Abstract
Objective
To investigate women's patterns of contraceptive use after delivery and the association between method use and risk of pregnancy within 18 months.
Methods
We used the 2006-2010 National Survey of Family Growth to examine women's contraceptive use after delivery, and at 3, 6, 12, and 18 months after giving birth. The sample included 3,005 births that occurred within 3 years of the survey date and for which information on contraceptive use was available. We estimated multivariable-adjusted Cox regression models to assess the association between women's method use and risk of pregnancy within 18 months after delivery. We also examined the percentage of pregnancies occurring ≤18 months after the index birth that were unintended.
Results
Between delivery and 3 months postpartum, contraceptive use increased from 21% to 72%. At 3 months, 13% of women used permanent contraception, 6% used long-acting reversible contraceptives, 28% used other hormonal methods and 25% relied on less-effective methods; the distribution of method use was similar in subsequent months. Among women using hormonal methods, 12.6% became pregnant ≤18 months of delivery compared to 0.5% using permanent and long-acting contraception (adjusted hazard ratio [HR]: 21.2, 95% confidence interval [CI]: 6.17-72.8). Additionally, 17.8% of women using less-effective methods (HR: 34.8, 95% CI: 9.26-131) and 23% using no method (HR: 43.2, 95% CI: 12.3-152) became pregnant ≤18 months. At least 70% of pregnancies within one year after delivery were unintended.
Conclusions
Few women use long-acting reversible contraceptives after delivery, and those using less-effective methods have an increased risk of unintended pregnancy.
Introduction
The postpartum period provides an important window of opportunity for women to initiate highly effective contraception because they are motivated to prevent another pregnancy and have access to health care and insurance coverage. Given the risks associated with closely spaced pregnancies, there has been considerable emphasis on the importance of counseling expectant or recent mothers about their contraceptive options and providing them with their chosen method on a timely basis.1, 2 Yet, over half of the unintended pregnancies experienced by parous women in the United States (US) occur within two years after delivery, and 35% of women have interpregnancy intervals less than 18 months, often referred to as short interpregnancy intervals.3, 4
Use of long acting reversible contraceptive (LARC) methods, such as the intrauterine device (IUD) and contraceptive implant, may reduce the incidence of short interpregnancy intervals and unintended pregnancy since these methods require minimal user effort to provide effective contraceptive coverage. The only recent nationally representative study of postpartum contraception found very low rates of IUD insertion in the hospital after delivery.5 However, several studies have found that women would like to use a LARC method soon after delivery,6-8 and a recent analysis of state-level data demonstrated wide variation in LARC use among postpartum women, ranging from 1.9% in Louisiana to >25% in Rhode Island and Colorado.9
The purpose of this analysis was to assess women's contraceptive use in the 18 months after delivery and the association between type of method used and risk of having a short interpregnancy interval using nationally representative data. We also examined the percentage of pregnancies occurring ≤18 months after delivery that were unintended.
Materials and Methods
We used the 2006-2010 National Survey of Family Growth (NSFG), a national probability survey of women and men aged 15-44 years conducted by the National Center for Health Statistics. Similar to previous cycles of the survey, participants were selected using a multistage, stratified, clustered sampling frame, and Black, Latino, and teenaged respondents were oversampled.10 However, unlike previous cycles, the 2006-2010 NSFG used continuous interviewing in which approximately 5,000 participants were surveyed each year in 33 different sampling units.11 The response rate was 78%,12 and a total of 12,279 female respondents completed a one-time in-person interview that collected detailed histories of their pregnancies, cohabiting and marital relationships and other important life events. Additionally, the survey included a contraceptive calendar in which women retrospectively reported the specific method used each month during the three years prior to the interview; women could report using up to four methods each month, and consistency checks for periods of sexual abstinence and pregnancy were used during data collection to improve accuracy of reporting.12 Although there were some changes in the survey questionnaire over the four-year data collection period,12 these revisions did not affect the variables used in our analysis. Approval from the University of Alabama at Birmingham's Institutional Review Board was not needed for use of this publicly available dataset.
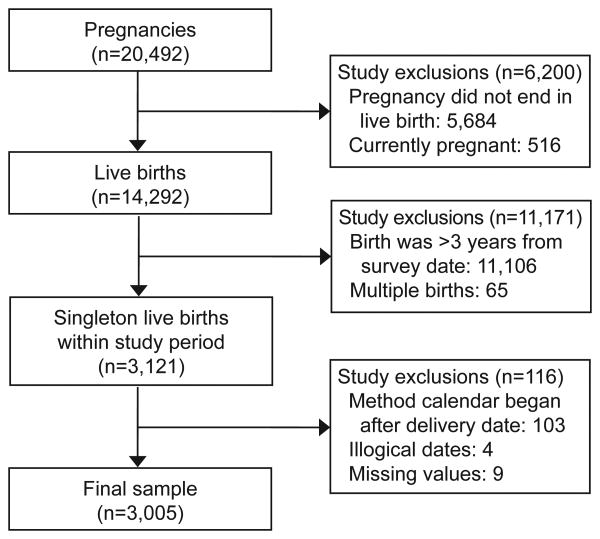
We identified a cohort of women who delivered a live-born singleton infant within three years of the survey date using the pregnancy file, which contains the date of conception, date the pregnancy ended, pregnancy outcome and maternal characteristics for each of the 20,492 pregnancies from female respondents (Figure 1). From these data, we also identified women having short pregnancy intervals, defined as conceptions resulting in live births that occurred ≤18 months after the index pregnancy.4 We focused on live births due to underreporting of miscarriage, abortions and stillbirths in the NSFG.12, 13
Figure 1.
Identification of a cohort of women in the 2006–2010 National Survey of Family Growth who delivered a live-born singleton infant within three years of the survey date.
Women's contraceptive method use in the 18 months after delivery was determined by matching women's delivery date to the contraceptive method calendar in the female respondent file. Following previous studies, we used the most effective method reported in each month of the calendar, which we then categorized as female sterilization, vasectomy, LARC, hormonal methods (e.g., oral contraceptive pills, injectables, hormonal patch, and vaginal ring), less-effective methods (e.g., diaphragm, male and female condoms, withdrawal and rhythm method) and no method.14, 15 We excluded observations in which the date of the index birth occurred before the start of the contraceptive calendar (n=103), as well as those with illogical dates (n=4) and missing values (n=9). The final sample included 3,005 births.
We examined the distribution of women's contraceptive use in the month and year of delivery, and at 3, 6, 12, and 18 months after delivery. Women who were pregnant at the interval or whose contraceptive calendar had ended before the interval were omitted.
Next, we calculated the percentage of women who had a short interpregnancy interval (≤18 months after delivery) according to age, parity, race–ethnicity, marital status, educational attainment and insurance status (i.e., Medicaid, private), all measured at the time of delivery. We then fit bivariate and multivariable-adjusted Cox proportional hazard models to compute hazard ratios and 95% confidence intervals (CI) for having a short interpregnancy interval, using the above covariates and women's contraceptive method use at the start of each interval (e.g., 3 months, 6 months). We combined female sterilization, vasectomy and LARC methods into a single category given their similar rates of effectiveness.16 Women were censored if they did not become pregnant or if their contraceptive calendar ended before 18 months after delivery. After fitting the model, we estimated the cumulative probability that a woman became pregnant by three, six, 12 and 18 months after delivery for each contraceptive method category.
As a final step, we examined women's pregnancy intentions for births that occurred ≤18 months after the index birth, according to the interval in which the pregnancy occurred. We computed the percentage of pregnancies that were intended (wanted then, occurring later than desired, indifferent), mistimed (wanted later), and unwanted.3, 17 We used negative binomial regression to assess whether women were more likely to report their pregnancy as unintended (i.e., mistimed or unwanted) if the pregnancy occurred earlier in 18-month interval after delivery. All analyses were conducted using Stata 13 and weighted to account for the complex sampling design of the NSFG.
Results
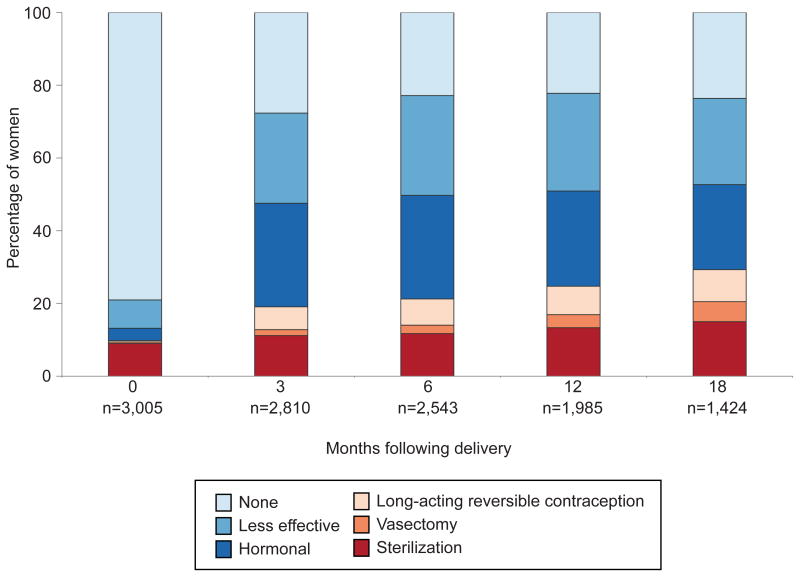
Among women in the initial cohort of 3,005 births, 621 (21% of the weighted sample) used a contraceptive method immediately postpartum (i.e., in the month and year of delivery), primarily female sterilization and less-effective methods (Figure 2). Contraceptive use increased to 72% by 3 months postpartum. Hormonal contraceptives were the most common methods (28%), followed by less-effective methods (25%), and female sterilization (11%). Only 6% of women reported using a LARC method at 3 months postpartum and 2% relied on their partner's vasectomy for contraception. The distribution of contraceptive method use was similar in subsequent months. By 18 months after delivery, 15% of women were using female sterilization and 9% were using LARC, while 24% of women were using less-effective methods or no method, respectively.
Figure 2.
Distribution of contraceptive method use, by months following delivery.
There were 434 pregnancies resulting in a live birth that were conceived ≤18 months after delivery. Compared to women aged 30-34 years, women who were 15-24 and 25-29 years old were more likely to have a short interpregnancy interval (8.2% versus 20.2% and 15.3%; Table 1). Additionally, short interpregnancy intervals were more common among women who had one child (20.0% versus 12.0% with two children), less than a high school level of education (19.2% versus 13.0% with high school or some college) and whose delivery was paid by Medicaid (16.4% versus 12.6% private insurance). After multivariable adjustment, age and education remained significantly associated with having a short interpregnancy interval. Additionally, compared to women who were married or cohabiting at the time of birth, women who were single were less likely to have a short pregnancy interval (hazard ratio [HR]: 0.73, 95% CI: 0.54-0.98).
Table 1. Characteristics associated with having a short interpregnancy interval*.
| Index births (n=3,005) | Bivariate | Multivariable | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Short interpregnancy interval (%)† | Hazard Ratio | (95% CI) | Hazard Ratio‡ | (95% CI) | ||
| Age at delivery, years | ||||||
| 15-24 | 1,202 | 20.2 | 2.91 | (1.91 - 4.45)‡ | 2.37 | (1.42 - 3.96)‡ |
| 25-29 | 882 | 15.3 | 1.99 | (1.29 - 3.05)‡ | 1.86 | (1.20 - 2.90)‡ |
| 30-34 | 594 | 8.2 | 1.00 | ref | 1.00 | ref |
| 35-44 | 327 | 8.0 | 0.98 | (0.56 - 1.73) | 0.98 | (0.56 - 1.71) |
| Parity at delivery | ||||||
| 1 child | 1,094 | 20.0 | 1.72 | (1.17 - 2.54)‡ | 1.30 | (0.87 - 1.95) |
| 2 children | 957 | 12.0 | 1.00 | ref | 1.00 | ref |
| 3 children or more | 954 | 10.1 | 0.90 | (0.57 - 1.41) | 1.17 | (0.73 - 1.87) |
| Race/ethnicity | ||||||
| White | 1,298 | 14.5 | 1.00 | ref | 1.00 | ref |
| Black | 696 | 16.2 | 1.21 | (0.85 - 1.73) | 0.96 | (0.68 - 1.34) |
| Latina | 783 | 13.0 | 0.95 | (0.67 - 1.33) | 0.84 | (0.58 - 1.21) |
| Other | 228 | 13.4 | 0.90 | (0.53 - 1.53) | 0.80 | (0.48 - 1.33) |
| Marital status at delivery | ||||||
| Single | 767 | 15.0 | 1.18 | (0.87 - 1.59) | 0.73 | (0.54 - 0.98)‡ |
| Married/cohabiting | 2,238 | 14.2 | 1.00 | ref | 1.00 | ref |
| Educational attainment | ||||||
| Less than high school | 863 | 19.2 | 1.53 | (1.13 - 2.06)‡ | 1.36 | (1.01 - 1.83)‡ |
| High school/some college | 1,371 | 13.0 | 1.00 | ref | 1.00 | ref |
| College degree | 771 | 12.7 | 0.88 | (0.62 - 1.24) | 1.12 | (0.75 - 1.66) |
| Payment for delivery | ||||||
| Private | 1,362 | 12.6 | 1.00 | ref | 1.00 | ref |
| Medicaid | 1,643 | 16.4 | 1.49 | (1.13 - 1.96)‡ | 1.18 | (0.83 - 1.69) |
| Contraceptive method§ | ||||||
| Sterilization, LARC | 621 | 0.5 | 1.00 | ref | 1.00 | ref |
| Hormonals | 724 | 12.6 | 26.5 | (8.00 - 87.6)‡ | 21.2 | (6.17 - 72.8 ‡ |
| Less effective | 746 | 17.8 | 38.6 | (10.5 - 141)‡ | 34.8 | (9.26 - 131)‡ |
| None | 914 | 23.0 | 50.0 | (14.5 - 172)‡ | 43.2 | (12.3 - 152)‡ |
These results are from our own calculations using the NSFG 2006–2010. See http://www.cdc.gov/nchs/nsfg/nsfg_2006_2010_puf.htm for more information.CI: confidence interval
Short interpregnancy interval pregnancies were conceived ≤18 months after the index birth (i.e., a live singleton birth that occurred within 3 years of the survey date [n=434]).
Percentages are weighted to reflect the sampling design of the NSFG.
p < 0.05. The p-value is based on Cox proportional hazard regression models.
Contraceptive method used at the last observed interval after delivery. Sterilization includes female sterilization and vasectomy. LARC (long-acting reversible contraception) includes intrauterine device and contraceptive implant. Hormonal methods include oral contraceptive pills, injectables, hormonal patch, and vaginal ring. Less effective methods include diaphragm, male or female condoms, withdrawal, and rhythm method.
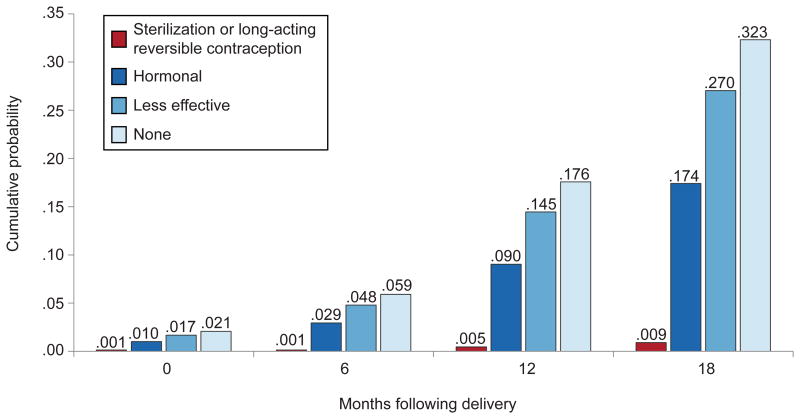
Contraceptive method use at the start of each interval also was significantly associated with becoming pregnant within 18 months after delivery. Compared to women using LARC or permanent methods, women using hormonal methods (HR: 21.2, 95% CI: 6.17-72.8), less-effective methods (HR: 34.8, 95% CI: 9.26-131) and no method (HR: 43.2, 95% CI: 12.3-152) were more likely to conceive a pregnancy resulting in a live birth. After cumulating the estimated hazard function for each method type, women using LARC or permanent methods had a 0.5% chance of getting pregnant by 12 months, whereas women using hormonal methods had a 9% chance and women using less-effective methods or no method had a 15% and 18% chance, respectively (Figure 3). Women's chances of getting pregnant using these methods were almost twice as high by 18 months.
Figure 3.
Cumulative probability pregnancy within 18 months following delivery, by contraceptive method.
Pregnancy intentions for the 434 pregnancies conceived within 18 months after delivery are presented in Table 2. Of the 61 pregnancies that occurred ≤2 months postpartum, 29 (54.4% of the weighted sample, 95% CI: 34.2-73.2) were mistimed and 22 (30.0% weighted sample, 95% CI: 15.3-50.5) were unwanted; the percentage of mistimed and unwanted pregnancies was similar for the 66 pregnancies that occurred between three and five months after delivery. Between 12-18 months, 148 women became pregnant, of which 39 (19.3% of the weighted sample, 95% CI: 11.9-27.9) reported the pregnancy was mistimed and 28 (13.4% weighted sample, 95% CI: 8.0-21.5%) reported it was unwanted. Overall, pregnancies that occurred ≤2 months postpartum, three to five months and six to 11 months after delivery were more likely to be reported as unintended compared to those occurring between 12 and 18 months after delivery (all p <0.001).
Table 2. Frequency of short interpregnancy interval pregnancies that were intended, mistimed and unwanted, by the period after delivery in which the pregnancy occurred *.
| 0-2 months | 3-5 months | 6-11 months | 12-18 months | |
|---|---|---|---|---|
|
| ||||
| n (%, 95% CI) | n (%, 95% CI) | n (%, 95% CI) | n (%, 95% CI) | |
| Intended | 10 (15.6, 5.9-35.3) | 12 (11.5, 5.3-23.3) | 45 (30.0, 21.2,41.3) | 81 (67.3, 56.2-76.8) |
| Unintended | 51 (84.4, 64.7-94.1)† | 54 (88.5, 76.7-94.7)† | 114 (70.0, 58.7-78.8)† | 67 (32.7, 23.2-43.8) |
| Mistimed | 29 (54.4, 34.2-73.2) | 29 (57.5, 41.3-72.3) | 87 (56.4, 46.3-66.1) | 39 (19.3, 11.9-29.7) |
| Unwanted | 22 (30.0, 15.3-50.5) | 25 (31.0, 18.2-47.5) | 27 (13.2, 7.6-22.0) | 28 (13.4, 8.0-21.5) |
These results are from our own calculations using the NSFG 2006–2010. See http://www.cdc.gov/nchs/nsfg/nsfg_2006_2010_puf.htm for more information.
CI: confidence interval
Short interpregnancy interval pregnancies were conceived ≤18 months after a live singleton birth that occurred within 3 years of the survey date (n=434). Percentages are weighted to reflect the sampling design of the NSFG.
p <0.001 compared to unintended pregnancy reported at 12-18 months after delivery. The p-value is based on negative binomial regression.
Discussion
This analysis shows that approximately half of US women rely on less-effective or no method of contraception in the 18 months after delivery. These national-level results support findings from a recent California study demonstrating that more than half of publicly insured women did not have a contraceptive claim within 90 days postpartum.18 Our study also demonstrates that unintended pregnancies are common in the 18 months after delivery, and at least 70% of these occur within the first year after the index birth. Finally, the regression analysis shows that less-effective contraceptive use was the leading predictor of having a short interpregnancy interval, after controlling for women's sociodemographic characteristics. Together, these results raise the question as to why US women do not make greater use of the most highly effective contraceptive methods in the months after delivery.
One plausible answer is that women have little interest in using more effective methods because of side effects or other perceived problems they associate with use of long-acting contraception.19, 20 They also may be opposed to using LARC because they do not like the idea of having a foreign object in their body or being unable to discontinue these methods without visiting a health care provider.21-23 Some women also may choose not to contracept because they plan to stay abstinent or underestimate their risk of pregnancy.24-27
However, recent studies indicate that postpartum women have a high demand for LARC methods. Among a pregnant adolescent cohort in Colorado, 43% chose to initiate the contraceptive implant immediately postpartum when offered, and more than one-third of women delivering in North Carolina said they planned to use a LARC method after delivery.7, 8 In a study of postpartum contraception in Texas, we found that 34% of women wanted to use a long-acting method after delivery, but many were unable to access their preferred method and instead relied on less-effective forms of contraception.6
Another potential explanation for the low use of highly effective methods after delivery is that women face insurance-related barriers. Women may be unable to access LARC in the hospital because the cost of the device and insertion are not included in the global fee for delivery and because few states have revised their Medicaid policies to permit separate billing.28 Uninsured, low-income women who are only eligible for Emergency Medicaid to cover the cost of delivery may also find it difficult to access contraception postpartum, since this is not an included service in most states.29 Additionally, some women may lose contraceptive coverage soon after delivery due to changes in employment 30 or if they are not automatically enrolled in their state's Medicaid family planning waiver. This may contribute to our finding that women's contraceptive method use changed relatively little after three months postpartum.
The importance of barriers to access has been demonstrated by the rapid uptake of LARC in several states where measures have been taken to make these methods more widely available. For example, state-wide and local initiatives to increase LARC access in Colorado, Iowa and St. Louis, Missouri resulted in substantially higher use of these methods and decreased rates of unintended pregnancy and abortion.31-33 Additionally, adolescents receiving contraceptive implants immediately postpartum had significantly lower rates of pregnancy within 12 months after delivery, compared to those who initiated contraception after hospital discharge.8
This study has several limitations. Our analysis relied on women's retrospective reporting of their contraceptive method use, and therefore may be subject to recall bias. However, the contraceptive calendar is a well-validated method, which when linked to other key life events and limited to three years preceding the survey should reduce reporting error.34 Also, we used women's contraceptive method at the start of each interval to assess the risk of having a short interpregnancy interval, and, therefore, may not have adequately captured women's contraceptive method use at the time of the pregnancy. Additionally, we defined an interpregnancy interval as the time between the index birth and conception of another pregnancy leading to a live birth and excluded pregnancies ending in miscarriage and abortion. This underestimates women's risk of becoming pregnant after delivery, but is more relevant to the maternal and neonatal health risks associated with a pregnancy carried to term. Finally, women's use of more effective methods after delivery may have changed since the period under study, which preceded the rollout of the Affordable Care Act and the Centers for Disease Control and Prevention's recommendation to delay initiation of combined hormonal contraception until 21 days postpartum.35
Despite these limitations, our study indicates that many US women rely on less-effective contraceptive methods – or use no method – in the 18 months after delivery, which results in short interpregnancy intervals and unintended pregnancies. In order to reduce adverse maternal and infant health outcomes associated with closely spaced births,4 programs and policies that remove barriers to initiating effective contraception are needed so that women can realize their contraceptive preferences and achieve their childbearing goals.
Acknowledgments
Infrastructural support for the study was provided by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R24 042849) to the Population Research Center, University of Texas at Austin.
Stephanie Teal has served on the scientific advisory boards of Actavis, Bayer Healthcare, and MicroCHIPs and the Data Safety and Monitoring Board for a Phase 4 study required by the U.S. Food and Drug Administration and funded by Merck; she also has received research support from Medicines360.
Footnotes
Financial Disclosure: The other authors did not report any potential conflicts of interest.
References
- 1.Teal SB. Postpartum contraception: Optimizing interpregnancy intervals. Contraception. 2014;89(6):487–8. doi: 10.1016/j.contraception.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Zapata LB, Murtaza S, Whiteman MK, Jamieson DJ, Robbins CL, Marchbanks PA, et al. Contraceptive counseling and postpartum contraceptive use. Am J Obstet Gynecol. 2015;212(2):171 e1–8. doi: 10.1016/j.ajog.2014.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gemmill A, Lindberg LD. Short interpregnancy intervals in the United States. Obstet Gynecol. 2013;122(1):64–71. doi: 10.1097/AOG.0b013e3182955e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services. Office of Disease Prevention and Health Promotion. Healthy people 2020. Available at: http://www.healthypeople.gov/2020/topics-objectives/topic/family-planning/objectives?topicId=13 Retrieved November 13, 2014.
- 5.Whiteman MK, Cox S, Tepper NK, Curtis KM, Jamieson DJ, Penman-Aguilar A, et al. Postpartum intrauterine device insertion and postpartum tubal sterilization in the United States. Am J Obstet Gynecol. 2012;206(2):127 e1–7. doi: 10.1016/j.ajog.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Potter JE, Hopkins K, Aiken AR, Hubert C, Stevenson A, White K, et al. Unmet demand for highly effective postpartum contraception in Texas. Contraception. 2014;90(5):488–95. doi: 10.1016/j.contraception.2014.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang JH, Dominik R, Re S, Brody S, Stewart GS. Characteristics associated with interest in long-acting reversible contraception in a postpartum population. Contraception. 2013;88(1):52–7. doi: 10.1016/j.contraception.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: Do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206(6):481 e1–7. doi: 10.1016/j.ajog.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 9.White K, Potter JE, Hopkins K, Grossman D. Variation in postpartum contraceptive method use: Results from the Pregnancy Risk Assessment Monitoring System (PRAMS) Contraception. 2014;89(1):57–62. doi: 10.1016/j.contraception.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lepkowski JM, Mosher WD, Groves RM, Wesst BT, Wagner J, Gu H. Responsive design, weighting and variance estimation in the 2006-2010 national survey of family growth. Vital and Health Statistics. 2013;2(158):1–42. [PubMed] [Google Scholar]
- 11.Groves RM, Mosher W, Lepkowski JM, Kirgis NG. Planning and development of the continous National Survey of Family Growth. Vital and Health Statistics. 2009;1(48):1–64. [PubMed] [Google Scholar]
- 12.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. Public use data file documentation: 2006-2010 National Survey of Family Growth, User's Guide. Hyattsville, MD: National Center for Health Statistics; 2011. [Google Scholar]
- 13.Jones RK, Kost K. Underreporting of induced and spontaneous abortion in the United States: An analysis of the 2002 National Survey of Family Growth. Stud Fam Plan. 2007;38(3):187–97. doi: 10.1111/j.1728-4465.2007.00130.x. [DOI] [PubMed] [Google Scholar]
- 14.Thiel de Bocanegra H, Chang R, Howell M, Darney P. Interpregnancy intervals: Impact of postpartum contraceptive effectiveness and coverage. Am J Obstet Gynecol. 2014;210(4):311 e1–8. doi: 10.1016/j.ajog.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Kavanaugh ML, Jerman J, Hubacher D, Kost K, Finer LB. Characteristics of women in the United States who use long-acting reversible contraceptive methods. Obstet Gynecol. 2011;117(6):1349–57. doi: 10.1097/AOG.0b013e31821c47c9. [DOI] [PubMed] [Google Scholar]
- 16.Hatcher RA, Trussell J, Nelson AL, Cates W, Stewart FH, Kowal D, editors. Contraceptive Technology. 19th. New York: Ardent Media, Inc.; 2007. [Google Scholar]
- 17.Finer LB, Zolna MR. Unintended pregnancy in the United States: Incidence and disparities, 2006. Contraception. 2011;84(5):478–85. doi: 10.1016/j.contraception.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiel de Bocanegra H, Chang R, Menz M, Howell M, Darney P. Postpartum contraception in publicly-funded programs and interpregnancy intervals. Obstet Gynecol. 2013;122(2 Pt 1):296–303. doi: 10.1097/AOG.0b013e3182991db6. [DOI] [PubMed] [Google Scholar]
- 19.Hladky KJ, Allsworth JE, Madden T, Secura GM, Peipert JF. Women's knowledge about intrauterine contraception. Obstet Gynecol. 2011;117(1):48–54. doi: 10.1097/AOG.0b013e318202b4c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming KL, Sokoloff A, Raine TR. Attitudes and beliefs about the intrauterine device among teenagers and young women. Contraception. 2010;82(2):178–82. doi: 10.1016/j.contraception.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White K, Hopkins K, Potter JE, Grossman D. Acceptability of long-acting reversible contraception among parous Latina women who do not want more children. Contraception. 2011;84(3):330. [Google Scholar]
- 22.Lessard LN, Karasek D, Ma S, Darney P, Deardorff J, Lahiff M, et al. Contraceptive features preferred by women at high risk of unintended pregnancy. Perspect Sex Reprod Health. 2012;44(3):194–200. doi: 10.1363/4419412. [DOI] [PubMed] [Google Scholar]
- 23.Borrero S, Abebe K, Dehlendorf C, Schwarz EB, Creinin MD, Nikolajski C, et al. Racial variation in tubal sterilization rates: Role of patient-level factors. Fertil Steril. 2011;95(1):17–22. doi: 10.1016/j.fertnstert.2010.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biggs MA, Karasek D, Greene Foster D. Unprotected intercourse among women wanting to avoid pregnancy: Attitudes, behaviors, and beliefs. Women's Health Issues. 2012;22(3):e311–8. doi: 10.1016/j.whi.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Vermont Department of Health. Vermont PRAMS data brief. Burlington, VT: Vermont Department of Health; 2010. [Google Scholar]
- 26.Virginia Department of Health. Postpartum contraceptive use in Virginia. Richmond, VA: Virginia Department of Health; 2010. [Google Scholar]
- 27.Arkansas Department of Health. Postpartum contraceptive use by Arkansas women who had live births: PRAMS, 2009-2011. Little Rock, AR: Arkansas Department of Health; 2013. [Google Scholar]
- 28.Aiken AR, Creinin M, Kaunitz AM, Nelson AL, Trussell J. Global fee prohibits postpartum provision of the most effective reversible methods. Contraception. 2014;90(5):466–7. doi: 10.1016/j.contraception.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez MI, Jensen JT, Darney P, Little SE, Caughey AB. The financial effects of expanding postpartum contraception for new immigrants. Obstet Gynecol. 2010;115(3):552–8. doi: 10.1097/AOG.0b013e3181d06f96. [DOI] [PubMed] [Google Scholar]
- 30.Dennis AE, Blanchard K, Córdova D, Wahlin B, Clark J, Edlund K, et al. What happens to the women who fall through the cracks of health care reform? Lessons from Massachusetts. J Health Polit Policy Law. 2013;38(2):393–419. doi: 10.1215/03616878-1966351. [DOI] [PubMed] [Google Scholar]
- 31.Ricketts S, Klingler G, Schwalberg R. Game change in Colorado: Widespread use of long-acting reversible contraceptives and rapid decline in births among young, low-income women. Perspect Sex Reprod Health. 2014 Sep;46(3):125–32. doi: 10.1363/46e1714. [DOI] [PubMed] [Google Scholar]
- 32.Peipert JF, Madden T, Allsworth JE, Secura GM. Preventing unintended pregnancies by providing no-cost contraception. Obstet Gynecol. 2012;120(6):1291–7. doi: 10.1097/aog.0b013e318273eb56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biggs MA, Roca C, Brindis CD, Hirsch H, Grossman D. Did increasing use of highly effective contraception contribute to declining abortions in Iowa? Contraception. 2015;91(2):167–73. doi: 10.1016/j.contraception.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Freedman D, Thornton A, Camburn D, Alwin D, Young-demarco L. The life history calendar: A technique for collecting retrospective data. Sociol Methodol. 1988;18:37–68. [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. U.S. Medical eligibility criteria for contraceptive use. Morbid Mortal Wkly Rep. 2010;59 [Google Scholar]