SUMMARY
Many types of approaches allow extra-capsular dissection in the deep parotid parenchyma in the treatment of benign tumours. A transcervical approach (TCA), transparotid approach (TPA) and a combined transcervical-transparotid approach (TPTCA) are the three main procedures performed to expose the deep parenchyma. We conducted a retrospective chart review enrolling 24 consecutive patients treated for benign tumours affecting the deep lobe of the parotid. Review of the surgical data was accompanied by careful follow-up to establish surgical morbidity, functional (Frey's Syndrome and first-bite syndrome) and aesthetical outcomes. A TPA was performed in the majority of cases; in 26% superficial parotidectomy was not required (selective deep parotidectomy). Minor's test showed a low rate of Frey's syndrome (3 cases of 23, 13%). No long-lasting first-bite syndrome was reported. Some additional procedures were easily performed in order to improve aesthetical results (rotational flap of sternocleidomastoid muscle, free abdominal fat transfer); these had the same results as selective deep parotidectomy. TCA (or TPTCA) ensures the best control of the facial nerve, providing good exposure and good functional and aesthetical results (without sparing the superficial parenchyma if additional techniques are performed with the aim of reducing skin depression in the treated area). The choice of the approach should have only the aim of safe resection and should not be influenced by aesthetical outcome; the craniocaudal level of the tumour seems to be the best indicator of the feasibility of the procedure also considering the branches of the facial nerve. In our experience, mandibulotomy can always be avoided.
KEY WORDS: Parotid, Deep lobe, Parapharyngeal space, Salivary gland tumours, Frey's syndrome
RIASSUNTO
Numerose sono le modalità di approccio chirurgico al parenchima parotideo profondo che permettono di trattare neoplasie benigne secondo il principio della dissezione extracapsulare. L'approccio transcervicale (TCA), il transparotideo (TPA) e una combinazione fra i due (transcervicale-transparotideo, TPTCA) sono le 3 principali procedure effettuate per esporre il parenchima profondo. Il nostro studio retrospettivo include 24 pazienti trattati per patologia neoplastica benigna del lobo profondo. La revisione dei dati chirurgici è stata associata ad una mirata visita di follow-up atta a stabilire vantaggi e svantaggi di ciascun approccio in termini di morbilità chirurgica sugli aspetti funzionali (sindrome di Frey e First-Bite Syndrome) ed estetici. TPA è stato eseguito nella maggioranza dei casi e nel 26% dei casi non ha previsto l'asportazione del lobo superficiale (parotidectomia profonda selettiva). Una sindrome di Frey clinicamente evidente (test di Minor) è stata documentata in 3 casi su 23 (13%) mentre nessuna first-bite syndrome di lunga durata è mai stata riportata. Procedure chirurgiche aggiuntive allo scopo di migliorare il risultato estetico (lembo di rotazione di sternocleidomastoideo, riempimento del minus chirurgico con grasso libero addominale) sono state facilmente eseguite e senza complicanze maggiori, assicurando un risultato estetico sovrapponibile a quello di una parotidectomia profonda selettiva. L'approccio transparotideo al lobo profondo assicura il controllo ideale sulle branche del nervo facciale e garantisce comunque buoni risultati estetici e funzionali (anche senza risparmio del parenchima superficiale a patto di eseguire semplici procedure aggiuntive allo scopo di minimizzare la depressione della pelle della zona trattata). La scelta del tipo di approccio non deve essere influenzata dal risultato estetico ma solo dalla fattibilità di una rimozione oncologicamente sicura; il livello cranio-caudale al quale si trova il tumore è il miglior indicatore delle difficoltà tecniche che possono presentarsi e delle branche nervose da dover preservare. Nella nostra esperienza la mandibulotomia può essere sempre evitata.
Introduction
The deep lobe of the parotid gland occupies the pre-styloid compartment of the parapharyngeal space (PPS). It has been estimated that almost half of all parapharyngeal tumours originate from a salivary gland (40-50%), while the remaining arise from lymphatic or nervous structures 1. Pleomorphic adenoma (PA) is the most common neoplasm of the PPS 2.
Many surgical approaches have been described to expose the deep parenchyma and allow a safe and appropriate removal of a tumour, usually by performing an extracapsular dissection (ECD) 3-5. A transoral approach is limited to small and benign lesions 6. A transcervical approach (TCA) (without mandibulotomy) is correlated with lower morbidity 7 8, but cannot be performed in all patients, especially in patients with large tumours, very poor exposure, or malignancy. Transoral robotic surgery (TORS) approach is an interesting and new technique; the experience of O'Malley and colleagues has confirmed the safety and feasibility of a TORS approach for PPS tumours in terms of local control and the low surgical complication rate 9.
A transparotid approach (TPA) is the most widely used, and can be combined with a TCA procedure. Mandibulotomy can be necessary in the presence of anatomical limitations which in most cases are represented by the ascending mandibular branch and posteriorly by the temporal bone and styloid apophysis.
Parotid surgery for benign pathologies can lead to several complications, such as Frey's syndrome (FS), first-bite syndrome (FBS), facial nerve deficits, or great auricular nerve anaesthesia. It also can lead to a poor aesthetical result due to severe asymmetry of the face.
The aim of this report is, after providing a brief description of surgical techniques, to evaluate the advantages and disadvantages of each approach to the deep lobe. These features have been investigated in terms of morbidity, aesthetic outcomes and oncologic results after long and careful follow-up.
Materials and methods
Patients
With the aim of examining a series of patients with longterm follow-up treated for a benign tumour of the parotid gland arising from the deep lobe, we conducted a retrospective study by searching through our digital archive (Ormaweb, Avelco s.r.l.) using specific numerical codes employed for salivary gland tumours. After exclusion of surgical procedures performed on the submandibulary gland, we reviewed 135 medical records starting from January 2009 to December 2011 to identify surgical procedures on the parotid gland. From the entire group, we excluded biopsies and surgical procedures performed for malignancy (19 cases, 14.07%) or for benign tumours affecting only the superficial lobe (92 cases, 79.14%). Of 135 procedures, we selected 24 records that identified 24 consecutive resections of benign tumours arising from the deep lobe of the parotid gland (17.7% of all procedures, 20.4% of benign tumours); MRI was available in all 24 cases. The study protocol was approved by the local Ethics committee and adhered to the principles outlined in the Declaration of Helsinki.
Surgical techniques
TPA: we perform a modified Blair incision, starting from the posterior edge of the tragus (or on a skin crease anteriorly to the tragus). A skin flap in the subplatysmal and sub-superficial musculoaponeurotic system (SMAS) plane is then elevated. The parotid capsule is separated from the external auditory canal and from the anterior aspect of the sternocleidomastoid muscle by sharp dissection. The posterior branch of the greater auricular nerve can be preserved, while the anterior branch must be sectioned. After exposing the posterior belly of the digastric muscle, we are able to identify the main trunk of the facial nerve and perform a superficial parotidectomy with an anterograde technique. We always employ a neural stimulator to identify and preserve the branches of the facial nerve until the deep lobe is completely exposed. At this point, we are able to perform extra-capsular dissection (ECD) of the deep lobe tumour by gently retracting the overlying neural branches of the facial nerve (Fig. 1). In order to improve the exposure (and to reduce the risk of damaging the structures of the PPS), fracture (or sectioning) of the styloid process is sometimes required.
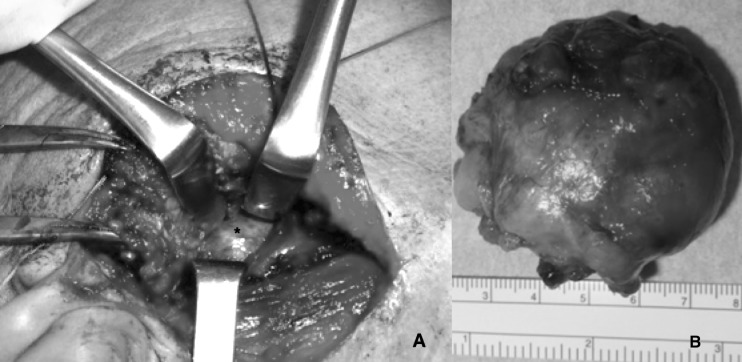
Fig. 1.
(A) tumour of the deep lobe (asterisk) under the superficial parenchyma, removed performing an ECD after TPA (a portion arising from the deep tumour occupies part of the superficial lobe and is surrounded by some branches of the facial nerve); (B) the tumour (pleomorphic adenoma) after the procedure.
TCA: we perform a small vertical cervical incision and subsequently elevate a skin flap in the subplatysmal plane, including the capsule of the submandibulary gland. The parotid is retracted posteriorly and superiorly; the submandibulary gland is retracted anteriorly. In some cases a section of the posterior belly of the digastric muscle is useful. This procedure allows to access the PPS and to identify the tumour. At this point, we are able to perform an ECD with blunt dissection and thus preserve any potential vascular structures surrounding the lesion.
We consider a transparotid-transcervical approach (TPTCA) as a combination of both TPA and TCA, and it is employed for large tumours or in cases of very poor exposure. TPA and TPTCA can lead to a depression over the mandibular angle or the pre-auricular region, causing poor aesthetical outcome due to facial asymmetry. In order to avoid this (the indication was given by the volume of the contralateral parotid lodge on the MRI), two types of additional surgical techniques are performed: sternocleidomastoid muscle (SCM) rotational flap and free abdominal fat transfer. An SCM muscle rotational flap is performed by sharply sectioning the most superficial aspect of the upper third of SCM muscle. A neural stimulator is employed to prevent damage to cranial nerve XI.
The transfer of free abdominal fat is performed by making a small horizontal skin incision 8-10 cm under the belly and removing an amount of abdominal fat tissue by sharp dissection. Alternatively, the skin incision can be carried out along the inferior aspect of the belly.
In selected cases, it is also possible to perform a selective deep lobe parotidectomy (SDP) by dividing the superficial lobe into two halves (upper and lower); this allows quick access to the deep lobe by leaving the superficial lobe pedicled anteriorly. The two halves are repositioned and sutured, preserving the symmetry of the face 10.
Three surgeons performed all surgical procedures as first operator.
All procedures were performed using an 0.6 mA direct nerve stimulator (Neuropacer®, FIAB S.p.A., Vicchio, Florence, Italy) that was also employed during preparation of SCM flaps.
All specimens were sent for histological examination where the dimensions of the lesion were measured, separating the neoplasm from the surrounding salivary tissue. On the basis of this data, we calculated the average size of the resected tumour.
Preoperative evaluation
Pre-operative scans were reviewed using an open-source DICOM file reader software (Osirix Imaging Software, Pixmeo, Geneve, Switzerland). Each scan was compared to identify a radiological pattern that could suggest one type of surgical approach over another.
In particular, after overlapping and comparing each MRI scan, a T1-weighted MRI sequence (enhanced with gadolinium) on the coronal plane was selected and employed to detect the tumour. The lateral pterygoid muscle (LPM) was detected as well, with the aim to assess the presence of healthy salivary tissue between the muscle and the lesion.
Follow-up and post-operative evaluation
Clinical examination, represented by a careful palpation of the treated area and critical inspection to detect facial asymmetry or facial nerve weakness, was initiated after administering a series of questionnaires.
The onset of Frey's syndrome (FS) was investigated by the Luna-Ortiz Questionnaire 11. Patients were asked to rate their aesthetic satisfaction on a simple scale from 1 to 5 (1: very poor satisfaction; 2: poor satisfaction; 3: sufficient; 4: good result; 5: excellent result).
Once a diagnosis of FBS was made, time of onset since surgery, location, duration and frequency of symptoms, and associating symptoms were noted.
The last MRI scan was examined to identify recurrences.
All patients underwent the Minor test (starch-iodine test) by applying an iodine solution (Lugol's Iodine) to the skin. Once air-dried, the area was dusted with cornstarch. Sweating was encouraged with the aid of lemon-flavoured candy. We considered the test as positive when the sweat reached the surface of the skin and the starch and iodine combined causing an evident colour change (from white to dark blue).
Results
The subjects in the present analysis included 11 males and 13 females, from 30 to 78 years old (mean 52.7 years). In most of patients, the tumour presented as a slow-growing, painless, non-ulcerated, deep nodule affecting the parotid region and not easily appreciable on palpation. In contrast, in 4 patients the tumour mass was well appreciable: this occurred in the 2 cases of multifocal involvement of the gland and in the 2 cases of massive involvement of the residual parenchyma (also superficial) by recurrence of disease. The neoplasm presented as a swelling of the oropharynx that displaced the tonsil in only one patient (Fig. 2). All patients underwent MRI imaging of the region with gadolinium (3 patients also underwent CT).
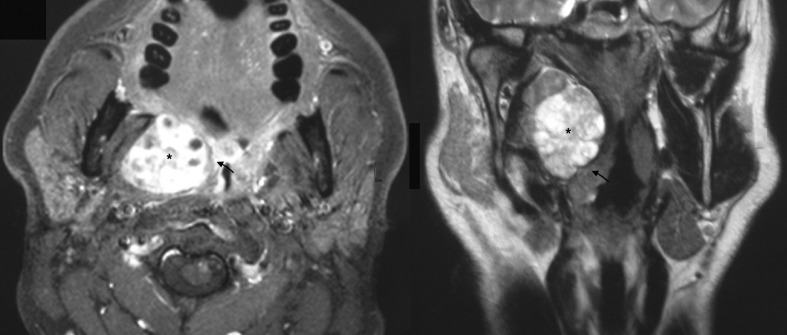
Fig. 2.
Large tumour of the deep lobe widely occupying the parapharyngeal space (black asterisk) and displacing the tonsil lodge (black arrow).
TCA was performed in 1 patient (4.2%) a; the tumour was 1.5 cm in diameter and completely extra-glandular, located in the prestyloid compartment of the PPS, with a clear manifestation of healthy salivary tissue between the tumour and the LPM on MRI (Fig. 3).
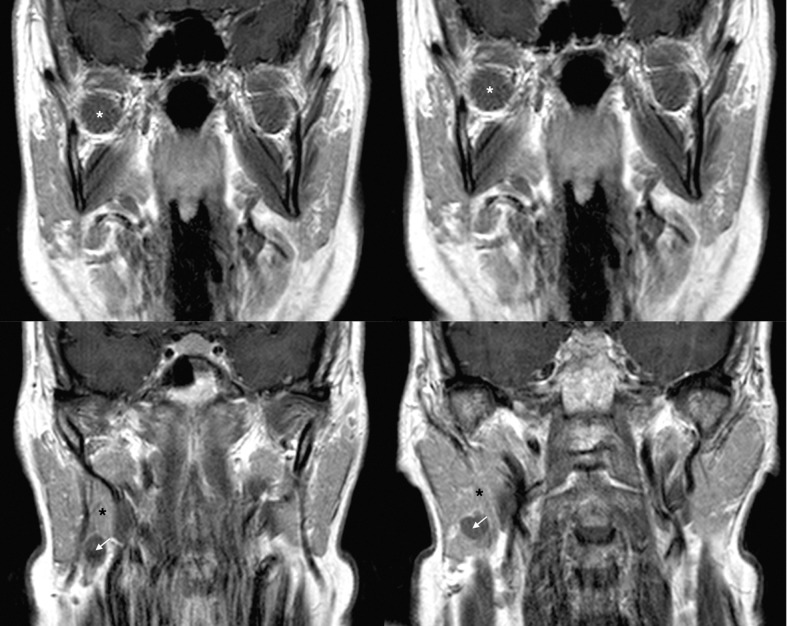
Fig. 3.
Four consecutive coronal preoperative MRI scans in a TCA; the images show the presence of healthy salivary tissue (black asterisk) between the tumour (white arrow) and the LPM (white asterisk).
TPA was performed in 19 patients (79.2%), of which 17 (70.8%) as first surgical procedure (in 5 an SDP was also feasible) and 2 (4.2%) as surgical revision after recurrence. Of 19 patients treated via a TPA, in 5 cases (20.8%) the tumour occupied both the superficial and the deep lobe, while in 14 cases (58.3%) the tumour was limited to the deep lobe, but no salivary tissue between the tumour and the LPM was detectable.
In 4 patients combined TPTCA was necessary (first operation in 3 cases and surgery for recurrence in 1 case); in all these cases, MRI revealed that the tumour widely occupied the entire deep lobe of the gland (in 1 case part of the superficial lobe as well) (Fig. 4), with a clear point of contact between the tumour and the LPM.
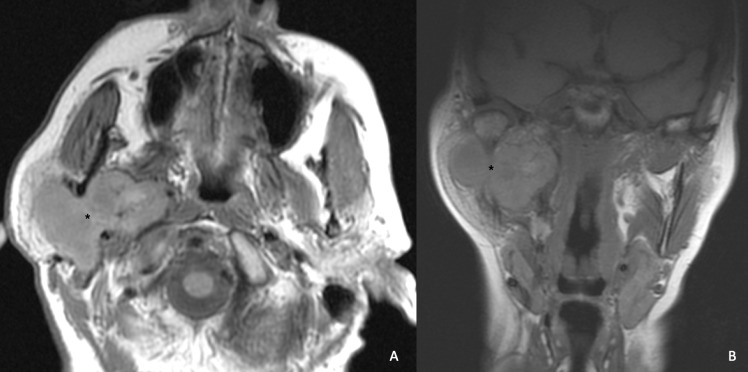
Fig. 4.
Pleomorphic adenoma (black asterisk), occupying both the superficial and the deep lobe treated via a TPTCA.
Mandibulotomy was not needed in any of the 24 patients. Fracture of the styloid process was performed in 2 of the 4 cases undergoing TPTCA to assure better exposure of all the edges of the tumour and allow a safer ECD procedure from the parapharyngeal space.
In 9 cases, during the same surgical session (7 TPA and 2 TPTCA), an additional procedure was performed to prevent important skin depression over and posterior to the mandibular angle and in the pre-auricular region. This prove minimised the asymmetry of the operated side compared to the contralateral side. In 3 cases we placed a rotational flap of sternocleidomastoid muscle. In 6 cases, we performed free abdominal fat grafting.
The mean size of the resected tumour was 3.4 x 2.9 x 2.2 cm (the superficial parenchyma removed to access the deep lobe, when not involved by neoplasm, was not considered). The results of pathological examination are shown in Table I.
Table I.
Clinical features and results of the surgical approach in the 24 patients.
| ID | SEX | Age | Year | Localisation of the tumour | Approach | Superficial lobe removed | Additional procedure | Pathology | Clinical and radiological follow-up | FBS | Positive minor's test | Luna- Ortiz scoring | Aesthetic outcome (1-5) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 65 | 2011 | DL | TPA | Yes | Abdominal fat transfe | Basal Cell Adenoma | NED | No | No | 0 | 5 |
| 2 | F | 50 | 2010 | DL | TPA | Yes | Abdominal fat transfe | Pleomorphic Adenoma, Lipoma | NED | No | Yes | 1 | 4 |
| 3 | F | 30 | 2011 | DL | TPA | No (selective deep parotidectomy) | No | Pleomorphic Adenoma | NED | No | No | 0 | 5 |
| 4 | M | 61 | 2011 | DL | TPA | No (selective deep parotidectomy) | No | Salivary Duct Cyst | NED | No | No | 0 | 4 |
| 5 | M | 62 | 2009 | DL | TPA | Yes | SCM flap | Warthin's Tumour | NED | No | No | 0 | 5 |
| 6 | F | 67 | 2011 | DL | TPA | Yes | Abdominal fat transfer | Pleomorphic Adenoma | NED | No | No | 0 | 5 |
| 7 | M | 45 | 2011 | SLDL | TPA | Yes | No | Multifocal Warthin's Tumour | Recurrence | No | No | 0 | 3 |
| 8 | F | 58 | 2011 | DL | TPTCA | Yes | Abdominal fat transfer | Pleomorphic Adenoma | NED | No | No | 0 | 5 |
| 9 | M | 49 | 2009 | DL | TPTCA | Yes | No | Pleomorphic Adenoma | NED | No | Yes | 6 | 4 |
| 10 | F | 45 | 2010 | DL | TPTCA | Yes | SCM flap | Pleomorphic Adenoma | NED | Yes | Yes | 5 | 5 |
| 11 | F | 47 | 2010 | DL | TPA | Yes | Abdominal fat transfer | Pleomorphic Adenoma | NED | No | No | 0 | 5 |
| 12 | F | 56 | 2011 | DL | TPA | Yes | No | Pleomorphic Adenoma | NED | No | No | 0 | 3 |
| 13 | F | 61 | 2010 | DL | TPA | No (selective deep parotidectomy) | No | Pleomorphic Adenoma | NED | No | No | 0 | 4 |
| 14 | F | 42 | 2011 | SLDL | TPA | Yes | No | Pleomorphic Adenoma | NED | No | No | 0 | 1 |
| 15 | M | 33 | 2009 | DL | TPA | No (selective deep parotidectomy) | No | Pleomorphic Adenoma | NED | No | No | 0 | 5 |
| 16 | F | 78 | 2010 | DL | TPA | Yes | SCM flap | Pleomorphic Adenoma | NED | No | No | 0 | 4 |
| 17 | M | 46 | 2011 | DL | TPA | Yes | Abdominal fat transfer | Warthin's Tumour | NED | No | No | 0 | 5 |
| 18 | F | 40 | 2011 | DL | TPA | No (selective deep parotidectomy) | No | Cystadenoma | NED | No | No | 0 | 5 |
| 19 | M | 58 | 2010 | DL | TPA | Yes | No | Schwannoma | NED | No | No | 0 | 3 |
| 20 | M | 52 | 2010 | SLDL | TPA | Yes | No | Multifocal Warthin's Tumour | NED | No | No | 0 | 4 |
| 21 | M | 66 | 2011 | SLDL | TPA | No (revision) | No | Pleomorphic Adenoma | NED | No | No | 0 | 4 |
| 22 | M | 38 | 2010 | DL | TPA | No (revision) | No | Salivary Duct Cyst | Recurrence | No | No | 0 | 2 |
| 23 | F | 51 | 2011 | SLDL | TPTCA | No (revision) | No | Oncocytoma | NED | Yes | No | 0 | 2 |
| 24 | M | 65 | 2011 | DL | TCA | No (TCA) | No | Pleomorphic Adenoma | NED | Yes | No | 0 | 5 |
DL: Deep Lobe; SL: Superficial Lobe; TPA: Trans Parotid Approach; TPTCA: combined Trans Parotid and Trans Cervical Approach; TCA: Trans Cervical Approach; SCM: Sternocleidomastoid muscle; NED: No Evidence of Disease.
An evident intraoperative complication was apparent in only one case, consisting in damage by stretching to the marginalis mandibulae branch of the facial nerve, also due to the large dimensions of the tumour. Intraoperative bleeding was controllable in all patients. No other major complications occurred during surgical procedures. In 3 patients a deficit of the marginalis mandibulae branch of the facial nerve was documented postoperatively; in 2 patients it recovered in a maximum of 12 weeks, while in the other (4.1%) patients it was permanent.
Regarding long-term follow-up, all 24 patients underwent yearly MRI scan. The mean period of follow-up was 2 years and 3 months after surgery. In 2 of 22 cases the onset of a new lesion was reported: in one case it was a new neoplasm in a patient affected by a multifocal Warthin's tumour; in the other case, recurrence was a salivary duct cyst (diagnosis by fine needle aspiration). In only 3 cases was onset of an FBS documented, although it completely disappeared within a few months. An FS was reported in 6 (25%) patients, but Minor's test was positive in only 3 cases (12.5%) (Luna-Ortiz questionnaire scoring respectively 1, 5 and 6). As such, no clinically silent FS was documented in our series.
Regarding aesthetic outcomes, the average rating was: 4.8 in the 6 patients (25%) who underwent the abdominal fat transfer; 4.6 in the 3 patients (12.5%) with the rotational SCM flap; 4.8 where an SDP was possible (5 patients, 20.8%) and 3.3 where the superficial lobe was removed with no additional procedures (10 patients, 41.6%). A summary of these results is reported in Table I.
Discussion
In parotid surgery, ECD, first described by Gleave 12, has been established to be the most appropriate treatment for benign tumours 3. The procedure is performed by carefully dissecting around the neoplasm without violating its capsule. This procedure allows sparing a sufficient amount of surrounding (healthy) salivary tissue around the capsule of the lesion. The technique appears to provide an acceptable rate of recurrence with low morbidity 4 5 13, especially where an extensive dissection could increase the risk of surgical complications (tumour surrounded by the facial nerve or involving the PPS).
Among recent studies, TCA (without mandibulotomy) has been correlated with lower morbidity but cannot be performed in all patients, especially in cases of large tumours, patients with very poor exposure, or with suspicion of malignancy 7 8. TPA is more invasive because it requires facial incision (Blair incision) extending to the upper part of the neck that can remain visible; alternatively, a standard face-lift incision can be employed in order to minimise the scar. Furthermore, the procedure may lead to a skin depression in the area of the parotid region where the superficial lobe has been removed. In addition, a traditional TPA can more easily cause the onset of Frey's syndrome 14. On the other hand, in our surgical experience, traditional TPA allows best control on the branches of the facial nerve and can be easily extended to a combined TPTCA, for example in the cases of poor exposure, large tumours, intra-operative suspicion of malignancy, or intra-operative complications such as uncontrollable bleeding. Regarding the choice of surgical approach, we retain that the possibility to perform a TCA must be considered only after accurate radiological study: in our experience, the presence of healthy salivary tissue between the LPM and the lesion is the best indicator of the feasibility of a TCA because it suggests a lower cranial-caudal level and a peripheral localisation within the deep lobe. Furthermore, our experience suggests that a preoperatively established high cranial-caudal level of the tumour can also provide some indications regarding the neural branches of the facial nerve that may require management: in this circumstance, direct control over the middle and the higher branches via a TPA (or TPTCA) is mandatory, also because these branches are often multiple with a relevant number of anastomoses 15. In summary, we believe that the choice of TCA should be limited to small or medium-sized benign tumours, located in the lower part of the deep lobe (and preferably characterised by an extra-glandular pattern of growth towards the PPS). In our experience, mandibulotomy can be avoided in the majority of cases.
As shown in the literature, some techniques can be easily added to a TPA (or TPTCA) in order to improve aesthetical outcomes, such as a standard face-lift skin incision, SDP and the filling of the lodge with abdominal fat or other tissues (SCM muscle or superficial muscle-aponeurotic system – SMAS - application) 16. These methods, in our experience, do not produce major complications and are able to minimise the scar, skin depression and facial asymmetry in the region with the same results as a selective deep parotidectomy.
This study has several limitations. Firstly, each type of lesion (similar tumours regarding histopathology, dimensions and location) was treated with the same type of approach, without a control group. Furthermore, the range of cases is heterogeneous, including both primary diagnosis and recurrences. The other important limitation is that the mean period of follow-up (2 years) is sufficient for assessment of surgical complications, but too short to obtain reliable oncological outcomes (PA needs at least 10 years of follow-up). The main aim of this study was to provide indications and short-term outcomes related to each surgical approach to the deep lobe, and not to establish the superiority of one approach in terms of oncological results. Additional studies are required to establish whether the employment of fat tissue or SMAS can effectively reduce the onset of an FS, especially correlating data with volume and localisation of the removed parenchyma.
The radiological parameters considered in our series have not been validated: further studies also involving radiologists are required to obtain a standardised method that allows the surgeon to stratify the choice of the right approach.
Conclusions
TPA (or TPTCA) ensures best control of the facial nerve providing good exposure and good functional and aesthetical results. In selected cases, the superficial parenchyma can be spared by performing selective deep parotidectomy with good functional outcomes. Aesthetic outcomes can be improved by adding simple additional techniques such as SCM flap and abdominal fat transfer. In our experience, mandibulotomy can always be avoided.
References
- 1.Khafif A, Segev Y, Kaplan D, et al. Surgical management of parapharyngeal space tumors: a 10-year review. Otolaryngol Head Neck Surg. 2005;132:401–406. doi: 10.1016/j.otohns.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 2.Oliai BR, Sheth S, Burroughs FH, et al. Parapharyngeal space tumors: a cytopathological study of 24 cases on Fineneedle aspiration. Diagn Cytopathol. 2005;32:11–15. doi: 10.1002/dc.20154. [DOI] [PubMed] [Google Scholar]
- 3.McGurk M, Thomas BL, Renehan AG. Extracapsular dissection for clinically benign parotid lumps: reduced morbidity without oncological compromise. Br J Cancer. 2003;89:1610–1613. doi: 10.1038/sj.bjc.6601281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith SL, Komisar A. Limited parotidectomy: the role of extracapsular dissection in parotid gland neoplasms. Laryngoscope. 2007;117:1163–1167. doi: 10.1097/MLG.0b013e31806009fe. [DOI] [PubMed] [Google Scholar]
- 5.Sergi B, Limongelli A, Scarano E, et al. Giant deep lobe parotid gland pleomorphic adenoma involving the parapharyngeal space. Report of three cases and review of the diagnostic and therapeutic approaches. Acta Otorhinolaryngol Ital. 2008;28:261–265. [PMC free article] [PubMed] [Google Scholar]
- 6.Ducic Y, Oxford L, Pontius AT. Transoral approach to the superomedialparapharyngeal space. Otolaryngol Head Neck Surg. 2006;134:466–470. doi: 10.1016/j.otohns.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Presutti L, Molteni G, Malvè L, et al. Parapharyngeal space tumors without mandibulotomy: our experience. Eur Arch Otorhinolaryngol. 2012;269:265–273. doi: 10.1007/s00405-011-1594-y. [DOI] [PubMed] [Google Scholar]
- 8.Yang TL, Hsiao TY, Wang CP, et al. Extracapsular dissection for minimal resection of benign parapharyngeal tumor. Eur Arch Otorhinolaryngol. 2012;269:2097–2102. doi: 10.1007/s00405-011-1855-9. [DOI] [PubMed] [Google Scholar]
- 9.O'Malley BW, Jr, Quon H, Leonhardt FD, et al. Transoral robotic surgery for parapharyngeal space tumors. ORL J Otorhinolaryngol Relat Spec. 2010;72:332–336. doi: 10.1159/000320596. [DOI] [PubMed] [Google Scholar]
- 10.Sesenna E, Bianchi B, Ferrari S, et al. Selective deep lobe parotidectomy for pleomorphic adenomas. Int J Oral Maxillofac Surg. 2013;42:1129–1133. doi: 10.1016/j.ijom.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Luna-Ortiz K, Sansón-RíoFrío JA, Mosqueda-Taylor A. Frey sindrome: a proposal for evaluating severity. Oral Oncol. 2004;40:501–505. doi: 10.1016/j.oraloncology.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Gleave EN. An alternative to superficial parotidectomy: extracapsular dissection. In: Burgh N, McGurk M, editors. Colour atlas and text of salivary glands: diseases, disorders and surgery. London: Mosby-Wolf; 1995. pp. 165–172. [Google Scholar]
- 13.Klintworth N, Zenk J, Koch M, et al. Postoperative complications after extracapsular dissection of benign parotid lesions with particular reference to facial nerve function. Laryngoscope. 2010;120:484–490. doi: 10.1002/lary.20801. [DOI] [PubMed] [Google Scholar]
- 14.Huang G, Yan G, Wei X, et al. Superficial parotidectomy versus partial superficial parotidectomy in treating benign parotid tumors. Oncol Lett. 2015;9:887–890. doi: 10.3892/ol.2014.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis RA, Anson BJ, Budinger JM, et al. Surgical anatomy of the facial nerve and the parotid gland based upon a study of 350 cervico-facial halves. Surg Gynecol Obstet. 1956;102:385–412. [PubMed] [Google Scholar]
- 16.Curry JM, Fisher KW, Heffelfinger RN, et al. Superficial musculoaponeurotic system elevation and fat graft reconstruction after superficial parotidectomy. Laryngoscope. 2008;118:210–215. doi: 10.1097/MLG.0b013e3181581f94. [DOI] [PubMed] [Google Scholar]