Highlights
-
•
Wolves are considered as definitive hosts for Echinococcus granulosus in Liguria, Italy.
-
•
Scats were examinated and taeniid eggs isolated.
-
•
Molecular species identification was performed through PCR analysis and sequencing.
-
•
Taeniid species diagnosed document domestic, semi-domestic and wildlife cycles.
Keywords: Liguria-Italy, Canis lupus italicus, Echinococcus granulosus, PCR, 12S, nad 1
Graphical Abstract

Abstract
Canids are definitive hosts of Taenia and Echinococcus species, which infect a variety of mammals as intermediate or accidental hosts including humans. Parasite transmission is based on domestic, semi-domestic and wildlife cycles; however, little is known of the epidemiological significance of wild large definitive hosts such as the wolf. In this study, 179 scats of wolves (Canis lupus italicus) collected throughout the Italian region of Liguria were analyzed for the detection of taeniid infection. Taeniid egg isolation was performed using a sieving/flotation technique, and the species level was identified by PCR (gene target: 12S rRNA and nad 1) followed by sequence analyses. Based on sequence homologies of ≥99%, Taenia hydatigena was identified in 19.6%, Taenia krabbei in 4.5%, Taenia ovis in 2.2%, Taenia crassiceps in 0.6%, Hydatigera taeniaeformis in 0.6% and Echinococcus granulosus in 5.6% of the samples. According to these results, Canis lupus italicus can be considered as involved in the wild (including cervids and rodents) and semi-domestic cycles (including sheep and goats) of taeniids in this area.
1. Introduction
The grey wolf Canis lupus (Linnaeus, 1758), a large carnivore of the Canidae family, is widely distributed throughout Eurasia and North America (Mech and Boitani, 2003). In Europe, at the end of the last century, the wolf survived in highly fragmented populations as a result of legal human persecution (Breitenmoser, 1998). The Italian wolf population Canis lupus italicus (Altobello, 1921) progressively increased after full legal protection and conservation action in 1976 (Boitani, 1992). From the Central Apennines, wolves recolonized the entire Apennine chain and the Western Alps, where they established stable packs (Fabbri et al., 2007; Marucco et al., 2012). Despite this positive trend, illegal killings are still one of the most important threats to the species (Boitani, 2000; Lovari et al., 2007), and thus it is still considered as endangered. Depredation on livestock is the main cause of conflict between human activities and the presence of wolves (Meriggi et al., 2011). These events have increased the probability of contact between wolves, domestic animals and humans, which has led to a higher risk of pathogen transmission (Daszak et al., 2000). However, due to the increase of human activity in wildlife habitats, a transmission of infectious agents from domestic animals to wild animals should be considered (Thompson, 2013). A European example for such scenario is the establishment of the Asian eye worm Thelazia callipaeda in fox populations (Malacrida et al., 2008).
In this study, we focused on Taeniidae (Cestoda), a family of tapeworms with a considerable medical and veterinary importance (Craig and Pawłowski, 2002; Hoberg, 2002). A previous study carried out on illegally killed wolves, from all over the Apennine chain (Guberti et al., 1993, 2004), identified the Italian wolf as a definitive host of many species of taeniids (Taenia hydatigena, Taenia multiceps, Taenia pisiformis, Taenia ovis and Echinococcus granulosus sensu lato). E. granulosus is the etiological agent of cystic echinococcosis (CE), an important parasitic zoonosis transmitted mainly in a domestic cycle, involving sheep and dog, in southern Europe (Eckert and Deplazes, 2004; Alvarez Rojas et al., 2014). The aim of this study was to evaluate with a non-invasive sampling the role of wolves as definitive hosts for the taeniid species.
2. Materials and methods
From 2011 to 2014, an investigation was carried out on six packs of wolves (approximately 66 individuals; Meriggi et al., 2013) living in the northern Apennines and southern Alps in Liguria (Italy).
The study area (5343 km2) was subdivided into 100 km2 sample units, each containing at least one transect (Fig. 1) randomly selected from among the existing footpaths for a total of 64 transects and 298 km. Every transect was covered four times a year (once per season) in order to collect wolf scats, georeferenced in the UTM WGS84 32N-coordinate system using ARCGIS 10.0 (ESRI, http://www.esri.com/software/arcgis/).
Fig. 1.
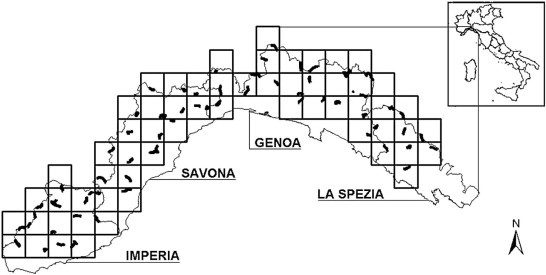
Study area, Liguria (Italy). Dark areas represent transects investigated to sample wolf scats. Each square represents a sample unit of 100 km2 over an area of 5343 km2.
A total of 179 fecal samples of wolves (44.75 ± 34.17 per year; 2.99 ± 5.39 per sample units) were collected from all over the region. Samples were stored at −80 °C for safety precautions (Eckert et al., 2001). In order to correctly discriminate wolf scats to those of dogs and foxes, we applied a critical analysis to reduce the probabilities of misinterpretation. Specifically, we evaluated size, shape, smell, composition and location (Mattioli et al., 1995; Bassi et al., 2012). Wolf adult droppings are 10–15 cm long and 3–5.5 cm thick, cylindrical, with sub-divisions, tapered at one of the extremities (Chame, 2003; Bang et al., 2007; Jedrzejewsky and Sidorovich, 2010). The secretion produced by the anal gland adheres to the feces during defecation, atrophied in most dog breeds, and give it an acrid and characteristic smell (Harrington and Asa, 2003). Moreover, wolves usually use feces in territorial marking. In the wild, feces are more common along trails and road, particularly at junctions, placed on conspicuous objects (Peters and Mech, 1975; Vilà et al., 1994). Finally, we also considered undigested prey remains (i.e., fur, snails and parts of bones), especially from large wild ungulates (i.e. wild boar and deer) (Meriggi et al., 2014) into wolf scats.
About 2 g of each fecal sample was placed in 15 ml tubes. Taeniid eggs were isolated using the flotation and sieving method described by Mathis et al. (1996), and morphological identification was carried out under an inverted microscope. DNA extraction of taeniid egg positive samples was performed as described by Štefanić et al. (2004), and species discrimination was carried out by multiplex PCR (for Taenia spp. and E. granulosus gene target: 12S rRNA, for E. multilocularis gene target: nad 1) and sequence analyses (Trachsel et al., 2007). Each E. granulosus positive sample was subsequently analyzed with E. granulosus “sheep strain” specific PCR (gene target: 12S rRNA) according to Štefanić et al. (2004).
In 8 out of 49 Taenia positive samples by multiplex PCR, the sequencing results were unclear and could not discriminate between T. serialis/T. multiceps. Therefore, further analyses of these samples using a PCR, targeting nad1 gene, according to Armua-Fernandez et al. (2011) were performed, which resulted in a 500 bp amplicon which allows further species discrimination by sequence analysis.
The amplicons were directly sequenced after purification of the PCR products using the MinElute PCR purification kit (Qiagen, Hilden, Germany). Sequencing was performed by Synergene Biotech GmbH, Biotech Center Zurich, Switzerland (http://www.synergene-biotech.com), using primers Cest5seq and Cest4 for Taenia spp. and E. granulosus amplicons obtained with multiplex PCR (Trachsel et al., 2007), and nad1T-Rv for Taenia spp. amplicons obtained with nad1 PCR (Armua-Fernandez et al., 2011).
Sequencing results were compared with entries in the GeneBank nucleotide database, using BLAST (http://www.blast.ncbi.nlm.nih.gov).
3. Results and discussion
Overall, 59 (33.0%) out of 179 samples were positive for eggs of taeniids (Table 1). Taenia hydatigena, T. ovis, T. crassiceps, Hydatigera (Taenia) taeniaeformis and E. granulosus identification was performed sequencing 12S amplicons obtained by multiplex PCR (Trachsel et al., 2007), while the 8 doubtful samples were correctly identified as T. krabbei by sequencing the amplicons obtained with nad1 PCR. In both genes, the sequence homology of the amplicons was ≥ 99% identical to the corresponding sequences in the GeneBank. By multiplex PCR in 6 of 59 positive samples, multiple infections with Taenia spp. and E. granulosus were detected (E. granulosus with T. hydatigena (3 cases), with T. krabbei (2 cases) and with unspecified Taenia sp. (one case)).
Table 1.
Frequency of taeniid findings in wolf fecal samples from the Liguria region (Italy).
| Taeniid species* | Frequency (%) | Confidence interval (95%) |
|---|---|---|
| Taenia hydatigena | 19.6 | 14.1–26.0 |
| Taenia krabbei | 4.5 | 2.0–8.5 |
| Taenia ovis | 2.2 | 0.8–5.4 |
| Taenia crassiceps | 0.6 | 0.0–2.9 |
| Hydatigera taeniaeformis | 0.6 | 0.0–2.9 |
| Echinococcus granulosus sensu lato | 5.6 | 2.9–9.9 |
| Total positive | 33.0 | 26.1–40.1 |
As determined by PCR (nad 1 and 12S rRNA gene).
The non-invasive sampling approach, based on collection of scats in the environment, is suitable for epidemiological studies on wild animals, and necessary when protected species are investigated without interfering in the existing structure of the population. The molecular identification of the parasite infections, obtained through the isolation of their eggs in feces, PCR analysis and sequencing, is a valuable tool to estimate the level of the environmental contamination, and it has been previously used for epidemiological investigation on wolves (Guerra et al., 2013). When stool are fresh, individual identification of the animal, using molecular analyses is possible (Galaverni et al., 2012). This study based on mostly old scats collected in the environment did not allow the individual animal identification and we cannot exclude that some stool samples belonged to the same animal. Therefore, we used the term frequency or occurrence and not prevalence to describe the proportion of taeniid infections in the wolf population investigated. Although the procedure used to identify wolf scats was useful to avoid collection of those of domestic dogs or foxes, we cannot completely exclude the occurrence of those of feral dogs. However, the presence of feral dogs has never been recorded during the sampling period in the study area by regional authorities (nor by Public Health or Police Departments). Furthermore, no evidence of the occurrence of feral dogs was recorded in our study area through other sampling methods, i.e., camera-trapping (Meriggi et al., 2013).
Taenia hydatigena was the most frequent (19.6%), which was distributed throughout the study area. This parasite is the most common gastrointestinal helminth in wolves in Europe (Craig and Craig, 2005). It is transmitted trophically between wolves and large wild and domestic ungulates, which are also the main prey of wolves in the study area (Meriggi et al., 2011).
Taenia krabbei (4.5%) and T. ovis (2.2%) were reported with a lower occurrence. Both parasites are known to occur in the wolf (Craig and Craig, 2005). T. ovis has been previously described in Italian wolves by Guberti et al. (1993). Current knowledge of T. krabbei identified roe deer (Capreolus capreolus), moose (Alces alces), red deer (Cervus elaphus), and fallow deer (Cervus dama) as intermediate hosts, indicating that the parasite might circulate mostly in wild cycles. T. ovis intermediate hosts are mostly Bovidae, above all sheep (Ovis aries), but also goats (Capra hircus), confirming that livestock is still part of a wolf's diet (Meriggi and Lovari, 1996) and therefore this can be considered as an example of transmission of parasites from domestic animals to wildlife. Adult worms of T. ovis and T. krabbei are very difficult to distinguish morphologically; however, molecular tools clearly differentiated them (Priemer et al., 2002; Lavikainen et al., 2008), suggesting possible identification mistakes in previous studies.
In this study, we found both H. taeniaeformis (0.6%) (one sample located at the border between the province of Imperia and France) and T. crassiceps (0.6%) (one sample in the province of Genoa). Both species are known to be wolf parasites and have been found in Canada and Eastern Europe (Craig and Craig, 2005) respectively. However, these species have never been reported in the Italian wolf population to date, although they have been found in foxes (Vulpes vulpes) in northern Italy (Di Cerbo et al., 2008).
Cystic echinococcosis (CE) is one of the most widespread zoonoses in the Mediterranean (Dakkak, 2010; Alvarez Rojas et al., 2014) with an important burden of diseases (Hoberg, 2002; Budke et al., 2006). In Italy, CE is endemic, and is more prevalent in the south of the peninsula and in Sicily and Sardinia (Garippa and Manfredi, 2009), where it is still of important public health concern (Brundu et al., 2014).
The first finding of E. granulosus in wolf was associated with the wolf–sheep predator/prey interaction (Guberti et al., 2004), and on this occasion no genotype was investigated. In our investigation, E. granulosus was recorded with a low occurrence (5.6%), compared to previous studies (prevalence 15–17%; Guberti et al., 1993, 2004). Most of the sequences obtained in this study were very short (almost 65 bp) and thus, it was not possible to obtain the genotype for all the PCR positive samples. Seven sequences out of ten revealed a 100% identity with E. granulosus (G1-G3), while they show less than 94% identity with other genotype (G4, G5, G6/7, G8-G10), and the remaining three samples (two located in Imperia and one in Savona) were also positive in the “sheep strain” specific PCR (Štefanić et al., 2004).
In Italy, E. granulosus sensu stricto (mainly G1 and G3) is known to occur in livestock such as sheep and cattle (Bos taurus; Busi et al., 2002, 2004, 2007; Capuano et al., 2006; Varcasia et al., 2006; Rinaldi et al., 2008; Casulli et al., 2008), but also in wild boar (Sus scrofa) (Busi et al., 2007), in which the E. granulosus “pig strain” (E. intermedius, G7) has also been found (Varcasia et al., 2007). Only a few investigations on the prevalence of the parasite in livestock (sheep and goat) have been conducted in the past in the north of Italy, and the values were very low, especially in Liguria (<0.1%) (Lorenzini and Ruggieri, 1987). Recent data on cystic echinococcosis prevalence in farm animals from northern regions are the lowest (<1%) registered in Italy (Garippa and Manfredi, 2009). Few data regarding cystic echinococcosis in wild animals are available for Liguria. Hydatid cysts (0.5–0.3%) have been found in wild boar and chamois (Rupicapra rupicapra) from Imperia, but no information on the fertility of the cysts was provided (Rossi et al., 2009). In Rossi et al.'s study, 19 wolf intestines were also investigated and no adult worms were found. More recently, three wolf intestines were investigated for the presence of adult worms, and no positive cases were found (Guidetti et al., 2014).
Our data confirm that Canis lupus italicus can be considered as definitive host for E. granulosus (G1-G3) in the north Apennine region. Spatial behavior of wolves characterized by large home ranges and long dispersion distances can promote the spread of this parasite throughout Europe. This could be a concern, given the current situation in other countries, such as France, where the south east, just near the border with Liguria, has the highest prevalences of ovine cystic echinococcosis in all France (ANSES, 2014).
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgments
This work represents part of the thesis of Francesca Gori, biologist. We thank Dr. A. Meriggi who supervised wolf monitoring and Dr. D. Signorelli, Dr. L. Schenone, Dr. E. Torretta, Dr. F. Puopolo, Dr. L. Guardone and students of the University of Pavia which collected wolves data, and Prof. A. Mathis from Institute für Parasitologie, Zürich, for advice in the molecular analysis of the samples.
References
- Altobello G. Fauna of Abruzzo and Molise. Mammiferi. 1921;4:38–45. in Italian. [Google Scholar]
- Alvarez Rojas C.A., Romig T., Lightowlers M.W. Echinococcus granulosus sensu lato genotypes infecting humans – review of current knowledge. Int. J. Parasitol. 2014;44:9–18. doi: 10.1016/j.ijpara.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail (ANSES) Bullettin Echinote num. 3. 2014. https://www.anses.fr/fr/content/bulletin-echinote in French.
- Armua-Fernandez M.T., Nonaka N., Sakurai T., Nakamura S., Gottstein B., Deplazes P. Development of PCR/dot blot assay for specific detection and differentiation of taeniid cestode eggs in canids. Parasitol. Int. 2011;60:84–89. doi: 10.1016/j.parint.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Bang P., Dahlstrøm P., Walters M. Oxford University Press; New York: 2007. Animal Tracks and Signs. [Google Scholar]
- Bassi E., Donaggio E., Marcon A., Scandura M., Apollonio M. Trophic niche overlap and wild ungulate consumption by red fox and wolf in a mountain area in Italy. Mamm. Biol. 2012;77:369–376. [Google Scholar]
- Boitani L. Wolf research and conservation in Italy. Biol. Conserv. 1992;61:125–132. [Google Scholar]
- Boitani L. 2000. Action plan for the conservation of wolves in Europe (Canis lupus) Nature and Environment Report, Council of Europe, Strasbourg. [Google Scholar]
- Breitenmoser U. Large predators in the Alps: the fall and rise of man's competitors. Biol. Conserv. 1998;83:279–289. [Google Scholar]
- Brundu D., Piseddu T., Stegel G., Masu G., Ledda S., Masala G. Retrospective study of human cystic echinococcosis in Italy based on the analysis of hospital discharge records between 2001 and 2012. Acta Trop. 2014;140:91–96. doi: 10.1016/j.actatropica.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Budke C.M., Deplazes P., Torgerson P.R. Global socioeconomic impact of cystic echinococcosis. Emerg. Infect. Dis. 2006;12:296–303. doi: 10.3201/eid1202.050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busi M., Snabel V., Denegri G., Dopchiz M., Elissondro M.C., D'Amelio S. Molecular epidemiology of cystic echinococcosis in Italy, Portugal, Slovak Republic and Argentina. Parassitologia. 2002;44:32. [Google Scholar]
- Busi M., Snabel V., De Liberato C., D'Amelio S. Molecular genotyping of Echinococcus granulosus hydatid cystis in Italy reveals the presence of three distinct genotypes. Parassitologia. 2004;46:164. [Google Scholar]
- Busi M., Snabel V., Varcasia A., Garippa G., Perrone V., De Liberato C. Genetic variation within and between G1 and G3 genotypes of Echinococcus granulosus in Italy revealed by multilocus DNA sequencing. Vet. Parasitol. 2007;150:75–83. doi: 10.1016/j.vetpar.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Capuano F., Rinaldi L., Maurelli M.P., Perugini A.G., Veneziano V., Garippa G. Cystic echinococcosis in water buffaloes: epidemiological survey and molecular evidence of ovine (G1) and buffalo (G3) strains. Vet. Parasitol. 2006;137:262–268. doi: 10.1016/j.vetpar.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Casulli A., Manfredi M.T., La Rosa G., Cerbo A.R.D., Genchi C., Pozio E. Echinococcus ortleppi and E. granulosus G1, G2 and G3 genotypes in Italian bovines. Vet. Parasitol. 2008;155:168–172. doi: 10.1016/j.vetpar.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Chame M. Terrestrial mammal feces: a morphometric summary and description. Mem. Inst. Oswaldo Cruz. 2003;98:71–94. doi: 10.1590/s0074-02762003000900014. [DOI] [PubMed] [Google Scholar]
- Craig H.L., Craig P.S. Helminth parasites of wolves (Canis lupus): a species list and an analysis of published prevalence studies in Nearctic and Palaearctic populations. J. Helminthol. 2005;79:95–103. doi: 10.1079/joh2005282. [DOI] [PubMed] [Google Scholar]
- Craig P., Pawłowski Z., editors. vol. 341. IOS Press; 2002. (Cestode Zoonoses: Echinococcosis and Cysticercosis: An Emergent and Global Problem). [Google Scholar]
- Dakkak A. Echinococcosis/hydatidosis: a severe threat in Mediterranean countries. Vet. Parasitol. 2010;174:2–11. doi: 10.1016/j.vetpar.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Daszak P., Cunningham A.A., Hyatt A.D. Emerging infectious diseases of wildlife–threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- Di Cerbo A.R., Manfredi M.T., Trevisiol K., Bregoli M., Ferrari N., Pirinesi F. Intestinal helminth communities of the red fox (Vulpes vulpes L.) in the Italian Alps. Acta Parasitol. 2008;53:302–311. [Google Scholar]
- Eckert J., Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 2004;17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert J., Gottstein B., Heath D., Liu F.J. Prevention of Echinococcosis in humans and safety precautions. In: Eckert J., Gemmell M.A., Meslin F.-X., Pawłowski Z.S., editors. Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. OIE and WHO; Paris, France: 2001. pp. 238–248. [Google Scholar]
- Fabbri E., Miquel C., Lucchini V., Santini A., Caniglia R., Duchamp C. From the Apennines to the Alps: colonization genetics of the naturally expanding Italian wolf (Canis lupus) population. Mol. Ecol. 2007;16:1661–1671. doi: 10.1111/j.1365-294X.2007.03262.x. [DOI] [PubMed] [Google Scholar]
- Galaverni M., Palumbo D., Fabbri E., Caniglia R., Greco C., Randi E. Monitoring wolves (Canis lupus) by non-invasive genetics and camera trapping: a small-scale pilot study. Eur. J. Wild. Res. 2012;58:47–58. [Google Scholar]
- Garippa G., Manfredi M.T. Cystic echinococcosis in Europe and in Italy. Vet. Res. Commun. 2009;33:35–39. doi: 10.1007/s11259-009-9245-0. [DOI] [PubMed] [Google Scholar]
- Guberti V., Stancampiano L., Francisci F. Intestinal helminth parasite community in wolves (Canis lupus) in Italy. Parassitologia. 1993;35:59–65. [PubMed] [Google Scholar]
- Guberti V., Bolognini M., Lanfranchi P., Battelli G. Echinococcus granulosus in the wolf in Italy. Parassitologia. 2004;46:425–427. [PubMed] [Google Scholar]
- Guerra D., Armua-Fernandez M.T., Silva M., Bravo I., Santos N., Deplazes P. Taeniid species of the Iberian wolf (Canis lupus signatus) in Portugal with special focus on Echinococcus spp. Int. J. Parasitol. 2013;2:50–53. doi: 10.1016/j.ijppaw.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidetti C., Robetto S., Notarnicola R., Mignone W., Orusa R. 2014. Diagnostic approach on Echinococcus spp. in wild canids in Italy. 3rd European EAVLD Congress, Pisa (Italy) [Google Scholar]
- Harrington F.H., Asa C.S. Wolf communication. In: Mech L.D., Boitani L., editors. Wolves: Behavior, Ecology, and Conservation. The University of Chicago Press; London: 2003. pp. 66–103. [Google Scholar]
- Hoberg E.P. Taenia tapeworms: their biology, evolution and socioeconomic significance. Microbes Infect. 2002;4:859–866. doi: 10.1016/s1286-4579(02)01606-4. [DOI] [PubMed] [Google Scholar]
- Jedrzejewsky W., Sidorovich V. Mammal Research Institute Polish Academy of Science; Bialowieza, Poland: 2010. The Art of Tracking Animals. [Google Scholar]
- Lavikainen A., Haukisalmi V., Lehtinen M.J., Henttonen H., Oksanen A., Meri S. A phylogeny of members of the family Taeniidae based on the mitochondrial cox1 and nad1 gene data. Parasitology. 2008;135:1457–1467. doi: 10.1017/S003118200800499X. [DOI] [PubMed] [Google Scholar]
- Lorenzini R., Ruggieri A. Distribution of echinococcosis/hydatidosis in Italy. J. Helminthol. 1987;61:261–267. doi: 10.1017/s0022149x00010130. [DOI] [PubMed] [Google Scholar]
- Lovari S., Sforzi A., Scala C., Fico R. Mortality parameters of the wolf in Italy: does the wolf keep himself from the door? J. Zool. 2007;272:117–124. [Google Scholar]
- Malacrida F., Hegglin D., Bacciarini L., Otranto D., Nägeli F., Nägeli C. Emergence of canine ocular thelaziosis caused by Thelazia callipaeda in southern Switzerland. Vet. Parasitol. 2008;157:321–327. doi: 10.1016/j.vetpar.2008.07.029. [DOI] [PubMed] [Google Scholar]
- Marucco F., Avanzinelli E., Boitani L. Non-invasive integrated sampling design to monitor the wolf population in Piemonte, Italian Alps. Hystrix, It. J. Mamm. 2012;23:5–13. [Google Scholar]
- Mathis A., Deplazes P., Eckert J. An improved test system for PCR-based specific detection of Echinococcus multilocularis eggs. J. Helminthol. 1996;70:219–222. doi: 10.1017/s0022149x00015443. [DOI] [PubMed] [Google Scholar]
- Mattioli L., Apollonio M., Mazzarone V., Centofanti E. Wolf food habits and wild ungulate availability in the Foreste Casentinesi National Park, Italy. Acta Theriol. 1995;40:387–402. [Google Scholar]
- Mech L.D., Boitani L. University of Chicago Press; Chicago: 2003. Wolves: Behavior, Ecology, and Conservation: Behavior, Ecology, and Conservation. [Google Scholar]
- Meriggi A., Lovari S. A review of wolf predation in southern Europe: does the wolf prefer wild prey to livestock? J. Appl. Ecol. 1996;33:1561–1571. [Google Scholar]
- Meriggi A., Brangi A., Schenone L., Signorelli D., Milanesi P. Changes of wolf (Canis lupus) diet in Italy in relation to the increase of wild ungulate abundance. Ethol. Ecol. Evol. 2011;23:195–210. [Google Scholar]
- Meriggi A., Milanesi P., Schenone L., Signorelli D., Serafini M., Torretta E. 2013. Status ed ecologia del lupo in Liguria. Dal monitoraggio alla gestione dei conflitti. Ed. Regione Liguria. [Google Scholar]
- Meriggi A., Dagradi V., Dondina O., Perversi M., Milanesi P., Lombardini M. Short-term responses of wolf feeding habits to changes of wild and domestic ungulate abundance in Northern Italy. Ethol. Ecol. Evol. 2014 [Google Scholar]
- Peters R.P., Mech L.D. Scent-marking in wolves. Am. Sci. 1975;63:628–637. [PubMed] [Google Scholar]
- Priemer J., Krone O., Schuster R. Taenia krabbei (Cestoda: Cyclophyllidea) in Germany and its delimitation from T. ovis. Zool. Anz. 2002;241:333–337. [Google Scholar]
- Rinaldi L., Maurelli M.P., Capuano F., Perugini A.G., Veneziano V., Cringoli S. Molecular update on cystic echinococcosis in cattle and water buffaloes of Southern Italy. Zoonoses Public Health. 2008;55:119–123. doi: 10.1111/j.1863-2378.2007.01101.x. [DOI] [PubMed] [Google Scholar]
- Rossi L., Mignone V., Grande D., Formisano F., Dalmasso S., Borgna V. Col du Marchairuz; Suisse: 2009. Echinococcosi/idatidosi e il ritorno del lupo sulle Alpi Occidentali. 27èmes Recontres du G.E.E.F.S.M., recueil des communications. [Google Scholar]
- Štefanić S., Shaikenov B.S., Deplazes P., Dinkel A., Torgerson P.R., Mathis A. Polymerase chain reaction for detection of patent infections of Echinococcus granulosus (“sheep strain”) in naturally infected dogs. Parasitol. Res. 2004;92:347–351. doi: 10.1007/s00436-003-1043-y. [DOI] [PubMed] [Google Scholar]
- Thompson R.A. Parasite zoonoses and wildlife: one health, spillover and human activity. Int. J. Parasitol. 2013;43:1079–1088. doi: 10.1016/j.ijpara.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel D., Deplazes P., Mathis A. Identification of taeniid eggs in the feces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology. 2007;134:911–920. doi: 10.1017/S0031182007002235. [DOI] [PubMed] [Google Scholar]
- Varcasia A., Canu S., Lightowlers M.W., Scala A., Garippa G. Molecular characterization of Echinococcus granulosus strains in Sardinia. Parasitol. Res. 2006;98:273–277. doi: 10.1007/s00436-005-0059-x. [DOI] [PubMed] [Google Scholar]
- Varcasia A., Tosciri G., Pedes T., Pipia A.P., Marrosu R., Scala A. 2007. Cystic echinococcosis in pigs and wild boars of Sardinia (Italy) pp. 11–13. October; In Proc 6th International Symposium on the Mediterranean Pig. [Google Scholar]
- Vilà C., Urios V., Castroviejo J. Use of faeces for scent marking in Iberian wolves (Canis lupus) Can. J. Zool. 1994;72:374–377. [Google Scholar]


