Abstract
Purpose of review
Macrophage activation syndrome (MAS), a major cause of morbidity and mortality in pediatric rheumatology, is most strongly associated with systemic juvenile idiopathic arthritis (SJIA). There are no validated diagnostic criteria and early diagnosis is difficult. This review summarizes the progress in understanding of MAS pathophysiology that may help define specific diagnostic biomarkers.
Recent findings
MAS is similar to the autosomal recessive disorders collectively known as familial hemophagocytic lymphohistiocytosis (FHLH), all associated with various genetic defects affecting the cytolytic pathway. Cytolytic function is profoundly depressed in SJIA with MAS as well. This immunologic abnormality distinguishes SJIA from other rheumatic diseases and is caused by both genetic and acquired factors. Phenotypic characterization of hemophagocytic macrophages has been another focus of research. These macrophages express CD163, a scavenger receptor that binds hemoglobin– haptoglobin complexes, and initiate pathways important for adaptation to oxidative stress induced by free iron. Expansion of these macrophages is seen in more than 30% of SJIA patients perhaps representing early stages of MAS. Recent gene expression studies linked expansion of these macrophages to distinct signatures.
Summary
Recent advances in understanding of pathophysiologic conditions that favor expansion of hemophagocytic macrophages provide a source of new MAS biomarkers with applicability to clinical practice.
Keywords: alternatively activated macrophages, CD163, hemophagocytic lymphohistiocytosis, macrophage activation syndrome, MUNC13-4, systemic juvenile arthritis
Introduction
Macrophage activation syndrome (MAS) is a severe, potentially fatal condition associated with excessive activation and expansion of macrophages and T cells (mainly CD8+) leading to an overwhelming inflammatory reaction. The main manifestations of MAS include fever, hepatosplenomegaly, lymphadenopathy, severe cytopenias, liver dysfunction, and coagulopathy consistent with disseminated intravascular coagulation [1–5]. The pathognomonic feature of MAS is the expansion of well differentiated macrophages exhibiting hemophagocytic activity. These macrophages are typically found in bone marrow or lymph nodes, but they may infiltrate almost any organ in the body and may account for many of the systemic features of this syndrome, including cytopenias and coagulopathy. In rheumatology, MAS is most strongly associated with the systemic form of juvenile idiopathic arthritis (SJIA). In fact, it accounts for much of the morbidity and mortality seen in this disease. About 10% of the patients with SJIA develop overt life-threatening MAS [6], and this may occur at any time point during the course of SJIA. Two recent studies [7,8] suggested that in a mild ‘subclinical’ form MAS may be occurring in as many as 25–30% of SJIA patients with active systemic disease. Systemic lupus erythematosus and Kawasaki disease are two other diseases in which MAS seems to occur more frequently compared with other rheumatic conditions [9•,10,11].
It is now widely recognized that MAS bears close resemblance to a group of histiocytic disorders collectively known as hemophagocytic lymphohistiocytosis (HLH) [4,12,13]. HLH is a term that describes a spectrum of disease processes characterized by abnormal accumulations of well differentiated mononuclear cells with a macrophage phenotype [14,15]. Currently, HLH includes the autosomal recessive genetic disorders known as familial HLH (FHLH) as well as the acquired forms known as reactive HLH. However, the distinction between genetic and acquired causes is becoming increasingly blurred as new genetic defects are identified, some of which lead to a relatively mild presentation of the syndrome that may occur later in life [15].
Cytolytic abnormalities in hemophagocytic syndromes
In genetic HLH, the uncontrolled proliferation of T cells and macrophages has been linked to decreased natural killer (NK) cell and cytotoxic T-lymphocyte (CTL) function [16]. The cytotoxic activity of these cells is mediated by the release of specialized cytotoxic granules that contain several classes of proteins expressed only in cytotoxic cells, including perforin and granzymes. Once cytotoxic cells are activated, these granules are delivered to the cell surface and their content is released at the immunologic synapse with the target cell. Perforin aids in delivery of the granule contents into the cytoplasm of the target cell, whereas granzymes trigger apoptosis once in the cytoplasm of the target cell. In 15–40% of patients with FHLH, cytolytic dysfunction is owing to mutations in the gene encoding perforin [17]. More recently, mutations in another gene, MUNC13-4, have been implicated in the development of hemophagocytic lymphohistiocytosis in about 10–30% of patients with inherited HLH [18]. The protein encoded by the MUNC13-4 gene is important for docking and fusion of the cytotoxic granules with the cytoplasmic membrane. Although the cytolytic cells of the patients with FHLH caused by MUNC13-4 mutations produce sufficient amounts of perforin, the poor ability to deliver the content of the cytolytic granules to the immunologic synapse with the target cell leads to profoundly decreased cytolytic activity. Defects in granule-dependent cytotoxic functions of lymphocytes have also been implicated in three other genetic diseases associated with the hemophagocytic syndrome. Mutations in the gene encoding Rab27a, one of the MUNC13-4 effector molecules, have been linked to the development of Griscelli syndrome type 2 [19]. Mutations in the Lyst gene have been identified as a cause of Chediak–Higashi syndrome [20]. Mutations in the SAP/SH2D1A gene, which encodes an adaptor protein critical for lymphocyte activation including granule-mediated cytotoxicity, are associated with X-linked lymphoproliferative disease [21].
Cytolytic dysfunction and T-cell/macrophage expansion
The presence of defects in the granule-dependent cytotoxic activity of lymphocytes in several diseases associated with hemophagocytic syndromes highlights the importance of this function in restraining some inflammatory responses. The specific mechanisms that link deficient NK cell and CTL functions with expansion of activated macrophages are not clear. One possible explanation is related to the fact that poor cytolytic activity seen in HLH/MAS patients may lead to diminished ability to control some infections [22]. More specifically, NK cells and cytotoxic T lymphocytes fail to kill infected cells and thus to remove the source of antigenic stimulation. Such persistent antigen stimulation leads, in turn, to persistent antigen-driven activation and proliferation of T cells associated with escalating production of cytokines that stimulate macrophages. It has also been hypothesized that abnormal cytotoxic cells may fail to provide appropriate apoptotic signals for removal of activated macrophages and T cells during the contraction stage of the immune response [23,24], leading to persistent expansion of T cells and macrophages that secrete proinflammatory cytokines. As a result of continuous stimulation with proinflammatory cytokines (most notably INF-γ), macrophages become hemophagocytic.
Cytolytic dysfunction in macrophage activation syndrome/systemic juvenile idiopathic arthritis
Given strong clinical similarities between MAS and FHLH, the cytolytic function in MAS was assessed by several groups and found to be profoundly depressed [25,26]. Furthermore, immunologic abnormalities similar to those seen in HLH and MAS, that is poor NK cell cytolytic activity often associated with abnormal levels of perforin expression, also have been reported to distinguish SJIA from other clinical forms of childhood arthritis [26,27]. Further, it has been proposed that the presence of such cytolytic dysfunction may identify patients at risk for MAS [27]. It appears that multiple factors, both genetic and acquired, are responsible for the development of the cytolytic dysfunction in SJIA patients. Two SJIA patients carrying biallelic FHLH-associated mutations in the MUNC13-4 gene have been recently reported [28]. Zhang et al. [29] also reported an association with single-nucleotide polymorphisms (SNPs) in the MUNC13-4 gene inherited as an extended haplotype in the majority of MAS patients. Vastert et al. [30•] described the association between MAS in SJIA and SNPs and/or heterozygous mutations in gene encoding perforin.
It has also been suggested that decreased NK cell function in SJIA could be induced by the rather unique cytokine and chemokine environment present in this disease. De Jager et al. [31] recently linked suppressed NK cell function in SJIA to defective phosphorylation and signaling by the IL-18 receptor β chain, which could explain reduced NK cell function in SJIA, despite high circulating levels of the NK cell stimulator, IL-18. Interestingly, a negative effect of IL-18 on NK cell homeostasis was also noted in HIV-infected patients [32]. Although the hypothesis that impaired cytotoxicity seen in SJIA is indeed linked to the expansion of hemophagocytic macrophages still remains to be proven, the extent of immunologic similarities between SJIA and HLH is quite striking.
Phenotype of hemophagocytic macrophages
Phenotypic characterization of the hemophagocytic macrophages in MAS has been another major focus of research [7,8,11,33].
An emerging paradigm is that macrophages are polarized by their environment, particularly the cytokine milieu, to distinct functional programs, much like Th1 and Th2 cells. The polarizing activation pathways of macrophages are referred to as the M1 and M2 pathways [34–36]. Classically activated M1 macrophages participate as inducers and effectors in polarized Th1 responses and TLR4 ligands alone or in combination with IFN-γ drive this pathway of macrophage differentiation. M2 macrophages, or alternatively activated macrophages, are more heterogeneous in terms of cytokines that induce their differentiation as well as the phenotypic characteristics acquired as a result of such differentiation. M2 macrophages have been implicated in tissue remodeling and repair, resistance to parasites, immunoregulation, and tumor promotion. Three M2 subclasses have been described (reviewed in [36]). The M2a phenotype is induced by IL-4 or IL-13. In these cells, production of proinflammatory mediators is downregulated, whereas expression of IL-1 receptor antagonist (IL1-ra) and decoy IL-1 receptor (IL-1RII) is enhanced. Caspase-1, which cleaves pro-IL-1β into active IL-1β, is reduced. Surface expression of CD14 and CCR5 also is reduced, whereas expression of several scavenger receptors, including mannose receptor C-1 (MRC1) and scavenger receptor-A (SR-A), and C-type membrane lectins is increased. The M2b phenotype is elicited after ligation of Fc receptors by immune complexes in the presence of TLR4 ligands or IL-1β. M2b cells express low IL-12 and high IL-10, favoring the development of Th2 responses and IgG1 responses. Unlike M2a cells, M2b cells produce significant amounts of TNFα, IL-1β and IL-6. They also produce the chemokine CCL1, which has been shown to recruit T regulatory cells. The M2c category includes phenotypes induced by IL-10, glucocorticoids or TGFβ and is itself heterogeneous, but the common feature is reduced pro-inflammatory cytokines and increased expression of scavenger receptors. One scavenger receptor, CD163 (see below), is particularly expressed on glucocorticoid-induced M2c cells. M2c cells are thought to represent de-activated macrophages that participate in the termination of inflammation.
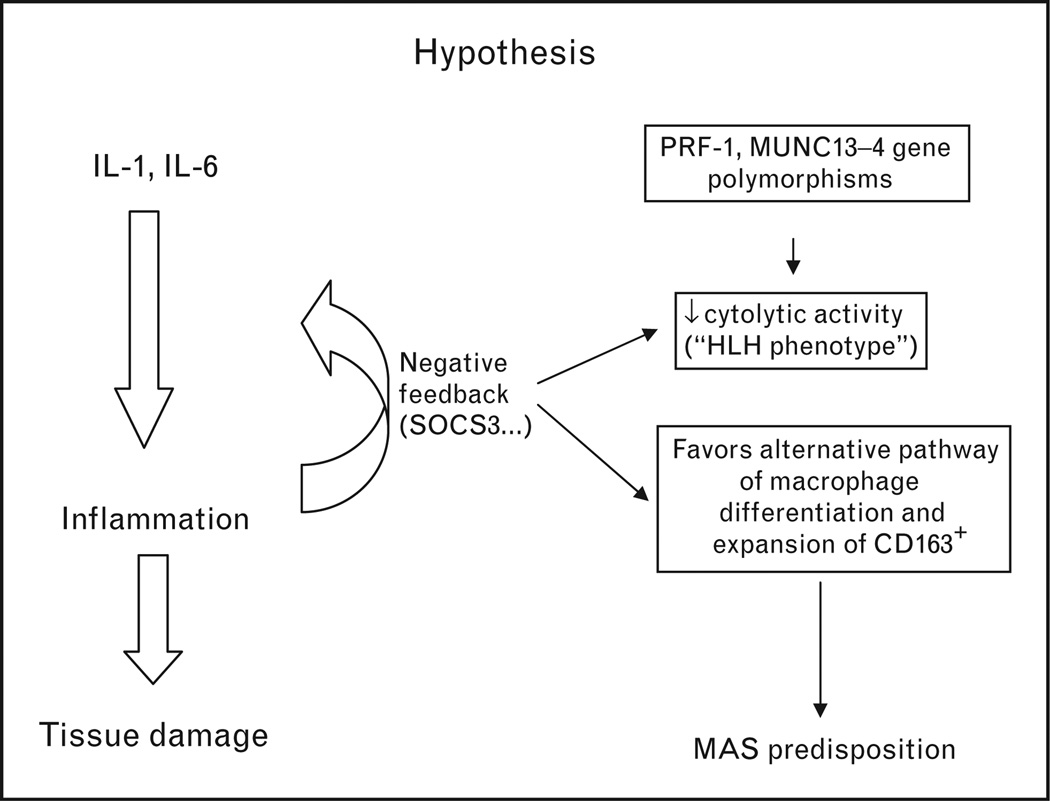
One perplexing feature of the hemophagocytic macrophages in MAS is that these cells exhibit many characteristics of alternatively activated (non-M1) macrophages including the expression of the scavenger receptor CD163 [7,8,11,33]. Given the highly inflammatory nature of SJIA and some evidence for increased IFN-γ [37,38], the emergence of alternatively activated M2 macrophages in this setting is surprising. One possible explanation is altered responsiveness of monocytes/macrophages to IFN-γ. Indeed, in the reported SJIA gene expression profiles, the IFN-induced gene expression signature is conspicuously absent. Another striking feature is consistently high levels of expression of SOCS3 in monocytes and T cells [39–41]. Interestingly, the pattern of expression of SOCS3 in SJIA parallels the expression of genes involved in negative regulation of innate immune responses, including those induced by IL-1 and IL-6, two cytokines that play the pivotal role in this disease. SOCS3 is a suppressor of cytokine signaling protein that interacts with the JAK/STAT signaling pathway leading to decreased responsiveness of immune cells to IFN-γ [42]. Supporting the model of resistance to IFN-γ, defective signaling in response to IFN-γ is observed in monocytes of SJIA patients compared to healthy controls (Macaubas et al., unpublished observation). Combined, these observations have led to the hypothesis that there may be a connection between the anti-inflammatory negative feedback loops in SJIA and conditions that would favor the expansion of CD163+ macrophages (see Fig. 1). In other words, altered responsiveness of monocytes to INF-γ, combined with the presence of cytokines implicated in alternative pathways of macrophage activation, such as IL-10, could lead to a net effect that favors the alternative activation. This view may be too simple; nonetheless, accumulating evidence suggests that mononuclear phagocytes in SJIA have a distinct phenotype that needs further characterization in future studies.
Figure 1. Proposed mechanisms that may lead to increased predisposition to macrophage activation syndrome in systemic juvenile idiopathic arthritis.
In active systemic juvenile idiopathic arthritis (SJIA), expression of the markers of the alternative pathway of macrophage differentiation parallels expression of negative regulators of innate immune responses including SOCS3 and IL-10. SOCS3 interacts with JAK/STAT signaling pathways leading to decreased responsiveness of monocytes/macrophages to IFN-γ. Altered responsiveness to IFN-γ combined with the presence of IL-10 may skew differentiation of monocytes towards the scavenger macrophages, which exhibit increased phagocytic activity and are CD163+. The presence of cytolytic dysfunction in SJIA patients that appears to be caused by both genetic and acquired factors may be another contributing factor.
Although there is some evidence to suggest that CD163 expressed on resident macrophages may act as innate immune sensor for bacteria and trigger production of pro-inflammatory cytokines [43], the only proven function of CD163 is related to its ability to bind hemoglobin–haptoglobin complexes and initiate pathways important for the adaptation to oxidative stress induced by free heme and iron [44]. Free heme is a source of redox active iron. To prevent cell damage caused by iron-derived reactive oxygen species, haptoglobin forms a complex with free hemoglobin. The haptoglobin–hemoglobin (Hp–Hb) complexes then bind to CD163 and are internalized by the macrophage. Endocytosis of Hp–Hb complexes leads to upregulation of heme-oxygenase enzymatic activity. Heme-oxygenase degrades the heme subunit of Hb into biliverdin that is subsequently converted to bilirubin, carbon monoxide, and free iron. The free iron is either sequestered in association with ferritin within the cell or transported and distributed to red blood cell precursors in the bone marrow. Increased uptake of Hp–Hb complexes by macrophages leads to increased synthesis of ferritin.
Because the sequestration of free iron by ferritin is an important component of these pathways, increased release of free hemoglobin associated with increased erythrophagocytosis would require increased ferritin production to sequestrate excessive amounts of free iron. Consistent with this, highly elevated level of serum ferritin is an important diagnostic feature of hemophagocytic syndromes.
Interestingly, a very high level of heme-oxygenase activity in freshly isolated PBMC appears to distinguish SJIA from other febrile illnesses [45•] and high serum ferritin levels are seen in a large proportion of patients with active SJIA without apparent MAS. These observations suggest that this pathway is important in the pathogenesis of not only MAS but SJIA in general. Consistent with this notion, expansion of CD163+ macrophages in the bone marrow may be seen not only in full-blown MAS but also in as many as 30–50% of patients with active SJIA without clinically apparent MAS [7,8].
As these macrophages appear to have the potential to become hemophagocytic, it has been suggested that such expansion may represent the early stages of MAS. Interestingly, in the report by Fall et al. [40], the signature of alternatively activated macrophages was particularly strong in the group of patients with very high levels of ferritin.
Macrophage activation syndrome diagnosis
Even with treatment in a timely manner, MAS can be fatal. The diagnosis, however, is very difficult in part owing to strong similarities between MAS and sepsis. There are no validated diagnostic criteria for MAS, and early diagnosis is often difficult. In general, in a patient with persistently active underlying rheumatologic disease, a fall in the ESR and platelet count, particularly in a combination with persistently high CRP and increasing levels of serum D-dimer and ferritin, should raise a suspicion of impending MAS. The diagnosis of MAS is usually confirmed by the demonstration of hemophagocytosis in the bone marrow. However, false negatives may occur owing to sampling errors, particularly at the early stages of the syndrome. In some patients, subsequent biopsies have revealed hemophagocytic macrophages in organs such as liver, lymph nodes, or lungs. In patients with negative bone marrow biopsies, assessment of the levels of sIL2Rα and sCD163 in serum may help with the timely diagnosis of MAS. Soluble IL2Rα receptors and soluble CD163 are now increasingly recognized as important biomarkers of hemophagocytic syndromes [33,46]. Because sIL2Rα and sCD163 are soluble molecules shed from the surfaces of activated T cells and macrophages, respectively, their levels are likely to increase in the serum regardless of the tissue localization of the cells. Although mild elevation of sIL2Rα has been reported in many rheumatic diseases including JIA and SLE [47], a several-fold increase in the levels of sIL2Rα in these diseases is highly suggestive of MAS [7,48]. Importantly, however, other clinical entities associated with high levels of sIL2Rα include malignancies and some viral infections, such as viral hepatitis, and these conditions should be considered in the differential diagnosis.
In contrast to MAS, the diagnosis of HLH is usually based on the diagnostic criteria developed by the International Histiocyte Society [49]. Unfortunately, the application of the HLH diagnostic criteria to SJIA patients with suspected MAS is problematic. Some of the HLH markers such as lymphadenopathy, splenomegaly, and hyperferritinemia are common features of active systemic JIA itself and therefore do not distinguish MAS from a conventional systemic JIA flare. Other HLH criteria, such as cytopenias and hypofibrinogenemia, become evident only at the late stages. This is related to the fact that SJIA patients often have increased white blood cell and platelet counts and elevated serum levels of fibrinogen as a part of the inflammatory response in this disease. Therefore, when they develop MAS, they demonstrate the degree of cytopenias and hypofibrinogenemia seen in HLH only at the late stages of the syndrome, when their management becomes challenging.
Diagnosis of MAS is even more problematic in SLE patients with autoimmune cytopenias, which are difficult to distinguish from cytopenias caused by MAS. In these patients, the presence of extreme hyperferritinemia and LDH elevation should raise suspicion of MAS [9•]. Attempts to modify the HLH criteria to increase their sensitivity and specificity for the diagnosis of MAS in rheumatic conditions have been initiated [50].
Conclusion
MAS remains a major cause of morbidity and mortality in pediatric rheumatology. There are still no validated diagnostic criteria and early diagnosis is difficult. However, the progress in understanding of the MAS pathophysiology and identification of the pathways associated with the early stages of this syndrome bring a promise to develop new biomarkers for clinical practice.
Acknowledgements
A.A.G. is supported by the NIH grant AR AR047784.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 619).
- 1.Silverman ED, Miller JJ, Bernstein B, Shafai T. Consumption coagulopathy associated with systemic juvenile rheumatoid arthritis. J Pediatr. 1983;103:872–876. doi: 10.1016/s0022-3476(83)80704-5. [DOI] [PubMed] [Google Scholar]
- 2.Hadchouel M, Prieur AM, Griscelli C. Acute hemorrhagic, hepatic, and neurologic manifestations in juvenile rheumatoid arthritis: possible relationship to drugs or infection. J Pediatr. 1985;106:561–566. doi: 10.1016/s0022-3476(85)80072-x. [DOI] [PubMed] [Google Scholar]
- 3.Stephan JL, Zeller J, Hubert P, et al. Macrophage activation syndrome and rheumatic disease in childhood: a report of four new cases. Clin Exp Rheumatol. 1993;11:451–456. [PubMed] [Google Scholar]
- 4.Grom AA. NK dysfunction: a common pathway in systemic onset juvenile rheumatoid arthritis, macrophage activation syndrome, and hemophagocytic lymphohistiocytosis. Arthritis Rheum. 2004;50:689–698. doi: 10.1002/art.20198. [DOI] [PubMed] [Google Scholar]
- 5.Stephan JL, Kone-Paut I, Galambrun C, et al. Reactive haemophagocytic syndrome in children with inflammatory disorders: a retrospective study of 24 patients. Rheumatology (Oxford) 2001;40:1285–1292. doi: 10.1093/rheumatology/40.11.1285. [DOI] [PubMed] [Google Scholar]
- 6.Sawhney S, Woo P, Murray KJ. Macrophage activation syndrome: a potentially fatal complication of rheumatic disorders. Arch Dis Child. 2001;85:421–426. doi: 10.1136/adc.85.5.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bleesing J, Prada A, Villanueva J, et al. The diagnostic significance of soluble CD163 and soluble IL2Ra chains in macrophage activation syndrome and untreated new onset systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007;56:965–971. doi: 10.1002/art.22416. [DOI] [PubMed] [Google Scholar]
- 8.Behrens EM, Beukelman T, Paessler M, Cron RQ. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J Rheumatol. 2007;34:1133–1138. [PubMed] [Google Scholar]
- 9. Parodi A, Davì S, Pringe AB, et al. Macrophage activation syndrome in juvenile systemic lupus erythematosus: multinational multicenter study of 38 patients. Arthritis Rheum. 2009;60:3388–3399. doi: 10.1002/art.24883. This is the first comprehensive evaluation of clinical features of MAS in pediatric systemic lupus erythematosus.
- 10.Muise A, Tallett SE, Silverman ED. Are children with Kawasaki disease and prolonged fever at risk for macrophage activation syndrome? Pediatrics. 2003;112:e495–e497. doi: 10.1542/peds.112.6.e495. [DOI] [PubMed] [Google Scholar]
- 11.Avcin T, Tse SML, Schneider R, et al. Macrophage activation syndrome as the presenting manifestation of rheumatic diseases in childhood. J Pediatr. 2006;148:683–686. doi: 10.1016/j.jpeds.2005.12.070. [DOI] [PubMed] [Google Scholar]
- 12.Athreya BH. Is macrophage activation syndrome a new entity? Clin Exp Rheumatol. 2002;20:121–123. [PubMed] [Google Scholar]
- 13.Ramanan AV, Baildam EM. Macrophage activation syndrome is hemophagocytic lymphohistiocytosis: need for the right terminology. J Rheumatol. 2002;29:1105. [PubMed] [Google Scholar]
- 14.Favara BE, Feller AC, Pauli M, et al. Contemporary classification of histiocytic disorders. The WHO Committee on Histiocytic/Reticulum Cell Proliferations. Reclassification Working Group of the Histiocyte Society. Med Pediatr Oncol. 1997;29:157–166. doi: 10.1002/(sici)1096-911x(199709)29:3<157::aid-mpo1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 15.Filipovich HA. Hemophagocytic lymphohistiocytosis and related disorders. Hematology Am Soc Hematol Educ Program. 2009:127–131. doi: 10.1182/asheducation-2009.1.127. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan KE, Delaat CA, Douglas SD, Filipovich AH. Defective natural killer cell function in patients with hemophagocytic lymphohistiocytosis and first degree relatives. Pediatr Res. 1998;44:465–468. doi: 10.1203/00006450-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Stepp SE, Dufourcq-Lagelouse R, Le Deist F, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286:1957–1959. [PubMed] [Google Scholar]
- 18.Feldmann J, Callebaut I, Raposo G, et al. MUNC13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–473. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 19.Menasche G, Pastural E, Feldman J, et al. Mutations in Rab27a cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genetics. 2000;25:173–176. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- 20.Barbosa MD, Nguyen QA, Tchernev VT, et al. Identification of the homologous beige and Chediak – Higashi syndromegenes (LYST) Nature. 1996;382:262–265. doi: 10.1038/382262a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coffey AJ, Brooksbank RA, Brandau O, et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet. 1998;20:129–135. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- 22.Menasche G, Feldmann J, Fischer A, de Saint Basile G. Primary hemophagocytic syndromes point to a direct link between lymphocyte cytotoxicity and homeostasis. Immunol Rev. 2005;203:165–179. doi: 10.1111/j.0105-2896.2005.00224.x. [DOI] [PubMed] [Google Scholar]
- 23.Kagi D, Odermatt B, Mak TW. Homeostatic regulation of CD8+ T cells by perforin. Eur J Immunol. 1999;29:3262–3272. doi: 10.1002/(SICI)1521-4141(199910)29:10<3262::AID-IMMU3262>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 24.Verbsky JW, Grossman WJ. Hemophagocytic lymphohistiocytosis: diagnosis, pathophysiology, treatment, and future perspectives. Ann Med. 2006;38:20–31. doi: 10.1080/07853890500465189. [DOI] [PubMed] [Google Scholar]
- 25.Grom AA, Villanueva J, Lee S, et al. Natural killer cell dysfunction in patients with systemic-onset juvenile rheumatoid arthritis and macrophage activation syndrome. J Pediatr. 2003;142:292–296. doi: 10.1067/mpd.2003.110. [DOI] [PubMed] [Google Scholar]
- 26.Wulffraat NM, Rijkers GT, Elst E, et al. Reduced perforin expression in systemic onset juvenile idiopathic arthritis is restored by autologous stem cell transplantation. Rheumatology (Oxford) 2003;42:375–379. doi: 10.1093/rheumatology/keg074. [DOI] [PubMed] [Google Scholar]
- 27.Villanueva J, Lee S, Giannini EH, et al. Natural killer cell dysfunction is a distinguishing feature of systemic onset juvenile rheumatoid arthritis and macrophage activation syndrome. Arthritis Res Ther. 2005;7:R30–R37. doi: 10.1186/ar1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hazen MM, Woodward AL, Hofman I, et al. Mutations of the hemophagocytosis-associated gene UNC13D in a patient with systemic juvenile idiopathic arthritis. Arthritis Rheum. 2008;58:567–570. doi: 10.1002/art.23199. [DOI] [PubMed] [Google Scholar]
- 29.Zhang K, Biroscak J, Glass DN, et al. Macrophage activation syndrome in systemic juvenile idiopathic arthritis is associated with MUNC13D gene polymorphisms. Arthritis Rheum. 2008;58:2892–2896. doi: 10.1002/art.23734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vastert SJ, Van Wijk R, D’Urbano LE, et al. Mutations in the perforin gene can be linked to macrophage activation syndrome in patients with systemic onset juvenile idiopathic arthritis. Rheumatology (Oxford) 2010;49:441–449. doi: 10.1093/rheumatology/kep418. The first study that linked genetic polymorphisms in the perforin gene to MAS presenting as a complication of systemic JIA.
- 31.De Jager W, Vastert SJ, Beekman JM, et al. Defective phosphorylation of IL-18 receptor beta causes impaired natural killer cell function in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2009;60:2782–2793. doi: 10.1002/art.24750. [DOI] [PubMed] [Google Scholar]
- 32.Iannello A, Samarani S, Debbeche O, et al. Potential role of interleukin-18 in the immunopathogenesis of AIDS: involvement in fratricidal killing of NK cells. J Virol. 2009;83:5999–6010. doi: 10.1128/JVI.02350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaer DJ, Schleiffenbaum B, Kurrer M, et al. Soluble hemoglobin-haptoglobin scavenger receptor CD163 as a lineage-specific marker in the reactive hemophagocytic syndrome. Eur J Haemotol. 2005;74:6–10. doi: 10.1111/j.1600-0609.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- 34.Porcheray F, Viaud S, Rimaniol A-C, et al. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142:481–489. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 36.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Ann Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 37.Imagawa T, Umebayashi H, Kurosawa R, et al. Differences between systemic onset juvenile idiopathic arthritis and macrophage activation syndrome from the standpoint of the proinflammatory cytokine profiles [abstract] Arthritis Rheum. 2004;50:S92. [Google Scholar]
- 38.De Jager W, Hoppenreijs EP, Wulffraat NM, et al. Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: a cross-sectional study. Ann Rheum Dis. 2007;66:589–598. doi: 10.1136/ard.2006.061853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pascual V, Allantaz F, Arce E, et al. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201:1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fall N, Barnes M, Thornton S, et al. Gene expression profiling of peripheral blood from patients with untreated new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis Rheum. 2007;56:3793–3804. doi: 10.1002/art.22981. [DOI] [PubMed] [Google Scholar]
- 41.Ogilvie EM, Khan A, Hubank M, et al. Specific gene expression profiles in systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007;56:1954–1965. doi: 10.1002/art.22644. [DOI] [PubMed] [Google Scholar]
- 42.Stoiber D, Kovarik P, Cohney S, et al. Lipopolysacharide induces in macrophages the synthesis of the suppressor of cytokine signaling 3 and suppresses signal transduction in response to the activationg factor IFN-γ. J Immunol. 1999;163:2640–2647. [PubMed] [Google Scholar]
- 43.Fabriek BO, Van Bruggen R, Deng DM, et al. The macrophage scavenger receptor CD163 functions as an immune sensor for bacteria. Blood. 2009;113:887–892. doi: 10.1182/blood-2008-07-167064. [DOI] [PubMed] [Google Scholar]
- 44.Kristiansen M, Graversen JH, Jacobsen C, et al. Identification of the hemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 45. Takahashi A, Mori M, Naruto T, et al. The role of heme oxygenase-1 in systemic onset juvenile idiopathic arthritis. Mod Rheumatol. 2009;19:302–308. doi: 10.1007/s10165-009-0152-6. This study confirmed the importance of the pathways involving heme oxygenases not only in MAS but in systemic JIA in general.
- 46.Komp DM, Mcnamara J, Buckley P. Elevated soluble interleukin-2 receptor in childhood hemophagocytic histiocytic syndromes. Blood. 1989;73:2128–2132. [PubMed] [Google Scholar]
- 47.Rubin LA. The soluble interleukin-2 receptor in rheumatic disease. Arthritis Rheum. 1990;33:1145–1148. doi: 10.1002/art.1780330814. [DOI] [PubMed] [Google Scholar]
- 48.Coca A, Bundy KW, Marston B, et al. Macrophage activation syndrome: serological markers and treatment with antithymocyte globulin. Clin Immunol. 2009;132:10–18. doi: 10.1016/j.clim.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Henter JI, Horne A, Arico M, et al. HLH-2004 diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatric Blood Cancer. 2007;48:124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 50.Ravelli A, Magni-Manzoni S, Pistorio A, et al. Preliminary diagnostic guidelines for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. J Pediatr. 2005;146:598–604. doi: 10.1016/j.jpeds.2004.12.016. [DOI] [PubMed] [Google Scholar]