Abstract
Objectives: Investigation of osteoblastic responses to oxidative stress, induced by C-reactive protein (CRP) and IL-6 and ameliorating effects of doxycycline (Dox); using assays for 5-alpha dihydrotestosterone (DHT) as an antioxidant marker of healing. IL-6 and CRP are risk markers of periodontitis and prevalent comorbidities in periodontitis subjects. Methods: Confluent monolayer cultures of osteoblasts were incubated with radiolabelled testosterone (14C-T) as substrate, in the presence or absence (Control) of pre-determined optimal concentrations of CRP, IL-6, Dox; alone and in combination (n=8) for 24h in MEM. The eluent was solvent-extracted for steroid metabolites. They were separated using TLC in a benzene/ acetone solvent system 4:1 v/v; and quantified using radioisotope scanning. The identity of formed metabolites was confirmed using the mobility of cold standards added to the samples and disclosed in iodine. Further confirmation of the authenticity of DHT was carried out by combined gas chromatrography-mass spectrometry, after derivatization to pentafluorobenzyloxime trimethyl silyl ether. Results: The yields of DHT from 14C-testosterone showed 2-fold and 1.8-fold- inhibition in response to IL-6 and CRP respectively and 28% stimulation in response to Dox, via the 5-alpha reductase pathway. The combination of IL-6 + CRP showed a 2-fold reduction in the yields of DHT, elevated to control values when combined with Dox (n=8; p<0.001). Yields of 4-androstenedione showed an inverse relationship to those of DHT, in response to the agents tested, in keeping with the 17-beta hydroxysteroid dehydrogenase pathway. Conclusions: Inhibition of DHT synthesis in osteoblasts by IL-6 and CRP was overcome by doxycycline. Oxidative actions of IL-6 and CRP; and antioxidant actions of Dox are reinforced by the metabolic yields of DHT in response to agents tested. Using a novel metabolically active model allows closer extrapolation to in vivo conditions; in the context of adjunctive therapeutic applications for periodontitis and prevalent comorbidities.
Keywords: Antioxidant responses, CRP, doxycycline, IL-6, DHT and AR, osteoblasts, periodontitis and systemic comorbidities, redox healing, risk markers
1. Introduction
This study aims to elucidate responses of osteoblasts to oxidative stress, induced by C-reactive protein (CRP) and interleukin-6 (IL-6); and the effects of doxycycline, using 5α-dihydrotestosterone (DHT) as a metabolically active steroid marker of redox status. Antioxidant and pro-anabolic effects of doxycycline are investigated in our osteoblastic cell culture model, relevant to their effects on periodontal diseases and their management as adjunctive agents. The importance of controlling periodontal disease for prevention of tooth loss may have wider implications in diabetes and cardiovascular disease as a result of systemic inflammatory loading, associated with the risk markers investigated. Background relevant to the agents tested and justification for the study are addressed here.
1.1. Implications of IL-6 and CRP on Periodontitis and Systemic Inflammatory Diseases
The rationale for using IL-6 and CRP in our study includes demonstrable links between progressive periodontitis
and high levels of CRP and IL-6; and elevated hazard ratios for cardiovascular incidents compared with low responders. It is relevant that there is significant reduction in IL-6, CRP and other cardiovascular risk markers following periodontal therapy, thus reducing cardiovascular risk in refractory hypertensive patients [1]. IL-6 could also have a modulatory effect on host responses in type 1 diabetes mellitus (DM) subjects [2]. Key pathogenic mechanisms associated with progression of insulin resistance (IR) are impaired metabolism of free fatty acids, plasminogen activator inhibitor 1 (PAI-1); and markers of inflammation IL-6 and CRP, which play a role. Amongst diabetic and non-diabetic subjects with myocardial infarction (MI), CRP and IL-6 levels are elevated, particularly amongst diabetics with MI [3]. These strong correlations between free fatty acids and IL-6 demonstrate the significance of non-specific inflammation in the development of IR in MI subjects; inflammatory cytokines could be the cause of IR in MI; with implications for links with the progression of periodontitis fuelled by these agents.
In addition to being an important risk marker for cardiovascular disease, CRP contributes to the development of atherosclerosis. 17β-Oestradiol (E2) reduces in vitro expression of pro-inflammatory molecules in endothelial cells. E2 attenuates CRP-mediated inflammatory responses by modulating endogenous production of CRP in endothelial cells. E2 also reduces the most potent agonist of CRP production, IL-6 [4]. These findings demonstrate attenuation of pro-inflammatory effects of CRP by E2, via a rapid non-genomic pathway, of importance for vascular repair. It is relevant that 5α-dihydrotestosterone (DHT) acting via the androgen receptor has similar anti-inflammatory actions; used as a marker in our study. DHT decreases the expression of cyclooxygenase-2 in vascular smooth muscle cells during cytokine or hypoxic stimulation. Typically DHT is a pure AR agonist; however it can be metabolized to 5α-androstane-3β, 17β-diol (3β-diol), a selective oestrogen receptor (ERβ) agonist [5]. DHT attenuates IL-1β-induced increases in COX-2; some of these actions could be mediated via AR and ERβ, reinforcing an anti-inflammatory role for DHT.
Study of RNA extracted from CRP-stimulated pulmonary arterial endothelial cells indicates genes related to NFkB-mediated signal transduction. It is relevant that CRP-induced expression of ICAM-1on the endothelial cell surface is impaired by an inhibitor of the NFkB pathway; it also inhibits the secretion of IL-6 by CRP-stimulated endothelial cells [6]. These findings suggest an involvement of the NFkB pathway in mediating different effects of CRP in these cells.
IL-6 directly regulates inflammation, implicated in several chronic diseases, including periodontitis. A common non-synonymous variant in the IL-6 receptor gene is a risk marker of several common diseases; the 358Ala allele confers protection from coronary heart disease, rheumatoid arthritis and other related conditions. The effect of the variant on IL-6 signalling is not entirely clear. Although 358Ala increases transcription of the soluble IL-6R isoform and not the membrane-bound isoform, it reduces surface expression of IL-6R on CD4+ T cells and monocytes [7]. Reduced expression of membrane-bound IL-6R results in impaired IL-6 responsiveness. These findings which clarify the regulation of IL-6 by IL-6 receptor, causally linked to several complex diseases identify new means of targeting the IL-6/IL-6R axis which could result in diverse responses based on the IL-6R variant. Polymorphism of genes for cytokines IL-6, TNF-α and IL-10 were studied in Type 2 DM subjects and controls. Analysis of genotypic, allelic and carriage rate frequency distribution in subjects and controls, demonstrate that individuals with haplotype combinations of AA, GG and CA for IL-6, TNF-α and IL-10 gene polymorphisms, show a greater susceptibility and risk of developing type 2 DM [8].
Elevated circulating levels of IL-6 are associated with increased risk of coronary heart disease. IL-6R blockage with the monoclonal antibody tocilixumab reduces systemic and articular inflammation in rheumatoid arthritis subjects. Applying the mendelian randomization principle, single nucleotide polymorphisms (SNPs) in the IL-6R gene were evaluated to define the efficacy of IL-6R inhibition for the primary prevention of coronary heart disease, for comparison with the effects of monoclonal antibody inhibition in RA subjects [9]. Based on genetic evidence in humans, IL-6R signalling appears to have a causal role in CHD. IL6R blockade could provide a novel therapeutic approach for the prevention of CHD. Large-scale genetic biomarker data confirm a causal relationship between IL-6-related pathways and coronary heart disease [10].
These concepts are reinforced by findings that correlate elevated levels of hs-CRP in periodontitis with low-grade systemic inflammation; periodontitis being a potential source of biomarkers, of relevance to metabolic syndrome and associated diseases. An inflammatory phenotype could link susceptibility to periodontitis with CHD, DM and rheumatoid arthritis (RA). Our in vitro investigative model is designed to study redox reactions of relevance to these concepts, using appropriate markers.
1.2. Androgen Receptor (AR)-mediated Anti-inflammatory and Matrix Stimulatory Pathways
ARs play a key role in maintaining tissue matrices. The AR of mature osteoblasts is essential for the maintenance of trabecular bone mass [11] demonstrated in mouse osteocytes when compared with mutants [12]. Altered gene expression for androgen responsiveness in osteoblasts due to androgen deprivation is overcome with replacement [13]; resulting in upregulation of osteoblast genes and enhanced matrix synthesis. The study also identified genes involved in metabolism (adiponectin and Dpp4) and growth (Tgfb, Tgfb2, Wnt4) as targets for AR in mineralizing osteoblasts. These findings are significant in reinforcing the relevance of AR-mediated actions of DHT as a marker of healing in a redox environment, utilized in our study.
The androgen receptor (AR) is a member of the nuclear receptor superfamily of ligand-inducible nuclear transcription factors. Ski-interacting protein (SKIP; SNW1, NCOA62) is one of the cofactors known to interact with several NRs. Interaction with key accessory cofactors determines cell- and gene-specific regulation of the AR. SKIP is considered to link mRNA splicing with transcription, as part of the spliceosome. It is relevant that SKIP enhanced DHT-induced N-terminal/C-terminal AR interaction significantly. On DHT-stimulation, a rapid translocation of AR from cytoplasm to the nucleus was demonstrated [14], indicating the relevance of cofactors for AR activation. Similarly, agents with antioxidant and proanabolic actions such as doxycycline could also be proactive via AR, using DHT as a marker, in our investigation.
Metabolism of the androgen substrate 14C-testosterone via the 5α-reductase pathway results in the formation of DHT; it is an effective marker of oxidative stress and healing, via its antioxidant and matrix stimulatory effects. Androgen receptor (AR) proteins are directly activated by DHT. They play a significant role as redox regulators via direct actions on glutathione S-transferase [15]. H2O2 decreases the level of DHT, cell cycle regulatory proteins and cell viability in mouse stem cells, as a result of its apoptotic effects. These effects are prevented by pre-treatment with DHT which increases levels of the antioxidant enzyme catalase; and reversed by the androgen receptor inhibitor flutamide, a non-steroidal androgen antagonist which competes for the same receptors as T and DHT due to its structural similarity [16] Androgen receptor (AR) proteins directly activated by DHT play an important role as redox regulators via direct actions on glutathione S-transferase. DHT as an effective marker of oxidative stress [17], is suitable for this application in our in vitro cell culture model.
1.3. Matrix-stimulatory Actions of Doxycycline
Tetracyclines have excellent non-antimicrobial pro-anabolic and anti-catabolic actions which are effective in the adjunctive management of periodontitis and associated systemic disorders [18]. Unique actions of tetracyclines in an over-exuberant inflammatory environment make them effective therapeutic adjuncts in the management of chronic inflammatory disorders. Their beneficial actions are effective in the adjunctive management of periodontitis subjects presenting with commonly prevalent comorbidities addressed here. Novel applications of subantimicrobial doxycycline dosing (SDD) in attenuating periodontal and systemic bone loss have been reviewed in this context. SDD is effective in enzyme inhibitory and anti-inflammatory actions. Its effects on oxidative stress have been evaluated in ligature-induced periodontitis in rats; alveolar bone loss was significantly greater in periodontits rats when compared with those supplemented with SDD [19]. Gingival tissue levels of lipid peroxidation enzymes were significantly reduced by SDD, with increased total antioxidant capacity, reduced oxidative stress and oxidant status. It is evident that SDD is effective in attenuating periodontal destruction by reducing local and systemic oxidative stress. In a similar periodontitis model in diabetic rats, it was demonstrated that a combination of SDD and biphosphonate clodronate resulted in a significant reduction in the expression of MMP-9 and IL-1β in rat gingivae [20]; these expression levels are significantly elevated in periodontitis rats prior to treatment.
Doxycycline is an effective inhibitor of MMPs; these anti-protease effects counteract tissue destruction in periodontitis. It is an effective free radical scavenger and anti-inflammatory agent [21]. Systemic SDD as an adjunct to periodontal treatment results in a significant reduction in serum biomarkers of inflammation [22] including CRP [23]. In view of the significance of IL-6 and CRP in chronic inflammatory diseases and the anti-inflammatory role of doxycycline, it is relevant to study the effects of these agents alone and in combination in a cell culture model of human osteoblasts, using DHT as a marker of inflammation and oxidative stress; using a novel metabolically active system which would be closer to in vivo conditions.
2. Materials and methods
Authentic steroids were supplied by Sigma Chemicals Co (Poole, Dorset, UK). Steroids were solubilised and redistilled in ethanol (supplied by Merk Chemicals Ltd; Dagenham, Essex, UK) at suitable concentrations and stored. The radioisotope 14C-testosterone (specific activity 58μCi/μmol) was purchased from Amersham International (Amersham, Bucks, UK). The organic solvents used for thin layer chromatography were benzene and acetone, ethyl acetate for solvent extraction of metabolites and chloroform to dissolve the dried bulk of extracts; these reagents were obtained from BDH Chemicals (Merck) (Dagenham, Essex, UK). The thin layer chromatography (TLC) plates were purchased from BDH Chemicals Ltd (Merck); their specifications are: pre-coated silicagel kiesegel 60 (20 x 20cm), Dagenham, Essex, UK. IL-6, C-reactive protein and doxycycline were obtained from Sigma Chemicals Ltd., Fancy Road, Poole Dorset. Components used for the preparation of cell culture media were, Eagle’s Minimum Essential Medium (MEM), with 10% foetal bovine serum (FBS), L-glutamine (200mM), penicillin (5000 IU/ml) and streptomycin (5mg/ml); media components and cell culture plastics were purchased from Invitrogen Ltd; Scotland.
2.1. Cell-Cultures
Human osteoblasts were derived from a permanent cell line isolated from human osteosarcoma, called MG-63 [24]. They were provided by the UCL Eastman Dental Institute, London, UK.
2.2. Experimental Design
The contents of a fully confluent 25cm2 flask (2.2x106 cells) were distributed amongst 24 wells of a multiwell dish in Eagle’s MEM for each incubation of osteoblasts; the cells were fully confluent before setting up experiments. Incubations were performed with 14C-testosterone and monolayer cultures of confluent cells, in the presence or absence of each testing agent; androgen metabolites were analysed for each incubation, for comparison with controls, in the absence of testing agents.
2.3. Establishing Optimal Concentrations of C-reactive Protein (CRP), IL-6 and Doxycycline (Dox) on the Metabolic Conversion of 14C-testosterone by Cultured Osteoblasts
In order to investigate the effects of agents tested, optimal effective concentrations were established, to be used in further experiments.
Three 24-well plates were prepared with serial concentrations of CRP: 1, 2, 5, 10 and 20 μg/ml; IL-6: 0.1, 0.5, 1, 5 and 10 ng/ml; doxycycline (Dox): 1, 5, 10, 15 and 20 μg/ml; and control incubations which did not contain testing agents. 4 replicates were used for each agent with individual controls for each of the three agents tested.
2.4. Effects of Optimal Concentrations of CRP, IL-6 and Doxycycline (Dox), Alone and in Combinations of CRP+ IL-6 and CRP+IL-6+Dox on the Metabolism of 14C-T by Cultured Osteoblasts
For each incubation eight replicates were done, using two 24-well plates with the optimal concentrations of CRP (10 μg/ml), IL-6 (1ng/ml) and doxycycline (10μg/ml) previously determined, alone and in combinations of CRP+IL-6 and CRP+IL-6+Dox; this was compared with controls which did not contain testing agents.
2.5. Detection and Quantification of Radioactive Steroid Metabolites
The 24-well multiwell plates were incubated for 24 hours in a CO2 humidified cell-culture incubator at 37ºC. This was established previously as the optimum incubation period (unpublished observations), as demonstrated here to establish the trends shown. Ethyl acetate was used for solvent extraction of the medium. The solvent extracts were evaporated until dry in a vortex evaporator (Gyrovap; Philip Harris House, London, UK). The formed metabolites were separated by thin layer chromatography (TLC). The TLC plates were run in a solvent system comprising benzene/acetone (4:1 v/v). The separated metabolites on the TLC plates were scanned and quantified using a radioisotope scanner linked to a computer.
The mobility of cold standards added to the samples, was used to confirm the identity of formed metabolites; by disclosing the TLC plates in iodine. The authenticity of DHT was further confirmed by combined gas chromatography-mass spectrometry (g.c-m.s; courtesy of Prof. A.I. Mallet, St. Thomas’ Hospital, London, UK), after derivatisation to pentafluorobenzyloxime trimethyl silyl ether (PFBO/TMS). The mass spectral fragmentation pattern of authentic PFBO/TMS ether of 5α-DHT was replicated in the derivatized biological material which had a molecular ion (557), but at lower levels, due to smaller amounts of steroid. There were characteristic ions noted, for example at m/z values of 542 [M-15]+ due to loss of a methyl group; 467 [M-90]+ due to loss of TMS ether; 452 [M-90-15]+ due to loss of TMS ether and a methyl group and at an m/z value of 360, due to loss of the pentafluorobenzyloxime group. Mechanisms involved in the staged ionic fragmentation pattern have been published previously, with the fragmentation pattern of ions represented in graphic form [25].
2.6. Statistical Analysis
Duplicate incubations of 4 or 8 sets of human osteoblasts were used, to obtain mean values for each of the metabolites isolated (n=4; n=8). Individual incubations were set up and analysed for each experimental replicate; there was no pooling of cells. For each experimental set-up, the control incubation which contained no testing agents served as the comparison for test incubations containing different agents. Standard deviations from the mean values are shown in the figures. One-way ANOVA was used for significance testing. It is used for testing differences between groups of data. It compares the means between groups of data and determines whether the means are significantly different from each other. It is considered to be a robust test against the normality assumption, particularly when p values are low. However, individual t rests between controls and agents/combinations tested were done as post hoc tests to validate significance of results tested. The observed variance in a particular variable is partitioned into components attributable to different sources of variation, in the context of a range of concentrations used, for comparison with controls (C); and for comparison of C with incubations of optimal concentrations of CRP, IL-6, Dox and their combinations.
3. Results
3.1. Establishing Effective Concentrations of CRP; Yields of DHT (pmol) in Response to Serial Concentrations of C-reactive Protein (μg/ml), using 14C-testosterone as Substrate (Fig.1)
Fig. (1).
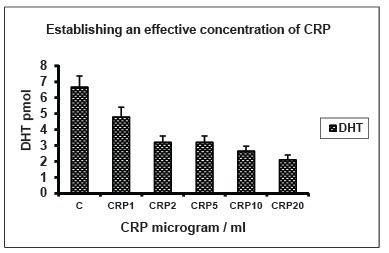
Establishing effective concentrations of CRP; Yields of DHT in response to serial concentrations of C-reactive protein (μg/ml), using 14C-testosterone as substrate. Yields of DHT (per well; 2.2x106 cells / 24 well- multiwell plate) isolated from monolayer cultures of osteoblasts in MEM are shown in response to serial concentrations of C-reactive protein (CRP) of 1, 2, 5, 10 and 20μg/ml, in order to determine its optimal concentration. C: Control; n=4, p<0.01. Standard deviations of means are shown for all figures and one-way ANOVA for significance testing. Yields of DHT / 4-A are per well in all figures.
When serial concentrations of C-reactive protein at 1, 2, 5, 10 and 20 μg/ml were incubated with 14C-T, it was metabolised to DHT, 4-androstenedione and diol. Yields of DHT are shown here as the main biologically active metabolite. Yields of diol and 4-A showed a direct or inverse relationship with that of DHT in keeping with enzymic pathways for their metabolism. There was progressive reduction in the yields of DHT, in response to the serial concentrations of CRP tested, from 1.4- fold to 3-fold (n=4; p<0.01). An optimal concentration of 10 μg/ml was established and used for subsequent experiments.
3.2. Establishing Effective Concentrations of IL-6; Yield of DHT (pmol) in Response to Serial Concentrations of IL-6 (ng/ml), Using 14C-testosterone as Substrate (Fig. 2)
Fig. (2).
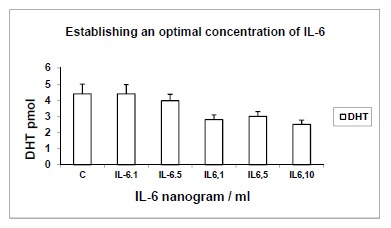
Establishing effective concentrations of IL-6; Yield of DHT (pmol) in response to serial concentrations of IL-6 (ng/ml), using 14C-testosterone as substrate. Yields of DHT are shown in response to serial concentrations of IL-6 of 0.1, 0.5, 1, 5 and 10 ng/ml, in order to determine its optimal concentration. C: Control; n=4, p<0.01.
When serial concentrations of IL-6 at 0.1, 0.5, 1, 5 and 10 ng/ml were incubated with 14C-T, it was metabolised to DHT, 4-A and diol. Yields of DHT are shown here as the main biologically active metabolite; the latter showed inverse and direct correlations respectively with the yield of DHT, in keeping with enzymic pathways. There was progressive reduction of 1.6-1.7-fold in the yields of DHT, compared with controls (duplicates of n=4; p<0.01), in response to IL-6. A concentration of IL-6 at 1ng/ml was established as the optimum concentration, which was used for subsequent experiments.
3.3. Establishing Effective Concentrations of Doxycycline (Dox); Yields of DHT (pmol) in Response to Serial Concentrations of Doxycycline (μg/ml), Using 14C-testosterone as Substrate (Fig. 3)
Fig. (3).
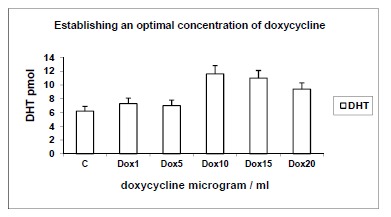
Establishing effective concentrations of doxycycline (Dox); Yields of DHT (pmol) in response to serial concentrations of doxycycline (μg/ml), using 14C-testosterone as substrate. Yields of DHT are shown in response to serial concentrations of doxycycline (Dox) of 1, 5, 10, 15 and 20μg/ml, in order to determine its optimal concentration. C: Control; n=4, p<0.01.
When serial concentrations of doxycycline (Dox) at 1, 5, 10, 15 and 20 μg/ml were incubated with 14C-T, it was metabolised to DHT, 4-A and diol. Yields of DHT are shown here; 4-A and diol showed inverse and direct correlations with yields of DHT, in keeping with 17β-hydroxysteroid dehydrogenase and 5α-reductase pathways respectively. There was a progressive increase in the yields of DHT in response to Dox from 1.2-fold to 1.8-fold (duplicates of n=4; p<0.01). An effective concentration of 10μg/ml was demonstrated to yield optimal amounts of DHT. This concentration was used for subsequent experiments.
3.4. Effects of CRP (10 μg/ml), IL-6 (1ng/ml) and Dox (10 μg/ml) Alone and in Combination on Yields of DHT (pmol) from 14C-testosterone as Substrate (Fig. 4)
Fig. (4).
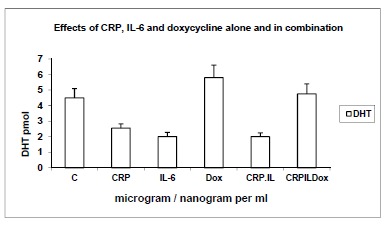
Effects of CRP (10μg/ml), IL-6 (1ng/ml) and Dox (10 μg/ml) alone and in combination on yields of DHT (pmol) from 14C-testosterone as substrate. Yields of DHT are shown in response to optimal concentrations of CRP (10μg/ml), IL-6 (1ng/ml) and doxycycline (Dox 10μg/ml) established above, alone and in combinations of CRP+IL-6, CRP+IL-6 and CRP+IL-6+Dox. C: Control; n=8, p<0.001.
Using 14C-testosterone as substrate, the effects of optimal concentrations of CRP, IL-6 and Dox as established above, were used alone and in combination to determine yields of the oxidative stress marker DHT. There were 1.8-fold and 2-fold decreases in yields of DHT, in response to CRP and IL-6 respectively and 28% stimulation in response to Dox, over control incubations. The combination of IL-6 + CRP showed a 2-fold reduction in the yields of DHT which was elevated to control values when combined with Dox, in the combined incubation of CRP+IL6+Dox (comparisons were significant, n=8; p<0.001; one way ANOVA). Yields of diol (not shown) showed similar trends in keeping with the 5α-reductase pathway, while those of 4-androstenedione are discussed below.
3.5. Effects of CRP (10 μg/ml), IL-6 (1ng/ml) and Dox (10 μg/ml) Alone and in Combination on Yields of 4-androstenedione (Fig.5)
Fig. (5).
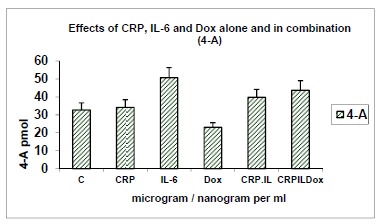
Effects of CRP (10μg/ml), IL-6 (1ng/ml) and Dox (10 μg/ml) alone and in combination on yields of 4-androstenedione (pmol) from 14C-testosterone as substrate. Yields of another metabolite 4-androstenedione (4-A) are shown in response to optimal concentrations of CRP, IL-6 and doxycycline (Dox) alone and in combinations of CRP+IL-6, CRP+IL-6 and CRP+IL-6+Dox. C: Control; n=8, p<0.001.
In the above incubation the metabolite 4-androstenedione was also isolated and quantified in response to the agents tested, alone and in combination. 4-A is formed from 14C-testosterone as substrate, via the 17β-hydroxysteroid dehydrogenase pathway (HSD), resulting in yields that are inversely related to those of DHT which is formed via the 5α-reductase enzyme system. In keeping with the 17β-HSD pathway, it is relevant that the yields of 4-A were increased over control values in response to CRP, IL-6 and their combinations; and significantly reduced in response to doxycycline (n=8; p<0.001).
4. Summary of results
IL-6 and CRP showed significant inhibitory effects on the synthesis of DHT. Concurrent incubation with doxycycline overcame these effects, reverting to control values. Yields of the weaker androgen 4-androstenedione showed inverse correlations with those of DHT, in keeping with enzymic pathways.
5. Discussion
When human MG63 osteoblasts were incubated with 14C-testosterone, it was metabolised mainly to DHT, 4-androstenedione and diol. Yields of 4-androstenedione bore an inverse relationship to those of DHT, in keeping with the 17β-HSD pathway from the substrate 14C-testosterone; while it is converted to DHT via the 5α-reductase pathway. The formed DHT acts as substrate for subsequent conversion to diol. IL-6 and CRP had significant inhibitory effects on the synthesis of DHT, an effective marker of inflammation [5] and oxidative stress [15-17]. Concurrent incubation with doxycycline, which has anti-inflammatory and pro-anabolic actions, overcame these effects to a significantly greater extent than incubation with doxycycline alone; demonstrating effective applications for adjunctive doxycycline in an inflammatory milieu. The significance of these findings in the context of markers and agents used, is discussed with regard to implications for systemic inflammatory diseases with IL-6 and CRP as risk markers [2, 3, 8, 10], as seen in periodontitis; and responses to doxycycline, using DHT as an effective marker of oxidative stress and its anti-inflammatory actions [5].
The role of DHT as a marker of inflammation has been shown by other workers, in reducing levels of nitric oxide (NO) and TNF-α, in a dose-dependent manner [26]. In the same study, the anti-inflammatory actions of DHT were significantly reduced in APOE4 targeted replacement mice when compared with APOE3 mice, due to androgen-regulated innate immune signalling pathways being altered in APOE4 microglia. These findings highlight the importance of genetic susceptibility on the outcome of inflammation. DHT could play an important role as a marker in this context. In our study, DHT was an effective marker of the antioxidant effects of doxycycline which overcame the oxidative effects of IL-6 and CRP as demonstrated by reduced yields of DHT in response to these agents, overcome by doxycycline. These actions are mediated via AR [11-13]. Agents tested are of significance in the progression of periodontitis, also relevant to cardiometabolic disorders [2, 3, 8, 10]; and serve as a useful tool in the context of our study, relevant to disease progression and response to treatment.
A 46% reduction in hs-CRP levels and a 32% reduction in IL-6 levels have been demonstrated in subjects, at six months of treatment with sub-antimicrobial doxycycline (SDD) [27]. The potential of a therapeutic agent with the ability to reduce CRP, IL-6 and MMPs has important clinical implications; considering that over 80% were also on simvastatin which has anti-inflammatory effects. In our in vitro study, validation of the inhibitory effects of IL-6 and CRP on yields of DHT, overcome and enhanced by doxycycline [17] is significant, considering the antioxidant and proanabolic actions of DHT [14]; SKIP, an interactive protein cofactor enhances DHT-induced AR activity. Similarly doxycycline could act as a cofactor via AR, relevant to the adjunctive management of periodontitis, with beneficial implications on prevalent comorbidities. These applications are bourne out in the 2-fold reduction in yields of DHT in response to a combination of IL-6 and CRP, increasing to control values when doxycycline was added to the incubation, effectively demonstrating a two-fold increase in response to doxycycline in the combined incubation, in our study. SDD as an adjunct to conventional periodontal treatment significantly improves HbA1c levels in diabetic subjects on stable medication, when compared with periodontal treatment alone [28]. This is a relevant finding in a population using prescribed medication for DM; thus SDD further improves oxidative stress via host-modulatory mechanisms [22, 23].
We have demonstrated the efficacy of doxycycline in overcoming oxidative stress induced by IL-6 and CRP using the metabolic marker DHT [29] with anabolic potential [11-13] acting via androgen receptor, in an in vitro culture of osteoblasts. A Cochrane review confirmed the effects of doxycycline in slowing down cartilage degeneration relevant to its actions as a disease modifying agent for the treatment of osteoarthritis [30]. We have used a suitable marker DHT in our study to validate anabolic and antioxidant actions of doxycycline, relevant to the above systemic disorders presenting as comorbidities of periodontitis. These actions of DHT against cytokines and inflammatory stimuli exerted via androgen receptor make it a valuable marker in our experimental inflammatory model comprising IL-6 and CRP. The yields of 4-androstenedione, a weaker androgen were inversely related to those of DHT in response to the above oxidants and the antioxidant effects of doxycycline. This mimics the in vivo 17β-hydroxysteroid dehydrogenase enzymic pathway, a reversible pathway between testosterone and 4-androstenedione. Distinct actions of 5α-reductase and 17β-hydroxysteroid dehydrogenase enzyme systems are reproduced, with implications on metabolic processes reported. The novel metabolically active in vitro model used is effective in reinforcing potential in vivo actions which may be cautiously interpreted from the pattern of metabolic yields in response to agents tested; and their implications that are addressed here.
These inflammatory markers are significantly reduced following periodontal treatment, reducing risk of cardiovascular incidents in subjects with refractory hypertension [1], and has implications on the host response in DM [2]. Multi-faceted susceptibility profiles and epigenetic effects make it difficult to apply generalizations universally. In our in vitro model, oxidative effects of IL-6 and CRP were overcome by the antioxidant effects of doxycycline, using DHT as a marker of oxidative stress. Some of these actions could be applied to in vivo clinical presentations of metabolic syndrome and DM as comorbidities in periodontitis subjects; and responses to adjunctive treatment with doxycycline. In the context of inflammatory diseases mediated by cytokines and acute phase reactions [31], clinically successful periodontal therapy impacts on improved outcome for systemic comorbidities by reducing serum levels of cytokines and systemic inflammation. This has been demonstrated with regard to circulating levels of hs-CRP, fibrinogen, interleukins-4, 6, 8, 10 and TNF-α associated with metabolic control in Type 2 DM patients with periodontitis [32]. These reports confirm the importance of risk markers such as IL-6 and CRP, utilised in this context in our experimental model; demonstrating 2- and 1.8 -fold reductions in the yields of DHT in response to IL-6 and CRP respectively.
The above markers are also common to other chronic inflammatory diseases such as RA [33] which prevails in periodontitis subjects. In synovial cells that over-express AR, DHT also inhibits the effects of TNF-α [34], implying that an optimally functional level of AR is required for this inhibition to occur. The actions of DHT in inhibiting IL-1α mRNA expression induced by TNF-α are overcome by the AR antagonist hydroxyflutamide, indicating a mechanism via AR; NF-kappaB-activation induced by TNF-α is inhibited by DHT via AR [35]. These findings validate the relevance of DHT as an effective marker of inflammatory networks operating in cells, also demonstrated in our study; which may be cautiously extrapolated to the in vivo mechanisms involved in periodontitis and associated co-morbidities, pivotal to their progression.
Oxidative stress and impaired antioxidant defences are characteristic features of chronic inflammatory diseases. CRP and IL-6, agents used in our experimental model imposed oxidative stress; demonstrating reduced yields of DHT alone and in combination, while co-incubation with doxycycline, resulted in improved yields, overcoming the diminished response to CRP and IL-6; reinforcing its capacity as an antioxidant and proanabolic agent. The efficacy of doxycycline as an anti-inflammatory agent is bourne out in our investigation. Our findings have applications in the adjunctive management of periodontitis, also relevant to curtailing chronic inflammatory loading, associated with IL-6 and CRP [36]; further substantiated in the restoration of free and total protein thiol levels in experimental diabetes, following combined treatment with doxycycline [37]. These actions substantiate the suitability of doxycycline in the context of our experimental model, with potential in vivo applications.
Doxycycline decreases MMP activity and oxidative stress induced by hypertension, with improved NO levels in aortic endothelial cells [38, 39]; with potential therapeutic applications. In this context, it is relevant that DHT enhances endothelial NO, due to due to rapid recruitment of extracellular signal-related kinase and the phosphotidylinositol 3-OH kinase / Akt cascades leading to phosphorylation of endothelial nitric oxide synthase [40]. Doxycycline and related non-antibiotic chemically modified tetracyclines such as CMT-3, are effective in reducing the production of cytokines and other inflammatory agents, in response to stimulants relevant to the pathogenesis of periodontitis and atherosclerotic cardiovascular disease [41]. The mechanism is partly due to suppression of phosphorylation/activation of the NFkB cell signalling pathway. SDD dramatically reduces hsCRP, IL-6 and MMP-9 levels in plasma of this patient population. It also causes significant elevation of serum levels of HDL cholesterol and its core molecule apolipoprotein A-1 which are cardioprotective, in periodontitis subjects susceptible to CHD. Tetracyclines including doxycycline are very effective in inhibiting chemiluminescence from an oxygen generating system [42], indicating their direct actions in influencing redox chemistry in aprotic media. Considering these actions, responses to doxycycline in our in vitro study provide scope for extrapolation to periodontitis and associated comorbidities such as DM, CHD, and arthritis in periodontitis subjects, addressed here. Actions of agents used in our investigation with DHT as a robust marker of inflammation and oxidative stress, provide some insight into the possible mechanisms involved.
The results of our study confirm these concepts using DHT as a marker of oxidative stress in our in vitro model, further substantiated by other workers. Our in vitro investigative model may be extrapolated to redox interactions in the context of periodontal and systemic co-morbidities, in the in vivo environment, with an adjunctive therapeutic role for doxycycline as discussed above.
6. Conclusions
A novel in vitro metabolically active model is used to reinforce potential for extrapolation to in vivo mechanisms associated with oxidant / antioxidant mechanisms relevant to periodontitis and associated systemic comorbidities. In view of current in vitro and in vivo documentation of oxidative stress-inducing mechanisms in response to IL-6 and CRP; and significant anti-inflammatory and proanabolic actions of doxycycline, this is a pertinent experimental model.
The study addresses the mechanism of action of doxycycline in an osteoblastic culture, using DHT as a marker of oxidative stress, inflammation and healing, in response to IL-6 and CRP, in the above context.
In this in vitro model, redox mechanisms exerted via AR in response to agents tested, using DHT as a marker of redox status and healing, may be cautiously extrapolated to the redox status in periodontitis, also relevant to systemic inflammatory diseases.
Adjunctive doxycycline plays a significant role in improving redox gradients in periodontal treatment. This could be extrapolated as a modifying factor for systemic diseases, considering common risk markers studied; patient susceptibility profiles and epigenetics make generalizations difficult to apply universally, although there are plausible mechanisms involved.
Acknowledgements
The authors wish to acknowledge Paula Coward for her assistance in the laboratory.
CONFLICT OF INTEREST
There is no conflict of interest associated with the contents.
REFERENCES
- 1.Vidal F., Cordovil I., Figueredo C.M., Fischer R.G. Non-surgical periodontal treatment reduces cardiovascular risk in refractory hypertensive patients: a pilot study. J. Clin. Periodontol. 2013;40(7):681–687. doi: 10.1111/jcpe.12110. [DOI] [PubMed] [Google Scholar]
- 2.Passoja A., Knuuttila M., Hiltunen L., Karttunen R., Niemelä O., Raunio T., Vainio O., Hedberg P., Tervonen T. Serum interleukin-6 may modulate periodontal inflammation in type 1 diabetic subjects. J. Clin. Periodontol. 2011;38(8):687–693. doi: 10.1111/j.1600-051X.2011.01731.x. [DOI] [PubMed] [Google Scholar]
- 3.Gruzdeva O., Uchasova E., Dyleva Y., Belik E., Shurygina E., Barbarash O. Insulin resistance and inflammation markers in myocardial infarction. J. Inflamm. Res. 2013;6:83–90. doi: 10.2147/JIR.S43081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cossette É., Cloutier I., Tardif K., DonPierre G., Tanguay J.F. Estradiol inhibits vascular endothelial cells pro-inflammatory activation induced by C-reactive protein. Mol. Cell. Biochem. 2013;373(1-2):137–147. doi: 10.1007/s11010-012-1482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuloaga K.L., O'Connor D.T., Handa R.J., Gonzales R.J. Estrogen receptor beta dependent attenuation of cytokine-induced cyclooxygenase-2 by androgens in human brain vascular smooth muscle cells and rat mesenteric arteries. Steroids. 2012;77(8-9):835–844. doi: 10.1016/j.steroids.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wynants M., Vengethasamy L., Ronisz A., Meyns B., Delcroix M., Quarck R.N. κB pathway is involved in CRP-induced effects on pulmonary arterial endothelial cells in chronic thromboembolic pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013;305(12):L934–L942. doi: 10.1152/ajplung.00034.2013. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira R.C., Freitag D.F., Cutler A.J., Howson J.M., Rainbow D.B., Smyth D.J., Kaptoge S., Clarke P., Boreham C., Coulson R.M., Pekalski M.L., Chen W.M., Onengut-Gumuscu S., Rich S.S., Butterworth A.S., Malarstig A., Danesh J., Todd J.A. Functional IL6R 358Ala allele impairs classical IL-6 receptor signalling and influences risk of diverse inflammatory diseases. PLoS Genet. 2013;9(4):e1003444. doi: 10.1371/journal.pgen.1003444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saxena M., Srivastava N., Banerjee M. Association of IL-6, TNF-α and IL-10 gene polymorphisms with type 2 diabetes mellitus. Mol. Biol. Rep. 2013;40(11):6271–6279. doi: 10.1007/s11033-013-2739-4. [DOI] [PubMed] [Google Scholar]
- 9.Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium, Hingorani, A.D., Casas, J.P. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379(9822):1214–1224. doi: 10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN, Gao P, Saleheen D, Rendon A, Nelson CP, Braund PS, Hall AS, Chasman DI, Tybjærg-Hansen A, Chambers JC, Benjamin EJ, Franks PW, Clarke R, Wilde AA, Trip MD, Steri M, Witteman JC, Qi L, van der Schoot CE, de Faire U, Erdmann J, Stringham HM, Koenig W, Rader DJ, Melzer D, Reich D, Psaty BM, Kleber ME, Panagiotakos DB, Willeit J, Wennberg P, Woodward M, Adamovic S, Rimm EB, Meade TW, Gillum RF, Shaffer JA, Hofman A, Onat A, Sundström J, Wassertheil-Smoller S, Mellström D, Gallacher J, Cushman M, Tracy RP, Kauhanen J, Karlsson M, Salonen JT, Wilhelmsen L, Amouyel P, Cantin B, Best LG, Ben-Shlomo Y, Manson JE, Davey-Smith G, de Bakker PI, O'Donnell CJ, Wilson JF, Wilson AG, Assimes TL, Jansson JO, Ohlsson C, Tivesten , Ljunggren , Reilly MP, Hamsten A, Ingelsson E, Cambien F, Hung J, Thomas GN, Boehnke M, Schunkert H, Asselbergs FW, Kastelein JJ, Gudnason V, Salomaa V, Harris TB, Kooner JS, Allin KH, Nordestgaard BG, Hopewell JC, Goodall AH, Ridker PM, Hólm H, Watkins H, Ouwehand WH, Samani NJ, Kaptoge S, Di Angelantonio E, Harari O, Danesh J. IL6R Genetics Consortium Emerging Risk Factors Collaboration, Interleukin-6 receptor pathways in coronary heart disease a collaborative meta-analysis of 82 studies. Lancet. 2012;379(9822): 1205–13. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manolagas S.C., O'Brien C.A., Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat. Rev. Endocrinol. 2013;9(12):699–712. doi: 10.1038/nrendo.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinnesael M., Claessens F., Laurent M., Dubois V., Boonen S., Deboel L., Vanderschueren D. Androgen receptor (AR) in osteocytes is important for the maintenance of male skeletal integrity: evidence from targeted AR disruption in mouse osteocytes. J. Bone Miner. Res. 2012;27(12):2535–2543. doi: 10.1002/jbmr.1713. [DOI] [PubMed] [Google Scholar]
- 13.Russell P.K., Clarke M.V., Skinner J.P., Pang T.P., Zajac J.D., Davey R.A. Identification of gene pathways altered by deletion of the androgen receptor specifically in mineralizing osteoblasts and osteocytes in mice. J. Mol. Endocrinol. 2012;49(1):1–10. doi: 10.1530/JME-12-0014. [DOI] [PubMed] [Google Scholar]
- 14.Abankwa D., Millard S.M., Martel N., Choong C.S., Yang M., Butler L.M., Buchanan G., Tilley W.D. Ueki, N.; Michael J Hayman, M.J.; Leong, G.M. Ski-interacting protein (SKIP) interacts with androgen receptor in the nucleus and modulates androgen-dependent transcription. BMC Biochem. 2013;14:10. doi: 10.1186/1471-2091-14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imperlini E., Mancini A., Spaziani S., Martone D., Alfieri A., Gemei M., del Vecchio L., Buono P., Orru S. Androgen receptor signalling induced by supra-physiological doses of dihydrotestosterone in human peripheral blood lymphocytes. Proteomics. 2010;10(17):3165–3175. doi: 10.1002/pmic.201000079. [DOI] [PubMed] [Google Scholar]
- 16.Lee S.H., Heo J.S., Lee M.Y., Han H.J. Effect of dihydrotestosterone on hydrogen peroxide-induced apoptosis of mouse embryonic stem cells. J. Cell. Physiol. 2008;216(1):269–275. doi: 10.1002/jcp.21402. [DOI] [PubMed] [Google Scholar]
- 17.Tinti F., Soory M. Oxidative actions of hydrogen peroxide in human gingival and oral periosteal fibroblasts: Responses to glutathione and nicotine, relevant to healing in a redox environment. Redox Biol. 2014;2:36–43. doi: 10.1016/j.redox.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tilakaratne A., Soory M. Anti-inflammatory actions of adjunctive tetracyclines and other agents in periodontitis and associated comorbidities. Open Dent. J. 2014;8:109–124. doi: 10.2174/1874210601408010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yağan A., Kesim S., Liman N. Effect of low-dose doxycycline on serum oxidative status, gingival antioxidant levels and alveolar bone loss in experimental periodontitis in rats. J. Periodontol. 2014;85(3):478–489. doi: 10.1902/jop.2013.130138. [DOI] [PubMed] [Google Scholar]
- 20.Ozdemir S.P., Kurtiş B., Tüter G., Bozkurt Ş., Gültekin S.E., Sengüven B., Watanabe K., Aydın S. Effects of low-dose doxycycline and bisphosphonate clodronate on alveolar bone loss and gingival levels of matrix metalloproteinase-9 and interleukin-1β in rats with diabetes: a histomorphometric and immunohistochemical study. J. Periodontol. 2012;83(9):1172–1182. doi: 10.1902/jop.2012.110459. [DOI] [PubMed] [Google Scholar]
- 21.Griffin M.O., Fricovsky E., Ceballos G., Villarreal F. Tetracyclines: a pleiotropic family of compounds with promising therapeutic properties. Review of the literature. Am. J. Physiol. Cell Physiol. 2010;299(3):C539–C548. doi: 10.1152/ajpcell.00047.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Payne J.B., Golub L.M., Stoner J.A., Lee H.M., Reinhardt R.A., Sorsa T., Slepian M.J. The effect of subantimicrobial-dose-doxycycline periodontal therapy on serum biomarkers of systemic inflammation: a randomized, double-masked, placebo-controlled clinical trial. J. Am. Dent. Assoc. 2011;142(3):262–273. doi: 10.14219/jada.archive.2011.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koppikar R.S., Agrawal S.V. The effect of sub-antimicrobial dose-doxycycline periodontal therapy on serum inflammatory biomarker C-reactive protein levels in post-menopausal Women: A 2-year, double-blinded, randomized clinical trial. Contemp. Clin. Dent. 2013;4(1):71–73. doi: 10.4103/0976-237X.111628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sollazzo V., Pezzetti F., Massari L., Palmieri A., Brunelli G., Zollino I., Lucchese A., Caruso G., Carinci F. Evaluation of gene expression in MG63 human osteoblast-like cells exposed to tantalum powder by microarray technology. Int. J. Periodontics Restorative Dent. 2011;31(4):e17–e28. [PubMed] [Google Scholar]
- 25.Soory M. Bacterial steroidogenesis by periodontal pathogens and the effect of bacterial enzymes on steroid conversions by human gingival fibroblasts in culture. J. Periodontal Res. 1995;30(2):124–131. doi: 10.1111/j.1600-0765.1995.tb01261.x. [DOI] [PubMed] [Google Scholar]
- 26.Brown C.M., Xu Q., Okhubo N., Vitek M.P., Colton C.A. Androgen-mediated immune function is altered by the apolipoprotein E gene. Endocrinology. 2007;148(7):3383–3390. doi: 10.1210/en.2006-1200. [DOI] [PubMed] [Google Scholar]
- 27.Brown D.L., Desai K.K., Vakili B.A., Nouneh C., Lee H.M., Golub L.M. Clinical and biochemical results of the metalloproteinase inhibition with subantimicrobial doses of doxycycline to prevent acute coronary syndromes (MIDAS) pilot trial. Arterioscler. Thromb. Vasc. Biol. 2004;24(4):733–738. doi: 10.1161/01.ATV.0000121571.78696.dc. [DOI] [PubMed] [Google Scholar]
- 28.Engebretson S.P., Hey-Hadavi J. Sub-antimicrobial doxycycline for periodontitis reduces hemoglobin A1c in subjects with type 2 diabetes: a pilot study. Pharmacol. Res. 2011;64(6):624–629. doi: 10.1016/j.phrs.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu Y., Tanaka T., Nawata H., Yanase T. Dihydrotestosterone inhibits lectin-like oxidized-LDL receptor-1 expression in aortic endothelial cells via a NF-κB/AP-1-mediated mechanism. Endocrinology. 2012;153(7):3405–3415. doi: 10.1210/en.2011-1993. [DOI] [PubMed] [Google Scholar]
- 30.da Costa B.R., Nüesch E., Reichenbach S., Jüni P., Rutjes A.W. Doxycycline for osteoarthritis of the knee or hip. 2012. [DOI] [PMC free article] [PubMed]
- 31.Soory M., El-Shinnawi U. Diagnostic value of acute phase proteins in periodontal, psychosomatic and cardiometabolic diseases: Response to treatment. In: Veas F., editor. Acute phase proteins as early non-specific biomarkers of human and veterinary diseases. In Tech. 2011. [Google Scholar]
- 32.Correa F.O., Gonçalves D., Figueredo C.M., Bastos A.S., Gustafsson A., Orrico S.R. Effect of periodontal treatment on metabolic control, systemic inflammation and cytokines in patients with type 2 diabetes. J. Clin. Periodontol. 2010;37(1):53–58. doi: 10.1111/j.1600-051X.2009.01498.x. [DOI] [PubMed] [Google Scholar]
- 33.Soory M. Association of periodontitis with rheumatoid arthritis and atherosclerosis: Novel paradigms in aetiopathogeneses and management? Rheumatol. Res. Rev. 2010;2:1–16. doi: 10.2147/oarrr.s10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J., Itoh Y., Hayashi H., Takii T., Miyazawa K., Onozaki K. Dihydrotestosterone inhibits interleukin-1α or tumor necrosis factor α-induced proinflammatory cytokine production via androgen receptor-dependent inhibition of nuclear factor-κB activation in rheumatoid fibroblast-like synovial cell line. Biol. Pharm. Bull. 2011;34(11):724–730. doi: 10.1248/bpb.34.1724. [DOI] [PubMed] [Google Scholar]
- 35.Itoh Y., Hayashi H., Xu J., Takii T., Miyazawa K., Ariga H., Akahoshi T., Waguri-Nagaya Y., Otsuka T., Okamoto T., Onozaki K. Dihydrotestosterone inhibits TNF-α-induced IL-1α mRNA expression in rheumatoid fibroblast-like synovial cells. Biol. Pharm. Bull. 2007;30(6):1140–1143. doi: 10.1248/bpb.30.1140. [DOI] [PubMed] [Google Scholar]
- 36.Nakajima T., Honda T., Domon H., Okui T., Kajita K., Ito H., Takahashi N., Maekawa T., Tabeta K., Yamazaki K. Periodontitis-associated up-regulation of systemic inflammatory mediator level may increase the risk of coronary heart disease. J. Periodontal Res. 2010;45(1):116–122. doi: 10.1111/j.1600-0765.2009.01209.x. [DOI] [PubMed] [Google Scholar]
- 37.Atalay M., Bilginoglu A., Kokkola T., Oksala N., Turan B. Treatments with sodium selenate or doxycycline offset diabetes-induced perturbations of thioredoxin-1 levels and antioxidant capacity. Mol. Cell. Biochem. 2011;351(1-2):125–131. doi: 10.1007/s11010-011-0719-3. [DOI] [PubMed] [Google Scholar]
- 38.Castro M.M., Rizzi E., Ceron C.S., Guimaraes D.A., Rodrigues G.J., Bendhack L.M., Gerlach R.F., Tanus-Santos J.E. Doxycycline ameliorates 2K-1C hypertension-induced vascular dysfunction in rats by a ttenuating oxidative stress and improving nitric oxide bioavailability. Nitric Oxide. 2012;26(3):162–168. doi: 10.1016/j.niox.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Nagareddy P.R., Rajput P.S., Vasudevan H., McClure B., Kumar U., Macleod K.M., McNeill J.H. Inhibition of matrix metalloproteinase-2 improves endothelial function and prevents hypertension in insulin-resistant rats. Br. J. Pharmacol. 2012;165(3):705–715. doi: 10.1111/j.1476-5381.2011.01583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goglia L., Tosi V., Sanchez A.M., Flamini M.I., Fu X.D., Zullino S., Genazzani A.R., Simoncini T. Endothelial regulation of eNOS, PAI-1 and t-PA by testosterone and dihydrotestosterone in vitro and in vivo. Mol. Hum. Reprod. 2010;16(10):761–769. doi: 10.1093/molehr/gaq049. [DOI] [PubMed] [Google Scholar]
- 41.Gu Y., Lee H.M., Sorsa T., Salminen A., Ryan M.E., Slepian M.J., Golub L.M. Non-antibacterial tetracyclines modulate mediators of periodontitis and atherosclerotic cardiovascular disease: a mechanistic link between local and systemic inflammation. Pharmacol. Res. 2011;64(6):573–579. doi: 10.1016/j.phrs.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 42.Kładna A., Kruk I., Michalska T., Berczyński P., Aboul-Enein H.Y. Characterization of the superoxide anion radical scavenging activity by tetracycline antibiotics in aprotic media. Luminescence. 2011;26(6):611–615. doi: 10.1002/bio.1283. [DOI] [PubMed] [Google Scholar]


