Abstract
Objectives
To examine the current approaches to cervical screening and points to consider for improving HPV vaccination acceptance and uptake in the US.
Methods
An expert forum was conducted September 12–13, 2008, by the Society of Gynecologic Oncologists including 56 experts in cervical cancer and titled “Future Strategies of Cervical Cancer Prevention: What Do We Need to Do Now to Prepare?”.
Results
Cervical cancer prevention has primarily relied on screening paradigms but vaccination against human papillomavirus (HPV), the cause of the disease, is a primary preventative measure that has been recommended by all cervical cancer screening stakeholders. Guidelines for vaccination are developed by national advisory groups, but successful implementation requires a supportive infrastructure and the cooperation of providers, clinicians, and patients. HPV vaccination has been available in the United States (US) since 2006 and screening practices have been updated to also include HPV genotyping. However, many clinicians fail to adhere to the guidelines for HPV testing (and HPV co-testing) as part of cervical cancer screening, and vaccination coverage has been poor among females aged 11 and 12, the group for which vaccination is recommended by all organizations.
Conclusions
The data reviewed and presented in this session of the “Future Strategies of Cervical Cancer Prevention. What Do We Need to do Now to Prepare?”. The Forum suggests that the policies influencing HPV vaccination and screening need to be reassessed at multiple levels in order to achieve more effective implementation and regular use.
Keywords: Cervical cancer, Screening, Vaccination
Introduction
Prevention of infection with oncogenic human papillomavirus (HPV) infection, and the potential development of cervical cancer, requires a comprehensive approach. Currently, the most effective means by which to accomplish this is via vaccination and continued cervical screening. This initiative has involved a coordinated effort amongst clinicians/providers, government agencies, and patients for successful implementation. In the United States (US), where HPV vaccination is not mandatory and there is no national cervical cancer screening program, there are considerable challenges for implementation and tracking use and nonuse. This review will provide an overview of cervical cancer screening practices, policies for implementation of vaccines, as well as provider and patient attitudes to HPV vaccination and cervical cancer screening in the US. The review will also reflect data presented at the Cervical Cancer Forum organized by the Society for Gynecologic Oncologists (SGO) and held in September, 2008, in which 56 experts were invited. Multiple organizations involved with vaccine delivery and monitoring participated in this session; however the views expressed in this publication were those of the individuals, not necessarily those of the organizations represented, including the SGO.
Provider and patient practices in cervical cancer screening
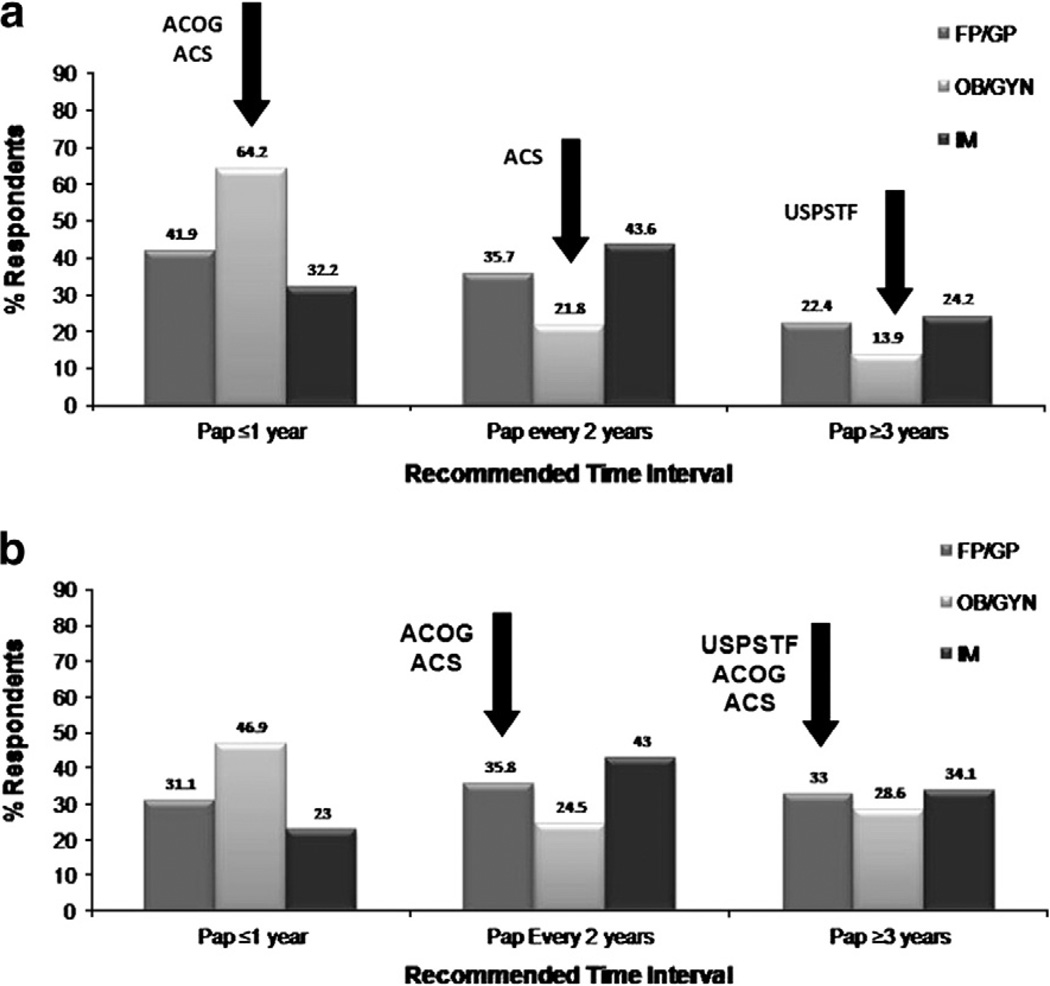
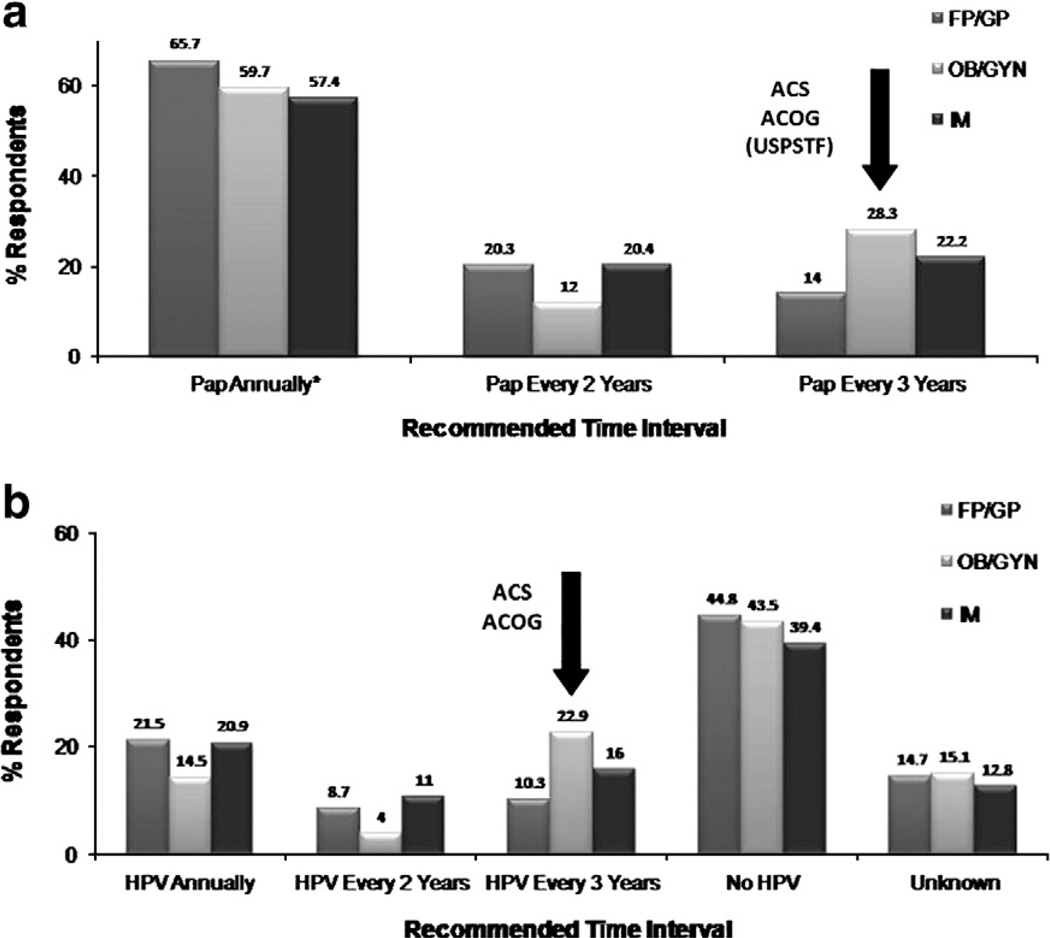
In the US, current cervical screening practices vary widely among physicians despite recommendations from the American Cancer Society (ACS), the United Stated Preventive Services Task Force (USPSTF), and American Congress of Obstetricians and Gynecologists (ACOG) (Table 1) [1,2,20]. Results from a 2006/2007 Primary Care Provider Survey (N = 1212; 68% response rate) showed that family/general practitioners (FP/GP), obstetricians and gynecologists (Ob–Gyns), and internal medicine (IM) clinicians differ in their recommendations for HPV testing and intervals for screening [3,4]. In this survey, the main outcome measure included self-reported data on timing of screening intervals for women with normal results using clinical vignettes that were closely linked with established guidelines, with some distractors. The categories were not mutually exclusive because a physician could respond ‘yes’ to both follow-up and co-testing. Currently, HPV testing is used as a reflex test to equivocal Papanicolaou (Pap) testing yet as per this survey, 28% of IMs, versus only 16% of Ob–Gyns, failed to recommend HPV testing [3,4]. Similarly, less than 44% of clinicians adhered to the ACS guidelines for screening of a low risk 25 year-old or 35 year-old female with three negative Pap tests (Figs. 1a and b) [3,4]. It was also observed that the majority of providers did not follow guidelines for HPV co-testing. Less that 30% of clinicians recommended the correct screening interval for a follow-up Pap test in a 35 year-old female who was HPV negative with no abnormal Pap tests (Fig. 2a) [3,4]. Similarly, less than 23% of clinicians recommended the correct interval for a follow-up HPV test (Fig. 2b) [3,4]. These results suggest that, among all clinicians, there is a moderate resistance in extension of screening intervals with sequential Pap testing, and that HPV co-testing has not changed current screening practices. Screening practices may also be influenced by the use of secondary testing facilities, and not at the level of the clinician. In 2006, a survey of HPV testing and reporting rates showed that only 9% of laboratories (N = 679) used HPV testing in cytology and that 45% of laboratories performed testing for non-cancer causing HPV types [5]. HPV testing may also be challenged by reimbursement issues [6]. Most insurance companies cover HPV testing for triage and co-testing but not all states mandate insurance coverage for HPV testing. Also, currently Medicaid does not fully reimburse for HPV co-testing, which affects the US population most at risk for the development of cervical cancer.
Table 1.
| ACS [1] | USPSTF [2] | ACOG [20] | |
|---|---|---|---|
| Age to start | Three years after initiation of sexual debut, or by the age of 21 | Begin at 21 years of age | |
| Intervals | |||
| Conventional Pap Test | Annually; every 2–3 years for women ≥30 years of age with three negative tests | At least every three years | Every 2 years for women between the ages of 21 years and 29 years; every 3 years for women ≥30 years of age with three negative tests and no history of CIN2/3, not HIV infected and not immunocompromised |
| Liquid-based cytology | Every 2 years; every 2–3 years for women ≥30 years of age with three negative tests | Insufficient evidence | Same as Conventional Pap Test |
| If HPV testing is used as an adjunct, women ≥30 | Every 3 years if cytology test is negative and HPV negative | Insufficient evidence | Every 3 years if cytology test is negative and HPV negative |
| Age to stop | Women >70 years of age with an intact cervix and ≥3 consecutive negative test in the past 10 years | Women >65 years of age with negative cytology and at low risk for cervical cancer | Women 65 to 70 years of age who have three or more negative cytology test results in a row and no abnormal test results in the past 10 years |
Fig. 1.
a. Recommended times for a follow-up Pap test for a 25 year-old female with no sexual partners in the last five years and three negative Pap tests [3,4]. Arrows indicate obstetrician/gynecologists responses. b. Recommended times for a follow-up Pap test for a 35 year-old female with no sexual partners in the last five years and three negative Pap tests [3,4].
Fig. 2.
a. Recommended times for a follow-up Pap test for a 35 year-old female with normal Pap tests and HPV negative [3,4]. b. Recommended times for a follow-up HPV test for a 35 year-old female with normal Pap tests and HPV negative [3,4].
Among patients, the role of the provider–patient relationship and continuity of care are more important reasons for an annual exam than the Pap test itself [7]. Patients also appear to feel more comfortable having Pap tests at more frequent intervals than what is recommended by their physicians. Sixty percent of women 40 years of age and older continue to get annual Pap testing even if their provider recommends against it [8]. Moreover, 35% of women would want to continue getting screened [8]. Another study reported similar results in women 50 years of age and older. The majority of these women wanted to use HPV testing as part of cervical cancer screening, with approximately 30% of these women wanting to continue receiving annual Pap tests, despite guidelines and prospective clinical trials to suggest that they would have little, if any, clinical benefit from such testing, with the potential for unnecessary harm due to abnormal, but clinically irrelevant, abnormal results [9]. The National Breast and Cervical Cancer Early Detection Program, led by the Center for Disease Control and Prevention (CDC), has initiated a study to assess the role of provider and patient education in improving appropriate use of HPV testing as an adjunct test and is examining its use in lengthening screening intervals [10]. Barriers to longer screening intervals include comfort level of providers and patients, fear of missing cancer and discouraging an annual exam, and financial concerns on the part of providers. It was surmised that the key to changing provider behavior was at the level of reimbursement, including positive or negative incentives [10]. Ob–Gyns have been resistant to changing their screening practices, in part due to financial disincentives to change screening frequency, and thus further education is indicated. Of interest, the 2006/2007 CDC Provider Survey showed that approximately 50% of Ob–Gyns and FP/GPs felt that vaccination against HPV would not impact the age at which screening is initiated or the frequency of screening among a fully vaccinated population [3]. This sentiment is in contradistinction to models that suggest that screening should be started later and intervals should be lengthened [11]. Recently ACOG has issued new screening guidelines that reflect these models as shown in Table 1.
HPV vaccine policy issues and implementation in the United States
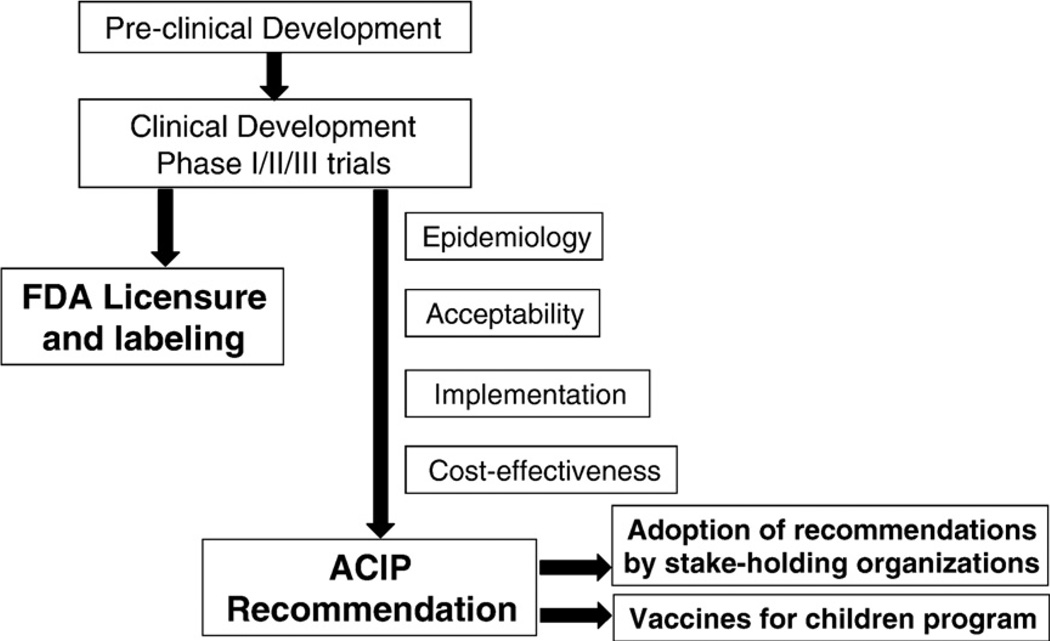
For implementation of vaccines, the Advisory Committee on Immunization Practices (ACIP) has sole authority to add vaccines to the US Vaccines for Children (VFC) program. Current legislation for school-based immunization programs is regulated at the state level [12]. For HPV vaccination, the ACIP has relied on clinical trial data, HPV epidemiology and related disease, sexual behavior patterns, vaccine acceptability, impact and cost effectiveness studies, and program/implementation issues (Fig. 3) [4]. The ACIP working group consisting of ACIP members, consultants and CDC staff, who have rigorously reviewed the available data and monitor progress in vaccine development and implementation. The group also develops recommendation options and drafts the ACIP recommendations. The full ACIP group considers and votes on options and approves written recommendations. These recommendations form the foundation for stake holding organizations to subsequently refine their recommendations for vaccine adoption and implementation. Currently in the US, there are two approved vaccines and routine HPV vaccination is recommended for females aged 11 to 12 years and can be started as early as age 9 or 10 with catch-up vaccination for females aged 13 to 26 years (Table 2) [13,19,21–25].
Fig. 3.
Steps to development of ACIP recommendations [4].
Table 2.
| ACIP | ACS | Other groups | |
|---|---|---|---|
| (AAP, AAFP, ACHAa) | |||
| 9 years | ✓ | ✓ | ✓ |
| 11–12 years | ✓ | ✓ | ✓ |
| 13–18 years | ✓ | ✓ | ✓ |
| 19–26 years | ✓ | Neither for nor against universal vaccination for this age group | ✓ |
AAP = American Academy of Pediatrics; AAFP = American Academy of Family Physicians; ACHA = American College Health Association.
Vaccine implementation is a complex dynamic that requires a fundamental understanding of the issues that surround policy development. Key steps include recommendations, financing, infrastructure, vaccine delivery, vaccine acceptance, communication and education, as well as monitoring and evaluation. There is also a need to address confounding issues in order to implement mandatory vaccination with the requisite financial infrastructure to sustain it. Financing for, and access to target age groups, have been identified as major challenges to implementation in the US. However, many believe instituting school-based mandates such as for elementary and high-school health classes is a strategy that would serve well to target the appropriate age for vaccination, and one that has already been adopted by Virginia and the District of Columbia, albeit with liberal opt-out clauses. HPV vaccination could be included as part of a group of standardized vaccines administered to adolescents 11 to 12 years of age in the same session. Such programs enhance convenience and improve vaccination uptake, while reducing some parental and infrastructure barriers to vaccination. The vaccine for children (VFC) provides the vaccine at no cost to eligible children less than 19 years of age. Through early 2010, approximately 25 million doses of the quadrivalent vaccine have been distributed. Currently, the VFC does not provide payment for vaccinating 19 to 26 year-olds and in some cases VFC providers may not be sufficient to reach all adolescents eligible for the program.
HPV vaccines are primarily being delivered in traditional primary care settings and complimentary settings are being explored. Typically, adolescents have fewer preventive health visits that younger children which suggests that a substantial increase in health care visits will be needed to provide three doses of the HPV vaccine. School immunization requirements have been credited for high childhood vaccination rates in the US but have generated debate about public health versus individual rights. For HPV vaccination, approximately 41 states have introduced legislation regarding HPV vaccination, and at least 17 have passed it into law [14]. Some states have allocated additional funds to cover the cost of vaccinating females 11 to 18 years of age [15]. Low rates of vaccination have caused some to propose more formalized programs to boost participation such as school immunization requirements or some form of mandates. Some forum participants indicated that “mandates” currently could be counterproductive until further acceptance of HPV vaccination occurs. Vaccine implementation is a complex process that requires adequate infrastructure, education, programmatic cost coverage, and scientific, community, and importantly parental acceptance that ideally should precede school-based requirements. Much of this activity has been conducted for HPV vaccination.
Post implementation, vaccine safety, coverage, patient behavior and provider practices as well as disease impact are monitored by various groups. HPV vaccine coverage has been assessed by the national surveys and databases such as the Behavioral Risk Factor Surveillance System as well as the Vaccine Safety Datalink and Immunization Information Systems. For safety, the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink are national monitoring systems supported by the CDC. The VAERS is a post-licensure safety surveillance system that is jointly operated by the FDA whereby reports are voluntarily submitted by clinicians, manufacturers, patients/parents and others. As of December 31, 2008, there have been 12,424 reports of adverse events following immunization [16]. There have also been 32 reports of deaths after vaccination, although none appear to have been caused by the vaccine. Of 42 reports of Guillian–Barre Syndrome (GBS), 12 were confirmed cases of which five received a meningococcal vaccine and the quadrivalent HPV vaccine, with one of these also receiving hepatitis A and one receiving varicella vaccine at the same time [16]. Studies are underway to evaluate the risk of GBS that may be associated with Menactra® but there has been no direct evidence that shows Menactra® causes GBS [17]. Furthermore, the CDC has consistently reported that the quadrivalent HPV vaccine is safe and effective, and that its benefits continue to outweigh its risks [18]. These recommendations have been fully supported by all stake-holding clinical and scientific member organizations, including SGO.
How can screening and vaccination policies be further implemented?
There are particular target groups which clearly need additional attention for improving screening and vaccination. Adolescents and their parents or guardians, specific geographic regions that have high incident rates of cervical cancer, adolescent medicine physicians (family practitioners), Ob–Gyns, local community-based health programs, and other provider associations and policy-making organizations are all essential for appropriately implementing widespread HPV vaccination. Accordingly, government-based organizations are integral for establishing overarching policies and recommendations for vaccination and screening, such as the CDC and ACIP. A competitive marketplace also generates potential financial incentives for providers. This result has downstream effects, ultimately benefitting the patient.
The second part of this equation is to generate ways to educate members of the aforementioned groups with the intent of improving screening and vaccination against oncogenic HPV. It might be worthwhile to consider sending letters to key organizations acknowledging the issues affecting the implementation of vaccination. Partnerships for educational efforts across disciplines and creating a universal voice based on the science are essential for moving forward with potentially paradigm-shifting best medical practices. As a result, this effort could be parlayed into transforming federal, state, and local policies, development of public service announcements, and increased initiatives for education. This strategy would likely be most effective in areas where the prevalence rates of cervical cancer are high and preventative healthcare measure utilization is low. It may also be beneficial to examine the factors that influence provider practices. This may determine strategies that facilitate use of appropriate cervical cancer prevention strategies and identify barriers such as delayed reimbursement, storage costs, record keeping and other fiscal concerns related to vaccine administration. It is also important to address physicians' understanding of financial disincentives such as new recommendations for less frequent screening. Accordingly, there is a need to increase access to educational materials for providers. This will help to facilitate adherence to recommended guidelines for screening and vaccination, regardless of subspecialty.
Conclusion
The landscape of public health is a dynamic process that requires cooperation among many disciplines. For cervical cancer prevention, HPV screening and vaccination have undergone many recent improvements in a relatively brief period. This has created some gaps in the knowledge and decision-making amongst clinicians. These gaps may be narrowed by education and influence from the appropriate organizations (both professional and public agencies), consistent implementation of guidelines, and frequent dissemination of new information. In the past several years, recommendations, vaccine financing, delivery, and monitoring have all been widely implemented in the US. Special populations defined by the CDC and others [19] require clarification and education for providers so that they can properly address such concerns with their patients. Since physicians play a major role in administering vaccines as well as educating the patient, they must be kept current on the data regarding vaccination. This will help further promote that vaccination is effective and safe and that the appropriate age groups are targeted. Physicians must also have access to data on adolescent sexual behavior, an important aspect in understanding the appropriate age to vaccinate, while addressing HPV vaccination as a preventive medicine issue with parents. When possible, relationships should be developed with legislators so that policies can be accurately reflected by the science. A well-informed clinician is a valuable resource for forming sound public health policies.
Appendix
The following individuals attended the Forum by invitation. The opinions expressed in this manuscript and at the Forum do not necessarily reflect the official opinions of any of the organizations represented at the Forum.
SGO Cervical Cancer Forum Organizing Committee
Levi Downs, MD, University of Minnesota
Mark Einstein, MD, MS, Albert Einstein College of Medicine
Thomas Herzog, MD, Columbia University College of Physicians & Surgeons
Warner Huh, MD, University of Alabama at Birmingham
Stewart Massad, MD, Washington University School of Medicine
Yvonne Collins, MD, University of Illinois-Chicago
Diljeet Singh, MD, DrPH, Northwestern Prentice Women's Hospital
Attendees
R. Marshall Austin, MD, University of Pittsburgh, Magee Women's Hospital
Vicki Benard, PhD, Centers for Disease Control and Prevention
Sharon Bisner, RN, FNP, New York State Department of Health
Xavier Bosch, MD, Catalan Institute of Oncology
Robert Burk, MD, Albert Einstein College of Medicine
David Chelmow, MD, Tufts Medical Center
Carmel Cohen, MD, Mount Sinai Medical Center
Rebecca Cowens-Alvarado, MPH, American Cancer Society
J. Thomas Cox, MD, University of California Santa Barbara
Amanda Dempsey, MD, PhD, MPH, University of Michigan, Dept. of Pediatrics
Charles Dunton, MD, Lankenau Hospital
Robert Edwards, MD, University of Pittsburgh
Donataus Ekwueme, PhD, Centers for Disease Control and Prevention
Lisa Flowers, MD, Emory University School of Medicine
Eduardo Franco, BSc, MPH, DrPH, McGill University
Anna Giuliano, PhD, Moffitt Cancer Center
Patti Gravitt, PhD, The Johns Hopkins Bloomberg School of Public Health
Richard Guido, MD, University of Pittsburgh, Magee Women's Hospital
Diane Harper, MD, MPH, MS, Dartmouth Medical School
Jody Hershey, MD, MPH, New River Health District
Maureen Killackey, MD, Memorial-Sloan Kettering Cancer Center
Kim Kobus, PhD, University of Chicago
Herschel Lawson, MD, Centers for Disease Control and Prevention
Joseph Lucci, MD, University of Miami, Sylvester Cancer Center
Lauri Markowitz, MD, Centers for Disease Control & Prevention
Edward Mayeaux, PhD, Louisiana State University
Anna-Barbara Moscicki, MD, University of California, San Francisco School of Medicine
Evan Myers, MD, MPH, Duke University Medical Center
Mark Pool, MD, University Pathologists, PC
Richard Roden, PhD, The Johns Hopkins University
Susan Rosenthal, PhD, University of Texas Medical Branch
Mary Rubin, NP, PhD, University of California, San Francisco Medical Center
Mona Saraiya, MD, MPH, Centers for Disease Control and Prevention
Isabel Scarinci, PhD, MPH, University of Alabama at Birmingham
Julian Schink, MD, Northwestern Prentice Women's Hospital
Jennifer Smith, PhD, MPH, University of North Carolina at Chapel Hill
Diane Solomon, MD, National Cancer Institute, Division of Cancer Prevention
Mark Spitzer, MD, Brookdale University Hospital & Medical Center
Mark Stoler, MD, University of Virgina Health System
Howard Strickler, MD, Albert Einstein College of Medicine
Edward Trimble, MD, MPH, National Cancer Institute
Elizabeth Unger, MD, PhD, Centers for Disease Control & Prevention
Ray Viscidi, MD, The Johns Hopkins University School of Medicine
Chastity Walker, MPH, Centers for Disease Control and Prevention
Joan Walker, MD, University of Oklahoma Health Sciences Center
Nicolas Wentzensen, MD, PhD, MSc, National Cancer Institute
Cosette Wheeler, PhD, University of New Mexico, Health Sciences Center
David Wilbur, PhD, Massachusettes General Hospital
Jason Wright, MD, Columbia University
Footnotes
On September 12–13, 2008, the Society of Gynecologic Oncologists (SGO) convened a symposium of 56 cervical cancer experts titled “Future Strategies of Cervical Cancer Prevention: What Do We Need to Do Now to Prepare?” to discuss evidence-based strategies in cervical cancer prevention and control, including HPV vaccination. This paper is the last in a series of manuscripts which highlight concepts, information, obstacles and approaches discussed during the Forum's sessions regarding cervical cancer prevention in the United States. This session focused on the impact of public policy with cervical cancer screening and HPV vaccination. No editorial support or input from the Forum supporters was received or included in this manuscript.
Conflict of interest statement
T. Herzog- Honoraria for educational programs from GSK and Merck M. Einstein- Dr. Einstein has advised or participated in educational speaking activities, but does not receive an honorarium from any companies. In specific cases, Montefiore Medical Center has received payment for time spent for these activities from Merck, GSK, Roche, Hologic, Advaxis, Aura Biosciences, and PDS Biotechnologies. Also, Montefiore has received grant funding for research related costs of clinical trials that Dr. Einstein has been the Montefiore PI from Merck, GSK, Roche, and Hologic. W. Huh- Consultant: Merck, GSK, Roche, Hologic, and Helix BioPharma; Speaker: Merck, GSK; Research Support: Merck, GSK and Roche.
References
- 1.FDA Approves Expanded Use of HPV Test. US Food and Drug Administration. [Accessed April 5, 2007]; http://www.fda.gov/bbs/topics/NEWS/2003/NEW00890.html.
- 2.Saslow D, Runowicz CD, Solomon D, et al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002 Nov-Dec;52(6):342–362. doi: 10.3322/canjclin.52.6.342. [DOI] [PubMed] [Google Scholar]
- 3.Saraiya M, Berkowitz Z, Yabroff R, Wideroff L, Kobrin S, Benard V. Cervical cancer screening with both human papillomavirus and papanicolaou testing vs Papanicolaou testing alone, what screening intervals are physicians recommending? Arch Intern Med. 2010;170(11):977–986. doi: 10.1001/archinternmed.2010.134. [DOI] [PubMed] [Google Scholar]
- 4.Yabroff KR, Saraiya M, Meissner HI, Haggstrom DA, Wideroff L, Yuan G, et al. Specialty differences in primary care physician reports of papanicolaou test screening practices: a national survey, 2006 to 2007. Ann Intern Med. 2009 Nov 3;151(9):602–611. doi: 10.7326/0003-4819-151-9-200911030-00005. [DOI] [PubMed] [Google Scholar]
- 5.Moriarty AT, Schwartz MR, Eversole G, et al. Human papillomavirus testing and reporting rates: practices of participants in the College of American Pathologists Interlaboratory Comparison Program in Gynecologic Cytology in 2006. Arch Pathol Lab Med. 2008 Aug;132(8):1290–1294. doi: 10.5858/2008-132-1290-HPTARR. [DOI] [PubMed] [Google Scholar]
- 6.CDC. National Breast and Cervical Cancer Early Detection Program. 2008 Oct 31; http://www.cdc.gov/cancer/NBCCEDP/
- 7.Becker HI, Longacre MR, Harper DM. Beyond the Pap: assessing patients' priorities for the annual examination. J Womens Health (Larchmt) 2004 Sep;13(7):791–798. doi: 10.1089/jwh.2004.13.791. [DOI] [PubMed] [Google Scholar]
- 8.Sirovich BE, Woloshin S, Schwartz LM. Screening for cervical cancer: will women accept less? Am J Med. 2005 Feb;118(2):151–158. doi: 10.1016/j.amjmed.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Huang AJ, Perez-Stable EJ, Kim SE, et al. Preferences for human papillomavirus testing with routine cervical cancer screening in diverse older women. J Gen Intern Med. 2008 Sep;23(9):1324–1329. doi: 10.1007/s11606-008-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CDC. CDC Cervical Cancer Study [Google Scholar]
- 11.Cuzick J, Arbyn M, Sankaranarayanan R, Tsu V, Ronco G, Mayrand MH, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008 Dec 9;26(52):6743–6744. doi: 10.1016/j.vaccine.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 12.CDC. VFC: The ACIP-VFC Vaccine Resolutions. http://www.cdc.gov/vaccines/programs/vfc/acip-vfc-resolutions.htm.
- 13.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007 Mar 23;56(RR-2):1–24. [PubMed] [Google Scholar]
- 14. [June 8, 2010]; as accessed via website on http://www.upenn.edu/pennnews/article.php?id=1286.
- 15.Herzog TJ, Huh WK, Downs LS, Smith JS, Monk BJ. Initial lessons learned in HPV vaccination. Gynecol Oncol. 2008 May;109(2 Suppl):S4–S11. doi: 10.1016/j.ygyno.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Slade BA, Leidel L, Velozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA. 2009;302(7):750–757. doi: 10.1001/jama.2009.1201. [DOI] [PubMed] [Google Scholar]
- 17.FDA and CDC Issue Alert on Menactra Meningococcal Vaccine and Guillain Barre Syndrome. [Accessed 07/21/10];2005 Sep 30; http://www.countyofsb.org/uploadedFiles/phd/dc/2005-10-04%20CD%20Menatra%20GBS%20Release%20Information.pdf. [Google Scholar]
- 18.CDC-Information from FDA and CDC on Gardasil and its Safety. 2008 Jul 22; [Google Scholar]
- 19.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER Centers for Disease Control and Prevention (CDC. Advisory Committee on Immunization Practices (ACIP) Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007 Mar 23;56(RR-2):1–24. [PubMed] [Google Scholar]
- 20.Obstet Gynecol. 2009 Dec;114(6):1409–1420. doi: 10.1097/AOG.0b013e3181c6f8a4. ACOG Practice Bulletin No. 109: Cervical Cytology Screening. [DOI] [PubMed] [Google Scholar]
- 21.American Academy of Pediatrics — HPV Policy Statement. 2007 Jun [Google Scholar]
- 22.American Academy of Family Practitioners Practice Guidelines for HPV Vaccination. 2007 May [Google Scholar]
- 23.American College of Health Association Guidelines. Recommendations for Institutional Prematriculation Immunizations. 2009 Jan; doi: 10.1080/07448481.2012.682826. [DOI] [PubMed]
- 24.Saslow D, Castle P, Cox JT, Davey D, Einstein M, Ferris DG, et al. American Cancer Society guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin. 2007;57:7–28. doi: 10.3322/canjclin.57.1.7. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Recommended adult immunization schedule — United States, 2009. MMWR. 2008;57(53) [Google Scholar]