SUMMARY
The role of corticosteroid in patients of chronic suppurative otitis media (CSOM) is unknown. In the present study, the efficacy and safety of ofloxacin alone (OA) and the ofloxacin + dexamethasone combination (ODC) is compared by studying clinical cure rates and adverse drug reactions in patients with CSOM. After prior permission from the Institutional Review Board and written informed consent from patients, pre-treatment clinical assessment and bacteriology of the middle ear discharge were done. The middle ear was categorised into active, mucoid or inactive according to the type of discharge. Grades of otorrhoea and size of tympanic membrane perforation were noted. CSOM with organisms sensitive to ofloxacin were treated either with OA or ODC eardrops for a period of 15 days. Post-treatment clinical cure (when grade of otorrhoea become 0) was recorded on the 5th, 10th and 15th days and bacteriological assessment was recorded at the last visit. All parameters were analysed using Fisher's exact test. A total 110 patients were randomised. The most common microorganism associated with CSOM was Pseudomonas aeruginosa (45.45 %). Clinical improvement was seen in 84.61% and 86.79% of cases, but bacteriological improvement in only 82.69% and 77.35% of cases treated with OA and ODC, respectively. Shift of middle ear discharge from active to inactive was noted in 71.15% and 64.15% patients by the 10th day in the OA and ODC groups, respectively. As there was no difference in clinical or bacteriological improvement, it may be unnecessary to combine steroids with topical antibiotic preparations for management of CSOM.
KEY WORDS: Antimicrobial agents, Ofloxacin, Ofloxacin + dexamethasone, Chronic suppurative otitis media
RIASSUNTO
Il ruolo dei farmaci corticosteroidei nei pazienti affetti da Otite Media Cronica Purulenta (OMCP) è sconosciuto. Nel presente studio sono state confrontate efficacia e sicurezza della terapia con Ofloxacina da sola (OS) con l'associazione di Ofloxacina + Desametasone (ODA), valutando il tasso di guarigione clinica e le reazioni avverse al farmaco in pazienti con OMCP. Previo consenso da parte del Institutional Review Board e adesione dei pazienti allo studio tramite consenso informato scritto, sono state effettuate valutazioni cliniche pre-trattamento ed esame colturale delle secrezioni provenienti dall'orecchio medio. In base alla tipologia di otorrea l'orecchio è stato classificato come attivo, mucoide o inattivo. Sono stati inoltre valutati il grado di otorrea e la dimensione della perforazione timpanica. I pazienti con OMCP in cui i microrganismi identificati risultavano sensibili all'Ofloxacina sono stati trattati in maniera randomizzata o con la sola Ofloxacina o con associazione Ofloxacina + Desametasone in gocce auricolari per 15 giorni. Il tasso di guarigione clinica alla fine del trattamento (ovvero quando il grado di otorrea risultava 0) è stato registrato al 5°, 10° e 15° giorno e in occasione dell'ultima visita è stata eseguito un esame colturale delle secrezioni. Tutti i parametri sono stati analizzati mediante il Test di Fisher. Sono stati reclutati in maniera randomizzata 110 pazienti. Il microrganismo associato più frequentemente al OMCP è risultato Pseudomonas aeruginosa (45,45%). È stato registrato un beneficio clinico in 84,61% dei casi trattati con OS e in 86,79% dei casi trattati con ODA ma la risoluzione dell'infezione all'esame batteriologico si è verificata solo nel 82,69% e nel 77,35% dei pazienti con OS e ODA rispettivamente. Il passaggio della forma attiva a quella inattiva è stato evidenziato al 10° giorno nel 71,15% e 64,15% dei pazienti trattati rispettivamente con OS e ODA. Dal momento che non è stata dimostrata nessuna differenza nel beneficio clinico e microbiologico, la combinazione di antibiotici topici e steroidi risulta sconsigliata nella terapia del OMCP.
Introduction
Chronic suppurative otitis media (CSOM) is one of the leading causes of acquired and preventable hearing loss. It is estimated to affect 65 to 330 million people worldwide mainly involving South East Asia, Western Pacific, Africa and ethnic minorities. Of these, 60% have significant hearing loss 1. CSOM is associated with mucoid or mucopurulent discharge that impairs healing of tympanic membrane perforation. Surgery is necessary for the definitive management of CSOM, and is also needed to convert wet ear into dry ear for good surgical outcomes 2. In addition, otorrhoea may become a complication of many surgical procedures such as tympanoplasty, myringoplasty and mastoidectomy 3. For these reasons, many ototopical antimicrobial with or without steroids are widely used for the treatment of otorrhoea in patients with CSOM 4. Antimicrobial and steroid combinations are also commonly used for the treatment of otorrhoea. It has been claimed that it reduces oedema of middle ear mucosa which prevents microbial colonisation within the middle ear and reduces the allergic sensitivity of the antibiotic component in ear drops 5. There are a few studies to claim the advantage of adding steroids in CSOM 6. The disadvantage of adding the steroid is that it increases fungal overgrowth in some steroid treated ears due to reduction in local tissue resistance 5. Thus, the role of steroids in the treatment of CSOM has always remained controversial. There is paucity of literature about the efficacy of a antimicrobialsteroid combination in CSOM. Hence, we designed the present study to compare the efficacy and safety of ofloxacin otic drops (0.3% w/v) with ofloxacin - dexamethasone otic drops (0.3% w/v & 0.1%w/v) for improved clinical status and bacteriological cure in CSOM patients.
Methods
Ethical approval was obtained from the Institutional Review Board, Government Medical College, Bhavnagar, Gujarat, India. Patients were thoroughly informed about the nature of the study and written informed consent was obtained prior to enrolment of subjects. This was a randomised, double blind, active control, parallel group, comparative study.
Subjects
Patients > 18 years of age including both genders, having ear discharge with tympanic membrane perforation and willing to give written informed consent were screened in the outpatient department of otorhinolaryngology. Patients with a history of sensitivity to any of the trial drugs, tuberculosis, diabetes mellitus, immunosuppressive disease, fungal or viral diseases, any clinically significant systemic diseases, chronic nasal obstruction, persistent rhinorrhoea, cholesteatoma, active atticoantral disease and pregnant or lactating women were excluded from the study. Patients with ear surgery in the preceding 12 months, congenital ear or hearing problems, middle ear obstruction (e.g., polyp) were also excluded. Patients who had taken systemic steroids, high dose of topical steroids and /or antibiotics in the previous one week and had temperature > 380C were also excluded.
Study design
Investigators were responsible for subject enrollment. Subjects were randomised by Rando software at a 1:1 ratio to receive either ofloxacin or ofloxacin + dexamethasone ear drops for 10 days. Containers of ofloxacin and ofloxacin + dexamethasone combination ear drops were identical in appearance. The label was covered and coded according to the randomisation sequence by a third person who was not involved in the study. Neither the participants nor the investigators knew the sequence. At the time of screening, a pus sample from the ear was collected from the middle ear in a sterile container for culture and sensitivity report. Those patients whose pus culture and sensitivity report showed sensitivity to ofloxacin were randomised and assigned to one of the two treatment groups. Group A patients received ofloxacin 0.3% w/v ear drops (12 drops twice a day); whereas, Group B patients received ofloxacin (0.3% w/v) + dexamethasone (0.1% w/v) combination (12 drops twice a day); for 10 days. The pus sample was again collected after 5 days of completion of treatment (on day 15th). After cleaning the ear discharge, the patient was instructed to instill five drops in the affected ear in a supine position with that ear facing the ceiling. The same position was maintained for 10 min, tragus was massaged repeatedly and whole procedure was repeated twice daily. Patients were assessed on day 0 (prior to treatment), day 5, day 10 and day 15.
Outcomes
The primary endpoint was clinical cure rate and secondary endpoints were subjective assessment of otorrhoea, change in size of perforation, isolated organisms, bacteriological improvement and safety analysis. Clinical cure was defined as conversion of wet ear into dry ear. According to grades of otorrhoea by otoscopic examination, patients were categorised into severe (the discharge totally obscuring any view of the tympanic membrane), moderate (partial obscuring tympanic membrane), mild (weeping ear with little to no obscuring of the tympanic membrane or middle-ear cavity) and no symptom categories on day 5, day 10 and day 15 3. Subjective assessment was categorised into active, mucoid and inactive ears by the investigator 7. Presence of purulent discharge was considered as an active discharge, the presence of mucopurulent and mucous discharge was considered as mucoid and absence of ear discharge was considered as an inactive ear. The different sizes of perforation were estimated and divided into categories of small (0-25%), medium (25-75%) and large (75-100%) according to its proportion to intact tympanic membrane. Adverse drug reactions were recorded at every follow-up visit and compared between groups.
Statistical analysis
Data were expressed in proportions. Sample size calculations by nMaster software indicated that 100 participants would be needed to achieve 80% power with an alpha level of 0.05 (two tailed), if the minimal expected difference in clinical cure rate is 20% and the anticipated clinical cure rate in ofloxacin group is 70%. Fisher's exact test was applied for comparison of all qualitative data. All subjects who had one follow-up visit after giving study medication were included in the efficacy analysis (intention-to-treat). All subjects who instilled ear drops at least once were included in the safety analysis. A P < 0.05 was considered statistically significant. All statistical calculations were performed using GraphPad InState 3 (version 3.06) software.
Results
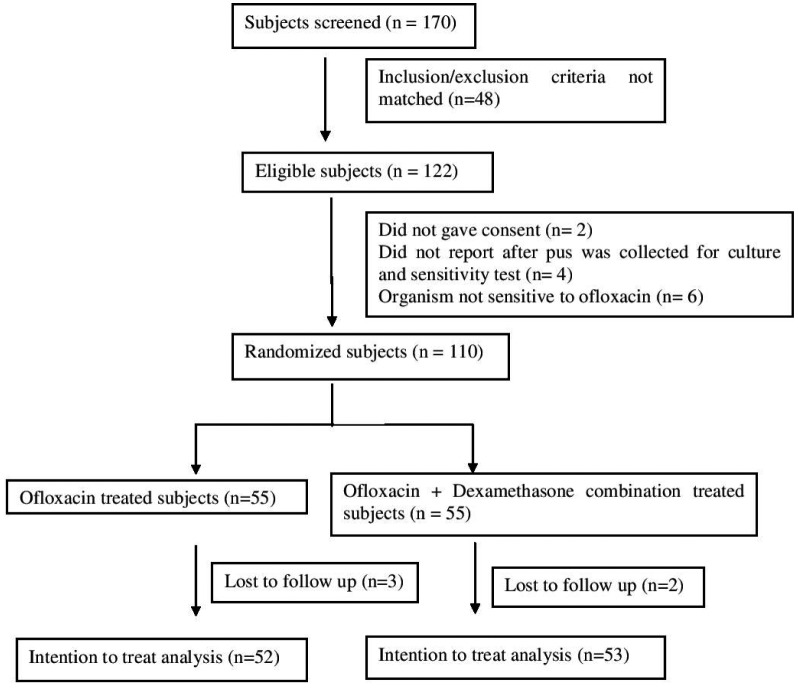
There were 55 subjects in each study arm (Fig. 1). Among these, 52 in the ofloxacin group and 53 in the ofloxacin + dexamethasone group were analysed in the intentionto- treat analysis. Table I shows baseline data and demographic characteristics of subjects in both groups. The prevalence of medium perforation was high (51/105 = 48.57%). 84.61% subjects in the ofloxacin group and 86.79% subjects in the ofloxacin + dexamethasone group were clinically cured. Subjective assessment shows the distribution of patients with active, mucoid and inactive disease (Table II).
Fig. 1.
CONSORT statement.
Table I.
Baseline demographic characteristics of patients in both groups.
| Ofloxacin (n =55) | Ofloxacin + Dexamethasone (n = 55) | P value | |
|---|---|---|---|
| Gender | |||
| Male | 24 (43.6%) | 26 (47.2%) | 0.5 |
| Female | 31 (56.3%) | 29 (52.7%) | |
| Side of ear | |||
| Right | 33 (60%) | 27 (49%) | 0.11 |
| Left | 22 (40%) | 28 (50.9%) | |
| Size of tympanic membrane perforation | |||
| Small | 04 (7.2%) | 09 (16.3%) | 0.63 |
| Medium | 32 (58.1%) | 28 (50.9%) | |
| Large | 19 (34.5%) | 18 (32.7%) | |
| Otorrhoea | |||
| Severe | 18 (32.8%) | 18 (32.8%) | 0.99 |
| Moderate | 26 (47.2%) | 26 (47.2%) | |
| Mild | 11 (20%) | 11 (20%) | |
| Status of middle ear | |||
| Active | 44 (80%) | 40 (72.7%) | 0.82 |
| Mucoid | 11 (20%) | 15 (27.2%) |
Data were expressed in absolute numbers. Groups were compared by Fisher's exact test.
Table II.
Cure rate of patients.
| Ofloxacin (n = 52) | Ofloxacin + Dexamethasone (n = 53) | p value | |
|---|---|---|---|
| Cured at day 5 | Grade 3 to 0 = 1 Grade 2 to 0 = 7 Grade 1 to 0 = 10 |
Grade 3 to 0 = 3 Grade 2 to 0 = 5 Grade 1 to 0 = 8 |
0.61 0.55 0.61 |
| Cured at day 10 | Grade 3 to 0 = 5 Grade 2 to 0 = 10 Grade 1 to 0 = 1 |
Grade 3 to 0 = 3 Grade 2 to 0 = 14 Grade 1 to 0 = 2 |
0.48 0.48 0.49 |
| Cured at day 15 | Grade 3 to 0 = 5 Grade 2 to 0 = 5 Grade 1 to 0 = 0 |
Grade 3 to 0 = 9 Grade 2 to 0 = 2 Grade 1 to 0 = 0 |
0.39 0.16 1.0 |
Data are expressed in absolute numbers. Groups were compared by Fisher's exact test.
Subjective assessment of active discharge did not show any significant difference in both the groups when compared at day 0, 5, 10 and 15 (Fig. 2).
Fig. 2.
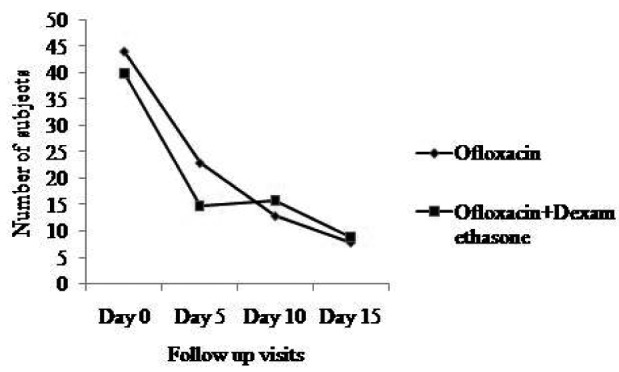
Comparison of subjective assessment of active discharge. Subjective assessment of active discharge at day 0 compared with day 5, day 10 and day 15 in both groups.
Shift of middle ear discharge from active to inactive was seen in 71.15% and 64.15% patients within the 10th day in the ofloxacin and ofloxacin + dexamethasone groups, respectively. The majority of patients were improved by day 10. A small number of patients continued to improve at day 15. The disease remained active in only 15 patients in the ofloxacin group and 20 patients in the ofloxacin + dexamethasone group at the end of the 15th day (p = 0.41, Fisher's exact test). No any serious adverse events were reported during the study and other adverse events were described in Table IV.
Table IV.
Adverse events in both groups.
| Adverse events | Ofloxacin (n = 52) | Ofloxacin + Dexamethasone (n = 53) |
|---|---|---|
| Headache | 2 | 3 |
| Vertigo | 1 | 2 |
| Local itchiness | 1 | 1 |
| Other | 1 | - |
| Total | 9.61% | 11.32% |
Bacteriological evaluation
Pseudomonas aeruginosa was found to be the most common organism associated with CSOM (40.95%) followed by Staphylococcus aureus (24.76%). Bacteriological improvement was seen in 82.69% and 77.35% of cases treated with ofloxacin and ofloxacin + dexamethasone, respectively. The organisms associated with CSOM are listed in Table III. The rate of bacterial eradication was higher in the ofloxacin group.
Table III.
Bacteria isolated and bacteriological cure rate.
| Organism | Number of patients (%) Ofloxacin (n = 52) |
Number of patients (%) Ofloxacin + Dexamethasone (n = 53) |
|---|---|---|
| Pseudomonas | 15/17 (88.2%) | 23/26 (88.4%) |
| Staphylococcus aureus | 16/18 (88.8%) | 6/8 (75%) |
| Coagulase –ve Staphylococcus aureus | 5/6 (83.3%) | 2/4 (50%) |
| Proteus | 1/2 (50%) | 1/2 (50%) |
| Klebsiella | 4/6 (66.6%) | 7/10 (70%) |
| E. coli | 2/3 (66.6%) | 2/3 (66.6%) |
Numerator indicates the number of patients with bacteriological cure. The denominator indicates total number of patients showing total number with that organism.
Discussion
CSOM is most commonly a pseudomonal / staphylococcal disease 8 9 which implicates the use of antipseudomonal drugs. Thus, fluroquinolones are the best choice for topical use 10-12. Yuen et al. have shown that only 26% patients had dry ears with amoxicillin + clavulanic acid, whereas 76% of patients had dry ears using topical ofloxacin 13. Our study also showed similar rates of improvement: 84.61% in the ofloxacin arm and 86.79% in the ofloxacin + dexamethasone arm. Esposito et al. compared oral ciprofloxacin with topical ciprofloxacin and found that 40% and 85% patients had cure of otorrhea, respectively, which favours the use of eardrops 14. Another study also showed the superiority of topical ciprofloxacin over intramuscular gentamicin 15. Accordingly, we used ototopical medications instead of oral medications. The susceptibility of microorganisms to ofloxacin and aminoglycosides are similar except that ofloxacin has a higher efficacy for staphylococcal and pseudomonal infections. Hence, we selected ofloxacin instead of other antimicrobials 16. The role of anaerobes in CSOM has recently gained widespread attention. The isolation rate of anaerobes in CSOM varies from no anaerobes 17 18 to 50% of isolated anaerobes 19 20. The reason for not finding anaerobes may be due to inclusion of only those cases whose microorganism was sensitive to ofloxacin.
There is no standardisation regarding the duration and type of eardrops in patients with CSOM. Indudharan et al. reported 82.5% and 75% of bacteriological improvement with gentamicin or gentamicin-steroid ear drops when used for 3 weeks. Our study had a similar bacteriological cure rate when topical ofloxacin with or without steroid was used for 10 days, which may be because of the higher efficacy of ofloxacin. Overall clinical improvement was 84.61% & 86.79%, and bacteriological improvement was 82.69% and 77.35%, respectively, with ofloxacin and ofloxacin-dexamethasone at 10 days. This confirms that 10 days of therapy demonstrates significant clinical cure and less bacteriological cure because of persistent of infection in CSOM. There was also no statistically significant difference in clinical or bacteriological improvement if ofloxacin or ofloxacin + dexamethasone was used. The size of tympanic membrane perforation was not reduced, which may be due to healing of tissue and may take a longer time, but inthis study there was a shorter duration of follow-up.
The most commonly isolated organisms in our study were gram-negative organisms, which were about twice as frequent as the gram positive agents isolated from middle ear discharge. The pattern of bacterial isolates showed that Pseudomonas aeruginosa was the most common organism found in middle ear discharge, which is similar to the findings of other studies 21-23. We also compared the antimicrobial response to ofloxacin alone and ofloxacin + dexamethasone by assessing the bacteriological cure at the end of visit. Because both medications contained the same antibiotic, the difference in bacteriological cure rates was not expected.
Crowther and Simpson demonstrated improvement in ear discharge in 29% of patients when betamethasone ear drops were used, while 80% improvement in ear discharge in of patients when gentamicin-hydrocortisone was used. Using only a topical steroid preparation is not better than placebo 24. Spandow demonstrated the possibility of ototoxicity by glucocorticoids related to an impaired auditory brainstem response threshold in the high frequency range in an animal model 25. However, our study indicates no advantage of using an ofloxacin – steroid combination. This could be due to inhibition of its entry into cochlear hair cells in CSOM patients. Clinical as well as bacteriological improvement also showed similar results whether ofloxacin alone or ofloxacin + dexamethasone combination was used. It shows that the addition of steroids does not have any advantage over ofloxacin ear drops alone. On the contrary, it may allow fungal colonisation in the external ear canal 5.
Lastly, some of the limitations of the study warrant consideration. Firstly, since the duration of follow-up was relatively short, recurrence of otorrhoea and healing of perforation was not evaluated. In addition, as patients were assessed on days 5, 10 and 15, the exact day when ear discharge stopped is not known. In this regard, patients should keep a symptom diary so that a more precise time can be obtained for resolution of ear discharge.
Conclusions
Our data suggest that Pseudomonas aeruginosa is the most common organism causing CSOM. Because clinical improvement of patients with topical ofloxacin eardrops was good in our study, we recommend its use for a period of 10 days, even if the ear becomes dry, to improve bacteriological cure. There is no added advantage of using a topical ofloxacin + dexamethasone combination over ofloxacin alone in terms of clinical and bacteriological cure.
Acknowledgements
The authors are very thankful to FDC Pvt. Ltd. Mumbai for the providing ear drops of ofloxacin and ofloxacin + dexamethasone used in the study.
References
- 1.Acuin J. Chronic suppurative otitis media: Burden of illness and management options. Geneve: World Health Organization (WHO); 2006. [Google Scholar]
- 2.Tong MC, Woo JK, Hasselt CA. A double-blind comparative study of ofloxacin otic drops versus neomycinpolymyxin B-hydrocortisone otic drops in the medical treatment of chronic suppurative otitis media. J Laryngol Otol. 1996;110:309–314. doi: 10.1017/s0022215100133523. [DOI] [PubMed] [Google Scholar]
- 3.Gydé MC. A double-blind comparative study of trimethoprim- polymyxin B versus trimethoprim-sulfacetamide-polymyxin B otic solutions in the treatment of otorrhea. J Laryngol Otol. 1981;95:251–259. doi: 10.1017/s002221510009068x. [DOI] [PubMed] [Google Scholar]
- 4.Louvois J, Gortvai P, Hurley R. Bacteriology of abscesses of central nervous system: a multicentric prospective study. Br Med J. 1977;2:981–984. doi: 10.1136/bmj.2.6093.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramunnikutty I, Kamaru Ambu V, Suraparaju Sivachandra R. Role of glucocorticoids in ototopical antibiotic–steroid preparations in the treatment of chronic suppurative otitis media. Arch Med Res. 2005;36:154–158. doi: 10.1016/j.arcmed.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Miró N. Controlled multicenter study on chronic suppurative otitis media treated with topical applications of ciprofloxacin 0.2% solution in single-dose containers or combination of polymyxin B, neomycin, and hydrocortisonesuspension. Otolaryngol Head Neck Surg. 2000;123:617–623. doi: 10.1067/mhn.2000.107888. [DOI] [PubMed] [Google Scholar]
- 7.Browning GG, Picozzi GL, Calder IT, et al. Controlled trial of medical treatment of active chronic otitis media. Br Med J (Clin Res Ed) 1983;287:1024–1024. doi: 10.1136/bmj.287.6398.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fliss DM, Dagan R, Meidan N, et al. Aerobic bacteriology of chronic suppurative otitis media without cholesteatoma in children. Ann Otol Rhinol Laryngol. 1992;101:866–869. doi: 10.1177/000348949210101011. [DOI] [PubMed] [Google Scholar]
- 9.Papastavros T, Giamarellou H, Varlejides S. Role of aerobic and anaerobic microorganism in chronic suppurative otitis media. Laryngoscope. 1986;96:438–442. doi: 10.1288/00005537-198604000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Browning GG, Gatehouse S, Calder IT. Medical management of active chronic otitis media: a controlled study. J Laryngol Otol. 1988;102:491–495. doi: 10.1017/s0022215100105444. [DOI] [PubMed] [Google Scholar]
- 11.Kasemsuwan L, Clongsuesuek P. A double blind, prospective trial of topical ciprofloxacin versus normal saline solution in the treatment of otorrhoea. Clin Otolaryngol. 1997;22:44–46. doi: 10.1046/j.1365-2273.1997.00857.x. [DOI] [PubMed] [Google Scholar]
- 12.ndudharan R, Haq JA, Aiyar S. Antibiotics in chronic suppurative otitis media—a bacteriologic study. Ann Otol Rhinol Laryngol. 1999;108:440–445. doi: 10.1177/000348949910800504. [DOI] [PubMed] [Google Scholar]
- 13.Yuen PW, Lau SK, Chau PY, et al. Ofloxacin eardrop treatment for active chronic suppurative otitis media. Prospective randomized study. Am J Otol. 1994;15:670–673. [PubMed] [Google Scholar]
- 14.Esposito S, D'Errico G, Montanaro C. Topical and oral treatment of chronic otitis media with ciprofloxacin. A preliminary study. Arch Otolaryngol Head Neck Surg. 1990;116:557–559. doi: 10.1001/archotol.1990.01870050057006. [DOI] [PubMed] [Google Scholar]
- 15.Esposito S, Noviello S, D'Errico G, et al. Topical ciprofloxacin vs intramuscular gentamicin for chronic otitis media. Arch Otolaryngol Head Neck Surg. 1992;118:842–844. doi: 10.1001/archotol.1992.01880080064014. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda K, Takasaka T. In vitro activity of ototopical drops against middle ear pathogens. Am J Otol. 1993;14:170–171. [PubMed] [Google Scholar]
- 17.Cooke ETM, Raghuvaran G. Clindamycin in conjuction with surgery of the chronic suppurative ear. Br J Clin Pract. 1974;28:57–59. [PubMed] [Google Scholar]
- 18.Deka RC, Kacker SK. Chronic otitis media: a clinical and bacteriological study. Eye Ear Nose Throat Man. 1975;54:198–201. [PubMed] [Google Scholar]
- 19.Brook I. Chronic otitis media in children: microbiological studies. Am J Dis Child. 1980;134:564–566. doi: 10.1001/archpedi.1980.02130180022007. [DOI] [PubMed] [Google Scholar]
- 20.Erkan M, Aslan T, Sevuk E, et al. Bacteriology of chronic suppurative otitis media. Ann Otol Rhinol Laryngol. 1994;103:771–774. doi: 10.1177/000348949410301005. [DOI] [PubMed] [Google Scholar]
- 21.Brook I, Frazier E. Microbial dynamics of persistent purulent otitis media in children. J Pediatrics. 1996;128:237–240. doi: 10.1016/s0022-3476(96)70397-9. [DOI] [PubMed] [Google Scholar]
- 22.Ologe FE, Nwawolo CC. Prevalence of chronic suppurative otitis media (CSOM) among school children in a rural community in Nigeria. Nig Postgrad Med J. 2002;9:63–66. [PubMed] [Google Scholar]
- 23.Bluestone CD. Pathogenesis and epidemiology of chronic otitis media. WHO/CIBA foundation workshop, report on prevention of hearing impairment for chronic otitis media held at CIBA foundation, London. 1996. pp. 14–17.
- 24.Crowther JA, Simpson D. Medical treatment of chronic otitis media: Steroid or antibiotic with steroid eardrops? Clin Otolaryngol Allied Sci. 1991;16:142–144. doi: 10.1111/j.1365-2273.1991.tb01963.x. [DOI] [PubMed] [Google Scholar]
- 25.Spandow O. Are glucocorticoids in ear drops ototoxic? Ada Oto-Laryngologica. 1992;493(suppl):89–91. [PubMed] [Google Scholar]