Abstract
Drugs of abuse have detrimental effects on homeostatic synaptic plasticity in the motivational brain network. Bidirectional plasticity at excitatory synapses helps keep neural circuits within a functional range to allow for behavioral flexibility. Therefore, impaired bidirectional plasticity of excitatory synapses may contribute to the behavioral hallmarks of addiction, yet this relationship remains unclear. Here we tracked excitatory synaptic strength in the oval bed nucleus of the stria terminalis (ovBNST) using whole-cell voltage-clamp recordings in brain slices from rats self-administering sucrose or cocaine. In the cocaine group, we measured both a persistent increase in AMPA to NMDA ratio (A:N) and slow decay time of NMDA currents throughout the self-administration period and after withdrawal from cocaine. In contrast, the sucrose group exhibited an early increase in A:N ratios (acquisition) that returned toward baseline values with continued self-administration (maintenance) and after withdrawal. The sucrose rats also displayed a decrease in NMDA current decay time with continued self-administration (maintenance), which normalized after withdrawal. Cocaine self-administering rats exhibited impairment in NMDA-dependent long-term depression (LTD) that could be rescued by GluN2B-containing NMDA receptor blockade. Sucrose self-administering rats demonstrated no impairment in NMDA-dependent LTD. During the maintenance period of self-administration, in vivo (daily intraperitoneally for 5 days) pharmacologic blockade of GluN2B-containing NMDA receptors did not reduce lever pressing for cocaine. However, in vivo GluN2B blockade did normalize A:N ratios in cocaine self-administrating rats, and dissociated the magnitude of ovBNST A:N ratios from drug-seeking behavior after protracted withdrawal. Altogether, our data demonstrate when and how bidirectional plasticity at ovBNST excitatory synapses becomes dysfunctional with cocaine self-administration and that NMDA-mediated potentiation of AMPA receptors in this region may be part of the neural circuits of drug relapse.
INTRODUCTION
Drugs of abuse cause long-lasting adaptations at excitatory synapses and contribute to the maladaptive behaviors that characterize addiction (Kauer and Malenka, 2007). Phenomena such as enhanced potentiation (LTP) and impaired depression or depotentiation (LTD) of excitatory synapses have been observed following reward-driven behaviors throughout motivational brain circuits (Chen et al, 2008; Dumont et al, 2005; Martin et al, 2006). Compared with natural rewards (ie, sucrose), the synaptic changes observed with drug rewards (ie, cocaine) are often exaggerated (Dumont et al, 2005), persistent, and resistant to behavioral extinction (Chen et al, 2008). This reduction in synaptic plasticity parallels the loss of behavioral flexibility observed with substance abuse (Kasanetz et al, 2010).
The NMDA receptor (NMDAR) seems critical in controlling both the drug-dependent changes in synaptic strength (Borgland et al, 2004; Liu et al, 2005; Ungless et al, 2001; Zweifel et al, 2008) and the expression of drug-induced behaviors (Kalivas and Alesdatter, 1993; Kauer, 2004; Schenk et al, 1993). Subunit composition (eg, GluN2A vs GluN2B) affects the neurophysiologic properties of NMDARs including their kinetics and intracellular protein–protein interactions, significantly influencing bidirectional plasticity of excitatory synapses (Yashiro and Philpot, 2008). Recent data suggest that deregulation of NMDAR subunit composition may contribute to impaired plasticity of excitatory synapses in the drug-exposed brain (Shen and Kalivas, 2013; Wills et al, 2012).
The capacity of synapses to undergo potentiation and depression/depotentiation depends on both the stimulus applied and previous synaptic activity or alterations (Abraham, 2008; Abraham and Bear, 1996). The ratio of GluN2A/GluN2B influences the threshold of postsynaptic activity that dictates the direction of synaptic plasticity; thus, anything that alters this ratio will affect the subsequent fate of the synapses (Liu et al, 2004; Morishita et al, 2007; Philpot et al, 2007). Under this model, a potentiating episode (LTP) would result in a greater GluN2A/2B ratio, raising the threshold of postsynaptic activity required for further potentiation, biasing synapses toward LTD. Drugs of abuse may impair this mechanism of homeostatic plasticity at excitatory synapses, yet the temporal changes in NMDAR-subunit composition throughout drug self-administration are unknown and have not been investigated for cocaine (Bellone and Luscher, 2012; Shen and Kalivas, 2013; Wills et al, 2012).
In this paper, we attempt to elucidate the cellular and behavioral significance of the GluN2B subunit in dysfunctional synaptic plasticity and cocaine self-administration. We used the rat model of intravenous cocaine self-administration to track excitatory synaptic strength temporally, from acquisition of the instrumental operant behavior, through the maintenance of the behavior, following a protracted withdrawal period and upon relapse to drug seeking behavior. In parallel, we compared excitatory synaptic strength at similar time-points in rats that self-administered sucrose orally (pellets). We investigated synaptic plasticity of excitatory synapses in the BNST, a brain region intrinsic to the motivation brain circuits and critically involved in self-administration and relapse to drug-seeking behaviors (Erb and Stewart, 1999; Jennings et al, 2013a, 2013b; Krawczyk et al, 2013; Leri et al, 2002). Our previous work demonstrated enhancement of synaptic strength in the BNST, but the exact time course, role, and underlying mechanisms are still unknown (Dumont et al, 2005). Here, we report impaired bidirectional plasticity of oval bed nucleus of the stria terminalis (ovBNST) synapses in cocaine (not sucrose) self-administering rats that could be rescued in vitro and in vivo with GluN2B antagonists. Plasticity at ovBNST synapses positively correlated with reinstatement of cocaine seeking after a 30-day withdrawal period and in vivo GluN2B blockade disrupted this relationship.
MATERIALS AND METHODS
Animals
One hundred and two male Long-Evans rats (Charles River Laboratories) weighing 250–300 g were included in the study. The rats were maintained on a 12 h reversed light–dark cycle (0900 hours lights off–2100 hours lights on) and all behavioral testing occurred during the dark cycle. The rats were allowed to acclimatize for a minimum of 7 days upon arrival to the facility. Rat chow and water were provided ad libitum in the home cages. All the experiments were conducted in accordance with the Canadian Council on Animal Care guidelines for use of animals in experiments and approved by the Queen's University Animal Care Committee. Forty-six rats were implanted with indwelling catheters for intravenous cocaine administration we included in the study.
Surgeries
Rats were weighted and anesthetized with isoflurane (2–3%, 3–5 l/min). We used manufactured indwelling catheters for intrajugular cannulations (Model IVSA28; Camcath). The end of the tubing was inserted 32 mm into the right jugular vein, toward the right atria, and tied with 4.0 suturing silks. The rest of the tubing was fed subcutaneously to a back-mounted 28 G cannula. All incisions were closed with 4-0 absorbable suturing silk. Upon surgery completion and recovery from anesthesia, rats were returned to the colony room. The rats received Metacam (5 mg/kg) injections, subcutaneously, for 3 days postoperatively and also received fruits to supplement normal chow diet during recovery. Intravenous cannulas were flushed daily with a sterile-heparin saline solution (20 IU heparin per ml) to prevent clots and conserve patency.
Acquisition of Instrumental Operant Behaviors
Behavioral testing for sucrose or cocaine self-administration was conducted in operant chambers, each equipped with a house light, a response lever with a secondary reinforcement cue light, and a food dispenser for sucrose pellet reinforcement (Med Associates). Rats were placed in the operant chambers for daily 4 h sessions. Rats learned sucrose- or cocaine-reinforced operant responding on a fixed ratio-1 (FR-1) schedule where each lever press illuminated the cue light and delivered the reward, either one sucrose pellet (75 mg) or a cocaine–HCl infusion (0.75 mg/kg in 0.12 ml of sterile saline over 4 s). Upon each reward delivery, the lever was retracted for 20 s, during which the cue light remained illuminated; no additional responses could occur during this time-out period. Training was considered acquired when the rats responded 25 times, in a titrated manner (infusions or pellet delivery at regular time intervals), for 3 consecutive days. Sucrose and cocaine trainings were equivalent as daily training sessions were terminated after the delivery of 25 rewards. After meeting the criteria for acquisition, 19 rats were killed 20 h following their last self-administration session for brain slice electrophysiology.
Maintenance of Instrumental Operant Behaviors
Seventy-nine rats were promoted to the maintenance of sucrose or cocaine self-administration after meeting the criteria for acquisition. During maintenance, the rats were switched to a progressive-ratio (PR) schedule of reinforcement for a minimum of 15 days. The PR series we used (response ratio=[5e(injection number × 0.2)]−5) starts with one lever press for the first reward delivery and escalates rapidly enough so that the rats reached break point (BP) within a 4-h daily session (Richardson and Roberts, 1996). We defined BP as the point in the series (ie, number of reinforcers) at which response ceases. We considered that BP occurred if a rat failed to receive a subsequent reward within a 1-h period. BP was the dependent variable used to quantify the PR schedule of reinforcement (Richardson and Roberts, 1996). Thirteen rats (6 sucrose, 7 cocaine) received daily (8 days) intraperitoneal treatments after 10 days on maintenance of self-administration. Intraperitoneal injections were carried out 30 min before self-administration and the rats were carefully monitored for any indications of drowsiness or motor impairment. Six rats (3 sucrose, 3 cocaine) received saline daily, while 7 rats received saline for 2 days and then the GluN2B blocker Ro 04-5595 (10 mg/kg) for 6 consecutive days. Twenty hours after their last self-administration session, the rats were killed for brain slice electrophysiology.
Withdrawal from Daily Sucrose or Cocaine Self-Administration
Ten rats maintaining daily sucrose or cocaine self-administration for 15 consecutive days were withdrawn from training and remained in their home cage for 30 days. Two cocaine self-administration rats remained in their home cage for 90 days. Following this withdrawal period, the rats were killed for brain slice electrophysiology.
Test of Reinstatement of Cocaine-Seeking Behaviors
Fifteen rats maintaining cocaine self-administration for 10 consecutive days received daily (8 days) intraperitoneal treatments 30 min before the session. Seven rats received saline daily (8 days), while 8 rats received saline for 2 days and then the GluN2B blocker Ro 04-5595 (10 mg/kg) for 6 consecutive days. Afterwards, the rats where withdrawn from self-administration and remained in their home cage for 30 days. Following this incubation or withdrawal period, rats were then reintroduced in the operant boxes for a 4-h test of cocaine seeking under the PR schedule where lever pressing resulted in illumination of the cue light followed by a 20-s time-out period but no cocaine was delivered. To quantify reinstatement of cocaine seeking, we compared BP achieved on PR day 10 (last day before intraperitoneal injections) to BP on reinstatement test day. Twenty hours after the test for reinstatement, the rats were killed for brain slice electrophysiology.
Slices Preparation and Electrophysiology
The rats were deeply anesthetized with isoflurane (5% at 3–5 l/min) and their brains rapidly removed and kept in ice-cold physiologic solution containing (in mM) 126 NaCl, 2.5 KCl, 1.2 MgCl2, 6 CaCl2, 1.2 NaH2PO4, 25 NaHCO3, and 12.5 D-glucose equilibrated with 95%O2/5%CO2. The brains were cut in coronal slices (250 μm) with a vibrating microtome (Leica VT-1000) in the physiologic solution maintained at 2 °C throughout the slicing procedure. Slices containing the BNST were incubated at 34 °C for at least 60 min and transferred to a chamber that was constantly perfused (3 ml/min) with the physiologic solution maintained at 34 °C and whole-cell voltage-clamp recordings were made using glass microelectrodes (3.5 MΩ). To measure A:N ratios, the recording electrodes contained (in mM) 130 Cs+MeSO3−, 1 EGTA, 5 HEPES, 2 Mg-ATP, 0.3 GTP, and 1 P-creatine. Neurons were initially voltage clamped at −70 mV until recordings were stable and steady. Excitatory postsynaptic currents (EPSCs) were electrically evoked (0.1 Hz) using bipolar tungsten electrodes positioned in the ovBNST, 100–500 μm dorsal from the recorded neurons. Neurons were then gradually depolarized and voltage clamped at +40 mV to relieve Mg2+ block of NMDA currents and obtain composite EPSCs. After 10 min of stable baseline, AMPA currents were isolated by bath applying the NMDAR blocker amino-5-phosphonopentanoic acid (AP-5) (50 μM) for 2–5 min. NMDA currents were obtained off-line by subtracting AMPA EPSCs from the total composite EPSCs. The peak of AMPA and NMDA currents were used to calculate A:N ratios. Measurement of low-frequency stimulation (LFS)-induced long-term depression of evoked EPSCs were carried out using the same internal solution than for A:N ratio but with the following modified Krebs solution (in mM): 151 NaCl, 3 KCl, 3.1 CaCl2, 1.4 NaH2PO4, 25 NaHCO3, and 12.5 D-glucose. Neurons were voltage clamped at −70 mV and EPSCs were electrically evoked at 0.1 Hz. After 10 min minimum of stable baseline, neurons were voltage clamped at −50 mV and submitted to an LFS protocol (900 pulses, 1 Hz). The neurons were then voltage clamped back to −70 mV for post-LFS recordings. Only neurons with high-quality 35-min post-LFS recordings were included in the study. Because recordings were carried out in a low Mg2+ solution, the evoked EPSCs contained both an AMPA and NMDA components. EPSCs were thus measured at the peak of the EPSCs and the potential effects of LFS on NMDA EPSCs were measured 75 ms after the stimulation, at a time where all AMPA currents returned to baseline. All recordings were carried out in the presence of the GABAA antagonist picrotoxin (100 μM). Drugs were bath applied through the perfusion system. Data were acquired and analyzed with Axograph X running on Apple computers.
Drugs
A stock solution of D-(−)-2-AP-5 (D-AP-5100 mM) was prepared in double-distilled water. Stock solutions of Ro 04-5595 (10 mM) and ifenprodil (10 mM) for electrophysiology were prepared in DMSO (100%). All drugs were further dissolved in physiologic solutions at desired concentrations (final DMSO concentration <0.1%). Unlike Ro 25-6981 or ifenprodil, Ro 04-5595 was soluble in sterile saline up to 10 mM; we favored this GluN2B antagonist for intraperitoneal injections and for consistency of our electrophysiology assay. Cocaine–HCl was dissolved at 2.5 mg/ml in sterile saline (pH: 7.3). Drugs were obtained from R&D Systems except cocaine–HCl (Medisca).
Statistical Analyses
We used ANOVAs to compare multiple means and conducted appropriate statistical tests for multiple comparisons when ANOVAs deemed significance. To control for type I error rates across multiple comparisons, a Bonferroni correction was applied to all p-values, unless otherwise stated. Data are reported as mean±SEM. All statistical analyses were carried out with JMP 10.0 (SAS Institute).
RESULTS
Effect of Self-Administration on the Strength of ovBNST Excitatory Synapses
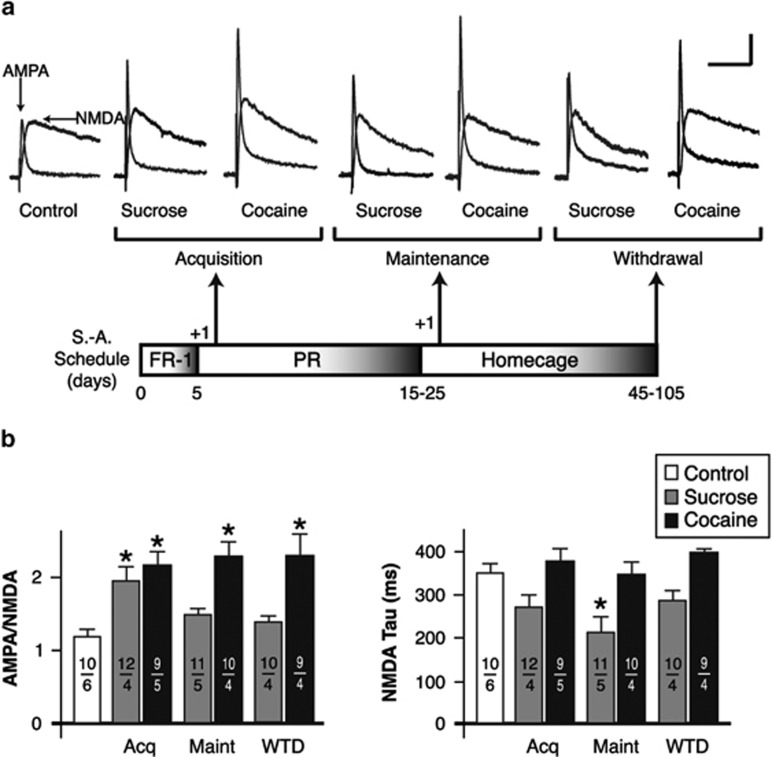
To track changes in ovBNST excitatory synaptic strength and NMDAR-mediated currents over the course of self-administration, we examined four key time-points using brain slices whole-cell voltage-clamp recordings: before operant training (control), after the operant response for sucrose or cocaine had been learned (acquisition), after continuous self-administration for 15–25 days (maintenance), and 30 days withdrawn from training (withdrawal), which followed a 15-day maintenance period. Both sucrose and cocaine self-administration exhibited increased A:N ratio in the ovBNST compared with control (one-way ANOVA, main effect of group, F(6,64)=5.9, p<0.0001; Figure 1a and b). However, in sucrose self-administering rats, the A:N ratio that had significantly increased after acquisition (p=0.04, Dunnett's method) stabilized at a slightly, but not significantly, higher value than control after 15 days of daily training (p=0.8, Dunnett's) and remained at this value after up to 30 days of withdrawal (p=0.7, Dunnett's; Figure 1a and b). In contrast, cocaine self-administration irreversibly increased A:N ratio (acquisition (p=0.003), maintenance (p=0.0003), and withdrawal (p=0.0006)). The A:N ratio remained higher than control even after 90 days of withdrawal from cocaine (A:N=1.9±0.1, n=20 neurons per 2 rats). The absolute values of NMDA were not changed by any of the conditions tested (one-way ANOVA, main effect of group, F(6,64)=2.2, p=0.06), suggesting that the increases in A:N ratio with both sucrose or cocaine self-administration resulted from increases in AMPA currents (one-way ANOVA, F(6,64)=2.7, p=0.02). Although the peak values of NMDA currents were not altered with self-administration training, their decay time did change under certain conditions (F(6,64)=7.3, p<0.0001; Figure 1b). Decay times became slightly, but not significantly, faster after acquisition (p=0.08, Dunnett's) of sucrose self-administration, but became faster with maintenance (p=0.0005, Dunnett's) and returned to baseline after withdrawal (p=0.3, Dunnett's; Figure 1b). In contrast, decay times remained completely unchanged at all time-points with cocaine self-administration (Figure 1b). Taken together, the data suggest that acquisition of operant training transiently strengthens ovBNST excitatory synapses and, with natural rewards (sucrose), further operant training leads to homeostatic adaptations and a reduction in synaptic strength. Such adaptations did not seem to occur with cocaine self-administration, resulting in enduring increases in AMPA-mediated transmission at ovBNST excitatory synapses.
Figure 1.
Effect of self-administration on the strength of oval bed nucleus of the stria terminalis (ovBNST) excitatory synapses. (a) Representative whole-cell recordings of ovBNST excitatory postsynaptic currents (EPSCs). Neurons were voltage clamped at +40 mV and the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) components were isolated pharmacologically (amino-5-phosphonopentanoic acid (AP-5), 50 μM). As depicted in the lower part of panel, recordings were obtained in brain slices prepared at three times-points and compared with those measured in age-matched untrained rats (control). Bar scale: 100 pA and 100 ms. (b) Summary of the effects of self-administration trainings on the ratio of AMPA to NMDA (left) and decay time of NMDA (right) currents. Numbers on each bar indicate the numbers of recorded neurons and rats. Asterisks indicate statistically significant difference compared with control, p<0.05.FR-1, fixed ratio-1; PR, progressive ratio; S-A, self-administration.
Effect of Self-Administration on LFS-Induced LTD of ovBNST Excitatory Synapses
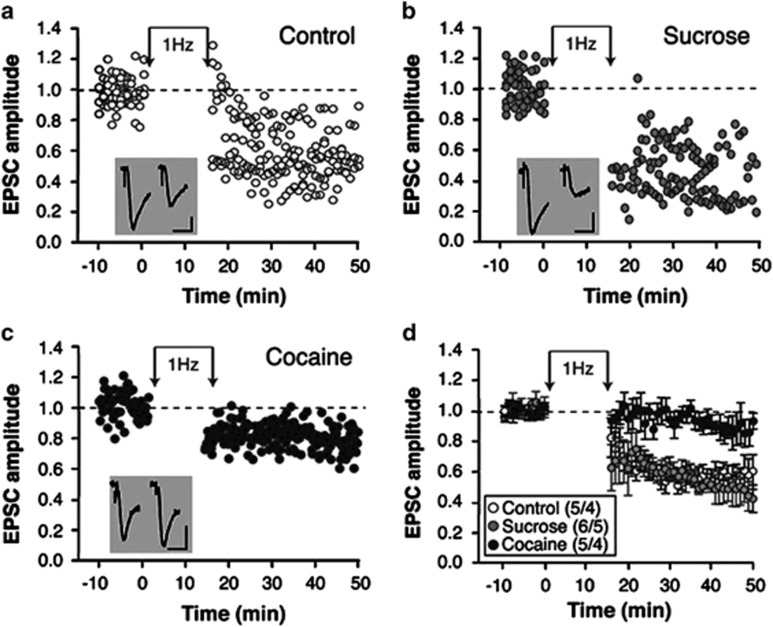
According to current hypotheses of homeostatic adaptation at excitatory synapses, synapses that underwent NMDAR-dependent potentiation shall be biased toward depression, perhaps because of NMDAR subunit substitution (Yashiro and Philpot, 2008). Therefore, we measured the ability of ovBNST synapses to undergo LTD in control conditions and after the maintenance of sucrose or cocaine self-administration. An LFS (900 pulses at 1 Hz) ovBNST synapses produced robust (42%) LTD in slices prepared from control rats (Figure 2a and d), or rats maintaining sucrose self-administration for 15 days (49% Figure 2b and d). In contrast, LFS-induced LTD was significantly reduced in slices prepared from rats that maintained cocaine self-administration (15%, two-way ANOVA with repeated measures, time × group interaction, F(11,74)=2.5, p=0.01; Figure 2c and d). A similar impairment of LTD occurs at nucleus accumbens synapses of cocaine self-administering rats (Martin et al, 2006), yet the underlying mechanism is still poorly understood. The experimental LTD we measured here was likely dependent on NMDAR activation as it required a pairing protocol (slight postsynaptic depolarization) and was more reliably observed when reducing the extracellular concentration of Mg2+ (not shown). Because NMDAR decay time increased with the maintenance of sucrose but not cocaine self-administration (Figure 1b), a reasonable assumption is that the ratio of GluN2A:2B subunit increases with sucrose but not with cocaine at ovBNST excitatory synapses. Under normal homeostatic plasticity conditions, increases in GluN2A:2B is reflected by a reduction in NMDAR decay time and shall occur following synaptic potentiation; that seemed to occur in the ovBNST of rats self-administering sucrose. In contrast, NMDAR decay time remain unchanged in the ovBNST of rats self-administering cocaine, indicative a potential deregulation of homeostatic plasticity.
Figure 2.
Effect of self-administration on low-frequency stimulation (LFS)-induced long-term depression (LTD) of oval bed nucleus of the stria terminalis (ovBNST) excitatory synapses. Representative time course of pool-evoked excitatory postsynaptic currents (EPSCs) in the ovBNST from brain slices prepared from control (a), sucrose-maintained (b), or cocaine-maintained (c) rats. Neurons were voltage clamped at −70 mV and EPSCs evoked at 0.1 Hz. During induction of LTD, neurons were voltage clamped at −50 mV for the duration of the induction protocol and EPSCs were evoked at 1 Hz for 15 min Insets show representative recordings of evoked EPSC pre- (left) and post- (right) induction in all three conditions. Bar scales: 50 pA and 10 ms. (d) Summary of the effects of LFS-induced LTD at ovBNST synapses measured at times 35–40 min and normalized to baseline. Numbers within parentheses display the number of neurons and rats.
Effect of GluN2B Blockade on LFS-Induced LTD of ovBNST Excitatory Synapses
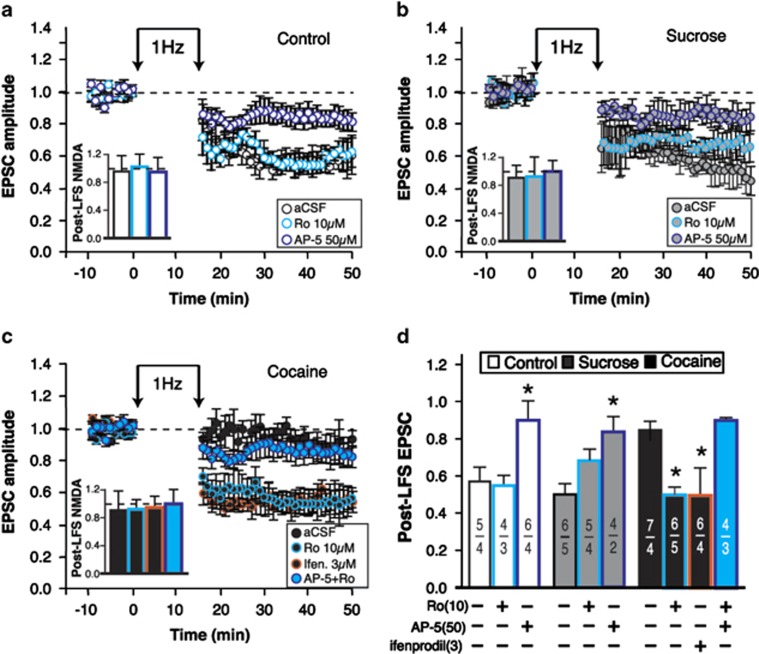
GluN2 subunit substitution is a key mechanism for the regulation of bidirectional plasticity at excitatory synapses (Liu et al, 2004; Morishita et al, 2007; Philpot et al, 2007). In certain cortical and subcortical area of the rat brain, potentiated synapses tend to increase the ratio of GluN2A:2B subunits, causing faster NMDAR-mediated decay currents and biasing synapses toward LTD (Morishita et al, 2007; Philpot et al, 2007). Here we hypothesized that the ratio of GluN2A:2B remains low at potentiated synapses in cocaine maintenance rats because decay times of NMDA currents remained slow (Figure 1b). We predicted that pharmacologic blockade of GluN2B subunits may mimic an increase in GluN2A:2B ratio and rescue LTD at ovBNST synapses of cocaine self-administering rats. We thus measured the effects of GluN2B blockade on ovBNST LTD in addition to determining whether this LTD was NMDAR-dependent, a sine qua non-condition for NMDAR subunit substitution to influence plasticity. In slices prepared from control rats, pharmacologic manipulations of NMDAR for the whole duration of the recordings (bath application) significantly influenced the magnitude of LFS-induced LTD of ovBNST synapses (one-way ANOVA, main effect of treatment, F(2,14)=24.8, p<0.0001; Figure 3a and d). Specifically, AP-5 significantly reduced LTD (p<0.0001, Dunnett's), whereas GluN2B blockade with Ro 04-5595 had no effect on LTD (p=0.7, Dunnett's; Figure 3a and d). Likewise, LTD was insensitive to GluN2B blockade (p=0.2, Dunnett's) and was totally abolished by AP-5 (p=0.006, Dunnett's) in slices prepared for the sucrose maintenance group (main effect of treatment, F(2,12)=6.7, p=0.01; Figure 3b and d). Pharmacologic blockade of NMDAR also influenced LTD in slices from cocaine maintenance rats (one-way ANOVA, main effect of treatment, F(3,19)=13.01, p<0.0001; Figure 3c and d). Specifically, GluN2B blockade with either Ro 04-5595 (p=0.001, Dunnett's) or ifenprodil (3 μM, p=0.0008, Dunnett's) rescued LTD in slices from cocaine maintenance rats (Figure 3c and d). This rescued LTD was NMDA-dependent because AP-5 completely blocked it (p=0.7, Dunnett's, nonsignificant compared with Krebs alone; Figure 3c and d). These results suggest that LFS-induced LTD of ovBNST synapses is NMDAR-dependent and that dysfunctional regulation of GluN2B-containing NMDARs impairs LTD in slices prepared from rats maintaining cocaine self-administration. The data also reveal that specific blockade of GluN2B-containing NMDAR produced very different effects than nonspecific blockade of NMDAR with a large dose of AP-5. We cannot rule out that a partial block of NMDAR with smaller concentration of AP-5 may produce similar effects than GluN2B blockers, which could suggest that a partial but nonspecific NMDAR blockade may also rescue LTD in our conditions. Nonetheless, GluN2B blockade rescued LTD in vitro and we hypothesized that GluN2B blockade in vivo may normalize plasticity of ovBNST excitatory synapses and perhaps reduce cocaine self-administration behaviors.
Figure 3.
Effect of GluN2B blockade on low-frequency stimulation (LFS)-induced long-term depression (LTD) of oval bed nucleus of the stria terminalis (ovBNST) excitatory synapses. Representative time course of average-evoked excitatory postsynaptic currents (EPSCs) in the ovBNST from brain slices prepared from control (a), sucrose-maintained (b), or cocaine-maintained (c) rats. Neurons were voltage clamped at −70 mV and EPSCs evoked at 0.1 Hz. LTD was induced at 1 Hz (15 min), while neurons were voltage clamped at −50 mV. Insets show the amplitude of N-methyl-D-aspartate receptor (NMDAR)-EPSCs (35–40 min) normalized to baseline values. (d) Summary of the effect of pharmacologic manipulations of NMDARs on LTD at ovBNST synapses measured at times 35–40 min and normalized to baseline. Numbers within parentheses display the number of neurons and rats. Asterisks indicate statistically significant difference compared with control, p<0.05.
Effect of In Vivo GluN2B Blockade on the Strength of ovBNST Excitatory Synapses and Maintenance of Self-Administration Behaviors
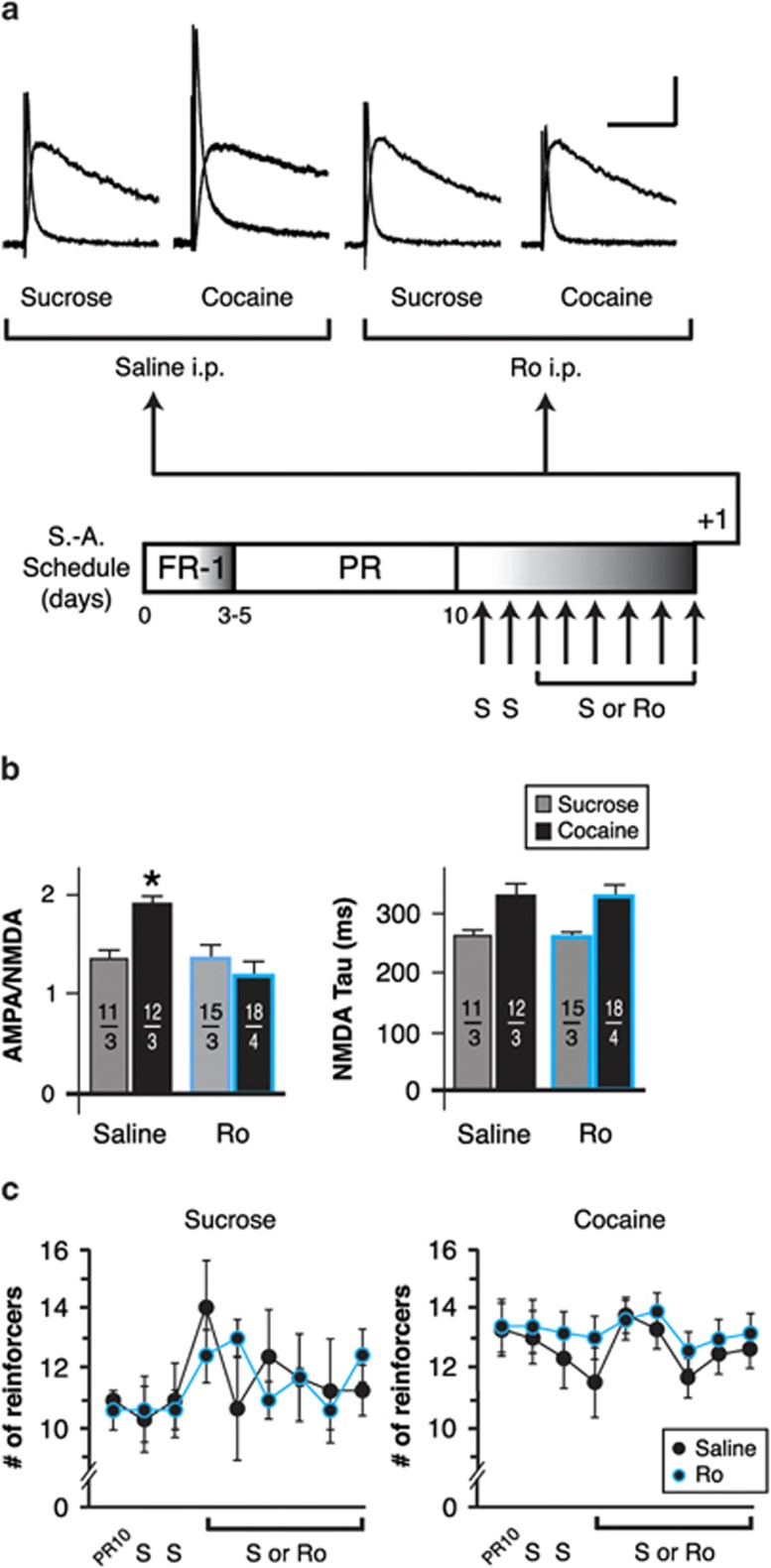
We sought to determine whether GluN2B-containing NMDAR could explain why A:N ratios remained elevated in the ovBNST of rats maintaining cocaine self-administration compared with the sucrose maintenance group (Figure 1). Rats underwent operant training acquisition followed by 10 days of maintenance. If the rats displayed stable behavior, they received intraperitoneal injections of saline 15 min before being introduced to the operant chamber on days 11 and 12. Rats were then randomly assigned to saline (8 days of total saline intraperitoneally) or GluN2B blockade (2 days of saline followed by 6 days of Ro 04-5595, 10 mg/kg, intraperitoneally; Figure 4a, bottom). The rats were killed 20 h after their last training session for brain slices recordings. In vivo blockade of GluN2B-containing NMDA receptors with Ro 04-5595 significantly reduced A:N ratios (compared with saline intraperitoneally) in cocaine but not in sucrose self-administering rats (two-way ANOVA, group × treatment interaction: F(3,52)=4.9, p=0.004; Figure 4a and b). In vivo GluN2B blockade had no effect of the kinetics of NMDA currents in either the sucrose or the cocaine maintenance group (two-way ANOVA, group × treatment interaction: F(3,52)=1.3, p=0.3; Figure 4b, right). The acute blockade of GluN2B-containing NMDAR during cocaine self-administration seemed sufficient to disrupt behavior-associated enhancement of AMPA currents, yet in vivo GluN2B blockade did not reduce lever pressing for either sucrose (two-way ANOVA with repeated-measures, group × treatment interaction: F(8,32)=0.8, p=0.5; Figure 4c, left) or cocaine (two-way ANOVA with repeated-measures, group × Treatment interaction: F(8,168)=0.4, p=0.9; Figure 4c, right). This challenges the assumption that enhanced AMPA-mediated excitatory transmission in the BNST is a major driving factor in the maintenance of cocaine self-administration (Dumont et al, 2005). However, the increases in A:N ratio measured with cocaine self-administration resisted up to 90 days of withdrawal from drug intake (Figure 1a and b), suggesting a contribution to relapse in cocaine seeking, a phenomena thought to involve the BNST (Erb et al, 2001).
Figure 4.
Effect of in vivo GluN2B blockade on the strength of oval bed nucleus of the stria terminalis (ovBNST) excitatory synapses and maintenance of self-administration behaviors. (a) Representative whole-cell recordings of ovBNST excitatory postsynaptic currents (EPSCs). Neurons were voltage clamped at +40 mV and the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) components were isolated pharmacologically (amino-5-phosphonopentanoic acid (AP-5), 50 μM). As depicted in the lower part of the panel, recordings were obtained in brain slices prepared from rats maintaining sucrose or cocaine self-administration, while receiving daily treatment with saline or the GluN2B blocker Ro 04-5595 (10 mg/kg, intraperitoneally). Bar scale: 100 pA and 100 ms. (b) Summary of the effects intraperitoneal treatments with saline or Ro on the ratio of AMPA to NMDA (left) and decay time of NMDA (right) currents. Numbers on each bar indicate the numbers of recorded neurons and rats. Asterisks indicate statistically significant difference between saline and Ro intraperitoneally, p<0.05. (c) Effect of repeated saline or Ro intraperitoneal injections on cocaine self-administration behaviors. FR-1, fixed ratio-1; PR, progressive ratio, S, saline; S-A, self-administration.
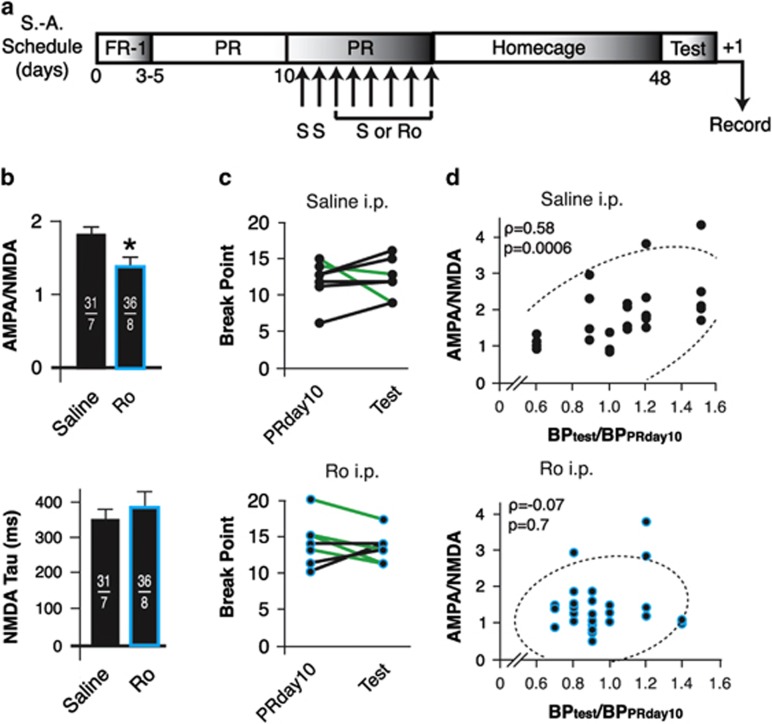
Effect of In Vivo GluN2B Blockade on the Strength of ovBNST Excitatory Synapses and Cocaine-Seeking Behavior after Protracted Withdrawal
We tested the hypothesis that potentiated ovBNST synapses may contribute in relapse to cocaine-seeking behavior after protracted withdrawal. Rats maintaining cocaine self-administration received the same intraperitoneal treatments with saline and/or Ro 04-5595 used in the previous series of experiment before being withdrawn from self-administration for 30 days (Figure 5a). We quantified reinstatement of cocaine-seeking behavior by comparing the BP reached during test for reinstatement with BP achieved by the rats on PR day 10. Rats were killed 20 h after testing for relapse for whole-cell patch clamp recordings. In vivo GluN2B blockade during the maintenance of cocaine self-administration significantly reduced A:N ratio 30 days after the last training session (two-tailed t-test, t(57)=2.1, p=0.03, saline vs Ro intraperitoneally; Figure 5b, top) This result shows that a short-term inhibition of GluN2B-mediated activity during self-administration (and perhaps for an undetermined period after training based on the pharmacokinetic of Ro) is sufficient to disrupt the mechanisms responsible for the enduring enhancement of A:N ratio in cocaine rats. Because in vivo GluN2B blockade did not normalize NMDAR kinetics (two-tailed t-test, t(58)=1.1, p=0.03; Figure 5b, bottom), the results suggest that updating processes in enhancement of A:N ratios at ovBNST synapses occur during or shortly after the daily operant session. Although interruption of this updating process with a GluN2B antagonist did not significantly alter relapse to cocaine-seeking behavior after 30 days of withdrawal, certain trends arose across individual rats. Overall, 30 days of withdrawal did not alter lever pressing either in saline- or Ro-treated rats (two-way ANOVA: F(3,26)=0.7, p=0.6; Figure 5c). When looked individually, however, four of seven (57%) saline-treated rats reached larger BP at reinstatement (ie, showed some incubation of cocaine craving) compared with BP achieved at PR day 10 (Figure 5c, top), whereas only two of eight (25%) Ro-treated rats incubated (Figure 5c, bottom). Conversely, five of eight (63%) Ro-treated rats lowered their BP at reinstatement compared with two of seven (27%) saline-treated rats. One rat in each group showed no change in BP at reinstatement. Accordingly, saline-treated rats had an 1.9 (95% CI: 0.7–5.2) risk of achieving a similar or higher BP at reinstatement than Ro-treated rats. We also measured a significant correlation between the magnitude of the ovBNST A:N ratio and reinstatement of drug-seeking behaviors in saline-treated rats (Spearman's ρ=0.6, p=0.0004; Figure 5d, top) and this relationship was reduced by in vivo GluN2B blockade, an effect most likely due to the effect of Ro on A:N ratios (Spearman's ρ=0.39, p=0.02; Figure 5d, bottom).
Figure 5.
Effect of in vivo GluN2B blockade on the strength of oval bed nucleus of the stria terminalis (ovBNST) excitatory synapses and cocaine-seeking behavior after protracted withdrawal. (a) Schematic representing the experimental procedures and time points. S, saline. (b) Summary of the effects of intraperitoneal treatments with saline or Ro on the ratio of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) to N-methyl-D-aspartate (NMDA) (top) and decay time of NMDA (bottom) currents after protracted withdrawal. Numbers on each bar indicate the numbers of recorded neurons and rats. Asterisks indicate statistically significant difference between saline and Ro intraperitoneally, p<0.05. (c) Comparison of break point (BP) reached when on the day tested for drug seeking after withdrawal (Test) and on the last day of maintenance (PRday10) before intraperitoneal injections of saline (top) or Ro 04-5595 (bottom). (d) Correlations between A:N ratio and indices of lever pressing after withdrawal from rats that received saline (top) or Ro 04-5595 (bottom) intraperitoneally. Each data point represents one recorded neuron.
DISCUSSION
In this study, we used the rat model of cocaine self-administration to track changes in excitatory synaptic strength in the ovBNST at various key time-points: from acquisition of the operant behavior to maintenance of the behavior to protracted withdrawal. By comparing results obtained from rats self-administering cocaine or a natural reward under identical behavioral regimens, we identified dysfunctional bidirectional plasticity of cocaine-exposed ovBNST excitatory synapses. Impairment in bidirectional plasticity was likely due to persistent GluN2B-containing NMDARs. Blocking GluN2B receptors in vitro rescued LFS-induced LTD of ovBNST synapses in cocaine self-administering rats. In vivo GluN2B receptor blockade normalized ovBNST synaptic strength and reduced the correlation between synaptic strength and reinstatement of cocaine-seeking behaviors after 30 days of withdrawal from cocaine intake. Despite some individual trends, plasticity of ovBNST synapses was not causally linked with the maintenance or reinstatement of cocaine self-administration and further experiments will be required to determine its exact contribution upon intake of natural or pharmacologic rewards.
Synaptic Plasticity in ovBNST and Temporal Unfolding of Sucrose or Cocaine Self-Administration Behaviors
In an earlier study, we showed an increase in A:N ratio at BNST synapses in rats self-administering cocaine daily for 10 consecutive days (Dumont et al, 2005). This effect required contingent cocaine intake because it did not occur in rats receiving cocaine passively (Yoked), a phenomena reproduced in other brain areas such as the nucleus accumbens (Martin et al, 2006). Likewise, we initially reported an increase in A:N ratio in rats trained to self-administer sucrose pellets, although the increase was only significant compared with the Yoked condition (where A:N ratios were slightly lower), but not compared with controls (Dumont et al, 2005). We clarify this observation here by adding several time-points including measures immediately following acquisition of the operant behaviors. We observed a substantial increase in A:N after acquisition of sucrose self-administration that stabilized at a slightly, but not statistically significant, higher level than control with the maintenance of the behavior or after a 1 month withdrawal period.
Taken together, the temporal unfolding of ovBNST plasticity in sucrose self-administration corroborates neural mechanisms of homeostatic regulation engaged to retain plasticity at excitatory synapses with long-term execution of an operant behavior toward a natural reward. A similar mechanism of homeostatic regulation is seen with the reorganization of synapses in limbic subcircuits in response to novel and rewarding stimuli (Groenewegen et al, 1996; Yin et al, 2006). Once the new stimuli has produced a behavioral response that is consistently and reliably associated with the desired outcome, the neural activity in the motor cortex becomes organized around task performance and synaptic flexibility returns to the limbic subcircuits (Barnes et al, 2005; Yin et al, 2006). In contrast, these potential homeostatic mechanisms seem altered with cocaine self-administration. We observed larger increases in AMPA currents that remained high with maintenance and resisted a relatively long withdrawal period. These strengthened synapses are thought to be an important neural correlate for pathologic drug-seeking behavior (Kalivas et al, 2009; Kauer and Malenka, 2007).
In vivo treatments with a GluN2B antagonist attenuated the increase in A:N ratio of ovBNST synapses, but were ineffective at concurrently reducing lever pressing during the maintenance phase of cocaine self-administration. This suggests that potentiation of ovBNST excitatory synapses is not or only minimally involved in reinforcement during the maintenance phase of cocaine self-administration. However, in vivo GluN2B blockade attenuated a rather robust correlation between the magnitude of ovBNST A:N ratios and indices of reinstatement of drug-seeking behaviors, suggesting a connection between the two parameters. However, we did not detect any significant effect of Ro on reinstatement ruling out a causal link between ovBNST plasticity and reinstatement of cocaine seeking. Accordingly, the exact role of ovBNST plasticity of excitatory synapses in reward seeking, taking, or both remains unknown. Nevertheless, we did observe some individual trends that may be related to the now well-demonstrated ‘addiction' phenotypes that can be discriminated among rat populations that are thought to correspond the human vulnerability to drug abuse (Deroche-Gamonet et al, 2004; Vanderschuren and Everitt, 2004). Although Ro did affect significantly drug-seeking after withdrawal, a majority of tested rats tended to seek cocaine less compared with rats receiving saline. Therefore, a much larger sample may confirm that GluN2B blockade effectively reduce cocaine seeking after behavior in a sub-population of rats, prone or not to an addiction phenotype. Further investigation could also determine whether ovBNST plasticity might causally link with other metrics of addiction in rats such as resistance to conditioned suppression or perseverance in drug-seeking behaviors (Deroche-Gamonet et al, 2004; Vanderschuren and Everitt, 2004).
Mechanisms of Bidirectional Plasticity: Role of NMDA Receptors Subunit Dynamics and Potential Effects of Cocaine Self-Administration
In sucrose self-administering rats, the decay time of NMDA EPSCs became significantly faster after acquisition of the behavior, mirroring the increase in A:N ratios. This observation is consistent with the mechanisms of homeostatic bidirectional regulation of plasticity at several excitatory synapses in the brain where LTP results in an adaptive increase in GluN2A:2B ratios (Philpot et al, 2007). AsGluN2A-containing NMDA currents decay faster than GluN2B, this substitution is thought to slide the postsynaptic activity threshold for LTP higher, biasing synapses toward LTD, helping circuits to remain within flexible range (Yashiro and Philpot, 2008).
In cocaine self-administering rats, the NMDA current decay times remain slow and GluN2B blockade, in vivo and in vitro, seemed to rescue impaired bidirectional plasticity. These findings may suggest that impaired homeostatic adaptations in GluN2A:2B ratio underlie the unrestrained potentiation accompanying cocaine self-administration. It is possible that cocaine interferes with synaptic insertion of GluN2A- or synaptic retention of GluN2B-containing NMDARs. Because GluN2B-containing NMDARs appear to be more mobile than GluN2A-containing ones (Groc and Choquet, 2006), it is more likely that cocaine prevents the departure of GluN2B-NMDAR from the postsynaptic density or impairs the mechanisms of lateral motility (towards peri- or extrasynaptic compartments) or internalization. Alternatively, cocaine may promote synaptic insertion of new GluN2B-NMDARs, although this possibility seems less likely given that NMDAR decay times were similar between controls and cocaine self-administering rats.
Several mechanisms may explain how cocaine could promote GluN2B synaptic retention. Cocaine may interfere with activity-dependent phosphorylation of GluN2B subunit PDZ-binding motif by casein kinase 2. This would normally allow for GluN2B subunit association with PSD scaffolding proteins and promote receptor clearance from the synapse (Chung et al, 2004; Sanz-Clemente et al, 2010). In contrast, phosphorylation of GluN2B subunits by protein kinase A, protein kinase C, c-Src tyrosine kinases, or cyclin-dependent kinase 5 promote receptor retention in the synapse (Salter and Kalia, 2004; Sanz-Clemente et al, 2013). Cocaine may also interfere with binding of the adaptor protein 2 complex to the C-terminal of GluN2B subunits, a process specific to GluN2B-containing NMDAR internalization (Groc and Choquet, 2006).
In a previous study, we showed that passive exposure to cocaine using a Yoked group of rats does not result in plastic changes at BNST excitatory synapses such as those produced by cocaine self-administration (Dumont et al, 2005). Similarly, changes in dopamine regulation of inhibitory transmission in the ovBNST resulted from cocaine self-administration but not Yoked exposure (Krawczyk et al, 2011, 2013). We did not include Yoked groups in the present study, but we predict that active drug intake would be required for ovBNST plasticity to occur at all measured time-points. Although we have not yet resolved this active vs passive intake distinction, mechanistically, obvious targets could be investigated. However, we recently showed increased c-Src intracellular signaling coupled to de novo D1 dopamine receptors in the ovBNST of cocaine self-administrating rats (Krawczyk et al, 2013). Given that c-Src tyrosine kinases promote GluN2B synaptic retention, it seems reasonable to suggest that accurately timed cue-induced dopamine release in the ovBNST could be a key event leading to dysfunctional plasticity in cocaine self-administering rats.
Role of ovBNST in Motivated Behaviors
The ovBNST is located in the dorsolateral region of the anterior to mid rostrocaudal portion of the BNST, and despite its small size, it contains 11 subtypes of GABA neurons (Larriva-Sahd, 2006), although none of these subtypes seem to have distinct neurophysiologic signatures, at least when recorded in vitro in our conditions. The ovBNST contains two distinct anatomic domains, a core and a shell, the core seemingly containing most projection neurons, whereas shell neurons are interconnected whilst sending centripetal short axon collaterals to the core region (Larriva-Sahd, 2006). Although we did observe variability in the magnitude of A:N ratios or LTD across neurons within single slices (eg, Figure 5d), we cannot determine whether plastic changes associated with our behavioral training were neuron- or domain-specific in the oval. In the past, we combined fluorescence-guided patch clamping of ventrolateral BNST neurons to identify specifically sub-populations of projecting neurons (Dumont et al, 2008; Dumont and Williams, 2004). However, the main target of the oval is within the BNST (the fusiform), making the combination tract tracing/patch clamping close to impossible, thus reducing our ability to distinguish specific sub-populations of ovBNST neurons.
Some of the variability we observed might also have resulted from the mixed excitatory inputs we recruited with our stimulating electrodes. The ovBNST receives its excitatory inputs from three main sources: the paraventricular thalamus (PVT), the dysgranular insular cortex, and anterior piriform cortex (Li and Kirouac, 2008; McDonald et al, 1999). In particular, the PVT triggers interoceptive-driven state-dependent arousal and is particularly important in locomotor and hormonal changes associated with anticipation of food reward and lesions of the PVT cause weight gains in rats (Bhatnagar and Dallman, 1999; Nakahara et al, 2004; Timofeeva and Richard, 2001). Furthermore, the insular cortex is well recognized in higher processing of interoceptive signals and has been implicated in drug craving in rats and humans (Contreras et al, 2007; Naqvi et al, 2007). Finally, the piriform cortex could carry contextual (exteroceptive) olfactory information to the ovBNST, contextualizing, for instance, the operant chamber. Altogether, the main excitatory inputs to the ovBNST are likely to contribute to motivationally driven behaviors, they could be differently altered through the processes of sucrose or cocaine self-administration, but only input-specific stimulation that can now be achieved in brain slices could resolve this issue (Britt et al, 2012).
Further supporting for a role of the ovBNST in motivational processes, the oval receives dopaminergic inputs from the ventral tegmental area, the periaqueducal gray region, and the retrorubral field and pharmacologic manipulations of dopamine receptors in this region affect sucrose, ethanol, and cocaine intake behaviors (Eiler et al, 2003; Epping-Jordan et al, 1998; Hasue and Shammah-Lagnado, 2002; Krawczyk et al, 2013; Meloni et al, 2006). Likewise, pharmacologic manipulations of the dorsolateral BNST reduce stress-induced reinstatement of cocaine seeking in rats (Erb et al, 2001; Erb and Stewart, 1999; Leri et al, 2002).
The ovBNST projects directly but mainly indirectly (through the fusiform BNST) to brain regions important in motivational processing such as the nucleus accumbens and lateral hypothalamus (Dong et al, 2001). In fact, optogenetic activation of lateral hypothalamus-projecting dorsal BNST neurons provokes voracious feeding behaviors in mice (Jennings et al, 2013a). On the other hand, the ovBNST (especially through the fusiform) sends projections to brain region more related to negative affects such as the paraventricular nucleus of the hypothalamus and the central amygdala (Dong et al, 2001) and in fact the oval and the downstream amBNST seem key in integrating aversion and motivation (Jennings et al, 2013a, 2013b,Kim et al, 2013). In corroboration, we show that excitatory inputs onto ovBNST neurons might contribute in the reinstatement of cocaine-seeking behaviors, a phenomenon thought to have both negative and positive reinforcement foundations. Nevertheless and although we observed similar and divergent neurophysiologic correlates of sucrose or cocaine intake in the ovBNST, we are still unsure of the exact role of these mechanisms in those motivated behaviors. It is possible that in both cases, the ovBNST provides a negative reinforcement signal related to deficit in either sugar or cocaine that would contribute to motivated drive toward potential sources of reward. In such case, it may be key to determine whether food deprivation, for instance, would produce neurophysiologic changes of more similar magnitude to those produced by cocaine.
Summary
Here we provide evidence of dysfunctional homeostatic NMDAR subunit substitution in the ovBNST of rats with a history of cocaine self-administration. GluN2B blockade in vitro and in vivo seem to re-establish plasticity of ovBNST synapses and disconnect it from indices of reinstatement to cocaine seeking after a withdrawal period. Our data shed more light unto how deregulated plasticity of excitatory synapses in rats with a history of cocaine intake and protracted withdrawal.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
This study was supported by the Canadian Institutes of Health Research (Research Operating Grant Nos MOP-79277 and MOP-25953 to ECD). ERH held a CIHR Fellowship (No. MFE-123712). CPN held a Master's Award: Frederick Banting and Charles Best Canada Graduate Scholarships (No. GSM-130017).
AUTHOR CONTRIBUTIONS
ÉCD contributed in all aspects of the project. JdB, ERH, CPN, and AAJ contributed in experiments, data analyses, and writing of the manuscript. CDP, EM, JGG, and SJH contributed in experiments and data analyses.
References
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–1161. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
- Bellone C, Luscher C. Drug-evoked plasticity: do addictive drugs reopen a critical period of postnatal synaptic development. Front Mol Neurosci. 2012;5:75. doi: 10.3389/fnmol.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman MF. The paraventricular nucleus of the thalamus alters rhythms in core temperature and energy balance in a state-dependent manner. Brain Res. 1999;851:66–75. doi: 10.1016/s0006-8993(99)02108-3. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, et al. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Huang YH, Lau LF, Huganir RL. Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J Neurosci. 2004;24:10248–10259. doi: 10.1523/JNEUROSCI.0546-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318:655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Mark GP, Mader S, Williams JT. Self-administration enhances excitatory synaptic transmission in the bed nucleus of the stria terminalis. Nat Neurosci. 2005;8:413–414. doi: 10.1038/nn1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Rycroft BK, Maiz J, Williams JT. Morphine produces circuit-specific neuroplasticity in the bed nucleus of the stria terminalis. Neuroscience. 2008;153:232–239. doi: 10.1016/j.neuroscience.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Williams JT. Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci. 2004;24:8198–8204. doi: 10.1523/JNEUROSCI.0425-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiler WJ, 2nd, Seyoum R, Foster KL, Mailey C, June HL. D1 dopamine receptor regulates alcohol-motivated behaviors in the bed nucleus of the stria terminalis in alcohol-preferring (P) rats. Synapse. 2003;48:45–56. doi: 10.1002/syn.10181. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Markou A, Koob GF. The dopamine D-1 receptor antagonist SCH 23390 injected into the dorsolateral bed nucleus of the stria terminalis decreased cocaine reinforcement in the rat. Brain Res. 1998;784:105–115. doi: 10.1016/s0006-8993(97)01190-6. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Choquet D. AMPA and NMDA glutamate receptor trafficking: multiple roads for reaching and leaving the synapse. Cell Tissue Res. 2006;326:423–438. doi: 10.1007/s00441-006-0254-9. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV. The nucleus accumbens: gateway for limbic structures to reach the motor system. Progr Brain Res. 1996;107:485–511. doi: 10.1016/s0079-6123(08)61883-x. [DOI] [PubMed] [Google Scholar]
- Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454:15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Rizzi G, Stamatakis AM, Ung RL, Stuber GD. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science. 2013a;341:1517–1521. doi: 10.1126/science.1241812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, et al. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013b;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Alesdatter JE. Involvement of N-methyl-D-aspartate receptor stimulation in the ventral tegmental area and amygdala in behavioral sensitization to cocaine. J Pharmacol Exp Therap. 1993;267:486–495. [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56 (Suppl 1:169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O, et al. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science. 2010;328:1709–1712. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- Kauer JA. Learning mechanisms in addiction: synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annu Rev Physiol. 2004;66:447–475. doi: 10.1146/annurev.physiol.66.032102.112534. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496:219–223. doi: 10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk M, Mason X, DeBacker J, Sharma R, Normandeau CP, Hawken ER, et al. D1 dopamine receptor-mediated LTP at GABA synapses encodes motivation to self-administer cocaine in rats. J Neurosci. 2013;33:11960–11971. doi: 10.1523/JNEUROSCI.1784-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk M, Sharma R, Mason X, Debacker J, Jones AA, Dumont EC. A switch in the neuromodulatory effects of dopamine in the oval bed nucleus of the stria terminalis associated with cocaine self-administration in rats. J Neurosci. 2011;31:8928–8935. doi: 10.1523/JNEUROSCI.0377-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larriva-Sahd J. Histological and cytological study of the bed nuclei of the stria terminalis in adult rat. II. Oval nucleus: extrinsic inputs, cell types, neuropil, and neuronal modules. J Comp Neurol. 2006;497:772–807. doi: 10.1002/cne.21011. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol. 2008;506:263–287. doi: 10.1002/cne.21502. [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, et al. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Chen BT, Hopf FW, Bowers MS, Bonci A. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat Neurosci. 2006;9:868–869. doi: 10.1038/nn1713. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann NY Acad Sci. 1999;877:309–338. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Gerety LP, Knoll AT, Cohen BM, Carlezon WA., Jr. Behavioral and anatomical interactions between dopamine and corticotropin-releasing factor in the rat. J Neurosci. 2006;26:3855–3863. doi: 10.1523/JNEUROSCI.4957-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita W, Lu W, Smith GB, Nicoll RA, Bear MF, Malenka RC. Activation of NR2B-containing NMDA receptors is not required for NMDA receptor-dependent long-term depression. Neuropharmacology. 2007;52:71–76. doi: 10.1016/j.neuropharm.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Nakahara K, Fukui K, Murakami N. Involvement of thalamic paraventricular nucleus in the anticipatory reaction under food restriction in the rat. J Vet Med Sci. 2004;66:1297–1300. doi: 10.1292/jvms.66.1297. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Cho KK, Bear MF. Obligatory role of NR2A for metaplasticity in visual cortex. Neuron. 2007;53:495–502. doi: 10.1016/j.neuron.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- Sanz-Clemente A, Matta JA, Isaac JT, Roche KW. Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron. 2010;67:984–996. doi: 10.1016/j.neuron.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Clemente A, Nicoll RA, Roche KW. Diversity in NMDA receptor composition: many regulators, many consequences. Neuroscientist. 2013;19:62–75. doi: 10.1177/1073858411435129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Valadez A, Worley CM, McNamara C. Blockade of the acquisition of cocaine self-administration by the NMDA antagonist MK-801 (dizocilpine) Behav Pharmacol. 1993;4:652–659. [PubMed] [Google Scholar]
- Shen H, Kalivas PW. Reduced LTP and LTD in prefrontal cortex synapses in the nucleus accumbens after heroin self-administration. Int J Neuropsychopharmacology. 2013;16:1165–1167. doi: 10.1017/S1461145712001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva E, Richard D. Activation of the central nervous system in obese Zucker rats during food deprivation. J Comp Neurol. 2001;441:71–89. doi: 10.1002/cne.1398. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Wills TA, Klug JR, Silberman Y, Baucum AJ, Weitlauf C, Colbran RJ, et al. GluN2B subunit deletion reveals key role in acute and chronic ethanol sensitivity of glutamate synapses in bed nucleus of the stria terminalis. Proc Natl Acad Sci USA. 2012;109:E278–E287. doi: 10.1073/pnas.1113820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Inactivation of dorsolateral striatum enhances sensitivity to changes in the action-outcome contingency in instrumental conditioning. Behav Brain Res. 2006;166:189–196. doi: 10.1016/j.bbr.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Argilli E, Bonci A, Palmiter RD. Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors. Neuron. 2008;59:486–496. doi: 10.1016/j.neuron.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]