Abstract
Considered signs of decreased welfare—abnormal behaviors such as self-injury and self-abuse among nonhuman primates housed in the laboratory—may put into question the validity and reliability of scientific research using these animals as models. Providing environmental enrichment decreases the incidence of some undesirable behaviors but is often unsuccessful at ameliorating the most severe types of abnormal behaviors. To prevent such behaviors from developing, it is important to identify risk factors that provide insight into the causes of certain abnormal behaviors. This study confirmed previous research identifying nursery rearing, single housing, and time spent in single housing as important risk factors. Results also indicate that the number of cage relocations affects the development of these behaviors. In addition, this study presents new data on comorbidity of several abnormal behaviors and discusses possible reasons for these patterns.
A variety of factors influence the early development of abnormal behaviors in nonhuman animals in captivity, but disruption of early rearing experience in captive nonhuman primates seems to be the most important factor contributing to the development of behavioral problems (Bellanca & Crockett, 2002; Lutz & Novak, 2005; Lutz, Well, & Novak, 2003; Novak, 2003; Novak & Petto, 1991; Novak & Suomi, 1988). This was first demonstrated by Harlow's classic study on the effects of early social isolation on infant rhesus macaque behavior (Harlow & Zimmermann, 1959). Self-injurious behavior and noninjurious self-abuse can develop when appropriate physical contact is restricted or deprived during the first months of life. Rearing infants in a nursery setting versus with their mothers seems to predispose them to the development of abnormal behaviors (Lutz et al., 2003; Novak, 2003). Previous studies indicate that other risk factors for abnormal behavior such as stereotypic behavior (motor, postural, self-stimulation) include individual housing, long time in individual housing, and high number of blood draws (Lutz et al., 2003; Novak, 2003).
A small proportion of individuals who engage in noninjurious self-abuse behaviors develop more severe forms of behavior resulting in physical injury. Animals in this category may go through bouts of self-injury, including severe self-biting, hair-plucking, or head-banging. These injuries can be severe enough to require veterinary care and represent a serious issue in nonhuman primate care.
Remediation for abnormal behavior in nonhuman primates spans from simple manipulanda and foraging devices to increased socialization. The degree to which these enrichment strategies promote species-typical behavior and ameliorate abnormal behavior depends upon the type of abnormal behavior exhibited (Lutz et al., 2003; Lutz & Novak, 2005; Novak, 2003). Generally, enrichment devices such as chew toys, foraging devices, and mirrors are provided to offer species-appropriate sensory stimuli and distract animals from engaging in abnormal behaviors. Whether these devices actually improve the psychological well being of the animal cannot be known directly; however, we can indirectly approximate this measure by evaluating whether these enrichment devices actually promote species-typical behavior and reduce abnormal behavior in an otherwise species-inappropriate environment (Garner, 2005).
The purpose of this study was to evaluate the risk factors associated with self-abuse behavior as well as its comorbidity with other types of abnormal behavior in indoor-housed rhesus macaques. We also examined the effectiveness of enrichment on mitigating abnormal behaviors in indoor-housed rhesus macaques showing self-injurious or other forms of self-abuse.
Methods
Subjects
Subjects for examining risk factors associated with self-abuse (noninjurious self-abuse and self-injurious behavior) were 231 adult rhesus macaques, of whom 132 exhibited self-abuse. Subjects for examining the mitigating effects of enrichment were the 132 adult rhesus macaques who exhibited self-abuse from this data set plus 3 additional animals who were not included in the risk-factor database. Individuals were paired or housed singly (Table 1 and Table 2).
Table 1. Definitions of Social Pairing Status.
| Pairing Condition | Definition |
|---|---|
| Continuous pairing | Paired 24 hr/day with compatible cagemate |
| Intermittent pairing | Paired ∼7 hr/day (usually paired by 8 a.m. and separated by 3 p.m.) with compatible cagemate |
| Continuous grate | Paired 24 hr/day with a 1-in. mesh metal grate between cages, which allows visual, auditory, olfactory, and tactile/grooming contact between compatible cagemates |
| Intermittent grate | Paired ∼7 hr/day (usually paired by 8 a.m. and separated by 3 p.m.) with a 1-in. mesh metal grate between cages, which allows visual, auditory, olfactory, and tactile/grooming contact between compatible cagemates |
| Singly housed | Never paired but have visual, auditory, and olfactory contact with other animals in room |
Table 2. Description of Study Subjects.
| A | |||||
|---|---|---|---|---|---|
|
|
|||||
| Self-Abusers | Controls | ||||
|
|
|
||||
| Pairing Type | Female | Male | Female | Male | Total |
| Continuous pair | 7 | 12 | 11 | 5 | 35 |
| Continuous grate | 0 | 4 | 0 | 1 | 5 |
| Intermittent pair | 14 | 18 | 27 | 1 | 60 |
| Intermittent grate | 2 | 2 | 0 | 0 | 4 |
| Single | 22 | 51 | 20 | 34 | 127 |
| Total | 45 | 87 | 58 | 41 | 231 |
|
| |||||
| B Self-Abusers Only | |||||
|
|
|||||
| Mother-Reared | Nursery-Reared | ||||
|
|
|
||||
| Pairing Type | Female | Male | Female | Male | Total |
|
| |||||
| Continuous pair | 2 | 3 | 3 | 5 | 13 |
| Continuous grate | 0 | 4 | 0 | 1 | 5 |
| Intermittent pair | 23 | 7 | 6 | 17 | 53 |
| Intermittent grate | 1 | 3 | 2 | 1 | 7 |
| Single | 9 | 18 | 8 | 22 | 57 |
| Total | 35 | 35 | 19 | 46 | 135 |
Data Collection
Data for examining the risk factors associated with self-abuse were extracted from California National Primate Research Center Webvitals, a colony database used to track husbandry and project assignment for each animal in the colony. Data were extracted for the following parameters:
Identity of dam and sire;
Sex and age;
Rearing type (mother reared, nursery reared);
Rearing location (large outdoor enclosure, small outdoor enclosure, indoors)
Number of relocations adjusted for age;
Pairing status; and
Number of pairings adjusted for age.
Data for examining comorbidity of abnormal behaviors with self-abuse and self-injurious behavior and for evaluating the mitigating effects of enrichment on these behaviors were collected on 135 individuals who exhibited self-abuse behavior. To collect the data, the study used a 5-min focal animal-sampling design and one-zero sampling every 15 s on a monthly basis across 2 years of study (Appendix).
Enrichment
Enrichment included perches, mirrors, puzzle balls, tube feeders, forage boards, kong toys, and coconuts as well as fresh fruits, vegetables, and tang pops. Tang pops were given monthly to each subject. Puzzle balls and tube feeders were given three times per week; fruits and vegetables, twice per week. Forage boards were given daily to each subject. Perches, mirrors, kong toys, and coconuts were available to each subject at all times.
Data Analysis
For the risk factor analysis, data were analyzed in Stata 9.0 using logistic regression with the outcome of behavioral status (self-abuse, no observed abnormal behavior). For the mitigating effects of enrichment, negative binomial regression was used with individual as the clustering variable to adjust for variation due to this repeated measure. Animals in the pairing categories of continuous grate and intermittent grate were removed before analysis for the second data set due to insufficient sample size of subjects (Table 2). Principal component analysis in S-Plus 6.0 was also used on the second data set to evaluate comorbidity of abnormal behavior in animals exhibiting self-abuse behavior. Alpha level was set at 0.05 for each analysis unless noted otherwise.
Results
Risk Factors
Mother-reared animals raised indoors were approximately 30 times more likely to exhibit self-abuse than mother-reared animals raised in smaller or larger outdoor enclosures (N = 294, z = 5.23, p < .0001). Nursery-reared animals were 11 times more likely to exhibit self-abuse such as self-biting than animals who were mother-reared indoors (N = 121, z = 3.01, p < .004); number of relocations to a different cage showed a significant positive relationship with self-abuse (N = 121, z = 3.06, p < .003) in these animals. Nursery-reared males were significantly more likely than nursery-reared females to be self-abusers (N = 99, z = 13.83, p < .0001). However, mother-reared males raised indoors and mother-reared females raised indoors were equally likely to be self-abusers (N= 195, z= 1.31,p = .19).
Comorbidity
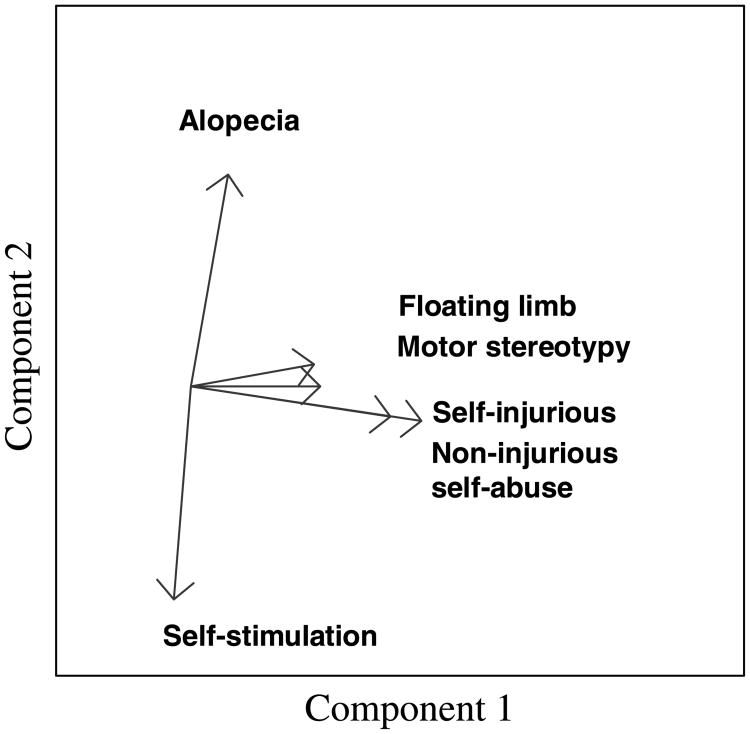
Floating limb, motor stereotypy, noninjurious self-abuse, and self-injurious behavior were highly correlated—load together on the same principal components— (Figure 1). Alopecia and self-stimulation did not load highly with these behaviors or with each other.
Figure 1.
Comorbidity of abnormal behaviors using principal component analysis. Graph comprises a plot of the loadings of the first two components for each abnormal behavior (data points were removed for better viewing). Behaviors showing similar loading for these two components indicate that they are colinear and thus comorbid.
Remediation for Abnormal Behavior
Animals identified with severe behavioral problems such as self-injurious behavior and noninjurious self-abuse exhibited less overall abnormal behavior (including motor stereotypies, postural stereotypies, self-stimulation, and ingestion disorders) when interacting with objects such as chew toys, coconuts, mirrors, perches, and foraging devices (N = 1041, z = −5.24, p < .0001) but did not specifically exhibit less self-injurious behavior or noninjurious self-abuse during interaction with enrichment (N = 1041, z = −0.06, p = .95). Rearing condition and social enrichment also had an effect on the expression of abnormal behavior in animals identified as self-abusers. Mother-reared animals raised indoors identified as self-abusers exhibited 34% less overall abnormal behavior than nursery-reared animals identified as self-abusers (N = 612, z = −1.98, p < .05). Mother-reared animals raised indoors who were continuously or intermittently paired as adults exhibited 90% less self-abuse and 39% less overall abnormal behavior than did mother-reared animals raised indoors who were singly housed (N = 53, z = −6.30, p < .0001; N = 53, z = −1.54, p < .09; note that alpha level here was set at 0.10). No significant differences were found in the nursery-reared animals with respect to differences in pairing status. No sex differences were found.
Discussion
Risk Factors
This study supports previous work (Lutz et al., 2003; Novak & Sackett, 2006), showing that nursery rearing and the rearing environment are important risk factors for development of self-abuse and other forms of abnormal behaviors. Most studies focusing on this issue have explored the effect of different types of socialization starting after a period in the incubator. However, ongoing research at the CNPRC suggests that some abnormal behaviors may become established before socialization begins. Hence, a lack of tactile stimulation early in life and response to infant distress may be factors in the development of early abnormal behaviors.
Previous studies also indicate that other risk factors for abnormal behavior such as stereotypic behavior (motor, postural, and self-stimulation) include individual housing and long time in individual housing (Anonymous, 2004; Bushong, Schapiro, & Bloomsmith, 1992; Chamove, Anderson, & Nash, 1984; Line, Morgan, Markowitz, Roberts, & Riddell, 1990; Lutz et al., 2003; Novak, 2003; Reinhardt, 1999) as well as a high number of blood draws (Lutz et al., 2003). Indeed, animals in individual housing most severely experience a lack of control over their environment, a condition that has been linked to the development of abnormal behaviors across species (Broom, 1991). Conspecifics may mitigate this by providing an environmental element responsive to the individual's behavior and acting as social buffers in anxiety-inducing situations (Heinrichs, Baumgartner, Kirschbaum, & Ehlert, 2003; Lepore, Allen, & Evans, 1997). Stress induced by procedures such as blood draws may also be reduced by employing positive-reinforcement techniques to train animals to cooperate with technicians (Reinhardt, 1997). Positive-reinforcement training may also be effective in providing captive animals with some control over their environment (Laule, Bloomsmith, & Schapiro, 2003). However, research investigating the effects of positive reinforcement on abnormal behavior is currently lacking and is needed to assess the utility of this technique as a potential method of prevention or intervention.
Comorbidity
The patterns found in the comorbidity of behavioral problems support findings in previous research (Lutz et al., 2003; Novak, 2003). There was a high correlation between floating limb and both self-abuse and self-injurious behavior (Figure 1). Floating limb is defined as a limb moving seemingly of its own accord, which often surprises the monkey who subsequently threatens or attacks the limb (Levin, Bushnell, & Baysinger, 1990). However, floating limb usually also includes a self-stroking pattern that resembles social grooming from a conspecific. This may lead us—in future studies—to reclassify floating limb from a postural stereotypy to a self-stimulation behavior. Self-stimulation behaviors such as self-sucking and self-clasping are most frequently observed in monkeys raised in limited social environments (Harlow & Harlow, 1962; Young, Suomi, Harlow, & Kinney, 1973) and have been hypothesized to result from the redirection of normal social behaviors toward the animal's own body (Mason & Berkson, 1975).
The comorbidity of motor stereotypies with both self-abuse and SIB in rhesus monkeys (Anonymous, 2004) may provide insight into the conditions under which these behavioral patterns develop. There is some evidence that the performance of repetitive motor patterns triggers a release of endorphins in the brain that may function as an analgesic agent (Cronin, Wiepkema, & van Ree, 1986). Furthermore, interesting studies in the field of human abnormal psychology provide evidence that self-abuse behaviors such as hand-biting target nonrandom sites on the body that are also associated with acupressure or autoanalgesic points (Anonymous, 2004; Wisely, Hare, & Fernandez-Ford, 2002). In addition, humans engaging in hand-biting are suggested to use pain as a means to outcompete unmanageable environmental inputs such as noise (Burton & Chapman, 2004). Hence, both motor stereotypies and self-abuse behaviors may function as an inappropriate attempt to cope with overwhelming and uncontrollable environmental stimuli or species-inadequate housing/handling conditions.
Remediation
Our data indicate that although enrichment devices such as chew toys, coconuts, mirrors, and foraging devices help ameliorate less severe forms of abnormal behavior—including motor and postural stereotypies and self-stimulation in animals diagnosed as self-abusers—these devices do not ameliorate the expression of the more severe forms of self-injurious and noninjurious self-abuse. Rearing condition and social interaction in contrast can reduce both overall abnormal behavior and self-abuse specifically in these animals. As demonstrated in the results, the use of intermittent pairing may be an effective measure in providing social enrichment to animals for whom—because of aggression or other social or experimental reasons—continuous pairing is not an option.
Conclusion
Because the developing brain exhibits greater plasticity and increased responsiveness to environmental changes than that of the adult (Kolb, Gibb, & Robinson, 2003), early detection may be the most effective strategy for remediation. This would involve monitoring infants, especially those raised in the nursery, for the early onset of abnormal behaviors and immediately intervening to prevent escalation of abnormal behaviors. Successful strategies for treatment of self-biting in humans include providing control over environmental stimuli and regular exercise (Burton & Chapman, 2004).
Hence, a combination of social enrichment, use of positive reinforcement— increasing animals' control over their environment and improving nursery-rearing techniques—may be most effective in the remediation of abnormal behaviors.
Acknowledgments
This project was funded in part by the National Institutes of Health Base Grant of the California National Primate Research Center (RR-00169). The research was conducted under the approved Institutional Animal Care and Use Committee protocol # 11843 at the University of California, Davis.
We thank our student employees and interns—Bryce Esch, Emily Gan, Jennifer Hayes, Kristin Hodge, Christina Maillard, Aiden McNeil, Courtney Sands, Aylssa Stark, and Marisol Whittaker—for their help on this project.
Appendix
An Ethogram of Abnormal Behaviors
Motor Stereotypies
Pacing
A behavior characterized by walking in the same pattern—either back and forth or in a circle—three or more repetitions.
Swinging
A behavior characterized by grasping a part of the cage with one or more hands or feet while moving in the same pattern—either back and forth or in a circular pattern—three or more repetitions.
Flipping
A behavior characterized by three or more repeated forward or backward somersaults.
Twirling
A behavior characterized by three or more repeated horizontal turns of the body.
Rocking
A behavior characterized by a rhythmic movement either side to side or forward and backward for at least three repetitions. This behavior is also observed while the monkey self-clasps.
Bouncing
A behavior characterized by bouncing up and down for three or more repetitions using a rigid posture. This behavior should not be confused with bouncing with a less rigid posture, which appears to serve only to make noise or shake the cage.
Head Twist
A behavior characterized by moving, lifting, or twisting the head in an exaggerated way. This behavior often occurs with pacing.
Postural Stereotypies
Floating Limb
A behavior characterized by a limb (either arm or leg) moving seemingly of its own accord. This often surprises the monkey, who subsequently threatens or attacks the limb.
Leg Lift
A behavior characterized by a leg wrapping around the back of the body or propped on the neck and staying in this position for at least 10 s.
Self-Abuse
Self-Bite
Any activity that involves biting the monkey's own body but that does not result in breaking the skin, bruising, or drawing blood. Monkeys will typically bite their arms, legs, shoulders, or genitals.
Threat Bite
An aggressive behavior that involves biting the monkey's own body—typically the hand, wrist, or forearm—while staring at the observer, mirror, or conspecific in a threatening manner.
SIB (Self-Injurious Behavior)
Any activity that involves biting or scratching the monkey's own body that results in breaking the skin and/or drawing blood. Monkeys typically bite their arms, legs, shoulders, or genitals.
Self-Hit
Any behavior that involves forcibly hitting or slapping oneself on any part of the body.
Self-Stimulation
Self-Clasp
A behavior characterized by grasping oneself with hands and/or feet for at least 5 s.
Self-Suck
A behavior characterized by sucking a part of the monkey's own body, including digits, tail, or genitals, for 3 or more s.
Eye Poke/Cover
A behavior that is typically characterized by a “saluting” gesture of the monkey's hand over the eye that may or may not be accompanied by pressing its knuckle or finger (typically the thumb) into the orbital space above the eye socket.
Hair Pluck
A behavior characterized by a jerking motion applied to the monkey's own hair with the hands or teeth, resulting in the removal of hair— sometimes becomes self-injurious.
References
- Anonymous. Self-injurious biting in laboratory animals: A discussion. Laboratory Primate Newsletter. 2004;43:11–13. [Google Scholar]
- Bellanca RU, Crockett CM. Factors predicting increased incidence of abnormal behavior in male pigtailed macaques. American Journal of Primatology. 2002;58:57–69. doi: 10.1002/ajp.10052. [DOI] [PubMed] [Google Scholar]
- Broom DM. Animal welfare: Concepts and measurement. Journal of Animal Science. 1991;69:4167–4175. doi: 10.2527/1991.69104167x. [DOI] [PubMed] [Google Scholar]
- Burton M, Chapman MJ. Problems of evidence based practice in community based services. Journal of Intellectual Disabilities. 2004;8(1):56. [Google Scholar]
- Bushong D, Schapiro S, Bloomsmith M. Self-aggression in nonhuman primates: A review of its development/possible causes, methods of therapeutic treatment, and its relevance to the zoo situation; American Zoo and Aquarium Association (AZA) Regional Conference Proceedings; 1992. pp. 723–728. [Google Scholar]
- Chamove A, Anderson J, Nash V. Social and environmental influences on self-aggression in monkeys. Primates. 1984;25:319–325. [Google Scholar]
- Cronin GM, Wiepkema PR, van Ree JM. Andorphins implicated in stereotypies of tethered sows. Cellular and Molecular Life Sciences. 1986;42(2):198–199. doi: 10.1007/BF01952467. [DOI] [PubMed] [Google Scholar]
- Garner JP. Stereotypies and other abnormal repetitive behaviors: Potential impact on validity, reliability, and replicability of scientific outcomes. ILAR Journal. 2005;46(2):106–117. doi: 10.1093/ilar.46.2.106. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Harlow M. Social deprivation in monkeys. Scientific American. 1962;207:136–146. doi: 10.1038/scientificamerican1162-136. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Zimmermann RR. Affectional responses in the infant monkey; orphaned baby monkeys develop a strong and persistent attachment to inanimate surrogate mothers. Science. 1959;130:421–432. doi: 10.1126/science.130.3373.421. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gibb R, Robinson TE. Brain plasticity and behavior. Current Directions in Psychological Science. 2003;12(1):1–5. [Google Scholar]
- Laule GE, Bloomsmith MA, Schapiro SJ. The use of positive reinforcement training techniques to enhance the care, management, and welfare of primates in the laboratory. Journal of Applied Animal Welfare Science. 2003;6:163–173. doi: 10.1207/S15327604JAWS0603_02. [DOI] [PubMed] [Google Scholar]
- Lepore SJ, Allen KAM, Evans GW. The availability of social support reduces cardiovascular reactivity to acute psychological stress. Psychosomatic Medicine. 1997;20(1):15–27. doi: 10.1023/a:1025583012283. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bushnell PJ, Baysinger CM. d-Amphetamine-induced “floating limb” syndrome in young rhesus monkeys. Psychopharmacology. 1990;101(1):112–117. doi: 10.1007/BF02253727. [DOI] [PubMed] [Google Scholar]
- Line S, Morgan K, Markowitz H, Roberts J, Riddell M. Behavioral responses of female long-tailed macaques (Macaca fascicularis) to pair formation. Laboratory Primate Newsletter. 1990;29:1–5. [Google Scholar]
- Lutz CK, Novak MA. Environmental enrichment for nonhuman primates: Theory and application. ILAR Journal. 2005;46(2):178–191. doi: 10.1093/ilar.46.2.178. [DOI] [PubMed] [Google Scholar]
- Lutz C, Well A, Novak M. Stereotypic and self-injurious behavior in rhesus macaques: A survey and retrospective analysis of environment and early experience. American Journal of Primatology. 2003;60:1–15. doi: 10.1002/ajp.10075. [DOI] [PubMed] [Google Scholar]
- Mason W, Berkson G. Effects of maternal mobility on the development of rocking and other behaviors in rhesus monkeys:A study with artificial mothers. Developmental Psychobiology. 1975;8:197–211. doi: 10.1002/dev.420080305. [DOI] [PubMed] [Google Scholar]
- Novak MA. Self-Injurious behavior in rhesus monkeys: New insights into its etiology, physiology, and treatment. American Journal of Primatology. 2003;59:3–19. doi: 10.1002/ajp.10063. [DOI] [PubMed] [Google Scholar]
- Novak MA, Petto AJ. Perspectives on psychological well-being in captive primates: Through the looking glass. In: Novak MA, Petto AJ, editors. Through the looking glass: Issues of psychological well-being in captive nonhuman primates. Washington, DC: American Psychological Association; 1991. pp. 1–7. [Google Scholar]
- Novak MA, Sackett GP. The effects of rearing experiences: The early years. In: Sackett GP, Ruppenthal GC, Elias K, editors. Nursery rearing of nonhuman primates in the 21st century. Chicago: Springer; 2006. pp. 5–19. [Google Scholar]
- Novak MA, Suomi SJ. Psychological well-being of primates in captivity. American Psychologist. 1988;43:765–773. doi: 10.1037//0003-066x.43.10.765. [DOI] [PubMed] [Google Scholar]
- Reinhardt V. Training non-human primates to cooperate during blood collection: A review. Laboratory Primate Newsletter. 1997;36:14. [Google Scholar]
- Reinhardt V. Pair-housing overcomes self-biting behavior in macaques. Laboratory Primate Newsletter. 1999;38:4. [Google Scholar]
- Wisely J, Hare DJ, Fernandez-Ford L. A study of the topography and nature of self-injurious behaviour in people with learning disabilities. Journal of Intellectual Disabilities. 2002;6(1):61. [Google Scholar]
- Young LD, Suomi SS, Harlow HF, McKinney WT. Early stress and later response to separation in rhesus monkeys. American Journal of Psychiatry. 1973;130:400–405. doi: 10.1176/ajp.130.4.400. [DOI] [PubMed] [Google Scholar]