Abstract
The largest-ever recorded outbreak of viral hemorrhagic fever is ongoing. Due to the epidemic and rural nature of outbreaks, little is published about the Filovirus infections Ebola virus disease and Marburg disease in pregnancy. This review of viral hemorrhagic fever focusing on Marburg and Ebola uses knowledge of disease in non-pregnant individuals and pregnancy-specific data to inform management for pregnant women. Filovirus infection presentation is similar between pregnant and non-pregnant patients, though infections may be more severe in pregnancy. Although labeled as hemorrhagic fevers, Marburg and Ebola do not commonly cause gross bleeding and should be conceptualized as diseases of high gastrointestinal losses. Early, aggressive supportive care is the mainstay of Filovirus infection management with massive fluid resuscitation as the key management principle. Patients often require 5–10 liters or more per day of intravenous or oral fluid to maintain circulating blood volume in the setting of ongoing gastrointestinal loss. Fluid shifts warrant aggressive monitoring and correction of potassium levels and acid-base disturbances to prevent life-threatening arrhythmias and metabolic complications. Regardless of maternal survival, fetal loss rates are nearly 100% in Filovirus infection, likely resulting from unchecked transplacental and hematogenous viral spread. High fetal loss rates support the placenta as a difficult-to-eradicate Filovirus infection reservoir. In conclusion, the management of Filovirus infection in pregnancy should focus on stabilizing the mother with intensive monitoring and aggressive fluid and electrolyte repletion, as well as maintaining strict infection control to minimize transmission to others.
Introduction
The largest-ever recorded outbreak of viral hemorrhagic fever is ongoing, with over 22,000 total cases and more than 8,000 fatalities reported (1, 2). Relevant to obstetrician–gynecologists, the majority of affected Ebola virus disease patients are of reproductive age between 15–44 years (2), with an overall 71% case fatality rate (2). This review focuses on Marburg and Ebola using knowledge gained in non-pregnant individuals to enhance knowledge about these viruses in pregnancy and to inform potential strategies to safely manage pregnant women.
Reservoirs and transmission of Filovirus Infections
The Filovirus infections Marburg disease and Ebola virus disease are negative-stranded, lipid enveloped ribonucleic acid (RNA) viruses (3), and are classic zoonotic diseases with index transmission occurring from an animal to human host (3). Though additional reservoirs may exist, bats are thought to be the primary asymptomatic host animal (4, 5). Even minimal contact with bats may lead to infection (4, 6, 7). Outside of laboratory and bush settings, Filovirus infections are transmitted by contact with body fluids of an infected individual (3, 6).
Clinical presentation and course
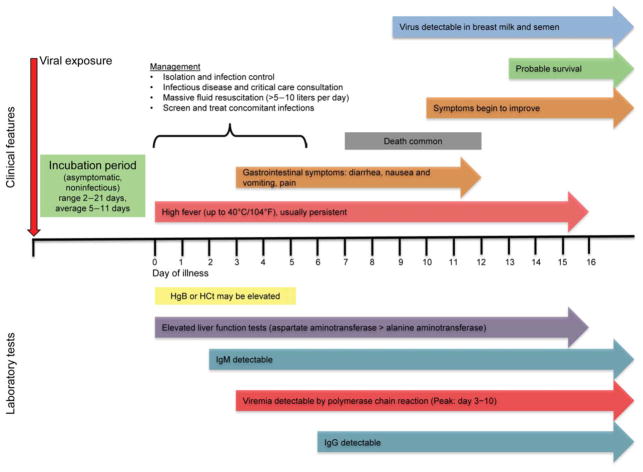
Though distinct viruses, the clinical presentation and course of Ebola and Marburg share significant overlap and can be managed similarly. The clinical presentation and disease course of Filovirus infections is presented in Figure 1 and is largely the same in pregnant and non-pregnant patients. Although labeled as hemorrhagic fevers, Marburg and Ebola should be conceptualized as gastrointestinal diseases (8, 9). Early symptoms include high fever, fatigue, malaise and muscle aches. While the viral incubation period ranges from 2–21 days (3, 10), most cases present within 5–7 days of viral transmission (2). Early in the course of disease, symptoms of Filovirus infection are non-specific and may mimic and even co-exist with more common illnesses such as cholera, typhoid, or malaria (3).
Figure 1.
The predilection of Filovirus infections for the gastrointestinal tract leads to life-threatening dehydration from severe diarrhea and emesis causing rapid intravascular volume depletion, accompanied by electrolyte and acid-base disorders (8). Hypoperfusion, shock, and multi-organ failure ensue, with a high mortality rate. Irrespective of the degree of dehydration, hypoperfusion of tissues is marked by elevated lactate (8) and other blood test abnormalities similar to those seen in bacterial sepsis. Symptoms improve in approximately 40% of patients by day 10 of illness (11) and most patients alive at two weeks ultimately survive (11).
Death from Filovirus infections usually occurs between one and two weeks after symptom onset, and results from persistent circulatory shock and multi-organ failure with marked hepatic and renal dysfunction (12, 13). Fatal cases are often heralded by high viral titers in blood (14), a marked increase in blood neutrophil counts, rapid decrease in blood lymphocytes and platelets (15) and markers of coagulopathy (16). Gross bleeding is reported in only 18% of all patients (2, 17) and with the exception of bloody stools, is a particularly ominous sign among women (2).
Diagnosis
Diagnosis of Marburg or Ebola is based on clinical presentation and travel or exposure history, and confirmed with laboratory testing. The most commonly used diagnostic tests are polymerase chain reaction blood tests, though these are often available only in reference laboratories or tertiary care centers (3). Antigen capture enzyme-linked immunosorbent assay (ELISA) or IgM ELISA can also be performed early in the course of disease (15). Filoviral antigen and nucleic acid are detectable in blood samples from as early as day 3 and at least through day 10 of symptomatic illness, and detectable virus persists for weeks in specific body tissues and fluids (3). While some patients will test positive for Filovirus infection within the first 24 hours after symptom onset, a negative polymerase chain reaction test cannot be relied on to rule out disease until >72 hours after symptoms begin (11). Antibodies develop as early as symptom day 2 (IgM) and day 6 (IgG) (3). IgG can persist for up to 10 years after infection, and IgM for 1–6 months (18).
Maternal-fetal features of Filovirus infection
To date 109 cases of Marburg or Ebola in pregnancy have been reported in the literature (Table 1). To compile and summarize reports to date, the PubMed database of the National Library of Medicine was last searched on December 17, 2014, for publications about Filovirus infections and pregnancy. Search terms “Marburg virus pregnancy,” “Marburg virus pregnant” and “Marburg fever pregnancy” yielded 12 unique results, none specific to pregnant women. “Filovirus pregnancy” and “filovirus pregnant” resulted 10 articles, none pregnancy-related. Search terms “viral hemorrhagic fever pregnancy” and “viral hemorrhagic fever pregnant” yielded 289 references, none unique to this search term and about Ebola or Marburg. Of these 289 results, 20 contained material on Filovirus infection in humans, including a 1999 case series describing 15 pregnant women with Ebola. Search terms “Ebola pregnancy” and “Ebola pregnant” yielded 16 unique articles including one clinical commentary reviewing some evidence on what obstetricians and gynecologists should know about Ebola. The search was expanded to include the term “Ebola” which yielded 2195 articles, 1000 of which were screened for relevance starting from 2007. Of these, 128 abstracts were deemed useful for full manuscript review. After reviewing these 128 articles, the authors’ scientific and clinical judgment was used to compile the best available evidence to guide management. Additional relevant manuscripts published after December 17, 2014 were included at the authors’ discretion. These series report maternal death rates ranging from 74–100%, not including the recent case report from Guinea of 2 pregnant women where both mothers survived but their infected fetuses died (19). Filovirus infection is more severe in pregnant than in non-pregnant adults (16, 20), possibly as a result of altered immune function or placental involvement. Despite high Filovirus infection fatality rates in pregnancy, there is currently no evidence that pregnant women are more susceptible to viral acquisition compared to others (21, 22).
Table 1.
Historical outbreaks of Ebola Virus Disease and Marburg Disease (Filovirus Infections) reporting pregnancy-related outcomes.
| Where When (Reference) | Virus | Women infected n (% of total) | Pregnant women n (% of women) | Maternal outcome | Fetal/neonatal outcome |
|---|---|---|---|---|---|
| Zaire 1976 (30) | Ebola | 177 (56%) | 82 (46%) | 89% mortality | 19 SAb (23%, all 1st/2nd trim) 7/11 with fever at birth (64%) 11/11 babies died by DOL 19 |
| Zaire 1995 (20) | Ebola | 105 (33%) | 15 (14%) | 15/15 vaginal bleeding 14/15 died (93% mortality) 1/15 lived (had 3rd trim SAb) |
10/15 SAb (67%) 4/5 born in 3rd trim died (80%) 1 live birth died on DOL 3 |
| Republic of Congo (31) | Marburg | 49 (32%) | 3 (6%) | 3/3 died (100% mortality) | 2 SAb 1 live birth died on DOL 1 |
| DRC 2014 (32) | Ebola | 36 (52%) | 1 (3%) | 1/1 died (100% mortality) | 1/1 SAb (100%) |
| Guinea 2014 (33) | Ebola | 13 (35%) | None reported | No data | No data |
| Sierra Leone 2014 (17) | Ebola | 59 (60%) | 1 (1.7%) | Not reported 74% mortality overall |
SAb prior to EVD diagnosis |
| Liberia 2014 (34) | Ebola | Not reported | 4 reported (% not reported) | 3/4 (75%) died after SAb in late 2nd/3rd trim | All resulted in SAb |
SAb = spontaneous abortion, DOL = day of life, trim = trimester, DRC = Democratic Republic of the Congo (formerly Zaire), EVD = Ebola virus disease.
Outbreaks with no published data on pregnant are not included.
Fetal loss rates are nearly 100% with or without maternal death (9, 17, 19, 23). Evidence from case reports confirms that hematogenous spread of the Filovirus infection through the placenta is likely, as high viral titers have been detected in placental tissue for Ebola and other hemorrhagic fever viruses (19, 24). In addition, a case report of Ebola in two pregnant women from Guinea describes one woman who recovered from Ebola but amniocentesis after her recovery still demonstrated a high viral load of Ebola, and the woman’s fetus died, despite maternal recovery (19). Such cases support the theory that transplacental or hematogenous spread of Filovirus infection is common and not halted by maternal immune defenses or the placenta.
Management—Supportive Care
Early, aggressive supportive care is the mainstay of Filovirus infection management. Despite clear differences in disease pathogenesis and clinical course between patients with viral hemorrhagic fever disease and other forms of sepsis, patients should be treated according to guidelines for severe sepsis management (25). Maternal vital signs should guide initial management, with massive fluid resuscitation as the main supportive treatment. Patients usually require 5–10 liters or more of intravenous or oral fluid daily to maintain circulating blood volume in the setting of ongoing gastrointestinal loss (8). Monitoring and aggressive correction of potassium levels, acid-base disturbances, and other electrolyte abnormalities can help prevent life-threatening arrhythmias and metabolic complications (8). Early administration of anti-emetics and anti-diarrheal therapy is recommended (11) and is safe in pregnancy. Blood product transfusion may also be beneficial (26).
The value of usual obstetric interventions such as fetal monitoring, cesarean delivery, induction of labor or pregnancy interruption is unclear during this time when women are at greatest risk for vascular collapse. A recent case report where labor was induced with misoprostol after intrauterine fetal demise was associated with a positive maternal outcome (19). Neonatal survival is universally poor in all viral hemorrhagic fevers, despite variable maternal mortality rates. High fetal loss rates underscore the need to focus efforts on treatment of the pregnant mother (22). Pregnant women with decompensated Ebola or Marburg disease may not survive surgical delivery. Current evidence does not provide a strong rationale for cesarean delivery, as transmission to the fetus is all but certain and fetal or neonatal survival is unlikely. Induction of labor is unlikely to benefit the baby and the effect on maternal morbidity is uncertain. At present, expectant management of labor seems the most appropriate strategy (22). Prevention and supportive care are the primary treatments of Ebola and Marburg disease while awaiting development of therapeutic drugs and vaccines.
Because co-infections with malaria, Strongyloides stercoralis and hepatitis B are common, these diseases should be considered and treated in patients at high risk based on exposure to endemic areas (8). Empiric use of broad-spectrum combination antimicrobials to treat gastrointestinal pathogens is recommended, due to loss of usual gastrointestinal barriers to bacterial translocation from the gut (8) and knowledge that gram-negative rod sepsis can complicate severe Ebola (27). Reactivation of tuberculosis can occur and should be evaluated in patients with significant respiratory symptoms or ventilator dependence. Finally, Ebola virus is also excreted in breast milk at least 15 days after recovery from infection (28). This finding leads us to recommend against breastfeeding during or after Filovirus infection (see below).
Prevention
Given the highly infectious nature of Filovirus infection, only providers trained in the use of personal protective equipment should care for potentially infected patients, and they should be monitored by additional staff trained in the use of personal protective equipment to avoid breaches in protocol (29). Soap and water hand washing and alcohol hand gel both can disrupt the lipid membrane of Filovirus infections and interrupt transmission, though hand hygiene is no substitute for correct use of personal protective equipment in suspect patients. Supplies used for patients with Filovirus infection should be disposable whenever possible, and the use of sharps should be minimized. Amniotic fluid, placenta, blood and aborted fetuses are highly infectious and likely remain high-risk for viral transmission even after the mother has recovered (19). Recognizing personal protective equipment protocol breaches may be challenging because amniotic fluid, urine and other body fluids may be colorless and complete elimination of fluid splatter is near impossible. Human remains should be handled as little as possible, and autopsies should be avoided (29). State and local authorities should be notified of Marburg and Ebola deaths and contaminated items and human remains should only be disposed of with their guidance.
Once a patient recovers from Ebola or Marburg, she is likely immune to future infection and is considered non-infectious to the general public. Ebola has been found in semen for up to three months after convalescence, and for this reason abstinence from sex, including oral sex, is recommended for at least three months (28). Condoms may help prevent the spread of disease, but this has not been studied. Convalescent mothers should avoid breast-feeding for a minimum of 15 days since virus has been detected in breast milk up to this time (28). There is no recommendation on when breast-feeding can be safely resumed after Filovirus infection due to lack of data. Though currently limited data are published on Marburg and Ebola in pregnancy, management principles can be extrapolated from non-pregnant patients with Filovirus infection. Analysis of existing data from the current Ebola outbreak will better inform disease expectations and care for pregnant women with Marburg or Ebola in the future. Therefore, it is imperative that resources be dedicated to gaining a greater understanding of the pathogenesis of this disease in pregnant women, where there is a tense balance between maternal and fetal survival.
Acknowledgments
Lisa M. Bebell’s salary is supported by NIH Research Training Grant # R25 TW009337 funded by the Fogarty International Center and the National Institute of Mental Health.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
References
- 1.CDC. 2014 Ebola Outbreak in West Africa: Outbreak Update 27 January 2015. Outbreak Update. 2015 [cited 2015 31 January 2015]; Available from: http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/case-counts.html.
- 2.Ebola virus disease in West Africa--the first 9 months of the epidemic and forward projections. N Engl J Med. 2014 Oct 16;371(16):1481–95. doi: 10.1056/NEJMoa1411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martines RB, Ng DL, Greer PW, Rollin PE, Zaki SR. Tissue and cellular tropism, pathology and pathogenesis of Ebola and Marburg Viruses. The Journal of pathology. 2014 Oct 9; doi: 10.1002/path.4456. [DOI] [PubMed] [Google Scholar]
- 4.Towner JS, Amman BR, Sealy TK, Carroll SA, Comer JA, Kemp A, et al. Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS pathogens. 2009 Jul;5(7):e1000536. doi: 10.1371/journal.ppat.1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogel G. Science (New York, NY) United States: 2014. Infectious disease. Are bats spreading Ebola across sub-Saharan Africa? p. 140. [DOI] [PubMed] [Google Scholar]
- 6.Smith DH, Johnson BK, Isaacson M, Swanapoel R, Johnson KM, Killey M, et al. Marburg-virus disease in Kenya. Lancet. 1982 Apr 10;1(8276):816–20. doi: 10.1016/s0140-6736(82)91871-2. [DOI] [PubMed] [Google Scholar]
- 7.Adjemian J, Farnon EC, Tschioko F, Wamala JF, Byaruhanga E, Bwire GS, et al. Outbreak of Marburg hemorrhagic fever among miners in Kamwenge and Ibanda Districts, Uganda, 2007. The Journal of infectious diseases. 2011 Nov;204( Suppl 3):S796–9. doi: 10.1093/infdis/jir312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fowler RA, Fletcher T, Fischer WA, 2nd, Lamontagne F, Jacob S, Brett-Major D, et al. Caring for critically ill patients with ebola virus disease. Perspectives from west Africa. American journal of respiratory and critical care medicine. 2014 Oct 1;190(7):733–7. doi: 10.1164/rccm.201408-1514CP. [DOI] [PubMed] [Google Scholar]
- 9.Mehedi M, Groseth A, Feldmann H, Ebihara H. Clinical aspects of Marburg hemorrhagic fever. Future virology. 2011 Sep;6(9):1091–106. doi: 10.2217/fvl.11.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chowell G, Nishiura H. Transmission dynamics and control of Ebola virus disease (EVD): a review. BMC medicine. 2014 Oct 10;12(1):196. doi: 10.1186/s12916-014-0196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chertow DS, Kleine C, Edwards JK, Scaini R, Giuliani R, Sprecher A. Ebola Virus Disease in West Africa - Clinical Manifestations and Management. N Engl J Med. 2014 Nov 5; doi: 10.1056/NEJMp1413084. [DOI] [PubMed] [Google Scholar]
- 12.Brauburger K, Hume AJ, Muhlberger E, Olejnik J. Forty-five years of Marburg virus research. Viruses. 2012 Oct;4(10):1878–927. doi: 10.3390/v4101878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2011 Mar 5;377(9768):849–62. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Towner JS, Rollin PE, Bausch DG, Sanchez A, Crary SM, Vincent M, et al. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. Journal of virology. 2004 Apr;78(8):4330–41. doi: 10.1128/JVI.78.8.4330-4341.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ansari AA. Clinical features and pathobiology of Ebolavirus infection. Journal of autoimmunity. 2014 Sep 23; doi: 10.1016/j.jaut.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Bwaka MA, Bonnet MJ, Calain P, Colebunders R, De Roo A, Guimard Y, et al. Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. The Journal of infectious diseases. 1999 Feb;179( Suppl 1):S1–7. doi: 10.1086/514308. [DOI] [PubMed] [Google Scholar]
- 17.Schieffelin JS, Shaffer JG, Goba A, Gbakie M, Gire SK, Colubri A, et al. Clinical Illness and Outcomes in Patients with Ebola in Sierra Leone. N Engl J Med. 2014 Oct 29; doi: 10.1056/NEJMoa1411680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowe AK, Bertolli J, Khan AS, Mukunu R, Muyembe-Tamfum JJ, Bressler D, et al. Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. Commission de Lutte contre les Epidemies a Kikwit. The Journal of infectious diseases. 1999 Feb;179( Suppl 1):S28–35. doi: 10.1086/514318. [DOI] [PubMed] [Google Scholar]
- 19.Baggi F, Taybi A, Kurth A, Herp MV, Caro AD, Wölfel R, et al. Management of pregnant women infected with Ebola virus in a treatment centre in Guinea, June 2014. Eurosurveillance. 2014;19(49):2–5. doi: 10.2807/1560-7917.es2014.19.49.20983. [DOI] [PubMed] [Google Scholar]
- 20.Mupapa K, Mukundu W, Bwaka MA, Kipasa M, De Roo A, Kuvula K, et al. Ebola hemorrhagic fever and pregnancy. The Journal of infectious diseases. 1999 Feb;179( Suppl 1):S11–2. doi: 10.1086/514289. [DOI] [PubMed] [Google Scholar]
- 21.Jamieson DJ, Uyeki TM, Callaghan WM, Meaney-Delman D, Rasmussen SA. What Obstetrician-Gynecologists Should Know About Ebola: A Perspective From the Centers for Disease Control and Prevention. Obstet Gynecol. 2014 Sep 8; doi: 10.1097/AOG.0000000000000533. [DOI] [PubMed] [Google Scholar]
- 22.CDC. Guidance for Screening and Caring for Pregnant Women with Ebola Virus Disease for Healthcare Providers in U.S. Hospitals. 2014 [cited 2014 November 20]; Available from: http://www.cdc.gov/vhf/ebola/hcp/guidance-maternal-health.html.
- 23.Jeffs B. A clinical guide to viral haemorrhagic fevers: Ebola, Marburg and Lassa. Tropical doctor. 2006 Jan;36(1):1–4. doi: 10.1258/004947506775598914. [DOI] [PubMed] [Google Scholar]
- 24.Walker DH, McCormick JB, Johnson KM, Webb PA, Komba-Kono G, Elliott LH, et al. Pathologic and virologic study of fatal Lassa fever in man. The American journal of pathology. 1982 Jun;107(3):349–56. [PMC free article] [PubMed] [Google Scholar]
- 25.Clark DV, Jahrling PB, Lawler JV. Clinical management of filovirus-infected patients. Viruses. 2012 Sep;4(9):1668–86. doi: 10.3390/v4091668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyon GM, Mehta AK, Varkey JB, Brantly K, Plyler L, McElroy AK, et al. Clinical Care of Two Patients with Ebola Virus Disease in the United States. N Engl J Med. 2014 Dec 18;371(25):2402–9. doi: 10.1056/NEJMoa1409838. [DOI] [PubMed] [Google Scholar]
- 27.Kreuels B, Wichmann D, Emmerich P, Schmidt-Chanasit J, de Heer G, Kluge S, et al. A Case of Severe Ebola Virus Infection Complicated by Gram-Negative Septicemia. N Engl J Med. 2014 Oct 22; doi: 10.1056/NEJMoa1411677. [DOI] [PubMed] [Google Scholar]
- 28.CDC. Review of Human-to-Human Transmission of Ebola Virus. 2014 [cited 2014 20 October]; Available from: http://www.cdc.gov/vhf/ebola/transmission/human-transmission.html-sixteen.
- 29.CDC. Infection Prevention and Control Recommendations for Hospitalized Patients with Known or Suspected Ebola Virus Disease in U.S. Hospitals. 2014 [cited 2014 3 November]; Available from: http://www.cdc.gov/vhf/ebola/hcp/infection-prevention-and-control-recommendations.html.
- 30.Ebola haemorrhagic fever in Zaire, 1976. Bull World Health Organ. 1978;56(2):271–93. [PMC free article] [PubMed] [Google Scholar]
- 31.Bausch DG, Nichol ST, Muyembe-Tamfum JJ, Borchert M, Rollin PE, Sleurs H, et al. Marburg hemorrhagic fever associated with multiple genetic lineages of virus. N Engl J Med. 2006 Aug 31;355(9):909–19. doi: 10.1056/NEJMoa051465. [DOI] [PubMed] [Google Scholar]
- 32.Maganga GD, Kapetshi J, Berthet N, Kebela Ilunga B, Kabange F, Mbala Kingebeni P, et al. Ebola virus disease in the Democratic Republic of Congo. N Engl J Med. 2014 Nov 27;371(22):2083–91. doi: 10.1056/NEJMoa1411099. [DOI] [PubMed] [Google Scholar]
- 33.Bah EI, Lamah MC, Fletcher T, Jacob ST, Brett-Major DM, Sall AA, et al. Clinical Presentation of Patients with Ebola Virus Disease in Conakry, Guinea. N Engl J Med. 2014 Nov 5; doi: 10.1056/NEJMoa1411249. [DOI] [PubMed] [Google Scholar]
- 34.Chertow DS, Kleine C, Edwards JK, Scaini R, Giuliani R, Sprecher A. Ebola virus disease in West Africa--clinical manifestations and management. N Engl J Med. 2014 Nov 27;371(22):2054–7. doi: 10.1056/NEJMp1413084. [DOI] [PubMed] [Google Scholar]