Abstract
Mesolimbic α6* nicotinic acetylcholine receptors (nAChRs) are thought to have an important role in nicotine behavioral effects. However, little is known about the role of the various α6*-nAChRs subtypes in the rewarding effects of nicotine. In this report, we investigated and compared the role of α6*-nAChRs subtypes and their neuro-anatomical locus in nicotine and cocaine reward-like effects in the conditioned place preference (CPP) paradigm, using pharmacological antagonism of α6β2* nAChRs and genetic deletion of the α6 or α4 subunits in mice. We found that α6 KO mice exhibited a rightward shift in the nicotine dose–response curve compared with WT littermates but that α4 KO failed to show nicotine preference, suggesting that α6α4β2*-nAChRs are involved. Furthermore, α6β2* nAChRs in nucleus accumbens were found to have an important role in nicotine-conditioned reward as the intra-accumbal injection of the selective α6β2* α-conotoxin MII [H9A; L15A], blocked nicotine CPP. In contrast to nicotine, α6 KO failed to condition to cocaine, but cocaine CPP in the α4 KO was preserved. Intriguingly, α-conotoxin MII [H9A; L15A], blocked cocaine conditioning in α4 KO mice, implicating α6β2* nAChRs in cocaine reward. Importantly, these effects did not generalize as α6 KO showed both a conditioned place aversion to lithium chloride as well as CPP to palatable food. Finally, dopamine uptake was not different between the α6 KO or WT mice. These data illustrate that the subjective rewarding effects of both nicotine and cocaine may be mediated by mesolimbic α6β2* nAChRs and that antagonists of these receptor subtypes may exhibit therapeutic potential.
INTRODUCTION
Reward is a component of addiction that motivates repeated drug-taking behavior and that intensifies stimulus drug associations (Di Chiara, 1999). Tobacco dependence remains the leading preventable cause of death worldwide. The current FDA-approved anti-smoking agents have only been modestly effective in maintaining abstinence and often cause undesirable side effects. There is a need for treatments with improved effectiveness and tolerability. Nicotine, a major psychoactive ingredient in tobacco, acts on a variety of nicotinic acetylcholine receptors (nAChRs) in the mammalian brain, including α4β2* (*denotes the presence of other subunits in the receptor composition) nAChRs subtypes. α4β2*-nAChRs, which comprise the most widely expressed high-affinity subtypes, have a major role in modulating the behavioral effects of nicotine. For that reason, identification of relevant α4β2* nAChR subtypes with a more restricted distribution in the brain is essential to finding more effective treatments for smoking cessation.
This study targeted α4* and α6* nAChRs, because these receptors are co-expressed with the β2 subunit, and β2*-nAChRs are known to be crucial for nicotine reinforcement and reward (Maskos et al, 2005; Picciotto et al, 1998; Walters et al, 2006). Intriguingly, β2* has also been shown to have a role in cocaine reward (Zachariou et al, 2001). Most DA terminals express a variety of nicotinic receptors, with the β2 subunit identified as the common subunit expressed (Salminen et al, 2007; Zoli et al, 2002). However, implications of the variety of nAChR subtypes expressed on DA terminals are not yet fully understood. Recent studies have shown that α6β2* nAChRs are expressed in catecholaminergic nuclei in midbrain regions thought to mediate drug reward and reinforcement in rodents and have a major role in presynaptic DA release (Grady et al, 2002; Whiteaker et al, 2000; Jackson et al, 2009; Pons et al, 2008; Brunzell et al, 2010). Of equal relevance, α4β2*-nAChRs are highly expressed in the midbrain (Klink et al, 2001). The α4* nicotinic receptor subtype has been shown to be sufficient (Tapper et al, 2004) and necessary (McGranahan et al, 2011; Pons et al, 2008) for nicotine reward and reinforcement as well as nicotine-induced DA release in rodents (Marubio et al, 2003; Salminen et al, 2007; Grady et al, 2007; Drenan et al, 2010).
Several studies in rodents and humans have revealed the involvement of nicotinic mechanisms in cocaine dependence. For example, Horger et al (1992) observed increased cocaine self-administration by rats that were preexposed to nicotine. Levine et al (2011) recently observed that nicotine pretreatment increased cocaine locomotor sensitization and conditioned place preference (CPP) in mice. Similarly, the nonselective nicotinic antagonist mecamylamine decreased cocaine self-administration in rats (Levin et al, 2000; Blokhina et al, 2005) and reduced cue-induced cocaine craving in cocaine-dependent and cigarette smoking humans (Reid et al, 1998). In addition, dihydro-beta-erythroidine (DHβE) (β2* nAChR antagonist), but not methyllycaconitine (α7* nAChR antagonist), microinjected into the VTA prevented cocaine locomotor sensitization (Champtiaux et al, 2006). Mice that received mecamylamine and mice null for the β2 nicotinic subunit displayed decreased place preference for cocaine compared with wild-type (WT) littermates (Zachariou et al, 2001). Interestingly, psychostimulants enhance release of acetylcholine (Ach) in the nucleus accumbens (NAc) and increase responsiveness of cholinergic neurons during acute and repeated drug exposure (Fiserová et al, 1999; Nestby et al, 1997). In humans, Budney et al (1993) reported co-morbidity of cigarette smoking in cocaine addicts. In addition, Sees and Clark (1993) reported that patients found that mentholated cigarettes prolonged the hedonic state induced by cocaine and alleviated cravings in the absence of cocaine. Moreover, administering a 2.5-mg tablet dose of mecamylamine to patients reduced reports of cocaine craving (Reid et al, 1999).
Because little is known about the role of α6β2* or α4β2* in the subjective rewarding effects of cocaine, we sought to investigate and compare the role of these nAChR subtypes in the acquisition and expression of nicotine and cocaine reward using the CPP paradigm. We set out to characterize the nicotinic subtype (α6β2*, α4α6β2*, and/or α4β2*), and the contribution of the nucleus accumbens (NAc) to both nicotine and cocaine reward-like effects using pharmacological antagonism of α6β2* nAChRs and genetic deletion of the α6 or α4 subunits in mice.
MATERIALS AND METHODS
Animals
Male C57BL/6J (B6) 8-weeks-old mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were 10–12 weeks of age at the start of the experiments and were group-housed (three to five per cage and received cage enrichment) under a 12-h light/dark cycle in a 21 °C humidity-controlled AAALAC-approved animal care facility with ad libitum access to food and water. Experiments were performed during the light cycle and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
For studies involving genetically modified mice, B6 provided the background strain for our α6 and α4 knockout (KO) and WTmice. Healthy viable mice null for the α6 nicotinic subunit were provided by Dr Uwe Maskos at Institut Pasteur (Paris, France) (Champtiaux et al, 2002). Viable mice null for the α4 subunit were provided by Dr Henry Lester at the California Institute of Technology, with the permission of Dr John Drago (Ross et al, 2000). Mutant and WT mice were obtained from crossing heterozygous (HET) mice. HET mice were back-crossed onto C57BL/6J background for at least 0 generations and were 10–12-weeks old at the beginning of testing.
Drugs
Lithium chloride (LiCl), DHβE, and (−)-nicotine hydrogen tartrate salt ((−)-1-methyl-2-(3-pyridyl)pyrrolidine (+)-bitartrate salt) was purchased from Sigma Chemical Co. (St. Louis, Mo). Cocaine HCl was obtained from the National Institute on Drug Abuse (Bethesda, MD, USA). All drugs were dissolved in physiological saline (0.9% sodium chloride) and administered at a volume of 10 ml/kg body weight. Nicotine, LiCl, and DHβE were administered subcutaneously (s.c.), and cocaine was administered intraperitoneally (i.p.). Doses are expressed as the free base of the drug. To study the role of the α6* nAChR in nicotine and cocaine reward, we used α-conotoxin MII [H9A;L15A], a highly selective α6* nicotinic antagonist. It is an analog of α-conotoxin MII that has a high selectivity for α6* nAChRs. For example, α-conotoxin MII [H9A;L15A] has up to a 2020-fold selectivity for α6* vs α3*, with little or no activity at other nAChRs (α2β2, α2β4, α3β4, α4β2, α4β4, and α7) (McIntosh et al, 2004). α-Conotoxin MII [H9A;L15A] was dissolved in small aliquots of saline and stored in an −18 to −20 °C freezer until use. The toxin was administered to each animal centrally at the following sites: lateral ventricles, nucleus accumbens, or septum. The doses used in our studies were calculated based on affinity and the potency of the compound at α6*-nAChRs (McIntosh et al, 2004).
Nicotine and Cocaine CPP Test
Nicotine and cocaine CPP were conducted using an unbiased design as previously described by Kota et al (2007). Mice were handled for 3 days before initiation of CPP testing. Briefly, place conditioning chambers consisted of two distinct compartments separated by a smaller intermediate compartment with openings that allowed access to either side of the chamber. On day 1, animals were confined to the intermediate compartment for a 5-min habituation period and then allowed to move freely between compartments for 15 min. Time spent in each compartment was recorded. These data were used to separate the animals into groups of approximately equal bias. Days 2–4 were conditioning days in which the saline group received saline in both compartments (20 min) and the drug groups received nicotine or cocaine in one compartment and saline in the opposite compartment. Control groups received saline in both compartments. On day 5, a drug-free test day, mice were allowed to move freely among the CPP chamber, and time spent in each side was recorded. Data were expressed as the time spent on the drug-paired side postconditioning minus time spent on the drug-paired side preconditioning.
For food-induced CPP, Kraft Classic Philadelphia Cheesecake (Deerfield, IL) was used to induce place preference in mice. For that, one extra day of conditioning was required in order to obtain significant place preference scores. Briefly, mice underwent preconditioning on day 1 and were divided into groups of equal bias, as described above, and were then immediately introduced to cheesecake (aka palatable food chow) for the next 4–6 h. The conditioning days followed the same experimental design as described in the paragraph above, but mice received cheesecake (or standard chow pellet) instead of drug (or saline), there was one extra day of conditioning (days 2–5), and each conditioning session lasted for 40 min. Control groups received the standard chow pellet in both compartments.
Lithium Chloride Induced Condition Place Aversion (CPA)
Following the same CPP paradigm as described above, mice were conditioned on days 2–4 during which the saline group received saline in both compartments and the drug groups received LiCl (150 mg/kg i.p.) in one compartment and saline in the opposite compartment. Drug-paired compartments were randomized among all groups. On day 5, animals were confined to the intermediate compartment for a 5-min habituation period, and then they were allowed to move freely between compartments for 15 min
Intracranial Cannula Implantation and Infusions for Studies with α-Conotoxin MII [H9A;L15A]
For cannulation surgeries, mice were anesthetized with 45 mg/kg sodium pentobarbital (i.p.). Once a mouse was readied for surgery, an incision was made to expose the skull.. Using the stereotaxic apparatus, the head was leveled, and a site of cannula implantation was found with the following coordinates for the lateral ventricle: −0.6 mm AP; +1.3 mm ML, with respect to bregma, and −2.1 mm DV from the skull's surface, the following coordinates for the NAc: +1.25 mm AP; ±0.75 mm ML, with respect to bregma, and −4.3 mm DV from the skull's surface, and the following coordinates for intra-septal injections: mm±0 AP; ±0 mm ML, with respect to bregma, and −3.0 mm DV from the skull's surface. A guide cannula was adhered to the skull using dental glue, which was then reinforced with dental cement. The cannulas used in our studies were 26 gauge, with an 8-mm pedestal height for the bilateral NAc cannulas and a 5-mm pedestal height for cannulas used in the lateral ventricle and septum. These cannulas fit 33 gauge internal cannulas for injections. A dummy cannula was inserted to maintain integrity of the guide. After completion of surgeries, animals were returned to clean home cages and were allowed to recover for 5 days before behavioral testing. At the end of the experiment, each brain was collected to evaluate accurate cannula placement.
Lateral ventricle infusions
During the 3 conditioning days of the CPP procedure, before both morning and afternoon conditioning sessions, mice received unilateral infusions of α-conotoxin MII [H9AL15A], or saline, 5 min before systemic injection of the psychoactive drug of interest or saline. Infusions were conducted using a microinfusion pump at a rate of 1.5 μl/min (for 2 min total, 3 μl total volume) through a sterile 33-gauge internal cannula extending 0.1 mm beyond the guide, which is attached to a Hamilton syringe via PE50 tubing.
NAc infusions
Before both morning and afternoon conditioning sessions, mice received bilateral infusions of α-conotoxin MII [H9AL15A] or saline. Infusions were done using a micro-infusion pump at a rate of 1 μl /min for 30 s (0.5 μl total volume) in a similar fashion to lateral ventricle infusions (described above).
Intra-septal infusions
During the 3 conditioning days of the CPP procedure, before both morning and afternoon conditioning sessions, mice received unilateral infusions of α-conotoxin MII [H9AL15A], or saline, 5 min before systemic injection of the psychoactive drug of interest or saline. Infusions were carried out using an internal connected to a micro-infusion pump via Hamilton syringe and PE50 tubing. Drug (or saline) was infused at a rate of 1 μl /min for 30 s.
Histology
To assess accurate cannula placement, methylene blue dye was injected centrally, followed by cervical dislocation, decapitation, and harvesting of the brain. Whole brain tissue was then fixed in a formalin/formaldehyde solution for 48 h before being sliced at thickness of 50–60 μm in a cryostat. Tissue slices were then stained with Nissl using a sequence of steps involving decreasing concentrations of ethanol in distilled water to hydrate tissue slices, followed by staining with cresyl violet, and then dehydrating the tissue slices using increasing concentrations of ethanol followed by clearing in xylene. Each site of injection was then reconstructed and marked on a worksheet of mouse bran coronal slice image for assessment.
Inhibition of [3H]-Dopamine Uptake by Cocaine
In order to demonstrate that deletion of α6 nicotinic subunit did not change the overall activity of dopamine uptake inhibition nor its sensitivity to cocaine, we measured the inhibition of [3H]-dopamine uptake by cocaine in α6 WT and KO mice. Crude synaptosomes were prepared from striata dissected from WT or α6 KO mice using by centrifugation of samples homogenized in 0.32 M sucrose containing 10 mM HEPES (pH=7.5) at 10 000 g for 20 min. The resulting pellet was suspended in isotonic incubation buffer (NaCl 128 mM; KCl 2.4 mM; CaCl2 3.2 mM; MgSO4 1.2 mM; HEPES 25 mM; glucose 10 mM; ascorbic acid 1 mM; pargyline 1 mM; pH=7.5), and the protein was measured using the Lowry assay. Uptake was initiated by the addition of [3H]dopamine (final concentration 0.075 μM, specific activity 13 Ci/mmol) to the synaptosomal suspension. After a 5-min incubation at 22 °C, uptake was terminated by filtration through glass fiber filters (top filter MFS Type B (Micro Filtration Systems) bottom filter Type A/E (Pall Life Sceinces)) that had been treated with 0.5% polyethylenimine using an Inotech Cell Harvester. Samples were subsequently washed four times with ice-cold buffer (incubation buffer minus glucose, ascorbic acid, and pargyline). Washed filters were transferred to a 96-well counting plate, 150 μl of Optiphase Supermix scintillation cocktail (Perkin-Elmer) was added to each well, and radioactivity was measured at 50% efficiency with a Trilux Microbeta scintillation counter (Perkin-Elmer). Blanks were determined by including 100 μM nomifensine. Cocaine inhibition was assessed by including one of the following concentrations of cocaine: 10, 30, 100, 300, 1, 3, 10, or 30 μM. Dopamine uptake was calculated as pmol/mg protein/5 min. Maximal specific dopamine uptake was measured for each sample, and IC50 values for cocaine inhibition were calculated for each curve using the following equation: Uptake at each [cocaine]=Control dopamine uptake/(1+[cocaine]/IC50).
Statistical Analyses
All CPP results were expressed as mean preference scores±SEM. Statistical analyses of all CPP studies were performed with an analysis of variance test (ANOVA) followed by a post-hoc analysis with Student–Newman–Keuls test when appropriate. p-Values of <0.05 were considered to be statistically significant. Maximal dopamine uptake and IC50 values for cocaine inhibition for WT and α6 null mutant mice were compared by t-test. All data were graphed, and statistical analyses performed using the GraphPad Prism version 5.00 (GraphPad Software; San Diego, CA).
RESULTS
Nicotine Place Preference in α6 and α4 KO Mice
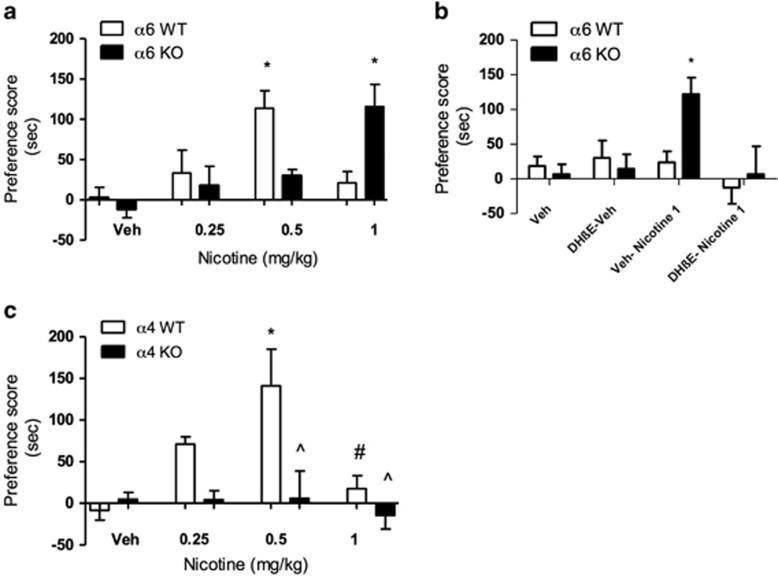
Male α6 KO mice and WT counterparts were conditioned with 0.25, 0.5, or 1 mg/kg nicotine (s.c.) for 3 days. Nicotine displayed a typical inverted U-shape curve CPP response in the WT mice (Figure 1a). Although the dose of 0.5 mg/kg nicotine (s.c) induced significant CPP in WT mice (F(7, 63)=4.803; p=0.0003), it failed to produce a CPP response in α6 KO mice. Interestingly, the higher dose of 1 mg/kg nicotine resulted in preference scores in α6 KO mice that were significantly higher than α6 WT littermates.
Figure 1.
(a) Nicotine place preference in α6 WT and KO mice. Mice were conditioned with saline or nicotine at 0.25, 0.5, or 1 mg/kg, s.c. Place preference scores for nicotine 0.5 mg/kg in α6 WT mice and nicotine 1 mg/kg in α6 KO mice were significantly greater than all other treatment and genotype groups (*p<0.05 compared with respective saline control group; *p<0.05 compared with correspondent WT or KO vehicle group. Results are expressed as mean preference scores±SEMs for 8–12 mice. (b) DHβE blockade of nicotine-induced place preference in α6 KO mice. α6 KO mice failed to show significant preference for 1 mg/kg nicotine when given a preinjection of 2 mg/kg DHβE (s.c.) (*p<0.01 compared with correspondent WT or KO vehicle group). Results are expressed as mean preference scores ±SEMs for 8–12 mice. (c) Nicotine place preference in α4 WT and KO mice. Mice were conditioned with saline or different doses of nicotine (0.25, 0.5, and 1 mg/kg, s.c.). Results are expressed as mean preference scores±SEMs for 8–12 mice. *p<0.05 compared with saline groups; #p<0.05 compared with α4 WT 0.5 mg/kg nicotine; ^p<0.1 compared with α4 WT 0.5 mg/kg nicotine.
Subsequently, we assessed the effect of DHβE, a selective β2* nAChRs antagonist, on place preference induced by 1 mg/kg nicotine in α6 KO mice to determine receptor subtype involvement (Figure 1b). A total of 2 mg/kg DHβE (s.c.) administered 5 min before 1 mg/kg nicotine injection on conditioning days resulted in a significant attenuation of nicotine place preference in α6 KO mice compared with the α6 KO group of mice that received saline pretreatment before nicotine exposure (F(7,72)=6.005; p=0.0003). Together these results suggest that higher doses of nicotine are possibly mediated by non-α6 β2*-containing nAChR subtypes.
We then investigated the possible involvement of α4 nicotinic subunits in nicotine place preference using α4 mutant mice. α4 KO and α4 WT male mice were conditioned with 0.25, 0.5, or 1 mg/kg nicotine (s.c.) (Figure 1c). The dose of 0.5 mg/kg nicotine (s.c) induced significant CPP in α4 WT mice, (F(7,45)=4.328; p=0.0014) compared with saline control and α4 KO mice. This was not due to a shift in the curve, as lower (0.25 mg/kg) and higher (1 mg/kg) doses of nicotine did not induce any place preference in α4 KO mice.
The Effect of α-Conotoxin MII [H9A; L15A] on the Acquisition of Nicotine CPP
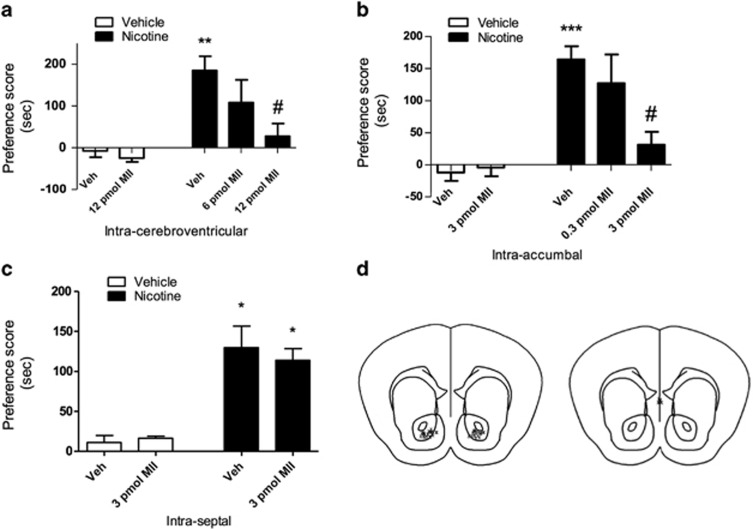
To confirm the role of α6β2* nAChRs in nicotine-conditioned reward, we evaluated the effect of α-conotoxin MII [H9A; L15A], a selective α6β2* antagonist, on the acquisition of nicotine (0.5 mg/kg) place preference (Figure 2a). Mice centrally infused with α-conotoxin MII [H9A; L15A] (i.c.v.) exhibited an attenuation of place preference scores for nicotine in a dose-related manner. More specifically, the group that received 12 pmol α-conotoxin MII [H9A; L15A] (i.c.v.) had significantly lower nicotine preference compared with the nicotine group that received only saline infusions (i.c.v.) (F(4, 27)=7.526; p=0.0010). These results suggest a critical role for brain α6β2* nAChRs in the acquisition of nicotine preference.
Figure 2.
(a) Unilateral infusions of α-conotoxin MII [H9A; L15A] into the lateral ventricle on nicotine conditioning days resulted in a dose-dependent decrease in the acquisition of nicotine place preference. The saline–nicotine 0.5 mg/kg group had significantly higher place preference for nicotine compared with the saline control groups (**p<0.01 compared with the saline groups) and compared with the 12-pmol α-conotoxin MII-nicotine 0.5 mg/kg group (#p<0.001 compared with saline–nicotine 0.5 mg/kg group). Results are expressed as mean preference scores±SEMs for 10–12 mice. (b) Intra-accumbal infusions of α-conotoxin MII [H9A; L15A] on nicotine conditioning days resulted in dose-dependent decrease in the acquisition of nicotine place preference. The saline–nicotine 0.5 mg/kg group had a significantly high place preference scores for nicotine compared with the 3-pmol α-conotoxin MII-nicotine 0.5 mg/kg group (*p<0.001 compared with saline groups, and #p<0.01 compared with the saline–nicotine 0.5 mg/kg group). Results are expressed as mean preference scores±SEMs for 10–12 mice. (c) Intra-septal infusions of α-conotoxin MII [H9A; L15A] had no effect on the acquisition of nicotine place preference. Both the saline–nicotine 0.5 mg/kg and 3-pmol α-conotoxin MII–nicotine 0.5 mg/kg groups had significant place preference scores for nicotine (*p<0.0001 compared with the saline groups). (d) Schematic of injection sites. Intra-accumbal and intra-septal microinjection sites are illustrated (left panel, NAc; right panel, Septum).
Subsequently, we examined the effect of intra-accumbal α-conotoxin MII [H9A; L15A] infusions (0.3 and 3 pmol) on the acquisition of nicotine (0.5 mg/kg) place preference (Figure 2b). The nicotine group that received intra-accumbal saline infusions showed significant place preference compared with the nicotine group that received intra-accumbal infusions of 3 pmol α-conotoxin MII [H9A; L15A] (F(4, 35)=7.38; p=0.0003), which had a significantly lower place preference scores. Although we have not directly measured the spread of the drug in our experiment, Figure 2d suggest that infusions of α-conotoxin MII [H9A; L15A] were local to the NAc and did not diffuse to nearby structures. Guide cannula further prevented α-CTX MII from traveling up the infusion cannula into the overlying brain structures. Furthermore, this is supported by earlier work and the properties of the antagonist/ligand itself. α-Conotoxin MII [H9A; L15A] is a peptide rather than a small molecule. Peptides are large, sticky molecules that exhibit limited diffusion characteristics in the brain and are also rapidly degraded. Importantly, the diffusion characteristics of an iodinated and similar conotoxin peptide that blocks α6β2* and α4β2* has already been performed in the brain. In these studies, Brunzell et al (2010) found that diffusion of 1 μl peptide was restricted to the rat medial nucleus accumbens shell. In our studies, 0.5 μl was micoinjected into the nucleus accumbens. Taken together, these data suggest that peptide diffusion out of the nucleus accumbens was minimal. These results suggest that NAc α6β2* nAChRs are important for the acquisition of nicotine place preference.
To assess specificity of NAc α6β2* blockade, we performed a neuroanatomical control by investigating the effect of intra-septal α-conotoxin MII [H9A; L15A] infusions on nicotine place preference as these infusions were of equal distance from the ventricles as the intra-accumbal infusions. As shown in Figure 2c, we observed no effect of intra-septal infusions of α-conotoxin MII [H9A; L15A] on nicotine-induced place preference (Figure 2c), as mice receiving either 3 pmol α-conotoxin MII [H9A; L15A] or saline infusions into the septum during conditioning days for nicotine displayed significant place preference for nicotine (F(3,19)=15.45; p<0.0001).
Cocaine Place Preference in α6 KO and α4 KO Mice
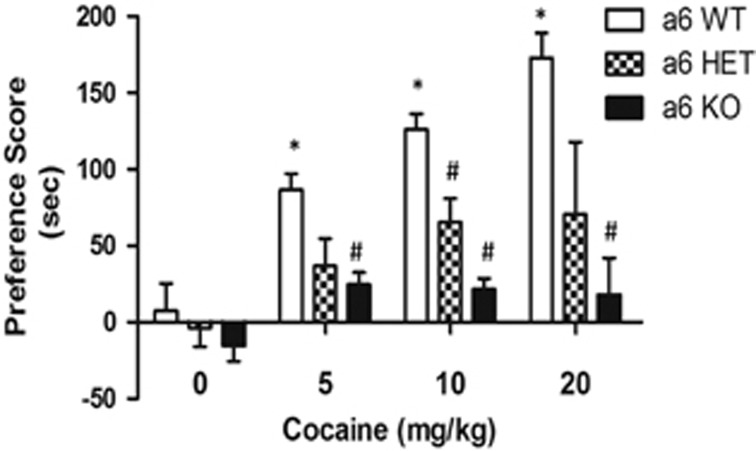
Previous work has shown that β2* nAChRs are involved in cocaine place preference (Zachariou et al, 2001). As the β2 subunit is often co-expressed with α6 and α4 subunits, we examined the involvement of α6* and α4* nAChRs in cocaine reward. To do this, we examined the capacity of 5, 10, or 20 mg/kg cocaine (i.p.) to induce CPP in α6 KO, HET, and WT male mice (Figure 3). There was a main effect of genotype (F(4, 32)=5.826; p=0.0030). Although WT mice displayed significant cocaine place preference in a dose-related manner, the effect was abolished in α6 KO littermates. The α6 HET mice also showed a decrease in cocaine preference scores but were only significantly different from the WT mice at the dose of 10 mg/kg of cocaine. These results indicate that the α6 nicotinic subunit has an important role in cocaine-induced place preference in mice. In a separate group of animals, α6 KO mice did not show a significant difference in acute cocaine-induced (10 mg/kg, i.p.) increase of locomotor activity (total number of interrupts) compared with WT mice over 60 min (WT saline=1017±109, WT cocaine=2876±190; KO saline=1198±139, KO cocaine=2886±455).
Figure 3.
α6 KO mice show significantly decreased place preference for cocaine compared with α6 WT mice. Mice were conditioned with saline or cocaine at 5, 10, and 20 mg/kg, i.p. in α6 WT, Het, and KO mice. Results are expressed as mean preference scores±SEMs for 8–12 mice. *p<0.05 compared with the saline group.
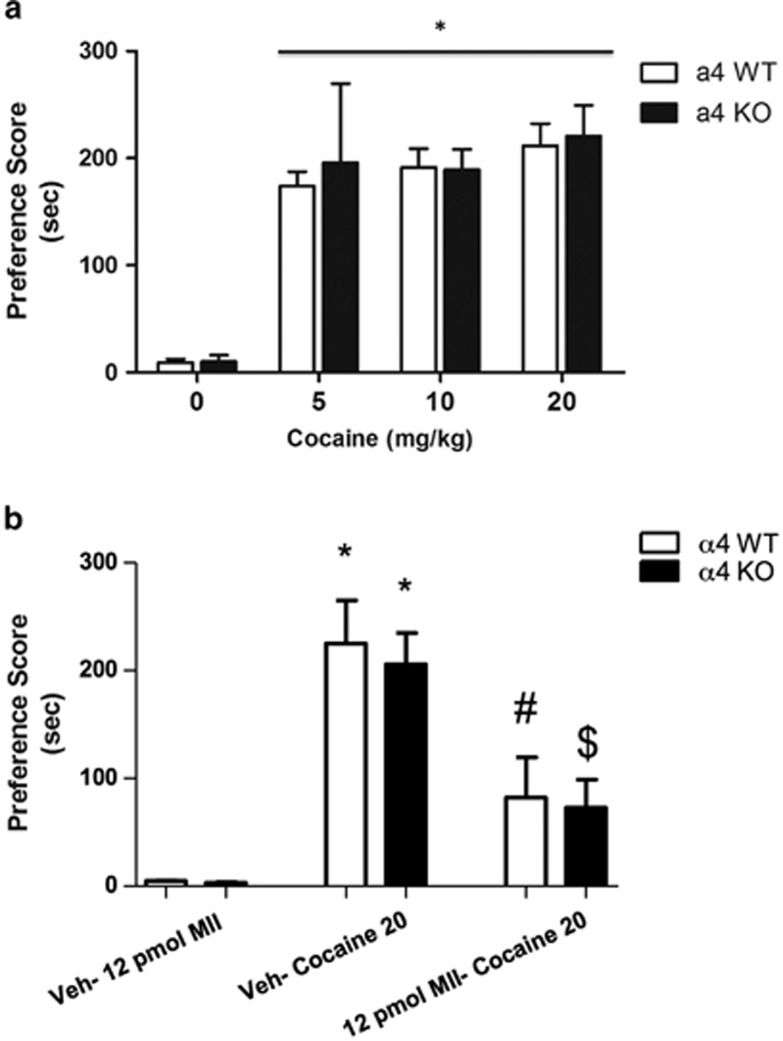
The role of α4 nicotinic subunit in cocaine CPP was investigated in the α4 KO and WT mice. These mice were conditioned with 5, 10, or 20 mg/kg cocaine (i.p.) for 3 days (Figure 4a). In contrast to nicotine-induced CPP, no genotypic effect on cocaine preference was observed; both α4 KO and WT mice displayed significant place preference for cocaine at all doses (F(9, 48)=3.04; p=0.0076). These results suggest that α4* nAChRs are not essential to cocaine place preference.
Figure 4.
(a) Cocaine place preference was induced by various doses of cocaine in α4 KO and WT mice. Mice were conditioned with saline or cocaine at 5, 10, and 20 mg/kg, i.p. Results are expressed as mean preference scores±SEMs for 8–12 mice. *p<0.05 compared with the saline groups. (b) α-Conotoxin MII [H9A; L15A] infusions into the lateral ventricle resulted in a decrease in the acquisition of cocaine place preference in α4 KO and WT mice. Both α4 KO and α4 WT saline–cocaine 20 mg/kg groups had significant place preference scores compared with the saline control groups (*p<0.05 compared with the saline groups) and compared with both the α4 KO and WT 12-pmol MII–cocaine 20 mg/kg groups (#p<0.05 compared with the α4 WT saline–cocaine 20 mg/kg group; $p<0.05 compared with the α4 KO saline–cocaine 20 mg/kg group). Results are expressed as mean preference scores±SEMs for 10–12 mice.
Next, we tested the effect of i.c.v. infusion of α-conotoxin MII [H9A; L15A] on cocaine CPP in α4 KO and WT mice to confirm the role of α6β2* nAChRs in these mice (Figure 4b). α-Conotoxin MII [H9A; L15A] (12 pmol) resulted in significantly attenuated cocaine (20 mg/kg) place preference scores in both α4 KO and WT mice (F(5,23)=6.506; p=0.0013) when compared with the nicotine groups that received saline infusions. These results confirm the role of α6β2* nAChRs in cocaine place preference and also suggests that α6β2* nAChRs are the main receptor subtypes mediating the effects of cocaine, which does not appear to require the α4 subunit.
The Effect of α-Conotoxin MII [H9A; L15A] on the Acquisition of Cocaine Place Preference
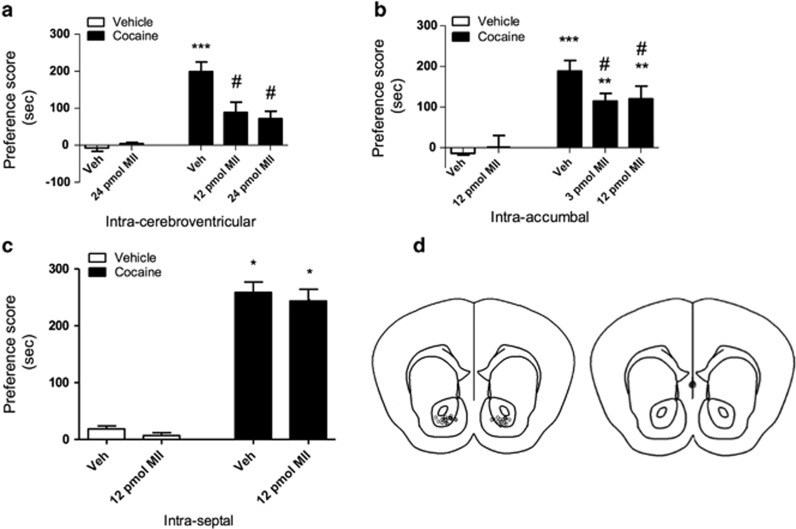
Figure 5a illustrates the effect of i.c.v. infusion of α-conotoxin MII [H9A; L15A] (12 and 24 pmol) on the acquisition of cocaine place preference. Mice that received saline infusions into the lateral ventricle followed by 20 mg/kg cocaine displayed significant place preference for cocaine on test day. On the other hand, mice that were infused with α-conotoxin MII [H9A; L15A] (i.c.v.) had significantly attenuated place preference scores on test day (F(4,35)=9.619; p<0.0001). Indeed, mice that received infusions of 12 or 24 pmol α-conotoxin MII [H9A; L15A] (i.c.v.) on conditioning days had significantly decreased acquisition of cocaine place preference compared with the cocaine group that received only saline infusions. These results suggest a critical role of α6β2* nAChRs in the reward-like effects of cocaine.
Figure 5.
(a) Unilateral infusions of α-conotoxin MII [H9A; L15A] into the lateral ventricle resulted in a decrease in the acquisition of cocaine place preference. The saline–cocaine 20 mg/kg group had significant place preference scores compared with saline control groups (***p<0.001 compared with the saline groups) and compared with the 12-pmol α-conotoxin MII-cocaine 20 mg/kg and 24-pmol α-conotoxin MII–cocaine 20 mg/kg groups (#p<0.01 compared with the saline–cocaine 20 mg/kg group). Results are expressed as mean preference scores±SEMs for 10–12 mice. (b) Intra-accumbal infusions of α-conotoxin MII [H9A; L15A] resulted in a decrease in the acquisition of cocaine place preference. The saline–cocaine 20 mg/kg group had significant place preference scores compared with the 3-pmol α-conotoxin MII–cocaine 20 mg/kg and 12-pmol α-conotoxin MII–cocaine 20 mg/kg groups (***p<0.001 compared with the saline groups; **p<0.01 compared with the saline groups; #p<0.05 compared with saline–cocaine 20 mg/kg group). Results are expressed as mean preference scores±SEMs for 10–12 mice. (c) Intra-septal infusions of α-conotoxin MII [H9A; L15A] had no effect on the acquisition of cocaine place preference. Both saline–cocaine 20 mg/kg and 12-pmol α-conotoxin MII–cocaine 20 mg/kg groups demonstrated significant place preference scores (*p<0.0001 compared with the saline groups). (d) Schematic of injection sites. Intra-accumbal and intra-septal microinjection sites are illustrated (left panel, NAc; right panel, Septum).
Subsequently, α-conotoxin MII [H9A; L15A] (3 and 12 pmol) was infused into the NAc on cocaine (20 mg/kg) conditioning days to assess its effect on the acquisition of cocaine place preference (Figure 5b). The cocaine group that received intra-accumbal infusions of 3 or 12 pmol α-conotoxin MII [H9A; L15A] had significantly lower place preference scores compared with the cocaine groups that received saline infusions (F(4, 30)=9.14; p<0.0001). Although place preference scores of mice administered α-conotoxin MII [H9A; L15A] were significantly lower than the cocaine group that received saline, these scores were also significantly greater than the control groups (veh-veh or veh-12 pmol). Therefore, there appears to be a significant but partial reduction for cocaine preference that is mediated by α6β2* nAChRs in NAc, suggesting that α6β2* nAChRs in other brain regions or other substrates are also contributing to cocaine CPP in mice.
Finally, to confirm the specificity of the effect of α-conotoxin MII [H9A; L15A] administration in NAc, we assessed the effect of intra-septal infusions of α-conotoxin MII [H9A; L15A] (12 pmol) (Figure 5c) on cocaine (20 mg/kg) place preference. Mice that received either saline or 12 pmol α-conotoxin MII [H9A; L15A] infusions during conditioning days for cocaine displayed significant place preference scores compared with the control groups (F(3,17)=76.49; p<0.0001). These results illustrate that α-conotoxin MII [H9A; L15A] infused 0.5 mm away from the ventricles does not spread to the ventricles, confirming our observations in the NAc.
Lithium Chloride-Induced Conditioned Place Aversion in α6 KO and α6 WT Mice
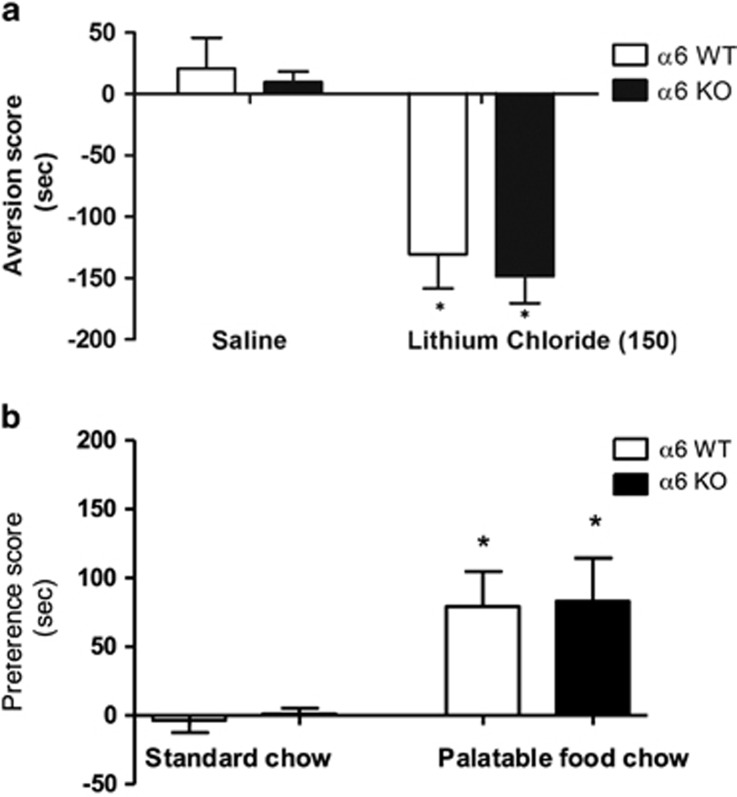
To rule out the possibility of a generalized impairment in learning or memory required for the acquisition or performance of CPP, we examined the associative process in place conditioning (memory recollection) not specific to reward, such as memory specific to aversion, with LiCl induced place aversion in α6 KO and α6 WT mice. Both α6 KO and α6 WT displayed an avoidance of the context that was associated with 150 mg/kg LiCl (F(3,35)=3.447; p=0.028) (Figure 6a). Similar results were observed with ic.v. α-conotoxin MII [H9A; L15A] (24 pmol/mouse) in the LiCl-induced CPA in WT mice (data not shown).
Figure 6.
(a) Both α6 KO and WT mice displayed conditioned place aversion induced by 150 mg/kg of LiCl (i.p.). (b) α6 KO and α6 WT displayed similar place preference scores for the context associated with the appetitive food stimulus. Results are expressed as mean preference scores±SEMs.
Food-Conditioned Preference in α6 KO and α6 WT Mice
We assessed palatable food-induced CPP in α6 KO and α6 WT mice (Figure 6b). After 4 days of conditioning, both α6 KO and α6 WT displayed similar place preference scores for the context associated with palatable food (F(3,18)=3.620; p=0.0381). Similar results were observed with ic.v. α-conotoxin MII [H9A; L15A] (24 pmol/mouse) on food-induced CPP in WT mice (data not shown).
Inhibition of Striatal [3H]Dopamine Synaptosomal Uptake by Cocaine in α6 KO and WT Mice
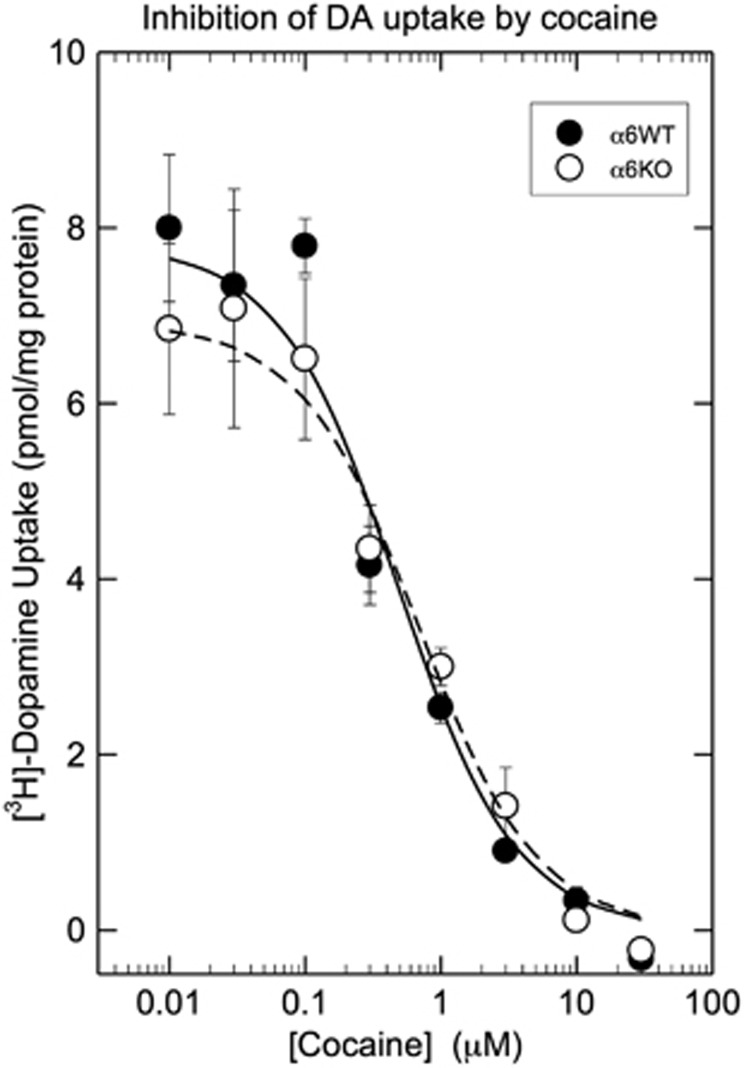
As cocaine inhibition of dopamine reuptake processes in the striatal region has a primary and an important role in reward and addiction (Kalivas, 2007), we determined the potency of cocaine at inhibiting this major cocaine target protein in native striatal synaptosomes prepared from α6 KO and WT mice. Dopamine uptake in the two genotypes was compared across saturation curves, as well as cocaine competition curves in the striatum. Comparison of total dopamine uptake between α6 KO and WT mice indicated no significant difference between the two genotypes (WT=6.84±0.94 and KO=6.32±0.70 pmol/mg/protein/5 min; t(6)=0.38, NS) (Figure 7). In addition, as seen from the concentration–response curves for cocaine inhibition of striatal [3H]dopamine synaptosomal uptake in both α6 KO and WT mice (Figure 7), little difference was observed in cocaine potency on dopamine uptake inhibition between the two genotypes. Indeed, the average IC50 values for cocaine at inhibiting uptake of [3H]dopamine uptake in α6 WT and KO mice were 0.50±0.14 and 0.69±13 μM, respectively (t(6)=0.99, NS).
Figure 7.
Cocaine competitively inhibited the uptake of [3H]-dopamine into crude synaptosomes prepared from either striata of either α6 WT or α6 KO mice. Each point represents the mean±SEM of data from four separate experiments. Curves were calculated as described in the Methods section.
DISCUSSION
Our overall results showed a critical role for α6α4β2* nAChRs in the NAc in nicotine-conditioned reward in mice. Furthermore, we provide the first evidence for an important role of α6β2* nicotinic receptor subtypes in the rewarding effects of cocaine in the mouse CPP test. These findings are consistent with the high expression of α6* nAChRs subtypes in midbrain catecholaminergic nuclei that can regulate dopamine release (Grady et al, 2002; Whiteaker et al, 2000) and mediate nicotine reward and reinforcement in rodents (Pons et al, 2008; Jackson et al, 2009, Brunzell et al, 2010; Gotti et al, 2010; Drenan et al, 2008).
α6* nAChRs Subtypes in Nicotine CPP
In CPP experiments, the magnitude of nicotine preference achieved differed between the α6 WT and KO mice following drug conditioning. Although the dose of 0.5 mg/kg nicotine induced significant preference in WT mice, it failed to produce a CPP response in α6 KO mice. These data are complementary to those observed with nicotine i.v. self-administration where α6 KO mice failed to self-administer nicotine when compared with WT littermates (Pons et al, 2008). However, the highest dose of nicotine (1 mg/kg) induced a significant preference in the α6 KO mice that was blocked by DHβE, a selective β2* nAChR antagonist. Although this residual nicotinic response could be due to issues related to the use of KO mice, the DHβE results suggest that place preference for the high dose of nicotine in α6 KO mice is mediated by β2* nAChRs not containing α6 subunits. This is consistent with the necessity of β2* nAChRs for nicotine reward and reinforcement in the CPP and self-administration procedures in rodents (Corrigall et al, 1994; Picciotto et al, 1998; Maskos et al, 2005; Pons et al, 2008; Walters et al, 2006). A similar phenotype was observed with nicotine-induced CPP at high doses in the α5 KO mouse (Jackson et al, 2010). Although α5 and α6 are co-expression in the substantia nigra and VTA, no direct evidence suggests co-assembly of these two subunits. Recent studies indicate possible functional interaction between α5 and α6 since with the genetic deletion of α5, there is an increase in α-conotoxinII-sensitive responses (dopamine release) (Salminen et al, 2007; Grady et al, 2007).
Our KO data suggest that α6β2* nAChRs mediate nicotine place preference, and this mediation was confirmed by that fact that intra-ventricular infusions of α-conotoxin MII [H9A; L15A] resulted in a dose-dependent decrease in nicotine place preference (Table 1). Furthermore, the α6β2* nAChR antagonist given into the NAc completely blocked the development of nicotine CPP. Collectively, our results showing that α6β2* nAChRs in the NAc have a critical role in nicotine-conditioned reward concur with several studies including that by Brunzell et al (2010) who observed that inhibiting α6β2* nAChRs in the NAc shell significantly reduced motivation to self-administer nicotine, and those of Exley et al (2008; 2011) who observed that α6β2* nAChRs responses dominate in the NAc. However, others studies showed that the VTA is the primary site for nicotine reinforcing effects (Pons et al, 2008, Gotti et al, 2010). Differences in the parameters under which these studies were conducted such as route of administration, self-administration procedures or species may explain the discrepancy between the results.
Table 1. Lack of Blockade of a High dose of Nicotine-Induced Place Preference in α6 KO Mice by α-Conotoxin MII [H9A; L15A].
| Treatment | α6 WT | α6 KO |
|---|---|---|
| Vehicle | 13±6 | 8±4 |
| Nicotine (1 mg/kg) | 35±8 | 155±10* |
| MII [H9A; L15A] (12 pmol) | −7±5 | 4±3 |
| MII [H9A; L15A] (12 pmol)+nicotine (1 mg/kg) | 12±6 | 146±13* |
α6 KO mice showed significant preference for 1 mg/kg nicotine when given a preinjection of 12 pmol of α-conotoxin MII [H9A; L15A] (i.c.v.) (*P<0.01 compared with the correspondent WT or KO vehicle group). Results are expressed as mean preference scores±SEMs for 8–12 mice.
Mice lacking the α4 nAChR subunit did not exhibit nicotine CPP at any of the doses tested. These results support previous reports suggesting that α4* nAChRs are necessary for nicotine reward, reinforcement, and striatal DA release (Marubio et al, 2003; Tapper et al, 2004; Salminen et al, 2007; Pons et al, 2008; Exley et al, 2011; McGranahan et al, 2011). In contrast to our results, Cahir et al, (2011) reported that α4 KO and WT mice showed similar nicotine CPP at 0.5 mg/kg. Although the two studies used the same α4 KO and WT progenitors (Ross et al, 2000) and they were back-crossed to C57BL6 mice for at least 10 generations, the C57BL6 substrain used for back-crossing in the Cahir et al (2011) study was not reported. This is an important distinction, as critical behavioral differences between the various C57BL6 substrains (in particular with C57BL6/J, the substrain used in our studies) have been reported (Mulligan et al, 2008; Mekada et al, 2009; Matsuo et al, 2010). Furthermore, the Cahir et al (2011) study used a different route of administration of nicotine (i.p. vs s.c.), performed a biased design where initial baseline preference scores were not included when calculating final preference scores on test day, and did not include a saline control.
Collectively, our results suggest a critical role for α4α6β2* nAChRs in nicotine reward using the CPP procedure. α4α6β2* nAChRs display the greatest sensitivity to nicotine (EC50=230 nM), with high affinity for nicotine and ACh binding (Salminen et al, 2007). Enhanced nicotine induced DA release in the α6 KO mice and was reduced when the α4 subunit was removed from their system, indicating that α4α6β2* nAChRs are key players in the cholinergic control of DA neurotransmission. Finally, both the α4 and the α6 subunits were necessary to maintain nicotine-sensitive cholinergic regulation of DA release in the NAc (Exley et al, 2011).
α6*-, but not α4* nAChRs, are Critical for Cocaine CPP
Using our CPP procedure, we found a genotype-dependent effect, where cocaine preference was reduced in α6 HET mice (which express half the amount of α6β2* nAChRs) but eliminated in α6 KO mice compared with α6 WT litermates. Our results with striatal [3H]dopamine synaptosomal uptake studies showed that the reduction of cocaine CPP was not due to an alteration of the dopamine transporter (DAT) functional activity in the α6 KO mice. The DAT is the primary target of cocaine. Furthermore, the involvement of α6β2* nAChRs was confirmed by the blockade of cocaine CPP with α-conotoxin MII [H9A; L15A] given i.c.v. These results are not surprising as previous studies have shown that nicotinic agonists and antagonists modulate cocaine reward, reinforcement, and sensitization (Champtiaux et al, 2006; Horger et al, 1992; Levine et al, 2011; Reid et al, 1998; Reid et al, 1999; Zachariou et al, 2001; Zanetti et al, 2007). Our results expand on a previous study implicating a role for β2 in cocaine place preference (Zachariou et al, 2001) by suggesting that α6 is the subunit co-expressed in the nicotinic subtype that is mediating the reward like effects of cocaine. Indeed, we found that both α4 KO and WT mice displayed similar and significant dose-dependent place preference scores for cocaine, suggesting that α4* nAChRs are not required for cocaine reward in the place preference test. Our conclusion is confirmed by the fact that the α6β2* nAChRs selective antagonist, α-conotoxin MII [H9A; L15A], mediated a similar decrease in cocaine place preference in both α4 KO and WT mice. Similarly, McGranahan et al (2011) reported that, while α4* nAChRs specifically on dopaminergic neurons were necessary for nicotine place preference, they were not for required for cocaine place preference.
When we targeted the NAc for inhibition by α-conotoxin MII [H9A; L15A], we observed a significant but partial reduction for cocaine preference that was mediated by α6β2* nAChRs. Our results implicating NAc α6β2* nAChRs in cocaine reward can be explained by the mechanisms underlying the reports of psychostimulants both enhancing the release of ACh in the NAc and increasing responsiveness of cholinergic neurons during acute and repeated drug exposure (Nestby et al, 1997). The partial reduction of cocaine-conditioned reward by the α6β2* antagonist suggests the involvement of other brain regions/substrates in cocaine CPP. Indeed the pedunculopontine tegmentum (PPTg) and laterodorsal tegmentum (LDTg) fibers supply heavy cholinergic input to the mesolimbic system that is robustly involved in excitation of DA neurons (Lanca et al, 2000). α4β2* nAChRs located on GABAergic terminals and DA cell bodies in midbrain and α4* and α6* nAChRs on dopaminergic terminals in midbrain neurons are all capable of responding to PPTg/LDTg-derived ACh (Calabresi et al, 1998). In the mesolimbic system, α4β2* nAChRs are expressed in cell bodies and axon terminals of midbrain and striatal DA and GABA neurons. In contrast, α6β2* nAChR expression is predominantly restricted to DA cell bodies and axon terminals and are therefore more exclusively involved in mediating DA neurotransmission when targeted in the whole system. A possible mechanism explaining our cocaine results would be that interfering with the cocaine-induced PPTg/LDTg excitation and cholinergic activation of α6β2* nAChRs on DA neurons in the mesolimbic system by inhibiting or removing the α6 subunit ultimately results in the disruption of an important neuronal signal involved in the attainment of the reward-like effect of cocaine.
Lithium-Conditioned Place Avoidance and Food Reward are not Altered by Pharmacological or Genetic Manipulations of α6* nAChRs
Our results with lithium-induced CPA show that lack of the α6 subunit decreased nicotine and cocaine place preference without having an effect on overall memory as indicated by the ability of the mice to associate the context paired with the aversive properties of lithium and recall this memory on the test day of CPP. Similarly, palatable food induced similar place preference profiles in α6 KO mice and WT littermates, suggesting that inactivation of the α6 subunit does not result in a general decrease in reward specifically pertaining to the natural incentive for food. Although unlikely, it is possible that genetic α6 ablation may have potentiated the aversive effects of nicotine and nicotine, which could explain the decrease in the CPP responses of these two drugs.
In summary, our results showed a critical role for α6β2* and α4β2* nAChR in nicotine reward, but only α6β2* were found to be required for cocaine reward-like effects in the CPP test. Given the neuroanatomical distribution of α6β2* nAChRs on catecholaminergic neurons and our behavioral assessments of this receptor subtype, targeting α6β2* nAChRs may be a valuable approach for treating nicotine and cocaine addiction.
FUNDING AND DISCLOSURE
This research was supported by the National Institute on Drug Abuse DA-019377 (to MID), P30 DA15663 (to MJM), DA003194 (to MJM) and National Institute of General Medical Sciences (GM48677 and GM103801) (to JMM). The authors declare no conflict of interest.
Acknowledgments
We greatly appreciate the technical assistance of Tie Han and Cindy Evans.
References
- Blokhina EA, Kashkin VA, Zvartau EE, Danysz W, Bespalov AY. Effects of nicotinic and NMDA receptor channel blockers on intravenous cocaine and nicotine self-administration in mice. Eur Neuropsychopharmacol. 2005;15:219–225. doi: 10.1016/j.euroneuro.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Boschen KE, Hendrick ES, Beardsley PM, Mcintosh JM. Alpha-conotoxin MII-sensitive nicotinic acetylcholine receptors in the nucleus accumbens shell regulate progressive ratio responding maintained by nicotine. Neuropsychopharmacology. 2010;35:665–673. doi: 10.1038/npp.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Hughes JR, Bickel WK. Nicotine and caffeine use in cocaine-dependent individuals. Subst Abuse. 1993;5:117–130. doi: 10.1016/0899-3289(93)90056-h. [DOI] [PubMed] [Google Scholar]
- Cahir E, Pillidge K, Drago J, Lawrence AJ. The necessity of α4* nicotinic receptors in nicotine-driven behaviors: dissociation between reinforcing and motor effects of nicotine. Neuropsychopharmacology. 2011;36:1505–1517. doi: 10.1038/npp.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Lacey MG, North RA. Nicotinic excitation of rat ventral tegmental neurones in vitro studied by intracellular recording. Br J Pharmacol. 1998;98:135–140. doi: 10.1111/j.1476-5381.1989.tb16873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Han ZY, Bessis A, Rossi FM, Zoli M, Marubio L, et al. Distribution and pharmacology of alpha 6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J Neurosci. 2002;22:1208–1217. doi: 10.1523/JNEUROSCI.22-04-01208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Kalivas PW, Bardo MT. Contribution of dihydro-beta-erythroidine sensitive nicotinic acetylcholine receptors in the ventral tegmental area to cocaine-induced behavioral sensitization in rats. Behav Brain Res. 2006;168:120–126. doi: 10.1016/j.bbr.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- Drenan RM, Grady SR, Whiteaker P, McClure-Begley T, McKinney S, Miwa JM, et al. In vivo activation of midbrain dopamine neurons via sensitized, high-affinity α6 nicotinic acetylcholine receptors. Neuron. 2008;60:123–136. doi: 10.1016/j.neuron.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenan RM, Grady SR, Steele AD, Mckinney S, Patzlaff NE, Mcintosh JM, et al. Cholinergic modulation of locomotion and striatal dopamine release is mediated by alpha6alpha4* nicotinic acetylcholine receptors. J Neurosci. 2010;30:9877–9889. doi: 10.1523/JNEUROSCI.2056-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, Mcintosh JM, Cragg SJ, Exley R, et al. Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology. 2008;33:2158–2166. doi: 10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- Exley R, Maubourguet N, David V, Eddine R, Evrard A, Pons S, et al. Distinct contributions of nicotinic acetylcholine receptor subunit alpha4 and subunit alpha6 to the reinforcing effects of nicotine. Proc Natl Acad Sci USA. 2011;108:7577–7582. doi: 10.1073/pnas.1103000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiserová M, Consolo S, Krsiak M, Fiserová M, Consolo S, Krsiak M. Chronic morphine induces long-lasting changes in acetylcholine release in rat nucleus accumbens core and shell: an in vivo microdialysis study. Psychopharmacology. 1999;142:85–94. doi: 10.1007/s002130050866. [DOI] [PubMed] [Google Scholar]
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, et al. Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci. 2010;30:5311–5325. doi: 10.1523/JNEUROSCI.5095-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, et al. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol. 2007;74:1235–1246. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Murphy KL, Cao J, Marks MJ, McIntosh JM, Collins AC. Characterization of nicotinic agonist-induced [3H] dopamine release from synaptosomes prepared from four mouse brain regions. J Pharmacol Exp Ther. 2002;301:651–660. doi: 10.1124/jpet.301.2.651. [DOI] [PubMed] [Google Scholar]
- Horger B, Giles M, Schenk S, Horger B, Giles A, Schenk M, et al. Preexposure to amphetamine and nicotine predisposes rats to self-administer a low dose of cocaine. Psychopharmacology. 1992;107:271–276. doi: 10.1007/BF02245147. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Marks MJ, Vann RE, Chen X, Gamage TF, Warner JA, et al. Role of alpha5 nicotinic acetylcholinereceptors in pharmacological and behavioral effects of nicotine in mice. J Pharmacol Exp Ther. 2010;334:137–146. doi: 10.1124/jpet.110.165738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI. The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. J Pharmacol Exp Ther. 2009;331:547. doi: 10.1124/jpet.109.155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. Neurobiology of cocaine addiction: implications for new pharmacotherapy. Am J Addict. 2007;16:71–78. doi: 10.1080/10550490601184142. [DOI] [PubMed] [Google Scholar]
- Klink R, d'Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Lanca AJ, Adamson KL, Coen KM, Chow BL, Corrigall WA. The pedunculopontine tegmental nucleus and the role of cholinergic neurons in nicotine self-administration in the rat: a correlative neuroanatomical and behavioral study. Neuroscience. 2000;96:735–742. doi: 10.1016/s0306-4522(99)00607-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Mead T, Rezvani AH, Rose JE, Gallivan C, Gross R. The nicotinic antagonist mecamylamine preferentially inhibits cocaine vs. food self-administration in rats. Physiol Behav. 2000;71:565–570. doi: 10.1016/s0031-9384(00)00382-6. [DOI] [PubMed] [Google Scholar]
- Levine A, Huang Y, Drisaldi B, EAJr Griffin, Pollak DD, Xu S, et al. Molecular mechanism for a gateway drug: epigenetic changes initiated by nicotine prime gene expression by cocaine. Sci Transl Med. 2011;3:107ra109. doi: 10.1126/scitranslmed.3003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marubio LM, Gardier AM, Durier S, David D, Klink R, Arroyo-Jimenez M, et al. Effects of nicotine in the dopaminergic system of mice lacking the alpha4 subunit of neuronal nicotinic acetylcholine receptors. Eur J Neurosci. 2003;17:1329–1337. doi: 10.1046/j.1460-9568.2003.02564.x. [DOI] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux J, et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- Matsuo N, Takao K, Nakanishi K, Yamasaki N, Tanda K, Miyakawa T. Behavioral profiles of three C57BL/6 substrains. Front Behav Neurosci. 2010;4:29. doi: 10.3389/fnbeh.2010.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGranahan TM, Patzlaff NE, Grady SR, Heinemann SF, Booker TK. a4b2 nicotinic acetylcholine receptors on dopaminergic neurons mediate nicotine reward and anxiety relief. J Neurosci. 2011;31:10891–10902. doi: 10.1523/JNEUROSCI.0937-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh JM, Azam L, Staheli S, Dowell C, Lindstrom JM, Kuryatov A, et al. Analogs of alpha-conotoxin MII are selective for alpha6-containing nicotinic acetylcholine receptors. Mol Pharmacol. 2004;65:944–952. doi: 10.1124/mol.65.4.944. [DOI] [PubMed] [Google Scholar]
- Mekada K, Abe K, Murakami A, Nakamura S, Nakata H, Moriwaki K, et al. Genetic differences among C57BL/6 substrains. Exp Anim. 2009;58:141–149. doi: 10.1538/expanim.58.141. [DOI] [PubMed] [Google Scholar]
- Mulligan MK, Ponomarev I, Boehm SL, 2nd, Owen JA, Levin PS, Berman AE, et al. Alcohol trait and transcriptional genomic analysis of C57BL/6 substrains. Genes Brain Behav. 2008;7:677–689. doi: 10.1111/j.1601-183X.2008.00405.x. [DOI] [PubMed] [Google Scholar]
- Nestby P, Vanderschuren Louk JMJ, De Vries TJ, Hogenboom F, Wardeh G, Mulder AH, et al. Ethanol, like psychostimulants and morphine, causes long-lasting hyperreactivity of dopamine and acetylcholine neurons of rat nucleus accumbens: possible role in behavioural sensitization. Psychopharmacology. 1997;133:69–76. doi: 10.1007/s002130050373. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, et al. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Hall SM, Berger SP. An acute dose of nicotine enhances cue-induced cocaine craving. Drug Alcohol Depend. 1998;49:95–104. doi: 10.1016/s0376-8716(97)00144-0. [DOI] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Berger SP. A nicotine antagonist, mecamylamine, reduces cue-induced cocaine craving in cocaine-dependent subjects. Neuropsychopharmacology. 1999;20:297–307. doi: 10.1016/S0893-133X(98)00076-1. [DOI] [PubMed] [Google Scholar]
- Ross SA, Wong JY, Clifford JJ, Kinsella A, Massalas JS, Horne MK, et al. Phenotypic characterization of an alpha 4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J Neurosci. 2000;20:6431–6441. doi: 10.1523/JNEUROSCI.20-17-06431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen O, Drapeau JA, Mcintosh JM, Collins AC, Marks MJ, Grady SR. Pharmacology of alpha-conotoxin MII-sensitive subtypes of nicotinic acetylcholine receptors isolated by breeding of null mutant mice. Mol Pharmacol. 2007;71:1563–1571. doi: 10.1124/mol.106.031492. [DOI] [PubMed] [Google Scholar]
- Sees KL, Clark HW. When to begin smoking cessation in substance abusers. J Subst Abuse Treat. 1993;10:189–195. doi: 10.1016/0740-5472(93)90044-3. [DOI] [PubMed] [Google Scholar]
- Tapper AR, Mckinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, et al. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 2006;184:339–344. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- Whiteaker PM, McIntosh JM, Luo S, Collins AC, Marks MJ. 125I-alpha-conotoxin MII identifies a novel nicotinic acetylcholine receptor population in mouse brain. Mol Pharmacol. 2000;57:913–925. [PubMed] [Google Scholar]
- Zachariou V, Caldarone BJ, Weathers-Lowin A, George TP, Elsworth JD, Roth RH, et al. Nicotine receptor inactivation decreases sensitivity to cocaine. Neuropsychopharmacology. 2001;24:576–589. doi: 10.1016/S0893-133X(00)00224-4. [DOI] [PubMed] [Google Scholar]
- Zanetti L, Picciotto M, Zoli M. Differential effects of nicotinic antagonists perfused into the nucleus accumbens or the ventral tegmental area on cocaine-induced dopamine release in the nucleus accumbens of mice. Psychopharmacology. 2007;190:189–199. doi: 10.1007/s00213-006-0598-6. [DOI] [PubMed] [Google Scholar]
- Zoli M, Moretti M, Zanardi A, Mcintos JM, Clementi F, Gotti C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci. 2002;22:8785–8789. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]