Abstract
Recent studies revealed a causal link between ventral tegmental area (VTA) phasic dopamine (DA) activity and pro-depressive and antidepressant-like behavioral responses in rodent models of depression. Cholinergic activity in the VTA has been demonstrated to regulate phasic DA activity, but the role of VTA cholinergic mechanisms in depression-related behavior is unclear. The goal of this study was to determine whether pharmacological manipulation of VTA cholinergic activity altered behavioral responding in the forced swim test (FST) in rats. Here, male Sprague-Dawley rats received systemic or VTA-specific administration of the acetylcholinesterase inhibitor, physostigmine (systemic; 0.06 or 0.125 mg/kg, intra-cranial; 1 or 2 μg/side), the muscarinic acetylcholine receptor (AChR) antagonist scopolamine (2.4 or 24 μg/side), or the nicotinic AChR antagonist mecamylamine (3 or 30 μg/side), prior to the FST test session. In control experiments, locomotor activity was also examined following systemic and intra-cranial administration of cholinergic drugs. Physostigmine administration, either systemically or directly into the VTA, significantly increased immobility time in FST, whereas physostigmine infusion into a dorsal control site did not alter immobility time. In contrast, VTA infusion of either scopolamine or mecamylamine decreased immobility time, consistent with an antidepressant-like effect. Finally, the VTA physostigmine-induced increase in immobility was blocked by co-administration with scopolamine, but unaltered by co-administration with mecamylamine. These data show that enhancing VTA cholinergic tone and blocking VTA AChRs has opposing effects in FST. Together, the findings provide evidence for a role of VTA cholinergic mechanisms in behavioral responses in FST.
Keywords: Ventral tegmental area, forced swim test, mecamylamine, scopolamine, physostigmine, acetylcholine
1. Introduction
Depression affects approximately 1 in 6 individuals in the United States [1] and there is a critical need for improved understanding of the neurocircuitry of depression and a need for more effective therapeutic interventions. Individuals with unipolar and bipolar depression commonly show impairments in goal-directed and motivated behaviors, exhibited as psychomotor slowing, anergia, and fatigue [2–4], that are highly resistant to treatment [5–7]. A large body of research in humans, non-human primates and rodents has shown that the mesolimbic dopamine (DA) system, including the ventral tegmental area (VTA) and the nucleus accumbens (NAc), plays a critical role in motivated behaviors in humans [8–10]. Further, mesolimbic dopamine deficits are associated with major depressive disorder (MDD) in humans and have also been observed in preclinical genetic and behavioral models of depression [11–13]. Recent findings in rodent models have revealed a causal link between phasic DA activity in the VTA to NAc pathway in specific pro-depressive and antidepressant-like behavioral phenotypes [14, 15]. Such findings raise the possibility that processes which regulate mesolimbic DA activity may mediate depression-related behavior. Indeed, previous work has demonstrated that midbrain cholinergic activity powerfully regulates DA activity [16–19] and DA-dependent drug-seeking behaviors [20–22]. However, the role of VTA cholinergic mechanisms in depression and depression-related behavior is poorly understood.
The cholinergic hypothesis of depression is strongly supported by extensive experimental evidence from both humans [23–27] and rodents [28–32]. Specifically, manipulations that increase brain cholinergic tone lead to pro-depressive effects [23–25, 29, 32, 33], while administration of either nicotinic or muscarinic acetylcholine receptor (AChR) antagonists leads to antidepressant-like effects [26, 28, 34–38]. Recent investigations of cholinergic mechanisms in depression have focused on key brain structures implicated in depression and other mood disorders, including the prefrontal cortex (PFC) [38–40], the hippocampus [28, 29, 41], and the NAc [42–44]. However, the field lacks understanding of the role of VTA cholinergic activity in depression, despite the fact that VTA cholinergic receptor mechanisms powerfully regulate phasic DA activity that is causally linked to depression-related behavioral responses to stress. Given that the VTA sends projections and receives input from the PFC, hippocampus, and NAc, examining VTA cholinergic mechanisms in depression-related behavior will also provide better understanding of the neurocircuitry of depression and the role of acetylcholine within this circuit.
Here, we sought to determine whether VTA acetylcholine and AChR activity in male Sprague-Dawley rats mediated behavioral responses in the forced swim test (FST). The goal of our study was to identify the role of midbrain mechanisms in behavioral responses in FST - in order to provide insight of the underlying neurobiological processes that mediate behavioral responses to stress. Using behavioral pharmacology, we found that intra-VTA infusion of nicotinic or muscarinic AChR antagonists decreased immobility time in FST – consistent with an antidepressant-like effect, while VTA infusion of the acetylcholinesterase inhibitor, physostigmine, led to an increase in immobility time that was dependent upon muscarinic AChR activation. The results of study reveal that VTA cholinergic manipulations robustly modulate behavioral responses in FST, independent of potential non-specific locomotor effects, and suggest that future investigation in this area can provide new understanding of the role of VTA cholinergic mechanisms in depression-related behaviors.
2. Materials and Methods
2.1. Animals and Surgery
Across all experiments, a total of 137 male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA) were used. Rats were housed in pairs in a colony maintained at 22–24°C with a 12 h light/12 dark cycle (lights on at 07:00) and were allowed one week to acclimate to the facility prior to any surgical procedures. Food and water were available ad libitum in the home cages at all times. Prior to surgery, rats were anesthetized with ketamine HCl (100 mg/kg, i.p., Sigma Aldrich, USA) and xylazine (10 mg/kg, i.p., Sigma Aldrich, USA) and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA) for implantation of intra-cranial cannula. All coordinates were obtained from the rat brain atlas [45] with anteroposterior (AP), mediolateral (ML) and dorsoventral (DV) positions referenced from Bregma. A bilateral cannula spaced 1 mm apart (Plastics One, Roanoke, VA, USA) was placed 1 mm above the VTA (AP −5.2 mm, ML ±0.5 mm, DV −7.0 mm from dura) and secured using screws (Gexpro, High Point, NC) and dental cement (Dentsply, Milford, DE, USA). For the dorsal control site experiment, bilateral cannula were placed 3 mm above the VTA (AP −5.2 mm, ML ± 0.5 mm, DV −5.0 mm from the dura). After surgery, rats were singly housed and allowed to recover for 5–7 days before testing began. Animal protocols were approved by Yale University Institutional Animal Care and Use Committee (IACUC) and performed in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
2.2. Drug administration
In preparation for brain-region specific drug delivery, bilateral internal cannula containing the drug were inserted into the guide cannula and extended 1 mm beyond the guide cannula to target the VTA (−8.0 mm from dura) or the dorsal control site (−6.0 mm from dura). Drugs were delivered in a 0.5 μL total volume over 1 min via a micro-infusion pump and syringe (25 gauge, Hamilton Syringe, Reno, NE, USA). After the 1 min drug infusion, the internal cannulae were left in place for an additional 1 min to allow for complete diffusion of drug into the brain tissue. The muscarinic AChR antagonist, scopolamine (Sigma Aldrich, St. Louis, MO), the nicotinic AChR antagonists, mecamylamine (Sigma Aldrich, St. Louis, MO), and the acetylcholinesterase inhibitor, physostigmine (VWR, Bridgeport, NJ) were each dissolved in 0.9% saline and infused at doses that we and others have previously shown to modulate behavior and phasic DA release when infused into the VTA [19, 20, 22, 46]. Physostigmine, which inhibits that acetylcholinesterase and prevents acetylcholine degradation, was used to enhance acetylcholine levels. Behaviorally relevant doses of systemic physostigmine were based on previously published work demonstrating pro-depressive effects of systemic administration and on pilot experiments to identify doses of physostigmine that did not alter baseline locomotor activity [29, 47, 48].
2.3. Forced Swim Test
The forced swim test (FST) was performed similar to protocols previously described by others [38, 43, 49–51]. During the pre-test, no pharmacological manipulation was given and behavior was not recorded. In the pre-test, rats were individually placed into a clear polypropylene, cylindrical water tank (diameter 30 cm; height 60 cm; water depth > 40 cm; water temperature between 23–26°C) for 15 min, to establish a stable baseline of immobility for the subsequent test. The FST test session occurred during the second swim session, which took place 24 hrs after the pre-test. For systemic drug administration, physostigmine or vehicle was administered by intraperitoneal (i.p.) injection 20 min prior to the 10 min FST (Fig. 1a). For VTA-specific infusion, rats were placed into the water tank immediately after drug infusion and immobility was scored during the last 6 min of the 10 min test session (Fig. 2a). Thus, there was a 4 min wait time between VTA drug infusion and the analysis of immobility time. This wait time is consistent with the time course for physiological responses to VTA drug infusion, as we have previously demonstrated that VTA infusion of cholinergic drugs alters dopamine release within 3 min of infusion - with effects that last up to 2 hr [19, 20]. Each rat was randomly assigned to a specific drug administration group. FST was recorded by video camera and immobility was defined as an interruption of swimming behavior, when rats showed a lack of hind and fore paw paddling. Thus, scoring of immobility time started when rats assumed a passive floating position, using only minimal movements required to keep their heads above water. For FST analysis, each test session was quantified by stopwatch by an experimenter blind to the treatment condition. Tank water was cleaned after each rat. At the end of the test session, rats were dried with a towel and placed in a warmed cage to completely dry off for 30 min before being returned to their home cages.
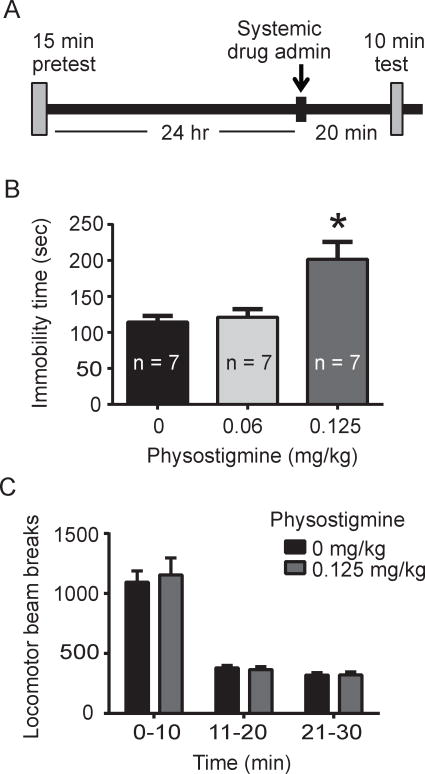
Fig. 1.
Effects of systemic physostigmine on immobility time in the FST and on total locomotor activity. A. Experimental timeline for the FST, including a 15 min pretest 24 hr prior to the FST test session. B. Administration of physostigmine led to increased immobility time in FST (p < 0.05, main effect of drug; p < 0.05, Tukey post-hoc for 0.125 mg/kg physostigmine versus saline). C. Systemic physostigmine administration did not alter locomotor activity as measured by locomotor photobeam breaks (p > 0.05, two-way repeated measures ANOVA).
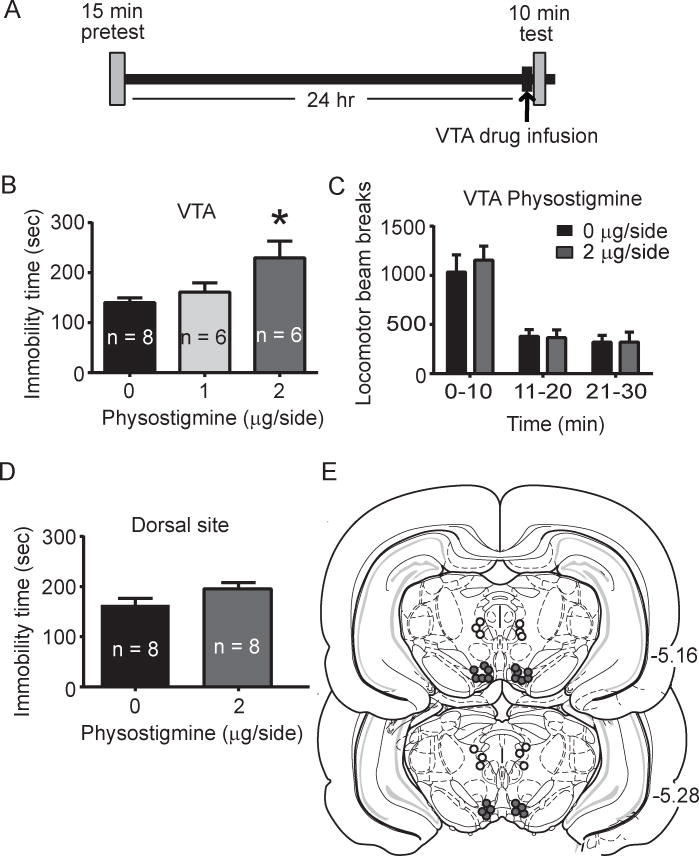
Fig. 2.
Brain-region specific physostigmine infusion: effects on immobility time in the FST and total locomotor activity. A. Experimental timeline for the FST experiment. Intra-VTA drug infusion was performed immediately prior to the 10 min FST test session. B. Administration of physostigmine increased immobility time in FST (p < 0.05, main effect of drug; p < 0.05, Tukey post-hoc for 2 μg/side physostigmine versus saline). C. Intra-VTA drug infusion did not significantly affect locomotor activity, as measured by photobeam breaks (p > 0.05, two-way repeated measured ANOVA). D. Immobility time in the FST following physostigmine infusion into a site 2 mm dorsal to the VTA. Administration of 2 μg/side physostigmine did not alter immobility time in the FST (p > 0.05, independent samples t-test). E. Histological verification of intra-cranial cannula placements. Filled circles indicate representative placements for intra-VTA infusions and open circles indicate representative placements for dorsal control infusions.
2.4. Locomotor Activity
Automated activity monitors (Digiscan animal activity monitor; Omnitech Electronics, Columbus OH) were used to assess locomotor activity. The monitors were equipped with two parallel rows of 16 sensors spaced 2.5 cm apart and were controlled by a computer using Micromax software (Omnitech Electronics). Rats that had previously undergone FST were subsequently examined in the locomotor activity assay and experiments were performed using a counterbalanced design to assign drug administration groups. For systemic administration, the drug was injected 20 min prior to the locomotor test, whereas VTA drug infusions were preformed immediately prior the test – consistent with the FST experimental design. Photobeam breaks were recorded, quantified and analyzed in 10 min bins.
2.5. Experimental procedures
2.5.1. Experiments 1 and 2: Effects of systemic and brain-region specific administration of the acetylcholinesterase inhibitor, physostigmine, on the forced swim test
In experiment 1, rats (n = 21) received one of the following i.p. delivered drugs prior to FST on the test day: 0.9% saline vehicle (20 min before testing), 0.06 mg/kg physostigmine (20 min before testing), or 0.125 mg/kg physostigmine (20 min before testing). In experiment 2a, a separate cohort of rats (n = 20 included, n = 2 excluded due to cannula misplacement) received one of the following intra-VTA drug infusions immediately prior to the FST test: 0.9% saline vehicle, 1 μg physostigmine, or 2 μg physostigmine. In experiment 2b, a different cohort of rats (n = 14 included, n = 2 excluded due to cannula misplacement) received drug infusions of 0.9% saline vehicle or 2 μg physostigmine into a site 2 mm dorsal to the VTA and were subsequently examined in FST.
2.5.2. Experiment 3: Effects of intra-VTA administration of scopolamine or mecamylamine on the forced swim test
In the scopolamine experiment, rats (n = 20 included, n = 1 excluded due to cannula misplacement) received one of the following intra-VTA drug infusions immediately prior to FST: 0.9% saline vehicle, 2.4 μg scopolamine, or 24 μg scopolamine. In the mecamylamine experiment, rats (n = 21 included, n = 2 excluded due to cannula misplacement) received one of the following VTA-infusions prior to the test: 0.9% saline vehicle, 3 μg mecamylamine, or 30 μg mecamylamine.
2.5.3. Experiments 4, 5 and 6.: Effects of co-administered physostigmine and scopolamine or co- administered physostigmine and mecamylamine in the forced swim test
In experiment 4, rats (n = 21 include, n = 3 excluded due to cannula misplacement) received intra-VTA administration of one of the following: 0.9% saline vehicle, 2 μg physostigmine alone, or 2 μg physostigmine + 24 μg scopolamine immediately prior to the test session. In experiment 5, rats (n = 20 included, n = 1 excluded due to cannula misplacement) received one of the following VTA-drug infusions immediately before FST: 0.9% saline vehicle, 2 μg physostigmine alone, or 2 μg physostigmine + 30 μg mecamylamine. In experiment 6, rats (n = 28 included, n = 4 excluded due to cannula misplacement) received i.p. administration of saline vehicle or 0.125 mg/kg physostigmine (20 min before testing) in combination with intra-VTA infusion of saline vehicle, 30 μg mecamylamine or 24 μg scopolamine (4 min before testing), and were then examined in the FST.
2.6 Histological Verification
At the end of all behavioral experiments, rats were given a lethal dose of pentobarbital (150 mg/kg i.p.) and perfused transcardially with 0.9% saline followed by 3.2% paraformaldehyde. After perfusion, the brains were removed and post-fixed for 24 hr in 3.2% paraformaldehyde, then stored in 30% sucrose. Coronal sections (50 μm) were taken on a cryostat and mounted on glass microscope slides. After mounting, slides were stained with cresyl violet for subsequent microscopic observation. Any subjects with misplaced cannula or significant damage around the injection site were excluded from the subsequent statistical analyses of the behavioral data.
2.7. Statistical analyses
For the forced swim experiments, time spent immobile was analyzed using a one-way analysis of variance (ANOVA), performed with GraphPad Prism 6 (Graph Pad Software, San Diego, CA). If the ANOVA revealed a significant main effect, a Tukey post-hoc analysis was performed to compare between specific drug-administration groups. Locomotor activity was analyzed using a two-way repeated measures analysis of variance (ANOVA), with time as the repeated measure.
3. Results
3.1. Experiments 1 and 2: Systemic and intra-VTA physostigmine increases immobility time in the FST
In experiment 1 (systemic administration of physostigmine), there was an overall significant effect of drug treatment on time spent immobile (F2, 18 = 9.06, p = 0.01, Fig. 1b). Specifically, post-hoc analysis revealed that 0.125 mg/kg physostigmine produced a significant increase in time spent immobile compared to saline (p < 0.05). Subsequent examination and analysis of locomotor behavior showed that systemic administration of physostigmine did not alter locomotor activity compared to saline controls (F1, 12 = 0.00834, p > 0.05, Fig. 1c). Similar to the effect of systemic physostigmine administration, analysis of the intra-VTA physostigmine experiment also revealed a main effect of drug administration on immobility time (F2, 17 = 5.0, p < 0.05, Fig. 2b). Post-hoc analysis showed that 2 μg/side dose of physostigmine produced a significant increase in time spent immobile compared to saline (p < 0.05, Fig. 2b). Further, the effect of 2 μg/side physostigmine on FST was not due to non-specific locomotor effects as VTA drug infusion led to no significant differences in locomotor activity (F1, 14 = 0.44, p > 0.05, Fig. 2c). In a separate cohort of rats where physostigmine was administered at a site dorsal to the VTA (experiment 2b), analysis revealed no statistically significant effect of drug treatment on time spent immobile (t(14) = 1.8, p > 0.05, Fig. 2d).
3.2 Experiments 3: VTA cholinergic receptor blockade with scopolamine or mecamylamine decreases immobility time in the FST
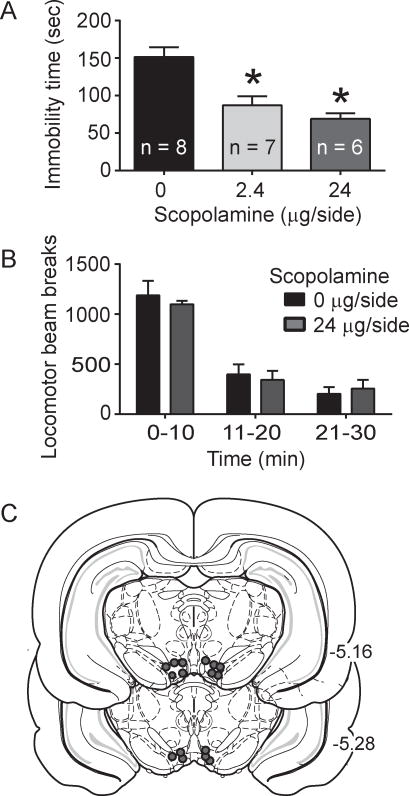
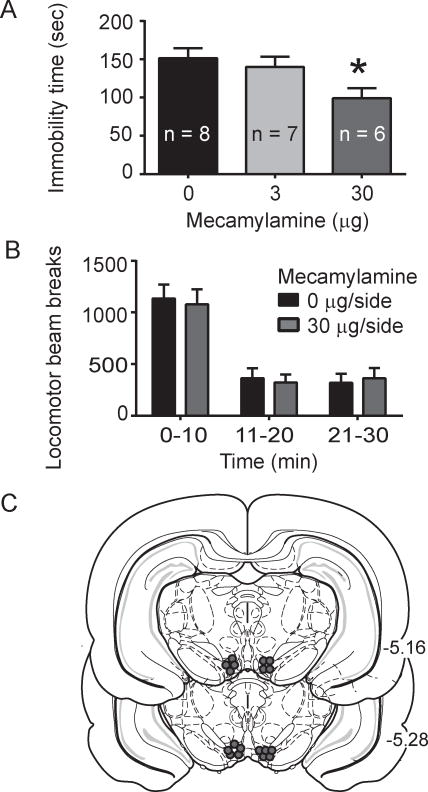
To determine the effects of VTA AChR blockade in the FST, the muscarinic AChR antagonist scopolamine or the nicotinic AChR antagonist mecamylamine was infused into the VTA immediately prior to the FST. In experiment 3, there was an overall significant effect of scopolamine on time spent immobile (F2, 18 = 14.08, p < 0.002, Fig. 3a). Post-hoc analysis revealed that both the 2.4 μg/side and 24 μg/side scopolamine doses, decreased time spent immobile compared to saline (p < 0.05, Fig. 3a). In addition, intra-VTA scopolamine administration did not alter locomotor activity compared to saline infused subjects, as there was no significant effect of treatment (F1, 14 = 0.15, p > 0.05, Fig. 3b). Examination of intra-VTA mecamylamine effects revealed an overall significant effect of drug on time spent immobile (F2,18 = 4.0, p < 0.05, Fig. 4a) and post-hoc revealed that the 30 μg/side dose produced a significant decrease in time spent immobile compared to saline (p < 0.05, Fig. 4a). Similar to scopolamine, intra-VTA mecamylamine infusion did not alter locomotor activity (F1, 14 = 0.035, p > 0.05, Fig. 4b).
Fig. 3.
Intra-VTA scopolamine effects on the FST immobility time and total locomotor activity. A. Scopolamine infusion into the VTA led to decreased immobility in the FST (p < 0.001, main effect of drug; p < 0.05, Tukey post-hoc for 2.4 μg/side scopolamine versus saline; p < 0.05, Tukey post-hoc for 24 μg/side scopolamine versus saline). B. Intra-VTA infusion of 24 μg/side scopolamine did not alter locomotor activity, as measured by photobeam breaks (p > 0.05, two-way repeated measures ANOVA). C. Representative cannula placements for intra-VTA infusions in the scopolamine experiment.
Fig. 4.
Intra-VTA mecamylamine effects on the FST immobility time and total locomotor activity. A. Mecamylamine infusion into the VTA leads to decreased immobility time in the FST (p < 0.05, main effect of drug; p < 0.05, Tukey post-hoc for 30 μg/side mecamylamine versus saline). B. Intra-VTA infusion of 30 μg/side mecamylamine did not alter locomotor activity as measured by locomotor beam breaks (p > 0.05, two-way repeated measures ANOVA). C. Representative VTA cannula placements for the mecamylamine infusion experiment.
3.3 Experiments 4: Effects of co-administered physostigmine and scopolamine or co-administered physostigmine and mecamylamine in the FST
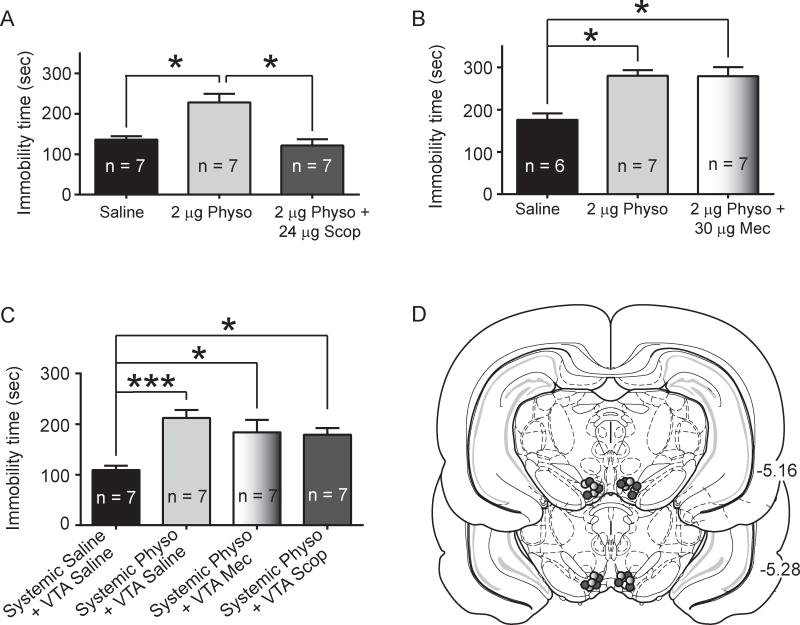
To determine if VTA AChR blockade could reverse the behavioral effects of VTA physostigmine in the FST, physostigmine was co-infused with either scopolamine or mecamylamine immediately prior to the FST session. In the scopolamine-physostigmine experiment, there was a significant effect of drug treatment on time spent immobile (F2,18 = 13.22, p < 0.001, Fig. 5a). Post-hoc analysis revealed that 2 μg/side physostigmine produced a significant increase in immobility time (p < 0.05, Fig. 5a), consistent with the results in experiment 2 (Fig. 2b). Further, co-administration of 24 μg/side scopolamine and 2 μg/side physostigmine led to decreased time spent immobile compared to the physostigmine alone group (p < 0.05, Fig 2b), and was not significantly different from the saline group (p > 0.05).
Fig. 5.
Effect of scopolamine plus physostigmine co-administration or mecamylamine plus physostigmine co-administration on immobility time in the FST. A. VTA scopolamine plus physostigmine experiment. Intra-VTA physostigmine administration increased immobility time in the FST (p < 0.05, Tukey post-hoc of 2 μg/side physostigmine versus saline), but co-administration with scopolamine blocked the physostigmine-induced increase in immobility (p < 0.05, Tukey post-hoc of 2 μg/side physostigmine plus 24 μg/side scopolamine versus scopolamine alone; p > 0.05, Tukey post-hoc of 2 μg/side physostigmine plus 24 μg/side scopolamine versus saline). B. VTA mecamylamine plus physostigmine experiment. Intra-VTA 2 μg/side physostigmine (in a separate cohort from that in panel A) increased immobility time in the FST (p < 0.05, Tukey post-hoc of 2 μg/side physostigmine versus saline). Intra-VTA 30 μg/side mecamylamine co-administration with 2 μg/side physostigmine did not alter the physostigmine-induced increase in immobility time (p > 0.05, Tukey post-hoc of 2 μg/side physostigmine plus 30 μg/side mecamylamine versus to 2 μg/side alone; p < 0.05, Tukey post-hoc of 2 μg/side physostigmine plus 30 μg/side mecamylamine versus saline). C. Systemic 0.125 mg/kg physostigmine plus intra-VTA mecamylamine or scopolamine experiment. Systemic administration of physostigmine led increased immobility time (p < 0.001, Tukey post-hoc of 2 μg/side physostigmine versus saline). Intra-VTA infusion of 30 μg/side mecamylamine co-administered with systemic 0.125 mg/kg physostigmine also increased immobility time compared to saline administered rats (p < 0.05, Tukey post-hoc), with no difference between physostigmine alone versus physostigmine plus mecamylamine (p > 0.05, Tukey post-hoc). Intra-VTA infusion of 24 μg/side scopolamine co-administered with systemic 0.125 mg/kg physostigmine increased immobility time compared to control rats (p < 0.05, Tukey post-hoc), with no difference between physostigmine alone versus physostigmine plus scopolamine (p > 0.05, Tukey post-hoc). D. Histological verification of cannula placements. Dark filled circles indicate representative placements for the scopolamine plus physostigmine experiments. Light filled circles indicate representative placements for the mecamylamine plus physostigmine experiments.
In the mecamylamine-physostigmine co-administration experiment, there was also an overall significant effect of drug treatment on time spent immobile (F2,17 = 11.24, p < 0.001, Fig. 5b) and post-hoc analysis revealed a significant increase in immobility in the 2 μg/side physostigmine group compared to saline (p < 0.05, Fig. 5b). Further, the 30 μg/side mecamylamine plus 2 μg/side physostigmine co-administration groups did not significantly differ from the physostigmine alone group (p > 0.05, Fig. 5b), but showed a significance increase in immobility time compared to saline (p < 0.05, Fig. 5b). Thus co-administration of intra-VTA mecamylamine did not alter the ability of intra-VTA physostigmine to increase immobility time.
To determine if VTA AChR blockade altered the behavioral effects of systemic physostigmine in the FST, VTA infusions of mecamylamine or scopolamine were performed in combination with systemic physostigmine. Drug administration altered immobility time, as revealed by a main effect of drug (F3,24 = 7.037, p < 0.01, Fig. 5c). Consistent with Experiment 1, systemic physostigmine increased immobility time in the FST (p < 0.001, post-hoc analysis, Fig. 5d). Rats that received co-administration of systemic physostigmine with VTA mecamylamine infusion also showed increased immobility time compared to saline control rats (p < 0.05, Fig. 5c), and did not alter immobility time compared to physostigmine alone (p > 0.05, Fig. 5c). In addition, co-administration of systemic physostigmine with VTA scopolamine infusion led to increased immobility compared to saline controls (p < 0.05, Fig. 5c), with no significant difference between scopolamine plus physostigmine infused rats compared to physostigmine infused rats (p > 0.05, Fig. 5c). Thus, VTA administration of mecamylamine or scopolamine did not alter the effects of systemic physostigmine in the FST.
4. Discussion
These experiments examined whether VTA cholinergic manipulations, which are known to modulate DA signals strongly implicated in depression, also mediate behavioral responses during the FST in rats. We found that blockade of either nicotinic or muscarinic AChRs in the VTA decreased immobility time in the FST. Our results also demonstrated that increasing cholinergic tone, with the acetylcholinesterase inhibitor physostigmine, with either systemic or VTA-specific administration, increased immobility time in FST. These opposing behavioral effects of VTA AChR activation (via prolonged acetylcholine tone) and VTA AChR blockade strongly support a role of VTA cholinergic activity in modulating behavioral responses to a stressor, such as the FST. Importantly, VTA cholinergic manipulations did not alter locomotor activity, suggesting that the observed modulation of behavioral responses in the FST was not due to non-specific locomotor effects. Moreover, we found that co-infusion of physostigmine with the muscarinic antagonist scopolamine, but not co-infusion with the nicotinic antagonist mecamylamine, fully reversed the behavioral effect of VTA physostigmine in the FST. Taken together, the results provide evidence for a role of VTA cholinergic mechanisms in behavioral responses to stress and demonstrate that VTA specific AChR blockade is sufficient to induce a behavioral response in FST similar to that induced by antidepressant administration.
Several preclinical and clinical studies have provided extensive evidence supporting the role of ACh in depressive-like behavior and demonstrating that administration of AChR antagonists produces antidepressant-like effects [29–31, 35–38, 52, 53]. Our findings strongly point to VTA AChR mechanisms as important modulators of behavioral phenotypes in the FST. However, it should be noted that we did not seek to determine whether VTA mechanisms are critical for the antidepressant effects of systemic AChR antagonist administration. Rather, our results demonstrate that VTA AChR blockade is sufficient to induce a behavioral response consistent with an antidepressant-like effect. Given the recent evidence for a causal role of phasic DA activity in the VTA to NAc pathway in depressive-like behavior [14, 15] and the ability of VTA nAChR or mAChR blockade to reduce phasic DA release in the NAc [16, 19, 20, 54, 55], it is possible that our observed decrease in immobility is mediated by the ability of the AChR antagonists to decrease phasic DA release in the NAc. Indeed, previous studies have also revealed alterations in phasic DA activity in preclinical genetic and behavioral models of depression [11, 12, 56–58]. While phasic DA mechanisms were not examined in the current study, previous work has shown increased DA burst firing following exposure to acute stressors [59–61]. If FST is sufficient to enhance phasic DA activity, we would predict that decreasing DA burst firing and phasic DA release would have an antidepressant-like effect in the FST. Consistent with this interpretation, we and others have shown that mecamylamine or scopolamine infusion into the VTA attenuates phasic DA release in the NAc [16, 19, 20] and our current findings confirm that VTA administration of these AChR antagonists decrease immobility time in the FST. Further, previous work using the social defeat model also showed increased DA burst firing and phasic DA release following social defeat stress [62]. Based on such findings, one would predict that decreasing DA burst firing and phasic DA release would also have an antidepressant-like effect following social defeat stress – as was recently demonstrated by Chaudhury and colleagues [14]. Thus, we propose that VTA cholinergic regulation of phasic DA activity and release is likely to mediate multiple depression-related behaviors, which should be further investigated in future work.
It should also be noted that recent investigations of the VTA to NAc pathway have shown somewhat inconsistent results concerning the causal role of phasic DA activity. Specifically, Chaudhury and colleagues found a pro-depressive effect of VTA to NAc phasic DA stimulation during social defeat stress [14], while Tye and colleagues found an antidepressant-like effect of VTA to NAc DA phasic activity following chronic mild stress [15]. These differences may have resulted from several factors; including the utilization of different stress paradigms, the potential activation of different VTA sub-regions, or the activation of functionally distinct DA cell populations between the studies. Indeed, recent examinations of reward processing have revealed functionally distinct populations of DA neurons that differentially encode either the valence or salience or reward-associated cues [63, 64]. In addition, numerous studies high revealed important differences between rostral versus caudal VTA [65–67] and medial versus lateral VTA [64, 68] in terms of cellular composition, input and output, and the regulation of behaviors related to reward, aversion, anxiety and depression (reviewed in [63, 69, 70]). While VTA sub-region specific effects have not been examined in the context of depression, through future studies, it will be important to consider whether there are sub-region specific contributions of VTA AChR mechanisms in depression-related behavior.
Our current physostigmine findings also suggest that VTA cholinergic mechanisms may contribute to pro-depressive behavioral responses to certain stressors. Previous studies have provided extensive evidence supporting the role of cholinergic mechanisms in depression-related behavior [25, 29–32]. To our knowledge, however, our findings are the first to demonstrate that increased cholinergic tone in the VTA is sufficient to increase immobility in the FST. Here, we also found that VTA infusion of mecamylamine or scopolamine did not alter the effect of systemic physostigmine in the FST. These findings are in contrast to the ability of systemic administration of mecamylamine or scopolamine to reverse the behavioral effects of systemic physostigmine [29]. Together, our current findings suggest that the effects of systemic physostigmine in FST are mediated primarily by AChR mechanisms outside of the VTA. Indeed, hippocampal AChR mechanisms have previously been shown to underlie the behavioral effects of systemic physostigmine [29]. Given that neither VTA mecamylamine nor scopolamine infusion reversed the effects of systemic physostigmine, it is striking VTA physostigmine robustly increased immobility time to a similar extent as systemic physostigmine. Our findings suggest that the robust ability of VTA AChR mechanisms to modulate FST behavior occurs independent of the effects of systemic physostigmine administration. Specifically, our findings suggest that VTA cholinergic mechanisms play an important role in mediating susceptibility or resilience to an FST stressor – independent of the effects of systemic physostigmine. One possible mechanistic explanation of our data is that stressors, like the FST, enhance VTA acetylcholine release and that the ability of VTA physostigmine to potentiate these acetylcholine effect leads to the observed increase in immobility. While no studies have examined VTA acetylcholine levels during or after the FST, a stress-induced increase in VTA acetylcholine would likely increase VTA DA neuronal activity. Indeed activation of VTA AChRs is sufficient to enhance DA burst firing [71] and DA burst firing is known to increase phasic DA release in terminal regions such as the NAc [72, 73]. Thus, a stress induced increase in VTA acetylcholine may be a mechanism by which stress could increase phasic DA in the NAc. In light of our current findings, future examinations of the interaction between VTA cholinergic and NAc dopaminergic processes in depression-related behavior will provide important understanding of the potential role of such mechanisms in depression.
Our results from the reversal experiment specifically point to VTA muscarinic AChRs as the critical AChR class that mediated the behavioral effects of VTA physostigmine. Specifically, the ability of intra-VTA scopolamine, but not mecamylamine, to reverse the physostigmine effect suggests that muscarinic AChR, but not nicotinic AChR, activation mediates the ability of enhanced acetylcholine to increase immobility. Importantly, the inability of mecamylamine to block the behavioral effects of physostigmine is not likely due to insufficiency of the 30μg dose. We have previously demonstrated that this 30 μg dose, when administered to the VTA, is sufficient to attenuate dopamine release [19, 20] and to modulate drug-seeking behavior [20]. In addition, this dissociation between VTA mAChR and nAChR mechanisms has been previously observed in other behaviors where VTA infusion of scopolamine, but not mecamylamine, disrupts operant responding for food [74], suggesting that this functional dissociation is not specific to behavioral responses to stress. One potential mechanism that could have contributed to the mAChR-mediated effect of VTA physostigmine is a preferential up-regulation of muscarinic AChRs that could have occurred in the FST. Indeed, previous work has shown that immobilization stress leads to increased muscarinic AChR binding in the striatum and hippocampus [75, 76], suggesting either an up-regulation or functional change in muscarinic receptors due to immobilization stress. However, additional work is required to determine whether changes in VTA muscarinic AChRs also occur in response to the FST. In future studies, it will be important to further investigate whether mild versus strong or acute versus chronic stressors lead to differential VTA acetylcholine release and whether muscarinic AChRs play a differential role in depressive behavioral responses to such stressors.
5. Conclusion
In summary, we have shown that enhancing acetylcholine tone or blocking AChRs in the VTA leads to increased or decreased immobility, respectively, in the FST. Together, our data provides strong evidence that VTA cholinergic mechanisms mediate behavioral responses in the FST. We hypothesize that such mechanisms may also mediate behavioral responses following acute or chronic stress in preclinical models of depression, as will be investigated in future studies. We also note that VTA AChR antagonist infusion that decreased immobility time in the current study, consistent with the effects of antidepressants, also attenuated cue-induced drug-seeking in our previous work [20]. Indeed, it has been suggested that substance abuse may increase susceptibility to depression and several preclinical studies have shown pro-depressive behavioral phenotypes during drug withdrawal [77–81]. Thus, further understanding of VTA AChR processes that mediate depression-related behavior and drug-seeking during withdrawal can guide investigation of AChRs that may serve as novel therapeutic targets to treat comorbid depression and addiction.
Highlights.
Systemic or VTA physostigmine increases immobility in the forced swim test (FST)
VTA mecamylamine or scopolamine decreases immobility time in the FST
VTA scopolamine blocks the effects of VTA physostigmine in the FST
VTA mecamylamine does not alter the effect of VTA physostigmine in the FST
Acknowledgments
This research was supported by the National Institutes of Health grant MH093897 Supplement (NAA), by National Institutes of Health grant MH014276 (EJN) and by a National Science Foundation Graduate Research Fellowship (RJW). We also thank Dr. Ronald Duman for reading the manuscript and providing helpful feedback.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Gold JM, et al. Cognitive effort avoidance and detection in people with schizophrenia. Cogn Affect Behav Neurosci. 2014 doi: 10.3758/s13415-014-0308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gold JM, et al. Negative symptoms and the failure to represent the expected reward value of actions: behavioral and computational modeling evidence. Arch Gen Psychiatry. 2012;69(2):129–38. doi: 10.1001/archgenpsychiatry.2011.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Treadway MT, et al. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol. 2012;121(3):55–8. doi: 10.1037/a0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bella R, et al. Clinical presentation and outcome of geriatric depression in subcortical ischemic vascular disease. Gerontology. 2010;56(3):298–302. doi: 10.1159/000272003. [DOI] [PubMed] [Google Scholar]
- 6.Demyttenaere K, De Fruyt J, Stahl SM. The many faces of fatigue in major depressive disorder. Int J Neuropsychopharmacol. 2005;8(1):93–105. doi: 10.1017/S1461145704004729. [DOI] [PubMed] [Google Scholar]
- 7.Stahl SM. The psychopharmacology of energy and fatigue. J Clin Psychiatry. 2002;63(1):7–8. doi: 10.4088/jcp.v63n0102. [DOI] [PubMed] [Google Scholar]
- 8.Arsenault JT, et al. Role of the primate ventral tegmental area in reinforcement and motivation. Curr Biol. 2014;24(12):1347–53. doi: 10.1016/j.cub.2014.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballard IC, et al. Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. J Neurosci. 2011;31(28):10340–6. doi: 10.1523/JNEUROSCI.0895-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76(3):470–85. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman A, et al. VTA dopamine neuron bursting is altered in an animal model of depression and corrected by desipramine. J Mol Neurosci. 2008;34(3):201–9. doi: 10.1007/s12031-007-9016-8. [DOI] [PubMed] [Google Scholar]
- 12.Valenti O, Gill KM, Grace AA. Different stressors produce excitation or inhibition of mesolimbic dopamine neuron activity: response alteration by stress pre-exposure. Eur J Neurosci. 2012;35(8):1312–21. doi: 10.1111/j.1460-9568.2012.08038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zangen A, et al. Association between depressive behavior and absence of serotonin-dopamine interaction in the nucleus accumbens. Psychopharmacology (Berl) 2001;155(4):434–9. doi: 10.1007/s002130100746. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhury D, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493(7433):532–6. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tye KM, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493(7433):537–41. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forster GL, Blaha CD. Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur J Neurosci. 2000;12(10):3596–604. doi: 10.1046/j.1460-9568.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- 17.Kitai ST, et al. Afferent modulation of dopamine neuron firing patterns. Curr Opin Neurobiol. 1999;9(6):690–7. doi: 10.1016/s0959-4388(99)00040-9. [DOI] [PubMed] [Google Scholar]
- 18.Maskos U. The cholinergic mesopontine tegmentum is a relatively neglected nicotinic master modulator of the dopaminergic system: relevance to drugs of abuse and pathology. Br J Pharmacol. 2008;153(Suppl 1):S438–45. doi: 10.1038/bjp.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wickham R, et al. Ventral tegmental area alpha6beta2 nicotinic acetylcholine receptors modulate phasic dopamine release in the nucleus accumbens core. Psychopharmacology (Berl) 2013;229(1):73–82. doi: 10.1007/s00213-013-3082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solecki W, et al. Differential role of ventral tegmental area acetylcholine and N-methyl-D-aspartate receptors in cocaine-seeking. Neuropharmacology. 2013;75:9–18. doi: 10.1016/j.neuropharm.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.You ZB, et al. Acetylcholine release in the mesocorticolimbic dopamine system during cocaine seeking: conditioned and unconditioned contributions to reward and motivation. J Neurosci. 2008;28(36):9021–9. doi: 10.1523/JNEUROSCI.0694-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou W, et al. Role of acetylcholine transmission in nucleus accumbens and ventral tegmental area in heroin-seeking induced by conditioned cues. Neuroscience. 2007;144(4):1209–18. doi: 10.1016/j.neuroscience.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gershon S, Shaw FH. Psychiatric sequelae of chronic exposure to organophosphorus insecticides. Lancet. 1961;1(7191):1371–4. doi: 10.1016/s0140-6736(61)92004-9. [DOI] [PubMed] [Google Scholar]
- 24.Janowsky DS, el-Yousef MK, Davis JM. Acetylcholine and depression. Psychosom Med. 1974;36(3):248–57. doi: 10.1097/00006842-197405000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Janowsky DS, et al. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2(7778):632–5. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- 26.Shytle RD, et al. Mecamylamine (Inversine): an old antihypertensive with new research directions. J Hum Hypertens. 2002;16(7):453–7. doi: 10.1038/sj.jhh.1001416. [DOI] [PubMed] [Google Scholar]
- 27.Shytle RD, et al. Nicotinic acetylcholine receptors as targets for antidepressants. Mol Psychiatry. 2002;7(6):525–35. doi: 10.1038/sj.mp.4001035. [DOI] [PubMed] [Google Scholar]
- 28.Caldarone BJ, et al. High-affinity nicotinic acetylcholine receptors are required for antidepressant effects of amitriptyline on behavior and hippocampal cell proliferation. Biol Psychiatry. 2004;56(9):657–64. doi: 10.1016/j.biopsych.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Mineur YS, et al. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc Natl Acad Sci U S A. 2013;110(9):3573–8. doi: 10.1073/pnas.1219731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Overstreet DH. Selective breeding for increased cholinergic function: development of a new animal model of depression. Biol Psychiatry. 1986;21(1):49–58. doi: 10.1016/0006-3223(86)90007-7. [DOI] [PubMed] [Google Scholar]
- 31.Overstreet DH. The Flinders sensitive line rats: a genetic animal model of depression. Neurosci Biobehav Rev. 1993;17(1):51–68. doi: 10.1016/s0149-7634(05)80230-1. [DOI] [PubMed] [Google Scholar]
- 32.Overstreet DH, Russell RW. Selective breeding for diisopropyl fluorophosphate-sensitivity: behavioural effects of cholinergic agonists and antagonists. Psychopharmacology (Berl) 1982;78(2):150–5. doi: 10.1007/BF00432254. [DOI] [PubMed] [Google Scholar]
- 33.Risch SC, Kalin NH, Janowsky DS. Cholinergic challenges in affective illness: behavioral and neuroendocrine correlates. J Clin Psychopharmacol. 1981;1(4):186–92. doi: 10.1097/00004714-198107000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Drevets WC, Zarate CA, Jr, Furey ML. Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol Psychiatry. 2013;73(12):1156–63. doi: 10.1016/j.biopsych.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furey ML, et al. Baseline mood-state measures as predictors of antidepressant response to scopolamine. Psychiatry Res. 2012;196(1):62–7. doi: 10.1016/j.psychres.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khajavi D, et al. Oral scopolamine augmentation in moderate to severe major depressive disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2012;73(11):1428–33. doi: 10.4088/JCP.12m07706. [DOI] [PubMed] [Google Scholar]
- 37.Rabenstein RL, Caldarone BJ, Picciotto MR. The nicotinic antagonist mecamylamine has antidepressant-like effects in wild-type but not beta2- or alpha7-nicotinic acetylcholine receptor subunit knockout mice. Psychopharmacology (Berl) 2006;189(3):395–401. doi: 10.1007/s00213-006-0568-z. [DOI] [PubMed] [Google Scholar]
- 38.Voleti B, et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry. 2013;74(10):742–9. doi: 10.1016/j.biopsych.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibbons AS, et al. Decreased muscarinic receptor binding in the frontal cortex of bipolar disorder and major depressive disorder subjects. J Affect Disord. 2009;116(3):184–91. doi: 10.1016/j.jad.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saricicek A, et al. Persistent beta2*-nicotinic acetylcholinergic receptor dysfunction in major depressive disorder. Am J Psychiatry. 2012;169(8):851–9. doi: 10.1176/appi.ajp.2012.11101546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pavlovsky L, et al. Stress-induced altered cholinergic-glutamatergic interactions in the mouse hippocampus. Brain Res. 2012;1472:99–106. doi: 10.1016/j.brainres.2012.05.057. [DOI] [PubMed] [Google Scholar]
- 42.Nunes EJ, et al. Nucleus accumbens neurotransmission and effort-related choice behavior in food motivation: effects of drugs acting on dopamine, adenosine, and muscarinic acetylcholine receptors. Neurosci Biobehav Rev. 2013;37(9 Pt A):2015–25. doi: 10.1016/j.neubiorev.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Rada P, et al. Behavioral depression in the swim test causes a biphasic, long-lasting change in accumbens acetylcholine release, with partial compensation by acetylcholinesterase and muscarinic-1 receptors. Neuroscience. 2006;141(1):67–76. doi: 10.1016/j.neuroscience.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 44.Warner-Schmidt JL, et al. Cholinergic interneurons in the nucleus accumbens regulate depression-like behavior. Proc Natl Acad Sci U S A. 2012;109(28):11360–5. doi: 10.1073/pnas.1209293109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. London: Elsevier; 2007. [Google Scholar]
- 46.Schmidt HD, Famous KR, Pierce RC. The limbic circuitry underlying cocaine seeking encompasses the PPTg/LDT. Eur J Neurosci. 2009;30(7):1358–69. doi: 10.1111/j.1460-9568.2009.06904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasey G, Hanin I. The cholinergic-adrenergic hypothesis of depression reexamined using clonidine, metoprolol, and physostigmine in an animal model. Biol Psychiatry. 1991;29(2):127–38. doi: 10.1016/0006-3223(91)90041-j. [DOI] [PubMed] [Google Scholar]
- 48.Mancinelli A, et al. Cholinergic drug effects on antidepressant-induced behaviour in the forced swimming test. Eur J Pharmacol. 1988;158(3):199–205. doi: 10.1016/0014-2999(88)90067-2. [DOI] [PubMed] [Google Scholar]
- 49.Duman CH. Models of depression. Vitam Horm. 2010;82:1–21. doi: 10.1016/S0083-6729(10)82001-1. [DOI] [PubMed] [Google Scholar]
- 50.Porsolt RD, et al. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47(4):379–91. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 51.Zangen A, Overstreet DH, Yadid G. High serotonin and 5-hydroxyindoleacetic acid levels in limbic brain regions in a rat model of depression: normalization by chronic antidepressant treatment. J Neurochem. 1997;69(6):2477–83. doi: 10.1046/j.1471-4159.1997.69062477.x. [DOI] [PubMed] [Google Scholar]
- 52.George TP, et al. Nicotinic antagonist augmentation of selective serotonin reuptake inhibitor-refractory major depressive disorder: a preliminary study. J Clin Psychopharmacol. 2008;28(3):340–4. doi: 10.1097/JCP.0b013e318172b49e. [DOI] [PubMed] [Google Scholar]
- 53.Lippiello PM, et al. TC-5214 (S-(+)-mecamylamine): a neuronal nicotinic receptor modulator with antidepressant activity. CNS Neurosci Ther. 2008;14(4):266–77. doi: 10.1111/j.1755-5949.2008.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lester DB, et al. Midbrain acetylcholine and glutamate receptors modulate accumbal dopamine release. Neuroreport. 2008;19(9):991–5. doi: 10.1097/WNR.0b013e3283036e5e. [DOI] [PubMed] [Google Scholar]
- 55.Sombers LA, et al. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci. 2009;29(6):1735–42. doi: 10.1523/JNEUROSCI.5562-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iniguez SD, et al. Extracellular signal-regulated kinase-2 within the ventral tegmental area regulates responses to stress. J Neurosci. 2010;30(22):7652–63. doi: 10.1523/JNEUROSCI.0951-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Razzoli M, et al. Increased phasic activity of VTA dopamine neurons in mice 3 weeks after repeated social defeat. Behav Brain Res. 2011;218(1):253–7. doi: 10.1016/j.bbr.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 58.Valenti O, et al. Antipsychotic drugs rapidly induce dopamine neuron depolarization block in a developmental rat model of schizophrenia. J Neurosci. 2011;31(34):12330–8. doi: 10.1523/JNEUROSCI.2808-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anstrom KK, Woodward DJ. Restraint increases dopaminergic burst firing in awake rats. Neuropsychopharmacology. 2005;30(10):1832–40. doi: 10.1038/sj.npp.1300730. [DOI] [PubMed] [Google Scholar]
- 60.Brischoux F, et al. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106(12):4894–9. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mantz J, Thierry AM, Glowinski J. Effect of noxious tail pinch on the discharge rate of mesocortical and mesolimbic dopamine neurons: selective activation of the mesocortical system. Brain Res. 1989;476(2):377–81. doi: 10.1016/0006-8993(89)91263-8. [DOI] [PubMed] [Google Scholar]
- 62.Anstrom KK, Miczek KA, Budygin EA. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience. 2009;161(1):3–12. doi: 10.1016/j.neuroscience.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68(5):815–34. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459(7248):837–41. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ikemoto S, Murphy JM, McBride WJ. Self-infusion of GABA(A) antagonists directly into the ventral tegmental area and adjacent regions. Behav Neurosci. 1997;111(2):369–80. doi: 10.1037//0735-7044.111.2.369. [DOI] [PubMed] [Google Scholar]
- 66.Olson VG, Nestler EJ. Topographical organization of GABAergic neurons within the ventral tegmental area of the rat. Synapse. 2007;61(2):87–95. doi: 10.1002/syn.20345. [DOI] [PubMed] [Google Scholar]
- 67.Perrotti LI, et al. DeltaFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment. Eur J Neurosci. 2005;21(10):2817–24. doi: 10.1111/j.1460-9568.2005.04110.x. [DOI] [PubMed] [Google Scholar]
- 68.Lammel S, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491(7423):212–7. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56(1):27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lammel S, Tye KM, Warden MR. Progress in understanding mood disorders: optogenetic dissection of neural circuits. Genes Brain Behav. 2014;13(1):38–51. doi: 10.1111/gbb.12049. [DOI] [PubMed] [Google Scholar]
- 71.Zhang L, Liu Y, Chen X. Carbachol induces burst firing of dopamine cells in the ventral tegmental area by promoting calcium entry through L-type channels in the rat. J Physiol. 2005;568(Pt 2):469–81. doi: 10.1113/jphysiol.2005.094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsai HC, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324(5930):1080–4. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wightman RM, Zimmerman JB. Control of dopamine extracellular concentration in rat striatum by impulse flow and uptake. Brain Res Brain Res Rev. 1990;15(2):135–44. doi: 10.1016/0165-0173(90)90015-g. [DOI] [PubMed] [Google Scholar]
- 74.Sharf R, McKelvey J, Ranaldi R. Blockade of muscarinic acetylcholine receptors in the ventral tegmental area prevents acquisition of food-rewarded operant responding in rats. Psychopharmacology (Berl) 2006;186(1):113–21. doi: 10.1007/s00213-006-0352-0. [DOI] [PubMed] [Google Scholar]
- 75.Mizukawa K, et al. Alterations of muscarinic cholinergic receptors in the hippocampal formation of stressed rat: in vitro quantitative autoradiographic analysis. Brain Res. 1989;478(1):187–92. doi: 10.1016/0006-8993(89)91496-0. [DOI] [PubMed] [Google Scholar]
- 76.Takayama H, et al. Regional repsonses of rat brain muscarinic cholinergic receptors to immobilization stress. Brain Research. 1987;436:291–95. doi: 10.1016/0006-8993(87)91673-8. [DOI] [PubMed] [Google Scholar]
- 77.Filip M, et al. Alterations in BDNF and trkB mRNAs following acute or sensitizing cocaine treatments and withdrawal. Brain Res. 2006;1071(1):218–25. doi: 10.1016/j.brainres.2005.11.099. [DOI] [PubMed] [Google Scholar]
- 78.Frankowska M, et al. Effects of imipramine or GABA(B) receptor ligands on the immobility, swimming and climbing in the forced swim test in rats following discontinuation of cocaine self-administration. Eur J Pharmacol. 2010;627(1–3):142–9. doi: 10.1016/j.ejphar.2009.10.049. [DOI] [PubMed] [Google Scholar]
- 79.Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4(1):17–26. [PubMed] [Google Scholar]
- 80.Renoir T, Pang TY, Lanfumey L. Drug withdrawal-induced depression: serotonergic and plasticity changes in animal models. Neurosci Biobehav Rev. 2012;36(1):696–726. doi: 10.1016/j.neubiorev.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 81.Zilkha N, et al. Induction of depressive-like effects by subchronic exposure to cocaine or heroin in laboratory rats. J Neurochem. 2014;130(4):575–82. doi: 10.1111/jnc.12753. [DOI] [PubMed] [Google Scholar]