Abstract
In genetically-modified Lmx1bf/f/p mice, selective deletion of LMX1B in Pet-1 expressing cells leads to failure of embryonic development of serotonin (5-HT) neurons. As adults, these mice have a decreased hypercapnic ventilatory response and abnormal thermoregulation. This mouse model has been valuable in defining the normal role of 5-HT neurons, but it is possible that developmental compensation reduces the severity of observed deficits. Here we studied mice genetically modified to express diphtheria toxin receptors (DTR) on Pet-1 expressing neurons (Pet-1-Cre/Floxed DTR or Pet1/DTR mice). These mice developed with a normal complement of 5-HT neurons. As adults, systemic treatment with 2 – 35 μg diphtheria toxin (DT) reduced the number of tryptophan hydroxylase immunoreactive (TpOH-ir) neurons in the raphe nuclei and ventrolateral medulla by 80%. There were no effects of DT on baseline ventilation (VE) or the ventilatory response to hypercapnia or hypoxia. At an ambient temperature (TA) of 24°C, all Pet1/DTR mice dropped their body temperature (TB) below 35°C after DT treatment, but the latency was shorter in males than females (3.0 ± 0.37 vs 4.57 ± 0.29 days, respectively; p < 0.001). One week after DT treatment, mice were challenged by dropping TA from 37°C to 24°C, which caused TB to decrease more in males than in females (29.7 ± 0.31°C vs 33.0 ± 1.3°C, p < 0.01). We conclude that the 20% of 5-HT neurons that remain after DT treatment in Pet1/DTR mice are sufficient to maintain normal baseline breathing and a normal response to CO2, while those affected include some essential for thermoregulation, in males more than females. In comparison to models with deficient embryonic development of 5-HT neurons, acute deletion of 5-HT neurons in adults leads to a greater defect in thermoregulation, suggesting that significant developmental compensation can occur.
Keywords: serotonin, chemoreception, thermoregulation, gender differences
Introduction
The 5-HT system is involved in mammalian respiratory drive, respiratory rhythm generation (Dekin et al., 1985, Pena and Ramirez, 2002, Feldman et al., 2003, Hodges and Richerson, 2008a, Hodges et al., 2008, Hodges et al., 2009, Ptak et al., 2009, Hodges and Richerson, 2010) and the ventilatory response to CO2 (Richerson, 1995, Wang et al., 1998, Wang and Richerson, 1999, Wang et al., 2001, Nattie et al., 2004, Li and Nattie, 2008, Corcoran et al., 2009, Ray et al., 2011). In addition, the 5-HT system, especially the raphe pallidus (RPa), has been associated with thermoregulation (Martin-Cora et al., 2000, Zaretsky et al., 2003, Morrison et al., 2008, Naumenko et al., 2009, Madden and Morrison, 2010, Hale et al., 2011, Morrison and Nakamura, 2011, Naumenko et al., 2011, Morrison et al., 2014). Adult mice genetically modified so that nearly all central 5-HT neurons are absent during adulthood (Lmx1bf/f/p mice) have previously been shown to breathe normally in room air (21% O2 and 0% CO2) and thermoregulate normally when TA is 22°C, but have a 50% decrease in the ventilatory response to CO2 and rapidly become hypothermic when exposed to a TA of 4°C (Hodges et al., 2008). Similarly, male Pet-1 null mice, in which there is a 70% decrease in 5-HT neurons, have normal baseline ventilation, whereas in 5-HT transporter (5-HTT) knockout mice baseline ventilation is increased (Gobbi et al., 2001, Li and Nattie, 2008, Hodges et al., 2011). However, in both of these strains thermoregulation is normal at a TA of 22°C. In each of the above mouse strains the defects are present from embryonic life onwards, giving an opportunity for developmental compensation. In contrast, RC::PDi transgenic mice express a modified muscarinic acetylcholine receptor under control of the promoter for the 5-HTT (Slc6a4), and 5-HT neurons are normal throughout development, but upon systemic treatment with the exogenous agonist clozapine-N-oxide (CNO) their membrane potential is reversibly hyperpolarized (Ray et al., 2011). This leads to a large drop in TB even when TA is 22°C, whereas there is no effect on baseline ventilation parameters. These results suggest that developmental compensation occurs in Lmx1bf/f/p, Pet-1 null and 5-HTT knockout mice. In contrast, there should not be any developmental compensation in RC::PDi mice. However, CNO treatment only suppresses 5-HT neuron firing by ≈50%, which could potentially explain why CNO only suppresses the hypercapnic ventilatory response by 50% in RC::PDi mice (Ray et al., 2011). Therefore, for different reasons each of these approaches could have given results that underestimated the actual contribution of 5-HT neurons to central respiratory chemoreception.
Our principal objective in the present work was to use the DTR/DT system to acutely delete a large percentage of central 5-HT neurons in adult mice and study the effect on thermoregulation and ventilation in the absence of compensatory developmental changes. DT is a protein with a molecular weight of ± 62,000 D that causes cell death by inhibition of protein synthesis, and must be transported into cells by DTR to be effective (Moskaug et al., 1989, Collier, 2001). Wild type mouse cells are at least 103–105 times more resistant to DT than human cells. Thus, engineered expression of the high-affinity human DTR by a particular cell type in mice is a powerful means of selectively depleting that population of cells in vivo upon systemic DT administration (Palmiter et al., 1987, Saito et al., 2001, Cha et al., 2003, Buch et al., 2005). In Pet1/DTR mice, we expected that DTRs would be expressed selectively in central 5-HT neurons. This was based on the previous demonstration that DTR is expressed selectively in cells that express Cre recombinase at some time in the life of floxed DTR mice (Palmiter et al., 1987, Buch et al., 2005), and the previous finding that Pet-1 is expressed selectively in central 5-HT neurons (Hendricks et al., 1999). We obtained evidence in support of this assumption using immunohistochemistry and Nissl staining, which confirmed that DT administration eliminated a large percentage of TpOH-ir neurons and an equally large number of Nissl stained neurons in the raphe nuclei.
Experimental procedures
All experiments were done in accordance with guidelines of the National Institutes of Health for animal care and use and were approved by the Yale University Animal Care and Use Committee.
Animals
Pet1/DTR mice were generated by mating ePet-Cre mice (Scott et al., 2005) with Cre-inducible DTR transgenic mice (iDTR) (Buch et al., 2005). In iDTR mice, DTR is expressed upon Cre recombinase-mediated excision of a STOP cassette (Buch et al., 2005). We expected that Cre recombinase would be expressed selectively in 5-HT neurons, because in ePet-Cre mice Cre expression is under control of the enhancer region of Pet-1. Genotyping of Pet1/DTR mice was done using the strategy as previously reported (Saito et al., 2001, Cha et al., 2003). Tail samples were digested and PCR was performed using the following Flox primers: DTR-1 sense, ACCATGAAGCTGCTGCCGTC and DTR-2 antisense, ATCAGTGGGAATTAGTCATGC. CRE primers: CRE-1 sense, 5′ATTTGCCTGCATTACCGGTCG 3′ and CRE-2 antisense, 5′CAGCATTGCTGCTGTCACTTGGTC 3′. The PCR product was analyzed by agarose gel electrophoresis. The band size for DTR is 600 bp and for Cre is 375 bp. Bands are absent for both wild-type (WT) genotypes.
Adult male Pet1/DTR mice (87 ± 4 days old; 27.9 ± 0.7 g; n = 24), female Pet1/DTR mice (104 ± 6 days old; 20.2 ± 0.8 g; n = 14) and their male WT (93 ± 5 days old; 28.5 ± 0.9 g; n = 11) and female WT (94 ± 9 days old; 21.1 ± 0.5 g; n = 7) littermates were studied. All animals received food and water ad libitum and were housed on a 12 hr light/dark cycle in the Yale Animal Care Facility.
Diphtheria toxin protocol
We tested the effects of systemic administration of DT (Sigma, DO564) using doses of 5, 50 and 250 μg/kg i.p. in 100 μl of normal saline solution. DT was administered 1, 3 or 5 times per week, for 2, 4 or 6 weeks in order to find a protocol for DT administration that caused a specific effect on Pet1/DTR mice without nonspecific effects on WT mice. The effects were independent of the dosing schedule and only on the cumulative dose so the results are presented as a function of the total amount of DT (DTT). Since DT led to a drop in TB, animals were housed at an TA of 30 C as needed to prevent hypothermia.
Immunohistochemistry
Mice were deeply anesthetized with pentobarbital and then perfused with phosphate buffered saline (PBS; 20 ml), followed by 4% paraformaldehyde in phosphate buffer (25 ml over 10 minutes). Brains were removed and left in fixative overnight, then for cryoprotection were placed in 30% sucrose in PBS for 2–3 days. Brainstems were sectioned in the coronal plane (25 μm) on a cryostat (CM30505, Leica). Sections were permeabilized and blocked with 3% horse serum and 0.4% Triton X-100 in PBS, then incubated overnight with a primary antibody against TpOH (1:2000, mouse monoclonal; Sigma T-0678) in blocking solution (3% horse serum in PBS). Sections were incubated with biotinylated horse anti-mouse IgG (1:1000; Vectastain Elite ABC kit, Vector Laboratories), followed by ABC reagent (Vectastain Elite ABC Kit, Vector Laboratories) for 50 min and then Nova Red peroxidase substrate (NovaRed™, Vector Laboratories) for 5–7 min. Sections were dehydrated with successive ethanols and xylene, and coverslipped with Permount mounting medium (UN1294, Fisher Scientific). The numbers of TpOH-ir neurons were counted from 17 Pet1/DTR (male = 11, female = 6) and 13 WT (male = 9, female = 4) mice using a brightfield microscope (DMI 6000B, Leica). One slice was studied out of every 4 slices from bregma −5.80 mm to bregma −7.64 mm (Paxinos and Franklin, 2001). TpOH-ir neurons were counted separately in the raphe obscurus (ROb), raphe magnus (RMg), RPa and ventrolateral medulla (VLM) as shown in Figure 1A.
Figure 1. Systemic diphtheria toxin reduces the number of TpOH-ir neurons in the brainstem of Pet1/DTR mice but not WT mice.
A: Schematic representation of the locations used to count the number of TpOH-ir neurons in the RMg, RPa ROb, and VLM using coordinates described in the text as distance from Bregma (B). Adapted from Paxinos & Franklin, 2001. B: TpOH-ir neurons in the RMg, ROb, RPa and VLM from WT and Pet1/DTR mice after 4 μg DTT. Black bars, 100 μm. C: Number of TpOH-ir neurons/25 μm thick slice in Pet1/DTR mice (red circles, n = 17) and WT mice (black circles, n = 13) as a function of dose of DTT from 2–36 μg. D: Summary of the number of TpOH-ir neurons from Pet1/DTR mice injected with DT (red bar; n = 17 compared with WT mice injected with DT (black bar; n = 13 and Pet1/DTR mice that did not receive any injection (empty bar; n = 5. ****, p < 0.0001). No differences were found between DT treated WT mice and untreated Pet1/DTR mice. (p > 0.05, 1 way ANOVA, Bonferroni’s multiple comparison test).
Nissl Staining
Every 4th section was mounted on a slide and used for Nissl staining. Slides were placed in 37% formalin and 100% ethyl alcohol (EtOH) 1:1 for 5 minutes, and rehydrated in 95% EtOH for 3 min, 70% EtOH for 2 min, rinsed twice in distilled water for 3 min and stained with Cresyl Violet (1 mg/ml) for 20 min. After staining, sections were rinsed twice in water for 2 min, then dehydrated in 70% EtOH for 2 min, in 1% glacial acetic acid in 95% EtOH for 5 min, in 95% EtOH for 3 min and twice in 100% EtOH for 3 min each. To lighten the sections, xylene was applied twice for 5 minutes each, after which slides were coverslipped with Permount mounting media (UN 1294, Fisher Scientific). The Nissl stained cells were counted using a brightfield microscope (DMI 6000B, Leica) using the same boundaries as shown in Figure 1A.
Whole-body plethysmography
Prior to the first injection of DT, and one week after the final injection of DT, standard whole-body plethysmography was used as described previously to measure VE, respiratory frequency (ƒR), tidal volume (VT) and oxygen consumption (VO2) in awake Pet1/DTR and WT mice of both sexes (Hodges and Richerson, 2008b, Hodges et al., 2008). All experiments were performed in the daytime (light) period and studies before and after DT administration were conducted at the same time of day for each mouse. VT was calibrated by injecting 300 μl of air into the plethysmography at a rate of 100 strokes/min using a mechanical ventilator (Minivent; Harvard Apparatus; Holliston, MA). The mice were monitored during more than 30 min in normoxia (21% O2- balance N2), 10 min in 5% hypercapnia (5% CO2-21% O2- balance N2), 10 min in 7% hypercapnia (7% CO2-21% O2- balance N2), 10 min in hyperoxic hypercapnia (7% CO2 −50% O2-balance N2), 20 min in normoxia and 10 min in hypoxia (10% O2- balance N2). TB was measured using a telemetric temperature probe (IPTT 6007) subcutaneously implanted between the scapulae under isoflurane anesthesia at least 5 days before the first experiment. Data were analyzed from the last 5 min of exposure to each gas using Matlab software off-line (The MathWorks; Natick, MA). Data were only analyzed during periods of quiet wakefulness when mice were not sleeping, walking, running, grooming, licking, eating or sniffing, and when the eyes were open.
Quantification of changes in ventilation
The ventilatory responses to changes in O2 and CO2 were quantified in three separate ways. 1) VE during the stimulus was calculated as a percentage of baseline VE. 2) The change in VE induced by the stimulus (in ml/min/gm) was the difference between VE during a stimulus and VE at baseline. 3) The mean value of VE during a stimulus was also compared to the mean value of VE at baseline. The same methods were used to compare changes of other parameters as well.
Statistics
Data are presented as mean values ± SEM. The threshold for statistical significance was set to p < 0.05 and determined by one-way or two-way ANOVA followed by Bonferroni’s multiple comparison test (Prism 4, GraphPad Software).
Results
DT decreased the number of TpOH-ir neurons in Pet1/DTR mice
DT injections resulted in a much smaller number of TpOH-ir neurons in Pet1/DTR mice (Fig. 1B). We tested three protocols: 1) DT 5 μg/kg i.p. 2 times daily for 2–4 weeks; 2) DT 50 μg/kg i.p. 1–2 times per week for 1–3 weeks, or; 3) DT 250 μg/kg i.p. 1–2 times per week for 1–2 weeks. The protocols using DTT from 2 to 35 μg all resulted in a decrease in the number of 5-HT neurons in each of the four nuclei studied (Fig. 1C); however, non-specific effects were seen when the higher doses of DT were administrated as a single injection, or when they were administered as 2 or 3 split doses. When the highest levels of DTT were given, undesirable effects occurred in Pet1/DTR mice, and surprisingly also in WT mice. In these cases, mice of both genotypes appeared sick, lost weight and had an increase in mortality. The protocol least likely to show nonspecific effects was 50 μg/kg DT once a week for 2–3 weeks (1.5 – 2 μg DTT). More than three injections at that dose or three injections at a higher dose caused an increase in mortality of both Pet1/DTR and WT mice. The mortality of Pet1/DTR mice was less than 10% with a DTT of 4 μg (n = 14), and increased progressively with DTT, being 23% and 57% with a DTT of 15 μg and 25 μg, respectively. The mortality of WT mice was 20% with a DTT of 30 μg (n = 10). No WT mice died after DTT less than 30 μg.
When data were pooled from animals with DTT injections between 2 and 35 μg it was found that there was a significant reduction in TpOH-ir neurons in Pet1/DTR mice by 85% in ROb, 83% in RMg, 77% in RPa and 81% in VLM (Fig. 1D). The number of TpOH-ir neurons present in Pet1/DTR mice (n = 17) was 2.5 ± 0.5, 2.8 ± 0.6, 1.8 ± 0.4 and 1.3 ± 0.3 per 25 μm thick slice in the ROb, RMg, RPa and VLM, respectively. In comparison, there were 16.7 ± 0.4, 16.6 ± 0.6, 7.7 ± 0.3 and 7.0 ± 0.2 TpOH-ir neurons/25 μm thick slice in WT mice injected with DT (n = 13, p < 0.0001). There were no differences in the numbers of TpOH-ir neurons between WT mice injected with DT versus Pet1/DTR mice that did not receive injections (n = 5; fig 1D). The highest doses of DT led to high mortality in Pet1/DTR mice as well as in WT mice, making it difficult to collect data from mice given the highest doses.
DT decreased the total number of neurons present in the raphe nuclei of Pet1/DTR mice
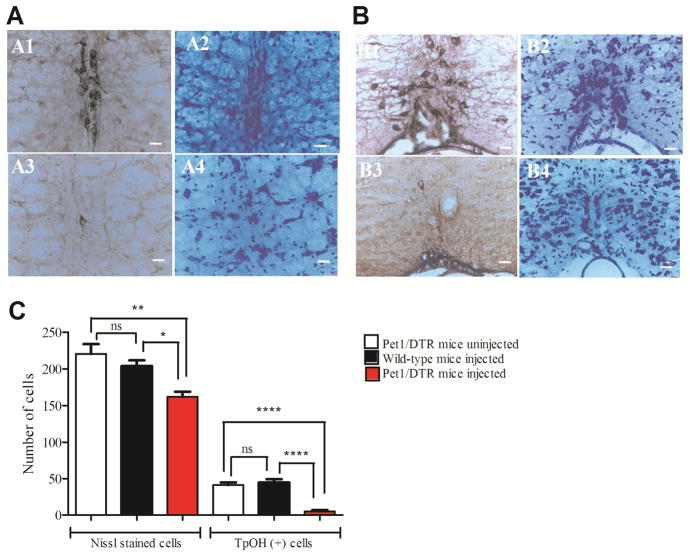
The mechanism of action of DT/DTR has been well-defined as causing cell death due to prevention of protein synthesis (Collier, 2001). However, it is theoretically possible that some neurons were still alive after our treatment, but their protein synthesis was so impaired that TpOH levels were too low to be detected using immunohistochemistry. Therefore, we examined whether the decrease in TpOH immunoreactivity after DT treatment was associated with a decrease in the total number of neurons in the medullary raphe. Nissl staining was performed on sections from the same brains that were used for immunohistochemistry. Photomicrographs of the ROb and RPa showed fewer Nissl stained cells in Pet1/DTR mice than in WT mice after both were treated with DT (Fig. 2A and B). We counted cells within 100 μm of the midline in sections from untreated Pet1/DTR mice (220 ± 13, n=5), DT treated WT mice (204 ± 8, n=5) and DT treated Pet1/DTR mice (162 ± 7, n=8) (Fig. 2C). There were many neurons in this region that were not serotonergic, but the average reduction in the number of Nissl stained cells was nearly equal to the average reduction in the number of TpOH-ir neurons, consistent with the reduction in TpOH-ir neurons being due to death of 5-HT neurons.
Figure 2. Nissl staining demonstrated that systemic DT caused death of neurons in the raphe nuclei of the medulla.
A & B: Photomicrographs of ROb (A) and RPa (B) showed fewer TpOH-ir neurons (A3 & B3) and Nissl stained cells (A4 & B4) in Pet1/DTR mice after systemic treatment with DT than in WT mice after the same treatment (A1, A2, B1 & B2). White bars 20 μm. C: Cells were counted within 100 μm of the midline in untreated Pet1/DTR mice (220 ± 13, n=5), WT mice treated with DT (204 ± 8, n=5) and Pet1/DTR mice treated with DT (162 ± 7, n=8). *, ** and **** p < 0.05, 0.01 and 0.0001 respectively. 1 way ANOVA, Bonferroni’s multiple comparison test.
Baseline ventilation and chemoreception were not affected by treatment with DT
There were no differences in resting ventilation between untreated male or female Pet1/DTR mice or WT mice (Table 1). There were no effects on VE, VO2, VT or ƒR after 4 μg DTT under baseline conditions in normoxia (Table 1). It was not possible to exceed that dose of DT without inducing non-specific effects such as a drop in temperature and death in WT mice, and yet it was ineffective in eliminating all serotonin neurons in Pet1/DTR mice, in that 20% of the TpOH-ir neurons were spared after that dose or larger doses (n = 17) (Fig. 1C and D).
Table 1.
Ventilation and oxygen consumption in room air at baseline and after 4 μg DTT for WT and Pet1/DTR mice.
| VO2 (ml min−1 g−1) | VE (ml min−1 g−1) | VT (ml g−1) | fR (min−1) | |
|---|---|---|---|---|
| Male WT | ||||
| Baseline | 0.056 ± 0.004 | 2.44 ± 0.32 | 0.015 ± 0.001 | 157 ± 12 |
| Post DT | 0.044 ± 0.005 | 2.11 ± 0.22 | 0.014 ± 0.001 | 153 ± 14 |
| Female WT | ||||
| Baseline | 0.047 ± 0.005 | 2.18 ± 0.14 | 0.016 ± 0.001 | 134 ± 9 |
| Post DT | 0.045 ± 0.004 | 2.38 ± 0.39 | 0.017 ± 0.002 | 140 ± 14 |
| Male Pet1/DTR | ||||
| Baseline | 0.056 ± 0.004 | 1.98 ± 0.13 | 0.013 ± 0.001 | 149 ± 6 |
| Post DT | 0.041 ± 0.004 | 1.94 ± 0.16 | 0.014 ± 0.001 | 141 ± 7 |
| Female Pet1/DTR | ||||
| Baseline | 0.058 ± 0.003 | 2.45 ± 0.18 | 0.016 ± 0.001 | 143 ± 4 |
| Post DT | 0.049 ± 0.003 | 2.09 ± 0.09 | 0.016 ± 0.001 | 134 ± 8 |
DTT, total amount of diphtheria toxin. Values are means ± SEM. VO2, oxygen consumption; VE, minute ventilation; VT, tidal volume, and; fR, respiratory frequency.
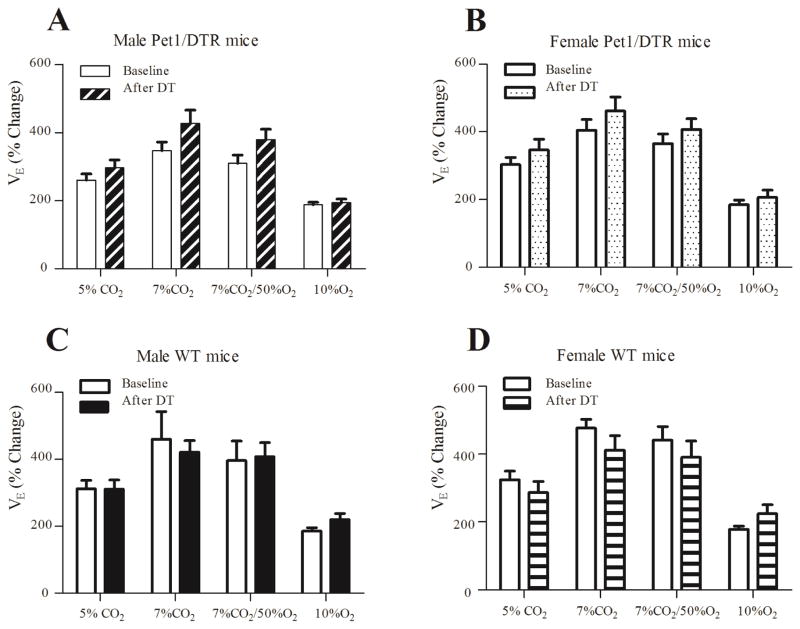
The ventilatory responses to chemoreceptor stimuli were also tested, and the responses were quantified using three different methods (see Methods). The absolute values of ventilation before and during hypoxia, hypercapnia and hyperoxic hypercapnia are shown in table 2 for the different groups before DT. There was no difference between groups for any of the measures. We also found no effect on changes in VE (Fig. 3; measured as percentage increase or the absolute change) or other ventilatory parameters (data not shown) in response to hypoxia, hypercapnia or hyperoxic hypercapnia after DT treatment among male or female Pet1/DTR mice (n = 12 each) in comparison to male or female WT mice (n = 7 each).
Table 2.
Baseline ventilatory response to hypercapnia or hypoxia for WT and Pet1/DTR mice.
| VO2 (ml min−1 g−1) | VE (ml min−1 g−1) | VT (ml g−1) | fR (min−1) | |
|---|---|---|---|---|
| Male WT | ||||
| 21% O2/5% CO2 | 0.049 ± 0.003 | 7.29 ± 0.62 | 0.024 ± 0.002 | 297 ± 9 |
| 21% O2/7% CO2 | 0.043 ± 0.002 | 10.10 ± 0.99 | 0.031 ± 0.003 | 325 ± 6 |
| 50% O2/7% CO2 | 0.034 ± 0.008 | 9.00 ± 1.02 | 0.029 ± 0.001 | 303 ± 9 |
| 10% O2/0% CO2 | 0.064 ± 0.007 | 3.79 ± 0.50 | 0.015 ± 0.001 | 248 ± 14 |
| Female WT | ||||
| 21% O2/5% CO2 | 0.039 ± 0.003 | 7.05 ± 0.64 | 0.028 ± 0.002 | 248 ± 7 |
| 21% O2/7% CO2 | 0.034 ± 0.004 | 10.27 ± 0.48 | 0.035 ± 0.002 | 292 ± 5 |
| 50% O2/7% CO2 | 0.030 ± 0.006 | 9.56 ± 0.93 | 0.034 ± 0.003 | 280 ± 6 |
| 10% O2/0% CO2 | 0.058 ± 0.004 | 3.81 ± 0.56 | 0.017 ± 0.002 | 223 ± 12 |
| Male Pet1/DTR | ||||
| 21% O2/5% CO2 | 0.049 ± 0.003 | 5.15 ± 0.47 | 0.019 ± 0.002 | 264 ± 6 |
| 21% O2/7% CO2 | 0.043 ± 0.003 | 6.69 ± 0.50 | 0.023 ± 0.002 | 291 ± 5 |
| 50% O2/7% CO2 | 0.045 ± 0.009 | 5.99 ± 0.46 | 0.022 ± 0.002 | 272 ± 5 |
| 10% O2/0% CO2 | 0.060 ± 0.006 | 2.90 ± 0.17 | 0.013 ± 0.001 | 225 ± 6 |
| Female Pet1/DTR | ||||
| 21% O2/5% CO2 | 0.049 ± 0.002 | 7.12 ± 0.38 | 0.027 ± 0.001 | 265 ± 8 |
| 21% O2/7% CO2 | 0.044 ± 0.002 | 9.44 ± 0.56 | 0.032 ± 0.002 | 294 ± 9 |
| 50% O2/7% CO2 | 0.055 ± 0.010 | 8.53 ± 0.53 | 0.031 ± 0.001 | 274 ± 9 |
| 10% O2/0% CO2 | 0.056 ± 0.006 | 3.44 ± 0.23 | 0.016 ± 0.001 | 217 ± 7 |
Values are means ± SEM. VO2, oxygen consumption; VE, minute ventilation; VT, tidal volume, and; fR, respiratory frequency.
Figure 3. The responses to hypercapnia and hypoxia were not affected after 80–85% of 5-HT neurons were deleted.
After two injections of DT (4 μg DTT), there was no effect on VE at baseline or in response to 5% and 7% hypercapnia, hypercapnia-hyperoxia or hypoxia. A: Pet1/DTR male mice. B: Pet1/DTR female mice. C: WT male mice. D: WT female mice. 1 way ANOVA, Bonferroni’s multiple comparison test.
Since Pet1/DTR mice decreased their TB after administration of DT it was necessary to raise TA in the plethysmography chamber from 30°C to 33 – 34°C in order to maintain a normal TB of Pet1/DTR mice. Before DT administration, TB during plethysmography for male and female Pet1/DTR mice was 37.5 ± 0.2°C (n=12) and 37.4 ± 0.2°C (n=12), respectively. After DT administration, with TA maintained at 33 – 34°C, TB during plethysmography was 36.8 ± 0.2°C (n=7) and 37.3 ± 0.1°C (n=7), for male and female Pet1/DTR mice, respectively.
DT caused male Pet1/DTR mice to be unable to maintain normal TB
Male Pet1/DTR mice housed at TA of 24°C dropped their TB below 35°C after one injection of DT at 50 μg/kg. A single injection of DT at 50 μg/kg caused TB of Pet1/DTR male mice (n = 9) to decrease from 37.4 ± 0.20°C to 34.8 ± 0.26°C (p = 0.02). After the first injection, animals were housed at TA of 30°C so they were able to maintain TB near normal, during which a second injection caused TB to decrease to 32.6 ± 0.59 °C (p < 0.0001). Neither of these two injections at 50 μg/kg had any effect on TB of male WT mice (n = 9). TB of male WT mice was 37.62 ± 0.23°C at baseline, 37.5 ± 0.19°C after the first DT injection and 37.3 ± 0.20°C after the second DT injection.
Acute deletion of 5-HT neurons affected thermoregulation in males more than in females
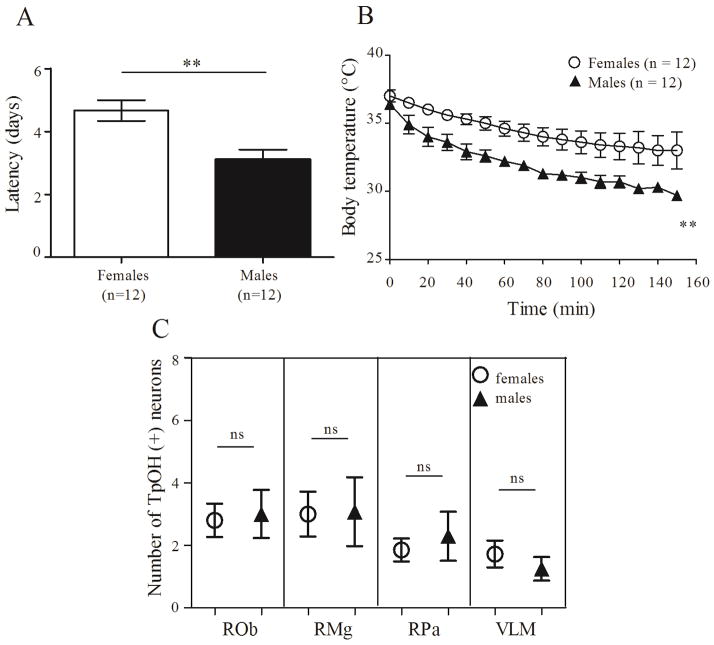
We measured the latency from the first DT injection (50 μg/kg i.p.) to when TB dropped below 35°C. As shown in Figure 4A, the latency in female Pet1/DTR mice (4.67 ± 0.33 days; n = 9) was longer than that in male Pet1/DTR mice (3.11 ± 0.31 days; n = 9; P < 0.001). After DT injections, if TB dropped below 35°C, TA was increased from 30°C first to 32°C, then 34°C and then 36.5°C, reaching the highest of these temperatures needed to maintain TB at ≈37°C.
Figure 4. Deletion of 5-HT neurons in adult Pet1/DTR mice affects thermoregulation in males more than in females.
A: Latency from the first injection of DT at 50 μg/kg to the time that TB dropped below 35°C. The latency in female Pet1/DTR mice was longer than in male Pet1/DTR mice (**, p = 0.0035, t = 3.421 and df = 16, t test). B: Body temperature dropped more in male than in female Pet1/DTR mice when TA was changed from 37°C to 24°C (****, p < 0.001, F = 110.9. 2 way ANOVA). C: There were no gender differences in the number of TpOH-ir neurons in ROb, RMg, RPa or VLM from Pet1/DTR mice (n=6 females and n=7 males. 1 way ANOVA, Bonferroni’s multiple comparison test).
Thermoregulation was then tested by decreasing TA from 36.5°C to 24°C and measuring changes in TB (Fig. 4B). Over a period of 2.5 hr, TB of male mice decreased to 29.7 ± 0.31°C (n=9), whereas that of females only decreased to 33.0 ± 1.34°C (n=9; p < 0.001). In contrast to this differential effect of 5-HT neuron deletion on thermoregulation in female vs male mice, there were no gender differences in the number of TpOH immunoreactive neurons between female Pet1/DTR mice (n = 6) and male Pet1/DTR mice (n = 7) after DT injections in any region studied (Fig. 4C).
Discussion
DTR mice were used as a genetic tool for temporal control of 5-HT neuron deletion
Acute deletion of 5-HT neurons allowed us to test their role in brain function, without compensation that often occurs with conventional knockout mice. These data show that 20% of central 5-HT neurons are capable of maintaining normal breathing in room air and a normal response to hypercapnia and hypoxia in adult Pet1/DTR mice. However, after 80% of central 5-HT neurons were lesioned with DT injections, mice were not able to maintain TB within a normal range, even when housed at an ambient temperature of 32 °C. 5-HT neurons were found to play a greater role in regulating TB in males than in females since there was a faster and larger fall in TB in males than in females after DT injection.
DT treatment had no effect on baseline breathing or chemoreception
The baseline ventilatory parameters presented here were consistent with previous data of fR, VT and VE in a range of 180 – 225 breath min−1, 8 – 15 μl g−1 and 1.5 – 3.5 ml min−1 g−1, respectively (Morin-Surun et al., 2001, Li and Nattie, 2008, Hodges et al., 2011). The 5-HT system is involved in respiratory drive and chemoreception (Dekin et al., 1985, Richerson, 1995, Wang et al., 1998, Wang and Richerson, 1999, Wang et al., 2001, Pena and Ramirez, 2002, Feldman et al., 2003, Nattie et al., 2004, Hodges and Richerson, 2008a, Hodges et al., 2008, Li and Nattie, 2008, Corcoran et al., 2009, Hodges et al., 2009, Ptak et al., 2009, Hodges and Richerson, 2010, Niebert et al., 2011, Ray et al., 2011). Nevertheless, we did not observe any significant change in breathing in normoxia, hypercapnia or hypoxia between male or female mice after deletion of 5-HT neurons with systemic administration of DT. The chemosensory response to CO2 and O2 of Pet1/DTR mice was similar to that previously observed in mice (Morin-Surun et al., 2001, Hodges et al., 2008, Li and Nattie, 2008, Ray et al., 2011). It is possible that this technique may lead to a result that is hard to interpret, because of the nonspecific effects and increased mortality with the highest doses of DT (Saito et al., 2001, Luquet et al., 2005, Bennett and Clausen, 2007). Unlike other genetically modified mice affecting the 5-HT system that are able to compensate during development, such as the Lmx1bf/f/p, 5-HTT knockout and Pet1 knockout mice (Gobbi et al., 2001, Hodges et al., 2008, Li and Nattie, 2008, Hodges et al., 2009, Cummings et al., 2010, Hodges et al., 2011), Pet1/DTR mice develop normally. However, the response of adult Pet1/DTR mice also differs from adult RC::PDi mice, which had a reduced response to CO2 after acute silencing of 5-HT neurons after normal development (Ray et al., 2011). One possibility for the difference is that a larger percentage of 5-HT neurons might have been affected in RC::PDi mice, but there are no data available on what percentage of 5-HT neurons are inhibited by CNO in that model. Another possibility is that the delay between DT treatment and loss of TpOH-ir neurons of a few days is long enough for there to be compensation, whereas the acute inhibition of 5-HT neurons in RC:PDi mice occurs so quickly that compensation does not occur. A third possibility is that the subset of 5-HT neurons that are spared in Pet1/DTR mice after DT treatment are a different subset than those that are unaffected by CNO in RC:PDi mice. This is feasible because DTR expression is driven by the enhancer region of Pet-1 in Pet1/DTR mice, whereas expression of Di is driven by the promoter for the 5-HTT in RC:PDi mice. The neurons affected by these two genetic influence may only be partially overlapping. In fact, the 30% of 5-HT neurons that are spared in Pet-1 knockout mice have somata that are distributed across the B1–B9 cell groups, but have projections to only a selective subset of brain regions, including the nucleus tractus solitarius, nucleus ambiguus, and ventrolateral medulla (Kiyasova et al., 2011), each of which is involved in breathing. This could explain why breathing is much less affected in Pet-1 knockout mice than in Lmx1bf/f/p mice even though 70% of 5-HT neurons are deleted in the former. This relatively lower dependence on Pet-1 of 5-HT neurons that project to respiratory nuclei may mean they express DTRs at a lower level than other 5-HT neurons, so that the effect of 5-HT neurons on breathing is relatively normal in Pet1/DTR mice after DT treatment.
Serotonergic mechanisms involved in thermoregulation
Pet1/DTR and RC::PDi mice both exhibited a decrease in TB. In RC::PDi mice the fall in TB occurred immediately after central 5-HT neurons were silenced, whereas in Pet1/DTR mice the fall in TB occurred over time in accordance with the time it took for 5-HT neurons to be deleted by the toxin. The data presented here support the hypothesis that the 5-HT system is required for normal thermoregulation. Several areas of the hypothalamus have been implicated as important thermosensitive regions that could evoke physiological responses (Adair, 1977, Boulant, 2006). Interactions between the hypothalamus and 5-HT system can explain why a decrease in 5-HT neurons produces abnormal regulation of TB. For example, there are serotonergic fibers and terminals throughout the hypothalamus, as well as 5-HT2C and 5-HT1B receptors (Steinbusch, 1981, Mirkes and Bethea, 2001, Makarenko et al., 2002). In addition, the RPa interacts with descending hypothalamic drive to control generation of heat by brown adipose tissue (BAT) (Madden and Morrison, 2010, Hale et al., 2011, Morrison and Nakamura, 2011), and there are direct projections to the spinal cord which help regulate the shivering response to cold (Cano et al., 2003). In fact, genetic deletion of 5-HT neurons in Lmx1bf/f/p mice leads to severely impaired BAT thermogenesis and abnormal shivering. Recently, it has been shown that there is potent modulation of BAT thermogenesis by orexin released from the lateral hypothalamus directly into the RPa (Tupone et al., 2011). Thus, there are several anatomical sites where dysfunction of 5-HT neurons could interfere with temperature sensation, BAT thermogenesis and shivering and lead to abnormal regulation of body temperature.
In contrast to the severe deficit seen here in adult Pet1/DTR mice after deletion of 80% of 5-HT neurons, there is a much less severe defect in adult Lmx1bf/f/p mice even though they lack ~100% of central 5-HT neurons, in Pet-1 null mice that lack ~70% of central 5-HT neurons and in 5-HTT knockout mice (Hodges et al., 2008, Li and Nattie, 2008, Hodges et al., 2011). The latter three models all maintain a normal TB when TA is at normal room temperature. One possible reason for the difference is that the brain in the latter three transgenic mouse lines may have compensated during development for the lack of normal 5-HT system function.
There is a decrease in number of 5-HT neurons in Pet1/DTR mice, Lmx1bf/f/p mice and Pet-1 null mice, presumably leading to a decrease in brain extracellular 5-HT levels, whereas 5-HTT knockout mice likely have an excess of brain extracellular 5-HT levels, and yet all four mouse lines have abnormal thermoregulation. The excess of 5-HT in 5-HTT knockout mice might lead to an increase in autoinhibition of 5-HT neurons due to activation of 5-HT1A inhibitory autoreceptors. This could explain why 5-HT neurons show reduced firing rate (Gobbi et al., 2001), as well as a reduction in the density, expression and desensitization of 5-HT1A receptors (Li et al., 2000, Bouali et al., 2003). Although this may seem paradoxical, treatment by 5-HTT blockers has been shown to cause such changes both in vivo (Gartside et al., 1995, Romero et al., 2003) and in brain slices (Evans et al., 2008). These papers show that even though SSRIs cause a decrease in firing rate of 5-HT neurons, there is still an increase in extracellular [5-HT]. The sustained increase in extracellular [5-HT] could lead to desensitization of some downstream receptors. The decrease in firing rate of 5-HT neurons could reduce the release of co-localized neuropeptide neurotransmitters. In both cases, the functional effects would be similar to those that occur with a decrease in the number of 5-HT neurons.
It is possible that compensatory changes occur in Lmx1bf/f/p mice, Pet-1 null mice and 5-HTT knockout mice, and may reduce the severity of the phenotypes resulting from these different defects in the 5-HT system. Compensatory changes could also occur in other neurotransmitter systems, such as norepinephrine or acetylcholine, to make up for the missing serotonin. If compensatory changes do occur in mice with a deficient 5-HT system, these changes might be expected to be greatest in the Lmx1bf/f/p mice since they have the most radical defect – a complete absence of central 5-HT neurons. Those compensatory changes could explain why Lmx1bf/f/p mice have normal TB at 22°C. However, there is no compensation by chronic metabolic acid/base changes, because arterial blood gases and pH are not different between Lmx1bf/f/p and WT mice (Hodges et al., 2008). There are also no changes in norepinephrine or dopamine levels in Lmx1bf/f/p mice, indicating that neither of these two systems have compensated for the absence of 5-HT (Zhao et al., 2006). There still remains the possibility that compensation occurs in other systems such as the cholinergic or glutamatergic systems. Further studies at different ages during development may define compensatory mechanisms in Lmx1bf/f/p and other transgenic mice, so that the magnitude of the contribution of the 5-HT system to thermoregulation, ventilation and chemoreception can be determined more accurately.
Thermoregulation is more dependent on 5-HT neurons in male than in female mice
Gender differences have been reported in rats for ventilatory control including the hypercapnic ventilatory response (HCVR), as well as ethanol-induced hypothermia and circadian temperature (Taylor et al., 2006, Behan and Wenninger, 2008, Taylor et al., 2009, Wenninger et al., 2009). Therefore, many respiratory and thermoregulatory studies have focused only on males to eliminate differences between the two genders as a confounding factor. Some studies have linked an altered 5-HT system with gender differences in ventilation or thermoregulation (Li and Nattie, 2008, Hodges et al., 2011). Here we found further evidence of gender differences in regulation of brain function by the 5-HT system as DT treatment has different effects on TB in males than in females. These findings suggest that 5-HT neurons are more important for thermoregulation in males compared to females. Several lines of evidence suggest possible interactions between sex hormones and the 5-HT system. It is possible that 5-HT neuron activity could be regulated by sex hormones, which could be acting directly on progestin receptor or estrogen receptor beta in several raphe nuclei (Bethea, 1993, Gundlah et al., 2001). Furthermore, ovarian steroids enhance serotonin neuron function by causing an increase in transcription and translation of TPH-2 and 5-HTT, and a decrease of 5HT1A receptors and monoamine oxidase A (Lu and Bethea, 2002, Lu et al., 2003, Sanchez et al., 2005, Tokuyama et al., 2008, Bethea et al., 2009). These changes could explain, in part, why the females in our study were less affected than males even though they had the same number of remaining 5-HT neurons. Moreover, convergence of hormones and 5-HT in the hypothalamus and release of different hormones from the hypothalamus after stimulation of 5-HT1A, 5-HT2A and 5-HT2C receptors has been shown (Bluet Pajot et al., 1995, Bagdy, 1996, Mirkes and Bethea, 2001), pointing out that not only is 5-HT activity regulated by sex hormones, but 5-HT neurons may also regulate hypothalamic activity, which is involved in homeostatic processes including hormonal release and thermoregulation.
Off target effects prevented deletion of more than 80–85% of 5-HT neurons
We expected that Pet1/DTR mice would express DTRs selectively in all 5-HT neurons, because the Pet-1 enhancer region causes Cre recombinase expression almost exclusively in 5-HT neurons (Scott et al., 2005), and Pet-1 is thought to be expressed selectively in all 5-HT neurons both during development and in adults (Hendricks et al., 1999). Even if Pet-1 was only expressed transiently during development that should have resulted in transient Cre recombinase expression, and thus permanent DTR expression. The DTR transgene should have only been expressed in cells that expressed Cre recombinase at some time in their life (Buch et al., 2005), so it should not have been expressed in cells other than 5-HT neurons.
DT injections at low doses impaired thermoregulation of Pet1/DTR mice but not that of WT mice. At these doses there was not any difference in baseline ventilation or in the ventilatory response to hypercapnia or hypoxia between Pet1/DTR and WT mice. As the dose was increased, nonspecific effects confounded the use of this genetic approach, because when doses were used that were high enough to lesion more than 85% of 5-HT neurons, defects in thermoregulation were induced in WT mice, as well as an increase in their mortality.
In this study, male and female mice both retained approximately 15% of 5-HT neurons in ROb, 17% in RMg, 23% in RPa and 19% in VLM. We did not find a difference in the effect of DT on survival of 5-HT neurons between different nuclei of the raphe. It was not determined whether the surviving 5-HT neurons were a subset characterized by specific projections, as has been observed in Pet-1 knockout mice (Kiyasova et al., 2011). As described above, the loss of 5-HT neuron cell bodies is distributed across many different raphe nuclei, but 5-HT innervation is preserved in medullary nuclei involved in control of breathing. Projections are also preserved to a variety of brain areas involved in stress responses, with dense innervation of the basolateral amygdala, the paraventricular nucleus of the hypothalamus, the intralaminar thalamic nuclei, the ventral part of the periaqueductal gray, and the parabrachial nucleus (Kiyasova et al., 2011). Defining whether the neurons that survived in Pet1/DTR mice after DT treatment have a specific phenotype (possibly the same subset as those that survive in Pet-1 knockouts) might contribute to understanding why there was a large effect on TB and not on breathing. It is possible that a subset of 5-HT neurons are thermosensitive and may reinforce the mechanisms of thermoregulatory control by the hypothalamus or modulate descending synaptic drive from the hypothalamus to effector organs involved in thermogenesis. It is already known that stimulation of 5-HT1A, 5-HT3 and 5-HT7 receptors can lead to hypothermia, and the mechanisms involved differ(Popova et al., 2008, Naumenko et al., 2009, 2011). However, it remains unknown how 5-HT neurons contribute to TB regulation and the underlying mechanisms for hypothermia produced by failure of the 5-HT system.
Conclusions
The data presented here confirm the importance of the 5-HT system in thermoregulation and demonstrate that failure of the 5-HT system affects the body temperature in males more than females. Acute deletion of 5-HT neurons by DT treatment of adult Pet1/DTR mice leads to a greater defect in thermoregulation than when 5-HT neurons are prevented from developing during embryogenesis (e.g. Lmx1bf/f/p and Pet-1 knockouts), pointing to the potential for significant compensation in the thermoregulatory system.
Highlights.
Pet1/DTR mice express diphtheria toxin receptors on serotonin neurons
Systemic diphtheria toxin causes loss of 80% of serotonin neurons
Ventilation was not affected after deletion of 80% of central serotonin neurons
Diphtheria toxin did cause a severe defect in thermogenesis
5-HT neurons that were spared may be those that are important for chemoreception
Acknowledgments
The authors wish to thank Xiuqiong Zhou for technical assistance. Research was supported by the NIH 2U54NS041069, P01HD36379, P20NS076916 and R01HD052772, by the VAMC and the Beth L. Tross Epilepsy Research Fund.
Footnotes
Author contributions: V.C. and G.B.R. designed the experiments; V.C and A.G. performed the experiments; V.C. analyzed the data; V.C. and G.B.R. wrote the paper.
We declare that we have read and abided by the statement of ethical standards for manuscripts submitted to Neuroscience.
No conflicts of interest, financial or otherwise, are declared by the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adair ER. Skin, preoptic, and core temperatures influence behavioral thermoregulation. J Appl Physiol Respir Environ Exerc Physiol. 1977;42:559–564. doi: 10.1152/jappl.1977.42.4.559. [DOI] [PubMed] [Google Scholar]
- Bagdy G. Role of the hypothalamic paraventricular nucleus in 5-HT1A, 5-HT2A and 5-HT2C receptor-mediated oxytocin, prolactin and ACTH/corticosterone responses. Behav Brain Res. 1996;73:277–280. doi: 10.1016/0166-4328(96)00112-x. [DOI] [PubMed] [Google Scholar]
- Behan M, Wenninger JM. Sex steroidal hormones and respiratory control. Respir Physiol Neurobiol. 2008;164:213–221. doi: 10.1016/j.resp.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CL, Clausen BE. DC ablation in mice: promises, pitfalls, and challenges. Trends Immunol. 2007;28:525–531. doi: 10.1016/j.it.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Bethea CL. Colocalization of progestin receptors with serotonin in raphe neurons of macaque. Neuroendocrinology. 1993;57:1–6. doi: 10.1159/000126334. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP, Tokuyama Y, Henderson JA, Lima FB. Protective actions of ovarian hormones in the serotonin system of macaques. Front Neuroendocrinol. 2009;30:212–238. doi: 10.1016/j.yfrne.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluet Pajot MT, Mounier F, di Sciullo A, Schmidt B, Kordon C. Differential sites of action of 8OHDPAT, a 5HT1A agonist, on ACTH and PRL secretion in the rat. Neuroendocrinology. 1995;61:159–166. doi: 10.1159/000126836. [DOI] [PubMed] [Google Scholar]
- Bouali S, Evrard A, Chastanet M, Lesch KP, Hamon M, Adrien J. Sex hormone-dependent desensitization of 5-HT1A autoreceptors in knockout mice deficient in the 5-HT transporter. Eur J Neurosci. 2003;18:2203–2212. doi: 10.1046/j.1460-9568.2003.02960.x. [DOI] [PubMed] [Google Scholar]
- Boulant JA. Neuronal basis of Hammel’s model for set-point thermoregulation. J Appl Physiol (1985) 2006;100:1347–1354. doi: 10.1152/japplphysiol.01064.2005. [DOI] [PubMed] [Google Scholar]
- Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, Jung S, Waisman A. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods. 2005;2:419–426. doi: 10.1038/nmeth762. [DOI] [PubMed] [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- Cha JH, Chang MY, Richardson JA, Eidels L. Transgenic mice expressing the diphtheria toxin receptor are sensitive to the toxin. Mol Microbiol. 2003;49:235–240. doi: 10.1046/j.1365-2958.2003.03550.x. [DOI] [PubMed] [Google Scholar]
- Collier RJ. Understanding the mode of action of diphtheria toxin: a perspective on progress during the 20th century. Toxicon. 2001;39:1793–1803. doi: 10.1016/s0041-0101(01)00165-9. [DOI] [PubMed] [Google Scholar]
- Corcoran AE, Hodges MR, Wu Y, Wang W, Wylie CJ, Deneris ES, Richerson GB. Medullary serotonin neurons and central CO2 chemoreception. Respir Physiol Neurobiol. 2009;168:49–58. doi: 10.1016/j.resp.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KJ, Li A, Deneris ES, Nattie EE. Bradycardia in serotonin-deficient Pet-1−/− mice: influence of respiratory dysfunction and hyperthermia over the first 2 postnatal weeks. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1333–1342. doi: 10.1152/ajpregu.00110.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekin MS, Richerson GB, Getting PA. Thyrotropin-releasing hormone induces rhythmic bursting in neurons of the nucleus tractus solitarius. Science. 1985;229:67–69. doi: 10.1126/science.3925552. [DOI] [PubMed] [Google Scholar]
- Evans AK, Reinders N, Ashford KA, Christie IN, Wakerley JB, Lowry CA. Evidence for serotonin synthesis-dependent regulation of in vitro neuronal firing rates in the midbrain raphe complex. Eur J Pharmacol. 2008;590:136–149. doi: 10.1016/j.ejphar.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartside SE, Umbers V, Hajos M, Sharp T. Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: effects on 5-HT cell firing and extracellular 5-HT. Br J Pharmacol. 1995;115:1064–1070. doi: 10.1111/j.1476-5381.1995.tb15919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Murphy DL, Lesch K, Blier P. Modifications of the serotonergic system in mice lacking serotonin transporters: an in vivo electrophysiological study. J Pharmacol Exp Ther. 2001;296:987–995. [PubMed] [Google Scholar]
- Gundlah C, Lu NZ, Mirkes SJ, Bethea CL. Estrogen receptor beta (ERbeta) mRNA and protein in serotonin neurons of macaques. Brain Res Mol Brain Res. 2001;91:14–22. doi: 10.1016/s0169-328x(01)00108-5. [DOI] [PubMed] [Google Scholar]
- Hale MW, Dady KF, Evans AK, Lowry CA. Evidence for in vivo thermosensitivity of serotonergic neurons in the rat dorsal raphe nucleus and raphe pallidus nucleus implicated in thermoregulatory cooling. Exp Neurol. 2011;227:264–278. doi: 10.1016/j.expneurol.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Hendricks T, Francis N, Fyodorov D, Deneris ES. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J Neurosci. 1999;19:10348–10356. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Best S, Richerson GB. Altered ventilatory and thermoregulatory control in male and female adult Pet-1 null mice. Respir Physiol Neurobiol. 2011;177:133–140. doi: 10.1016/j.resp.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: neuromodulatory and trophic effects. Respir Physiol Neurobiol. 2008a;164:222–232. doi: 10.1016/j.resp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Interaction between defects in ventilatory and thermoregulatory control in mice lacking 5-HT neurons. Respir Physiol Neurobiol. 2008b;164:350–357. doi: 10.1016/j.resp.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. The role of medullary serotonin (5-HT) neurons in respiratory control: contributions to eupneic ventilation, CO2 chemoreception, and thermoregulation. J Appl Physiol. 2010;108:1425–1432. doi: 10.1152/japplphysiol.01270.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J Neurosci. 2009;29:10341–10349. doi: 10.1523/JNEUROSCI.1963-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyasova V, Fernandez SP, Laine J, Stankovski L, Muzerelle A, Doly S, Gaspar P. A genetically defined morphologically and functionally unique subset of 5-HT neurons in the mouse raphe nuclei. J Neurosci. 2011;31:2756–2768. doi: 10.1523/JNEUROSCI.4080-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Nattie E. Serotonin transporter knockout mice have a reduced ventilatory response to hypercapnia (predominantly in males) but not to hypoxia. J Physiol. 2008;586:2321–2329. doi: 10.1113/jphysiol.2008.152231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Lesch KP, Murphy DL. Reduction in the density and expression, but not G-protein coupling, of serotonin receptors (5-HT1A) in 5-HT transporter knock-out mice: gender and brain region differences. J Neurosci. 2000;20:7888–7895. doi: 10.1523/JNEUROSCI.20-21-07888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu NZ, Bethea CL. Ovarian steroid regulation of 5-HT1A receptor binding and G protein activation in female monkeys. Neuropsychopharmacology. 2002;27:12–24. doi: 10.1016/S0893-133X(01)00423-7. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Eshleman AJ, Janowsky A, Bethea CL. Ovarian steroid regulation of serotonin reuptake transporter (SERT) binding, distribution, and function in female macaques. Mol Psychiatry. 2003;8:353–360. doi: 10.1038/sj.mp.4001243. [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Endogenous activation of spinal 5-hydroxytryptamine (5-HT) receptors contributes to the thermoregulatory activation of brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2010;298:R776–783. doi: 10.1152/ajpregu.00614.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarenko IG, Meguid MM, Ugrumov MV. Distribution of serotonin 5-hydroxytriptamine 1B (5-HT(1B)) receptors in the normal rat hypothalamus. Neurosci Lett. 2002;328:155–159. doi: 10.1016/s0304-3940(02)00345-2. [DOI] [PubMed] [Google Scholar]
- Martin-Cora FJ, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic medullary and pontine raphe neurons to environmental cooling in freely moving cats. Neuroscience. 2000;98:301–309. doi: 10.1016/s0306-4522(00)00133-0. [DOI] [PubMed] [Google Scholar]
- Mirkes SJ, Bethea CL. Oestrogen, progesterone and serotonin converge on GABAergic neurones in the monkey hypothalamus. J Neuroendocrinol. 2001;13:182–192. doi: 10.1046/j.1365-2826.2001.00612.x. [DOI] [PubMed] [Google Scholar]
- Morin-Surun MP, Boudinot E, Dubois C, Matthes HW, Kieffer BL, Denavit-Saubie M, Champagnat J, Foutz AS. Respiratory function in adult mice lacking the mu-opioid receptor: role of delta-receptors. Eur J Neurosci. 2001;13:1703–1710. doi: 10.1046/j.0953-816x.2001.01547.x. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 2014;19:741–756. doi: 10.1016/j.cmet.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci (Landmark Ed) 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskaug JO, Sletten K, Sandvig K, Olsnes S. Translocation of diphtheria toxin A-fragment to the cytosol. Role of the site of interfragment cleavage. J Biol Chem. 1989;264:15709–15713. [PubMed] [Google Scholar]
- Nattie EE, Li A, Richerson GB, Lappi DA. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol. 2004;556:235–253. doi: 10.1113/jphysiol.2003.059766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumenko VS, Kondaurova EM, Popova NK. Central 5-HT3 receptor-induced hypothermia in mice: interstrain differences and comparison with hypothermia mediated via 5-HT1A receptor. Neurosci Lett. 2009;465:50–54. doi: 10.1016/j.neulet.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Naumenko VS, Kondaurova EM, Popova NK. On the role of brain 5-HT7 receptor in the mechanism of hypothermia: comparison with hypothermia mediated via 5-HT1A and 5-HT3 receptor. Neuropharmacology. 2011;61:1360–1365. doi: 10.1016/j.neuropharm.2011.08.022. [DOI] [PubMed] [Google Scholar]
- Niebert M, Vogelgesang S, Koch UR, Bischoff AM, Kron M, Bock N, Manzke T. Expression and function of serotonin 2A and 2B receptors in the mammalian respiratory network. PLoS One. 2011;6:e21395. doi: 10.1371/journal.pone.0021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Behringer RR, Quaife CJ, Maxwell F, Maxwell IH, Brinster RL. Cell lineage ablation in transgenic mice by cell-specific expression of a toxin gene. Cell. 1987;50:435–443. doi: 10.1016/0092-8674(87)90497-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. New York, NY: Academic Press; 2001. [Google Scholar]
- Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova NK, Tibeikina MA, Amstislavskaya TG. Hypothermic effect of 5-HT1A receptor agonist: comparison of intranasal, intraperitoneal, and subcutaneous routes of administration. Bull Exp Biol Med. 2008;146:433–435. doi: 10.1007/s10517-009-0304-x. [DOI] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci. 2009;29:3720–3737. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB. Response to CO2 of neurons in the rostral ventral medulla in vitro. J Neurophysiol. 1995;73:933–944. doi: 10.1152/jn.1995.73.3.933. [DOI] [PubMed] [Google Scholar]
- Romero L, Celada P, Martin-Ruiz R, Diaz-Mataix L, Mourelle M, Delgadillo J, Hervas I, Artigas F. Modulation of serotonergic function in rat brain by VN2222, a serotonin reuptake inhibitor and 5-HT1A receptor agonist. Neuropsychopharmacology. 2003;28:445–456. doi: 10.1038/sj.npp.1300062. [DOI] [PubMed] [Google Scholar]
- Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, Mekada E, Kimata Y, Tsuru A, Kohno K. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol. 2001;19:746–750. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- Sanchez RL, Reddy AP, Centeno ML, Henderson JA, Bethea CL. A second tryptophan hydroxylase isoform, TPH-2 mRNA, is increased by ovarian steroids in the raphe region of macaques. Brain Res Mol Brain Res. 2005;135:194–203. doi: 10.1016/j.molbrainres.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, Jiang W, Conlon RA, Strowbridge BW, Deneris ES. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci USA. 2005;102:16472–16477. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Tio DL, Bando JK, Romeo HE, Prolo P. Differential effects of alcohol consumption and withdrawal on circadian temperature and activity rhythms in Sprague-Dawley, Lewis, and Fischer male and female rats. Alcohol Clin Exp Res. 2006;30:438–447. doi: 10.1111/j.1530-0277.2006.00048.x. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Tio DL, Bando JK, Truong AH, Prolo P. Sex differences in ethanol-induced hypothermia in ethanol-naive and ethanol-dependent/withdrawn rats. Alcohol Clin Exp Res. 2009;33:60–69. doi: 10.1111/j.1530-0277.2008.00811.x. [DOI] [PubMed] [Google Scholar]
- Tokuyama Y, Reddy AP, Bethea CL. Neuroprotective actions of ovarian hormones without insult in the raphe region of rhesus macaques. Neuroscience. 2008;154:720–731. doi: 10.1016/j.neuroscience.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupone D, Madden CJ, Cano G, Morrison SF. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J Neurosci. 2011;31:15944–15955. doi: 10.1523/JNEUROSCI.3909-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol. 1998;511 (Pt 2):433–450. doi: 10.1111/j.1469-7793.1998.433bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Richerson GB. Development of chemosensitivity of rat medullary raphe neurons. Neuroscience. 1999;90:1001–1011. doi: 10.1016/s0306-4522(98)00505-3. [DOI] [PubMed] [Google Scholar]
- Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol. 2001;85:2224–2235. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- Wenninger JM, Olson EB, Jr, Cotter CJ, Thomas CF, Behan M. Hypoxic and hypercapnic ventilatory responses in aging male vs. aging female rats. J Appl Physiol (1985) 2009;106:1522–1528. doi: 10.1152/japplphysiol.90802.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretsky DV, Zaretskaia MV, DiMicco JA. Stimulation and blockade of GABAA receptors in the raphe pallidus: effects on body temperature, heart rate, and blood pressure in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R110–116. doi: 10.1152/ajpregu.00016.2003. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau RWt, Johnson RL, Deneris ES, Chen ZF. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci. 2006;26:12781–12788. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]