Abstract
Differential thermal nociception across inbred mouse strains has genetic determinants. Thermal nociception is largely attributed to the heat/capsaicin receptor transient receptor potential vanilloid 1 (TRPV1); however, the contribution of this channel to the genetics of thermal nociception has not been revealed. In this study we compared TRPV1 expression levels and electrophysiological properties in primary sensory neurons and thermal nociceptive behaviors between two (C57BL/6 and BALB/c) inbred mouse strains. Using immunofluorescence and patch-clamp physiology methods, we demonstrated that TRPV1 expression was significantly higher in isolectin B4 (IB4)-positive trigeminal sensory neurons of C57BL/6 relative to BALB/c; the expression in IB4-negative neurons was similar between the strains. Furthermore, using electrophysiological cell classification (current signature method), we showed differences between the two strains in capsaicin sensitivity in IB4-positive neuronal cell types 2 and 13, which were previously reported as skin nociceptors. Otherwise electrophysiological membrane properties of the classified cell types were similar in the two mouse strains. In publicly available nocifensive behavior data and our own behavior data from the using the two mouse strains, C57BL/6 exhibited higher sensitivity to heat stimulation than BALB/c, independent of sex and anatomical location of thermal testing (the tail, hind paw, and whisker pad). The TRPV1-selective antagonist JNJ-17203212 inhibited thermal nociception in both strains; however, removing IB4-positive trigeminal sensory neurons with IB4-conjugated saporin inhibited thermal nociception on the whisker pad in C57BL/6 but not in BALB/c. These results suggest that TRPV1 expression levels in IB4-positive type 2 and 13 neurons contributed to differential thermal nociception in skin of C57BL/6 compared with BALB/c.
Keywords: capsaicin, heat pain sensitivity, TRPV1, electrophysiological property, trigeminal ganglion
pain perception varies across individuals. Systematic reviews of twin studies on pain demonstrate large effects of genetic factors on experimental pain and clinical pain syndromes (Nielsen et al. 2008, 2012; MacGregor et al. 1997). Genetic heritability of nociception has also been reported among inbred mouse strains (Mogil and Adhikari 1999a; Mogil et al. 1999b,c; Lariviere et al. 2002; Furuse et al. 2002). Previous studies on genetic heritability of pain have identified several genes whose variable expression on nociceptors determines pain traits. For example, calcitonin gene-related polypeptide (CGRP) in dorsal root ganglion (DRG) neurons affects thermal nociception in inbred mouse strains (Mogil et al. 2005). Lower expression of P2X3 (a nociceptive adenosine 5′-triphosphate-sensitive channel) on DRG neurons correlates with lower sensitivity to a P2X3-selective agonist, a noxious stimulus (Tsuda et al. 2002).
The transient receptor potential vanilloid 1 (TRPV1) channel is an important transducer for noxious heat in primary sensory neurons, but the relationship between TRPV1 expression and thermal nociceptive traits in inbred mouse strains is unknown. TRPV1 responds to both noxious heat (>43°C) and capsaicin and is expressed in many mouse peptidergic sensory neurons that contain CGRP and do not bind isolectin B4 (IB4) (Dinh et al. 2003; Price and Flores 2007). However, early studies that measure TRPV1 expression in IB4-positive neurons are in disagreement. Electrophysiological studies confirm capsaicin sensitivity in IB4-positive DRG neurons of C57BL/6 (Dirajlal et al. 2003; Breese et al. 2005), while immunofluorescent studies demonstrate lack of TRPV1 expression in IB4-positive DRG neurons of BALB/c and C3H/Bl6 (Zwick et al. 2002: Woodbury et al. 2004). The reasons for the inconsistent results are not clear. Technical or mouse strain differences might be responsible: however, a systematic study has not been performed to address the question. C57BL/6 has been reported to have higher thermal pain sensitivity and aversion to capsaicin solution than BALB/c (Mogil and Adhikari 1999a; Mogil et al. 1999b,c; Lariviere et al. 2002; Furuse et al. 2002). Since IB4-positive afferents predominantly innervate the epidermal layer of the skin (Price and Flores 2007), differences in TRPV1 expression level in IB4-binding neurons may contribute to strain differences in cutaneous thermal nociceptive sensitivity.
In the present study, we hypothesized that TRPV1 expression on sensory neurons in the C57BL/6 and BALB/c inbred mouse strains determines thermal nociceptive traits. To reveal differences in TRPV1 expression levels in IB4-positive neurons, we investigated differences in TRPV1 immunoreactivity and capsaicin sensitivity with IB4 staining in mouse trigeminal ganglion (TG) neurons from the two strains. Furthermore, we classified TG neurons into several internally homogeneous subpopulations based on electrophysiological classification using a current signature method. The method was initially used to identify four neuron types from rat DRG neurons (Cardenas et al. 1995). Subsequently, DRG neurons have been further classified into 18 cell groups by Cooper and colleagues (Petruska et al. 2000b, 2002; Jiang et al. 2006; Rau et al. 2011, 2014). The electrophysiologic-based cell classification system has been applied to rat TG neurons; nine cell groups (types 1–5, 7–9, and 13) demonstrated similar electrophysiological properties and capsaicin sensitivity to matched DRG cell types (Xu et al. 2010). The electrophysiological cell classification has the substantial advantage of being able to predict histochemical and innervation properties. Next, we used a publicly available database of nocifensive behaviors in inbred mouse strains and compared the results to our own behavioral assays. Finally, we examined the contribution of TRPV1 and IB4-positive neurons to thermal nocifensive behavior.
METHODS
Animals.
C57BL/6 and BALB/c (females, 6–10 wk old; Charles River Laboratories, NY) weighing 16–20 g were used in the present study. Mice were exposed to a 12-h light-dark cycle and kept in a temperature-controlled room with food and water ad libitum. All procedures were approved by the New York University Institutional Animal Care and Use Committee.
Quantitative reverse transcription PCR analysis.
Ten milligrams of each fresh frozen TG (5 samples from 5 animals in each strain) were homogenized with a Mini Beadbeater-1 (BioSpec Products, OK, USA) and subject to RNA/DNA extraction with AllPrep DNA/RNA Kit (Qiagen, Valencia, CA). mRNA was reverse transcribed with Random Hexamers (Applied Biosystems). Five replicates were performed in each strain. A 2-μl cDNA aliquot was amplified on the Mx3005P qPCR system (Agilent Technologies, Santa Clara, CA) according to manufacturer's recommendations with the Taqman gene expression assay for Trpv1 (TRPV1 gene; Mm01246302 m1; Applied Biosystems), which did not detect residual genomic DNA. Mouse ACTB gene (Applied Biosystems) was used as the endogenous control. The delta-delta CT method was used to quantify relative expression. The assays were carried out in duplicate, and the given relative amounts of Trpv1 to ACTB were averaged in each sample.
Immunofluorescence.
For immunohistochemistry, the TG were dissected from three animals in each strain after perfusion with 4% paraformaldehyde in phosphate-buffered saline (PBS) and cut in the horizontal plane at 8-μm thickness in a cryostat. After being sectioned, TG sections were rinsed with PBS and blocked with SuperBlock solution (Thermoscientific) for 1 h and then incubated overnight in the primary antibody (rabbit anti-TRPV1; 1:400; Alomone Labs, Jerusalem, Israel). After incubation with the primary antibody, sections were rinsed in PBS five times for 5 min each and then incubated in the chicken anti-rabbit Alexa-594 (1:1,000; Invitrogen, Carlsbad, CA) and IB4-fluorescein isothiocyanate (5 μg/ml; Sigma-Aldrich, MO) for 2 h at room temperature. Thereafter, sections were washed with PBS and coverslipped with UltraCruz mounting medium (Santa Cruz Biotechnologies). Control tissue sections incubated with secondary antibody only or preabsorbed primary antibody showed no positivity (data not shown). Images were taken under an epifluorescence microscope (Eclipse Ti; Nikon, Tokyo, Japan). Six random sections from each slide were used for quantification of each staining. Data analysis was performed using Nikon Element software, which allowed both single and merged picture acquisitions.
TG neuron dissociation.
TG neurons were dissociated according to an established protocol (Malin et al. 2007). After the animals were deeply anesthetized with isoflurane, the TG were removed (17 and 16 animals in C57BL/6 and BALB/c, respectively) and transferred into a culture medium (Ca2+ and Mg2+-free Hanks' balanced salt solution; Invitrogen). After the tissue was minced into 10–12 pieces, the tissues were incubated in collagenase type 2 (3.3 mg/ml; Worthington Biochemical) and dispase II (4.7 mg/ml; Roche) for 20 min and then in papain (20 U/ml; Worthington Biochemical) for 20 min at 37°C. After trituration and centrifugation, the cell pellet was resuspended with F-12 (Invitrogen) containing 5% fetal calf serum and plated on laminin-coated coverslips. The cells were incubated at 37°C in a humidified 5% CO2 chamber until whole cell patch-clamp recordings. All TG neurons were stained with IB4-fluorescein isothiocyanate (5 μg/ml, Sigma) for 20 min before recording.
Whole cell patch-clamp recording.
Electrophysiological procedures were described in our previous study (Ye et al. 2014b). Within 3–8 h of plating the neurons, coverslips with neurons were transferred to a recording chamber superfused continuously with external solution containing the following (in mM): 140 NaCl, 4 KCl, 2 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES (pH 7.3 with NaOH, 320 mosmol/kgH2O with sucrose), at room temperature. Clearly identifiable IB4-positive and IB4-negative TG neurons were selected for the recording. Patch pipettes were double-pulled (P-2000; Sutter) from quartz glass capillaries (Q100-50-10; Sutter). Pipettes were adjusted to 3–6 MΩ when filled with a pipette solution containing the following (in mM): 145 KCl, 3 MgCl2, 2.25 CaCl2, 1 EGTA, and 10 HEPES (pH 7.3 with KOH, 310 mosmol/kgH2O with sucrose). After the whole cell configuration was established, the voltage was clamped at −60 mV using Axopatch 200B amplifier (Molecular Devices) and controlled by Clampex software (pClamp 10.2; Molecular Devices). The values of membrane potentials were not corrected for the liquid junction potential (3 mV) present in the pipette. Series resistance was not compensated, and cells with a series resistance over 15 MΩ were eliminated from the present study. As stock solutions, capsaicin (Sigma-Aldrich) was dissolved in dimethyl sulfoxide. The stock solution was diluted to 0.1% with the perfusion solution (final concentration of capsaicin was 1 μM). Capsaicin was applied for 5 s, as a probe test to detect TRPV1 expression, using a fast-step SF-77B superfusion system (Warner Instrument) with a three-barreled pipette placed near the cell. Dimethyl sulfoxide alone at 0.1% was confirmed to have no effect on base currents in five neurons. Current amplitudes were measured at the peak of the inward component. If the recorded cells showed capsaicin-induced current of >1 pA/pF, the cells were deemed to be sensitive to capsaicin. The concentration, application time, and detection threshold were selected from previous rat TG studies (Xu et al. 2010; Ono et al. 2010).
Electrophysiological cell classification.
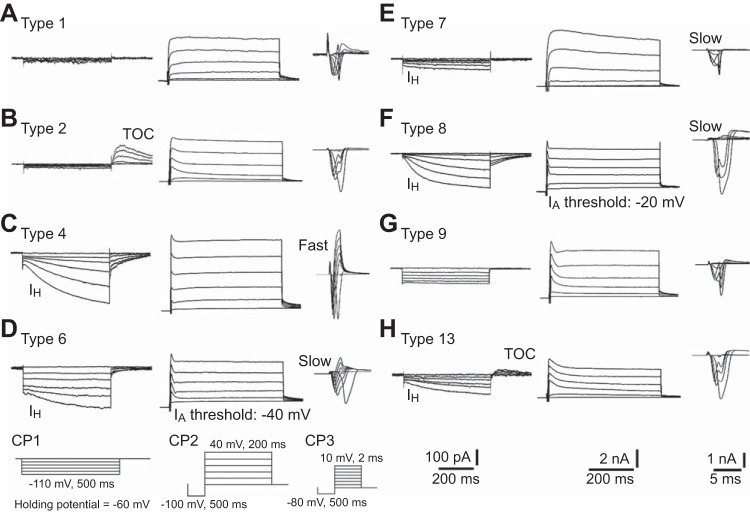
In addition to dichotomizing TG neurons based on IB4 binding, the current signature method was used to further classify TG neurons into functionally distinct cell groups, similar to previous studies in rat TG and DRG neurons (Cardenas et al. 1995; Petruska et al. 2000b, 2002; Xu et al. 2010; Ono et al. 2010). The cell classification is based on cell size and parameters from three kinds of classification protocols (CP1-3; see Fig. 4, insets). Since a previous study comparing DRG neurons in mice and rats used cell size limits that were 10% smaller for mouse neurons than rat neurons (Barabas et al. 2012), we used the 10% smaller cell size category (small: 11–22 μm; medium: 23–34 μm) than that used in rat TG studies (Xu et al. 2010; Ono et al. 2010). This method of cell size categorization led to the highest homogeneity of capsaicin sensitivity and electrophysiological properties in each cell type. Because larger-sized sensory neurons are generally considered to be nonnociceptive neurons (Basbaum et al. 2009), data from the cells of >34 μm diameter were excluded from this study to focus on nociceptive sensory neurons. CP1 was used to produce transient outward current (TOC) and hyperpolarization-activated inward current (IH). A series of hyperpolarizing command steps (10-mV steps, 500-ms duration, 5-s intervals) was applied from the holding potential to a final potential of −110 mV. Peak amplitude of the TOC, which was observed in repolarization from the hyperpolarization step of −110 mV, was evaluated. The amplitude of IH was evaluated by subtracting an instantaneous inward current at the beginning of the hyperpolarization step of −110 mV from a current at the end of the voltage step. CP2 was used to produce depolarization-activated outward currents that involve A-type transient outward current (IA). After a 500-ms conditioning pulse to −100 mV, a series of depolarizing command steps (20-mV steps, 200-ms duration, 5-s intervals) were applied to a final potential of 40 mV. Threshold voltage of IA (AT) was obtained from outward current traces. CP3 was used to produce an inward current. After a 500-ms conditioning pulse to −80 mV, a series of depolarizing command steps (10-mV steps, 2-ms duration, 5-s intervals) was applied to a final potential of 10 mV. CP3-induced inward currents are divided into fast and slow current kinetics: fast inward current is low-threshold fast-activating and -decaying currents and slow inward current is high-threshold slow-activating and -decaying currents.
Fig. 4.
Current signatures in the classified TG cell types. Current signatures evoked by the classification protocols (CP1, CP2, and CP3, as indicated in D, bottom) are shown for TG cell types 1–13 (A-I, respectively) as well as early rat studies (Petruska et al. 2000b; Xu et al. 2010; Ono et al. 2010). The CP1 was run by applying a series of hyperpolarizing command steps (10-mV steps). The CP2 was run with a conditioning pulse to −100 mV, followed by a series of depolarizing command steps (20-mV steps). The CP3 was run with a conditioning pulse to −80 mV, followed by a series of depolarizing command steps (10-mV steps). Scales for all TG cell types are indicated at bottom right.
First, CP1 and the cell size ranges were used to delineate cell types 1, 2, 9, and 13. If neurons expressed TOC (>30 pA), they were divided into types 2 and 13 based on the absence or presence of IH (>30 pA). Furthermore, neurons with neither TOC nor IH were divided into types 1 and 9 based on whether they were small or medium in size. Second, CP3 and cell size ranges were used to determine cell types 3, 4, and 7. In small sized neurons that expressed IH without TOC during CP1, they were divided into types 3 and 7 by virtue of expressing either fast decaying (fast) or slow decaying (slow) inward currents during CP3. If medium-sized neurons that expressed IH without TOC showed fast inward currents, they were determined to be type 4. Third, CP2 was used to determine cell types 5, 6, and 8, which were defined by the presence of IH without TOC during CP1, slow inward currents during CP3, and medium cell size. Types 5, 6, and 8 were delineated based on whether the AT during CP2 was 0, −40, or −20 mV. After electrophysiological classification, the cell types were compared with previously reported TG and DRG cell types based on IB4-binding and current properties (Xu et al. 2010; Ono et al. 2010; Petruska et al. 2000b, 2002; Jiang et al. 2006; Rau et al. 2011, 2014).
Publicly available database for thermal pain tests.
Data from the “Heritability of Nociception Project,” which is publicly available on The Jackson Laboratory's Mouse Phenome Database Website (http://www.jax.org/phenome; Projects: Mogil1 and Jaxwest1), were used for comparison of thermal pain thresholds between C57BL/6 and BALB/c. Detailed experimental methods, the protocol, and comparative results can be found in previous publications (Mogil et al. 1999b,c; Lariviere et al. 2002) and at The Jackson Laboratory's Mouse Phenome Database Website. Tail withdrawal latencies to water bath at 47.5°C and 49°C (tail-flick test) and paw withdrawal latencies to lamp radiant heat stimulation (Hargreaves' test) and to 53°C (hot-plate test) in male C57BL/6 and BALB/c were used from the Mogil1 project. Paw withdrawal latencies to 55°C (hot-plate test) in male and female C57BL/6 and BALB/c were used from the Jaxwest1 project.
Thermal sensitivity tests for the hind paw and whisker pad.
The pain tests were performed in female mice. Thermal sensitivity in the hind paw was measured using a thermal stimulator (Hargreaves' Apparatus; Dept. of Anesthesiology, University of California, San Diego, CA), according to the general method of Hargreaves' test (Hargreaves et al. 1988). Mice were placed in plastic chambers on a glass surface (25°C) and acclimated for 30 min before applying a heat source to the right hind paw. To apply heat stimulation to the whisker pad, mice were restrained in a plastic tube during measurements (37 animals in each strain; 5 animals were tested in the hind paw). The retention tube was made from a 50-ml conical tube by creating a hole of 13 mm in diameter at the bottom of the tube and then cutting at the 30-ml graduation line. Mice were habituated to enter and stay in the retention tube for three sessions (once per day) prior to thermal testing. For whisker pad testing, a thick-paper cone with a 7-mm diameter opening on the top was attached to the thermal stimulator to keep a constant distance (8 cm) and heat-filament position from the whisker pad. A radiant heat source was focused on the hind paw or whisker pad, and latency to withdrawal was measured as the average of four trials per animal taken >5 min apart. A cutoff latency was set at 20 s to avoid tissue damage.
Drug treatments for mice.
To examine involvement of TRPV1 in withdrawal behavior following radiant heat stimulation, mice were intraperitoneally administrated a TRPV1 selective antagonist JNJ-17203212 (40 mg/kg in saline, 6 animals in each strain; Tocris, Bristol, UK). For the control, the same volume of saline was administered (6 animals in each strain). Thermal pain tests at the whisker pad were performed before and 1 h after the treatments; the experimenter was blinded to the drug treatments. The drug concentration and observation time were decided from previous studies (Huang et al. 2011; Ye et al. 2014a).
To examine involvement of IB4-positive neurons in withdrawal behavior, IB4-saporin (2 μg in 10 μl PBS) or unconjugated saporin (Advanced Targeting Systems) was administrated into the right infraorbital foramen under 2.5% isoflurane anesthesia (5 animals for each drug administration in each strain). According to a previous study (Ye et al. 2012), the thermal pain test for the whisker pad was performed 15 days after the treatment; the experimenter was blinded to the drug treatment. To confirm removal of IB4-positive neurons after the treatment of IB4-saporin, the right TG was removed after the pain test, cut in the horizontal plane at a 10-μm thickness, and stained with IB4-fluorescein isothiocyanate, as described above. As a preliminary experiment, the neuronal tracer fluorogold (10% in PBS: 10 μl) was injected similar to the IB4- saporin injection (C57BL/6: 2 animals and BALB/c: 1 animal) and the specific labeling to the second branch area of the TG (V2) was confirmed. Therefore, mean fluorescence intensities in the V2 were compared with those of the first branch area of the TG (V1) on the same section. Two random sections from each mouse were used for quantification of each staining.
Statistical analyses.
Data are presented as the means ± SE; n represents the number of neurons tested. The levels of Trpv1 mRNA were calculated as a ratio change relative to the mean value for C57BL/6. Mann-Whitney U-test was used to compare the different groups (C57BL/6 vs. BALB/c, IB4 positive vs. IB4 negative, 2 electrically subclassified cell types and control vs. drug treatment) when cell numbers in the groups were over four. Fisher's exact test was used to compare percentages of responders between the two groups. To compare parameters between greater than two electrically subclassified cell types in the same strains, the Dunn's multiple comparison post hoc test was used following the Kruskal-Wallis test. Significance was accepted at P < 0.05.
RESULTS
TRPV1 expression in TG neurons.
To begin, we performed quantitative reverse transcription PCR (qRT-PCR) to determine whether there were transcript differences in Trpv1 mRNA in isolated whole TGs. With qRT-PCR, we failed to detect significant differences between the two strains in Trpv1 transcript levels in TGs (5 animals in each; Fig. 1A). Since previous studies showed differences in TRPV1 expression between IB4-positive and IB4-negative neurons (Dinh et al. 2003; Price and Flores 2007; Dirajlal et al. 2003; Breese et al. 2005; Zwick et al. 2002: Woodbury et al. 2004), we next compared TRPV1 expression in these two neuronal subtypes in the TG neurons isolated from the two strains. As our qRT-PCR results did not allow us to quantify the specific expression levels of Trpv1 in IB4-positive and IB4-negative subpopulations, we relied on immunofluorescence from three animals in each strain (Fig. 1B). In our immunofluorescent studies, TRPV1 immunoreactivity was higher in IB4-positive neurons of C57BL/6 than those of BALB/c (P < 0.05; n = 10/47, 21.3% and 2/48, 4.2%, respectively; Fig. 1C). IB4-binding in TRPV1-immunoreactive neurons were also significantly higher in C57BL/6 than BALB/c (P < 0.05; n = 10/56, 17.9% and 2/48, 4.2%, respectively; Fig. 1C).
Fig. 1.
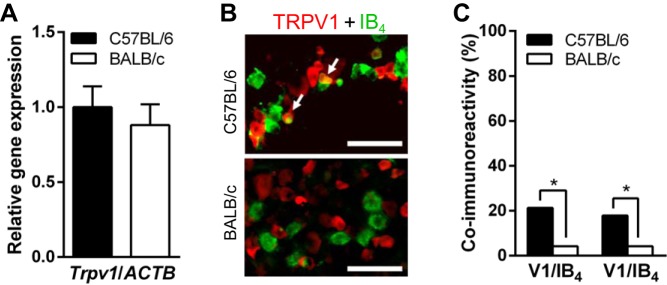
Expression levels of transient receptor potential vanilloid 1 (TRPV1) in mouse trigeminal ganglion (TG) neurons of C57BL/6 and BALB/c. A: similar levels of Trpv1 mRNA in the TG between C57BL/6 and BALB/c strains (5 animals in each). Data are represented as ratio changes against mean value of C57BL/6. B: immunofluorescence of TRPV1 and isolectin B4 (IB4) in the TG from C57BL/6 and BALB/c strains. Arrows indicate coimmunoreactivity of TRPV1 (red) and IB4 (green). C: coimmunoreactivity of TRPV1 in IB4-positive neurons (V1/IB4; C57BL/6, n = 10/47 and BALB/c, n = 2/48, from 3 animals in each strain) and IB4 binding in TRPV1-immunopositive neurons (IB4/V1; C57BL/6, n = 10/56 and BALB/c, n = 2/48). *P < 0.05, Fisher's exact test.
Capsaicin sensitivity in IB4-positive vs IB4-negative TG neurons.
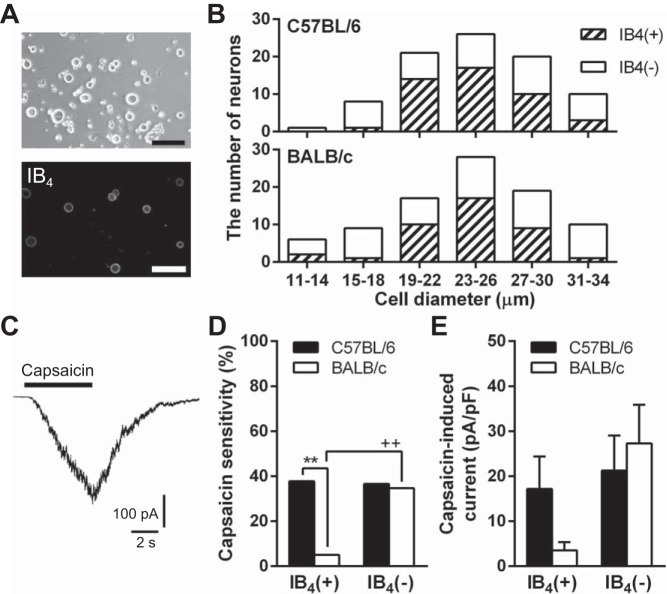
Given the differences in TRPV1 immunoreactivity in the IB4-positive subpopulations, we next determined whether there were differences in sensitivity to the TRPV1-selective agonist capsaicin in the subpopulations of the two strains. In freshly dissociated TG neurons, IB4-positive neurons accounted for ∼30% of total neurons (C57BL/6: n = 28/90, 31% and BALB/c: n = 37/108, 34%, from 3 animals in each strain). To optimize the potential of finding differences in capsaicin sensitivity, we ensured that the IB4-positive and -negative subpopulations were equally represented in whole cell patch-clamp recordings (C57BL/6: n = 45 and 40, respectively, and BALB/c: n = 41 and 49, respectively). There were no discernable differences in cell shape or IB4 staining of acutely dissociated TG neurons between C57BL/6 and BALB/c strains (Fig. 2A). Cell size distributions of IB4-positive and IB4-negative neurons between C57BL/6 and BALB/c were similar (Fig. 2B), indicating morphological equivalence of collected neurons between both strains in our experiments. In 32 of 85 C57BL/6 neurons and 19 of 90 BALB/c neurons, application of 1 μM capsaicin for 5 s induced inward currents with the peak after the end point of the application (Fig. 2C). TG neurons from C57BL/6 had the same proportion of IB4-positive and -negative neurons responding to capsaicin (n = 17/45, 37.8% and 15/40, 37.5%, respectively), but TG neurons from BALB/c had fewer IB4-positive neurons responding to capsaicin than IB4-negative neurons (P < 0.01; n = 2/41, 4.9% and 17/49, 34.7%, respectively; Fig. 2D). There were significantly more capsaicin-sensitive IB4-positive neurons in C57BL/6 than BALB/c (P < 0.01), but there was no strain difference in the proportion of capsaicin-sensitive IB4-negative neurons (Fig. 2D). The electrophysiological results were consistent with the present immunofluorescence results. As shown in Fig. 2E, densities of capsaicin-induced current in IB4-positive neurons seemed to be higher in C57BL/6 (n = 17) than BALB/c (n = 2), but those in IB4-negative neurons were similar between C57BL/6 and BALB/c (n = 15 and 17, respectively).
Fig. 2.
Capsaicin sensitivity in TG neurons of C57BL/6 and BALB/c. A: images are representative bright-field and phase contrast images of TG cells from C57BL/6 and fluorescent IB4 labeling. Scale bars indicate = 100 μm. B: cell size distributions of IB4-positive [IB4(+), shaded bars] and IB4-negative [IB4(−), white bars] neurons of C57BL/6 and BALB/c. C: image depicts a representative capsaicin-induced current in a TG neuron of C57BL/6. The concentration of capsaicin was 1 μM. D: capsaicin sensitivity in IB4(+) and IB4(−) neurons in C57BL/6 (n = 17/45 and 15/40, respectively) and BALB/c (n = 2/41 and 17/49, respectively). **P < 0.01 and ++P < 0.01, between strains and IB4-binding properties in the same strain, respectively, using Fisher's exact test. E: mean densities of capsaicin-induced currents in IB4(+) and IB4(−) neurons in C57BL/6 (n = 17 and 15, respectively) and BALB/c (n = 2 and 17, respectively). Statistical comparison between IB4(+) neurons was not performed because the cell number for BALB/c was only 2.
Electrophysiological properties in IB4-positive vs. IB4-negative TG neurons.
We compared electrophysiological properties, membrane resistance (Rm), resting membrane potential (Em), TOC, and IH (see CP1-induced traces in Fig. 4, B and C, respectively) of dissociated TG neurons between C57BL/6 and BALB/c. These electrophysiological properties in IB4-positive or IB4-negative neurons were similar between the strains (Fig. 3). When comparing IB4-positive and IB4-negative neurons in both strains, we observed some significant differences, namely, higher Rm (P < 0.01; Fig. 3A), lower IH-expressing cell populations (P < 0.01 for both strains; C57BL/6: n = 9/45, 20.0% and 24/40, 60.0% and BALB/c: n = 8/41, 19.5% and 26/49, 53.1%, respectively; Fig. 3C), smaller current densities of IH (P < 0.01 for both strains; C57BL/6: n = 9 and 24 and BALB/c: n = 8 and 26, respectively; Fig. 3D), and higher TOC-expressing cell populations (P < 0.01 for both strains; C57BL/6: n = 35/45, 77.8% and 1/40, 2.5% and BALB/c: n = 31/41, 75.6% and 5/49, 10.2%, respectively; Fig. 3E) in IB4-positive neurons than IB4-negative neurons. The relationships of electrophysiological properties between the two IB4 populations of the two mouse strains were comparable to those of rat TG and DRG neurons, which were classified based on the current signature (Petruska et al. 2000b; 2002; Xu et al. 2010; Ono et al. 2010).
Fig. 3.
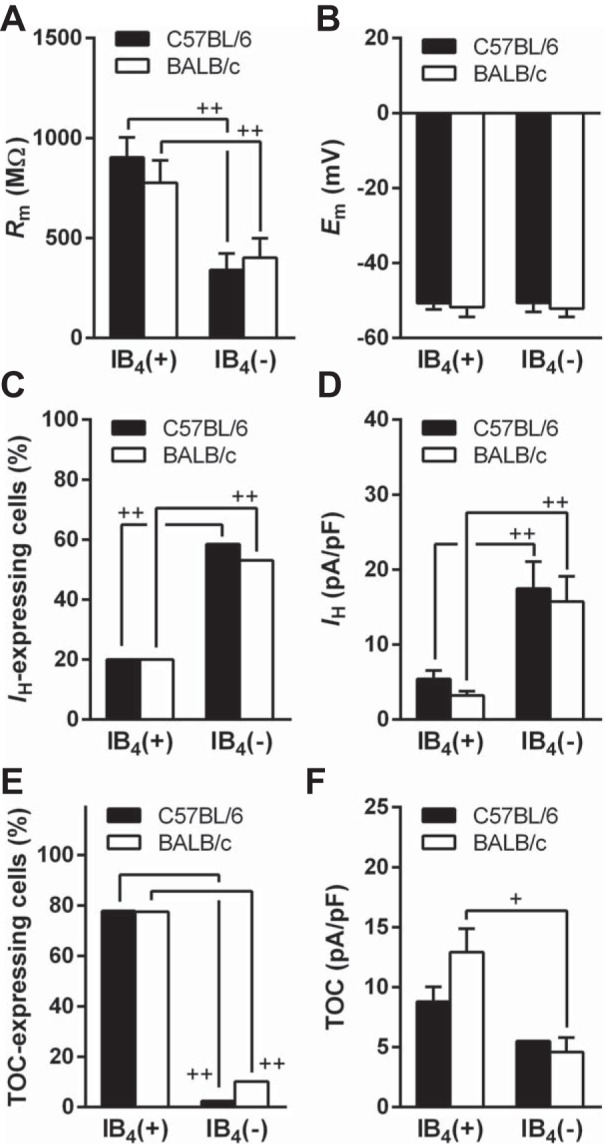
Electrophysiological properties in IB4(+) and IB4(−) TG neurons of C57BL/6 and BALB/c. A: membrane resistance (Rm). IB4(+) and IB4(−) neurons in C57BL/6: n = 45 and 40, respectively, and in BALB/c: n = 41 and 49, respectively. B: resting membrane potential (Em). Cell numbers are the same of A. C: lower hyperpolarization-activated inward current (IH)-showing cell population in IB4(+) neurons than that in IB4(−) neurons of C57BL/6 (n = 9/45 and 24/40, respectively) and BALB/c (n = 8/41 and 26/49, respectively). D: IH current densities of IB4(+) and IB4(−) neurons. IB4(+) and IB4(−) neurons in C57BL/6: n = 9 and 24, respectively, and BALB/c: n = 8 and 26, respectively. E: transient outward current (TOC) in IB4(+) and IB4(−) neurons of C57BL/6 (n = 35/45 and 1/40, respectively) and BALB/c (n = 31/41 and 5/49, respectively). F: TOC densities. +P < 0.05, between IB4(+) and IB4(−) neurons in same strain (C57BL/6: n = 35 and 1, respectively, and BALB/c: n = 31 and 5, respectively). Statistical comparison between IB4(−) neurons was not performed because the cell number for C57BL/6 was only one. In A, D, and F, +P < 0.05 and ++P < 0.01, between IB4(+) and IB4(−) neurons in same strain (Mann-Whitney U-test). In C and E, ++P < 0.01, between IB4(+) and IB4(−) neurons in same strain (Fisher's exact test).
Cell classification by current signature.
We subclassified the mouse TG neurons into 10 cell types (1–9 and 13, similar to rat subclassifications) by modifying the size cutoff to accommodate for the fact that mouse neurons are smaller than rat neurons (Barabas et al. 2012). Because few cells classified to the type 3 (n = 1 in each strain) and the type 5 (C57BL/6: n = 2, and BALB/c: n = 1), these two cell types were eliminated from the following analysis. Representative current signatures in classified cell types of mouse TG neurons are illustrated in Fig. 4.
All type 1 cells (C57BL/6: n = 16 and BALB/c: n = 19) were small in size, did not exhibit TOC or IH, and expressed a slow inward current evoked by CP3 (Fig. 4A). Half of the cells expressed no clear IA component in CP2-evoked current (C57BL/6: n = 10 and BALB/c: n = 8; Fig. 4A) and others expressed a slowly inactivated IA component (data not shown). Approximately 40% of them were IB4 positive in both C57BL/6 (n = 7/16) and BALB/c (n = 7/19) strains. Since the current and IB4-binding profiles were similar to those of rat TG type 1 (Xu et al. 2010; Ono et al. 2010), all these cells were characterized as type 1.
Type 2 cells (C57BL/6: n = 28 and BALB/c: n = 27) were both small and medium in size and displayed TOC expression without IH (Fig. 4B). All cells expressed slow inward current evoked by CP3 and nearly all (C57BL/6: n = 24/28 and BALB/c: n = 26/27) expressed a slowly inactivated IA component in CP2-evoked current (Fig. 4B). The current profiles corresponded to what has been reported for rat TG and DRG type 2 (Petruska et al. 2000b; Xu et al. 2010). Almost all type 2 cells of C57BL/6 (n = 28/28) and BALB/c (n = 24/27) were IB4 positive. Since all rat type 2 cells have been reported to be IB4 positive (Petruska et al. 2000b; Ono et al. 2010), the three IB4-negative cells in BALB/c were excluded from further analyses.
The type 4 cells (C57BL/6: n = 6 and BALB/c: n = 8) were classified as medium in size and displayed IH and fast inward current expressions without TOC (Fig. 4C). All cells were IB4 negative and expressed rapidly inactivated IA component in CP2-evoked current, corresponding to rat TG and DRG type 4 cells (Petruska et al. 2000b; Xu et al. 2010; Ono et al. 2010). Many of the CP2-evoked currents were −40 mV at AT (C57BL/6: n = 4 and BALB/c: n = 6; Fig. 4C), but a few were −20 mV at AT. The latter cases were similar to rat DRG types 14 and 16 in a recent study (Rau et al. 2014) rather than those of rat type 4 cells. Therefore, only the former cases were analyzed as type 4 cells.
Type 6 cells (C57BL/6: n = 6 and BALB/c: n = 3) were defined as medium in size with IH, −40 mV at AT, and slow inward current expressions without TOC (Fig. 4D); this cell type has not been reported in rat TG studies (Xu et al. 2010; Ono et al. 2010). All cells were IB4 negative and many cells expressed rapidly inactivated IA component in CP2-evoked current (C57BL/6: n = 4/6 and BALB/c: n = 2/3; Fig. 4D), which matched to reported rat DRG type 6 cells (Petruska et al. 2000b, 2002). However, a few cells expressed slowly inactivated IA component (C57BL/6: n = 2 and BALB/c: n = 1). Since the number of reliable type 6 cells of BALB/c was not enough (n = 2) to perform statistical analyses, the cell type was excluded from further analyses.
Type 7 cells (C57BL/6: n = 5 and BALB/c: n = 6) were classified as small in size with IH and slow inward current expressions without TOC (Fig. 4E). Some cells showed rounded outward current without clear IA in CP2-evoked current (C57BL/6: n = 4/5 and BALB/c: n = 3/6; Fig. 4E), and others showed slowly inactivated IA expressing a clear peak with AT at −20 or −40 mV. The rounded outward currents were similar to reported properties of rat DRG types 7, 11 and 17 (Petruska et al. 2000b; Rau et al. 2011, 2014) and the latter cases matched to those of rat TG type 7 (Xu et al. 2010). All these cells were IB4 negative, similar to rat TG type 7 (Ono et al. 2010) and DRG types 11 and 17 (Rau et al. 2011, 2014) but inconsistent with rat DRG type 7 (Petruska et al. 2000b). Because of the possibility of misclassification, the type 7 was excluded from further analyses.
Type 8 cells (C57BL/6: n = 5 and BALB/c: n = 6) were classified as medium in size with IH, −20 mV at AT, and slow inward current expressions without TOC (Fig. 4F). Two cells in each strain expressed rapidly inactivated IA component in CP2-evoked current (Fig. 4F), corresponding to rat TG and DRG type 8 cells (Petruska et al. 2000b, 2002; Xu et al. 2010), while others expressed a slowly inactivating IA component. Furthermore, one cell expressing rapidly inactivated IA in each strain was IB4 positive, which is different from rat DRG and TG type 8 cells (Petruska et al. 2000b, 2002; Ono et al. 2010). Since the number of reliable type 8 cells was only one in each strain, the cell type 8 was excluded from further analyses.
All type 9 cells (C57BL/6: n = 9 and BALB/c: n = 9) were defined as medium in cell size with neither TOC nor IH and expressed slow inward current evoked by CP3 (Fig. 4G). Almost all of them were IB4 negative (n = 8/9 in each strain), but one neuron in each strain was IB4 positive, although all TG and DRG type 9 cells have been reported to be IB4 negative (Petruska et al. 2000b, 2002; Ono et al. 2010). Many of them expressed slowly inactivating IA component, showing 0, −20, or −40 mV at ATs in CP2-evoked current (C57BL/6: n = 6/9 and BALB/c: n = 5/9; Fig. 4G) and others expressed no clear IA. The former cases matched to reported rat TG and DRG type 9 (Petruska et al. 2000b; Xu et al. 2010). Therefore, IB4-negative cells expressing slowly inactivated IA were analyzed as type 9 cells in following analyses.
All type 13 cells (C57BL/6: n = 8 and BALB/c: n = 9), except for one C57BL/6 neurons, was classified as medium in size. The cell size is the same as previously reported for TG cell classification (Xu et al. 2010; Ono et al. 2010). All cells exhibited both IH and TOC, slow inward current and slowly inactivated IA component and 0, −20, or −40 mV at ATs, in CP2-evoked current (Fig. 4H). Almost all type 13 cells were IB4 positive in both C57BL/6 and BALB/c (n = 7/8 and 7/9, respectively; Fig. 5). Since the current, cell size and IB4-binding profiles were similar to the parameters reported for rat TG and DRG type 13 (Jiang et al. 2006; Xu et al. 2010), all these cells were analyzed as type 13.
Fig. 5.
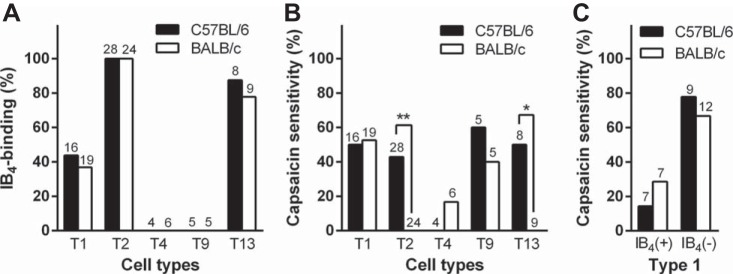
IB4-binding property and capsaicin sensitivity in each TG cell type. A: bar graph depicts IB4-positivity within the different populations of TG neurons for each mouse strain. B: bar graph depicts capsaicin sensitivity within the different populations of TG neurons for each mouse strain. There were strain differences in capsaicin-sensitivity in types 2 and 13 cells. *P < 0.05 and **P < 0.01, between strains (Fisher's exact test). C: capsaicin sensitivity in IB4(+) and IB4(−) subpopulations in type 1. Numbers on the top of bars or open sites on x-axis represent the number of total cells.
Finally, the five reliable cell types in the both mouse strains (types 1, 2, 4, 9, and 13: n = 16, 28, 4, 5, and 8 in C57BL/6 and n = 19, 24, 6, 5, and 9 in BALB/c) were compared by cell sizes and electrophysiological properties. In both C57BL/6 and BALB/c, ∼40% of type 1 cells (n = 7/16 and 7/19, respectively), all type 2 cells (n = 28 and 24, respectively), and ∼80% of type 13 cells (n = 7/8 and 7/9, respectively) were IB4 positive and all type 4 cells (n = 4 and 6, respectively) and type 9 cells (n = 5 and 5, respectively) were IB4 negative (Fig. 5A).
Capsaicin sensitivity in the electrically classified cell types.
In C57BL/6, 40–60% of type 1, 2, 9, and 13 neurons (n = 8/16, 12/28, 3/5 and 4/8, respectively) were capsaicin sensitive and all type 4 neurons (n = 4) were capsaicin insensitive (Fig. 5B). In BALB/c, 40–50% of type 1 and 9 neurons (n = 10/19 and 2/5, respectively) were capsaicin sensitive and almost all type 4 neurons (n = 5/6) were capsaicin insensitive (Fig. 5B), similar to C57BL/6. Different from C57BL/6, all types 2 and 13 neurons from BALB/c were capsaicin insensitive (Fig. 5B). There were strain differences in capsaicin sensitivity for type 2 (P < 0.01) and 13 neurons (P < 0.05); these types showed IB4-binding. In type 1, there was no significant difference in current densities of capsaicin-induced currents between C57BL/6 and BALB/c (29.8 ± 14.6 and 36.0 ± 13.1 pA/pF, respectively). Since the type 1 was a mixed group with different IB4-binding properties (Fig. 5A), the cell type was further divided into IB4-positive and IB4-negative subgroups, similar to a previous rat TG study (Ono et al. 2010). In both IB4-positive and IB4-negative type 1 subgroups, the proportions of capsaicin sensitivity were similar (C57BL/6: n = 1/7, 14.3% and 7/9, 77.8%, respectively and BALB/c: n = 2/7, 28.6% and 8/12, 66.7%, respectively; Fig. 5C). The current densities of capsaicin-induced currents in IB4-negative type 1 subgroup between C57BL/6 and BALB/c were similar (33.6 ± 16.3 pA/pF, n = 7 and 44.1 ± 15.1 pA/pF, n = 8, respectively).
Electrophysiological properties in the electrically classified cell types.
With regard to cell size and electrophysiological parameters, we failed to detect any statistical significances between C57BL/6 and BALB/c (Table 1), indicating no large strain differences in cell size and basic electrophysiological properties. Cell sizes of type 1 in both C57BL/6 and BALB/c were significantly smaller than many other cell types (P < 0.01 against types 2, 4, 9, and 13 in both strains). Similarly, membrane capacitance (Cm) of type 1 was significantly smaller than that of other cell types (P < 0.01 against types 2, 4, and 13 in both strains and against type 9 in C57BL/6). Since Rm is relatively dependent on cell membrane surface, small-sized type 1 and small/medium-sized type 2 showed higher Rm than other cell types of medium sizes (P < 0.01 for type 2 against types 4 and 13 in C57BL/6; P < 0.05 for type 2 against type 4 in BALB/c). Em of type 4 in both strains showed the lowest value among these cell types. Current densities of IH in type 13 in both strains were significantly lower than those of type 4 (P < 0.01 for both stains). Current densities of TOC in type 13 were smaller than those in type 2 (P < 0.01 and 0.05 in C57BL/6 and BALB/c, respectively).
Table 1.
Size and electrophysiological properties in TG neurons classified based on current signature
| Type/Strains | n | Size (range), μm | Cm, pF | Rm, MΩ | Em, mV | IH, pA/pF | TOC, pA/pF |
|---|---|---|---|---|---|---|---|
| Type 1 | |||||||
| C57BL/6 | 16 | 18.8 ± 0.5 (15–22) | 8.5 ± 0.8 | 740 ± 213 | −49.5 ± 3.4 | — | — |
| BALB/c | 19 | 17.2 ± 0.8 (12–22) | 8.3 ± 1.1 | 946 ± 219 | −56.2 ± 3.0 | — | — |
| Type 2 | |||||||
| C57BL/6 | 28 | 25.2 ± 0.7 (20–32)* | 15.1 ± 0.7* | 1178 ± 97 | −54.6 ± 1.9 | — | 9.9 ± 1.4‡ |
| BALB/c | 24 | 24.5 ± 0.5 (20–29)* | 14.9 ± 1.3* | 937 ± 137 | −52.5 ± 3.7 | — | 14.9 ± 2.3‡ |
| Type 4 | |||||||
| C57BL/6 | 4 | 30.3 ± 2.3 (24–34)* | 24.0 ± 3.2* | 48 ± 7† | −61.8 ± 3.9 | 23.9 ± 5.6‡ | — |
| BALB/c | 6 | 28.7 ± 1.2 (26–34)* | 20.4 ± 1.9* | 87 ± 34† | −56.5 ± 5.8 | 29.2 ± 11.6‡ | — |
| Type 9 | |||||||
| C57BL/6 | 5 | 27.2 ± 1.3 (25–32)* | 19.4 ± 2.0* | 288 ± 115 | −54.8 ± 7.8 | — | — |
| BALB/c | 5 | 29.4 ± 1.8 (23–33)* | 16.1 ± 3.0 | 100 ± 33 | −41.4 ± 3.8 | — | — |
| Type 13 | |||||||
| C57BL/6 | 8 | 26.8 ± 1.1 (22–32)* | 17.4 ± 1.0* | 196 ± 87† | −46.9 ± 3.2 | 4.7 ± 0.8 | 4.6 ± 1.3 |
| BALB/c | 9 | 28.0 ± 0.9 (23–31)* | 17.6 ± 1.1* | 290 ± 124 | −46.0 ± 4.6 | 3.4 ± 0.5 | 7.6 ± 1.4 |
Values represent means ± SE; n is the cell number for each cell type in either the C57BL/6 or BALB/c mouse strain.
TG, trigeminal ganglion; Cm, membrane capacitance; Rm, membrane resistance; Em, resting membrane potential; TOC, transient outward current; IH, hyperpolarization-activated inward current.
There were no significant differences in these electrophysiological parameters of each cell type between strains (Mann-Whitney U-test).
Significantly larger than type 1 (Dunn's multiple post hoc test following Kruskal-Wallis test).
Significantly smaller than type 2 (Dunn's multiple post hoc test following Kruskal-Wallis test).
Significantly larger than type 13 (Mann-Whitney U-test).
Thermal nociceptive thresholds.
We analyzed the public database to identify C57BL/6 and BALB/c differences in thermal thresholds. In the Mogil1 project, tail and paw withdrawal latencies to heat in male C57BL/6 were significantly shorter than those in male BALB/c (Table 2). In the Jaxwest1 project, paw withdrawal latencies in male and female C57BL/6 were significantly shorter than those in male and female BALB/c (Table 2). To validate these strain differences, we performed our own thermal nociceptive testing (paw and head: 5 and 15 animals, respectively, in each strain). Withdrawal latencies of the paw and the head in female C57BL/6 were significantly shorter than those in female BALB/c (Table 2). From these collective results, C57BL/6 universally demonstrated higher thermal sensitivity than BALB/c, independent of assay, anatomical location, and sex. Furthermore, by systemic injection of the TRPV1-selective antagonist JNJ-17203212, the head withdrawal latencies to heat stimulation were significantly prolonged in both C57BL/6 and BALB/c (P < 0.01 and P < 0.05, respectively, 6 animals in each strain; Fig. 6A). Percent changes of the withdrawal latencies were similar between C57BL/6 and BALB/c (144 ± 10 and 142 ± 10%, respectively), and they were significantly larger than control injection groups (P < 0.01, 96 ± 7% and P < 0.05, 99 ± 10%, respectively, 6 animals in each strain). The results suggest that the thermal pain behavior is mediated by activation of TRPV1 in both mouse strains.
Table 2.
Comparison of thermal pain sensation between C57BL/6 and BALB/c
| C57BL/6J (s) |
BALB/cJ (s) |
|||||
|---|---|---|---|---|---|---|
| Project/Pain Tests | Sex | Regions | n | Means ± SE | n | Means ± SE |
| Mogil1 | ||||||
| Tail-flick test, 47.5°C | Male | Tail | 5 | 5.8 ± 0.6 | 5 | 8.7 ± 0.5* |
| Tail-flick test, 49°C | Male | Tail | 10 | 2.2 ± 0.1 | 10 | 4.6 ± 0.2* |
| Hargreaves' test | Male | Hind paw | 6 | 8.2 ± 0.3 | 6 | 16.3 ± 1.7* |
| Hot-plate test | Male | Hind paw | 8 | 19.3 ± 1.7 | 8 | 32.7 ± 3.7* |
| Jaxwest1 | ||||||
| Hot-plate test | Male | Hind paw | 8 | 5.5 ± 0.7 | 8 | 11.1 ± 1.3* |
| Hot-plate test | Female | Hind paw | 8 | 6.4 ± 0.6 | 8 | 10.6 ± 1.1* |
| This study | ||||||
| Hargreaves' test | Female | Hind paw | 15 | 3.4 ± 0.2 | 15 | 7.5 ± 0.3* |
| Hargreaves' test | Female | Whisker pad | 15 | 2.4 ± 0.1 | 15 | 8.3 ± 0.5* |
Values represent means ± SE; n = number of animals. Thermal pain behavior data from the “Heritability of Nociception Project,” which is publicly available (http://www.jax.org/phenome).
P < 0.01, with Mann-Whitney U test.
Fig. 6.
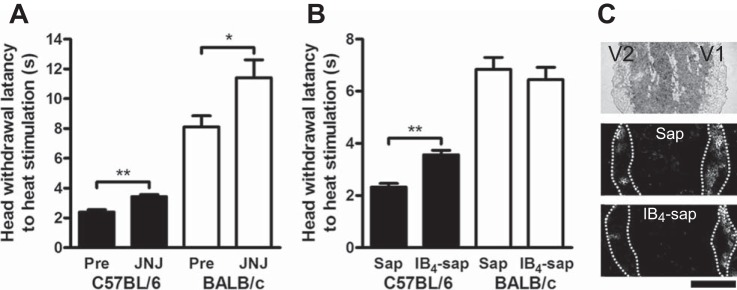
Thermal nociception following antagonism of TRPV1 and IB4-positive neurons. A: effects of a selective TRPV1 antagonist on thermal pain sensation. Compared with preinjection levels (Pre), head withdrawal latencies to heat stimulation were significantly prolonged in both C57BL/6 and BALB/c (6 animals in each) after systemic injection of the selective TRPV1 antagonist JNJ-17203212 (JNJ). *P < 0.05 and **P < 0.01, respectively, between Pre and JNJ (Mann-Whitney U-test). B: effects of a selective reduction of IB4-neurons on thermal pain sensation in C57BL/6 and BALB/c (5 animals per each drug group in each strain). Compared with unconjugated saporin-treated mice (Sap), IB4-saporin-treated (IB4-sap) C57BL/6 showed significantly longer head withdrawal latency to heat stimulation. **P < 0.01, between Sap and IB4-sap (Mann-Whitney U test). C: relative fluorescent intensities of the trigeminal second branch area (V2) and the first branch area (V1) in IB4-stained TG sections in unconjugated saporin-treated mice (5 animals in each strain) were 98 ± 16 and 101 ± 20% in C57BL/6 and BALB/c, respectively. However, relative fluorescent intensities in IB4-saporin-treated mice (5 animals in each strain) were low (29 ± 8 and 37 ± 7%, P < 0.01 and 0.05, respectively, Mann-Whitney U-test). Scale bar = 1 mm.
Contributions of IB4-positive neurons to heat nociception.
From the immunofluorescence and electrophysiological results, differential TRPV1 expression in IB4-positive neurons might contribute to thermal pain sensation differences between C57BL/6 and BALB/c. To examine the possibility, we investigated the effect of the selective IB4-binding cell toxin IB4-saporin on thermal nociceptive behavior. Compared with unconjugated saporin-treated mice (5 animals in each group), IB4-saporin-treated C57BL/6 showed significantly longer head withdrawal latency to heat stimulation (P < 0.01), but IB4-saporin-treated BALB/c showed similar head withdrawal latency (5 animals in each group; Fig. 6C). From the all animals, the horizontal sections of ipsilateral TG were stained with IB4-fluorescein isothiocyanate to confirm removal of the IB4-positive sensory neurons in the V2 area by the IB4-saporin treatment. Although relative fluorescent intensities of the V2 vs. V1 in IB4-stained TG sections in unconjugated saporin-treated mice were 98 ± 16 and 101 ± 20% in C57BL/6 and BALB/c, respectively, those in IB4-saporin-treated mice were lower (29 ± 8 and 37 ± 7%, P < 0.01 and 0.05, respectively; Fig. 6C). The result indicates that treatment with IB4-saporin into the infraorbital foramen largely reduced IB4-positive neurons in V2 area, which innervates the whisker pad.
DISCUSSION
In this study, we evaluated TRPV1 expression and function in subsets of TG neurons from two inbred mouse strains, C57BL/6 and BALB/c, which exhibited differences in thermal nociceptive behavior. Our immunofluorescence and electrophysiological studies demonstrated that TRPV1 was expressed significantly higher in IB4-positive TG neurons of C57BL/6 than BALB/c; however, cell morphology, IB4-binding properties, and electrophysiological parameters were similar between the two strains. Thermal nociceptive behavior was determined using data from two publicly available datasets and then validated using our original behavioral data. The finding that C57BL/6 had higher thermal nociception than BALB/c regardless of the nocifensive behavioral assay used, the region tested or the sex of the animal suggests that cutaneous thermal nociception among inbred mouse strains is genetically mediated. Removal of IB4-binding TG neurons using IB4-conjugated saporin reduced thermal pain sensitivity in the whisker pad of only C57BL/6, suggesting that IB4-positive sensory afferents contribute to cutaneous nociceptive heat sensation in C57BL/6 but not BALB/c.
Regulation of TRPV1 expression.
Although there was a difference in detection levels of TRPV1 in IB4-positive neurons between immunocytochemistry (21.3%) and electrophysiology (37.8%), the two different methods demonstrated higher expression of TRPV1 in IB4-positive neurons of C57BL/6 than those of BALB/c. These results suggest that the inconsistency in TRPV1 expression in IB4-positive DRG neurons in early studies using electrophysiological or immunofluorescence (Dirajlal et al. 2003; Breese et al. 2005; Zwick et al. 2002: Woodbury et al. 2004) is due to strain differences rather than methodological differences. According to the mouse genome database (http://phenome.jax.org/), there are two nonsynonymous and 16 synonymous single nucleotide polymorphisms (SNPs) in the Trpv1 gene of C57BL/6 and BALB/c mice. We failed to detect a significant difference in Trpv1 levels in the TG between the strains. Possibly, the SNPs do not affect transcriptional levels of the gene. If the SNPs impact protein translation, stability, or translocation to cell membrane, then differences in TRPV1 expression would be detected in IB4-negative neurons and not just IB4-positive neurons of C57BL/6 and BALB/c. Therefore, the SNPs of C57BL/6 and BALB/c do not explain strain difference in TRPV1 expression in IB4-positive neurons. In a previous study (Zwick et al. 2002) the species difference in TRPV1 expression in IB4-positive neurons between mice and rats has been attributed to differential expression of glial cell-derived neurotrophic factor (GDNF) receptors. GDNF receptor activation on IB4-positive neurons induces upregulation of TRPV1 protein in rats (Amaya et al. 2004). The absence of the GDNF receptor accessory protein of c-Ret has been proposed to explain differences in hair follicles between BALB/c and C57BL/6 (Kato et al. 2001). Therefore, such impairment of c-Ret signaling in BALB/c may be related to the lower TRPV1 expression in IB4-positive neurons. However, further experiments are needed to determine the mechanism in which c-Ret controls TRPV1 protein expression in IB4-positive neurons in mice.
Characteristics of mouse cell types.
We classified mouse primary sensory neurons using the current signature method. However, when comparing to previously reported TG and DRG cell types, we found some exceptions or misclassified cells in each classified cell group. Recent DRG studies have reported six additional new cell types, 11, 12, 14, 16, 17, and 18; although the current signature method still cannot classify all sensory neurons (Rau et al. 2011 2014). Therefore, not surprisingly, there were exceptions in some cell types in the present study. Misclassified cells in types 4 and 7 likely could be reclassified into newly defined cell types (Rau et al. 2014). Finally, we were able to compare the five reliable cell groups, types 1, 2, 4, 9, and 13; moreover, we had enough cell numbers for statistical analyses, in both C57BL/6 and BALB/c strains. Table 3 shows a summary of properties of the mouse cell types with rat cell types. Based on higher Rm and Em values in mouse unmyelinated TG neurons relative to myelinated neurons that were identified by conduction velocities (López de Armentia et al. 2000), the types 1 and 2 cells that exhibit high Rm and Em values are presumed to be unmyelinated neurons and the type 4 cells that exhibit low values of Rm and Em are presumed to be myelinated neurons (Table 3). Based on these presumptions and IB4-binding properties, we suggest that types 1, 2, and 13 cells are unmyelinated neurons and the types 4 and 9 are myelinated neurons. Furthermore, DRG types 2 and 4 have been reported to be immunoreactive negative and immunoreactive positive for neurofilament, a myelination marker, respectively (Petruska et al. 2000a,b; 2002), supporting the presumed myelination status. In DRG studies (Petruska et al. 2000b, 2002), type 1 and 9 cells express CGRP and/or substance P (SP) and type 2 and 4 do not express these neuropeptides; there are no reports on CGRP and/or SP expression available in type 13 cells (Table 3). Together with presumed myelination status, type 1 and 9 cells are probably CGRP-expressing unmyelinated and myelinated neurons, respectively, and type 2 and 4 cells are CGRP/SP-negative unmyelinated and myelinated neurons, respectively. Approximately half of the types 1, 2, 9, and 13 cells were capsaicin sensitive. Since the capsaicin sensitivities in these cell types were considerably lower than reported proportions in rat TG and DRG cell types (70–100%; Petruska et al. 2000b, 2002; Xu et al. 2012; Ono et al. 2012), TRPV1 expression in sensory neurons show species, and not only strain, differences. Almost all type 4 cells, except for one neurons in BALB/c, were capsaicin insensitive, corresponding to rat TG and DRG type 4 cells (Petruska et al. 2000b, 2002; Xu et al. 2010; Ono et al. 2010).
Table 3.
Summary of mouse TG cell types and previously reported properties in rats
| Capsaicin Sensitivity |
Previously Reported Properties |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Cell Size | Rm | Em | IB4 | C57BL/6 | BALB/c | Rats | CGRP | SP | Innervation |
| 1 | S | High | High | ± | ± | ± | ± | + | − | NR |
| 2 | S/M | High | High | + | ± | −* | +† | − | − | H-skin |
| 4 | M | Low | Low | − | − | − | − | − | − | H-skin |
| 9 | M | Low | High | − | ± | ± | +† | + | ± | H-skin |
| 13 | M | Low | High | + | ± | −* | +† | NR | NR | G-skin |
For cell size, 11- to 22-μm and 23- to 34-μm cell diameter represent small (S) and medium (M), respectively. Findings for capsaicin sensitivity are relative to previous studies for rat TG neurons (Xu et al. 2010; Ono et al. 2010). The remaining properties are from previous studies on rat dorsal root ganglion (DRG) neurons (Petruska et al. 2000a,b, 2002; Rau et al. 2005, 2006, 2007, 2014; Jiang et al. 2006, 2013; Jiang and Cooper 2011).
IB4, isolectin B4; CGRP, calcitonin gene-related polypeptide; SP, substance P.
“+” And “−” indicate the presence and absence, respectively. NR indicates no report. H-skin and G-skin indicate hairy and glabrous skins, respectively. Note that there is a difference in capsaicin sensitivity in type 1 between Wister TG(±) and Sprague-Dawley DRG(+).
Difference for C57BL/6.
Difference for both mouse strains.
Heat-sensitive sensory afferents in skin.
Rat DRG type 2, 4, and 9 cells have been reported to predominantly innervate the hairy skin and rat type 13 cells have been reported to innervate the glabrous skin; rat DRG type 1 cells have not been traced from skin (Table 3; Jiang et al. 2006). Hence, types 2, 4, 9, and 13 cells likely contribute to heat sensitivity in cutaneous tissues. Therefore, TRPV1 activation in unmyelinated afferents of types 2 and 13 cells likely results in higher cutaneous heat nociception in C57BL/6 than BALB/c. In the present study, strain differences in thermal nociception remained even after administration of the TRPV1 antagonist and after treatment of IB4-saporin. The results suggest that an additional mechanism not involving TRPV1 in IB4-positive neurons contributes to cutaneous thermal nociception. Since TRPV2 and TRPM3 (>30°C) have been reported to impact thermal nociception (Caterina et al. 1999; Vriens et al. 2011), differential expression of these channels might also contribute to strain differences in thermal thresholds. Other alternative mechanism might involve CGRP. CGRP accounts for thermal nociceptive differences in the hind paw between C57BL/6 and AKR mouse strains (Mogil et al. 2005). Therefore, while there were no strain differences in capsaicin sensitivity in type 9 cells, which are presumed CGRP-expressing myelinated afferents (Table 3), differences in central and peripheral CGRP release from type 9 cells might partly contribute to skin thermal nociceptive differences between C57BL/6 and BALB/c.
Conclusion.
Based on our results we infer that differential TRPV1 expression in IB4-positive unmyelinated neurons, types 2 and 13, account for differences in cutaneous thermal nociception between C57BL/6 and BALB/c. Such high genetic diversity in IB4-positive neurons may correlate to high adaptability in skin nociceptive function for variable living conditions. TRPV1 expression in IB4-positive neurons in C57BL/6 has been reported to be upregulated by inflammation without a change in IB4-negative neurons (Breese et al. 2005), suggesting that IB4-positive neurons can respond to changes in environmental conditions. On the other hand, electrophysiological properties in sensory neurons are highly conserved among mice and rats, compared with nociceptive channel expressions. These results help us understand the mechanism underlying individual pain differences in humans.
GRANTS
This work was funded by National Institute of Dental and Craniofacial Research Grants R21-DE-018561 and R01-DE-19796 (to B. L. Schmidt).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.O., Y.Y., C.T.V., and D.D. performed experiments; K.O., Y.Y., and C.T.V. analyzed data; K.O. prepared figures; K.O., Y.Y., and C.T.V. drafted manuscript; Y.Y. and B.L.S. interpreted results of experiments; B.L.S. conception and design of research; B.L.S. edited and revised manuscript; B.L.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Ronald R. Campbell for technical assistance and Kiyotoshi Inenaga for English proofing the electrophysiological parts.
REFERENCES
- Amaya F, Shimosato G, Nagano M, Ueda M, Hashimoto S, Tanaka Y, Suzuki H, Tanaka M. NGF and GDNF differentially regulate TRPV1 expression that contributes to development of inflammatory thermal hyperalgesia. Eur J Neurosci 20: 2303–2310, 2004. [DOI] [PubMed] [Google Scholar]
- Barabas ME, Kossyreva EA, Stucky CL. TRPA1 is functionally expressed primarily by IB4-binding, non-peptidergic mouse and rat sensory neurons. PLoS One 7: e47988, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 139: 267–284, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese NM, George AC, Pauers LE, Stucky CL. Peripheral inflammation selectively increases TRPV1 function in IB4-positive sensory neurons from adult mouse. Pain 115: 37–49, 2005. [DOI] [PubMed] [Google Scholar]
- Cardenas CG, Del Mar LP, Scroggs RS. Variation in serotinergic inhibition of calcium channel currents in four types of rat sensory neurons differentiated by membrane properties. J Neurophysiol 74: 1870–1879, 1995. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398: 436–441, 1999. [DOI] [PubMed] [Google Scholar]
- Dinh QT, Groneberg DA, Mingomataj E, Peiser C, Heppt W, Dinh S, Arck PC, Klapp BF, Fischer A. Expression of substance P and vanilloid receptor (VR1) in trigeminal sensory neurons projecting to the mouse nasal mucosa. Neuropeptides 37: 245–250, 2003. [DOI] [PubMed] [Google Scholar]
- Dirajlal S, Pauers LE, Stucky CL. Differential response properties of IB4-positive and -negative unmyelinated sensory neurons to protons and capsaicin. J Neurophysiol 89: 513–524, 2003. [DOI] [PubMed] [Google Scholar]
- Furuse T, Blizard DA, Moriwaki K, Miura Y, Yagasaki K, Shiroishi T, Koide T. Genetic diversity underlying capsaicin intake in the Mishima battery of mouse strains. Brain Res Bull 57: 49–55, 2002. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32: 77–88, 1988. [DOI] [PubMed] [Google Scholar]
- Huang SM, Li X, Yu Y, Wang J, Caterina MJ. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol Pain 7: 37, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N, Rau KK, Johnson RD, Cooper BY. Proton sensitivity Ca2+ permeability and molecular basis of acid-sensing ion channels expressed in glabrous and hairy skin afferents. J Neurophysiol 95: 2466–2478, 2006. [DOI] [PubMed] [Google Scholar]
- Kato M, Takeda K, Kawamoto Y, Tsuzuki T, Dai Y, Nakayama S, Toriyama K, Tamada Y, Takahashi M, Nakashima I. RET tyrosine kinase enhances hair growth in association with promotion of melanogenesis. Oncogene 20: 7536–7541, 2001. [DOI] [PubMed] [Google Scholar]
- Lariviere WR, Wilson SG, Laughlin TM, Kokayeff A, West EE, Adhikari SM, Wan Y, Mogil JS. Heritability of nociception. III. Genetic relationships among commonly used assays of nociception and hypersensitivity. Pain 97: 75–86, 2002. [DOI] [PubMed] [Google Scholar]
- López de Armentia M, Cabanes C, Belmonte C. Electrophysiological properties of identified trigeminal ganglion neurons innervating the cornea of the mouse. Neuroscience 101: 1109–1115, 2000. [DOI] [PubMed] [Google Scholar]
- MacGregor AJ, Griffiths GO, Baker J, Spector TD. Determinants of pressure pain threshold in adult twins: evidence that shared environmental influences predominate. Pain 73: 253–257, 1997. [DOI] [PubMed] [Google Scholar]
- Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc 2: 152–160, 2007. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Adhikari SM. Hot and cold nociception are genetically correlated. J Neurosci 19: RC25, 1999a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Miermeister F, Seifert F, Strasburg K, Zimmermann K, Reinold H, Austin JS, Bernardini N, Chesler EJ, Hofmann HA, Hordo C, Messlinger K, Nemmani KV, Rankin AL, Ritchie J, Siegling A, Smith SB, Sotocinal S, Vater A, Lehto SG, Klussmann S, Quirion R, Michaelis M, Devor M, Reeh PW. Variable sensitivity to noxious heat is mediated by differential expression of the CGRP gene. Proc Natl Acad Sci USA 102: 12938–12943, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain 80: 67–82, 1999b. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. Heritability of nociception II. “Types” of nociception revealed by genetic correlation analysis. Pain 80: 83–93, 1999c. [DOI] [PubMed] [Google Scholar]
- Nielsen CS, Knudsen GP, Steingrímsdóttir ÓA. Twin studies of pain. Clin Genet 82: 331–340, 2012. [DOI] [PubMed] [Google Scholar]
- Nielsen CS, Stubhaug A, Price DD, Vassend O, Czajkowski N, Harris JR. Individual differences in pain sensitivity: genetic and environmental contributions. Pain 136: 21–29, 2008. [DOI] [PubMed] [Google Scholar]
- Ono K, Xu S, Inenaga K. Isolectin B4 binding in populations of rat trigeminal ganglion cells. Neurosci Lett 486: 127–131, 2010. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Cooper BY, Gu JG, Rau KK, Johnson RD. Distribution of P2X1, P2X2, and P2X3 receptor subunits in rat primary afferents: relation to population markers and specific cell types. J Chem Neuroanat 20: 141–162, 2000a. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Napaporn J, Johnson RD, Cooper BY. Chemical responsiveness and histochemical phenotype of electrophysiologically classified cells of the adult rat dorsal root ganglion. Neuroscience 115: 15–30, 2002. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Napaporn J, Johnson RD, Gu JG, Cooper BY. Subclassified acutely dissociated cells of rat DRG: histochemistry and patterns of capsaicin-, proton-, and ATP-activated currents. J Neurophysiol 84: 2365–2379, 2000b. [DOI] [PubMed] [Google Scholar]
- Price TJ, Flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain 8: 263–272, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau KK, Jiang N, Johnson RD, Cooper BY. Tissue specific distribution of nociceptors with nicotinic acetylcholine receptors. In: Pharmacology of Nicotinic Acetylcholine Receptors from the Basic and Therapeutic Perspectives, edited by Arias HR. Kerala, India: Research Signpost, 2011, p. 223–245. [Google Scholar]
- Rau KK, Petruska JC, Cooper BY, Johnson RD. Distinct subclassification of DRG neurons innervating the distal colon and glans penis/distal urethra based on the electrophysiological current signature. J Neurophysiol 112: 1392–1408, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Ueno S, Koizumi S, Ueda H, Iwanaga T, Inoue K. Downregulation of P2X3 receptor-dependent sensory functions in A/J inbred mouse strain. Eur J Neurosci 15: 1444–1450, 2002. [DOI] [PubMed] [Google Scholar]
- Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, Benoit M, Xue F, Janssens A, Kerselaers S, Oberwinkler J, Vennekens R, Gudermann T, Nilius B, Voets T. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 70: 482–494, 2011. [DOI] [PubMed] [Google Scholar]
- Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci 24: 6410–6415, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Ono K, Inenaga K. Electrophysiological and chemical properties in subclassified acutely dissociated cells of rat trigeminal ganglion by current signatures. J Neurophysiol 104: 3451–3461, 2010. [DOI] [PubMed] [Google Scholar]
- Ye Y, Bae SS, Viet CT, Troob S, Bernabé D, Schmidt BL. IB4(+) and TRPV1(+) sensory neurons mediate pain but not proliferation in a mouse model of squamous cell carcinoma. Behav Brain Funct 10, 5, 2014a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Dang D, Viet CT, Dolan JC, Schmidt BL. Analgesia targeting IB4-positive neurons in cancer-induced mechanical hypersensitivity. J Pain 13: 524–531, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Ono K, Bernabé DG, Viet CT, Pickering V, Dolan JC, Hardt M, Ford AP, Schmidt BL. Adenosine triphosphate drives head and neck cancer pain through P2X2/3 heterotrimers. Acta Neuropathol Commun 2: 62, 2014b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick M, Davis BM, Woodbury CJ, Burkett JN, Koerber HR, Simpson JF, Albers KM. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci 22: 4057–4065, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]