Abstract
Previous reports have indicated that with aging, intrinsic brain tissue changes in cellular bioenergetics may hamper the brain’s ability to cope with metabolic stress. Therefore, we analyzed the effects of age on neuronal sensitivity to glucose deprivation by monitoring changes in field excitatory postsynaptic potentials (fEPSPs), tissue Po2, and NADH fluorescence imaging in the CA1 region of hippocampal slices obtained from F344 rats (1–2, 3–6, 12–20, and >22 months). Forty minutes of moderate low glucose (2.5 mM) led to approximately 80% decrease of fEPSP amplitudes and NADH decline in all 4 ages that reversed after reintroduction of 10 mM glucose. However, tissue slices from 12 to 20 months and >22-month-old rats were more vulnerable to low glucose: fEPSPs decreased by 50% on average 8 minutes faster compared with younger slices. Tissue oxygen utilization increased after onset of 2.5 mM glucose in all ages of tissue slices, which persisted for 40 minutes in younger tissue slices. But, in older tissue slices the increased oxygen utilization slowly faded and tissue Po2 levels increased toward baseline values after approximately 25 minutes of glucose deprivation. In addition, with age the ability to regenerate NADH after oxidation was diminished. The NAD+/NADH ratio remained relatively oxidized after low glucose, even during recovery. In young slices, glycogen levels were stable throughout the exposure to low glucose. In contrast, with aging utilization of glycogen stores was increased during low glucose, particularly in hippocampal slices from >22 months old rats, indicating both inefficient metabolism and increased demand for glucose. Lactate addition (20 mM) improved oxidative metabolism by directly supplementing the mitochondrial NADH pool and maintained fEPSPs in young as well as aged tissue slices, indicating that inefficient metabolism in the aging tissue can be improved by directly enhancing NADH regeneration.
Keywords: Hippocampus, NADH, Glycogen, Aging, Oxygen, Hypoglycemia
1. Introduction
Aging can be associated with a decreased ability to respond to metabolic challenges resulting, for example, in fatigue on cognitive tasks and increased susceptibility to substrate deprivation, such as during hypoglycemia (Deary et al., 2003; McNay, 2005). Episodes of hypoglycemia can occur in older individuals as either a common, iatrogenic complication of diabetes treatment (McCrimmon and Sherwin, 2010), or during prolonged cognitive tasks or training with enhanced substrate utilization (Gold, 2005; McNay et al., 2000). Aging individuals are more likely to suffer from lower glucose levels and experience neuroglycopenic symptoms than younger individuals, because mechanisms to help guard against hypoglycemia-induced complications may be blunted. For example, neuroendocrine and glucoregulatory responses that help to maintain blood glucose levels within the normal range can be attenuated with aging (Kirsh and Aron, 2011; Zammitt and Frier, 2005), compromising both self-detection of low-glucose conditions and appropriate endogenous and exogenous treatment.
Although episodes of severe hypoglycemia are potentially life-threatening, moderate hypoglycemia (defined as blood glucose levels of 2.4–4.0 mM) occurs far more frequently during routine diabetic treatment (Kirsh and Aron, 2011). Even moderate hypoglycemia can cause electroencephalogram abnormalities, impairment of cognitive function, and falls, further compromising the ability of individuals to both recognize and treat the low-glucose condition, especially in the elderly population (Bramlage et al., 2012; Whitmer et al., 2009). If these episodes recur frequently, there may be resulting long-term cognitive dysfunction and neuronal damage (Choi et al., 2013), which can be exacerbated in aging individuals.
The hippocampus is a brain region involved in normal learning and memory (Axmacher et al., 2010; Campo and Poch, 2012) that is particularly vulnerable to substrate deprivation (McCall, 2005). Therefore, early cognitive impairment in response to hypoglycemia may be correlated to acute dysfunction of hippocampal neurons. Several studies have also demonstrated that in aging individuals extracellular glucose levels may drop in the hippocampus by as much as 30%–40% during a behavioral testing period (McNay and Gold, 2001); in contrast, young individuals demonstrate increased blood and maintained brain levels of glucose in response to either training or stress because of compensatory epinephrine release (Morris et al., 2010). This aging-related insensitivity to appropriate neuroendocrine responses to metabolic stress can exacerbate fatigue and decrease cognitive performance, especially during prolonged high demand cognitive tasks or training, even in the absence of iatrogenic hypoglycemia (i.e., with therapeutic diabetes treatment).
Although vascular alterations and deficient systemic responses to hypoglycemia associated with aging can contribute to enhanced vulnerability to substrate deprivation (Aanerud et al., 2012), it is possible that in aging intrinsic brain tissue changes in cellular bioenergetics may also decreased ability to cope with metabolic stress. Therefore, we analyzed the effects of age on neuronal sensitivity to moderately low glucose conditions by assessing changes in synaptic activity (by measuring field excitatory postsynaptic potential [fEPSP]), tissue Po2, and NADH fluorescence imaging. Because astrocytic glycogen is critical in providing additional energy substrates that can support neuronal function during glucose deprivation (Shetty et al., 2012; Suh et al., 2007), we have also investigated alteration in glycogen turnover and utilization as a function of aging and during moderate glucose deprivation. Analyzing critical aspects of age-related metabolism may lead to long-term preventative treatments, which could be used to improve metabolic buffering in the central nervous system (Dzuira et al., 2009; Malaguarnera et al., 2007; Page et al., 2009).
2. Methods
2.1. Tissue slice preparation
Hippocampal slices were obtained from 1 to 2 months old (mo) (young adult), 3 to 6 months (adult),12 to 20 months (older adult), and >22 months (senescent) F344 rats, (Harlan, Indianapolis, IN, USA). All animal use was approved by the Duke University and Durham VAMC Animal Care and Use Committees. Rats were anesthetized with isofluorane (Abbott Laboratories, North Chicago, IL, USA), decapitated, and the brain rapidly removed from the skull and placed in ice-cold artificial cerebrospinal fluid (ACSF) oxygenated with 95% O2/5% CO2 for 2 minutes. The ACSF solution consisted of (in mM): NaCl, 124; KCl, 3.0; NaH2PO4, 1.25; NaHCO3, 24; CaCl2, 2.00; MgSO4, 2.00; dextrose, 10; and pH 7.4. The hippocampus was rapidly dissected and transverse slices (400 mm) were cut on a manual tissue chopper (Galeffi et al., 2007, 2011). Slices were transferred immediately to an oxygenated holding chamber maintained at 22 °C, and allowed to recover for at least 3 hours. Slices were then transferred to a recording chamber, and partly submerged (approximately 1 mm) in ACSF buffer, which was continuously perfused (1.5 mL/min), and aerated with 95% O2 and 5% CO2. The temperature in the chamber was kept at 36.0 °C–36.5 °C. Experimental conditions such changes of glucose concentration with or without addition of lactate to the ACSF were all accompanied by adjustments of NaCl to keep osmolality constant.
Based on our previous study (Sadgrove et al., 2007), we simulated conditions of moderate glucose deprivation by lowering glucose concentration in the buffer from 10 mM to 2.5 mM. Euglycemic plasma glucose concentration of 7–8 mM correlates with a brain extracellular glucose concentration ranging between 1 and 2.5 mM in humans (Abi-Saab et al., 2002; Gruetter et al., 1992; Magnoni et al., 2003) as well as in rodents (McNay et al., 2000; Silver and Erecinska, 1994), resulting in a linear 3:1 ratio between plasma and tissue glucose concentrations (Magnoni et al., 2003). During moderate glucose deprivation in vivo, with plasma glucose concentrations of about 3 mM, extracellular brain glucose levels are estimated to drop to <1 mM glucose, leading to impaired cognitive function (Blackman et al., 1990; Boyle, 1997) and limitations of glycolysis (Gruetter et al., 1992, 1998). Hippocampal slices maintained in an interface chamber in vitro are normally exposed to excess glucose (10 mM glucose) and 95% oxygen by most investigators to ensure slice viability and responses. Despite this excess of substrates, with limited substrate diffusion between the surface and the interior of the slice (Kann and Kovacs, 2007; Lund-Andersen and Kjeldsen, 1976; McNay et al., 2000, 2006; Tekkok et al., 2002) and persistent metabolic demand, the level of glucose and oxygen drops sharply in the interior of the slice (Foster et al., 2005; Galeffi et al., 2011). By adapting the calculations given by Tekkok, et al. (2002) the glucose concentration at a depth of 200 mm can be estimated for various glucose concentrations: 10 mM in ACSF decreases to 7.24 mM; 5 mM ACSF to 2.24 mM; and 2.5 mM ACSF would likely result in <1 mM glucose concentration in the tissue interior, which is closer to the brain glucose concentration reported in vivo. The observed decline in fEPSP amplitude in slices exposed to 2.5 mM ACSF glucose in the present study suggests a significant limitation in glucose availability. Therefore, our slice model provides experimental glucose tissue levels comparable with that expected during iatrogenic hypoglycemia.
2.2. Electrophysiological recording and synaptic stimulation
The Schaffer collateral-commissural pathway was stimulated with a bipolar electrode situated in the stratum radiatum of the CA1 hippocampal region using single pulses (100 ms, 0.1–0.3 Hz). After performing an I/O curve to establish the saturation values for fEPSP, we adjusted the stimulus current to produce a fEPSP of approximately 50%–60% maximal amplitude, recorded using glass microelectrodes filled with 0.2 M NaCl (4–8 mU) placed in the stratum radiatum.
2.3. NADH fluorescence imaging and analysis
NADH fluorescence in hippocampal slices was monitored using a 290–370 nm excitation filter and a 420 nm long-pass filter for the emission (Omega Optical, Brattleboro, VT, USA), as previously described by our laboratory (Foster et al., 2008; Galeffi et al., 2011). The light source was a Lambda DG-4 (Sutter Instruments, Novato, CA, USA) equipped with a stabilized xenon arc lamp. Slices were epi-illuminated with an incident angle of 45° and imaged through a Nikon upright microscope (UM-2) with a compound lens (4x, N.A. 0.13) (Nikon, Melville, NY, USA). Slices were imaged using a linear, cooled 12-bit CCD camera (Cooke Instruments Sensicam QE, Auburn Hills, MI, USA) with 1280 × 1024 digital spatial resolution. Because of the low level of fluorescence emission, NADH images were acquired every 5 seconds as 8 × 8 binned images (effective spatial resolution of 160 × 128 pixels). These imaging specifications resulted in stable digital images with high-quality signal to noise ratios, as well as shorter exposure time (approximately 300 ms) to avoid tissue damage or bleaching. Each binned pixel corresponded to a slice region of 144 mm2. Only those slices with a stable baseline, ≤5% drift of fluorescence level during the initial control period (10 minutes), and a fluorescence intensity between 1000 and 2000 optical density levels (camera gain of 4 photons/level) were used for data analysis. Changes in NADH calculated for ROI were expressed as the percentage changes in fluorescence over the baseline levels prior to experimental manipulation ([DF/Fb] × 100). Because there was a slow decrease of NADH fluorescence in control slices overtime, the percentage change for any experimental condition was also compared with the percentage change in control slices perfused with 10 mM glucose buffer for equivalent amount of time.
2.4. Direct measurements of tissue level of NAD+ and NADH
For direct measurement of tissue levels of NAD+ and NADH, hippocampal slices were allowed to recover in an incubation chamber for 1 hour at room temperature and then kept at 36.0 °C–36.5 °C for 2 hours before any further experimental manipulation. Total NAD+ and NADH (NAD[H]) content was measured using NAD+/NADH assay kit (from Abcam # ab65348), as previously described (Shetty et al., 2012). Hippocampal slices were collected at different time intervals before and after exposure to low-glucose conditions; (2 slices each) and homogenized in ice cold NAD+/NADH extraction buffer and centrifuged at 16,000g for 5 minutes at 4 °C. The supernatant was filtered immediately through 10 kDa cutoff microspin column to separate the NADH consuming enzymes at 4 °C. Ultra filtrates (50 mL) were heated at 60 °C for 30 minutes in a heating block to decompose NAD+ for NADH measurement. Both the heated (NADH) and unheated samples (total NAD+) were processed for NAD+/NADH cycling assay reaction for 5 minutes to convert NAD+ into NADH in a 96-well microplate. The color was developed with NADH developer solution, and the absorbance was measured at 450 nm (microplate reader) after 2 hours. The concentration of total NAD+ and NADH were expressed in nmol per 100 mg protein based on standard NADH readings.
2.5. Tissue Po2 monitoring
A Clark-style oxygen microelectrode (OX10, Unisense, Aarhus, Denmark) was used to measure brain tissue Po2. The electrode consisted of a glass-insulated Ag/AgCl reference anode with guard cathode. The electrode was connected to a polarographic amplifier (PA2000 picoammeter, Unisense, Aarhus, Denmark), and the cathode was polarized at −800 mV in normal saline at 36 °C for up to 12 hours before use. A 2-point calibration (in nA) was performed following polarization by inserting the electrode in normal saline solution (at 36 °C) equilibrated with 95% O2 and 5% CO2 or room air at 21% O2, and 95% N2, 5% CO2, and 0% O2 (medical grade). Calibrations were repeated after every slice to determine the Po2 values, calibrated to mmHg. Electrode drift was generally linear over the course of an experiment. The current (in nA) values obtained from the 2 calibration points in 95% and 0% O2 during an experiment varied by 3.7% ± 2.7% hr −1 and 8.8% ± 7.4% hr −1, respectively. Following calibration, the oxygen electrode was positioned in the stratum radiatum in close proximity to the recording electrode and was then manually lowered into the tissue at 50 mm intervals using a micrometer to a depth at which the Po2 was at the minimum (nadir). The amplitude of the change in tissue Po2 due to experimental manipulation was calculated by the equation: ΔPo2 = (Po2 (baseline) - Po2 (stim)), where “baseline” refers to the level immediately before the response, not the initial control, and all readings are in mmHg.
2.6. Glycogen measurements
Glycogen measurements were performed on intact hippocampus immediately after dissection to assess age-dependent baseline levels. To measure changes in glycogen homeostasis after metabolic stress hippocampal slices were collected from the medium at different time intervals after slicing or exposure to low-glucose conditions (2.5 mM glucose); then tissue was rapidly processed for measurements as previously described (Shetty et al., 2012). Slices were immediately placed in ice-cold 85% ethanol containing 15% of 30 mM HCl to arrest both glycogenesis and glycogenolysis and frozen in liquid N2. Frozen slices were dried on Whatman #1 filter paper and homogenized in a volume (110 mL/3 slices) of 0.1 M NaOH with 0.01% SDS and 1 mM EDTA, using a sonicator. Homogenates were neutralized with 0.3 N HCl (32 mL). Then, 50 mL of paired homogenates were added to 250 mL of 50 mM sodium acetate buffer (pH 5.5) for glycogen hydrolysis with and without 1 unit of amyloglucosidase to measure total and free glucose levels, respectively. Samples were incubated at 32 ° C for 2 hours, cooled on ice, and then centrifuged at 14,000 rpm for 20 minutes. Supernatant (100 mL) from the digested samples were used for glucose measurements (Swanson et al., 1989).
The free and total glucose content were measured by a glucose assay kit according to the manufacturer instructions (Sigma Aldrich). In brief, glucose is phosphorylated by adenosine triphosphate (ATP) in the reaction catalyzed by hexokinase. Glucose-6-phosphate is then oxidized to 6-phosphogluconate in the presence of oxidized nicotinamide adenine dinucleotide (NAD+) in a reaction catalyzed by glucose-6-phosphate dehydrogenase. During this oxidation, an equimolar amount of NAD+ is reduced to NADH. The consequent increase in absorbance at 340 nm is directly proportional to glucose concentration. The protein concentrations of the homogenates were estimated by Bradford dye binding method to normalize the glycogen content (nmol) per mg protein. A standard glucose plot was established to calculate the glycogen content, using a conversion factor of 180 g/mol free glucose = 162 g/mol glycosyl units of glycogen. The glycogen units are presented as mmol glycosyl unit/g wet tissue weight by considering the fact that protein content in the brain constitutes 11.7% of wet brain weight for consistency with our previous study (Shetty et al., 2011), as well as numerous studies in the literature (Benzi et al., 1984; Choi et al., 2003; Gruetter et al., 1998; Zilberter et al., 2013). Statistical analysis performed on glycogen levels expressed as either glycosyl units nmol/mg protein or mmol/g of wet brain weight has given comparable results.
3. Results
3.1. Effects of age on susceptibility to moderate glucose deprivation
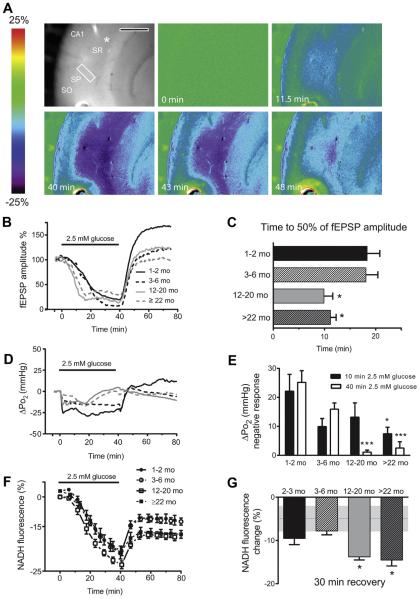
Slices were incubated for a minimum of 3 hours before physiological experimentation to allow for adequate recovery of astrocytic glycogen stores (see the following). We then exposed hippocampal slices to moderate glucose deprivation (2.5 mM glucose) for 40 minutes, allowing slices to recover in 10 mM glucose buffer for an additional 40 minutes. Exposure to 2.5 mM glucose buffer resulted in an acute suppression of fEPSP amplitudes in all 4 age groups (Fig. 1B and C), but evoked potentials were partially maintained (Sadgrove et al., 2007). Hippocampal slices of rats from different age groups showed a similar reduction in fEPSP amplitude after 40 minutes of 2.5 mM glucose (about 80%, Fig. 1B). However, synaptic responses in slices obtained from older rats (12–20 months and >22 months) appeared to be more sensitive to glucose deprivation compared with the younger age groups. The amplitude of the fEPSPs decreased to 50% of baseline values in 9.9 ± 1.7 minutes and 11.2 ± 1.1 minutes in 12–20 months and >22 months old rats, respectively, significantly faster than in younger animals: 18.3 ± 2.5 minutes in 1–2 months and 18.1 ± 2.3 minutes in 3–6 months (Fig. 1B). The suppression of fEPSP caused by glucose deprivation was completely reversible after reintroducing 10 mM glucose to the perfusion buffer; the fEPSP values recovered rapidly to baseline values in all 4 age groups (Fig. 1B).
Fig. 1.
Effect of moderate glucose deprivation (2.5 mM) on field excitatory post-synaptic potentials, NADH fluorescence, and tissue Po2 n the CA1 region of hippocampal slices, at 4 different ages across the life span. (A) The top left shows an unsubtracted camera image of the hippocampal CA1 region. The recording electrode is positioned next to the oxygen sensor, indicated by the white asterisk. Scale bar = 500 mm. The imaging region of interest (ROI; a white rectangle) is positioned in the stratum radiatum between the stimulating and recording electrode. The stratum pyramidale (SP), the stratum oriens (SO), and stratum radiatum (SR) are also indicated. Next are a series of pseudocolor images of NADH fluorescence at different times during and after exposure to 2.5 mM glucose in hippocampal slices exposed to 95% O2. Low-glucose conditions resulted in a decline of NADH fluorescence because of a decrease in substrate availability (at 11.5 minutes). Then NADH fluorescence starts to recovers toward baseline levels after the slices were returned to the control buffer (after 40 minutes). (B) Mean time-course of fEPSP amplitudes during 40 minutes low glucose (2.5 mM) condition and recovery. (C) Data summary: in hippocampal slices obtained from 12- to20-month-old rats fEPSP amplitudes declined significantly earlier compared with 1- to 2-month-old rats (*p < 0.05 vs. 1–2 months; 1-way ANOVA followed by Tukey multiple comparisons test). Data are the mean ± SEM of 10, 5, 5, and 7 slices/condition in 1–2, 3-6, 12-20, and >22 months rats, respectively. (D) Mean time-course of tissue Po2 during 40 minutes low-glucose (2.5 mM) condition and recovery. (E) Data summary of tissue Po2 decline at 10 and 40 minutes after glucose deprivation (*p < 0.05 and ***p < 0.001 vs. 1–2 months; 2-way ANOVA followed by Tukey multiple comparisons test). Data are the mean ± SEM of 6, 3, 4, and 5 slices/condition in 1–2, 3–6, 12–20, and >22 months rats, respectively. (F) Averaged traces of NADH fluorescence changes over time. (G) Data summary: changes of NADH fluorescence from baseline were calculated 30 minutes after recovery in control buffer (10 mM glucose). In hippocampal slices obtained from 12 to 20 and >22 months old rats NADH levels remain lower than in younger rats (*p < 0.05 vs. 1–2 months; 1-way ANOVA followed by Tukey multiple comparisons test). Data are the mean ± SEM of 9, 5, 9, and 6 slices/condition in 1–2, 3–6, 12–20, and >22 months rats, respectively. The gray dotted line indicates average NADH fluorescence changes ± SEM in control slices at equivalent time. Abbreviations: ANOVA, analysis of variance; fEPSP, field excitatory postsynaptic potential; SEM, standard error of the mean. (For interpretation of the references to color in this Figure, the reader is referred to the web version of this article.)
3.2. Tissue Po2 levels during moderate glucose deprivation
The Po2 was measured at the nadir of oxygen tension (200–250 mm depth from the surface) within each slice. In control slices, tissue Po2 levels averaged 270.9 ± 20 mm Hg and 280 ± 10 mm Hg in 1–2 months and 3–6 months rats, respectively and were significantly higher in hippocampal slices of older rats: 350 ± 22 mm Hg and 348 ± 10 mm Hg in 12–20 months and >22 months rats, respectively (p < 0.05 vs. 1–2 months after 1-way analysis of variance (ANOVA) followed by Tukey multiple comparisons test), suggesting an overall decline in oxidative metabolism and oxygen utilization within the older slices (Foster et al., 2005). Tissue Po2 levels remained stable during at least 10 minutes baseline recording in 10 mM glucose buffer but decreased significantly at the onset of 2.5 mM glucose condition, by 22.1 ± 5.8 mm Hg (p < 0.05 paired t test baseline vs. 2.5 glucose at 10 minutes) indicating an enhanced rate of oxygen utilization (Fig. 1D). This rapid oxygen decrease from the baseline is shown in Fig. 1D and E. This metabolic switch to enhanced oxidative metabolism is likely because of more pyruvate entering the TCA cycle to prevent ATP loss during limited glucose supply, with a relative reduction in aerobic glycolysis (Swerdlow et al., 2013). In hippocampal slices from young adult and adult rats, the net drop in tissue Po2 normally persisted for the duration of the moderate glucose deprivation, then at 40 minutes on reintroduction of 10 mM glucose buffer, Po2 rapidly returned to baseline levels, with a net increase of Po2 of 25.2 ± 4.1 mm Hg (Fig. 1D and E). It should also be noted that during 40 minutes of 2.5 mM glucose condition the fEPSP was not fully suppressed in any age group and neuronal demand for substrate was maintained, although lessened by the substrate reduction.
In hippocampal slices from aging and senescent rats, the initial decrease of tissue Po2 was smaller, in particular in the older aged group: ΔPo2 at 10 minutes 13.2 ± 4.9 and 7.45 ± 2.25 in 12–20 and >22 months, respectively (p < 0.05, 1–2 months vs. 22 months, 2-way ANOVA followed by Tukey multiple comparisons test) (Fig. 1D and E). In addition, in older tissue slices the initial increase in oxygen utilization did not persist and Po2 levels slowly returned to baseline values after approximately 25–40 minutes of glucose deprivation (Fig. 1D). Therefore, the ΔPo2 after 40 minutes (on restoration of 10 mM glucose) in aging rats was significantly smaller because of the progressive decline in oxygen utilization observed in slices from older animals (Fig. 1D and E).
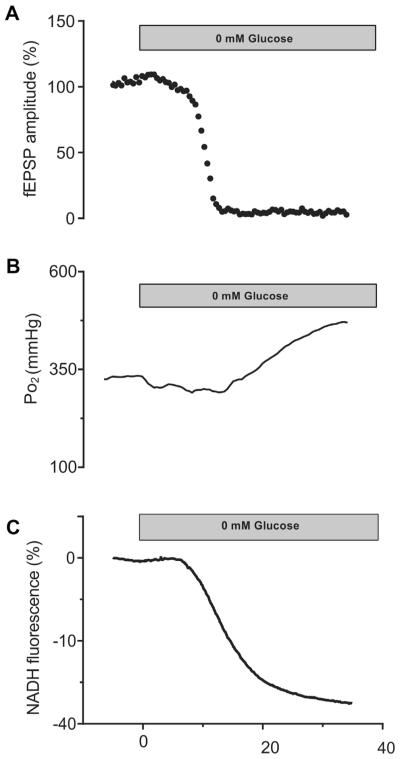
To further investigate the relationship among tissue oxygen utilization, metabolic substrate availability, and local cellular metabolic demand, we also exposed slices of 1- to 2-month-old rats to complete glucose deprivation (i.e., 0 mM glucose) (Fig. 2). Under these conditions, tissue oxygen levels initially declined below baseline levels (DPo2 at 10 minutes was 25.6 ± 5.2, n = 5), similar to the 2.5 mM glucose condition. However, after 17.5 ± 1.7 minutes (n = 5) of exposure to zero glucose buffer, tissue Po2 levels drifted back to baseline levels, concurrent with the rapid decline of the fEPSP amplitude. Then, once the synaptic responses were completely suppressed (Fig. 2A) the Po2 rose above the baseline levels (Fig. 2B), indicating a decline in oxygen utilization because of compromised cellular function and reduced metabolic demand (Shetty et al., 2012). In comparison with the 2.5 mM glucose condition, where the fEPSP was not fully suppressed and limited neuronal demand for substrate was maintained, the decline in oxygen utilization with aging is clearly more related to changes in efficiency of oxidative metabolism rather than simply a decrease in metabolic demand.
Fig. 2.
Representative traces of 1 experiment of glucose deprivation (0 mM) in a young slice (1–2 months of age), showing fEPSP amplitude (A), NADH fluorescence (B), and tissue Po2 (C). Severe glucose deprivation results in the eventual complete suppression of fEPSP (A) and a severe decline of NADH fluorescence. Tissue Po2 decreased immediately on onset of the low-glucose condition because of a moderate increase in oxygen utilization. But, with the severe substrate deprivation both oxygen utilization and metabolic demand declined as the fEPSP failed. Abbreviation: fEPSP, field excitatory postsynaptic potential.
3.3. NAD+/NADH redox state and total NAD(H) content during 2.5 mM glucose
The NAD+/NADH redox balance in neurons and astrocytes strongly depends on availability of both energy substrates (i.e., glucose and oxygen) and metabolic demand. Examining NADH levels can reveal age-dependent imbalances between NADH to NAD+ turnover through the electron transport chain and NADH generation via dehydrogenase activity. Therefore, we have monitored real time changes of NADH with fluorescence imaging during 40 minutes glucose deprivation (2.5 mM) and up to 40 minutes recovery. In slices incubated at 36 °C, there was a progressive, mild decline in the NADH baseline fluorescence over time in 10 mM glucose control buffer; therefore, NADH ΔF/Fb (change in fluorescence with respect to the baseline) for any experimental condition was compared with ΔF/Fb of control slices perfused with 10 mM glucose buffer for an equivalent amount of time. First, we have determined that ΔF/Fb over an average of 80 minutes recording was not significantly different in hippocampal slices from 1 to 2 months compared with rats >12 months (DF/Fb at 40 minutes recording = −5.6 ± 0.8 and −4.8 ± 1.2 in 1–2 months and >12 months rats, respectively, and at 80 minutes recording = −6.5 ± 1.0 and −7.7 ± 1.6 in 1–2 months and >12 months rats, respectively; n = 4 slice per condition: p > 0.05; paired t test; >12 months rats vs. 1–2 months). Decreasing glucose from the control value of 10 mM–2.5 mM for 40 minutes resulted in a significant decline of NADH fluorescence that was similar for all 4 age groups (Fig. 1A, F, and G) (DF/Fb = −20.5 ± 1.5 in 1–2 months, −19.1 ± 0.5 in 3–6 months, −23.1 ± 1.2 for 2–20 months, and −21 ± 2.3, >22 months) (p > 0.05; 2.5 mM glucose vs. 10 mM glucose).
On the reintroduction of 10 mM glucose, NADH fluorescence recovered toward baseline levels and reached maximum recovery after approximately 12 minutes, and then NADH fluorescence levels remained stable for the rest of the recording period, which was extended to 40 minutes (Fig. 1F and G). In 1–2 months and 3–6 months tissue slices, NADH fluorescence had recovered to ΔF/Fb = −9.54% ± 14.0% and −7.8% ± 0.08% of baseline levels (not different than the expected time-dependent change in control slices, see values previously mentioned), but a more limited recovery was observed in hippocampal slices from aging rats: ΔF/Fb = −13.8% ± 0.07% (12–20 months) and −14.5% ± 1.4% (>22 months), respectively (Fig. 1G) (p < 0.05; 1–2 months vs. 12–20 months and >22 months; 1-way ANOVA followed by Tukey multiple comparisons test).
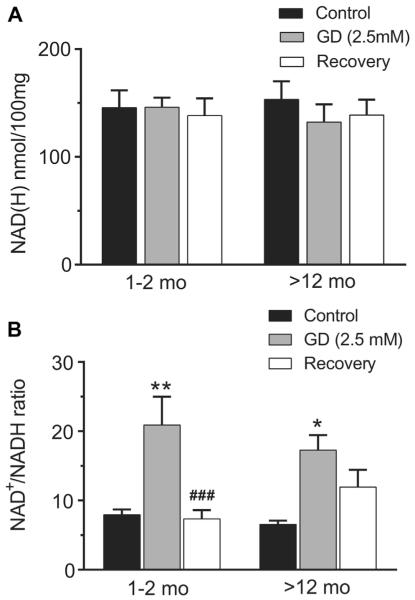
Several factors can affect NADH fluorescence over a long period of recording time, including changes in the redox level as well as possible loss from the total NAD(H) pool. To further validate our findings, we have performed direct measurements of tissue level using biochemical analysis of NAD+ and NADH separately to estimate changes in the NAD+ to NADH ratio and total NAD(H) in acutely isolated hippocampal slices (Fig. 3) collected at different time points before, during, and after exposure to moderate glucose deprivation. For these measurements, we directly compared changes in the NAD+ to NADH ratio and total NAD(H) between 1–2 months rats and rats >12 months, as the diminished NADH fluorescence recovery after glucose deprivation was observed in both 12–20 and >22-month-old rats. The total level of NAD(H) in slices maintained at 36 °C for at least 2 hours were similar in young and aged rats before the insult: (145.5 ± 16.2 nmol and 153.3 ±16.7 nmol/100 mg of protein in young and aged rats, respectively) and remained stable after 40 minutes of moderate glucose deprivation and subsequent recovery in both young and aged rats: (138.2 ± 16 nmol and 138.7 ± 14.47 nmol/100 mg of protein in young and aged rats, respectively) (Fig. 3A). Thus, there was no net loss of NAD+ from the tissue under these conditions.
Fig. 3.
Effect of glucose deprivation (2.5 mM) on total NAD(H) content (A) and the NAD+/NADH ratio (B) in hippocampal slices of young adult (1–2 months) and aging rats (12–20, and >22 months). (A) Total NAD(H) content remained stable in hippocampal slices after 40 minutes 2.5 mM glucose condition and 40 minutes 10 mM glucose recovery. (B) The NAD+/NADH ratio increased significantly after moderate glucose deprivation (2.5 mM) but recovered completely after 40 minutes of 10 mM glucose in young slices. In contrast, the NAD+/NADH ratio remained relatively oxidized in hippocampal slices from aging rats after return to 10 mM glucose compared with the control levels (*p < 0.05, **p < 0.01; GD vs. control. ###p <0.01; recovery vs. GD; 2-way ANOVA followed by Sidak multiple comparisons test, n = 6 [young] and 5 [aging]).
The average NAD+/NADH ratio in control slices incubated in 10 mM glucose buffer was 7.9 ± 1.1 for hippocampal slices from young adult (1–2 months) and for aging rats (>12 months) 6.5 ±0.6, respectively. These values were consistent with slice measurements reported by Garofalo et al. (1988) under similar experimental conditions. Similar to the NADH imaging, exposure to 40 minutes 2.5 mM glucose deprivation resulted in a significant shift of the redox state toward oxidation: the NAD+/NADH ratio increased to 20.9 ± 4.1 and 17.3 ± 2.2 in hippocampal slices from young adult and aging rats, respectively (p < 0.01 and p < 0.05; 2.5 mM glucose vs. control; 1–2 months and >12 mo, respectively). However, after 40 minutes recovery in 10 mM glucose the NAD+/NADH ratio was restored to control levels in the young age group (7.3 ± 2.3, 1–2 months), whereas the older groups did not recover completely (11.9 ± 2.4, >12 months) (p < 0.001 and p > 0.05; recovery vs. 2.5 mM glucose, 1–2 months and >12 months, respectively, 2-way ANOVA followed by Turkey multiple comparisons test) (Fig. 3B). These finding confirmed the observations made with NADH imaging analysis that suggested limited recovery of NADH with aging after the metabolic challenge.
3.4. Age-related increase of glycogen turnover in hippocampal slices during low glucose
Glycogen stored in astrocytes can provide energy to support neuronal homeostasis during glucose deprivation (Shetty et al., 2012). Specifically, investigators have hypothesized that lactate or pyruvate derived from glycogen metabolism can be shuttled to neurons to support oxidative metabolism (Tekkok et al., 2005). Therefore, we investigated age-related changes of glycogen levels and turnover during low-glucose conditions in relationship to the enhanced vulnerability of aging brain tissue to glucose deprivation.
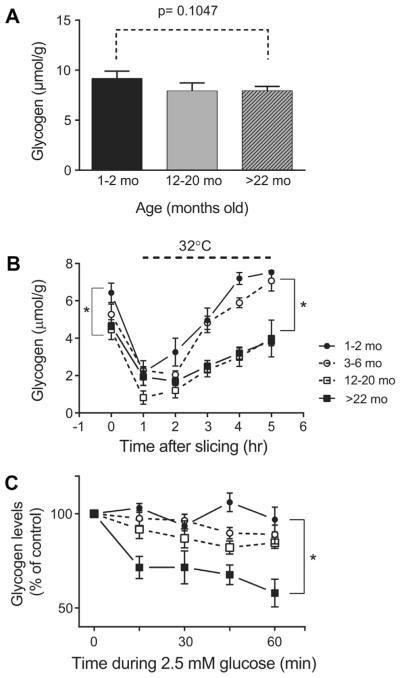
First, we measured glycogen levels under control conditions in the intact hippocampus and hippocampal slices at different time points after preparation, as well as after exposure to moderate glucose deprivation. Glycogen levels measured in intact hippocampal tissue were not significantly different between “young adult,” “older adult,” and “senescent” rats: 9.17 ± 0.42, 7.92 ± 0.46, 7.94 ± 0.25 (mmol/g) in 1–2 months, 12–20 months, and >22 months old, respectively (p = 0.105, 1-way ANOVA followed by Tukey multiple comparisons test) (Fig. 4A). Consistent with previous observations (Abdelmalik et al., 2008; Shetty et al., 2012), tissue glycogen levels declined immediately following slice preparation due to rapid glycogenolysis, and continued to drop during the first hr of incubation. Then, glycogen levels began to recover toward control values during continuous incubation at 32 °C with 10 mM glucose buffer (Fig. 4B). Immediately after slicing, glycogen levels at time 0 (Fig. 4B) were overall lower than those from the intact hippocampus for all the age groups but very low in slices from 12 to 20 months and >22 months animals, compared with the younger age group: 6.43 ± 0.52 for 1–2 months old versus 4.46 ± 0.27 12–20 months, and 4.65 ± 0.18 >22 months (mmol/g), respectively (p < 0.01, 1-way ANOVA followed by Tukey multiple comparisons test). In addition, the rate of glycogen recovery over hours was slower in the slices from older animals, although glycogen levels did eventually recover to near their initial control values in all the age groups by 4 hours of incubation at 32 °C (Fig. 4B).
Fig. 4.
Age-dependent changes in glycogen storage in intact hippocampi and hippocampal slices. (A) Glycogen levels were comparable in intact hippocampus in the 4 age groups. (B) Glycogen content in hippocampal slices at different time points after slice preparation. After preparation slices were kept at room temperature during the first hour of recovery and then transferred to ACSF maintained at 32 ° C to allow further recovery of the glycogen stores. Data are mean ± SEM (n = 4), *p < 0.05, 12–20 months and >22 months versus 1–2 months. Two-way ANOVA followed by Tukey multiple comparisons test. (C) The utilization of glycogen stores is significantly enhanced during moderate glucose deprivation in aged hippocampal slices; comparing slices from 1 to 2, 3 to 6, 12 to 20, and >22 months rats after exposure to 2.5 mM glucose for 60 minutes and collected at different time. Data are the mean ± SEM values n =4. *p < 0.05; >22 months versus 1–2 months. Two-way ANOVA followed by Tukey multiple comparisons test. Abbreviations: ACSF, artificial cerebrospinal fluid; ANOVA, analysis of variance; SEM, standard error of the mean.
During moderate glucose deprivation (2.5 mM glucose), glycogen levels remained stable in hippocampal slices of young adult rats (1–2 months and 3–6 months; Fig. 4C) up to 60 minutes after the beginning of the insult. In contrast, slices from the older age groups demonstrated a net decrease in glycogen levels during moderate glucose deprivation; although not significantly different from baseline levels in 3–6 and 12–20 months hippocampal slices, there was trend toward a decrease. There was a significant drop of glycogen in >22 months rats to 60% control levels after 60 minutes (Fig. 4C). Thus, the aged-tissue slices showed enhanced glycogen utilization during the moderate glucose deprivation compared with the younger tissue slices.
3.5. Lactate supplementation
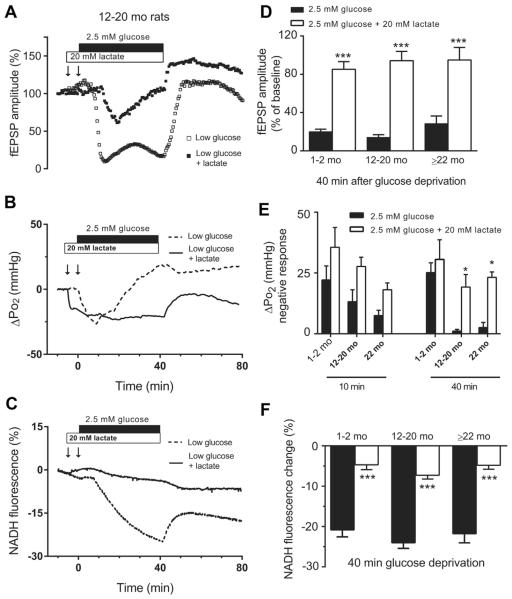
Metabolic substrates other than glucose can be utilized in the brain for energy production. In particular, lactate has been suggested as an alternate brain fuel under certain conditions, especially during hypoglycemia, as a critical glycogen derived substrate (Herzog et al., 2013; Seaquist and Oz, 2013). Therefore, we have evaluated whether supplementation with lactate could help maintain oxidative metabolism and neuronal activity during moderate glucose deprivation. Lactate (20 mM, slightly above an equicaloric concentration of 10 mM glucose) (Galeffi et al., 2007; Schurr et al., 1997) was incubated with the hippocampal slices 5 minutes before and during 40 minutes exposure to glucose deprivation (2.5 mM). Supplementation of low-glucose buffer with sodium lactate significantly prevented the depression of the fEPSP amplitude (Fig. 5A and D). In some experiments, addition of lactate before 2.5 mM glucose deprivation led to transient depression of the fEPSP amplitude to approximately 50% of baseline, which then recovered nearly to approximately 90% by the end of the glucose deprivation period, in all 4 age groups (Fig. 5A and D).
Fig. 5.
Lactate supplementation improves neuronal dysfunction induced by moderate glucose deprivation in hippocampal slices across the life span. (Left) Representative traces of fEPSP (A), NADH response (B), and tissue Po2 (C) recorded in hippocampal slices from12- to 20-month-old animal exposed to 2.5 mM glucose with or without (±) lactate 20 mM. (Right) Data summary: (D) lactate supplementation significantly prevented the suppression of fEPSP during low-glucose condition. (E) Lactate increased the rate of oxygen utilization (F) and prevented the decline of NADH fluorescence in aging rats. (*p < 0.05, ***p < 0.001; 2.5 mM glucose + lactate vs. 2.5 mM glucose; 2-way ANOVA followed by Sidak multiple comparisons test; data are mean ± SEM [n = 5]). Abbreviations: ANOVA, analysis of variance; fEPSP, field excitatory postsynaptic potential; SEM, standard error of the mean.
Lactate supplementation enhanced oxygen utilization early during 5 minutes of preincubation, even in the presence of 10 mM glucose (Fig. 5B and E), consistent with the previous observation that lactate supplementation decreases the rate of glucose uptake and alters metabolism with a shift toward enhanced oxidative metabolism (McKenna, 2012). In the presence of excess lactate, enhanced oxygen utilization was maintained throughout the exposure to low glucose, even in the aged slices. In the presence of the exogenous lactate, the ΔPo2 values recorded at both 10 and 40 minutes into the low-glucose condition were similar in all 4 age groups (Fig. 5 E).
Lactate supplementation enhanced NADH fluorescence early during 5 minutes of preincubation, even in the presence of 10 mM glucose. During 2.5 mM glucose buffer, lactate also significantly prevented the decline in NADH fluorescence compared with hippocampal slices exposed to 2.5 mM glucose alone, in a similar manner in all 4 age groups (Fig. 5C and F). After 40 minutes exposure to low glucose, in the presence of lactate the mean NADH decrease was ΔF/Fb = −4.6% ± 1.2% in 1–2 months slices, −7.3% ± 0.9% in 12–20 months slices, and −4.8% ± 1% in >22 months slices (p < 0.001; 2.5 mM glucose + lactate vs. 2.5 mM glucose: 2-way ANOVA followed by Sidak multiple comparisons test; data are mean ± SEM, n = 5).
4. Discussion
Exposure to even mild hypoglycemia causes electroencephalogram abnormalities (Cox and Bachelard, 1982) and cognitive dysfunction (Deary et al., 2003), under conditions where ATP and phosphocreatine are maintained at normal levels (Cox and Bachelard, 1982; Ghajar et al., 1982). Transient suppression of neuronal activity in the hippocampus and other vulnerable brain regions in response to hypoglycemia can impair performance on complex tasks and affect simple daily activity (Deary et al., 2003). Although autonomic responses to hypoglycemia are triggered at higher glucose levels in young adults compared with aged individuals, allowing for earlier recognition of low glucose and faster self-treatment, neurologic function (i.e., reaction time) deteriorates faster and to a greater degree in older individuals (Matyka et al., 1997). For example, reaction time deteriorated at higher low glucose blood levels in older men, at 3.0 ±0.1 mM, versus 2.6 ± 0.1 mM in younger men, indicating enhanced susceptibility to neuronal dysfunction during even moderate hypoglycemia (Matyka et al., 1997).
Consistent with these observations in vivo, results from our experiments, together with previous observations using hippocampal slices (Tekkök et al., 1998), have confirmed a significant age-dependent increase of brain tissue vulnerability to glucose deprivation, with a faster decline of synaptic activity, enhanced glycogen utilization, and fatigue of oxidative metabolism with the sustained low glucose. Similar to our results, Tekkök et al. (1998) specifically showed that hippocampal slices from 12-month-old control rats demonstrated a reduced time to transmission block in glucose-free ASCF of 11 minutes, versus 23 minutes in 4-month-old rats (Tekkök et al., 1998). However, in the present study synaptic responses were not completely suppressed over the 40 minutes of 2.5 mM glucose exposure, suggesting that energy substrates (such as ATP) were not fully depleted.
Tissue oxygen levels rapidly declined at the onset of the low-glucose condition, indicating increased oxygen utilization (Galeffi et al., 2011). This initial switch to enhanced oxidative metabolism indicates that in the presence of sufficient oxygen neuronal and glial metabolism can rapidly adapt to changes in glucose availability, to maintain stable ATP levels and neuronal activity. But, when glucose drops to a very low level and neuronal activity becomes completely suppressed (Nehlig, 1997), the tissue oxygen levels rise above the baseline because of severe loss of metabolic demand (Fig. 2B). Analogous to our findings, a previous study using XF24 flux analyzer (Seahorse) demonstrated that oxygen utilization is dependent on glucose availability, by showing a 25% increase in oxygen utilization when glucose concentration was reduced from 10 mM to 2.5 mM in neuroblastoma cells (Swerdlow et al., 2013). These authors concluded that under constant energy demand pyruvate generated from glycolysis may be preferentially diverted from lactic acid production and into the TCA cycle in mitochondria, to generate reducing equivalents sufficient to sustain oxidative phosphorylation.
The pyruvate dehydrogenase/pyruvate dehydrogenase kinase system (PDH/PDK) system is a key regulator of mitochondrial activity, particularly in response to levels of NAD+/NADH and ADP/ATP in the central nervous system (Patel et al., 2014). Therefore, an increase in NAD+ and ADP levels in response to lowered glucose can inhibit PDK, which rapidly activates PDH activity and facilitates pyruvate entry into the TCA cycle; this shift can generate reducing equivalents sufficient to sustain oxidative phosphorylation and preserve energy high energy substrates until carbohydrate stores are exhausted (Ghajar et al., 1982; Nehlig, 1997). Consistent with this hypothesis investigators have reported both an increase in brain PDH activity in response to hypoglycemia induced by injection of insulin in mice (Jope and Blass, 1976), as well as rats (Cardell et al., 1991) and a progressive decrease in extracellular lactate levels in brain tissue (Galeffi and Turner, unpublished observations).
Interestingly, although the initial tissue oxygen decrease was similar in both young and aged tissue slices, the young tissue slices may show more efficient oxidative metabolism because the oxygen decrease (and heightened oxidative metabolism) is maintained throughout the entire period of 2.5 mM glucose, whereas aging brain tissue showed a progressive fading of oxygen utilization during the low-glucose condition, in spite of relative preservation of fEPSP. Several factors can lead to the progressive decline in oxygen utilization in aging tissue during energetic challenges. For example, investigators have found that PDH activity is reduced with aging in both 12 and 24 months old rats (Zhou et al., 2009); this decrease was associated with an enhanced expression of brain specific isoform PDK2 (which inhibits PDH activity), leading to phosphorylation of PDH1a regulatory subunit. This finding indicates that increased phosphorylation of PDH by PDK2 with aging (Zhou et al., 2009) may limit the ability of the brain to adapt to energetic challenges, such as low glucose, for sufficient ATP production to meet metabolic demand.
During the low-glucose condition with intact oxygen availability, the NAD+/NADH redox ratio increased significantly, because of oxidation of available mitochondrial NADH to NAD+ in the electron transport chain and limited substrate availability for NADH regeneration (Bryan and Jobsis, 1986; Sadgrove et al., 2007). Interestingly, we have found that the shift of NAD+/NADH toward oxidation was similar in slices of young and aging rats despite the lower oxygen utilization in aging rats compared with younger rats during moderate glucose deprivation.
Changes in NADH fluorescence or NAD+/NADH represent only the net balance between NADH oxidation and NAD+ reduction in the cytosol during glycolysis and mitochondria TCA cycle, rather than the absolute oxidative capacity of mitochondria (Turner et al., 2007). Therefore, NADH dynamics and tissue oxygen utilization are not always correlated (Galeffi et al., 2011). Likely with aging, a decreased ability to regenerate NADH contributes to a shift of the NAD+/NADH toward oxidation (Ghosh et al., 2012) leading to a lower NADH level despite a slow decrease in the rate of oxygen utilization observed here. Using combined 13 C/1H magnetic resonance spectroscopy with infusions of [1–13C] glucose and [2–13C] acetate, investigators have found that neuronal TCA metabolism was 30% lower in elderly subjects compared with young subjects (Boumezbeur et al., 2009). Here, the limited recovery of NADH after reintroduction of 10 mM glucose is consistent with these observations and confirms that in aging rat tissue the ability to regenerate NADH is significantly compromised (Ghosh et al., 2014).
Glycogen utilization is crucial to delay synaptic failure during severe glucose deprivation (Shetty et al., 2011), and glycogen levels drop rapidly when fEPSP becomes completely suppressed. However, in the presence of normal glucose, balanced glycogen metabolism, and glycolysis are coactivated (Hertz et al., 2007) to support energy requiring processes associated with synaptic activity in astrocytes as well as neurons. Particularly at times of low energy charge (i.e., intense neuronal activation and low glucose conditions), glycogen-derived glucose-6-phosphate can directly fuel glycolysis without the initial ATP expenditure required for the utilization of glucose imported from the extracellular compartment to generate glucose 6-phosphate.
Our finding in young hippocampal tissue that glycogen levels recovered quickly after slicing and remained stable during moderate glucose deprivation indicates that under these conditions there is sufficient ATP and extracellular glucose to support glycogen recycling in astrocytes. In contrast, in aging hippocampal tissue, we have found enhanced glycogen turnover after slicing and during moderate glucose deprivation, suggesting that substrate utilization is increased to compensate for mitochondrial dysfunction and inefficient oxidative metabolism. Consistent with our observations, 12- and 24-month-old rats experience a higher glucose drop within the brain during hypoglycemia in vivo (Benzi et al., 1984). These investigators have also reported that during recovery from hypoglycemia both glycogen stores and ATP levels showed diminished recovery in older brains. Therefore, in aging rats during low glucose there is both inefficient ATP production and higher glucose demand, which together impair glycogen synthesis, increase glycogenolysis, and lead to a net drop of glycogen stores.
In addition, investigators have proposed that lactate derived from glycogen in astrocytes is extruded into the extracellular space, where it can be used by neurons, therefore providing additional substrate to support neuronal function especially during low-glucose condition (Pellerin et al., 2007). Lactate levels in the brain can increase significantly as a result of stimulation and glycogenolysis (Itoh et al., 2003), and under this condition, lactate metabolism can support neuronal function. Furthermore, most of the lactate release was from astrocytes, but under conditions of mitochondrial antagonism (with the mitochondrial poison dinitrophenol), neurons can also release lactate (Walz and Mukerji, 1988). With aging, metabolism of glycogen and glycogen-derived lactate, although key in preventing further deregulation of neuronal homeostasis (Shetty et al., 2012) appears to be insufficient to fully compensate for energy deficits and to fully support oxidative metabolism, as evidenced by the faster decrease in fEPSP and oxygen utilization during low glucose.
In the presence of excess lactate during normal glucose conditions, we recorded a transient net shift of the redox state toward increased NADH fluorescence in all 4 age groups. Because both glucose and lactate compete for NAD+ in the cytosol high extracellular lactate levels will likely inhibit the glyceraldehyde3-phosphate dehydrogenase step in glycolysis. However, a reduced redox state in mitochondria will increase NADH turnover at complex I and increase oxygen utilization (Wilson et al., 1979), which will rapidly regenerate NAD+ (Cerdán et al., 2006) necessary for glucose and lactate metabolism in the cytosol. This finding confirms previous observations reporting that neurons and astrocytes can actively oxidize glucose or lactate for energy depending on substrate availability and the metabolic state of the cell (McKenna et al., 1994), and that glucose consumption in neural cells can be modulated by extracellular lactate concentration (Cerdán et al., 2006; Garcia-Espinosa et al., 2004). Note that, during lactate supplementation lactate levels in the extracellular fluid are well above lactate levels the brain in vivo, ranging from 1 to 2 mmol/L (Hertz et al., 2007).
Interestingly, lactate supplementation increases the rate of oxygen utilization and supports neuronal function during glucose deprivation in all age groups, possibly as a shift away from baseline glycolytic activity. This finding is somehow surprising, because aging individuals often demonstrate higher lactate levels in the brain because of mitochondrial dysfunction and decreased oxidative metabolism, possibly as a compensation mechanism with increased glycolysis (Ross et al., 2010). Although the aging brain may not tolerate additional lactate supplementation because of already higher levels, some investigators have hypothesized that lactate addition may exert different effects depending on the time of administration relative to the insult and the metabolic state of the cell (Berthet et al., 2009).
We propose that during euglycemic conditions long term exposure to high lactate levels may not be desirable (especially in aging tissue), whereas during hypoglycemia changes in the metabolic state in the brain (i.e., a net NAD+/NADH redox shift toward oxidation) may facilitate lactate metabolism. Therefore, lactate supplementation under this condition can offset energy failure and support neuronal function. Lactate metabolism both at cytosolic level (with conversion to pyruvate via lactate dehydrogenase then reducing equivalents transferred to the mitochondria via the malate-aspartate shuttle) as well as at the mitochondrial level (with enhanced pyruvate oxidation in the tricarboxylic acid cycle) can help correct the relative deficit in NADH formation, especially in aging rats and maintain electron flow and offset energy deficit.
Acknowledgements
The authors are grateful to Prof. C.A. Piantadosi for very helpful discussion and to Prof. D. Calvetti for critical reading of the manuscript. This research was supported by National Institutes of Health Grant NIA RO1AG037599 and VAMC Merit Review Award.
Footnotes
Disclosure statement
The authors have no actual or potential conflicts of interest.
References
- Aanerud J, Borghammer P, Chakravarty MM, Vang K, Rodell AB, Jonsdottir KY, Moller A, Ashkanian M, Vafaee MS, Iversen P, Johannsen P, Gjedde A. Brain energy metabolism and blood flow differences in healthy aging. J. Cereb. Blood Flow Metab. 2012;32:1177–1187. doi: 10.1038/jcbfm.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmalik PA, Liang P, Weisspapir M, Samoilova M, Burnham WM, Carlen PL. Factors which abolish hypoglycemic seizures do not increase cerebral glycogen content in vitro. Neurobiol. Dis. 2008;29:201–209. doi: 10.1016/j.nbd.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Abi-Saab WM, Maggs DG, Jones T, Jacob R, Srihari V, Thompson J, Kerr D, Leone P, Krystal JH, Spencer DD, During MJ, Sherwin RS. Striking differences in glucose and lactate levels between brain extracellular fluid and plasma in conscious human subjects: effects of hyperglycemia and hypoglycemia. J. Cereb. Blood Flow Metab. 2002;22:271–279. doi: 10.1097/00004647-200203000-00004. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Henseler MM, Jensen O, Weinreich I, Elger CE, Fell J. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc. Natl. Acad. Sci. U.S.A. 2010;107:3228–3233. doi: 10.1073/pnas.0911531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzi G, Pastoris O, Villa RF, Giuffrida AM. Effect of aging on cerebral cortex energy metabolism in hypoglycemia and posthypoglycemic recovery. Neurobiol. Aging. 1984;5:205–212. doi: 10.1016/0197-4580(84)90064-2. [DOI] [PubMed] [Google Scholar]
- Berthet C, Lei H, Thevenet J, Gruetter R, Magistretti PJ, Hirt L. Neuroprotective role of lactate after cerebral ischemia. J. Cereb. Blood Flow Metab. 2009;29:1780–1789. doi: 10.1038/jcbfm.2009.97. [DOI] [PubMed] [Google Scholar]
- Blackman JD, Towle VL, Lewis GF, Spire JP, Polonsky KS. Hypoglycemic thresholds for cognitive dysfunction in humans. Diabetes. 1990;39:828–835. doi: 10.2337/diab.39.7.828. [DOI] [PubMed] [Google Scholar]
- Boumezbeur F, Mason GF, de Graaf RA, Behar KL, Cline GW, Shulman GI, Rothman DL, Petersen KF. Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. J. Cereb. Blood Flow Metab. 2009;30:211–221. doi: 10.1038/jcbfm.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PJ. Alteration in brain glucose metabolism induced by hypoglycaemia in man. Diabetologia. 1997;40(Suppl. 2):S69–S74. doi: 10.1007/s001250051408. [DOI] [PubMed] [Google Scholar]
- Bramlage P, Gitt AK, Binz C, Krekler M, Deeg E, Tschope D. Oral antidiabetic treatment in type-2 diabetes in the elderly: balancing the need for glucose control and the risk of hypoglycemia. Cardiovasc. Diabetol. 2012;11:122. doi: 10.1186/1475-2840-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan RM, Jr., Jobsis FF. Insufficient supply of reducing equivalents to the respiratory chain in cerebral cortex during severe insulin-induced hypoglycemia in cats. J. Cereb. Blood Flow Metab. 1986;6:286–291. doi: 10.1038/jcbfm.1986.50. [DOI] [PubMed] [Google Scholar]
- Campo P, Poch C. Neocortical-hippocampal dynamics of working memory in healthy and diseased brain states based on functional connectivity. Front. Hum. Neurosci. 2012;6:36. doi: 10.3389/fnhum.2012.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardell M, Siesjo BK, Wieloch T. Changes in pyruvate dehydrogenase complex activity during and following severe insulin-induced hypoglycemia. J. Cereb. Blood Flow Metab. 1991;11:122–128. doi: 10.1038/jcbfm.1991.14. [DOI] [PubMed] [Google Scholar]
- Cerdán S, Rodrigues TB, Sierra A, Benito M, Fonseca LL, Fonseca CP, García-Martín ML. The redox switch/redox coupling hypothesis. Neurochem. Int. 2006;48:523–530. doi: 10.1016/j.neuint.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Choi BY, Kim JH, Kim HJ, Yoo JH, Song HK, Sohn M, Won SJ, Suh SW. Pyruvate administration reduces recurrent/moderate hypoglycemia-induced cortical neuron death in diabetic rats. PLoS One. 2013;8:e81523. doi: 10.1371/journal.pone.0081523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Seaquist ER, Gruetter R. Effect of hypoglycemia on brain glycogen metabolism in vivo. J. Neurosci. Res. 2003;72:25–32. doi: 10.1002/jnr.10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DWG, Bachelard HS. Attenuation of evoked field potentials from dentate granule cells by low glucose, pyruvate + malate, and sodium fluoride. Brain Res. 1982;239:527–534. doi: 10.1016/0006-8993(82)90527-3. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Sommerfield AJ, McAulay V, Frier BM. Moderate hypoglycaemia obliterates working memory in humans with and without insulin treated diabetes. J. Neurol. Neurosurg. Psychiatry. 2003;74:278–279. doi: 10.1136/jnnp.74.2.278-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzuira J, McCrimmon RJ, McNay EC, Page KA, Sherwin RS, Williamson A, Yu N. Medium-chain fatty acids improve cognitive function in intensively treated type 1 diabetic patients and support in vitro synaptic transmission during acute hypoglycemia. Diabetes. 2009;58:1237–1244. doi: 10.2337/db08-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KA, Beaver CJ, Turner DA. Interaction between tissue oxygen tension and NADH imaging during synaptic stimulation and hypoxia in rat hippocampal slices. Neuroscience. 2005;132:645–657. doi: 10.1016/j.neuroscience.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Foster KA, Margraf RR, Turner DA. NADH hyperoxidation correlates with enhanced susceptibility of aged rats to hypoxia. Neurobiol. Aging. 2008;29:598–613. doi: 10.1016/j.neurobiolaging.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeffi F, Foster KA, Sadgrove MP, Beaver CJ, Turner DA. Lactate uptake contributes to the NAD(P)H biphasic response and tissue oxygen response during synaptic stimulation in area CA1 of rat hippocampal slices. J. Neurochem. 2007;103:2449–2461. doi: 10.1111/j.1471-4159.2007.04939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeffi F, Somjen GG, Foster KA, Turner DA. Simultaneous monitoring of tissue PO2 and NADH fluorescence during synaptic stimulation and spreading depression reveals a transient dissociation between oxygen utilization and mitochondrial redox state in rat hippocampal slices. J. Cereb. Blood Flow Metab. 2011;31:626–639. doi: 10.1038/jcbfm.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Espinosa MA, Rodrigues TB, Sierra A, Benito M, Fonseca C, Gray HL, Bartnik BL, Garcia-Martin ML, Ballesteros P, Cerdan S. Cerebral glucose metabolism and the glutamine cycle as detected by in vivo and in vitro 13C NMR spectroscopy. Neurochem. Int. 2004;45:297–303. doi: 10.1016/j.neuint.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Garofalo O, Cox DW, Bachelard HS. Brain levels of NADH and NAD+ under hypoxic and hypoglycaemic conditions in vitro. J. Neurochem. 1998;51:172–176. doi: 10.1111/j.1471-4159.1988.tb04851.x. [DOI] [PubMed] [Google Scholar]
- Ghajar JBG, Plum F, Duffy TE. Cerebral oxidative metabolism and blood flow during acute hypoglycemia and recovery in unanesthetized rats. J. Neurochem. 1982;38:397–409. doi: 10.1111/j.1471-4159.1982.tb08643.x. [DOI] [PubMed] [Google Scholar]
- Ghosh D, LeVault KR, Barnett AJ, Brewer GJ. A reversible early oxidized redox state that precedes macromolecular ROS damage in aging nontransgenic and 3xTg-AD mouse neurons. J. Neurosci. 2012;32:5821–5832. doi: 10.1523/JNEUROSCI.6192-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D, Levault KR, Brewer GJ. Relative importance of redox buffers GSH and NAD(P)H in age-related neurodegeneration and Alzheimer disease-like mouse neurons. Aging Cell. 2014;13:631–640. doi: 10.1111/acel.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE. Glucose and age-related changes in memory. Neurobiol. Aging. 2005;26(Suppl 1):60–64. doi: 10.1016/j.neurobiolaging.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Novotny EJ, Boulware SD, Rothman DL, Mason GF, Shulman GI, Shulman RG, Tamborlane WV. Direct measurement of brain glucose concentrations in humans by 13C NMR spectroscopy. Proc. Natl. Acad. Sci. U.S.A. 1992;89:1109–1112. doi: 10.1073/pnas.89.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruetter R, Ugurbil K, Seaquist ER. Steady-state cerebral glucose concentrations and transport in the human brain. J. Neurochem. 1998;70:397–408. doi: 10.1046/j.1471-4159.1998.70010397.x. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J. Cereb. Blood Flow Metab. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Herzog RI, Jiang L, Herman P, Zhao C, Sanganahalli BG, Mason GF, Hyder F, Rothman DL, Sherwin RS, Behar KL. Lactate preserves neuronal metabolism and function following antecedent recurrent hypoglycemia. J. Clin. Invest. 2013;123:1988–1998. doi: 10.1172/JCI65105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Esaki T, Shimoji K, Cook M, Law MJ, Kaufman E, Sokoloff L. Dichloroacetate effects on glucose and lactate oxidation by neurons and astroglia in vitro and on glucose utilization by brain in vivo. Proc. Natl. Acad. Sci. U.S.A. 2003;100:4879–4884. doi: 10.1073/pnas.0831078100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope R, Blass JP. The regulation of pyruvate dehydrogenase in brain in vivo. J. Neurochem. 1976;26:709–714. doi: 10.1111/j.1471-4159.1976.tb04441.x. [DOI] [PubMed] [Google Scholar]
- Kann O, Kovacs R. Mitochondria and neuronal activity. Am. J. Physiol. Cell Physiol. 2007;292:C641–C657. doi: 10.1152/ajpcell.00222.2006. [DOI] [PubMed] [Google Scholar]
- Kirsh SR, Aron DC. Choosing targets for glycaemia, blood pressure and low-density lipoprotein cholesterol in elderly individuals with diabetes mellitus. Drugs Aging. 2011;28:945–960. doi: 10.2165/11594750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Lund-Andersen H, Kjeldsen CS. Uptake of glucose analogues by rat brain cortex slices: a kinetic analysis based upon a model. J. Neurochem. 1976;27:361–368. doi: 10.1111/j.1471-4159.1976.tb12254.x. [DOI] [PubMed] [Google Scholar]
- Magnoni S, Ghisoni L, Locatelli M, Caimi M, Colombo A, Valeriani V, Stocchetti N. Lack of improvement in cerebral metabolism after hyperoxia in severe head injury: a microdialysis study. J. Neurosurg. 2003;98:952–958. doi: 10.3171/jns.2003.98.5.0952. [DOI] [PubMed] [Google Scholar]
- Malaguarnera M, Cammalleri L, Gargante MP, Vacante M, Colonna V, Motta M. L-carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians: a randomized and controlled clinical trial. Am. J. Clin. Nutr. 2007;86:1738–1744. doi: 10.1093/ajcn/86.5.1738. [DOI] [PubMed] [Google Scholar]
- Matyka K, Evans M, Lomas J, Cranston I, Macdonald I, Amiel SA. Altered hierarchy of protective responses against severe hypoglycemia in normal aging in healthy men. Diabetes Care. 1997;20:135–141. doi: 10.2337/diacare.20.2.135. [DOI] [PubMed] [Google Scholar]
- McCall AL. Altered glycemia and brain-update and potential relevance to the aging brain. Neurobiol. Aging. 2005;26(Suppl 1):70–75. doi: 10.1016/j.neurobiolaging.2005.08.009. [DOI] [PubMed] [Google Scholar]
- McCrimmon RJ, Sherwin RS. Hypoglycemia in type 1 diabetes. Diabetes. 2010;59:2333–2339. doi: 10.2337/db10-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MC. Substrate competition studies demonstrate oxidative metabolism of glucose, glutamate, glutamine, lactate and 3-hydroxybutyrate in cortical astrocytes from rat brain. Neurochem. Res. 2012;37:2613–2626. doi: 10.1007/s11064-012-0901-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MC, Tildon JT, Stevenson JH, Hopkins IB. Energy metabolism in cortical synaptic terminals from weanling and mature rat brain: evidence for multiple compartments of tricarboxylic acid cycle activity. Dev. Neurosci. 1994;16:291–300. doi: 10.1159/000112122. [DOI] [PubMed] [Google Scholar]
- McNay EC. The impact of recurrent hypoglycemia on cognitive function in aging. Neurobiol. Aging. 2005;26(Suppl 1):76–79. doi: 10.1016/j.neurobiolaging.2005.08.014. [DOI] [PubMed] [Google Scholar]
- McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2881–2885. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay EC, Gold PE. Age-related differences in hippocampal extracellular fluid glucose concentration during behavioral testing and following systemic glucose administration. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:B66–B71. doi: 10.1093/gerona/56.2.b66. [DOI] [PubMed] [Google Scholar]
- McNay EC, Williamson A, McCrimmon RJ, Sherwin RS. Cognitive and neural hippocampal effects of long-term moderate recurrent hypoglycemia. Diabetes. 2006;55:1088–1095. doi: 10.2337/diabetes.55.04.06.db05-1314. [DOI] [PubMed] [Google Scholar]
- Morris KA, Chang Q, Mohler EG, Gold PE. Age-related memory impairments due to reduced blood glucose responses to epinephrine. Neurobiol. Aging. 2010;31:2136–2145. doi: 10.1016/j.neurobiolaging.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlig A. Cerebral energy metabolism, glucose transport and blood flow: changes with maturation and adaptation to hypoglycaemia. Diabetes Metab. 1997;23:18–29. [PubMed] [Google Scholar]
- Page KA, Arora J, Qiu M, Relwani R, Constable RT, Sherwin RS. Small decrements in systemic glucose provoke increases in hypothalamic blood flow prior to the release of counterregulatory hormones. Diabetes. 2009;58:448–452. doi: 10.2337/db08-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MS, Nemeria NS, Furey W, Jordan F. The pyruvate dehydrogenase complexes: structure-based function and regulation. J. Biol. Chem. 2014;289:16615–16623. doi: 10.1074/jbc.R114.563148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- Ross JM, Öberg J, Brené S, Coppotelli G, Terzioglu M, Pernold K, Goiny M, Sitnikov R, Kehr J, Trifunovic A, Larsson N-G, Hoffer BJ, Olson L. High brain lactate is a hallmark of aging and caused by a shift in the lactate dehydrogenase A/B ratio. Proc. Natl. Acad. Sci. U.S.A. 2010;107:20087–20092. doi: 10.1073/pnas.1008189107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadgrove MP, Beaver CJ, Turner DA. Effects of relative hypoglycemia on LTP and NADH imaging in rat hippocampal slices. Brain Res. 2007;1165:30–39. doi: 10.1016/j.brainres.2007.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurr A, Payne RS, Miller JJ, Rigor BM. Brain lactate is an obligatory aerobic energy substrate for functional recovery after hypoxia: further in vitro validation. J. Neurochem. 1997;69:423–426. doi: 10.1046/j.1471-4159.1997.69010423.x. [DOI] [PubMed] [Google Scholar]
- Seaquist ER, Oz G. Diabetes: does lactate sustain brain metabolism during hypoglycaemia? Nat. Rev. Endocrinol. 2013;9:386–387. doi: 10.1038/nrendo.2013.104. [DOI] [PubMed] [Google Scholar]
- Shetty PK, Galeffi F, Turner DA. Age-induced alterations in hippocampal function and metabolism. Aging Dis. 2011;2:196–218. [PMC free article] [PubMed] [Google Scholar]
- Shetty PK, Sadgrove MP, Galeffi F, Turner DA. Pyruvate incubation enhances glycogen stores and sustains neuronal function during subsequent glucose deprivation. Neurobiol. Dis. 2012;45:177–187. doi: 10.1016/j.nbd.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver IA, Erecinska M. Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J. Neurosci. 1994;14:5068–5076. doi: 10.1523/JNEUROSCI.14-08-05068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh SW, Bergher JP, Anderson CM, Treadway JL, Fosgerau K, Swanson RA. Astrocyte glycogen sustains neuronal activity during hypoglycemia: studies with the glycogen phosphorylase inhibitor CP-316,819 ([R-R*,S*]-5-chloro-N-[2-hydroxy-3-(methoxymethylamino)-3-oxo-1-(phenylmethyl)pro pyl]-1H-indole-2-carboxamide) J. Pharmacol. Exp. Ther. 2007;321:45–50. doi: 10.1124/jpet.106.115550. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Sagar SM, Sharp FR. Regional brain glycogen stores and metabolism during complete global ischaemia. Neurol. Res. 1989;11:24–28. doi: 10.1080/01616412.1989.11739856. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, E L, Aires D, Lu J. Glycolysiserespiration relationships in a neuroblastoma cell line. Biochim. Biophys. Acta. 2013;1830:2891–2898. doi: 10.1016/j.bbagen.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekkök S, Krifiž J, Padjen AL, Krnjević K. Higher sensitivity of CA1 synapses to aglycemia in streptozotocin-diabetic rats is age-dependent. Brain Res. 1998;813:268–273. doi: 10.1016/s0006-8993(98)01028-2. [DOI] [PubMed] [Google Scholar]
- Tekkok SB, Brown AM, Westenbroek R, Pellerin L, Ransom BR. Transfer of glycogen-derived lactate from astrocytes to axons via specific monocarboxylate transporters supports mouse optic nerve activity. J. Neurosci. Res. 2005;81:644–652. doi: 10.1002/jnr.20573. [DOI] [PubMed] [Google Scholar]
- Tekkok SB, Godfraind JM, Krnjevic K. Moderate hypoglycemia aggravates effects of hypoxia in hippocampal slices from diabetic rats. Neuroscience. 2002;113:11–21. doi: 10.1016/s0306-4522(02)00154-9. [DOI] [PubMed] [Google Scholar]
- Turner DA, Foster KA, Galeffi F, Somjen GG. Differences in O2 availability resolve the apparent discrepancies in metabolic intrinsic optical signals in vivo and in vitro. Trends Neurosci. 2007;30:390–398. doi: 10.1016/j.tins.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz W, Mukerji S. Lactate release from cultured astrocytes and neurons: a comparison. Glia. 1988;1:366–370. doi: 10.1002/glia.440010603. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Jr., Selby JV. HYpoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301:1565–1572. doi: 10.1001/jama.2009.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DF, Erecinska M, Drown C, Silver IA. The oxygen dependence of cellular energy metabolism. Arch. Biochem. Biophys. 1979;195:485–493. doi: 10.1016/0003-9861(79)90375-8. [DOI] [PubMed] [Google Scholar]
- Zammitt NN, Frier BM. Hypoglycemia in type 2 diabetes: pathophysiology, frequency, and effects of different treatment modalities. Diabetes Care. 2005;28:2948–2961. doi: 10.2337/diacare.28.12.2948. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Lam PY, Han D, Cadenas E. Activation of c-Jun-N-terminal kinase and decline of mitochondrial pyruvate dehydrogenase activity during brain aging. FEBS Lett. 2009;583:1132–1140. doi: 10.1016/j.febslet.2009.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter M, Ivanov A, Ziyatdinova S, Mukhtarov M, Malkov A, Alpar A, Tortoriello G, Botting CH, Fulop L, Osypov AA, Pitkanen A, Tanila H, Harkany T, Zilberter Y. Dietary energy substrates reverse early neuronal hyperactivity in a mouse model of Alzheimer’s disease. J. Neurochem. 2013;125:157–171. doi: 10.1111/jnc.12127. [DOI] [PubMed] [Google Scholar]