Abstract
Prior research has found inconsistent evidence regarding the association among childhood adversity, inflammation, and internalizing symptoms, perhaps because previous studies have yet to adequately integrate important factors such as the timing of the adversity, genetic variation, and other relevant processes such as neuroendocrine regulation. The aims of the present study were threefold: 1) Determine whether the effect of the timing of child maltreatment on C-reactive protein (CRP), an inflammatory marker, varies by CRP gene variation; 2) Explore whether links between salivary CRP and childhood internalizing symptoms depend on the presence and timing of maltreatment experiences; 3) Investigate the role of CRP in the relations between child neuroendocrine regulation and internalizing symptoms and examine whether these associations are moderated by the presence and timing of child maltreatment. Participants included a sample of 267 maltreated and 222 nonmaltreated children (M age= 9.72, SD=0.99; 52.4% male; 66% African-American) who attended a summer day camp research program designed for school-aged low-income children. Department of Human Services records were examined to determine the onset and recency of maltreatment for children in the maltreated group. Results indicated that among children with recent onset maltreatment, those with at least one A allele from CRP SNP rs1417938 evidenced significantly higher CRP levels compared to recently maltreated children carrying the TT genotype. Moreover, higher levels of CRP were associated with higher levels of internalizing symptoms only for recently maltreated children. Finally, we did not find support for salivary CRP as a mechanism in the relation between neuroendocrine regulation and childhood internalizing symptoms. Our findings highlight the importance of the timing of child maltreatment and have important implications for characterizing variability in inflammation and internalizing symptoms among youth.
Substantial research has shown that child maltreatment exerts dire effects on the developmental course. Child abuse and neglect progressively contribute to compromised adaptation on a number of developmental domains and issues central to successful adjustment. These developmental failures pose significant risk for psychopathology (Cicchetti & Toth, in press; Cicchetti & Valentino, 2006).
The deleterious sequelae accompanying child maltreatment not only result in adverse consequences during childhood, but also often initiate a negative developmental cascade that continues throughout the life course (Cicchetti & Tucker, 1994; Masten & Cicchetti, 2010). The proximal environment involving the nuclear family, as well as more distal factors associated with the community and culture more broadly, transact to undermine normal biological and psychological developmental processes in children who have experienced maltreatment (Cicchetti & Lynch, 1993, 1995; Rogosch, Cicchetti, Shields, & Toth, 1995). In fact, child maltreatment may constitute the greatest failure of the caregiving environment to provide opportunities for normal development (Cicchetti & Lynch, 1995). Moreover, maltreatment experiences may provide serious challenges to the species-typical organism-environment “coactions” that play important roles in the emergences and timing of normal developmental change (Cicchetti & Lynch, 1995; Gottlieb, 1992).
Findings from the Adverse Childhood Experiences (ACE) study (Anda, Dube, & Giles, 2006), one of the largest and arguably most important public health studies conducted to date in the United States, demonstrate that childhood adversity is associated with an extraordinary number of adult mental and physical health risk behaviors. The ACE factors that were examined included exposure to childhood emotional, physical, and sexual abuse, substance abuse in the family, parental mental illness, domestic violence, and adult criminal behavior. Adults with elevated ACEs were not only at increased risk for psychosocial and substance abuse problems, but also for diseases, including cardiovascular, diabetic, and immunological problems, and early morbidity.
Strikingly, over two thirds of the adults surveyed in the ACE study had experienced one or more types of adverse childhood experiences; 87% of these individuals had experienced two or more types of trauma. According to the Center for Disease Control (Fang, Brown, Florence, & Mercy, 2012), one year of confirmed cases of child maltreatment (including cases of sexual, physical, and emotional abuse, and neglect) costs $124 billion over the life span of the abused and neglected children. The public health implications of the adverse effects accompanying child maltreatment are enormous. Thus, the magnitude of the effects of child maltreatment and the toll they exert on individuals, families, and society should not be underestimated.
Previously, research on the sequelae of child maltreatment has focused predominately on psychosocial outcomes. Developmental psychopathologists have advocated research on normal and atypical populations incorporating multiple levels of analyses (Cicchetti & Dawson, 2002; Cicchetti & Toth, 2009). In recent decades, research on the effects of maltreatment increasingly has incorporated multilevel investigations of biological and psychosocial sequelae (Curtis & Cicchetti, 2013; DeBellis, 2001, 2005; Hart & Rubia, 2012; McCrory et al., 2010; Pollak, Cicchetti, Klorman, & Brumaghin, 1997).
As toxic stressors (Shonkoff, Boyce, & McEwen, 2009), child abuse and neglect are implicated in the disruption of biological systems, including neuroendocrine and immune functioning, physical and mental health outcomes, and neurobiology. Physiological and behavioral responses to maltreatment are interrelated and contribute to an organization of developmental systems that may produce pathological development. Early maltreatment may alter young neural networks, resulting in cascading effects through the course of later development, possibly constraining the child's capacity to adapt flexibly to new challenging situations and environmental demands (Cicchetti & Tucker, 1994).
The concepts of allostasis and allostatic load provide an integrative framework for understanding how exposure to chronic stress, such as child maltreatment, potentiates long-term liabilities for physical and mental health (Danese & McEwen, 2012; Juster et al., 2011). Allostasis is a process that involves the activation of multiple interactive physiological systems (e.g., cardiovascular, neuroendocrine, immune, and metabolic systems). Epinephrine, norepinephrine, cortisol, dehydroepiandosterone (DHEA), and pro- and anti-inflammatory cytokines are considered the primary mediators of allostatic load (AL) because they operate on the cellular level (McEwen, 2003; Rogosch, Dackis, & Cicchetti, 2011).
In the short term, mobilization of these systems exerts a protective effect on the body and promotes an adaptive response to stress; however, with chronic activation, physiological reactions to stress become less efficient in protecting the individual. Ensuing damage to the body results in allostatic overload, which in turn, contributes to change in brain structure and function and the development of various disease states (Hostinar, Sullivan, & Gunnar, 2013). It is the collective impact of alterations across multiple systems that contributes most strongly to morbidity, rather than changes within any one system (Juster et al., 2011; Juster, McEwen, & Lupien, 2010; Rogosch et al., 2011).
Cicchetti and Rogosch (2001) examined the extent to which maltreated children vary with respect to cortisol regulation, a biomarker of AL. Although no differences in cortisol regulation were found between the maltreated and nonmaltreated groups, findings revealed significant within-group variation as a function of maltreatment subtype. In particular, children who had experienced both physical and sexual abuse, in combination with neglect and/or emotional maltreatment, exhibited substantial elevations in morning cortisol levels (i.e., hypercortisolism). In addition, a subgroup of physically abused children showed a trend toward lower morning cortisol relative to nonmaltreated children (i.e. hypocortisolism). Furthermore, the neglected and emotionally maltreated groups did not differ from nonmaltreated children in terms of cortisol regulation.
Relatedly, Cicchetti, Rogosch, and Oshri (2011), within an allostatic load framework, investigated the effect of GxE interactions on diurnal cortisol regulation and internalizing symptomatology. Variation in the CRHR1 TAT haplotype and 5-HTTLPR was identified in a sample of maltreated and nonmaltreated children. GxE effects for CRHR1 and maltreatment and early abuse (before Age 5) on diurnal cortisol regulation were observed; CRHR1 variation was related to cortisol dysregulation only among maltreated children. Early abuse and high internalizing symptoms also interacted to predict atypical diurnal cortisol regulation. The interaction of CRHR1, 5-HTTLPR, and child maltreatment (GxGxE) identified a subgroup of maltreated children with high internalizing symptoms who shared the same combination of the genetic variants (2 copies of the CRHR1 TAT haplotype and the LL 5-HTTLPR genotype). The findings provide support for an allostatic-load perspective on the effects of the toxic stress associated with child maltreatment on cortisol regulation and internalizing symptomatology as moderated by genetic variation.
There are different models of how the effects of excesses, deficits, and dysregulation in the primary mediators of AL, particularly cortisol, may affect brain structure and function. Further, developmental considerations are important. In this regard, the timing of periods of severe stress may be critical. For example, variation in the age of maturation of brain structures, notably the hippocampus, prefrontal cortex, and the amygdala, may result in differential effects of functioning and health (Lupien, McEwen, Gunnar, & Heim, 2009; Shonkoff et al., 2009). Sensitive periods in the development of these brain structures may generate heightened vulnerability to the neurotoxic effects of excess glucocorticoids, thereby creating a long-term liability as development proceeds. Alternatively, the cumulative exposure to stressful experiences and concomitant dysregulation of the HPA axis may contribute to ongoing wear and tear on these brain structures. These effects of chronic stress are heightened, given that these areas of the brain have dense concentrations of glucocorticoid receptors, thereby promulgating progressive inefficiency in brain structure and function (Gunnar & Vasquez, 2006). For children in low-income environments and those subjected to abuse and neglect, the consequences may be particularly salient for health outcomes across the life span.
Consistent with the concept of AL, Rogosch et al. (2011) conducted a multidomain assessment of stress-sensitive systems among low-income maltreated and nonmaltreated comparison children. An AL composite was created from measurements of salivary cortisol and dehydroepiandosterone (DHEA), body mass index (BMI), waist-hip ratio, and blood pressure. Results indicated that maltreatments and AL independently predicted psychopathology and health difficulties (i.e., parent report of child's physical health status and utilization of health care systems). As AL increased, the level of the child health and psychological problems increased for all low-income children. Child maltreatment had an additive effect, contributing to the degree of physical and mental health problems beyond that accounted for by the AL composite. Therefore, children with both high AL and a history of maltreatment had the most health problems.
In addition to cortisol dysregulation, maltreatment has been found to be associated with other adverse health outcomes. For example, maltreatment predicts increased risk for hospital-based treatment of asthma, cardiorespiratory, and infectious diseases in childhood. (Lanier, Jonson-Reid, Stahlschmidt, Drake, & Constantino, 2010). Early child abuse has been linked to more health-related symptoms such as sleep and eating difficulties, higher BMI, high blood pressure, low high density lipoprotein, leptin deficiency, obesity, fibromyalgia, and compromised immune system functioning in childhood and adolescence (Danese et al., 2011; Danese & Tan, 2014; Danese, Pariante, Caspi, Taylor, & Poulton, 2007; Lee, 2010; Rogosch et al., 2011; Shirtcliff, Coe, & Pollak, 2009). Furthermore, health liabilities extend into adulthood, as child maltreatment has been found to predict adult cardiovascular disease, elevated inflammation levels, type II diabetes, HIV risk, self-reported physical symptoms across a range of organ systems, and decreased longevity (Danese et al., 2007; Felitti et al, 1998; Wilson & Widom, 2011). Moreover, child maltreatment has been linked to shortened leucocyte telomere length, whereby it is thought that maltreatment may influence cellular aging (Tyrka, et al, 2010).
Miller, Chen, and Parker (2011) proposed a conceptual model to explain why those who experience child maltreatment have an enhanced proinflammatory phenotype. Miller and colleagues (2011) state that stress that occurs during a sensitive developmental period, i.e., when immune function is highly malleable/plastic, becomes embedded in the functioning of the cells that regulate inflammation. Coelho, Viola, Walss-Bass, Brietzke, and Grassi-Oliveira (2014) reviewed twenty studies that examined the association between adults' recollections of child maltreatment and inflammatory markers. Coelho and colleagues (2014) concluded that a history of child maltreatment was associated with increased levels of C-reactive protein (CRP), an inflammatory biomarker that is indicative of systemic inflammation. Reichlin (1993) proposed that the neuroendocrine and immune systems interact. Immune responses are thought to alter neural and endocrine function. In turn, neural and endocrine activity are seen as modifying the function of the immune system. The dysregulation of the HPA axis in maltreated persons, who manifest pituitary hyporesponsiveness to corticotropic releasing hormone (CRH) and/or increased glucocorticoid sensitivity to cortisol, has the potential to bring about a systemic proinflammatory state.
Danese, Moffitt et al. (2008) studied adults who were currently depressed and had a history of child maltreatment. They found that individuals with both depression and a maltreatment history were more likely to have high levels of high-sensitivity CRP compared to control subjects. In contrast, individuals who were currently depressed, but who had no history of child maltreatment, had a non-significant elevation of CRP. In another study, Danese, Moffitt et al. (2009) investigated adults who were exposed to adverse psychosocial experiences during their childhood. Danese, Moffitt, and colleagues (2009) found that these adults were at an elevated risk of depression and had high inflammation levels and metabolic risk markers. These metabolic risks included high blood pressure, high total cholesterol, and low high density lipoprotein cholesterol. Adults who had not been exposed to child maltreatment did not exhibit elevations in depression, inflammation, and metabolic risks.
Slopen et al. (2013) conducted a prospective longitudinal study in which they examined acute adverse events at 7 time points prior to age 8 and inflammation outcomes at ages 10 (CRP; IL-6) and 15 (CRP). Investigations have rarely examined the links between child adversity and inflammation prospectively. Inflammation may be an early mechanism by which adverse experiences in childhood become biologically embedded and adversely impact health throughout the lifespan (cf. Miller et al., 2011). Exposure to adverse events before age 8 is associated with elevated levels of inflammation at age 10 and in mid-adolescence. Slopen and colleagues (2013) discovered that the timing of the exposure to adverse events appears to matter with respect to later development of inflammation. They found that adverse events experienced in early childhood and in relatively close proximity to the measure of inflammation were most strongly associated in inflammatory levels.
Bufalino, Hepgul, Aguglia, and Pariante (2012) reviewed the clinical studies literature on the role of immune genes in the association between depression and inflammation. Dysregulation of the immune system in the pathogenesis of depressive disorders commonly occurred and research has begun to implicate the role of an underlying genetic vulnerability. Functional allelic variants of genes for C-reactive protein were among the genes that were found to augment the risk for depression. Genetic variants influence the biological mechanisms by which the immune system contributes to the development of depression (Hadler et al., 2010).
Given the paucity of studies conducted on the effects of child maltreatment on inflammation in children, and evidence accumulating that variation in the CRP gene is implicated in vulnerability to depression, in the present multilevel investigation we examine the association between timing of child maltreatment, variation in CRP genotype, neuroendocrine regulation, salivary C-reactive protein, and levels of internalizing symptoms.
Hypotheses
The present study is guided by the following hypotheses:
The effect of child maltreatment experiences on inflammation will vary by CRP genotype.
Higher levels of inflammation will relate to higher levels of internalizing symptoms more strongly for individuals with recent maltreatment experiences.
C-reactive protein inflammation levels will mediate the association between neuroendocrine regulation and internalizing symptomatology.
These relations will be moderated by maltreatment timing.
Method
Participants
Participants included 489 children aged 8 to 12 (M age= 9.72, SD=0.99; 52.4% male) who attended a summer day camp research program designed for school-aged low-income children. The sample included both maltreated children (n= 267) and nonmaltreated children (n=222) and was racially and ethnically diverse (66.0% African-American, 10.0% Caucasian, 19.6% Hispanic, and 4.5% another race/ethnicity). Informed consent was obtained from parents of maltreated and nonmaltreated children for their child's participation in the summer camp program and for examination of any Department of Human Services (DHS) records pertaining to the family.
Children in the maltreated group were recruited through a DHS liaison who examined Child Protective Services reports to identify children who had been maltreated. Children living in foster care were not recruited for the current investigation. The DHS liaison contacted eligible families and explained the study. Parents who were interested in having their child participate, provided signed permission for the contact information to be shared with project staff. These families were representative of those receiving services through DHS. Comprehensive reviews of all DHS records for each family were conducted. Coding is based on all available information and does not rely on DHS determinations. Maltreatment information was coded by trained research staff, doctoral students, and clinical psychologists, using the Maltreatment Classification System (MCS; Barnett, Manly, and Cicchetti, 1993), a nosological system for classifying child maltreatment. Adequate reliability has been obtained (weighted ks=0.86-0.98; Manly, 2005; Manly et al., 2001).
Because maltreating families primarily have low socioeconomic status (National Incidence study – NIS-4; Sedlak et al., 2010), nonmaltreating families were recruited from those receiving Temporary Assistance to Needy Families (TANF) in order to ensure socioeconomic comparability between maltreated and nonmaltreated families. A DHS liaison contacted eligible nonmaltreating families and described the project. Parents who were interested in participating signed a release allowing their contact information to be given to project staff for recruitment. Families who received preventative DHS services due to concerns over risk for maltreatment were not included with the nonmaltreated comparison group. In order to further verify a lack of DHS involvement, trained research assistants interviewed the mothers of children recruited for the nonmaltreated group using the Maternal Child Maltreatment Interview (Cicchetti, Toth & Manly, 2003) and reviewed DHS records in the year following camp participation to assure that all information had been assessed.
As shown in Table 1, children in the maltreated and nonmaltreated groups were comparable on a number of demographic variables including child age, maternal education, maternal marital status, and history of receiving public assistance. Groups differed on gender such that more males comprised the maltreated group (χ2 (1) =5.78, p=.02). Groups also differed on ancestry informed race/ethnicity such that there were more African-American children in the nonmaltreated group (χ2 (3) = 16.77, p=.001).
Table 1.
Demographic and descriptive characteristics of sample
| Nonmaltreated (n=222) | Maltreated(n=267) | |
|---|---|---|
| M (SD) or % | M (SD) or % | |
| Age | 9.67 (.94) | 9.77 (1.03) |
| Gender* | ||
| Male | 46.3% | 57.3% |
| Female | 53.7% | 42.7% |
| Ancestry informed race/ethnicity** | ||
| African-American | 74.2% | 59.1% |
| Caucasian | 4.7% | 14.3% |
| Hispanic | 17.8% | 21.0% |
| Other | 3.3% | 5.5% |
| Maternal education | ||
| Did not graduate high school | 30.8% | 38.3% |
| High school degree or higher | 69.2% | 61.7% |
| Maternal marital status | ||
| Never married | 42.2% | 36.1% |
| Married | 20.4% | 17.3% |
| Living with partner | 16.6% | 22.1% |
| No longer married | 20.9% | 24.4% |
| History of receiving public assistance | 100% | 100% |
Notes.
p<.05;
p<.001
Procedure
Families were approached and asked if they would agree to have their child attend a week-long day camp and participate in the research (for detailed descriptions of the camp procedures, see Cicchetti & Manly, 1990). Children were transported by bus to the camp each day, with travel time averaging 45 minutes. At the camp, children were assigned to groups of 10 (5 maltreated, 5 nonmaltreated) same-age and same-sex peers. Each group was led by three trained camp counselors, who were unaware of the maltreatment status of children and the hypotheses of the study. Camp lasted 7 hours per day for 5 days, providing 35 hours of interaction between children and counselors. In addition to the recreational activities, after providing assent, children participated in various research assessments and provided morning and afternoon saliva samples. Trained research assistants, who also were unaware of research hypotheses and maltreatment status, conducted individual research sessions with children, in which questionnaires and other research measures were administered. If any concerns over danger to self or others emerged during research sessions, then clinical psychologists were available for consultation and referrals were made as needed. The counselors, who had been trained extensively for 2 weeks prior to the camp, also completed assessment measures on individual children, based on their 35 hours of observations and interactions with the children in their respective groups.
Measures
Maltreatment classification
The Maltreatment Classification System (MCS; Barnett et al., 1993) was used to delineate diverse features of child maltreatment that individual children experienced. The MCS utilized DHS records detailing investigations and findings regarding maltreatment occurrences in identified families. Rather than relying on official designations and case dispositions, the MCS codes all information available on a designated family from DHS records, making independent determinations of maltreatment experiences. In particular, the MCS codes all incidents that have been documented in DHS records and, based on operational criteria, designates the subtypes of maltreatment individual children have experienced (i.e. emotional maltreatment, neglect, physical abuse, sexual abuse), the severity of each type of maltreatment, the frequency of occurrence, the developmental periods in which maltreatment occurred, and the perpetrators of maltreatment.
Children were classified into 4 groups based on the timing of their maltreatment experiences. Children who experienced maltreatment before age 5, but not since then were classified as “early, not recently maltreated.” Children who experienced maltreatment both before age 5 and since then were classified as “early and recently maltreated.” Children whose first maltreatment experience occurred between age 5 and the time of study, were classified as “recent onset maltreated.” Children without maltreatment histories were classified as “nonmaltreated.”
DNA collection, extraction, and genotyping
Using an established protocol, trained research assistants obtained DNA samples from participants by collecting saliva using the Oragene DNA Self-Collection kits. DNA was purified from 0.5 ml of Oragene-DNA solution using the DNAgenotek protocol for manual sample purification using prepIT-L2P. Sample concentrations were determined using the Quant-iT PicoGreen dsDNA Assay Kit (P7589, Invitrogen).
Variations in the gene encoding for C-reactive protein were determined using assays for SNP rs1417938 purchased from Applied Biosystems, Inc. (ABI, Bedford, MA) as C7479322 10. Individual allele discriminations were made using Taq Man Genotyping Master Mix (ABI Catalog No. 4371357) with amplification in an ABI 9700 thermal cycler and analyzing the endpoint fluorescence using a Tecan M200. If a genotype could not be determined after the first run, then it was repeated up to four times. The call rate for the CRP SNP was 99.2%. CRP SNP distribution did not deviate from Hardy-Weinberg equilibrium (χ2 (1) = .89, p=n.s.). The frequency distribution of the CRP SNP was as follows: TT=66.9% AT=25.4%; AA=3.1%. Genotypes AA and AT were combined in these analyses because of the low frequency of AA.
Ancestry
For ancestral proportion testing, DNA from study participants was subjected to SNP genotyping of the Burchard et al panel of 106 SNPs (Lai et al., 2009; Yaeger et al., 2008), known to be informative for ancestry from Africa, Europe, and Native America. The SNPs were genotyped using the iPLEX platform from Sequenom Bioscience, Inc which uses the Sequenom MassArray. Samples are subjected to single base primer extension (SBE) with fluorophore labeled nucleotides from primers designed for SNPs of interest. The samples including the SBE products were placed on the iPLEX platform and MALDI-TOF was used to identify the allele based on the fluorophore passing the detector at the expected time associated with the mass of the SBE primer. The SNP genotyping results were then recoded and uploaded into STRUCTURE v2.3.4 which uses algorithms developed by Pritchard and colleagues (Falush Stephens, & Pritchard, 2003, 2007; Hubisz, Falush, Stephens, & Pritchard, 2009). Three SNP tests were excluded based on high allele call rates of the non-DNA containing wells. The data from remaining 103 loci were uploaded into the software and set to analyze with an Admixture model of ancestry and initialization of the simulation on the GALA cohort (initialize of POPINFO). The simulation was set to run with a Burn-in of 10,000, MCMC Reps of 1,000 and assuming 3 populations within the group. The results of the simulations were subsequently identified as percent association to each ancestry group based on the known ancestry of the GALA cohort.
To facilitate gene × race/ethnicity and maltreatment × race/ethnicity interaction tests, a grouping variable using ancestral proportion continuous scores was created using multinomial logistic regression to classify cases. This was done because an ancestry-informed race/ethnicity grouping variable is more readily interpretable in the case of any potentially statistically significant interactions. Parent-reported race/ethnicity (coded 1=African-American, 2=Caucasian, 3=Hispanic, 4=other race/ethnicity) was predicted from proportion African ancestry and proportion Native American ancestry. We did not include proportion European ancestry because of collinearity concerns with the other ancestral proportion scores. The parent-identified Caucasian group was selected as the reference group. The overall multinomial logistic regression was significant (LRχ2 (6) = 606.60, p<.001) and together the 2 ancestry proportion scores explained a very large amount of variance in parent-identified group membership (Nagelkerke R2 = .81). Results indicated that higher African ancestry scores predicted a significantly greater likelihood of being in the parent-identified African-American versus Caucasian group (B=.48, OR=1.62, 95% CI = 1.21; 2.17), Hispanic versus Caucasian group (B=.38, OR=1.46, 95% CI = 1.09; 1.95), and other race/ethnicity group versus Caucasian group (B=.39, OR=1.48, 95% CI = 1.10; 1.97). Higher Native American ancestry scores significantly predicted a greater likelihood of being in the parent-identified Hispanic versus Caucasian group (B=.06, OR=1.06, 95% CI; 1.09) and other race/ethnicity group versus Caucasian group (B=.04, OR=1.04, 95% CI; 1.06). Native American ancestry did not significantly differentiate between parent-identified African-American and Caucasian groups. The mean probability of predicted group membership from the two ancestry proportion scores was .84 (SD=.18) with probabilities ranging from .28 (SD= .28; among those parent-identified as “other”) to .90 (SD= .16) among those parent-identified as African-American). Ancestral proportion scores were more likely to reclassify individuals who parent-identified as Hispanic or “other race/ethnicity” compared to those who parent-identified as African-American or Caucasian.
Cortisol and DHEA
The camp context allowed for a setting where a consistent collection of saliva samples could occur at uniform times across the camp week. Each child arrived at camp via camp bus transportation. Each day upon arrival to the camp at 9:00 a.m., trained research assistants obtained saliva samples from each child. Given the approximate 45 minute camp bus transportation time and time spent being greeted by camp staff, children had been awake at least 1 hour prior to providing the morning saliva samples, thus avoiding the period of the dynamic cortisol awakening response (Susman et al., 2007). Children had not consumed food or drink for at least 30 minutes before each saliva sample was obtained. Samples were collected following the method recommended by Granger and colleagues (1999). The children were asked to chew Trident® sugarless original flavor gum to stimulate saliva flow and then passively drool through a short drinking straw into a 20-ml plastic vial. The samples were immediately frozen and stored at -40°C. Each week, the samples were shipped overnight on dry ice for next day delivery to Salimetrics Laboratories (State College, PA) for assay. After thawing, each sample was processed by placing four to five 1 ml aliquots into 1.8 ml cryogenic storage vials and frozen at -80°C. Upon assay, samples were thawed to room temperature and centrifuged at 3000 rpm for 15 minutes. The clear top plastic of the sample was pipetted into appropriate test tubes/wells.
Salivary cortisol (in micrograms/deciliter) was assayed using an enzyme immunoassay kit (Salimetrics, State College, PA). This kit is commercially available and uses 25 μl of saliva. Its lower limit of sensitivity is 0.007 μg/dl (range up to 1.8 μg/dl) with average intra- and interassay coefficient of variation of <5.0 and 10.0% respectively. Cortisol was assayed from saliva for each day across the week that it was collected.
Salivary dehydroepiandrosterone (DHEA) (in picograms/milliliter) was also processed using an enzyme immunoassay kit (Salimetrics, State College, PA). This kit uses 550 μl of saliva. Its lower limit of sensitivity is 10.0 pg/ml (range up to 1000 pg/ml) with average intra- and interassay coefficient of variation of <5.0 and 15.0%, respectively. DHEA was assayed from saliva for two days, Tuesday and Thursday, because of less variability in DHEA levels.
To calculate the cortisol/DHEA ratio, cortisol values in micrograms/deciliter and DHEA values in pictograms/milliliter were transformed into nanomoles/liter values. A cortisol/DHEA ratio was then calculated for Tuesday morning and Thursday morning. These ratio values evidenced high skew and kurtosis and were log-transformed. The two log-transformed values were averaged to yield the cortisol/DHEA ratio value for analyses. See Table 2 for descriptive statistics across maltreatment groups.
Table 2.
Descriptive statistics of study variables by maltreatment timing groups
| Nonmaltreated(n=222) | Early, not recent maltreated (n=115) | Early and recent maltreatment(n=83) | Recent onset maltreatment(n=69) | |
|---|---|---|---|---|
| M (SD) or % | M (SD) or % | M (SD) or % | M (SD) or % | |
| CRP rs1417938 | ||||
| TT | 71.0% | 74.5% | 63.4% | 69.1% |
| AT/AA | 29.0% | 25.5% | 36.6% | 30.9% |
| Salivary CRP* | 3.49 (.64) | 3.36 (.57) | 3.28 (.52) | 3.50 (.68) |
| Cortisol/DHEA ratio | 1.44 (.23) | 1.46 (.25) | 1.51 (.23) | 1.43 (.22) |
| Internalizing T-score | 47.82 (7.46) | 48.86 (7.89) | 48.84 (7.71) | 48.20 (7.70) |
Notes.
p<.05
Salivary C-reactive protein
Saliva samples were collected via passive drool once per day for three days in the afternoon. All samples were assayed for C-reactive protein (CRP) using a commercially available immunoassay without modification to the manufacturer's recommended protocol. The test volume was 15 μL, with a range of standards from 93.75 to 3000 pg/mL. Samples were thawed to room temperature, centrifuged at 3000 rpm for 15 minutes to remove mucins, and diluted 1:10 prior to assay. Intra- and inter-assay coefficients of variation were less than 10% and 15% respectively. Log-transformations were conducted on each value to address skew and kurtosis and were then averaged across days resulting in a mean CRP value. Importantly, previous research has demonstrated a moderate-to-strong association between salivary CRP correlates with plasma CRP (Ouellet-Morin, Danese, Williams, & Arseneault, 2011). See Table 2 for descriptive statistics across maltreatment groups.
Internalizing symptoms
The Teacher Report Form of the Child Behavior Checklist (TRF; Achenbach, 1991) was used to assess children's internalizing symptomatology. After observing and interacting with children in their respective groups over the course of the camp week, camp counselors completed the TRF on individual children in their group. The camp counselors were unaware of the maltreatment status of the children in their group, as well as the research hypotheses. The TRF is a widely used and validated instrument to assess symptomatology by teachers. In the present study, because counselors are able to observe similar behavior to that of teachers, the camp counselors' ratings were used to provide an analogous assessment of child functioning by an adult external to the family. The TRF contains 118 total items rated for frequency that assess two broadband dimensions of child psychopathology-internalizing behavior problems and externalizing behavior problems. In this study we employed the internalizing behavior problems broadband scale (comprised of withdrawal, somatic complaints, anxiety/depression subscales). Average inter-rater reliability (kappa) among pairs of counselors' was .67. The counselors' scores for each child were averaged to obtain individual children's raw and T-scores for the internalizing dimension. The mean internalizing T-score was 48.29 (SD=7.63). See Table 2 for descriptive statistics for each maltreatment group.
Results
The data analytic strategy for this investigation involved three sets of analyses. The first set utilized analyses of covariance (ANCOVAs) to investigate the GxE interactive effects of maltreatment and CRP genetic variation on salivary CRP levels. The second set of analyses employed OLS regression to examine whether the relation between salivary CRP levels and children's internalizing symptoms varied by maltreatment experiences. The final set of analyses were multiple group path models used to explore associations between neuroendocrine functioning (as measured by cortisol/DHEA ratio), inflammatory processes (as measured by salivary CRP) and internalizing symptoms and to investigate whether these relations were moderated by maltreatment timing.
Preliminary analyses are presented in Table 2. No differences in CRP SNP rs1417938 allele frequencies (TT vs. AT/AA) were found between the 4 maltreatment timing groups (nonmaltreated, early and not recent maltreatment, early and recent maltreatment, and recent onset maltreatment). Thus, we did not find evidence for a gene-environment correlation (rGE) for CRP and maltreatment timing. Moreover, the 4 maltreatment timing groups did not differ on cortisol/DHEA ratio and internalizing symptoms. Results indicated a statistically significant difference between maltreatment timing groups on salivary CRP levels (F (3, 476) =3.34, p=.03); however, none of the Bonferroni contrasts were statistically significant.
Interactive effects of maltreatment and CRP gene on salivary CRP levels
First, the unique and interactive effects of child maltreatment and CRP genetic variation were examined using a 2 (CRP TT vs. AT/AA) × 2 (maltreated vs. nonmaltreated) ANCOVA with age, gender, and ancestry-informed race/ethnicity included as covariates. All covariate by CRP gene and covariate by maltreatment interactions were included in this model as recommended by Keller (2014). There were 21 children who were identified as having a race/ethnicity other than African American, Caucasian, or Hispanic. Small cell sizes precluded the investigation of race/ethnicity by gene and race/ethnicity by maltreatment when including these children. Therefore, the 21 children were excluded from GxE analyses. Results indicated that younger children evidenced higher salivary CRP levels (F (1, 423) = 12.24, p = .02). Main effects of race/ethnicity (F (1, 423) = .02), gender (F (1, 423) = .01), maltreatment (F (1, 423) = .35), and CRP genotype (F (1, 423) = .10), were all non-significant. Moreover, none of the covariate by CRP gene or covariate by maltreatment interactions was statistically significant. However, the interaction of maltreatment and CRP genotype was significant (F (1, 423) = 5.26, p=.02) indicating that the association between maltreatment and salivary CRP levels depends on CRP genetic variation.
Because main effects of race/ethnicity and gender as well as interactions of race/ethnicity by maltreatment, race/ethnicity by CRP gene, gender by maltreatment, gender by CRP gene, age by maltreatment, and age by CRP gene were all non-significant, a second model was tested which trimmed these effects from the model. This allowed for the inclusion of the 21 children in the “other” race/ethnicity group. Results of this ANCOVA with age included as a covariate were similar to those presented above; however, the interaction of maltreatment and CRP genotype was marginally significant (F (1, 454) = 3.01, p=.08).
To clarify the extent to which timing of maltreatment may be influential in these associations, a 2 (CRP TT vs. AT/AA) × 4 (1- nonmaltreated; 2-early, not recent maltreatment; 3-early and recent maltreatment; 4-recent onset maltreatment) ANCOVA was tested again including age, gender, and ancestry-informed race/ethnicity included as covariates. As above, all covariate by CRP gene and covariate by maltreatment interactions were included in this model. Younger children evidenced higher salivary CRP levels (F (1, 411) = 12.13, p=.001). Consistent with the above model, the main effects of race/ethnicity (F (1, 411) = .42), gender (F (1, 411) = .34), maltreatment timing (F (1, 411) = .34), and CRP genotype (F (1, 411) = 1.27), were all non-significant. The interaction of maltreatment timing and CRP genotype was significant (F (3, 411) = 4.90, p=.002) indicating that the relation between maltreatment timing and salivary CRP levels depends on CRP genetic variation. See Figure 1 for graphical representation of results. As above, none of the covariate by gene and covariate by maltreatment timing interactions was statistically significant.
Figure 1.
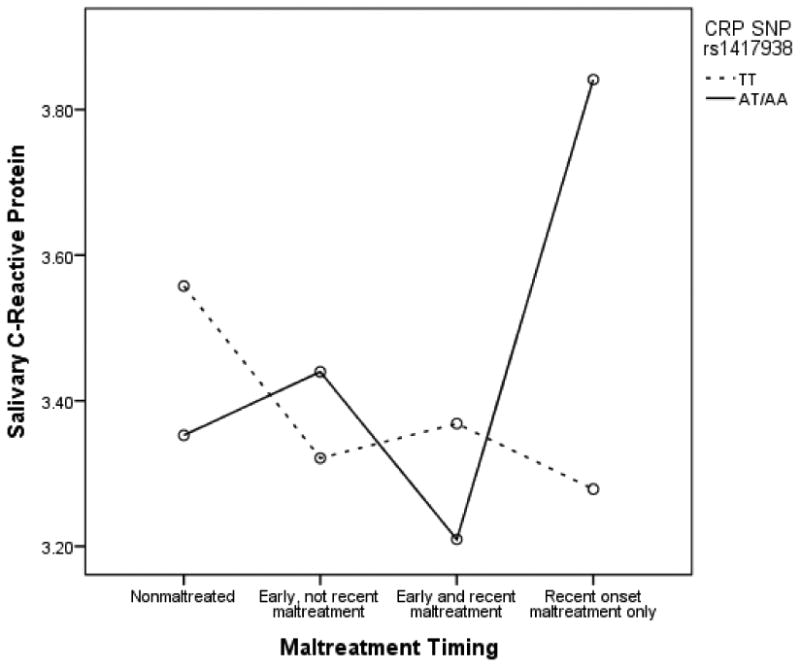
CRP SNP rs1417938 moderates association between maltreatment timing and salivary CRP
Because main effects of race/ethnicity and gender as well as interactions of race/ethnicity by maltreatment, race/ethnicity by CRP gene, gender by maltreatment, gender by CRP gene, age by maltreatment, and age by CRP gene were all non-significant, an additional model was tested which trimmed these effects from the model. This allowed for the inclusion of the 21 children in the “other” race/ethnicity group. Results of this ANCOVA with age included as a covariate were similar to those presented above; however, the main effect of maltreatment timing was significant (F (1, 450) = 3.42, p=.02) which is consistent with the results presented in Table 2. The interaction of maltreatment timing and CRP gene remained significant in this model (F (3, 450) = 3.48, p=.02).
Follow-up analyses revealed that among children with recent onset maltreatment, those with at least one A allele (AT/AA group) evidenced significantly higher salivary CRP levels than those children in the TT group (t (62) = -2.56, p=.01). There were no significant differences between genotypes for children in any of the other maltreatment timing groups (i.e. nonmaltreated, early, not recent maltreated, and early and recent maltreated).
Salivary CRP and internalizing symptoms: moderation by maltreatment
We also sought to examine the association between salivary CRP and child internalizing symptoms and to determine the role of maltreatment in this relation. We employed OLS regression with age, maltreatment (binary variable: maltreated vs. nonmaltreated), salivary CRP (mean centered), and the interaction of salivary CRP and maltreatment included as predictors of children's internalizing T-scores. Gender and race/ethnicity were initially included as covariates but were not significantly related to internalizing scores and were therefore trimmed from the final model. Results indicated a marginally significant association between age and internalizing scores such that younger children evidenced marginally significantly higher internalizing T-scores (B=-.64 (SE=.36), p=.08). None of the other main effects were significant. The interaction of maltreatment status and salivary CRP was marginally significant (B=1.95 (SE=1.15), p=.09).
Because of the potential importance of maltreatment timing in these relations, an additional regression was conducted with the 4 group maltreatment timing variable (1. nonmaltreated, 2. early, not recent maltreated, 3. early and recent maltreated, 4. recent onset maltreated). Three dummy codes were created for the 4 groups such that the recent onset maltreated children were the reference group. Age, the 3 dummy coded maltreatment timing variables, salivary CRP (mean centered variable), and the 3 interaction terms (dummy code by salivary CRP) were included in this model. None of the dummy codes were significantly related to internalizing and consistent with the above model, younger children demonstrated marginally significantly higher internalizing T-scores (B=-.65 (SE=.36), p=.07). The interaction of the dummy code comparing recent onset maltreated to nonmaltreated children by salivary CRP was significant (B=-5.23 (SE=1.62), p=.001) as was the interaction of the dummy code comparing recent onset maltreated to early onset, not recently maltreated children by salivary CRP (B=-5.69 (SE=1.88), p=.003). The interaction of dummy code comparing recent only to early and recently maltreated children by salivary CRP was non-significant.
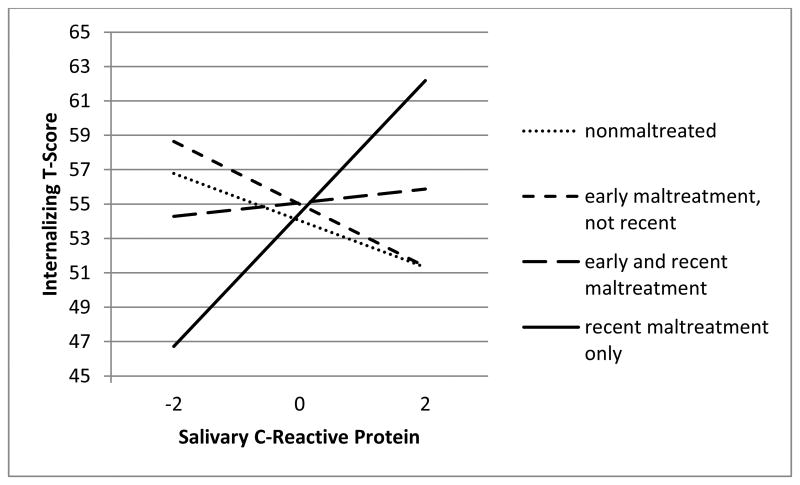
To probe these interactions, three new sets of three dummy codes were created such that each maltreatment timing group was coded as the reference group in one set. Three additional regression models were tested in which a different maltreatment timing group was the reference group in each model and new interaction terms were created with the new dummy codes in each model. The simple slope for recent onset maltreated children was statistically significant (Y=3.86X + 54.45; p=.006). However, simple slopes for the 3 other maltreatment timing groups were non-significant (early and recent maltreated: Y=.40X + 55.08; early, not recent maltreated: Y=-1.82X + 55.00; nonmaltreated: Y=-1.37 + 54.04). Thus, results indicate that higher levels of salivary CRP are associated with higher levels of internalizing symptoms for recently maltreated children only. See Figure 2 for graphical representation.
Figure 2.
Maltreatment timing moderates the relation between salivary CRP levels and internalizing symptomatology
We also investigated the effect of child internalizing symptoms on salivary CRP levels (reverse direction of effect) and moderation by maltreatment timing. Results indicated that higher child internalizing symptoms were associated with higher levels of salivary CRP only for recently maltreated children. We chose to present the model in which we explore the effect of salivary CRP on internalizing symptoms given prior literature suggesting that inflammation may temporally precede depressive symptoms (Gimeno, Kivimaki, Brunner, Elovainio, De Vogli, Steptoe, et al., 2009).
Relations between neuroendocrine functioning, immune functioning, and internalizing symptoms
To examine relations between neuroendocrine functioning and immune functioning and internalizing symptoms among maltreated and nonmaltreated children, multiple group path analysis was conducted in Mplus Version 7.0 (Muthén and Muthén, 1998–2012). Missing data were handled using full information maximum likelihood (FIML). Model fit was estimated with the chi-square statistic, comparative fit index (CFI), root mean square error of approximation (RMSEA), and standardized root mean square residual (SRMR). Child age, gender, race/ethnicity, and cortisol/DHEA ratio were entered as exogenous variables, salivary CRP was entered as a mediator, and internalizing T-score was the endogenous variable. Because child gender and race/ethnicity did not uniquely predict salivary CRP or internalizing symptoms, they were trimmed from the final model.
To test for moderation of these associations by timing of maltreatment, a model which fully constrained all paths to be equal across the four maltreatment groups (1- nonmaltreated; 2-early, not recent maltreatment; 3-early and recent maltreatment; 4-recent onset maltreatment) was tested first. This model evidenced inadequate fit to the data (χ2 (19) = 27.73 p=.09, CFI = .80, RMSEA .06, SRMR=.09). Next a model was tested which relaxed the following constraints across the 4 groups (i.e., allowed them to vary across the 4 groups): 1) relation between cortisol/DHEA ratio and salivary CRP; 2) relation between cortisol/DHEA ratio and internalizing symptoms; 3) relation between salivary CRP and internalizing symptoms. This model evidenced good model fit (χ2 (10) = 9.10, p=.53, CFI = 1.00, RMSEA =.001, SRMR=.06) which was significantly better than the fully-constrained model: (Δχ2 (9) = 18.63, p<.05). This suggests significant moderation of the associations by maltreatment timing. Results of the partially unconstrained model are presented in Figure 3. Consistent with early models described above, younger children evidenced higher salivary CRP levels (b=-.23, p<.001). Among nonmaltreated children and early, not recent, maltreated children, lower cortisol/DHEA ratios were associated with higher salivary CRP levels (bnon=-.20, bearly=-.25; ps<.01); however, among early and recent maltreated children and recent only maltreated children, cortisol/DHEA ratios were unrelated to salivary CRP levels. Consistent with earlier models, higher salivary CRP levels were significantly associated with higher internalizing symptoms among recent onset maltreated children only (b=.39, p<.001). Among nonmaltreated children, early, not recent maltreated children, and early and recent maltreated children, salivary CRP was unrelated to internalizing symptoms. Finally, higher cortisol/DHEA ratios were marginally associated with higher internalizing symptoms among recent maltreated children only (b=.22, p=.06). Results were not indicative of a mediational process for any of the subgroups of children. Instead, results suggest that associations between neuroendocrine functioning, immune functioning, and internalizing symptoms depend on the occurrence and developmental timing of maltreatment.
Figure 3.
Multiple-group model of relations between neuroendocrine functioning, immune functioning and internalizing symptomatology Notes: Standardized estimates shown. N=nonmaltreated; E=early, not recent maltreatment; ER=early and recent maltreatment; R=recent maltreatment. tp=.06, *p<.05, **p<.01, ***p<.001.
Discussion
All maltreated children are not affected in the same way by their experiences of abuse and neglect (Cicchetti & Rizley, 1981). Maltreated children display heterogeneity in their responses to abuse and neglect (Cicchetti & Manly, 2001). An examination of a multiple-levels-of-analysis approach, such as that employed in the present study, has the potential to serve not only to advance scientific understanding of the developmental sequelae of child maltreatment, but also to inform efforts to prevent and ameliorate the negative consequences that often ensue (Cicchetti & Dawson, 2002; Cicchetti & Toth, 2009). As is characteristic of research on the sequelae of child maltreatment, the investigation of a large sample allows for identification of where effects are operating, particularly for specific variation in the timing of maltreatment experiences.
Over the past several decades, a number of studies have found considerable importance for the consequences of maltreatment that occurs during the early years of life (Cicchetti, Rogosch, Gunnar, & Toth, 2010; Curtis & Cicchetti, 2013; Dunn, McLaughlin, Slopen, Rosand, & Smoller, 2013; Kaplow & Widom, 2007; Manly, Kim, Rogosch, & Cicchetti, 2001). This research on the effects of early child maltreatment has important implications for understanding the process of brain development.
Brain development is a dynamic, self-organizing process (Cicchetti & Tucker, 1994). Environmental perturbations, such as child maltreatment, that take place during early brain development may potentiate a cascade of maturational and structural changes that eventuate in the neural system proceeding along a trajectory that deviates from that usually taken in normal neurobiological development (Cicchetti, 2002; Cicchetti & Tucker, 1994; Courchesne, Chisum, & Townsend, 1994). In keeping with the principle of multifinality (Cicchetti & Rogosch, 1996), the neurobiological development of maltreated children is not affected in the same way in all abused and neglected individuals. The multilevel investigation of maltreated children presents a unique opportunity for comprehending how environmental experiences can bring about individual differences in neurobiological development.
The most notable aspect of the present study is that all significant results pertain to variation in the developmental timing of maltreatment. Specifically, it was the group of maltreated children who experienced a recent onset of maltreatment who evinced relations among inflammatory and internalizing pathology. In terms of C-reactive protein, in particular, the major finding is more of an acute response to maltreatment occurring for the first time during the childhood/school-age years.
C-reactive protein genotype variation
Prior to investigating our primary hypotheses, we conducted a number of important preliminary analyses. We found no evidence for gene-environment correlation (rGE) for CRP genotype and maltreatment timing. Therefore, CRP genetic variation was unrelated to maltreatment timing and children with different CRP genotypes were not differentially more likely to experience maltreatment at various onset and recency time-points. In terms of GxE effects, we demonstrated that the CRP associated gene was in fact related to variation in salivary CRP levels. However, this finding was only observed in the recent onset maltreatment group. Specifically, children in the recent onset only group who had a minor allele of the gene (AA or AT) showed significantly higher levels of CRP compared to recently maltreated children with the TT genotype. No genotype differences in CRP levels were obtained in the remaining onset/recency groups suggesting that CRP genotype variation affects inflammation in the context of recent onset maltreatment only.
In keeping with the suggestion proffered by Keller (2014) regarding the importance of including covariate main and interactive effects in gene by environment models, our test of CRP genotype by maltreatment included age, gender, and ancestry informative race/ethnicity as covariates as well as all covariate by gene and covariate by maltreatment interactions. The main effects of race/ethnicity, gender, maltreatment, and CRP genotypes all were non-significant. In addition, none of the covariate by CRP gene or covariate by maltreatment interactions were significant. Importantly, we found a significant interaction between CRP genotype and maltreatment timing even with the inclusion of these covariate main and interactive effects, thus indicating that our results are not driven by confounders.
Inflammation, neuroendocrine regulation, and internalizing symptoms
Empirical studies exist in the literature that provide evidence that inflammation may be related to depressive internalizing symptomatology (Bufalino et al., 2012; Danese, Moffitt, et al., 2008; Danese, Moffitt, et al., 2009; Hadler et al, 2010). In the present investigation, we advance this literature by demonstrating the importance of timing of maltreatment in this association. Specifically, it was only among children with recent onset of maltreatment that higher CRP levels were related to higher internalizing symptoms. CRP was not associated with internalizing symptoms in nonmaltreated children or in maltreated children with different onset/recency patterns.
Dysregulation of the HPA axis has been shown to be related to inflammation and, probabilistically, to later internalizing depressive symptoms (Hostinar et al., 2013; Miller et al., 2011). Different patterns of association were found among these levels of analysis depending on patterns of onset and recency of maltreatment. Consistent with expected relations; for nonmaltreated and early not recent maltreated children, lower cortisol/DHEA ratios were associated with higher salivary CRP levels. Conceivably, the HPA axis of the early onset and not recently maltreatment group either had adapted or recalibrated over time so that expected associations between cortisol/DHEA ratio and CRP levels were obtained. Interestingly, among the early and recent maltreatment and recent only maltreatment groups, cortisol/DHEA ratios were unrelated to CRP levels suggesting that in the context of a recent maltreatment experience these processes may be less interrelated.
Contrary to our hypothesis, we did not find evidence of CRP mediating the relation between the cortisol/DHEA ratio and internalizing symptoms for any of the developmental timing groups. However, for the recent onset maltreatment group, both CRP and, marginally (p=.06), the cortisol/DHEA ratio, were independently or additively related to internalizing symptomatology, rather than functioning in a coordinated, interdependent manner. These findings indicate that among children with recent onset maltreatment experiences, disruption in both inflammatory and neuroendocrine processes may uniquely contribute to internalizing symptoms and again highlight the criticality of examining the developmental timing of child maltreatment when investigating the associations between neuroendocrine functioning, immune functioning, and internalizing symptomatology.
Summary and future directions
The comparisons between the maltreatment timing groups are informative. Within these comparisons, the recent onset maltreatment group stands out as unique, compared to the other developmental timing groups, when considering immune system response and associations with genetic variation, neuroendocrine regulation, and internalizing symptomatology. The recent onset group was the only developmental timing group that evinced genetic moderation by the CRP gene. Whereas this group did not show the expected association between cortisol/DHEA ratio and CRP levels, as observed in the nonmaltreated children and the early onset only maltreatment group, it was the only developmental timing group to demonstrate expected positive relations between CRP and internalizing symptoms and marginal effects between the cortisol/DHEA ratio and internalizing symptoms. In fact, there was support for the independent contributions of CRP and cortisol/DHEA in jointly influencing internalizing symptoms for the recent onset maltreatment group only. These results demonstrate that all neurobiological effects of maltreatment are not confined to those individuals who had onset in the early years of life. Avoiding maltreatment during these early periods of neurological development does not inoculate children from the effects of maltreatment that begins during the school-age years. Rather, different systems, specifically the immune system, appear more uniquely responsive to later emerging maltreatment. It also is important to note that children who experience both early and recent maltreatment did not show the same pattern of relations. It is not clear how earlier adaptations to maltreatment occurring early in life alter the way in which developmental systems are affected when subsequent maltreatment occurs in childhood. However, the effects appear different from that of the more acute response to maltreatment when it occurs for the first time during the school-age years.
Our findings are based on cross-sectional assessments of cortisol, DHEA, CRP, and internalizing symptoms. Accordingly, we do not know how these different domains of biological and behavioral functioning have adapted previously in development, or what the prospective implications of the current findings are. Moreover, we are not able to determine if onset of maltreatment specifically during the childhood years has a unique impact on immune, neuroendocrine, and behavioral systems, or whether similar acute effects may have occurred if assessments were available earlier or later in development. Future research incorporating longitudinal designs and obtaining assessments closer to the onset of maltreatment for individuals first maltreated in different developmental periods will provide important insights into these questions.
Implications
Biomarkers of neuroendocrine dysregulation and of immune inflammation can be used to identify children and adolescents who are at enhanced risk for later health problems. Additionally, these biomarkers can be used to evaluate the efficacy of interventions to prevent chronic disease risk among high-risk children and adolescents (Cicchetti, Rogosch, Toth & Sturge-Apple, 2011; Dozier, Peloso, Lewis, Laurenceau, & Levine, 2008; Miller, Brody, Yu, & Chen, 2014; Slopen et al., 2013).
Efforts to reduce the effects of poverty are vital (Heckman, 2006); however, interventions that are targeted to advance cognitive functioning and capacities for self-regulation (Blair & Diamond, 2008) are critical for enhancing self-righting processes and promoting resilience (cf. Cicchetti & Rogosch, 1997; Cicchetti, Rogosch, Lynch & Holt, 1993). Intensive interventions that aim to develop more sensitive and nurturant parenting, autonomous self-development, and neurobiological reorganization in maltreated children also are promising for promoting positive developmental trajectories (Cicchetti, Rogosch, & Toth, 2006; Dozier et al., 2008; Toth, Maughan, Manly, Spagnola & Cicchetti, 2002). The current study suggests that interventions for those experiencing maltreatment at different developmental periods, and not exclusively for those experiencing maltreatment early in life, are necessary. A multiple-levels-of analysis perspective suggests that the implementation of multisystem approaches to intervention would be important for reducing the stress accompanying poverty, decreasing child maltreatment, and alleviating allostatic overload in order to enhance mental and physical health across the life course (Cicchetti & Gunnar, 2008; Cicchetti et al., 2011)
Acknowledgments
We are grateful to the Jacobs Foundation and the National Institute of Mental Health (R01-MH83979) for their support of this work.
References
- Achenbach TM. Manual for the teacher's report form and 1991 profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Anda RF, Dube SR, Giles WH. The enduring effects of abuse and related adverse experiences in childhood. European Archives of Psychiatry and Clinical Neuroscience. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett D, Manly JT, Cicchetti D. Defining child maltreatment: The interface between policy and research. In: Cicchetti D, Toth SL, editors. Child abuse, child development, and social policy. Norwood, NJ: Ablex; 1993. pp. 7–74. [Google Scholar]
- Blair C, Diamond A. Biological processes in prevention and intervention: The promotion of self-regulation as a means of preventing school failure. Development and Psychopathology. 2008;20(3):899–911. doi: 10.1017/S0954579408000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufalino C, Hepgul N, Aguglia E, Pariante CM. The role of immune genes in the association between depression and inflammation: A review of recent clinical studies. Brain, Behavior, and Immunity. 2012;31:31–47. doi: 10.1016/j.bbi.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Cicchetti D. How a child builds a brain: Insights from normality and psychopathology. In: Hartup W, Weinberg R, editors. Minnesota symposia on child psychology: Child psychology in retrospect and prospect. Vol. 32. Mahwah, NJ: Lawrence Erlbaum Associates; 2002. pp. 23–71. [Google Scholar]
- Cicchetti D, Dawson G. Multiple levels of analysis. Development and Psychopathology. 2002;14:417–420. doi: 10.1017/s0954579402003012. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Gunnar MR. Integrating biological processes into the design and evaluation of preventive interventions. Development and Psychopathology. 2008;20(3):737–743. doi: 10.1017/S0954579408000357. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Lynch M. Toward an ecological/transactional model of community violence and child maltreatment: Consequences for children's development. Psychiatry. 1993;56:96–118. doi: 10.1080/00332747.1993.11024624. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Lynch M. Failures in the expectable environment and their impact on individual development: The case of child maltreatment. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Risk, disorder, and adaptation. Vol. 2. New York, NY: John Wiley & Sons, Inc; 1995. pp. 32–71. [Google Scholar]
- Cicchetti D, Manly JT. A personal perspective on conducting research with maltreating families: Problems and solutions. In: Brody G, Sigel I, editors. Methods of family research: Families at risk. Vol. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1990. pp. 87–133. [Google Scholar]
- Cicchetti D, Manly JT, editors. Operationalizing child maltreatment: Developmental processes and outcomes [Special Issue] Development and Psychopathology. 2001;13(4):755–1048. [PubMed] [Google Scholar]
- Cicchetti D, Rizley R. Developmental perspectives on the etiology, intergenerational transmission and sequelae of child maltreatment. New Directions for Child Development. 1981;11:31–55. [Google Scholar]
- Cicchetti D, Rogosch FA. Equifinality and multifinality in developmental psychopathology. Development and Psychopathology. 1996;8:597–600. [Google Scholar]
- Cicchetti D, Rogosch FA. The role of self-organization in the promotion of resilience in maltreated children. Development and Psychopathology. 1997;9:797–815. doi: 10.1017/s0954579497001442. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch F. Diverse patterns of neuroendocrine activity in maltreated children. Developmental Psychopathology. 2001;13(3):677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Lynch M, Holt K. Resilience in maltreated children: Processes leading to adaptive outcome. Development and Psychopathology. 1993;5:629–647. [Google Scholar]
- Cicchetti D, Rogosch FA, Gunnar MR, Toth SL. The differential impacts of early abuse on internalizing problems and diurnal cortisol activity in school-aged children. Child Development. 2010;25:252–269. doi: 10.1111/j.1467-8624.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti DC, Rogosch FA, Oshri A. Interactive effects of corticotropin releasing hormone receptor 1, serotonin transporter linked polymorphic region, and child maltreatment on diurnal cortisol regulation and internalizing symptomatology. Development and Psychopathology. 2011;23:1125–1138. doi: 10.1017/S0954579411000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Toth SL. Fostering secure attachment in infants in maltreating families through preventive interventions. Development and Psychopathology. 2006;18(3):623–650. doi: 10.1017/s0954579406060329. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Toth SL, Sturge-Apple ML. Normalizing the development of cortisol regulation in maltreated infants through preventive interventions. Development and Psychopathology. 2011;23:789–800. doi: 10.1017/S0954579411000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. Child Maltreatment. In: Lamb M, Garcia Coll C, editors. Handbook of child psychology and developmental science, 7th ed, Vol 3: Socioemotional process. New York: Wiley; in press. [Google Scholar]
- Cicchetti D, Toth SL. A developmental psychopathology perspective on adolescent depression. In: Nolen-Hoeksema S, Hilt L, editors. Handbook of adolescent depression. New York, NY: Taylor & Francis; 2009. pp. 3–31. [Google Scholar]
- Cicchetti D, Toth SL, Manly JT. Maternal Maltreatment Classification Interview. Mt. Hope Family Center; Rochester, NY: 2003. Unpublished measure. [Google Scholar]
- Cicchetti D, Tucker D. Development and self-regulatory structures of the mind. Development and Psychopathology. 1994;6:533–549. [Google Scholar]
- Cicchetti D, Valentino K. An ecological transactional perspective on child maltreatment: Failure of the average expectable environment and its influence upon child development. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology. Vol. 3. New York, NY: Wiley; 2006. pp. 129–201. [Google Scholar]
- Coelho R, Viola TW, Walss-Bass C, Brietzke E, Grassi-Oliveira R. Childhood maltreatment and inflammatory markers: A systematic review. Acta Psychiatrica Scandinavica. 2014;129(3):180–192. doi: 10.1111/acps.12217. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum H, Townsend J. Neural activity-dependent changes in development: Implications for psychopathology. Development and Psychopathology. 1994;6:741–758. [Google Scholar]
- Curtis WJ, Cicchetti D. Affective facial expression processing in 15 month-old infants who have experienced maltreatment: An event-related study. Child Maltreatment. 2013;18(3):140–154. doi: 10.1177/1077559513487944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Caspi A, Williams B, Ambler A, Sugden K, Mika J, … &, Arseneault L. Biological embedding of stress through inflammation processes in childhood. Molecular Psychiatry. 2011;16:244–246. doi: 10.1038/mp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & Behavior. 2012;106(1):29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, … &, Caspi A. Adverse childhood experiences and adult risk factors for age-related disease: Depression, inflammation, and clustering of metabolic risk markers. Archives of Pediatric and Adolescent Medicine. 2009;163(12):1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Archives of General Psychiatry. 2008;65(4):409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(4):1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Tan M. Childhood maltreatment and obesity: a systematic review and meta-analysis. Molecular Psychiatry. 2014;19(5):544–554. doi: 10.1038/mp.2013.54. [DOI] [PubMed] [Google Scholar]
- DeBellis MD. Developmental traumatology: The psychobiological development of maltreated children and its implications for research, treatment, and policy. Development and Psychopathology. 2001;13:539–564. doi: 10.1017/s0954579401003078. [DOI] [PubMed] [Google Scholar]
- DeBellis MD. The psychobiology of neglect. Child Maltreatment. 2005;10(2):150–172. doi: 10.1177/1077559505275116. [DOI] [PubMed] [Google Scholar]
- Dozier M, Peloso E, Lewis E, Laurenceau JP, Levine S. Effects of an attachment-based intervention on the cortisol production of infants and toddlers in foster care. Development and Psychopathology. 2008;20:845–859. doi: 10.1017/S0954579408000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn EC, McLaughlin KA, Slopen N, Rosand J, Smoller JW. Developmental timing of maltreatment and symptoms of depression and suicidal ideation in young adulthood: Results from the National Longitudinal Study of Adolescent Health. Depression and Anxiety. 2013;30:955–964. doi: 10.1002/da.22102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: Linked loci and correlated allele frequencies. Genetics. 2003;164(4):1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: dominant markers and null alleles. Molecular Ecology Notes. 2007;7(4):574–578. doi: 10.1111/j.1471-8286.2007.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Brown DS, Florence CS, Mercy JA. The economic burden of child maltreatment in the United States and implications for prevention. Child Abuse & Neglect. 2012;36(2):156–165. doi: 10.1016/j.chiabu.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, … &, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) study. American Journal of Preventive Medicine. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Gimeno D, Kivimaki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, … &, Ferrie JE. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychological Medicine. 2009;39(3):413–423. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G. Individual development and evolution: The genesis of novel behavior. New York, NY: Oxford University Press; 1992. [Google Scholar]
- Gunnar MR, Vasquez D. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology. Vol. 3. New York, NY: Wiley; 2006. pp. 533–577. [Google Scholar]
- Halder I, Marsland AL, Cheong J, Muldoon MF, Ferrell RE, Manuck SB. Polymorphisms in the CRP gene moderate an association between depressive symptoms and circulating levels of C Reactive Protein. Brain, Behavior, and Immunity. 2010;24(1):160–167. doi: 10.1016/j.bbi.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Rubia K. Neuroimaging of child abuse: A critical review. Frontiers in Human Neuroscience, 6(52) 2012 doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman JJ. Skill formation and the economics of investing in disadvantaged children. Science. 2006;312:1900–1902. doi: 10.1126/science.1128898. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan R, Gunnar MR. Psychobiological mechanisms underlying the social buffering of stress: A review of animal models and human studies across development. Psychological Bulletin e-pub ahead of print. 2013 doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubisz MJ, Falush D, Stephens M, Pritchard JK. Inferring weak population structure with the assistance of sample group information. Molecular Ecology Resources. 2009;9(5):1322–1332. doi: 10.1111/j.1755-0998.2009.02591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster RP, Bizik G, Picard M, Arsenault-Lapierre G, Sindi S, Trepanier L, … &, Lupien SJ. A transdisciplinary perspective of chronic stress in relation to psychopathology throughout life span development. Development and Psychopathology. 2011;23:725–776. doi: 10.1017/S0954579411000289. [DOI] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stressand impact on health and cognition. Neuroscience and Biobehavioral Reviews. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kaplow JB, Widom CS. Age of onset of maltreatment predicts long-term mental health outcomes. Journal of Abnormal Psychology. 2007;116:176–187. doi: 10.1037/0021-843X.116.1.176. [DOI] [PubMed] [Google Scholar]
- Keller MC. Gene× environment interaction studies have not properly controlled for potential confounders: the problem and the (simple) solution. Biological Psychiatry. 2014;75(1):18–24. doi: 10.1016/j.biopsych.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CQ, Tucker KL, Choudhry S, Parnell LD, Mattei J, Garcia-Bailo B, et al. Population admixture associated with disease prevalence in the Boston Puerto Rican health study. Human Genetics. 2009;125:199–205. doi: 10.1007/s00439-008-0612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier P, Jonson-Reid M, Stahlschmidt M, Drake B, Constantino J. Child maltreatment and pediatric health outcomes: A longitudinal study of low-income children. Journal of Pediatric Psychology. 2010;35(5):511–522. doi: 10.1093/jpepsy/jsp086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. Fibromyalgia and childhood abuse: Exploration of stress reactivity as a developmental mediator. Developmental Review. 2010;30:294–307. [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behavior and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Manly JT. Advances in research definitions of child maltreatment. Child Abuse & Neglect. 2005;29(5):425–439. doi: 10.1016/j.chiabu.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Manly JT, Kim JE, Rogosch FA, Cicchetti D. Dimensions of child maltreatment and children's adjustment: Contributions of developmental timing and subtype. Development and Psychopathology. 2001;13:759–782. [PubMed] [Google Scholar]
- Masten AS, Cicchetti D. Developmental cascades. Development and Psychopathology. 2010;22:491–495. doi: 10.1017/S0954579410000222. [DOI] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E. Research review: The neurobiology and genetics of maltreatment and adversity. The Journal of Child Psychology and Psychiatry. 2010;51:1079–1095. doi: 10.1111/j.1469-7610.2010.02271.x. [DOI] [PubMed] [Google Scholar]
- McEwen B. Mood disorders and allostatic load. Biological Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- Miller GE, Brody GH, Yu T, Chen E. Mitigating the effects of childhood disadvantage: Family-oriented intervention reduces inflammation in low-SES African American youth. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:11287–11292. doi: 10.1073/pnas.1406578111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User's Guide. 7th. Los Angeles, CA: Muthén & Muthén; 1998-2012. [Google Scholar]
- Ouellet-Morin I, Danese A, Williams B, Arseneault L. Validation of a high-sensitivity assay for C-reactive protein in human saliva. Brain, Behavior, and Immunity. 2011;25(4):640–646. doi: 10.1016/j.bbi.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Cicchetti D, Klorman R, Brumaghim J. Cognitive brain event-related potentials and emotion processing in maltreated children. Child Development. 1997;68:773–787. doi: 10.1111/j.1467-8624.1997.tb01961.x. [DOI] [PubMed] [Google Scholar]
- Reichlin S. Neuroendocrine-immune interactions. The New England Journal of Medicine. 1993;329(17):1246–1253. doi: 10.1056/NEJM199310213291708. [DOI] [PubMed] [Google Scholar]
- Rogosch FA, Cicchetti D, Shields A, Toth SL. Parenting dysfunction in child maltreatment. In: Bornstein MH, editor. Handbook of parenting: Applied and practical parenting. Vol. 4. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1995. pp. 127–159. [Google Scholar]
- Rogosch FA, Dackis MN, Cicchetti D. Child maltreatment and allostatic load: Consequences for physical and mental health in children from low-income families. Development and Psychopathology. 2011;23:1107–1124. doi: 10.1017/S0954579411000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz EB, Granger DA, Susman EJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child Development. 1998;69(6):1503–1513. [PubMed] [Google Scholar]
- Sedlak AJ, Mettenburg J, Basena M, Petta I, McPherson K, Greene A, Spencer L. Fourth National Incidence Study of child abuse and neglect (NIS–4): Report to Congress. Washington, D.C: U.S. Department of Health and Human Services, Administration for Children and Families; 2010. [Google Scholar]
- Shirtcliff EA, Coe CL, Pollak SD. Early childhood stress is associated with elevated antibody levels to herpes simplex virus type 1. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2963–2967. doi: 10.1073/pnas.0806660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. The Journal of the American Medical Association. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Slopen N, Kubzansky LD, McLaughlin KA, Koenen KC. Childhood adversity and inflammatory processes in youth: A prospective study. Psychoneuroendocrinology. 2013;38(2):188–200. doi: 10.1016/j.psyneuen.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman EJ, Dockray S, Schiefelbein VL, Herwehe S, Heaton JA, Dorn LD. Morningness/eveningness, morning-to-afternoon cortisol ratio, and antisocial behavior problems during puberty. Developmental Psychology. 2007;43:811–822. doi: 10.1037/0012-1649.43.4.811. [DOI] [PubMed] [Google Scholar]
- Toth SL, Maughan A, Manly JT, Spagnola M, Cicchetti D. The relative efficacy of two interventions in altering maltreated preschool children's representational models: Implications for attachment theory. Development and Psychopathology. 2002;14:877–908. doi: 10.1017/s095457940200411x. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: Preliminary support for an effect of early stress on cellular aging. Biological Psychiatry. 2010;67:531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HW, Widom CS. Pathways from childhood abuse and neglect to HIV-risk sexual behavior in middle adulthood. Journal of Consulting & Clinical Psychology. 2011;79(2):236–246. doi: 10.1037/a0022915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaeger R, Alvial-Bront A, Abdul K, Nolan PC, Grann VR, Birchette MG, et al. Comparing genetic ancestry and self-described race in African Americans born in the United States and in Africa. Cancer Epidemiology, Biomarkers & Prevention. 2008;17:1329–1338. doi: 10.1158/1055-9965.EPI-07-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]