Abstract
Purpose
To determine the effect of anti-vascular endothelial growth factor (VEGF) therapy on choroidal thickness in eyes with diabetic macular edema (DME)
Design
A retrospective, cohort analysis of 59 eyes from 59 patients with DME without prior anti-VEGF therapy
Methods
Choroidal thickness was measured using semi-automated segmentation of enhanced-depth imaging optical coherence tomography (EDI-OCT) images at 0.5mm intervals from 2.5mm nasal to 2.5mm temporal to the fovea. Changes in choroidal thickness with and without anti-VEGF treatment over 6 months were compared. Best-corrected visual acuity (BCVA) and central foveal thickness (CFT) were analyzed to evaluate the association of choroidal thickness with functional and anatomical outcomes.
Results
Of the 59 eyes with DME, 26 eyes were observed without treatment, while 33 underwent intravitreal anti-VEGF therapy (mean number of injections = 2.73) over 6 months. In untreated eyes, there was no significant change in BCVA (p=0.098), CFT (p=0.472), or choroidal thickness at all measurements along the macula (p=0.057 at the fovea). In eyes treated with anti-VEGF injections, choroidal thickness significantly decreased at the fovea (246.6μm to 224.8μm; p<0.001) and at 0.5 mm nasal (240.9μm to 221.9μm; p = 0.002) and 0.5 mm temporal (249.3μm to 224.8μm; p=0.011) to the fovea. The decrease in subfoveal choroidal thickness after anti-VEGF treatment was not associated with the cumulative number of anti-VEGF injections (R2=0.031, p=0.327), or to changes in BCVA (R2=0.017; p=0.470) or CFT (R2=0.040; p=0.263).
Conclusions
Central choroidal thickness decreases after anti-VEGF therapy for DME after 6 months, but may not be associated with functional or anatomical outcomes in eyes with DME.
INTRODUCTION
The pathogenesis of diabetic macular edema (DME) has long been attributed to retinal vascular hyperpermeability, which is associated with focal leakage from microaneurysms or diffuse leakage from incompetent capillaries when visualized on fluorescein angiography. However, histopathological studies have also implicated choroidal dysfunction in diabetics. These changes include loss of the choriocapillaris, increased tortuosity, narrowing and dilation of vessels, and sinus-like structure formation between choroidal lobules.1-3 Functional imaging studies also showed a reduction in choroidal blood flow in eyes with diabetic retinopathy.4, 5 Yet, the role of choroidal perfusion in the pathophysiology of diabetic macular edema remains unclear.
The recent use of enhanced-depth imaging optical coherence tomography (EDI-OCT), which utilizes the increased depth of field from the inverted images obtained by placing the OCT device closer to the eye, has allowed researchers to examine the anatomical changes in the choroid in diabetic eyes.6-8 Choroidal thickness studies in diabetes have produced diverging results, however, with some reports suggesting choroidal thickening,9 thinning,10-12 and no change13-15 in eyes with diabetic retinopathy. Choroidal thickness measurements in eyes with DME have been similarly inconsistent.9-11, 14 One important explanation for these variable results is the significant variability of choroidal thickness in these retrospective, cross-sectional studies. Choroidal thickness has been shown to vary with age,7 refractive error,16, 17 and even time of day.18, 19 Moreover, many of these studies include eyes that received treatment with intravitreal anti-vascular endothelial growth factor (ant-VEGF) therapy, which has been shown to cause choroidal thinning in other diseases such as age-related macular degeneration (AMD).20,21
To address these limitations, we performed a cohort analysis evaluating choroidal thickness changes over 6 months in DME eyes without prior anti-VEGF therapy. We hypothesized that choroidal thickness may be associated with clinical outcomes in eyes with DME, and that anti-VEGF therapy may affect choroidal anatomy. Using a semi-automated choroidal segmentation software, we measured choroidal thickness changes in eyes with DME after anti-VEGF treatment, compared with DME eyes that were observed without treatment over the same time period. Finally, we evaluate potential associations of choroidal thickness with functional (visual acuity) and anatomical (retinal thickness) outcomes in DME.
METHODS
A retrospective, non-randomized cohort analysis was performed on 59 consecutive patients with treatment-naïve diabetic macular edema identified by a database search for all patients with the diagnosis of diabetic macular edema (ICD-9 code 362.07) who were evaluated at Duke University Eye Center Retina department between 2011 and 2013. This retrospective study was approved by the Institutional Review Board of Duke University and was conducted in accordance with the tenets of the Declaration of Helsinki.
Only eyes with center-involving diabetic macular edema as seen on OCT, without prior anti-VEGF therapy, and had undergone at least two EDI-OCT imaging 6 months apart were included for analysis. Eyes that received intravitreal steroid therapy, focal laser, or panretinal photocoagulation (PRP) in the 3 months before the first time point were excluded. Other exclusion criteria include high myopia >6 diopters, history of glaucoma, presence of vitreomacular traction, and history of vitreoretinal surgery. Since choroidal thickness has been shown to be unaffected by focal laser therapy,22 eyes with prior focal laser treatment were not excluded. Only one eye from each subject was included for analysis. The study eye was chosen if only one eye qualified based on inclusion/exclusion criteria. If both eyes qualified, the eye with worse best-corrected visual acuity (BCVA) was selected. If BCVA was equal between the two eyes, the right eye was designated the study eye for subjects with an even number birth month, and the left eye was selected for those with an odd number birth month.
For patients who met all inclusion and exclusion criteria, the initial visit was defined as the first visit at which an EDI-OCT image was obtained, or in the subset of patients who received intravitreal anti-VEGF therapy, the first visit at which the injection was given. EDI-OCT images obtained at 6 months from the initial visit were also collected for analysis. Charts were reviewed to collect clinical history, including hemoglobin A1c level (HbA1c), type of diabetic retinopathy including nonproliferative (NPDR) and proliferative diabetic retinopathy (PDR), and prior DME treatments at baseline. Visual acuity (logMAR), intraocular pressure (mmHg), and time of EDI-OCT image at each visit were also recorded. Due to the retrospective nature of this study, the criteria for anti-VEGF therapy and retreatment were made at the discretion of the treating physician and not addressed in this analysis.
Complete ophthalmic examination and imaging with Heidelberg Spectralis SD-OCT (870nm) device (Heidelberg Engineering, Heidelberg, Germany) were performed as part of standard evaluation for patients with DME. EDI-OCT scans were captured using Spectralis EDI mode, a preset, software-driven algorithm that places the retinal pigment epithelium (RPE) near the zero-delay line while producing an upright enhanced choroidal image. In EDI-OCT mode, a single 30-degree horizontal line scan (approximately 8.9 mm) captures 1536 A-scans per B-scan with 40 averaged B-scans per image, using the automatic averaging and eye tracking features. Images were always captured before any intravitreal injections were given on the same day.
Choroidal thickness was measured semi-automatically using the Duke Optical Coherence Tomography Retinal Analysis Program (DOCTRAP) software.23, 24 Originally developed for automated segmentation applications,25 DOCTRAP has a custom graphical user interface (GUI) for manual adjustments, which has enabled it to be reliably utilized as a semi-automatic OCT layer segmentation tool in several large-scale clinical trials.26 The inner boundary is defined by the outer border of the hyperreflective line corresponding to the RPE, while the outer boundary is defined by the outer border of the choroid stroma, corresponding to the “stromal choroidal thickness” as defined recently.27 Choroidal thickness measurements were recorded at the subfoveal location, and at 0.5 mm intervals from 2.5 mm nasal to 2.5 mm temporal to the fovea (Figure 1). Poor quality images where the segmentation software could not detect the choroid boundaries were excluded from analysis. Central foveal thickness (CFT) of the retina was also measured by the DOCTRAP program.
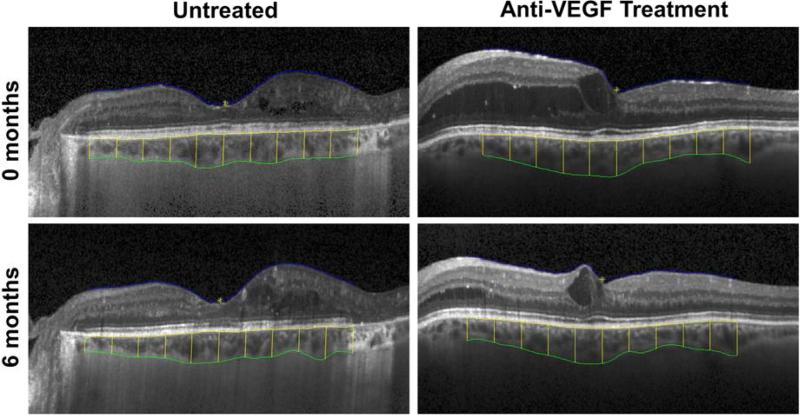
Figure 1. Semi-automated Segmentation of Retinal and Choroidal Thickness on Enhanced-Depth Imaging Optical Coherence Tomography.
Images show the inner border of the retinal surface (blue), outer border of the RPE (yellow), and outer border of the stromal choroid (green), with vertical lines indicating choroidal thickness measurements at 0.5mm intervals from 2.5mm nasal to 2.5mm temporal to the fovea. Images are taken from a sample eye with untreated diabetic macular edema at 0 and 6 months (top left and bottom left) and another eye treated with anti-VEGF at 0 and 6 months (top right and bottom right).
Statistical analysis was performed using Statistical Package for Social Sciences (version 20, SPSS Inc, Chicago, IL, USA). Differences between DME eyes with and without anti-VEGF treatment were compared using Student's t-tests for scale variables (age, HbA1c, BCVA, IOP, CFT, and subfoveal choroidal thickness) and Chi-square test for nominal variables (gender, eye laterality, diabetic retinopathy type, lens status). Difference in median time of day when the images were obtained was evaluated by Wilcoxon rank sum analysis. Changes in BCVA, CFT, subfoveal choroidal thickness, or other choroidal thickness measurements over time were compared using paired-samples T-tests. Univariate linear regression analysis was used to determine the association of subfoveal choroidal thickness or subfoveal choroidal thickness change with other variables tested, including the cumulative number of anti-VEGF treatments, BCVA, CFT, and change in BCVA or CFT.
RESULTS
Baseline characteristics
Fifty-nine eyes of 59 patients (mean age 62.5 ± 12.8 years) were included in this cohort analysis. Of these, 27 eyes (45.8%) were right eyes, and most eyes were phakic (72.9%). There were 28 eyes (47.4%) classified as PDR and 31 eyes (52.5%) classified as NPDR, with mean HbA1c of 7.9 ± 2.1%. At baseline, 24 eyes (40.7%) had prior focal laser therapy, 3 eyes (5.1%) had prior intravitreal triamcinolone (Kenalog, Bristol-Myers Squibb, New York, NY), and 18 eyes (30.5%) had prior PRP. No eyes had prior anti-VEGF therapy, as these were excluded from the study. Complete baseline characteristics are listed in Table 1.
Table 1.
Comparison of Patient Demographics and Baseline Eye Characteristics of Eyes with Diabetic Macular Edema with and without Anti-Vascular Endothelial Growth Factor Therapy.
| All Eyes (n=59) | Untreated (n=26) | Anti-VEGF Treated (n=33) | P-value | |
|---|---|---|---|---|
| Age (years) | 62.5 ± 12.8 | 62.1 ± 15.6 | 62.8 ± 10.3 | 0.828a |
| Gender (male / female) | 29 / 30 | 14 / 12 | 15 / 18 | 0.522b |
| Hemoglobin A1c (%) | 7.9 ± 2.1 | 7.6 ± 2.1 | 8.3 ± 2.1 | 0.222a |
| Laterality (right / left) | 27 / 32 | 16 / 10 | 11 / 22 | 0.031b |
| Retinopathy type (NPDR / PDR) | 31 / 28 | 11 / 15 | 20 / 13 | 0.128b |
| Lens (phakic/pseudophakic/aphakic) | 43 / 15 / 1 | 18 / 7 / 1 | 25 / 8 / 0 | 0.498b |
| Baseline BCVA (logMAR) | 0.422 ± 0.285 | 0.321 ± 0.299 | 0.501 ± 0.250 | 0.014a |
| Baseline IOP (mmHg) | 16.2 ± 3.6 | 15.9 ± 3.6 | 16.4 ± 3.6 | 0.541a |
| Baseline CFT (μm) | 389.3 ± 151.1 | 324.6 ± 123.9 | 441.1 ± 152.0 | 0.002a |
| Baseline subfoveal choroidal thickness (μm) | 251.0 ± 93.0 | 256.6 ± 96.8 | 246.6 ± 91.2 | 0.687a |
Student's t-test
Chi-square test
c Abbreviations: NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; BCVA, best-corrected visual acuity; IOP, intraocular pressure; CFT, central foveal thickness
Of the cohort, 33 eyes (55.9%) received at least one anti-VEGF treatment during the study period, while the remaining 26 eyes (44.1%) were observed without treatment. The majority of eyes treated with anti-VEGF received bevacizumab (Genentech, San Francisco, CA) (30 eyes), while the remainder (3 eyes) had ranibizumab (Genentech, San Francisco, CA). As expected, untreated eyes had significantly greater mean BCVA (0.321 vs. 0.501; p=0.014) and lower mean CFT (324.6 μm vs. 441.1 μm; p = 0.002) at baseline compared with eyes that underwent anti-VEGF therapy during the study period, which likely explained the decision for conservative management in the untreated group. Mean baseline subfoveal choroidal thickness, however, was similar between the two groups (256.6 μm vs. 246.6 μm; p = 0.687). The untreated group had more right eyes (61.5% vs 33.3%; p=0.031), with otherwise no statistical difference in mean age, HbA1c, and IOP, as well as similar proportion of gender, diabetic retinopathy type, and lens status among untreated and treated eyes with DME (Table 1). The median time at which the EDI-OCT was obtained did not differ significantly (10:53 a.m. vs. 10:22 a.m.; p = 0.891) between the two groups at baseline.
Baseline Factors Associated with Choroidal Thickness
At baseline, subfoveal choroidal thickness was not associated with BCVA (R2 = 0.010; p = 0.458) or CFT (R2 = 0.009; p = 0.484), in contrast to the significant association between CFT and BCVA (R2 = 0.205, p < 0.001) (Supplemental Figure 1). However, subfoveal choroidal thickness was associated with age (R2 = 0.234; p < 0.001), consistent with previous reports,7 and not with gender (R2 = 0.002, p = 0.773), HbA1c (R2 = 0.021; p = 0.335), eye laterality (R2 = 0.015; p = 0.362), diabetic retinopathy type (R2 = 0.004; p = 0.620), lens status (R2 = 0.030; p = 0.186), or IOP (R2 = 0.014; p = 0.379). These results suggest that in eyes that have never received anti-VEGF therapy, subfoveal choroidal thickness may not be associated with functional (BCVA) or anatomic (CFT) measures of DME severity.
Effect of Anti-VEGF on Choroidal Thickness
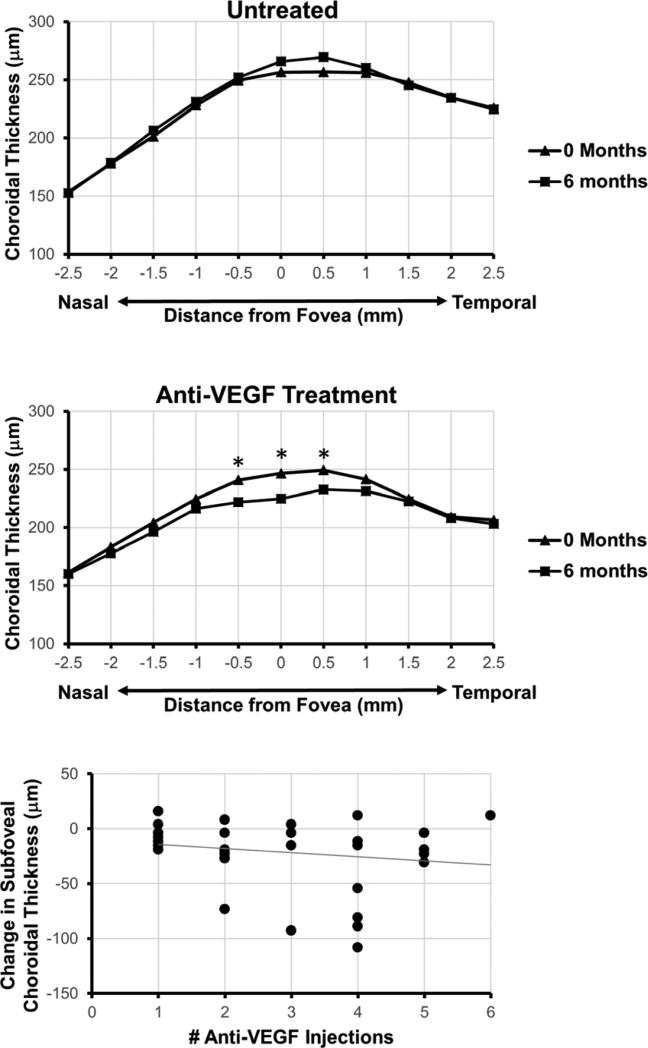
Due to the variability of choroidal thickness between individuals at a single time point, we evaluated whether choroidal thickness changes in eyes with DME over time, and if such changes are affected by anti-VEGF therapy. At 6 months follow-up, eyes that were observed without treatment showed a slight, but non-significant increase in subfoveal choroidal thickness from 256.6 μm to 266.0 μm (p = 0.057), but not at other locations measured along the macula (Figure 2, Table 2). In contrast, eyes that received anti-VEGF therapy (mean number of injections 2.73) had a significant decrease in subfoveal choroidal thickness from 246.6 μm to 224.8 μm (p < 0.001), as well as two adjacent choroidal thickness measurements at 0.5 mm nasal (240.9 μm to 221.9 μm; p = 0.002) and 0.5 mm temporal (249.3 μm to 224.8 μm; p = 0.011) to the fovea, but not at other locations measured along the macula (Figure 2, Table 2). Greater number of anti-VEGF treatments showed a possible trend towards a greater decrease in subfoveal choroidal thickness (Figure 2), but this relationship was not significant (R2 = 0.031, p = 0.327). Together, these results suggest that anti-VEGF therapy may be associated with central choroidal thinning in eyes with DME, although there was no significant dose relationship detected in this cohort study.
Figure 2. Effect of Anti-Vascular Endothelial Growth Factor Therapy on Choroidal Thickness.
Line graphs demonstrate mean choroidal thickness measured at 0.5mm intervals from 2.5mm nasal to 2.5mm temporal to the fovea at 0 and 6 months in eyes with DME without treatment (top) and after anti-VEGF treatment (middle). A scatterplot showing relationship of change in subfoveal choroidal thickness with cumulative number of anti-VEGF injections, along with the corresponding trend line (bottom).
Table 2.
6 Month Changes in Choroidal Thickness in Eye with Diabetic Macular Edema with and without Anti-Vascular Endothelial Growth Factor Therapy
| Untreated (n=26) | Anti-VEGF Treatment (n=33) | |||||
|---|---|---|---|---|---|---|
| Baseline | 6 Month | P-value | Baseline | 6 Month | P-value | |
| BCVA (logMAR) | 0.321 ± 0.299 | 0.296 ± 0.285 | 0.248 | 0.501 ± 0.250 | 0.424 ± 0.211 | 0.026 |
| CFT (μm) | 323.6 ± 123.9 | 320.0 ± 148.0 | 0.745 | 453.6 ± 125.1 | 375.2 ± 122.8 | <0.001 |
| CT @ −2.5mm (μm)a | 153.7 ± 81.1 | 152.6 ± 81.0 | 0.798 | 161.6 ± 67.8 | 160.1 ± 69.0 | 0.859 |
| CT @ −2.0mm (μm) | 178.1 ± 84.0 | 178.4 ± 90.3 | 0.944 | 183.1 ± 76.3 | 177.7 ± 73.4 | 0.112 |
| CT @ −1.5mm (μm) | 201.3 ± 96.2 | 206.4 ± 96.1 | 0.220 | 204.2 ± 79.2 | 196.4 ± 81.0 | 0.092 |
| CT @ −1.0mm (μm) | 228.0 ± 98.9 | 231.1 ± 103.5 | 0.417 | 224.6 ± 81.4 | 216.3 ± 81.3 | 0.204 |
| CT @ −0.5mm (μm) | 249.6 ± 105.4 | 252.3 ± 108.7 | 0.598 | 240.9 ± 84.6 | 221.9 ± 79.3 | 0.002 |
| CT @ fovea (μm) | 256.6 ± 96.8 | 266.0 ± 108.1 | 0.057 | 246.6 ± 91.1 | 224.8 ± 83.3 | <0.001 |
| CT @ +0.5mm (μm) | 256.7 ± 90.9 | 269.4 ± 107.5 | 0.071 | 249.3 ± 81.1 | 232.8 ± 84.6 | 0.011 |
| CT @ +1.0mm (μm) | 256.1 ± 88.6 | 260.3 ± 95.5 | 0.373 | 241.6 ± 65.6 | 231.5 ± 80.2 | 0.158 |
| CT @ +1.5mm (μm) | 247.8 ± 81.0 | 245.3 ± 85.5 | 0.647 | 224.6 ± 66.4 | 222.4 ± 78.1 | 0.790 |
| CT @ +2.0mm (μm) | 234.7 ± 72.8 | 234.4 ± 81.9 | 0.974 | 209.1 ± 62.4 | 208.1 ± 71.3 | 0.897 |
| CT @ +2.5mm (μm) | 225.7 ± 65.8 | 224.6 ± 74.1 | 0.905 | 206.5 ± 55.7 | 203.2 ± 55.5 | 0.592 |
negative distances denote nasal to fovea; positive distances denote temporal to fovea
b Abbreviations: BCVA, best-corrected visual acuity; CFT, central foveal thickness; CT, choroidal thickness; SFCT, subfoveal choroidal thickness
Relation of Choroidal Thickness Change to Outcomes
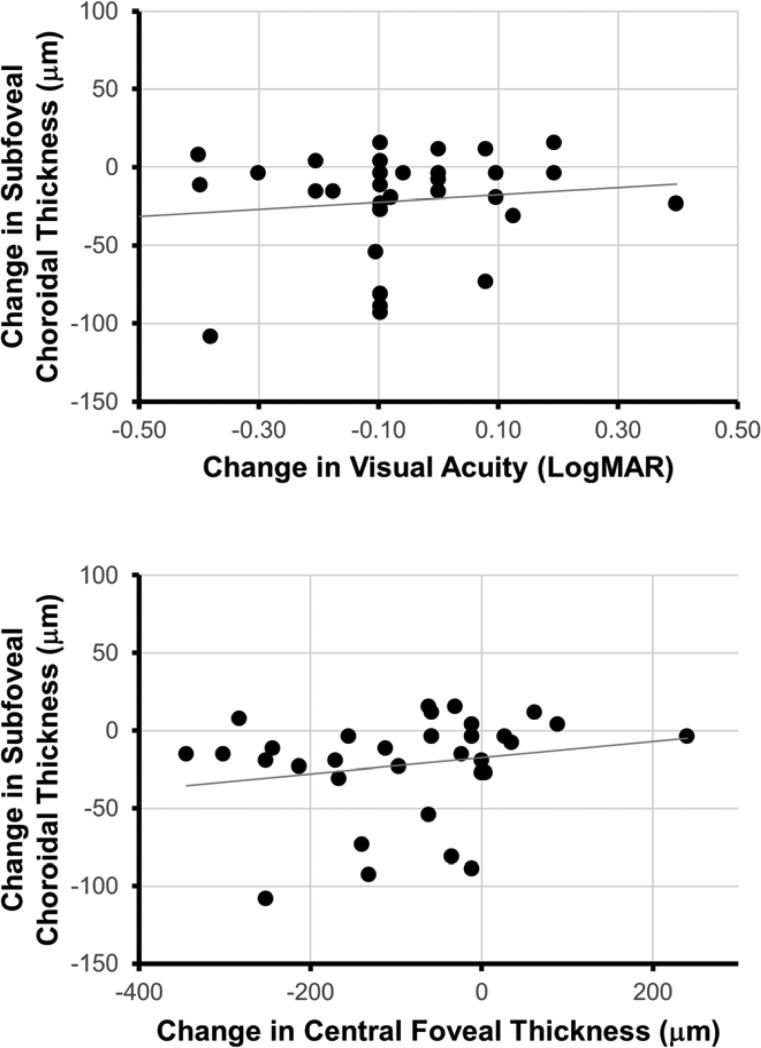
Although choroidal thickness appears to decrease with anti-VEGF treatment, it is unclear if choroidal thinning may be involved in DME pathogenesis or rather as a non-specific effect of anti-VEGF therapy. To address this question, we evaluated the association of subfoveal choroidal thickness change at 6 months with visual and anatomic outcomes in eyes with DME. In contrast to untreated eyes, which showed no significant change in BCVA (p = 0.248) or CFT (p = 0.745), eyes treated with anti-VEGF injections showed an improvement in mean BCVA from 0.501 to 0.424 (p = 0.026) and CFT from 453.6 μm to 375.2 μm (p < 0.001). However, the decrease in subfoveal choroidal thickness was not associated with the improvement in BCVA (R2 = 0.017; p = 0.470) or CFT (R2 = 0.040; p = 0.263) (Figure 3). These results suggest that choroidal thickness changes after anti-VEGF therapy in DME may not be associated with the degree of functional (BCVA) or anatomic (CFT) improvement after treatment.
Figure 3. Relation of Choroidal Thickness Change with Visual and Anatomic Outcomes.
Scatterplots demonstrate the relationship of subfoveal choroidal thickness change after 6 months of anti-VEGF therapy with change in best-corrected visual acuity (top) and central foveal thickness (bottom), along with corresponding trend lines.
DISCUSSION
The recent availability of EDI-OCT has spurred a renewed interest in understanding the role of the choroid in various diseases. While the pathogenesis of DME has been attributed mainly to hyperpermeability of retinal vessels, the choroidal vasculature may play a potential role in modulating disease severity by affecting the hydrostatic or osmotic pressures that determine the absorption rate of intraretinal fluid. At the same time, choroidal ischemia may also play a role in the VEGF-driven response resulting in DME. Therefore, we designed this study to evaluate the relationship between choroidal thickness and DME, to evaluate the potential use of choroidal thickness as a biomarker for disease progression or treatment response, and to understand the effect of anti-VEGF therapy on choroidal anatomy.
In this study, we demonstrated that in a cohort of DME eyes without prior anti-VEGF therapy, choroidal thickness significantly decreases over 6 months after anti-VEGF treatment, but remains unchanged and perhaps even increased slightly in untreated eyes. The decrease in choroidal thickness is not associated with greater number of anti-VEGF injections, or with visual or anatomic outcomes. Based on these results, it is difficult to determine whether the choroidal thinning is a desired drug effect that directly modulates DME pathophysiology, or a non-specific secondary effect. Several lines of evidence suggest the latter. First, the degree of choroidal thinning in treated eyes do not correlate with the amount of visual or anatomic improvement. In addition, although retinal thickness at baseline was greater in eyes that were treated compared with those that were observed, choroidal thickness was similar between the two groups, suggesting no clear relationship between choroidal thickness and disease severity at baseline. Also, focal laser treatment has been shown to improve vision and anatomy without choroidal thinning.22 Finally, various studies indicate that anti-VEGF injections may result in choroidal thinning in AMD also,20, 21 further implying that the effect is not specific to the disease process in DME. VEGFa has a known trophic effect on maintaining the choroidal vasculature. Targeted deletion of VEGFa in adult mice results in loss of the choriocapillaris.28 Therefore, the implication of our results may support a more cautious approach to aggressive use of anti-VEGF therapy for the management of DME, although further studies are necessary to clarify this possibility.
The current study is strengthened by the stringent inclusion criteria of only eyes without prior anti-VEGF treatments and by the longitudinal nature of the analysis over time. This design overrides the confounding factor of individual variability in choroidal thickness common in most cross-sectional studies, as well as prior anti-VEGF treatments that may result in choroidal thinning prior to inclusion in analysis. The semi-automated method of choroidal segmentation also allows choroidal thickness measurements across the macula, rather than just the subfoveal location, and eliminates observer and interobserver variability in manual measurements.
Choroidal thickness measurements using EDI-OCT can be limited in cases of severe intraretinal and/or subretinal fluid, where the underlying choroid-scleral junction may become obscured, resulting in a selection bias toward eyes with less severe disease. In addition, eyes with a thinner choroid at baseline may not undergo additional thinning due to a “floor effect.” In this study, the eyes that received 5-6 anti-VEGF injections had little to no decrease in choroidal thickness compared with eyes that received 4 injections, possibly due to a lower baseline subfoveal choroidal thickness in these eyes (Supplemental Table 1). This inherent limitation limits the sensitivity of this study to detect a possible relationship between choroidal thinning and frequency of anti-VEGF treatments, which was suggested by the trend in our results but did not reach statistical significance. The small number of eyes within each of these subgroups also limits the interpretation of these results. The fact that the choroid becomes thinner at locations farther away from the fovea also limits detectable choroidal thickness changes to the very central region of the macula. Finally, this study is also limited by its retrospective nature. In particular, the untreated eyes had less severe disease at baseline, and did not exhibit significant change in retinal or choroidal thickness. Thus, untreated eyes do not serve as a true comparison arm against eyes treated with anti-VEGF. Eyes treated with focal laser treatment alone would serve as a superior control group, although cases are uncommon since anti-VEGF therapy has now become the standard of care for DME.29 Either way, a larger prospective study with a more uniform baseline subfoveal choroidal thickness or that includes axial length measurements to adjust for variability between individuals could yield more conclusive results. Further studies to evaluate choroidal microvasculature or choroidal perfusion may prove valuable in delineating the role of the choroid in the pathophysiology of DME.
Supplementary Material
ACKNOWLEDGMENTS
a. Funding/Support: National Institutes of Health R01-EY022691 (Drs. Farsiu and Chiu). The sponsor or funding organization had no role in the design or conduct of this research.
Biography
 Glenn C. Yiu, MD, PhD is completing a fellowship in Vitreoretinal Surgery at Duke University. He obtained a Bachelor of Arts from Columbia University, followed by an MD-PhD degree from Harvard Medical School, medical internship at Brigham & Women's Hospital, and residency at Massachusetts Eye & Ear Infirmary. Dr. Yiu's research interests include choroidal vascular biology, ocular imaging, neuro-regeneration, and the pathophysiology of retinal diseases. He will be joining the faculty at University of California, Davis as an Assistant Professor in fall 2014.
Glenn C. Yiu, MD, PhD is completing a fellowship in Vitreoretinal Surgery at Duke University. He obtained a Bachelor of Arts from Columbia University, followed by an MD-PhD degree from Harvard Medical School, medical internship at Brigham & Women's Hospital, and residency at Massachusetts Eye & Ear Infirmary. Dr. Yiu's research interests include choroidal vascular biology, ocular imaging, neuro-regeneration, and the pathophysiology of retinal diseases. He will be joining the faculty at University of California, Davis as an Assistant Professor in fall 2014.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
c. Contribution of Authors: Design of the study (GY, TM); Conduct of the study (GY, VM); Data collection and management (GY, VM, SJC); Data analysis and interpretation (GY, VM, SJC, SF, TM); Preparation of manuscript (GY, VM); Review and approval of manuscript (GY, VM, SJC, SF, TM).
DISCLOSURE
b. Financial Disclosures: Dr. Farsiu and Ms. Chiu have patents pending in OCT imaging and analysis. Dr. Mahmoud is a consultant for Alcon, Alimera Sciences, and Allergan. Drs. Yiu, and Manjunath have no financial disclosures.
REFERENCES
- 1.Cao J, McLeod S, Merges CA, Lutty GA. Choriocapillaris degeneration and related pathologic changes in human diabetic eyes. Arch Ophthalmol. 1998;116(5):589–97. doi: 10.1001/archopht.116.5.589. [DOI] [PubMed] [Google Scholar]
- 2.Fryczkowski AW, Sato SE, Hodes BL. Changes in the diabetic choroidal vasculature: scanning electron microscopy findings. Ann Ophthalmol. 1988;20(8):299–305. [PubMed] [Google Scholar]
- 3.Hidayat AA, Fine BS. Diabetic choroidopathy. Light and electron microscopic observations of seven cases. Ophthalmology. 1985;92(4):512–22. [PubMed] [Google Scholar]
- 4.Nagaoka T, Kitaya N, Sugawara R, et al. Alteration of choroidal circulation in the foveal region in patients with type 2 diabetes. Br J Ophthalmol. 2004;88(8):1060–3. doi: 10.1136/bjo.2003.035345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schocket LS, Brucker AJ, Niknam RM, Grunwald JE, DuPont J, Brucker AJ. Foveolar choroidal hemodynamics in proliferative diabetic retinopathy. Int Ophthalmol. 2004;25(2):89–94. doi: 10.1023/b:inte.0000031744.93778.60. [DOI] [PubMed] [Google Scholar]
- 6.Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol. 2010;150(3):325–329. e1. doi: 10.1016/j.ajo.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147(5):811–5. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146(4):496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 9.Kim JT, Lee DH, Joe SG, Kim JG, Yoon YH. Changes in choroidal thickness in relation to the severity of retinopathy and macular edema in type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2013;54(5):3378–84. doi: 10.1167/iovs.12-11503. [DOI] [PubMed] [Google Scholar]
- 10.Adhi M, Brewer E, Waheed NK, Duker JS. Analysis of morphological features and vascular layers of choroid in diabetic retinopathy using spectral-domain optical coherence tomography. JAMA Ophthalmol. 2013;131(10):1267–74. doi: 10.1001/jamaophthalmol.2013.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regatieri CV, Branchini L, Carmody J, Fujimoto JG, Duker JS. Choroidal thickness in patients with diabetic retinopathy analyzed by spectral-domain optical coherence tomography. Retina. 2012;32(3):563–8. doi: 10.1097/IAE.0b013e31822f5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vujosevic S, Martini F, Cavarzeran F, Pilotto E, Midena E. Macular and peripapillary choroidal thickness in diabetic patients. Retina. 2012;32(9):1781–90. doi: 10.1097/IAE.0b013e31825db73d. [DOI] [PubMed] [Google Scholar]
- 13.Esmaeelpour M, Povazay B, Hermann B, et al. Mapping choroidal and retinal thickness variation in type 2 diabetes using three-dimensional 1060-nm optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(8):5311–6. doi: 10.1167/iovs.10-6875. [DOI] [PubMed] [Google Scholar]
- 14.Querques G, Lattanzio R, Querques L, et al. Enhanced depth imaging optical coherence tomography in type 2 diabetes. Invest Ophthalmol Vis Sci. 2012;53(10):6017–24. doi: 10.1167/iovs.12-9692. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Xu L, Du KF, et al. Subfoveal choroidal thickness in diabetes and diabetic retinopathy. Ophthalmology. 2013;120(10):2023–8. doi: 10.1016/j.ophtha.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Fujiwara T, Imamura Y, Margolis R, Slakter JS, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009;148(3):445–50. doi: 10.1016/j.ajo.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 17.Ikuno Y, Tano Y. Retinal and choroidal biometry in highly myopic eyes with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50(8):3876–80. doi: 10.1167/iovs.08-3325. [DOI] [PubMed] [Google Scholar]
- 18.Tan CS, Ouyang Y, Ruiz H, Sadda SR. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(1):261–6. doi: 10.1167/iovs.11-8782. [DOI] [PubMed] [Google Scholar]
- 19.Usui S, Ikuno Y, Akiba M, et al. Circadian changes in subfoveal choroidal thickness and the relationship with circulatory factors in healthy subjects. Invest Ophthalmol Vis Sci. 2012;53(4):2300–7. doi: 10.1167/iovs.11-8383. [DOI] [PubMed] [Google Scholar]
- 20.Branchini L, Regatieri C, Adhi M, et al. Effect of intravitreous anti-vascular endothelial growth factor therapy on choroidal thickness in neovascular age-related macular degeneration using spectral-domain optical coherence tomography. JAMA Ophthalmol. 2013;131(5):693–4. doi: 10.1001/jamaophthalmol.2013.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamazaki T, Koizumi H, Yamagishi T, Kinoshita S. Subfoveal choroidal thickness after ranibizumab therapy for neovascular age-related macular degeneration: 12-month results. Ophthalmology. 2012;119(8):1621–7. doi: 10.1016/j.ophtha.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Adhi M, Alwassia AA, Duker JS. Analysis of choroidal thickness in eyes treated with focal laser photocoagulation using SD-OCT. Can J Ophthalmol. 2013;48(6):535–8. doi: 10.1016/j.jcjo.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Chiu SJ, Izatt JA, O'Connell RV, Winter KP, Toth CA, Farsiu S. Validated automatic segmentation of AMD pathology including drusen and geographic atrophy in SD-OCT images. Invest Ophthalmol Vis Sci. 2012;53(1):53–61. doi: 10.1167/iovs.11-7640. [DOI] [PubMed] [Google Scholar]
- 24.Chiu SJ, Li XT, Nicholas P, Toth CA, Izatt JA, Farsiu S. Automatic segmentation of seven retinal layers in SDOCT images congruent with expert manual segmentation. Opt Express. 2010;18(18):19413–28. doi: 10.1364/OE.18.019413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JY, Chiu SJ, Srinivasan PP, et al. Fully automatic software for retinal thickness in eyes with diabetic macular edema from images acquired by cirrus and spectralis systems. Invest Ophthalmol Vis Sci. 2013;54(12):7595–602. doi: 10.1167/iovs.13-11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farsiu S, Chiu SJ, O'Connell RV, et al. Quantitative Classification of Eyes with and without Intermediate Age-related Macular Degeneration Using Optical Coherence Tomography. Ophthalmology. 2014;121(1):162–72. doi: 10.1016/j.ophtha.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yiu G, Pecen P, Sarin N, et al. Characterization of the Choroid-Scleral Junction and Suprachoroidal Layer in Healthy Individuals on Enhanced-Depth Imaging Optical Coherence Tomography. JAMA Ophthalmol. 2013 doi: 10.1001/jamaophthalmol.2013.7288. [DOI] [PubMed] [Google Scholar]
- 28.Kurihara T, Westenskow PD, Bravo S, Aguilar E, Friedlander M. Targeted deletion of Vegfa in adult mice induces vision loss. J Clin Invest. 2012;122(11):4213–7. doi: 10.1172/JCI65157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diabetic Retinopathy Clinical Research N. Elman MJ, Aiello LP, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–1077. e35. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.