Abstract
1. Organisms can respond to changing climatic conditions in multiple ways including changes in phenology, body size or morphology, and range shifts. Understanding how developmental temperatures affect insect life-history timing and morphology is crucial because body size and morphology affect multiple aspects of life history, including dispersal ability, while phenology can shape population performance and community interactions.
2. We experimentally assessed how developmental temperatures experienced by aquatic larvae affected survival, phenology, and adult morphology of dragonflies (Pachydiplax longipennis). Larvae were reared under 3 environmental temperatures: ambient, +2.5 °C, and +5 °C, corresponding to temperature projections for our study area 50 and 100 years in the future, respectively. Experimental temperature treatments tracked naturally-occurring variation.
3. We found clear effects of temperature in the rearing environment on survival and phenology: dragonflies reared at the highest temperatures had the lowest survival rates, and emerged from the larval stage approximately 3 weeks earlier than animals reared at ambient temperatures. There was no effect of rearing temperature on overall body size. Although neither the relative wing nor thorax size was affected by warming, a non-significant trend towards an interaction between sex and warming in relative thorax size suggests that males may be more sensitive to warming than females, a pattern that should be investigated further.
4. Warming strongly affected survival in the larval stage and the phenology of adult emergence. Understanding how warming in the developmental environment affects later life-history stages is critical to interpreting the consequences of warming for organismal performance.
Keywords: Pachydiplax, larvae, freshwater systems, Libellulidae, thermal performance
Introduction
Most habitats are experiencing increasing temperatures as a consequence of climate change (IPCC, 2007). Environmental warming affects the thermal regime experienced by the organisms that occupy these habitats, and developmental processes are likely to be especially sensitive to warming because multiple aspects of development are affected by temperature. Two of the three widely-recognized biological responses to climate change, phenological shifts (Parmesan, 2006; Forrest & Miller-Rushing, 2010) and reduced body size (Gardner et al., 2011; Sheridan & Bickford, 2011), can be directly affected by thermal influences on the rate and progress of development. Further, the combined effects of thermal influences on developmental processes could indirectly affect the third major biological response to climate change, range shifts, through effects of body size on dispersal ability (McCauley & Mabry, 2011). Understanding how environmental warming affects developmental timing, growth, and morphology can provide critical insights into how climate change will affect the performance of species.
Phenological shifts appear to be one of the most common responses to climate change across taxa, with many species having key spring life-history events (e.g. flowering, emergence from diapause) shifted forward seasonally (Parmesan, 2006; Diez et al., 2012). Phenology can have a range of effects on ecological processes, and phenological shifts may results in species being exposed to abiotic conditions which they have not previously experienced. For example, advanced phenologies can negatively affect species by exposing them to late season winter weather events (Inouye, 2008). When interacting species have different phenological responses to climate change, the nature and strength of those interactions may also be affected (Parmesan, 2006; Yang & Rudolf, 2010). Species that experience large phenological shifts may interact with a different suite of species, or with different developmental stages of species with which they have previous interacted, and these changes can alter the outcome of interspecific interactions (Yang & Rudolf, 2010). Species phenological patterns can arise from the effects of multiple cues interacting with genotypic and phenotypic differences among individuals within a population (Visser et al., 2010; Wilczek et al., 2010), but the increased pace of development in warmer environments is expected to be especially important for many ectotherms (Gillooly et al., 2002; Angilletta et al., 2004) including odonates (Doi, 2008). It has been estimated that 80% of metazoan animal species have complex life-cycles (Werner, 1988), and one of the most critical life-history events for these animals is the transition to the adult stage. Because many animals with complex life-cycles are also ectothermic, these animals are especially likely to exhibit changes in growth and developmental rates that alter the phenology of metamorphosis or transition to the adult stage.
The effects of temperature on developmental processes in ectotherms often have lasting consequences for adult body size, and declines in body size appear to be a general response to environmental warming across taxa (Gardner et al., 2011; Sheridan & Bickford, 2011). The dominant pattern in ectotherms is a negative relationship between developmental temperature and body size (a “hotter is smaller” rule; Atkinson, 1994, 1995; Kingsolver & Huey, 2008), although exceptions do exist. The mechanisms driving this pattern may vary between species and are still not completely understood for many groups, but accelerated development under warmer conditions is a critical component (Forster et al., 2011; Callier & Nijhout, 2013; Ghosh et al., 2013). Declining body sizes can affect diverse processes, from individual performance to energy transfers within ecosystems.
Absolute body size affects many aspects of performance, but allometry and patterns of morphological allocation also critically affect the ability of organisms to perform different functions. Studies examining responses of body size to temperature often use a single measure of body size rather than considering allometric variation in response to the thermal environment. However, in studies in which multiple aspects of morphology have been measured, differential responses across morphological features have been observed (Shingleton et al., 2009; Reiskind & Zarrabi, 2012). Organisms may respond adaptively by increasing allocation to structures related to dispersal when conditions in the developmental environment are sub-optimal; such modifications could be an adaptive response allowing escape from adverse conditions (Dingle, 1996; Zera & Denno, 1997). Alternatively, developmental constraints such as time limitations may change allometric patterns without increasing fitness (Tobler & Nijhout, 2010). Patterns of allocation to dispersal related morphology can generate maintenance costs (Dudley 2000) and generate trade-offs with other functions (Hill et al., 1999a,b; Karlsson & Johansson, 2008).
The effects of temperature on developmental timing, growth, and morphological allocation all affect individual performance, and may indirectly affect population-level processes. However, temperature can also directly affect populations by altering birth and death rates. For populations of reproductively immature organisms, birth rates are a potential future consequence of the carry-over effects of the developmental thermal environment. In addition, death rates in such populations are often high and can be influenced by factors that do not directly cause death, but can increase the risk of mortality in response to other mortality agents (e.g. McCauley et al., 2011). For example, both low and high temperatures can be stressors that make developing organisms more sensitive to nutritional deficits (Petter et al., 2014), disease or parasites (Fellowes et al., 1999; Thomas & Blanford, 2003), and other stressors (Janssens & Stoks, 2013; Kimberly & Salice, 2013; Rohr & Palmer, 2013). Further, these different stressors may also act synergistically to increase mortality risk (Darling & Côté, 2008).
We examined how environmental warming affects multiple life history and morphological traits in dragonflies (Odonata: Anisoptera), including larval survival, timing of metamorphosis to the adult stage, and adult body size and morphology. Adults emerging from this experiment were also individually marked and released - we attempted to resight these individuals to follow their dispersal behaviour and assess how larval developmental conditions and adult traits affect dispersal and survival of adults. Freshwater organisms such as dragonfly larvae appear to be especially sensitive to body size declines induced by environmental warming (Forster et al., 2012), but significant gaps remain in our understanding of how warming affects most freshwater species. Evidence that dragonflies are affected by and responding to climate change is growing (Hassall & Thompson, 2008), and includes substantial evidence of advancing phenologies in natural populations (Hassall et al., 2007; Dingemanse & Kalkman, 2008; Richter et al., 2008) and experimental work showing a strong link between temperature and phenology for odonates (Richter et al., 2008). Further, the ranges of many European species are shifting north, apparently tracking changing temperatures (Hickling et al., 2005; Flenner & Sahlén, 2008; Grewe et al., 2013).
The response of dragonflies to environmental warming will have effects that extend beyond these populations. Disruption of top consumer populations by climate change can have effects that permeate entire communities, and are expected to be especially disruptive to community structure (Zarnetske et al., 2012). Therefore, understanding the effects of environmental warming on dragonflies, which are the top predator in many freshwater habitats that lack fish (McPeek, 1990), may have far-reaching implications for community structure. Dragonflies have a critical role as a link between freshwater and terrestrial communities (Knight et al., 2005), suggesting that effects of climate change on dragonflies may extend beyond the freshwater environment and have the potential to alter processes across ecosystem boundaries. To determine the effects of warming in this system, we reared larvae under experimental conditions simulating the thermal environment predicted for 50 and 100 years in the future (Cayan et al., 2009). By examining multiple responses to the thermal conditions of the freshwater habitats in which these animals develop, we can make predictions about how warming from climate change will affect performance at the individual level including the effects on dispersal performance, and the implications of this for the communities in which these species are embedded.
Materials
Study species
We conducted this experiment with the dragonfly species Pachidiplax longipennis (Burmeister, 1839), which is widely distributed across North America and abundant in northern California, where our study was conducted. While P. longipennis can vary in its life-cycle length including semi-, uni-, and bi-voltine strategies (reviewed in Corbet et al., 2006), observations over several years indicate that in our study region P. longipennis is uni-voltine; breeding and egg laying occur during the summer and individuals overwinter in an aquatic larval stage, and emerging as aerial adults the following summer (McCauley, personal observation). Lifetime dispersal occurs across the summer flight season, and male breeding dispersal distance averages 400 m (McCauley, 2010).
Egg collection
We collected P. longipennis eggs during June and July 2012 from females flying at ponds on the Wantrup Wildlife Sanctuary (Napa County, California, 38°35’58.07”N, 122°22’13.10”W). We captured adult females or mating pairs with aerial insect nets, then collected eggs by gently holding the female's wings together while dipping her abdomen into a cup filled with pond water until eggs were released. After egg collection was complete, females were marked with permanent ink on the wing to prevent repeated sampling from the same individuals and then released. Eggs were then transported to our rearing facility at the Quail Ridge Reserve (Napa County, California, 38°28’58.72” N, 122°8’58.17” W, hereafter: QRR), approximately 40 km southeast of the collection site, where they were kept overnight in a climate-controlled room. The following morning clutches were checked to see if they had tanned, an indication that clutches were fertilized, before being placed into rearing tanks. A total of 27 fertilized clutches were collected and placed in rearing tanks.
Rearing conditions
Clutches were initially placed into a 1,153-L cattle tank filled with well water, leaf litter (primarily oak, Quercus spp. the dominant trees in this region which form an important component of the allochthonous debris entering local ponds), and structure provided by polypropylene rope tied to stainless steel washers. On August 5, 2012, larvae were transferred to 416-L experimental rearing tanks. Fifty larvae were randomly assigned to each rearing tank. This is a lower density than has been estimated for natural populations of larval P. longipennis (Morin, 1984). During the experimental set-up, 100 larvae were randomly selected, preserved, and later photographed. Head-width was calculated from these photographs using imageJ (Rasband, 2014). There was variation in both larval size and instar at the start of the experiment, but all larvae entered the experiment early in development and at small sizes (mean head width ± 1 SD: 1.8 ± 0.05 mm). Each rearing tank was filled with aged well water, and contained polypropylene rope and aquarium rocks for structure as well as leaf litter (again primarily oak) to provide structure and slowly released nutrients. All tanks were inoculated with a zooplankton culture established from the collection site, and provided with rabbit chow for nutrients.
Rearing tanks were set up in 8 blocks with each block containing three tanks: one ambient temperature, one + 2.5 °C above the ambient tank's temperature, and one + 5 °C above the ambient tank's temperature. Tanks within blocks were randomly assigned a location across a north-south gradient, for a total of 24 tanks. These temperature differentials were chosen to represent the 50 and 100-year climate projections for the study region (Cayan et al., 2009). Temperature was continuously monitored in each tank using a temperature sensor (CR1000; Campbell Scientific Inc., Logan UT); each experimental tank was equipped with a heavy-duty aquarium heater (JBJ 500Watt True Temp for 100-160 gallons) which adjusted water temperature once/hour based on the temperature reading from the ambient tank. This experimental set-up allowed us to simulate warming in a realistic fashion, incorporating both daily and seasonal variation in temperature. Experimental warming began in late November 2012 so that winter and spring developmental conditions could be manipulated. To minimize heat loss from the tanks, stabilize within tank temperature variation and avoid temperature gradients near tank edges, each tank was insulated using fiberglass insulation covered in Tyvek® HomeWrap® (DuPont, Wilmington, DE). To prevent colonization and minimize temperature gradients from surface warming, tanks were covered with 70% shade cloth attached to a wooden frame that held the cloth above the water. These shade cloth lids were secured tightly to the tanks to prevent colonization by tree frogs or other animals capable of climbing tank walls. Well water was added to tanks as necessary to keep tanks at a consistent water level until winter rains began; rainfall penetrating the shade cloth eliminated the need to add additional water during the winter rainy season.
Adult morphology and survival to emergence
In April 2013, shade cloth lids were removed and each tank was covered with a mosquito net (Bryne nets, Inter IKEA Systems B.V., Delft, The Netherlands) to capture emerging dragonflies. Nets were checked at least once/day (twice/day when temperatures were especially warm), and all dragonflies that had emerged were gently removed by hand and placed into mesh cages (Minifångst, Inter IKEA Systems B.V., Delft, The Netherlands). Individuals were housed overnight in a climate-controlled room (21 °C) to allow the exoskeleton to harden and their wings to dry before additional handling. On the day after emergence, each individual was sexed, individually marked with a number on the wing using a permanent marker (Sharpie™ Ultra-Fine Point), and the width of the head, length of the thorax, and length of a forewing (the right or left wing was chosen haphazardly) measured with digital calipers (accurate to ± 0.001 mm). Thorax length was measured because it is a good proxy for thoracic mass in dragonflies that can be measured non-destructively (McCauley, 2012). After one week in which no more emergers arose from the tanks, tanks were searched for remaining larvae and none were found. Nets were removed six days later. Survival was calculated in two ways for each tank: 1) the proportion of individuals that survived the larval stage, and 2) the proportion of those individuals that survived the larval stage, but failed to successfully emerge as adults (i.e., died during the process of metamorphosis).
Adult survival and dispersal
Extensive attempts to resight released adults did not produce sufficient data to make inferences about the effects of warming on adult survival or dispersal. Additional information on resighting methods and results are provided in the supplementary information (Methods/Results S1) and are not discussed further in text.
Statistical analysis
We first examined the rearing tank temperatures using repeated-measures analysis of variance (rmANOVA) on the daily average of each tank within each block to confirm effects of warming treatments on temperature. Next, we examined the response variables of larval survival, proportion of larval survivors that died during metamorphosis, emergence date (as ordinal day of the year), body size (a principal component score that included head width, thorax length, and forewing length, all of which loaded positively and strongly [all factor loadings 0.75-0.79] on a single axis that explained 60% of the variation in the data) and flight morphology by generated tank means within replicates. To assess whether flight morphology, particularly the relative size of the wings or thorax, was affected by warming we compared two ratios in individuals that successfully emerged across these treatments: forewing length:head width and thorax length:head width. We were unable to measure the mass of these individuals, another measure of relevance to flight performance, because accurate weight measurements required anesthetizing animals which can increase mortality, particularly in newly emerged odonates such as the ones we were working with (McCauley, personal observation). It was necessary to transform one variable (proportion of failed emergers) by adding a constant then applying a log transformation to attain a normal distribution. All other response variables were normally distributed and all variables met the assumption of equal variance across treatments. Two blocks were removed from the analysis due to significant divergent survival effects in their ambient temperature tanks (we normalized survival within temperature, then examined the resulting distribution, with both of these ambient tanks appearing as outliers). At least one of these tanks became anoxic over the winter. Although the cause of mass mortality in the other tank is unknown, it is possible that we did not detect anoxic conditions due to intermittent winter sampling.
To test for effects of temperature on each response variable, we used rmANOVA with each experimental block acting as a single replicate (N = 6 replicates (sets of 3 treatment tanks) used). For every variable except survival, we also included an effect of the interaction between sex and temperature to determine if males and females responded differently within each temperature treatment, and to account for variation due to sexual dimorphism (Moore, 1990). This approach creates an equally weighted average between males and females, regardless of the actual proportion of individuals of each sex within each tank. Because we do not know the sex ratio of the larvae that initially went into each tank, we could not test for an effect of sex on survival. Statistically significant rmANOVAs were followed up with post-hoc paired t-tests with Bonferroni corrections to allow for interpretation of which treatments were different from each other. This approach preserved the linked nature and variation present between the temperature treatments, with all based on the 6 ambient temperature tanks. The alpha level for statistical significance was set at 0.05 for all analyses.
Results
Temperature differences among treatments
Temperatures fluctuated naturally within treatments, because all experimental warming was based on ambient conditions (Fig S1a), but consistent differences of 2.5 °C and 5 °C above ambient were maintained in treatments (Fig. S1b).
Survival
Temperature had a statistically significant effect on larval survival, but no effect on the proportion of larval survivors that failed to complete emergence (rmANOVA, larval survival: F2,4 = 7.83, P = 0.041; proportion failing to survive through metamorphosis: F2,4 = 0.37 P = 0.712). Larval survival was significantly lower in the high temperature treatment as compared to the ambient and medium treatments but did not differ between the ambient and medium treatments (paired t-tests: ambient vs. medium, t5 = 0.91, P = 0.405; ambient vs. high, t5 = 3.69, P = 0.014, medium vs. high, t5 = 4.19, P = 0.009, Bonferroni-adjusted P-value = 0.017). For the proportion of individuals failing to successfully emerge, no pair-wise differences were present (paired t-tests: ambient vs. medium, t5 = 0.93, P = 0.394; ambient vs. high, t5 = 0.94, P = 0.389, medium vs. high, t5 = 0.19, P = 0.860).
Phenology
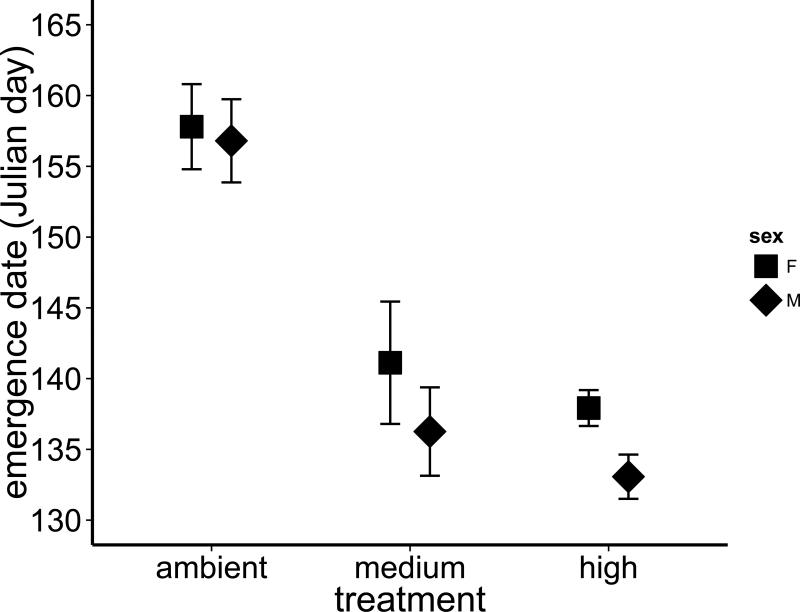
Warming accelerated development and shifted the timing of emergence into the adult stage forward in the season. There was a statistically significant effect of treatment on emergence date (rmANOVA, F3,3 = 17.6, P = 0.021), with individuals from the high temperature treatment emerging earliest, followed by the medium temperature treatment and finally the ambient treatment (Fig. 1, paired t-tests: ambient vs. medium, t5 = 3.98, P = 0.011; ambient vs. high, t5 = 5.16, P = 0.004, medium vs. high, t5 = 5.33, P = 0.003, Bonferroni-adjusted P-value = 0.017). There was also a significant effect of the interaction between temperature and sex on emergence date (rmANOVA, F3,3 = 14.13, P = 0.028), but this interaction effect is both less interpretable and more peripheral to our primary questions than the effect of temperature. Both average date of emergence and first date of emergence were dramatically shifted forward in warmed tanks relative to the ambient tanks (Fig. S2). Compared to the ambient treatment, the average date of emergence was 22 days earlier in the high treatment and 19 days earlier in the medium treatment. There were 12 days between the first emergence from the medium and high treatments (which had their first emergence events on the same day) and the emergence of the first individual from the ambient treatment.
Fig. 1.
Warming of the aquatic developmental environment significantly advanced the timing of P. longipennis emergence (mean Julian day ± 1SE), the metamorphosis from the larval to adult stage, relative to the ambient thermal environment (all P < 0.02). Relative to the ambient treatment the mean date of emergence was 22 days earlier from tanks warmed 5°C above ambient and 19 days earlier in tanks warmed to 2.5°C above ambient.
Body size
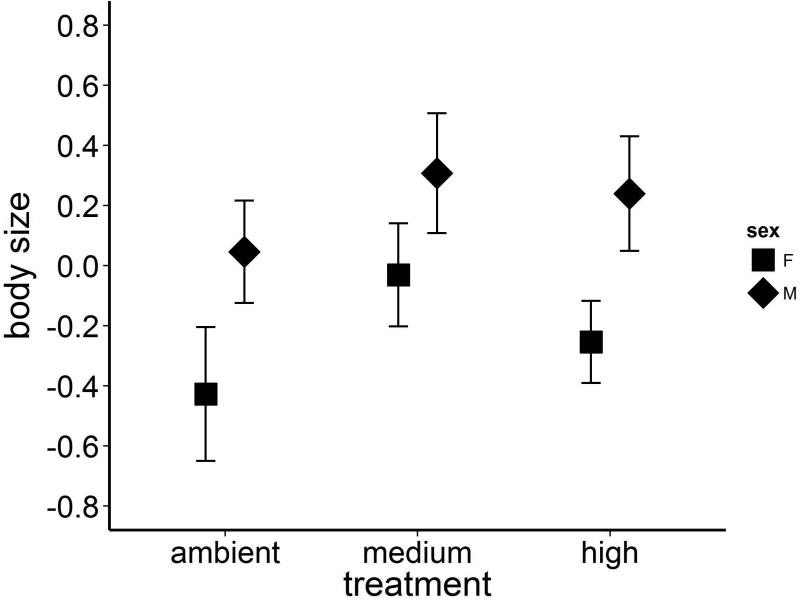
There was no effect of temperature treatment on our composite measure of body size (Fig. 2, rmANOVA, F3,3 = 1.04, P = 0.488). However, there was a statistically significant interaction effect between sex and temperature (rmANOVA, F3,3 = 14.83, P = 0.026), with the trend for larger size with increasing temperature being stronger in females than males (Fig. 2). There were no statistically significant pair-wise comparisons between the sexes within treatments, so as with emergence date, we do not consider this interaction effect further.
Fig 2.
The body size (mean ± 1SE ) of adult P. longipennis emerging from experimental tanks did not differ between the three treatments (all P > 0.05); however, there is a non-significant trend of increasing adult body size with warming. Our size metric used a PCA to combine head-width, thorax length, and forewing length into a composite measure of body size.
Flight morphology
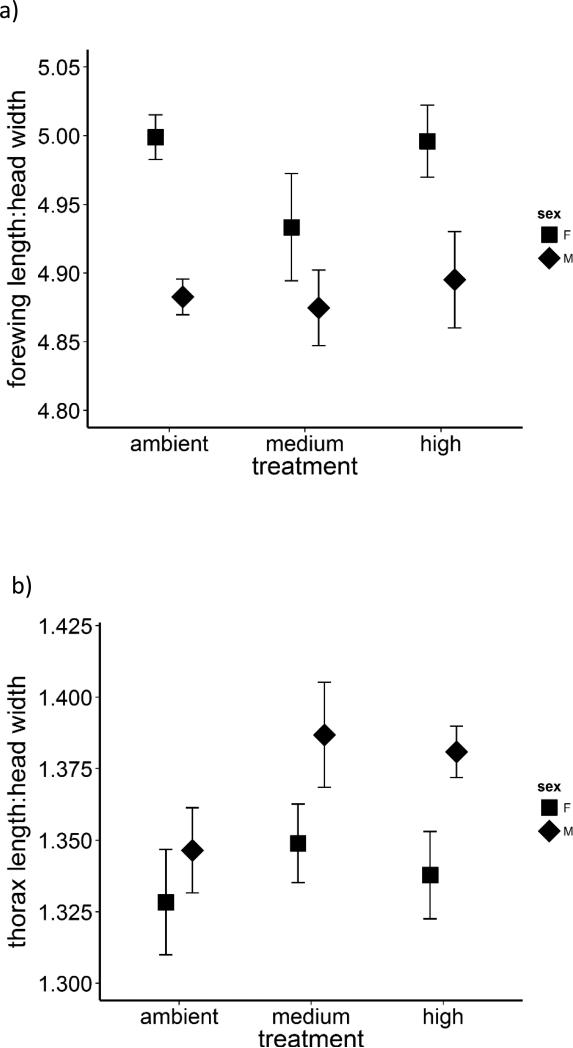
There was no significant effect of temperature on the ratio of forewing length to head width (Fig. 3a, rmANOVA, F3,3 = 0.41, P = 0.756) or thorax length to head width (Fig. 3b, F3,3 = 1.72, P = 0.333). These ratios exhibited no significant interaction between sex and temperature (rmANOVA, forewing length:head width: F3,3 = 5.83, P = 0.091; thorax length:head width: F3,3 = 6.53, P = 0.079) although there are modest trends in these data suggesting that relative thorax size may be affected more strongly by temperature in males than females.
Fig. 3.
a) Relative wing size (mean ratio ± 1SE of forewing to head width) was unaffected by the thermal conditions experienced by larvae (all P > 0.05). There was a non-significant trend of an interaction between sex and treatment (P = 0.091), but the pattern is difficult to interpret. b) Relative thorax size (mean ratio ± 1SE of thorax length to head width) was unaffected by the thermal conditions experienced by larvae (all P > 0.05). There was a non-significant trend of an interaction between sex and treatment (P = 0.079) with relative thorax size in males appearing to be more affected by warming than in females.
Discussion
We experimentally manipulated temperature in the developmental habitat of P. longipennis, simulating the expected thermal conditions for this region 50 (ambient +2.5 °C) and 100 (ambient +5 °C) years in the future (Cayan et al., 2009) while incorporating the natural variation in temperature that organisms will continue to experience. Pachydiplax longipennis were strongly affected by the thermal environment of their developmental habitats: temperatures experienced in the aquatic environment affected larval survival and phenology. However, we did not observe the expected influence of temperature on overall body size: warmer treatments did not result in smaller adults at emergence. Not all of the observed responses to changes in the thermal environment are necessarily negative (e.g., phenology shifts could be negative, neutral or even beneficial; O'Regan et al., 2014), but others, such as increased larval mortality at higher temperatures, clearly have the potential to negatively affect populations of this species. Our results provide evidence that the survival and development of larval dragonflies, and likely many other aquatic and semi-aquatic insects developing in pond environments, are altered by thermal regime shifts equivalent to the changes expected in this region over the next 100 years.
Experimental warming increased larval mortality. In the highest temperature treatment (+5 °C above ambient), fewer than 50% of larvae survived to metamorphosis, while approximately 60% of larvae survived in the ambient and medium temperature treatments (medium was +2.5 °C above ambient; Fig. 2). This 10% difference in survival can have consequences for adult population size as well as for the magnitude of aquatic to terrestrial energy and nutrient transfers (Wesner, 2010) and other cross-ecosystem trophic connections (Knight et al., 2005). However, the underlying proximate causes of death in these larvae are unknown, as is typical in studies of mortality in response to stressors or in the wild (McCauley et al., 2011; Klaassen et al., 2014). One possibility is that mortality was a direct effect of temperatures exceeding the upper lethal limit (ULL) for the species. This scenario is unlikely based on the temperatures reached in this experiment and the generally high ULLs documented for dragonflies (~ 42 °C, Dallas & Rivers-Moore, 2012; Stewart et al., 2013), including P. longipennis (44.5-45.8 °C, Garten & Gentry 1976). The highest temperature reached in our rearing tanks during the course of this study was 33.3 °C. Thus, it is unlikely that direct effects of temperature were primarily responsible for the mortality observed in this study.
If the increase in mortality observed in our highest temperature treatment is not due to exceeding the upper temperature maxima of the species, other factors related to temperature must be contributing to increase the risk of mortality. Changes in food resources are another possible explanation, but again, this appears to be unlikely to be a major explanatory factor. The primary food resource for larvae in our study was zooplankton. Although we did not quantify zooplankton abundance, qualitative observations across the season did not suggest any declines in zooplankton associated with temperature. Further, a recent laboratory study that manipulated both temperature and food abundance for larval P. longipennis found increased mortality at the higher temperature, but no effect of food abundance on mortality (M.Y. Chavez, J.I. Hammond, S.J. McCauley and K.E. Mabry, unpublished). Similarly, Janssens and Stoks (2013) found no interaction between food and temperature on mortality rates of damselfly larvae. Taken together, these results suggest that differences in food resources among treatments are unlikely to be driving our observation of increased mortality with temperature. Additionally, in the Chavez et al. (unpublished) study, larvae were housed individually so that cannibalism or intraguild predation could not be the driver of increased mortality. While trophic interactions among larvae may also change in response to temperature, and possibly contributed to the decreased survival in our high temperature treatment, it appears individual responses can be sufficient to increase mortality.
A remaining possibility is that temperature interacts with other stressors to increase the net risk of mortality. In other invertebrates, warming to temperatures below the ULL have been associated with increased mortality (Folt et al., 1999; Flenner et al., 2010; Shama et al., 2011; Pelini et al., 2012), and temperature can exacerbate the effects of other stressors (Folt et al., 1999; Schisler et al., 2000; Verbeck & Colosi, 2012) and decrease immune responses even when enhancing other aspects of performance (Karl et al., 2011). The presence of an intraguild predator can increase mortality with warming in some but not all species of odonates (Suhling & Suhling, 2013). For species that diapause in the larval stage, warming may prevent them from entering this diapause stage and may elevate metabolic costs and increase mortality (Hassall & Thompson, 2008). It is unknown whether P. longipennis in this region undergo larval diapause, and given the relatively warm winter temperatures of the Mediterranean climate where this study took place, this diapause is unlikely to be extensive. However, if even brief periods of diapause are broken this could contribute to the observed increase in mortality with warming. Larval dragonflies are subject to mortality from multiple causes: even our ambient treatment, which had the lowest mortality rate, had a larval mortality of nearly 40%. The increase in mortality observed with warming is likely to result from larvae becoming more sensitive to the factors causing mortality throughout the experiment when they are also subject to temperature stress. This suggests that understanding how multiple stressors, including temperature, interact to affect mortality may be critical to assessing the potential impacts of warming on population dynamics (Janssens & Stoks, 2013; Kimberly & Salice, 2013; Rohr & Palmer, 2013).
One of the most dramatic responses to warming that we observed was advancing phenology. Larvae from warmed tanks emerged significantly earlier than those from the ambient tanks, with a mean difference in emergence date between ambient and high temperature treatments of more than three weeks (Fig. 3), and 12 days elapsed between the first emergences from the warmed tanks (both high and medium) and the ambient tanks. The magnitude of the phenology shift observed here (onset of emergence was shifted forward 2.4 or 4.8 days/1°C of warming in the and medium and high treatments, respectively) is similar to that observed in a previous study of the effects of experimental warming on odonates, where a 6°C increase above ambient shifted the onset of emergence forward 28 days (a shift of 4.7 days/1°C of warming; Richter et al., 2008). In the British odonates the phenology of the first quartile of adult emergence has been estimated to have advanced an average of 3.08 days/1°C of warming since 1960 (Hassall et al. 2008), a level of phenological advance similar to what we observed. These strong seasonal shifts indicate that the timing of larval development in odonates is plastic and responsive to realistic changes in environmental warming expected within the next century. Differential phenological responses among species may be critical in determining the outcome of their interactions in the larval stage (Hassall & Thompson, 2008; Rasmussen et al., 2014).What are the potential consequences of such seasonal shifts for these animals? First of all, earlier emergence may expose fragile teneral individuals to unpredictable abiotic conditions, including weather events such as wind and rain. The Mediterranean climate of our study region has warm, dry summers that are generally hospitable to dragonflies. However, earlier emergence can expose individuals to late spring storms and adverse weather (e.g. Augspurger, 2013) that can negatively affect survival during metamorphosis and after emergence. Phenological shifts can also have consequences for interactions with other species (Yang & Rudolf, 2009) which may differ in their phenological responses to warming (Nilsson-Örtman et al., 2013). This may include altering the size at which larvae interact and changing the outcome of intraguild predation (Suhling & Suhling, 2013; Rasmussen et al., 2014), a common interaction in odonates. Phenological shifts are often non-optimal (Miller-Rushing et al., 2010): species affected by climate change often shift their phenologies either too much or too little in relation to their prey, resulting in mismatches with species at other trophic levels (Visser & Both, 2005). Dragonflies are important predators in both the aquatic and terrestrial environments; larval P. longipennis prey upon zooplankton and other small invertebrates in the aquatic environment, and adults are major predators upon other insects. These predator-prey relationships may be affected by predicted climatic changes, because earlier emergence means that these dragonflies are transitioning from aquatic to terrestrial predators earlier in the year, altering the timing of nutrient exports to the terrestrial environment (Wesner, 2010) and the potential for trophic cascades across the aquatic-terrestrial divide (Knight et al., 2005) .
We did not find the predicted effect of rearing temperature on overall body size at emergence – warmed dragonflies were not smaller than those reared under ambient thermal conditions, and the trend was actually in the opposite direction from predicted. It may be that the temperatures we reached in this experiment fall below those at which growth is maximized. A similar result was found in studies of European damselflies occurring across a broad range of latitude (Nilsson-Örtman et al., 2012) and may reflect the neotropical origins of P. longipennis (Corser et al., 2014). Alternatively, if small or slow-growing dragonflies are differentially vulnerable to the increased mortality observed with warming, selection through mortality could play a part in this pattern, but we do not currently know the traits associated with the dead individuals.
Our results indicate that environmental warming of an extent predicted to occur across the next century will affect freshwater insects such as the dragonflies we studied in both their larval aquatic phase and the number and timing of adults crossing into terrestrial environments. Exposure to temperatures 5 °C above ambient resulted in increased larval mortality, and increases of 2.5 °C or more led to more rapid development of larvae, and an advance in the phenology of the transition into the adult stage. Although the expected pattern of decreasing body size with increasing temperature was not observed, there are suggestions that warming may negatively impact adult survival and/or dispersal. This suite of changes suggests that the response of ectotherms developing in increasingly warm freshwater environments are multi-faceted and will likely affect population dynamics and community interactions, including connections between freshwater and terrestrial environments.
Supplementary Material
Acknowledgements
We are grateful to Shane Waddell, Virginia Boucher, Chris Hill, Marge Hill, the University of California Natural Reserve System, the Quail Ridge Reserve, and the Wantrup Wildlife Refuge of the Napa Land Trust for research support and access to research sites. Thanks to Maria Chavez, Jackson Deen, Renee Pardee, Michelle Rampulla, Grant Reed, Stephan Schneider, and Bekah Rottenberg for research assistance in the field. We had permits from the California Department of Fish and Game (SC-9036) to conduct this work as well as permission from the UC-NRS system and the Napa Land Trust. J. I. Hammond was supported by Grant Number K12GM088021 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health. We received financial support for this project from a NMSU College of Arts and Sciences Mini-grant and the National Science Foundation (DEB 1245415).
Footnotes
Contributions of authors
SJM and KEM designed this research. DNF, SJM, and KEM contributed to data collection while JIH, SJM, and KEM were involved in data analysis. All authors contributed to paper writing.
References
- Angilletta MJ, Steury TD, Sears MW. Temperature, growth rate, and body size in ectotherms: fitting pieces of a life-history puzzle. Integrative and Comparative Biology. 2004;44:498–509. doi: 10.1093/icb/44.6.498. [DOI] [PubMed] [Google Scholar]
- Atkinson D. Temperature and organism size - a biological law for ectotherms. Advances in Ecological Research. 1994;25:1–58. [Google Scholar]
- Atkinson D. Effects of temperature on the size of aquatic ectotherms - exceptions to the general rule. Journal of Thermal Biology. 1995;20:61–74. [Google Scholar]
- Augspurger CK. Reconstructing patterns of temperature, phenology, and frost damage over 124 years: Spring damage risk is increasing. Ecology. 2013;94:41–50. doi: 10.1890/12-0200.1. [DOI] [PubMed] [Google Scholar]
- Callier V, Nijhout HF. Body size determination in insects: a review and synthesis of size- and brain-dependent and independent mechanisms. Biological Reviews. 2013;88:944–954. doi: 10.1111/brv.12033. [DOI] [PubMed] [Google Scholar]
- Cayan D, Tyree M, Dettinger M, Hidalgo H, Das T, Maurer E, Bromirski P, Graham N, Flick R. Climate change scenarios and sea level rise estimates for the California 2008 climate change scenarios assessment. California Climate Change Center; 2009. [Google Scholar]
- Corbet PS, Suhling F, Soendgerath D. Voltinism of Odonata: a review. International Journal of Odonatology. 2006;9:1–44. [Google Scholar]
- Corser JD, White EI, Schlesinger MD. Odonata origins, biogeography, and diversification in an Eastern North American hotspot: multiple pathways to high temperate forest insect diversity. Insect Conservation and Diversity. 2014 In press. [Google Scholar]
- Dallas HF, Rivers-Moore NA. Critical thermal maxima of aquatic macroinvertebrates: towards identifying bioindicators of thermal alteration. Hydrobiologia. 2012;679:61–76. [Google Scholar]
- Darling ES, Cote IM. Quantifying the evidence for ecological synergies. Ecology Letters. 2008;11:1278–1286. doi: 10.1111/j.1461-0248.2008.01243.x. [DOI] [PubMed] [Google Scholar]
- Diez JM, Ibanez I, Miller-Rushing AJ, Mazer SJ, Crimmins TM, Crimmins MA, Bertelsen CD, Inouye DW. Forecasting phenology: from species variability to community patterns. Ecology Letters. 2012;15:545–553. doi: 10.1111/j.1461-0248.2012.01765.x. [DOI] [PubMed] [Google Scholar]
- Dingemanse NJ, Kalkman VJ. Changing temperature regimes have advanced the phenology of Odonata in the Netherlands. Ecological Entomology. 2008;33:394–402. [Google Scholar]
- Dingle H. Migration: the biology of life on the move. Oxford University Press; New York: 1996. [Google Scholar]
- Doi H. Delayed phenological timing of dragonfly emergence in Japan over five decades. Biology Letters. 2008;4:388–391. doi: 10.1098/rsbl.2008.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley R. The Biomechanics of Insect Flight. Princeton University Press; Princeton, NJ: 2000. [Google Scholar]
- Fellowes MDE, Kraaijeveld AR, Godfray HCJ. Association between feeding rate and parasitoid resistance in Drosophila melanogaster. Evolution. 1999;53:1302–1305. doi: 10.1111/j.1558-5646.1999.tb04544.x. [DOI] [PubMed] [Google Scholar]
- Flenner I, Richter O, Suhling F. Rising temperature and development in dragonfly populations at different latitudes. Freshwater Biology. 2010;55:397–410. [Google Scholar]
- Flenner I, Sahlen G. Dragonfly community re-organisation in boreal forest lakes: rapid species turnover driven by climate change? Insect Conservation and Diversity. 2008;1:169–179. [Google Scholar]
- Folt CL, Chen CY, Moore MV, Burnaford J. Synergism and antagonism among multiple stressors. Limnology and Oceanography. 1999;44:864–877. [Google Scholar]
- Forrest J, Miller-Rushing AJ. Toward a synthetic understanding of the role of phenology in ecology and evolution. Philosophical Transactions of the Royal Society B-Biological Sciences. 2010;365:3101–3112. doi: 10.1098/rstb.2010.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster J, Hirst AG, Atkinson D. How do organisms change size with changing temperature? The importance of reproductive method and ontogenetic timing. Functional Ecology. 2011;25:1024–1031. [Google Scholar]
- Forster J, Hirst AG, Atkinson D. Warming-induced reductions in body size are greater in aquatic than terrestrial species. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:19310–19314. doi: 10.1073/pnas.1210460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JL, Peters A, Kearney MR, Joseph L, Heinsohn R. Declining body size: a third universal response to warming? Trends in Ecology & Evolution. 2011;26:285–291. doi: 10.1016/j.tree.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Garten CT, Gentry JB. Thermal tolerance of dragonfly nymphs. 2. Comparison of nymphs from control and thermally altered environments. Physiological Zoology. 1976;49:206–213. [Google Scholar]
- Ghosh SM, Testa ND, Shingleton AW. Temperature-size rule is mediated by thermal plasticity of critical size in Drosophila melanogaster. Proceedings of the Royal Society B-Biological Sciences. 2013;280 doi: 10.1098/rspb.2013.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly JF, Charnov EL, West GB, Savage VM, Brown JH. Effects of size and temperature on developmental time. Nature. 2002;417:70–73. doi: 10.1038/417070a. [DOI] [PubMed] [Google Scholar]
- Grewe Y, Hof C, Dehling DM, Brandl R, Braendle M. Recent range shifts of European dragonflies provide support for an inverse relationship between habitat predictability and dispersal. Global Ecology and Biogeography. 2013;22:403–409. [Google Scholar]
- Hassall C, Thompson DJ. The effects of environmental warming on Odonata: a review. International Journal of Odonatology. 2008;11:131–153. [Google Scholar]
- Hassall C, Thompson DJ, French GC, Harvey IF. Historical changes in the phenology of British Odonata are related to climate. Global Change Biology. 2007;13:933–941. [Google Scholar]
- Hickling R, Roy DB, Hill JK, Thomas CD. A northward shift of range margins in British Odonata. Global Change Biology. 2005;11:502–506. [Google Scholar]
- Hill JK, Thomas CD, Blakeley DS. Evolution of flight morphology in a butterfly that has recently expanded its geographic range. Oecologia. 1999a;121:165–170. doi: 10.1007/s004420050918. [DOI] [PubMed] [Google Scholar]
- Hill JK, Thomas CD, Lewis OT. Flight morphology in fragmented populations of a rare British butterfly, Hesperia comma. Biological Conservation. 1999b;87:277–283. [Google Scholar]
- Inouye DW. Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology. 2008;89:353–362. doi: 10.1890/06-2128.1. [DOI] [PubMed] [Google Scholar]
- IPCC . Climate Change 2007: The physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. United Kingdom and New York, USA; Cambridge: 2007. [Google Scholar]
- Janssens L, Stoks R. Fitness effects of chlorpyrifos in the damselfly Enallagma cyathigerum strongly depend upon temperature and food level and can bridge metamorphosis. Plos One. 2013;8 doi: 10.1371/journal.pone.0068107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl I, Stoks R, De Block M, Janowitz SA, Fischer K. Temperature extremes and butterfly fitness: conflicting evidence from life history and immune function. Global Change Biology. 2011;17:676–687. [Google Scholar]
- Karlsson B, Johansson A. Seasonal polyphenism and developmental trade-offs between flight ability and egg laying in a pierid butterfly. Proceedings of the Royal Society B-Biological Sciences. 2008;275:2131–2136. doi: 10.1098/rspb.2008.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberly DA, Salice CJ. Interactive effects of contaminants and climate-related stressors: High temperature increases sensitivity to cadmium. Environmental Toxicology and Chemistry. 2013;32:1337–1343. doi: 10.1002/etc.2198. [DOI] [PubMed] [Google Scholar]
- Kingsolver JG, Huey RB. Size, temperature, and fitness: three rules. Evolutionary Ecology Research. 2008;10:251–268. [Google Scholar]
- Klaassen RHG, Hake M, Strandberg R, Koks B, Trierweiler C, Exo KM, Bairlein F, Alerstam T. When and where does mortality occur in migratory birds? Direct evidence from long-term satellite tracking of raptors. Journal of Animal Ecology. 2014;83:176–184. doi: 10.1111/1365-2656.12135. [DOI] [PubMed] [Google Scholar]
- Knight TM, McCoy MW, Chase JM, McCoy KA, Holt RD. Trophic cascades across ecosystems. Nature. 2005;437:880–883. doi: 10.1038/nature03962. [DOI] [PubMed] [Google Scholar]
- McCauley SJ. Body size and social dominance influence breeding dispersal in male Pachydiplax longipennis (Odonata). Ecological Entomology. 2010;35:377–385. [Google Scholar]
- McCauley SJ. Relationship between morphology, dispersal and habitat distribution in three species of Libellula (Odonata: Anisoptera). Aquatic Insects. 2012;34:195–204. [Google Scholar]
- McCauley SJ, Mabry KE. Climate change, body size, and phenotype dependent dispersal. Trends in Ecology & Evolution. 2011;26:554–555. doi: 10.1016/j.tree.2011.06.017. [DOI] [PubMed] [Google Scholar]
- McCauley SJ, Rowe L, Fortin MJ. The deadly effects of “nonlethal” predators. Ecology. 2011;92:2043–2048. doi: 10.1890/11-0455.1. [DOI] [PubMed] [Google Scholar]
- McPeek MA. Determination of species composition in the Enallagma damselfly assemblages of permanent lakes. Ecology. 1990;71:83–98. [Google Scholar]
- Miller-Rushing AJ, Hoye TT, Inouye DW, Post E. The effects of phenological mismatches on demography. Philosophical Transactions of the Royal Society B-Biological Sciences. 2010;365:3177–3186. doi: 10.1098/rstb.2010.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AJ. The evolution of sexual dimorphism by sexual selection - the separate effects of intrasexual selection and intersexual selection. Evolution. 1990;44:315–331. doi: 10.1111/j.1558-5646.1990.tb05201.x. [DOI] [PubMed] [Google Scholar]
- Morin PJ. The impact of fish exclusion on the abundance and species composition of larval odonates - results of short-term experiments in a North Carolina farm pond. Ecology. 1984;65:53–60. [Google Scholar]
- Nilsson-Ortman V, Stoks R, De Block M, Johansson F. Generalists and specialists along a latitudinal transect: patterns of thermal adaptation in six species of damselflies. Ecology. 2012;93:1340–1352. doi: 10.1890/11-1910.1. [DOI] [PubMed] [Google Scholar]
- Nilsson-Ortman V, Stoks R, De Block M, Johansson F. Latitudinal patterns of phenology and age-specific thermal performance across six Coenagrion damselfly species. Ecological Monographs. 2013;83:491–510. [Google Scholar]
- O'Regan SM, Palen WJ, Anderson SC. Climate warming mediates negative impacts of rapid pond drying for three amphibian species. Ecology. 2014;95:845–855. doi: 10.1890/13-0916.1. [DOI] [PubMed] [Google Scholar]
- Parmesan C. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology Evolution and Systematics. 2006;37:637–669. [Google Scholar]
- Pelini SL, Diamond SE, MacLean H, Ellison AM, Gotelli NJ, Sanders NJ, Dunn RR. Common garden experiments reveal uncommon responses across temperatures, locations, and species of ants. Ecology and Evolution. 2012;2:3009–3015. doi: 10.1002/ece3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petter G, Weitere M, Richter O, Moenickes S. Consequences of altered temperature and food conditions for individuals and populations: a dynamic energy budget analysis for Corbicula fluminea in the Rhine. Freshwater Biology. 2014;59:832–846. [Google Scholar]
- Rasband WS. ImageJ, U. S. National Institutes of Health. Bethesda, Maryland, USA: 2014. http://imagej.nih.gov/ij/, 1997-2014. [Google Scholar]
- Rasmussen NL, Van Allen BG, Rudolf VHW. Linking phenological shifts to species interactions through size-mediated priority effects. Journal of Animal Ecology. 2014;83:1206–1215. doi: 10.1111/1365-2656.12203. [DOI] [PubMed] [Google Scholar]
- Reiskind MH, Zarrabi AA. Is bigger really bigger? Differential responses to temperature in measures of body size of the mosquito, Aedes albopictus. Journal of Insect Physiology. 2012;58:911–917. doi: 10.1016/j.jinsphys.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Richter O, Suhling F, Mueller O, Kern D. A model for predicting the emergence of dragonflies in a changing climate. Freshwater Biology. 2008;53:1868–1880. [Google Scholar]
- Rohr JR, Palmer BD. Climate change, multiple stressors, and the decline of ectotherms. Conservation Biology. 2013;27:741–751. doi: 10.1111/cobi.12086. [DOI] [PubMed] [Google Scholar]
- Schisler GJ, Bergersen EP, Walker PG. Effects of multiple stressors on morbidity and mortality of fingerling rainbow trout infected with Myxobolus cerebralis. Transactions of the American Fisheries Society. 2000;129:859–865. [Google Scholar]
- Shama LNS, Campero-Paz M, Wegner KM, De Block M, Stoks R. Latitudinal and voltinism compensation shape thermal reaction norms for growth rate. Molecular Ecology. 2011;20:2929–2941. doi: 10.1111/j.1365-294X.2011.05156.x. [DOI] [PubMed] [Google Scholar]
- Sheridan JA, Bickford D. Shrinking body size as an ecological response to climate change. Nature Climate Change. 2011;1:401–406. [Google Scholar]
- Shingleton AW, Estep CM, Driscoll MV, Dworkin I. Many ways to be small: different environmental regulators of size generate distinct scaling relationships in Drosophila melanogaster. Proceedings of the Royal Society B-Biological Sciences. 2009;276:2625–2633. doi: 10.1098/rspb.2008.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart BA, Close PG, Cook PA, Davies PM. Upper thermal tolerances of key taxonomic groups of stream invertebrates. Hydrobiologia. 2013;718:131–140. [Google Scholar]
- Suhling I, Suhling F. Thermal adaptation affects interactions between a range-expanding and a native odonate species. Freshwater Biology. 2013;58:705–714. [Google Scholar]
- Thomas MB, Blanford S. Thermal biology in insect-pathogen interactions. Trends in Ecology & Evolution. 2003;18:344–350. [Google Scholar]
- Tobler A, Nijhout HF. Developmental constraints on the evolution of wing-body allometry in Manduca sexta. Evolution & Development. 2010;12:592–600. doi: 10.1111/j.1525-142X.2010.00444.x. [DOI] [PubMed] [Google Scholar]
- Verberk W, Calosi P. Oxygen limits heat tolerance and drives heat hardening in the aquatic nymphs of the gill breathing damselfly Calopteryx virgo (Linnaeus, 1758). Journal of Thermal Biology. 2012;37:224–229. [Google Scholar]
- Visser ME, Both C. Shifts in phenology due to global climate change: the need for a yardstick. Proceedings of the Royal Society B-Biological Sciences. 2005;272:2561–2569. doi: 10.1098/rspb.2005.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser ME, Caro SP, van Oers K, Schaper SV, Helm B. Phenology, seasonal timing and circannual rhythms: towards a unified framework. Philosophical Transactions of the Royal Society B-Biological Sciences. 2010;365:3113–3127. doi: 10.1098/rstb.2010.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner EE. Size, scaling, and the evolution of complex life-cycles. In: Ebenman B, Persson L, editors. Size-structured populations: ecology and evolution. Springer; Berlin: 1988. pp. 60–81. [Google Scholar]
- Wesner JS. Aquatic predation alters a terrestrial prey subsidy. Ecology. 2010;91:1435–1444. doi: 10.1890/09-1532.1. [DOI] [PubMed] [Google Scholar]
- Wilczek AM, Burghardt LT, Cobb AR, Cooper MD, Welch SM, Schmitt J. Genetic and physiological bases for phenological responses to current and predicted climates. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365:3129–3147. doi: 10.1098/rstb.2010.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LH, Rudolf VHW. Phenology, ontogeny and the effects of climate change on the timing of species interactions. Ecology Letters. 2010;13:1–10. doi: 10.1111/j.1461-0248.2009.01402.x. [DOI] [PubMed] [Google Scholar]
- Zarnetske PL, Skelly DK, Urban MC. Biotic multipliers of climate change. Science. 2012;336:1516–1518. doi: 10.1126/science.1222732. [DOI] [PubMed] [Google Scholar]
- Zera AJ, Denno RF. Physiology and ecology of dispersal polymorphism in insects. Annual Review of Entomology. 1997;42:207–230. doi: 10.1146/annurev.ento.42.1.207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.