Abstract
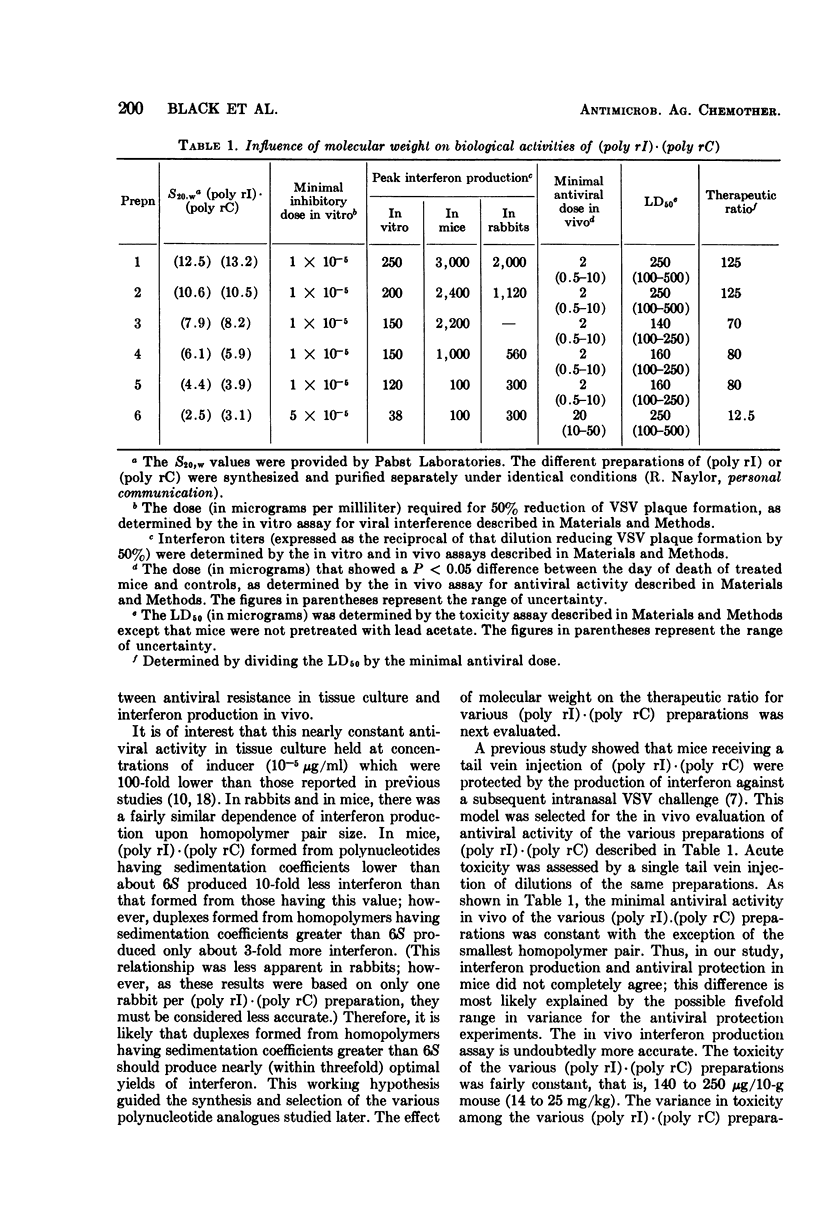
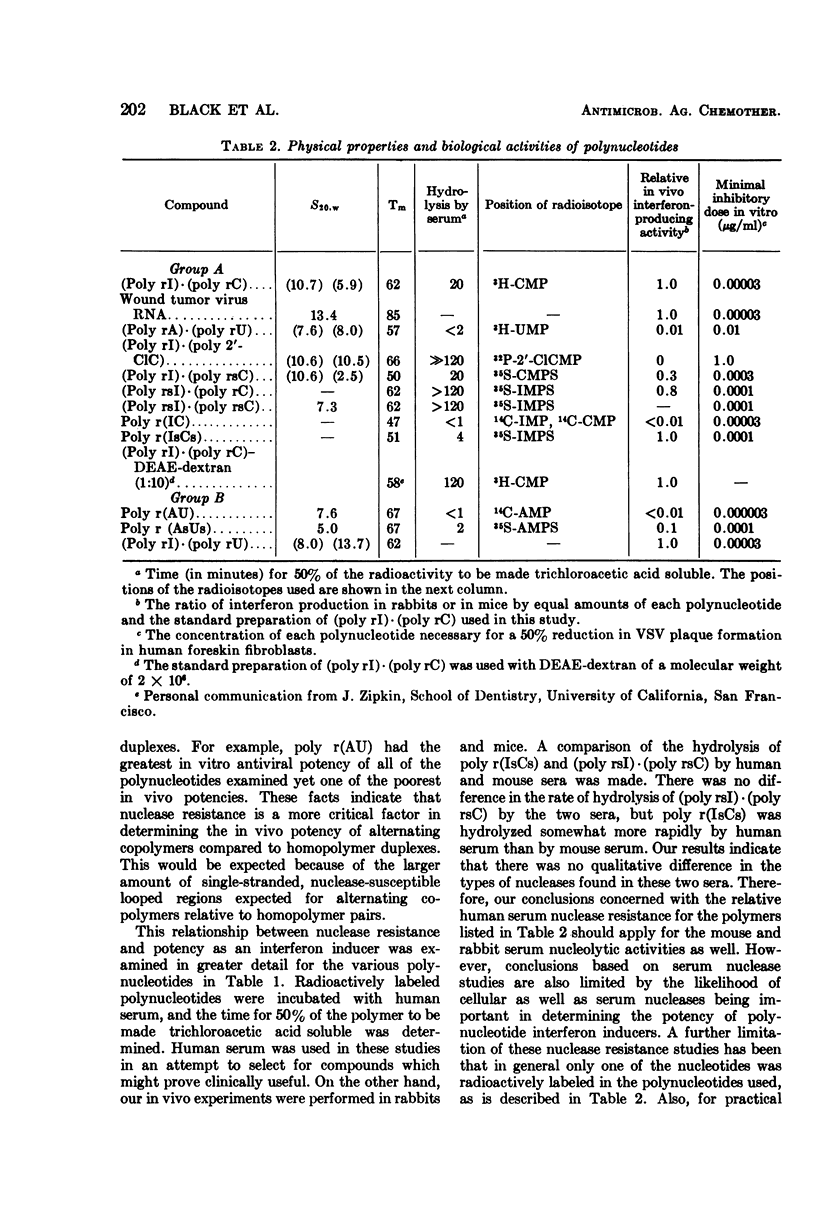
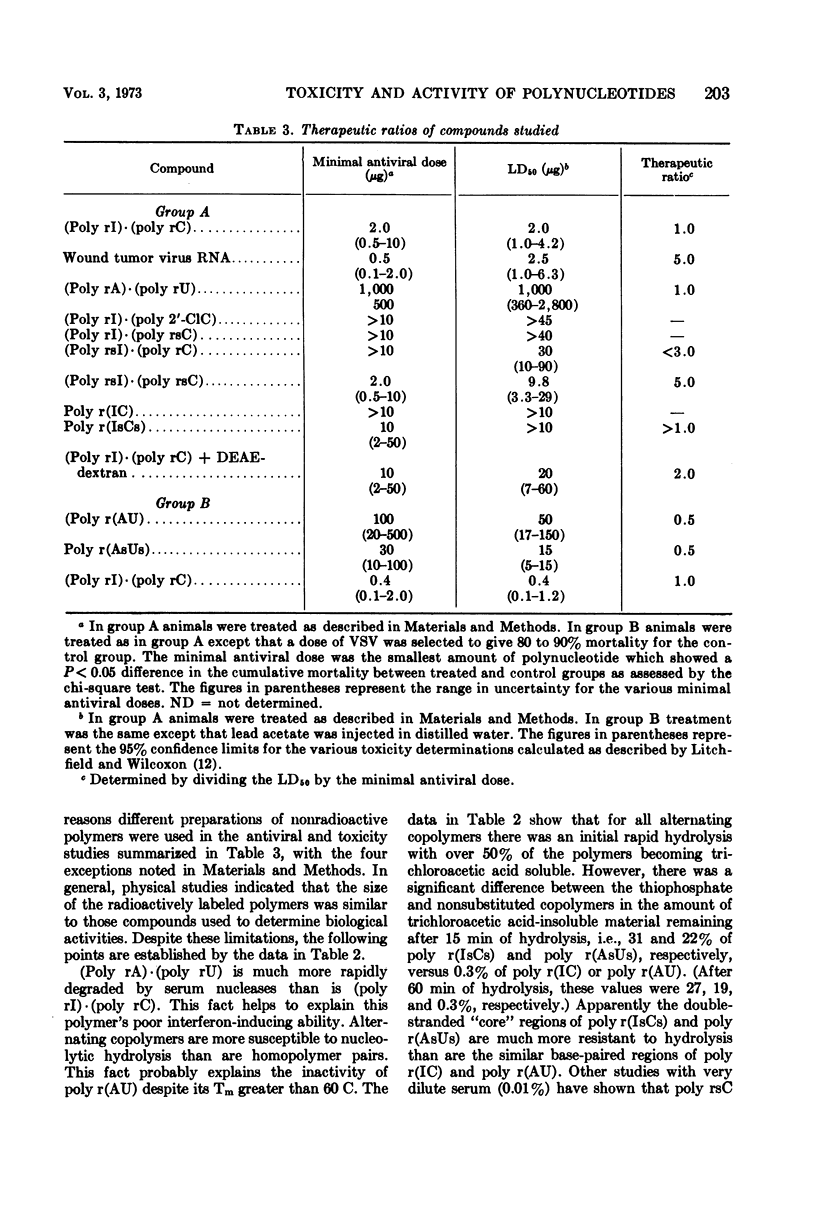
Various polynucleotides were examined for antiviral activity and toxicity in mice. Although the antiviral potency of the various interferon inducers varied, there was a concomitant variation in toxicity. This was reflected by a fivefold range in therapeutic ratio for the various compounds. In addition, no polynucleotide proved to be a more potent interferon inducer than polyinosinic·polycytidylic acid [(poly rI)·(poly rC)]. Our results suggest that there may be intrinsic limitations to the development of polynucleotide interferon inducers having improved therapeutic ratios.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Absher M., Stinebring W. R. Toxic properties of a synthetic double-stranded RNA. Endotoxin-like properties of poly I. poly C, an interferon stimulator. Nature. 1969 Aug 16;223(5207):715–717. doi: 10.1038/223715a0. [DOI] [PubMed] [Google Scholar]
- Black D. R., Eckstein F., Hobbs J. B., Sternbach H., Merigan T. C. The antiviral activity of certain thiophosphate and 2'-chloro substituted polynucleotide homopolymer duplexes. Virology. 1972 May;48(2):537–545. doi: 10.1016/0042-6822(72)90064-5. [DOI] [PubMed] [Google Scholar]
- Black D. R., Knight C. A. Ribonucleic acid transcriptase acitvity in purified wound tumor virus. J Virol. 1970 Aug;6(2):194–198. doi: 10.1128/jvi.6.2.194-198.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDe Clercq E., Eckstein F., Sternbach H., Merigan T. C. The antiviral activity of thiophosphate-substituted polyribonucleotides in vitro and in vivo. Virology. 1970 Oct;42(2):421–428. doi: 10.1016/0042-6822(70)90285-0. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Eckstein E., Merigan T. C. [Interferon induction increased through chemical modification of a synthetic polyribonucleotide]. Science. 1969 Sep 12;165(3898):1137–1139. doi: 10.1126/science.165.3898.1137. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Nuwer M. R., Merigan T. C. The role of interferon in the protective effect of a synthetic double-stranded polyribonucleotide against intranasal vesicular stomatitis virus challenge in mice. J Clin Invest. 1970 Aug;49(8):1565–1577. doi: 10.1172/JCI106374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. K., Tytell A. A., Lampson G. P., Hilleman M. R. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1004–1010. doi: 10.1073/pnas.58.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs J., Sternbach H., Eckstein F. Poly 2'-deoxy-2'-chlorouridylic and -cytidylic acids. FEBS Lett. 1971 Jul 8;15(5):345–348. doi: 10.1016/0014-5793(71)80330-7. [DOI] [PubMed] [Google Scholar]
- Lampson G. P., Field A. K., Tytell A. A., Nemes M. M., Hilleman M. R. Relationship of molecular size of rIn:rCn (poly I:C) to induction of interferon and host resistance. Proc Soc Exp Biol Med. 1970 Dec;135(3):911–916. doi: 10.3181/00379727-135-35169. [DOI] [PubMed] [Google Scholar]
- Lindahl-Magnusson P., Leary P., Gresser I. Interferon and cell division. VI. Inhibitory effect of interferon on the multiplication of mouse embryo and mouse kidney cells in primary cultures. Proc Soc Exp Biol Med. 1971 Dec;138(3):1044–1050. doi: 10.3181/00379727-138-36047. [DOI] [PubMed] [Google Scholar]
- Michelson A. M., Monny C. Polynucleotides. X. Oligonucleotides and their association with polynucleotides. Biochim Biophys Acta. 1967 Nov 21;149(1):107–126. doi: 10.1016/0005-2787(67)90695-8. [DOI] [PubMed] [Google Scholar]
- Morahan P. S., Munson A. E., Regelson W., Commerford S. L., Hamilton L. D. Antiviral activity and side effects of polyriboinosinic-cytidylic acid complexes as affected by molecular size. Proc Natl Acad Sci U S A. 1972 Apr;69(4):842–846. doi: 10.1073/pnas.69.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niblack J. F., McCreary M. B. Relationship of biological activities of poly I-poly C to homopolymer molecular weights. Nat New Biol. 1971 Sep 8;233(36):52–53. doi: 10.1038/newbio233052a0. [DOI] [PubMed] [Google Scholar]
- Tytell A. A., Lampson G. P., Field A. K., Nemes M. M., Hilleman M. R. Influence of size of individual homopolynucleotides on the physical and biological properties of complexed rIn:rCn (poly I:C). Proc Soc Exp Biol Med. 1970 Dec;135(3):917–921. doi: 10.3181/00379727-135-35170. [DOI] [PubMed] [Google Scholar]



