Summary
Schizophrenia is associated with disruptions in N-methyl-D-aspartate glutamate receptor subtype (NMDAR)-mediated excitatory synaptic signaling. The metabotropic glutamate receptor subtype 5 (mGlu5) is a closely associated signaling partner with NMDARs and regulates NMDAR function in forebrain regions implicated in the pathology of schizophrenia. Efficacy of mGlu5 positive allosteric modulators (PAMs) in animal models of psychosis and cognition was previously attributed to potentiation of NMDAR function. To directly test this hypothesis, we identified VU0409551 as a novel mGlu5 PAM that exhibits distinct stimulus bias and selectively potentiates mGlu5 coupling to Gαq–mediated signaling but not mGlu5 modulation of NMDAR currents or NMDAR-dependent synaptic plasticity in the rat hippocampus. Interestingly, VU0409551 produced robust antipsychotic-like and cognition-enhancing activity in animal models. These data provide surprising new mechanistic insights into the actions of mGlu5 PAMs and suggest that modulation of NMDAR currents is not critical for in vivo efficacy.
Introduction
Multiple clinical and genetic studies have led to the hypothesis that hypofunction of the N-methyl-D-aspartate subtype of glutamate receptor (NMDAR) plays an important role in the pathophysiology of schizophrenia and that administration of agents that enhance NMDAR function can provide symptomatic improvement in schizophrenic patients (Coyle et al., 2012; Field et al., 2011; Timms et al., 2013). Based on these findings, multiple efforts have focused on developing approaches to enhance NMDAR signaling as a potential novel treatment strategy for schizophrenia. Metabotropic glutamate receptor subtype 5 (mGlu5) is a closely associated signaling partner with the NMDAR and activation of mGlu5 potentiates NMDAR responses and NMDAR-mediated synaptic plasticity in forebrain regions implicated in the pathology of schizophrenia (Ayala et al., 2009; Collingridge et al., 1983; Doherty et al., 1997; Homayoun et al., 2004; Mannaioni et al., 2001; Marino and Conn, 2002). Consistent with this, deletion or blockade of mGlu5 exacerbates psychotomimetic-like and cognition-disrupting effects of NMDAR antagonists (Brody et al., 2004; Campbell et al., 2004; Henry et al., 2002; Kinney et al., 2003), and highly selective mGlu5 positive allosteric modulators (PAMs) potentiate mGlu5-mediated regulation of NMDAR currents and have cognition-enhancing and antipsychotic-like effects in preclinical models (Ayala et al., 2009; Darrah et al., 2008; Gastambide et al., 2012; Gregory et al., 2013a; Kinney et al., 2005; Liu, 2008; Noetzel, 2012; Parmentier-Batteur et al., 2013; Rodriguez, 2010). However, activation of mGlu5 has multiple actions in forebrain circuits and the hypothesis that mGlu5-induced potentiation of NMDAR currents is required for in vivo efficacy of mGlu5 PAMs has not been directly tested.
The majority of actions of mGlu5 are mediated by activation of the Gαq GTP-binding protein subunit (Niswender and Conn, 2010) whereas mGlu5-induced potentiation of NMDAR currents in forebrain neurons is mediated by interactions of mGlu5 with NMDARs through adaptor proteins that are independent of signaling through Gαq, (Gao et al., 2013; Hu et al., 2012; Park et al., 2013; Shepherd and Huganir, 2007). We now report discovery and characterization of a novel mGlu5 PAM, VU0409551, that induces a robust potentiation of mGlu5 coupling to Gαq-mediated calcium mobilization and other signaling pathways but does not enhance mGlu5 modulation of NMDAR currents in hippocampal neurons. In addition, VU0409551 potentiates NMDAR-independent long-term depression (LTD) but does not potentiate NMDAR-dependent long-term potentiation (LTP) in the rat hippocampus. Interestingly, VU0409551 produces robust, dose-dependent efficacy in preclinical rodent models of psychosis and cognitive function. These studies challenge the prevailing hypothesis that the in vivo effects of mGlu5 PAMs in models related to schizophrenia and cognitive function are mediated by potentiation of mGlu5 modulation of NMDAR currents and provide important new insights into modulation for specific effects of mGlu5 activation.
Results
VU0409551 is a potent, highly selective potentiator of mGlu5
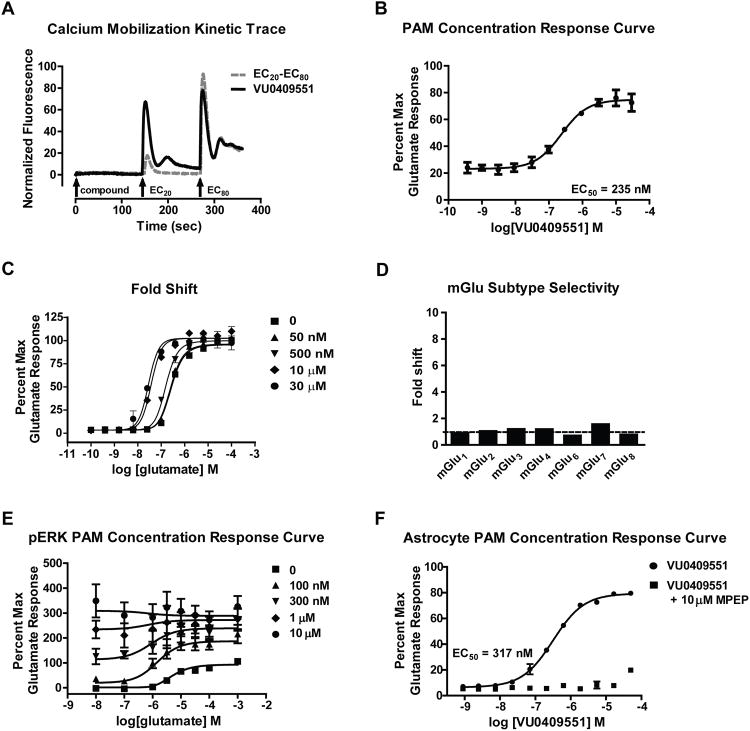
We have identified multiple mGlu receptor PAMs, including PAMs that display striking stimulus bias and selectively potentiate coupling of mGlu receptors to specific signaling pathways (Hammond et al., 2010; Noetzel et al., 2013; Sheffler and Conn, 2008; Zhang et al., 2005). Based on these studies, we initiated an effort to optimize novel mGlu5 PAMs that potentiate mGlu5-mediated Gαq signaling and calcium mobilization but do not potentiate coupling of mGlu5 to modulation of NMDAR currents and possess physicochemical and pharmacokinetic properties suitable for systemic dosing, a characteristic not achieved with previous biased mGlu5 PAMs. This culminated in the discovery of VU0409551 as a potent mGlu5 PAM (Figure S1). VU0409551 behaved as a classic mGlu5 PAM (Conn et al., 2014) in rat mGlu5-expressing HEK293A cells and did not possess intrinsic agonist activity (Figure 1A) but potentiated the response to an EC20 concentration of glutamate with an EC50 of 235 nM (Figure 1B). Increasing concentrations of VU0409551 resulted in progressive leftward shifts in the glutamate concentration-response curve with a maximum fold shift of 11 at a 30 μM concentration of VU0409551 (Figure 1C). VU0409551 (10 μM) is highly selective for mGlu5 and had no effect at the other mGlu receptor subtypes (Figure 1D). Additionally, radioligand competition binding assays revealed that VU0409551 (10 μM) displays weak affinity for α2A adrenergic receptors (IC50 8.9 μM) but has no activity at any of 66 other receptors and ion channels (Table S1). These studies indicate that VU0409551 is a potent and highly selective pure PAM of mGlu5-mediated calcium mobilization.
Figure 1. VU0409551 is a potent mGlu5 PAM in HEK293A-mGlu5 rat cells and native systems.
(A) Representative raw calcium traces following the addition of 30 μM VU0409551 and the subsequent additions of EC20 and EC80 concentrations of glutamate. (B) VU0409551 potentiates an EC20 concentration of glutamate with a potency of 235 nM in mGlu5-expressing R10A rat cells (C) VU0409551 shifts the glutamate concentration response curve with a maximum fold shift of 11 at 30 μM (D) VU0409551 did not shift the agonist concentration response curve in mGlu1,2,3,4,6,7,8-expressing cells, displaying its high selectivity for mGlu5. (E) VU0409551 potentiates glutamate-stimulated ERK1/2 phosphorylation and exhibits robust agonist activity in this assay. (F) VU0409551 potentiates glutamate-induced calcium mobilization in rat cortical astrocytes with a potency of 317 nM and maximum glutamate response of 79.5%, which is blocked by addition of 10 μM of the mGlu5 antagonist MPEP. Data represent mean ± S.E.M. from 2-4 independent experiments performed in duplicate or triplicate.
VU0409551-induced ERK phosphorylation
VU0409551 also potentiated glutamate-induced phosphorylation of extracellular signal-regulated kinase 1/2 (pERK1/2) in mGlu5-expressing cells (Figure 1E). In addition, VU0409551 increased pERK1/2 in the absence of added glutamate. This is similar to effects of other mGlu5 PAMs, which can also display agonist activity in this assay with greater efficacy than glutamate (Gregory et al., 2013b; Gregory et al., 2012). Analysis of these data with an operational model of allosterism (Gregory et al., 2012) allowed for quantification of VU0409551 agonist efficacy (τB: 1.04), affinity (KB: 89 nM) and cooperativity (β: 1.43) with glutamate.
VU0409551-induced modulation of mGlu5 in native systems
We next assessed the ability of VU0409551 to potentiate mGlu5 signaling in CNS preparations. Similar to its effects in HEK293A-mGlu5 cells, VU0409551 had no intrinsic agonist activity in cultured rat cortical astrocytes (Peavy et al., 2002) but potentiated glutamate-induced calcium mobilization in these cells (EC50 = 317 ± 1 nM, Figure 1F). This effect was blocked by the mGlu5 negative allosteric modulator (NAM) MPEP (10 μM, Figure 1F). Activation of mGlu5 also plays an important role in induction of an NMDAR-independent form of long-term depression (LTD) at the hippocampal Schaffer collateral-CA1 (SC-CA1) synapse (Huber et al., 2001; Rammes et al., 2003). To assess the effect of VU0409551 on LTD induced by the group I mGlu receptor agonist, DHPG, field excitatory postsynaptic potentials (fEPSPs) were recorded from the dendritic layer of CA1 after stimulation of the Schaffer collaterals (Figure 2). In agreement with our previous studies (Noetzel et al., 2013; Noetzel, 2012), DHPG (75 μM) induced robust LTD at the hippocampal SC-CA1 synapse measured 55 min after washout of DHPG (Figure 2B, 54.9% ± 5.3% of baseline fEPSP slope), whereas a lower concentration of DHPG (25 μM) induced only slight depression of fEPSPs (Figure 2B, 78.9% ± 5.7% of baseline fEPSP slope). As observed previously with other mGlu5 PAMs (Ayala et al., 2009; Noetzel et al., 2013; Noetzel, 2012), VU0409551 potentiated the response to 25 μM DHPG, inducing robust LTD (Figure 2B, 43.7% ± 4.6% baseline). Although VU0409551 alone induced a slight depression in the fEPSP slope, this effect was transient and returned to baseline within 10 min of washout (Figure S2). Taken together, these data suggest that VU0409551 potentiates multiple responses to mGlu5 activation in recombinant and native systems.
Figure 2. VU0409551 potentiates NMDAR-independent synaptic plasticity in the hippocampus.
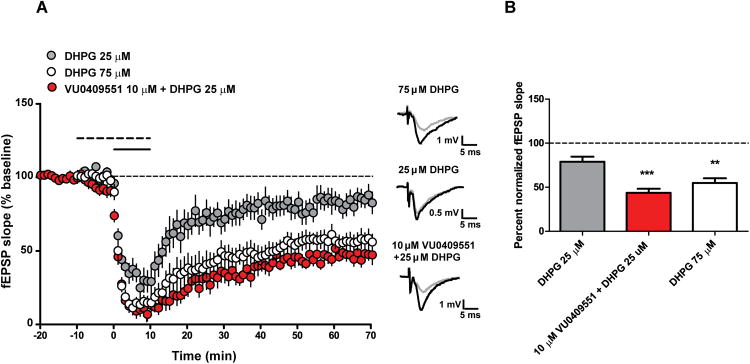
(A-B) VU0409551 potentiates DHPG-induced long term depression (LTD). (A) Bath application of 75 μM DHPG for 10 min (open circle, solid line) resulted in LTD of the fEPSP slope. In contrast, bath application of 25 μM DHPG for 10 min (gray circles, solid line) resulted in a slight decrease in fEPSP slope 55 min after compound washout. Application of 10 μM VU0409551 for 10 min (dashed line), first alone and then in combination with 25 μM DHPG (solid line) for 10 min (black circles), resulted in a significant LTD in fEPSP slope (p = 0.0005, n = 6 - 7). Inset shows representative fEPSP traces for each condition for baseline (black trace) and 55 min after compound washout (gray trace). (B) Quantification of the change in fEPSP slope measured 55 min after compound washout. Data represent mean ± S.E.M. ** p < 0.01, *** p < 0.001 when compared with 25 μM DHPG.
VU0409551 does not potentiate mGlu5 modulation of NMDAR currents in CA1 pyramidal cells
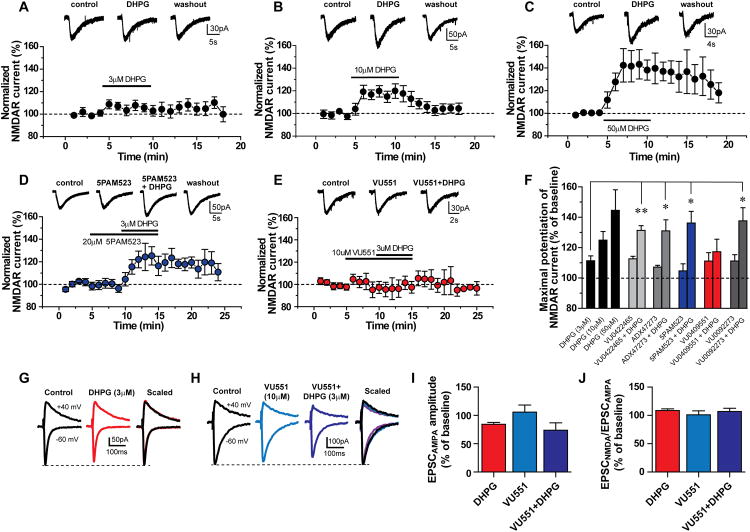
Activation of mGlu5 potentiates NMDAR currents in multiple cell types, including hippocampal CA1 pyramidal cells (Awad et al., 2000; Doherty et al., 1997; Fitzjohn et al., 1996; Mannaioni et al., 2001; O'Brien et al., 2004). Consistent with previous studies, whole-cell patch clamp recordings from CA1 pyramidal cells revealed that DHPG induced a concentration-dependent increase in inward currents evoked by application of NMDA through a patch pipette positioned adjacent to the recorded cell (Figure 3A-C, F). Application of the recently reported mGlu5 PAM 5PAM523 (Parmentier-Batteur et al., 2013) had no effect on NMDA-evoked currents when added alone but potentiated the effect of 3 μM DHPG on NMDAR currents (Figure 3D and F). Likewise, structurally distinct mGlu5 PAMs, VU0422465 (Rook et al., 2012), ADX47273 (Liu et al., 2008) and VU0092273 (Noetzel et al., 2012), resulted in a similar potentiation of DHPG-induced modulation of NMDAR currents (Figure 3F). In contrast, VU0409551 had no effect on NMDAR currents either alone or in the presence of 3 μM DHPG (Figure 3E and F). Consistent with these results, current clamp studies showed VU0409551 had no effect on NMDA-evoked responses when applied alone (Figure S3B, C and D) and did not potentiate 3 μM DHPG-induced depolarization or increase in NMDAR response (Figure S3A-D). We also assessed the effect of VU0409551 on NMDAR-mediated fEPSPs at the SC-CA1 synapse and found that VU0409551 had no effect on the NMDAR-fEPSP slope (Figure S3F-G). Finally, voltage clamp recordings revealed that VU0409551 (10 μM) had no effects on the EPSCNMDA/EPSCAMPA ratio when applied alone (Figure 3G-J), and did not have effects on 3 μM DHPG-induced inhibition of EPSCampa or the DHPG-induced slight increase in the EPSCNMDA/EPSCAMPA ratio (Figure 3G-J). Taken together, these results indicate that VU0409551 represents a unique molecular probe and behaves as an mGlu5 PAM, potentiating multiple responses to mGlu5 activation in HEK293A-mGlu5 cells, astrocytes and hippocampal slices but does not potentiate mGlu5-mediated increases in NMDAR currents.
Figure 3. Differential effects of mGlu5 PAMs on potentiation of NMDAR currents in CA1 pyramidal cells of the hippocampus.
(A-C) DHPG induces a concentration-dependent increase in NMDAR currents. Top: representative traces of NMDA-evoked currents in hippocampal CA1 pyramidal cells and Bottom: time courses of normalized NMDAR current amplitude before, during and after application of DHPG at different concentrations. (D-E) Top: representative traces of NMDA-evoked currents and Bottom: time courses of normalized NMDAR current amplitude in control, during applications of mGlu5 PAM alone and a combination of mGlu5 PAM and DHPG (3 μM), and after washout of the compounds. (D) The mGlu5 PAM 5PAM523 (20 μM) potentiates the effect of DHPG on NMDAR currents. (E) VU0409551 (10 μM), a highly selective mGlu5 PAM, does not significantly potentiate NMDAR currents. (F) Summary of DHPG and mGlu5 PAMs (p < 0.05 n = 5 - 8) alone and in the presence of 3 μM DHPG on NMDAR currents. (G-J) VU0409551 does not have a significant effect on the EPSCNMDA/EPSCAMPA ratio at SC-CA1 synapses. (G-H) Representative traces of EPSCsAMPA and EPSCsNMDA at holding potentials of −60 mV and +40 mV in controls, during application of 3 μM DHPG, 10 μM VU0409551, or co-application of VU0409551 and DHPG, respectively. Traces in right panels in G and H are scaled to the peak of AMPAR-EPSCs and superimposed, demonstrating that VU0409551 did not potentiate the EPSCNMDA/EPSCAMPA ratio when applied alone or co-applied with DHPG. (I-J) Summary of the effects of 3 μM DHPG, 10 μM VU0409551, and 10 μM VU0409551 along with 3 μM DHPG on EPSCAMPA amplitude and the EPSCNMDA/EPSCAMPA ratio, respectively (n = 4 - 6). Data represent mean ± S.E.M., * p < 0.05, ** p < 0.005.
VU0409551 does not potentiate LTP at the SC-CA1 synapse
Previous studies reveal that mGlu5 PAMs enhance theta burst stimulation (TBS)-induced LTP at the SC-CA1 synapse and this effect has been postulated to be mediated by potentiation of mGlu5-induced modulation of NMDAR currents (Ayala et al., 2009; Noetzel et al., 2013). To determine the effects of VU0409551 on induction of LTP, maximal LTP was initiated using a standard TBS protocol (saturation TBS; four trains of 10 Hz TBS), which resulted in a 141.1% ± 8.4% increase in fEPSP slope, compared with baseline, when measured 35 min after stimulation (Figure 4A-B). In contrast, a single train of lower frequency bursts resulted in a slight potentiation of the fEPSP slope (Figure 4A-B; 116.2% ± 3.3% of baseline) and was used as a threshold protocol for induction of TBS LTP. Consistent with previous reports (Ayala et al., 2009; Noetzel et al., 2013), pretreatment of slices with the mGlu5 PAM VU0092273 resulted in a significant potentiation of the fEPSP slope in response to threshold TBS (Figure 4B). In contrast, treatment of hippocampal slices with VU0409551 followed by threshold TBS, had no effect on fEPSP slope, compared with threshold TBS alone (Figure 4B; 110.4% ± 4.0% of baseline). Similar to the LTD studies, a slight decrease in the fEPSP slope observed following application of VU0409551 resolved within 25 min of application, which is before LTP quantification (Figure S2). The ability of VU0409551 to potentiate NMDAR-independent mGlu-LTD but not NMDAR-dependent LTP is consistent with the lack of effect of this mGlu5 PAM on modulation of NMDAR currents.
Figure 4. In contrast to other mGlu5 PAMs, VU0409551 does not potentiate NMDAR-mediated hippocampal synaptic plasticity.
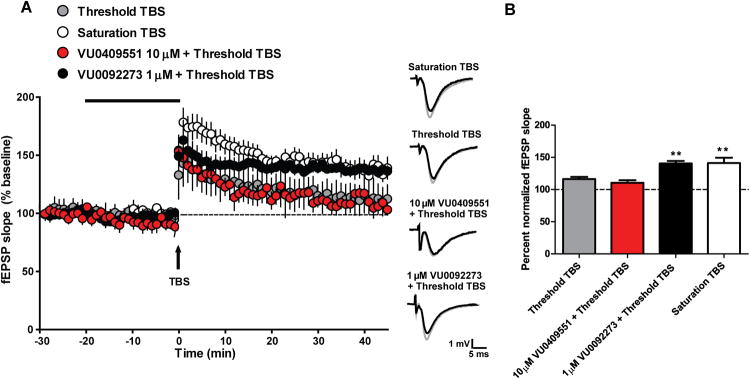
(A) VU0409551 does not potentiate NMDAR-mediated, theta burst stimulation-induced long term potentiation (LTP). Saturation TBS resulted in a significant increase in fEPSP slope 35 min after stimulation. Threshold TBS resulted in a small increase in fEPSP slope measured 35 min after stimulation. Bath application of 10 μM VU0409551 for 20 min, followed by threshold TBS, resulted in no change in fEPSP slope from threshold TBS alone. In contrast, bath application of 1 μM VU0092273 for 20 min, followed by threshold TBS, resulted in a significant enhancement of fEPSP slope measured 35 min after stimulation. Inset shows representative fEPSP traces for each condition for baseline (black trace) and 35 min after TBS (gray trace). (B) Quantification of the change in fEPSP slope measured 35 min after TBS stimulation. In contrast to VU0092273, bath application of VU0409551 for 20 min followed by threshold TBS did not result in significant LTP 35 min after stimulation (p = 0.0002, n = 6 - 11). Data represent mean ± S.E.M. ** p < 0.01, when compared with threshold TBS.
Rat pharmacokinetics (PK) and brain distribution of VU0409551
Before using this compound for in vivo studies, we evaluated the pharmacokinetic (PK) profile and brain distribution of VU0409551 after systemic administration. VU0409551 exhibited a moderate clearance from plasma (CLp; 33 mL/min/kg) with a large volume of distribution at steady-state (Vss; 9.6 L/kg) and a moderate half-life (t1/2; 3.9 hr) following a single intravenous (i.v.) administration in rats (2.5 mg/kg bodyweight, Figure S4). VU0409551 biotransformation experiments in rat hepatocytes revealed moderate turnover through multiple oxidative pathways with mono-hydroxylation of the phenyl ring (para-position) producing the predominant metabolite, which was inactive at mGlu5 in in vitro assays (data not shown). A single oral (p.o.) administration (3 mg/kg) of VU0409551 revealed high oral bioavailability (%F = 63), a maximum concentration in plasma (Cmax,p) of 270 nM, a time to reach maximum concentration in plasma (Tmax,p) of 1.3 hours, and an area-under-the-curve from 0-last (AUC0-last) of 2.9 μM*hr (Figure S5). The in vitro fraction of unbound VU0409551 in rat plasma (fup) of 0.07 and in rat brain (fubr) of 0.04 suggested a brain to plasma partition coefficient (Kp) at unrestricted equilibrium ([fup]/[fubr]) of 1.8 (Table S2). Distribution of VU0409551 to brain in vivo at 1.5 hr following a single p.o. administration (3, 10, 30, 100 mg/kg) revealed a brain to plasma Kp of 2.3 and corresponding unbound brain to plasma Kp (Kp,uu) of 1.3, suggesting the compound freely permeates the blood-brain barrier (BBB), and unbound concentrations in brain and plasma reach unrestricted equilibrium (Table S2). Thus, VU0409551 exhibits excellent PK and brain exposure for use in in vivo studies.
VU0409551 induces wake-promoting effects in rats
Previous studies reveal that mGlu5 PAMs increase wakefulness in rodents (Gilmour et al., 2012; Parmentier-Batteur et al., 2012) and this provides an excellent measure of their in vivo CNS activity. Acute oral administration of VU0409551 exerts clear central activity immediately following administration, as expressed by a dose-dependent decrease in sleep. Specifically, VU0409551 dose-dependently decreased the total time spent asleep, leading to significant reduction in non-rapid eye movement (NREM) and REM sleep (Figure 5). A 10 mg/kg dose had a modest effect on vigilance states, decreasing time asleep for 2 hr post-administration. A dose of 60 mg/kg VU0409551 significantly decreased the total time spent in NREM and REM sleep for up to 7 hr post-administration (Figure 5).
Figure 5. VU0409551 displays wake-promoting effects in vivo.

Sleep-wake electroencephalography (EEG) studies demonstrate that VU0409551 (10 or 60 mg/kg), administered at the 2nd hr of the light cycle, dose-dependently decreases the percent time spent asleep (p < 0.0001, n=8). Sleep polygraphic variables were measured in rats for 24 hr beginning at the start of the light cycle. Data represent mean ± S.E.M.. * p < 0.05.
Antipsychotic-like and procognitive efficacy of VU0409551
Discovery and optimization of VU0409551 as an mGlu5 PAM that has an excellent PK profile and does not potentiate coupling of mGlu5 to NMDAR currents provides an unprecedented opportunity to directly assess the importance of potentiation of NMDAR signaling in antipsychotic-like and cognition-enhancing effects of mGlu5 PAMs in rodent models. Two primary models used to assess antipsychotic-like activity of mGlu5 PAMs include reversal of NMDAR antagonist- or amphetamine-induced hyperlocomotion (AHL) and reversal of pharmacologically-induced disruptions in prepulse inhibition (PPI) of the acoustic startle reflex. Interestingly, pretreatment (30 min) of rats with VU0409551 (10-100 mg/kg) reversed the increased locomotor activity induced by the NMDAR antagonist MK-801 (0.2 mg/kg, s.c., Figure 6A). The minimal effective dose (MED) of VU0409551 was 10 mg/kg with a maximum reversal of 49.7% at the highest dose (Figure 6A). In addition, pretreatment (30 min) with VU0409551 (3-100 mg/kg) dose-dependent reversed increased locomotor activity induced by administration of amphetamine (1 mg/kg, s.c.) with an MED of 3 mg/kg, an ED50 of 5.9 mg/kg and a maximum reversal of 78.1% (Figure S6A-B). VU0409551 terminal (1.5 hr) unbound brain concentration data from rat AHL studies were used to determine an in vivo EC50 using individual animal concentration-efficacy data from all dose groups. This analysis provided an EC50 of 97 nM with an Emax of 82% for reversal of AHL. Additionally, reversal of AHL by VU0409551 was blocked by the mGlu5 antagonist, MTEP, supporting mGlu5-mediated antipsychotic-like efficacy (data not shown). Finally, pretreatment (30 min) with VU0409551 (56.6 and 100 mg/kg) reversed amphetamine-induced disruptions in PPI in rats (Figure S6C).
Figure 6. VU0409551 demonstrates antipsychotic-like activity and cognition enhancement in rodent behavioral models.
(A) VU0409551 (10 – 100 mg/kg) reverses MK-801-induced hyperlocomotion in rats with a minimum effective dose of 10 mg/kg and maximum reversal of 49.7% at 100 mg/kg. Data are expressed as the mean total number of beam breaks per 5-min intervals ± S.E.M.. Area under the curve from time interval t = 60 to 120 min was quantified for each treatment group and expressed as total ambulations. Percent reversal was calculated after normalizing to the vehicle + MK-801 treatment group. (p < 0.0001, n = 10 - 12). (B) VU0409551 dose-dependently enhances acquisition of contextual fear conditioning in rats. Pretreatment with 1, 3, 5.6 and 10 mg/kg VU0409551 prior to conditioning significantly enhanced acquisition of fear conditioning assessed 24 hr later (p = 0.0005, n = 10-13). Data are expressed as mean ± S.E.M. *** p < 0.001, ** p < 0.01 and * p < 0.05. (C) VU0409551 dose-dependently enhances recognition memory in rats. Pretreatment with 3 and 10 mg/kg VU0409551 prior to exposure to identical objects significantly enhanced recognition memory assessed 24 hr later (p = 0.0014, n = 19). Data are expressed as mean ± S.E.M.. *** p < 0.001 and * p < 0.05. (D) VU0409551 demonstrates pro-cognitive effects in the delayed non-matching to position task in rats. Pretreatment with VU0409551 (10 – 60 mg/kg) prior to testing significantly increased the proportion of correct responses at 10 sec and 20 sec delays, respectively (p = 0.0236, n = 15 – 35). Data are expressed as mean ± S.E.M. * p < 0.05.
In addition to its antipsychotic-like activity, VU0409551 demonstrated robust cognition enhancement. VU0409551 induced a dose-dependent enhancement of contextual fear conditioning acquisition in rats. Pretreatment (30 min) with VU0409551 (0.3 - 10 mg/kg) prior to conditioning significantly enhanced acquisition of fear memory assessed 24 hr later (Figure 6B), demonstrating efficacy of this novel mGlu5 PAM in enhancing an established form of hippocampal-dependent cognitive function. Moreover, administration of VU0409551 (1-10 mg/kg) 30 min prior to exposure to two identical objects resulted in a dose-dependent increase in recognition memory in the novel object recognition task, another commonly used rodent learning and memory paradigm dependent upon hippocampal function (Figure 6C). To evaluate the effects of VU0409551 on working memory and executive function, the operant delayed non-matching to position (DNMTP) task was used. Pretreatment (60 min) with VU0409551 (10-60 mg/kg) prior to the testing session increased the proportion of correct responses at a 10 and 20 sec delay (60 mg/kg; Figure 6D) with no change in total number of trials initiated (Figure S6D). Based on the PK analysis, the doses of VU0409551 required to elicit antipsychotic-like and cognition-enhancing effects provide brain concentrations that fit well with those required to potentiate mGlu5-mediated responses in cell lines and in brain slices (100 nM–30 μM). These data demonstrate that VU0409551 has robust efficacy in rodent models used to predict antipsychotic-like and cognition-enhancing effects that are similar to that described for previous mGlu5 PAMs that potentiate mGlu5 modulation of NMDAR currents.
Repeated administration of VU0409551 does not induce detectable changes in Fluoro-Jade C staining
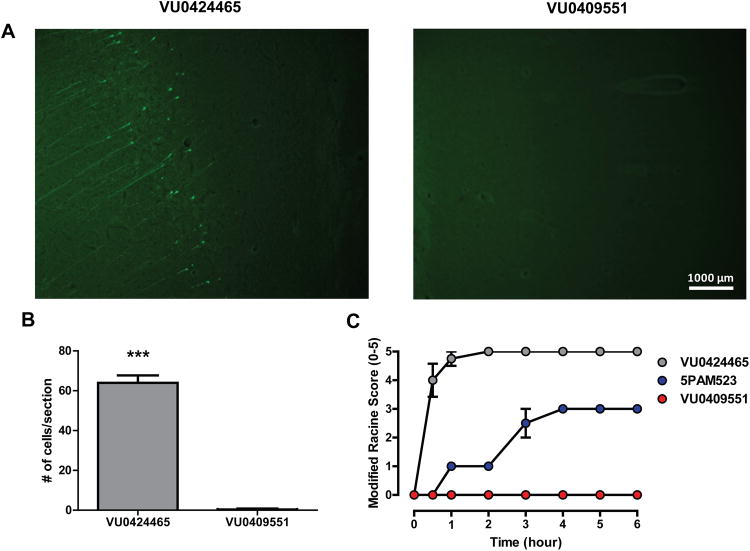
Previous studies reveal that some mGlu5 PAMs can induce severe epileptiform activity, behavioral convulsions (Parmentier-Batteur et al., 2013; Rook et al., 2012), and neuronal death (Parmentier-Batteur et al., 2013) in rodents as assessed by measuring increases in staining for Fluoro-Jade C (FJC), which selectively stains degenerating neurons (Schmued et al., 1997). Similar to previous reports with 5PAM523 (Parmentier-Batteur et al., 2013), we found that VU0424465 induced cell death, as assessed by the number of FJC-positive neurons in the auditory cortex and adjacent cortical regions 24 hr following a single relatively low dose (3 mg/kg, intraperitoneal (i.p), Figure 7A-B). In contrast, daily administration of a high dose of VU0409551 (120 mg/kg, p.o.) for four consecutive days did not induce in FJC staining in sections harvested from rats 24 hr following the last dose (Figure 7A-B).
Figure 7. Repeat administration of VU0409551 does not result in positive Fluoro-Jade C staining in the rat brain.
(A-B) mGlu5 ago-PAM VU0424465 (3 mg/kg) induces positive Fluoro-Jade C (FJC) staining in the auditory cortex 24 hr following acute dosing. Unlike VU0424465, VU0409551 does not induce neurotoxicity in rats at doses above those required for robust in vivo efficacy in behavioral assays as demonstrated by negative FJC staining following repeat dosing of 120 mg/kg once daily for four consecutive days (p < 0.0001, n = 3). Data represent mean ± S.E.M. *** p < 0.0001, when compared with VU0409551. (C) mGlu5 PAMs VU0424465 (3 mg/kg) and PAM 5PAM523 (56.6 mg/kg) induce robust behavioral convulsions as measured by the modified Racine scale (0-5). VU0409551 is void of seizure activity following acute or repeat dosing (120 mg/kg) once daily for four consecutive days. Data represent mean ± S.E.M.
In order to determine the PK of VU0409551 following repeat (once-daily for 4 days) administration (120 mg/kg, p.o., 75% PEG 400 in water) used in FJC studies, a satellite PK study was performed using an identical design; plasma concentrations were obtained at 3, 7, and 24 hr post-administration on day 1 and day 4 (Figure S7). In this study, VU0409551 displayed similar, high Cmax,p and exposure on day 1 (Cmax,p, 7.0 μM; AUC0-last, 96 μM*hr) and day 4 (Cmax,p, 7.5 μM; AUC0-last, 95 μM*hr). Thus, the exposure was maintained at levels far in excess of those required to achieve maximal efficacy over this 4 day dosing period.
Finally, consistent with previous reports (Parmentier-Batteur et al., 2013; Rook et al., 2012), VU0424465 and 5PAM523 induced behavioral convulsions in rats that could be assessed using the Racine scale of behavioral convulsions (Figure 7C). In contrast VU0409551, at doses up to 100 times that required for measureable efficacy in behavioral models (120 mg/kg), did not induce behavioral convulsions in rats (Figure 7C). In addition, while chronic administration of previous mGlu5 PAMs, such as 5PAM523, led to progressive worsening of seizure activity (Parmentier-Batteur et al., 2013), VU0409551 did not induce behavioral convulsions after repeated once-daily administration of high doses (450 mg/kg) over 14 days (data not shown). Thus, VU0409551 does not have detectable toxic effects at the doses well above those used for these behavioral studies.
Discussion
Converging evidence suggests that NMDAR hypofunction is a primary risk factor for schizophrenia (Coyle et al., 2012; Field et al., 2011; Matosin and Newell, 2013; Timms et al., 2013). NMDAR and mGlu5 are co-localized at postsynaptic sites implicated in the pathology of schizophrenia and their intracellular domains are physically connected via scaffold proteins (Gao et al., 2013; Hu et al., 2012; Matosin and Newell, 2013; Park et al., 2013; Shepherd and Huganir, 2007), facilitating the potentiation of NMDAR activity by mGlu5 activation (Ayala et al., 2009; Homayoun et al., 2004; Mannaioni et al., 2001; Marino and Conn, 2002). Numerous reports demonstrate that selective mGlu5 PAMs have efficacy in animal models of psychosis and cognition (Darrah et al., 2008; Gastambide et al., 2012; Gregory et al., 2013a; Kinney et al., 2003;Liu, 2008; Noetzel, 2012; Rodriguez, 2010), and these effects have been hypothesized to result from direct potentiation of NMDAR currents. In support of this hypothesis, previous studies revealed that mGlu5 PAMs potentiate mGlu5 modulation of NMDAR currents in pyramidal cells of the hippocampus (O'Brien et al., 2004), and we now confirm these previous findings using multiple, structurally unrelated mGlu5 PAMs. However, potentiation of mGlu5 coupling to Gαq in postsynaptic neurons also facilitates activity in these forebrain circuits through effector systems independent of NMDAR activation. Discovery of VU0409551 as a potent mGlu5 PAM that displays signaling bias and does not potentiate mGlu5 coupling to NMDAR currents while exhibiting robust in vivo efficacy in rodent models provides an exciting advance in our understanding of mGlu5 pharmacology and suggests that mGlu5 acts in concert with NMDARs to modulate brain circuits that are impacted in these models rather than exerting actions that are fully dependent on mGlu5 modulation of NMDAR signaling.
While these data are consistent with a clear stimulus bias and lack of effect of VU0409551 on mGlu5 modulation of NMDAR currents, it is important to note that VU0409551 could cause an indirect potentiation of NMDAR function in some neuronal populations by depolarizing neurons, reducing the voltage-dependent Mg2+ block of NMDAR channels and thereby increase NMDAR currents indirectly. However, DHPG-induced depolarization of pyramidal cells in hippocampal area CA1 in rats is mediated by mGlu1 rather than mGlu5 (Mannaioni et al., 2001). Thus, selective potentiation of mGlu5 is not likely to potentiate NMDAR function by depolarizing the pyramidal cells at this particular synapse. However, mGlu5 activation inhibits the slow AHP current (IAHP) and thereby increases excitability in CA1 pyramidal cells (Mannaioni et al., 2001). Thus, the overall physiological effects of mGlu5 activation in forebrain circuits include multiple actions that are independent of direct modulation of NMDAR currents but act in concert with NMDAR signaling to enhance transmission through circuits that are impacted in schizophrenia and relevant animal models. This and other mechanisms by which mGlu5 activation can enhance transmission at synapses where NMDARs play important roles would likely enhance transmission through these circuits and may be involved in the efficacy of mGlu5 PAMs.
Similar to previously reported mGlu5 PAMs, VU0409551 exhibits selective, potent potentiation of mGlu5-mediated calcium mobilization. Importantly, VU0409551 does not possess intrinsic allosteric agonist activity in induction of calcium mobilization in cell-based assays or native systems but potentiates glutamate-induced increases in intracellular calcium, therefore displaying pure PAM activity at mGlu5 in this assay. Previous studies suggest that allosteric agonist or “ago-PAM” activity in inducing calcium mobilization is a primary factor that can contribute to adverse effects of mGlu5 PAMs (Conn et al., 2014; Rook et al., 2012). Thus, the lack of ago-PAM activity in VU0409551 in this assay is a critical property for avoiding adverse effects. However, mGlu5 PAMs that lack allosteric agonist activity can still lead to seizures and cell death after chronic administration of high doses (Parmentier-Batteur et al., 2013). This, coupled with the established role of NMDAR activation in excitotoxicity throughout the CNS (Stone and Burton, 1988) raises the possibility that agents that do not potentiate mGlu5 modulation of NMDAR currents could provide the desired efficacy while reducing the risk of neurotoxic effects. Thus, the stimulus bias provided by VU0409551 that allows potentiation of mGlu5 signaling through Gαq without potentiation of mGlu5 coupling to NMDAR current modulation could be a significant factor that leads to the improved safety profile that we observe for this novel mGlu5 PAM in which no toxicity is observed in the dose range that provides preclinical efficacy in rats. However, it is important to note that there can be species differences in the PAM-induced activation of specific signaling cascades (Conn et al., 2014; Croy et al., 2014) or pharmacokinetic differences across species, all of which may affect their net in vivo effects. Thus, this type of stimulus bias can be species-dependent and we cannot extrapolate beyond the current studies to make predictions regarding effects of VU0409551 on NMDAR currents and likely safety of this compound in other species. Indeed, when administered chronically at very high doses (360 mg/kg, once daily for 1 month) we have found evidence that VU0409551 can induce FJC staining (unpublished findings).
It was especially interesting to find that VU0409551 does not potentiate TBS-induced LTP in the hippocampus. This is in contrast to previous mGlu5 PAMs, which induce robust potentiation of TBS-LTP (Ayala et al., 2009; Noetzel et al., 2013). Induction of LTP at this synapse is known to be NMDAR-dependent (Ben-Ari et al., 1992; Harris et al., 1984; Muller et al., 2013). Thus, this finding, together with the lack of ability of VU0409551 to potentiate mGlu5 modulation of NMDAR currents, is consistent with the hypothesis that mGlu5-induced potentiation of hippocampal LTP is dependent on the ability of mGlu5 to potentiate NMDAR currents (Ben-Ari et al., 1992; Collingridge et al., 2013; Mannaioni et al., 2001). Interestingly, VU0409551 did enhance fear conditioning and novel object recognition, which are established models of hippocampal-dependent cognitive function (Franca et al., 2014; Maren and Holt, 2000). VU0409551 also improved performance in an operant DNMTP task, which assesses cortical-dependent working memory and executive function. These data suggest that the cognition-enhancing effects of mGlu5 PAMs in are not dependent upon potentiation of NMDAR currents or LTP. However, as mentioned above, mGlu5 activation has a broad range of effects in the hippocampus and cortical circuits that are unrelated to modulation of NMDAR currents but increase overall transmission through the hippocampal circuit. In the future, it will be important to perform further studies to better understand the mechanisms by which mGlu5 PAMs enhance cognitive function.
Experimental Procedures
In Vitro Assays
Cell Culture and in vitro Assays
HEK293A cells stably expressing rat mGlu5 or mGlu1 and primary rat cortical astrocytes (Lonza) were maintained and measurement of mGlu5- or mGlu1-mediated intracellular Ca2+ mobilization was performed as previously described (Hammond et al., 2010; Rodriguez, 2010; Rook et al., 2012). For selectivity screening, calcium flux in HEK293 cells stably expressing rat mGlu1 or thallium flux through GIRK channels in HEK-293-GIRK cells expressing mGlu subtype 2, 3, 4, 6, 7 or 8 was measured as described in supplemental experimental procedures (Hammond et al., 2010; Niswender et al., 2008; Rodriguez, 2010). Measurement of mGlu5-mediated ERK phosphorylation was performed in HEK293A cells stably expressing rat mGlu5 using the ALPHAScreen-based ERK Surefire kit (Perkin-Elmer, TGR Biosciences) as described in supplemental experimental procedures and previously (Gregory et al., 2012).
In Vivo Studies
Animals
All present studies used male Sprague Dawley (SD) rats (Harlan, Indianapolis, IN) with the exception of the delayed non-matching to position task [male Lister-hooded rats (Harlan, Netherlands)], which were cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All experimental procedures were approved by the Vanderbilt University Animal Care and Use Committee. Unless otherwise stated, all VU0409551 dosing was performed orally in 20% β-cyclodextrin at 3 ml/kg. Additional detailed methods for animal studies below can be found in the supplemental experimental procedures.
Brain Slice Electrophysiology
Whole-cell voltage and current clamp recordings
Transverse hippocampal slices were prepared from rats (age 17–28 days) (Charles River, Wilmington, MA). Whole-cell recordings were made from hippocampal CA1 pyramidal neuron soma. NMDA receptor mediated responses were induced by pressure ejection of 0.5-1 mM NMDA to the dendritic field near the soma of the recorded cell every 30 sec through a patch pipette using a Picospritzer II (General Valve, Fairfield, NJ). This experiment was carried out in the presence of tetrodotoxin (1 μM), and the cell was voltage-clamped at -65 to -70 mV or recorded at resting membrane potential under current clamp. For the studies of synaptic NMDAR currents, EPSCsAMPA and EPSCsNMDA were evoked by electrical stimulation of Schaffer collaterals every 30 sec and recorded at holding potential of -60 mV and +40mV, respectively, in the presence of 20 μM bicuculline. All drugs were bath applied. Data were analyzed using Clampfit 10.2, Origin 6 (OriginLab, Northampton, MA) and Prism 5.0 (GraphPad Software, La Jolla, CA)..
Extracellular field potential recordings
Transverse hippocampal slices were prepared from young adult (age 29–36 days) rats (Charles River, Wilmington, MA) (Ayala et al., 2009; Noetzel et al., 2012). A bipolar-stimulating electrode was utilized to stimulate the Schaffer collaterals and field potential recordings were acquired from the CA1. mGlu5 compounds were applied to the bath for 10-20 min and chemically induced mGlu LTD was initiated by the application of DHPG for 10 min. Threshold LTP was induced by one train of theta burst stimulation (TBS; nine bursts of four pluses at 100 Hz, 230-millisecond interburst interval). Saturated LTP was induced by four trains of 10 Hz TBS (nine bursts of four pulses at 100 Hz, 100-millisecond interburst interval). Data were analyzed using Clampfit 10.2 and GraphPad Prism 5.0 (Noetzel et al., 2012).
Drug Metabolism and Pharmacokinetics in Rat
The in vitro and in vivo rat drug metabolism and pharmacokinetic properties of VU0409551 were determined essentially as previously described (Bridges et al., 2013; Gregory et al., 2013a; Jones et al., 2012).
Rat Chronic Dosing Satellite PK for Fluoro-Jade Studies
In order to determine the PK of VU0409551 following chronic administration (once-daily [QD] for 4 days; 120 mg/kg, p.o., vehicle: 70% PEG 400 in water) to non-fasted rats used in Fluoro-Jade studies, serial blood samples were obtained and plasma concentrations determined at 3, 7, and 24 hr post-administration on day 1 and day 4. Samples and data were analyzed as described above via LC-MS/MS quantification.
Behavioral Studies
Sleep-wake electroencephalography studies
Electroencephalography (EEG) and electromyography (EMG) data were recorded from rats (250-275 g) beginning at the start of the light cycle. VU0409551 was then administered (vehicle, 10, or 60 mg/kg) at the 2nd hour of the light period. Sleep polygraphic variables were measured for a period of 22 hr after compound administration in rats. EEG and EMG waveform data were collected using Dataquest A.R.T. 4.3 software (DSI, Minneapolis, MN) using a continuous sampling method. Telemetric data were sampled at a rate of 500 Hz and transmitted via a receiver (RPC-2, DSI, MN) placed below the cage of each rat. Data was scored manually by a blinded observer into 10-second epoch using Neuroscore 3.0 software (Data Sciences International, St. Paul, MN) to determine changes in sleep-wake stages following dosing based upon accepted characteristic oscillatory patterns. Sleep-wake stages were scored in 10 sec epochs then NREM and REM sleep time was combined into 60-min bins to examine the percent of time spent in total sleep across the first 22 hr cycle following compound administration.
MK-801-induced hyperlocomotion
Effects of VU0409551 on MK-801-induced hyperlocomotion in rats were determined (Rodriguez, 2010). using an open-field chamber (KinderScientific, San Diego, CA). At t = 30, rats were administered vehicle or VU0409551 (10 – 100 mg/kg) followed by MK-801 (0.2 mg/kg, s.c., saline, 1 ml/kg) at t = 60 min. Locomotor activity was measured for an additional 60 min. Changes in locomotor activity were measured as the total number of photobeam breaks per 5-min bins.
Contextual fear conditioning
Effects of VU0409551 on acquisition of contextual fear conditioning were evaluated in rats (275-300 g) (Lebois et al., 2009). Rats were given a 30 min pretreatment of VU0409551 (0.3 - 10 mg/kg) and placed in a sound-attenuating conditioning chamber (Med Associates, St. Albans, VT) in the presence of 1 mL of a 10% vanilla extract solution. Following a 2 min habituation period, rats received a one shock-context pairing trials (1 s, 0.5 mA) and after 45 sec were returned to their home cages. Twenty-four hr later, the fear response was assessed in the same conditioning environment for 2 min 45 sec, measuring freezing behavior in the absence of any shock stimuli or drug. Testing sessions were recorded and time spent freezing was scored by blinded personnel (MED-VFC-RS, MedAssociates).
Novel object recognition
Effects of VU0409551 on recognition memory were evaluated in rats (275-300 g). Rats were habituated in an empty novel object recognition (NOR) arena for 10 min for 2 consecutive days. On day 3, rats were administered vehicle, 1, 3, or 10 mg/kg of VU0409551 30 min before being placed into the NOR arena containing 2 identical objects. Following a 10 min exposure period, rats were placed back into their home cages, and 24 hr later returned to the arena in which one of the previously exposed (familiar) objects was replaced by a novel object. The rats were video recorded for 10 min while they explored the objects. Time spent exploring each object was scored by a blinded observer.
Delayed non-matching to position
Effects of VU0409551 on working memory and executive function were evaluated in the delayed non-matching to position (DNMTP) task using male Lister-hooded rats (350-450 g). Rats were trained to ensure a high level of stable and accurate performance was obtained. Rats were administered vehicle or VU0409551 (10 – 60 mg/kg) 60 min prior to testing. Four delay lengths (1, 10, 20 and 30 sec) were used in this study with 15 trials occurring at each delay (60 trials total). Rats were allowed a maximum of 45 minutes to complete these trials.
Fluoro-Jade C staining
Twenty-four hours after the last dose of VU0409551 or VU0424465, Fluoro-jade C staining was performed on rat brains. Fluoro-jade C positive neurons within the auditory cortex were counted under 20× magnification. A total of five sections per animal was quantified and the data are presented as mean number of cells per section.
Supplementary Material
Highlights.
VU0409551 is a potent, selective mGlu5 PAM
VU0409551 induces stimulus bias in mGlu5 signaling.
mGlu5 PAM in vivo efficacy does not require potentiation of NMDAR modulation.
Acknowledgments
The authors would like to thank Dina McGinnis, Kiran Gogi, Rocco D. Gogliotti, Mark Turlington and Tom Van De Casteele for their technical expertise. Fluoro-Jade C data analysis and presentation were performed in part through the use of the Vanderbilt University Medical Center Cell Imaging Shared Resource. PNV, SRS, CMN, JSD, CKJ, CWL, PJC have received compensation from Johnson and Johnson and are inventors on patents that protect multiple classes of mGlu5 PAMs. HL, CM, SC, JA, JMB, GJM, and TS are employees and shareholders of Janssen Research and Development. JMR, ZX, XL, AG, JWD, TMB, KAJ, DJF, KJG, ADT, NB, RLC, MB, MTN, RWG, have no conflict of interest to disclose. This work was supported by the National Institutes of Health (R01 MH062646, R01 MH074953 R01 NS031373 (PJC); U54 MH084659 (CWL) and by Janssen Research and Development.
Footnotes
Supplemental Information: Supplemental information includes Supplemental Experimental Procedures, eight figures and two tables.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci. 2000;20:7871–7879. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, Telk AN, Watson NL, Xiang Z, Zhang Y, Jones PJ, et al. mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology. 2009;34:2057–2071. doi: 10.1038/npp.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Aniksztejn L, Bregestovski P. Protein kinase C modulation of NMDA currents: an important link for LTP induction. Trends Neurosci. 1992;15:333–339. doi: 10.1016/0166-2236(92)90049-e. [DOI] [PubMed] [Google Scholar]
- Bridges TM, Rook JM, Noetzel MJ, Morrison RD, Zhou Y, Gogliotti RD, Vinson PN, Xiang Z, Jones CK, Niswender CM, et al. Biotransformation of a novel positive allosteric modulator of metabotropic glutamate receptor subtype 5 contributes to seizure-like adverse events in rats involving a receptor agonism-dependent mechanism. Drug Metab Dispos. 2013;41:1703–1714. doi: 10.1124/dmd.113.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody SA, Conquet F, Geyer MA. Effect of antipsychotic treatment on the prepulse inhibition deficit of mGluR5 knockout mice. Psychopharmacology (Berl) 2004;172:187–195. doi: 10.1007/s00213-003-1635-3. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Lalwani K, Hernandez L, Kinney GG, Conn PJ, Bristow LJ. The mGluR5 antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) potentiates PCP-induced cognitive deficits in rats. Psychopharmacology (Berl) 2004;175:310–318. doi: 10.1007/s00213-004-1827-5. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Volianskis A, Bannister N, France G, Hanna L, Mercier M, Tidball P, Fang G, Irvine MW, Costa BM, et al. The NMDA receptor as a target for cognitive enhancement. Neuropharmacology. 2013;64:13–26. doi: 10.1016/j.neuropharm.2012.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Meiler J, Niswender CM. Opportunities and Challenges in Discovery of Allosteric Modulators of GPCRs for Treatment of CNS Disorders. Nature Reviews Drug Discovery. 2014;13:692–708. doi: 10.1038/nrd4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT, Basu A, Benneyworth M, Balu D, Konopaske G. Glutamatergic synaptic dysregulation in schizophrenia: therapeutic implications. Handb Exp Pharmacol. 2012:267–295. doi: 10.1007/978-3-642-25758-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy CH, Schober DA, Xiao H, Quets A, Christopoulos A, Felder CC. Characterization of the novel positive allosteric modulator, LY2119620, at the muscarinic M(2) and M(4) receptors. Mol Pharmacol. 2014;86:106–115. doi: 10.1124/mol.114.091751. [DOI] [PubMed] [Google Scholar]
- Darrah JM, Stefani MR, Moghaddam B. Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behav Pharmacol. 2008;19:225–234. doi: 10.1097/FBP.0b013e3282feb0ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty AJ, Palmer MJ, Henley JM, Collingridge GL, Jane DE. (RS)-2-chloro-5-hydroxyphenylglycine (CHPG) activates mGlu5, but no mGlu1, receptors expressed in CHO cells and potentiates NMDA responses in the hippocampus. Neuropharmacology. 1997;36:265–267. doi: 10.1016/s0028-3908(97)00001-4. [DOI] [PubMed] [Google Scholar]
- Field JR, Walker AG, Conn PJ. Targeting glutamate synapses in schizophrenia. Trends Mol Med. 2011;17:689–698. doi: 10.1016/j.molmed.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzjohn SM, Irving AJ, Palmer MJ, Harvey J, Lodge D, Collingridge GL. Activation of group I mGluRs potentiates NMDA responses in rat hippocampal slices. Neurosci Lett. 1996;203:211–213. doi: 10.1016/0304-3940(96)12301-6. [DOI] [PubMed] [Google Scholar]
- Franca AS, do Nascimento GC, Lopes-Dos-Santos V, Muratori L, Ribeiro S, Lobao-Soares B, Tort AB. Beta2 oscillations (23-30 Hz) in the mouse hippocampus during novel object recognition. The European journal of neuroscience. 2014;40:3693–3703. doi: 10.1111/ejn.12739. [DOI] [PubMed] [Google Scholar]
- Gao C, Tronson NC, Radulovic J. Modulation of behavior by scaffolding proteins of the post-synaptic density. Neurobiol Learn Mem. 2013;105:3–12. doi: 10.1016/j.nlm.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastambide F, Gilmour G, Robbins TW, Tricklebank MD. The mGlu(5) positive allosteric modulator LSN2463359 differentially modulates motor, instrumental and cognitive effects of NMDA receptor antagonists in the rat. Neuropharmacology. 2012;64:240–247. doi: 10.1016/j.neuropharm.2012.07.039. [DOI] [PubMed] [Google Scholar]
- Gilmour G, Broad LM, Wafford KA, Britton T, Colvin EM, Fivush A, Gastambide F, Getman B, Heinz BA, McCarthy AP, et al. In vitro characterisation of the novel positive allosteric modulators of the mGlu(5) receptor, LSN2463359 and LSN2814617, and their effects on sleep architecture and operant responding in the rat. Neuropharmacology. 2012;64:224–239. doi: 10.1016/j.neuropharm.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Gregory KJ, Herman EJ, Ramsey AJ, Hammond AS, Byun NE, Stauffer SR, Manka JT, Jadhav S, Bridges TM, Weaver CD, et al. N-aryl piperazine metabotropic glutamate receptor 5 positive allosteric modulators possess efficacy in preclinical models of NMDA hypofunction and cognitive enhancement. J Pharmacol Exp Ther. 2013a;347:438–457. doi: 10.1124/jpet.113.206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory KJ, Nguyen ED, Reiff SD, Squire EF, Stauffer SR, Lindsley CW, Meiler J, Conn PJ. Probing the metabotropic glutamate receptor 5 (mGlu(5)) positive allosteric modulator (PAM) binding pocket: discovery of point mutations that engender a “molecular switch” in PAM pharmacology. Mol Pharmacol. 2013b;83:991–1006. doi: 10.1124/mol.112.083949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory KJ, Noetzel MJ, Rook JM, Vinson PN, Stauffer SR, Rodriguez AL, Emmitte KA, Zhou Y, Chun AC, Felts AS, et al. Investigating metabotropic glutamate receptor 5 allosteric modulator cooperativity, affinity, and agonism: enriching structure-function studies and structure-activity relationships. Mol Pharmacol. 2012;82:860–875. doi: 10.1124/mol.112.080531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond AS, Rodriguez AL, Townsend SD, Niswender CM, Gregory KJ, Lindsley CW, Conn PJ. Discovery of a Novel Chemical Class of mGlu(5) Allosteric Ligands with Distinct Modes of Pharmacology. ACS Chem Neurosci. 2010;1:702–716. doi: 10.1021/cn100051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EW, Ganong AH, Cotman CW. Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res. 1984;323:132–137. doi: 10.1016/0006-8993(84)90275-0. [DOI] [PubMed] [Google Scholar]
- Henry SA, Lehmann-Masten V, Gasparini F, Geyer MA, Markou A. The mGluR5 antagonist MPEP, but not the mGluR2/3 agonist LY314582, augments PCP effects on prepulse inhibition and locomotor activity. Neuropharmacology. 2002;43:1199–1209. doi: 10.1016/s0028-3908(02)00332-5. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B. Functional Interaction Between NMDA and mGlu5 Receptors: Effects on Working Memory, Instrumental Learning, Motor Behaviors, and Dopamine Release. Neuropsychopharmacology. 2004;29:1259–1269. doi: 10.1038/sj.npp.1300417. [DOI] [PubMed] [Google Scholar]
- Hu JH, Yang L, Kammermeier PJ, Moore CG, Brakeman PR, Tu J, Yu S, Petralia RS, Li Z, Zhang PW, et al. Preso1 dynamically regulates group I metabotropic glutamate receptors. Nat Neurosci. 2012;15:836–844. doi: 10.1038/nn.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Roder JC, Bear MF. Chemical induction of mGluR5- and protein synthesis--dependent long-term depression in hippocampal area CA1. J Neurophysiol. 2001;86:321–325. doi: 10.1152/jn.2001.86.1.321. [DOI] [PubMed] [Google Scholar]
- Jones CK, Bubser M, Thompson AD, Dickerson JW, Turle-Lorenzo N, Amalric M, Blobaum AL, Bridges TM, Morrison RD, Jadhav S, et al. The metabotropic glutamate receptor 4-positive allosteric modulator VU0364770 produces efficacy alone and in combination with L-DOPA or an adenosine 2A antagonist in preclinical rodent models of Parkinson's disease. J Pharmacol Exp Ther. 2012;340:404–421. doi: 10.1124/jpet.111.187443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney GG, Burno M, Campbell UC, Hernandez LM, Rodriguez D, Bristow LJ, Conn PJ. Metabotropic glutamate subtype 5 receptors modulate locomotor activity and sensorimotor gating in rodents. J Pharmacol Exp Ther. 2003;306:116–123. doi: 10.1124/jpet.103.048702. [DOI] [PubMed] [Google Scholar]
- Kinney GG, O'Brien JA, Lemaire W, Burno M, Bickel DJ, Clements MK, Chen TB, Wisnoski DD, Lindsley CW, Tiller PR, et al. A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychoticlike effects in rat behavioral models. J Pharmacol Exp Ther. 2005;313:199–206. doi: 10.1124/jpet.104.079244. [DOI] [PubMed] [Google Scholar]
- Lebois EP, Bridges TM, Lewis LM, Dawson ES, Kane AS, Xiang Z, Jadhav SB, Yin H, Kennedy JP, Meiler J, et al. Discovery and characterization of novel subtype-selective allosteric agonists for the investigation of M(1) receptor function in the central nervous system. ACS Chem Neurosci. 2009;1:104–121. doi: 10.1021/cn900003h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Grauer S, Kelley C, Navarra R, Graf R, Zhang G, Atkinson PJ, Popiolek M, Wantuch C, Khawaja X, et al. ADX47273 [S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]-piperidin-1- yl}-methanone]: a novel metabotropic glutamate receptor 5-selective positive allosteric modulator with preclinical antipsychotic-like and procognitive activities. J Pharmacol Exp Ther. 2008;327:827–839. doi: 10.1124/jpet.108.136580. [DOI] [PubMed] [Google Scholar]
- Liu F, Grauer S, Kelley C, Navarra R, Graf R, Zhang G, Atkinson PJ, Popiolek M, Wantuch C, Khawaja X, Smith D, Olsen M, Kouranova E, Lai M, Pruthi F, Pulicicchio C, Day M, Gilbert A, Pausch MH, Brandon NJ, Beyer CE, Comery TA, Logue S, Rosenzweig-Lipson S, Marquis KL. ADX47273 [S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]-piper idin-1-yl}-methanone]: a novel metabotropic glutamate receptor 5-selective positive allosteric modulator with preclinical antipsychotic-like and procognitive activities. J Pharmacol Exp Ther. 2008;327:827–839. doi: 10.1124/jpet.108.136580. [DOI] [PubMed] [Google Scholar]
- Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J Neurosci. 2001;21:5925–5934. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Holt W. The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behav Brain Res. 2000;110:97–108. doi: 10.1016/s0166-4328(99)00188-6. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Conn PJ. Direct and indirect modulation of the N-methyl D-aspartate receptor. Curr Drug Targets CNS Neurol Disord. 2002;1:1–16. doi: 10.2174/1568007023339544. [DOI] [PubMed] [Google Scholar]
- Matosin N, Newell KA. Metabotropic glutamate receptor 5 in the pathology and treatment of schizophrenia. Neurosci Biobehav Rev. 2013;37:256–268. doi: 10.1016/j.neubiorev.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Muller L, Tokay T, Porath K, Kohling R, Kirschstein T. Enhanced NMDA receptor-dependent LTP in the epileptic CA1 area via upregulation of NR2B. Neurobiol Dis. 2013;54:183–193. doi: 10.1016/j.nbd.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Johnson KA, Luo Q, Ayala JE, Kim C, Conn PJ, Weaver CD. A novel assay of Gi/o-linked G protein-coupled receptor coupling to potassium channels provides new insights into the pharmacology of the group III metabotropic glutamate receptors. Mol Pharmacol. 2008;73:1213–1224. doi: 10.1124/mol.107.041053. [DOI] [PubMed] [Google Scholar]
- Noetzel MJ, Gregory KJ, Vinson PN, Manka JT, Stauffer SR, Lindsley CW, Niswender CM, Xiang Z, Conn PJ. A novel metabotropic glutamate receptor 5 positive allosteric modulator acts at a unique site and confers stimulus bias to mGlu5 signaling. Mol Pharmacol. 2013;83:835–847. doi: 10.1124/mol.112.082891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noetzel MJ, Rook JM, Vinson PN, Cho HP, Days E, Zhou Y, Rodriguez AL, Lavreysen H, Stauffer SR, Niswender CM, et al. Functional impact of allosteric agonist activity of selective positive allosteric modulators of metabotropic glutamate receptor subtype 5 in regulating central nervous system function. Mol Pharmacol. 2012;81:120–133. doi: 10.1124/mol.111.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noetzel MJ, Rook JM, Vinson PN, Cho HP, Days E, Zhou Y, Rodriguez AL, Lavreysen H, Stauffer SR, Niswender CM, Xiang Z, Daniels JS, Jones CK, Lindsley CW, Weaver CD, Conn PJ. Functional impact of allosteric agonist activity of selective positive allosteric modulators of metabotropic glutamate receptor subtype 5 in regulating central nervous system function. Mol Pharmacol. 2012;81:120–133. doi: 10.1124/mol.111.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JA, Lemaire W, Wittmann M, Jacobson MA, Ha SN, Wisnoski DD, Lindsley CW, Schaffhauser HJ, Rowe B, Sur C, et al. A novel selective allosteric modulator potentiates the activity of native metabotropic glutamate receptor subtype 5 in rat forebrain. J Pharmacol Exp Ther. 2004;309:568–577. doi: 10.1124/jpet.103.061747. [DOI] [PubMed] [Google Scholar]
- Park JM, Hu JH, Milshteyn A, Zhang PW, Moore CG, Park S, Datko MC, Domingo RD, Reyes CM, Wang XJ, et al. A prolyl-isomerase mediates dopamine-dependent plasticity and cocaine motor sensitization. Cell. 2013;154:637–650. doi: 10.1016/j.cell.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier-Batteur S, Hutson PH, Menzel K, Uslaner JM, Mattson BA, O'Brien JA, Magliaro BC, Forest T, Stump CA, Tynebor RM, et al. Mechanism based neurotoxicity of mGlu5 positive allosteric modulators - Development challenges for a promising novel antipsychotic target. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Parmentier-Batteur S, O'Brien JA, Doran S, Nguyen SJ, Flick RB, Uslaner JM, Chen H, Finger EN, Williams TM, Jacobson MA, Hutson PH. Differential effects of the mGluR5 positive allosteric modulator CDPPB in the cortex and striatum following repeated administration. Neuropharmacology. 2012;62:1453–1460. doi: 10.1016/j.neuropharm.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Peavy RD, Sorensen SD, Conn PJ. Differential regulation of metabotropic glutamate receptor 5-mediated phosphoinositide hydrolysis and extracellular signal-regulated kinase responses by protein kinase C in cultured astrocytes. J Neurochem. 2002;83:110–118. doi: 10.1046/j.1471-4159.2002.01113.x. [DOI] [PubMed] [Google Scholar]
- Rammes G, Palmer M, Eder M, Dodt HU, Zieglgansberger W, Collingridge GL. Activation of mGlu receptors induces LTD without affecting postsynaptic sensitivity of CA1 neurons in rat hippocampal slices. J Physiol. 2003;546:455–460. doi: 10.1113/jphysiol.2002.033514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AL, Grier MD, Jones CK, Herman EJ, Kane AS, Smith RL, Williams R, Zhou Y, Marlo JE, Days EL, Blatt TN, Jadhav S, Menon UN, Vinson PN, Rook JM, Stauffer SR, Niswender CM, Lindsley CW, Weaver CD, Conn PJ. Discovery of novel allosteric modulators of metabotropic glutamate receptor subtype 5 reveals chemical and functional diversity and in vivo activity in rat behavioral models of anxiolytic and antipsychotic activity. Mol Pharmacol. 2010;78:1105–1123. doi: 10.1124/mol.110.067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook JM, Noetzel MJ, Pouliot WA, Bridges TM, Vinson PN, Cho HP, Zhou Y, Gogliotti RD, Manka JT, Gregory KJ, et al. Unique signaling profiles of positive allosteric modulators of metabotropic glutamate receptor subtype 5 determine differences in in vivo activity. Biol Psychiatry. 2012;73:501–509. doi: 10.1016/j.biopsych.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Albertson C, Slikker W., Jr Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- Sheffler DJ, Conn PJ. Allosteric potentiators of metabotropic glutamate receptor subtype 1a differentially modulate independent signaling pathways in baby hamster kidney cells. Neuropharmacology. 2008;55:419–427. doi: 10.1016/j.neuropharm.2008.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Stone TW, Burton NR. NMDA receptors and ligands in the vertebrate CNS. Prog Neurobiol. 1988;30:333–368. doi: 10.1016/0301-0082(88)90027-5. [DOI] [PubMed] [Google Scholar]
- Timms AE, Dorschner MO, Wechsler J, Choi KY, Kirkwood R, Girirajan S, Baker C, Eichler EE, Korvatska O, Roche KW, et al. Support for the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia from exome sequencing in multiplex families. JAMA Psychiatry. 2013;70:582–590. doi: 10.1001/jamapsychiatry.2013.1195. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Rodriguez AL, Conn PJ. Allosteric potentiators of metabotropic glutamate receptor subtype 5 have differential effects on different signaling pathways in cortical astrocytes. J Pharmacol Exp Ther. 2005;315:1212–1219. doi: 10.1124/jpet.105.090308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.