Abstract
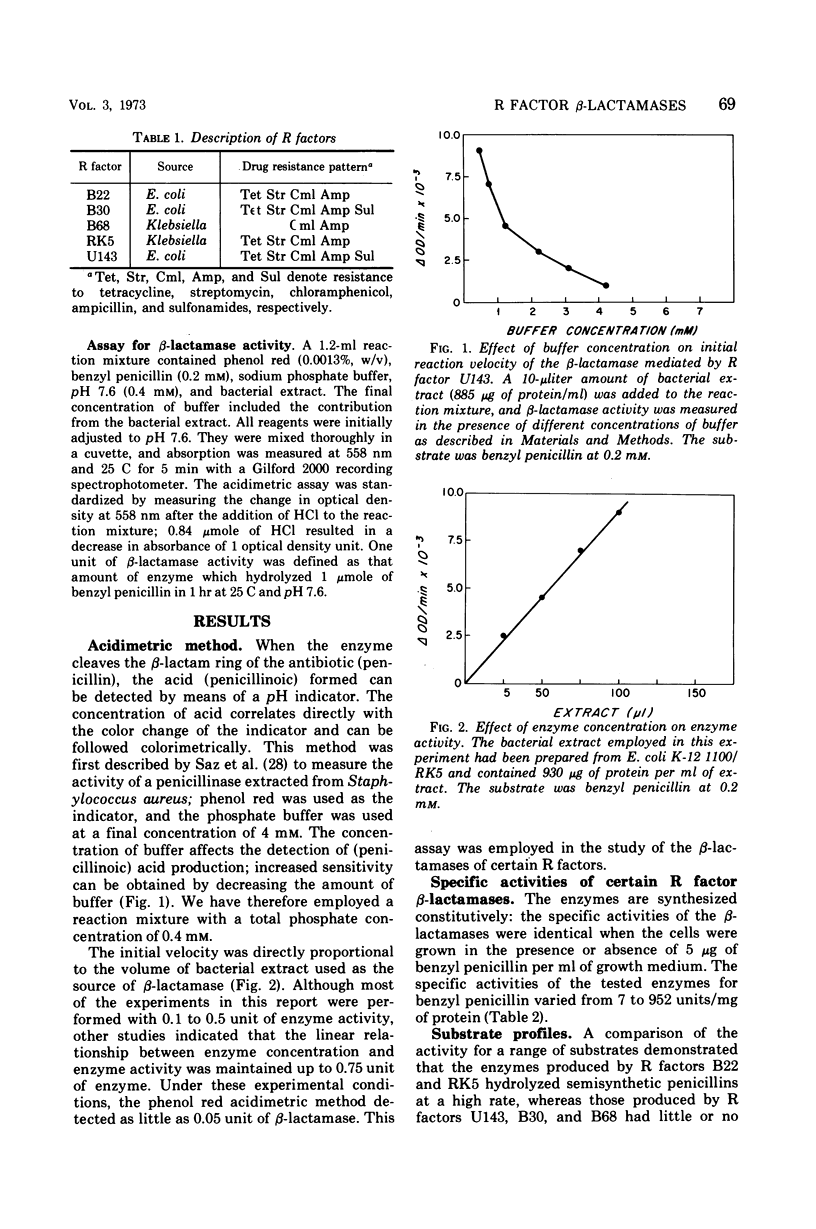
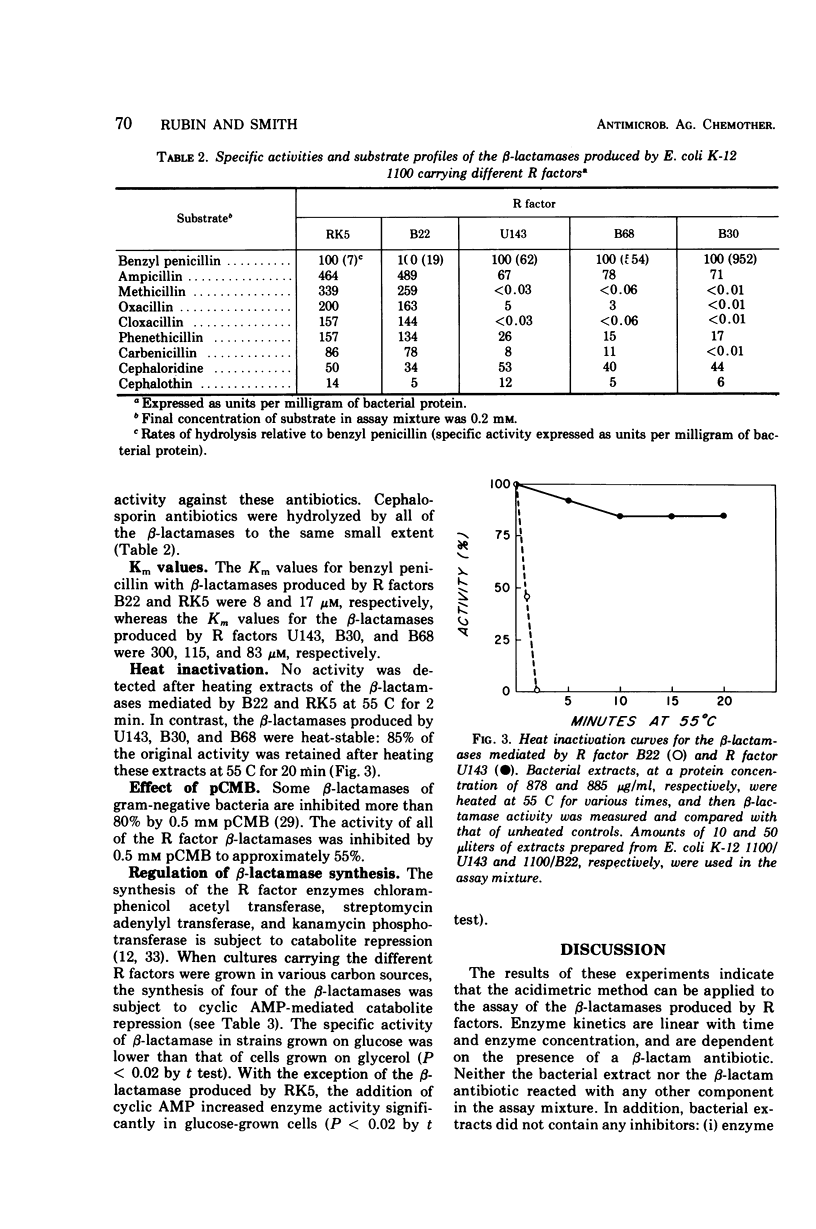
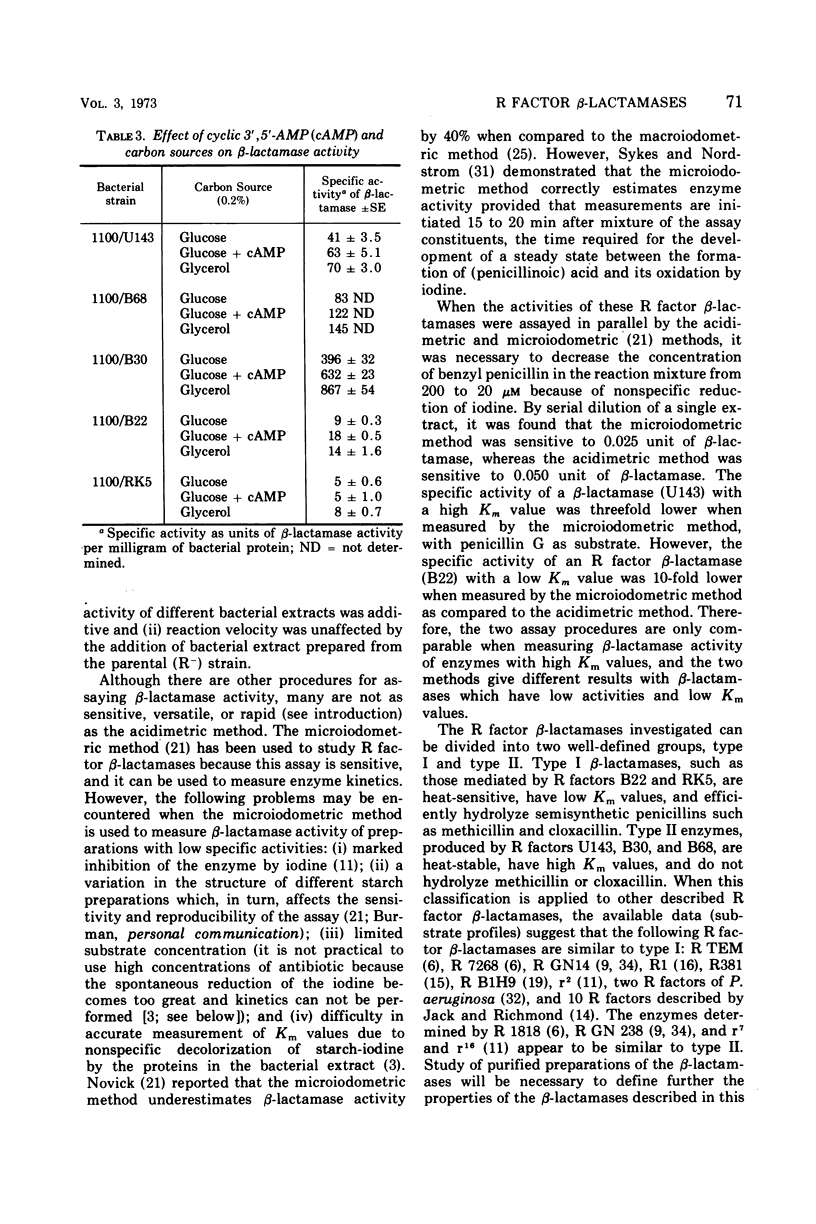
The properties and regulation of β-lactamases produced by certain R factors derived from ampicillin-resistant strains of Escherichia coli and Klebsiella were characterized. β-Lactamase activity was determined by the acidimetric method with phenol red as indicator. The sensitivity of this assay was increased by employing phosphate buffer at a final concentration of 0.4 mm; as little as 0.05 unit (1 unit equals the amount of β-lactamase that hydrolyzes 1 μmole of benzyl penicillin per hr at pH 7.6 and 25 C) could be detected. This assay is rapid and convenient and appears to be superior to other methods currently employed to assay R factor β-lactamases. Two classes of β-lactamases were distinguished on the basis of substrate profile, heat inactivation, and Km values. The regulation of most of these R factor β-lactamases, like certain other R factor enzymes, is subject to cyclic adenosine monophosphate-mediated catabolite repression.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess R. R., Travers A. A., Dunn J. J., Bautz E. K. Factor stimulating transcription by RNA polymerase. Nature. 1969 Jan 4;221(5175):43–46. doi: 10.1038/221043a0. [DOI] [PubMed] [Google Scholar]
- Burman L. G., Nordström K., Boman H. G. Resistance of Escherichia coli to penicillins. V. Physiological comparison of two isogenic strains, one with chromosomally and one with episomally mediated ampicillin resistance. J Bacteriol. 1968 Aug;96(2):438–446. doi: 10.1128/jb.96.2.438-446.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. W. Characterization of the -lactamase specified by the resistance factor R-1818 in E. coli K12 and other Gram-negative bacteria. Biochem J. 1971 Jul;123(4):501–505. doi: 10.1042/bj1230501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. W., Smith J. T. Some relationships between R-factor and chromosomal -lactamase in Gram-negative bacteria. Biochem J. 1971 Jul;123(4):507–512. doi: 10.1042/bj1230507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. W., Smith J. T. The purification and properties of the -lactamase specified by the resistance factor R-1818 in Escherichia coli and Proteus mirabilis. Biochem J. 1971 Jul;123(4):493–500. doi: 10.1042/bj1230493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Kontomichalou P. Penicillinase synthesis controlled by infectious R factors in Enterobacteriaceae. Nature. 1965 Oct 16;208(5007):239–241. doi: 10.1038/208239a0. [DOI] [PubMed] [Google Scholar]
- Datta N., Richmond M. H. The purification and properties of a penicillinase whose synthesis is mediated by an R-factor in Escherichia coli. Biochem J. 1966 Jan;98(1):204–209. doi: 10.1042/bj0980204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer M., deCrombrugghe B., Pastan I., Perlman R. Cyclic AMP receptor protein of E. coli: its role in the synthesis of inducible enzymes. Proc Natl Acad Sci U S A. 1970 Jun;66(2):480–487. doi: 10.1073/pnas.66.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J., Galindo E., Olarte J., Falkow S. Beta-lactamase of R factors. J Bacteriol. 1968 Oct;96(4):1441–1442. doi: 10.1128/jb.96.4.1441-1442.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood J., Smith D. H. Catabolite repression of chloramphenicol acetyl transferase synthesis in E. coli K12. Biochem Biophys Res Commun. 1971 Jan 8;42(1):57–62. doi: 10.1016/0006-291x(71)90361-5. [DOI] [PubMed] [Google Scholar]
- Jack G. W., Richmond M. H. A comparative study of eight distinct beta-lactamases synthesized by gram-negative bacteria. J Gen Microbiol. 1970 Apr;61(1):43–61. doi: 10.1099/00221287-61-1-43. [DOI] [PubMed] [Google Scholar]
- Jenkins P. H., Drabble W. T. -lactamases of R factors derived from Shigella and Salmonella strains. J Bacteriol. 1971 Oct;108(1):159–165. doi: 10.1128/jb.108.1.159-165.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindqvist R. C., Nordström K. Resistance of Escherichia coli to penicillins. VII. Purification and characterization of a penicillinase mediated by the R factor R1. J Bacteriol. 1970 Jan;101(1):232–239. doi: 10.1128/jb.101.1.232-239.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. Micro-iodometric assay for penicillinase. Biochem J. 1962 May;83:236–240. doi: 10.1042/bj0830236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Winshell E. B. Purification and characterization of penicillinases from Salmonella typhimurium and Escherichia coli. Arch Biochem Biophys. 1970 Aug;139(2):278–290. doi: 10.1016/0003-9861(70)90479-0. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Kirby S. M., Wishart D. R. Microbiological assay of mixed cephalosporins. Antimicrob Agents Chemother (Bethesda) 1967;7:716–722. [PubMed] [Google Scholar]
- Ooka T., Hashimoto H., Mitsuhashi S. Comparison of penicillinases produced by R factors isolated from ampicillin-resistant gram-negative bacteria. Jpn J Microbiol. 1970 Mar;14(2):123–128. doi: 10.1111/j.1348-0421.1970.tb00499.x. [DOI] [PubMed] [Google Scholar]
- PERRET C. J. Iodometric assay of penicillinase. Nature. 1954 Nov 27;174(4439):1012–1013. doi: 10.1038/1741012a0. [DOI] [PubMed] [Google Scholar]
- Perlman R. L., Pastan I. Regulation of beta-galactosidase synthesis in Escherichia coli by cyclic adenosine 3',5'-monophosphate. J Biol Chem. 1968 Oct 25;243(20):5420–5427. [PubMed] [Google Scholar]
- Richmond M. H., Jack G. W., Sykes R. B. Mechanisms of drug resistance. The beta-lactamases of gram-negative bacteria including pseudomonads. Ann N Y Acad Sci. 1971 Jun 11;182:243–257. doi: 10.1111/j.1749-6632.1971.tb30661.x. [DOI] [PubMed] [Google Scholar]
- SAZ A. K., LOWERY D. L., JACKSON L. J. Staphylococcal penicillinase. I. Inhibition and stimulation of activity. J Bacteriol. 1961 Aug;82:298–304. doi: 10.1128/jb.82.2.298-304.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Takahashi K., Yamagishi S., Mitsuhashi S. Variant of penicillinase mediated by an R factor in Escherichia coli. J Bacteriol. 1970 Nov;104(2):620–629. doi: 10.1128/jb.104.2.620-629.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. T. R-factor gene expression gram-negative bacteria. J Gen Microbiol. 1969 Jan;55(1):109–120. doi: 10.1099/00221287-55-1-109. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Nordström K. Microiodometric determination of beta-lactamase activity. Antimicrob Agents Chemother. 1972 Feb;1(2):94–99. doi: 10.1128/aac.1.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Richmond M. H. Intergeneric transfer of a beta-lactamase gene between Ps. aeruginosa and E. coli. Nature. 1970 Jun 6;226(5249):952–954. doi: 10.1038/226952a0. [DOI] [PubMed] [Google Scholar]
- Tsukada I., Yagisawa M., Umezawa M., Hori M., Umezawa H. Stimulation of kanamycin phosphotransferase synthesis in Escherichia coli by 3',5'-cyclic AMP. J Antibiot (Tokyo) 1972 Feb;25(2):144–146. doi: 10.7164/antibiotics.25.144. [DOI] [PubMed] [Google Scholar]
- Yamagishi S., O'Hara K., Sawai T., Mitsuhashi S. The purification and properties of penicillin beta-lactamases mediated by transmissible R factors in Escherichia coli. J Biochem. 1969 Jul;66(1):11–20. doi: 10.1093/oxfordjournals.jbchem.a129111. [DOI] [PubMed] [Google Scholar]
- Zubay G., Schwartz D., Beckwith J. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc Natl Acad Sci U S A. 1970 May;66(1):104–110. doi: 10.1073/pnas.66.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]



