica 1986;112:1101‐5. [Google Scholar]
Sellitti 1982 {published data only}
- Sellitti G, Mastroberti A, Scarpa M, Santamarina M. Methisoprinol eye drops in herpetic dendritic keratitis [Il metisoprinolo in collirio nella cheratite erpetica dendritica]. Annali di Ottalmologia e Clinica Oculistica 1982;108:417‐23. [Google Scholar]
Shimomura 1987 {published data only}
- Shimomura Y, Ohashi Y, Maeda N, Matsuda M, Hamano T, Manabe R. Herpetic keratitis therapy to reduce recurrence. Current Eye Research 1987;6:105‐10. [DOI] [PubMed] [Google Scholar]
Shiota 1979 {published data only}
- Shiota H, Inoue S, Imai M, Yamane S, Ogawa T. Clinical evaluation of trifluorothymidine and adenine arabinoside against ulcerative herpetic keratitis [Trifluorothymidineおよび adenine arabinoside による角膜ヘルペスの治療成績]. Japanese Review of Clinical Ophthalmology [Ganka Rinsho Iho] 1979;33(2):145‐50. [Google Scholar]
Shiota 1988 {published data only}
- Shiota H, Naito T, Kusujima K, Katan T, Mimura Y. Topical interferon in the treatment of herpetic keratitis [角膜ヘルペスの治療に対するインターフェロンの応用]. Japanese Journal of Clinical Ophthalmology [Rinsho Ganka] 1988;42(5):542‐3. [Google Scholar]
Sozen 2006 {published data only}
- Sozen E, Avunduk AM, Akyol N. Comparison of efficacy of oral valacyclovir and topical acyclovir in the treatment of herpes simplex keratitis: a randomized clinical trial. Chemotherapy 2006;52:29‐31. [DOI] [PubMed] [Google Scholar]
Stambuk 1995 {published data only}
- Stambuk M, Stambuk V, Pesic MC. Effect of adjuvant immunotherapy with thymic extract thymex‐L in herpetic keratitis. International Journal of Thymology 1995;3:252‐7. [Google Scholar]
Su 2010 {published data only}
- Su YY, He YZ, Wen TS. Clinical observation about ganciclovir ophthalmic gel combined interferon in the treatment of herpes simplex viral keratitis [更昔洛韦眼用凝胶联合干扰素治疗单纯疱疹病毒性角膜炎疗效观察]. China Modern Medicine [Zhongguo Dangdai Yiyao] 2010;3(10):53‐4. [Google Scholar]
Sun 2012 {published data only}
- Sun H. Efficacies of yanhuning combined with acyclovir and single use of acyclovir in treatment of herpes simplex virus keratitis: a comparative study [炎琥宁联合阿昔洛韦与单独采用阿昔洛韦治疗单疱病毒性角膜炎疗效比较]. Chinese Journal of Nosocomiology [Zhonghua Yiyuanganranxue Zazhi] 2012;17(23):5376‐8. [Google Scholar]
Sun 2014 {published data only}
- Sun L, Zhang YQ, Zhong YH, Gao RH. Clinical observation of corneal scarring after herpes simplex virus keratitis with large doses of vitamin C treatment [大剂量维生素C治疗单纯疱疹病毒性角膜炎后角膜瘢痕的临床观察]. Guide of China Medicine 2014;12(5):145‐6. [Google Scholar]
Tamburi 1990 {published data only (unpublished sought but not used)}
- Tamburi S, Palombaro P, Micozzi I, Florio FR, Abdulaziz MA. Alpha interferon therapy for superficial herpetic keratitis [L'interferon alfa naturale nel trattamento della cheratite erpetica superficiale]. Atti del LXX Congresso della Societa del Ottalmologia Italiano, Roma. 1990:369‐74.
Tarakji 1978 {published data only}
- Tarakji MS, Matta CS, Shammas HF. Cryotherapy of herpes simplex keratitis. Annals of Ophthalmology 1978;10:1557‐9. [PubMed] [Google Scholar]
Tommila 1963 {published data only}
- Tommila V. Treatment of dendritic keratitis with interferon. Acta Ophthalmologica 1963;41:478‐82. [DOI] [PubMed] [Google Scholar]
Topciu 1992 {published data only}
- Topciu V, Chercotă G, Mihăilescu R, Dressler S. Immunologic and antiviral therapy in herpetic keratitis. Roumanian Archives of Microbiology and Immunology 1992;51:233‐7. [PubMed] [Google Scholar]
Wan 2014 {published data only}
- Wan JL, Kang B. Treatment of epithelial herpes simplex viral keratitis by recombinant human interferon α‐2b eye drops [重组人干扰素 α‐2b 滴眼液治疗上皮型单纯疱疹病毒性角膜炎]. International Eye Science [Guoji Yanke Zazhi] 2014;14(2):276‐8. [Google Scholar]
Wang 2009 {published data only}
- Wang SL, Xu M. Ganciclovir ophthalmic gel and acyclovir eye drops: comparative efficacy in treatment of viral keratitis [更昔洛韦眼用凝胶与阿昔洛韦眼液治疗病毒性角膜炎疗效比较]. China Modern Medicine [Zhongguo Dangdai Yiyao] 2009;16(17):53. [Google Scholar]
Wang 2010a {published data only}
- Wang QL. Clinical observation of integrated traditional Chinese and western medical therapy on herpes simplex keratitis [中西医结合治疗单纯疱疹病毒性角膜炎临床观察]. Hebei Journal of Traditional Chinese Medicine [He Bei Zhong Yi] 2010;22(9):1388‐9. [Google Scholar]
Wang 2010b {published data only}
- Wang ZP. Clinical experience in herpes simplex viral keratitis treated with integrated Chinese and western medicine [中西医结合治疗单纯疱疹病毒性角膜炎临床体会]. World Journal of Integrated Traditional and Western Medicine [Shijie Zhongxiyi Jiehe Zazhi] 2010;5(9):780‐1. [Google Scholar]
Wang 2011 {published data only}
- Wang ZY, Wang F. Clinical analysis of herpes simplex keratitis treated by Chinese herbs [中药治疗单纯疱疹性角膜炎临床分析]. Journal of Practical Traditional Chinese Medicine [Shiyong Zhongyiyiao Zazhi] 2011;27(5):294‐5. [Google Scholar]
Wang 2014b {published data only}
- Wang YZ, Xu Y, Wang LQ, Xin YF, Yang XS, Gu GX. Clinical study of yiqi jiedu granules combined with wester medicine for 107 cases of herpes simplex keratitis [益气解毒冲剂联合西药治疗单纯疱疹病毒性角膜炎107例临床研究]. Journal of Traditional Chinese Medicine [Zhongyi Zazhi] 2014;55(2):133‐6. [Google Scholar]
Wei 2012 {published data only}
- Wei XH, Li L, Su JL, Cui GD, Wang XM, Wang XJ. Polyinosinic acid combined with ganciclovir gel in treating herpes simplex keratitis [聚肌胞联合更昔洛韦眼用凝胶治疗单疱病毒性角膜炎]. Medical Journal of Trauma and Disability [Shangcan Yixue Zazhi] 2012;20(9):27‐8. [Google Scholar]
Wen 2011 {published data only}
- Wen HL. Observation of the curative effect on herpes simplex keratitis treated by the combination of Chinese traditional medicine and western medicine [中西医结合治疗单疱病毒性角膜炎疗效观察]. Chinese and Foreign Women Health [Zhong Wai Fu Er Jian Kang] 2011;2(9):4‐5. [Google Scholar]
Weng 2014 {published data only}
- Weng CY, Hong B. Recombinant human interferon combined with acyclovir in the treatment of herpes simplex virus keratitis and the prevention of recurrence [重组人干扰素联合无环鸟苷滴眼液治疗单纯疱疹病毒性角膜炎的效果及预防复发作用]. Chinese Remedies and Clinics [Zhongguo Yaowu Yu Linchuang] 2014;14(1):76‐7. [Google Scholar]
Whitcher 1976 {published data only}
- Whitcher JP, Dawson CR, Hoshiwara I, Daghfous T, Messadi M, Triki F, et al. Herpes simplex keratitis in a developing country. Natural history and treatment of epithelial ulcers in Tunisia. Archives of Ophthalmology 1976;94:587‐92. [DOI] [PubMed] [Google Scholar]
Xiao 1998 {published data only}
- Xiao AY, Li Y, Li JY, Zhang XH, Shen XF. Clinical study of curing herpes simplex keratitis with human recombinant IL‐2 [hrIL‐2治疗单疱病毒性角膜炎临床研究]. Chinese Ophthalmic Research [Yanke Yan Jiu] 1998;16(1):46‐7. [Google Scholar]
Xie 2005 {published data only}
- Xie W, Jiang S, Xie Y, Xie K. Clinical study of frost‐snow eye drops in the treatment of herpes simplex keratitis [冰霜雪眼药水治疗单纯疱疹性病毒性角膜炎的临床研究]. China Journal of Modern Medicine [Zhongguo Xiandai Yixue Zazhi] 2005;7(15):2344‐6. [Google Scholar]
Xu 2009b {published data only}
- Xu JJ. Clinical study on treating herpes simplex keratitis with recombinant human interleukin 2 [重组白细胞介素Ⅱ治疗单纯疱疹性角膜炎的临床观察]. Modern Preventive Medicine [Xiandai Yufang Yixue] 2009;36(6):1182‐3. [Google Scholar]
Xu 2012 {published data only}
- Xu GY. Houttuynia cordata as a treatment method for herpes simplex keratitis [鱼腥草治疗单纯疙疹病毒性角膜炎]. Guide of China Medicine [Zhongguo Yiyao Xuekan] 2012;10(24):15,17. [Google Scholar]
Yamamoto 1984 {published data only}
- Yamamoto K, Fujiwara H, Kohno M, Kurimoto R, Hanafusa M. Effectiveness of topical zinc sulfate for herpes simplex keratitis [単純ヘルペス性角膜炎に対する硫酸亜鉛点眼の有効性]. Japanese Journal of Clinical Ophthalmology [Rinsho Ganka] 1984;38(7):743‐6. [Google Scholar]
Yang 2012 {published data only}
- Yang XB. Compound Chinese medicine eye drops contrasted with acyclovir eye drops in the treatment of herpes simplex keratitis [复方中药滴眼液与无环鸟苷滴眼液治疗单纯疱疹性角膜炎的效果对比]. Lishizhen Medicine and Materia Medica Research [Zhizhen Guoyi Guoyao] 2012;23(8):2067‐8. [Google Scholar]
Yi 2011 {published data only}
- Yi YM, Yi JL, Wang HZ, Luo JY, Gong JM, Li RJ, Wang HY. Curative effect of qinggan xiehuo combined with acupuncture in the treatment of herpes simplex virus keratitis [清肝泻火法联合针刺治疗单纯疱疹病毒性角膜炎的疗效]. Practical Clinical Medicine [Shiyong Linchuang Yixue] 2011;12(9):77‐8,82. [Google Scholar]
Yu 1999 {published data only}
- Yu XL, Zeng QH, Li X, Wang MF. A clinical study on the efficacy of miedulin eye drops in the treatment of herpes simplex keratitis [灭毒灵滴眼液治疗单纯疱疹性角膜炎的临床研究]. Journal of Traditional Chinese Ophthalmology [Zhongguo Zhongyi Yanke Zazhi] 1999;9(1):29‐31. [Google Scholar]
Yu 2012b {published data only}
- Yu J, Zhang MC, Xiang QY. Clinical observation of epithelial herpes simplex keratitis treated by live attenuated measles vaccine [麻疹减毒活疫苗治疗上皮型单纯疱疹病毒性角膜炎临床观察]. Journal of Xinxiang Medical College [Xinxiang Yixueyuan Xuebao] 2012;29(6):454‐6. [Google Scholar]
Yu 2014 {published data only}
- Yu AZ. Clinical efficacy of Chinese herbs combined with acyclovir in treatment of herpes simplex keratitis [中药联合无环鸟苷治疗单纯疱疹性角膜炎临床疗效观察]. Journal of Guiyang College of Traditional Chinese Medicine [Guiyang Zhongyi Xueyuan Xuebao] 2014;36(2):79‐81. [Google Scholar]
Yuan 2011 {published data only}
- Yuan HY. Effects of ganciclovir ophthalmic gel combined with interferon in the treatment of herpes simplex viral keratitis [更昔洛韦眼用凝胶联合干扰素治疗单纯疱疹病毒性角膜炎的疗效分析与评价]. Chinese Journal of Clinical Rational Drug Use [Linchuang Heli Yongyao Zazhi] 2011;18(4):43‐4. [Google Scholar]
Zagaigora 1971 {published data only}
- Zagaigora EP. Comparative effectiveness of gamma‐globulin, deoxyribonuclease and Kerecid in herpetic keratitis [Сравнение эффективности гамма‐глобулина, дезоксирибонуклеазы и Keрeцида в герпетическиx кератитаx]. Oftalmologicheskii Zhurnal 1971;26(1):29‐32. [PubMed] [Google Scholar]
Zajácz 1968 {published data only}
- Zajácz M, Kovács M. Superficial herpetic keratitis (statistical assessment of 146 cases) [Felületes herpes‐keratitis (146 eset statisztikai értékelése)]. Szemészet 1968;105:294‐8. [Google Scholar]
Zhai 2007 {published data only}
- Zhai N, Chen Y. Integrated traditional Chinese and Western medicine in the treatment of 58 cases of herpes simplex virus keratitis [中西医结合治疗单纯疱疹病毒性角膜炎58例]. Journal of Shaanxi College of Traditional Chinese Medicine [Shaanxi Zhong Yixue Yuan Xuebao] 2007;30(4):34‐5. [Google Scholar]
Zhan 2011 {published data only}
- Zhan YH, Xiao YQ, Wu ZL. Traditional Chinese medicine compared with conventional western medicine, each in the treatment of 50 cases of herpes simplex keratitis [消翳方配合常规西药治疗单纯疱疹性角膜炎50例临床观察]. Guiding Journal of Traditional Chinese Medicine and Pharmacy [Zhongyiyao Daobao] 2011;8(3):50‐2. [Google Scholar]
Zhang 1992 {published data only}
- Zhang MD, Luan XH, Meng W, Zhao XZ. Clinical and immunologic evaluation of combination therapy for herpes simplex keratitis with antiviral drugs and immunopotentiating agent [抗病毒药联合免疫增强剂治疗单疱病毒性角膜炎的临床与免疫学评价]. Chinese Ophthalmic Research [Yanke Yan Jiu] 1992;19(2):38‐40. [Google Scholar]
Zhang 1995 {published data only}
- Zhang H, Li X, Li X, Liang Q, Xiang Y. Clinical observations on herpes simplex keratitis treated with qing gan xie huo decoction [清肝泻火汤治疗单纯疱疹性角膜炎的临床观察]. Bulletin of Hunan Medical University [Hunan Yike Daxue Xuebao] 1995;20(5):439‐41. [Google Scholar]
Zhang 1997 {published data only}
- Zhang H, Song HW, Guo RX, Sun NX. Detection of subtribe of T‐lymphocyte before and after traditional Chinese medicine treatment in simple herpetic keratitis with applied OKT‐McAb [应用OKT单克隆抗体检测单纯疱疹性角膜炎中药治疗前后外周血T细胞亚群]. Journal of Xi'an Medical University [Xi'an Yike Daxue Xuebao] 1997;18(4):433‐5, 452. [Google Scholar]
Zhang 2003 {published data only}
- Zhang XF, Li WH, Zhang JM, Yao J, Yang J, Sheng W. The comparison of interferon‐α‐1b and interferon‐α‐2b on the treatment of herpes simplex virus keratitis [α1b、α2b型干扰素滴眼剂治疗单纯疱疹病毒性角膜炎临床对比研究]. Chinese Journal of Practical Ophthalmology [Zhongguo Shiyong Erke Zazhi] 2003;21(2):828‐9. [Google Scholar]
Zhang 2008 {published data only}
- Zhang P. Integrated treatment of Chinese and Western medicine of 100 patients with herpes simplex virus keratitis [中西医结合治疗单纯疱疹病毒性角膜炎100例]. Chinese Archives of Traditional Chinese Medicine [Zhonghua Zhongyiyao Xuekan] 2008;26(7):1595‐7. [Google Scholar]
Zhang 2010 {published data only}
- Zhang YY, Chen GY. Effect of combined treatment of traditional Chinese medicine and western medicine on recurrent herpes simplex keratitis [复发性单纯痕莎性角膜炎中西医结合治疗的疗效分析]. Journal of Jinggangshan University (Natural Science) [Jinggangshan Daxue Xuebao (Ziranban)] 2010;6(1):106‐8. [Google Scholar]
Zhao 2001 {published data only}
- Zhao X, Feng Y, Ding Y, Guo Y, Tang Y, Feng D. Clinical observation of the effect of acyclovir with interferon and diclofenac on treatment of herpes simplex keratitis [无环鸟苷联合干扰素、双氯芬酸钠治疗单纯疱疹病毒性角膜炎]. Journal of Clinical Ophthalmology [Linchuang Yanke Zazhi] 2001;9(5):400‐1. [Google Scholar]
Zhao 2007 {published data only}
- Zhao Y, Wu L, Liang Q. Clinical observation of herpes simplex keratitis during treatment with yinhuang eye drop [银黄滴眼液治疗单纯疱疹性角膜炎临床研究]. Journal of Traditional Chinese Ophthalmology [Zhongguo Zhongyi Yanke Zazhi] 2007;4(8):187‐90. [Google Scholar]
Zhao 2010 {published data only}
- Zhao AX, Sun MQ, Sun HY. Chinese medicine combined with eyebrow injection in the treatment of 54 cases of recurrent herpes simplex keratitis [中药结合眉弓注射治疗复发性单疱病毒性角膜炎54 例]. Chinese Journal of Experimental Traditional Medical Formulae [Zhongguo Shiyan Fangjixue Zazhi] 2010;16(8):206‐7. [Google Scholar]
Zhao 2013 {published data only}
- Zhao SQ, Zhao L, Sun ZX. Randomized controlled research of herpes simplex keratitis treated with modified yupingfeng powder [玉屏风散加味治疗单纯疱疹性角膜炎的随机对照研究]. World Journal of Integrated Traditional and Western Medicine [Shijie Zhongxiyi Jiehe Zazhi] 2013;8(8):812‐4. [Google Scholar]
Zheng 2008 {published data only}
- Zheng LM, Cai HY, Lin RS. Therapeutic effect of Chinese medicine combined with iontophoresis of Radix astragali for herpes simplex keratitis [观察中药配合电离子导入治疗单纯疱疹病毒性角膜炎的疗效]. Journal of Guangzhou University of Traditional Chinese Medicine [Guangzhou Zhong Yiyao Daxue Xuebao] 2008;25(4):289‐91. [Google Scholar]
Zheng 2014 {published data only}
- Zheng HY. Effects observation on treating 45 cases of herpes simplex in the integrative medicine [中西医结合治疗45例单纯疱疹性角膜炎疗效观察]. Clinical Journal of Chinese Medicine [Zhongyi Linchuang Yanjiu] 2014;6(7):100‐1. [Google Scholar]
Zhi 2001 {published data only}
- Zhi S, Yang F, Liang C, Sun X. Clinical study of curing herpes simplex keratitis with recombinant lL‐2 [rIL‐2治疗单纯疱疹性角膜炎的临床研究]. Chinese Journal of Practical Ophthalmology [Zhongguo Shiyong Yanke Zazhi] 2001;19(8):604‐5. [Google Scholar]
Zhong 2003 {published data only}
- Zhong LY, Wang XM, Lai J, Liu YQ. Ultrasonic nebulization of reduqing eye drop in clinical treatment of herpes simplex keratitis [热毒清眼药水雾化治疗单疱性角膜炎临床研究]. Bulletin of the Academy of Military Medical Sciences [Junshi Yixue Kexueyuan Yuankan] 2003;15(3):209‐12. [Google Scholar]
Zhou 2007 {published data only}
- Zhou LS, Wu DL. Clinical observation on the treatment of 38 cases of herpes simplex virus keratitis with atomization of traditional Chinese medicine and acyclovir [中药雾化联合无环鸟苷治疗单纯疱疹病毒性角膜炎38例临床观察]. Guiding Journal of Traditional Chinese Medicine and Pharmacy [Zhong Yi Yao Dao Bao] 2007;13(11):44‐5. [Google Scholar]
Zhou 2008 {published data only}
- Zhou Q, Chen K, Fang L, Yu J, Yue X. Interferon combined with acyclovir for herpes simplex keratitis [干扰素联合无环鸟苷滴眼液治疗单纯疱疹病毒性角膜炎]. Chinese Journal of General Practice [Zhongguo Quanke Yixue] 2008;6(10):1009‐10. [Google Scholar]
Zirm 1981 {published data only}
- Zirm M, Söser M, Ogriseg M. Dendritic keratitis—a comparison of different therapy forms with special emphasis on antibody‐therapy. In: Sundmacher R editor(s). Herpetische Augenerkrankungen. München: JF Bergmann, 1981:365‐72. [Google Scholar]
References to studies awaiting assessment
Ajanta Pharma 2013 {published data only}
- Singh S. A clinical trial in patients with herpes keratitis: clinical trial is to study efficacy, safety and tolerability of ganciclovir ophthalmic gel 0.15% vs acyclovir ophthalmic ointment 3% in patients with herpes keratitis. Ajanta Pharma Ltd (protocol APL/CT/09/031) 2013. [CTRI/2010/091/000198]
Bausch & Lomb 2003 {published data only}
- Anonymous. Safety and efficacy of cidofovir topical ophthalmic solution in the treatment of unilateral epithelial keratitis due to HSV: comparative study between cidofovir (0.3% and 0.5%) vs Viroptic 1%. Unpublished clinical trial sponsored by Bausch and Lomb and Gilead Sciences 2003.
Additional references
AAO 2013
- American Academy of Ophthalmology. Herpes simplex virus epithelial keratitis-2013. one.aao.org/clinical‐questions/herpes‐simplex‐virus‐epithelial‐keratitis (accessed 21 August 2013).
Adhin 2012
- Adhin MR, Grunberg MG, Labadie‐Bracho M, Pawiroredjo J. Incidence of alpha‐herpes virus induced ocular disease in Suriname. Journal of Medical Virology 2012;84(12):1937‐42. [DOI] [PubMed] [Google Scholar]
Ahrens 2013
- Ahrens KA, Anderka MT, Feldkamp ML, Canfield MA, Mitchell AA, Werler MM, et al. Antiherpetic medication use and the risk of gastroschisis: findings from the National Birth Defects Prevention Study, 1997‐2007. Pediatric and Perinatal Epidemiology 2013;27(4):340‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alarcón 1984
- Alarcón B, González ME, Carrasco L. Antiherpesvirus action of atropine. Antimicrobial Agents and Chemotherapy 1984;26(5):702‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aldave 2007
- Aldave AJ, Nguyen A. Ocular surface toxicity associated with topical interferon α‐2b. British Journal of Ophthalmology 2007;91(8):1087‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alm 2008
- Alm A, Grierson I, Shields MB. Side effects associated with prostaglandin analog therapy. Survey of Ophthalmology 2008;53 Suppl 1:93‐105. [DOI] [PubMed] [Google Scholar]
Ambrósio 2001
- Ambrósio Jr R, Moro F, Fonseca VC, Noleto S, Cvintal T. Herpetic keratitis after penetrating keratoplasty for cases not related to herpes simplex infection [Ceratite herpética em transplante de córnea por causas não ligadas ao Herpes]. Revista Brasileira de Oftalmologia 2001;60(11):828‐31. [Google Scholar]
Ashaye 2008
- Ashaye A, Aimola A. Keratitis in children as seen in a tertiary hospital in Africa. Journal of the National Medical Association 2008;100(4):386‐90. [DOI] [PubMed] [Google Scholar]
Barker 2008
- Barker NH. Ocular herpes simplex. BMJ Clinical Evidence 2008; Vol. 0707. [www.clinicalevidence.bmj.com] [PMC free article] [PubMed]
Bauer 1985
- Bauer DJ. A history of the discovery and clinical application of antiviral drugs. British Medical Bulletin 1985;41:309‐14. [DOI] [PubMed] [Google Scholar]
Behrens‐Baumann 2010
- Behrens‐Baumann W. Herpes simplex keratitis. A short overview of current therapy [Herpes‐simplex‐Keratitis. Ein kurzer Überblick zur aktuellen Therapie]. Klinische Monatsblätter für Augenheilkunde 2010;227:388‐92. [DOI] [PubMed] [Google Scholar]
Bhatt 2009
- Bhatt UK, Abdul Karim MN, Prydal JI. Oral antivirals for preventing recurrent herpes simplex keratitis in people with corneal grafts. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007824] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bierly 1994
- Bierly JR, Ostler HB. Varicella dendritic keratitis. Journal of Pediatric Ophthalmology and Strabismus 1994;31:53‐6. [DOI] [PubMed] [Google Scholar]
Boisjoly 1983
- Boisjoly HM, Pavan‐Langston D, Kenyon KR, Backer AS. Superinfections in herpes simplex keratitis. American Journal of Ophthalmology 1983;96:354‐61. [DOI] [PubMed] [Google Scholar]
Borderie 2004
- Borderie VM, Méritet JF, Chaumeil C, Rozenberg F, Baudrimont M, Touzeau O, et al. Culture‐proven herpetic keratitis after penetrating keratoplasty in patients with no previous history of herpes disease. Cornea 2004;23:118‐24. [DOI] [PubMed] [Google Scholar]
Borkar 2014
- Borkar DS, Gonzales JA, Tham VM, Esterberg E, Vinoya AC, Parker JV, et al. Association between atopy and herpetic eye disease: results from the Pacific Ocular Inflammation Study. JAMA Ophthalmology 2014;132(3):326‐31. [DOI] [PubMed] [Google Scholar]
Boto‐de‐los‐Bueis 2013
- Boto‐de‐los‐Bueis A, Romero Gómez MP, Hierro Zarzuelo A, Sanchez EG, Mediero S, Noval S. Recurrent ocular surface inflammation associated with human herpesvirus 6 infection. Eye & Contact Lens 2014;40:in press. [DOI] [PubMed] [Google Scholar]
Brandt 2005
- Brandt CR. The role of viral and host genes in corneal infection with herpes simplex virus type 1. Experimental Eye Research 2005;80:607‐21. [DOI] [PubMed] [Google Scholar]
Bucher 1997
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50:683‐91. [DOI] [PubMed] [Google Scholar]
Burrel 2013
- Burrel S, Boutolleau D, Azar G, Doan S, Deback C, Cochereau I, et al. Phenotypic and genotypic characterization of acyclovir‐resistant corneal HSV‐1 isolates from immunocompetent patients with recurrent herpetic keratitis. Journal of Clinical Virology 2013;58(1):321‐4. [DOI] [PubMed] [Google Scholar]
Callaghan 2006
- Callaghan T, Khain E, Sander LM, Ziff RM. A stochastic model for wound healing. Journal of Statistical Physics 2006;122:909‐24. [Google Scholar]
Campoli‐Richards 1986
- Campoli‐Richards DM, Sorkin EM, Heel RC. Insoine pranobex: a preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs 1986;32(5):383‐424. [DOI] [PubMed] [Google Scholar]
Cantell 1995
- Cantell K. Development of antiviral therapy with alpha interferons: promises, false hopes, and accomplishments. Annals of Medicine 1995;27:23‐8. [DOI] [PubMed] [Google Scholar]
Carmine 1982
- Carmine AA, Brogden RN, Heel RC, Speight TM, Avery GS. Trifluridine: a review of its antiviral activity and therapeutic use in the topical treatment of viral eye infections. Drugs 1982;23:329‐53. [DOI] [PubMed] [Google Scholar]
Cernik 2008
- Cernik C, Gallina K, Brodell RT. The treatment of herpes simplex infections: an evidence‐based review. Archives of Internal Medicine 2008;168:1137‐44. [DOI] [PubMed] [Google Scholar]
Chaimani 2013
- Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta‐analysis in STATA. PLoS One 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chanzy 2002
- Chanzy B, Braig S, Morand P. Debates and updates regarding the virologic diagnosis of herpes [Débats et actualités dans le diagnostic virologique de l'herpès]. Pathologie Biologie 2002;50:419‐24. [DOI] [PubMed] [Google Scholar]
Chen 1989
- Chen CW. Adverse side effects caused by topically applied antiviral agents in herpetic keratitis [抗病毒藥物對單純疱疹角膜炎治療之副作用]. Kaohsiung Journal of Medical Sciences [Gaoxiong Yixue Kexue Zazhi] 1989;5(8):416‐29. [PubMed] [Google Scholar]
Chentoufi 2012
- Chentoufi AA, Kritzer E, Yu DM, Nesburn AB, BenMohamed L. Towards a rational design of an asymptomatic clinical herpes vaccine: the old, the new, and the unknown. Clinical and Developmental Immunology 2012;2012:187585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chodosh 1995
- Chodosh J, Miller D, Stroop WG, Pflugfelder SC. Adenovirus epithelial keratitis. Cornea 1995;14:167‐74. [PubMed] [Google Scholar]
Choong 2010
- Choong K, Walker NJ, Apel AJG, Whitby M. Aciclovir‐resistant herpes keratitis. Clinical and Experimental Ophthalmology 2010;38:309‐13. [DOI] [PubMed] [Google Scholar]
Chou 2014
- Chou TY, Hong B. Ganciclovir ophthalmic gel 0.15% for the treatment of acute herpetic keratitis: background, effectiveness, tolerability, safety, and future applications. Therapeutics and Clinical Risk Management 2014;10:665‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chung 1998
- Chung JH. Correlation between epithelial healing rate and initial wound size in contact lens‐induced central epithelial abrasion. Ophthalmologica 1998;212:46‐9. [DOI] [PubMed] [Google Scholar]
Chung 2004
- Chung CY, Kwok AKH, Chung KL. Use of ophthalmic medications during pregnancy. Hong Kong Medical Journal 2004;10:191‐4. [PubMed] [Google Scholar]
Coster 1977b
- Coster DJ, Jones BR, Falcon MG. Role of debridement in the treatment of herpetic keratitis. Transactions of the Ophthalmological Societies of the United Kingdom 1977;97:314‐7. [PubMed] [Google Scholar]
Croxtall 2011
- Croxtall JD. Ganciclovir ophthalmic gel 0.15%: in acute herpetic keratitis (dendritic ulcers). Drugs 2011;71(5):603‐10. [DOI] [PubMed] [Google Scholar]
Dantas 1997
- Dantas PEC, Dantas MCN, Holzchuchk N. Recurrence of corneal herpetic disease after argon laser treatment [Recorrência de doença herpética corneal após tratamento para neovascularizaçäo de córnea com argon laser]. Arquivos Brasileiros de Oftalmologia 1997;60(1):103‐5. [Google Scholar]
Darougar 1985
- Darougar S, Wishart MS, Viswalingam ND. Epidemiological and clinical features of primary herpes simplex virus ocular infection. British Journal of Ophthalmology 1985;69(1):2‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Clerq 2005
- Clerq E. (E)‐5‐(2‐bromovinyl)‐2'‐deoxyuridine (BVDU). Medical Research Reviews 2005;25:1‐20. [DOI] [PubMed] [Google Scholar]
de la Parra 2013
- Parra‐Colin P, Garza‐Leon M, Ortiz‐Nieva G, Barrientos‐Gutierrez T, Lindsley K. Oral antivirals for preventing recurrence of herpes simplex virus keratitis. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD010556] [DOI] [Google Scholar]
Deeks 2002
- Deeks JJ. Issues in the selection of a summary statistic for meta‐analysis of clinical trials with binary outcomes. Statistics in Medicine 2002;21:1575‐600. [DOI] [PubMed] [Google Scholar]
Di 2011
- Di Y, Zhang Y. Diagnosis and treatment of herpetic keratitis after laser‐assisted sub‐epithelial keratomileusis [LASEK 术后单纯疱疹病毒性角膜炎的诊断及治疗]. Recent Advances in Ophthalmology [Yanke Xin Jinzhan] 2011;22(10):948‐51. [Google Scholar]
Du 2010
- Du ZN, Yang YY, Zhu QW. Six cases of cataract surgery complicated by herpes simplex keratitis [白内障术后并发单纯疱疹性角膜炎6例]. Chinese Journal of Ophthalmology and Otorhinolaryngology [Zhongguo Yan Er Bi Houke Zazhi] 2010;10(4):238. [Google Scholar]
Duan 2009
- Duan R, Vries RD, Dun JM, Leonen FB, Osterhaus ADME, Remeijer L, et al. Acyclovir susceptibility and genetic characteristics of sequential herpes simplex virus type 1 corneal isolates from patients with recurrent herpetic keratitis. Journal of Infectious Diseases 2009;200:1402‐14. [DOI] [PubMed] [Google Scholar]
Elbadawy 2012
- Elbadawy HM, Gailledrat M, Desseaux C, Ponzin D, Ferrari S. Targeting herpetic keratitis by gene therapy. Journal of Ophthalmology 2012;2012:594869. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Emmert 1885
- Emmert E. Keratitis dendritica exulcdrans mycotica: an undescribed form of ulcerative keratitis [Keratitis dendritica exulcerans mycotica: Eine noch nicht beschriebene Form ulcerirender Hornhautentzündung]. Zentralblatt für praktische Augenheilkunde 1885;9:302‐11. [Google Scholar]
Epstein 1989
- Epstein RJ, Wilhelmus KR. Dendritic keratitis—will wiping it off wipe it out?. In: Deutsch TA editor(s). Ophthalmic Clinical Debates. Chicago: Year Book Medical Publishers, 1989:85‐99. [Google Scholar]
Falcon 1981
- Falcon MG, Jones BR, Williams HP, Wilhelmus K, Coster DJ. Adverse reactions in the eye from topical therapy with idoxuridine, adenine arabinoside and trifluorothymidine. In: Sundmacher R editor(s). Herpetische Augenerkrankungen. München: JF Bergmann Verlag, 1981:263‐8. [Google Scholar]
Farhatullah 2004
- Farhatullah S, Kaza S, Athmanathan S, Garg P, Reddy SB, Sharma S. Diagnosis of herpes simplex virus‐1 keratitis using Giemsa stain, immunofluorescence assay, and polymerase chain reaction assay on corneal scrapings. British Journal of Ophthalmology 2004;88(1):142‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farooq 2012
- Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Survey of Ophthalmology 2012;57(5):448‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Felton 1992
- Felton DT. Bias in meta‐analytic research. Journal of Clinical Epidemiology 1992;45(8):885‐92. [DOI] [PubMed] [Google Scholar]
Field 1995
- Field AJ, Gottsch JD. Persisting epithelial herpes simplex keratitis while on cyclosporin‐A ointment. Australian and New Zealand Journal of Ophthalmology 1995;23(4):333‐4. [DOI] [PubMed] [Google Scholar]
Friedenwald 1900
- Friedenwald H. On the treatment of dendritic keratitis and of marginal ulcer of the cornea with tincture of iodine. Transactions of the American Ophthalmological Society 1900;9:129‐33. [PMC free article] [PubMed] [Google Scholar]
Garcia‐Medina 2011
- Garcia‐Medina JJ, del‐Rio‐Vellosillo M, Garcia‐Medina M. Herpetic blepharitis and inhaled budesonide. Ophthalmology 2011;118(12):2520.e5‐7. [DOI: 10.1016/j.ophtha.2011.07.054] [DOI] [PubMed] [Google Scholar]
Glenny 2005
- Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D'Amico R, et al. Indirect comparisons of competing interventions. Health Technology Assessment 2005;9:1‐134. [DOI] [PubMed] [Google Scholar]
Gold 1965
- Gold JA, Stewart RC, McKee J. The epidemiology and chemotherapy of herpes simplex keratitis and herpes simplex skin infections. Annals of the New York Academy of Sciences 1965;130(1):209‐12. [DOI] [PubMed] [Google Scholar]
Goldschmidt 2006
- Goldschmidt P, Rostane H, Saint‐Jean C, Batellier L, Alouch C, Zito E, et al. Effects of topical anaesthetics and fluorescein on the real‐time PCR used for the diagnosis of Herpesviruses and Acanthamoeba keratitis. British Journal of Ophthalmology 2006;90(11):1354‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gordon 2000
- Gordon YJ. The evolution of antiviral therapy for external ocular viral infections over twenty‐five years. Cornea 2000;19:673‐80. [DOI] [PubMed] [Google Scholar]
Grüter 1920
- Grüter W. Experimental and clinical studies on so‐called corneal herpes [Experimentelle und klinische Untersuchungen über den sogenannten Herpes corneæ]. Berichte über die Versammlung der Deutschen Ophthalmologischen Gesellschaft 1920;42:162‐7. [Google Scholar]
Gu 2014
- Gu ZS, Lu SL. Observation on diagnosis and treatment of new‐onset herpes simplex virus keratitis after cataract surgery [白内障术后初发性单纯疱疹病毒性角膜炎的诊疗观察]. Journal of Shanghai Jiaotong University (Medical Science) [Shanghai Jiaotong Daxue Xuebao (Yixue Ban)] 2014;34(5):677‐80. [Google Scholar]
Guess 2007
- Guess S, Stone DU, Chodosh J. Evidence‐based treatment of herpes simplex virus keratitis: a systematic review. The Ocular Surface 2007;5:240‐50. [DOI] [PubMed] [Google Scholar]
Guess 2010
- Guess SM, Butt AL, Neely SB, Wild RC, Chou AF, Chodosh J. Dissemination of knowledge from randomized clinical trials for herpes simplex virus keratitis. Archives of Ophthalmology 2010;128(12):1624‐5. [DOI] [PubMed] [Google Scholar]
Gulkilik 2007
- Gulkilik G, Demirci G, Ozdamar AM, Muftuoglu GT. A case of herpetic keratitis after intravitreal triamcinolone injection. Cornea 2007;26(8):1000‐1. [DOI] [PubMed] [Google Scholar]
Gómez García 2004
- Gómez García S, Piñero Bustamente AM, Gutiérrez Sánchez E, Piñero Bustamente A. Herpes simplex keratitis following laser in situ keratomileusis [Queratitis por herpes simplex tras laser in situ keratomileusis]. Archivos de la Sociedad Española de Oftalmología 2004;79(3):139‐41. [DOI] [PubMed] [Google Scholar]
Hamoudi 2013
- Hamoudi H. Picture of the month: Epithelial herpes simplex virus keratitis [Månedens billede]. Ugeskrift for Læger 2013;175:744. [PubMed] [Google Scholar]
Hamroush 2014
- Hamroush A, Welch J. Herpes simplex epithelial keratitis associated with daily disposable contact lens wear. Contact Lens and Anterior Eye 2014;37(3):228‐9. [DOI] [PubMed] [Google Scholar]
Hansen Grut 1886
- Hansen Grut E. Two typical forms of keratitis [Sur deux formes typiques de kératite]. Compte‐Rendu: Congrès Périodique International des Sciences Médicales. 8me Session, Copenhague, 1884. 1886; Vol. 3:34‐40.
Harris 2008
- Harris RJ, Bradburn MJ, Deeks JJ, Harbord RM, Altman DG, Sterne JAC. Metan: fixed‐ and random‐effects meta‐analysis. The Stata Journal 2008;8(1):3‐28. [Google Scholar]
Hashizume 2009
- Hashizume K, Nabeshima T, Fujiwara T, Machida S, Kurosaka D. A case of herpetic epithelial keratitis after triamcinolone acetonide subtenon injection. Cornea 2009;28:463‐4. [DOI] [PubMed] [Google Scholar]
Hassan 2009
- Hassan M, Patel DK, Subrayan V. Primary herpes virus keratitis after penetrating keratoplasty. Annals of Ophthalmology 2009;41(3‐4):203‐5. [PubMed] [Google Scholar]
Hayashi 2008
- Hayashi T, Ishioka M, Ito N, Kato Y, Nakagawa H, Hatano H, et al. Bilateral herpes simplex keratitis in a patient with chronic graft‐versus‐host disease. Clinical Ophthalmology 2008;2(2):457‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
HEDS Group 2000a
- Herpetic Eye Disease Study Group. Psychological stress and other potential triggers for recurrences of herpes simplex virus eye infections. Archives of Ophthalmology 2000;118:1617‐25. [DOI] [PubMed] [Google Scholar]
HEDS Group 2000b
- Herpetic Eye Disease Study Group. Oral acyclovir for herpes simplex virus eye disease: effect on prevention of epithelial keratitis and stromal keratitis. Archives of Ophthalmology 2000;118:1030‐6. [PubMed] [Google Scholar]
HEDS Group 2001
- Herpetic Eye Disease Study Group. Predictors of recurrent herpes simplex virus keratitis. Cornea 2001;20:123‐8. [DOI] [PubMed] [Google Scholar]
Hergeldzhieva 2012
- Hergeldzhieva T. Treatment: medical therapy. Acta Ophthalmologica 2012;90(Suppl 249):0. [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Hlinomazová 2012
- Hlinomazová Z, Loukotová V, Horáčková M, Šerý O. The treatment of HSV1 ocular infections using quantitative real‐time PCR results. Acta Ophthalmologica 2012;90(5):456‐60. [DOI] [PubMed] [Google Scholar]
Hodge 1997
- Hodge WG, Margolis TP. Herpes simplex virus keratitis among patients who are positive or negative for human immunodeficiency virus: an epidemiologic study. Ophthalmology 1997;104:120‐4. [DOI] [PubMed] [Google Scholar]
Horner 1871
- Horner F. On corneal herpes [Ueber Herpes cornealis]. Klinische Monatsblätter für Augenheilkunde 1871;9:321‐37. [Google Scholar]
Hosmer 2009
- Hosmer DW, Lemeshow S. Survival analysis: applications to ophthalmic research. American Journal of Ophthalmology 2009;147:957‐8. [DOI] [PubMed] [Google Scholar]
Hou 2004
- Hou YC, Chen CC, Wang IJ, Hu FR. Recurrent herpetic keratouveitis following YAG laser peripheral iridotomy. Cornea 2004;23(6):641‐2. [DOI] [PubMed] [Google Scholar]
Hu 2010
- Hu AY, Strauss EC, Holland GN, Chan MF, Yu F, Margolis TP. Late varicella‐zoster virus dendriform keratitis in patients with histories of herpes zoster ophthalmicus. American Journal of Ophthalmology 2010;149:214‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Inoue 2014
- Inoue H, Suzuki T, Joko T, Inoue T, Ohashi Y. A case of herpetic keratitis after subconjunctival triamcinolone acetonide injection. Case Reports in Ophthalmology 2014;5(3):277‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Itoi 1975
- Itoi M, Gefter JW, Kaneko N, Ishii Y, Ramer RM, Gasset AR. Teratogenicities of ophthalmic drugs. I. Antiviral ophthalmic drugs. Archives of Ophthalmology 1975;93:46‐51. [DOI] [PubMed] [Google Scholar]
Jewell 2009
- Jewell NP. Risk comparisons. American Journal of Ophthalmology 2009;148:484‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jezegabel 1967
- Jezegabel C, Rossazza C, Delplace MP. Recurrence of herpes on a lamellar keratoplasty [Les récidives d'herpes sur kératoplastie lamellaire]. Bulletin des Sociétés d'Ophtalmologie de France 1967;67(9):759‐62. [PubMed] [Google Scholar]
Jones 1967
- Jones BR. Prospects in treating viral disease of the eye. Transactions of the Ophthalmological Societies of the United Kingdom 1967;87:537‐79. [PubMed] [Google Scholar]
Jones 1977
- Jones BR, Coster DJ, Falcon MG. Prospects of prevention of recurrent herpetic eye disease. Transactions of the Ophthalmological Society of the United Kingdom 1977;97:350‐5. [PubMed] [Google Scholar]
Joseph 2005
- Joseph MA, Kaufman HE, Insler M. Topical tacrolimus ointment for treatment of refractory anterior segment inflammatory disorders. Cornea 2005;24(4):417‐20. [DOI] [PubMed] [Google Scholar]
Kaiserman 2005
- Kaiserman I, Kaiserman N, Nakar S, Vinker S. Herpetic eye disease in diabetic patients. Ophthalmology 2005;112:2184‐8. [DOI] [PubMed] [Google Scholar]
Kaiserman 2006
- Kaiserman I, Kaiserman N, Elhayany A, Vinker S. Increased risk for herpetic eye disease in patients with allergic conjunctivitis. Current Eye Research 2006;31:721‐5. [DOI] [PubMed] [Google Scholar]
Kaneko 2008
- Kaneko H, Kawana T, Ishioka K, Ohno S, Aoki K, Suzutani T. Evaluation of mixed infections cases with both herpes simplex virus types 1 and 2. Journal of Medical Virology 2008;80:883‐7. [DOI] [PubMed] [Google Scholar]
Kastner 1984
- Kastner JK, Weiss SM, Kulikowski CA, Dawson CR. Therapy selection in an expert medical consultation system for ocular herpes simplex. Computers in Biology and Medicine 1984;14:285‐301. [DOI] [PubMed] [Google Scholar]
Kaufman 1962
- Kaufman HE. Clinical cure of herpes simplex keratitis by 5‐iodo‐2'‐deoxyuridine. Proceedings of the Society for Experimental Biology and Medicine 1962;109:251‐2. [DOI] [PubMed] [Google Scholar]
Kaufman 1972
- Kaufman HE. The natural history and diagnosis of herpes cornea. Israel Journal of Medical Sciences 1972;8:1212‐5. [PubMed] [Google Scholar]
Kaufman 2002
- Kaufman HE. Can we prevent recurrences of herpes infections without antiviral drugs?. Investigative Ophthalmology & Visual Science 2002;43:1325‐9. [PubMed] [Google Scholar]
Kaufman 2012
- Kaufman HE, Haw WH. Ganciclovir ophthalmic gel 0.15%: safety and efficacy of a new treatment for herpes simplex keratitis. Current Eye Research 2012;37(7):654‐60. [DOI] [PubMed] [Google Scholar]
Khalili 2009
- Khalili MR, Mehdizadeh M, Mehryar M. Herpetic epithelial keratitis after intravitreal injection of bevacizumab (Avastin). Cornea 2009;28:360‐1. [DOI] [PubMed] [Google Scholar]
Kip 2001
- Kip KE, Cohen F, Cole SR, Wilhelmus KR, Patrick DL, Blair RC, et al. Recall bias in a prospective cohort study of acute time‐varying exposures: example from the Herpetic Eye Disease Study. Journal of Clinical Epidemiology 2001;54(5):482‐7. [DOI] [PubMed] [Google Scholar]
Kipp 1880
- Kipp CJ. On keratitis from malarial fever. Transactions of the American Ophthalmological Society 1880;3:91‐3. [PMC free article] [PubMed] [Google Scholar]
Kipp 1890
- Kipp CJ. Further observations on malarial keratitis. Transactions of the American Ophthalmological Society 1890;5:331‐40. [PMC free article] [PubMed] [Google Scholar]
Knickelbein 2009
- Knickelbein JE, Hendricks RL, Charukamnoetkanok P. Management of herpes simplex virus stromal keratitis: an evidence‐based review. Survey of Ophthalmology 2009;54:226‐34. [DOI] [PubMed] [Google Scholar]
Kowalski 1993
- Kowalski RP, Gordon YJ, Romanowski EG, Araullo‐Cruz T, Kinchington PR. A comparison of enzyme immunoassay and polymerase chain reaction with the clinical examination for diagnosing ocular herpetic disease. Ophthalmology 1993;100(4):530‐3. [DOI] [PubMed] [Google Scholar]
Kremer 1991
- Kremer I, Wagner A, Shmuel D, Yussim A, Shapira Z. Herpes simplex keratitis in renal transplant patients. British Journal of Ophthalmology 1991;75(2):94‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kymionis 2007
- Kymionis GD, Portaliou DM, Bouzoukis DI, Suh LH, Pallikaris AI, Markomanolakis M, et al. Herpetic keratitis with iritis after corneal crosslinking with riboflavin and ultraviolet A for keratoconus. Journal of Cataract and Refractive Surgery 2007;33(11):1982‐4. [DOI] [PubMed] [Google Scholar]
Labetoulle 2005
- Labetoulle M, Auquier P, Conrad H, Crochard A, Daniloski M, Bouée S, et al. Incidence of herpes simplex virus keratitis in France. Ophthalmology 2005;112:888‐95. [DOI] [PubMed] [Google Scholar]
Labetoulle 2012
- Labetoulle M, Colin J. Current concepts in the treatment of herpetic keratitis [Aspects actuels du traitement des kératites herpétiques]. Journal Français d'Ophtalmologie 2012;35(4):292‐307. [DOI] [PubMed] [Google Scholar]
Lairson 2003
- Lairsen DR, Begley CE, Reynolds TF, Wilhelmus KR. Prevention of herpes simplex virus eye disease: a cost‐effectiveness analysis. Archives of Ophthalmology 2003;121:108‐12. [DOI] [PubMed] [Google Scholar]
Langeland 1987
- Langeland N, Holmsen H, Lillehaug JR, Haarr L. Evidence that neomycin inhibits binding of herpes simplex virus type 1 to the cellular receptor. Journal of Virology 1987;61(11):3388‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larrañaga Fragoso 2015
- Larrañaga Fragoso P, Boto de Los Bueis A, Bravo Ljubetic L, Hierro Zarzuelo A, Romero Gómez MP, Mora Rillo M. Herpes simplex keratitis in rheumatoid arthritis patients. Ocular Immunology and Inflammation 2015;23:in press. [DOI] [PubMed] [Google Scholar]
Lass 1984
- Lass JH, Langston RH, Foster CS, Pavan‐Langston D. Antiviral medications and corneal wound healing. Antiviral Research 1984;4:143‐57. [DOI] [PubMed] [Google Scholar]
Li 2014d
- Li JY, Li SQ. Systematic reviews of ganciclovir versus acyclovir for herpes simplex virus keratitis [更昔洛韦对比阿昔洛韦治疗单纯疱疹病毒性角膜炎的系统评价]. International Eye Science [Guoji Yanke Zazhi] 2014;14(9):1590‐3. [Google Scholar]
Liesegang 1989a
- Liesegang TJ. Epidemiology of ocular herpes simplex. Natural history in Rochester, Minn, 1950 through 1982. Archives of Ophthalmology 1989;107:1160‐5. [DOI] [PubMed] [Google Scholar]
Liesegang 1989b
- Liesegang TJ, Melton LJ III, Daly PJ, Ilstrup DM. Epidemiology of ocular herpes simplex. Incidence in Rochester, Minn, 1950 through 1982. Archives of Ophthalmology 1989;107(8):1155‐9. [DOI] [PubMed] [Google Scholar]
Liesegang 2001
- Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea 2001;20:1‐13. [DOI] [PubMed] [Google Scholar]
Lingua 1985
- Lingua RW. Sequelae of botulinum toxin injection. American Journal of Ophthalmology 1985;100(2):305‐7. [DOI] [PubMed] [Google Scholar]
Lu 2006
- Lu CK, Chen KH, Lee SM, Hsu WM, Lai JY, Li YS. Herpes simplex keratitis following excimer laser application. Journal of Refractive Surgery 2006;22(5):509‐11. [DOI] [PubMed] [Google Scholar]
Lu 2011
- Lu JY. Observation on diagnosis and treatment of herpes simplex keratitis after phacoemulsification surgery [超声乳化白内障吸除术后单纯疱疹病毒性角膜炎的诊疗观察]. Journal of Clinical and Experimental Medicine [Linchuang He Shiyan Yixue Zazhi] 2011;10(9):666‐7. [Google Scholar]
Ludema 2014a
- Ludema C, Cole SR, Poole C, Smith JS, Schoenbach VJ, Wilhelmus KR. Association between unprotected ultraviolet radiation exposure and recurrence of ocular herpes simplex virus. American Journal of Epidemiology 2014;179(2):208‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ludema 2014b
- Ludema C, Cole SR, Hudgens MG, Poole C, Smith JS, Schoenbach VJ, et al. Prophylactic oral acyclovir and multiple herpes simplex virus recurrences. PLOS One 2014;9:in press. [Google Scholar]
Luzi 1983
- Luzi G, Voci MC, Bonomo R, Aiuti F, Pivetti‐Pezzi P, Aziz M. Levamisole therapy: clinical and immunological evaluation in herpetic keratitis. International Journal of Immunopharmacology 1983;5:197‐9. [DOI] [PubMed] [Google Scholar]
Ma 2006b
- Ma YD. Analysis of the integration of Chinese and western medical treatment of herpes simplex keratitis using evidence‐based medicine [中西医结合治疗单纯疱疹性角膜炎的循证医学分析]. Journal of Traditional Chinese Ophthalmology [Zhongguo Zhongyi Yanke Zazhi] 2006;16(2):103‐4. [Google Scholar]
Maudgal 1979
- Maudgal PC, Deuren H, Missotten L. Therapeutic effect of corneal replicas in herpetic keratitis. Bulletin de la Société Belge d'Ophtalmologie 1979;185:39‐45. [PubMed] [Google Scholar]
McAllum 2003
- McAllum PJ, McGhee CNJ. Prescribing trends in infectious keratitis: a survey of New Zealand ophthalmologists. Clinical & Experimental Ophthalmology 2003;31:496‐504. [DOI] [PubMed] [Google Scholar]
McDonald 2015
- McDonald EM, Patel DV, McGhee CN. A prospective study of the clinical characteristics of patients with herpes simplex and varicella zoster keratitis, presenting to a New Zealand emergency eye clinic. Cornea 2015;34:in press. [DOI] [PubMed] [Google Scholar]
Michiels 2005
- Michiels S, Piedbois P, Burdett S, Syz N, Stewart L, Pignon JP. Meta‐analysis when only the median survival times are known: a comparison with individual patient data results. International Journal of Technology Assessment in Health Care 2005;21:119‐25. [DOI] [PubMed] [Google Scholar]
Mills 2012
- Mills EJ, Ioannidis JPA, Thorlund K, Schünemann HJ, Puhan MA, Guyatt GH. How to use an article reporting a multiple treatment comparison meta‐analysis. JAMA 2012;308(12):1246‐53. [DOI] [PubMed] [Google Scholar]
Minkovitz 1995
- Minkovitz JB, Pepose JS. Topical interferon alpha‐2a treatment of herpes simplex keratitis resistant to multiple antiviral medications in an immunosuppressed patient. Cornea 1995;14(3):326‐30. [DOI] [PubMed] [Google Scholar]
Miyajima 2003
- Miyajima S, Sano Y, Sotozono C, Yokoi N, Ishino Y, Kinoshita S. Herpes simplex after ophthalmic surgery [眼科手術後に発症した角膜ヘルペス]. Japanese Journal of Ophthalmology [Nihon Ganka Gakkai Zasshi] 2003;107(9):538‐42. [PubMed] [Google Scholar]
Mortensen 1979
- Mortensen KK, Sjølie AK. Keratitis dendritica. An epidemiological investigation. Acta Ophthalmologica 1979;57:750‐4. [DOI] [PubMed] [Google Scholar]
Mucci 2009
- Mucci JJ, Utz VM, Galor A, Feuer W, Jeng BH. Recurrence rates of herpes simplex virus keratitis in contact lens and non‐contact lens wearers. Eye and Contact Lens 2009;35:185‐7. [DOI] [PubMed] [Google Scholar]
Musch 2006
- Musch DC, Gillespie BW. The state of being noninferior. Ophthalmology 2006;113:1‐2. [DOI] [PubMed] [Google Scholar]
Nagy 2003
- Nagy ZZ, Keleman E, Kovács A. Herpes simplex keratitis after photorefractive keratectomy. Journal of Cataract and Refractive Surgery 2003;29(1):222‐3. [DOI] [PubMed] [Google Scholar]
Naito 1987
- Naito T, Shiota H, Mimura Y. Side effects in the treatment of herpetic keratitis. Current Eye Research 1987;6:237‐9. [DOI] [PubMed] [Google Scholar]
Nataneli 2013
- Nataneli N, Chai JSM, Donnenfeld ED, Perry HD. Recurrent herpes simplex keratitis adjacent to femtosecond laser arcuate keratotomies. JAMA Ophthalmology 2013;131(10):1372. [DOI] [PubMed] [Google Scholar]
Neumann‐Haefelin 1978
- Neumann‐Haefelin D, Sundmacher R, Wochnik G, Bablok B. Herpes simplex virus types 1 and 2 in ocular disease. Acta Ophthalmologica 1978;96:64‐9. [DOI] [PubMed] [Google Scholar]
Ng 1998
- Ng P, McCluskey P, McCaughan G, Glanville A, MacDonald P, Keogh A. Ocular complications of heart, lung, and liver transplantation. British Journal of Ophthalmology 1998;82(4):423‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Okuno 2011
- Okuno T, Hooper LC, Ursea R, Smith J, Nussenblatt R, Hooks JJ, et al. Role of human herpes virus 6 in corneal inflammation alone or with human herpesviruses. Cornea 2011;30(2):204‐7. [DOI] [PubMed] [Google Scholar]
Oshry 1998
- Oshry T, Lifshitz T. Intravenous acyclovir treatment for extensive herpetic keratitis in a liver transplant patient. International Ophthalmology 1998;21(5):265‐8. [DOI] [PubMed] [Google Scholar]
Pan 2014
- Pan D, Kaye SB, Hopkins M, Kirwan R, Hart IJ, Coen DM. Common and new acyclovir resistant herpes simplex virus‐1 mutants causing bilateral recurrent herpetic keratitis in an immunocompetent patient. Journal of Infectious Diseases 2014;209(3):345‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Panagiotou 2013
- Panagiotou OA, Contopoulos‐Ioannidis DG, Ioannidis JP. Comparative effect sizes in randomised trials from less developed and more developed countries: meta‐epidemiological assessment. BMJ 2013;346:f707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [Addendum: Freels S. Statistics in Medicine 2004;23:1817.] [DOI] [PubMed] [Google Scholar]
Pasternak 2010
- Pasternak B, Hviid A. Use of acyclovir, valacyclovir, and famciclovir in the first trimester of pregnancy and the risk of birth defects. JAMA 2010;304(8):859‐66. [DOI] [PubMed] [Google Scholar]
Patel 2009
- Patel NN, Teng CC, Sperber LT, Dodick JM. New‐onset herpes simplex virus keratitis after cataract surgery. Cornea 2009;28:108‐10. [DOI] [PubMed] [Google Scholar]
Pavan‐Langston 1973
- Pavan‐Langston D, McCulley JP. Herpes zoster dendritic keratitis. Archives of Ophthalmology 1973;89:25‐9. [DOI] [PubMed] [Google Scholar]
Pflugfelder 1990
- Pflugfelder SC, Huang A, Crouse C. Epstein‐Barr virus keratitis after a chemical facial peel. American Journal of Ophthalmology 1990;110:571‐3. [DOI] [PubMed] [Google Scholar]
Pollard 1982
- Pollard RB. Interferons and interferon inducers: development of clinical usefulness and therapeutic promise. Drugs 1982;23(1‐2):37‐55. [DOI] [PubMed] [Google Scholar]
Prabriputaloong 2006
- Prabriputaloong T, Margolis TOP, Lietman TM, Wong IG, Mather R, Gritz DC. Atopic disease and herpes simplex eye disease: a population based case‐control study. American Journal of Ophthalmology 2006;142:745‐9. [DOI] [PubMed] [Google Scholar]
Pramod 1999
- Pramod NP, Rajendran P, Kannan KA, Thyagarajan SP. Herpes simplex keratitis in South India: clinico‐virological correlation. Japanese Journal of Ophthalmology [Nihon Ganka Gakkai Zasshi] 1999;43:303‐7. [DOI] [PubMed] [Google Scholar]
Prasher 2009
- Prasher P, Muftuoglu O. Herpetic keratitis after descemet stripping automated endothelial keratoplasty for failed graft. Eye and Contact Lens 2009;35(1):41‐2. [DOI] [PubMed] [Google Scholar]
Rao 2009
- Rao A, Tandon R, Sharma N, Sihota R, Gupta V, Dada T. Herpetic keratitis and keratouveitis after mitomycin‐C use in glaucoma filtering surgery: a short case series. European Journal of Ophthalmology 2009;19(6):1088‐90. [PubMed] [Google Scholar]
Remeijer 1997
- Remeijer L, Doornenbal P, Geerards AJ, Rijneveld WA, Beekhuis WH. Newly acquired herpes simplex virus keratitis after penetrating keratoplasty. Ophthalmology 1997;104:648‐52. [DOI] [PubMed] [Google Scholar]
Remeijer 2002
- Remeijer L, Maertzdorf J, Buitenwerf J, Osterhaus ADME, Verjans GMGM. Corneal herpes simplex virus type 1 superinfection in patients with recrudescent herpetic keratitis. Investigative Ophthalmology & Visual Science 2002;43(2):358‐63. [PubMed] [Google Scholar]
Revere 2013
- Revere K, Davidson SL. Update on management of herpes keratitis in children. Current Opinion in Ophthalmology 2013;24(4):343‐7. [DOI] [PubMed] [Google Scholar]
Rezende 2004
- Rezende RA, Uchoa UB, Raber IM, Rapuano CJ, Laibson PR, Cohen EJ. New onset of herpes simplex virus epithelial keratitis after penetrating keratoplasty. American Journal of Ophthalmology 2004;137:415‐9. [DOI] [PubMed] [Google Scholar]
Rezende 2006
- Rezende RA, Hammersmith K, Bisol T, Lima AL, Webster GF, Freitas JF, et al. Comparative study of ocular herpes simplex virus in patients with and without self‐reported atopy. American Journal of Ophthalmology 2006;141:1120‐5. [DOI] [PubMed] [Google Scholar]
Ribaric 1976
- Ribarić V. The incidence of herpetic keratitis among population. Ophthalmologica 1976;173(1):19‐22. [DOI] [PubMed] [Google Scholar]
Richards 1983
- Richards DM, Carmine AA, Brogden RN, Heel RC, Speight TM, Avery GS. Acyclovir. A review of its pharmacodynamic properties and therapeutic efficacy. Drugs 1983;26:378‐438. [DOI] [PubMed] [Google Scholar]
Roat 1987
- Roat MI, Romanowski E, Araullo‐Cruz T, Gordon YJ. The antiviral effects of rose bengal and fluorescein. Archives of Ophthalmology 1987;105:1415‐7. [DOI] [PubMed] [Google Scholar]
Robert 2005
- Robert PY, Adenis JP, Pleyer U. How "safe" is corneal transplantation? A contribution on the risk of HSV‐transmission due to corneal transplantation [Wie "sicher" ist das Hornhauttransplantat? Ein Beitrag zum Risiko der HSV‐Transmission durch Hornhauttransplantation]. Klinische Monatsblätter für Augenheilkunde 2005;222:870‐3. [DOI] [PubMed] [Google Scholar]
Romano 1988
- Romano A, Sadan Y. Ten years of experience with human fibroblast interferon in treatment of viral ophthalmic infections. Metabolic, Pediatric, and Systemic Ophthalmology 1988;11:43‐6. [PubMed] [Google Scholar]
Ruiz 2007
- Ruiz L, Rodriguez I, Baez R, Aldana R. Stability of an extemporaneously prepared recombinant human interferon alfa‐2b eye drop formulation. American Journal of Health‐System Pharmacy 2007;64(16):1716‐9. [DOI] [PubMed] [Google Scholar]
Sahin 2012
- Sahin A, Hamrah P. Acute herpetic keratitis: what is the role for ganciclovir ophthalmic gel?. Ophthalmology and Eye Diseases 2012;4:23‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Santos 1983
- Santos CR. Herpetic corneal ulcer following radial keratotomy. Annals of Ophthalmology 1983;15(1):82‐5. [PubMed] [Google Scholar]
Satpathy 2011
- Satpathy G, Mishra AK, Tandon R, Sharma MK, Sharma A, Nayak N, et al. Evaluation of tear samples for herpes simplex virus 1 (HSV) detection in suspected cases of viral keratitis using PCR assay and conventional laboratory diagnostic tools. British Journal of Ophthalmology 2011;95(3):415‐8. [DOI] [PubMed] [Google Scholar]
Seitzman 2006
- Seitzman GD, Cevallos V, Margolis TP. Rose bengal and lissamine green inhibit detection of herpes simplex virus by PCR. American Journal of Ophthalmology 2006;141:756‐8. [DOI] [PubMed] [Google Scholar]
Shtein 2007
- Shtein RM, Stahl RM, Saxe SJ, Mian SI. Herpes simplex keratitis after intravitreal triamcinolone acetonide. Cornea 2007;26(5):641‐2. [DOI] [PubMed] [Google Scholar]
Siddique 2011
- Siddique SS, Gonzalez‐Gonzalez LA, Amorese L, Shaikh M, Foster CS. Herpes keratitis in a patient undergoing treatment with topical mitomycin C. Cornea 2011;30(4):466‐7. [DOI] [PubMed] [Google Scholar]
Smith 2002
- Smith JS, Robinson NJ. Age‐specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. Journal of Infectious Diseases 2002;186 Suppl 1:S3‐28. [DOI] [PubMed] [Google Scholar]
Song 2003
- Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta‐analyses. BMJ (Clinical research ed.) 2003;326(7387):472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2009
- Song F, Loke YK, Walsh T, Glenny AM, Eastwood AJ, Altman DG. Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews. BMJ 2009;338:b1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Souza 2003
- Souza PMF, Holland EJ, Huang AJW. Bilateral herpetic keratoconjunctivitis. Ophthalmology 2003;110:493‐6. [DOI] [PubMed] [Google Scholar]
Stan 2000
- Stan C, Bedeoan S, Stan R. The influence of meteorological factors for frequency of herpes simplex keratitis [Influenta factorilor meteorologici asupra frecventei de aparitie a cheratitei herpetice]. Oftalmologia 2000;50:32‐6. [PubMed] [Google Scholar]
Stanzel 2014
- Stanzel TP, Diaz JD, Mather R, Wong IG, Margolis TP, Gritz DC. The epidemiology of herpes simplex virus eye disease in northern California. Ophthalmic Epidemiology 2014;21(6):370‐7. [DOI] [PubMed] [Google Scholar]
Sun 2013b
- Sun L, Chen W. Clinical characteristics and treatment of herpes simplex keratitis after phacoemulsification [白内障超声乳化吸除术后并发单纯疱疹病毒性角膜炎的特点及治疗]. Ophthalmology in China [Yanke] 2013;22(3):161‐4. [Google Scholar]
Sundmacher 1977
- Sundmacher R, Mackensen G. Herpes therapy and prophylaxis. II. Results of a survey of practicing ophthalmologists [Herpestherapie und‐prophylaxe. II. Das Ergebnis einer Umfrage bei niedergelassenen Augenarzten]. Klinische Monatsblätter für Augenheilkunde 1977;171(1):90‐7. [PubMed] [Google Scholar]
Sundmacher 1978c
- Sundmacher R. Trifluorothymidin‐prophylaxis of dendritic keratitis in steroid‐treated herpetic keratouveitis [Trifluorthymidinprophylaxe bei der Steroidtherapie herpetischer Keratouveitiden]. Klinische Monatsblätter für Augenheilkunde 1978;173(4):516‐9. [PubMed] [Google Scholar]
Sundmacher 1982
- Sundmacher R. Interferon in ocular viral diseases. In: Gresser I editor(s). Interferon. London: Academic Press, 1982:177‐200. [PubMed] [Google Scholar]
Sundmacher 1983
- Sundmacher R. Use of nucleoside analogues in the treatment of herpes simplex virus eye diseases. Metabolic, Pediatric, and Systemic Ophthalmology 1983;7:89‐94. [PubMed] [Google Scholar]
Sundmacher 1984b
- Sundmacher R. The role of interferon in prophylaxis and treatment of dendritic keratitis. In: Blodi F editor(s). Herpes Simplex Infections of the Eye. New York: Churchill Livingstone, 1984:129‐46. [Google Scholar]
Sundmacher 1986
- Sundmacher R. Dendritic keratitis following corneal foreign body [Keratitis dendritica nach Hornhautfremdkorper]. Klinische Monatsblätter für Augenheilkunde 1986;188:307‐9. [DOI] [PubMed] [Google Scholar]
Sundmacher 2009
- Sundmacher R. Color Atlas of Herpetic Eye Disease: A Practical Guide to Clinical Management. Heidelberg: Springer, 2009:13‐9. [Google Scholar]
Tabbara 2010
- Tabbara KF, Al Balushi N. Topical ganciclovir in the treatment of acute herpetic keratitis. Clinical Ophthalmology 2010;4:905‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tabery 2010
- Tabery HM. Herpes Simplex Virus Epithelial Keratitis: In Vivo Morphology in the Human Cornea. Heidelberg: Springer, 2010:1‐24. [Google Scholar]
Thygeson 1976
- Thygeson P. Historical observations on herpetic keratitis. Survey of Ophthalmology 1976;21(2):82‐90. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2006
- Turner A, Rabiu M. Patching for corneal abrasion. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD004764.pub2] [DOI] [PubMed] [Google Scholar]
Turner 2013
- Turner LD, Beckingsale P. Acyclovir‐resistant herpetic keratitis in a solid‐organ transplant recipient on systemic immunosuppression. Clinical Ophthalmology 2013;7:229‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uchio 1994
- Uchio E, Hatano H, Mitsui K, Sugita M, Okada K, Goto K, et al. A retrospective study of herpes simplex keratitis over the last 30 years. Japanese Journal of Ophthalmology [Nihon Ganka Gakkai Zasshi] 1994;38:196‐201. [PubMed] [Google Scholar]
Uchoa 2003
- Uchoa UB, Rezende RA, Carrasco MA, Rapuano CJ, Laibson PR, Cohen EJ. Long‐term acyclovir use to prevent recurrent ocular herpes simplex virus infection. Archives of Ophthalmology 2003;121:1702‐4. [DOI] [PubMed] [Google Scholar]
Ukety 1991
- Ukety TO, Maertens K. Ocular ulcerative herpes following measles in Kinshasa, Zaire. Current Eye Research 1991;10(Suppl):131‐7. [DOI] [PubMed] [Google Scholar]
Van Spall 2007
- Spall HGC, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high‐impact general medical journals: a systematic sampling view. JAMA 2007;297:1233‐40. [DOI] [PubMed] [Google Scholar]
van Velzen 2013
- Velzen M, Vijver DA, Loenen FB, Osterhaus AD, Remeijer L, Verjans GM. Acyclovir prophylaxis predisposes to antiviral resistant recurrent herpetic keratitis. Journal of Infectious Diseases 2013;208(9):1359‐65. [DOI] [PubMed] [Google Scholar]
Verhoeff 1909
- Verhoeff FH. Neuropathic keratitis and some allied conditions, with special reference to treatment. Journal of the American Medical Association 1909;53:191‐8. [Google Scholar]
Villegas 2008
- Villegas VM, Diaz L, Izquierdo NJ. Herpetic keratitis in a patient who used two different prostaglandin analogue ophthalmic solutions: a case report. Puerto Rico Health Sciences Journal 2008;27(4):348‐9. [PubMed] [Google Scholar]
Wagoner 1984
- Wagoner MD, Kenyon KR, Gipson IK, Hanninen LA, Seng WL. Polymorphonuclear neutrophils delay corneal epithelial wound healing in vitro. Investigative Ophthalmology & Visual Science 1984;25:1217‐20. [PubMed] [Google Scholar]
Wagstaff 1994
- Wagstaff AJ, Faulds D, Goa KL. Aciclovir. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs 1994;47:153‐205. [DOI] [PubMed] [Google Scholar]
Webre 2012
- Webre JM, Hill JM, Nolan NM, Clement C, McFerrin HE, Bhattacharjee PS, et al. Rabbit and mouse models of HSV‐1 latency, reactivation, and recurrent eye diseases. Journal of Biomedicine and Biotechnology 2012;2012:Article ID 612316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wei 2008
- Wei X, Chen XM, Liu XY, Deng YP. Evaluation of the effectiveness of the common drugs for herpes simplex keratitis by using the evidence‐based medicine method [关于单纯疱疹性角膜炎药物治疗的循证证据]. International Journal of Ophthalmology [Guoji Yanke Zazhi] 2008;8(10):2084‐6. [Google Scholar]
Weinberg 1977
- Weinberg RJ, Oh JO. The effect of a topical anesthetic on the recovery of herpes simplex virus. Annals of Ophthalmology 1977;9(8):977‐83. [PubMed] [Google Scholar]
Wells 2009 [Computer program]
- Wells GA, Sultan SA, Chen L, Khan M, Coyle D. Indirect treatment comparison. Version 1.0. Ottawa: Canadian Agency for Drugs and Technologies in Health, 2009. [www.cadth.ca/media/pdf/H0462_itc_tr_e.pdf]
Wheeler 1959
- Wheeler CE, Canby CM. Effect of temperature on the growth curves of herpes simplex virus in tissue cultures. Journal of Immunology 1959;83:392‐6. [PubMed] [Google Scholar]
White 2012
- White IR, Barrett JK, Jackson D, Higgins JPT. Consistency and inconsistency in network meta‐analysis: model estimation using multivariate meta‐regression. Research Synthesis Methods 2012;3(2):111‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2014
- White ML, Chodosh J. Herpes simplex virus keratitis: a treatment guideline ‐ 2014. ONE Network [The Ophthalmic News & Education Network] 2014; Vol. American Academy of Ophthalmology, issue http://one.aao.org/clinical‐statement/herpes‐simplex‐virus‐keratitis‐treatment‐guideline.
Whitley 1980
- Whitley R, Alford C, Hess F, Buchanan R. Vidarabine: a preliminary review of its pharmacological properties and therapeutic use. Drugs 1980;20:267‐82. [DOI] [PubMed] [Google Scholar]
Wilhelmus 1981b
- Wilhelmus KR, Coster DJ, Donovan HC, Falcon MG, Jones BR. Prognostic indicators of herpetic keratitis. Analysis of a five‐year observation period after corneal ulceration. Archives of Ophthalmology 1981;99:1578‐82. [DOI] [PubMed] [Google Scholar]
Wilhelmus 1981c
- Wilhelmus KR, Falcon MG, Jones BR. Bilateral herpetic keratitis. British Journal of Ophthalmology 1981;65:385‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilhelmus 1982
- Wilhelmus KR. Suppurative corneal ulceration following herpetic keratitis. Documenta Ophthalmologica 1982;53:17‐36. [DOI] [PubMed] [Google Scholar]
Wilhelmus 1983
- Wilhelmus KR, Jones DB. What's new: acyclovir for treatment of ocular viral infections. Texas Medicine 1983;70:27‐9. [PubMed] [Google Scholar]
Wilhelmus 1989
- Wilhelmus KR. Dendritic keratitis: will wiping it off wipe it out?. In: Deutsch TA editor(s). Ophthalmic Clinical Debates. Chicago: Year Book Medical Publishers, 1989:94‐9. [Google Scholar]
Wilhelmus 1996a
- Wilhelmus KR, Dawson CR, Barron BA, Bacchetti P, Gee L, Jones DB, et al. Risk factors for herpes simplex virus epithelial keratitis recurring during treatment of stromal keratitis or iridocyclitis. British Journal of Ophthalmology 1996;80:969‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilhelmus 1996b
- Wilhelmus KR, Font RL, Lehmann RP, Cernoch PL. Cytomegalovirus keratitis in acquired immunodeficiency syndrome. Archives of Ophthalmology 1996;114:869‐72. [DOI] [PubMed] [Google Scholar]
Wilhelmus 2000
- Wilhelmus KR. The treatment of herpes simplex virus epithelial keratitis. Transactions of the American Ophthalmological Society 2000;98:505‐32. [PMC free article] [PubMed] [Google Scholar]
Wilhelmus 2007a
- Wilhelmus KR. Redundant publication of clinical trials on herpetic keratitis. American Journal of Ophthalmology 2007;144:222‐6. [DOI] [PubMed] [Google Scholar]
Wilhelmus 2008a
- Wilhelmus KR. The epidemiology of ocular infections. In: Jaeger EA, Tasman W editor(s). Duane's Foundations of Clinical Ophthalmology. Philadelphia: Lippincott‐Raven, 2008. [Google Scholar]
Williams 1977
- Williams HP, Falcon MG, Jones BR. Corticosteroids in the management of herpetic eye disease. Transactions of the Ophthalmological Societies of the United Kingdom 1977;97:341‐4. [PubMed] [Google Scholar]
Wood 1936
- Wood CA. Memorandum Book of a Tenth‐Century Oculist for the Use of Modern Ophthalmologists: A Translation of the Tadhkirat of Ali ibn Isa of Baghdad (cir. 940‐1010 A.D.), the Most Complete Practical and Original of All the Early Textbooks on the Eye and its Diseases. Chicago: Northwestern University, 1936:154. [Google Scholar]
Wu 2002
- Wu X, Chen X. Acyclovir for the treatment and prevention of recurrent infectious herpes simplex keratitis. Chinese Medical Journal [Zhonghua Yixue Zazhi] 2002;115:1569‐72. [PubMed] [Google Scholar]
Wu 2009
- Wu T, Li Y, Bian Z, Liu G, Moher D. Randomized trials published in some Chinese journals: how many are randomized?. Trials 2009;10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamagami 1998
- Yamagami H, Takamura E. The effect of acyclovir ointment on herpes simplex keratitis [角膜ヘルペスの軽症化に対するアシク口ビル限軟膏の効果]. Japanese Review of Clinical Ophthalmology [Ganka Rinsho Iho] 1998;92(8):170‐2. [Google Scholar]
Yang 2011
- Yang YY. Seven cases of cataract surgery complicated by herpes simplex virus keratitis [白内障术后并发单纯疱疹病毒性角膜炎7 例]. Journal of Practical Medicine [Shiyong Yixue Zazhi] 2011;27(3):551‐2. [Google Scholar]
Yao 1996
- Yao YF, Inoue Y, Kase T, Uchihori Y, Mori Y, Ohashi Y. Clinical characteristics of acyclovir‐resistant herpetic keratitis and experimental studies of isolates. Graefe's Archive for Clinical and Experimental Ophthalmology 1996;234(Suppl 1):S126‐32. [DOI] [PubMed] [Google Scholar]
Yoon 2010
- Yoon KC, Im SK, Park HY. Recurrent herpes simplex keratitis after verteporfin photodynamic therapy for corneal neovascularization. Cornea 2010;29(4):465‐7. [DOI] [PubMed] [Google Scholar]
Yorston 1992
- Yorston D, Foster A. Herpetic keratitis in Tanzania: association with malaria. British Journal of Ophthalmology 1992;76(10):582‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2010
- Young RC, Hodge DO, Liesegang TJ, Baratz KH. Incidence, recurrence, and outcomes of herpes simplex virus eye disease in Olmsted County, Minnesota, 1976‐2007: the effect of oral antiviral prophylaxis. Archives of Ophthalmology 2010;128(9):1178‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yuksel 2011
- Yuksel N, Bilgihan K, Hondur AM. Herpetic keratitis after corneal collagen cross‐linking with riboflavin and ultraviolet‐A for progressive keratoconus. International Ophthalmology 2011;31(6):513‐5. [DOI] [PubMed] [Google Scholar]
Zhang 2007
- Zhang W, Suzuki T, Shiraishi A, Shimamura I, Inoue Y, Ohashi Y. Dendritic keratitis caused by an acyclovir‐resistant herpes simplex virus with frameshift mutation. Cornea 2007;26:105‐6. [DOI] [PubMed] [Google Scholar]
Ziahosseini 2009
- Ziahosseini K, Ikram K. Current clinical practice of consultant ophthalmologists in treating herpetic eye disease in the United Kingdom. Eye 2009;23(4):993‐4. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Wilhelmus 2001
- Wilhelmus KR. Interventions for herpes simplex virus epithelial keratitis. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD002898] [DOI] [PubMed] [Google Scholar]
Wilhelmus 2003
- Wilhelmus KR. Interventions for herpes simplex virus epithelial keratitis. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD002898] [DOI] [PubMed] [Google Scholar]
Wilhelmus 2007
- Wilhelmus KR. Therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD002898.pub2] [DOI] [PubMed] [Google Scholar]
Wilhelmus 2008
- Wilhelmus KR. Therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD002898.pub3] [DOI] [PubMed] [Google Scholar]
Wilhelmus 2010
- Wilhelmus KR. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD002898.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]


