Abstract
The fastigial oculomotor region is the output by which the medioposterior cerebellum influences the generation of saccades. Recent inactivation studies reported observations suggesting an involvement in their dynamics (velocity and duration). In this work, we tested this hypothesis in the head-restrained monkey with the electrical microstimulation technique. More specifically, we studied the influence of duration, frequency, and current on the saccades elicited by fastigial stimulation and starting from a central (straight ahead) position. The results show ipsilateral or contralateral saccades whose amplitude and dynamics depend on the stimulation parameters. The duration and amplitude of their horizontal component increase with the duration of stimulation up to a maximum amplitude. Varying the stimulation frequency mostly changes their latency and the peak velocity (for contralateral saccades). Current also influences the metrics and dynamics of saccades: the horizontal amplitude and peak velocity increase with the intensity, whereas the latency decreases. The changes in peak velocity and in latency observed in contralateral saccades are not correlated. Finally, we discovered that contralateral saccades can be evoked at sites eliciting ipsilateral saccades when the stimulation frequency is reduced. However, their onset is timed not with the onset but with the offset of stimulation. These results corroborate the hypothesis that the fastigial projections toward the pontomedullary reticular formation (PMRF) participate in steering the saccade, whereas the fastigiocollicular projections contribute to the bilateral control of visual fixation. We propose that the cerebellar influence on saccade generation involves recruiting neurons and controlling the size of the active population in the PMRF.
Keywords: brain stem, cerebellum, dynamics, primate, saccade
the normal functioning of the medioposterior cerebellum (MPC) is required for accurately orienting gaze toward a visual target (Pélisson et al. 2003; Robinson and Fuchs 2001). In primates, the critical regions are the oculomotor vermis (OV), located in the vermal lobules VIc–VII, and the two caudal fastigial nuclei (cFNs). The cFNs are the output by which the MPC influences the foveation of a visual target, through their ascending projections toward the deep superior colliculus (May et al. 1990) and their descending projections toward the contralateral pontine and medullary reticular formation (PMRF), where excitatory and inhibitory premotor neurons are respectively located (Noda et al. 1990; Sato and Noda 1991). The cFNs receive inhibitory afferents from Purkinje cells in the OV (Yamada and Noda 1987) and mossy fiber inputs from pontine and reticular medial nuclei (Noda et al. 1990). A group of neurons in the cFNs burst during every saccade, regardless of the amplitude and direction (Fuchs et al. 1993; Kleine et al. 2003; Ohtsuka and Noda 1991). Finally, unilateral inactivation of cFN with muscimol alters the horizontal component of fixational (Guerrasio et al. 2010) and regular saccades made with (Quinet and Goffart 2005, 2007) or without a concurrent movement of the head (Goffart et al. 2004; Iwamoto and Yoshida 2002; Robinson et al. 1993): its amplitude is increased for all ipsilesional saccades and reduced for all contralesional saccades, whereas vertical saccades are deviated toward the inactivated side (ipsipulsion) with a magnitude that increases with saccade duration (Goffart et al. 2004).
Because the saccade velocities were found unchanged after very large cerebellar lesions (Aschoff and Cohen 1971; Optican and Robinson 1980; Selhorst et al. 1976; Zee et al. 1976) and most recording studies failed to report any relation between the firing rate of cFN neurons and saccade velocity (Fuchs et al. 1993; Helmchen et al. 1994; Ohtsuka and Noda 1991), an influence of MPC on the dynamics (velocity and duration) of saccades was not unanimously admitted (see e.g., Marsden and Harris 2011). Yet, in the monkey, when they are acute, lesions in the cFN (Buzunov et al. 2013; Goffart et al. 2004; Quinet and Goffart 2007; Robinson et al. 1993) or in the OV (Kojima et al. 2010; Takagi et al. 1998) severely affect the velocity of saccades in addition to making them dysmetric (not in the cat; Goffart et al. 1998a). The difficulty in relating the discharge of cFN neurons to the dynamics of saccades could be due to the fact that their time course is highly stereotyped. Only one thorough study reported some correlations, although weak, between the peak velocity of saccades and the maximum firing rate of cFN neurons (Kleine et al. 2003), but these relations could be the consequence of a change in the animal's level of alertness, since the neurons were recorded during long and likely monotonous sessions that involved a large number of saccades of the same size and toward the same targets. Differences in alertness or attention can indeed account for variations in the discharge of saccade-related fastigial neurons (Fuchs et al. 1993). A comparable “arousal” factor can also explain why the peak burst activity of cFN neurons is weaker (62%) during saccades made in darkness than during saccades made in the light (Helmchen et al. 1994). Thus the suggestion of an involvement of cFN activity in the dynamics of saccades is essentially based on observations that were made after inactivation (or lesion) in the cFN (or OV), but the interpretation of these observations is not straightforward either, for two reasons. First, if one considers that the two cFNs modulate the balance of activity between the excitatory and inhibitory burst neurons in the PMRF, any lesion will introduce asymmetric activities between the left and right parts of the saccade-related reticular regions. In turn, these asymmetries will inevitably alter the burst discharge of agonist motoneurons in the abducens nucleus (van Gisbergen et al. 1981), and consequently, the usual time course of saccades. In other words, the dynamics will be altered after lesion, not because the cFN activity regulates the time progression of saccades but merely because the bilateral control of saccade generation is altered (Goffart et al. 2004). Second, the binary (all-or-nothing) effect of lesion/inactivation techniques on neuronal activities does not afford the opportunity to relate the strength of cFN activity to the velocity of saccades. Electrical microstimulation with different current strengths or different interpulse intervals (frequency of stimulation) would be an appropriate technique for testing such an influence.
It is known that cFN microstimulation elicits saccades in the head-restrained (Cogdell et al. 1977; Noda et al. 1988) and unrestrained monkey (Quinet and Goffart 2009). The direction of elicited saccades is ipsilateral when its dorsocaudal part is stimulated and contralateral when the stimulation is applied in the ventromedial region. The contralateral saccades would result from the recruitment of axons that innervate the contralateral PMRF (Noda et al. 1988), presumably activating the excitatory and inhibitory burst neurons (Fuchs et al. 1993; Ohtsuka and Noda 1991, 1995) that drive the burst discharge of motor and internuclear neurons in the abducens nucleus (Henn et al. 1984). Until now, no studies have tested whether variations in stimulation frequency or intensity would be associated with variations in the characteristics of evoked saccades. The present study bridges this gap and demonstrates that cFN activity influences the dynamics of saccades. This demonstration corroborates the hypothesis that the cerebellum is involved in steering the saccades (Goffart et al. 2003; Keller et al. 1983).
METHODS
Subjects and Surgical Procedures
Four adult macaque rhesus monkeys were used for this experiment. Two surgical procedures were performed under isoflurane anesthesia and aseptic conditions. First, a titanium post for immobilizing the head was secured with stainless steel screws and bone cement on the top front center of the skull. For the monitoring of eye movements, a three-turn search coil (Cooner Wire AS 632) was sutured to the sclera under the conjunctiva of one eye (monocular recording). Leads were passed under the skin to a connector located on the top of the skull. Oculomotor training was initiated after full recovery. In a second surgical procedure, a craniotomy was made and a recording chamber implanted for stimulating the fastigial nuclei. The chamber was placed in the frontal plane, centered stereotaxically between the two cFNs (9 mm posterior and 7 mm above the interaural line), and tilted 20° to the right with respect to the animal's sagittal plane. All surgical procedures and experiments were approved by the Baylor College of Medicine Animal Care and Use Committee (monkeys I and M) and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the guidelines from the French Ministry of Agriculture (87/848) and the European Community (86/609/EEC) (monkeys B and E). Care and maintenance of the animals were under the auspices of full-time veterinarians.
Behavioral Tasks
Experiments were conducted in a dimly illuminated room. The monkeys were seated in a primate chair with the head-restrained. In two animals (B and E), the chair was fitted with foam cushions (front and back) that gently but firmly positioned the animal's trunk in front of a concave spherical surface (located 110 cm ahead) that we designed for studies made in head-unrestrained conditions (Quinet and Goffart 2005, 2007, 2009). In the other animals, the target display was flat and located 145 cm ahead (Goffart et al. 2004). Eye position was measured with a phase angle detection system (CNC Engineering; 3-ft. coil frame), and signals were calibrated by having the head-restrained animal foveate stationary targets located at ±20° along the horizontal and vertical meridians.
Each animal was trained to perform a saccade task that shifted gaze from a central (located straight ahead) light-emitting diode (LED) toward a peripheral one. For each trial, a warning tone preceded the onset of the central LED. The monkey's task was to maintain gaze within a spatial window around it (3° radius) for a variable interval (400–1,200 ms varied in increments of 200 ms). After this interval, the central LED was extinguished, and after a gap interval (200 ms), the peripheral LED was flashed for 100 ms (25% of the trials) or remained on for the duration of the trial (35–45%). The subjects were not required to maintain fixation during the gap interval. This period was used as a catch to test eventual expectancies about target locations. The absence of premature saccades teaches us that the monkeys did not make such expectancies. Reward was delivered after the animal looked at the peripheral LED within a spatial window (6–10° radius) for a minimum interval of 300 ms. The location of peripheral LEDs was pseudorandomly selected among several predefined positions with various horizontal (8, 16, and 24° to the left or to the right of the central LED) and vertical eccentricities (8 or 16° above or below). In the remaining trials (30–40%), a stimulation train was delivered to the fastigial nucleus immediately after the gap interval. Five hundred milliseconds after the offset of the stimulation train, a peripheral LED was lit at a location that was also pseudorandomly selected among the same positions as defined above, and the monkey was rewarded for generating a saccade toward its location and foveating it for a minimum interval of 300 ms. These stimulation trials were pseudorandomly interleaved with the other trials.
Microstimulation Parameters
Electrical microstimulation was delivered through a tungsten microelectrode (Microprobe WE5003; impedance = 0.7–1.2 MΩ, tapered tip) using a stimulus generator (Grass S48) and photoelectric isolation unit (Grass PSIU6). It consisted of a train of cathodal (negative) monopolar pulses (0.2 ms duration). Several frequencies (from 100 to 600 pulses/s) and durations (from 10 to 300 ms) were tested. Current intensities were varied between the threshold (T) for evoking a saccadic eye movement (elicitation of a saccade in 75% of the cases with a 60-ms train of 600 pulses/s) and four times the threshold (4T); they never exceeded 120 μA. Stimulation was applied only at sites where saccade-related neurons were identified, at nearby sites (strong saccade-related background activity), and where unilateral inactivation was performed during subsequent experiments in the head-restrained (Goffart et al. 2003, 2004, 2005) and -unrestrained conditions (Quinet and Goffart 2005, 2007). We want to stress that the electrical stimulation elicits artificial activity that does not mimic any natural signal. The duration of stimulation likely affects the time during which activity is injected into the stimulated region, whereas the current determines the volume of neural tissue (axons and cells) that is activated. The effects of frequency on neural activity are more difficult to appreciate. In addition to influencing the firing rate of axons and neurons, the frequency of stimulation also contributes to the volume of activated neural mass, because it excites inhibitory afferents and interneurons. In this work, the stimulation frequency and intensity must be considered only as means to experimentally test the effects of different strengths of fastigial activity.
Data Analysis
A total of 118 sites were tested with electrical microstimulation in the head-restrained condition (monkey B, 46 sites; monkey E, 22 sites; monkey I, 19 sites; monkey M, 31 sites). Data were digitized online and analyzed offline using a software program that detected the onset and offset of saccades on the basis of a velocity threshold (15°/s). The results of the automatic detection were checked by inspecting each trial individually and adjusted manually when necessary. Several parameters such as the amplitude, duration, and peak velocity of the horizontal and vertical components were measured automatically. When the stimulation train was long enough to elicit a sequence of saccades (staircase saccades), only the first evoked saccade was measured. Its amplitude stayed approximately stable for some stimulation train duration before the second saccade (in the staircase) was evoked. To facilitate the reading of the quantitative results and figures, the horizontal amplitudes are expressed so that positive values correspond to ipsilateral saccades and negative values to contralateral saccades (exception is made in the qualitative Figs. 1 and 12). Positive values of vertical amplitude correspond to upward saccades and negative values to downward saccades. For each parameter of stimulation, a correlation (Pearson correlation) was performed whenever possible (at least 3 sets of measures). We report only correlations (positive or negative) that are statistically significant (P < 0.05). These correlations were graphically inspected to verify that they were not attributed to outliers. Statistical values reported in results correspond to means ± SD.
Fig. 1.
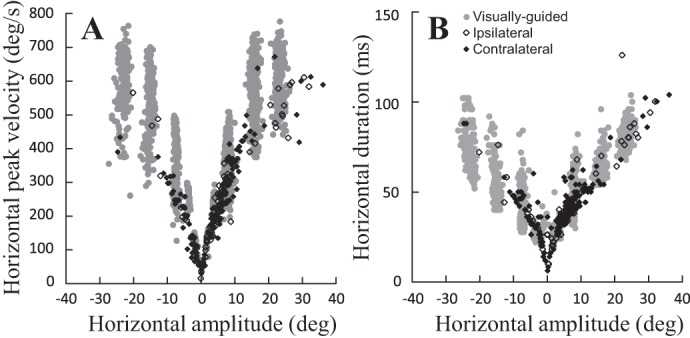
Main sequence relationships. Amplitude-peak velocity (A) and amplitude-duration (B) relationships of the horizontal component of visually guided (gray circles) and electrically elicited saccades [100 ms, 400 pps, 2 times threshold (2T)] are shown. Open diamonds, ipsilateral saccades (elicited at 13 sites tested in monkeys B and E); filled diamonds, contralateral saccades (20 sites in monkeys B, E, and I). The visual saccades (recorded in B) correspond to horizontal and oblique saccades aimed at a target located −16, −8, 8, or 16° horizontally and −8, 0, 8, or 16° vertically.
Fig. 12.
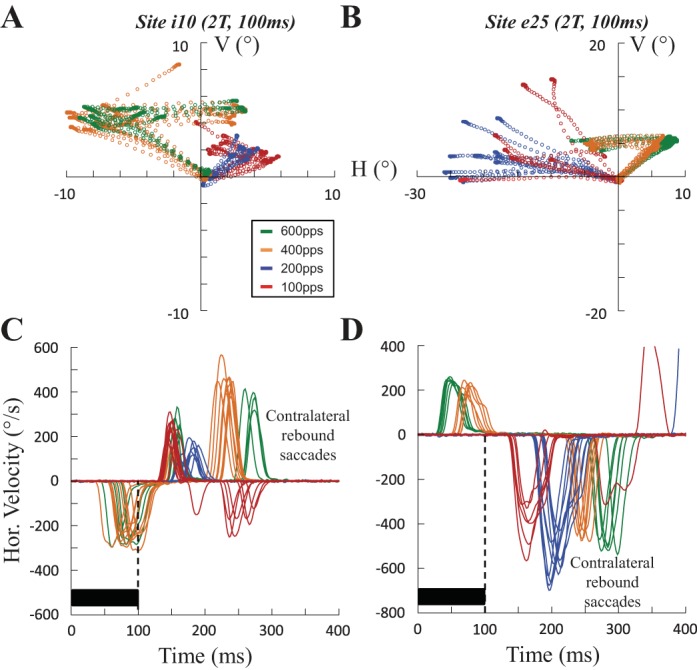
Rebound saccade when the stimulation frequency is reduced. Spatial trajectory (A and B) and time course of the horizontal velocity (C and D) of saccades evoked at sites i10 (100 ms, 2T = 40 μA) and e25 (100 ms, 2T = 60 μA) are shown with different stimulation frequencies (red, 100 pps; blue, 200 pps; orange, 400 pps; green, 600 pps). At a high frequency of stimulation (400 and 600 pps), an ipsilateral saccade is first evoked, followed by a rebound saccade. At a lower frequency of stimulation (100 and 200 pps), the ipsilateral saccade has disappeared. A contralateral saccade is evoked with a latency that is timed to the stimulation offset. Note at site i10 the “counter-rebound” saccade (negative velocity values in C), which is absent at site e25 (D).
Localization of Stimulation Sites
After termination of all experiments (unit recording, electrical microstimulation and pharmacological inactivation), two monkeys (monkey B and I) were euthanized by an overdose of pentobarbital sodium and perfused transcardially with saline, followed by 10% formalin. Standard techniques were used to prepare slices of 60-μm thickness on a freezing microtome. The observation of electrode tracks in coronal sections confirmed that our electrode penetrations were all made in the deep medial cerebellum (Goffart et al. 2004; see also Fig. 1 in Quinet and Goffart 2007 for the histology of monkey B). The distance along the mediolateral axis that separated the two most eccentric tracks toward the left and right fastigial nuclei was ∼5mm. In the other monkeys, histological reconstruction could not be made. However, when muscimol was injected at the stimulated sites (Goffart et al. 2003, 2004, 2005; Quinet and Goffart 2005, 2007) or when a low-frequency electrical stimulation was applied in the dorsocaudal part of the nuclei (e.g., Goffart et al. 2003) during visually guided saccades, the typical horizontal hypermetria of ipsilesional saccades/hypometria of contralesional saccades and the ipsipulsion of vertical saccades were always observed. The fact that the ipsipulsion changed from leftward to rightward when these functional perturbations were applied at the sites that were electrically stimulated in this article confirms that the microstimulations described here were applied in the fastigial nuclei and not in the interpositus nucleus. When the latter was stimulated, the current threshold to evoke saccades was much higher and no ipsipulsion was observed during pharmacological inactivation.
RESULTS
Depending on the site, electrical microstimulation with a high-frequency train (600 pulses/s) elicited rapid eye movements whose directions were either contralateral or ipsilateral with respect to the stimulated side. Figure 1 shows the horizontal amplitude-peak velocity (A) and horizontal amplitude-duration relationships (B) for electrically evoked (100 ms, 400 pulses/s, 2T) and various visually guided saccades. The fact that the main sequence relationships of electrically evoked movements overlap with those of visual saccades shows that the eye movements elicited by fastigial stimulation are saccadic. They are slower than visual saccades, possibly because the electrical stimulation did not evoke a phosphene as salient as the target LEDs. Moreover, it is worth noting that the spontaneous saccades and those aimed at auditory, somatosensory, or imaginary targets are slower than visual saccades (Goffart 2009). Furthermore, since only one eye was recorded, we do not know whether the elicited saccades were binocularly balanced or unbalanced. In the following, we first describe the contralateral saccades because they were proposed to result from the recruitment of axons of cFN neurons en route toward the contralateral reticular formation where preoculomotor neurons are located (Batton et al. 1977; Noda et al. 1988, 1990). The description of ipsilateral saccades follows. For each group of saccades, we successively describe the effects of the duration, the frequency and the current intensity on the electrically evoked saccades.
Contralateral Saccades
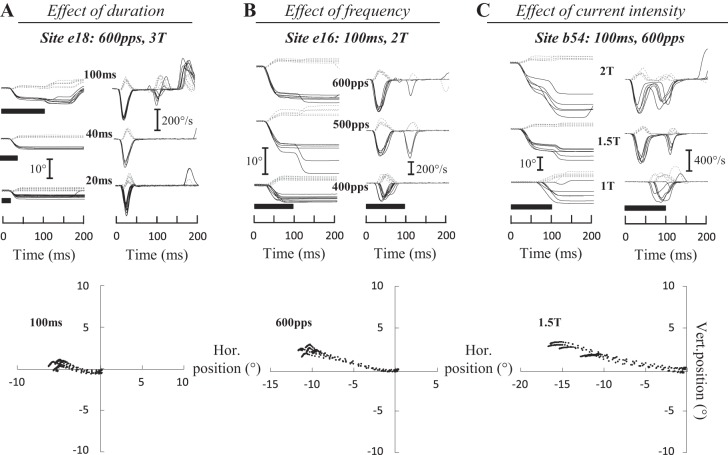
In our study, 51 sites evoked contralateral saccades (25 sites in monkey B, 8 in monkey E, 11 in monkey I, and 7 in monkey M). Figure 2 shows the time course of typical contralateral saccades evoked at three sites (e18, e16, and b54). In each panel, the horizontal and vertical components of evoked saccades are superimposed and aligned with the onset of stimulation. Increasing the train duration (Fig. 2A) enhanced the amplitude and peak velocity of the horizontal component without changing the vertical component. Similar increases were observed when the frequency (Fig. 2B) or current intensity (Fig. 2C) was increased. Manipulating these two parameters of stimulation also influenced the latency of evoked saccades. In the illustrated examples, the latency diminished from 29 ± 5 to 15 ± 1 ms when the frequency was increased from 400 to 600 pulses/s (Fig. 2B) and from 68 ± 6 to 15 ± 1 ms when the current increased from 1T to 2T (Fig. 2C). Figure 2, bottom, illustrates that the trajectory of contralateral saccades was not “abnormally” curved.
Fig. 2.
Electrically evoked contralateral saccades. The time course of eye position (horizontal, solid black lines; vertical, dashed gray lines) is shown after electrical microstimulation in the fastigial nucleus for 3 sites and as a function of the duration (A), frequency (B), and intensity (C) of stimulation. A: for site e18 [600 pulses/s (pps), 3T = 45 μA], when the stimulation was increased from 20 to 100 ms, the amplitude of the horizontal component increased from 2.7 ± 0.5 to 4.9 ± 0.3° and its peak velocity from 221 ± 34 to 286 ± 14°/s. The amplitude of the vertical component increased from 0.7 ± 0.5 to 1.1 ± 0.3° and its peak velocity from 65 ± 31 to 85 ± 17°/s. B: for site e16 (100 ms, 2T = 30 μA), when the stimulation frequency was increased from 400 to 600 pps, the amplitude of the horizontal component increased from 5.8 ± 2.1 to 10.4 ± 0.7° and its peak velocity from 233 ± 51 to 442 ± 30°/s. The amplitude of the vertical component decreased from 2.7 ± 1.7 to 2.5 ± 0.4°, whereas peak velocity increased from 117 ± 38 to 120 ± 13°/s. C: for site b54 (100 ms, 600 pps), when the current intensity was doubled from 40 to 80 μA (i.e., from 1T to 2T), the horizontal amplitude and peak velocity increased from 10.7 ± 3.4 to 16.8 ± 4.1° and from 334 ± 64 to 597 ± 66°/s, respectively, whereas the amplitude of the vertical component decreased from 4.5 ± 0.8 to 2.8 ± 1.4° and its peak velocity from 140 ± 23 to 138 ± 48°/s. The trajectory of saccades is illustrated at bottom. Values are means ± SD. Hor., horizontal; Vert., vertical.
For 15 sites where contralateral saccades were elicited, Table 1 gives the amplitude, peak velocity, and duration of the horizontal and vertical components, and also the latency of saccades. The evoked saccades were mostly horizontal with mean amplitudes that ranged from −1.9 ± 0.7 (b47) to −21 ± 10.9° (b17). At two sites (b7 and b109), the vertical amplitude was larger than 5° (8.0 ± 3.8 and 12.4 ± 4.6°, respectively). The horizontal peak velocity ranged from 135 ± 40 (b47) to 597 ± 66°/s (b54), and the vertical peak velocity ranged from 41 ± 17 (b12) to 301 ± 90°/s (b109). The horizontal duration ranged from 21 ± 3 (b47) to 77 ± 34 ms (b17), and the vertical duration ranged from 17 ± 3 (e18) to 61 ± 9 ms (b109). The values for latency ranged from 15 ± 1 (b54 and e16) to 52 ± 27 ms (b109). We next quantitatively document the effects of varying stimulation duration, frequency, and current.
Table 1.
Effects of stimulation frequency on contralateral and ipsilateral evoked saccades
| Site | Direction | n | Parameters, pulses/s, ms, μA | Horizontal Amplitude, ° | Vertical Amplitude, ° | Horizontal Peak Velocity, °/s | Vertical Peak Velocity, °/s | Horizontal Duration, ms | Vertical Duration, ms | Latency, ms |
|---|---|---|---|---|---|---|---|---|---|---|
| b7 | Contra | 6 | 600, 100, 50 | −17.2 ± 6.7 | 8.0 ± 3.8 | 542 ± 52 | 226 ± 14 | 54 ± 22 | 52 ± 22 | 16 ± 1 |
| b7 | Contra | 7 | 400, 100, 50 | −13.6 ± 7.6 | 8.9 ± 4.8 | 345 ± 56 | 214 ± 42 | 58 ± 21 | 56 ± 21 | 28 ± 4 |
| b12 | Contra | 7 | 600, 100, 50 | −7.0 ± 1.6 | 0.3 ± 0.5 | 339 ± 54 | 41 ± 17 | 43 ± 6 | 33 ± 16 | 20 ± 1 |
| b12 | Contra | 9 | 400, 100, 50 | −6.2 ± 2.5 | 1.5 ± 1.5 | 251 ± 55 | 77 ± 50 | 39 ± 8 | 24 ± 11 | 38 ± 6 |
| b17 | Contra | 5 | 600, 200, 60 | −19.1 ± 8.6 | 2.3 ± 0.5 | 534 ± 93 | 110 ± 29 | 66 ± 21 | 29 ± 3 | 21 ± 2 |
| b17 | Contra | 14 | 400, 200, 60 | −21 ± 10.9 | 1.7 ± 3.6 | 438 ± 95 | 120 ± 48 | 77 ± 34 | 52 ± 35 | 39 ± 5 |
| b19 | Contra | 6 | 600, 100, 80 | −5.0 ± 0.6 | 1.1 ± 0.4 | 205 ± 35 | 73 ± 23 | 39 ± 3 | 18 ± 3 | 21 ± 2 |
| b19 | Contra | 6 | 400, 100, 80 | −7.7 ± 2.3 | 2.3 ± 1.2 | 278 ± 50 | 131 ± 52 | 46 ± 11 | 25 ± 5 | 50 ± 10 |
| b54 | Contra | 5 | 600, 100, 80 | −16.8 ± 4.1 | 2.8 ± 1.4 | 597 ± 66 | 138 ± 48 | 49 ± 4 | 29 ± 8 | 15 ± 1 |
| b54 | Contra | 14 | 400, 100, 80 | −12.0 ± 7.6 | 2.6 ± 1.1 | 355 ± 101 | 120 ± 48 | 54 ± 15 | 31 ± 10 | 23 ± 3 |
| b63 | Contra | 8 | 600, 100, 50 | −8.6 ± 1.8 | 1.1 ± 0.9 | 387 ± 45 | 83 ± 48 | 55 ± 6 | 22 ± 14 | 17 ± 1 |
| b63 | Contra | 7 | 400, 100, 50 | −7.2 ± 2.2 | 2.2 ± 0.9 | 295 ± 71 | 111 ± 33 | 43 ± 5 | 26 ± 4 | 31 ± 7 |
| b96 | Contra | 5 | 600, 200, 60 | −12.0 ± 2.0 | 4.2 ± 0.9 | 459 ± 36 | 178 ± 29 | 46 ± 5 | 36 ± 3 | 19 ± 2 |
| b96 | Contra | 5 | 400, 200, 60 | −9.7 ± 2.0 | 4.3 ± 1.0 | 322 ± 31 | 174 ± 31 | 46 ± 5 | 36 ± 4 | 29 ± 2 |
| e16 | Contra | 6 | 600, 100, 30 | −10.4 ± 0.7 | 2.5 ± 0.4 | 442 ± 30 | 120 ± 13 | 40 ± 2 | 29 ± 2 | 15 ± 1 |
| e16 | Contra | 31 | 400, 100, 30 | −7.0 ± 4.6 | 3.1 ± 2.8 | 258 ± 79 | 119 ± 44 | 40 ± 12 | 32 ± 15 | 29 ± 5 |
| e18 | Contra | 7 | 600, 100, 45 | −4.9 ± 0.3 | 1.1 ± 0.3 | 286 ± 14 | 85 ± 17 | 30 ± 1 | 17 ± 3 | 18 ± 0 |
| e18 | Contra | 5 | 400, 100, 45 | −4.1 ± 0.4 | 1.4 ± 0.4 | 227 ± 17 | 89 ± 21 | 29 ± 1 | 20 ± 3 | 27 ± 2 |
| b47 | Contra | 6 | 600, 100, 100 | −1.9 ± 0.7 | 4.2 ± 1.2 | 135 ± 40 | 224 ± 48 | 21 ± 3 | 29 ± 3 | 30 ± 10 |
| b109 | Contra | 5 | 600, 200, 20 | −9.5 ± 1.7 | 12.4 ± 4.6 | 305 ± 21 | 301 ± 90 | 55 ± 10 | 61 ± 9 | 52 ± 27 |
| e11 | Contra | 5 | 600, 100, 60 | −9.2 ± 2.5 | 3.6 ± 1.0 | 397 ± 75 | 136 ± 22 | 38 ± 4 | 37 ± 6 | 17 ± 2 |
| e21 | Contra | 9 | 400, 200, 50 | −5.1 ± 1.2 | 2.6 ± 0.6 | 268 ± 33 | 145 ± 26 | 34 ± 4 | 29 ± 4 | 28 ± 4 |
| e56 | Contra | 15 | 600, 200, 50 | −6.8 ± 2.4 | 2.1 ± 0.4 | 347 ± 74 | 126 ± 21 | 35 ± 4 | 26 ± 3 | 17 ± 2 |
| e57 | Contra | 5 | 600, 200, 20 | −6.3 ± 0.9 | 2.0 ± 0.3 | 359 ± 44 | 123 ± 20 | 33 ± 2 | 27 ± 1 | 23 ± 1 |
| b66 | Ipsi | 6 | 600, 100, 60 | 9.3 ± 1.8 | 3.4 ± 0.9 | 445 ± 79 | 175 ± 47 | 43 ± 3 | 30 ± 2 | 14 ± 1 |
| b66 | Ipsi | 8 | 400, 100, 60 | 6.9 ± 1.7 | 2.9 ± 1.0 | 292 ± 47 | 136 ± 30 | 39 ± 5 | 30 ± 6 | 24 ± 3 |
| b69 | Ipsi | 5 | 600, 200, 60 | 26.6 ± 4.8 | 2.8 ± 1.8 | 515 ± 74 | 170 ± 40 | 90 ± 7 | 69 ± 20 | 55 ± 10 |
| b69 | Ipsi | 10 | 400, 200, 60 | 19.2 ± 7.2 | 6.7 ± 2.8 | 414 ± 124 | 241 ± 62 | 76 ± 20 | 56 ± 21 | 63 ± 10 |
| b71 | Ipsi | 10 | 600, 200, 80 | 7.5 ± 3.3 | 8.6 ± 3.1 | 246 ± 58 | 261 ± 55 | 49 ± 12 | 55 ± 14 | 42 ± 17 |
| b71 | Ipsi | 8 | 400, 200, 80 | 6.8 ± 3.2 | 10.8 ± 4.9 | 153 ± 35 | 267 ± 72 | 56 ± 19 | 60 ± 21 | 76 ± 21 |
| e38 | Ipsi | 6 | 600, 200, 100 | 11.9 ± 1.8 | 4.5 ± 0.8 | 295 ± 36 | 184 ± 42 | 64 ± 10 | 54 ± 13 | 35 ± 8 |
| e38 | Ipsi | 7 | 400, 200, 100 | 13.5 ± 6.4 | 4.0 ± 0.7 | 283 ± 8 | 143 ± 24 | 75 ± 28 | 50 ± 15 | 79 ± 5 |
| m53 | Ipsi | 15 | 600, 120, 80 | 2.4 ± 0.7 | 5.2 ± 0.9 | 117 ± 39 | 250 ± 30 | 29 ± 4 | 32 ± 2 | 52 ± 17 |
| m53 | Ipsi | 14 | 400, 120, 80 | 1.5 ± 0.9 | 4.9 ± 1.1 | 81 ± 46 | 229 ± 47 | 28 ± 3 | 32 ± 2 | 62 ± 12 |
| b55 | Ipsi | 5 | 400, 200, 60 | 0.7 ± 1.6 | −12.1 ± 4.3 | 71 ± 33 | 308 ± 65 | 53 ± 33 | 68 ± 23 | 52 ± 9 |
| b62 | Ipsi | 16 | 600, 200, 100 | 2.0 ± 2.3 | 7.9 ± 6.3 | 92 ± 52 | 283 ± 99 | 24 ± 18 | 42 ± 22 | 50 ± 25 |
| e14 | Ipsi | 7 | 400, 100, 100 | 1.8 ± 0.2 | 3.1 ± 0.3 | 127 ± 11 | 159 ± 19 | 21 ± 1 | 28 ± 1 | 28 ± 3 |
| e17 | Ipsi | 7 | 600, 100, 60 | 1.1 ± 0.3 | 2.0 ± 0.3 | 83 ± 13 | 110 ± 16 | 18 ± 2 | 25 ± 2 | 25 ± 2 |
| e48 | Ipsi | 6 | 400, 200, 80 | 7.9 ± 1.7 | 5.4 ± 1.8 | 332 ± 74 | 273 ± 104 | 40 ± 4 | 35 ± 3 | 84 ± 31 |
| i4 | Ipsi | 5 | 400, 100, 80 | 5.6 ± 3.3 | 11.3 ± 5.4 | 245 ± 115 | 450 ± 191 | 39 ± 13 | 52 ± 10 | 23 ± 10 |
| m25 | Ipsi | 7 | 400, 120, 50 | 11.3 ± 1.3 | −0.7 ± 1.3 | 466 ± 36 | 81 ± 74 | 47 ± 5 | 18 ± 15 | 56 ± 8 |
| m51 | Ipsi | 6 | 600, 100, 35 | 18.9 ± 2.7 | 4.7 ± 1.2 | 429 ± 70 | 172 ± 35 | 77 ± 8 | 47 ± 7 | 49 ± 8 |
| m54 | Ipsi | 11 | 600, 120, 50 | 12.0 ± 1.1 | −0.9 ± 0.8 | 516 ± 35 | 53 ± 27 | 48 ± 3 | 33 ± 16 | 37 ± 3 |
| m67 | Ipsi | 7 | 600, 100, 50 | 15.5 ± 1.8 | 0.2 ± 0.4 | 585 ± 43 | 78 ± 15 | 47 ± 3 | 19 ± 13 | 32 ± 2 |
Summary data represent 30 stimulation sites. For each site, the direction (ipsi- or contralateral) of evoked movements, the number of stimulation trials (n) and the means ± SD of horizontal or vertical amplitude, peak velocity, and duration are reported. Latency is also documented. All movements were evoked with current intensity equal to 2 times the threshold (2T).
Effects of train duration.
When they stimulated the fastigial nucleus in the head-restrained monkey, Noda et al. (1988) only tested a short train with a fixed frequency (20 ms at 600 pulses/s). Figure 3 shows that the amplitude of evoked saccades was likely truncated during these testing conditions, since an increase in amplitude was observed when the stimulation duration exceeded 20 ms. For three typical sites, the average amplitude (Fig. 3A), peak velocity (Fig. 3B), latency (Fig. 3C), and duration (Fig. 3D) of evoked saccades are plotted as a function of the train duration. The amplitude of the horizontal component increased with the duration of stimulation (Fig. 3A). At two sites (e18 and b54), a maximum amplitude was reached when the stimulation duration exceeded 40 ms. For the site m46, the horizontal amplitude increased beyond this duration, likely because of saccade latencies that were longer than those evoked at the two other sites (Fig. 3C). Note that at this site, the amplitude increased only because the electrical excitation lasted longer (Fig. 3D), whereas the peak velocity reached a maximum value (Fig. 3B). Concerning the vertical component of evoked movements, an effect of increasing the duration of stimulation was barely visible.
Fig. 3.
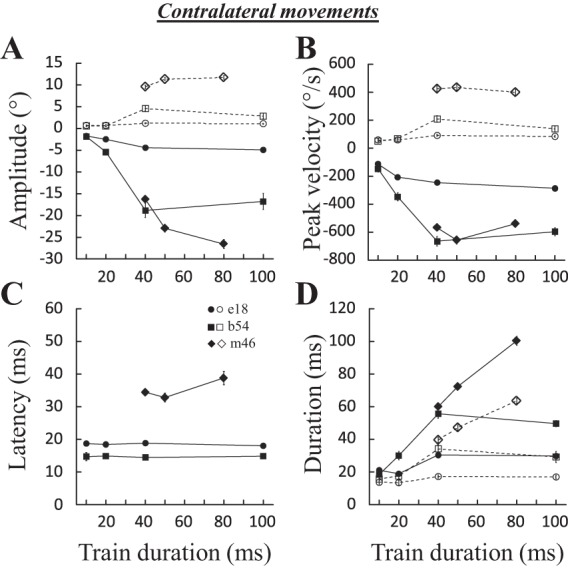
Effects of stimulation duration on contralateral saccades. The amplitude (A), peak velocity (B), latency (C), and duration (D) of evoked saccades are documented for 3 sites: e18 (600 pps, 3T = 45 μA), b54 (600 pps, 2T = 80 μA), and m46 (600 pps, 2T = 50 μA). Means and SE are represented for each tested stimulation duration. Filled symbols and solid lines, horizontal component; open symbols and dashed lines, vertical component.
For 15 sites, a correlation analysis could be performed to test the effect of stimulation duration because we had a minimum of 3 durations tested per site. The horizontal amplitude was positively correlated with the stimulation duration at 12 (80%) sites (Spearman correlation coefficients: 0.41 < R < 0.88, P < 0.05), the peak velocity and the duration of the horizontal component were positively correlated at 9 (60%; 0.37 < R < 0.75) and 13 (87%; 0.43 < R < 0.97) sites, respectively. For the vertical component, the correlations were less frequent: increasing the stimulation duration increased its amplitude, peak velocity, and duration at 7 (46%; 0.34 < R < 0.72), 4 (26%; 0.33 < R < 0.64), and 7 (46%; 0.32 < R < 0.96) sites, respectively. In summary, our study demonstrates that saccade amplitude increases with stimulation duration until reaching a maximum value, which indicates that the contralateral movements documented by Noda et al. (1988) were most likely truncated. Consistent with anatomic and inactivation studies, it also confirms that the cFN axons target neural processes that are primarily involved in the generation of horizontal saccades (Goffart et al. 2004; Sato and Noda 1991; see also below). Increasing the duration of stimulation primarily influences the duration and amplitude of contralateral saccades.
Effects of stimulation frequency.
As shown in Fig. 2, the duration was not the only parameter of stimulation that influenced the properties of evoked contralateral saccades. Figure 4 shows the effects of increasing the frequency for three sites. For each site, the horizontal amplitude (Fig. 4A) and peak velocity (Fig. 4B) increased, whereas the latency decreased, with the frequency of stimulation. At two sites (b7 and b63), increasing the frequency from 400 to 600 pulses/s augmented the horizontal amplitude by 26% (b7) and 21% (b63) and the horizontal peak velocity by 56% (b7) and 31% (b63). The duration of saccades barely changed (6% at b7, 28% at b63, and 0% at e16). The frequency of stimulation also influenced the latency of evoked saccades: the higher the rate, the shorter the latency (Fig. 4C). The effect of stimulation frequency could be tested with a correlation analysis at 13 sites: at 8 (61%) sites, the horizontal amplitude was positively correlated with the frequency of stimulation (0.43 < R <0.84); the peak velocity and the duration of the horizontal component were correlated at 10 (77%; 0.49 < R < 0.92) and 8 sites [61%; positively at 5 sites (0.37 < R < 0.77) and negatively at 3 sites (−0.51 < R < −0.74)], respectively. The latency of evoked saccades was negatively correlated in 12 sites (92%; −0.38 < R < −0.91). We could not find any significant correlation between the increases in peak velocity and the decreases in latency (using the percentage values) as the stimulation frequency was increased from 300 to 600 pulses/s (P = 0.40). At some sites, increasing the frequency of stimulation decreased the amplitude [6 sites (46%); 0.49 < R < 0.82], peak velocity [5 sites (38%); 0.52 < R < 0.75], and duration [6 sites (46%); 0.37 < R < 0.82] of the vertical component. In summary, the frequency of stimulation primarily influences the peak velocity and the latency of contralateral saccades. These influences on the dynamics and triggering of saccades are not correlated. Faster saccades are evoked with higher frequencies of stimulation. This result is consistent with the reduction of horizontal peak velocity of contralateral saccades after muscimol injection in the cFN (Buzunov et al. 2013; Goffart et al. 2004; Robinson et al. 1993).
Fig. 4.
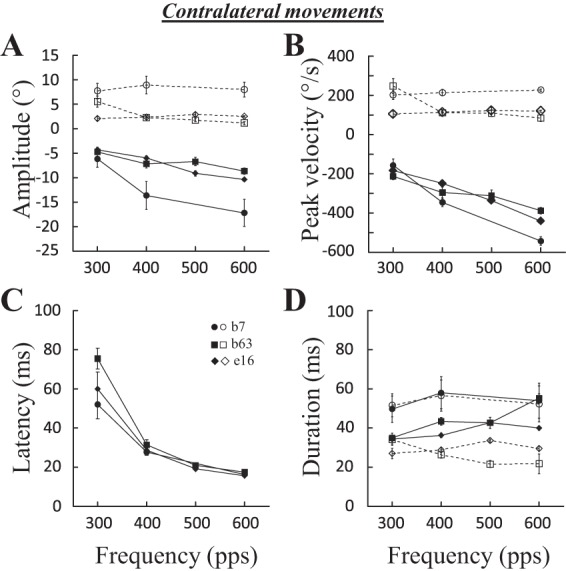
Effects of stimulation frequency on contralateral saccades. The amplitude (A), peak velocity (B), latency (C), and duration (D) of evoked saccades are documented for 3 sites: b7 (100 ms, 2T = 50 μA), b63 (100 ms, 2T = 50 μA), and e16 (100 ms, 2T = 30 μA). Means and SE are represented for each tested stimulation frequency. Filled symbols and solid lines, horizontal component; open symbols and dashed lines, vertical component.
Effects of current intensity.
Figure 5 shows that the amplitude (A), peak velocity (B), and duration (D) of the horizontal component also increased with the intensity of the stimulation current, whereas those of the vertical component did not. For the three illustrated sites, the horizontal amplitude (Fig. 5A) and peak velocity (Fig. 5B) increased, whereas the saccade latency decreased, with the intensity (Fig. 5C). It was possible to perform a correlation analysis for 16 sites: the horizontal amplitude, peak velocity, and duration were positively correlated with the stimulation intensity at 9 (56%; 0.55 < R < 0.91), 10 (62%; 0.55 < R < 0.98), and 7 (44%; 0.52 < R < 0.92) sites, respectively. The latency was negatively correlated with the intensity at 15 sites (94%; −0.92< R <−0.52). We could not find any significant correlation between the increases in peak velocity and the decreases in latency as the stimulation intensity was increased from 1T to 2T (P = 0.17). The amplitude, peak velocity, and duration of the vertical component were decreased when the current intensity was increased at 6 (37%; 0.47 < R < 0.73), 4 (25%; 0.57 < R < 0.88), and 3 (18%; 0.50 < R < 0.76) sites, respectively.
Fig. 5.
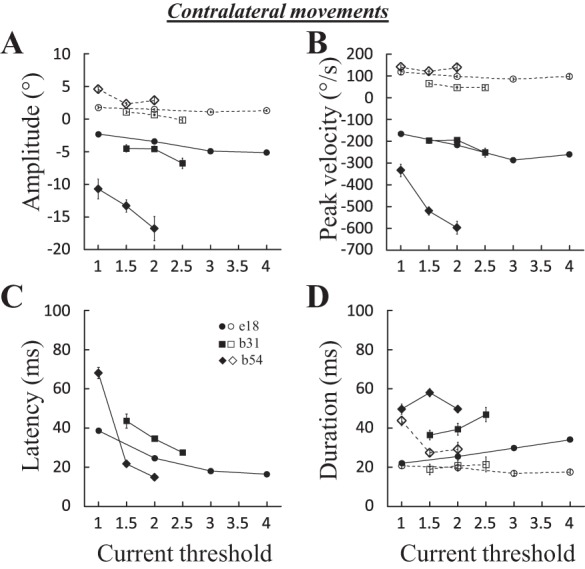
Effects of stimulation current on contralateral saccades. The amplitude (A), peak velocity (B), latency (C), and duration (D) of evoked saccades are documented for 3 sites: e18 (100 ms, 600 pps), b31 (100 ms, 400 pps), and b54 (100 ms, 600 pps). Means and SE are represented for each tested stimulation current. Filled symbols and solid lines, horizontal component; open symbols and dashed lines, vertical component.
In summary, these results are altogether another demonstration that the cFN predominantly influences the neural processes involved in the generation of horizontal component of saccades (Goffart et al. 2004; Sato and Noda 1991). The intensity of the stimulation primarily affects processes involved in the control of saccade velocity. An influence on their triggering is also observed, but these influences on the dynamics and triggering of saccades are not correlated.
To better appreciate the specific influence of increasing fastigial activity on the horizontal component, Fig. 6 shows the effect of doubling the stimulation current (A; 15 tested sites) and frequency (B; 8 tested sites) on the average horizontal and vertical peak velocities. Augmenting the current significantly increased the horizontal peak velocity at 11 (73%) sites and the vertical peak velocity at 5 (33%) sites. The paired comparison (Wilcoxon nonparametric test) revealed a statistically significant increase in horizontal peak velocity (average difference = 137 ± 152°/s; P < 0.01) and no significant change in the vertical peak velocity (average difference = −11 ± 41°/s; P = 0.33). When the frequency was increased (from 300 to 600 pulses/s), the horizontal peak velocity was increased at 6 (75%) sites and the vertical peak velocity at 3 (37%) sites. The paired comparison revealed a statistically significant increase in horizontal peak velocity (average difference = 186 ± 123°/s; P < 0.01) and a reduction of vertical peak velocity (average difference = −56 ± 84°/s; P < 0.05). Table 1 also shows 9 sites where the effect of frequency on the horizontal and vertical peak velocities can be examined. The horizontal peak velocity increased at 8 of 9 (89%) sites, whereas the vertical peak velocity decreased at 4 (44%) sites, when the frequency of stimulation increased from 400 to 600 pulses/s.
Fig. 6.
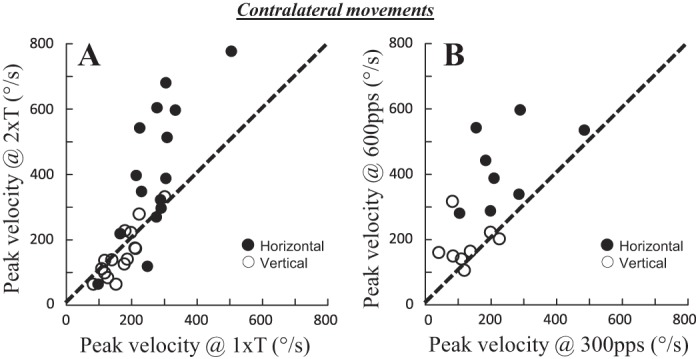
Effect of increased fastigial activity on peak velocity of contralateral saccades. The effects of doubling the stimulation current (A) and frequency (B) on the average horizontal (filled circles) and vertical peak velocities (open circles) are shown. In A, the stimulation current was increased from 1T to 2T (15 tested sites), whereas in B, the stimulation frequency was increased from 300 to 600 pps (8 sites).
Ipsilateral Saccades
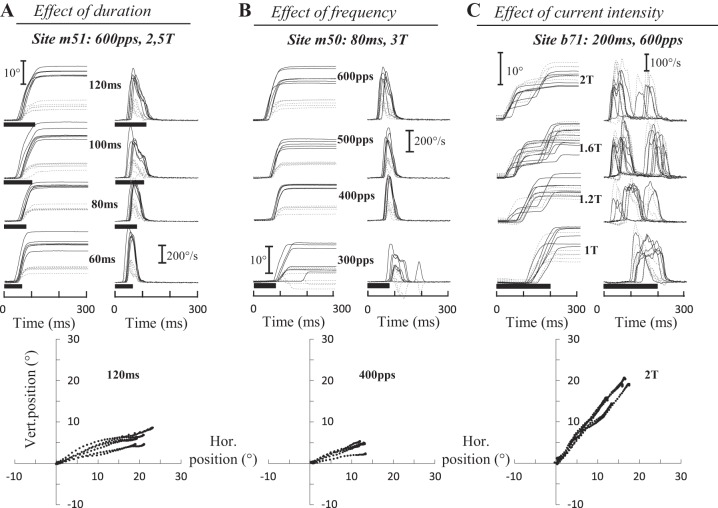
Noda et al. (1988) showed that stimulation in the fastigial nucleus can also elicit ipsilateral saccades, mostly when its dorsocaudal part is electrically excited. In our experiments, ipsilateral saccades were evoked at 67 sites (21, 14, 9, and 23 sites in monkeys B, E, I, and M, respectively). Figure 7 shows the time course of typical ipsilateral saccades evoked at three sites (m51, m50, and b71) when the duration (A), frequency (B), and current intensity (C) of stimulation were increased. Increasing the duration of stimulation (Fig. 7A) increased the amplitude and peak velocity of the horizontal component of evoked saccades; no vivid effect could be observed on the vertical component. The same dependency was observed when the frequency (Fig. 7B) was increased. The latency of evoked saccades was also influenced by the frequency and current intensity. For the illustrated sites, the latency diminished from 96 ± 33 to 46 ± 8 ms when the frequency increased from 300 to 600 pulses/s (Fig. 7B) and from 116 ± 16 to 29 ± 11 ms when the current intensity increased from 1T to 2T (Fig. 7C). Like contralateral evoked saccades, the horizontal amplitude and the peak velocity of ipsilateral saccades varied with all three parameters of stimulation, whereas the latency was influenced by the frequency and current intensity. Figure 7, bottom, illustrates that the trajectory of ipsilateral saccades was not abnormally curved.
Fig. 7.
Electrically evoked ipsilateral saccades. Time course of eye position (horizontal, black; vertical, gray) after electrical microstimulation in the fastigial nucleus for 3 sites and as a function of the duration (A), frequency (B), and intensity (C) of stimulation. A: for site m51 (600 pps, 2.5T = 35 μA), when the stimulation duration was doubled (from 60 to 120 ms), the horizontal amplitude increased from 16.1 ± 2.5 to 20.1 ± 1.9° and the peak velocity decreased from 534 ± 42 to 496 ± 40°/s. The amplitude of the vertical component increased from 4.5 ± 2.3 to 5.3 ± 0.9° and its peak velocity from 190 ± 73 to 203 ± 53°/s. B: for site m50 (80 ms, 3T = 21 μA), when the stimulation frequency was doubled (from 300 to 600 pps), the horizontal amplitude increased from 8.1 ± 4.4 to 15.6 ± 2.6° and the peak velocity from 219 ± 74 to 443 ± 44°/s. The amplitude of the vertical component increased from 1.6 ± 2.0 to 5.5 ± 1.4° and its peak velocity from 118 ± 47 to 226 ± 51°/s. C: for site b71 (200 ms, 600 pps), when the current intensity was increased from 1T to 2T (T = 50 μA), the amplitude of the horizontal component decreased from 16.9 ± 3.1 to 8.6 ± 2.9° and its peak velocity from 255 ± 43 to 241 ± 50°/s. The amplitude of the vertical component barely changed from 10.7 ± 3.6 to 10.1 ± 3.3°, whereas its peak velocity increased from 219 ± 70 to 338 ± 39°/s. The trajectory of saccades is illustrated at bottom. Values are means ± SD.
Table 1 shows the amplitude, peak velocity, and duration of the horizontal and vertical components of ipsilateral saccades, as well as their latency, for 15 sites. At 47% of sites, the evoked movement had vertical amplitude larger than 5°. At one site only, the vertical component was directed downward (b55: −12.1 ± 4.3°). The horizontal amplitude ranged from 0.7 ± 1.6 (b55) to 26.6 ± 4.8° (b69). The horizontal peak velocity ranged from 71 ± 33 (b55) to 585 ± 43°/s (m67), whereas the vertical peak velocity ranged from 53 ± 27 (m54) to 450 ± 191°/s (i4). The duration of the horizontal component ranged from 18 ± 2 (e17) to 90 ± 7 ms (b69), and the duration of the vertical component ranged from 18 ± 15 (m25) to 69 ± 20 ms (b69). Finally, the latencies ranged from 14 ± 1 (b66) to 84 ± 31 ms (e48).
Effects of train duration.
Figure 8 shows how the train duration influences the amplitude (A), peak velocity (B), latency (C), and duration (D) of saccades evoked at three sites. The amplitude of the horizontal component increased with the duration of stimulation (Fig. 8A). When the peak velocity seemed to have reached a plateau (Fig. 8B, i7 and m67), the saccade amplitude increased because of longer duration. Concerning the vertical component, there was no obvious change as the duration of stimulation was increased. For 18 sites, we tested the statistical significance of the influence of stimulation duration with a correlation analysis: the stimulation duration was positively correlated with the horizontal amplitude at 12 sites (67%; 0.49 < R < 0.93, P < 0.05), the peak velocity at 4 sites (22%; 0.41 < R < 0.64), the duration of the horizontal component at 14 sites (77%; 0.41 < R < 0.94), and the latency at 11 sites (61%; 0.41< R < 0.75). Increasing the duration of stimulation was also found to increase the amplitude, peak velocity, and duration of the vertical component at 10 (0.41 < R < 0.79), 5 (0.44 < R < 0.72), and 9 (0.31 < R <0.94) sites, respectively. In summary, the duration of stimulation primarily drives the duration and amplitude of evoked saccades up to a maximum amplitude.
Fig. 8.
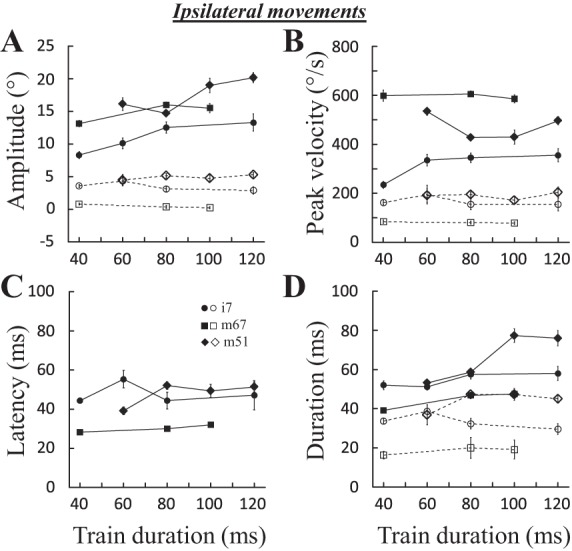
Effects of stimulation duration on ipsilateral saccades. The amplitude (A), peak velocity (B), latency (C), and duration (D) of evoked saccades are documented for 3 sites: i7 (400 pps, 2.5T = 38 μA), m67 (600 pps, 2.5T = 50 μA), and m51 (600 pps, 2.5T = 37 μA). Mean and SE are represented for each tested stimulation duration. Filled symbols and solid lines, horizontal component; open symbols and dashed lines, vertical component.
Effect of stimulation frequency.
Figure 9 shows the effects of stimulation frequency for three sites (b66, i16, and m51). Increasing the frequency increased the horizontal amplitude (Fig. 9A), peak velocity (Fig. 9B), and duration (Fig. 9D) of evoked saccades. As with contralateral evoked saccades, the latency of ipsilateral saccades decreased when the frequency of stimulation was increased (Fig. 9C). Effects on their vertical component were barely visible. For 16 sites where the correlation could be performed, a positive correlation was found between the frequency and the horizontal amplitude at only 5 sites (31%; 0.42 < R < 0.77), the peak velocity at 6 sites (37%; 0.37 < R < 0.75), and the duration of the horizontal component at 5 sites (31%; 0.42 < R < 0.84). The latency was negatively correlated with the frequency of stimulation in 11 sites (68%; −0.50 < R < −0.85). Increasing the frequency of stimulation also influenced the vertical component in a few sites (amplitude and peak velocity: 3 sites, 0.39 < R < 0.73 and 0.63 < R < 0.73, respectively; duration: 2 sites, 0.68 < R < 0.88). In summary, the frequency of stimulation primarily influences the latency of evoked saccades.
Fig. 9.
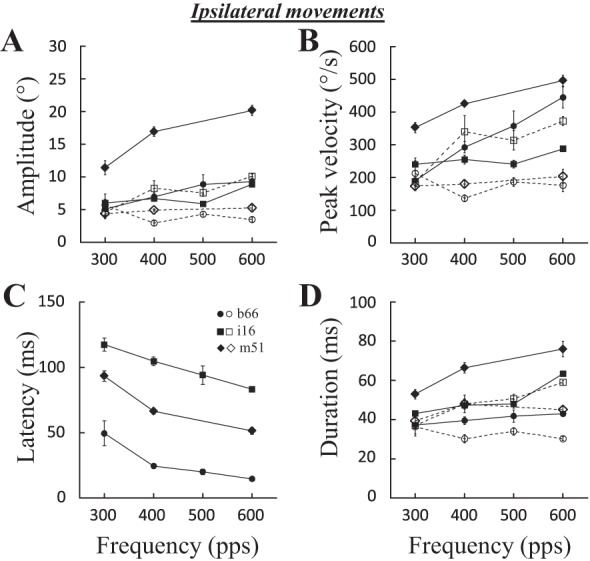
Effects of stimulation frequency on ipsilateral saccades. The amplitude (A), peak velocity (B), latency (C), and duration (D) of evoked saccades are documented for 3 sites: b66 (100 ms, 2T = 60 μA), i16 (200 ms, 2T = 50 μA), and m51 (120 ms, 2.5T = 35 μA). Means and SE are represented for each tested stimulation frequency. Filled symbols and solid lines, horizontal component; open symbols and dashed lines, vertical component.
Effect of current intensity.
Figure 10 shows the effects of increasing the current intensity for three sites (b69, e25, and m63). At some sites (b69 and e25), increasing the current intensity increased the amplitude of both horizontal and vertical components, whereas at some other sites (e.g., m63), the changes in horizontal and vertical amplitude were opposite. As expected, current intensity also influenced the latency of evoked saccades, decreasing it with larger current values (Fig. 10C). For 8 sites where an analysis of correlation could be made, stimulation intensity was positively correlated with the horizontal amplitude and peak velocity at 7 sites (87%; 0.53 < R < 0.92 and 0.57 < R < 0.95, respectively) and with the duration at 6 sites (75%; 0.46 < R < 0.92). A negative correlation was found between the latency of evoked saccades and current intensity at 7 sites (87%; −0.93 < R < −0.54). Increasing the current intensity also increased the peak velocity of the vertical component at 6 sites (75%; 0.47 < R < 0.83), whereas the vertical amplitude was increased at only 3 (37%) sites. In summary, like contralateral evoked saccades, current intensity has an effect on the amplitude, peak velocity, and duration of evoked movements. The latency is also influenced by this parameter, increasing the current decreases the latency of ipsilateral saccades.
Fig. 10.
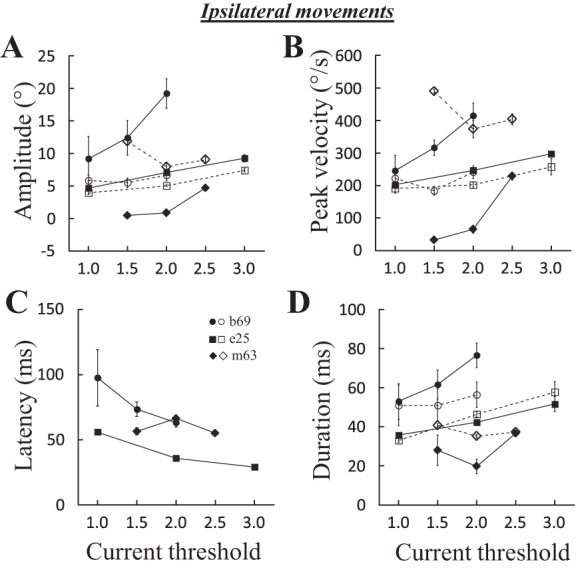
Effects of stimulation intensity on ipsilateral saccades. The amplitude (A), peak velocity (B), latency (C), and duration (D) of evoked saccades are documented for 3 sites: b69 (200 ms, 400 pps), e25 (100 ms, 600 pps), and m63 (60 ms, 600 pps). Means and SE are represented for each tested stimulation current. Filled symbols and solid lines, horizontal component; open symbols and dashed lines, vertical component.
Contralateral Rebound Saccades
In 52% of sites, the ipsilateral saccades were followed by a saccade directed toward the contralateral side. Figure 11A shows this phenomenon, which was also previously reported by Ron and Robinson (1973) when they stimulated in the OV (lobules VI–VII). Ipsilateral saccades were elicited with a latency that diminished (from 56 ± 3 to 29 ± 4 ms) and an amplitude that increased (from 4.7 ± 0.4 to 9.2 ± 1.4°) when the current of stimulation increased from 1T to 3T. Approximately 156 ms after the stimulation end (current intensity = 1T), a contralateral saccade was observed with timing and direction properties indicating that its origin lay in effects of the stimulation that cannot be appreciated under these behavioral testing conditions. The amplitude of the rebound saccades also increased (from −7.8 ± 0.9 to −16.8 ± 3.0°) when the current intensity increased from 1T to 3T.
Fig. 11.
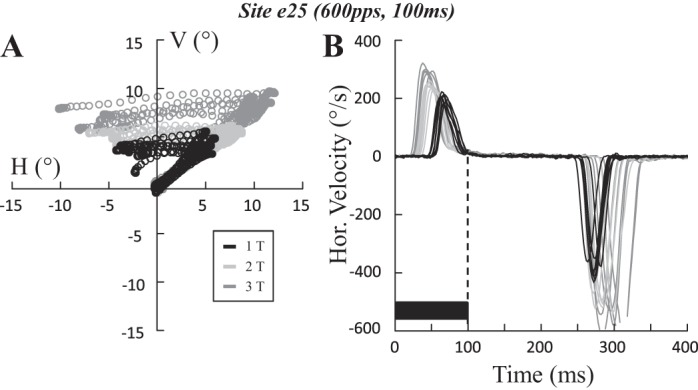
Rebound saccade after the ipsilateral saccade. Spatial trajectory (A) and time course of the horizontal velocity (B) of saccades evoked at site e25 (100 ms, 600 pps) are shown when the stimulation current was increased from one (1T = 15 μA; black) to two (2T; light gray) and 3 times threshold (3T; dark gray). The ipsilateral saccade is followed by a contralateral rebound saccade. Note how the onset of the rebound is timed to the offset of stimulation. The horizontal amplitude of ipsilateral saccades increased with the stimulation current, from 4.6 ± 0.2 (1T) to 7 ± 0.3 (2T) and 9.2 ± 0.6° (3T). Similarly, that of the rebound saccades increased from −7.8 ± 0.3 (1T) to −12 ± 0.6 (2T) and −16.8 ± 1.2° (3T). The latency of ipsilateral saccades decreased with the stimulation current, from 56 ± 1 (1T) to 35 ± 1 (2T) and 29 ± 2 ms (3T). Latency for the rebound saccades increased from 156 ± 2 (1T) to 164 ± 3 (2T) and 173 ± 4 ms (3T) with respect to the end of the stimulation train. Values are means ± SD. H, horizontal; V, vertical.
In a preliminary report, Goffart et al. (2003) showed that when a lower frequency of stimulation (100 pulses/s) was applied at a site where a train of 600 pulses/s evoked an ipsilateral saccade, this saccade was no longer elicited. A contralateral saccade was instead produced after the offset of the stimulation train. Figure 12 depicts this transition at 2 sites (i10 and e25) when the stimulation frequency was reduced from 600 to 100 pulses/s. With the highest frequencies of stimulation (400 or 600 pulses/s), the ipsilateral saccade was first evoked and followed by a contralateral rebound saccade with a direction (mostly horizontal) and a timing that were rather consistent across the trials. When lower frequencies (100 and 200 pulses/s) were tested at the same sites, the ipsilateral saccades disappeared; only the rebound saccades were elicited after the offset of the electrical stimulation train. Interestingly, their latency decreased from 180 ± 7 to 158 ± 46 ms while their direction became more variable when the stimulation frequency decreased from 200 to 100 pulses/s (Fig. 12D). This change in the direction of saccades when the frequency of stimulation was reduced from 600 to 100 pulses/s was observed at 18 of the 23 (78%) sites that elicited ipsilateral saccades and for which low-frequency stimulation trains were tested.
DISCUSSION
During the last decade, several unit recording studies could not provide any evidence that the MPC is involved in the control of saccade dynamics, whereas lesion and inactivation studies reported acute observations that suggested the contrary (see Introduction). However, the interpretation of the latter ought to be made cautiously. Lesion/inactivation techniques can indeed introduce asymmetries between the left and right parts of the reticular formation, which in turn would be responsible for the altered dynamics of saccades. Moreover, their binary (all-or-nothing) effect on the activity of neurons does not give the opportunity to test whether the strength of cFN activity influences the dynamics of saccades. The purpose of our work was to test this possibility with the microstimulation technique. Thus, in addition to confirming the elicitation of contralateral or ipsilateral saccades (Noda et al. 1988) and that the fastigial influence concerns primarily the horizontal component of saccades (Goffart et al. 2004; Sato and Noda 1991), we present several other new findings. First, the amplitude and dynamics of evoked saccades depend on the stimulation parameters. Second, varying the stimulation frequency or intensity evokes saccades with different velocity and latency. Third, these changes in latency and velocity are not correlated. Finally, contralateral saccades can be evoked at sites eliciting ipsilateral saccades, but their onset is timed with the end and not with the beginning of the stimulation. Altogether, this work demonstrates an influence of cFN activity on the dynamics of saccades, consistent with the hypothesis of a cerebellar involvement in steering the saccades (Goffart et al. 2003; Keller et al. 1983).
Neural Processes Leading to the Elicitation of Ipsilateral Saccades
Microstimulation in the MPC elicits ipsilateral saccades with amplitude and direction that depend on the stimulated region (Cogdell et al. 1977; McElligott and Keller et al. 1984; Murakami et al. 1991; Noda et al. 1988; Ron and Robinson 1973). When it is applied in lobules VIc–VII of the cerebellar cortex, the saccades have amplitude that depends on the stimulated layer and the stimulation duration (Fujikado and Noda 1987; Noda and Fujikado 1987a; Ron and Robinson 1973). Stimulation in the molecular layer elicits small and curved saccades, regardless of the starting eye position, whereas stimulation in the granular layer elicits large and mostly straight saccades (Fujikado and Noda 1987). In both cases, the amplitude and duration of saccades increase with the stimulation duration. Noda's group demonstrated that the elicitation of ipsilateral saccades is due to the orthodromic activation of Purkinje cells in the lobules VIc-VII. Indeed, the lesion of Purkinje cells by local injection of kainic acid suppresses their occurrence (Noda and Fujikado 1987b). Since GABAA is the primary neurotransmitter by which the cerebellar cortex modulates the activity of deep cerebellar nuclei (Ito et al. 1970), the ipsilateral saccades are likely produced by the recruitment of Purkinje cells' axons, because they disappear when bicuculline (GABAA antagonist) is injected in the fastigial nucleus (Sato and Noda 1992). Ipsilateral saccades are also elicited when the stimulation is applied in the dorsocaudal fastigial region (Noda et al. 1988; Quinet and Goffart 2009), i.e., in the region where the axons of Purkinje cells pass before synaptically contacting the fastigial neurons.
The elicitation of ipsilateral saccades cannot be attributed to the recruitment of axons of neurons projecting to the deep superior colliculus. Otherwise, their excitation would not be sensitive to the (presynaptic) disinhibition of fastigial neurons by local injection of bicuculline. Indeed, Noda's group also showed that local injection of bicuculline in the cFN suppressed the elicitation of ipsilateral saccades (Noda et al. 1988; Sato and Noda 1992), demonstrating that the stimulation recruited afferent axons from Purkinje cells rather than efferent projections from the fastigial oculomotor region (FOR). Moreover, if the ipsilateral saccades involved the fastigiocollicular projections, one should expect to observe some movement of the head (Freedman et al. 1996; Walton et al. 2007). Yet, in the head-unrestrained monkey, the electrical stimulation of the fastigial nucleus barely moves the head (Quinet and Goffart 2009).
Furthermore, when long (100–300 ms) and relatively low-frequency (100 Hz) stimulation trains are applied to the dorsocaudal portion of the fastigial nucleus, microstimulation does not evoke ipsilateral saccades as one would expect if saccade-related neurons in the contralateral deep superior colliculus were anterogradely activated (Stanford et al. 1996). Instead, contralateral saccades were evoked, and their onset was timed to the stimulation offset rather than to its onset (Fig. 12; see also Fig. 2 in Goffart et al. 2003). For 78% of sites, we indeed found that the ipsilateral saccade is replaced by a contralateral one when a lower frequency (< 200 pulses/s) is used. When the same train is applied during visual saccades, their trajectory is altered in a way similar to that observed after muscimol injection in the cFN: the horizontal amplitude is increased for ipsilateral saccades and decreased for contralateral saccades, and an ipsipulsion deflects the trajectory of vertical saccades (see Fig. 3 in Goffart et al. 2003). This muscimol-like effect is compatible with the hypothesis that the stimulation activates Purkinje cells axons that innervate the FOR (Noda et al. 1988). Thus the contralateral rebound saccades, whether generated after an ipsilateral saccade (Fig. 11) or after the offset of a low-frequency train (Fig. 12), likely result from a rebound depolarization and spike bursting by cFN neurons following a prolonged stimulation-induced hyperpolarization (Aizenman and Linden 1999). Studying the relation between the cFN discharges and the rebound saccades should tell whether the instantaneous firing rate of cFN neurons also participates in steering the saccade, more specifically during this period that follows the end of the stimulation train.
Interestingly, the rebound saccades elicited after the high-frequency trains occur later than those elicited after the lower frequencies (e.g., compare green and blue traces in Fig. 12). This delay could result from the recruitment of a fastigio-olivo-cortico-fastigial loop (Ikeda et al. 1989). Indeed, the high-frequency stimulation could exert a powerful excitation over all cFN neurons, even those that project to the contralateral medial accessory olive (cMAO) and that are GABAergic (de Zeeuw et al. 1989), presumably also in the monkey. The inhibition of cMAO neurons would then lead to a prolonged enhancement of the firing rate of Purkinje cells (Benedetti et al. 1984) in the OV, which in turn would prolong the hyperpolarization of neurons (Benedetti et al. 1983) in the fastigial nucleus. The rebound saccade would then happen once the fastigial saccade-related neurons recover from the inhibition exerted by the OV.
Coming back to the elicitation of ipsilateral saccades, they may result from the activity in the opposite FOR that is unopposed by the reduced activity in the stimulated FOR. In other words, they would be elicited by sudden unilateral changes in the balance of activity between the left and right FOR. A prediction of this hypothesis is that bilateral cFN inactivation suppresses the electrical elicitation of ipsilateral saccades. Concerning the triggering per se, for both ipsilateral and contralateral saccades, it would be interesting to test whether the premotor burst neurons fire because the omnipause neurons (Iwamoto et al. 2009) are inhibited by the electrophysiological recruitment of glycinergic fastigioreticular neurons (Bagnall et al. 2009) and/or the recruitment of inhibitory burst neurons contralateral to the stimulated cFN (Shinoda et al. 2011).
Neural Processes Leading to the Elicitation of Contralateral Saccades
When the ventromedial fastigial region is stimulated, contralateral saccades are generated. Again, several observations refute the view that these saccades involve a transcollicular route. The most convincing evidence comes from perturbation experiments that tested the compensation for an unexpected change in eye position preceding a saccade toward a flashed target. Such compensation has been shown when the change in eye position is induced by electrical microstimulation in the deep superior colliculus (Sparks and Mays 1983). When the change in eye position is elicited by fastigial stimulation, the correction saccade does not compensate for the perturbation and misses the target by an error equal to the change in eye position (Noda et al. 1991). In other words, fastigial stimulation activates neural elements that do not include the deep superior colliculus but the pontine reticular formation (Sparks et al. 1987).
According to Noda, contralateral saccades result from the recruitment of axons of fastigial neurons that project toward the contralateral PMRF (Noda et al. 1988, 1990; Sato and Noda 1992). The same process is likely involved in the rebound saccades discussed above. Although synaptic contacts to the premotor excitatory burst neurons (EBN) have not yet been documented (Takahashi et al. 2014), two sets of observations suggest this possibility. The first set concerns the latency of evoked saccades. In our study, it ranged from 15 to 35 ms with suprathreshold microstimulations. This range is compatible with the 17-ms latency reported by Noda et al. (1991) (see also Cogdell et al. 1977) and exceeds the latency range of movements evoked by stimulation in the pontine reticular formation (7–18 ms; Gandhi et al. 2008). The second set combines observations made after muscimol injection in the cFN. Indeed, the reduced peak velocity that affects the horizontal component of contralateral saccades (Buzunov et al. 2013; Goffart et al. 2004, 2005) suggests that the cFN participates in the excitatory input that drives the activity of motor and internuclear neurons in the abducens nucleus. Moreover, the saccade duration-dependent ipsipulsion of vertical saccades (Goffart et al. 2004) indicates an excitatory drive on the abducens neurons that is unopposed by the input that ipsilateral inhibitory burst neurons (IBN) emit during vertical saccades (Scudder et al. 1988). The absence of ipsipulsion during bilateral inactivation (Robinson et al. 1993; unpublished observations) demonstrates that the unaffected cFN is the origin of the excitatory drive responsible for the ipsipulsion. Altogether, these results are consistent with the suggestion that the activity of cFN neurons, visually or electrically triggered, contributes to the firing of contralateral EBN.
More recently, Kojima et al. (2014) proposed that the cFN output normally increases the activity of antagonist muscles during ipsilateral saccades. This conjecture could explain the contralateral direction of electrically evoked saccades. However, it is not compatible with the fact that EBN do not burst during off-direction saccades (Cromer and Waitzman 2006; Keller et al. 2000; Sparks and Hu 2006; Strassman et al. 1986a), contrary to IBN (Cullen and Guitton 1997; Kojima et al. 2008; Scudder et al. 1988; Strassman et al. 1986b).
Fastigial Oculomotor Region and the Vertical Component of Saccades
In the present study, oblique movements with somewhat large vertical components were evoked (see Fig. 2, e16 and b54; Fig. 3A, m46; Fig. 4A, b7; Fig. 8A, i16; Fig. 9A, b69, e25, and m63; Fig. 10A; Fig. 11). Several observations lead us to assert that these vertical components should not be considered as evidence for a fastigial involvement in the adjustment of the vertical component of saccades. First, the vertical component of elicited saccades was directed upward, regardless of whether the head was restrained or free to move (see Figs. 2, 3, and 7 in Quinet and Goffart 2009). Second, the stimulation elicited a downward saccade at only 1 site (see Table 1, b55) of the 118 sites we tested so far. We do not think that this failure to evoke oblique downward saccades results from the inexistence of neurons projecting toward the reticular regions involved in the generation of downward saccades. These neurons likely exist, but they are located outside the fastigial saccade-related region described here. With the use of our microstimulation perturbation technique, a saccade-related region was indeed discovered in the deep cerebellar nuclei (unpublished observations), where the low-frequency microstimulation (100 pulses/s) deflected the trajectory of visual saccades vertically and not horizontally. At one site, the ipsilateral and contralateral saccades toward a visual target located along the horizontal meridian (horizontal saccades) had an upward deflection, whereas saccades toward a target located on the vertical meridian were hypermetric when the target was in the upper visual field (upward saccades) and hypometric when the target was in the lower visual field (downward saccades). At another site, located at the same stereotaxic coordinates and tested the next day, the electrically induced bias was downward, i.e., the horizontal saccades were deflected downward, upward saccades were hypometric, and downward saccades were hypermetric. The proximity of the two sites explains why their electrical microstimulation did not evoke any saccade. These sites were not located near the sites described in the present article, and whose functional perturbation (with the same microstimulation parameters or with a local injection of muscimol) affects the horizontal component of all saccades, as reported elsewhere (Goffart et al. 2003, 2004, 2005; Quinet and Goffart 2005, 2007).
Concerning the upward deflection of saccades observed in this study, two explanations can be proposed for their occurrence. First, it can result from the uncontrolled diffusion of the electrical excitation (because of current spread or retrograde activation) to cerebellar neurons (or axons) projecting to reticular neurons involved in the generation of upward saccades. Anatomic studies are clearly required to locate these cerebellar regions. Second, the upward deflection is reminiscent of the bias that affects the saccades when they are made toward a visual target presented in a dark background. We have indeed shown that in a dark background, horizontal and vertical saccades made toward a small target LED exhibit an upward deflection in their trajectory with a magnitude that increases with the saccade size (Goffart et al. 2006). Thus it is possible that the electrically evoked saccades reported in the present study missed a component that the visual background normally brings to the neural processes involved in the generation of saccades. It is worth noting that the peak burst activity of cFN neurons is significantly weaker during saccades made in darkness than during saccades in the light (Helmchen et al. 1994).
In addition to these observations, other lines of evidence indicate that the FOR is involved in adjusting the horizontal component of saccades. First, perturbing unilaterally the activity of the FOR, either with muscimol injection (Goffart et al. 2004) or with electrical microstimulation (Goffart et al. 2003), primarily alters the horizontal component of all saccades (horizontal, oblique, and vertical). Second, when the inactivation is bilateral, the amplitude of the horizontal components is again perturbed, whereas the vertical components are comparatively spared (Robinson et al. 1993; unpublished results). Finally, Sato and Noda (1992) provided convincing anatomic evidence that the FOR projections to the midbrain (where the vertical saccade generator is located) are very rare.
Fastigial Influence on Saccade Dynamics
Compared with the study of Noda et al. (1988), the saccades evoked in the present study have more variable amplitudes. This difference is due to the fact that Noda and colleagues used much shorter stimulation (20 ms): their saccades were smaller and less variable because they were truncated, i.e., interrupted by the stopping of the stimulation. We have shown that with longer stimulation durations, the amplitude of evoked saccades increases up to a maximum. It increases because the peak velocity and the duration increase with the stimulation duration. As for the observation that the amplitude saturates at different values, a possible explanation is that different FOR neurons establish synaptic contacts to different subsets of premotor neurons in the PMRF and that our stimulations did not always recruit the same FOR neurons from one site to the other. Finally, the fact that very short stimulation durations (<40 ms; see Fig. 3) influence the peak velocity and duration without changing the latency indicates that the fastigial stimulation activates neural elements that participate in the dynamics of saccades after they are triggered.
This involvement of the cFN in the processes that determine the dynamics of saccades is also supported by our results showing that increasing the frequency or the intensity of stimulation increased the peak velocity of evoked saccades. One may argue that the variation in these stimulation parameters would influence the conjugacy of saccadic responses that we would have incorrectly interpreted as changes in (conjugate) saccadic dynamics. This scenario is unlikely because it would imply that when the current is increased, the microstimulation recruits either neurons that activate the far-response (like those located in the posterior interpositus nucleus, Zhang and Gamlin 1998; Gamlin 1999) or neurons that inhibit the near-response. Neurons increasing their firing rate during convergence and accommodation have indeed been reported in the fastigial nucleus (Zhang and Gamlin 1996), but it seems that these neurons do not inhibit but rather activate the near-response. Indeed, muscimol injection in the fastigial nucleus induces a divergent change in eye alignment in both the normal (Gamlin and Zhang 1996) and strabismic monkeys (Joshi and Das 2013). Thus we would expect that increasing the current would spread toward fastigial neurons involved in the near response and slow down the saccades because of the inclusion of a convergence component. This is clearly not what we observed when the current or the frequency was increased: the saccade peak velocity was enhanced, not diminished.
In addition to the velocity, the frequency and intensity of stimulation also influenced the latency of saccades: the higher the frequency or the intensity, the lower the latency. This association between the changes in latency and the changes in peak velocity is reminiscent of observations made when the activity in the deep layers of the superior colliculus (dSC) is altered. When a dSC site is stimulated with a lower frequency, the peak velocity of evoked saccades is reduced whereas their latency is increased (Stanford et al. 1996). This association, which is also suggested by results reported by Sparks et al. (1990), was recently demonstrated to involve a common neural process in the dSC. Indeed, when small amounts of muscimol are injected in the dSC, the increase in the latency of visual saccades is correlated with the decrease in their peak velocity (see Fig. 12 in Goffart et al. 2012). These stimulation and inactivation results are consistent with the hypothesis that the size of the population of active neurons in the dSC determines both the latency and the velocity of saccades (Goffart 2009). By contrast, the latency of saccades is not increased during cFN inactivation in the monkey (Quinet and Goffart 2007), and we did not find any correlation between the latency reduction and the peak velocity increase as the stimulation frequency (or intensity) of stimulation increased. Again, these observations refute the view of a participation of the fastigiocollicular pathway in the control of saccade dynamics.
Conclusion
Altogether, the results presented in this study are consistent with a cerebellar involvement in steering the trajectory of saccades (Goffart et al. 2003; Keller et al. 1983) through the bilateral fastigial projections toward the saccade-related neurons in the pontomedullary reticular formation (Goffart et al. 2004) and in the bilateral control of visual fixation through the fastigial projections toward the rostral superior colliculus (Goffart et al. 2012; Guerrasio et al. 2010). Because the saccade-related bursts of fastigial neurons do not correlate with the peak velocity of saccades (Fuchs et al. 1993; Helmchen et al. 1994; Ohtsuka and Noda 1991), the cerebellar influence on the saccadic dynamics most likely involves recruiting neurons (more neurons during faster saccades) and controlling the size of the active population in the pontomedullary reticular formation. Many more experiments are still required to precisely determine whether this dynamical control specifies the location where the saccades are ultimately aimed (Goffart et al. 1998b) and how it guides the trajectory of gaze toward an object that is moving (Büttner et al. 1991; Fleuriet and Goffart 2012; Goffart et al. 2011), even within this so-called “3D space” (van Horn et al. 2013). Monkeys are indeed endowed with the capability to keep track of a target that moves with diverse dynamics (Bourrelly et al. 2014; Goffart et al. 2014) probably because the “parallel, distributed structuro-functional features of neural networks do furnish the central nervous system with an innate a priori propensity to implement [any kind of] geometries” (Pellionisz and Llinas 1982).
GRANTS
This work was fully supported by the Centre National de la Recherche Scientifique, partly by Agence Nationale de la Recherche Grant VISAFIX and European Research Council Grant POSITION (to P. Cavanagh). Part of this work was also made possible thanks to support from National Eye Institute (Grant EY-01189 to D. L. Sparks), Fondation pour la Recherche Médicale Grant FDT 20041202949 and Fondation de France Berthe Fouassier Grant 0039352 (to J. Quinet), and Human Frontier Science Program Organization Fellowship LT58/96 (to L. Goffart).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.Q. and L.G. performed experiments; J.Q. and L.G. analyzed data; J.Q. and L.G. interpreted results of experiments; J.Q. and L.G. prepared figures; J.Q. and L.G. drafted manuscript; J.Q. and L.G. edited and revised manuscript; J.Q. and L.G. approved final version of manuscript; L.G. conception and design of research.
ACKNOWLEDGMENTS
We thank Prof. Jean-Marie Coquery and Dr. David L. Sparks for continuous support, Kathy Pearson for software programming support, Dr. Anthony Dron for participation in the data analysis, and Dr. Lewis L. Chen for comments on a previous draft.
REFERENCES
- Aizenman CD, Linden DJ. Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J Neurophysiol 82: 1697–1709, 1999. [DOI] [PubMed] [Google Scholar]
- Aschoff JC, Cohen B. Changes in saccadic eye movements produced by cerebellar cortical lesions. Exp Neurol 32: 123–133, 1971. [DOI] [PubMed] [Google Scholar]
- Bagnall MW, Zingg B, Sakatos A, Moghadam SH, Zeilhofer HU, du Lac S. Glycinergic projection neurons of the cerebellum. J Neurosci 29: 10104–10110, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batton RR, Jayaraman A, Ruggiero D, Carpenter MB. Fastigial efferent projections in the monkey: an autoradiographic study. J Comp Neurol 174: 281–306, 1977. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Montarolo PG, Strata P, Tempia F. Inferior olive inactivation decreases the excitability of the intracerebellar and lateral vestibular nuclei in the rat. J Physiol 340: 195–208, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Montarolo PG, Rabacchi S. Inferior olive lesion induces long-lasting functional modification in the Purkinje cells. Exp Brain Res 55: 368–371, 1984. [DOI] [PubMed] [Google Scholar]
- Bourrelly C, Quinet J, Goffart L. Unsupervised dynamic morphing of a spatiotemporal visual event during its oculomotor tracking. J Vis 14: 492, 2014. [Google Scholar]
- Büttner U, Fuchs AF, Markert-Schwab G, Buckmaster P. Fastigial nucleus activity in the alert monkey during slow eye and head movements. J Neurophysiol 65: 1360–1371, 1991. [DOI] [PubMed] [Google Scholar]
- Buzunov E, Mueller A, Straube A, Robinson FR. When during horizontal saccades in monkey does cerebellar output affect movement? Brain Res 1503: 33–42, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogdell B, Hassul M, Kimm J. Fastigial evoked eye movement and brain stem neuronal behavior in the alert monkey. Arch Otolaryngol 103: 658–666, 1977. [DOI] [PubMed] [Google Scholar]
- Cromer JA, Waitzman DM. Neurones associated with saccade metrics in the monkey central mesencephalic reticular formation. J Physiol 570: 507–523, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KE, Guitton D. Analysis of primate IBN spike trains using system identification techniques. I. Relationship to eye movement dynamics during head-fixed saccades. J Neurophysiol 78: 3259–3282, 1997. [DOI] [PubMed] [Google Scholar]
- de Zeeuw CI, Holstege JC, Ruigrok TJ, Voogd J. Ultrastructural study of the GABAergic, cerebellar, and mesodiencephalic innervation of the cat medial accessory olive: anterograde tracing combined with immunocytochemistry. J Comp Neurol 284: 12–35, 1989. [DOI] [PubMed] [Google Scholar]
- Fleuriet J, Goffart L. Saccadic interception of a moving visual target after a spatiotemporal perturbation. J Neurosci 32: 452–461, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman EG, Stanford TR, Sparks DL. Combined eye-head gaze shifts produced by electrical stimulation of the superior colliculus in rhesus monkeys. J Neurophysiol 76: 927–952, 1996. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson FR, Straube A. Role of the caudal fastigial nucleus in saccade generation. I. Neuronal discharge patterns. J Neurophysiol 70: 1712–1740, 1993. [DOI] [PubMed] [Google Scholar]
- Fujikado T, Noda H. Saccadic eye movements evoked by microstimulation of lobule VII of the cerebellar vermis of macaque monkeys. J Physiol 394: 573–594, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi NJ, Barton EJ, Sparks DL. Coordination of eye and head components of movements evoked by stimulation of the paramedian pontine reticular formation. Exp Brain Res 189: 35–47, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamlin P. Subcortical neural circuits for ocular accommodation and vergence in primates. Ophthalmic Physiol Opt 19: 81–89, 1999. [DOI] [PubMed] [Google Scholar]
- Gamlin PD, Zhang H. Effect of muscimol blockade of posterior fastigial nucleus on vergence and accommodation in the primate. Soc Neurosci Abstr 22: 2034, 1996. [Google Scholar]
- Goffart L. Saccadic eye movements. In: Encyclopedia of Neuroscience, edited by Squire LR. Oxford: Academic, 2009, p. 437–444. [Google Scholar]
- Goffart L, Akao T, Kurkin S, Fukushima J, Fukushima K. Neural control of saccades to moving targets: contribution of the fastigial oculomotor region. Program 699.14 2011 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2011. [Google Scholar]
- Goffart L, Chen LL, Sparks DL. Saccade dysmetria during functional perturbation of the caudal fastigial nucleus in the monkey. Ann NY Acad Sci 1004: 220–228, 2003. [DOI] [PubMed] [Google Scholar]
- Goffart L, Chen LL, Sparks DL. Deficits in saccades and fixation during muscimol inactivation of the caudal fastigial nucleus in the rhesus monkey. J Neurophysiol 92: 3351–3367, 2004. [DOI] [PubMed] [Google Scholar]
- Goffart L, Guillaume A, Pélisson D. Compensation for gaze perturbation during inactivation of the caudal fastigial nucleus in the head-unrestrained cat. J Neurophysiol 80: 1552–1557, 1998b. [DOI] [PubMed] [Google Scholar]
- Goffart L, Hafed ZM, Krauzlis RJ. Visual fixation as equilibrium: evidence from superior colliculus inactivation. J Neurosci 32: 10627–10636, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffart L, Koene A, Quinet J. Coupling between horizontal and vertical saccade generators after muscimol injection in the monkey caudal fastigial nucleus. Program 475.2. 2005 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2005. [Google Scholar]
- Goffart L, Pélisson D, Guillaume A. Orienting gaze shifts during muscimol inactivation of caudal fastigial nucleus in the cat. II. Dynamics and eye-head coupling. J Neurophysiol 79: 1959–1976, 1998a. [DOI] [PubMed] [Google Scholar]
- Goffart L, Quinet J, Bourrelly C. Foveating a moving target here-and-now. J Vis 14: 495, 2014. [Google Scholar]
- Goffart L, Quinet J, Chavane F, Masson GS. Influence of background illumination on fixation and visually guided saccades in the rhesus monkey. Vision Res 46: 149–162, 2006. [DOI] [PubMed] [Google Scholar]
- Guerrasio L, Quinet J, Büttner U, Goffart L. Fastigial oculomotor region and the control of foveation during fixation. J Neurophysiol 103: 1988–2001, 2010. [DOI] [PubMed] [Google Scholar]
- Helmchen C, Straube A, Büttner U. Saccade-related activity in the fastigial oculomotor region of the macaque monkey during spontaneous eye movements in light and darkness. Exp Brain Res 98: 474–482, 1994. [DOI] [PubMed] [Google Scholar]
- Henn V, Lang W, Hepp K, Reisine H. Experimental gaze palsies in monkeys and their relation to human pathology. Brain 107: 619–636, 1984. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Noda H, Sugita S. Olivocerebellar and cerebelloolivary connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J Comp Neurol 284: 463–488, 1989. [DOI] [PubMed] [Google Scholar]
- Ito M, Yoshida M, Obata K, Kawai N, Udo M. Inhibitory control of intracerebellar nuclei by the Purkinje cell axons. Exp Brain Res 10: 64–80, 1970. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y, Yoshida K. Saccadic dysmetria following inactivation of the primate fastigial oculomotor region. Neurosci Lett 325: 211–215, 2002. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y, Kaneko H, Yoshida K, Shimazu H. Role of glycinergic inhibition in shaping activity of saccadic burst neurons. J Neurophysiol 101: 3063–3074, 2009. [DOI] [PubMed] [Google Scholar]
- Joshi AC, Das VE. Muscimol inactivation of caudal fastigial nucleus and posterior interposed nucleus in monkeys with strabismus. J Neurophysiol 110: 1882–1891, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller EL, Slakey DP, Crandall WF. Microstimulation of the primate cerebellar vermis during saccadic eye movements. Brain Res 288: 131–143, 1983. [DOI] [PubMed] [Google Scholar]
- Keller EL, McPeek RM, Salz T. Evidence against direct connections to PPRF EBNs from SC in the monkey. J Neurophysiol 84: 1303–1313, 2000. [DOI] [PubMed] [Google Scholar]
- Kleine JF, Guan Y, Büttner U. Saccade-related neurons in the primate fastigial nucleus: what do they encode? J Neurophysiol 90: 3137–3154, 2003. [DOI] [PubMed] [Google Scholar]
- Kojima Y, Iwamoto Y, Robinson FR, Noto CT, Yoshida K. Premotor inhibitory neurons carry signals related to saccade adaptation in the monkey. J Neurophysiol 99: 220–230, 2008. [DOI] [PubMed] [Google Scholar]
- Kojima Y, Robinson FR, Soetedjo R. Cerebellar fastigial nucleus influence on ipsilateral abducens activity during saccades. J Neurophysiol 111: 1553–1563, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Soetedjo R, Fuchs AF. Effects of GABA agonist and antagonist injections into the oculomotor vermis on horizontal saccades. Brain Res 1366: 93–100, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden J, Harris C. Cerebellar ataxia: pathophysiology and rehabilitation. Clin Rehabil 25: 195–216, 2011. [DOI] [PubMed] [Google Scholar]
- May PJ, Hartwich-Young R, Nelson J, Sparks DL, Porter JD. Cerebellotectal pathways in the macaque: implications for collicular generation of saccades. Neuroscience 36: 305–324, 1990. [DOI] [PubMed] [Google Scholar]
- McElligott JG, Keller EL. Cerebellar vermis involvement in monkey saccadic eye movements: microstimulation. Exp Neurol 86: 543–558, 1984. [DOI] [PubMed] [Google Scholar]
- Murakami S, Noda H, Warabi T. Converging eye movements evoked by microstimulation of the fastigial nucleus of macaque monkeys. Neurosci Res 10: 106–117, 1991. [DOI] [PubMed] [Google Scholar]
- Noda H, Fujikado T. Involvement of Purkinje cells in evoking saccadic eye movements by microstimulation of the posterior cerebellar vermis of monkeys. J Neurophysiol 57: 1247–1261, 1987a. [DOI] [PubMed] [Google Scholar]
- Noda H, Fujikado T. Topography of the oculomotor area of the cerebellar vermis in macaques as determined by microstimulation. J Neurophysiol 58: 359–378, 1987b. [DOI] [PubMed] [Google Scholar]
- Noda H, Murakami S, Yamada J, Tamada J, Tamaki Y, Aso T. Saccadic eye movements evoked by microstimulation of the fastigial nucleus of macaque monkeys. J Neurophysiol 60: 1036–1052, 1988. [DOI] [PubMed] [Google Scholar]
- Noda H, Sugita S, Ikeda Y. Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J Comp Neurol 302: 330–348, 1990. [DOI] [PubMed] [Google Scholar]
- Noda H, Murakami S, Warabi T. Effects of fastigial stimulation upon visually-directed saccades in macaque monkeys. Neurosci Res 10: 188–199, 1991. [DOI] [PubMed] [Google Scholar]
- Ohtsuka K, Noda H. Saccadic burst neurons in the oculomotor region of the fastigial nucleus of macaque monkeys. J Neurophysiol 65: 1422–1434, 1991. [DOI] [PubMed] [Google Scholar]
- Ohtsuka K, Noda H. Discharge properties of Purkinje cells in the oculomotor vermis during visually guided saccades in the macaque monkey. J Neurophysiol 74: 1828–1840, 1995. [DOI] [PubMed] [Google Scholar]
- Optican LM, Robinson DA. Cerebellar-dependent adaptive control of primate saccadic system. J Neurophysiol 44: 1058–1076, 1980. [DOI] [PubMed] [Google Scholar]
- Pélisson D, Goffart L, Guillaume A. Control of saccadic eye movements and combined eye/head gaze shifts by the medio-posterior cerebellum. Prog Brain Res 142: 69–89, 2003. [DOI] [PubMed] [Google Scholar]
- Pellionisz A, Llinas R. Space-time representation in the brain: the cerebellum as a predictive space-time metric tensor. Neuroscience 7: 2949–2970, 1982. [DOI] [PubMed] [Google Scholar]
- Quinet J, Goffart L. Saccade dysmetria in head unrestrained gaze shifts after muscimol inactivation of the caudal fastigial nucleus in the monkey. J Neurophysiol 93: 2343–2349, 2005. [DOI] [PubMed] [Google Scholar]
- Quinet J, Goffart L. Head-unrestrained gaze shifts after muscimol injection in the caudal fastigial nucleus of the monkey. J Neurophysiol 98: 3269–3283, 2007. [DOI] [PubMed] [Google Scholar]
- Quinet J, Goffart L. Electrical microstimulation of the fastigial oculomotor region in the head-unrestrained monkey. J Neurophysiol 102: 320–336, 2009. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Fuchs AF. The role of the cerebellum in voluntary eye movements. Annu Rev Neurosci 24: 981–1004, 2001. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Straube A, Fuchs AF. Role of the caudal fastigial nucleus in saccade generation. II. Effect of muscimol inactivation. J Neurophysiol 70: 1741–1758, 1993. [DOI] [PubMed] [Google Scholar]
- Ron S, Robinson DA. Eye movements evoked by cerebellar stimulation in the alert monkey. J Neurophysiol 36: 1004–1022, 1973. [DOI] [PubMed] [Google Scholar]
- Sato H, Noda H. Divergent axon collaterals from fastigial oculomotor region to mediodiencephalic junction and paramedian pontine reticular formation in macaques. Neurosci Res 11: 41–44, 1991. [DOI] [PubMed] [Google Scholar]
- Sato H, Noda H. Saccadic dysmetria induced by transient functional decortication of the cerebellar vermis. Exp Brain Res 88: 455–458, 1992. [DOI] [PubMed] [Google Scholar]
- Scudder CA, Fuchs AF, Langer TP. Characteristics and functional identification of saccadic inhibitory burst neurons in the alert monkey. J Neurophysiol 59: 1430–1454, 1988. [DOI] [PubMed] [Google Scholar]
- Selhorst JB, Stark L, Ochs AL, Hoyt WF. Disorders in cerebellar ocular control. I. Saccadic overshoot dysmetria: an oculographic, control system and clinic-anatomical analysis. Brain 99: 497–508, 1976. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Sugiuchi Y, Takahashi M, Izawa Y. Neural substrate for suppression of omnipause neurons at the onset of saccades. Ann NY Acad Sci 1233: 100–106, 2011. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Hu X. Saccade initiation and the reliability of motor signals involved in the generation of saccadic eye movements. Novartis Found Symp 270: 75–91, 2006. [PubMed] [Google Scholar]
- Sparks DL, Lee C, Rohrer WH. Population coding of the direction, amplitude, and velocity of saccadic eye movements by neurons in the superior colliculus. Cold Spring Harb Symp Quant Biol 55: 805–811, 1990. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Mays LE. Spatial localization of saccade targets. I. Compensation for stimulation-induced perturbations in eye position. J Neurophysiol 49: 45–63, 1983. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Mays LE, Porter JD. Eye movements induced by pontine stimulation: interaction with visually triggered saccades. J Neurophysiol 58: 300–318, 1987. [DOI] [PubMed] [Google Scholar]
- Stanford TR, Freedman EG, Sparks DL. Site and parameters of microstimulation: evidence for independent effects on the properties of saccades evoked from the primate superior colliculus. J Neurophysiol 76: 3360–3381, 1996. [DOI] [PubMed] [Google Scholar]
- Strassman A, Highstein SM, McGrea RA. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. I. Excitatory burst neurons. J Comp Neurol 249: 337–357, 1986a. [DOI] [PubMed] [Google Scholar]
- Strassman A, Hightstein SM, McCrea RA. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. II. Inhibitory burst neurons. J Comp Neurol 249: 358–380, 1986b. [DOI] [PubMed] [Google Scholar]
- Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol 80: 1911–1931, 1998. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Sugiuchi Y, Shinoda Y. Convergent synaptic inputs from the caudal fastigial nucleus and the superior colliculus onto pontine and pontomedullary reticulospinal neurons. J Neurophysiol 111: 849–967, 2014. [DOI] [PubMed] [Google Scholar]
- van Gisbergen JAM, Robinson DA, Gielen S. A quantitative analysis of generation of saccadic eye movements by burst neurons. J Neurophysiol 45: 417–442, 1981. [DOI] [PubMed] [Google Scholar]
- van Horn MR, Waitzman DM, Cullen KE. Vergence neurons identified in the rostral superior colliculus code smooth eye movements in 3D space. J Neurosci 33: 7274–7284, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton MM, Bechara B, Gandhi NJ. Role of the primate superior colliculus in the control of head movements. J Neurophysiol 98: 2022–2037, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J, Noda H. Afferent and efferent connections of the oculomotor cerebellar vermis in the macaque monkey. J Comp Neurol 265: 224–241, 1987. [DOI] [PubMed] [Google Scholar]
- Zee DS, Yee RD, Cogan DG, Robinson DA, Engel WK. Ocular motor abnormalities in hereditary cerebellar ataxia. Brain 99: 207–234, 1976. [DOI] [PubMed] [Google Scholar]
- Zhang H, Gamlin PD. Neurons in the posterior interposed nucleus of the cerebellum related to vergence and accommodation. I. Steady-state characteristics. J Neurophysiol 79: 1255–1269, 1998. [DOI] [PubMed] [Google Scholar]
- Zhang H, Gamlin PD. Single-unit activity within the posterior fastigial nucleus during vergence and accommodation in the alert primate. Soc Neurosci Abstr 22: 2034, 1996. [Google Scholar]