Abstract
Purpose
Paraneoplastic disorders are autoimmune diseases associated with risks for specific cancers and marked by specific autoantibodies. They cause diverse clinical syndromes of the central or peripheral nervous systems.
Recent Findings
In the peripheral nervous system, autoimmunity to synaptic or axonal proteins has long been recognized to associate with specific cancers. In these disorders, typified by myasthenia gravis, the antibodies are directly toxic, and recovery with immunotherapy is the rule. In contrast, the “classical” paraneoplastic syndromes involve a higher risk of cancer, autoantibodies to intracellular proteins (e.g. Hu proteins), T-cell dependent disease mechanisms targeting the CNS or PNS, and a poor response to treatment. Following the discovery of NMDAR antibodies, there has emerged a new and expanding group of disorders involves autoantibodies to CNS synaptic and neuronal membrane proteins, and a favorable response to immunotherapy. A final group involves antibodies to intracellular synaptic proteins such as GAD65, and both antibody and T-cell mechanisms have been proposed.
Summary
Neurologists should recognize the clinical syndromes associated with paraneoplastic disorders, utilize autoantibody and other testing to confirm the diagnosis, understand the pathological basis of the diseases, and promptly give appropriate therapies.
Introduction
Paraneoplastic disorders are associated with tumors but are not caused by direct tumor invasion of the target tissue. While paraneoplastic disorders can affect many organ systems, some of the most striking and debilitating affect the nervous system. Numerous mechanisms were initially considered, but many paraneoplastic disorders now have a proven or likely autoimmune mechanism. The same immune processes may occur in other patients without tumors, and the risk of particular tumors is highly variable among different syndromes. Paraneoplastic disorders should therefore be considered autoimmune disorders affecting various regions of the nervous system, each conveying different risks for specific cancers. The evaluation of these patients is complex and usually involves recognizing the clinical syndrome, excluding other potential causes, testing for autoantibodies, and a search for tumors. Clinical care may involve immunotherapy, which often must be coordinated with tumor therapy.
Paraneoplastic disorders can be divided into four groups, roughly in order of their recognition. (1) The neuromuscular paraneoplastic disorders, typified by myasthenia gravis, generally involve autoantibodies to neuromuscular junction or peripheral nerve membrane proteins that have direct pathophysiological effects. For this reason, and due the strong reparative abilities of the PNS, these disorders respond to immunotherapy. (2) The classical paraneoplastic disorders, such as anti-Hu, involve T-cell processes targeting neurons of the CNS or PNS. Cancer associations are relatively strong and prognosis is poorer. (3) The next group involves autoantibodies to intracellular synaptic proteins such as GAD65 and amphiphysin. These both associate with stiff person syndrome and other CNS disorders. It is controversial whether these antibodies are directly pathogenic, or mark a T-cell response, or both. (4) The newest group involves antibodies to CNS synaptic and other neuronal membrane proteins such as the NMDA receptor. These disorders involve direct effects of the antibodies, have variable tumor associations, and tend to improve with immunotherapy.
Diagnosis of the paraneoplastic disorders
The evaluation of a suspected paraneoplastic syndrome is always dependent upon the recognition of a clinical syndrome through a careful history and detailed neurological examination. Once the syndrome is clear, the next steps are to recognize how various paraneoplastic disorders can enter into the differential diagnosis, perform autoantibody and other testing, search for relevant tumors, and give appropriate treatments.
In the case of encephalitis, brainstem encephalitis, or cerebellitis, brain MRI may show signs of inflammation affecting the limbic system, brainstem, or cerebellum, respectively, but is often normal. CSF analysis may show non-specific evidence of inflammation such as mild CSF pleocytosis, elevated protein, or oligoclonal bands, but is often normal. Typically, multiple other causes are evaluated in parallel to the paraneoplastic diseases. In the case of encephalitis, this may involve excluding various types of viral encephalitis and other infections such as toxoplasmosis and Cryptococcus. Nutritional (Wernicke's syndrome) and toxic causes (drug overdose, ingestions) also may be considered initially. In patients with tumors, a direct effect of the tumor (e.g. carcinomatous meningitis) should also be considered. The neuromuscular paraneoplastic disorders, such as myasthenia gravis, may have specific electrodiagnostic features that assist with diagnosis. Since multiple autoantibodies may be associated with the same clinical syndrome, a broad approach to testing is reasonable. In the case of CNS disorders, especially the CNS synaptic autoantibody disorders, testing CSF may be more sensitive than testing serum.
Testing for autoantibodies
Testing for specific autoantibodies frequently provides the key evidence supporting the diagnosis of a paraneoplastic disorder, and will often strongly influence the course of treatment. For example, physicians may or may not try immunotherapy in a patient with unexplained encephalitis, but a patient with encephalitis and NMDAR antibodies should be treated with a progressively stronger immunotherapy until clinical improvement occurs.
A positive autoantibody test has several possible meanings. (1) Patients may have pathogenic antibodies that cause or probably cause disease, such as antibodies to the nicotinic acetylcholine receptor (AchR) in myasthenia. These antibodies generally target surface epitopes of neuronal proteins and have functional effects on their targets. (2) Antibodies may also be the markers of a specific immune process but not directly pathogenic. This is most likely the case with the classic onconeuronal antibodies such as anti-Hu, anti-Ma/Ta, etc. In the appropriate clinical setting these antibodies mark a cell-mediated immune response but do not directly cause disease. Many of these antibodies target intracellular proteins and could not access the antigens on living neurons. (3) Antibodies may be a response to tumor but not indicate any significant autoimmune process. For instance, up to 40% of patients with certain types of lung cancer have anti-Hu, a number vastly in excess of those with the anti-Hu paraneoplastic syndromes. (4) Antibodies may indicate a general predisposition to autoimmunity but not be directly relevant to the disease process at hand. For instance anti-thyroid antibodies may be found in patients with NMDAR, GABA-BR and other synaptic autoimmune syndromes. In the past these patients may have been thought to have “Hashimoto's encephalitis” and there was some confusion over whether the thyroid antibodies somehow caused the encephalitis.
Paraneoplastic syndromes and paraneoplastic antibodies associate with specific tumors
While paraneoplastic disorders may occur in patients with known tumors, it is more common for the neurological syndrome to lead to the diagnosis of an otherwise asymptomatic tumor. A prompt and appropriate cancer screening in patients with these syndromes should therefore be undertaken. Both the clinical syndrome and specific autoantibody testing can guide the search for tumors. For instance, a patient with paraneoplastic cerebellar degeneration (PCD) is at risk for several types of tumors. A patient with PCD and antibodies to mGluR1 or DNER is likely to have lymphoma, but a patient with identical symptoms and antibodies to GAD65 is much less likely to have a tumor. In cases where several different types of cancer are plausible, CT-PET may be useful as a screening test. Dedicated ovarian imaging, such as with transvaginal ultrasound, may be used for diseases associated with ovarian neoplasm such as the anti-NMDAR syndrome or stiff person syndrome with amphiphysin antibodies. Testicular ultrasound may be particularly useful in male patients with Ma2 antibodies. Breast imaging with mammogram is the minimum screening for syndromes linked to breast cancer, and breast MRI could be considered. Age-appropriate cancer screening should always be brought up to date.
In addition to imaging, some practitioners recommend screening for serum cancer markers such as CA-125, CA15.3, PSA, and/or CEA.
If initial screening for cancer is negative, our center usually conducts repeat surveillance at increasing intervals. Most paraneoplastic tumors manifest within the first year after the neurological syndrome, and the likelihood of detecting a tumor declines dramatically after the first year. Worsening of a known autoimmune syndrome, or the occurrence of a new autoimmune syndrome can herald a return or worsening of a tumor.
Neuromuscular Paraneoplastic Disorders
A full review of the autoimmune neuromuscular disorders is outside the scope of this article, but they should be understood in the overall framework of paraneoplastic disease (Table 1). In most of these disorders, the autoantibodies directly affect ion channels and related proteins on peripheral nerves or NMJs, altering the physiology of the targeted systems. These disorders generally respond to immunotherapy such as steroids, IVIg, plasmapheresis, etc. Due to the strong regenerative abilities of muscle and neuromuscular junction, patients with myasthenia gravis or inflammatory myopathy, to give two examples, may have a good recovery once the immune response is controlled. The degree of recovery in paraneoplastic neuropathies depends on the degree of axonal damage, and is more variable. In addition, symptomatic treatments can help compensate for the pathophysiological effects of the antibodies: pyridostigmine increases synaptic acetylcholine for myasthenia gravis or autoimmune autonomic neuroapathy, 4-aminopyridine (4-AP) augments presynaptic calcium influx in LEMS, and sodium channel blockers such as phenytoin decrease peripheral nerve excitability in Isaacs’ syndrome.
Table 1.
Paraneoplastic and autoantibody disorders of the PNS
| Disease | Antigen(s) | Clinical Syndromes | Tumors |
|---|---|---|---|
| Myasthenia Gravis | AChR MUSK (10% of systemic MG is seronegative) |
Fatigable muscle weakness, including bulbar muscles (diploplia, dysphagia, dysarthria, neck weakness, ptosis) and respiratory muscles | 10% of anti-AchR positives have thymoma |
| Lambert-Eaton Myasthenic Syndrome (LEMS) | VGCC (85%) Unknown in 15% |
Fatigable weakness that often resembles a myopathy. Dry mouth, sexual dysfunction, constipation. | 50% have tumors, mostly small cell lung cancer. Thymoma is rarely reported |
| Autoimmune Autonomic Neuropathy | Ganglionic acetylcholine receptors (containing α3β4 subunits) | Subacute pan-dysautonomia (hypotension, anhydrosis, dry mouth/eyes, sexual dysfunction, GI dysmotility, fixed pupils, fixed heart rate) | 10% thymoma |
| Isaacs’ syndrome | VGKC complex (Caspr2 or unknown subtype) | Diffuse myokymia, cramps, hyperhidrosis, stiffness, muscle hypertrophy | Thymoma in a few patients |
| Inflammatory myopathy | Most patients with malignancy do not have Jo-1 antibodies | Muscle pain, weakness, elevated muscle enzyme levels. Dermatomyositis patients also have rash, Gottron's papules, skin and nail bed inflammation | 29% of dermatomyositis patients have breast, bladder, lung, or hemotologic cancer |
Myasthenia gravis (MG) is a disease of fatigable weakness associated with antibodies to the post-synaptic neuromuscular junction. The acetylcholine receptor (AChR) is the most common antigen, although a smaller group as antibodies to MUSK. Thymoma is present in approximately 15% of myasthenics with AchR antibodies, and about 40% of thymoma patients have MG (Marx et al., 2013). The autoantibodies are directly pathogenic.
Autoimmune Autonomic Neuropathy (AAN) is a syndrome of acquired autonomic dysfunction associated with antibodies to the acetylcholine receptors found in autonomic ganglia. Both parasympathetic (dry eyes, sexual dysfunction, fixed heart rate, sluggish pupils) and sympathetic (orthostasis, anhydrosis) symptoms peak within several months, and may partially improve with treatment (Vernino and Lennon, 2003). There is a risk of thymoma similar to that is MG (about 10%), and there are patients with both AAN and MG.
Lambert-Eaton Myasthenic Syndrome (LEMS) is a disorder of slowly progressive weakness, often with dry mouth, constipation, and sexual dysfunction (Titulaer et al., 2011). Approximately 85% of patients have antibodies to P/Q-type voltage-gated calcium channels (VGCC antibodies). About half of patients have tumors, most often small cell lung cancers. A smaller group of patients with VGCC antibodies have cerebellar syndromes, most often with LEMS but rarely in isolation (Burk et al., 2010). The antibodies are thought to be directly pathogenic.
Isaacs’ syndrome (acquired neuromyotonia) is a disorder of peripheral nerve hyperexcitability that manifests with cramps, fasciculations, myokymia, and stiffness. Some patients may show hyperhidrosis and in severe, prolonged cases muscle hypertrophy may occur. Some patients have autoantibodies to the VGKC complex, and particularly Caspr2 (see below) but in many patients the autoimmune basis of the disease is not well understood. Symptomatic treatment with sodium channel blockers, such as phenytoin or carbamazepine is first line. Immunotherapy, such as plasmapheresis, may be attempted in patients who do not respond sufficiently.
Paraprotein-associated neuropathies most often are clinically and electrophysiologically similar to chronic inflammatory demyelinating polyneuropathy (CIDP). The condition is paraneoplastic when the paraprotein is associated with multiple myeloma, solitary plasmacytoma, chronic lymphocytic leukemia, or lymphoma. But other patients with a monoclonal gammopathy of unclear significance (MGUS) have clinically identical syndromes. POEMS (polyneuropathy, organomegaly, endocrinopathy, m-spike, skin changes) affects multiple organ systems, although neuropathy is often the presenting problem, and is usually associated with osteosclerotic myeloma. The paraproteins probably cause some of these neuropathies, although a role for vascular endothelial growth factor, which is secreted by some myelomas, in causing POEMS syndrome is possible. Detailed guidelines for evaluation and treatment have been published (European Federation of Neurological et al., 2006).
Inflammatory myopathy presents with muscle pain, weakness, and elevated muscle enzymes. Approximately 3% of patients with polymyositis and 29% of patients with dermatomyositis have tumors, most commonly of the breast, ovary, or bladder (Andras et al., 2008). These tumors may precede or follow presentation of the myositis.
CNS paraneoplastic disorders
Paraneoplastic encephalomyelitis (PEM) affects multiple regions of the nervous system, causing altered consciousness and behavioral problems (limbic involvement), cranial neuropathies, vertigo and ataxia (brainstem and cerebellar involvement), and myelopathy (spinal cord involvement). Symptom onset is most often subacute (weeks to months). Anti-Hu is he most common antibody response associated with this phenotype, and sensory neuronopathy (involvement of the dorsal root ganglia) may occur in these patients. Cancer associations are strong and immunotherapy (see treatment of classical paraneoplastic disorders, below) usually can only stop the disorder from worsening. The Ma2 response is more common in young men with testicular cancer, and has a somewhat better response to therapy.
Limbic encephalitis causes subacute psychiatric manifestations (hallucinations, delusions, abnormal behaviors) and memory impairment, especially poor encoding of new memories. Seizures, especially of temporal lobe origin, are common. Brain MRI may show increased T2 signal in one or both temporal lobes that may increase with symptoms and become less prominent during remission. EEG may show abnormal excitability originating form the temporal lobes (spike-wave discharges), electrographic seizures, or even non-convulsive status epilepticus. Many different immune responses are associated with this phenotype (Tables 2, 3, and 4). The cancer associations and response to treatment are accordingly variable. Patients with antibodies to cell surface and synaptic proteins, such as the NMDA receptor, may recover well even after profound coma and unresponsiveness. Seizures and behavioral issues often improve rapidly with appropriate immunotherapy in this group, but memory deficits recover over many months.
Table 2.
Classical Paraneoplastic Disorders with antibodies to intracellular antigens
| Antibody and Antigen | Patient demographics | Clinical Syndromes | Tumors |
|---|---|---|---|
|
ANNA-1 (Hu) Target HuD and related nuclear proteins |
75% males, median 63 years | Sensory Neuropathy/neuronopathy Encephalomyelitis Cerebellar degeneration Autonomic dysfunction |
83% have tumors; small cell lung cancer most common |
|
ANNA-2 (Ri) Target the NOVA proteins. |
66% female, mean age 65 | Cerebellar degeneration Encephalomyelitis Opsoclonus-myoclonus |
86% have cancer, especially lung or breast |
| ANNA-3 | Male and female, ages 8-83 | Often multifocal and including: neuropathy, myelopathy, brainstem or limbic encephalitis | Cancer very common, especially lung |
|
PNMA-1 (Ma) Target PNMA-1 and PMNA-2, expressed in brain/testes and also in tumors |
Males and females, middle aged | Encephalitis, cerebellar ataxia, opthalmoplegia, dementia | High risk of diverse tumors (lung, breast, colon, renal, etc.) |
|
PNMA2 (Ma2) (Also known at Ta) Target PNMA-2 |
Mostly males (median age 34) with fewer females (median age 64) | Limbic encephalitis Brainstem encephalitis Cerebellar degeneration Neuropathy |
Young men with Ma2 often have germ cell tumors |
|
PCA-1 (Yo) Target cdr2, a cytoplasmic protein expressed in brain and in tumors |
Almost all female, young adult-elderly | Cerebellar degeneration | >90% have breast or ovarian cancers |
|
PCA-2 Bind a cytoplasmic protein in neurons, especially cerebellar neurons |
Cerebellar degeneration, encephalitis, autonomic dysfunction, motor neuropathy | Small cell cancers | |
|
CRMP5 CRMP5 is a neuronal protein critical for growth cone function. |
Men and women, older adults | Dementia, ataxia, myelopathy, chorea, seizures, cranial neuropathies, peripheral neuropathy, retinopathy – often multifocal | Lung cancer, thymoma |
Table 3.
Antibodies to intracellular synaptic antigens
| Antibody and Antigen | Patient demographics | Clinical Syndromes | Tumors |
|---|---|---|---|
|
GAD-65 Glutamic acid decarboxylase-65 is a synaptic enzyme that synthesizes the neurotransmitter GABA |
33-80 years, 82% female (Ariño H and Saiz A, 2014) | Cerebellar degeneration Stiff Person Syndrome (Type 1 diabetes) * may co-exist with other autoantibodies |
Usually none |
|
Amphiphyisin Amphiphysins regulate the recycling of synaptic vesicles |
Mean age 64, 60% male (Pittock et al., 2005) | Stiff person syndrome (when present in isolation) * may co-exist with other autoantibodies |
Breast and Ovarian Cancers are common |
Table 4.
The synaptic and other neuronal surface autoantibody disorders.
| Antigen | Patient demographics | Clinical Syndromes | Tumors |
|---|---|---|---|
|
NMDAR N-methyl-D-aspartate receptor is an ionotropic glutamate receptor important for a form of synaptic plasticity |
80% female, mostly children, teens, and young adults | Early hallucinations, delusions, bizarre behaviors. Evolves into seizures, abnormal movements, coma, dysautonomia, respiratory arrest. | Ovarian teratoma |
|
AMPAR 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl) propanoic acid receptor is an ionotropic glutamate receptor |
90% female, Adults-elderly | Limbic encephalitis, psychiatric manifestations | Lung, breast, thymus |
|
GABA-B-R γ-amino-butyric acid receptor B is a metabotropic inhibitory receptor |
60% male, mean age 62 but younger in non-paraneoplastic cases | Limbic encephalitis, often with severe seizures or status epilepticus. More rarely opsoclonus-myoclonus or ataxia |
50% have small cell lung cancer |
|
GABA-A-R γ-amino-butyric acid receptor A is a the primary ionotropic inhibitory receptor in the adult brain |
5 of 6 male, median age 22 years | Refractory status epilepticus or epilepsia partialis continua (Low titer responses may be be associated with diverse syndromes and other autoantibodies) |
1 of 6 high titer patients had Hodgkin's lyphoma |
|
LGI1 Leucine-rich, glioma-inactivated 1 is a secreted synaptic protein that organizes AMPA receptors and Kv1 channels at CNS synapses |
65% male, median age 60 years | Encephalitis, relatively less severe than with the above syndrome. Fasciobrachial dystonic seizures may precede encephalitis. Myoclonus (40%) may initially suggest CJD. NB: usually can be detect with VGKC radioassay |
Usually none |
|
Caspr2 Contactin-associated protein-like 2 organizes Kv1 channels one myelinated CNS and PNS axons |
85% male, median age 60 | Neuromyotonia, encephalitis or Morvan's syndrome. CNS and PNS symptoms may occur in either order. About 20% may have comorbid myasthenia gravis. |
Thymoma |
|
DPPX Dipeptidyl-peptidase-like protein 6 is a regulatory subunit of Kv4.2 potassium channels |
50% male, ages 45-76 | Encephalitis with CNS hyperexcitability (tremor, seizures, myoclonus, agitation, PERM). Prodrome of unexplained diarrhea is common (due to DPPX in myenteric plexus) | None so far |
|
GlyR Glycine Receptor is the main inhibitory ionotropic receptor in spinal cord |
6 of 11 female and ages 5-69 years in one series | Stiff person syndrome, PERM Many patients have co-existing GAD65 antibodies |
1 of 11 had Hodgkin's lymphoma |
|
mGluR5 metabotropic glutamate receptor 5 mediates certain types of synaptic plasticity in hippocampus |
So far one 15-year-old male and one 46-year-old female | Ophelia syndrome | Hodgkin's lymphoma |
|
mGluR1 metabotropic glutamate receptor 1 mediates synaptic transmission in cerebellum |
Males and females 19-69 (total of 5 cases since year 2000) | Paraneoplastic cerebellar degeneration | Hodgkin's lymphoma, prostate adenocarcinoma |
|
Homer-3 Homer-3 organizes mGluR1s at cerebellar synapses |
Single case of 38-year-old man | Paraneoplastic cerebellar degeneration | None reported to date |
|
DNER Delta/notch-like epidermal growth factor-related receptor is a notch ligand on cerebellar Purkinje neurons |
50% males, ages 12-73 in one series | Paraneoplastic cerebellar degeneration Anti-DNER was previously known as “anti-Tr”, and commercial testing often uses that name. |
90% have Hodgkin's lympohma |
Paraneoplastic cerebellar degeneration (PCD) usually presents with progressive cerebellar dysfunction over a period of several weeks. Patients may experience dysarthria, vertigo, gait instability, and limb ataxia. Visual symptoms include diploplia, nystagmus (especially up-beating), skew deviation, ocular-motor ataxia (apraxia of gaze), and hypo- or hyper-metric saccades. Visual symptoms may evolve during illness and recovery; for instance there may be prominent nystagmus early in the disease, but only hypometric saccades during recovery. Dysarthria most often has a hypophonic, strained, rasping, or harsh quality; the classic staccato dysarthria associated with cerebellar lesions is less common. The particular domains affected vary substantially among patients; some may have prominent ocular symptoms and gait instability, while others may have limb symptoms and dysarthria. Brain MRI is often normal, although some patients show enhancement or other signal abnormalities near the cerebellum. In the long term, patients may show cerebellar atrophy. Since other causes of subacute cerebellar impairment without an obvious are relatively rare, searching for a paraneoplastic or autoimmune cause is relatively high-yield compared to the other clinical syndromes associated with paraneoplastic diseases. In many patients recovery with this disorder is slow or partial, possibly due to the permanent loss of cerebellar Purkinje neurons that has been found in some postmortem studies.
Morvan's syndrome patients have symptoms affecting both the CNS (encephalitis, insomnia) and PNS (fasciculations, myokymia, neuromyotonia, pain, dysautonomia) (Irani et al., 2012). Morvan's syndrome can be thought of as Isaacs’ syndrome plus encephalitis. Patients may show profound sleep disruption, manifesting as prolonged insomnia. Some patients have thymoma. Antibodies to the VGKC complex, specifically to Caspr2 (see below), are found in some patients with Morvan's syndrome. In other patients the relevant antigens are not known.
Stiff Person Syndrome (SPS) presents with subacute stiffness of the limb and axial muscles. Patients may have painful and debilitating muscle spasms and increased tone. The reflexes may be hyperactive. Onset may be in any limb and may spread from to other body regions over weeks to months. EMG/NCS is notable for continuous discharges of voluntary motor units with attempted relaxation. Imaging is typically not informative. SPS is due to hyperexcitability at the spinal cord and brainstem level, and should be distinguished from the myotonic disorders and Isaacs’ syndrome. GAD65, amphiphysin, and glycine receptor antibodies may be found in some patients.
Progressive encephalomyelitis with rigidity and myoclonus (PERM) may be considered a variant of SPS. In addition to rigidity, myoclonus and/or encephalitis, these patients also show pathologically increased startle responses with even mild stimuli. (In some cases this may be so severe as to resemble strychnine toxicity, an observation that may have lead investigators to detect glycine receptor antibodies in some of these patients). Improvement with immunotherapy has been reported, particularly in patients with glycine receptor antibodies.
CLASSICAL PARANEOPLASTIC DISORDERS
These disorders involve T-cell immune responses to the intracellular or nuclear proteins expressed in the CNS and sometimes also the PNS. Antibody responses in these disorders are probably just markers of the underlying disease process and not directly pathogenic. The demographics, syndromes and tumor associations of these diseases are summarized in table 2. Case 1 illustrates one of the many ways these disorders may manifest.
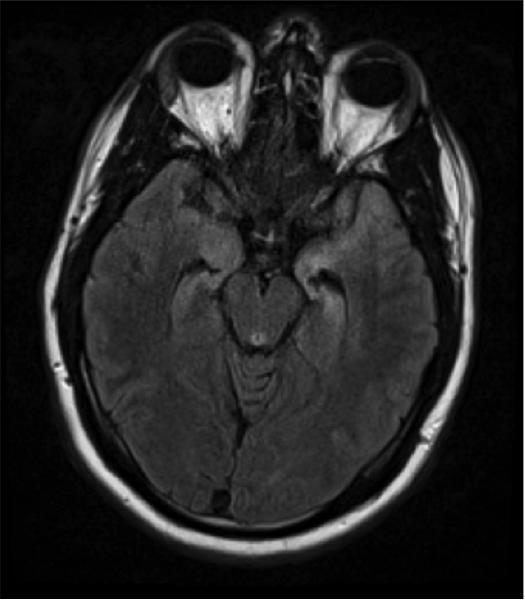
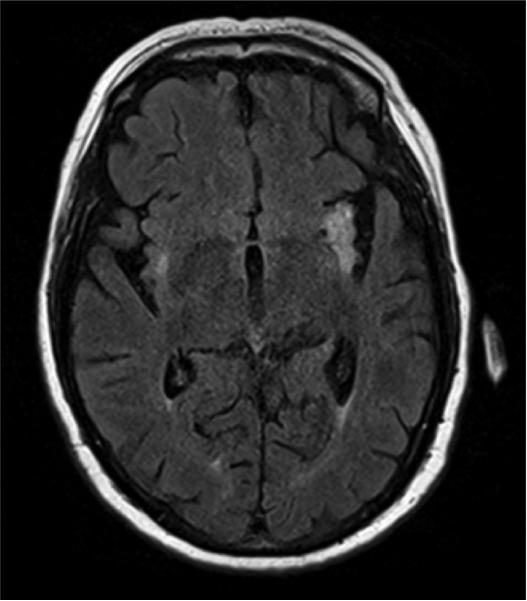
Case 1 Figure.
Brain MRI of a patient with paraneoplastic encephalomyelitis showing increased T2 signal in the medial temporal lobes.
Anti-Hu (ANNA-1) patients show diverse neurological syndromes. (Graus et al., 2001). PNS manifestations (sensory-motor neuropathy or sensory neuronopathy) are most common. Sensory neuronopathy, with profound and non-length-dependent sensory loss is most characteristic and should prompt consideration of a paraneoplastic disorder. (Slowly progressive sensory neuropathy by itself is unlikely to be paraneoplastic.) Gastroparesis may occur due to involvement of the enteric nervous system. CNS involvement may include limbic encephalitis, PEM, or PCD. Multifocal involvement is common. Patients are more often male and elderly, and anti-Hu is strongly associated with lung cancers, with other tumors being less frequent.
Anti-Ma (PNMA-1) and the anti-Ma2 responses are closely related. Patients with Anti-Ma have antibodies to two proteins, PNMA-1 and PMNA-2. Anti-Ma are associated with diverse tumors and clinical syndromes, including limbic encephalitis, memory deficits, behavioral changes, ataxia, dyarthria and opthalmoplegia (Hoffmann et al., 2008). Patients are usually middle aged and either sex. MRI may show changes in the temporal lobes, thalamus or other brain regions. Response to immunotherapy is often poor.
Anti-Ma2 (Anti-Ta; PNMA-2) associate with limbic encephalitis, brainstem encephalitis, PCD or neuropathy (Dalmau et al., 2004). Symptoms resembling narcolepsy-cataplexy may occur. Patients with brainstem involvement usually have eye movement impairments, especially impaired upward gaze. This immune response is seem most often in young men with germ cell tumors, so screening for testicular tumors is critical. Brain MRI abnormalities reflect the clinical diversity, and may involve the temporal lobes, brainstem, diencephalon or other regions. Response to immunotherapy tends to be somewhat better than for patients with Anti-Hu, but not as good as patients with autoantibodies to cell-surface antigens.
Anti-Yo (PCA-1) are found in women with PCD. A pan-cerebellar syndrome with ataxia of gaze, limb movements, and the trunk is most typical. Most patients lose the ability to stand or sit. The risk of breast or ovarian cancer is approximately 90%, and the tumors are usually detected only after the neurological manifestations occur (Peterson et al., 1992).
Anti-Ri (ANNA-2) are found in patients with brainstem encephalitis, PCD, PEM, or, more rarely, other syndromes (Pittock et al., 2003). The brainstem syndrome may include opsoclonus and/or myoclonus, cranial nerve palsies, and dysphagia. There is a high risk of breast or lung cancers. Most patients improve at least partially with treatment.
Anti-CRMP-5 associates with PCD, chorea, uveitis/retinitis, and/or neuropathy (Honnorat et al., 2009). Anti-CRMP5 may coexist with anti-Hu, and some patients have comorbid LEMS or MG. Lung and thymic tumors are common. Since CRMP-5 is an intracellular protein but closely associated with the neuronal membrane, it could reasonably be classified with the intracellular synaptic antibody disorders.
Other rarer syndromes include PCA-2 (Vernino and Lennon, 2000) and ANNA-3 (Chan et al., 2001), which are outlined briefly in table 2.
Treatment of classical paraneoplastic disorders
Evaluation for associated cancers is critical, since the neurological syndrome often leads to recognition of a new or recurrent tumor. Treatment of the tumor is crucial for stopping the immune response, not just for oncological reasons. Immunotherapy with steroids, cyclophosphamide, or other immunosuppression should be coordinated with cancer therapies and attempted promptly. Therapies aimed at the antibodies directly, such as plasmapheresis or IVIG, are unlikely to be effective. Due to the nature of the immune response, many patients do not respond to therapy, and stabilization may be the best that can be achieved in other cases.
SYNAPTIC INTRACELLULAR ANTIBODY DISORDERS
Since the antigens in these disorders are intracellular, but at areas of dynamic membrane turnover, it is unclear whether the antibodies can exert direct effects. Patients’ antibodies recognize the target synaptic proteins, but cannot under most conditions penetrate living neurons to do so (Figure 1). Responses to immunotherapy are mixed. Case 2 illustrates some of the difficulties of these situations.
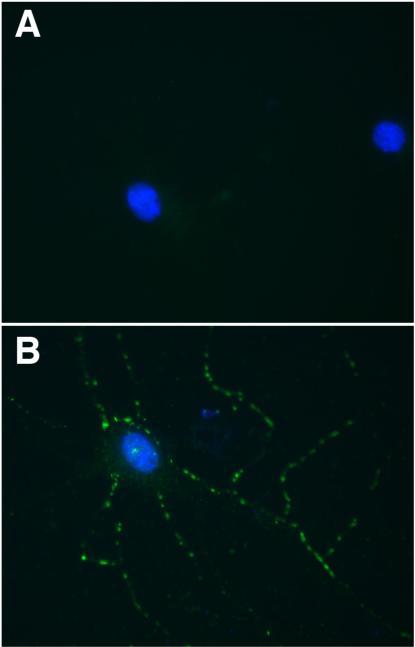
Figure 1.
Cultured rat embryonic hippocampal neurons were immunostained live with CSF of a patient with GAD65 antibodies (green), with nuclei visualized with DAPI (blue), but patients’ antibodies are unable to bind to the neurons (A).
Neurons immunostained with the same CSF after these neurons were fixed and permeabilized show reactivity of the CSF with GAD65 (B).
GAD65 antibodies have been reported in patients with stiff-person syndrome, paraneoplastic cerebellar degeneration, or seizures (Pittock et al., 2006; Ariño H and Saiz A, 2014). GAD65 is a soluble intracellular synaptic protein that produces the inhibitory neurotransmitter GABA in response to synaptic activity. While the antibodies of some patients have effects in various animal or tissue model systems, other investigators have proposed that the antibodies are merely a marker of a CD8+ T-cell immune response targeting neurons (Burton et al., 2010). GAD65 antibodies are found in many patients with type 1 diabetes, although these responses are usually lower titer and target a slightly different epitope on the protein. For this reason, GAD65 antibodies in a patient with known type 1 diabetes should be interpreted with caution. Conversely, patients with GAD65 antibodies may manifest diabetes at the same time or even after the neurological disorder. GAD65 antibodies are also found to co-exist in patients with multiple other autoantibodies, including GABA-B-R and AMPAR. This phenomenon may contribute to the different phenotypes seen with GAD65 autoimmunity. GAD65 patients only rarely have tumors.
Amphiphysin antibodies, when found in isolation, occur mostly in women with stiff person syndrome or encephalomyelitis and breast cancer or lung cancer (Pittock et al., 2005). However, amphiphysin antibodies may co-exist with other autoantibodies and, particularly in that context, occur in men and women with sensory neuropathy/neuronopathy, myelopathy, brainstem syndromes, Lambert-Eaton syndrome, and other disorders. Amphiphysin is an intracellular synaptic protein that helps with recycling synaptic vesicles. As with GAD65 antibodies, some studies have suggested a direct functional role for the antibodies, but postmortem pathology shows CD8+ T-cell infiltrates. So it is unclear whether the antibodies are primarily a marker or directly toxic.
CNS SYNAPTIC / NEURONAL SURFACE AUTOANTIBODY DISORDERS
These disorders involve antibodies to a diverse range of neuronal membrane proteins. The antigens include ionotropic receptors (NMDAR, AMPAR, GABA-AR, GlyR), metobotropic receptors (GABA-B-R, mGluR1, mGluR5, DRD2), a secreted synaptic protein (LGI1), a cell adhesion molecule that organizes potassium channels on axons (Caspr2), a synaptic scaffolding protein (Homer-3) and a Notch signaling ligand (DNER). New antigens have recently been added at a rate of approximately 2 per year, making this one of the most dynamic areas in neurology. The diagnostic tests for these disorders typically involve screening CSF samples for reactivity to cells transfected to express the antigens (“Cell-based assays”; Figure 2). Although serum can tested in this manner as well, the sensitivity and specificity is lower. In contrast to the intracellular synaptic antibodies, these cell surface antibodies recognize the target epitopes on living neurons (Figure 3). Identification of these diseases should prompt immunotherapy since most patients improve considerably. Each immune response is associated with characteristic syndromes. Cases 3 and 4 explore some of these manifestations.
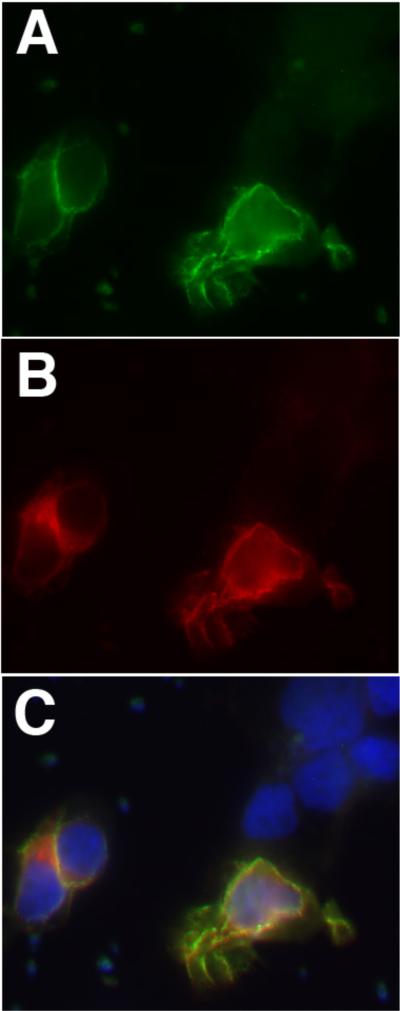
Figure 2.
Cells transfected to express a synaptic antigen (in this example Caspr2) were immunostained with patient CSF (in green; A) and a commercial antibody to Caspr2 (in red; B). Merge images show colocalization, with the nuclei of transfected and untransfected cells labeled blue with DAPI (C).
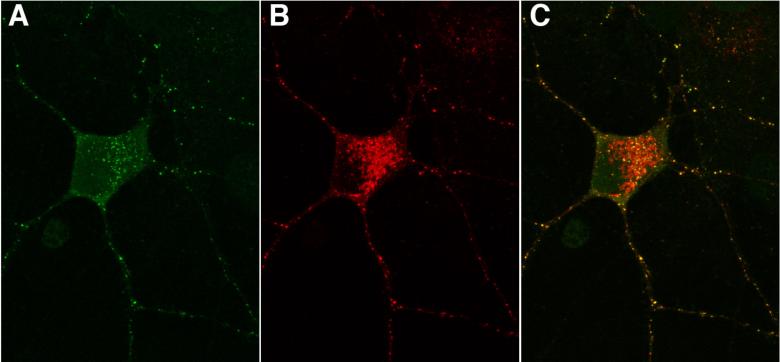
Figure 3.
Cultured rat embryonic hippocampal neurons were immunostained live with CSF of a patient with cell surface antibodies (in this case to DNER) (A). The same neurons were immunostaining with a commercial antibody to DNER (B). Merged images demonstrate the colocalization (C).
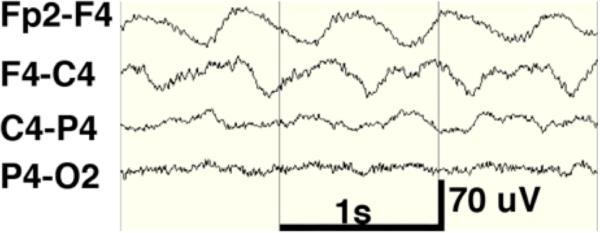
Case 3 Figure.
The extreme delta brush EEG pattern.
Case 4.
Brain MRI of a patient with autoimmune encephalitis, showing involvement of the insular cortex, especially on the left side.
NMDAR antibodies may be found in patients with non-specific encephalitis but more often associate with a multi-stage clinical syndrome that may begin after a viral prodrome. The initial neuropsychiatric symptoms include behavioral change, memory loss, hallucinations and delusions, often resulting in psychiatric evaluation. Seizures, autonomic instability, decreased responsiveness, dyskinesias (especially writhing movements of the face, tongue, and limbs), and eventually respiratory failure emerge as the disease progresses. In many cases patients become catatonic and unresponsive to all stimuli, ventilator dependent and very difficult to manage due to autonomic instability. Many patients in the comatose phase show dystonic postures and continuous movements of the lips and face. EEG of patients in coma may show a characteristic pattern, “extreme delta brush”. MRI may show increased T2 signal in the medial temporal lobes in some patients. CSF may show signs of inflammation (pleocytosis, increased IgG index, oligoclonal bands, increased protein). Treatment protocols typically start with IVIg and IV steroids, followed by cyclophosphamide and/or rituximab if the first line treatments are not effective (Dalmau et al., 2011). Treatment should escalate and the most effective treatment continued until recovery. While a minority of patients relapse, it is not clear whether ongoing immunosuppression with agents such as mycophenylate mofetil prevents this. In general, early treatment associates with better outcomes. Patients typically pass through the stages of the disorder in reverse during recovery, so a repeat of the psychiatric symptoms may occur as patients awake from coma. Recovery is usually slow, occurring over many months or even years. Seizures and psychotic symptoms typically fully resolve during the recovery phase, so medications for these symptoms are usually gradually discontinued. Difficulty consolidating new memories may be the last symptom to recover. Ovarian teratoma is the only tumor strongly associated with NMDAR antibodies; pelvic ultrasound and pelvic MRI are helpful tests.
NMDAR antibodies were the first of the CNS synaptic antibodies to be characterized in patients with encephalitis and appear to be more common than the other disorders of this type, even among patients with encephalitis but not the other characteristic symptoms associated with NMDAR antibodies. The antibodies have direct effects on the target receptors (Moscato et al., 2010).
AMPAR antibodies are found in patients with encephalitis, particularly in older women with breast, lung, or other cancers (Lai et al., 2009). As with NMDAR antibodies, in some patients the initial symptoms may be psychiatric. AMPAR antibodies cause cross-linking and internalization of receptors resulting in decreased AMPAR signaling (Gleichman et al., 2014).
Voltage-gated potassium channel complex (VGKC) antibodies were initially reported in patients with Isaacs’ syndrome, but soon found in other patients with encephalitis or Morvan's syndrome. A recent advance in the field is the discovery that these antibodies do not actually target voltage-gated potassium channel subunits (Kv1.1/Kv1.2 containing potassium channels) but rather proteins that associate with these channels (Irani et al., 2010). The two primary target antigens are LGI1 and Caspr2, although a significant subset of patients may target other antigens yet to be identified. The pathophysiological significant of a low titer response that does not target LGI1 or Caspr2, especially when found only in serum, is uncertain; such results should be interpreted with care.
LGI1 antibodies associate with a relatively mild form of encephalitis (Lai et al., 2010). Patients may have myoclonus. Seizures are common, although only a subset show the fasciobrachial dystonic seizures (very rapid spasms involving the face, neck and upper limb on one side) that are characteristic of this disease. LGI1 antibodies do not convincingly associate with PNS symptoms. LGI1 is a secreted protein that binds to both presynaptic and post-synaptic proteins (ADAM22 and ADAM23) to organize AMPARs and VGKCs at CNS synapses. LGI1 antibodies have recently been shown to block the interactions of LGI1 with the ADAM proteins, resulting in improper expression of AMPARs, which may be important to the pathogenesis of this disease (Ohkawa et al., 2013).
GABA-B-R antibodies associate with encephalitis, often with severe seizures or status epilepticus. A recent series of 20 patients with this disorder confirmed that limbic encephalitis is the primary phenotype, that about half of patients have small cell lung cancer, and that most patients responded to immunotherapies and/or tumor therapy (Hoftberger et al., 2013b).
GABA-A-R antibodies have recently been reported at high titer in 6 patients with encephalitis. All six patients developed status epilepticus or epilepsia partialis contiinua (Petit-Pedrol et al., 2014). Since GABA-A is the most prevalent inhibitory ionotropic receptor in the adult CNS, and the antibodies selectively decreased GABA-A receptors at synapses, severe epilepsy is a logical outcome in this disease. Low titer responses may be found in patients with other autoimmune neurological disorders and are of uncertain pathophysiological significance.
mGluR5 antibodies are found in patients with Ophelia syndrome, a rare disorder of memory and cognition in patients with Hodgkin's lymphoma that reverses with treatment of the tumor (Lancaster et al., 2011). In most patients, the psychiatric manifestations are much quieter than those seem in patients with NMDAR antibodies. The neurological symptoms are most often the presenting symptom of the tumor.
mGluR1 antibodies are found in patients with cerebellitis, and about half of reported patients have Hodgkin's lymphoma (Coesmans et al., 2003). Interesting, mGluR1 is the closest homolog of mGluR5, but mGluR1 is important for synaptic transmission in the cerebellum while mGluR5 is more important for synaptic signaling in the hippocampus. The antibodies studied to date are always specific for either mGluR1 or mGluR5. (This appears to be a general pattern with synaptic autoantibodies; they are highly specific for one individual protein).
Homer-3 antibodies target post-synaptic scaffolding protein important for modulating the group 1 metabotropic glutamate receptors (mGluR1, mGluR5). Two patients with a pattern of Purkinje cell antibody reactivity similar to mGluR1 and cerebellar ataxia have been reported to have Homer-3 antibodies (Zuliani et al., 2007; Hoftberger et al., 2013a). These patients did not have tumors, and Homer-3 antibodies do not appear to co-exist in patients with other antibodies.
DNER (delta/notch-like EGF-related receptor) antibodies are found in patients with paraneoplastic cerebellar degeneration. About 90% of reported cases associate with Hodgkin's lymphoma. This antibody response was recognized decades ago by Trotter and colleagues and cases were characterized by a pattern of reactivity with cerebellar sections (“anti-Tr”). Recently, deGraaf et al. have shown the DNER is the true target epitope of this antibody response (de Graaff et al., 2012), a finding we have confirmed in our laboratory. DNER is a Notch ligand expressed on cerebellar Purkinje neurons, and the antibodies may have functional effects on DNER-Notch signaling between Purkinje neurons and apposed Bergmann glia, which express Notch. While the true disease mechanisms have not been established, DNER antibodies target surface epitopes of DNER and react with live neurons, and therefore should not be grouped with conventional paraneoplastic antibodies.
Dopamine D2 receptor (DRD2) antibodies have been reported in children with basal ganglia encephalitis. Patients typically have both psychiatric (psychosis, disturbed attention, emotional liability) and movement (chorea, parkinsonism, dystonia) symptoms (Dale et al., 2012). Some patients have responded to treatment with IVIG and/or steroids, and regained normal function. These antibodies have also been reported in patients with Sydenham chorea and the antibodies are thought to have direct functional effects on the receptor (Cox et al., 2013).
Glycine receptor (GlyR) antibodies are found in patients with stiff person syndrome or PERM (Hutchinson et al., 2008). Genetic mutations affecting GlyR result in hyperekplexia (human startle disease), so the association with PERM, which is often marked by a pathologically exaggerated startle response, is intriguing. GlyR antibodies may co-exist with GAD65 or other autoantibodies.
Treatment of the CNS synaptic and cell surface autoimmune disorders
In addition to tumor therapy (when a tumor is present), synaptic autoimmune disorders are usually treated initially with IVIg and IV steroids. Plasmapheresis is generally less effective in reducing CNS antibodies compared to peripheral antibodies in disorders such as myasthenia, and our group generally does not use it. In hospitalized patients who don't respond to first line treatments, rituximab and/or cyclophosphamide should be considered. Rituximab may take weeks to exert its full effects since it does not affect matured plasma cells. Cyclophosphamide can cause infertility, particularly with repeated doses; GNRH agonist treatment may reduce the risk of infertility for reproductive-aged women (Clowse et al., 2009). Sperm or egg donation should be addressed in some patients as this treatment is planned in case multiple doses are needed. Some practitioners use mycophenolate mofetil or azathioprine to prevent relapses, but there is no evidence concerning the efficacy of these treatments.
Most patients with anti-NMDAR encephalitis have a monophasic illness but the others disorders may be more prone to relapse. The decision to give more immunotherapy should be guided primarily by the clinical examination.
Antibody titers in autoimmune encephalitis
CSF NMDAR antibody titers have been shown to correlate with disease activity (e.g. they are higher in active disease or during relapses and lower in remission) (Gresa-Arribas et al., 2014). Before titers can be applied to clinical practice, several factors should be considered. First, titers in serum are not predictive, and only CSF titers seem to correlate with disease. Second, only in disorders where antibodies are pathogenic does it make sense to follow titers. Third, the comparison of the patient CSF samples should be done side-by-side in the same laboratory, and only significant changes in titers (at least 2 units) should be considered meaningful. There may therefore be some use to banking CSF from different stages of the disease (acute presentation, recovery) for use in analyzing potential future relapses. When there is a clear clinical relapse, titers are usually not needed to guide treatment. But titers may be helpful for cases where it is not clear whether a clinical decline represents a true relapse or has some other cause. Advice for evaluating a suspected relapse is included in Table 5.
Table 5.
Evaluation of new symptoms in patients with prior autoimmune encephalitis. This is a difficult area but several guidelines can be offered:
| • The clinical findings are paramount. Worsening of the specific neurological findings without an alternative cause trumps antibody tests, radiology, and CSF analysis. |
| • Progression of findings on MRI (such as T2 signal changes in the temporal lobes) often supports disease activity. |
| • Seizures usually resolve with treatment of the autoimmune encephalitis. Therefore new or worsened seizures may suggest relapse. |
| • If the CSF is bland and the antibody response is no longer present, the patients’ symptoms are less likely to be due to the autoimmune encephalitis. |
| • If the CSF shows signs of inflammation, this favors and active disease process. |
| • CSF results may be ambiguous: the antibody response persists but there is no evidence of inflammation. |
| • Sera titers are not generally useful since antibody response can persist in the sera and not the CSF or vice versa. |
| • CSF titers may be useful with these caveats: samples from different time points must be studied carefully side by side in a skilled lab, the titer must change by at least two points of measurement (e.g. not 1:10 to 1:20). A significant increase in titer compared to CSF obtained during a remission favors a relapse. |
Conclusions
The paraneoplastic and autoimmune disorders represent a rapidly evolving field of neurology. Neurologist can help these patients by providing clear diagnoses, finding associated cancers, and giving the appropriate treatments. New diseases continue to be discovered, so patients who do not fit known disorders should have research-based studies. Many aspects of these diseases are not completely understood, including the pathophysiology of many disorders, and the optimal treatments.
CASE 1
A 22-year-old man presented with 3 years of vertigo, and 2 years of generalized tonic-clonic seizures. Over the previous 6 months he also has had slurred speech and disconjugate gaze, and increased stiffness in the legs. He had periods of staring and confusion, as well as worsening memory and concentration. Examination showed that he was alert and cooperative. He was oriented to month and city, but not date, day of the week, or place. He had dysarthria but no aphasia. Recall was 1/3 at 5 minutes. There was continuous down-beating nystagmus that was worse with left gaze. Bilateral cranial nerve 6 palsies were noted, and there was also decreased upward gaze for both eyes. There was mild right facial, arm and leg weakness, as well as subtle slowing of right-sided movements compared to the left. Limb reflexes were 3+ except 4+ (non-sustained clonus) at the ankles. Gait was spastic and he could not perform a tandem gait.
Lumbar puncture showed protein 33 mg/dL, glucose 63 mg/dL, 1 WBC/ul, 2 RBC/ul, 5 oligoclonal bands, and elevated IgG index (1.77). Brain MRI showed increased T2 signal in the medial temporal lobes (Figure 3). PET/CT showed increased radionucleotide uptake in the left temporal lobe. Serology showed positive Ma2 antibodies in CSF and serum.
He was treated with steroids, IVIG, and cyclophosphamide. Symptoms stabilized and his gaze problems improved slightly (less nystagmus and partial improvement in the 6th nerve palsies) over the next six months. Seizures also became controlled on a single anti-epileptic medication. Repeat PET/CT scans for cancer and 6 months and 1 year showed no malignancy. Repeated testicular ultrasound should a small nodule that was biopsied and shown to be a benign cyst, and no further abnormalities were detected on repeated scans 2 years after diagnosis.
Comment
Encephalitis involving the limbic system and brainstem is a common phenotype with anti-Ma2. Eye movement abnormalities may affect approximately 90% of patients, and multiple cranial neuropathies may occur with these syndrome. Due to the strong association of anti-Ma2 with testicular tumors in young men, periodic testicular ultrasound is a prudent precaution.
CASE 2
A 65-year-old previously healthy woman developed diploplia and difficulty reading over 2 weeks. As her visual symptoms continue to worsen she developed dysarthria, dysphagia, and gait instability over the following 8 weeks. Examination was remarkable for down-beating nyastagmus in all stages of gaze, dysarthria and ataxia of lower extremity movements. Severe vertigo limited her ability to travel by car or plane. MRI showed faint enhancement of the cerebellar folia. Lumbar puncture showed 15 WBC/ul, 3 RBC/ul, and protein of 65 mg/dl. Testing for HIV, syphilis, lyme disease, HSV pcr, VZV pcr, CMV pcr, and CSF cultures were negative. GAD65 antibodies were strongly positive in CSF and serum. PET/CT was performed and showed no evidence of malignancy. Treatment with steroids was considered but at this time she was diagnosed with type 1 diabetes. IVIg (2 g/kg) was given in monthly cycles for 3 months without benefit. Cyclophosphamide (750 mg/m2) was given in month cycles for 3 months, also without evident benefit. She continued to have severe vertigo and difficulty seeing due to constant nystagmus. She had slowly progressive upper extremity ataxia that limited the ability to use the right hand slightly. Lower extremity ataxia slow progressed to the point where transfers were very difficult and she was entirely wheel-chair bound.
Comment
This case of paraneoplastic cerebellar degeneration associated with GAD65 antibodies highlights several aspects of this disorder, including the relatively poor response to many patients to immunotherapy. GAD65 antibodies are associated with type 1 diabetes, which may manifest at the same time as the neurological symptoms. Most GAD65 positive patients do not have tumors, but the is higher risk for other autoantibodies associated with this phenotype.
CASE 3
A 28-year-old healthy woman developed confusion, hallucinations, and bizarre behaviors over 2 weeks. She was hospitalized on psychiatry service and treated with risperidone. Over the following 2 weeks her mental status worsened: she became less responsive to external stimuli, had rigid posturing of the upper extremities, and periods of tachycardia were noted. She had a generalized convulsion and was transferred to a neurology service. EEG showed slow delta with superimposed fast beta (extreme delta brush; Figure 1). Lumbar puncture showed 13 WBC/uL, 2 RBC/uL, protein 35 mg/dL, normal glucose and 3 oligoclonal bands. NMDAR antibodies were positive in CSF. Brain MRI with and without gadolinium contrast was normal. Ovarian ultrasound and pelvic MRI showed no evidence of teratoma.
Seizures were controlled with phenytoin, but she remained unresponsive (even to pain) with occasional writing movements of the lips and tongue, and periods of rigid posturing of the upper extremities. She continued to have periods of tachycardia up to 200 beats/min and hypertension. IVIG (2 grams/kg over 5 days) and solumedrol were given but no improvement was noted over the following 2 weeks. Rituximab (375 mg/m2 weekly for 4 week) and cyclophosphamide (750 mg/m2 once with the first dose of rituximab) were given. 20 days after the start of rituximab, she opened her eyes and in the subsequent days started following commands. Over the following 7 months she regained the ability to live independently, but had some persistent difficulties with memory. These gradually improved and she returned to work until 18 months after the onset of illness.
Comment
This case illustrates the typical clinical course of anti-NMDAR encephalitis. Many patients do not respond to first line treatments but may still have good recovery when second-line treatments are given. The extreme delta brush EEG finding is seen in some patients during the comatose phase of the illness. Recovery is often good but may take many months.
CASE 4
A 55-year-old man had 4 complex partial seizures over 4 weeks. He had increasing difficulty remembering appointments, irritability, and lethargy. He had previously kept meticulous records and financial accounts for his small business, but these were “a complete mess” according to his wife. Examination was notable only for decreased short-term memory (1/3 recall at 5 minutes even with prompting) and impaired concentration tasks (serial 7s, spelling WORLD backwards). Brain MRI was normal. EEG showed rare spike and wave discharges from the left temporal region. Serum B12, TSH, RPR, HIV, folate, electrotyles, complete blood count, renal function tests, and liver function tests were normal. Over the next 2 weeks he developed more significant confusion, and angry verbal outbursts on a daily basis. He had a total of 10 seizures over these two weeks despite titration of levetiracetam to 1500 mg bid. Repeat examination at that time showed periods of agitation alternating with periods of somnolence, extremely poor memory (with no ability to register new information), and confabulation. Repeat brain MRI showed increased T2 signal in the left more than right medial temporal lobes extending upwards to involve the insular cortex. EEG monitoring showed increased spike and wave discharges from the left temporal region and a complex partial seizure originating from this region. Lumbar puncture showed elevated CSF protein (62 mg/dL, normal 20-45 mg/dL), normal glucose, 2/uL WBC, 4/uL RBC, and no Oligoclonal bands. A panel of antibody tests showed VGKC complex antibodies (Caspr2 subtype). He was treated with 5 days of IV solumedrol (1 gram/day) and then oral prednisone at 60 mg/day.
He recovered to normal mental status over 3 months, but had a brief relapse with steroid taper at 1 year after onset that responded to increased prednisone dose.
Comment
The encephalitis associated with antibodies to LGI1 or Caspr2 tends to be milder than that associated with NMDAR antibodies, rarely associates with tumors (a risk of thymoma in the case of Caspr2 antibodies) and often responds to immunotherapy. Many patients have a good recovery. However, some cases can have relapses, necessitating prolonged immunotherapy.
KEY POINT.
Autoantibody testing can be extremely helpful in diagnosing paraneoplastic disorders.
Antibodies to surface epitopes of neuronal receptors and other membrane proteins may have direct pathogenic effects, but antibodies to intracellular proteins are more likely just markers of other autoimmune mechanisms.
A positive antibody test may sometimes be an incidental finding that does not directly explain the patient's symptoms. Antibodies to non-neuronal proteins, such as thyroid antibodies or anti-nuclear antibodies, may indicate a general predisposition to autoimmunity.
Cancer screening should be informed by the paraneoplastic syndrome and the autoantibody findings.
A paraneoplastic syndrome in a patient with a known tumor, even one thought to be cured, should prompt an investigation into whether the tumor has returned or spread.
Antibodies to GAD65 and Amphiphysin target intracellular synaptic proteins and associate with stiff person syndrome or other CNS disorders.
Amphiphysin antibodies are strongly associated with breast or gynecologic cancers.
Patients with NMDAR antibodies often have psychiatric manifestations first and progress to develop seizures, dyskinesias, catatonia, autonomic instability and respiratory failure.
Memory impairment (inability to consolidate new memories) is nearly universal in anti-NMDAR encephalitis, and is often the last symptom to recover.
Patients recovering from NMDAR antibodies may awake from coma and then pass through the psychotic phase of the illness again as they become more responsive.
VGKC complex antibodies do not actually target potassium channels but rather associated proteins.
VGKC complex antibodies that do not target LGI1 or Caspr2, especially those that are low titer and present only in serum, are of uncertain significance.
Antibodies to either GABA-A-R or GABA-B-R target inhibitory neurotransmitter receptors and associate with encephalitis, particularly with severe seizures, status epilepticus or epilepsia partialis continua.
Antibodies to mGluR5 are found in Ophelia syndrome, a disorder of memory and cognition in the setting of Hodgkin's lymphoma that responds to tumor treatment.
Antibodies to GAD65, mGluR1, DNER, VGCC, and Homer-3 have been associated with cerebellar syndromes. Of these, mGluR1 and DNER are strongly associated with Hodgkin's lymphoma.
Treatment of synaptic autoimmune disorders should be guided primarily by the patient's symptoms. There may be a role for comparing CSF titers from different phases of illness in evaluating suspect relapses.
Footnotes
Disclosures: None.
REFERENCES
- Andras C, Ponyi A, Constantin T, Csiki Z, Szekanecz E, Szodoray P, Danko K. Dermatomyositis and polymyositis associated with malignancy: a 21-year retrospective study. The Journal of rheumatology. 2008;35:438–444. [PubMed] [Google Scholar]
- Ariño HG-AN, Blanco Y, Martínez-Hernández E, Sabater L, Petit-Pedrol M, Rouco I, Bataller L, Dalmau JO, Saiz AGF. Cerebellar Ataxia and Glutamic Acid Decarboxylase Antibodies Immunologic Profile and Long-term Effect of Immunotherapy. JAMA neurology. 2014 doi: 10.1001/jamaneurol.2014.1011. Epub June 16, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk K, Wick M, Roth G, Decker P, Voltz R. Antineuronal antibodies in sporadic late-onset cerebellar ataxia. Journal of neurology. 2010;257:59–62. doi: 10.1007/s00415-009-5262-8. [DOI] [PubMed] [Google Scholar]
- Burton AR, Baquet Z, Eisenbarth GS, Tisch R, Smeyne R, Workman CJ, Vignali DA. Central nervous system destruction mediated by glutamic acid decarboxylase-specific CD4+ T cells. J Immunol. 2010;184:4863–4870. doi: 10.4049/jimmunol.0903728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KH, Vernino S, Lennon VA. ANNA-3 anti-neuronal nuclear antibody: marker of lung cancer-related autoimmunity. Annals of neurology. 2001;50:301311. doi: 10.1002/ana.1127. [DOI] [PubMed] [Google Scholar]
- Clowse ME, Behera MA, Anders CK, Copland S, Coffman CJ, Leppert PC, Bastian LA. Ovarian preservation by GnRH agonists during chemotherapy: a meta-analysis. Journal of women's health. 2009;18:311–319. doi: 10.1089/jwh.2008.0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coesmans M, Smitt PA, Linden DJ, Shigemoto R, Hirano T, Yamakawa Y, van Alphen AM, Luo C, van der Geest Jn, Kros JM, Gaillard CA, Frens MA, de Zeeuw CI. Mechanisms underlying cerebellar motor deficits due to mGluR1-autoantibodies. Annals of neurology. 2003;53:325–336. doi: 10.1002/ana.10451. [DOI] [PubMed] [Google Scholar]
- Cox CJ, Sharma M, Leckman JF, Zuccolo J, Zuccolo A, Kovoor A, Swedo SE, Cunningham MW. Brain human monoclonal autoantibody from sydenham chorea targets dopaminergic neurons in transgenic mice and signals dopamine D2 receptor: implications in human disease. J Immunol. 2013;191:5524–5541. doi: 10.4049/jimmunol.1102592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale RC, Merheb V, Pillai S, Wang D, Cantrill L, Murphy TK, Ben-Pazi H, Varadkar S, Aumann TD, Horne MK, Church AJ, Fath T, Brilot F. Antibodies to surface dopamine-2 receptor in autoimmune movement and psychiatric disorders. Brain : a journal of neurology. 2012;135:3453–3468. doi: 10.1093/brain/aws256. [DOI] [PubMed] [Google Scholar]
- Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet neurology. 2011;10:63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau J, Graus F, Villarejo A, Posner JB, Blumenthal D, Thiessen B, Saiz A, Meneses P, Rosenfeld MR. Clinical analysis of anti-Ma2-associated encephalitis. Brain : a journal of neurology. 2004;127:1831–1844. doi: 10.1093/brain/awh203. [DOI] [PubMed] [Google Scholar]
- de Graaff E, Maat P, Hulsenboom E, van den Berg R, van den Bent M, Demmers J, Lugtenburg PJ, Hoogenraad CC, Sillevis Smitt P. Identification of delta/notch-like epidermal growth factor-related receptor as the Tr antigen in paraneoplastic cerebellar degeneration. Annals of neurology. 2012;71:815–824. doi: 10.1002/ana.23550. [DOI] [PubMed] [Google Scholar]
- European Federation of Neurological S. Peripheral Nerve S, Hadden RD, Nobile-Orazio E, Sommer C, Hahn A, Illa I, Morra E, Pollard J, Hughes RA, Bouche P, Cornblath D, Evers E, Koski CL, Leger JM, Van den Bergh P, van Doorn P, van Schaik IN. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of paraproteinaemic demyelinating neuropathies: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2006;13:809–818. doi: 10.1111/j.1468-1331.2006.01467.x. [DOI] [PubMed] [Google Scholar]
- Gleichman AJ, Panzer JA, Baumann BH, Dalmau J, Lynch DR. Antigenic and mechanistic characterization of anti-AMPA receptor encephalitis. Annals of clinical and translational neurology. 2014;1:180–189. doi: 10.1002/acn3.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus F, Keime-Guibert F, Rene R, Benyahia B, Ribalta T, Ascaso C, Escaramis G, Delattre JY. Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain : a journal of neurology. 2001;124:1138–1148. doi: 10.1093/brain/124.6.1138. [DOI] [PubMed] [Google Scholar]
- Gresa-Arribas N, Titulaer MJ, Torrents A, Aguilar E, McCracken L, Leypoldt F, Gleichman AJ, Balice-Gordon R, Rosenfeld MR, Lynch D, Graus F, Dalmau J. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet neurology. 2014;13:167–177. doi: 10.1016/S1474-4422(13)70282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann LA, Jarius S, Pellkofer HL, Schueller M, Krumbholz M, Koenig F, Johannis W, la Fougere C, Newman T, Vincent A, Voltz R. Anti-Ma and anti-Ta associated paraneoplastic neurological syndromes: 22 newly diagnosed patients and review of previous cases. Journal of neurology, neurosurgery, and psychiatry. 2008;79:767–773. doi: 10.1136/jnnp.2007.118588. [DOI] [PubMed] [Google Scholar]
- Hoftberger R, Sabater L, Ortega A, Dalmau J, Graus F. Patient with homer-3 antibodies and cerebellitis. JAMA neurology. 2013a;70:506–509. doi: 10.1001/jamaneurol.2013.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoftberger R, Titulaer MJ, Sabater L, Dome B, Rozsas A, Hegedus B, Hoda MA, Laszlo V, Ankersmit HJ, Harms L, Boyero S, de Felipe A, Saiz A, Dalmau J, Graus F. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology. 2013b;81:1500–1506. doi: 10.1212/WNL.0b013e3182a9585f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honnorat J, Cartalat-Carel S, Ricard D, Camdessanche JP, Carpentier AF, Rogemond V, Chapuis F, Aguera M, Decullier E, Duchemin AM, Graus F, Antoine JC. Onco-neural antibodies and tumour type determine survival and neurological symptoms in paraneoplastic neurological syndromes with Hu or CV2/CRMP5 antibodies. Journal of neurology, neurosurgery, and psychiatry. 2009;80:412–416. doi: 10.1136/jnnp.2007.138016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson M, Waters P, McHugh J, Gorman G, O'Riordan S, Connolly S, Hager H, Yu P, Becker CM, Vincent A. Progressive encephalomyelitis, rigidity, and myoclonus: a novel glycine receptor antibody. Neurology. 2008;71:1291–1292. doi: 10.1212/01.wnl.0000327606.50322.f0. [DOI] [PubMed] [Google Scholar]
- Irani SR, Alexander S, Waters P, Kleopa KA, Pettingill P, Zuliani L, Peles E, Buckley C, Lang B, Vincent A. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain : a journal of neurology. 2010;133:2734–2748. doi: 10.1093/brain/awq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani SR, Pettingill P, Kleopa KA, Schiza N, Waters P, Mazia C, Zuliani L, Watanabe O, Lang B, Buckley C, Vincent A. Morvan syndrome: clinical and serological observations in 29 cases. Annals of neurology. 2012;72:241–255. doi: 10.1002/ana.23577. [DOI] [PubMed] [Google Scholar]
- Lai M, Huijbers MG, Lancaster E, Graus F, Bataller L, Balice-Gordon R, Cowell JK, Dalmau J. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet neurology. 2010;9:776–785. doi: 10.1016/S1474-4422(10)70137-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M, Hughes EG, Peng X, Zhou L, Gleichman AJ, Shu H, Mata S, Kremens D, Vitaliani R, Geschwind MD, Bataller L, Kalb RG, Davis R, Graus F, Lynch DR, Balice-Gordon R, Dalmau J. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Annals of neurology. 2009;65:424–434. doi: 10.1002/ana.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster E, Martinez-Hernandez E, Titulaer MJ, Boulos M, Weaver S, Antoine JC, Liebers E, Kornblum C, Bien CG, Honnorat J, Wong S, Xu J, Contractor A, Balice-Gordon R, Dalmau J. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. 2011 doi: 10.1212/WNL.0b013e3182364a44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx A, Pfister F, Schalke B, Saruhan-Direskeneli G, Melms A, Strobel P. The different roles of the thymus in the pathogenesis of the various myasthenia gravis subtypes. Autoimmunity reviews. 2013;12:875–884. doi: 10.1016/j.autrev.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Moscato EH, Jain A, Peng X, Hughes EG, Dalmau J, Balice-Gordon RJ. Mechanisms underlying autoimmune synaptic encephalitis leading to disorders of memory, behavior and cognition: insights from molecular, cellular and synaptic studies. The European journal of neuroscience. 2010;32:298309. doi: 10.1111/j.1460-9568.2010.07349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa T, Fukata Y, Yamasaki M, Miyazaki T, Yokoi N, Takashima H, Watanabe M, Watanabe O, Fukata M. Autoantibodies to epilepsy-related LGI1 in limbic encephalitis neutralize LGI1-ADAM22 interaction and reduce synaptic AMPA receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:18161–18174. doi: 10.1523/JNEUROSCI.3506-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K, Rosenblum MK, Kotanides H, Posner JB. Paraneoplastic cerebellar degeneration. I. A clinical analysis of 55 anti-Yo antibody-positive patients. Neurology. 1992;42:1931–1937. doi: 10.1212/wnl.42.10.1931. [DOI] [PubMed] [Google Scholar]
- Petit-Pedrol M, Armangue T, Peng X, Bataller L, Cellucci T, Davis R, McCracken L, Martinez-Hernandez E, Mason WP, Kruer MC, Ritacco DG, Grisold W, Meaney BF, Alcala C, Sillevis-Smitt P, Titulaer MJ, Balice-Gordon R, Graus F, Dalmau J. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet neurology. 2014;13:276286. doi: 10.1016/S1474-4422(13)70299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittock SJ, Lucchinetti CF, Lennon VA. Anti-neuronal nuclear autoantibody type 2: paraneoplastic accompaniments. Annals of neurology. 2003;53:580–587. doi: 10.1002/ana.10518. [DOI] [PubMed] [Google Scholar]
- Pittock SJ, Yoshikawa H, Ahlskog JE, Tisch SH, Benarroch EE, Kryzer TJ, Lennon VA. Glutamic acid decarboxylase autoimmunity with brainstem, extrapyramidal, and spinal cord dysfunction. Mayo Clinic proceedings Mayo Clinic. 2006;81:1207–1214. doi: 10.4065/81.9.1207. [DOI] [PubMed] [Google Scholar]
- Pittock SJ, Lucchinetti CF, Parisi JE, Benarroch EE, Mokri B, Stephan CL, Kim KK, Kilimann MW, Lennon VA. Amphiphysin autoimmunity: paraneoplastic accompaniments. Annals of neurology. 2005;58:96–107. doi: 10.1002/ana.20529. [DOI] [PubMed] [Google Scholar]
- Titulaer MJ, Lang B, Verschuuren JJ. Lambert-Eaton myasthenic syndrome: from clinical characteristics to therapeutic strategies. Lancet neurology. 2011;10:1098–1107. doi: 10.1016/S1474-4422(11)70245-9. [DOI] [PubMed] [Google Scholar]
- Vernino S, Lennon VA. New Purkinje cell antibody (PCA-2): marker of lung cancer-related neurological autoimmunity. Annals of neurology. 2000;47:297–305. [PubMed] [Google Scholar]
- Vernino S, Lennon VA. Neuronal ganglionic acetylcholine receptor autoimmunity. Annals of the New York Academy of Sciences. 2003;998:211–214. doi: 10.1196/annals.1254.023. [DOI] [PubMed] [Google Scholar]
- Zuliani L, Sabater L, Saiz A, Baiges JJ, Giometto B, Graus F. Homer 3 autoimmunity in subacute idiopathic cerebellar ataxia. Neurology. 2007;68:239240. doi: 10.1212/01.wnl.0000251308.79366.f9. [DOI] [PubMed] [Google Scholar]