Abstract
Normal brain function depends on a dynamic balance between local specialization and large-scale integration. It remains unclear, however, how local changes in functionally specialized areas can influence integrated activity across larger brain networks. By combining transcranial magnetic stimulation with resting-state functional magnetic resonance imaging, we tested for changes in large-scale integration following the application of excitatory or inhibitory stimulation on the human motor cortex. After local inhibitory stimulation, regions encompassing the sensorimotor module concurrently increased their internal integration and decreased their communication with other modules of the brain. There were no such changes in modular dynamics following excitatory stimulation of the same area of motor cortex nor were there changes in the configuration and interactions between core brain hubs after excitatory or inhibitory stimulation of the same area. These results suggest the existence of selective mechanisms that integrate local changes in neural activity, while preserving ongoing communication between brain hubs.
Keywords: connectivity, modularity, brain network, fMRI, TMS, hubs
the human brain is a complex network segregated into functionally specialized neural populations. Those populations with strong internal interactions and weak external associations are defined as modules (Park and Friston 2013). A module includes a subset of nodes (regions) of the network that show a high level of internal communication and a relatively low level of association with other modules of the brain (Fig. 1) (van den Heuvel and Sporns 2013b). Brain modules emerge at an intermediate scale between regional specialization and large-scale integration (Park and Friston 2013; Raichle 2011) and as such are crucial for normal brain function (Crossley et al. 2013). For example, noninvasive neuroimaging studies in healthy humans have shown that the reconfiguration of interactions between brain modules is critical for cognitive flexibility, including task-based recollection (Fornito et al. 2012).
Fig. 1.
Nodes, modules, and rich club hubs. Nodes are network elements (e.g., brain regions, depicted as gray dots). In the context of this article, modules (in blue, brown, and yellow) are defined as clusters of brain regions (nodes) with strong internal interactions and weak external associations. Interactions between modules is supported by brain hubs (purple dots), i.e., regions that possess dense interconnections with other areas of the brain. Recent findings further suggest that communication across brain modules is disproportionately supported by a relatively small set of highly interconnected brain hubs, known as the “rich club” (van den Heuvel et al. 2011, 2012).
The mechanisms underlying functional reorganization of whole brain dynamics at the modular scale remain unclear. Specifically, it is not known whether transient changes in the specialized activity of a local brain region are sufficient to induce reorganization of intrinsic modular dynamics in the human brain. An intriguing possibility is that opposing effects of local inhibition vs. excitation can selectively alter intrinsic neural dynamics between and within functionally specialized large-scale brain modules. This hypothesis is supported by the results of empirical and simulation studies, which suggest that the functional effects of focal changes in neural activity may extend outside a functionally segregated network (Alstott et al. 2009; Bestmann et al. 2004; Massimini et al. 2005; Valero-Cabre et al. 2005; van Dellen et al. 2013). Likewise, a number of findings from human (Bestmann et al. 2010; Eldaief et al. 2011) and animal (Logothetis et al. 2010; Sato et al. 2014; Tolias et al. 2005) research have suggested that local changes in neural activity may impact integration within segregated functional neural networks. Because large-scale modules are positioned at an intermediate scale between local and global integration, they may play a critical role in integrating local changes without reorganizing the backbone of large-scale brain communication. Such module-specific plasticity in brain dynamics may be an important mechanism for optimally integrating local changes in neural activity without influencing other critical brain processes.
Here we combined resting-state functional magnetic neuroimaging (rsfMRI), transcranial magnetic stimulation (TMS), and network-based analyses to examine the impact of local changes in brain activity on intrinsic whole brain dynamics. Specifically, we applied inhibitory or excitatory theta-burst stimulation (TBS) (Huang et al. 2005) to the right primary motor cortex in healthy human participants and measured consequent changes in rsfMRI brain dynamics at the modular scale (see Fig. 1). The motor cortex was selected as the stimulation site because it can yield a measure of TMS-induced changes in local neural excitability, the motor-evoked potential (MEP) (Hoogendam et al. 2010). To test the hypothesis that modular dynamics are critical for integrating local peripheral changes in neural activity while preserving ongoing global communication across the brain, we also verified the impact of focal TMS on “rich club” topology and dynamics (Fig. 1). The rich club is a set of densely interconnected and energetically costly brain regions (hubs) that support the bulk of large-scale brain communication (Collin et al. 2014; van den Heuvel et al. 2012; van den Heuvel and Sporns 2011). Rich club hubs are thus positioned at the highest level of the connectional hierarchy. We predicted that TMS-induced changes in neural activity would significantly impact at the intermediate level of modular dynamics, while leaving unaffected the rich club backbone on which these are supported.
MATERIALS AND METHODS
Participants
Twenty-six right-handed healthy participants commenced the study, but two were excluded due to claustrophobia (n = 1) and intolerance to theta TBS (n = 1). An additional participant was excluded following preliminary data quality checks (i.e., head movement >2 mm during resting-state fMRI). For the remaining 23 participants (average age 22 yr, ±SD 3 yr; 12 females), resting-state fMRI (rsfMRI) data were obtained to assess the effect of TMS on large-scale brain dynamics.
For each experimental session (Fig. 2), participants were reimbursed 20 AUD. The study was approved by The University of Queensland Human Research Ethics Committee and was conducted according to the Declaration of Helsinki. Written informed consent was obtained for all participants.
Fig. 2.

Experimental design for the theta-burst stimulation (TBS) and resting-state functional neuroimaging (rsfMRI) protocols. Participants undertook 2 experimental sessions comprising rsfMRI and TBS. The 2 sessions were scheduled at least 24 h apart. MEPs, motor-evoked potentials; cTBS, continuous TBS (inhibitory); iTBS, intermittent TBS (excitatory); T1, T1-weighted image.
General Experimental Design
Resting state fMRI data were acquired using a Siemens TRIO 3 Tesla MR scanner fitted with a 32-channel head coil. TMS was delivered using a Magstim Super Rapid TMS machine (Magstim, UK). Electromyography data were acquired with a data acquisition interface (BNC-2110; National Instruments) and an amplifying and filtering system (NeuroLog; Digitimer). These were located in an experimental room annexed to the MR scanner. Participants walked (∼10 m) to the annexed room after the first resting-state acquisition and walked back to the scanner for the post-TMS acquisition. An overview of the experimental design is shown in Fig. 2. The two experimental sessions were counterbalanced and scheduled at least 24 h apart to avoid carry-over effects of the TBS (Huang et al. 2005). The sessions were also arranged so that each participant was tested at approximately the same time of day to minimize any circadian variability in susceptibility to TBS (Sale et al. 2008).
TMS
As anticipated above, we targeted the thumb region of the right primary motor cortex because it permits a quantification of TBS-induced changes in local cortical excitability through measurement of MEPs from muscles in the contralateral hand. TMS was administered using a figure-of-eight coil (70-mm diameter). During stimulation, the coil handle was held at a 45° angle to the sagittal plane over the hand area of the right motor cortex. Once the optimal stimulation site was established, the location was marked on the participant's scalp with a felt tip pen. A fiducial marker (fish oil capsule) was also glued to the scalp over the optimal stimulation site to assist with the offline localization of the stimulation site using T1 images.
Assessment of motor cortical excitability.
Participants were comfortably seated to ensure a relaxed muscle state. MEPs were recorded using disposable surface electromyography (EMG) electrodes (Ag-AgCl) from the abductor pollicis brevis (APB) muscle. EMG signals were amplified (×1,000) and filtered (5–500 Hz) using a Neurolog system (Digitimer) and digitized (20 kHz) using a data acquisition interface (BNC-2110; National Instruments) and custom Matlab software (MathWorks).
Single pulses of TMS to the right hemisphere established the optimal cortical site to induce a motor response in a muscle of the left hand (the APB). The scalp region at which the largest MEP was consistently evoked was used as the stimulation site. Following identification of the optimal stimulation site, stimulus intensity for the two TBS paradigms was determined. This stimulus intensity was set at 80% of the active motor threshold. The active motor threshold was defined as the minimum TMS intensity required to evoke an MEP >200 μV in at least three out of five consecutive trials while participants were actively contracting their hand muscle (using a pincer grip) at a level equivalent to ∼20% of their maximum voluntary contraction. As depicted in Fig. 2, cortical excitability was quantified at three time points: immediately before TBS (baseline), 5 min post-TBS (but before entry into the scanner), and ∼20 min post-TBS (after exiting the second session in the scanner). For each time point participants received 20 single pulses of TMS at their individual suprathreshold test intensity (established before TBS, Fig. 2). The test intensity was defined as the TMS intensity that consistently produced an MEP between ∼0.5 and 1.0 mV (peak-to-peak) before the inhibitory/excitatory TBS protocol, with the hand relaxed.
Theta-burst stimulation.
Standard continuous (inhibitory) and intermittent (excitatory) TBS protocols were used to induce local changes in cortical activity (Huang et al. 2005). The 5- and 20- min post-TBS data were averaged for each participant to best represent each participant's motor cortex excitability while in the scanner (Fig. 2). Both inhibitory [continuous TBS (cTBS)] and excitatory [intermittent TBS (iTBS)] protocols involved bursts of three TMS pulses delivered at 50 Hz, repeated at 200-ms intervals at 80% of active motor threshold stimulus intensity. The inhibitory protocol consisted of 40 s of uninterrupted stimulation. In contrast, in the excitatory protocol, the stimulation was delivered for 2 s followed by an 8-s delay, repeated for a total of 192 s. Importantly, in both TBS protocols, participants received the same number of TMS pulses overall. To minimize the effect of muscle activity on changes in cortical excitability induced by TBS, participants avoided hand movements following TBS (Goldsworthy et al. 2012).
Analysis of MEPs.
MEP data were analyzed using standard univariate analyses implemented in the software IBM SPSS 19.0.
Imaging
Data acquisition.
During brain scanning, participants were instructed to keep their eyes open and to fixate on a central white cross on a black background. Participants were monitored via eye tracking video to ensure that eyes remained open during data acquisition and they did not fall asleep (Tagliazucchi and Laufs 2014). This display was back projected onto a screen positioned at the head end of the scanner by a liquid crystal display (LCD) projector. To avoid systematic changes in cortical activity arising from specific cognitive processing or motor imagery, participants were instructed to not think of anything in particular. Whole brain images were acquired using an echo-planar imaging sequence [38 axial slices, slice thickness = 3 mm, 180 volumes, gap = 10%, in-plane resolution = 64 × 64, time repetition = 2.02 s, time echo = 30 ms, flip angle = 90°, and field of view (FOV) = 220 × 220 mm].
Imaging preprocessing and analyses.
PREPROCESSING.
Preprocessing of rsfMRI data was performed using the Matlab (MathWorks) toolbox Data Processing Assistant for Resting-State fMRI A 2.2 (DPARSF; Chao-Gan and Yu-Feng 2010). The data preprocessing pipeline was established based on recent advances in the field (Keller et al. 2013; Power et al. 2012; Van Dijk et al. 2012; Yan et al. 2013). The first 10 image volumes (20 s) were discarded to allow tissue magnetization to reach a steady-state and participant adaptation to the MR scanner environment. DICOM images were converted to Nifti format, corrected for differences in acquisition time, normalized to standard Montreal Neurological Institute (MNI) space, and smoothed using a Gaussian function with a 6-mm full-width at half-maximum (FWHM) kernel. Data processing steps also involved filtering (0.01–0.08 Hz), the exclusion of undesired linear trends, and the regression of nuisance covariates. Specifically, signals from the six head motion parameters, volume-level mean of frame-to-frame displacements >0.5 mm (including the preceding and the two subsequent frames), global signal, white matter signal, and cerebrospinal fluid signal were regressed from each voxel's time series. Further analyses indicated that the average number of regressed volumes following head motion correction was similar and small across the four rsfMRI sessions [F(3,91) = 0.59, P = 0.6; note that the total number of regressed volumes in each session was <4%]. While global signal regression may be problematic in between-group analyses (Hahamy et al. 2014; Yang et al. 2014), such an approach is unlikely to mask meaningful differences in neural activity in within-subject analyses. As a precaution, however, we tested whether the regressed global signal differed between sessions. As expected, the global signal was not reliably different between sessions [within-subjects ANOVA: F(3,66) = 0.1, 0.96; cTBS: t(22) = 0.3, P = 0.76; iTBS: t(22) = 0.6, P = 0.5].
ANALYSES OF CHANGES IN BRAIN MODULAR DYNAMICS FOLLOWING TBS.
Preprocessed images were parcellated into 200 spatially coherent and volumetrically similar regions using a validated whole brain parcellation atlas (Craddock et al. 2012). fMRI signals were extracted for each region by averaging the signal across all voxels comprising the region. The degree of statistical dependency (i.e., functional connectivity) for a pair of regions was quantified using Pearson's correlation coefficient for the regionally averaged fMRI signals. Repeating for all pairs of regions resulted in a separate 200 × 200 functional connectivity matrix for each participant (n = 23), TBS type (cTBS or iTBS), and session (pre- or post-TBS).
Before the application of the algorithm (see below) to detect the community structure of the data, the connectivity matrices were binarized, preserving the top 5, 10, and 15% correlation values. The optimal number of modules depends in general on the sparsity of the network. On average, six, four, and three modules were isolated when connectivity matrices contained the top 5, 10, and 15% of functional connectivity values, respectively (Table 1). There were no significant main effects of session or TBS type and no significant session by TBS type interaction across the considered thresholds (P > 0.05). Analyses assessing connectivity changes within and between modules were performed using the 10% matrices. In fact, this threshold provided the most consistent modular decomposition across baseline conditions and participants (Fig. 3). Also, the modular segregation at 10% density was more consistent with prior analyses of resting state modularity than at 5% (i.e., >2 nodes in each module). Finally, according to previous studies, the 10% threshold provides an optimal trade-off between reducing spurious connections and retaining true connections (Dosenbach et al. 2010; Lord et al. 2012).
Table 1.
Number of modules as a function of sparsity
| 5% Sparsity | 10% Sparsity | 15% Sparsity | |
|---|---|---|---|
| Pre-iTBS | 5.91 (±1.68); Q = 0.60 | 3.91 (±1.04); Q = 0.50 | 3.26 (±0.69); Q = 0.45 |
| Post-iTBS | 5.87 (±1.42); Q = 0.64 | 3.65 (±0.98); Q = 0.50 | 3.30 (±0.70); Q = 0.45 |
| Pre-cTBS | 6.14 (±1.66); Q = 0.67 | 3.71 (±0.83); Q = 0.57 | 3.29 (±0.47); Q = 0.50 |
| Post-cTBS | 6.07 (±1.14); Q = 0.67 | 4.21 (±0.58); Q = 0.55 | 3.43 (±0.51); Q = 0.51 |
Values are means ± SD. Q, maximized modularity; iTBS, intermittent theta-burst stimulation; cTBS, continuous theta-burst stimulation; ± SD.
Fig. 3.
Representative modular decomposition for participant 9.
The modularity for each participant was identified using the widely used Newman's spectral algorithm (Newman 2006), implemented in the igraph package in the software R. Networks were first thresholded to 10% and then binarized. In the form,
where Avw is an element in the adjacency matrix. The proportion of connections present within modules can thus be calculated as,
where m is the total number of connections. The equation δ(Cv, Cw) = 1 if Cv and Cw both belong to the same module and 0 otherwise. However, (Newman) states this is not in itself a good measure of community structure. To improve this measure, the expected value of connection by random chance is subtracted. The expected connection chance is therefore defined as the average of degree for the connecting nodes, resulting in a goodness of modularity equation such as,
where the degree for node v (kv) is defined as:
For each participant and session, one decomposition was obtained. The plausibility and relative stability of this approach across participants was controlled manually after aligning the nodes. A full description of the method is provided by Clauset et al. (2004).
Two complementary metrics were investigated to assess whether TBS induced a temporary reorganization of brain modular dynamics: 1) out-degree participation index (PI), and 2) within module degree (WMD). These metrics were calculated using an algorithm implemented in the Brain Connectivity Toolbox (Rubinov and Sporns 2010; https://sites.google.com/site/bctnet/) and computed independently for each of the 200 regions of interest:
1) Out-degree participation index (PI)
where PI(i) is the out-degree participation index for node i, M is the list of modules, ki is the out-degree of node i and ki(m) is the out-degree of node i associated with module m. The PI of a brain region can thus take values between 0 and 1. A PI value of 0 is obtained when all connections are within the same module as the region of interest and will approach 1 when no connections are within the same module. As such, in this context, out-degree is an index of how a node is interconnected with nodes outside its own module.
2) Within module degree (WMD)
where WMDi is the within module degree for node i, ki(mi) is the total number of connections (k) for node i within its module (m). Is the mean of the WMD for module mi. σk(mi) Is the standard deviation for WMD for all nodes in module mi. The WMD is a (z-transformed) measure of within-module centrality and hence allows the assessment of whether cTBS influenced the extent to which regions were integrated within their modules (Guimera and Nunes Amaral 2005; Rubinov and Sporns 2010). Importantly, measures of PI and WMD are not necessarily inversely correlated. For example, a region may have both high PI and WMD.
Cluster-based statistics were used to localize statistically significant changes in the PI for the main effect of session (i.e., pre-TBS vs. post-TBS). Specifically, a paired samples t-statistic was computed for each region to assess the null hypothesis of equality in the PI values between pre- and post-TBS. Any pair of regions with a t-statistic exceeding a preliminary exploratory threshold were clustered together if and only if 1) they shared a common border (i.e., they were physical neighbors); or 2) they were contralateral homologues (i.e., they comprised nonzero overlap if one of the regions was reflected about the left-right plane and overlaid on top of the other region). The number of regions comprising each cluster identified based on these two clustering rules was recorded. Permutation testing was subsequently used to assess the statistical significance of each cluster and to control the false positive rate across the family of all regions. Specifically, the PI values were randomly permuted such that for some participants the pre-TBS PI value was swapped with the post-TBS PI value. Then, with the use of the same two clustering rules described above, clusters were identified in the permuted data and the number of regions comprising the largest cluster was recorded. This was repeated for 10,000 permutations to generate an empirical null distribution for the largest cluster size. For a cluster recorded in the original nonpermuted data comprising k regions, a P value corrected for the familywise error rate (FWE) was determined as the proportion of permutations containing a cluster comprising k or more regions (Nichols and Holmes 2002). This procedure was repeated for the WMD.
Finally, for each region showing a significant difference in the PI or WMD, any of the 200-1 = 199 possible connections originating from that node that was present in at least 50% of participants was included in a group-averaged connectivity profile. This was repeated for each session (i.e., baseline and post-TBS, for iTBS, and cTBS). The group-averaged connectivity profiles for each node were then compared qualitatively to characterize further the changes in functional brain connectivity following TBS.
To determine whether the modular architecture was modulated by inhibitory and/or excitatory TBS, we first assessed if all nodes were assigned to the same module at baseline and following stimulation. We generated matrices indexing the modular assignments of pairs of nodes, separately for each participant and each session (pre- vs. post-TBS). For each node pair, a value of 1 indicated that the two nodes belonged to the same module, and a value of zero indicated that they belonged to different modules. Cluster-based statistics were used to investigate significant changes in the assignment of the nodes following stimulation. A paired-samples t-statistic was computed for each node to assess the null hypothesis of unchanged modular location between pre- and post-TBS. A preliminary exploratory threshold was used to identify pairs of regions that had a change in modular assignment following TBS. Statistical significance was inferred via permutation testing.
ANALYSES OF RICH CLUB CONFIGURATION AND DYNAMICS.
The hypothetical effect of local TMS on large-scale neural dynamics was further assessed by testing for changes in the configuration and/or connectivity of the rich club (Collin et al. 2013; van den Heuvel et al. 2012; van den Heuvel and Sporns 2011). These analyses were performed by using the binarized, undirected rich club algorithm (van den Heuvel and Sporns 2011), as implemented in the brain connectivity toolbox (Rubinov and Sporns 2010).
First, for each participant, the functional connectivity matrices were thresholded and binarized. To allow a direct comparison with the modularity results, the connectivity matrices were thresholded at 10% (i.e., the matrices that contained the top 10% of functional connectivity values). The degree of each region of interest was calculated as the sum of functional interactions between the node and the rest of the brain. Second, the rich club coefficient Φ (Colizza et al. 2006; McAuley et al. 2007) was calculated for all levels of degree k. This was done by selecting a subgraph S of nodes with a degree > k and assessing the number of connections EK present within S (Collin et al. 2013). Formally, the (unweighted) rich club coefficient is defined as:
where NK is the number of regions in the subgraph S and EK is the total number of connections within S. As such, the rich club coefficient at level of k, Φ(k), is the fraction of connections between nodes that have a degree equal to or higher than k out of the maximum number of connections that such nodes could share. The rich club of the empirical network was compared with the distribution obtained from a set of random graphs generated by randomizing the connections within the whole network while preserving the original sparsity and degree sequence (i.e., rich club surrogates). For each rsfMRI session, Φrandom(k) was calculated as the mean over the set of 1,000 random graphs. Conversely, Φnormalized(k) was computed as the ratio of Φ(k) and Φrandom(k). The rich club organization within a network is defined as Φnomralized(k) > 1 (Colizza et al. 2006; McAuley et al. 2007). Significance of rich club organization was established with a one-sample t-test at each size of S (P < 0.05 FWE corrected; Collin et al. 2013; van den Heuvel et al. 2013).
Once the rich club organization was defined, a permutation testing procedure was used to assess statistically significant changes in rich club topology as a function of resting-state sessions. Specifically, for each participant, brain regions were first ranked as a function of their degree. The mean ranking of each region across all participants was then computed. The resulting top 20 and 40 regions (corresponding to the top 10 and 20% of all regions) were considered as rich clubs. These thresholds were chosen to allow comparability with previous work (e.g., van den Heuvel et al. 2011) and also to ensure that rich club properties were stable. Finally, the indexes of rich club regions encompassing the subgraphs Sbaselines and Spost-TBSs were permuted to assess hypothetical statistical changes in rich club composition (topology) across sessions or TBS protocols. We also assessed whether PI and WMD values associated with rich club hubs changed following stimulation.
Next, changes in functional connectivity within the rich clubs (i.e., rich clubs encompassing the top 20 and 40 regions) following TBS were assessed using the network-based statistic (NBS) (Zalesky et al. 2010). NBS has been extensively described elsewhere (Cocchi et al. 2012; Zalesky et al. 2010, 2012a). In brief, a two-sample t-statistic was calculated for each pair of rich club regions to test the null hypothesis of equality in mean functional connectivity between baselines and post-TBS sessions. All possible pairs of regions were tested: (20 × 19)/2 = 190 (for S = 20) and (40 × 39)/2 = 780 (for S = 40). The size of the identified network component at different testing thresholds (between t = 1 and t = 5) was measured by the number of suprathreshold connections it comprised. Permutation of connectivity matrices (baseline and post-TBS, for iTBS and cTBS) used to build the null model was subsequently performed to establish a corrected P value for each network. Specifically, for each of the 5,000 permutations, the size of the largest network was recorded to generate a null distribution that was used to calculate the FWE-corrected statistical thresholds. An FWE-corrected P value for a network identified in the actual data was subsequently estimated by the proportion of permutations for which a network of equal or greater size was identified. The same procedure was applied for the definition of networks based on the average values of functional connectivity. Compared with the definition of brain networks based on their spatial extent, this second method has the advantage of being sensitive to large changes in functional connectivity within small numbers of rich club hubs. Results did not differ significantly between the two methods (see results).
THUMB ACTIVATION TASK.
Ten participants (counterbalanced across the 2 TBS sessions) undertook a 4-min task to isolate brain regions associated with abduction of the left thumb (TBS target). The task was performed after acquisition of the resting-state baseline. Brain activity was measured by adopting the following T2* sequence: 98 volumes, in-plane resolution = 64 × 64, time repetition = 2.67 s, time echo = 28 ms, flip angle = 90°, and FOV = 220 × 220 mm. Participants were instructed to rapidly abduct their left thumb in synchrony with a flashing (2 Hz) white cross presented to them in the scanner on a uniform black background. The thumb movements were undertaken repeatedly for 16 s, followed by a 16-s rest period. This sequence was repeated eight times. Image preprocessing and analysis were performed in SPM8 (Wellcome Department of Cognitive Neurology, London, UK). Functional images were first corrected for acquisition time (slice timing), spatially realigned to the middle volume, and normalized to a standard neuroanatomical space defined by the MNI template. The normalized images were resampled to 3-mm isotropic voxels and then smoothed with a FWHM Gaussian kernel of 8 mm. Time series were high-pass filtered with a 128-s cutoff period and were additionally corrected for first-order serial autocorrelations. Brain regions involved in left thumb abduction in each participant (first level statistics) were isolated using the general linear model framework implemented in SPM8 (contrast: task > rest). Group level results were thresholded at P < 0.05 FDR, and corrected at the cluster level (Chumbley et al. 2010).
RESULTS
We first assessed the effect of TBS on cortical excitability. Analysis of MEPs obtained from the contralateral APB muscle showed a significant main effect of stimulation type [F(1,22) = 4.6, P = 0.04] and a significant interaction between rsfMRI session and stimulation type [F(1,22) = 4.5, P = 0.04; Fig. 4, A and B]. Post hoc analyses showed that cortical excitability was significantly increased following iTBS (t22 = 2.18, P = 0.04, Cohen's d = 0.91), but not significantly decreased, even though a relatively large effect was detected, after cTBS (t22 = 0.80, P = 0.42, Cohen's d = 0.65).
Fig. 4.
Changes in motor cortex excitability after TBS. A: representative mean MEPs (participant 21) obtained from abductor pollicis brevis (APB), showing the effect of cTBS (red) and iTBS (green) on baseline (black) MEP amplitude. B: MEP amplitude (±SE) for each session (pre/post-TBS) and TBS type (iTBS/cTBS).
We next analyzed rsfMRI data to investigate whether TBS altered the number of brain modules relative to the prestimulation baseline. To achieve this, functional connectivity was assessed independently for each participant, stimulation type (iTBS and cTBS), and session (pre- and post-TBS). This provided a network characterization of the patterns of pairwise associations in neural activity across the whole brain. As mentioned above, the pattern of connections between brain regions decomposed optimally into four modules (Table 1 and Figs. 3 and 6). Importantly, this decomposition is consistent with the one obtained by previous studies that adopted different whole brain parcellation atlas (e.g., Zalesky et al. 2014). There were no statistically significant main effects of session or stimulation type, and no interaction between these factors in terms of the number of modules.
Fig. 6.
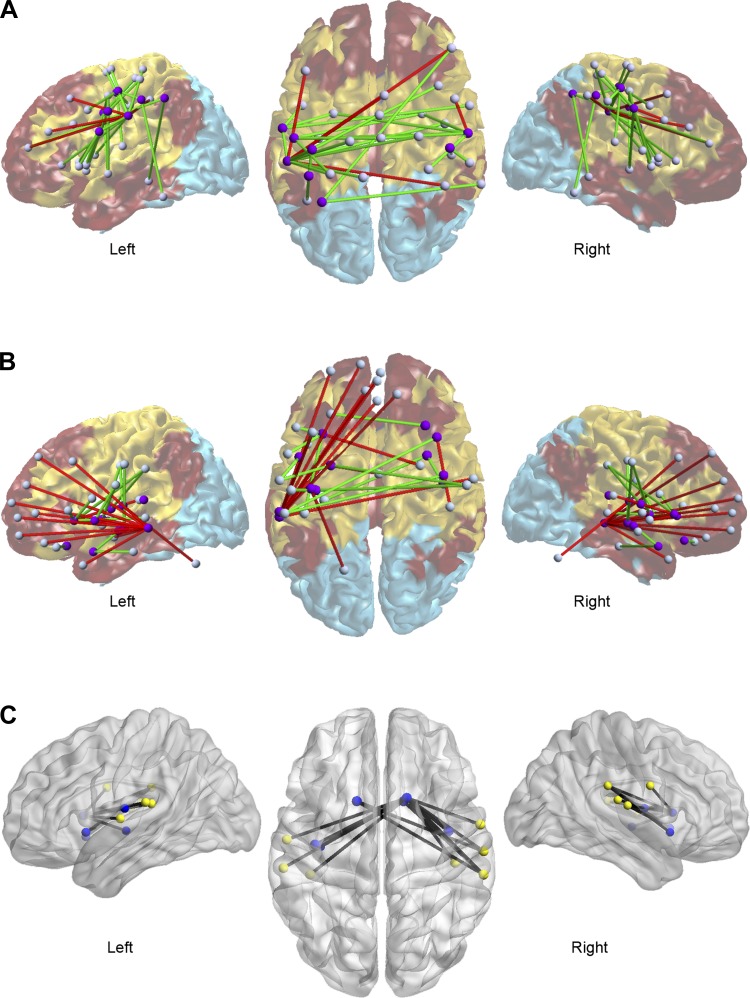
Changes in functional connectivity as a function of inhibitory stimulation (cTBS). Spheres represent the centroid of brain regions. A and B: dominant modular decomposition is projected on a reconstruction of the cortical surface shown in the background (note that this representation does not show the subcortical module; see Fig. 3). This decomposition was obtained by calculating the mode (across all participants and sessions) of the modular assignment for each region after alignment. A: purple regions showed a decrease in PI (i.e., between-module communication) and/or an increase in WMD (i.e., intramodular connectivity) following cTBS. Results showed increased functional connectivity (in green) within the sensorimotor module (in yellow) and decreased connectivity (in red) between this module and other modules of the brain. B: purple regions showed increased PI and/or reduced WMD following cTBS. Regions represented with purple spheres (insula, striatum, and left temporal cortex) increased their functional connectivity with the sensorimotor module and decreased their intra-modular connectivity. C depicts changes in the node assignment as a function of cTBS stimulation (inhibition). Following inhibitory stimulation of the right motor cortex pairs of nodes encompassing the sensorimotor (yellow spheres) and the subcortical (blue spheres) modules change their assignment.
To assess whether brain modular dynamics were influenced by stimulation type, we calculated the PI of each brain region (see materials and methods). PI decreased in motor and somatosensory cortices following cTBS (i.e., inhibition; red clusters in Fig. 5A). Interestingly, such changes occurred in cortical areas involved in the abduction of the left thumb (purple in Fig. 5A; P = 0.04 FWE corrected) and were statistically significant (red in Fig. 5A; P = 0.04, FWE corrected). An increase in the PI following inhibitory TBS (cTBS) was also found in nonmotor regions involved in abduction of the left thumb (depicted in purple in Fig. 5B), including the insula, striatum, and left temporal cortex (red clusters in Fig. 5B). However, while overlapping with brain areas involved in abduction of the left thumb (purple in Fig. 5B) and changes in WMD (see below), increases in PI did not quite reach statistical significance according to a strict correction for multiple comparisons (P < 0.08, FWE corrected; P < 0.02, uncorrected).
Fig. 5.
Changes in brain modular dynamics as a function of local changes in neural excitability. A: n = 23. Red: clusters showing a higher participation index (PI) at baseline. Green: clusters showing a lower within module degree (WMD) at baseline [all P < 0.05 familywise error rate (FWE) corrected]. Purple: brain activity induced by abduction of the left thumb (task > rest, P < 0.05 FDR correction at cluster level). Note that changes in PI and WMD largely overlap with the brain regions involved in abduction of the left thumb. This result suggests that connectivity changes occur mainly in areas that are functionally related to the targeted region (i.e., right motor cortex). B: red clusters represent a trend-level increase in PI following cTBS (P = 0.08, FWE). Clusters in which the WMD was decreased following cTBS are depicted in green (P = 0.04, FWE corrected). Brain regions that showed a change in both PI and WMD are shown in yellow. Purple: regions activated by abduction of the left thumb (task > rest, P < 0.05, FDR corrected at cluster level).
Interestingly, the WMD increased in sensorimotor regions that also showed a decrease in PI following inhibitory TBS [i.e., the WMD was lower at baseline than after inhibition (cTBS), P < 0.05 FWE; green in Fig. 5A]. The opposite pattern (decreased WMD after inhibitory TBS) was found in the insula, striatum, and left temporal cortex, regions also involved in abduction of the left thumb and showing qualitative changes in the PI (P = 0.04 FWE; green and yellow in Fig. 5B). Together, changes in the PI and WMD indicate temporary variations in modular interactions following cTBS. Specifically, the motor cortex (bilaterally) became functionally dissociated from external (nonmotor) modules but functionally integrated with regions within its own module.
Analysis of the baseline resting-state data showed a significant negative correlation between PI and WMD values in the regions of interest (P < 0.05). However, calculating a Pearson's correlation between the mean node PI (mean per node across participants) at baseline and the WMD counterpart showed a weak and non-significant relationship (r = −0.1, P > 0.05). Likewise, some regions (e.g., 48) showed a high ratio between PI and WMD values. These findings suggest that while PI and WMD can be negatively correlated under some circumstances, they can also provide complementary information on brain modular dynamics.
Further analyses of functional connectivity confirmed that changes in PI and WMD following cTBS were related to increased connectivity between sensorimotor cortexes (Fig. 6A). After cTBS, regions within the insula, temporal cortex, and striatum (predominantly located in close proximity to the sensorimotor module) also became more integrated with regions in the sensorimotor module (Fig. 6B). These findings are in line with the results showing a significant switch in modular assignment between striatal and insular nodes, and nodes comprising the sensorimotor module after local inhibitory stimulation (Fig. 6C).
To exclude the possibility that changes in brain modular dynamics might be related to the execution of the thumb abduction task, we reperformed the analyses including only the 13 individuals who did not perform this task. Results from this analysis replicated the original findings. These findings confirm that differences in MEPs were similar between participants that did and did not perform the motor task.
Although iTBS (excitatory) exerted a stronger influence on MEPs than cTBS (inhibitory; Fig. 4, A and B), no significant differences in regional PI or WMD were found between the baseline and post-iTBS conditions. These results were confirmed when restricting the analysis to participants who did not perform the thumb abduction task (n = 13). Likewise no changes in functional modular architecture were detected between the baseline and post-iTBS session.
Finally, we assessed if TBS-induced changes in modular dynamics occurred in parallel with changes in the organization and dynamics of rich club hubs. Rich club regions are nodes with high connection density with the rest of the brain (nodal degree) and are more strongly interconnected with each other than expected by chance (van den Heuvel and Sporns 2011). Our analyses revealed the presence of rich club hubs in the four datasets (i.e., 2 baselines, post-cTBS, and post-iTBS; Fig. 7A). The right primary motor cortex (TBS target) was not part of the rich club (Fig. 7, C and D). Permutation testing showed that the rich club encompassed the same regions across the four rsfMRI sessions, highlighting the stability of rich club hubs across different resting-state acquisitions. However, while the rich club was comprised of a stable set of regions, the assignment of a number of its hubs (Fig. 7, C and D) to subcortical or sensorimotor modules was dynamic (Fig. 6C). Analysis of the connections within the rich club using NBS (see materials and methods) further indicated that functional connectivity within the rich club network was not significantly changed by focal cTBS or iTBS of the right primary motor cortex. Likewise, the PI and WMD values associated with rich club regions were similar at baseline and after local stimulation (iTBS and cTBS). Thus, by contrast with modularity, the rich club dynamics revealed by our rsfMRI data were not reliably influenced by local inhibitory or excitatory TBS and remained stable across the four resting state sessions.
Fig. 7.
Rich club dynamics and effect of TBS. A: rich club coefficient (Φ) at level of k is defined as the fraction of connections between nodes that have a degree (number of connections) equal to or higher than k out of the maximum number of connections that such nodes could share. The curve shown in blue is the Φ averaged over participants (n = 23, cTBS baseline. Similar results were found in the three additional conditions, see text). The black line represents the rich club of degree-preserving random reference graphs (mean of 1,000 random networks per participant). The red line shows the normalized rich club curve (i.e., ratio between actual rich club and surrogate rich clubs). A indicates the existence of rich clubs in the functional connectivity of the brain (i.e., red line is significantly greater than unity, as indicated by *P < 0.05 Bonferroni corrected one-tailed t-test). Note that above k > 35 the subgraph disconnected for a number of participants, suggesting that in the matrices considered, k > 35 represents the maximum degree. B: ranking of brain regions (x-axis) as a function of nodal degree (y-axis). The figure indicates the existence of a non-Gaussian distribution of brain region degrees, with few regions having a very high degree (i.e., rich club, at left. This observation was consistent across baselines and post-TBS resting-state periods). The anatomical location of brain regions showing the highest degree is presented in C (20 regions with the highest degree across sessions, in purple) and D (top 40 regions, in purple). Permutation testing showed a similar distribution of rich clubs across the four fMRI resting-state sessions. Likewise, the network-based statistic (NBS) showed that functional connectivity between rich clubs (top 20 and 40 regions) did not differ significantly between baseline and post-TBS sessions.
DISCUSSION
We studied the influence of local inhibitory and excitatory theta-burst TMS over the primary motor cortex on whole brain resting state connectivity. To this end, we used two validated theta-burst TMS protocols (TBS) to selectively increase (iTBS) or decrease (cTBS) the excitability of neurons within the right primary motor cortex and resting-state fMRI to assess causal changes in integrative brain activity at the scale of modules. We also tested whether focal stimulation of the primary motor cortex influenced dynamic interactions at the scale of rich club. The rich club represents an aggregation of densely interconnected brain regions that support the bulk of integration. As such, rich club hubs are positioned at the highest level of the connectivity hierarchy (Sporns 2012). Results showed that dynamics within and between the sensorimotor module were highly responsive to transient local inhibition. Moreover, our analyses suggest that selective changes in sensorimotor-module dynamics occurred in the context of globally preserved topology and dynamics of modules and the rich club. These observations provide support for the hypothesis that a local change (disruption) of neural activity is progressively integrated into whole brain dynamics by hierarchical mechanisms. More broadly, results from this study highlight the complex neural changes involved in managing the balance between local neural specialization and global integration.
Local TBS to the thumb area of the right motor cortex did not significantly change the overall pattern of large-scale integration across the brain. However, inhibitory TBS over the same area in a separate session selectively changed within- and between-module dynamics. Specifically, following local cTBS, regions within the sensorimotor module became more integrated. This is consistent with recent results in healthy humans showing that inhibitory TMS of the left motor cortex enhances endogenous functional connectivity with the contralateral motor cortex (Watanabe et al. 2014). Likewise, the observed increase in resting-state connectivity within the sensorimotor module is reminiscent of previous findings in stroke patients, which suggest that recovery of motor function relies on enhanced inter-hemispheric connectivity between motor cortexes (Carter et al. 2010; Grefkes and Fink 2011, 2014; van Meer et al. 2010; Wang et al. 2010). Our findings also add to previous knowledge by showing that increases in intrinsic functional integration within the sensorimotor module can occur rapidly after localized reduction in neural functions and in the context of a general decrease in integration between this module and other modules of the brain. In fact, the enhanced integration between right motor cortex and other regions supporting motor function was paralleled by reduced connectivity between the sensorimotor module and other modules of the brain. These results suggest that transitory inhibition of local neural activity reorganizes intrinsic module dynamics to support functional specialization, albeit at the cost of integration with other brain processes.
Complex reorganization of global brain dynamics following virtual lesions has been suggested by previous computational work (Alstott et al. 2009; Honey and Sporns 2008). In agreement with recent advances in characterizing the core brain regions that facilitate large-scale integration (i.e., hubs; Collin et al. 2013; Leech et al. 2012; Power et al. 2013; van den Heuvel et al. 2012; van den Heuvel and Sporns 2013a; 2011), results from these modeling studies indicate that the extent of change in brain dynamics is related to the centrality of the affected region within the connectional hierarchy. In line with the hypothesis of a critical role of the rich club in cross-modular communication, our findings reveal that regions shifting modular assignment following inhibitory stimulation (cTBS) of the right motor cortex were mainly rich club hubs (Figs. 6C and 7, C and D). Likewise, in agreement with previous studies, our findings show that the primary motor cortex is an output region with a low participation coefficient; i.e., it is not a brain hub (Power et al. 2013). While inhibitory TBS (cTBS) had complex effects on the specific dynamics of the sensorimotor module, TBS did not significantly affect the general configuration and communication between modules and within rich club hubs. Benchmarking functional networks against degree-preserving random networks is likely to overestimate the richness of the rich club hubs in edge strengths, because the empirical networks are transitive whereas the random ones are not (Zalesky et al. 2012b). However, this factor is present before and after TMS and is hence unable to explain the absence of a change in the rich club configuration. Thus this null result suggests that although brain dynamics may adapt to a focal reduction in neural functions, ongoing large-scale integration may be generally preserved by mechanisms operating at different levels of the connectional hierarchy. Such level-specific plasticity in neural dynamics may be critical for containing the impact of local neural insults on brain function. Consistent with the proposition that the rich club provides the backbone of brain integration, our results highlight the within-subject stability of rich club configuration and dynamics across resting-state sessions performed on different days. Future investigations should explore the efficiency of the hierarchical, adaptive brain mechanisms highlighted in this study by targeting brain regions with different functional contributions to brain dynamics. Such research will require new probes for measuring the effect of TMS on local neural activity and behavior. Here we assume that TBS altered local excitability within the stimulated motor cortex, but it remains possible that our stimulation regimes also induced changes in spinal cord excitability, and that these changes were reflected in altered MEPs measured following stimulation.
In contrast to the effects of inhibitory TBS on brain modular dynamics, excitatory TBS did not significantly alter intrinsic integration within the sensorimotor module, nor the segregation between this module and other nonmotor modules. There was also no influence of excitatory TBS on the topology or dynamics of the rich club. These findings indicate that while endogenous local processes may be transiently enhanced, the resulting facilitation of motor function (indexed by MEPs) is not necessarily related to a significant reorganization of neural dynamics at the level of large-scale modules and rich clubs. As such, the significant increase in MEPs following excitatory TBS of right motor cortex is likely to be related to more circumscribed changes in endogenous neural activity. This suggestion is supported by previous TMS-neuroimaging studies that have shown activity and connectivity increases between the targeted motor cortex and confined sensorimotor regions (Bestmann et al. 2004; Fox et al. 2006; Moisa et al. 2010; Nettekoven et al. 2014; but see Watanabe et al. 2014). Moreover, findings from previous TMS research suggest that large-scale effects of focal neural enhancement are context dependent (Bestmann and Feredoes 2013; Bestmann et al. 2008; Moisa et al. 2012; Ruff et al. 2009). Thus, while there may be common mechanisms underpinning large-scale neural plasticity, the net effects of local changes in neural excitability on global neural dynamics may depend on specific network states (e.g., rest vs. task).
In summary, our findings highlight the complex functional consequences of local alterations in neural excitability on intrinsic whole brain dynamics. The results suggest greater functional resilience of whole brain dynamics to local increases in neural excitability than to local decreases. The observed plasticity of large-scale brain network activity following focal reduction in neural excitability supports the existence of hierarchical mechanisms that integrate these focal changes at intermediate levels of the connectivity hierarchy, while preserving the backbone of the network. By highlighting some of the principles underlying plasticity of large-scale brain dynamics, our results have important implications for guiding the development of brain stimulation protocols aimed at selectively restoring dysfunctional integration across brain systems in neurological and psychiatric disorders (Fox et al. 2012; Hoy and Fitzgerald 2010).
GRANTS
This study was supported by a grant from the National and International Research Alliance Program (NIRAP), Queensland State Government, Australia. J. B. Mattingley was supported by an Australian Research Council (ARC) Australian Laureate Fellowship (FL110100103), the ARC Science of Learning Research Centre (SR120300015), and the ARC Centre of Excellence for Integrative Brain Function (ARC Centre Grant CE140100007). M. V. Sale and A. Zalesky were supported by the Australian National Health and Medical Research Council (APP1028210 to M. V. Sale and APP1047648 to A. Zalesky).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.C., M.V.S., and J.B.M. conception and design of research; L.C. and M.V.S. performed experiments; L.C., M.V.S., A.L., A.Z., and M.B. analyzed data; L.C., M.V.S., A.L., A.Z., M.B., and J.B.M. interpreted results of experiments; L.C., M.V.S., and A.L. prepared figures; L.C. drafted manuscript; L.C., M.V.S., A.L., A.Z., M.B., and J.B.M. edited and revised manuscript; L.C., M.V.S., A.L., A.Z., M.B., and J.B.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Luke Hearne and Amy Taylor for assistance with data collection.
REFERENCES
- Alstott J, Breakspear M, Hagmann P, Cammoun L, Sporns O. Modeling the impact of lesions in the human brain. PLoS Comput Biol 5: e1000408, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci 19: 1950–1962, 2004. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Feredoes E. Combined neurostimulation and neuroimaging in cognitive neuroscience: past, present, and future. Ann NY Acad Sci 1296: 11–30, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Swayne O, Blankenburg F, Ruff CC, Haggard P, Weiskopf N, Josephs O, Driver J, Rothwell JC, Ward NS. Dorsal premotor cortex exerts state-dependent causal influences on activity in contralateral primary motor and dorsal premotor cortex. Cereb Cortex 18: 1281–1291, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Swayne O, Blankenburg F, Ruff CC, Teo J, Weiskopf N, Driver J, Rothwell JC, Ward NS. The role of contralesional dorsal premotor cortex after stroke as studied with concurrent TMS-fMRI. J Neurosci 30: 11926–11937, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, Pope DL, Shulman GL, Corbetta M. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol 67: 365–375, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Front Syst Neurosci 4: 13, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumbley J, Worsley K, Flandin G, Friston K. Topological FDR for neuroimaging. Neuroimage 49: 3057–3064, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauset A, Newman ME, Moore C. Finding community structure in very large networks. Phys Rev E Stat Nonlin Soft Matter Phys 70: 066111, 2004. [DOI] [PubMed] [Google Scholar]
- Cocchi L, Bramati IE, Zalesky A, Furukawa E, Fontenelle LF, Moll J, Tripp G, Mattos P. Altered functional brain connectivity in a non-clinical sample of young adults with attention-deficit/hyperactivity disorder. J Neurosci 32: 17753–17761, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colizza V, Flammini A, Serrano M, Vespignani A. Detecting rich-club ordering in complex networks. Nat Phys 2: 110–115, 2006. [Google Scholar]
- Collin G, Sporns O, Mandl RC, van den Heuvel MP. Structural and functional aspects relating to cost and benefit of rich club organization in the human cerebral cortex. Cereb Cortex 24: 2258–2267, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock RC, James GA, Holtzheimer PE, Hu XP 3rd, Mayberg HS. A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum Brain Mapp 33: 1914–1928, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Vertes PE, Winton-Brown TT, Patel AX, Ginestet CE, McGuire P, Bullmore ET. Cognitive relevance of the community structure of the human brain functional coactivation network. Proc Natl Acad Sci USA 110: 11583–11588, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, Barnes KA, Dubis JW, Feczko E, Coalson RS, Pruett JR Jr, Barch DM, Petersen SE, andSchlaggar BL. Prediction of individual brain maturity using fMRI. Science 329: 1358–1361, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldaief MC, Halko MA, Buckner RL, Pascual-Leone A. Transcranial magnetic stimulation modulates the brain's intrinsic activity in a frequency-dependent manner. Proc Natl Acad Sci USA 108: 21229–21234, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Harrison BJ, Zalesky A, Simons JS. Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proc Natl Acad Sci USA 109: 12788–12793, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry 72: 595–603, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Narayana S, Tandon N, Fox SP, Sandoval H, Kochunov P, Capaday C, Lancaster JL. Intensity modulation of TMS-induced cortical excitation: primary motor cortex. Hum Brain Mapp 27: 478–487, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsworthy MR, Pitcher JB, Ridding MC. The application of spaced theta burst protocols induces long-lasting neuroplastic changes in the human motor cortex. Eur J Neurosci 35: 125–134, 2012. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. Connectivity-based approaches in stroke and recovery of function. Lancet Neurol 13: 206–216, 2014. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. Reorganization of cerebral networks after stroke: new insights from neuroimaging with connectivity approaches. Brain 134: 1264–1276, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimera R, Nunes Amaral LA. Functional cartography of complex metabolic networks. Nature 433: 895–900, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahamy A, Calhoun V, Pearlson G, Harel M, Stern N, Attar F, Malach R, Salomon R. Save the global: global signal connectivity as a tool for studying clinical populations with functional magnetic resonance imaging. Brain Connect 4: 395–403, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Sporns O. Dynamical consequences of lesions in cortical networks. Hum Brain Mapp 29: 802–809, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul 3: 95–118, 2010. [DOI] [PubMed] [Google Scholar]
- Hoy KE, Fitzgerald PB. Brain stimulation in psychiatry and its effects on cognition. Nat Rev Neurol 6: 267–275, 2010. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron 45: 201–206, 2005. [DOI] [PubMed] [Google Scholar]
- Keller CJ, Bickel S, Honey CJ, Groppe DM, Entz L, Craddock RC, Lado FA, Kelly C, Milham M, Mehta AD. Neurophysiological investigation of spontaneous correlated and anticorrelated fluctuations of the BOLD Signal. J Neurosci 33: 6333–6342, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. J Neurosci 32: 215–222, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Augath M, Murayama Y, Rauch A, Sultan F, Goense J, Oeltermann A, Merkle H. The effects of electrical microstimulation on cortical signal propagation. Nat Neurosci 13: 1283–1291, 2010. [DOI] [PubMed] [Google Scholar]
- Lord A, Horn D, Breakspear M, Walter M. Changes in community structure of resting state functional connectivity in unipolar depression. PLoS One 7: e41282, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science 309: 2228–2232, 2005. [DOI] [PubMed] [Google Scholar]
- McAuley J, da Fontoura Costa L, Caetano T. Rich-club phenomena accross complex network hierarchies. Appl Physics Lett 91: 2007. [Google Scholar]
- Moisa M, Pohmann R, Uludag K, Thielscher A. Interleaved TMS/CASL: comparison of different rTMS protocols. Neuroimage 49: 612–620, 2010. [DOI] [PubMed] [Google Scholar]
- Moisa M, Siebner HR, Pohmann R, Thielscher A. Uncovering a context-specific connectional fingerprint of human dorsal premotor cortex. J Neurosci 32: 7244–7252, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettekoven C, Volz LJ, Kutscha M, Pool EM, Rehme AK, Eickhoff SB, Fink GR, Grefkes C. Dose-dependent effects of theta burst rTMS on cortical excitability and resting-state connectivity of the human motor system. J Neurosci 34: 6849–6859, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman ME. Modularity and community structure in networks. Proc Natl Acad Sci USA 103: 8577–8582, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15: 1–25, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Friston K. Structural and functional brain networks: from connections to cognition. Science 342: 1238411, 2013. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59: 2142–2154, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Lessov-Schlaggar CN, Petersen SE. Evidence for hubs in human functional brain networks. Neuron 79: 798–813, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. The restless brain. Brain Connect 1: 3–12, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52: 1059–1069, 2010. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Driver J, Bestmann S. Combining TMS and fMRI: from “virtual lesions” to functional-network accounts of cognition. Cortex 45: 1043–1049, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale MV, Ridding MC, Nordstrom MA. Cortisol inhibits neuroplasticity induction in human motor cortex. J Neurosci 28: 8285–8293, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TK, Hausser M, Carandini M. Distal connectivity causes summation and division across mouse visual cortex. Nat Neurosci 17: 30–32, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. Discovering the Human Connectome. Cambridge, MA: MIT Press; 2012, p. 232. [Google Scholar]
- Tagliazucchi E, Laufs H. Decoding wakefulness levels from typical fMRI resting-state data reveals reliable drifts between wakefulness and sleep. Neuron 82: 695–708, 2014. [DOI] [PubMed] [Google Scholar]
- Tolias AS, Sultan F, Augath M, Oeltermann A, Tehovnik EJ, Schiller PH, Logothetis NK. Mapping cortical activity elicited with electrical microstimulation using fMRI in the macaque. Neuron 48: 901–911, 2005. [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Payne BR, Rushmore J, Lomber SG, Pascual-Leone A. Impact of repetitive transcranial magnetic stimulation of the parietal cortex on metabolic brain activity: a 14C-2DG tracing study in the cat. Exp Brain Res 163: 1–12, 2005. [DOI] [PubMed] [Google Scholar]
- van Dellen E, Hillebrand A, Douw L, Heimans JJ, Reijneveld JC, Stam CJ. Local polymorphic delta activity in cortical lesions causes global decreases in functional connectivity. Neuroimage 83: 524–532, 2013. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Kahn RS, Goni J, Sporns O. High-cost, high-capacity backbone for global brain communication. Proc Natl Acad Sci USA 109: 11372–11377, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. An anatomical substrate for integration among functional networks in human cortex. J Neurosci 33: 14489–14500, 2013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. Network hubs in the human brain. Trends Cogn Sci 17: 683–696, 2013b. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. Rich-club organization of the human connectome. J Neurosci 31: 15775–15786, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, van Soelen IL, Stam CJ, Kahn RS, Boomsma DI, Hulshoff Pol HE. Genetic control of functional brain network efficiency in children. Eur Neuropsychopharmacol 23: 19–23, 2013. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity. MRI Neuroimage 59: 431–438, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer MP, van der Marel K, Wang K, Otte WM, El Bouazati S, Roeling TA, Viergever MA, Berkelbach van der Sprenkel JW, Dijkhuizen RM. Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. J Neurosci 30: 3964–3972, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yu C, Chen H, Qin W, He Y, Fan F, Zhang Y, Wang M, Li K, Zang Y, Woodward TS, Zhu C. Dynamic functional reorganization of the motor execution network after stroke. Brain 133: 1224–1238, 2010. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Hanajima R, Shirota Y, Ohminami S, Tsutsumi R, Terao Y, Ugawa Y, Hirose S, Miyashita Y, Konishi S, Kunimatsu A, Ohtomo K. Bidirectional effects on interhemispheric resting-state functional connectivity induced by excitatory and inhibitory repetitive transcranial magnetic stimulation. Hum Brain Mapp 35: 1896–1905, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo XN, Castellanos FX, Milham MP. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage 76: 183–201, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GJ, Murray JD, Repovs G, Cole MW, Savic A, Glasser MF, Pittenger C, Krystal JH, Wang XJ, Pearlson GD, Glahn DC, Anticevic A. Altered global brain signal in schizophrenia. Proc Natl Acad Sci USA 111: 7438–7443, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Cocchi L, Fornito A, Murray MM, Bullmore E. Connectivity differences in brain networks. Neuroimage 60: 1055–1062, 2012a. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore E. On the use of correlation as a measure of network connectivity. Neuroimage 60: 2096–2106, 2012b. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage 53: 1197–1207, 2010. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Cocchi L, Gollo LL, Breakspear M. Time-resolved resting-state brain networks. Proc Natl Acad Sci USA 111: 10341–10346, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]