Abstract
Purpose
To determine if selected computed tomography (CT) characteristics of pulmonary nodules in pediatric patients with osteosarcoma can help distinguish the nodules as benign or malignant.
Methods
The institutional review board approved this HIPAA-compliant, retrospective study of 30 pediatric osteosarcoma patients (median age 14 years, range 8-22) who underwent chest CT with resection of 117 pulmonary nodules from January 2001 to December 2006. Two pediatric radiologists and one chest radiologist independently and retrospectively reviewed the CT scans and classified nodules as benign, malignant, or indeterminate on the basis of nodule size, laterality, number, location, growth, density, margin appearance and calcification. Generalized estimating equations were used to examine which characteristics were independent predictors of nodule malignancy.
Results
Of the 117 nodules, 80 (68%) were malignant and 37 (32%) were benign by pathologic review. The readers correctly classified 93-94% of the malignant nodules. For benign lesions, the results were not as accurate, with the readers correctly classifying only 11-30% of lesions. The majority of benign lesions were classified as indeterminate by the readers (54-65%). Nodule size (≥ 5 mm) and the presence of calcifications were associated with an increased probability of malignancy (p<0.05).
Conclusion
On chest CT, nodule size ≥ 5 mm and the presence of calcifications are associated with an increased probability of malignant nodule histology in pediatric patients with osteosarcoma. However, nodule characteristics, apart from size and calcification, at chest CT cannot reliably distinguish benign from malignant pulmonary nodules in these patients.
Keywords: Radiology, Computed Tomography, Pulmonary Nodules, Pediatric, Cancer, Osteosarcoma, Chest
INTRODUCTION
The correct determination of malignant potential discovery of pulmonary nodules in children with known sarcoma is a clinical problem that radiologists face on a routine basis. Often the assumption is that pulmonary nodules identified in children with sarcomas indicate metastatic disease. However, benign causes for these nodules can include granulomata, infection, inflammation, intrapulmonary lymph node, vascular malformations, hamartomas, and atelectasis (1, 2).
Identifying a pulmonary nodule as malignant or benign is essential for accurate staging, selecting the appropriate treatment and determining prognosis (3-5). The prognosis of children with sarcoma has improved due to better staging, use of neoadjuvant chemotherapy and aggressive surgical resection of all suspected disease (6-8).
Computed tomography (CT) is the imaging modality of choice for detecting pulmonary nodules (9-14). The development of helical CT and more recently multi-detector CT has improved the quality of chest CTs and the sensitivity in detecting small pulmonary nodules (15-20).
Children with sarcomas are at risk for pulmonary metastases and are scanned to identify any nodules both at the time of diagnosis and sequentially during their follow up. When pulmonary nodules are detected an invasive procedure such as CT-guided needle biopsy, video assisted thoracoscopy (VATS) or even thoracotomy may be necessary to establish the histopathologic diagnosis. Although resection of pulmonary metastases is a safe and effective treatment option (5), these procedures do carry risks. Moreover a substantial percentage of patients undergo biopsy or surgery that ultimately reveals only benign nodules (21). Furthermore, surgical recovery may delay needed administration of chemotherapy.
The purpose of this study was to evaluate CT characteristics of pulmonary nodules in pediatric patients with osteosarcoma and determine if there are any associated characteristics that can help distinguish malignant from benign nodules.
MATERIALS AND METHODS
Our institutional review board approved and waived the informed consent requirement for this retrospective study, which was compliant with the Health Insurance Portability and Accountability Act. Through a review of our institutional database, we identified 298 pediatric patients (median age 12 years, range 0.1–18 years) with confirmed sarcoma who had at least one CT of the chest between January 2001 and December 2006. The CT scans were performed at the time of diagnosis and sequentially during follow up. Thirty osteosarcoma patients in whom the nodule was resected at thoracotomy performed after CT scanning were included in our study. In all of these patients a previous CT scan, that indicated potential lesion enlargement, was available.
CT scans were obtained with a 4–detector row (LightSpeed QX/i; GE Healthcare, Milwaukee, Wis), 8–detector row (LightSpeed Ultra; GE Healthcare) or 16–detector row (LightSpeed 16; GE Healthcare) scanner. Images were obtained in the craniocaudal direction with a slice thickness of 5 mm using either a 1:1 or 1:1.5 pitch. All patients were scanned using the standard radiation safety principle, ALARA (As Low As Reasonable Achievable). Children were classified by weight and size according to the Broselow-Luten System, and dose was determined using the GE Color Coding for Kids CT protocols. Forty-eight of the 59 scans included in our review were obtained without an intravenous contrast agent.
CT images were reviewed using a workstation (Advanced Workstation; GE Healthcare) of our enterprise-wide picture archiving and communication system (PACS). Image analysis was performed using standard lung window settings (width, –50 HU; level, 1500 HU) and mediastinal window settings (width, 350 HU; level, 50 HU). CT scans were retrospectively and independently reviewed by three radiologists (one thoracic radiologist with 14 years experience in chest CT and two pediatric radiologists with 33 and 30 years of pediatric radiology experience).
To ensure accurate comparisons, before imaging analysis, a fourth year radiology resident reviewed all scans and correlated the sampled nodule(s) on each scan with the pathology results as determined from the medical record. Only if this correlation was clearly indicated was the nodule was included in our study. This investigator then numbered the corresponding nodules on the CT images by using the annotation function in the PACS. This numbering system allowed the individual nodules to be tracked among the readers and enabled the readers’ findings to be correlated with the histologic nodule analyzed. On the most recent CT scan before resection, each of the three readers independently assessed the total number of nodules present, unilateral versus bilateral distribution of nodules, lobar site of nodules, and the development and number of new nodules since the previous CT scan. The size, margin appearance (smooth, spiculated, round, or lobulated), growth (any change in size), density (solid, ground glass), and calcification (present or absent) of each biopsy-sampled nodule were assessed. Each reviewer blindly and independently classified the sampled nodules as benign, malignant, or indeterminate on the basis of these features and their own knowledge and experience.
Inter-reader agreement of nodule classification as benign, malignant, or indeterminate was measured using the Kappa statistic. To test for associations between each feature and nodule status, a generalized estimating equations model was fit for each characteristic using a logit link function and exchangeable working covariance matrix, in order to account for the correlation due to multiple nodules per patient. To examine which features were independently associated with nodule status, a multivariate model was created for each reader in which all predictors with univariate p values < 0.20 were entered into the model. The model was reduced until only those predictors with a p value < 0.05 remained.
RESULTS
Nodule Classification Results
Thirty pediatric osteosarcoma patients underwent CT of the chest followed by resection of one or more pulmonary nodules (n = 117) during the study period (Table 1).
Table 1.
Patient characteristics.
| Characteristic | Number |
|---|---|
| Sex | |
| Male | 18 (60.0%) |
| Female | 12 (40.0%) |
| Median age (range) at biopsy | 14 (8.0 - 22.0) |
| Number of nodules | |
| 1 | 5 (16.7%) |
| 2 | 4 (13.3%) |
| 3 | 6 (20.0%) |
| 4 | 5 (16.7%) |
| 5 | 3 (10.0%) |
| 6 | 4 (13.3%) |
| 7 | 1 (3.3%) |
| 10 | 2 (6.7%) |
| Median (range) | 4 (1.0 - 10.0) |
| Time from scan to biopsy | 24 (2.0 - 193.0) |
Of the 117 nodules resected, 80 (68%) were malignant and 37 (32%) were benign (Table 2). Twenty-five (83%) of the 30 patients had at least one malignant mass, while 5 (17%) of the 30 patients had only benign nodules. Seventeen (57%) had a combination of benign and malignant nodules. In seventeen (57%) patients, nodules were distributed bilaterally.
Table 2.
Histologic types of 37 benign nodules.
| Histologic Type | Number (%) |
|---|---|
| Fibrosis | 16 (43.2%) |
| Lymph node | 10 (27.0%) |
| Atelectasis (normal lung tissue) | 4 (10.8%) |
| Calcified nodule | 3 (8.1%) |
| Chronic inflammation | 2 (5.4%) |
| Calcified thrombi | 1 (2.7%) |
| Organized pneumonia | 1 (2.7%) |
Each reader classified each nodule as benign, malignant or indeterminate. Readers 1 and 3 correctly classified 75 of 80 malignant nodules (94%), and reader 2 correctly classified 74 (93%). The readers correctly classified only 11-30% of benign nodules, classifying 54-65% as indeterminate and 76-84% as either benign or indeterminate. Overall, readers 1, 2, and 3 correctly classified 86 (74%), 83 (71%), and 79 (68%)nodules, respectively (Table 3).
Table 3.
Nodule classification results by reader among Osteosarcoma patients.
| Biopsy result | Malignant (N=80) | Benign (N=37) | ||||
|---|---|---|---|---|---|---|
| Reader result | Malignant | Indeterminate | Benign | Malignant | Indeterminate | Benign |
| Reader 1 | 75 (93.8%) | 4 (5.0%) | 1 (1.3%) | 6 (16.2%) | 20 (54.1%) | 11 (29.7%) |
| Reader 2 | 74 (92.5%) | 5 (6.3%) | 1 (1.3%) | 7 (18.9%) | 21 (56.8%) | 9 (24.3%) |
| Reader 3 | 75 (93.8%) | 5 (6.3%) | 0 (0.0%) | 9 (24.3%) | 24 (64.9%) | 4 (10.8%) |
Reader agreement was moderate, with kappa=0.52 between reader 1 and reader 2, kappa=0.54 between reader 1 and reader 3, and kappa=0.73 between reader 2 and reader 3.
Nodule Characteristics (Univariate Results)
The results of the univariate analysis of nodule characteristics as predictors of nodule status at biopsy are provided in Table 4. For all three readers, size and calcifications were both associated with nodule status (malignant versus benign). Malignant lesions were more likely to be larger and to have calcifications.
Table 4.
A: Nodule imaging features as predictors of malignant histology. Univariate analysis among Osteosarcoma patients
| Imaging feature | OR (95% CI) | p-value |
|---|---|---|
| Reader 1 | ||
| Size | 0.02 | |
| < 3 mm | reference | |
| 3-5 mm | 1.67 (0.65, 4.30) | |
| ≥ 5 mm | 7.03 (1.57, 31.48) | |
| Calcifications | 9.05 (2.68, 30.60) | <0.01 |
| Growth | 1.75 (0.77, 3.96) | 0.18 |
| Solid density | 11.55 (0.53, 252.94) | 0.12 |
| Smooth margins | 1.35 (0.72, 2.54) | 0.34 |
| Spiculated margins | 0.54 (0.04, 6.72) | 0.63 |
| Round margins | 0.62 (0.34, 1.14) | 0.12 |
| Lobulated margins | 1.61 (0.71, 3.67) | 0.26 |
| Reader 2 | ||
| Size | 0.04 | |
| < 3 mm | reference | |
| 3-5 mm | 1.37 (0.59, 3.21) | |
| ≥ 5 mm | 6.49 (1.47, 28.74) | |
| Calcifications | 8.01 (2.41, 26.65) | <0.01 |
| Growth | 1.47 (0.60, 3.59) | 0.39 |
| Solid density | 11.68 (0.18, 743.33) | 0.25 |
| Smooth margins | 0.70 (0.39, 1.25) | 0.22 |
| Spiculated margins | 0.62 (0.03, 13.67) | 0.76 |
| Round margins | 1.00 (0.54, 1.88) | 0.99 |
| Lobulated margins | 1.29 (0.55, 2.99) | 0.56 |
| Reader 3 | ||
| Size | 0.01 | |
| < 3 mm | reference | |
| 3-5 mm | 0.96 (0.47, 1.98) | |
| ≥ 5 mm | 5.60 (1.31, 24.04) | |
| Calcifications | 7.97 (2.50, 25.42) | <0.01 |
| Growth | 1.30 (0.56, 3.00) | 0.55 |
| Solid density | 11.68 (0.18, 743.33) | 0.25 |
| Smooth margins | 1.12 (0.56, 2.24) | 0.75 |
| Spiculated margins | 0.62 (0.03, 13.67) | 0.76 |
| Round margins | 0.81 (0.39, 1.66) | 0.56 |
| Lobulated margins | 1.41 (0.57, 3.47) | 0.45 |
| 4B: Nodule imaging features as predictors of malignant histology. Multivariate analysis among Osteosarcoma patients. | ||
| OR (95% CI) | p-value | |
| Reader 1 | ||
| Size | 0.03 | |
| < 3 mm | ||
| 3-5 mm | 1.16 (0.38, 3.47) | |
| ≥ 5 mm | 6.77 (1.25, 36.69) | |
| Calcifications | 17.44 (4.11, 74.08) | <0.01 |
| Growth | 2.57 (1.04, 6.37) | 0.04 |
| Reader 2 | ||
| Size | 0.04 | |
| < 3 mm | ||
| 3-5 mm | 1.42 (0.55, 3.67) | |
| ≥ 5 mm | 7.98 (1.54, 41.44) | |
| Calcifications | 9.25 (2.49, 34.44) | <0.01 |
| Reader 3 | ||
| Size | <0.01 | |
| < 3 mm | ||
| 3-5 mm | 0.78 (0.32, 1.86) | |
| ≥ 5 mm | 6.09 (1.24, 29.89) | |
| Calcifications | 8.47 (2.35, 30.49) | <0.01 |
Nodule Characteristics (Multivariate Results)
In a multivariate analysis that incorporated all features showing a univariate trend, nodule size and the presence of calcifications remained significantly associated with nodule status for all three readers, while nodule growth was also significantly associated with nodule status as determined by reader 1. Specifically, nodule size ≥ 5 mm and the presence of calcifications were associated with an increased probability of malignancy (Table 4).
DISCUSSION
The solitary pulmonary nodule is defined as a relatively spherical opacity that is ≤ 3 cm diameter and surrounded by lung parenchyma. CT imaging characteristics of pulmonary nodules used for determining their nature in adults include nodule contour, margins and internal characteristics. However, only two characteristics are considered to be sufficiently predictive to preclude further evaluation in adult patients and classify the nodule as benign: calcification in a benign pattern and stability in size for > 2 years (22).
In children with osteosarcomas the detection of pulmonary nodules often suggests metastatic disease that may not always be present. Even in studies of high-risk oncologic populations, relatively high percentages (35-44%) of the pulmonary lesions in pediatric patients have proved to be benign (21, 23). In our study, 32% of all nodules assessed were benign, and 17% of the patients evaluated had only benign nodules. At present, there is no algorithm for making the distinction between benign and malignant pulmonary nodules based on radiologic findings in pediatric patients with sarcomas.
Analyzing the interpretations of two experienced pediatric radiologists and one experienced thoracic radiologist, we found that only two of seven evaluated imaging parameters were consistently useful in predicting the malignancy of nodules: nodule size and the presence of calcifications. Nodules that were ≥ 5mm or calcified were more likely to be malignant. The correlation between malignancy and calcification is interesting, as it is the opposite of what is observed in the adult population, in which nodules with calcification are more likely to be benign (22). To get a homogeneous patient population we only included patients in our study whose primary tumors were osteosarcomas. In this study population the osteoid matrix produced by the osteosarcoma cell may form bone and lead to calcification in pulmonary nodules (24 - 26). However, studies of similar pediatric patient cohorts with similar distribution of primary tumors found a negative correlation between malignancy and calcification (21, 27, 28).
We expected the growth of nodules to be predictive of nodules’ histologic status, since studies conducted by Pass et al and Gross et al (14, 29) have suggested that nodules that enlarge over time are much more likely to be metastatic than are nodules that remain stable. However, there was a significant association between nodule growth and malignant histology for only one of the three readers in this study. These findings with respect to nodule growth are in agreement with those of McCarville et al. (21), who found no relation between nodule growth and malignancy.
McCarville et al. (21) found that sharply defined pulmonary nodules were more likely to be malignant. They speculated that this was due to the metastatic character of the nodules and the biologic behavior of primary tumors that metastasize to the lung in pediatric age groups. However, in our study, no correlation was found between nodule margins and histology.
In our study, multivariate analysis revealed that nodule size ≥ 5mm indicated a greater probability of malignancy. This finding is in agreement with the results of a study by Ginsberg et al. (30) that involved 315 adults with cancer, in whom 685 pulmonary nodules were assessed. Ginsberg et al. found that smaller nodules resected at VATS were significantly more likely to be benign.
Univariate analyses showed that for all three readers in our study, size and the presence of calcification were associated with malignant nodule status at histology.
None of the benign nodules in our study were granulomas (Table 2). However, depending on the prevalence of benign granulomatous disease in the subject populations the distinction between benign and malignant calcified nodules can be challenging (31).
In our study, two experienced pediatric radiologists and one experienced thoracic radiologist were able to correctly identify and predict nodule histology type on the basis of radiologic appearance for only 68-74% of the nodules resected. However, the readers correctly predicted malignancy for 93-94% of the malignant lesions. Of the 37 histologically benign lesions evaluated, the radiologists classified only 11-30% correctly and classified 54-65% as indeterminate. These data show that our three experienced radiologists were more effective in predicting malignant histology than in predicting benign histology.
Our study was limited by our patient population. We are a specialized cancer center where patients are often referred for treatment of advanced malignancies. Therefore in our study population, the incidence of benign nodules may be lower than at other institutions. This lower incidence of benign nodules limits the statistical power of our study. CT scans also identify fewer nodules than the number of lesions identified at thoracotomy. Kayton et al. (32) showed that in more than one third of thoracotomies in their study, metastases would have been missed by any tactic besides manual palpation of the lung during open thoracotomy. Finally, the estimates are potentially subject to verification bias in the study design arising inclusion of only patients with a biopsy.
In addition the authors think that for future studies, it would also be interesting to evaluate the added values of FDG - PET/CT and dynamic MRI, because these modalities may potentially add more information for the differentiation of malignant or benign pulmonary nodules in children with known sarcoma and therefore may also play a complementary role in the management of pulmonary nodules.
In conclusion, we found an important difference between CT imaging characteristics of pulmonary nodules in children – namely, in children with osteosarcoma, the presence of calcification appears to be associated with malignant histology. We also found that, as in adults, nodule size is associated with histologic type. We found no correlation between histologic type and nodule density or margins and only evidence from one reader of a correlation between histologic type and nodule growth. Although experienced readers can correctly classify the histologic status of > 90% of malignant nodules seen on CT, they cannot reliably classify benign nodules. Further research is needed to develop means to distinguish benign from malignant nodules on imaging.
Figure 1. Example of a malignant pulmonary nodule.
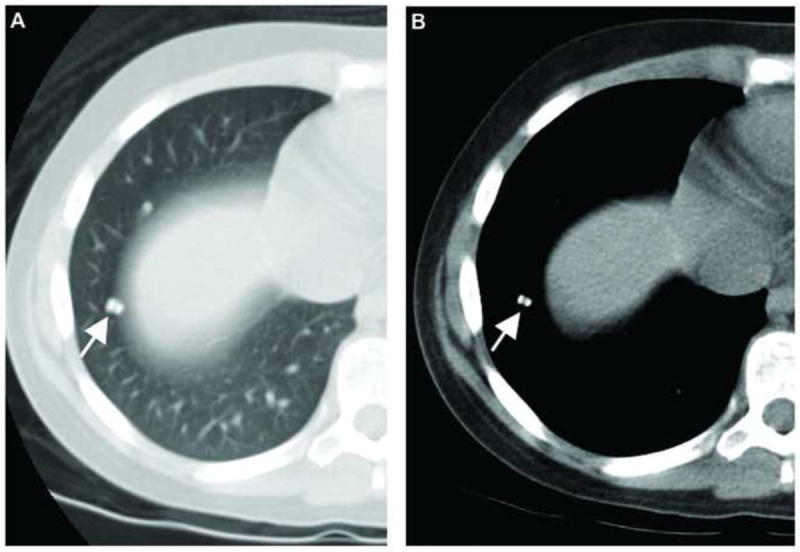
Axial CT images in (A) lung and (B) mediastinal window settings of a pulmonary nodule in an 11-year-old female patient with pathologically proven metastasis from osteosarcoma. Typical calcification is seen in the nodule.
Figure 2. Example of benign and malignant nodules in a single patient.
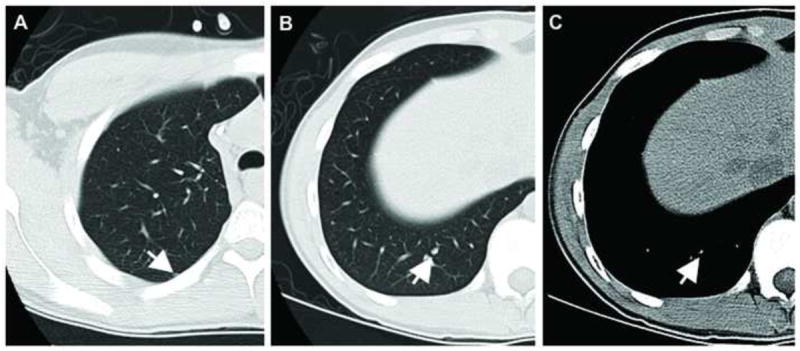
Axial CT images from a 17-year-old male patient with osteosarcoma. Image (A) shows a pathologically proven benign nodule which was rated by all three readers as either indeterminate or benign. The calcified nodule in (B) and (C) (lung and mediastinal window settings, respectively) was rated by all three readers as malignant and proved to be malignant at resection.
Figure 3. Example of a malignant nodule read as benign or indeterminate.
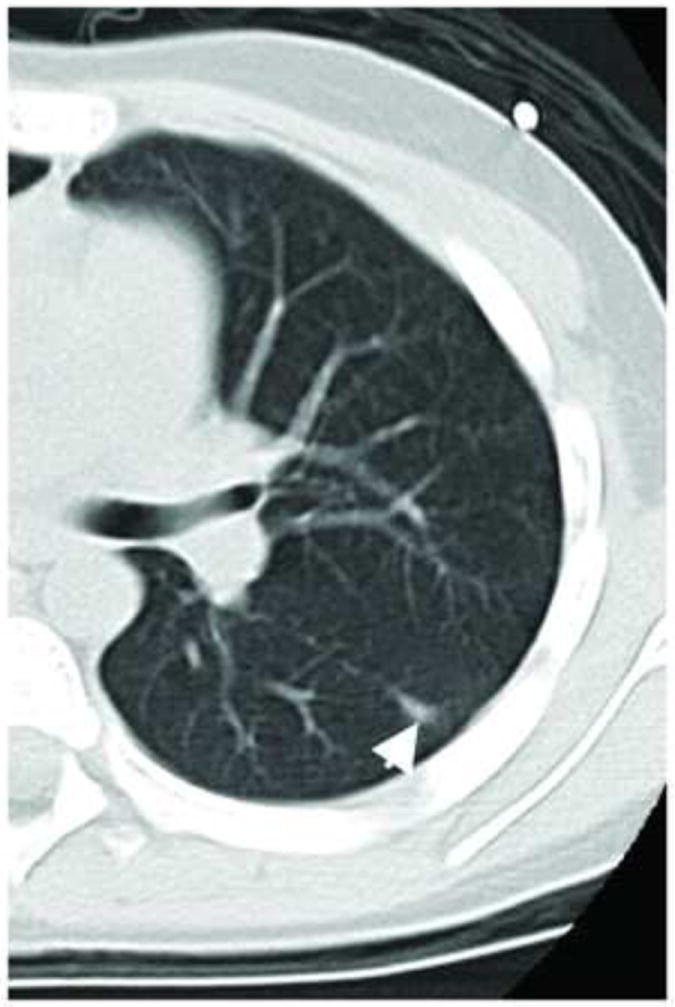
Axial CT image shows a pathologically-proven malignant pulmonary nodule from an 18-year-old male patient with osteosarcoma which was rated by the three readers as either benign or indeterminate.
Acknowledgments
We thank Ada Muellner, B.A., for editing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cahan WG, Shah JP, Castro EB. Benign solitary lung lesions in patients with cancer. Ann Surg. 1978;187:241–244. doi: 10.1097/00000658-197803000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenfield NS, Keller MS, Markowitz RI, Touloukian R, Seashore J. CT differentiation of benign and malignant lung nodules in children. J Pediatr Surg. 1992;27:459–461. doi: 10.1016/0022-3468(92)90336-6. [DOI] [PubMed] [Google Scholar]
- 3.Meyer WH, Schell MJ, Kumar AP, et al. Thoracotomy for pulmonary metastatic osteosarcoma. An analysis of prognostic indicators of survival. Cancer. 1987;59:374–379. doi: 10.1002/1097-0142(19870115)59:2<374::aid-cncr2820590235>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Rissing S, Rougraff BT, Davis K. Indeterminate pulmonary nodules in patients with sarcoma affect survival. Clin Orthop Relat Res. 2007;459:118–121. doi: 10.1097/BLO.0b013e31805d8606. [DOI] [PubMed] [Google Scholar]
- 5.Tronc F, Conter C, Marec-Berard P, et al. Prognostic factors and long-term results of pulmonary metastasectomy for pediatric histologies. Eur J Cardiothorac Surg. 2008;34:1240–1246. doi: 10.1016/j.ejcts.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari A, Miceli R, Meazza C, et al. Soft tissue sarcomas of childhood and adolescence: the prognostic role of tumor size in relation to patient body size. J Clin Oncol. 2009;27:371–376. doi: 10.1200/JCO.2007.15.4542. [DOI] [PubMed] [Google Scholar]
- 7.Granowetter L, Womer R, Devidas M, et al. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: a Children’s Oncology Group Study. J Clin Oncol. 2009;27:2536–2541. doi: 10.1200/JCO.2008.19.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oberlin O, Rey A, Lyden E, et al. Prognostic factors in metastatic rhabdomyosarcomas: results of a pooled analysis from United States and European cooperative groups. J Clin Oncol. 2008;26:2384–2389. doi: 10.1200/JCO.2007.14.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang AE, Schaner EG, Conkle DM, Flye MW, Doppman JL, Rosenberg SA. Evaluation of computed tomography in the detection of pulmonary metastases: a prospective study. Cancer. 1979;43:913–916. doi: 10.1002/1097-0142(197903)43:3<913::aid-cncr2820430319>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 10.Jablons D, Steinberg SM, Roth J, Pittaluga S, Rosenberg SA, Pass HI. Metastasectomy for soft tissue sarcoma. Further evidence for efficacy and prognostic indicators. J Thorac Cardiovasc Surg. 1989;97:695–705. [PubMed] [Google Scholar]
- 11.Muhm JR, Brown LR, Crowe JK, Sheedy PF, 2nd, Hattery RR, Stephens DH. Comparison of whole lung tomography and computed tomography for detecting pulmonary nodules. AJR Am J Roentgenol. 1978;131:981–984. doi: 10.2214/ajr.131.6.981. [DOI] [PubMed] [Google Scholar]
- 12.Parsons AM, Detterbeck FC, Parker LA. Accuracy of helical CT in the detection of pulmonary metastases: is intraoperative palpation still necessary? Ann Thorac Surg. 2004;78:1910–1916. doi: 10.1016/j.athoracsur.2004.05.065. discussion 1916-1918. [DOI] [PubMed] [Google Scholar]
- 13.Parsons AM, Ennis EK, Yankaskas BC, Parker LA, Jr, Hyslop WB, Detterbeck FC. Helical computed tomography inaccuracy in the detection of pulmonary metastases: can it be improved? Ann Thorac Surg. 2007;84:1830–1836. doi: 10.1016/j.athoracsur.2007.06.069. [DOI] [PubMed] [Google Scholar]
- 14.Pass HI, Dwyer A, Makuch R, Roth JA. Detection of pulmonary metastases in patients with osteogenic and soft-tissue sarcomas: the superiority of CT scans compared with conventional linear tomograms using dynamic analysis. J Clin Oncol. 1985;3:1261–1265. doi: 10.1200/JCO.1985.3.9.1261. [DOI] [PubMed] [Google Scholar]
- 15.Kalender WA, Seissler W, Klotz E, Vock P. Spiral volumetric CT with single-breath-hold technique, continuous transport, and continuous scanner rotation. Radiology. 1990;176:181–183. doi: 10.1148/radiology.176.1.2353088. [DOI] [PubMed] [Google Scholar]
- 16.Meisel JA, Guthrie KA, Breslow NE, Donaldson SS, Green DM. Significance and management of computed tomography detected pulmonary nodules: a report from the National Wilms Tumor Study Group. Int J Radiat Oncol Biol Phys. 1999;44:579–585. doi: 10.1016/s0360-3016(99)00086-3. [DOI] [PubMed] [Google Scholar]
- 17.Owens CM, Veys PA, Pritchard J, Levitt G, Imeson J, Dicks-Mireaux C. Role of chest computed tomography at diagnosis in the management of Wilms’ tumor: a study by the United Kingdom Children’s Cancer Study Group. J Clin Oncol. 2002;20:2768–2773. doi: 10.1200/JCO.2002.02.147. [DOI] [PubMed] [Google Scholar]
- 18.Remy-Jardin M, Remy J, Giraud F, Marquette CH. Pulmonary nodules: detection with thick-section spiral CT versus conventional CT. Radiology. 1993;187:513–520. doi: 10.1148/radiology.187.2.8475300. [DOI] [PubMed] [Google Scholar]
- 19.Rydberg J, Buckwalter KA, Caldemeyer KS, et al. Multisection CT: scanning techniques and clinical applications. Radiographics. 2000;20:1787–1806. doi: 10.1148/radiographics.20.6.g00nv071787. [DOI] [PubMed] [Google Scholar]
- 20.Jennings SG, Winer-Muram HT, Tarver RD, Farber MO. Lung tumor growth: assessment with CT-comparison of diameter and cross-sectional area with volume measurements. Radiology. 2004;231:866–871. doi: 10.1148/radiol.2313030715. [DOI] [PubMed] [Google Scholar]
- 21.McCarville MB, Lederman HM, Santana VM, et al. Distinguishing benign from malignant pulmonary nodules with helical chest CT in children with malignant solid tumors. Radiology. 2006;239:514–520. doi: 10.1148/radiol.2392050631. [DOI] [PubMed] [Google Scholar]
- 22.Khan A. ACR Appropriateness Criteria on solitary pulmonary nodule. J Am Coll Radiol. 2007;4:152–155. doi: 10.1016/j.jacr.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Cohen M, Smith WL, Weetman R, Provisor A. Pulmonary pseudometastases in children with malignant tumors. Radiology. 1981;141:371–374. doi: 10.1148/radiology.141.2.7291560. [DOI] [PubMed] [Google Scholar]
- 24.Maile CW, Rodan BA, Godwin JD, Chen JT, Ravin CE. Calcification in pulmonary metastases. Br J Radiol. 1982;55:108–113. doi: 10.1259/0007-1285-55-650-108. [DOI] [PubMed] [Google Scholar]
- 25.Morse D, Jr, Reed JO, Bernstein J. Sclerosing osteogenic sarcoma. Am J Roentgenol Radium Ther Nucl Med. 1962;88:491–495. [PubMed] [Google Scholar]
- 26.Seo JB, Im JG, Goo JM, Chung MJ, Kim MY. Atypical pulmonary metastases: spectrum of radiologic findings. Radiographics. 2001;21:403–17. doi: 10.1148/radiographics.21.2.g01mr17403. [DOI] [PubMed] [Google Scholar]
- 27.McCarville MB, Kaste SC, Cain AM, Goloubeva O, Rao BN, Pratt CB. Prognostic factors and imaging patterns of recurrent pulmonary nodules after thoracotomy in children with osteosarcoma. Cancer. 2001;91:1170–1176. doi: 10.1002/1097-0142(20010315)91:6<1170::aid-cncr1114>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 28.Picci P, Vanel D, Briccoli A, et al. Computed tomography of pulmonary metastases from osteosarcoma: the less poor technique. A study of 51 patients with histological correlation. Ann Oncol. 2001;12:1601–1604. doi: 10.1023/a:1013103511633. [DOI] [PubMed] [Google Scholar]
- 29.Gross BH, Glazer GM, Bookstein FL. Multiple pulmonary nodules detected by computed tomography: diagnostic implications. J Comput Assist Tomogr. 1985;9:880–885. doi: 10.1097/00004728-198509000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Ginsberg MS, Griff SK, Go BD, Yoo HH, Schwartz LH, Panicek DM. Pulmonary nodules resected at video-assisted thoracoscopic surgery: etiology in 426 patients. Radiology. 1999;213:277–282. doi: 10.1148/radiology.213.1.r99oc08277. [DOI] [PubMed] [Google Scholar]
- 31.Berger WG, Erly WK, Krupinski EA, Standen JR, Stern RG. The solitary pulmonary nodule on chest radiography: can we really tell if the nodule is calcified? AJR Am J Roentgenol. 2001;176:201–204. doi: 10.2214/ajr.176.1.1760201. [DOI] [PubMed] [Google Scholar]
- 32.Kayton ML, Huvos AG, Casher J, et al. Computed tomographic scan of the chest underestimates the number of metastatic lesions in osteosarcoma. J Pediatr Surg. 2006;41:200–206. doi: 10.1016/j.jpedsurg.2005.10.024. [DOI] [PubMed] [Google Scholar]


