SUMMARY
We report a rare case of a large intraparotid facial nerve schwannoma (IFNS) in a 51-year-old female who presented with a painless, slow growing left parotid mass without peripheral facial nerve palsy, with non-specific findings at preoperative diagnostic work-up, that was treated with conservative surgery. Management of IFNS is very challenging because the diagnosis is often made intra-operatively, and in most cases resection may lead to severe facial nerve paralysis, with important aesthetic sequelae. Our experience suggests a new surgical option, namely intra-capsular enucleation using a microscope, currently used for schwannomas arising from a major peripheral nerve, which should be a safe and reliable treatment for IFNS. This surgical technique is the first experience of intracapsular microenucleation of facial nerve schwannoma described in the literature and allows preservation of the nerve without resection and reconstruction.
KEY WORDS: Parotid tumour surgery, Intraparotid facial nerve schwannoma, Facial nerve palsy, Microenucleation in peripheral nerve
RIASSUNTO
Descriviamo un raro caso di un neurinoma intraparotideo del nervo facciale in una donna di 51 anni giunta alla nostra osservazione per una voluminosa neoformazione parotidea sinistra, non dolente, senza deficit periferico del nervo facciale. L'agoaspirato e la RM della regione parotidea, eseguiti prima dell'intervento chirurgico, non si sono dimostrati diagnostici, pertanto poiché l'esame istologico estemporaneo rilevava cellule fusiformi compatibili con neurinoma la paziente è stata sottoposta a trattamento conservativo. La gestione degli schwannomi intraparotidei è molto difficile perché la diagnosi viene spesso fatta intraoperatoriamente e nella maggior parte dei casi, l'asportazione può procurare lesione del nervo facciale, con importanti conseguenze estetiche. La nostra esperienza suggerisce una nuova opzione chirurgica: l'utilizzo di microdissezione ed enucleazione con preservazione dell'epinevrio, eseguita con microscopio, attualmente utilizzata per gli schwannomi dei grandi nervi periferici. Questa tecnica potrebbe rivelarsi utile ed applicabile anche ai neurinomi intraparotidei del nervo facciale. Tale opzione chirurgica è la prima esperienza di microenucleazione intracapsulare che consente di preservare il nervo facciale senza resezione e ricostruzione descritta in letteratura.
Introduction
Facial nerve schwannoma (FNS) is a benign tumour arising from Schwann cells of the facial nerve sheath. Intraparotid FNS is very rare and comprises 0.2-1.5% of all parotid tumours 1. From an histological point of view, schwannomas usually grow eccentrically from the nerve sheath and do not contain nerve fibres intramurally. The clinical symptoms of a schwannoma of the facial nerve vary depending on the location. Within the parotid gland, schwannomas may reach significant size without causing any symptoms other than parotid swelling. In the majority of cases, these tumours present as an asymptomatic intraparotid mass, mobile to the skin and lower layers. However, signs of facial paralysis have been reported as the initial presenting symptom in a small number of cases, mainly when the tumour is located close to the stylomastoid foramen 3. Preoperative fine-needle aspiration cytology (FNAC) is very often not diagnostic, and in some cases can give an incorrect diagnosis 3.
MRI diagnosis of intraparotid facial nerve schwannoma (IFNS) remains difficult. MRI characterises such heterogeneous lesions with isointensity in T1-weighted images and hyperintensity in T2-weighted images, which are not specific for schwannoma and can be found in other neoplasms, such as pleomorphic adenomas 4. Alicandri-Ciufelli et al. 3 in 2009 published a classification of FNS based on position and adherences of the neoplasm with respect to the nerve and post-operative function of the facial nerve. This classification includes type A neoplasms (tumour that can be resected without any sacrifice of the nerve), type B neoplasms (tumour that cannot be resected without sacrifice of the nerve, involving a main division or a distal branch of the nerve), type C neoplasms (tumour that cannot be resected without sacrifice of the nerve, involving the main trunk of the nerve), type D neoplasms (tumour that cannot be resected without sacrifice of the nerve, involving either the main trunk and its main divisions).
During surgical treatment, if these tumours are recognised, the surgeon is faced with a number of dilemmas regarding treatment policy. Because of its rarity, only a few clinicians have experience in the surgical management of intraparotid schwannomas. In addition, the results of facial nerve repair after resection of schwannoma are rarely described 2. It is critical to have a diagnostic and management algorithm when a suspected facial nerve tumour is encountered during parotidectomy 5. We present such a case and discuss the management strategy used.
Case report
A 51-year-old woman presented with a 6-year history of a slowly growing, asymptomatic, mass in her left parotid gland. There was no history of facial nerve weakness. Physical examination revealed a large, 4 cm, painless, slightly mobile mass, just inferior to the cartilaginous auditory canal. Fine needle aspiration cytology (FNAC) was not diagnostic, describing a spindle–cell morphology possibly of mesenchymal origin. MRI showed a well defined lesion that appeared hyperintense on fat-suppressed T2-weighted images (T2WI) and hypointense on T1-weighted images (T1WI), with marked enhancement on gadolinium-enhanced T1WI and cystic changes inside the lesion localised in the superficial and deep lobe of the parotid gland, which measured approximately 3.2 × 3.5 cm (Fig. 1). The patient underwent parotidectomy using a modified Blair incision. After elevation of the superficial musculoaponeurotic system (SMAS), flap a bulky, yellowish tumour appeared in the parotid gland with prolongation to the deep lobe. Intraoperatively under microscopic view (using conventional landmarks: i.e. pointer) problems were encountered in identifying and isolating the proximal main trunk of the facial nerve, so we attempted to identify it by retrograde dissection of the peripheral branches using nerve stimulation. Finally, we observed that the tumour was adherent to the facial nerve trunk and that the peripheral branches could not be separated from the tumour mass. It became apparent that the facial nerve branches in this location were intimately associated with the mass and were not simply lying on its surface. The nerve stimulator was used again, and facial motion occurred with stimulation of the mass. Intraoperative frozen section analysis from an incisional biopsy revealed spindle cells compatible with schwannoma. We carefully made a longitudinal incision in the epineurium, and the uninvolved nerve fibres that splayed around the tumour were dissected and retracted extracapsularly (Fig. 2). The onion skin-like epineurial tissue layers were meticulously peeled out until the shiny surface of the tumour was exposed. Gentle dissection along the plane of the tumour capsule from the epineurial layers allowed the tumour to be shelled out en bloc without disturbing the nerve fascicles. Intra-operative nerve stimulation during the dissection was performed. Haemostasis was carefully checked by delicate bipolar to prevent secondary compression of the affected nerve by haematoma. Suction drainage was positioned at the end of the procedure and closure was made in layers taking care to not entrap the nerve, and finally an aesthetic intradermal suture was used.
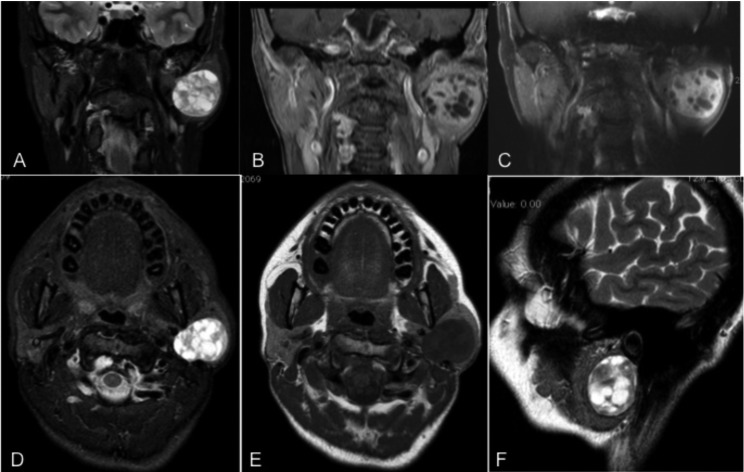
Fig. 1.
MRI images showing left parotid lesion with extension in the deep parotid lobe close to the stylomastoid process (as appears in the axial, coronal and sagittal plane). The lesion appears on hyperintense fat-suppressed T2-weighted images (T2WI) and hypointense on T1-weighted images (T1WI), with marked enhancement on gadolinium-enhanced T1WI with cystic changes inside the lesion. All these features are similar to those observed in cases of pleomorphic adenoma.
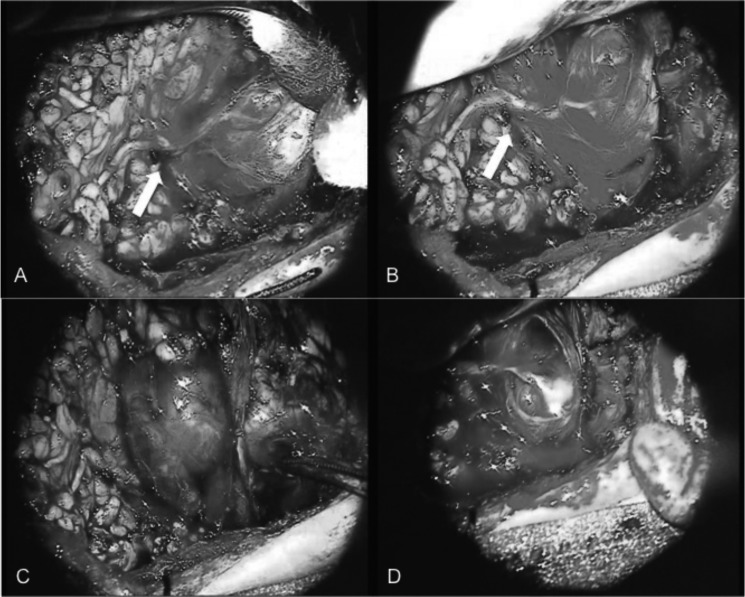
Fig. 2.
Intra-operative microscopic view of the lesion dissected from the peripheral branch (inferior branch) of the facial nerve (arrow) after carefully dissected preserving the pseudocapsule (epinevrium) (A-B). Intraneural microdissection of the nerve at both the proximal and distal poles of the tumour with preserved perineurium of fascicles (C-D).
Histological features showed spindle shaped Schwann cells arranged in interlacing fascicles, and immunohistochemical analysis confirmed strong expression of S-100 protein by tumour cells, low expression of CD99, while AE1/AE3, CAM 5.2, SMA, HHF-35, caldesmone, calponine and CD 34 were negative; these confirmed diagnosis of schwannoma.
Postoperatively the patient developed facial nerve dysfunction on her left side (House-Brackmann grade IV facial nerve paralysis). Three months later, after physiotherapy rehabilitation, facial nerve function improved to grade II-IIIa on the House- Brackmann grading system. No recurrence was observed after 6 months.
Discussion
In this report, FNS is described as a slow growing, painless parotid mass, mimicking at preoperative imaging the most common benign parotid tumour, pleomorphic adenoma. Preoperatively, it is very difficult to differentiate intraparotid FNS from other parotid tumours, but preoperative diagnosis of FNS is essential for treatment planning 6. From an imaging point of view, in diagnosing intraparotid FNS there is no evidence that any radiological method is of particular assistance. CT usually shows a smooth, sharply circumscribed lesion in the parotid, which is difficult to differentiate from other tumours. It is not the preferred imaging modality for FNS, but it may reveal osseous changes especially in the extension through the stylomastoid foramen 7. MRI shows intraparotid FNS as a mass with an isointense signal to muscle on T1 and hyperintense to muscle on T2 sequence, respectively, and also gadolinium-enhancement on T1 sequences, although with MRI it is not always possible to make preoperative diagnosis of FNS, especially in lesions located purely in the gland 6.
A diagnosis of FNS is often made intraoperatively, and is frequently only recognised while exploring a "parotid mass". When the surgeon, using landmarks, encounters an intraparotid tumour in which the main trunk of the facial nerve is not easily identified, with retrograde dissection the presence of a facial nerve schwannoma should also be considered. Macroscopically, nerve fibres that disappear entering into the mass and also displaced over it strongly suggest the presence of this tumour. Generally, in case of schwannoma electrical stimulation of the tumour will elicit facial movement. In this event, frozen section analysis should always be performed 2. If presence of schwannoma is confirmed, according to a critical literature review on the management of FNS, the surgeon should opt in the case of type A or B neoplasms, or in the case of a pre-operative FN HB grade IV or worse, for resection and (where necessary possible primary reconstruction of the FN with nerve graft. In the case of pre-operative HB grade III or higher and in type C or D neoplasms, patients should undergo an intra-operative biopsy to rule out malignancy, and conservative management should be considered 3.
Stereotactic radiosurgery shows promising preliminary results for treatment of intra-temporal FN schwannomas, even if at the moment the role of stereotactic radiotherapy remains to be determined 8. If sacrifice of the nerve is necessary, a sural nerve interposition graft or a hypoglossal– facial nerve anastomosis can be performed. Better results are obtained in young patients and when three is a short time interval between paralysis and reconstructive surgery 9.
Our experience suggests another possible surgical option: intra-capsular enucleation by microscope, which is currently used for schwannomas arising from a major peripheral nerve, and should be a safe and reliable treatment for FNS (Fig. 3). The surgical technique generally used in peripheral nerves involves exposure of the nerve beginning in a region of normal anatomy and extends into a region of tumour formation. Loop magnification or an operating microscope is essential for identification of the fascicles coursing over the tumour. Electrical nerve stimulation is also used to differentiate fascicles from tumour. A dissection plane is now established by gently separating and elevating the fascicles from the surface of the tumour. There may be a thin capsule (epinevrium) that requires opening to allow easier dissection of tumour from the nerve. As the tumour is mobilised, there may be single fascicles seen entering and exiting the mass. Electrical stimulation will determine if these are motor fascicles and still functional. Most schwannomas can be removed en bloc. Although often splayed and thinned, the intact fascicles should still function. The tumour bed is inspected for any evidence of divided fascicles. No attempt is made to remove remaining tumour capsule because the presence of tumour capsule is not associated with tumour recurrence 10 11.
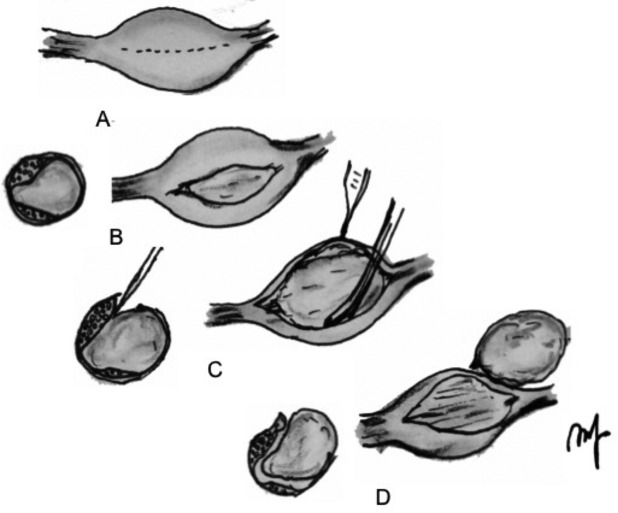
Fig. 3.
Axial cuts of a neuroma and nerve are shown. A, longitudinal incision is made on the capsule of the mass; B schwannoma is seen and on axial plane the fascicles are displaced laterally by the tumour mass. C, the nerve fascicles must be separated or dissected from the abnormal tumour mass. D, fascicles that have been dissected away and spared are seen below the tumour, which is elevated away from the tumour bed. The tumour has been elevated away from the fascicular bed, leaving the coagulated ends of the entering and leaving fascicles and spared fascicles behind.
In our case report, after 3 months neurological deficits following enucleation are lower using the intra-capsular compared with a resection and reconstruction of the facial nerve. Therefore, further reports on additional cases might help to better understand if this conservative option is feasible in treating schwannomas of the facial nerve.
When dealing with lesions of the parotid gland suspected to be FNS, informed consent of the patient should include this possibility (intracapsular microenucleation by microscope) to preserve the nerve without resection and reconstruction, considering the benign nature of the lesion as suggested by frozen sections, and in particular when there is no preoperative facial nerve palsy.
The surgical technique used in our patient is the first reported experience of intracapsular microenucleation by microscope of facial nerve schwannoma. Informed consent and good clinical judgement may lead the surgeon to subtotal excision or to retreat after biopsy without any definitive surgery, preserving nerve functionality and avoiding medico-legal issues.
References
- 1.Fyrmpas G, Konstantinidis I, et al. Hatzibougias D, Intraparotid facial nerve schwannoma: management options. Eur Arch Otorhinolaryngol. 2008;265:699–703. doi: 10.1007/s00405-007-0521-8. [DOI] [PubMed] [Google Scholar]
- 2.Kreeft A, Schellekens PP, Leverstein H, et al. Intraparotid facial nerve schwannoma. What to do? Clin Otolaryngol. 2007;32:125–129. doi: 10.1111/j.1365-2273.2007.01352.x. [DOI] [PubMed] [Google Scholar]
- 3.Alicandri-Ciufelli M, Marchioni D, Mattioli F, et al. Critical literature review on the management of intraparotid facial nerve schwannoma and proposed decision-making algorithm. Eur Arch Otorhinolaryngol. 2009;266:475–479. doi: 10.1007/s00405-008-0893-4. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu K, Iwai H, Ikeda K, et al. Intraparotid facial nerve schwannoma: a report of five cases and an analysis of MR imaging results. Am J Neuroradiol. 2005;26:1328–1330. [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta RP, Deschler DG. Intraoperative diagnosis of facial nerve schwannoma at parotidectomy. Am J Otolaryngol. 2008;29:126–129. doi: 10.1016/j.amjoto.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Ma Q, Song H, Zhang P, et al. Diagnosis and management of intraparotid facial nerve schwannoma. J Craniomaxillofac Surg. 2010;38:271–273. doi: 10.1016/j.jcms.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Jäger L, Reiser M. CT and MR imaging of the normal and pathologic conditions of the facial nerve. Eur J Radiol. 2001;40:133–146. doi: 10.1016/s0720-048x(01)00381-3. [DOI] [PubMed] [Google Scholar]
- 8.Gross BC, Carlson ML, Moore EJ. The intraparotid facial nerve schwannoma: a diagnostic and management conundrum. Am J Otolaryngol. 2012;33:497–504. doi: 10.1016/j.amjoto.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Salemis NS, Karameris A, Gourgiotis S, et al. Large intraparotid facial nerve schwannoma: case report and review of the literature. Int J Oral Maxillofac Surg. 2008;37:679–681. doi: 10.1016/j.ijom.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Belzberg AJ, Dorsi MJ. Neoplasms of peripheral nerves. In: Fessel RG, Sekhar L, editors. Atlas of neurosurgical techinques - Spine and perupheral nerves. New York: Thieme; 2007. [Google Scholar]
- 11.Kim DH, Hudson AR, Kline DG, et al. Surgical technique for nerve tumors. In: Atlas of Peripheral nerve surgery. Amsterdam: Elsevier-Saunders; 2012. [Google Scholar]