Abstract
Impairments in social cognition are now recognized as core illness features in psychotic and affective disorders. Despite the significant disability caused by social cognitive abnormalities, treatments for this symptom dimension are lacking. Here, we describe the evidence demonstrating abnormalities in social cognition in schizophrenia, major depressive disorder, and bipolar disorder, as well as the neurobiology of social cognition including the role of oxytocin. We then review clinical trials of oxytocin administration in psychotic and affective disorders and the impact of this agent on social cognition. To date, several studies have demonstrated that oxytocin may improve social cognition in schizophrenia; too few studies have been conducted in affective disorders to determine the effect of oxytocin on social cognition in these disorders. Future work is needed to clarify which aspects of social cognition may be improved with oxytocin treatment in psychotic and affective disorders.
Keywords: oxytocin, schizophrenia, major depressive disorder, bipolar disorder, social cognition
1. Introduction
Social cognitive abnormalities have recently been recognized as a core feature of mood and psychotic disorders (Billeke and Aboitiz, 2013; Cusi et al., 2013; Millan and Bales, 2013; Wolkenstein et al., 2011). Indeed, they are a critical obstacle to recovery and function (Brune et al., 2007) (Couture et al., 2006; Harvey and Bowie, 2012; Roncone et al., 2002), may cause more disability than psychosis (Doop and Park, 2009; Hooker and Park, 2002; Malaspina and Coleman, 2003; Perlick et al., 1992), and are only modestly improved by currently available medications (Goldberg et al., 2007; Green, 2006; Harvey and Bowie, 2012; Maat et al., 2014; Millan et al., 2014). Moreover, social cognitive impairments persist during remission (Inoue et al., 2004; Montag et al., 2010) and are present in those with subclinical symptoms (Cusi et al., 2013) as well as in drug-naïve patients (Wang et al., 2008). Evidence suggests that abnormalities in social cognition may be distinct from broader cognitive and perceptual deficits known to exist in affective and psychotic disorders (Billeke and Aboitiz, 2013; Harvey and Bowie, 2012; Lee et al., 2005; Montag et al., 2010).
Intact social cognition is critical for social functioning and interpersonal relationships (Tomasello et al., 2005). In addition, social cognitive abilities are important for successful engagement in many psychological interventions (Inoue et al., 2004). Although social cognitive impairment has been found to persist during periods of remission, there is also evidence to suggest that this impairment may become worse as the illness progresses. For example, social cognition impairment correlates with illness load (i.e., illness duration and symptom severity) (McKinnon et al., 2010), supporting the need for early intervention. It is also associated with lower social functioning (Couture et al., 2006; Inoue et al., 2006), higher disability (Cusi et al., 2013), and poor prognosis. Specifically, alterations in social cognition are associated with lower social adjustment (Couture et al., 2006) and global functioning, as well as higher relapse rates (Inoue et al., 2006). As these deficits are known to persist in the remitted state, patients may still have poor social adjustment due to impairments in social cognition even during symptomatic remission from affective or psychotic episodes (Inoue et al., 2004).
Despite the clinical significance of social cognitive impairment, pharmacological treatment for this core illness feature is not currently available. Several lines of evidence suggest that the neuropeptide oxytocin may be a potential treatment for social cognitive deficits across diagnoses (Bakermans-Kranenburg and van, 2013; Gumley et al., 2014). Here, we review the evidence for social cognitive abnormalities in mood and psychotic disorders as well as the clinical trials of intranasal oxytocin administration across diagnoses and evaluate the evidence for improvement of social cognition across disorders.
1.1. Definitions and Components of Social Cognition
Social cognition may be defined as the “psychological processes that enable individuals to take advantage of being part of a social group” (Frith, 2008), and it is crucial to maintaining social relationships (Eisenberg and Miller, 1987). Therefore, it is not surprising that social cognitive abnormalities are associated with impaired social functioning, and, more broadly, decreased global functioning and disability. Social cognition can be conceptualized as a multidimensional construct encompassing different subcomponents which can be broadly summarized into five areas: theory of mind (ToM), social perception, social knowledge, emotion recognition and causal attribution style (Green et al., 2008, van Hooren et al., 2008, Ochsner et al., 2008, Mancuso et al., 2011).
Theory of Mind (ToM), also called mentalization or mentalizing, refers to the ability to represent others’ mental states and to make inferences about others’ intentions. It is a broad ability which involves the capacity to understand feelings, intentions, beliefs and metaphors (Bruno et al., 2005), and is closely related to the Research Domain Criteria (RDoC) (Insel et al., 2010) subconstruct “Understanding Mental States” within the Perception and Understanding of Others Construct in the Social Processes Domain.
Social perception refers to the ability to infer social roles as well as rules in complex and/or ambiguous situations from nonverbal and paraverbal social cues (Penn et al., 2002; Toomey et al., 2002). It also includes the capacity to determine the nature of the relationship between people such as professional, friendly, or romantic.
Partially overlapping with social perception, social knowledge refers to the awareness of rules and behaviors that are expected in social situations and/or interactions. Social perception and social knowledge are closely linked as a correct perception of social rules is necessary in order to determine what rules to adhere to in different social contexts (Green et al., 2008).
Emotion recognition indicates the ability to perceive and identify emotion by facial expression and/or vocal prosody (Edwards et al., 2002). Emotion recognition closely overlaps with the RDoC (Insel et al., 2010) subconstruct “Reception of Facial Communication,” within the Social Communication Construct in the Social Processes Domain.
Attributional Style refers to the tendency of an individual to assign causality to events in his or her life, including whether this causality is internal or external. Healthy people usually have a self-serving bias in which they tend to attribute positive events to personal, internal factors and negative events to external causes (Miller and Ross, 1975).
Although these domains are generally accepted as representing the construct of social cognition, their boundaries cannot be considered absolute and considerable overlap exists between them (Green et al., 2008). For example, the concept of empathy, which is considered to be a component of social cognition, is a complex multidimensional process that involves both cognitive and affective mechanisms (Achim et al., 2011) and taps into several of the above mentioned social cognitive domains.
Overlapping neural structures and systems subserve social cognitive processes. Primary sensory areas and more specialized structures (e.g. the fusiform face area) are involved in social perceptual processes (e.g. Sabatinelli et al., 2011). Emotion processing is regulated in part by the amygdala which, in turn, interacts with the insula and with the anterior cingulate cortex and the orbitofrontal cortex (e.g. Meyer-Lindenberg and Tost, 2012). Social attribution processes are partly mediated by the ventral premotor cortex, the superior temporal sulcus, the amygdala and the insula, whereas ToM abilities are subserved by the anterior medial prefrontal cortex and the temporoparietal junction (Meyer-Linderberg and Tost 2012). In addition to these somewhat distinct features, there are many more common structures and circuits that are involved in each domain of social cognition, and the ways in which these circuits interact are a focus of much current research.
2. Social Cognition Abnormalities Across Diagnoses
2.1 Social Cognition Abnormalities in Schizophrenia
Neurocognitive impairment characterizes individuals with schizophrenia (SZ) (Heinrichs & Zakzanis 1998; Fioravanti et al., 2005; Elvevag & Goldeberg, 2000) and it has been considered a core feature of the disorder since it was first described (Kraepelin, 1921). SZ patients are characterized by a more prominent level of neurocognitive impairment compared to other affective psychoses; data showed that differences in neurocognitive functioning between schizophrenia, schizoaffective and bipolar disorder are largely quantitative with disorders presenting the same pattern of deficits across neurocognitive domains that vary only on a quantitative level (Martinez-Aran et al 2002; Reichenberg et al. 2009).
Social cognition has become an area of interest in SZ research only in the last decade, with an increasing number of studies assessing social cognitive constructs in SZ (Penn et al., 1997; Green and Leitman, 2008). Meta-analytic studies have shown that social cognition is impaired in individuals with SZ (Brune, 2005; Hoeckert et al., 2007) and that there is a direct relationship between social cognition and functional outcome (Couture et al., 2006; Brune et al., 2007; Fett et al., 2011).
The inclusion of a social cognitive domain as part of the neurocognitive battery developed for clinical trials in SZ, the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) (Nuechterlein et al., 2004), confirms the importance of social cognition as a crucial aspect to be considered in clinical and research settings. Moreover, the CNTRICS (Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia) initiative has identified the development of social cognition measurement paradigms that are translatable across animals and humans as one of its top priorities (Millan and Bales, 2013).
The majority of studies in SZ have been conducted on three of the social cognition domains described above: theory of mind (ToM), emotion perception/recognition and attributional style (Penn et al., 2008).
Theory of Mind (ToM)
Meta-analytic studies have reported large effect sizes when comparing the performance of SZ patients with healthy controls (Sprong et al., 2007; Bora et al., 2009). Specifically, Bora and colleagues (2009) reported large effect sizes (Cohen’s d = 0.9 – 1.08). When the analysis was restricted to remitted patients a moderate to large effect size (d = 0.69 – 0.80) remained. Data on ToM in first episode SZ patients demonstrates that deficits exist and are comparable to those present in chronic SZ samples (Bertrand et al., 2007; Kettle et al., 2008; Green et al., 2012; Koelkbeck et al., 2010). Green and colleagues (2012), in their cross-sectional study examining ToM across different phases of the illness (prodromal, first episode and chronic), found that deficits were present and comparable across all three phases and with no evidence of either progression or improvement that was dependent upon illness phase.
Findings are still mixed regarding whether ToM deficits are trait- or state-related. Although meta-analyses (Sprong et al., 2007; Bora et al., 2009) haven shown that impairments are present during symptom remission, thus supporting a state-independent hypothesis, some studies have shown that performance is worse during acute phases of the illness (Drury et al., 1998; Corcoran 2003) and particularly in relation to specific illness features such as negative symptoms (Corcoran et al., 1995; Mitchley et al., 1998) and disorganization (Sarfati et al., 1999; Mazza et al., 2001). A recent meta-analysis showed that both negative symptoms and disorganization are moderately associated with social cognition (Pearson correlations ranged between −0.2 and −0.3 for disorganization and negative symptoms) (Ventura et al., 2011). Correlations between dimensional psychosis proneness/schizotypy and ToM impairments (Fyfe et al., 2008; Gooding and Pflum, 2011; Pflum et al., 2013), as well as the presence of social cognitive abnormalities in patients with schizotypal personality disorder (Ripoll et al., 2013) (a non-psychotic disorder within the schizophrenia spectrum), also support the trait-like, clinical state-independent nature of social cognition deficits. Taken together, it may be that baseline social cognitive deficits are present during remission but that they worsen during acute psychotic episodes.
Emotion recognition
It has been shown that patients with SZ demonstrate striking impairments in emotion recognition compared to healthy controls as well as to patients with other psychiatric disorders (Gaebel and Wolwer, 1992; Addington and Addington, 1998; Chan et al., 2010; Kohler et al., 2010). It has also been reported that emotion recognition abnormalities are associated with both positive (Shea et al., 2007) and negative symptoms (Chan et al., 2010). Results from a recent meta-analysis (Tseng et al., 2013) show that the association between symptoms and the recognition of certain emotions appears to be specific; in particular, these authors reported an inverse correlation between the ability to recognize the vocal presentation of happiness and the severity of psychotic symptoms. Evidence suggests that emotion recognition is negatively associated with negative symptoms, particularly among negative emotions; ‘fear’ seems to be the most difficult to be identified (Edwards et al., 2001; Tseng et al., 2013). Abnormalities in emotional prosody recognition were found to be associated with worse cognitive functioning (Bozikas et al., 2004) and with more severe positive symptoms (i.e. auditory hallucinations) (Shea et al., 2007). Data also show that emotion recognition deficits are present in subjects at high-risk for SZ, as well as during the prodromal phase and early onset of the illness (Addington et al., 2012; Edwards et al., 2001; Pinkham et al., 2007; Amminger et al., 2012). Amminger and colleagues (2012) found that first episode patients and subjects at high-risk for SZ demonstrated deficits in emotion recognition. In particular, participants in both groups had difficulty identifying negative emotions, such as fear and sadness, in faces and were impaired in detecting anger in voices. Longitudinal studies show that both acute and remitted SZ patients demonstrate deficits in facial affect recognition (Wolwer et al., 1996; Addington et al., 2012). Finally, there is evidence that abnormal (restricted) visual scanpaths to faces and facial expressions can be involved in deficits in emotional recognition; data suggest that patients with SZ spend less time looking at the eyes and mouth, which are critical areas for inferring emotions (Streit et al., 1997; Loughland et al., 2002).
Attributional Style
Research on attributional style in SZ has mainly focused on paranoid SZ patients. The evidence suggests that, in general, SZ patients are characterized by an exaggerated self-serving bias (Kaney and Bentall, 1989; Garety and Freeman, 1999) which may serve to preserve their self-esteem (Bentall et al., 2001; Penn et al., 2008). This exaggerated bias may be state-related, as it has been most strongly demonstrated in the context of paranoid delusions (Akre et al., 2009; Lincoln et al., 2010) and it does not appear to be evident in subjects at high-risk for psychosis (DeVylder et al., 2013). Nevertheless, this bias does appear to be present even in the first episode of psychosis, where it was found to be associated with more severe suspiciousness (Krstev et al., 1999).
2.2. Social Cognition Abnormalities in Depression
Although less well studied than social cognition in schizophrenia, a wealth of evidence suggests that patients experiencing significant depressive symptoms also demonstrate impairments in social cognition (Ladegaard et al., 2013; Wolkenstein et al., 2011). The majority of the research in social cognition in unipolar depression conducted to date has focused on ToM deficits, with an emphasis on empathy, as well as facial emotion perception/recognition and attributional style.
Empathy
Several studies have used the Interpersonal Reactivity Index (Davis, 1983) to assess empathy in clinical and subclinical depression. This self-report measure quantifies the degree to which one tends to feel empathically concerned (i.e., a general feeling of compassion for those who are helpless or less fortunate) and empathically distressed (i.e., feeling upset when specific negative events happen to others). A recent meta-analysis reported a significant effect size for empathic distress but not empathic concern in samples experiencing major depressive disorder (MDD) compared to controls; that is, depression was associated with an increased level of empathic distress but no differences were found in the level of empathic concern (Schreiter et al., 2013). However, an association between depression and a decreased ability to tolerate negative events such as emergencies or tragedies, even if one is not directly involved, may not reflect an alteration in social cognition, per se. The lack of a difference in empathic concern is perhaps more surprising and suggests that alterations in social cognition in depression may be related to other facets of this construct.
Emotion Recognition/Perception
The majority of studies that have assessed emotion recognition in major depressive disorder have found that patients are impaired relative to healthy samples (Kohler et al., 2011). For example, Leppänen et al., (2004) compared depressed patients with matched controls and tested their ability to identify happy, sad, or neutral faces. Both depressed patients and healthy controls accurately identified happy and sad faces, but the depressed participants were less accurate and slower when presented with the neutral faces. Similar deficits have been reported in studies of depressed samples performing emotion discrimination tasks (Bourke et al., 2010). Gollan et al., (2008) found depressed patients rated neutral faces as sad and had a longer reaction time for sad faces when compared to healthy controls. A recent meta-analysis of facial emotion perception in major depressive disorder and bipolar disorder reported a moderate effect size for impairment across the two disorders, with no statistically significant difference in effect size between emotion identification or discrimination tasks and no difference between depression and bipolar disorder (Kohler et al., 2011).
Theory of Mind (ToM)
The Reading the Mind in the Eyes Test (RMET; Baron-Cohen et al., 2001) has frequently been used to assess ToM, although the distinction between ToM and emotion recognition/perception when assessed using this measure is unclear. Results in depression using this task have been mixed; several groups have reported impairments in depressed samples relative to controls (Lee et al., 2005; Wang et al., 2008), whereas others have not (Wolkenstein et al., 2011). Wang and colleagues (2008) compared performance on the RMET in depressed patients with and without psychotic symptoms and found that there were no differences in performance between the groups, although both depressed groups were impaired relative to controls. Kettle and colleagues (2008) reported that depressed patients were impaired on the RMET compared to a sample of university students but they showed no differences in performance when compared with demographically matched community controls, suggesting that deficits in ToM as assessed by this measure are likely to be subtle in MDD.
Social Perception and Knowledge
Several other groups have examined so-called “higher order” social cognitive functioning in depression; the focus of these studies has been on skills such as humor appreciation, the ability to detect a social faux pas, and the capacity to understand implied and subtle social cues. The evidence to date suggests that patients with current MDD tend to demonstrate impairments on such tasks (Ladegaard et al., 2014). Uekermann and colleagues (2008) reported that patients with MDD performed less well than controls on a task measuring humor understanding and appreciation, and found that deficits on this humor task were significantly associated with deficits in executive functioning. A similar study found that patients with MDD were impaired on a test of faux pas detection, and that depressed patients with psychotic features performed worse than both controls and depressed patients without psychotic features (Wang et al., 2008). Two studies have tested patients with MDD using the Movie for the Assessment of Social Cognition (MASC; Dzobiek et al., 2006). This task measures mentalizing capacity and social perception and knowledge by requiring participants to watch a short video featuring several actors in social interactions and then to answer questions based on what they have seen. Wilbertz and colleagues (2010) did not find any differences on MASC performance between 16 depressed patients and 16 controls; a more recent study of acutely depressed patients reported that they were impaired on this task compared to controls (Wolkenstein et al., 2011), suggesting that current symptom severity may be related to social cognitive impairment. Most recently, Ladegaard and colleagues (2014) conducted a comprehensive study of higher order social cognition in medication-naïve patients experiencing their first major depressive episode. Results indicated that the depressed patients were significantly impaired across all tasks compared to controls; moreover, these performance differences remained after controlling for non-social neurocognitive impairment.
Attributional Style
Decades of research have generally supported the notion that individuals with MDD are more likely to have an attributional style in which negative events are interpreted as due to internal, stable, and global factors (Alloy et al., 1992; Sweeney et al., 1986). Recent work suggests that acutely depressed patients demonstrate the most severely depressed attributional style, but that remitted patients tend to persist in attributing negative events to personal factors more than participants who have never been depressed (Ball et al., 2008). These data suggest that a depressive attributional style is both state- and trait-like, becoming less prominent in the remitted state and more severe during acute mood episodes.
Although it is very likely that social cognitive impairments are implicated in decreased social functioning in affective disorders, the direct connection between social cognition and psychosocial outcomes has rarely been tested (Van Rheenan et al., 2014). Nevertheless, it is clear that social functioning is seriously impaired in patients with MDD (Hirschfeld et al., 2000) and bipolar disorder (Miklowitz, 2011). By targeting social cognition deficits directly, it may be possible to decrease affective symptoms as well as improve psychosocial functioning.
2.3. Social Cognition Abnormalities in Bipolar Disorder
Unlike the majority of the studies assessing social cognition in MDD, many social cognition studies in bipolar disorder (BD) have tested participants during euthymia. As such, it is difficult to determine whether social cognition is differentially impaired between BD and MDD. The evidence to date suggests that, even in the absence of frank mood symptoms, many patients with BD tend to demonstrate subtle yet persistent deficits in social cognition (Samamé et al., 2012). As with the studies in depression, the focus of social cognition investigations in BD has been on emotion recognition/perception and ToM, with fewer studies examining social knowledge and perception.
Emotion Recognition/Perception
A recent meta-analysis of social cognition studies in euthymic BD reported a small but significant effect size (d = .35) for impairments in facial emotion perception (Samamé et al., 2012); this is in line with a previous meta-analysis that reported similar results (Kohler et al., 2011). There were no significant differences for the effect sizes between any of the six most commonly presented emotions in these tasks (happiness, fear, disgust, anger, sadness, and surprise) (Samamé et al., 2012). The relatively small number of studies and the large degree of heterogeneity within and across studies makes it difficult to draw firm conclusions regarding this potential impairment. Moreover, very few studies controlled for more general, non-social neurocognitive impairment. In fact, one study reported that the differences in facial emotion recognition between patients and controls were not statistically significant after general neurocognitive impairments were controlled for (Martino et al., 2011). Another study that reported differences in facial emotion perception reported that deficits in the patient group were correlated with more broad neuropsychological deficits (Bora et al., 2005), again suggesting that this impairment may not be particularly social in nature but rather a more general cognitive impairment. In addition, mood state is likely to affect facial emotion perception; it appears that manic patients demonstrate deficits in this area relative to both controls and euthymic patients (Lembke et al., 2002).
Theory of Mind (ToM)
As appears to be the case for MDD, it may be that the social cognitive deficits in BD are related to higher order skills such as mentalizing. There is evidence that euthymic BD patients demonstrate hypomentalization (Bora et al., 2009), and that the degree of impairment may be related to the number of previous (hypo)manic episodes (Montag et al., 2010). Little is known about the impact of a history of psychosis on social cognition in BD. One study found that although BD patients performed worse than controls on a naturalistic ToM task (Happé, 1994), there were no differences between patients with and without a history of psychosis (Lahera et al., 2008). Mood state is likely to play a role in ToM functioning, although few studies have assessed this. Wolf and colleagues (2010) reported no differences between euthymic, manic, and depressed BD patients on a battery of neurocognitive and ToM tests, although the patient group as a whole demonstrated deficits compared to controls. In contrast, symptomatic patients have been found to perform worse than remitted patients and controls in several studies of ToM (Kerr et al., 2003; Bazin et al., 2009).
Attributional Style
Less is known about attributional style in BD compared to unipolar depression, although there is evidence that remitted patients with unipolar depression evidence a more vulnerable (i.e. internal, stable, and global) style for negative events compared to both remitted patients with BD and controls (Tracey et al., 1992). Nevertheless, there is some evidence to suggest that extremely negative or positive attributions, regardless of valence, are associated with affective episodes in BD (Strange et al., 2013). Recent work has also demonstrated that depressed patients with BD are more likely to ascribe angry and intentional biases toward neutral events; these attributional biases are associated with decreased social functioning (Lahera et al., 2008). Few studies of social cognition in BD have assessed or controlled for non-social cognitive impairment; of those that did, social cognitive deficits appeared to be only partially explained by general neurocognitive deficits, suggesting that social cognitive impairment is a distinct target for treatment (Lahera et al., 2008; Wolf et al., 2010).
3. Oxytocin and Social Cognition
Because of its anxiolytic, prosocial, and social cognitive-enhancing effects (Bakermans-Kranenburg and van, 2013; Bos et al., 2012; Gumley et al., 2014; Macdonald and Macdonald, 2010; Meyer-Lindenberg et al., 2011; Zink and Meyer-Lindenberg, 2012), oxytocin has been suggested as a promising novel treatment for patients with psychiatric and developmental disorders associated with severe deficits in social interactions and social information processing, such as schizophrenia (Millan et al., 2014), borderline personality disorder and autism (Meyer-Lindenberg et al., 2011).
3.1. Biology and mechanism of intranasal oxytocin
Oxytocin is a neuropeptide hormone synthesized in magnocellular and parvocellular neurons in the paraventricular and supraoptic nuclei of the hypothalamus. Oxytocin is stored in secretory vesicles in axonal terminals in the posterior lobe of the pituitary and is released into the peripheral circulation. There are also direct neuronal projections of oxytocinergic neurons to other brain regions including the amygdala, hippocampus, striatum, suprachiasmatic nucleus, bed nucleus of stria terminalis and brainstem, where it acts as a neuromodulator and neurotransmitter. Finally, oxytocin exerts effects on both local and distant brain targets through dendritic release and diffusion into the extracellular space (Meyer-Lindenberg et al., 2011). Evidence suggests that a dynamic balance between the activities of brain oxytocin and vasopressin plays a key role in regulating multiple aspects of social cognition and behavior (Neumann and Landgraf, 2012). Humans have four different receptors for oxytocin and vasopressin: the oxytocin receptor (OXTR), the vasopressin receptor 1A (AVPR1A), 1B (AVPR1B) and 2 (AVPR2). The distribution of these receptors in the human brain has not yet been fully characterized (Meyer-Lindenberg et al., 2011).
The oxytocin system modulates multiple social cognitive domains, such as trust, attachment behavior, stress response, social memory, and the ability to recognize emotions and understand mental states in others (Bartz et al., 2011b; Bos et al., 2012; Gumley et al., 2014; Meyer-Lindenberg et al., 2011). Exogenous oxytocin modulates social perception, decreases social fears and promotes social approach behavior and trust in others (Meyer-Lindenberg et al., 2011; Zink and Meyer-Lindenberg, 2012).
Early experiments with intravenous oxytocin yielded disappointing results (Bakermans-Kranenburg and van, 2013), likely due to the blood–brain barrier (BBB). Intranasal administration seems to circumvent the BBB given replicable changes in brain function (Perry et al., 2010; Riem et al., 2011), perception and behavior (Bakermans-Kranenburg and van, 2013). The nasal mucosa directly connects the central nervous system (CNS) and the environment. Intranasal administration causes systemic effects similar to those observed after peripheral administration, and potentially higher delivery to CNS targets (Zink and Meyer-Lindenberg, 2012). Nonhuman research has shown behavioral effects after direct injection of oxytocin to specific brain targets (Bielsky and Young, 2004) and increased brain (Neumann et al., 2013) and CSF (Meera and Young, personal communication) oxytocin after intranasal administration, leading to assumptions that administration in humans also causes effects through direct central action (Guastella et al., 2013), although research on bioavailability of intranasal oxytocin in humans is lacking (Guastella et al., 2013). However, animal studies are not always predictive of data in humans, and further studies are needed to elucidate target engagement and mechanisms of action of oxytocin in humans.
Taken together, the evidence suggests that intranasal oxytocin likely acts centrally, through modulation of an integrated dopaminergic/oxytonergic social cognitive network (Love, 2014) centered on the amygdala (Li et al., 2010; Pinkham et al., 2011), which is strongly correlated with social functioning (Pinkham et al., 2008). Oxytocin modulates frontolimbic networks involved in social cognition and emotion regulation, including the amygdala, medial prefrontal cortex and anterior cingulate cortex (Meyer-Lindenberg et al., 2011). In healthy controls, oxytocin significantly increased connectivity between both amygdalae and rostral medial frontal cortex, regions critical to social cognition and emotion regulation (Sripada et al., 2012). In fact, amygdala–medial frontal cortex connectivity, which is believed to be abnormal in mood disorders, is a putative brain-based biomarker of social-affective functioning (Sripada et al., 2012).
Oxytocin also modulates stress reactivity, has anxiolytic and antidepressant properties, and regulates stress-coping style (Neumann and Landgraf, 2012) through interactions with monoaminergic, especially serotonergic, and corticotropin-releasing factor systems (Neumann and Landgraf, 2012). Indeed, data suggest that oxytocin reduces limbic (amygdala) and hypothalamic-pituitary-adrenal (HPA) axis reactivity to social stressors (Ditzen et al., 2009; Quirin et al., 2011; Zink and Meyer-Lindenberg, 2012), and that it mediates the anxiolytic and stress-protective effects of positive social interaction (Meyer-Lindenberg et al., 2011).
Initial reports estimating the half-life of oxytocin at under two hours raised questions about its clinical utility, since frequent administrations throughout the day would be required for a sustained effect on social cognition. More recently, reports of longer effects after intranasal oxytocin administration (up to 7 hours) (Bakermans-Kranenburg and van, 2013) support its suitability for sustained long-term use in clinical settings (Bakermans-Kranenburg and van, 2013). Moreover, exogenous oxytocin may stimulate a ‘feedforward’ release of endogenous oxytocin, prolonging the effect beyond its half-life (Bakermans-Kranenburg and van, 2013). Another potential clinical application of oxytocin, in which a short half-life would be desirable, is in combination with psychotherapy (e.g., administration immediately before a therapy session to enhance its effect [Bryant and Hung, 2013; Guastella et al., 2009; MacDonald et al., 2013]), as is done with other drugs such as D-cycloserine (Andero and Ressler, 2012). Of note, other psychotropic medications used in clinical settings have very short half-lives as well (e.g., stimulants, some benzodiazepines) and are used during specific periods of time or on an as-needed basis to target intermittent symptoms.
Changes in emotion recognition and understanding of mental states after oxytocin may be mediated by changes in the salience or rewarding properties of social stimuli (Groppe et al., 2013; Stavropoulos and Carver, 2013). Recent evidence suggests that oxytocin-induced increases in social cognitive ability are correlated with increased pupil dilation, which is in turn coupled with increased firing of the locus coeruleus (Prehn et al., 2013); (Leknes et al., 2013). In addition, increased attention toward the eye region of facial stimuli (measured by eye gaze or reaction time) has been found after oxytocin administration (Bertsch et al., 2013; Brune et al., 2013; Domes et al., 2013; Tollenaar et al., 2013). There is also evidence that oxytocin affects social cognition through modulation of subcortical attention/orienting networks and amygdala activity (Li et al., 2010; Pinkham et al., 2011).
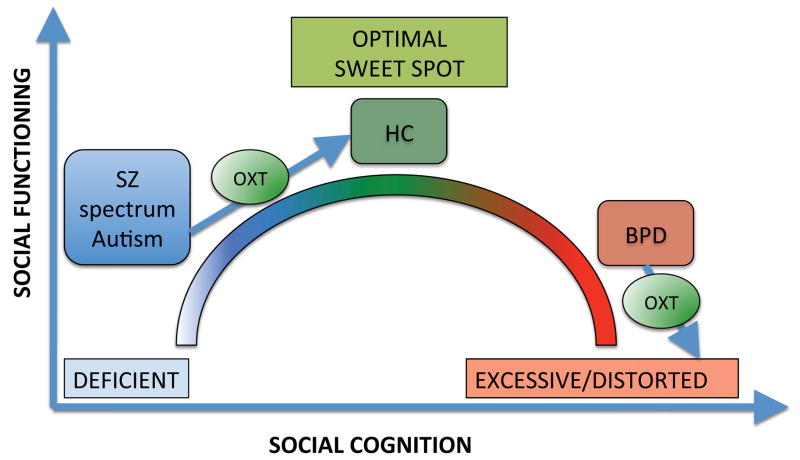
There are, in general, two theoretical models of the effect of intranasal oxytocin on social cognition (Bartz et al., 2011b; Meyer-Lindenberg et al., 2011). The “optimizing” model (Meyer-Lindenberg et al., 2011; Simeon et al., 2011) postulates that oxytocin optimizes social cognitive functioning in all populations, regardless of baseline social cognitive skills (Meyer-Lindenberg et al., 2011). Conversely, the “interactionist” model (Bartz et al., 2011b) suggests that oxytocin’s effects on social cognition are modulated by baseline social cognition skills (Bartz et al., 2010; Fischer-Shofty et al., 2013; Leknes et al., 2013). According to the interactionist model (see Figure 1), there is a “sweet spot” of oxytocinergic tone/social cognition (i.e., optimal emotion recognition, mentalizing, salience, and attention to emotional stimuli), below which social cognition is deficient (with positive effects of exogenous oxytocin) and beyond which social cognition is excessive/distorted (with negative effects of exogenous oxytocin) (Bartz et al., 2011b).
Figure 1. Interactionist Model of the Effect of Oxytocin on Social Cognition.
Oxytocin’s effects on social cognition and functioning are modulated by baseline social cognition skills. There is a sweet spot of oxytocinergic tone/social cognition (i.e., optimal emotion recognition, mentalizing, salience and attention to emotional stimuli) and social functioning, below which social cognition is deficient (as in schizophrenia and autism, with positive effects of exogenous oxytocin) and beyond which social cognition is excessive/distorted (as in borderline personality disorder, with potentially negative effects of exogenous oxytocin).
Per the interactionist model, individuals can be classified into three distinct groups according to baseline social cognitive skills. The first group includes those with baseline optimal social cognition (e.g., healthy controls). They have optimal social cognitive brain network activity, emotion recognition, mentalizing accuracy, salience/reward processing and attention towards social stimuli. The second group is characterized by social cognitive deficits at baseline (e.g., SZ spectrum, autism). These individuals have low activity and altered emotional modulation of social cognitive networks, poor emotion recognition, low mentalizing accuracy, hypomentalizing errors (Montag et al., 2011), deficient salience/reward processing of social information, and low attention to social cues. These deficits are improved by oxytocin (Feifel et al., 2012; Feifel et al., 2010; Goldman et al., 2011; Leknes et al., 2013; Macdonald and Feifel, 2012; Pedersen et al., 2011). Finally, the third group includes those with baseline social cognitive distortions (e.g., borderline personality disorder). They are characterized by poor mentalizing accuracy, excessive, distorted mentalizing (hypermentalizing) (Sharp et al., 2011) and excessive salience/attention towards social stimuli, with attentional biases (Bartz et al., 2011a; Bartz et al., 2011b). In these populations, the effects of oxytocin are mixed, ranging from positive (decreased emotional responses and cortisol levels after social stress induction, decreased attentional biases and emotional reactions to angry faces, etc.) to negative (decreased trust and cooperation) (Bartz et al., 2011a; Bertsch et al., 2013; Brune et al., 2013; Ebert et al., 2013; Simeon et al., 2011).
Despite some evidence of attentional biases to emotional stimuli and social cognitive distortions (e.g., extremely negative or positive attributions associated with affective episodes in BD [Strange et al., 2013] or an exaggerated self-serving bias in paranoid patients [Kaney and Bentall, 1989; Garety and Freeman, 1999]), whether those individuals with bipolar disorder and actively psychotic paranoid schizophrenia belong to this third group remains largely unexplored.
Furthermore, the effects of oxytocin appear to be modulated by other factors, including gender, context, attachment style, and history of childhood adversity (Bakermans-Kranenburg and van, 2013; Bartz et al., 2011b; Domes et al., 2010). For example, the effects of intranasal oxytocin on functional connectivity between the posterior cingulate cortex and the cerebellum and postcentral gyrus were only observed among those healthy individuals with supportive family backgrounds (i.e., those who had experienced low levels of maternal love withdrawal in childhood) (Riem et al., 2013).
3.2. Clinical Trials of Intranasal Oxytocin Administration in Schizophrenia
To date, approximately ten clinical trials (summarized in Table 1) have been conducted in patients with SZ to test the efficacy of oxytocin in ameliorating social cognitive deficits and psychopathology.
Table 1.
Overview of Clinical Trials of the Effect of Oxytocin on Social Cognition and Psychopathology in Schizophrenia
| Study | Sample* | Oxytocin (OT) Intranasal Dose | Design | Outcome Measure | Results |
|---|---|---|---|---|---|
| Goldman et al. (2008) | 13 SZ
11 HCs
|
10 IU or 20 IU once a week | Double-blind, placebo-controlled crossover 3-week trial |
|
Facial affect intensity was greater in patients with PD compared to HCs across the treatment conditions but there was no difference between the two patients groups. The low OT dose (10 IU) increased intensity in the PD group compared to HCs but not to the nonPD group. The 20 IU dose showed a decrease in intensity in the PD group relative to the nonPD group but there were no differences compared to the HCs. Among emotions, only ‘fear’ was altered by OT: PD patients showed a reduction in fear compared to the nonPD patients. Recognition deteriorated in both SZ groups on the 10 IU dose but improved with 20 IU dose in SZ with PD. Overall emotion recognition improved with the 20 IU dose in the PD patients compared to the nonPD patients. |
| Feifel et al. (2010) | 15 SZ
Sample age=48+/−8.9; males=12 |
20 IU twice a day (first week); 40 IU twice a day for the subsequent two weeks | Double-blind, placebo-controlled crossover 3-week trial |
|
Reduction of symptoms with improvement of PANSS and CGI scores at the 3 week endpoint in OT group vs the placebo group. Study supports the hypothesis that OT has antipsychotic properties with effects on both negative and positive symptoms but more statistically robust for positive symptoms |
| Pederson et al. 2011 | 20 SZ
|
24 IU twice daily | Randomize d, double-blind, placebo-controlled 2-week trial |
|
The OT group improved in accurate identification in second order false beliefs (Brune Task) but there were no other significant differences in other measures of the Brune Task. The OT group also showed reduction in PANSS (positive, total, general subscale and suspiciousness/persecutory items) and Paranoia Scale scores. |
| Averback et al 2012 | 21 SZ
Sample age=38.2+/− 1.8; males=21 |
24 IU single dose | Double-blind, placebo-controlled crossover 2-week trial |
|
The OT group showed an overall better performance on facial emotion recognition, but none of the individual emotions showed a significant difference. The OT group performance remained lower compared to that of a control group used in a previous experiment in the study. |
| Feifel 2012 | 15 SZ
Sample age=48+/−8.9; males=12 |
20 IU twice a day (first week); 40 IU twice a day for the other two weeks | Double-blind, placebo-controlled crossover study |
|
The OT group vs the placebo groups showed significant effect on CVLT but not on LNS. The effect of OT was significant in CVLT total recall trials 1–5, short delayed free recall and total recall discrimination. However, it was stronger for short-term recall than for long-term. Authors suggest that this might be due to the fact that OT modulates only neural processes involved in short-term verbal memory. |
| Modabbernia et al 2012 | 40 SZ
|
20 IU twice daily for the 1st week; 40 IU twice daily for the following 7 weeks | Randomize d, double-blind, placebo-controlled 8-week trial |
|
OT group showed improvement in PANSS total score by week 4 with a total 11.2% symptoms reduction from baseline. Positive and negative symptoms and general psychopathology subscale scores showed that in the OT group at the study endpoint there was a symptoms reduction (of 20%, 7% and 8% respectively) starting from week 6. No changes were detected in ESRS score between the OT and the placebo group. |
| Davis et al 2013 | 23 SZ
|
Single dose of 40 IU | Randomize d, double-blind, placebo-controlled study |
|
OT did not significantly improve performance either in the social composite measure (combination of all the social tasks) or in the low-level processes score (TASIT- Part III detection of lies, Half-PONS and Facial Affect Recognition Task). However, OT significantly improved performance on high-level social cognition (TASIT-Part III detection of sarcasm, EPTT) with a large effect size (d=1.0). No improvement on positive, negative or general symptoms. |
| Fischer- Shoftly et al. 2013a | 35 SZ
48 HCs
|
24 IU single dose | Double-blind, within-subjects crossover 2-week trial |
|
There was no general effect of OT on mood. However a positive effect on social perception performance was found after administration in the OT group compared to placebo. In patients, the OT and placebo group significantly differed in the identification of kinship relationships but not intimacy while no such effect was detected in the control group. |
| Fischer- Shoftly et al. 2013b | 30 SZ
35 HCs
|
24 IU single dose | Double-blind, within-subjects crossover 2-week trial |
|
There was no general effect of OT on mood. In all participants, regardless of their psychiatric status, the OT administration showed higher accuracy recognition for the ‘fearful’ but not for ‘happy’ faces. Also, independently of psychiatric status, OT administration improved the performance of those participants whose basic fear recognition was below the median. |
| Lee et al. (2013) | 28 SZ or SAD
|
20 IU twice daily | Randomize d, double-blind, placebo-controlled 3-week trial |
|
Improvement in the OT group in the total UPSIT score and in the subscore for the pleasant smells (no effects for neutral or unpleasant smells). The placebo group showed improved global symptomatology (BPRS). The inpatient group that received OT had a better total SANS (d=.85) at week 3 while no differences were detected for the outpatient group. The motivational/pleasure subscale of the SANS showed improvement in the OT inpatient group (d=.74). |
SZ= Schizophrenia; SAD=Schizoaffective; HCs= Healthy Controls; PD= polydipsia OT=oxytocin; IU=International Unit
All SZ subjects across the clinical trials reported in the table maintained their pre-study antipsychotic medication
The most commonly administered doses of oxytocin in clinical trials in SZ were 20–24 IU (International Units) and 40 IU. Most of the studies showed that intranasal oxytocin enhanced facial affect recognition in SZ individuals compared to placebo groups (Goldman et al., 2008; Averback et al., 2012; Fischer-Shoftly et al., 2013b) and a specificity for the recognition of fearful faces was found in one study (Fischer-Shoftly et al., 2013b). Only Davis et al., (2013) failed to find significant improvements in facial affect recognition. However, this group found a large effect size (d = 1.0) for high level social cognition processes as measured by a ToM task (the sarcasm subscale of The Awareness of Social Inference Test [TASIT-III; McDonald et al., 2006]) and an empathy task (the Emotional Perspective Taking Task [EPTT; Derntl et al., 2009]). Similarly, a positive effect on ToM was also detected by Pederson et al., (2011), but only within more complex tasks of second order beliefs. Fischer-Shoftly and colleagues (2013a) reported an improvement in the ability to discriminate the correct nature of a presented relationship (i.e. kinship compared to romantic) after oxytocin administration. Regarding oxytocin’s effect on nonsocial cognition, one study examined the influence of oxytocin on memory in SZ (Feifel et al., 2012) and found that oxytocin has a beneficial effect on short-term verbal memory whereas no such effect was present in working memory.
With regard to the antipsychotic effect of oxytocin, results are mixed. Improvement in overall psychopathology (Lee et al., 2010; Feifel et al., 2010; Pederson et al., 2011; Modabbernia et al., 2012) seems to be linked to a higher oxytocin dosage and to a longer duration of treatment. Studies that administered a single dose of oxytocin did not report any amelioration of symptoms (Davis et al., 2013; Fischer-Shoftly et al., 2013a, b).
3.3. Clinical Trials of Intranasal Oxytocin Administration in Depression
Although several studies have demonstrated a relationship between depressive symptoms and endogenous oxytocin (e.g. Cyranowski et al., 2008; Scantamburlo et al., 2007; Gordon et al., 2008), reports have been small and inconsistent. Nevertheless, given the evidence for social cognitive impairments in depression, it may be worthwhile to examine the effect of intranasal oxytocin on social cognition and related symptoms. To date, only two placebo-controlled trials of oxytocin have been conducted in MDD. Mah and colleagues (2013) administered a single dose of 24 IU oxytocin or placebo, about one week apart, to 25 women with post-partum depression and measured mood and expressed emotion 45 minutes after administration. The results of this within-subject crossover design showed that in the oxytocin condition, the participants rated their mood as significantly sadder than in the control condition. Moreover, in the oxytocin condition, participants were more likely to describe their babies as difficult while at the same time, they reported that their relationship with their babies was more positive relative to the placebo condition. These somewhat conflicting results are difficult to interpret but seem to suggest that oxytocin has little effect on mood but that it may enhance salient relationship features, at least in mothers regarding their infants. A subset of these participants underwent a test of parental protectiveness under oxytocin and placebo conditions; the results indicate that oxytocin significantly enhanced parental protectiveness (Mah et al., 2014). Only one other study to date has investigated the effects of intranasal oxytocin on social cognition in major depressive disorder. MacDonald and colleagues (2013) administered a single-dose of oxytocin 40 IU or placebo immediately before a mock therapy session. Intranasal oxytocin was associated with improvement in social cognition (increased accuracy in a task measuring the understanding of mental states) and increased prosocial behavior (reduction in non-verbal behaviors that cut off social contact). However, oxytocin was also associated with higher anxiety during the psychotherapy session. Of note, higher anxiety during an initial therapy session may be understood as a positive sign of engagement in the therapy (MacDonald et al., 2013). Moreover, the therapists in this study had been instructed to provide “minimal or neutral interaction with the subjects,” which may have contributed to increased anxiety in the context of increased prosociality by oxytocin (MacDonald et al., 2013).
A study of nonclinical university students reported that there was a significant interaction between depressive symptoms and the ability to inhibit the processing of sad faces after a single administration of 24 IU of intranasal oxytocin (Ellenbogen et al., 2013). Using an affective priming paradigm, results suggested that participants with higher levels of depressive symptoms were unable to inhibit the processing of sad faces in the oxytocin condition compared to the placebo condition; there was no significant interaction between inhibition and oxytocin among the participants with lower levels of depressive symptoms. These results raise the question of whether oxytocin may enhance salient social stimuli in patients with depression, thereby improving social cognition. Conversely, it is possible that the bias toward negative social stimuli already present in individuals with depression (e.g. Leppänen, 2006) may be exacerbated by oxytocin, as per the interactionist model.
3.4. Clinical Trials of Intranasal Oxytocin Administration in Bipolar Disorder
There is very little information regarding oxytocin and BD. Intranasal oxytocin has not been administered to BD patients as of yet, and only one study to date has examined the relationship between endogenous serum oxytocin and BD (Turan et al., 2013). Turan and colleagues (2013) reported that serum oxytocin was significantly higher in patients compared to controls, and that patients experiencing a manic episode demonstrated significantly higher levels of oxytocin compared to remitted and depressed patients with BD. Oxytocin was measured at baseline and at the point at which patients had achieved a 50% reduction of symptoms after treatment with appropriate medications; no differences in pre- and post-treatment oxytocin were found, and post-treatment levels of oxytocin in the patients continued to be higher than baseline oxytocin levels in controls. Clearly, more work is needed to understand the relationship between endogenous oxytocin levels and symptoms of BD. Nevertheless, given the social cognitive deficits in BD, it may be worthwhile to explore intranasal oxytocin as a potential treatment for these deficits. Future work is required to test whether the administration of oxytocin to patients with BD would improve social cognitive impairments in BD, or whether the potentially increased salience of social stimuli resulting from oxytocin administration may perhaps exacerbate deficits in social cognition in BD.
3.5. Clinical Trials Combining Oxytocin with Psychotherapy
While there is a growing literature regarding oxytocin’s effects on social cognition and affiliation in clinical populations, to date only two studies have examined the effect of oxytocin in combination with psychotherapy (MacDonald et al., 2013; Guastella et al., 2009; Table 2). One additional study looked at the effect of oxytocin combined with hypnosis (Bryant and Hung, 2013). These studies suggest that augmentation of psychotherapy with oxytocin as compared to placebo leads to improvements in social cognition, as well as in self-perceptions during the induction of social anxiety. The evidence indicates that oxytocin may also result in an enhancement of hypnotizability, which may have implications for disorders that are improved through this treatment modality (e.g. chronic pain, smoking cessation). While not a study of a formal psychotherapy, a recent study examining the combination of oxytocin with social support found evidence that this combination exerted a more beneficial effect on cortisol responses to social stress than either intervention administered individually (Heinrichs et al., 2003). However, these studies have several limitations: 1) No study has combined oxytocin with an evidence-based psychotherapy for the treatment of depression or bipolar disorder; 2) No studies of therapy plus oxytocin have examined the effect of sustained treatment with daily oxytocin doses; and 3) No studies have measured oxytocin plus psychotherapy effects on social cognition using a standardized, validated assessment of complex social interactions using real-life naturalistic social cognition tasks before and after treatment. Comprehensive studies combining oxytocin and psychotherapy are needed in order to identify effects of treatments individually and in combination, and to explore the possible neural mechanisms underlying clinical change.
Table 2.
Studies examining combination treatment with oxytocin with psychotherapy.
| Study | Population | N | Intervention | Results: Oxytocin effect vs. Placebo | Limitations |
|---|---|---|---|---|---|
| Macdonald et al., 2013 | Major Depressive Disorder | 18 | Single 20-minute psychotherapy session + pretreatment with double-blind intranasal oxytocin (40IU) vs. placebo | Greater improvement in social cognition on “reading the mind in the eyes test”, greater reduction in non-verbal behaviors that cut off social contact, but increased anxiety during psychotherapy session. | Single-dose oxytocin (vs sustained use). Therapists instructed to provide “minimal or neutral interaction with the subjects.” |
| Guastella et al., 2009 | Social Anxiety Disorder | 25 | 5 weekly sessions of group exposure therapy + pretreatment with double-blind intranasal oxytocin (24IU) vs. placebo | Oxytocin yielded greater improvements in self-perceived performance and appearance during social performance (speech) than placebo. No difference in anxiety outcomes between groups. | Single-dose oxytocin (vs sustained use). |
| Bryant and Hung, 2013 | Healthy volunteers | 28 | Single session of hypnosis + intranasal oxytocin (24IU) vs. placebo | Greater hypnotizability observed following oxytocin than placebo | Single-dose, single-session. Not a clinical population, hypnosis not for therapeutic purposes |
4. Social Cognitive Remediation in Schizophrenia and Affective Disorders
Non-pharmacological interventions for social cognitive deficits have been developed and tested over many years in patients with SZ, with far fewer studies in MDD and BD. Social cognitive remediation treatments have sought to target the social cognitive deficits in SZ, namely emotion perception, ToM, and attributional style. Meta-analytic findings from an examination of 19 studies with a total of 692 participants found moderate to large effects of social cognitive remediation on facial affect recognition, small to moderate effects on ToM, and non-significant effects on social cue perception and attributional bias (Kurtz and Richardson, 2012). Social Cognition and Interaction Training (SCIT; Penn et al., 2005) is a relatively recently developed manualized group treatment for social cognition in SZ and has had generally positive findings (Combs et al., 2007; Roberts et al., 2009; Chan et al., 2010; Wang et al., 2013; Roberts et al., 2014). SCIT contains both skills training and an integration phase for practicing the skill set.
Overall, although social cognitive remediation is beneficial and appears to improve many important social cognitive domains in SZ, the skills generated may not generalize to real-world situations. In order for the trainings to be of clinical utility, they will need to also demonstrate sustainability over time and translate into functional improvement outside the laboratory (Henderson, 2013). More recently, oxytocin has been used as an augmentation for social cognitive training in SZ (Davis et al., 2014). Participants underwent 12 sessions of social cognitive remediation over 6 weeks; oxytocin (40 IU) or placebo was administered 30 minutes before each training session. Participants who received oxytocin demonstrated significant improvements in empathic accuracy compared to the placebo condition at the end of treatment and one month after treatment; no other social cognitive or neurocognitive variables differed between the placebo and treatment groups. Empathic accuracy has been traditionally difficult to improve in SZ; thus, these results suggest that combining oxytocin with social cognitive remediation may lead to improved outcomes compared with either treatment alone.
Social cognitive remediation has not been well-studied in affective disorders. A recent trial of SCIT for outpatients with BD demonstrated that this treatment is efficacious in improving ToM, emotion perception, and depressive symptoms and has smaller but still significant effects in reducing hostile attributional biases (Lahera et al., 2013). To date, no randomized controlled trials of social cognitive remediation have been conducted in MDD.
5. Limitations, Challenges, and Future Directions
Current challenges in the assessment of exogenous oxytocin’s effect on social cognition include the lack of standardized social cognition measures; the incomplete characterization of the human central oxytocin receptor (e.g., lack of quantification of the oxytocin receptor in the living brain, limited data regarding its distribution in the human brain and scarce genetic association data [Smith et al., 2013]) and neural circuitry of oxytocinergic networks (Churchland and Winkielman, 2012; Millan et al., 2014); and a lack of clarity regarding the intranasal formulation of oxytocin (Guastella et al., 2013) and its absorption into relevant brain areas (Born et al., 2002; Neumann et al., 2013). The development of a PET ligand would represent a significant advance in this area of research (Smith et al., 2012; Smith et al., 2013).
Efforts to examine associations between oxytocin levels and neural/behavioral effects and clinical symptoms are limited by the challenges of accurate, reliable peripheral quantification of oxytocin (Apter-Levy et al., 2013; Keri and Kiss, 2011; Kim et al., 2013). Moreover, it is unknown whether peripheral oxytocin levels are correlated with central (brain or CSF) levels in humans. Further work is required to understand the relationship between peripheral oxytocin and central levels.
None of the clinical trials of intranasal oxytocin administration described above have investigated the mechanism by which oxytocin enhances social cognition, and thus the two models (optimizing and interactionist) remain untested in SZ, MDD and BD. This is critically important work, as it is unclear whether oxytocin may help or hinder social cognitive functioning in patients with affective disorders. Future work is sorely needed investigating which specific social cognitive domains are affected by oxytocin and in which populations, as well as the nature and mechanism of this effect. The evidence to date suggests that oxytocin has subtle and extremely person- and context-dependent effects; clarification of who may benefit and under what circumstances is a promising and important next step.
Acknowledgments
This research is supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Medical Research Service of the Veterans Affairs James J Peters VAMC; the Department of Veterans Affairs NY/NJ (VISN3) Mental Illness Research, Education, and Clinical Center (MIRECC); a Brain and Behavior Research Foundation NARSAD Young Investigator Award to Dr. Perez Rodriguez, and the NIMH (R01 MH100125 to Dr. Burdick, CTSA grant UL1TR000067 awarded to the Mt. Sinai School of Medicine).
Role of Funding Source
This research is supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Medical Research Service of the Veterans Affairs James J Peters VAMC; the Department of Veterans Affairs NY/NJ (VISN3) Mental Illness Research, Education, and Clinical Center (MIRECC); a Brain and Behavior Research Foundation NARSAD Young Investigator Award to Dr. Perez Rodriguez, and the NIMH (R01 MH100125 to Dr. Burdick, CTSA grant UL1TR000067 awarded to the Mt. Sinai School of Medicine).
Footnotes
Conflict of Interest
All authors declare that they have no conflicts of interest
Contributors
Authors MMPR, MR, AKU and KM managed the literature searches; MMPR, MR, KM, AKU and KEB wrote the initial draft, MMPR, MR, AKU and KM addressed the reviewers’ comments, and KEB and MMPR revised the final manuscript critically. All authors contributed to and have approved the final manuscript.
The funding sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the VA MIRECC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aakre JM, Seghers JP, St-Hilaire A, Docherty N. Attributional style in delusional patients: a comparison of remitted paranoid, remitted nonparanoid, and current paranoid patients with nonpsychiatric controls. Schizophr Bull. 2009;35:994–1002. doi: 10.1093/schbul/sbn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott GR, Green MJ. Facial affect recognition and schizotypal personality characteristics. Early Interv Psychiatry. 2012;7:58–63. doi: 10.1111/j.1751-7893.2012.00346.x. [DOI] [PubMed] [Google Scholar]
- Abdi Z, Sharma T. Social cognition and its neural correlates in schizophrenia and autism. CNS Spectr. 2004;9:335–343. doi: 10.1017/s1092852900009317. [DOI] [PubMed] [Google Scholar]
- Addington J, Addington D. Facial affect recognition and information processing in schizophrenia and bipolar disorder. Schizophr Res. 1998;32:171–181. doi: 10.1016/s0920-9964(98)00042-5. [DOI] [PubMed] [Google Scholar]
- Addington J, Piskulic D, Perkins D, Woods SW, Liu L, Penn DL. Affect recognition in people at clinical high risk of psychosis. Schizophr Res. 2012;140:87–92. doi: 10.1016/j.schres.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Lipman AJ, Abramson LY. Attributional style as a vulnerability factor for depression: Validation by past history of mood disorders. Cognitive Therapy and Research. 1992;16:391–407. [Google Scholar]
- Amminger GP, Schäfer MR, Papageorgiou K, Klier CM, Schlögelhofer M, Mossaheb N, Werneck-Rohrer S, Nelson B, McGorry PD. Emotion recognition in individuals at clinical high-risk for schizophrenia. Schizophr Bull. 2012;38:1030–1039. doi: 10.1093/schbul/sbr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andero R, Ressler KJ. Fear extinction and BDNF: translating animal models of PTSD to the clinic. Genes Brain Behav. 2012;11:503–512. doi: 10.1111/j.1601-183X.2012.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. text rev. [Google Scholar]
- Apter-Levy Y, Feldman M, Vakart A, Ebstein RP, Feldman R. Impact of Maternal Depression Across the First 6 Years of Life on the Child’s Mental Health, Social Engagement, and Empathy: The Moderating Role of Oxytocin. Am J Psychiatry. 2013;170:1161–1168. doi: 10.1176/appi.ajp.2013.12121597. [DOI] [PubMed] [Google Scholar]
- Averbeck BB, Bobin T, Evans S, Shergill SS. Emotion recognition and oxytocin in patients with schizophrenia. Psychol Med. 2012;42:259–266. doi: 10.1017/S0033291711001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJMH. Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl Psychiatry. 2013;3:e258. doi: 10.1038/tp.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball HA, McGuffin P, Farmer AE. Attributional style and depression. Br J Psychiatry. 2008;192:275–278. doi: 10.1192/bjp.bp.107.038711. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42:241–251. [PubMed] [Google Scholar]
- Bartz J, Simeon D, Hamilton H, Kim S, Crystal S, Braun A, Vicens V, Hollander E. Oxytocin can hinder trust and cooperation in borderline personality disorder. Soc Cogn Affect Neurosci. 2011a;6:556–563. doi: 10.1093/scan/nsq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Hollander E, Ludwig NN, Kolevzon A, Ochsner KN. Oxytocin selectively improves empathic accuracy. Psychol Sci. 2010;21:1426–1428. doi: 10.1177/0956797610383439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011b;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bazin N, Brunet-Gouet E, Bourdet C, Kayser N, Falissard B, Hardy-Baylé MC, Passerieux C. Quantitative assessment of attribution of intentions to others in schizophrenia using an ecological video-based task: a comparison with manic and depressed patients. Psychiatry Res. 2009;167:28–35. doi: 10.1016/j.psychres.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Bentall RP, Corcoran R, Howard R, Blackwood N, Kinderman P. Persecutory delusions: a review and theoretical integration. Clin Psychol Rev. 2001;21:1143–1192. doi: 10.1016/s0272-7358(01)00106-4. [DOI] [PubMed] [Google Scholar]
- Bertrand MC, Sutton H, Achim AM, Malla AK, Lepage M. Social cognitive impairments in first episode psychosis. Schizophr Res. 2007;95:124–33. doi: 10.1016/j.schres.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Bertsch K, Gamer M, Schmidt B, Schmidinger I, Walther S, Kastel T, Schnell K, Buchel C, Domes G, Herpertz SC. Oxytocin and reduction of social threat hypersensitivity in women with borderline personality disorder. Am J Psychiatry. 2013;170:1169–1177. doi: 10.1176/appi.ajp.2013.13020263. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Billeke P, Aboitiz F. Social cognition in schizophrenia: from social stimuli processing to social engagement. Front Psychiatry. 2013;4:4. doi: 10.3389/fpsyt.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Vahip S, Gonul AS, Akdeniz F, Alkan M, Ogut M, Eryavuz A. Evidence for theory of mind deficits in euthymic patients with bipolar disorder. Acta Psychiatrica Scandinavica. 2005;112:110–116. doi: 10.1111/j.1600-0447.2005.00570.x. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Dis. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr Res. 2009;109:1–9. doi: 10.1016/j.schres.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Bos PA, Panksepp J, Bluthe RM, van Honk J. Acute effects of steroid hormones and neuropeptides on human social-emotional behavior: a review of single administration studies. Front Neuroendocrinol. 2012;33:17–35. doi: 10.1016/j.yfrne.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Bourke C, Douglas K, Porter R. Processing of facial emotion expression in major depression: a review. Aust N Z J Psychiatry. 2010;44:681–696. doi: 10.3109/00048674.2010.496359. [DOI] [PubMed] [Google Scholar]
- Bozikas VP, Kosmidis MH, Anezoulaki D, Giannakou M, Karavatos A. Relationship of affect recognition with psychopathology and cognitive performance in schizophrenia. J Int Neuropsychol Soc. 2004;10:549–558. doi: 10.1017/S1355617704104074. [DOI] [PubMed] [Google Scholar]
- Braff DL, Light GA. The use of neurophysiological endophenotypes to understand the genetic basis of schizophrenia. Dialogues Clin Neurosci. 2005;7:125–135. doi: 10.31887/DCNS.2005.7.2/dlbraff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüne M. Emotion recognition, ‘theory of mind,’ and social behavior in schizophrenia. Psychiatry Res. 2005;133:135–47. doi: 10.1016/j.psychres.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Brune M, Abdel-Hamid M, Lehmkamper C, Sonntag C. Mental state attribution, neurocognitive functioning, and psychopathology: what predicts poor social competence in schizophrenia best? Schizophr Res. 2007;92:151–159. doi: 10.1016/j.schres.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Brune M, Ebert A, Kolb M, Tas C, Edel MA, Roser P. Oxytocin influences avoidant reactions to social threat in adults with borderline personality disorder. Hum Psychopharmacol. 2013;28:552–561. doi: 10.1002/hup.2343. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Hung L. Oxytocin enhances social persuasion during hypnosis. PloS one. 2013;8:e60711. doi: 10.1371/journal.pone.0060711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RC, Li H, Cheung EF, Gong QY. Impaired facial emotion perception in schizophrenia: a meta-analysis. Psychiatry Res. 2010;178:381–390. doi: 10.1016/j.psychres.2009.03.035. [DOI] [PubMed] [Google Scholar]
- Churchland PS, Winkielman P. Modulating social behavior with oxytocin: how does it work? What does it mean? Horm Behav. 2012;61:392–399. doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran R, Frith CD. Autobiographical memory and theory of mind: evidence of a relationship in schizophrenia. Psychol Med. 2003;33:897–905. doi: 10.1017/s0033291703007529. [DOI] [PubMed] [Google Scholar]
- Corcoran R, Mercer G, Frith CD. Schizophrenia, symptomatology and social inference: investigating “theory of mind” in people with schizophrenia. Schizophr Res. 1995;17:5–13. doi: 10.1016/0920-9964(95)00024-g. [DOI] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32(Suppl 1):S44–63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusi AM, Nazarov A, Macqueen GM, McKinnon MC. Theory of mind deficits in patients with mild symptoms of major depressive disorder. Psychiatry Res. 2013;210:672–674. doi: 10.1016/j.psychres.2013.06.018. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Hofkens TL, Frank E, Seltman H, Cai HM, Amico JA. Evidence of dysregulated peripheral oxytocin release among depressed women. Psychosom Med. 2008;70:967–975. doi: 10.1097/PSY.0b013e318188ade4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH. Measuring individual differences in empathy: Evidence for a multidimensional approach. J Pers Soc Psychol. 1983;44:113–126. [Google Scholar]
- Davis MC, Lee J, Horan WP, Clarke AD, McGee MR, Green MF, Marder SR. Effects of single dose intranasal oxytocin on social cognition in schizophrenia. Schizophr Res. 2013;147:393–397. doi: 10.1016/j.schres.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Derntl B, Finkelmeyer A, Toygar TK, Hülsmann A, Schneider F, Falkenberg DI, Habel U. Generalized deficit in all core components of empathy in schizophrenia. Schizophr Res. 2009;108:197–206. doi: 10.1016/j.schres.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Devylder JE, Ben-David S, Kimhy D, Corcoran CM. Attributional style among youth at clinical risk for psychosis. Early Interv Psychiatry. 2013;7:84–88. doi: 10.1111/j.1751-7893.2012.00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diforio D, Walker EF, Kestler LP. Executive functions in adolescents with schizotypal personality disorder. Schizophr Res. 2000;42:125–134. doi: 10.1016/s0920-9964(99)00119-x. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol Psychiatry. 2009;65:728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Domes G, Sibold M, Schulze L, Lischke A, Herpertz SC, Heinrichs M. Intranasal oxytocin increases covert attention to positive social cues. Psychol Med. 2013;43:1747–1753. doi: 10.1017/S0033291712002565. [DOI] [PubMed] [Google Scholar]
- Doop ML, Park S. Facial expression and face orientation processing in schizophrenia. Psychiatry Res. 2009;170:103–107. doi: 10.1016/j.psychres.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Drury VM, Robinson EJ, Birchwood M. ‘Theory of mind’ skills during an acute episode of psychosis and following recovery. Psychol Med. 1998;28:1101–1112. doi: 10.1017/s0033291798006850. [DOI] [PubMed] [Google Scholar]
- Dziobek I, Fleck S, Kalbe E, Rogers K, Hassenstab J, Brand M, Convit A. Introducing MASC: a movie for the assessment of social cognition. J Autism Dev Disord. 2006;36:623–636. doi: 10.1007/s10803-006-0107-0. [DOI] [PubMed] [Google Scholar]
- Ebert A, Kolb M, Heller J, Edel MA, Roser P, Brune M. Modulation of interpersonal trust in borderline personality disorder by intranasal oxytocin and childhood trauma. Soc Neurosci. 2013;8:305–313. doi: 10.1080/17470919.2013.807301. [DOI] [PubMed] [Google Scholar]
- Edwards J, Pattison PE, Jackson HJ, Wales RJ. Facial affect and affective prosody recognition in first-episode schizophrenia. Schizophr Res. 2001;48:235–253. doi: 10.1016/s0920-9964(00)00099-2. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Miller PA. The relation of empathy to prosocial and related behaviors. Psychol Bull. 1987;101:91–119. [PubMed] [Google Scholar]
- Ellenbogen MA, Linnen AM, Cardoso C, Joober R. Intranasal oxytocin impedes the ability to ignore task-irrelevant facial expressions of sadness in students with depressive symptoms. Psychoneuroendocrinology. 2013;38:387–398. doi: 10.1016/j.psyneuen.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Elvevåg B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- Farmer CM, O’Donnell BF, Niznikiewicz MA, Voglmaier MM, McCarley RW, Shenton ME. Visual perception and working memory in schizotypal personality disorder. Am J Psychiatry. 2000;157:781–788. doi: 10.1176/appi.ajp.157.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Macdonald K, Cobb P, Minassian A. Adjunctive intranasal oxytocin improves verbal memory in people with schizophrenia. Schizophr Res. 2012;139:207–210. doi: 10.1016/j.schres.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, Minassian A, Becker O, Cooper J, Perry W, Lefebvre M, Gonzales J, Hadley A. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry. 2010;68:678–680. doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]
- Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35:573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Fioravanti M, Carlone O, Vitale B, Cinti ME, Clare L. A meta-analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychol Rev. 2005;15:73–95. doi: 10.1007/s11065-005-6254-9. [DOI] [PubMed] [Google Scholar]
- Fischer-Shofty M, Brüne M, Ebert A, Shefet D, Levkovitz Y, Shamay-Tsoory SG. Improving social perception in schizophrenia: the role of oxytocin. Schizophr Res. 2013a;146:357–362. doi: 10.1016/j.schres.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Fischer-Shofty M, Shamay-Tsoory SG, Levkovitz Y. Characterization of the effects of oxytocin on fear recognition in patients with schizophrenia and in healthy controls. Front Neurosci. 2013b;7:127. doi: 10.3389/fnins.2013.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD. Social cognition. Philos Trans R Soc Lond B Biol Sci. 2008;363:2033–2039. doi: 10.1098/rstb.2008.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe S, Williams C, Mason OJ, Pickup GJ. Apophenia, theory of mind and schizotypy: perceiving meaning and intentionality in randomness. Cortex. 2008;44:1316–1325. doi: 10.1016/j.cortex.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Gaebel W, Wölwer W. Facial expression and emotional face recognition in schizophrenia and depression. Eur Arch Psychiatry Clin Neurosci. 1992;242:46–52. doi: 10.1007/BF02190342. [DOI] [PubMed] [Google Scholar]
- Garety PA, Freeman D. Cognitive approaches to delusions: a critical review of theories and evidence. Br J Clin Psychol. 1999;38:113–154. doi: 10.1348/014466599162700. [DOI] [PubMed] [Google Scholar]
- Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry. 1997;54:159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Goldman RS, Burdick KE, Malhotra AK, Lencz T, Patel RC, Woerner MG, Schooler NR, Kane JM, Robinson DG. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch Gen Psychiatry. 2007;64:1115–1122. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biol Psychiatry. 1999;46:650–661. doi: 10.1016/s0006-3223(99)00130-4. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Gomes AM, Carter CS, Lee R. Divergent effects of two different doses of intranasal oxytocin on facial affect discrimination in schizophrenic patients with and without polydipsia. Psychopharmacology. 2011;216:101–110. doi: 10.1007/s00213-011-2193-8. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Pflum MJ. Theory of Mind and psychometric schizotypy. Psychiatry Res. 2011;188:217–223. doi: 10.1016/j.psychres.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Schneiderman I, Leckman JF, Weller A, Feldman R. Oxytocin and cortisol in romantically unattached young adults: associations with bonding and psychological distress. Psychophysiology. 2008;45:349–352. doi: 10.1111/j.1469-8986.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67:e12. [PubMed] [Google Scholar]
- Green MF, Bearden CE, Cannon TD, Fiske AP, Hellemann GS, Horan WP, Kee K, Kern RS, Lee J, Sergi MJ, Subotnik KL, Sugar CA, Ventura J, Yee CM, Nuechterlein KH. Social cognition in schizophrenia, Part 1: performance across phase of illness. Schizophr Bull. 2012;38:854–864. doi: 10.1093/schbul/sbq171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Leitman DI. Social cognition in schizophrenia. Schizophr Bull. 2008;34:670–672. doi: 10.1093/schbul/sbn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppe SE, Gossen A, Rademacher L, Hahn A, Westphal L, Grunder G, Spreckelmeyer KN. Oxytocin influences processing of socially relevant cues in the ventral tegmental area of the human brain. Biol Psychiatry. 2013;74:172–179. doi: 10.1016/j.biopsych.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Hickie IB, McGuinness MM, Otis M, Woods EA, Disinger HM, Chan HK, Chen TF, Banati RB. Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology. 2013;38:612–625. doi: 10.1016/j.psyneuen.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Howard AL, Dadds MR, Mitchell P, Carson DS. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology. 2009;34:917–923. doi: 10.1016/j.psyneuen.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Gumley A, Braehler C, Macbeth A. A meta-analysis and theoretical critique of oxytocin and psychosis: Prospects for attachment and compassion in promoting recovery. Br J Clin Psychol. 2014;53:42–61. doi: 10.1111/bjc.12041. [DOI] [PubMed] [Google Scholar]
- Happé FG. An advanced test of theory of mind: understanding of story characters’ thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. J Autism Dev Disord. 1994;24:129–154. doi: 10.1007/BF02172093. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Bowie CR. Cognitive enhancement in schizophrenia: pharmacological and cognitive remediation approaches. Psychiatr Clin North Am. 2012;35:683–698. doi: 10.1016/j.psc.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RM, Montgomery SA, Keller MB, Kasper S, Schatzberg AF, Möller HJ, Bourgeois M. Social functioning in depression: a review. J Clin Psychiatry. 2000;61:268–275. doi: 10.4088/jcp.v61n0405. [DOI] [PubMed] [Google Scholar]
- Hoekert M, Kahn RS, Pijnenborg M, Aleman A. Impaired recognition and expression of emotional prosody in schizophrenia: review and meta-analysis. Schizophr Res. 2007;96:135–145. doi: 10.1016/j.schres.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Hooker C, Park S. Emotion processing and its relationship to social functioning in schizophrenia patients. Psychiatry Res. 2002;112:41–50. doi: 10.1016/s0165-1781(02)00177-4. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Tonooka Y, Yamada K, Kanba S. Deficiency of theory of mind in patients with remitted mood disorder. J Affect Disord. 2004;82:403–409. doi: 10.1016/j.jad.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Yamada K, Kanba S. Deficit in theory of mind is a risk for relapse of major depression. J Affect Disord. 2006;95:125–127. doi: 10.1016/j.jad.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Kaney S, Bentall RP. Persecutory delusions and attributional style. Br J Med Psychol. 1989;62:191–198. doi: 10.1111/j.2044-8341.1989.tb02826.x. [DOI] [PubMed] [Google Scholar]
- Keri S, Kiss I. Oxytocin response in a trust game and habituation of arousal. Physiol Behav. 2011;102:221–224. doi: 10.1016/j.physbeh.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Kerr N, Dunbar RIM, Bentall RP. Theory of mind deficits in bipolar affective disorder. J Affect Disord. 2003;73:253–259. doi: 10.1016/s0165-0327(02)00008-3. [DOI] [PubMed] [Google Scholar]
- Kettle JW, O’Brien-Simpson L, Allen NB. Impaired theory of mind in first-episode schizophrenia: comparison with community, university and depressed controls. Schizophr Res. 2008;99:96–102. doi: 10.1016/j.schres.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Kim S, Fonagy P, Koos O, Michael K, Strathearn L. Maternal Oxytocin Response Predicts Mother-to-Infant Gaze. Brain Res. 2013 doi: 10.1016/j.brainres.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelkebeck K, Pedersen A, Suslow T, Kueppers KA, Arolt V, Ohrmann P. Theory of Mind in first-episode schizophrenia patients: correlations with cognition and personality traits. Schizophr Res. 2010;119:115–123. doi: 10.1016/j.schres.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Hoffman LJ, Eastman LB, Healey K, Moberg PJ. Facial emotion perception in depression and bipolar disorder: a quantitative review. Psychiatry Res. 2011;188:303–309. doi: 10.1016/j.psychres.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull. 2010;36:1009–1019. doi: 10.1093/schbul/sbn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E. In: Manic Depressive Insanity and Paranoia. Robertson George M., editor. Edinburgh: E.& S. Livingston; 1921. [Google Scholar]
- Krstev H, Jackson H, Maude D. An investigation of attributional style in first-episode psychosis. Br J Clin Psychol. 1999;38:181–194. doi: 10.1348/014466599162737. [DOI] [PubMed] [Google Scholar]
- Ladegaard N, Larsen ER, Videbech P, Lysaker PH. Higher-order social cognition in first-episode major depression. Psychiatry Res. 2013;216:37–43. doi: 10.1016/j.psychres.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Lahera G, Montes JM, Benito A, Valdivia M, Medina E, Mirapeix I, Saiz-Ruiz J. Theory of mind deficit in bipolar disorder: is it related to a previous history of psychotic symptoms? Psychiatry Res. 2008;161:309–317. doi: 10.1016/j.psychres.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Lee L, Harkness KL, Sabbagh MA, Jacobson JA. Mental state decoding abilities in clinical depression. J Affect Disord. 2005;86:247–258. doi: 10.1016/j.jad.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Lee MR, Wehring HJ, McMahon RP, Linthicum J, Cascella N, Liu F, Bellack A, Buchanan RW, Strauss GP, Contoreggi C, Kelly DL. Effects of adjunctive intranasal oxytocin on olfactory identification and clinical symptoms in schizophrenia: results from a randomized double blind placebo controlled pilot study. Schizophr Res. 2013;145:110–115. doi: 10.1016/j.schres.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S, Wessberg J, Ellingsen DM, Chelnokova O, Olausson H, Laeng B. Oxytocin enhances pupil dilation and sensitivity to ‘hidden’ emotional expressions. Soc Cogn Affect Neurosci. 2013;8:741–749. doi: 10.1093/scan/nss062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembke A, Ketter TA. Impaired recognition of facial emotion in mania. Am J Psychiatry. 2002;152:302–304. doi: 10.1176/appi.ajp.159.2.302. [DOI] [PubMed] [Google Scholar]
- Leppänen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr Opin Psychiatry. 2006;19:34–39. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- Li H, Chan RC, McAlonan GM, Gong QY. Facial emotion processing in schizophrenia: a meta-analysis of functional neuroimaging data. Schizophr Bull. 2010;36:1029–1039. doi: 10.1093/schbul/sbn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln TM, Lange J, Burau J, Exner C, Moritz S. The effect of state anxiety on paranoid ideation and jumping to conclusions. An experimental investigation. Schizophr Bull. 2010;36:1140–1148. doi: 10.1093/schbul/sbp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughland CM, Williams LM, Gordon E. Schizophrenia and affective disorder show different visual scanning behavior for faces: a trait versus state-based distinction? Biol Psychiatry. 2002;52:338–348. doi: 10.1016/s0006-3223(02)01356-2. [DOI] [PubMed] [Google Scholar]
- Love TM. Oxytocin, motivation and the role of dopamine. Pharmacol Biochem Behav. 2014;119C:49–60. doi: 10.1016/j.pbb.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald K, Feifel D. Oxytocin in schizophrenia: a review of evidence for its therapeutic effects. Acta Neuropsychiatr. 2012;24:130–146. doi: 10.1111/j.1601-5215.2011.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald K, Macdonald TM. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harv Rev Psychiatry. 2010;18:1–21. doi: 10.3109/10673220903523615. [DOI] [PubMed] [Google Scholar]
- MacDonald K, MacDonald TM, Brune M, Lamb K, Wilson MP, Golshan S, Feifel D. Oxytocin and psychotherapy: a pilot study of its physiological, behavioral and subjective effects in males with depression. Psychoneuroendocrinology. 2013;38:2831–2843. doi: 10.1016/j.psyneuen.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Mah BL, Bakermans-Kranenburg MJ, Van Ijzendoorn MH, Smith R. Oxytocin promotes protective behavior in depressed mothers: a pilot study with the enthusiastic stranger paradigm. Depress Anxiety. 2014 doi: 10.1002/da.22245. (in press) [DOI] [PubMed] [Google Scholar]
- Mah BL, Van Ijzendoorn MH, Smith R, Bakermans-Kranenburg MJ. Oxytocin in postnatally depressed mothers: its influence on mood and expressed emotion. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:267–272. doi: 10.1016/j.pnpbp.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Coleman E. Olfaction and social drive in schizophrenia. Arch Gen Psychiatry. 2003;60:578–584. doi: 10.1001/archpsyc.60.6.578. [DOI] [PubMed] [Google Scholar]
- Martínez-Arán A, Penadés R, Vieta E, Colom F, Reinares M, Benabarre A, Salamero M, Gastó C. Executive function in patients with remitted bipolar disorder and schizophrenia and its relationship with functional outcome. Psychother Psychosom. 2002;71:39–46. doi: 10.1159/000049342. [DOI] [PubMed] [Google Scholar]
- Martino DJ, Strejilevich SA, Fassi G, Marengo E, Igoa A. Theory of mind and facial emotion recognition in euthymic bipolar I and bipolar II disorders. Psychiatry Res. 2011;189:379–384. doi: 10.1016/j.psychres.2011.04.033. [DOI] [PubMed] [Google Scholar]
- Matsui M, Yuuki H, Kato K, Takeuchi A, Nishiyama S, Bilker WB, Kurachi M. Schizotypal disorder and schizophrenia: a profile analysis of neuropsychological functioning in Japanese patients. J Int Neuropsychol Soc. 2007;13:672–682. doi: 10.1017/S135561770707083X. [DOI] [PubMed] [Google Scholar]
- Mazza M, De Risio A, Surian L, Roncone R, Casacchia M. Selective impairments of theory of mind in people with schizophrenia. Schizophr Res. 2001;47:299–308. doi: 10.1016/s0920-9964(00)00157-2. [DOI] [PubMed] [Google Scholar]
- McDonald S, Bornhofen C, Shum D, Long E, Saunders C, Neulinger K. Reliability and validity of The Awareness of Social Inference Test (TASIT): a clinical test of social perception. Disabil Rehabil. 2006;28:1529–1542. doi: 10.1080/09638280600646185. [DOI] [PubMed] [Google Scholar]
- McKinnon MC, Cusi AM, Macqueen GM. Impaired theory of mind performance in patients with recurrent bipolar disorder: Moderating effect of cognitive load. Psychiatry Res. 2010;177:261–262. doi: 10.1016/j.psychres.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Miklowitz DJ. Functional impairment, stress, and psychosocial intervention in bipolar disorder. Curr Psychiatry Rep. 2011;13:504–512. doi: 10.1007/s11920-011-0227-x. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Bales KL. Towards improved animal models for evaluating social cognition and its disruption in schizophrenia: the CNTRICS initiative. Neurosci Biobehav Rev. 2013;37:2166–2180. doi: 10.1016/j.neubiorev.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Fone K, Steckler T, Horan WP. Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur Neuropsychopharmacol. 2014;24:645–92. doi: 10.1016/j.euroneuro.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Mitchley NJ, Barber J, Gray YM, Brooks N, Livingston MG. Comprehension of irony in schizophrenia. Cogn Neuropsychiatry. 1998;3:127–138. [Google Scholar]
- Modabbernia A, Rezaei F, Salehi B, Jafarinia M, Ashrafi M, Tabrizi M, Hosseini SM, Tajdini M, Ghaleiha A, Akhondzadeh S. Intranasal oxytocin as an adjunct to risperidone in patients with schizophrenia : an 8-week, randomized, double-blind, placebo-controlled study. CNS Drugs. 2013;27:57–65. doi: 10.1007/s40263-012-0022-1. [DOI] [PubMed] [Google Scholar]
- Montag C, Dziobek I, Richter IS, Neuhaus K, Lehmann A, Sylla R, Heekeren HR, Heinz A, Gallinat J. Different aspects of theory of mind in paranoid schizophrenia: Evidence from a video-based assessment. Psychiatry Res. 2011;186:203–209. doi: 10.1016/j.psychres.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Montag C, Ehrlich A, Neuhaus K, Dziobek I, Heekeren HR, Heinz A, Gallinat J. Theory of mind impairments in euthymic bipolar patients. J Affect Disord. 2010;123:264–269. doi: 10.1016/j.jad.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL, Leserman J, Jarskog LF, Penn DL. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophr Res. 2011;132:50–53. doi: 10.1016/j.schres.2011.07.027. [DOI] [PubMed] [Google Scholar]
- Penn DL, Corrigan PW, Bentall RP, Racenstein JM, Newman L. Social cognition in schizophrenia. Psychol Bull. 1997;121:114–132. doi: 10.1037/0033-2909.121.1.114. [DOI] [PubMed] [Google Scholar]
- Penn DL, Sanna LJ, Roberts DL. Social cognition in schizophrenia: an overview. Schizophr Bull. 2008;34:408–411. doi: 10.1093/schbul/sbn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlick D, Stastny P, Mattis S, Teresi J. Contribution of family, cognitive and clinical dimensions to long-term outcome in schizophrenia. Schizophr Res. 1992;6:257–265. doi: 10.1016/0920-9964(92)90009-t. [DOI] [PubMed] [Google Scholar]
- Perry A, Bentin S, Shalev I, Israel S, Uzefovsky F, Bar-On D, Ebstein RP. Intranasal oxytocin modulates EEG mu/alpha and beta rhythms during perception of biological motion. Psychoneuroendocrinology. 2010;35:1446–1453. doi: 10.1016/j.psyneuen.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Pflum MJ, Gooding DC, White HJ. Hint, hint: theory of mind performance in schizotypal individuals. J Nerv Ment Dis. 2013;201:394–399. doi: 10.1097/NMD.0b013e31828e1016. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Hopfinger JB, Ruparel K, Penn DL. An investigation of the relationship between activation of a social cognitive neural network and social functioning. Schizophr Bull. 2008;34:688–697. doi: 10.1093/schbul/sbn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham AE, Loughead J, Ruparel K, Overton E, Gur RE, Gur RC. Abnormal modulation of amygdala activity in schizophrenia in response to direct- and averted-gaze threat-related facial expressions. Am J Psychiatry. 2011;168:293–301. doi: 10.1176/appi.ajp.2010.10060832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham AE, Penn DL, Perkins DO, Graham KA, Siegel M. Emotion perception and social skill over the course of psychosis: a comparison of individuals “at-risk” for psychosis and individuals with early and chronic schizophrenia spectrum illness. Cogn Neuropsychiatry. 2007;12:198–212. doi: 10.1080/13546800600985557. [DOI] [PubMed] [Google Scholar]
- Prehn K, Kazzer P, Lischke A, Heinrichs M, Herpertz SC, Domes G. Effects of intranasal oxytocin on pupil dilation indicate increased salience of socioaffective stimuli. Psychophysiology. 2013;50:528–537. doi: 10.1111/psyp.12042. [DOI] [PubMed] [Google Scholar]
- Quirin M, Kuhl J, Dusing R. Oxytocin buffers cortisol responses to stress in individuals with impaired emotion regulation abilities. Psychoneuroendocrinology. 2011;36:898–904. doi: 10.1016/j.psyneuen.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Harvey PD, Bowie CR, Mojtabai R, Rabinowitz J, Heaton RK, Bromet E. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr Bull. 2009;35:1022–1029. doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riem MM, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MA, Vermeiren RR, van Ijzendoorn MH, Rombouts SA. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: a randomized controlled trial. Biol Psychiatry. 2011;70:291–297. doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Riem MM, van IJzendoorn MH, Tops M, Boksem MA, Rombouts SA, Bakermans-Kranenburg MJ. Oxytocin effects on complex brain networks are moderated by experiences of maternal love withdrawal. Eur Neuropsychopharmacol. 2013;23:1288–95. doi: 10.1016/j.euroneuro.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Ripoll LH, Zaki J, Perez-Rodriguez MM, Snyder R, Strike KS, Boussi A, Bartz JA, Ochsner KN, Siever LJ, New AS. Empathic accuracy and cognition in schizotypal personality disorder. Psychiatry Res. 2013;210:232–241. doi: 10.1016/j.psychres.2013.05.025. [DOI] [PubMed] [Google Scholar]
- Roncone R, Falloon IR, Mazza M, De Risio A, Pollice R, Necozione S, Morosini P, Casacchia M. Is theory of mind in schizophrenia more strongly associated with clinical and social functioning than with neurocognitive deficits? Psychopathology. 2002;35:280–288. doi: 10.1159/000067062. [DOI] [PubMed] [Google Scholar]
- Samamé C, Martino DJ, Strejilevich SA. Social cognition in euthymic bipolar disorder: systematic review and meta-analytic approach. Acta Psychiatr Scand. 2012;125:266–280. doi: 10.1111/j.1600-0447.2011.01808.x. [DOI] [PubMed] [Google Scholar]
- Sarfati Y, Hardy-Baylé MC, Brunet E, Widlöcher D. Investigating theory of mind in schizophrenia: influence of verbalization in disorganized and non-disorganized patients. Schizophr Res. 1999;37:183–190. doi: 10.1016/s0920-9964(98)00154-6. [DOI] [PubMed] [Google Scholar]
- Scantamburlo G, Hansenne M, Fuchs S, Pitchot W, Maréchal P, Pequeux C, Legros JJ. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology. 2007;32:407–410. doi: 10.1016/j.psyneuen.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Schreiter S, Pijnenborg GHM, Aan Het Rot M. Empathy in adults with clinical or subclinical depressive symptoms. J Affect Disord. 2013;150:1–16. doi: 10.1016/j.jad.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Sharp C, Pane H, Ha C, Venta A, Patel AB, Sturek J, Fonagy P. Theory of mind and emotion regulation difficulties in adolescents with borderline traits. J Am Acad Child Adolesc Psychiatry. 2011;50:563–573. e561. doi: 10.1016/j.jaac.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Sharp C, Pane H, Ha C, Venta A, Patel AB, Sturek J, Fonagy P. Theory of mind and emotion regulation difficulties in adolescents with borderline traits. J Am Acad Child Adolesc Psychiatry. 2011;50:563–573. e561. doi: 10.1016/j.jaac.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Shea TL, Sergejew AA, Burnham D, Jones C, Rossell SL, Copolov DL, Egan GF. Emotional prosodic processing in auditory hallucinations. Schizophr Res. 2007;90:214–220. doi: 10.1016/j.schres.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Simeon D, Bartz J, Hamilton H, Crystal S, Braun A, Ketay S, Hollander E. Oxytocin administration attenuates stress reactivity in borderline personality disorder: a pilot study. Psychoneuroendocrinology. 2011;36:1418–1421. doi: 10.1016/j.psyneuen.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Smith AL, Freeman SM, Stehouwer JS, Inoue K, Voll RJ, Young LJ, Goodman MM. Synthesis and evaluation of C-11, F-18 and I-125 small molecule radioligands for detecting oxytocin receptors. Bioorg Med Chem. 2012;20:2721–2738. doi: 10.1016/j.bmc.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AL, Freeman SM, Voll RJ, Young LJ, Goodman MM. Investigation of an F-18 oxytocin receptor selective ligand via PET imaging. Bioorg Med Chem Lett. 2013;23:5415–5420. doi: 10.1016/j.bmcl.2013.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprong M, Schothorst P, Vos E, Hox J, van Engeland H. Theory of mind in schizophrenia: meta-analysis. Br J Psychiatry. 2007;191:5–13. doi: 10.1192/bjp.bp.107.035899. [DOI] [PubMed] [Google Scholar]
- Sripada CS, Phan KL, Labuschagne I, Welsh R, Nathan PJ, Wood AG. Oxytocin enhances resting-state connectivity between amygdala and medial frontal cortex. Int J Neuropsychopharmacol. 2013;16:255–260. doi: 10.1017/S1461145712000533. [DOI] [PubMed] [Google Scholar]
- Stange JP, Sylvia LG, da Magalhães PVS, Frank E, Otto MW, Miklowitz DJ, Deckersbach T. Extreme attributions predict transition from depression to mania or hypomania in bipolar disorder. J Psychiatr Res. 2013;47:1329–1336. doi: 10.1016/j.jpsychires.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavropoulos KK, Carver LJ. Research review: Social motivation and oxytocin in autism--implications for joint attention development and intervention. J Child Psychol Psychiatry. 2013;54:603–618. doi: 10.1111/jcpp.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit M, Wölwer W, Gaebel W. Facial-affect recognition and visual scanning behaviour in the course of schizophrenia. Schizophr Res. 1997;24:311–317. doi: 10.1016/s0920-9964(96)00126-0. [DOI] [PubMed] [Google Scholar]
- Sweeney PD, Anderson K, Bailey S. Attributional style in depression: A meta-analytic review. J Per Soc Psychol. 1986;50:974–991. doi: 10.1037//0022-3514.50.5.974. [DOI] [PubMed] [Google Scholar]
- Tollenaar MS, Chatzimanoli M, van der Wee NJ, Putman P. Enhanced orienting of attention in response to emotional gaze cues after oxytocin administration in healthy young men. Psychoneuroendocrinology. 2013;38:1797–1802. doi: 10.1016/j.psyneuen.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Carpenter M, Call J, Behne T, Moll H. Understanding and sharing intentions: the origins of cultural cognition. Behav Brain Sci. 2005;28:675–691. doi: 10.1017/S0140525X05000129. discussion 691–735. [DOI] [PubMed] [Google Scholar]
- Tracy A, Bauwens F, Martin F, Pardoen D, Mendlewicz J. Attributional style and depression: a controlled comparison of remitted unipolar and bipolar patients. Br J Clin Psychol. 1992;31:83–84. doi: 10.1111/j.2044-8260.1992.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Trestman RL, Keefe RS, Mitropoulou V, Harvey PD, deVegvar ML, Lees-Roitman S, Davidson M, Aronson A, Silverman J, Siever LJ. Cognitive function and biological correlates of cognitive performance in schizotypal personality disorder. Psychiatry Res. 1995;59:127–136. doi: 10.1016/0165-1781(95)02709-2. [DOI] [PubMed] [Google Scholar]
- Tseng HH, Chen SH, Liu CM, Howes O, Huang YL, Hsieh MH, Liu CC, Shan JC, Lin YT, Hwu HG. Facial and prosodic emotion recognition deficits associate with specific clusters of psychotic symptoms in schizophrenia. PLoS One. 2013;8:e66571. doi: 10.1371/journal.pone.0066571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan T, Uysal C, Asdemir A, Kiliç E. May oxytocin be a trait marker for bipolar disorder? Psychoneuroendocrinology. 2013;38:2890–2896. doi: 10.1016/j.psyneuen.2013.07.017. [DOI] [PubMed] [Google Scholar]
- Uekermann J, Channon S, Lehmkämper C, Abdel-Hamid M, Vollmoeller W, Daum I. Executive function, mentalizing and humor in major depression. J Int Neuropsychol Soc. 2008;14:55–62. doi: 10.1017/S1355617708080016. [DOI] [PubMed] [Google Scholar]
- Van Rheenen TE, Rossell SL. Phenomenological predictors of psychosocial function in bipolar disorder: is there evidence that social cognitive and emotion regulation abnormalities contribute? Aust N Z J Psychiatry. 2014;48:26–35. doi: 10.1177/0004867413508452. [DOI] [PubMed] [Google Scholar]
- Ventura J, Wood RC, Hellemann GS. Symptom domains and neurocognitive functioning can help differentiate social cognitive processes in schizophrenia: a meta-analysis. Schizophr Bull. 2013;39:102–111. doi: 10.1093/schbul/sbr067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmaier MM, Seidman LJ, Niznikiewicz MA, Dickey CC, Shenton ME, McCarley RW. A comparative profile analysis of neuropsychological function in men and women with schizotypal personality disorder. Schizophr Res. 2005;74:43–49. doi: 10.1016/j.schres.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YG, Wang YQ, Chen SL, Zhu CY, Wang K. Theory of mind disability in major depression with or without psychotic symptoms: a componential view. Psychiatry Res. 2008;161:153–161. doi: 10.1016/j.psychres.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Wilbertz G, Brakemeier EL, Zobel I, Härter M, Schramm E. Exploring preoperational features in chronic depression. J Affect Disord. 2010;124:262–269. doi: 10.1016/j.jad.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Wolf F, Brüne M, Assion HJ. Theory of mind and neurocognitive functioning in patients with bipolar disorder. Bipolar Disord. 2010;12:657–666. doi: 10.1111/j.1399-5618.2010.00854.x. [DOI] [PubMed] [Google Scholar]
- Wolkenstein L, Schonenberg M, Schirm E, Hautzinger M. I can see what you feel, but I can’t deal with it: Impaired theory of mind in depression. J Affect Disord. 2011;132:104–111. doi: 10.1016/j.jad.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Wölwer W, Streit M, Polzer U, Gaebel W. Facial affect recognition in the course of schizophrenia. Eur Arch Psychiatry Clin Neurosci. 1996;246:165–170. doi: 10.1007/BF02189118. [DOI] [PubMed] [Google Scholar]
- Zink CF, Meyer-Lindenberg A. Human neuroimaging of oxytocin and vasopressin in social cognition. Horm Behav. 2012;61:400–409. doi: 10.1016/j.yhbeh.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]